Development of Flow Fields for Zinc Slurry Air Flow Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Air Cathode
2.2. Zinc Slurry Preparation
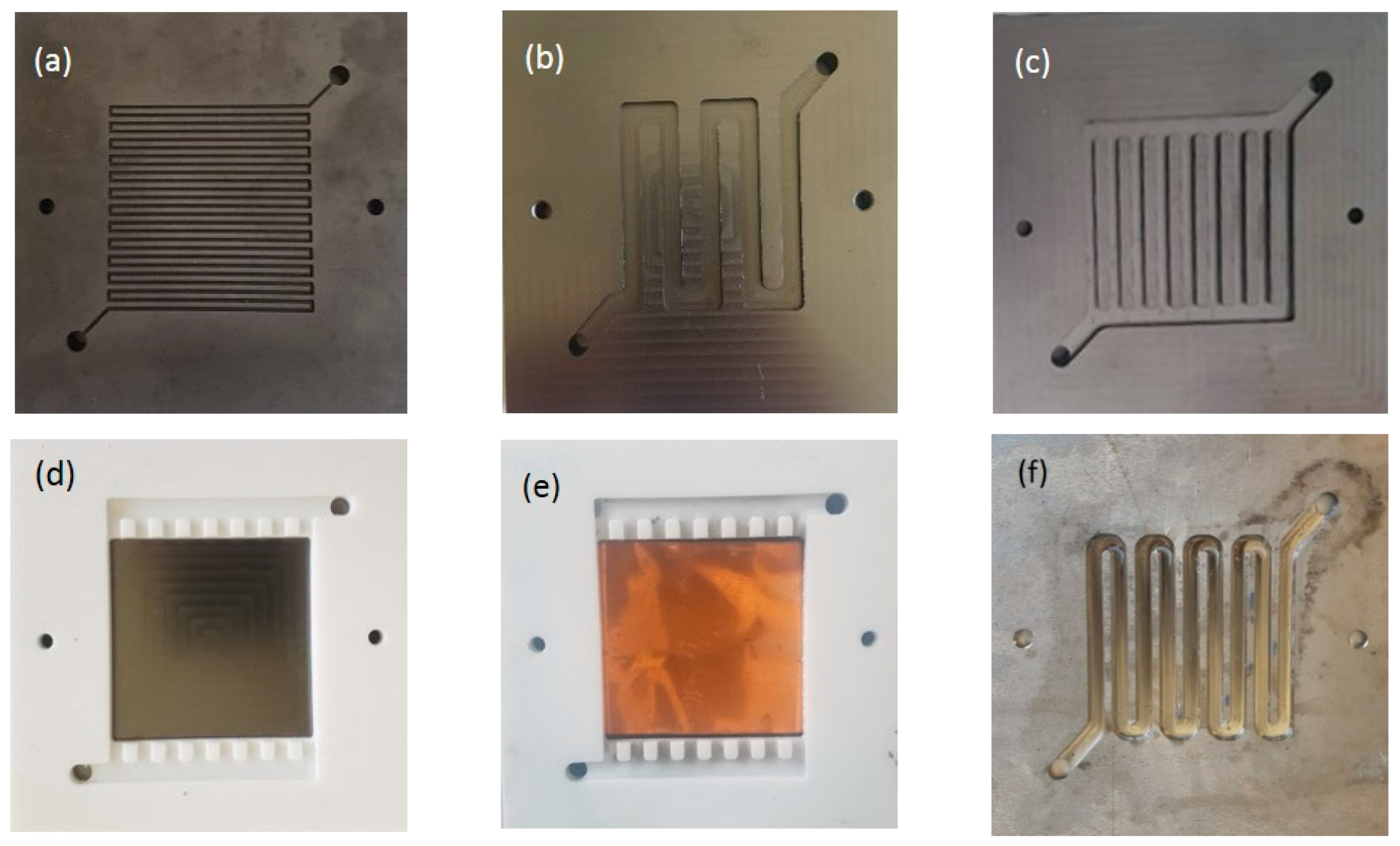
2.3. Flow Field Design
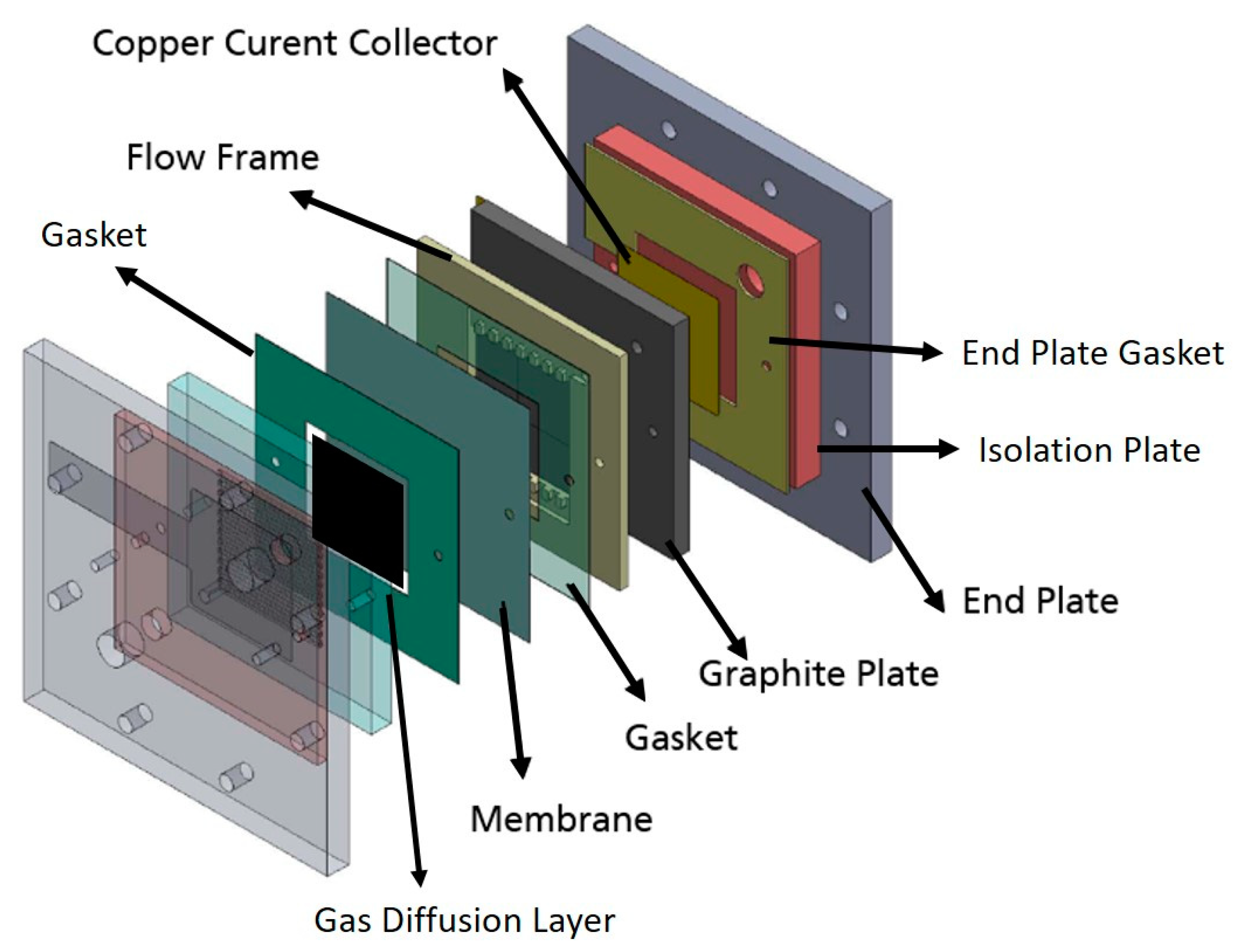
2.4. Single Cell Assembly and Electrochemical Performance
3. Results and Discussion
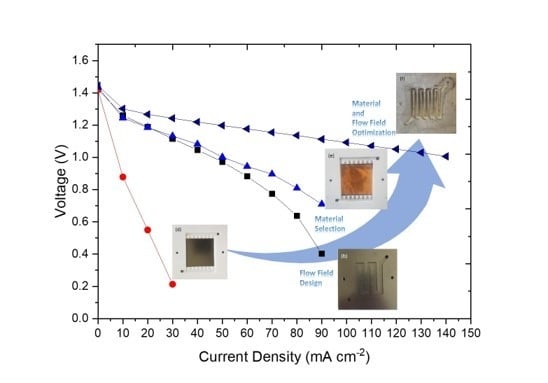
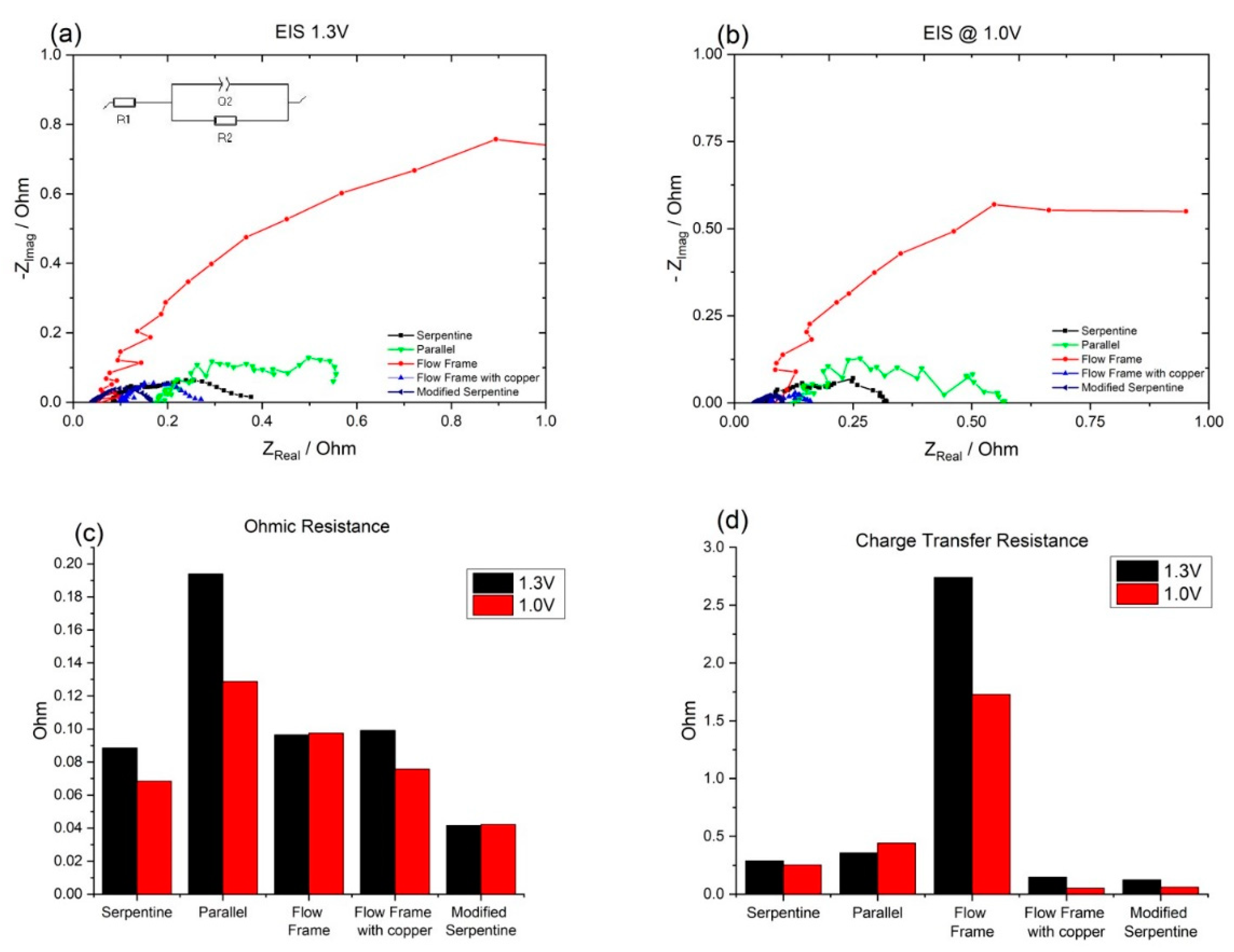
3.1. Effect of Flow Fields
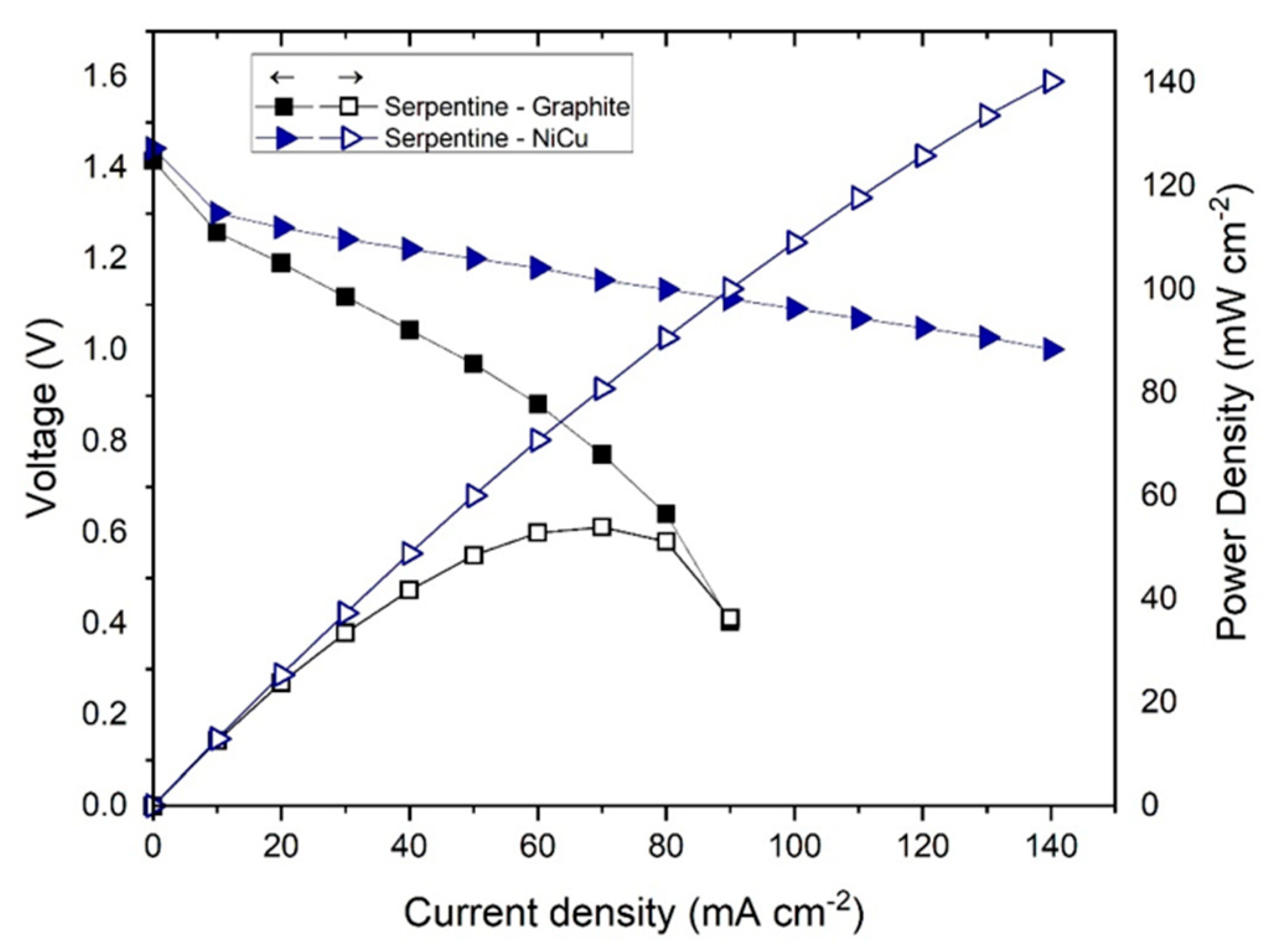
3.2. Effect of the Bipolar Plate Material
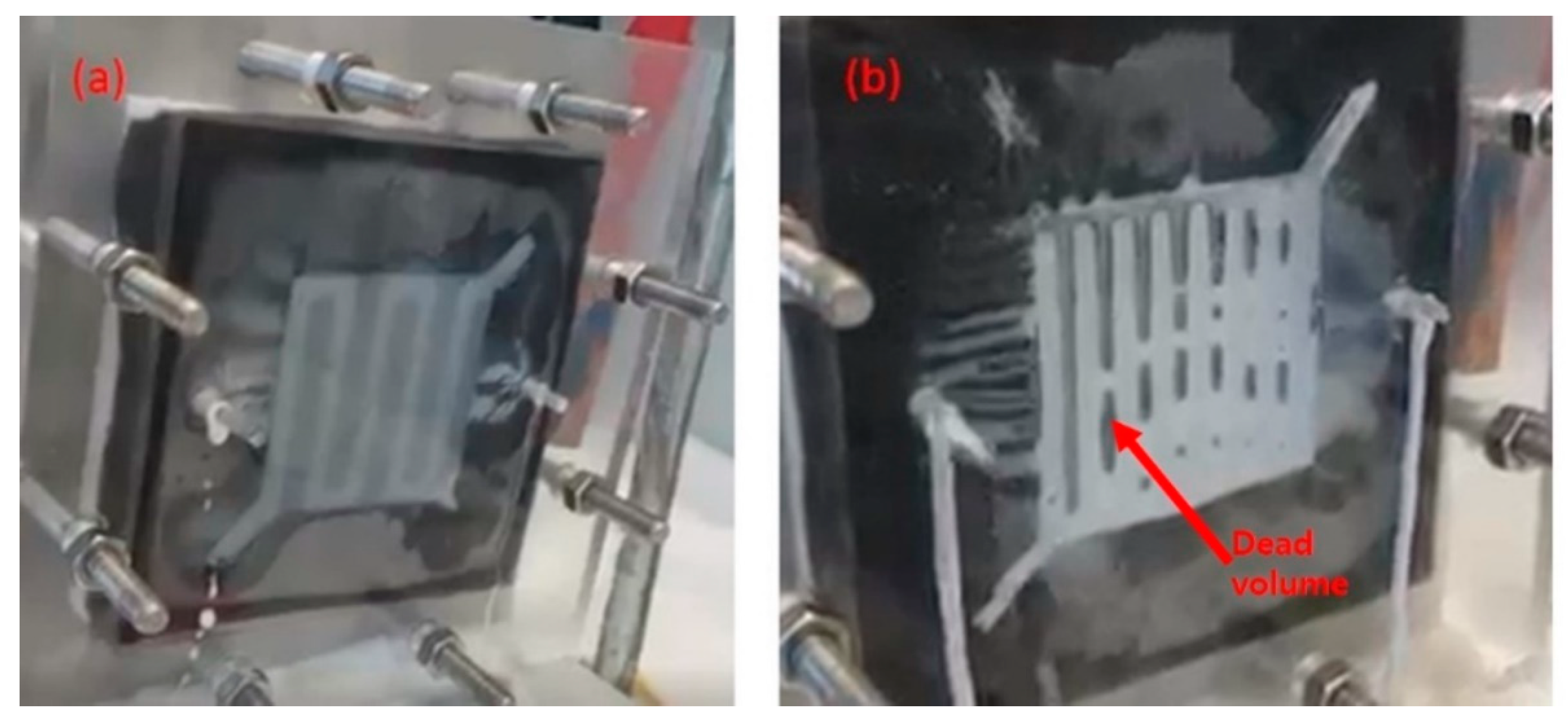
3.3. Modifying a Flow Field with a New Material
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caramia, V.; Bozzini, B. Materials science aspects of zinc-air batteries: A review. Mater. Renew. Sustain. Energy 2014, 3, 28. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Want, W.; Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2016, 2, 16080. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal-Air Batteries: From static to flow system. Adv. Energy Mater. 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Bockelmann, M.; Kunz, U.; Turek, T. Electrically rechargeable zinc-oxygen flow battery with high power density. Electrochem. Commun. 2016, 69, 24–27. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q. Redox Species of Redox Flow Batteries: A Review. Molecules 2015, 20, 20499–20517. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Blázquez, J.A. Alkaline aqueous electrolytes for secondary zinc–air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiratchayamaethasakul, C.; Srijaroenpramong, N.; Bunyangyuen, T.; Arpavate, W.; Wongyao, N.; Therdthianwong, A.; Therdthianwong, S. Effects of anode orientation and flow channel design on performance of refuelable zinc-air fuel cells. J. Appl. Electrochem. 2014, 44, 1205–1218. [Google Scholar] [CrossRef]
- McLarnon, F.R.; Cairns, E.J. The Secondary Alkaline Zinc Electrode. J. Electrochem. Soc. 1991, 138, 645. [Google Scholar] [CrossRef]
- Chakkaravarthy, C.; Waheed, A.K.A.; Udupa, H.V.K. Zinc-Air alkaline batteries—A Review. J. Power Sources 1981, 6, 203–228. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress Challenges and Perspectives. Adv. Mater. 2016, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Harting, K.; Kunz, U.; Turek, T. Zinc-air batteries: Prospects and challenges for future improvement. Z. Phys. Chem. 2012, 226, 151. [Google Scholar] [CrossRef]
- Sapkota, P.; Kim, H. Zinc air fuel cell a potential candidate for alternative energy. J. Ind. Eng. Chem. 2009, 15, 445–450. [Google Scholar] [CrossRef]
- Mainar, A.; Iruin, E.; Colmenares, L.; Blázquez, J.; Grande, H.-J. Systematic cycle life assessment of a secondary zinc–air battery as a function of the alkaline electrolyte composition. Energy Sci. Eng. 2018, 6, 174–186. [Google Scholar] [CrossRef]
- Kupsch, C.; Feierabend, L.; Nauber, R.; Büttner, L.; Czarske, J. Ultrasound flow investigations at a zinc-air flow battery model. In Proceedings of the ICEFM 2018 Munich, Munich, Germany, 2–4 July 2018. [Google Scholar]
- Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies 2014, 7, 6548–6557. [Google Scholar] [CrossRef]
- Ma, Z.; Pei, P.; Wang, K.; Wang, X.; Xu, H.; Liu, Y.; Peng, G. Degradation characteristics of air cathode in zinc air fuel cells. J. Power Sources 2015, 274, 56–64. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent Progress in Oxygen Electrocatalysts for Zinc–Air Batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Mainar, A.; Iruin, E.; Colmenares, L.; Kvasha, A.; Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Liu, S.; Han, W.; Cui, B.; Liu, X.; Sun, H.; Zhang, J.; Lefler, M.; Licht, S. Rechargeable Zinc Air Batteries and Highly Improved Performance through Potassium Hydroxide Addition to the Molten Carbonate Eutectic Electrolyte. J. Electrochem. Soc. 2018, 165, A149–A154. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.M.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. The Influence of Electrode and Channel Configuration on Flow Battery Performance. J. Electrochem. Soc. 2014, 161, A1381–A1387. [Google Scholar] [CrossRef]
- Houser, J.; Clement, J.; Pezeshki, A.; Mench, M.M. Influence of architecture and material properties on vanadium redox flow battery performance. J. Power Sources 2016, 302, 369–377. [Google Scholar] [CrossRef]
- Houser, J.; Pezeshki, A.; Clement, J.T.; Aaron, D.; Mench, M.M. Architecture for improved mass transport and system performance in redox flow batteries. J. Power Sources 2017, 351, 96–105. [Google Scholar] [CrossRef]
- Ke, X.; Alexander, J.I.D.; Prahl, J.M.; Savinell, R.F. Flow distribution and maximum current density studies in redox flow batteries with a single passage of the serpentine flow channel. J. Power Sources 2014, 270, 646. [Google Scholar] [CrossRef]
- Ke, X.; Alexander, J.I.D.; Prahl, J.M.; Savinell, R.F. Mathematical modeling of electrolyte flow in a segment of flow channel over porous electrode layered system in vanadium flow battery with flow field design. J. Electrochim. Acta 2017, 223, 124. [Google Scholar] [CrossRef]
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Wainright, J.S.; Zawodzinski, T.A.; Savinell, R.F. Rechargeable redox flow batteries: Flow fields, stacks and design considerations. Chem. Soc. Rev. 2018, 47, 8721–8743. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Leung, P.K. Numerical investigations of flow field designs for vanadium redox flow batteries. Appl. Energy 2013, 105, 47–56. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Zhang, C. Performance of a vanadium redox flow battery with and without flow field. Electrochim. Acta 2014, 142, 61–67. [Google Scholar] [CrossRef]
- Dennison, C.R.; Agar, E.; Akuzum, B.; Kumbur, E.C. Enhancing Mass Transport in Redox Flow Batteries by Tailoring Flow Field and Electrode Design. J. Electrochem. Soc. 2016, 161, A5163–A5169. [Google Scholar] [CrossRef]
- Cho, K.T.; Ridgway, P.; Haussener, W.S.; Battaglia, V.; Srinivasan, V. High performance hydrogen/bromine redox flow battery for grid-scale energy storage. J. Electrochem. Soc. 2012, 159, A1806–A1815. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Academic Press: Cambridge, MA, USA, 1963; pp. 411–496. [Google Scholar]
- Vijayaratnam, V.; Natter, H.; Grandthyll, S.; Neurohr, J.U.; Jacobs, K.; Müller, F.; Hempelmann, R. Unwanted electroless zinc plating on current collectors in zinc air batteries. arXiv 2017, arXiv:1706.05929. [Google Scholar]

| Mass Fraction (wt%) | |||
|---|---|---|---|
| Zinc | ZnO | Carbopol | KOH + Water |
| 33.8 | 4 | 0.7 | 61.5 |
| Flow Field | Geometric Area (cm2) | Area of Walls (cm2) | Corrected Geometric Area (cm2) |
|---|---|---|---|
| Serpentine | 25 | 10.5 | 35.5 |
| Parallel | 25 | 14.4 | 39.4 |
| Flow frame | 25 | 0 | 25 |
| Flow frame with copper base | 25 | 0 | 25 |
| Modified serpentine | 25 | 17.82 | 42.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.H.; del Olmo, D.; Fischer, P.; Pinkwart, K.; Tübke, J. Development of Flow Fields for Zinc Slurry Air Flow Batteries. Batteries 2020, 6, 15. https://doi.org/10.3390/batteries6010015
Choi NH, del Olmo D, Fischer P, Pinkwart K, Tübke J. Development of Flow Fields for Zinc Slurry Air Flow Batteries. Batteries. 2020; 6(1):15. https://doi.org/10.3390/batteries6010015
Chicago/Turabian StyleChoi, Nak Heon, Diego del Olmo, Peter Fischer, Karsten Pinkwart, and Jens Tübke. 2020. "Development of Flow Fields for Zinc Slurry Air Flow Batteries" Batteries 6, no. 1: 15. https://doi.org/10.3390/batteries6010015
APA StyleChoi, N. H., del Olmo, D., Fischer, P., Pinkwart, K., & Tübke, J. (2020). Development of Flow Fields for Zinc Slurry Air Flow Batteries. Batteries, 6(1), 15. https://doi.org/10.3390/batteries6010015