Design Principles and Engineering Strategies for Stabilizing Ni-Rich Layered Oxides in Lithium-Ion Batteries
Abstract
1. Introduction
2. Goals and Issues
2.1. Why Decrease the Co Concentration?
2.2. Problems Limiting the Electrochemical Performance
2.2.1. Cation Mixing
2.2.2. Phase Stability
2.2.3. Oxygen Release
2.2.4. Modifications During Cycling
2.2.5. Microcracks
3. Synthesis Methods
3.1. Single Crystals Versus Polycrystals
3.2. Synthesis of Single Crystals
4. Coating
4.1. Li-Free Surface Layers
4.2. Li-Based Surface Layers
4.3. Organic Layers
4.4. Dual Coating
5. Doping
5.1. Experimental Results
5.2. Theoretical Calculations
5.3. Morphology Aspects
5.4. Synergetic Effects of Co-Doping and-or Multiple Coating
5.4.1. Dual Doping
5.4.2. Doping Plus Coating
5.4.3. Multiple Coatings
6. Core–Shell and Gradient Structures
6.1. Core–Shell Structures
6.2. Gradient Layers
7. Cells with Ni-Rich Cathodes
7.1. Solid Electrolytes
7.2. Liquid Electrolytes
7.2.1. Additives
7.2.2. Electrolyte Formulations
7.3. Recycling Ni-Rich Batteries
7.4. Safety Issues
8. Conclusions
- The interaction between the electrolytes and the Ni-rich materials and the related CEI needs further investigation. Newly-developed experimental set-ups, such as cryogenic transmission electron microscopy revealing atomic-resolution CEI structures, should help for this purpose. An example is the dynamic evolution of over cycling and its impact on the performance of NMC811, revealed by the ultrafine images of CEI obtained by this process in presence of FEC-based electrolytes [948].
- Safety issues with high-voltage cathode materials would plead in favor of the use of solid electrolytes. As shown in this review, some attempts are promising, but the research should continue in the next years. The space charge layer between the cathode and a ceramic solid electrolyte may result in high polarization and capacity degradation. Another difficulty comes from the deterioration of the contact between the cathode and solid electrolyte upon cycling, due to the rigid ceramic nature of the solid electrolyte and the change in the lattice parameters of the cathode material, in particular at high charge. The columnar shape of the Ni-rich particles or actually any elongated form will help [949], pointing to the need for further investigation on the correlations between morphology, synthesis, and electrochemical performance Elongated polymer electrolytes have already made possible the commercialization of LiFePO4-based batteries, due to the low voltage (3.5 V) of the cells. With higher voltage cells, the same polymers cannot be used, because of their poor antioxidative ability. Further investigations on polymer electrolytes with wide electrochemical window are thus needed, and under studies [950]. Further research is needed to pair Ni-rich cathodes with lithium metal or silicon anodes. The reaction of the electrolytes with the Ni-rich cathode and the anode materials is not well understood, and further studies on the additives able to generate both a SEI and a CEI protecting the electrodes against side reactions with an electrolyte stable at high voltage are still needed.
- The application of AI to the research on Li-rich batteries is needed and most promising. It is possible today to predict material degradation pathways according to operating environments, which will help to optimize the design and composition of electrode materials for enhanced performance and durability of the Ni-rich cathode materials. Machine learning (ML) can accelerate the design of new Ni-rich cathode chemistries, and can also be used to build next-generation battery architectures. Many trials have been made to dope Ni-rich cathode materials with different single and multiple doping elements. ML models can predict dopants that improve stability without expensive trial-and-error experiments. In particular, deep learning models trained on large materials databases (e.g., Materials Project) predict stable Ni-rich structures with minimal degradation. ML will also help designing single-crystal vs. polycrystalline Ni-rich cathodes to reduce cracking. Computer vision models will be used to analyze SEM/TEM images to detect defects and suggest improvements in synthesis processes.
- Surface coating to prevent electrolyte attack, suppress transition metal (TM) dissolution, and stabilize the cathode–electrolyte interface. The benefits are the reduction of surface reactivity, minimization of HF attack, and improvement of the cycling stability. To fulfill this goal, however, the coating must be uniform, thin (<10 nm), and ionically conductive to avoid impeding Li+ transport.
- Doping, to enhance structural stability, suppress phase transitions, and reduce oxygen release. The cation doping stabilizes the layered structure and suppresses Li/Ni disorder, while anion doping, in particular F− (fluorination), strengthens TM–O bonds and suppresses oxygen evolution. The benefits are improved structural integrity, better capacity retention, and reduced gas generation.
- Single-crystal morphology minimizes grain boundaries and microcracks, which are common initiation sites for degradation. The benefits are improved mechanical integrity, longer cycle life, and better thermal stability. However, there are drawbacks: this morphology is more difficult and costly to synthesize. It may require higher sintering temperatures.
- Gradient composition or core–shell design combines a Ni-rich core for capacity with a stable outer shell (lower-Ni or doped) to buffer against side reactions. These architectures enhance thermal and chemical stability and delay impedance rise.
- Optimized electrolyte formulations reduce parasitic reactions, stabilize CEI (cathode–electrolyte interface), and suppress gas generation.
- Particle size and morphology engineering is useful to reduce mechanical stress and optimize Li+ diffusion. Spherical secondary particles with controlled porosity or dense, crack-resistant structures are preferred, resulting in improved structural stability and rate capability.
- Thermal and pressure management during cycling, to reduce gas generation and mechanical damage. The strategy for this purpose is to use stack pressure or thermal management in cell design.
- Advanced synthesis techniques, to achieve controlled doping, precise morphology, and homogeneous element distribution.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALD | atomic layer deposition |
| ASSLBs | all-solid-state lithium batteries |
| CB | carbon black |
| CBDA | cyclobutane-tetracarboxylic dianhydride |
| CEI | cathode electrolyte interphase |
| COF | covalent organic framework |
| DFT | density functional theory |
| DMC | dimethyl carbonate |
| DTBQ | di-tert-butyl-o-benzoquinone |
| EPD | electrophoretic deposition |
| EV | electric vehicle |
| HOMO | Highest Occupied Molecular Orbital |
| HoMS | hollow multi-shell structure |
| LIBs | Lithium-ion batteries |
| LIPON | lithium phosphorus oxynitride |
| LLAO | Li0.5La2Al0.5O4 |
| LUMO | Lowest Unoccupied Molecular Orbital |
| NASICON | sodium (Na) superionic conductor |
| NATM | LiNi0.93Al0.05Ti0.01Mg0.01O2 |
| NCA | LiNixCoyAlzO2 |
| NMC | LiNi1–x–yMxCoyxO2 |
| NMCA | LiNixMnyCozAl(1–x–y–z)O2 |
| NMC811 | LiNi0.8Mn0.1Co0.1O2 |
| NMC333 | LiNi1/3Mn1/3Co1/3O2 |
| NMC622 | LiNi0.6Mn0.2Co0.2O2 |
| PANI | polyaniline |
| PEG | polyethylene glycol |
| PPC | pyrrole-co-citral nitrile |
| PPy | polypyrrole |
| SEI | solid–electrolyte interphase |
| SEM | scanning electron microscopy |
| SOC | state-of-charge |
| TM | transition metal |
| VC | vinylene carbonate |
| XRD | X-ray diffraction |
References
- You, L.Z.; Chu, B.B.; Li, G.X.; Huang, T.; Yu, A.S. H3BO3 washed LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance and storage characteristics. J. Power Sources 2021, 482, 228940. [Google Scholar] [CrossRef]
- Yu, H.F.; Cao, Y.Q.; Chen, L.; Hu, Y.J.; Duan, X.Z.; Dai, S.; Jiang, H. Surface enrichment and diffusion enabling gradient-doping and coating of Ni-rich cathode toward Li ion batteries. Nat. Commun. 2021, 12, 4564. [Google Scholar] [CrossRef] [PubMed]
- Rajkamal, A.; Sharma, A.; Pullagura, B.K.; Thapa, R.; Kim, H. Engineering lithium nickel cobalt manganese oxides cathodes: A computational and experimental approach to bridging gaps. Chem. Eng. J. 2024, 481, 148223. [Google Scholar] [CrossRef]
- Ding, H.; Wang, X.; Wang, J.; Zhang, H.; Liu, G.; Yu, W.; Wang, J. Morphology-controllable synthesis and excellent electrochemical performance of Ni-rich layered NCM622 as cathode materials for lithium-ion batteries via glycerin-assisted solvothermal method. J. Power Sources 2023, 553, 232307. [Google Scholar] [CrossRef]
- Kim, J.; Lee, I.; Kim, Y.-H.; Bae, J.H.; Hwang, K.; Kang, H.; Yoon, S. Ni-rich cathode material with isolated porous layer hindering crack propagation under 4.5 V high cut-off voltage cycling. Chem. Eng. J. 2023, 455, 140578. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhu, Z.Y.; Yan, Q.Z.; Yu, S.C.; He, X.; Chen, Y.; Liu, P. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63–67. [Google Scholar] [CrossRef]
- Xie, H.; Peng, H.; Jiang, D.; Xiao, Z.; Liu, X.; Liang, H.; Wu, M.; Liu, D.; Li, Y.; Sun, Y.; et al. Structures, issues, and optimization strategies of Ni-rich and Co-low cathode materials for lithium-ion battery. Chem. Eng. J. 2023, 470, 144051. [Google Scholar] [CrossRef]
- Tian, X.; Guo, R.; Bai, Y.; Li, N.; Wang, X.; Wang, J.; Wu, C. High-performance high-nickel multi-element cathode materials for lithium-ion batteries. Batteries 2023, 9, 319. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Namkoong, B.; Kim, J.H.; Belharouak, I.; Yoon, C.S.; Sun, Y.K. Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes. ACS Energy Lett. 2021, 6, 2726–2734. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, R.; Wang, L.; Cheng, K.; Zhao, Z.; Mu, D.; Wu, B. A short review on layered LiNi0.8Mn0.1Co0.1O2 positive electrode material for lithium-ion batteries. Energy Proc. 2017, 105, 2941–2952. [Google Scholar] [CrossRef]
- Mubarac, S.; Silva, M.N.; Silva, G.T.; Freitas, B.; Gonçalves, J.M.; Zanin, H. Super Ni-rich and Co-poor LiNixCoyMn1-x-yO2, LiNixCoyAl1-x-yO2, and LiNixCoyMnzAl(1-x-y-z)O2 (x ≥ 0.85) based cathodes for lithium-ion batteries: A review on emerging trends, recent developments, and future perspectives. J. Energy Storage 2024, 96, 112612. [Google Scholar] [CrossRef]
- Li, J.; Manthiram, A. A comprehensive analysis of the interfacial and structural evolution over long-term cycling of ultrahigh-nickel cathodes in lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1902731. [Google Scholar] [CrossRef]
- Cui, Z.; Xie, Q.; Manthiram, A. Zinc-doped high-nickel, low-cobalt layered oxide cathodes for high-energy-density lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 15324–15332. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Xia, J.; Cameron, A.R.; Nie, M.; Botton, G.A.; Dahn, J.R. The impact of electrolyte additives and upper cut-off voltage on the formation of a rocksalt surface layer in LiNi0.8Mn0.1Co0.1O2 electrodes. J. Electrochem. Soc. 2017, 164, A655–A665. [Google Scholar] [CrossRef]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Mehdi, B.L.; et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 84–92. [Google Scholar] [CrossRef]
- Li, J.; Downie, L.E.; Ma, L.; Qiu, W.; Dahn, J.R. Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 Cathode material for lithium ion batteries. J. Electrochem. Soc. 2015, 162, A1401–A1408. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, electrochemical, and thermal properties of nickel-rich LiNixMnyCozO2 materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review: Recent advances and remaining challenges for lithium-ion battery cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Kim, M.; Zhu, J.; Li, L.; Wang, C.; Chen, G. Understanding reactivities of Ni-rich Li[NixMnyCo1-x-y]O2 single-crystal cathode materials. ACS Appl. Energy Mater. 2020, 3, 12238–12245. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. A 2017, 5, 874–901. [Google Scholar] [CrossRef]
- Duh, Y.S.; Sun, Y.; Lin, X.; Zheng, J.; Wang, M.; Wang, Y.; Lin, X.; Jiang, X.; Zheng, Z.; Zheng, S.; et al. Characterization on thermal runaway of commercial 18650 lithium-ion batteries used in electric vehicles: A review. J. Energy Storage 2021, 41, 102888. [Google Scholar] [CrossRef]
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Zizebert, C. Thermal and mechanical safety assessment of type 21700 lithium-ion batteries with NMC, NCA and LFP cathodes–investigation of cell abuse by means of accelerating rate calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar] [CrossRef]
- Huggins, R.A. Energy Storage: Fundamentals, Materials and Applications, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 181–208. [Google Scholar]
- Manthiram, A. An outlook on lithium-ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Devnani, H.; Sharma, A.; Lim, W.C.; Dhyani, A.; Chae, K.H.; Lee, S. Challenges and opportunities using Ni-rich layered oxide cathodes in Li-ion rechargeable batteries: The case of nickel cobalt manganese oxides. Energy Adv. 2024, 3, 1869–1893. [Google Scholar] [CrossRef]
- Ahangari, M.; Szalai, B.; Lujan, J.; Zhou, M.; Luo, H. Advancements and challenges in high-capacity Ni-rich cathode materials for lithium-ion batteries. Materials 2024, 17, 801. [Google Scholar] [CrossRef]
- Butt, A.; Ali, G.; Kubra, K.T.; Sharif, R.; Salman, A.; Bashir, M.; Jamil, S. Recent advances in enhanced performance of Ni-rich cathode materials for Li-ion batteries: A review. Energy Technol. 2022, 10, 2100775. [Google Scholar] [CrossRef]
- Jamil, S.; Wang, G.; Fasehullah, M.; Xu, M. Challenges and prospects of nickel-rich layered oxide cathode material. J. Alloys Compd. 2022, 909, 164727. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Britala, L.; Marinaro, M.; Kucenskis, G. A review of the degradation mechanisms of NCM cathodes and corresponding mitigation strategies. J. Energy Storage 2023, 73A, 108875. [Google Scholar] [CrossRef]
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Hou, P.Y.; Yin, J.M.; Ding, M.; Huang, J.; Xu, X. Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: Advances and perspectives. Small 2017, 13, 1701802. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2(NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Strehle, B.; Kleiner, K.; Jung, R.; Chesneau, F.; Mendez, M.; Gasteiger, H.A.; Piana, M. The role of oxygen release from Li- and Mn-rich layered oxides during the first cycles investigated by on-line electrochemical mass spectrometry. J. Electrochem. Soc. 2017, 164, A400–A406. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Pan, Q.-L.; Wei, H.-X.; Huang, Y.-D.; Tang, L.-B.; Wang, Z.-Y.; He, Z.-J.; Yan, C.; Mao, J.; Dai, K.-H.; et al. Towards Ni-rich layered oxides cathodes with low Li/Ni intermixing by mild molten-salt ion exchange for lithium-ion batteries. Nano Energy 2022, 102, 107626. [Google Scholar] [CrossRef]
- Xiao, P.; Li, W.-H.; Chen, S.; Li, G.; Dai, Z.-J.; Feng, M.-D.; Chen, X.; Yang, W.-S. Effects of Oxygen pressurization on Li+/Ni2+ cation mixing and the oxygen vacancies of LiNi0.8Co0.15Al0.05O2 cathode materials. ACS Appl. Mater. Interfaces 2022, 14, 31851–31861. [Google Scholar] [CrossRef]
- Chen, J.-N.; Yang, Y.; Tang, Y.-S.; Wang, Y.-F.; Li, H.; Xiao, X.-H.; Wang, S.-N.; Mariyam, S.D.D.; Etter, M.; Missyul, A.; et al. Constructing a thin disordered self-protective layer on the LiNiO2 primary particles against oxygen release. Adv. Funct. Mater. 2022, 33, 2211515. [Google Scholar] [CrossRef]
- Li, X.-H.; Wang, Q.; Guo, H.-Y.; Artrith, N.; Urban, A. Understanding the onset of surface degradation in LiNiO2 cathodes. ACS Appl. Energy Mater. 2022, 5, 5730–5741. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, S.P.; Zhang, L.; Guo, L.; Shi, F.N. Review on oxygen release mechanism and modification strategy of nickel-rich NCM cathode materials for lithium-ion batteries: Recent advances and future directions. Energy Fuel 2024, 38, 5607–5631. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Piana, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC impedance. J. Electrochem. Soc. 2015, 162, A2737–A2746. [Google Scholar] [CrossRef]
- Komagata, S.; Itou, Y.; Kondo, H. Impact of surface layer formation during cycling on the thermal stability of the LiNi0.8Co0.1Mn0.1O2 cathode. ACS Appl. Mater. Interfaces 2022, 14, 8931–8937. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, P.; Zhou, X.; Wu, W.; Liu, X.; Liu, Y.; Feng, G.; Zhang, B.; Xing, W.; Zuo, M.; et al. Slightly Li-enriched chemistry enabling super stable LiNi0.5Mn0.5O2 cathodes under extreme conditions. Chem. Sci. 2024, 15, 14415–14424. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, Y.; Wang, L.; Fu, N.; Li, Y.; Luo, Y.; Wang, J.; Xiao, X.; Wang, X.; Yang, X.; et al. Challenges of thermal stability of high-energy layered oxide cathode materials for lithium-ion batteries: A review. Mater. Today 2023, 69, 236–261. [Google Scholar] [CrossRef]
- Lim, J.M.; Hwang, T.; Kim, D.; Park, M.-S.; Cho, K.; Cho, M. Intrinsic origins of crack generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 2017, 7, 39669. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Li, W.; Dolocan, A.; Celio, H.; Park, H.; Warner, J.H.; Manthiram, A. In-depth analysis of the degradation mechanisms of high-nickel, low/no-cobalt layered oxide cathodes for lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2100858. [Google Scholar] [CrossRef]
- Laakso, E.; Efimova, S.; Colalongo, M.; Kauranen, P.; Lahtinen, K.; Napolitano, E.; Ruiz, V.; Moskon, J.; Gaberscek, M.; Park, J.; et al. Aging mechanisms of NMC811/Si-Graphite Li-ion batteries. J. Power Sources 2024, 599, 234159. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Yuan, F.; Lyu, M.; Yang, P.; Zhang, L.; Zhou, M.; Wang, L.; Zhang, S.; Wang, L. Ni-rich cathode materials for stable high-energy lithium-ion batteries. Nano Energy 2024, 126, 109620. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, D. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, X.; Zhang, J.; Manthiram, A.; Meng, Y.S.; Xu, W. A Review on the stability and surface modification of layered transition-metal oxide cathodes. Mater. Today 2021, 46, 155–182. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.; Yuan, W.; Zhang, G.; Zheng, H.; Chen, Z. Advances in multi-scale design and fabrication processes for thick electrodes in lithium-ion batteries. Energy Rev. 2024, 3, 100066. [Google Scholar] [CrossRef]
- Das, J.; Kleiman, A.; Rehman, A.U.; Verma, R.; Young, M.H. The cobalt supply chain and environmental life cycle impacts of lithium-ion battery energy storage systems. Sustainability 2024, 16, 1910. [Google Scholar] [CrossRef]
- The Organisation for Economic Co-Operation and Development (OECD). Interconnected Supply Chains: A Comprehensive Look at Due Diligence Challenges and Opportunities Sourcing Cobalt and Copper from the Democratic Republic of the Congo. In Responsible Business Conduction; OECD: Paris, France, 2019; Available online: https://mneguidelines.oecd.org/Interconnected-supply-chains-a-comprehensive-look-at-due-diligence-challenges-and-opportunities-sourcing-cobalt-and-copper-from-the-DRC.pdf (accessed on 1 January 2019).
- U.S. Geological Survey (USGS). Mineral Commodity Summaries 2022-Cobalt; U.S. Geological Survey: Reston, VA, USA, 2022. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-cobalt.pdf (accessed on 25 May 2023).
- Cobalt Institute. State of Cobalt Market Report. 2021. Available online: https://www.cobaltinstitute.org/wp-content/uploads/2021/09/Cobalt-Institute-State-of-the-Cobalt-Market-Report_2020.pdf (accessed on 25 May 2023).
- U.S. Department of Energy. Energy Storage Grand Challenge: Energy Storage Market Report; U.S. Department of Energy: Washington, DC, USA, 2020. Available online: https://www.energy.gov/sites/prod/files/2020/12/f81/Energy%20Storage%20Market%20Report%202020_0.pdf (accessed on 1 December 2020).
- The White House. FACT SHEET: Securing a Made in America Supply Chain for Critical Minerals. 2022. Available online: https://bidenwhitehouse.archives.gov/briefing-room/statements-releases/2022/02/22/fact-sheet-securing-a-made-in-america-supply-chain-for-critical-minerals/ (accessed on 22 February 2022).
- Nriagu, J.O. Toxic metal pollution in Africa. Sci. Total. Environ. 1992, 121, 1–37. [Google Scholar] [CrossRef]
- Banza, C.L.N.; Nawrot, T.S.; Haufroid, V.; Decrée, S.; De Putter, T.; Smolders, E.; Kabyla, B.I.; Luboya, O.N.; Ilunga, A.N.; Mutombo, A.M.; et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009, 109, 745–752. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Water Issues in the Democratic Republic of Congo: Challenges and Opportunities—Technical Report. 2011. Available online: https://www.ircwash.org/sites/default/files/Partow-2011-Water.pdf (accessed on 1 January 2011).
- Slack, J.F.; Kimball, B.E.; Shedd, K.B. Cobalt Report 1802F. Professional Paper; USGS Publications Warehouse: Reston, VA, USA, 2017. Available online: https://pubs.er.usgs.gov/publication/pp1802F (accessed on 25 May 2023).
- Shengo, M.; Kime, M.-B.; Mambwe, M.; Nyembo, T. A review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J. Sustain. Min. 2019, 18, 226–246. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A.; Nkulu, C.B.L.; Mwitwa, J.; Kampemba, F.M.; Nabuyanda, M.M.; Haufroid, V.; Smolders, E.; Nemery, B. Contamination of water and food crops by trace elements in the African Copperbelt: A collaborative cross-border study in Zambia and the Democratic Republic of Congo. Environ. Adv. 2021, 6, 100103. [Google Scholar] [CrossRef]
- Schmidt, T.; Buchert, M.; Schebek, L. Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour. Conserv. Recycl. 2016, 112, 107–122. [Google Scholar] [CrossRef]
- Voronina, N.; Sun, Y.-K.; Myung, S.-T. Co-free layered cathode materials for high energy density lithium-ion batteries. ACS Energy Lett. 2020, 5, 1814–1824. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Sun, H.H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energy Environ. Sci. 2021, 14, 844–852. [Google Scholar] [CrossRef]
- Gent, W.E.; Busse, G.M.; House, K.Z. The predicted persistence of cobalt in lithium-ion batteries. Nat. Energy 2022, 7, 1132. [Google Scholar] [CrossRef]
- Chu, B.; Guo, Y.-J.; Shi, J.-L.; Yin, Y.-X.; Huang, T.; Su, H.; Yu, A.; Guo, Y.-G. Cobalt in high-energy-density layered cathode materials for lithium-ion batteries. J. Power Sources 2022, 544, 231873. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Liu, J.; Lu, J.; Bi, X.; Dai, A.; Li, M.; Li, M.; Hu, Z.; Ma, L.; et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 2021, 6, 277–286. [Google Scholar] [CrossRef]
- Ge, H.; Shen, Z.; Wang, Y.; Sun, Z.; Cao, X.; Wang, C.; Fan, X.; Bai, J.; Li, R.; Yang, T.; et al. Design of high-performance and sustainable Co-free Ni-rich cathodes for next-generation lithium-ion batteries. SusMat 2024, 4, 48–71. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Fan, Y.; Yang, W.; Zhan, C.; Liu, G. Controversy on necessity of cobalt in nickel-rich cathode materials for lithium-ion batteries. J. Ind. Eng. Chem. 2022, 110, 120–130. [Google Scholar] [CrossRef]
- Mallick, S.; Patel, A.; Sun, X.-G.; Paranthaman, M.P.; Mou, M.; Mugumya, J.H.; Jiang, M.; Rasche, M.L.; Lopez, H.; Gupta, R.B. Low-cobalt active cathode materials for high-performance lithium-ion batteries: Synthesis and performance enhancement methods. J. Mater. Chem. A 2023, 11, 3789–3821. [Google Scholar] [CrossRef]
- Hussain, S.K.; Bang, J.H. Recent progress in Co-free, Ni-rich cathode materials for lithium-ion batteries. Bull. Korean Chem. Soc. 2024, 45, 4–15. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.-L.; Yin, L.; Hwang, I.; Li, Y.; Lu, L.; Xu, W.; Zhang, X.; Chen, Y.; Ren, Y.; et al. Probing the thermal-driven structural and chemical degradation of Ni-rich layered cathodes by Co/Mn exchange. J. Am. Chem. Soc. 2020, 142, 19745. [Google Scholar] [CrossRef]
- Choi, J.U.; Voronina, N.; Sun, Y.-K.; Myung, S.-T. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: Yesterday, today, and tomorrow. Adv. Energy Mater. 2020, 10, 2002027. [Google Scholar] [CrossRef]
- Song, Y.; Cui, Y.; Geng, L.; Li, B.; Ge, L.; Zhou, L.; Qiu, Z.; Nan, J.; Wu, W.; Xu, H.; et al. Li/Ni intermixing: The real origin of lattice oxygen stability in Co-free Ni-rich cathode materials. Adv. Energy Mater. 2024, 14, 2303207. [Google Scholar] [CrossRef]
- Kim, Y.; Seong, W.M.; Manthiram, A. Cobalt-free, high-nickel layered oxide cathodes for lithium-ion batteries: Progress, challenges, and perspectives. Energy Storage Mater. 2021, 34, 250–259. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, T.; Liu, J.; He, L.; Chen, J.; Zhang, J.; Chen, H. Insight into the origin of lithium/nickel ions exchange in layered Li(NixMnyCoz)O2 cathode materials. Nano Energy 2018, 49, 77–85. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, F.; Pan, F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Wang, D.; Xin, C.; Zhang, M.; Bai, J.; Zheng, J.; Kou, R.; Ko, J.Y.P.; Huq, A.; Zhong, G.; Sun, C.-J.; et al. Intrinsic role of cationic substitution in tuning Li/Ni mixing in high-Ni layered oxides. Chem. Mater. 2019, 31, 2731–2740. [Google Scholar] [CrossRef]
- Ben Kamel, K.; Amdouni, N.; Abdel-Ghany, A.; Zaghib, K.; Mauger, A.; Gendron, F.; Julien, C. Local structure and electrochemistry of LiNiyMnyCo1-2yO2 electrode materials for Li-ion batteries. Ionics 2008, 14, 89–97. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Yang, X.; Liu, Y.; Wu, Z.; Li, H.; Indris, S.; Ehrenberg, H.; Hua, W. Unravelling the peculiar role of Co and Al in highly Ni-rich layered oxide cathode materials. Chem. Eng. J. 2024, 484, 149599. [Google Scholar] [CrossRef]
- Lin, J.; Lian, X.; Wang, X.; Ma, Y.; Fang, L.; Suo, X. Effects of Al content on the electrochemical performance and thermal stability of Li(Ni0.9Co0.06Mn0.04)1–xAlxO2 cathode materials for Li-ion batteries. Ionics 2023, 29, 2153–2162. [Google Scholar] [CrossRef]
- Aishova, A.; Park, G.; Yoon, C.S.; Sun, Y. Cobalt-free high-capacity Ni-rich layered Li[Ni0.9Mn0.1]O2 cathode. Adv. Energy Mater. 2020, 10, 1903179. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Kan, W.H.; Kuai, C.; Yang, Z.; Li, L.; Sun, C.-J.; Sainio, S.; Avdeev, M.; Nordlund, D.; Lin, F. Structural and electrochemical impacts of Mg/Mn dual dopants on the LiNiO2 cathode in Li-metal batteries. ACS Appl. Mater. Interfaces 2020, 12, 12874–12882. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Parejiya, A.; Amin, R.; Bai, Y.; Du, Z.; Belharouak, I. LiNixFeyAlzO2, a new cobalt-free layered cathode material for advanced Li-ion batteries. J. Power Sources 2020, 471, 228389. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, R.; Kan, W.H.; Zhang, Y.; Li, L.; Kuai, C.; Zydlewski, B.; Rahman, M.M.; Sun, C.-J.; Sainio, S.; et al. Dopant distribution in Co-free high-energy layered cathode materials. Chem. Mater. 2019, 31, 9769–9776. [Google Scholar] [CrossRef]
- Yang, Z.; Mu, L.; Hou, D.; Rahman, M.M.; Xu, Z.; Liu, J.; Nordlund, D.; Sun, C.; Xiao, X.; Lin, F. Probing dopant redistribution, phase propagation, and local chemical changes in the synthesis of layered oxide battery cathodes. Adv. Energy Mater. 2020, 11, 2002719. [Google Scholar] [CrossRef]
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is cobalt needed in Ni-rich positive electrode materials for lithium ion batteries? J. Electrochem. Soc. 2019, 166, A429–A439. [Google Scholar] [CrossRef]
- Cui, Z.; Xie, Q.; Manthiram, A. A cobalt- and manganese-free high-nickel layered oxide cathode for long-life, safer lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2102421. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and back again—The journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 2019, 58, 10434–10458. [Google Scholar] [CrossRef]
- Cui, J.; Ding, X.; Luo, D.; Xie, H.; Zhang, Z.; Zhang, B.; Tan, F.; Liu, C.; Lin, Z. Effect of cationic uniformity in precursors on Li/Ni mixing of Ni-rich layered cathodes. Energy Fuels 2021, 35, 1842–1850. [Google Scholar] [CrossRef]
- Wang, S.; Hua, W.; Missyul, A.; Darma, M.S.D.; Tayal, A.; Indris, S.; Ehrenberg, H.; Liu, L.; Knapp, M. Kinetic control of long-range cationic ordering in the synthesis of layered Ni-rich oxides. Adv. Funct. Mater. 2021, 31, 2009949. [Google Scholar] [CrossRef]
- Paidi, A.K.; Lee, A.T.; Paidi, V.K.; Ahn, H.; Lim, J.; Lee, K.-S.; Lee, S.; Ahn, D. Atomic-level insights into the first cycle irreversible capacity loss of Ni-rich layered cathodes for Li-ion batteries. J. Mater. Chem. A 2023, 11, 12002–12012. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.Z.; Ren, D.S.; Liao, J.Y.; Song, Y.Z.; Liang, H.M.; Wang, A.P.; He, Y.F.; Wang, L.; Chen, Z.H.; et al. Revisiting the initial irreversible capacity loss of LiNi0.6Co0.2Mn0.2O2 cathode material batteries. Energy Storage Mater. 2022, 50, 373–379. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Lu, Y.; Xie, W.; Yan, Z.; Chen, J. Insights into cation migration and intermixing in advanced cathode materials for lithium-ion batteries. Adv. Energy Mater. 2024, 14, 2402068. [Google Scholar] [CrossRef]
- Wu, K.; Ran, P.; Yin, W.; He, L.; Wang, B.; Wang, F.; Zhao, E.; Zhao, J. Dynamic evolution of antisite defect and coupling anionic redox in high-voltage ultrahigh-Ni cathode. Angew. Chem. Int. Ed. 2024, 63, e202410326. [Google Scholar]
- Shi, C.-G.; Peng, X.-X.; Dai, P.; Xiao, P.H.; Zheng, W.-C.; Li, H.-Y.; Li, H.; Indris, S.; Mangold, S.; Hong, Y.-H.; et al. Investigation and suppression of oxygen release by LiNi0.8Co0.1Mn0.1O2 cathode under overcharge conditions. Adv. Energy Mater. 2022, 12, 2200569. [Google Scholar] [CrossRef]
- Gabrisch, H.; Yi, T.H.; Yazami, R. Transmission electron microscope studies of LiNi1/3Mn1/3Co1/3O2 before and after long-term aging at 70 °C. Electrochem. Solid State Lett. 2008, 11, A119–A124. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2 Mn0.3O2 cathode material in lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Robert, R.; Villevieille, C.; Novák, P. Enhancement of the high potential specific charge in layered electrode materials for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 8589–8598. [Google Scholar] [CrossRef]
- Konishi, H.; Yoshikawa, M.; Hirano, T. The effect of thermal stability for high-Ni-content layer-structured cathode materials, LiNi0.8Mn0.1–xCo0.1MoxO2 (x = 0, 0.02, 0.04). J. Power Sources 2013, 244, 23–28. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.-H.; Wang, Z.X.; Guo, H.; Huang, Z.; Kong, L.; Ru, J. Improved high voltage electrochemical performance of Li2ZrO3-coated LiNi0.5Co0.2Mn0.3O2 cathode material. J. Alloys Compd. 2015, 647, 612–619. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Hwang, S.; Chang, W.; Kim, S.M.; Su, D.; Kim, D.Y.; Lee, J.Y.; Chung, K.Y.; Stach, E.A. Investigation of changes in the surface structure of LixNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem. Mater. 2014, 26, 1084–1092. [Google Scholar] [CrossRef]
- Yang, J.; Hou, M.Y.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. Improving the cycling performance of the layered Ni-rich oxide cathode by introducing low content Li2MnO3. Electrochim. Acta 2016, 189, 101–110. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.Y. Enhancement on the cycling stability of the layered Ni-rich oxide cathode by in situ fabricating nano-thickness cation-mixing layers. J. Electrochem. Soc. 2016, 163, A2665–A2672. [Google Scholar] [CrossRef]
- Liu, J.; Du, Z.; Wang, X.; Tan, S.; Wu, X.; Geng, L.; Song, B.; Chien, P.-H.; Everett, S.M.; Hu, E. Anionic redox induced anomalous structural transition in Ni-rich cathodes. Energy Environ. Sci. 2021, 14, 6441–6454. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, J.; Zhang, Q.; Wong, D.; Yin, W.; Zhang, N.; Chen, Z.; Gu, L.; Hu, Z.; Liu, X. Oxygen anion redox chemistry correlated with spin state in Ni-rich layered cathodes. Adv. Sci. 2023, 10, 2206442. [Google Scholar] [CrossRef]
- Chu, Y.; Mu, Y.; Zou, L.; Wu, F.; Yang, L.; Feng, Y.; Zeng, L. Oxygen release in Ni-rich layered cathode for lithium-ion batteries: Mechanisms and mitigating strategies. ChemElectroChem 2024, 11, e20230065. [Google Scholar] [CrossRef]
- Flores, E.; Vonrüti, N.; Novák, P.; Aschauer, U.; Berg, E.J. Elucidation of LixNi0.8Co0.15Al0.05O2 redox chemistry by operando raman spectroscopy. Chem. Mater. 2018, 30, 4694–4703. [Google Scholar] [CrossRef]
- Li, J.; Hua, H.; Kong, X.; Yang, H.; Dai, P.; Zeng, J.; Zhao, J. In-situ probing the near-surface structural thermal stability of high-nickel layered cathode materials. Energy Storage Mater. 2022, 46, 90–99. [Google Scholar] [CrossRef]
- Lee, E.; Muhammad, S.; Kim, T.; Kim, H.; Lee, W.; Yoon, W.S. Tracking the influence of thermal expansion and oxygen vacancies on the thermal stability of Ni-rich layered cathode materials. Adv. Sci. 2020, 7, 1902413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, A.; Tang, Z.; Meng, F.; Shang, T.; Guo, S.; Ding, J.; Luo, Y.; Xiao, D.; Wang, X.; et al. Robust surface reconstruction induced by subsurface Ni/Li antisites in Ni-rich cathodes. Adv. Funct. Mater. 2021, 31, 2010291. [Google Scholar] [CrossRef]
- Chae, B.G.; Park, S.Y.; Song, J.H.; Lee, E.; Jeon, W.S. Evolution and expansion of Li concentration gradient during charge-discharge cycling. Nat. Commun. 2021, 12, 3814. [Google Scholar] [CrossRef]
- Yin, S.; Deng, W.; Chen, J.; Gao, X.; Zou, G.; Hou, H.; Ji, X. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 2021, 83, 105854. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, S.M.; Bak, S.-M.; Chung, K.Y.; Chang, W. Investigating the reversibility of structural modifications of LixNiyMnzCo1–y–zO2 cathode materials during initial charge/discharge, at multiple length scales. Chem. Mater. 2015, 27, 6044–6052. [Google Scholar] [CrossRef]
- Ghanty, C.; Markovsky, B.; Erickson, E.M.; Talianker, M.; Haik, O.; Tal-Yossef, Y.; Mor, A.; Aurbach, D.; Lampert, J.; Volkov, A.; et al. Li+-ion extraction/insertion of Ni-rich Li1+x(NiyCozMnz)wO2 (0.005 < x < 0.03; y:z = 8:1, w ≈ 1) electrodes: In situ XRD and Raman spectroscopy study. ChemElectroChem 2015, 2, 1479–1486. [Google Scholar]
- Wang, X.L.; An, K.; Cai, L.; Feng, Z.; Nagler, S.E.; Daniel, C.; Rhodes, K.J.; Stoica, A.D.; Skorpenske, H.D.; Liang, C.; et al. Visualizing the chemistry and structure dynamics in lithium-ion batteries by in situ neutron diffraction. Sci. Rep. 2012, 2, 747. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.Y. Suppressing the phase transition of the layered Ni-rich oxide cathode during high-voltage cycling by introducing low-content Li2MnO3. ACS Appl. Mater. Interfaces 2016, 8, 1297–1308. [Google Scholar] [CrossRef]
- Li, J.; Shunmugasundaram, R.; Doig, R. In situ X-ray diffraction study of layered Li–Ni–Mn–Co oxides: Effect of particle size and structural stability of core–shell materials. Chem. Mater. 2016, 28, 162–171. [Google Scholar] [CrossRef]
- Yoon, S.; Park, H.G.; Koo, S.; Hwang, J.; Lee, Y.; Park, K.; Kim, D. An in-depth understanding of chemomechanics in Ni-rich layered cathodes for lithium-ion batteries. J. Alloys Compd. 2023, 939, 168531. [Google Scholar] [CrossRef]
- Laubach, S.; Laubach, S.; Schmidt, P.; Ensling, D.; Schmid, S.; Jaegermann, W.; Thissen, A.; Nikolowski, K.; Ehrenberg, H. Changes in the crystal and electronic structure of LiCoO2 and LiNiO2 upon Li intercalation and de-intercalation. Phys. Chem. Chem. Phys. 2009, 11, 3278–3289. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Makimura, Y.; Ohzuku, T. Solid-state chemistry and electrochemistry of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Electrochem. Soc. 2007, 154, A314–A321. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yan, M.Y.; Li, Y.; Vinado, C.; Yang, J. Separating electronic and ionic conductivity in mix-conducting layered lithium transition-metal oxides. J. Power Sources 2018, 393, 75–82. [Google Scholar] [CrossRef]
- Cui, S.; Wei, Y.; Liu, T.; Deng, W.; Hu, Z.; Su, Y.; Li, H.; Li, M.; Guo, H.; Duan, Y.; et al. Optimized temperature effect of Li-ion diffusion with layer distance in Li(NixMnyCoz)O2 cathode materials for high performance Li-ion battery. Adv. Energy Mater. 2016, 6, 1501309. [Google Scholar] [CrossRef]
- Wu, F.; Liu, N.; Chen, L.; Su, Y.; Tan, G.; Bao, L.; Zhang, Q.; Lu, Y.; Wang, J.; Chen, S.; et al. Improving the reversibility of the H2–H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy 2019, 59, 50–57. [Google Scholar] [CrossRef]
- Yoon, C.S.; Ryu, H.H.; Park, G.T.; Kim, J.H.; Kim, K.H.; Sun, Y.K. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries. J. Mater. Chem. A 2018, 6, 4126–4132. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, H.; Park, J.; Roh, Y.; Byun, S.; Lim, J.; Jung, S.; Kim, N.; Lee, K.-T.; Lee, Y.-M. A microcrack propagation-based life prediction model for lithium-ion batteries with Ni-rich cathode materials. J. Energy Storage 2023, 58, 106420. [Google Scholar] [CrossRef]
- Park, N.-Y.; Kim, M.-C.; Han, S.-M.; Park, G.-T.; Kim, D.-H.; Kim, M.-S.; Sun, Y.-K. Mechanism behind the loss of fast charging capability in nickel-rich cathode materials. Angew. Chem. Int. Ed. 2024, 63, e202319707. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, L.; Lu, J.; Zhou, T.; Huang, X.; Cai, Z.; Dai, A.; Gim, J.; Ren, Y.; Xiao, X.; et al. Rational design of mechanically robust Ni-rich cathode materials via concentration gradient strategy. Nat Commun. 2021, 12, 6024. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Ryu, H.H.; Park, N.Y.; Yoon, C.S.; Sun, Y.K. Tungsten doping for stabilization of Li[Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage. J. Power Sources 2019, 442, 227242. [Google Scholar] [CrossRef]
- Lv, C.; Li, Z.; Ren, X.; Li, K.; Ma, J.; Duan, X. Revealing the degradation mechanism of Ni-rich cathode materials after ambient storage and related regeneration method. J. Mater. Chem. A 2021, 9, 3995–4006. [Google Scholar] [CrossRef]
- Su, M.; Chen, Y.; Liu, H.; Li, J.; Fu, K.; Zhou, Y.; Dou, A.; Liu, Y. Storage degradation mechanism of layered Ni-rich oxide cathode material LiNi0.8Co0.1Mn0.1O2. Electrochim. Acta 2022, 422, 140559. [Google Scholar] [CrossRef]
- Yang, J.; Liang, X.; Ryu, H.-H.; Yoon, C.S.; Sun, Y.-K. Ni-rich layered cathodes for lithium-ion batteries: From challenges to the future. Energy Storage Mater. 2023, 63, 102969. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Zhao, Y.; Ray, S.; Lu, X.; Hou, Y.; Zhang, J. A review of nickel-rich layered oxide cathodes: Synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects. Appl. Energy 2022, 305, 117849. [Google Scholar] [CrossRef]
- Quilty, C.-D.; West, P.-J.; Li, W.-Z.; Dunkin, M.-R.; Wheeler, G.-P.; Ehrlich, S.; Ma, L.; Jaye, C.; Fischer, D.-A.; Takeuchi, E.-S.; et al. Multimodal electrochemistry coupled microcalorimetric and X-ray probing of the capacity fade mechanisms of Nickel rich NMC–progress and outlook. Phys. Chem. Chem. Phys. 2022, 24, 11471–11485. [Google Scholar] [CrossRef]
- Sun, H.-H.; Manthiram, A. Impact of microcrack generation and surface degradation on a nickel-rich layered Li[Ni0.9Co0.05Mn0.05]O2 cathode for lithium-ion batteries. Chem. Mater. 2017, 29, 8486–8493. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; Sui, T.; Song, B. Explicit formulae for the internal stress in spherical particles of active material within lithium-ion battery cathodes during charging and discharging. Mater. Des. 2015, 69, 247–252. [Google Scholar] [CrossRef]
- Brandt, L.R.; Marie, J.-J.; Moxham, T.; Förstermann, D.P.; Salvati, E.; Besnard, C.; Papadaki, C.; Wang, Z.; Bruce, P.G.; Korsunsky, A.M. Synchrotron X-ray quantitative evaluation of transient deformation and damage phenomena in a single nickel-rich cathode particle. Energy Environ. Sci. 2020, 13, 3556–3566. [Google Scholar] [CrossRef]
- Park, N.-Y.; Park, G.-T.; Kim, S.-B.; Jung, W.; Park, B.-C.; Sun, Y.-K. Degradation mechanism of Ni-rich cathode materials: Focusing on particle interior. ACS Energy Lett. 2022, 7, 2362–2369. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, J.; Guo, H.; Li, X.; Gui, W.; Chen, N.; Yan, G. Anchoring K+ in Li+ sites of LiNi0.8Co0.15Al0.05O2 cathode material to suppress its structural degradation during high-voltage cycling. Energy Technol. 2018, 6, 2358–2366. [Google Scholar] [CrossRef]
- Lee, E.J.; Chen, Z.; Noh, H.-J.; Nam, S.C.; Kang, S.; Kim, D.H.; Amine, K.; Sun, Y.-K. Development of microstrain in aged lithium transition metal oxides. Nano Lett. 2014, 14, 4873–4880. [Google Scholar] [CrossRef]
- Xu, G.-L.; Liu, Q.; Lau, K.K.S.; Liu, Y.; Liu, X.; Gao, H.; Zhou, X.; Zhuang, M.; Ren, Y.; Li, J.; et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 2019, 4, 484–494. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Hu, E.; Chae, S.; Jia, H.; Liu, T.; Wu, B.; Bi, J.; Amine, K.; Wang, C.; et al. Mesoscale-architecture-based crack evolution dictating cycling stability of advanced lithium-ion batteries. Nano Energy 2020, 79, 105420. [Google Scholar] [CrossRef]
- Bi, Y.J.; Tao, J.H.; Wu, Y.Q.; Li, L.Z.; Xu, Y.B.; Hu, E.Y.; Wu, B.B.; Hu, J.T.; Wang, C.M.; Zhang, J.G.; et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 2020, 370, 1313–1317. [Google Scholar] [CrossRef]
- Tian, H.; Gao, L.T.; Guo, Z.-S. Microstructural adjusting crack Evolution of polycrystalline NCM particle during charge/discharge cycle. J. Electrochem. Soc. 2022, 169, 090513. [Google Scholar] [CrossRef]
- Han, G.-M.; Kim, Y.-S.; Ryu, H.-H.; Sun, Y.-K.; Yoon, C.S. Structural stability of single-crystalline Ni-rich layered cathode upon delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Su, Y.F.; Zhang, Q.Y.; Chen, L.; Bao, L.Y.; Lu, Y.; Chen, S.; Wu, F. Stress accumulation in Ni-rich layered oxide cathodes: Origin, impact, and resolution. J. Energy Chem. 2022, 65, 236–253. [Google Scholar] [CrossRef]
- Koo, J.K.; Ran, W.T.A.; Yun, Y.; Seo, J.K.; Kim, M.; Lee, J.; Lee, S.; Kim, J.; Hwang, S.M.; Kim, Y.-J. Probing intraparticle heterogeneity in Ni-rich layered cathodes with different carbon black contents using scanning probe microscopy. J. Energy Storage 2022, 51, 104395. [Google Scholar] [CrossRef]
- Lee, S.; Song, G.; Yun, B.; Kim, T.; Choi, S.H.; Kim, H.; Doo, S.W.; Lee, K.T. Revealing the nanoscopic corrosive degradation mechanism of nickel-rich layered oxide cathodes at low state-of-charge levels: Corrosion cracking and pitting. ACS Nano 2024, 18, 10566–10581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Vasconcelos, L.S.; Hassan, S.; Zhao, K. Asynchronous-to-synchronous transition of li reactions in solid-solution cathodes. Nano Lett. 2022, 22, 5883–5890. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Cao, T.; Wu, R.; Wang, M.; Liu, H.; Liu, X.; Lu, J.; Zhang, Y. Real-time observation of chemomechanical breakdown in a layered nickel-rich oxide cathode realized by in situ scanning electron microscopy. ACS Energy Lett. 2021, 6, 1703–1710. [Google Scholar] [CrossRef]
- Mu, L.; Lin, R.; Xu, R.; Han, L.; Xia, S.; Sokaras, D.; Steiner, J.D.; Weng, T.C.; Nordlund, D.; Doeff, M.M.; et al. Oxygen release induced chemomechanical breakdown of layered cathode materials. Nano Lett. 2018, 18, 3241–3249. [Google Scholar] [CrossRef]
- Kim, U.H.; Ryu, H.H.; Kim, J.H.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles. Adv. Energy Mater. 2019, 9, 1803902. [Google Scholar] [CrossRef]
- Morzy, J.K.; Dose, W.M.; Vullum, P.E.; Lai, M.C.; Mahadevegowda, A.; De Volder, M.F.L.; Ducati, C. Origins and importance of intragranular cracking in layered lithium transition metal oxide cathodes. ACS Appl. Energy Mater. 2024, 7, 3945–3956. [Google Scholar] [CrossRef]
- Park, K.-J.; Hwang, J.-Y.; Ryu, H.-H.; Maglia, F.; Kim, S.-J.; Lamp, P.; Yoon, C.S.; Sun, Y.-K. Degradation mechanism of Ni-enriched NCA cathode for lithium batteries: Are microcracks really critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef]
- Li, W.; Asl, H.-Y.; Xie, Q.; Manthiram, A. Collapse of LiNi1-x-yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 2019, 141, 5097–5101. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Bi, X.; Chen, Z.; Amine, K.; Zhong, C.; Lu, J. Cationic and anionic redox in lithium-ion based batteries. Chem. Soc. Rev. 2020, 49, 1688–1705. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich Li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, G.T.; Yoon, C.S.; Sun, Y.K. Microstructural degradation of Ni-rich Li[NixCoyMn1-x-y]O2 cathodes during accelerated calendar aging. Small 2018, 14, 1803179. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Zhong, S.; Li, Z.; Zeng, M.; Fan, F.; Yan, T. Extebded fast-charging life of ultrahigh-Ni layered oxides by adjusting the current density during the H2 -> H3 phase transition. Energy Storage Mater. 2022, 50, 757–759. [Google Scholar]
- Dai, P.P.; Kong, X.B.; Yang, H.Y.; Li, J.Y.; Zeng, J.; Zhao, J.B. Single-crystal Ni-rich layered LiNi0.9Mn0.1O2 enables superior performance of Co-free cathodes for lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 4381–4390. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lee, S.B.; Yoon, C.S.; Sun, Y.K. Morphology-dependent battery performance of Ni-rich layered cathodes: Single-crystal versus refined polycrystal. ACS Energy Lett. 2022, 7, 3072–3079. [Google Scholar] [CrossRef]
- Niu, S.; Xu, J.; Wu, K.; Liang, C.; Zhu, G.; Qu, Q.; Zheng, H. Different mechanical and electrochemical behavior between the two major Ni-rich cathode materials in Li-ion batteries. Mater. Chem. Phys. 2021, 260, 124046. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Shi, L.; Wang, Z.; Yuan, S. Spheroidization: The impact of precursor morphology on solid-state lithiation process for high-quality ultrahigh-nickel oxide cathodes. Angew. Chem. Int. Ed. 2024, 63, e202407477. [Google Scholar] [CrossRef]
- Zou, L.; Zhao, W.; Jia, H.; Zheng, J.; Li, L.; Abraham, D.P.; Chen, G.; Croy, J.R.; Zhang, J.-G.; Wang, X.C. The role of secondary particle structures in surface phase transitions of Ni-rich cathodes. Chem. Mater. 2020, 32, 2884–2892. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, J.-H.; Aishova, A.; Ribas, R.M.; Monteiro, R.S.; Griffith, K.J.; Yoon, C.S.; Sun, Y.-K. Microstructure engineered Ni-rich layered cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 2100884. [Google Scholar] [CrossRef]
- Park, G.-T.; Yoon, D.R.; Kim, U.-H.; Namkoong, B.; Lee, J.; Wang, M.M.; Lee, A.C.; Gu, X.W.; Chueh, W.C.; Yoon, C.S.; et al. Ultrafine-grained Ni-rich layered cathode for advanced Li-ion batteries. Energy Environ. Sci. 2021, 14, 6616–6626. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wu, T.; Lu, J. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 2022, 611, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xu, Z.; Yang, Z.; Kuai, C.; Du, Z.; Sun, C.-J.; Ren, Y.; Liu, J.; Xiao, X.; Lin, F. Effect of the grain arrangements on the thermal stability of polycrystalline nickel-rich lithium-based battery cathodes. Nat. Commun. 2022, 13, 3437. [Google Scholar] [CrossRef]
- Namkoong, B.; Park, N.-Y.; Park, G.-T.; Shin, J.-Y.; Beierling, T.; Yoon, C.S.; Sun, Y.-K. High-energy Ni-rich cathode materials for long-range and long-life electric vehicles. Adv. Energy Mater. 2022, 12, 2200615. [Google Scholar] [CrossRef]
- Trevisanello, E.; Ruess, R.; Conforto, G.; Richter, F.H.; Janek, J. Polycrystalline and single crystalline NCM cathode materials—Quantifying particle cracking, active surface area, and lithium diffusion. Adv. Energy Mater. 2021, 11, 2003400. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, R.; Zhou, K.; Gao, Z.; He, W.; Zhang, L.; Han, C.; Kang, F.; Li, B. A comparative investigation of single crystal and polycrystalline Ni-rich NCMs as cathodes for lithium-ion batteries. Energy Environ. Mater. 2022, 6, e12331. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Ge, M.; Xin, H.L. Accelerated degradation in a quasi-single-crystalline layered oxide cathode for lithium-ion batteries caused by residual grain boundaries. Nano Lett. 2022, 22, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yao, Z.; Zheng, J.; Fu, M.; Cen, J.; Hwang, S.; Jin, H.; Orlov, A.; Gu, L.; Wang, S.; et al. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode. Angew. Chem. Int. Ed. 2020, 59, 22092–22099. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, W.; Zhou, J.; Meng, J.; Huang, W.; Chen, T.; Gao, Q.; Wei, X.; Zeng, Y.; Li, J.; et al. Ni–Li anti-site defect induced intragranular cracking in Ni-rich layer-structured cathode. Nano Energy 2020, 76, 105021. [Google Scholar] [CrossRef]
- Meng, X.H.; Lin, T.; Mao, H.; Shi, J.L.; Sheng, H.; Zou, Y.G.; Fan, M.; Jiang, K.; Xiao, R.J.; Xiao, D.; et al. Kinetic origin of planar gliding in single-crystalline Ni-rich cathodes. J. Am. Chem. Soc. 2022, 144, 11338–11347. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhang, R.; Lei, T.; Kisslinger, K.; Xin, H.L. Resolving complex intralayer transition motifs in high-Ni-content layered cathode materials for lithium-ion batteries. Nat. Mater. 2023, 22, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cao, X.; Yang, H.; He, P.; Dato, M.A.; Cabana, J.; Zhou, H. The origin of high-voltage stability in single-crystal layered Ni-rich cathode materials. Angew. Chem. 2022, 134, e202207225. [Google Scholar] [CrossRef]
- Saaid, F.I.; Kasim, M.F.; Winie, T.; Elong, K.A.; Azahidi, A.; Basri, N.D.; Yaakob, M.K.; Mastuli, M.S.; Shaffee, S.N.A.; Yaakob, M.K.; et al. Ni-rich lithium nickel manganese cobalt oxide cathode materials: A review on the synthesis methods and their electrochemical performances. Heliyon 2024, 10, e23968. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Du, K.; Cao, Y.B.; Lu, Y.; Peng, Z.D.; Fan, J.; Hu, G. Hydrothermal preparing agglomerate LiNi0.8Co0.1Mn0.1O2 cathode material with submicron primary particle for alleviating microcracks. J. Power Sources 2020, 477, 228701. [Google Scholar] [CrossRef]
- Li, Y.; He, J.J.; Luo, L.; Li, X.; Chen, Z.; Zhang, Y.B.; Deng, L.; Dong, P.; Yang, S.; Wu, K.; et al. Highly dispersed micrometer nickel-rich single-crystal construction: Benefits of supercritical reconstruction during hydrothermal synthesis. ACS Appl. Energy Mater. 2022, 5, 6302–6312. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Wang, Z.; Tong, J.; Qi, N.; Han, G.; Zhang, M. Self-assembled porous LiNi0.8Co0.1Mn0.1O2 cathode materials with micro/nano-layered hollow morphologies for high-power lithium-ion batteries. Appl. Surf. Sci. 2021, 539, 148034. [Google Scholar] [CrossRef]
- Li, S.Y.; Liang, W.B.; Xie, J.; Wei, Y.; Cui, X.L. Synthesis of hollow microspheres LiNi0.5Mn1.5O4 coated with Al2O3 and characterization of the electrochemical capabilities. J. Electrochem. Energy Convers. Storage 2020, 17, 031007. [Google Scholar]
- Guo, J.; Li, W. Synthesis of single-crystal LiNi0.7Co0.15Mn0.15O2 materials for Li-ion batteries by a sol-gel method. ACS Appl. Energy Mater. 2022, 5, 397–406. [Google Scholar] [CrossRef]
- Bai, X.; Wei, A.J.; He, R.; Li, W.; Li, X.H.; Zhang, L.H.; Liu, Z. The structural and electrochemical performance of Mg-doped LiNi0.85Co0.10Al0.05O2 prepared by a solid-state method. J. Electroanal. Chem. 2020, 858, 113771. [Google Scholar] [CrossRef]
- Zheng, L.; Bennett, J.C.; Obrovac, M.N. All-dry synthesis of single crystal NMC cathode materials for Li-ion batteries. J. Electrochem. Soc. 2020, 167, 130536. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Xu, C.; Cheng, J.; Zhao, J.; Huang, Q.; Yang, C. Advances in reactive co-precipitation technology for preparing high-performance cathodes. Green Carbon 2023, 1, 193–209. [Google Scholar] [CrossRef]
- He, F.R.; Tian, Z.Q.; Xiang, W.; Yang, W.; Zheng, B.P.; Cai, J.Y.; Guo, X.D. Insight into the surface reconstruction-induced structure and electrochemical performance evolution for Ni-rich cathodes with postannealing after washing. ACS Appl. Mater. Interfaces 2023, 15, 9160–9170. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Shi, L.; Wang, Z.; Wang, Y.; Zhang, M.; Yuan, S. Advances of high-performance LiNi1-x-yCoxMyO2 cathode materials and their precursor particles via co-precipitation process. Particuology 2024, 86, 67–85. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Q.; Zhan, C.; Liu, G. One-step solid-state synthesis of Ni-rich cathode materials for lithium-ion batteries. Materials 2023, 16, 3079. [Google Scholar] [CrossRef]
- El Kouihen, F.; Chakir, M.; Faik, A. The effect of synthesis methods on cation mixing degree in cobalt-free, nickel-rich NMA materials. Chem. Pap. 2025, 79, 2411–2420. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Wang, S.; Wang, M.; Jiang, F.; Liu, Y.; Huang, Y.; Zheng, J. Optimal synthetic conditions for a novel and high-performance Ni-rich cathode material of LiNi0.68Co0.10Mn0.22O2. Sustain. Energy Fuels 2018, 2, 1772–1780. [Google Scholar]
- Ying, B.X.; Fitzpatrick, J.R.; Teng, Z.J.; Chen, T.X.; Lo, T.W.B.; Siozios, V.; Murray, C.A.; Brand, H.E.A.; Day, S.; Tang, C.C.; et al. Monitoring the formation of nickel-poor and nickel-rich oxide cathode materials for lithium-ion batteries with synchrotron radiation. Chem. Mater. 2023, 35, 1514–1526. [Google Scholar] [CrossRef]
- Han, Q.; Cai, L.L.; Yang, Z.F.; Hu, Y.J.; Jiang, H.; Li, C.Z. New insights into the pre-lithiation kinetics of single-crystalline Ni-rich cathodes for long-life Li-ion batteries. Green Energy Environ. 2024, 9, 556–564. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, L.; Li, J.T.; Zhang, B.; Hu, Q.; Liu, J.L.; Wu, Y.Q.; Gao, J.H.; Chen, Y.B.; Song, S.L.; et al. Rational synthesis of high-performance Ni-rich layered oxide cathode enabled via probing solid-state lithiation evolution. Nano Energy 2023, 113, 108528. [Google Scholar] [CrossRef]
- Liu, J.J.; Yuan, Y.F.; Zheng, J.H.; Wang, L.G.; Ji, J.; Zhang, Q.; Yang, L.; Bai, Z.Y.; Lu, J. Understanding the synthesis kinetics of single-crystal Co-free Ni-rich cathodes. Angew. Chem. Int. Ed. 2023, 62, e202302547. [Google Scholar] [CrossRef] [PubMed]
- Wolfman, M.; Wang, X.P.; Garcia, J.C.; Barai, P.; Stubbs, J.E.; Eng, P.J.; Kahvecioglu, O.; Kinnibrugh, T.L.; Madsen, K.E.; Iddir, H.; et al. Spheroidization: The impact of precursor morphology on solid-state lithiation process for high-quality ultrahigh-nickel oxide cathodes. Adv. Energy Mater. 2022, 12, 2102951. [Google Scholar] [CrossRef]
- Xu, Z.R.; Jiang, Z.S.; Kuai, C.G.; Xu, R.; Qin, C.D.; Zhang, Y.; Rahman, M.M.; Wei, C.; Nordlund, D.; Sun, C.-J.; et al. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials. Nat. Commun. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Polierer, S.; Guse, D.; Wild, S.; Delgado, K.H.; Otto, T.N.; Zevaco, T.A.; Kind, M.; Sauer, J.; Studt, F.; Pitter, S. Enhanced direct dimethyl ether synthesis from CO2 rich syngas with Cu/ZnO/ZrO2 catalysts prepared by continuous co-precipitation. Catalysts 2020, 10, 816. [Google Scholar] [CrossRef]
- Ahmed, R.R.; Mubarak, T.H.; Mohamed, I.H. A study of structural and chemical properties of Ni1-xZnxFe2O4 ferrite powder prepared by co-precipitation method. Digest J. Nanomater. Biostruct. 2022, 17, 741–748. [Google Scholar] [CrossRef]
- Entwistle, T.; Sanchez-Perez, E.; Murray, G.J.; Anthonisamy, N.; Cussen, S.A. Co-precipitation synthesis of nickel-rich cathodes for Li-ion batteries. Energy Rep. 2022, 8, 67–73. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, K.Y.; Choi, B.K.; Yoo, H.D. A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for lithium-ion batteries. J. Electroanal. Chem. 2022, 924, 116828. [Google Scholar] [CrossRef]
- Heo, K.; Lee, J.S.; Kim, H.S.; Kim, M.Y.; Jeong, H.; Kim, J.; Lim, J. Ionic conductor-LiNi0.8Co0.1Mn0.1O2 composite synthesized by simultaneous co-precipitation for use in lithium-ion batteries. J. Electrochem. Soc. 2018, 165, A2955–A2960. [Google Scholar] [CrossRef]
- Xu, L.P.; Zhou, F.; Kong, J.Z.; Zhou, H.B.; Zhang, Q.C.; Wang, Q.Z.; Yan, G. Influence of precursor phase on the structure and electrochemical properties of LiNi0.6Mn0.2Co0.2O2 cathode materials. Solid State Ion. 2018, 324, 49–58. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, G.R.; Peng, Z.D.; Cao, Y.B.; Li, L.Y.; Tan, C.P.; Du, K. Synthesis and characterization of mono-dispersion LiNi0.8Co0.1Mn0.1O2 micrometer particles for lithium-ion batteries. Ceram. Int. 2021, 47, 25680–25688. [Google Scholar] [CrossRef]
- Pollen, H.N.; Tolchard, J.R.; Svensson, A.M.; Wagner, N.P. A single-pot co-precipitation synthesis route for Ni-rich layered oxide materials with high cycling stability. ChemElectroChem 2022, 9, e202200859. [Google Scholar] [CrossRef]
- Para, M.L.; Alidoost, M.; Shiea, M.; Boccardo, G.; Buffo, A.; Barresi, A.A.; Marchisio, D. A modelling and experimental study on the co-precipitation of Ni0.8Mn0.1Co0.1(OH)2 as precursor for battery cathodes. Chem. Eng. Sci. 2022, 254, 117634. [Google Scholar] [CrossRef]
- Zhang, H.W.; Cen, T.; Tian, Y.H.; Zhang, X.J. Synthesis of high-performance single-crystal LiNi0.8Co0.1Mn0.1O2 cathode materials by controlling solution super-saturation. J. Power Sources 2022, 532, 231037. [Google Scholar] [CrossRef]
- Kumar, D.; Kurian, E.; Ramesha, K. Synthesis of quasi-spherical LiNi0.86Mn0.1Co0.04O2 cathode particles via hydroxide coprecipitation: Influence of pH on precursor particle size in enhancing capacity and stability of Ni-rich cathode for lithium-ion batteries. Energy Technol. 2025, 2402418. [Google Scholar] [CrossRef]
- Duan, J.H.; Zhang, R.C.; Zhu, Q.H.; Xiao, H.; Huang, Q.S. The effect of controlling strategies of pH and ammonia concentration on preparing full concentration gradient Ni0.8Co0.1Mn0.1(OH)2 via co-precipitation in a pilot-scale reactor. Energy Technol. 2020, 8, 1901437. [Google Scholar] [CrossRef]
- Park, B.H.; Kim, T.; Park, H.; Sohn, Y.; Shin, J.; Kang, M. Electrochemical performance of layer-structured Ni0.8Co0.1Mn0.1O2 cathode active materials synthesized by carbonate co-precipitation. Nanomaterials 2022, 12, 3610. [Google Scholar] [CrossRef]
- Shen, W.-Z.; Ma, Y.; Yao, C.; Liang, F. Controlling the precursor morphology of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode for lithium-ion battery. Nano 2019, 14, 100–108. [Google Scholar] [CrossRef]
- Hu, K.H.; He, Y.Y.; Zhu, C.Q.; Zhou, K.; Chen, Q.; Yang, Z.M.; Wan, B.-R.; Yang, E.-Q.; Zhang, T.-T.; Qin, Y.-D. Insight into the evolution of precursor and electrochemical performance of Ni-rich cathode modulated by ammonia during hydroxide precipitation. J. Alloys Compd. 2019, 803, 538–545. [Google Scholar] [CrossRef]
- Liang, H.L.; Yuan, S.; Shi, L.Y.; Zhao, Y.; Wang, Z.Y.; Zhu, J.F. Highly-ordered microstructure and well performance of LiNi0.6Mn0.2Co0.2O2 cathode material via the continuous microfluidic synthesis. Chem. Eng. J. 2020, 394, 124846. [Google Scholar] [CrossRef]
- Zou, L.; Li, J.; Liu, Z.; Wang, G.; Manthiram, A.; Wang, C. Lattice doping regulated interfacial reactions in cathode for enhanced cycling stability. Nat. Commun. 2019, 10, 3447. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Hwang, J.U.; Im, J.S.; Lee, J.D. Electrochemical properties of LiNi0.9Co0.1O2 cathode material prepared by co-precipitation using an eco-friendly chelating agent. J. Solid State Electrochem. 2022, 26, 1567–1576. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Zhang, B.; Zhu, H.; Liu, Q.; Liu, J.; Zhu, M. A nanorod-like Ni-rich layered cathode with enhanced Li+ diffusion pathways for high-performance lithium-ion batteries. J. Mater. Chem. 2021, 9, 2830–2839. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Ren, L.; Wu, W.; Zuo, M.; Xing, W.; Zhang, B.; Fan, W.; He, Z.; Yu, Z.; et al. Micro-structure tuning and evolution of hydroxide precursor with radially oriented grains during industrial-scale continuous precipitation process. J. Alloys Compd. 2024, 977, 173458. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, F.; Wang, S.; Zhao, T.; Peng, J.; Zhang, B.; Fan, W.; Xing, W.; Zuo, M.; Zhang, P.; et al. Precision engineering of high-performance Ni-rich layered cathodes with radially aligned microstructure through architectural regulation of precursors. eScience 2024, 4, 100276. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Xiao, H.; Zhang, R.C.; Geng, S.J.; Huang, Q.S. Effect of impeller type on preparing spherical and dense Ni1-x-yCoxMny(OH)2 precursor via continuous co-precipitation in pilot scale: A case of Ni0.6Co0.2Mn0.2(OH)2. Electrochim. Acta 2019, 318, 1–13. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Liu, Y.; Ye, Y.; Yang, Y. Facile synthesis and electrochemical performance of lithium-rich layered oxides with stable hierarchical structure through HEPES-assisted co-precipitation method. Electrochim. Acta 2022, 401, 139485. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, X.; Zhao, T.; Yang, X.; Wang, Q.; Chen, Z.; Chen, M.-C.; Ma, J.-J.; Lu, Y.-R.; Hung, S.-F.; et al. What impact does ammonia have on the microstructure of the precursor and the electrochemical performance of Ni-rich layered oxides? J. Mater. Chem. A 2025, 13, 1181–1190. [Google Scholar] [CrossRef]
- Kim, E.; Cho, Y.H.; Shin, J.H.; Eun, H.J.; Song, H.I.; Lee, S.-H.; Suk, J.; Moon, S. Unlocking the superior performance of Ni-rich layered materials through precise precursor engineering. J. Eur. Ceram. Soc. 2025, 45, 117280. [Google Scholar] [CrossRef]
- Lao, J.; Lu, Y. Synthesis of nano cubic microstructure LiNi0.8Co0.1Mn0.1O2 cathode materials via rapid precipitation assisted with hydrothermal treatment. Heliyon 2024, 10, e40201. [Google Scholar] [CrossRef]
- Chen, A.; Wang, K.; Li, J.; Mao, Q.; Xiao, Z.; Zhu, D.; Wang, G.; Liao, P.; He, J.; You, Y.; et al. The formation, detriment and solution of residual lithium compounds on Ni-rich layered oxides in lithium-ion batteries. Front. Energy Res. 2020, 8, 593009. [Google Scholar] [CrossRef]
- Li, W.D.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Dai, A.; De Andrade, V.; Ren, Y.; Xu, W.; Lee, S.; Zhang, Q.; Gu, L.; Wang, S.; et al. Reaction inhomogeneity coupling with metal rearrangement triggers electrochemical degradation in lithium-rich layered cathode. Nat. Commun. 2021, 12, 5370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, X.; Zhang, Y.; Zhou, J.; Wang, J.; Xu, S. Grain size regulation for balancing cycle performance and rate capability of LiNi0.9Co0.055Mn0.045O2 single crystal nickel-rich cathode materials. J. Energy Chem. 2022, 65, 681–687. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Seenivasan, M.; Wu, Y.-S.; Wu, S.-H.; Chang, J.-K.; Jose, R.; Yang, C.-C. Preparation of long-term cycling stable Ni-rich concentration-gradient NCMA cathode materials for li-ion batteries. J. Colloid Interface Sci. 2023, 639, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Jin, F.; Zhao, Y.; Shi, L.; Liu, Q.; Wang, Z.; Wang, Y.; Zhang, M.; Zhu, J.; Yuan, S. Synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 materials for Li-ion batteries by microfluidic technology. Chem. Eng. J. 2023, 464, 142656. [Google Scholar] [CrossRef]
- Riewald, F.; Kurzhals, P.; Bianchini, M.; Sommer, H.; Janek, J.; Gasteiger, H.A. The LiNiO2 cathode active material: A comprehensive study of calcination conditions and their correlation with physicochemical properties part II. Morphology. J. Electrochem. Soc. 2022, 169, 020529. [Google Scholar] [CrossRef]
- Yu, Z.; Qu, X.; Wan, T.; Dou, A.; Zhou, Y.; Peng, X.; Su, M.; Liu, Y.; Chu, D. Synthesis and mechanism of high structural stability of nickel-rich cathode materials by adjusting Li-excess. ACS Appl. Mater. Interfaces 2020, 12, 40393–40403. [Google Scholar] [CrossRef]
- Kan, H.; Yang, Z.; Meng, Q.; Dong, P.; Zhang, Y. Preparation and electrochemical performance of the Ni-rich Co-free cathode material LiNi0.94Mn0.04Al0.02O2. Energy Fuels 2024, 38, 6420–6426. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Warner, J.H.; Manthiram, A. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Lett. 2021, 6, 941–948. [Google Scholar] [CrossRef]
- Li, J.; Liang, G.; Zheng, W.; Zhang, S.; Davey, K.; Pang, W.K.; Guo, Z. Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review. Nano Mater. Sci. 2023, 5, 404–420. [Google Scholar] [CrossRef]
- Li, X.Q.; Xiong, X.; Wang, Z.; Chen, Q. Effect of sintering temperature on cycling performance and rate performance of LiNi0.8Co0.1Mn0.1O2. Trans. Nonferr. Met. Soc. China 2014, 24, 4023–4029. [Google Scholar] [CrossRef]
- Ding, Y.; Mu, D.; Wu, B.; Wang, R.; Zhao, Z.; Wu, F. Recent progresses on nickel-rich layered oxide positive electrode materials used in lithium-ion batteries for electric vehicles. Appl. Energy 2017, 195, 586–599. [Google Scholar] [CrossRef]
- Wang, L.; Lei, X.; Liu, T.; Dai, A.; Su, D.; Amine, K.; Lu, J.; Wu, T. Regulation of surface defect chemistry toward stable Ni-rich cathodes. Adv. Mater. 2022, 34, 2200744. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Tan, X.; Guo, H.; Peng, W.; Li, X.; Wang, J.; Yan, G. Correlating morphological and structural evolution with the electrochemical performance of nickel-rich cathode materials: From polycrystal to single crystal. J. Electrochem. Soc. 2022, 169, 090520. [Google Scholar] [CrossRef]
- Allen, E.; Shin, Y.; Judge, W.; Wolfman, M.; De Andrade, V.; Cologna, S.M.; Cabana, J. 3D quantification of elemental gradients within heterostructured particles of battery cathodes. ACS Energy Lett. 2023, 8, 1371–1378. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.; Kim, Y.; Choi, W.; Yoon, W.-S. Enhancing the structural durability of Ni-rich layered materials by post-process: Washing and heat-treatment. J. Mater. Chem. A 2020, 8, 10206–10216. [Google Scholar] [CrossRef]
- Karger, L.; Korneychuk, S.; Sicolo, S.; Li, H.; van der Bergh, W.; Zhang, R.; Indris, S.; Kondrakov, A.; Janek, J.; Brezesinski, T. Decoupling substitution effects from point defects in layered Ni-rich oxide cathode materials for lithium-ion batteries. Adv. Funct. Mater. 2024, 34, 2402444. [Google Scholar] [CrossRef]
- Wang, T.; Ren, K.; Xiao, W.; Dong, W.; Qiao, H.; Duan, A.; Pan, H.; Yang, Y.; Wang, H. Tuning the Li/Ni disorder of the NMC811 cathode by thermally driven competition between lattice ordering and structure decomposition. J. Phys. Chem. C 2020, 124, 5600–5607. [Google Scholar] [CrossRef]
- Mo, Y.; Liu, S.; Yuan, G.; Li, Z.; Zhang, M.; Guo, L. Enhancing the reversibility of the chemical evolution of the Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode via a simple pre-oxidation process. RSC Adv. 2024, 14, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huo, H.; Jian, J.-Y.; Wang, L.-G.; Zhu, H.; Xu, S.; He, X.-S.; Yin, G.-P.; Du, C.-Y.; Sun, X.-L. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1803963. [Google Scholar] [CrossRef]
- Wang, T.; Ren, K.; He, M.; Dong, W.; Xiao, W.; Pan, H.; Yang, J.; Yang, Y.; Liu, P.; Cao, Z.; et al. Synthesis and manipulation of single-crystalline lithium nickel manganese cobalt oxide cathodes: A review of growth mechanism. Front. Chem. 2020, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Harlow, J.; Dahn, J. Microstructural observations of “single crystal” positive electrode materials before and after long term cycling by cross-section scanning electron microscopy. J. Electrochem. Soc. 2020, 167, 020512. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Peng, S.; Zeng, J.; Zhao, J. Superiority of single crystal to polycrystalline LiNixCoyMn1-x-yO2 cathode materials in storage behaviors for lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 14938–14948. [Google Scholar] [CrossRef]
- Lüther, M.J.; Jiang, S.-K.; Lange, M.A.; Buchmann, J.; Martin, A.G.; Schmuch, R.; Placke, T.; Hwang, B.J.; Winter, M.; Kasnatscheew, J. Systematic “apple-to-apple” comparison of single-crystal and polycrystalline Ni-rich cathode active materials: From comparable synthesis to comparable electrochemical conditions. Small 2024, 5, 2400119. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Chen, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Fan, X.-M.; Hu, G.-R.; Zhang, B.; Ou, X.; Zhang, J.-F.; Zhao, W.-G.; Jia, H.-P.; Zou, L.F.; Li, P.; Yang, Y. Crack-free single crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim. Acta 2021, 365, 137380. [Google Scholar] [CrossRef]
- Han, Y.; Xu, J.; Wang, W.; Long, F.; Qu, Q.; Wang, Y.; Zheng, H. Implanting an electrolyte additive on a single crystal Ni-rich cathode surface for improved cycle ability and safety. J. Mater. Chem. A 2020, 8, 24579–24589. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Fan, C.; Han, S. A highly stabilized single crystalline nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode through a novel surface spinel-phase modification. Electrochim. Acta 2020, 341, 136075. [Google Scholar] [CrossRef]
- Ge, M.; Wi, S.; Liu, X.; Bai, J.; Ehrlich, S.; Lu, D.; Lee, W.K.; Chen, Z.; Wang, F. Kinetic limitations in single-crystal high-nickel cathodes. Angew. Chem. Int. Ed. 2021, 60, 17350–17355. [Google Scholar] [CrossRef]
- You, B.; Wang, Z.; Shen, F.; Chang, Y.; Peng, W.; Li, X.; Guo, H.; Hu, Q.; Deng, C.; Yang, S.; et al. Research progress of single-crystal nickel-rich cathode materials for lithium-ion batteries. Small Methods 2021, 5, 2100234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, E.; Zhang, X.; Yu, H. High-voltage “single-crystal” cathode materials for lithium-ion batteries. Energy Fuels 2021, 35, 918–932. [Google Scholar] [CrossRef]
- Han, Y.; Lei, Y.; Ni, J.; Zhang, Y.; Geng, Z.; Ming, P.; Zhang, C.; Tian, X.; Shi, J.-L.; Guo, Y.; et al. Single-crystalline cathodes for advanced Li-ion batteries: Progress and challenges. Small 2022, 18, 2107048. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, L.; Zhang, L.; Fan, X.; Zhang, H.; Pagani, F.; Brack, E.; Seidl, L.; Ou, X.; Egorov, K.; et al. Assessing long-term cycling stability of single-crystal versus polycrystalline nickel-rich NCM in pouch cells with 6 mAh cm−2 electrodes. Small 2022, 18, 2107357. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, S.; Li, S.; Yu, Z.; Liu, Q.; Dai, A.; Cao, C.; Toney, M.F.; Ge, M.; Xiao, X.; et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 2020, 11, 3050. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Bi, Y.; Tao, J.; Lochala, J.; Liu, D.; Wu, B.; Cao, X.; Chae, S.; Wang, C.; et al. Locking oxygen in lattice: A quantifiable comparison of gas generation in polycrystalline and single crystal Ni-rich cathodes. Energy Storage Mater. 2022, 47, 195–202. [Google Scholar] [CrossRef]
- Ge, J.; Xie, M.; Zhao, Q.; Zhang, S.; Sun, H. Advances in Co-free layered cathode materials for Li-ion batteries. Int. J. Electrochem. Sci. 2023, 18, 100292. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Tian, H.; Yan, J.; Tong, J.; Liu, X.; Zhang, H.; Huang, H.; Hao, S.; Gao, J.; et al. Pulse high temperature sintering to prepare single-crystal high nickel oxide cathodes with enhanced electrochemical performance. Adv. Energy Mater. 2023, 13, 2203188. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Song, Y.; Zhang, Z.; Du, A.; Tang, Y.; Wang, J.; He, X. Why the synthesis affects performance of layered transition metal oxide cathode materials for Li-ion batteries. Adv. Mater. 2024, 36, 2312292. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lv, F.; Wang, Z.; Feng, J.; Wu, M.; Liu, Z.; Chen, L.; Gu, Y. Open-channel [011] facet CeO2 with a shorter pathway of Li+ migration as a modification material for LiNi0.8Co0.1Mn0.1O2 toward high-rate lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 8795–8802. [Google Scholar] [CrossRef]
- Liu, H.-W.; Parthasarathi, S.-K.; Thi, S.; Weng, Y.-T.; Bolloju, S.; Chen, C.C.; Jeng, R.-J.; Wu, N.-L. A comparative study of polycrystal/single-crystal LiNi0.8Co0.1Mn0.1O2 in all-solid-state Li-ion batteries with halide-based electrolyte under low stacking pressure. Energy Technol. 2023, 11, 2201439. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Deng, Q.; Chu, Y.; Dong, P.; Chen, C.; Wang, Z.; Xia, Z.; Yang, C. In situ and real-time monitoring the chemical and thermal evolution of lithium-ion batteries with single-crystalline Ni-rich layered oxide cathode. Angew. Chem. Int. Ed. 2024, 63, e202401716. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Zhao, J.; Zhao, W.; Liang, Z.; Su, Y.; Xie, C.; Zhou, K.; Xiang, Y.; Zhu, J.; et al. Electrochemo-mechanical effects on structural integrity of Ni-rich cathodes with different microstructures in all solid-state batteries. Adv. Energy Mater. 2021, 11, 2003583. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, Y.; Wiaderek, K.M.; Borkiewicz, O.J.; Ren, Y.; Zhang, J.; Ren, J.; Fan, L.; Li, C.C.; Li, D.; et al. Spontaneous strain buffer enables superior cycling stability in single-crystal nickel-rich NCM cathode. Nano Lett. 2021, 21, 9997–10005. [Google Scholar] [CrossRef]
- Ran, A.; Chen, S.; Cheng, M.; Liang, Z.; Li, B.; Zhou, G.; Kang, F.; Zhang, X.; Wei, G. A single-crystal nickel-rich material as a highly stable cathode for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 19680–19689. [Google Scholar] [CrossRef]
- Han, Q.; Yu, H.; Cai, L.; Jiang, H. Unique insights into the design of low-strain single-crystalline Ni-rich cathodes with superior cycling stability. Proc. Natl. Acad. Sci. USA 2024, 121, e2317282121. [Google Scholar] [CrossRef]
- Cha, H.; Kim, J.; Lee, H.; Kim, N.; Hwang, J.; Sung, J.; Yoon, M.; Kim, K.; Cho, J. Boosting reaction homogeneity in high-energy lithium-ion battery cathode materials. Adv. Mater. 2020, 32, 2003040. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Li, J.; Yang, H.; Dai, P.; Zeng, J.; Zhao, J. Single-crystal structure helps enhance the thermal performance of Ni-rich layered cathode materials for lithium-ion batteries. Chem. Eng. J. 2022, 434, 134638. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Siu, C.; Ge, M.; Kisslinger, K.; Shin, Y.; Xin, H.L. Chemomechanically stable ultrahigh-Ni single-crystalline cathodes with improved oxygen retention and delayed phase degradations. Nano Lett. 2021, 21, 9797–9804. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, W.; Karger, L.; Murugan, S.; Janek, J.; Kondrakov, A.; Brezesinski, T. Single crystal layered oxide cathodes: The relationship between particle size, rate capability, and stability. ChemElectroChem 2023, 10, e202300165. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, S.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. A comparative investigation of single crystal and polycrystalline Ni-rich layered cathodes. Energy Environ. Mater. 2022, 12, 2201510. [Google Scholar]
- Zhou, X.; Wang, X.; Xu, Z.; Wang, Q.; Lu, C.; Ma, R.; Zheng, Y.; Ruan, M.; Mao, Q.; Xu, J.; et al. Facile ball-milling synthesis of pseudo-single-crystalline nickel-rich layered oxide cathodes towards long-lifespan lithium-ion batteries. J. Electron. Mater. 2024, 53, 7355–7366. [Google Scholar] [CrossRef]
- Leng, J.; Wang, J.; Peng, W.; Tang, Z.; Xu, S.; Liu, Y.; Wang, J. Highly-dispersed submicrometer single-crystal nickel-rich layered cathode: Spray synthesis and accelerated lithium-ion transport. Small 2021, 17, e2006869. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, M.; Guo, Z.; Cao, L.; Xiao, Y.; Chen, L.; Cheng, Y.-J.; Xia, Y. Simultaneous enhancement of ordered layered structure and inhibition of micro-cracks via porous structure to improve stability for Ni-rich cathode. J. Energy Storage 2024, 76, 109936. [Google Scholar] [CrossRef]
- Chen, J.; Feng, S.; Deng, J.; Zhou, Y. Insights into the porosity and radial structure for precursor of Ni-rich LiNi0.9Co0.05Mn0.05O2 cathode materials. Chem. Engin. Sci. 2025, 304, 121092. [Google Scholar] [CrossRef]
- Zou, K.; Xie, S.; Jiang, M.; Wang, P.; Ning, T.; Tan, L.; Li, H.; Zhou, Y.; Wang, W.; Li, L. Insights into the precursor specific surface area for engineering Co-free Ni-rich cathodes with tailorable properties. Chem. Eng. J. 2024, 483, 149189. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, H.S.; Hong, S.; Jeon, H.; Lim, J.; Jung, Y.H.; Ahn, H.; Jang, J.D.; Kim, M.-H.; Seo, J.H.; et al. Toward a nanoscale-defect-free Ni-rich layered oxide Cathode through Regulated pore evolution for long-lifespan Li rechargeable batteries. Adv. Func. Mater. 2024, 34, 2306654. [Google Scholar] [CrossRef]
- Lv, F.; Zhang, Y.; Wu, M.; Gu, Y. A molten-salt method to synthesize ultrahigh-nickel single-crystalline LiNi0.92Co0.06Mn0.02O2 with superior electrochemical performance as cathode material for lithium-ion batteries. Small 2022, 18, 2201946. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Liu, X.; Gao, J.; Hao, S.; Yang, Y.; Qiu, J. Perspective on the preparation methods of single crystalline high nickel oxide cathode materials. Adv. Energy Mater. 2023, 13, 2300378. [Google Scholar] [CrossRef]
- Yoo, Y.-W.; Kang, C.-Y.; Kim, H.-K.; Lee, J.-K.; Kumar, R.V.; Kim, K.-N.; Yoon, J.-R.; Lee, S.-H. Enhanced structure/interfacial properties of single-crystal Ni-rich LiNi0.92Co0.04Mn0.04O2 cathodes synthesized via LiCl-NaCl molten-salt method. Energy Environ. Mater. 2025, 8, e12778. [Google Scholar] [CrossRef]
- Kim, S.; Park, K. Electrode design to mitigate the kinetic issue of cathodes in high energy lithium-ion batteries. J. Power Sources 2022, 547, 231916. [Google Scholar] [CrossRef]
- Guo, F.; Xie, Y.; Zhang, Y. Low-temperature strategy to synthesize single-crystal LiNi0.8Co0.1Mn00.1O2 with enhanced cycling performances as cathode material for lithium-ion batteries. Nano Res. 2022, 15, 2052–2059. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.; Lee, E.; Choi, M.; Thangavel, R.; Lee, Y.; Yoon, W.-S. Destabilization of the surface structure of Ni-rich layered materials by water-washing process. Energy Storage Mater. 2022, 44, 441–451. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Lim, H.-W.; Lee, S.G.; Sun, Y.-K. Near-surface reconstruction in Ni-rich layered cathodes for high-performance lithium-ion batteries. Nat. Energy 2024, 9, 47–56. [Google Scholar] [CrossRef]
- Hu, B.-R.; Yuan, Y.-Y.; Wang, Y.-C.; Xiong, X.-H. Simultaneously enhanced electrochemical performance and air stability of Ni-rich cathode with a modified washing process. Rare Met. 2023, 43, 87–97. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Wang, F.; Ding, J.; An, B.; Rua, J.; Sun, D.; Fang, F.; Wang, F. One-step molten-salt-assisted approach for direct preparation and regeneration of LiNi0.6Co0.2Mn0.2O2 cathode. Small 2024, 20, 2400762. [Google Scholar] [CrossRef]
- Guo, Z.; Jian, Z.; Zhang, S.; Feng, Y.; Kou, W.; Ji, H.; Yang, G. The effect of Ni oxidation state on the crystal structure and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material for highly reversible lithium storage. J. Alloys Compd. 2021, 882, 160642. [Google Scholar] [CrossRef]
- Gao, C.; Liu, H.; Zhang, J.; Guo, Z.; Huang, H. Simplified crystal grain boundary engineering of solid electrolyte-infused LiNi0.5Mn1.5O4 cathodes for high cycling stability lithium-ion batteries. J. Power Sources 2023, 582, 233434. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Deng, J.; Han, Z.; Xiao, X.; Wang, L.; Chen, Z.; Deng, Y.; He, X. Insight of synthesis of single crystal Ni-rich LiNi1–x–yCoxMnyO2 cathodes. Adv. Energy Mater. 2024, 14, 2303758. [Google Scholar] [CrossRef]
- Tayal, A.; Barai, P.; Zhong, H.; Kahvecioglu, O.; Wang, X.; Pupek, K.Z.; Ma, L.; Ehrlich, N.; Srinivasan, V.; Qu, X.; et al. In situ insights into cathode calcination for predictive synthesis: Kinetic crystallization of LiNiO2 from hydroxides. Adv. Mater. 2024, 36, 2312027. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, H.; Gao, J.; Wang, J.; Shao, M.; Zhou, W. Grain-growth inhibitor with Three-section-sintering for highly dispersed single-crystal NCM90 cubes. Angew. Chem. Int. Ed. 2024, 136, e202314457. [Google Scholar] [CrossRef]
- Choi, J.H.; Embleton, T.J.; Ko, K.; Yang, H.; Son, Y.; Park, J.; Lee, S.; Oh, P. A perspective on the requirements of Ni-rich cathode surface modifications for application in lithium-ion batteries and all-solid-state lithium-ion batteries. ChemElectroChem 2024, 11, e202300705. [Google Scholar] [CrossRef]
- Yu, H.; Pei, D. A review of the origin, adverse influence and modification method of residual surface lithium in Ni-rich cathodes. Int. J. Electrochem. Sci. 2024, 19, 100391. [Google Scholar] [CrossRef]
- Han, B.; Key, B.; Lipton, A.S.; Vaughey, J.T.; Hughes, B.; Trevey, J.; Dogan, F. Influence of coating protocols on alumina-coated cathode material: Atomic layer deposition versus wet-chemical coating. J. Electrochem. Soc. 2019, 166, A3679–A3684. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, J.; Lu, J.; Li, Y.; Yan, P.; Zhang, Y. Realizing superior cycling stability of Ni-rich layered cathode by combination of grain boundary engineering and surface coating. Nano Energy 2019, 62, 30–37. [Google Scholar] [CrossRef]
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. 2020, 10, 11114. [Google Scholar] [CrossRef]
- Chen, G.; Peng, B.; Han, R.; Chen, N.; Wang, Z.; Wang, Q. A robust carbon coating strategy toward Ni-rich lithium cathodes. Ceram. Int. 2020, 46, 20985. [Google Scholar] [CrossRef]
- Qiu, Y.; Wei, X.; Liu, N.; Song, Y.; Bi, L.; Long, X.; Chen, Z.; Wang, S.; Liao, J. Plasma-induced amorphous N-nano carbon shell for improving structural stability of LiNi0.8Co0.1Mn0.1O2 cathode. Electrochim. Acta 2022, 428, 140973. [Google Scholar] [CrossRef]
- Jan, S.S.; Nurgul, S.; Shi, X.; Xia, H.; Pang, H. Improvement of chemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by graphene nanosheets modification. Electrochim. Acta 2014, 149, 86–93. [Google Scholar] [CrossRef]
- Du, Z.; Li, J.; Wood, M.; Mao, C.; Daniel, C.; Wood, D.L. Three-dimensional conductive network formed by carbon nanotubes in aqueous processed NMC electrode. Electrochim. Acta 2018, 270, 54–61. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Zheng, J.; Wei, Y.; Qiao, R.; Shen, F.; Dai, J.; Hu, L.; Xu, K.; Lin, Y.; et al. Depolarized and fully active cathode based on Li(Ni0.5Co0.2Mn0.3)O2 embedded in carbon nanotube network for advanced batteries. Nano Lett. 2014, 14, 4700–4706. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Pham, H.Q.; Kang, D.-H.; Park, H.-Y.; Song, S.-W. Improved rate capability of highly loaded carbon fiber-interwoven LiNi0.6Co0.2Mn0.2O2 cathode material for high-power Li-ion batteries. J. Alloys Compd. 2016, 657, 464–471. [Google Scholar] [CrossRef]
- Ning, R.; Yuan, K.; Zhang, K.; Shen, C.; Xie, K. A scalable snowballing strategy to construct uniform rGO-wrapped LiNi0.8Co0.1Mn0.1O2 with enhanced processability and electrochemical performance. Appl. Surf. Sci. 2021, 542, 148663. [Google Scholar] [CrossRef]
- Poursalehi, F.; Javanbakht, M.; Daryakenari, A.A.; Gao, B. Preparing a binder-free cathode composed of NMC811/sulfonated reduced graphene oxide sheets/carbon black via in-situ electrophoretic deposition to enhance cycleability of Li-ion batteries. J. Electroanal. Chem. 2024, 971, 118593. [Google Scholar] [CrossRef]
- She, S.; Zhou, Y.; Hong, Z.; Huang, Y.; Wu, Y. Surface coating of NCM-811 cathode materials with g-C3N4 for enhanced electrochemical performance. ACS Omega. 2022, 7, 24851–24857. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.-H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.-J. Graphene collage on Ni-rich layered oxide cathodes for advanced lithium-ion batteries. Nat. Commun. 2021, 12, 2145. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lim, H.-W.; Kang, G.-C.; Park, N.-Y.; Sun, Y.-K. Long-lasting Ni-rich NCMA cathodes via simultaneous microstructural refinement and surface modification. ACS Energy Lett. 2023, 8, 1354–1361. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.-M.; Song, J.-H.; Yim, T.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Han, B.; Xu, S.; Zhao, S.; Lin, G.-X.; Feng, Y.-Z.; Chen, L.-B.; Ivey, G.-D.; Wang, P.; Wei, W.-F. Enhancing the structural stability of Ni-rich layered oxide cathodes with a preformed Zr-concentrated defective nanolayer. ACS Appl. Mater. Interfaces 2018, 10, 39599–39607. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liang, F.; Jin, J.; Chowdari, B.V.R.; Yang, J.; Wen, Z. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via in-situ ZrO2 coating for high energy density lithium-ion batteries. Chem. Eng. J. 2020, 389, 124403. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Hemmelmann, H.; Walther, F.; Bianchini, M.; Kondrakov, A.; Janek, J.; Brezesinski, T. Atomic layer deposition derived zirconia coatings on Ni-rich cathodes in solid-state batteries: Correlation between surface constitution and cycling performance. Small Sci. 2023, 3, 2200073. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, B.; Wang, Z.; Lu, C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sources 2014, 256, 20–27. [Google Scholar] [CrossRef]
- Li, W.-W.; Zhang, X.-J.; Si, J.-J.; Yang, J.; Sun, X.-Y. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met. 2021, 40, 1719–1726. [Google Scholar] [CrossRef]
- Wang, W.-C.; Lee, C.-H.; Yu, D.; Kondo, Y.; Miyahara, Y.; Abe, T.; Miyazaki, K. Effects of a solid solution outer layer of TiO2 on the surface and electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathodes for lithium-ion batteries through the use of thin-film electrodes. ACS Appl. Energy Mater. 2022, 5, 5117–5126. [Google Scholar] [CrossRef]
- Su, S.; Zheng, W.; Qin, Y.; Wu, J.; Ke, J.; Sun, Y.; Lin, X. Controllable construction of CeO2 shells with a nanoscale thickness to enhance the high-voltage electrochemical performance of Ni-rich cathodes. ACS Appl. Nano Mater. 2025, 8, 3039–3049. [Google Scholar] [CrossRef]
- Arumugam, R.S.; Ma, L.; Li, J.; Xia, X.; Paulsen, J.M.; Dahn, J.R. Special synergy between electrolyte additives and positive electrode surface coating to enhance the performance of Li[Ni0.6Mn0.2Co0.2]O2/graphite cells. J. Electrochem. Soc. 2016, 163, A2531–A2538. [Google Scholar] [CrossRef]
- Kong, J.Z.; Chen, Y.; Cao, Y.Q.; Wang, Q.-Z.; Li, A.-D.; Li, H.; Zhou, F. Enhanced electrochemical performance of Ni-rich LiNi0.6Co0.2Mn0.2O2 coate;d by molecular layer deposition derived dual-functional C-Al2O3 composite coating. J. Alloys Compd. 2019, 799, 89–98. [Google Scholar] [CrossRef]
- Neudeck, S.; Strauss, F.; Garcia, G.; Wolf, H.; Janek, J.; Hartmann, P.; Brezesinski, T. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material. Chem. Commun. 2019, 55, 2174–2177. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.F.; Xiong, D.B.; Hao, Y.C.; Li, J.W.; Kou, H.R.; Yan, B.; Li, D.J.; Lu, S.G.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium-ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Li, M.L.; Wahyudi, W.; Sheng, G.; Miao, X.H.; Anthopoulos, T.D.; Huang, K.W.; Li, Y.X.; Lai, Z.P. Performance and stability improvement of layered NCM lithium-ion batteries at high voltage by a microporous Al2O3 sol–gel coating. ACS Omega 2019, 4, 13972–13980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycle ability at extended voltage ranges. Coatings 2019, 9, 92. [Google Scholar] [CrossRef]
- Hu, D.; Du, F.; Cao, H.; Zhou, Q.; Sun, P.; Xu, T.; Mei, C.; Hao, Q.; Fan, Z.; Zheng, J. An effective strategy to control thickness of Al2O3 coating layer on nickel-rich cathode materials. J. Electroanal. Chem. 2021, 880, 114910. [Google Scholar] [CrossRef]
- Anh, V.T.; Vu, H.-A.T.; Nguyen, V.; Nang, H.X.; Do, L.T.; Dao, V.-D.; Vu, N.H. Enhancing electrochemical performance of Ni-rich cathodes for Li-ion batteries through spatial atomic layer deposition of ZnO coatings. Mater. Chem. Phys. 2025, 336, 130544. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Wei, G.; Qiu, S.; Qu, J. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via wet-chemical coating of MgO. J. Solid State Electrochem. 2019, 23, 2213–2224. [Google Scholar] [CrossRef]
- Liu, W.; Gao, F.; Zang, Y.; Qu, J.; Xu, J.; Ji, S.; Huo, Y.; Qiu, J. Boosting cycle stability of NMC811 cathode material via 2D Mg-Al-LDO nanosheet coating for lithium-ion battery. J. Alloys Compd. 2021, 867, 159079. [Google Scholar] [CrossRef]
- Mohanty, D.; Dahlberg, K.; King, D.-M.; David, L.-A.; Sefat, A.-S.; Wood, D.-L.; Daniel, C.; Dhar, S.; Mahajan, V.; Lee, M.; et al. Modification of Ni-rich FCG NMC and NCA cathodes by atomic layer deposition: Preventing surface phase transitions for high-voltage lithium-ion batteries. Sci. Rep. 2016, 6, 26532. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Zeng, T.; Yu, Z.; Qu, X.; Peng, X.; Zhou, Y.; Duan, X.; Dou, A.; Su, M.; et al. Improving the structure stability of LiNi0.8Co0.15Al0.05O2 by double modification of tantalum surface coating and doping. ACS Appl. Energy Mater. 2021, 4, 8641–8652. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, A.; Wang, K.; Mao, Q.; Huang, H.; Zhang, J.; He, X.; Gan, Y.; Xiao, Z.; Zhang, W. Industrial modification comparison of Ni-Rich cathode materials towards enhanced surface chemical stability against ambient air for advanced lithium-ion batteries. Chem. Eng. J. 2022, 450, 138382. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.L.; Jin, E.M.; Moon, S.-G.; Jeong, S.M. Optimization of B2O3 coating process for NCA cathodes to achieve long-term stability for application in lithium-ion batteries. Energy 2021, 222, 119913. [Google Scholar] [CrossRef]
- Cui, X.; Ai, L.; Mao, L.; Xie, Y.; Liang, Y.; Zhang, N.; Feng, Y.; Wang, S.; Li, S. Enhanced electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathode material by the diffusional Al2O3 coating layer. Ionics 2019, 25, 411–419. [Google Scholar] [CrossRef]
- Negi, R.S.; Elm, M.T. Reproducible long-term cycling data of Al2O3 coated LiNi0.70Co0.15Mn0.15O2 cathodes for lithium-ion batteries. Sci. Data 2022, 9, 127. [Google Scholar] [CrossRef]
- Ahangari, M.; Xia, F.; Szalai, B.; Zhou, M.; Luo, H. Advancing lithium-ion batteries’ electrochemical performance: Ultrathin alumina coating on Li(Ni0.8Co0.1Mn0.1)O2 cathode materials. Micromachines 2024, 15, 894. [Google Scholar] [CrossRef]
- Woo, S.G.; Han, J.H.; Kim, K.J.; Kim, J.-H.; Yu, J.-S.; Kim, Y.-J. Surface modification by sulfated zirconia on high-capacity nickel-based cathode materials for Li-ion batteries. Electrochim. Acta 2015, 153, 115–121. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, D.; Li, Z.; Huan, Y.; Cheng, G.; Ren, Z.; Liu, X.; Wang, K.; Chen, L.; Gu, H.; et al. Breaking the cycle of heterogeneous degradation: Surface-targeted protection for Ni-rich cathodes in practical high-energy batteries. ACS Energy Lett. 2025, 10, 1457–1465. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, T.; Qin, D.; Leng, L.; Tian, J.; Sun, T.; Lu, J.; Liu, X.; Zhang, Y. Optimizing annealing strategy to achieve effective grain boundary modification with aluminum oxide for stable cycling Ni-rich cathodes. ACS Appl. Mater. Interfaces 2025, 17, 13988–13996. [Google Scholar] [CrossRef]
- Mitra, S.; Mudiyanselage, T.R.; Wang, X.; Lohar, G.; Dubal, D. Bolstering the rate performance of Co-free Ni-rich layered oxide cathode through a rapid heating method. Batter. Supercaps 2025, e202400782. [Google Scholar] [CrossRef]
- Meng, K.; Wang, Z.; Guo, H.; Li, X.; Wang, D. Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3. Electrochim. Acta 2016, 211, 822–831. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Mu, D.; Li, L.; Wu, F.; Chen, R. Surface reconstruction reduces internal stress of Ni-rich cathode particles. Small 2025, 21, 2406495. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-Z.; Wang, S.-S.; Tai, G.-A.; Zhu, L.; Wang, L.-G.; Zhai, H.-F.; Wu, D.; Li, A.-D.; Li, H. Enhanced electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by ultrathin ZrO2 coating. J. Alloys Compd. 2016, 657, 593–600. [Google Scholar] [CrossRef]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From Surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Guan, P.; Min, J.; Chen, F.; Zhang, S.; Hu, L.; Ma, Z.; Han, Z.; Zhou, L.; Jia, H.; Liu, Y.; et al. Enhancing the electrochemical properties of nickel-rich cathode by surface coating with defect-rich strontium titanate. ACS Appl. Mater. Interfaces 2023, 15, 29308–29320. [Google Scholar] [CrossRef]
- Lou, F.; Xie, Q.; Luo, X.; Xie, Y.; Wang, M.; Hao, H.; Wang, Z.; Yang, L.; Wang, G.; Chen, J.; et al. Preventing structural collapse and thermal runaway to improve the electrochemical performance and safety of LiNi0.8Co0.1Mn0.1O2 by a negative-thermal-expansion material of Al2(WO3)4. Ind. Eng. Chem. Res. 2022, 61, 4588–4600. [Google Scholar] [CrossRef]
- Wustrow, A.; Hancock, J.C.; Incorvati, J.T.; Vaughey, J.T.; Poeppelmeier, K.R. Effect of fluoride doping on lithium diffusivity in layered molybdenum oxide. ACS Appl. Energy Mater. 2019, 2, 2080–2086. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Bin Sapari, M.A.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Park, S.-B.; Kang, C.-Y.; Hwang, D.-Y.; Yoo, Y.-W.; Park, H.-J.; Park, J.-H.; Kim, H.-S.; Lee, S.-H. Effect of MoO3 surface coating on electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode. Current Appl. Phys. 2022, 44, 1–5. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Sun, Q.; Nie, W.; Chang, L.; Chen, S.; Cheng, H. Architecting versatile NiFe2O4 coating for enhancing structural stability and rate capability of layered Ni-rich cathodes. Chem. Eng. J. 2023, 470, 144210. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Chang, L.; Liu, Y.; Zhang, J.; Yuan, Y.; Lu, X.; Cheng, H. Promoting durability of Ni-rich layered oxide via inverse spinel NiFe2O4 interface layer for high-voltage lithium-ion batteries. ACS Appl. Energy Mater. 2024, 7, 6236–6247. [Google Scholar] [CrossRef]
- Wang, L.; Mu, D.; Wu, B.; Yang, G.; Gai, L.; Liu, Q.; Fan, Y.; Peng, Y.; Wu, F. Enhanced electrochemical performance of lithium metasilicate-coated LiNi0.6Co0.2Mn0.2O2 Ni-rich cathode for Li-ion batteries at high cutoff voltage. Electrochim. Acta 2016, 222, 806–813. [Google Scholar] [CrossRef]
- Qu, X.; Huang, H.; Wan, T.; Hu, L.; Yu, Z.; Liu, Y.; Dou, A.; Zhou, Y.; Su, M.; Peng, X.; et al. An integrated surface coating strategy to enhance the electrochemical performance of nickel-rich layered cathodes. Nano Energy 2022, 91, 16665. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, A.; Lu, C.; Ma, R.; Fang, R.; Gan, Y.; Wang, G.; Xu, J.; Mao, Q.; Lu, X.; et al. Ultra-low-dose Nb2O5 coating promotes electrochemical kinetics and rate capability of Ni-rich oxide cathode. J. Solid State Electrochem. 2024, 1–10. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Miikkulainen, V.; Mäntimäki, M.; Mousavihashemi, S.; Lahtinen, J.; Lide, Y.; Yang, H.; Mizohata, K.; Kankaanpää, T.; Kallio, T. Understanding the stabilizing effects of nanoscale metal oxide and Li-metal oxide coatings on lithium-ion battery positive electrode materials. ACS Appl. Mater. Interfaces 2021, 13, 42773–42790. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Ryu, H.-H.; Kim, U.-H.; Weeks, J.A.; Heller, A.; Sun, Y.-K.; Mullins, C.B. Beyond doping and coating: Prospective strategies for stable high-capacity layered Ni-rich cathodes. ACS Energy Lett. 2020, 5, 1136–1146. [Google Scholar] [CrossRef]
- Lee, D.-J.; Scrosati, B.; Sun, Y.-K. Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55 °C. J. Power Sources 2011, 196, 7742–7746. [Google Scholar] [CrossRef]
- Kim, T.; Goh, M.S.; Moon, H.; Shin, H.; Lee, J.; Jeong, H.; Joo, S.W.; Kim, Y.S.; Im, Y.; Kang, M. Enhanced battery capacity and cycle life due to suppressed side reactions on the surface of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials coated with Co3(PO4)2. J. Colloid Int. Sci. 2024, 670, 729–741. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Wang, Z.; Guo, H. A facile process for coating amorphous FePO4 onto LiNi0.8Co0.15Al0.05O2 and the effects on its electrochemical properties. Mater. Lett. 2014, 131, 210–213. [Google Scholar] [CrossRef]
- Zha, G.; Luo, Y.; Hu, N.; Ouyang, C.; Hou, H. Surface modification of the LiNi0.8Co0.1Mn0.1O2 cathode material by coating with FePO4 with a yolk–shell structure for improved electrochemical performance. ACS Appl. Mater. Interfaces. 2020, 12, 36046–36053. [Google Scholar] [CrossRef]
- Qi, R.; Shi, J.; Zhang, X.; Zeng, X. Improving the stability of LiNi0.80Co0.15Al0.05O2 by AlPO4 nanocoating for lithium-ion batteries. Sci. China Chem. 2017, 60, 1230–1235. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, W.; Hao, S.; Li, L.; Ran, Q.; Liu, L.; Ji, Y.; Huo, J.; Liu, X. Utilizing dual functions of LaPO4 to enhance the electrochemical performance of LiNi0.87Co0.09Al0.04O2 cathode material. Nanotechnology 2023, 34, 075706. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Lin, Z.; Fan, M.; Wang, F.; Liu, Y.; Xiong, X. Facile and efficient fabrication of Li3PO4-coated Ni-rich cathode for high-performance lithium-ion battery. Appl. Surf. Sci. 2020, 504, 144506. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Li, X.; Luo, J.; Deng, S.; Zhao, Y.; Sun, Y.; Wu, D.; Hu, Y.; Li, W.; et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. X. Sun. Nat. Commun. 2023, 14, 146. [Google Scholar] [CrossRef]
- Park, H.G.; Kwon, D.; Cho, W.; Yoon, S.; Kim, D.; Park, K. Correlation between grain boundary coating and chemomechanics in Ni-rich layered Li cathodes. Chem. Eng. J. 2023, 452, 139442. [Google Scholar] [CrossRef]
- Luo, Z.; Ding, C.; Wang, W.; Hu, G.; Du, K.; Cao, Y.; Peng, Z. Improving storage performance and lattice oxygen stability of single crystal LiNi0.925Co0.03Mn0.045O2 by integrating utilization of surface residual lithium and lattice modulation. Appl. Surf. Sci. 2024, 665, 160238. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.T.; Guang, Y.; Bresser, D.; Diemant, Y.; Huang, M.; Copley, R.; Behm, J.; Passerini, S.; Shen, Z. Manganese phosphate coated Li[Ni0.6Co0.2Mn0.2]O2 cathode material: Towards superior cycling stability at elevated temperature and high voltage. J. Power Sources 2018, 402, 263–271. [Google Scholar] [CrossRef]
- Peng, Z.-D.; Huang, M.; Wang, W.-G.; Du, K.; Guan, D.-C.; Hu, G.; Cao, Y.-B. Enhancing the structure and interface stability of LiNi0.83Co0.12Mn0.05O2 cathode material for Li-ion batteries via facile CeP2O7 coating. ACS Sustain. Chem. Eng. 2022, 10, 4881–4893. [Google Scholar] [CrossRef]
- Min, K.; Park, K.; Park, S.Y.; Seo, S.W.; Choi, B.; Cho, E. Improved electrochemical properties of LiNi0.91Co0.06Mn0.03O2 cathode material via Li-reactive coating with metal phosphates. Sci. Rep. 2017, 7, 7151. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, L.; Yang, G.; Li, Y.; Pan, Q.; Li, Y.; Hu, S.; Zheng, F.; Wang, H.; Li, Q. Nanoscale surface modification to suppress capacity fade of Ni-rich layered oxide material at high cut-off voltage. Chem. Eng. J. 2023, 451, 138911. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, K.; Qiu, B.; Liang, H.; Li, J.; Zhao, W.; Li, S.; Zhang, W.; Liu, Z.; Liu, Q. Hydrophobic surface coating against chemical environmental instability for Ni-rich layered oxide cathode materials. Chem. Eng. J. 2022, 437, 135276. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.-Y.; Wei, H.-X.; Luo, Y.-H.; Tang, L.-B.; Chen, H.-Z.; Zhang, X.; Zheng, J. Suppress moisture sensitivity of Ni-rich cathode materials by bioinspired self-assembly hydrophobic layer. Chem. Eng. J. 2023, 477, 146850. [Google Scholar] [CrossRef]
- Jo, M.; Oh, P.; Kim, J.; Choi, J.-H.; Kim, S.; Ha, S.; Son, Y. Electrochemical lithium storage performance at high voltage and temperature of LiNi0.6Co0.2Mn0.2O2 cathode for Lithium-ion batteries by facile Mn3(PO4)2 dry coating. Appl. Surf. Sci. 2013, 613, 156018. [Google Scholar] [CrossRef]
- Yang, C.; Shao, R.; Mi, Y.; Shen, L.; Zhao, B.; Wang, Q.; Wu, K.; Lui, W.; Gao, P.; Zhou, H. Stable interstitial layer to alleviate fatigue fracture of high nickel cathode for lithium-ion batteries. J. Power Sources 2018, 376, 200–206. [Google Scholar] [CrossRef]
- Ahn, J.; Jang, E.K.; Yoon, S.; Lee, S.-J.; Sung, S.-J.; Kim, D.-H.; Cho, K.Y. Ultrathin ZrO2 on LiNi0.5Mn0.3Co0.2O2 electrode surface via atomic layer deposition for high-voltage operation in lithium-ion batteries. Appl. Surf. Sci. 2019, 484, 701–709. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Ma, J.; Yu, X.; Zhang, S.; Zhou, X.; Cui, G. A bifunctional chemomechanics strategy to suppress electrochemo-mechanical failure of Ni-rich cathodes for all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2022, 14, 17674–17681. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Wu, Y.; Wu, S.; Jose, R.; Yang, C. Surface-modified quaternary layered Ni-rich cathode materials by Li2ZrO3 for improved electrochemical performance for high-power Li-ion batteries. ACS Appl. Energy Mater. 2022, 5, 4796–4807. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, F.; Zhu, C.; Qiu, L.; Zhou, J.; Deng, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Effective and low-cost in situ surface engineering strategy to enhance the interface stability of an ultrahigh Ni-rich NCMA cathode. ACS Appl. Mater. Interfaces 2022, 14, 51835–51845. [Google Scholar] [CrossRef]
- Qiao, Y.; Hao, R.; Shi, X.; Li, Y.; Wang, Y.; Zhang, Y.; Tang, C.; Li, G.; Wang, G.; Liu, J.; et al. Improving the cycling stability of LiNi0.8Co0.1Mn0.1O2 by enhancing the structural integrity via synchronous Li2SiO3 coating. ACS Appl. Energy Mater. 2022, 5, 4885–4892. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, W.; Yao, J.; Bu, L.; Li, X.; Tian, K.; Lu, H.; Quan, C.; Xu, S.; Xu, K.; et al. Suppressing structural degradation of Ni-rich cathode materials towards improved cycling stability enabled by a Li2MnO3 coating. J. Mater. Chem. A 2020, 8, 17429–17441. [Google Scholar] [CrossRef]
- Dong, L.; Guan, X.; Zhou, Y.; Tang, S.; Chen, F. Supplying active lithium to single-crystal Li(Ni0.90Co0.05Mn0.05)0.98Ta0.02O2 with Li2MnO3 coating served as cathode for Li-ion batteries. Particuology 2024, 95, 303–318. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Strauss, F.; Bartsch, T.; Teo, J.-H.; Janek, J.; Brezesinski, T. Effect of surface carbonates on the cyclability of LiNbO3-coated NCM622 in all-solid-state batteries with lithium thiophosphate electrolytes. Sci. Rep. 2021, 11, 5367. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, S.; Hu, Y.; He, G.; Quo, L.; Parkin, B.P.; Iang, H. Lithium-conductive LiNbO3 coated high-voltage LiNi0.5Co0.2Mn0.3O2 cathode with enhanced rate and cyclability. Green Energy Environ. 2022, 7, 266–274. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Xie, Y.; Luo, S.; Wang, Z.; Zhu, L.; Zhang, X. Insights into capacity fading mechanism and coating modification of high-nickel cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 55491–55502. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, J.; Duan, R.; Bai, Y.; Xu, K.; Zhang, K.; Wang, J.; Zhang, K.; Yang, Z.; Li, M.; et al. Grain binding derived reinforced interfacial mechanical behavior of Ni-rich layered cathode materials. Nano Energy 2024, 121, 109214. [Google Scholar] [CrossRef]
- Xie, J.; Sendek, A.-D.; Cubuk, E.-D.; Zhang, X.; Lu, Z.; Gong, Y.; Wu, T.; Shi, F.; Liu, W.; Reed, E.-J.; et al. Atomic layer deposition of stable LiAlF4 lithium-ion conductive interfacial layer for stable cathode cycling. ACS Nano 2017, 11, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Susai, F.A.; Sclar, H.; Maiti, S.; Burstein, L.; Perkal, O.; Grinblat, J.; Talianker, M.; Ruthstein, S.; Erk, C.; Hartmann, P.; et al. Stabilized behavior of LiNi0.85Co0.10Mn0.05O2 cathode materials induced by their treatment with SO2. ACS Appl. Energy Mater. 2020, 3, 3609–3618. [Google Scholar] [CrossRef]
- Tan, X.; Peng, W.; Duan, H.; Wang, Z.; Guo, H.; Luo, G.; Yuan, R.; Li, X.; Wang, J. A scalable dry chemical method for lithium borate coating to improve the performance of LiNi0.90Co0.06Mn0.04O2 cathode material. Solid State Ion. 2022, 28, 2073–2082. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Xi, X.; Zheng, J.; Yu, J.; He, Z. Suppressed internal intrinsic stress engineering in high-performance Ni-Rich cathode via multilayered in situ coating structure. Energy Environ. Mater. 2022, 24, 12574. [Google Scholar] [CrossRef]
- Llanos, S.; Ahaliabadeh, Z.; Miikkulainen, V.; Kong, X.; Obrezkov, F.; Lahtinen, J.; Yao, L.; Jiang, H.; Lassi, U.; Kallio, T. Structural and interfacial stability of a coated Ni-rich layered oxide cathode at high-voltage operation. Mater. Today Energy 2025, 50, 101862. [Google Scholar] [CrossRef]
- Byeon, Y.S.; Lee, H.B.; Hong, Y.; Kim, H.; Cho, W.; Park, M.-S. Cation-deficient LixWO3 surface coating on Ni-rich cathodes materials for lithium-ion batteries. ACS Appl. Interfaces 2025, 17, 9322–9331. [Google Scholar] [CrossRef]
- Yu, J.; Peng, J.; Huang, W.; Wang, L.; Wei, Y.; Yang, N.; Li, L. Inhibition of excessive SEI-forming and improvement of structure stability for LiNi0.8Co0.1Mn0.1O2 by Li2MoO4. Ionics 2021, 27, 2867–2876. [Google Scholar] [CrossRef]
- You, M.J.; Jung, J.; Byeon, Y.S.; Jung, J.Y.; Hong, Y.; Park, M.-S. Controlled crystallinity of LiTaO3 surface layer for single-crystalline Ni-rich cathodes for lithium-ion batteries and all-solid-state batteries. Chem. Engin. J. 2024, 483, 149199. [Google Scholar] [CrossRef]
- Herzog, M.J.; Gauquelin, N.; Esken, D.; Verbeeck, J.; Janek, J. Increased performance improvement of lithium-ion batteries by dry powder coating of high-nickel NMC with nanostructured fumed ternary lithium metal oxides. ACS Appl. Energy Mater. 2021, 4, 8832–8848. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Xiong, F.; Chen, F.; Huang, C.; Gao, Q.; Wang, T.; Yang, Z.; Zhang, W. An effective etching-induced coating strategy to shield LiNi0.8Co0.1Mn0.1O2 electrode materials by LiAlO2. J. Power Sources 2019, 412, 246–254. [Google Scholar] [CrossRef]
- Ran, Q.; Zhao, H.; Hu, Y.; Shen, Q.; Liu, W.; Liu, J.; Shu, X.; Zhang, M.; Liu, S.; Tan, M.; et al. Enhanced electrochemical performance of dual-conductive layers coated Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode for Li-ion batteries at high cut-off voltage. Electrochim. Acta 2018, 289, 82–93. [Google Scholar] [CrossRef]
- Wang, H.; Chu, Y.; Pan, Q.; Yang, G.; Lai, A.; Liu, Z.; Zheng, F.; Hu, S.; Huang, Y.; Li, Q. Enhanced interfacial reaction interface stability of Ni-rich cathode materials by fabricating dual-modified layer coating for lithium-ion batteries. Electrochim. Acta 2021, 366, 137476. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Zhang, J. Surface-coated LiNi0.8Co0.1Mn0.1O2 (NMC811) cathode materials by Al2O3, ZrO2, and Li2O-2B2O3 thin-layers for improving the performance of lithium-ion batteries. Front. Mater. 2019, 6, 309. [Google Scholar] [CrossRef]
- Jamil, S.; Ran, Q.; Yang, L.; Huang, Y.; Cao, S.; Yang, X.; Wang, X. Improved high-voltage performance of LiNi0.87Co0.1Al0.03O2 by Li+-conductor coating. Chem. Eng. J. 2021, 407, 126442. [Google Scholar] [CrossRef]
- Wu, K.; Li, Z.; Chen, X. Designing a corrosion inhibiting layer to enhance cycling stability of 4.7 V Ni-rich cathode. Adv. Func. Mater. 2024, 34, 15327. [Google Scholar] [CrossRef]
- Du, F.; Sun, P.; Zhou, Q.; Zeng, D.; Hu, D.; Fan, Z.; Hao, Q.; Mei, C.; Xu, T.; Zheng, J. Interlinking primary grains with lithium boron oxide to enhance the stability of LiNi0.8Co0.15Al0.05O2. ACS Appl. Mater. Interfaces 2020, 12, 56963–56973. [Google Scholar] [CrossRef]
- Lin, J.; Guo, H.; Sun, K.; Fang, L. A nickel-rich oxide, Li(Ni0.90Co0.06Mn0.04)0.995Al0.005O2, with a dual conductive coating. J. Alloys Compd. 2023, 940, 168870. [Google Scholar] [CrossRef]
- Xiao, P.; Cao, Y.; Li, W.; Li, G.; Yu, Y.; Dai, Z.; Du, Z.; Chen, X.; Sun, J.; Yang, W. Simple strategy for synthesizing LiNi0.8Co0.15Al0.05O2 using Co Al-LDH nanosheet-coated Ni(OH)2 as the precursor: Dual effects of the buffer layer and synergistic diffusion. ACS Appl. Mater. Interfaces 2021, 13, 29714–29725. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, L.; Jia, D.; Jiang, X.; Wu, Y.; Hao, Q.; Xia, X.; Ouyang, Y.; Peng, L.; Tang, W.; et al. LiNi0.8Co0.15Al0.05O2 cathodes exhibiting improved capacity retention and thermal stability due to a lithium iron phosphate coating. Electrochim. Acta 2019, 312, 179–187. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, Z.; Xi, R.; Li, Y.; Zhang, C. The cycle stability and rate performance of LiNi0.8Mn0.1Co0.1O2 enhanced by Mg doping and LiFePO4 coating. ChemElectroChem 2022, 9, e202101654. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Liu, J.; Wang, B.; Cheng, X.; Zhang, Y.; Sun, C.; Wang, X.; Zhang, J. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600–605. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Wang, Z.; Li, Z.; Zhu, Y.; Li, Y.; Gu, S.; Yuan, H.; Luo, W.; Lu, L.; et al. Revealing mechanism of Li3PO4 coating suppressed surface oxygen release for commercial Ni-rich layered cathodes. ACS Appl. Energy Mater. 2020, 3, 7445–7455. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.; Jin, B.; Kim, H. Dual function Li-reactive coating from residual lithium on Ni-rich NCM cathode material for Lithium-ion batteries. Sci. Rep. 2021, 11, 18590. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, J.; Zhou, X.; Chen, J.; Shi, Y.; Kalimudina, G.; Wang, F.; Belgibayeva, A.; Kong, L. Basicity regulation of Ni-rich layered oxide cathodes for all-solid-state Li-ion batteries. J. Energy Chem. 2025, 105, 454–460. [Google Scholar] [CrossRef]
- Cheng, W.; Hao, S.; Ji, Y.; Li, L.; Liu, L.; Xiao, Y.; Wu, Y.; Huo, J.; Tang, F.; Liu, X. Optimizing surface residual alkali and enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode by LiH2PO4. Nanotechnology 2022, 33, 045404. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Xu, C.; Wu, K.; Zou, F.; Hu, X.; Hu, Z. LiZr2(PO4)3 surface coating towards stable layer structure Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials with long cycle performance. Nano Res. 2023, 16, 2373–2382. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, X.; Dai, W.; Yao, C.; Qian, J.; Chen, Z.; Liu, C. Optimized Ni-rich LiNi0.83Co0.06Mn0.06Al0.05O2 cathode material with a Li1.3Al0.3Ti1.7(PO4)3 fast ion conductor coating for Lithium-ion batteries. J. Alloys Compd. 2022, 923, 166277. [Google Scholar] [CrossRef]
- Lv, Z.C.; Wang, F.F.; Wang, J.C.; Wang, P.F.; Yi, T.F. Durable lithium-ion insertion/extraction and migration behavior of LiF-encapsulated cobalt-free lithium-rich manganese-based layered oxide cathode. J. Colloid Interface Sci. 2023, 649, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A. Functional behavior of AlF3 coatings for high-performance cathode materials for lithium-ion batteries. AIMS Mater. Sci. 2019, 6, 406–440. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Lu, X.; Jiang, H.; Zhang, Q.; Wang, B.; Lai, C. Enhancing high-potential stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode with PrF3 coating. Ceram. Int. 2021, 47, 6341–6351. [Google Scholar] [CrossRef]
- Kaufman, L.A.; McCloskey, B.D. Surface lithium carbonate influences electrolyte degradation via reactive oxygen attack in lithium-excess cathode materials. Chem. Mater. 2021, 33, 4170–4176. [Google Scholar] [CrossRef]
- Kang, W.; Jiang, A.; Chen, S.; Cao, X.; Yu, X.; Luo, Y.; Deng, W. High-performance Ti-doped V2O5 coating on the Ni-rich layered cathode: Construction and theoretical calculation. J. Alloys Compd. 2024, 970, 172700. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Yao, Q.; Chen, Z.; Song, L.; Zhang, Z.; Gao, C.; Wang, P.; Yu, Z.; Lai, Y. Alleviating surface degradation of nickel-rich layered oxide cathode material by encapsulating with nanoscale Li-ions/electrons superionic conductors hybrid membrane for advanced Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 30879–30889. [Google Scholar] [CrossRef]
- Qu, J.; Sadighi, Z.; Yuan, H.; Zuin, L.; Goward, G.R.; Botton, G.A. Effective recovery of the air-exposed Ni-rich lithium transition metal oxide cathodes with a coating-stabilized surface. J. Power Sources 2024, 614, 234983. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Pham, Q.-T.; Yang, C.-C.; Chern, C.-S.; Babulal, L.M.; Seenivasan, M.; Jeyakumar, J.; Mengesha, T.H.; Placke, T.; Brunklaus, G.; et al. Coating of a novel lithium-containing hybrid oligomer additive on nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for high-stability and high-safety lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 7394–7408. [Google Scholar] [CrossRef]
- Nie, H.; Shang, C.; Hu, P.; Li, Y. In-situ electrochemical polymerization of polypyrrole on LiNi0.8Co0.1Mn0.1O2 cathode with improved performance for lithium-ion batteries. Mater. Lett. 2023, 335, 133768. [Google Scholar] [CrossRef]
- Kim, H.I.; Jang, H.J.; Park, E.J.; Kim, S.-J.; Son, J.-T. Dramatic enhancement of the electrochemical property of nano-fiber Li[Ni0.9Co0.05Al0.05]O2 as a lithium-ion battery cathode material using a polypyrrole coating. J. Korean Phys. Soc. 2021, 79, 567–572. [Google Scholar] [CrossRef]
- Han, Y.; Heng, S.; Wang, Y.; Qu, Q.; Zheng, H. Anchoring interfacial nickel cations on single-crystal LiNi0.8Co0.1Mn0.1O2 cathode surface via controllable electron transfer. ACS Energy Lett. 2020, 5, 2421–2433. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Zhu, Y.; Huang, Z.; Zhang, F.; Gu, S.; Xie, J.; Zhang, K.; Lu, L.; Lu, Z. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-rich. LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 12594–12604. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, S.; Shin, M.W. Fundamental understanding of the effect of a polyaniline coating layer on cation mixing and chemical states of LiNi0.9Co0.085Mn0.015O2 for Li-ion batteries. ACS Appl. Polym. Mater. 2023, 5, 1344–1353. [Google Scholar] [CrossRef]
- He, P.; Zhang, M.; Wang, S.; Wan, M.; Wang, D.Y.; Wang, Y.; Yan, D.; Zhang, X. Sun. Enhanced high-rate and cyclic performance of Co-free and Ni-rich LiNi0.95Mn0.05O2 cathodes by coating electronic/Li+ conductive PANI-PEG layer. J. Solid State Electrochem. 2024, 28, 4259–4272. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Tang, H.; Huang, S.; Tang, Y.; Ma, J.; Huang, B.; Li, Y.; Sun, Y.; Xiao, S.; et al. A Mo/PANI co-modified ultra-high nickel NCM9622 cathode composite with excellent cycle stability and high-rate performance for power batteries. J. Mater. Chem. A 2024, 12, 21412–21424. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, M.; Mo, L.; Zhang, L.; Su, D.; Jiang, J.; Pan, Q.; Hu, S.; Wang, H.; Li, Q.; et al. Organic coating strategy with oxidized oxygen anion capture to suppress lattice oxygen evolution of Ni-rich cathode materials at high voltage. Chem. Eng. J. 2024, 493, 152525. [Google Scholar] [CrossRef]
- Li, Z.-W.; Lin, F.; Zhang, X.-D.; Zhang, X.-S.; Wen, R.; Li, X.; Zheng, Z.-J. Stress-relieving protective elastomeric interphase for stable Ni-rich cathodes. Adv. Func. Mater. 2025, 35, 2415035. [Google Scholar] [CrossRef]
- Liu, C.; Liao, C.M.; Li, Z.; Nie, S.; Yi, Z.; Xiao, W. Revealing the origin of enhanced structural stability for nickel-rich LiNi0.83Co0.11Mn0.06O2 cathodes in situ modified with multifunctional polysiloxane. Chem. Eng. J. 2024, 491, 152078. [Google Scholar] [CrossRef]
- Lin, K.; Yang, S.; Shi, Z.; Fan, Q.; Liu, Z.; Liu, L. Knitting a sweater with UV-induced in situ polymerization of poly(pyrrole-co-citral nitrile) on Ni-rich layer oxide cathode materials for lithium ion batteries. J. Power Sources 2022, 520, 230768. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, M.W. Cationic covalent organic framework coating of the Ni-rich cathode surface for the significant improvement of the interfacial Li+ transport and structural stability. ACS Appl. Energy Mater. 2024, 7, 5244–5252. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode properties at high operating voltage by double coating layer of Al2O3 and AlPO4. J. Alloys Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Wu, F.; Shi, Q.; Chen, L.; Dong, J.; Zhao, J.; Wang, H.; Gao, F.; Liu, J.; Zhang, H.; Li, N.; et al. New insights into dry-coating-processed surface engineering enabling structurally and thermally stable high-performance Ni-rich cathode materials for lithium-ion batteries. Chem. Eng. J. 2023, 470, 144045. [Google Scholar] [CrossRef]
- Bai, H.; Yuan, K.; Zhang, C.; Zhang, W.; Tang, X.; Jiang, S.; Jin, T.; Ma, Y.; Kou, L.; Shen, C.; et al. Advantageous surface engineering to boost single-crystal quaternary cathodes for high-energy-density lithium-ion batteries. Energy Storage Mater. 2023, 61, 102879. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, T.; Li, G.; Wan, T.; Li, M.; Zhang, X.; Li, M.; Su, M.; Dou, A.; Zeng, W.; et al. The surface double-coupling on single-crystal LiNi0.8Co0.1Mn0.1O2 for inhibiting the formation of intragranular cracks and oxygen vacancies. Energy Storage Mater. 2022, 52, 534–546. [Google Scholar] [CrossRef]
- Mohsen, M.; Rahim, L.; Hossein, E.; Manesha, M. Preparation and characteristics of graphene/Y2O3/LiNi0.8Co0.15Al0.05O2 composite for the cathode of lithium-ion battery. J. Electroanal. Chem. 2020, 862, 113971. [Google Scholar]
- Yua, J.; Zhang, J.; Zhang, Y.; Wang, Z.; Chen, Z.; Gao, A.; Zhang, J.; Wang, Y.; Zhao, R. Electrostatic adsorption facilitating dual-layer coating for stabilized cathode-electrolyte interphases and boosted lithium intercalation thereof in LiNi0.8Co0.1Mn0.1O2 cathode. Appl. Surf. Sci. 2022, 577, 151716. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Zeng, L.; Lin, J.; Zheng, B.; Liu, H.; Chen, L.; Wu, F. Single-crystal Ni-rich layered oxide cathodes with LiNbO3-Li3BO3 coating for sulfide all-solid-state batteries. Nano Energy 2025, 137, 110798. [Google Scholar] [CrossRef]
- Yang, G.; Liu, B.; Lai, F.; Xue, K.; Zhang, X.; Wang, H.; Xie, M.; Wang, C. Low-temperature synthesis of amorphous LiF/Li3BO3 iterfaces with F, B co-doped subsurface for long-cycling and high-rate Ni-rich cathodes. Nano Energy 2025, 140, 111009. [Google Scholar] [CrossRef]
- Rambukwella, I.; Ponnuru, H.; Yan, C. The role of dopants in mitigating the chemo-mechanical degradation of Ni-rich cathode: A critical review. EcoEnergy 2025, 3, 321–353. [Google Scholar] [CrossRef]
- Dang, R.; Qu, Y.; Ma, Z.; Yu, L.; Duan, L.; Lü, W. The effect of elemental doping on nickel-rich NCM cathode materials of lithium ion batteries. J. Phys. Chem. C 2022, 126, 151–159. [Google Scholar] [CrossRef]
- Byeon, Y.S.; Lee, W.; Park, S.; Kim, D.; Jung, J.; Park, M.-S.; Yoon, W.-S. Comprehensive understanding of elemental doping and substitution of Ni-rich cathode materials for lithium-ion batteries via in situ operando analyses. Small Sci. 2024, 4, 2400165. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, U.-H.; Yoon, C.S.; Sun, Y.-K. Enhanced cycling stability of Sn-doped Li[Ni0.90Co0.05Mn0.05]O2 via optimization of particle shape and orientation. Chem. Eng. J. 2021, 405, 126887. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, E.Y.; Chen, D.F.; Wu, M.; Han, S.; Huang, Q.; Yang, L.; Xiao, X.; Hu, Z. Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg. Chem. 2017, 56, 8355–8362. [Google Scholar] [CrossRef]
- Ko, G.; Jeong, S.; Park, S.; Lee, J.; Kim, S.; Shin, Y.; Kim, W.; Kwo, K.J. Doping strategies for enhancing the performance of lithium nickel manganese cobalt oxide cathode materials in lithium-ion batteries. Energy Storage Mater. 2023, 60, 102840. [Google Scholar] [CrossRef]
- Susai, F.A.; Kovacheva, D.; Kravchuk, T.; Ravikumar, R.; Talianker, M.; Grinblat, J.; Burstein, L.; Kauffmann, Y.; Major, D.T.; Markovski, B.; et al. Improving performance of LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries by doping with molybdenum-ions: Theoretical and experimental studies. ACS Appl. Energy Mater. 2019, 2, 4521–4534. [Google Scholar] [CrossRef]
- Sattar, T.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Influence of Mo addition on the structural and electrochemical performance of Ni-rich cathode material for lithium-ion batteries. Sci. Rep. 2020, 10, 8562. [Google Scholar] [CrossRef]
- Park, G.-T.; Namkoong, B.; Kim, S.-B.; Liu, J.; Yoon, C.-S.; Sun, Y.-K. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 2022, 7, 946–954. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Lim, H.-W.; Lee, S.G.; Sun, Y.-K. Optimization of molybdenum-doped Ni-rich layered cathodes for long-term cycling. Energy Storage Mater. 2023, 59, 102771. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, G.-T.; Yoon, C.-S.; Sun, Y.-K. Suppressing detrimental phase transitions via tungsten doping of LiNiO2 cathode for next generation lithium-ion batteries. J. Mater. Chem. A 2019, 7, 18580–18588. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, D.-R.; Aishova, A.; Yoon, C.-S.; Sun, Y.-K. Li[Ni0.9Co0.09W0.01]O2: A new type of layered oxide cathode with high cycling stability. Adv. Energy Mater. 2019, 9, 1902698. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, N.-Y.; Park, G.-T.; Kim, H.; Yoon, C.-S.; Sun, Y.-K. High-energy W-doped Li[Ni0.95Co0.04Al0.01]O2 cathodes for next-generation electric vehicles. Energy Storage Mater. 2020, 33, 399–407. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, C.-J.; Wang, Q.; Xu, G.-L.; Miao, C.; Xu, M.-B.; Wang, C.-J.; Xiao, W. Investigation of W6+-doped in high-nickel LiNi0.83Co0.11Mn0.06O2 cathode materials for high-performance lithium-ion batteries. J. Colloid Interface Sci. 2022, 628, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Leng, Q.; Yang, X.; Jiao, T.; Gong, Z.; Wang, M.-S.; Yang, Y. Boosting electrochemical performance of Co-free Ni-rich cathodes by combination of Al and high-valence elements. Chem. Eng. J. 2023, 474, 145869. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Jiang, M.; Ning, T.; Tan, L.; Zhang, X.; Zheng, J.; Wang, J.; Wu, Q.; Ji, X.; et al. Uncovering mechanism behind tungsten bulk/grain-boundary modification of Ni-rich cathode. Energy Storage Mater. 2025, 75, 104016. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Yu, R.; Cao, S.; Pei, Y.; Luo, Z.; Zhao, Q.; Chang, B.; Wang, Y.; Wang, X. Tellurium surface doping to enhance the structural stability and electrochemical performance of layered Ni-rich cathodes. ACS Appl. Mater. Interfaces 2019, 11, 40022–40033. [Google Scholar] [CrossRef]
- Jamil, S.; Yu, R.; Wang, Q.; Fasehullah, M.; Huang, Y.; Yang, Z.; Yang, X.; Wang, X. Enhanced cycling stability of nickel-rich layered oxide by tantalum doping. J. Power Sources 2020, 473, 228597. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, G.-T.; Son, B.-K.; Nam, G.-W.; Liu, J.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat. Energy 2020, 5, 860–869. [Google Scholar] [CrossRef]
- Chu, B.; Liu, S.; You, L.; Liu, D.; Huang, T.; Li, Y.; Yu, A. Enhancing the cycling stability of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode at a high cutoff voltage with Ta doping. ACS Sustain. Chem. Eng. 2020, 8, 3082–3090. [Google Scholar] [CrossRef]
- Sun, H.-H.; Kim, U.-H.; Park, J.-H.; Park, S.-W.; Seo, D.-H.; Heller, A.; Mullins, C.-B.; Yoon, C.-S.; Sun, Y.-K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef]
- Li, X.; Ge, W.; Zhang, K.; Zhang, K.; Peng, G.; Fu, Y.; Ma, X. Comprehensive study of tantalum doping on morphology, structure, and electrochemical performance of Ni-rich cathode materials. Electrochem. Acta 2022, 403, 139653. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zeng, T.; Dou, A.; Zhang, P.; Su, M.; Zhou, Y.; Liu, Y. Conversion of residual lithium into fast ionic conductor coating to achieve one-step double modification strategy in LiNi0.8Co0.15Al0.05O2. J. Alloys Compd. 2023, 931, 167638. [Google Scholar] [CrossRef]
- Monsees, F.; Misiewicz, C.; Dalkilic, M.; Diddens, D.; Heuer, A. Enhancing the stability and performance of Ni-rich cathode materials through Ta doping: A combined theoretical and experimental study. Phys. Chem. Chem. Phys. 2025, 27, 834–843. [Google Scholar] [CrossRef]
- Fan, F.; Zheng, R.; Zeng, C.; Zeng, C.; Xu, H.; Wang, X.; Tian, G.; Wang, S.; Liu, P.; Shu, C. Synergistically dissipating the local strain and restraining lattice oxygen escape by fine-tuning of microstructure enabling Ni-rich cathodes with superior cyclabilities. J. Energy Chem. 2025, 105, 24–34. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Liu, Z.; Monteiro, R.S.; Ribas, R.M.; Gao, P.; Zhu, Y.; Yu, H.; Ben, L.; Huang, X. Investigation of structure and cycling performance of Nb5+ doped high-nickel ternary cathode materials. Solid State Ion. 2021, 359, 115520. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Zhang, D.; Yan, Y.; Li, Z. An effective doping strategy to improve the cyclic stability and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode. Chem. Eng. J. 2020, 402, 126195. [Google Scholar] [CrossRef]
- Liu, L.-H.; Li, M.-C.; Chu, L.-H.; Jiang, B.; Lin, R.-X.; Zhu, X.-P.; Cao, G.-Z. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar]
- Kim, U.-H.; Lee, S.-B.; Park, N.-Y.; Kim, S.-J.; Yoon, C.-S.; Sun, Y.-K. High-energy-density Li-ion battery reaching full charge in 12 min. ACS Energy Lett. 2022, 7, 3880–3888. [Google Scholar] [CrossRef]
- Wang, J.-L.; Yi, Z.-C.; Liu, C.-J.; He, M.-Y.; Miao, C.; Li, J.-Q.; Xu, G.-L.; Xiao, W. Revealing the effect of Nb5+ on the electrochemical performance of nickel-rich layered LiNi0.83Co0.11Mn0.06O2 oxide cathode for lithium-ion batteries. J. Colloid Interface Sci. 2023, 635, 295–304. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Chen, L.; Lu, Y.; Su, Y.; Bao, L.; Chen, R.; Chen, S. Use of Ce to reinforce the interface of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries under high operating voltage. ChemSusChem 2019, 12, 935–943. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Wu, S.; Wang, Z.; Liu, X.; Li, W.; Li, N.; Wang, J.; Zhuang, W. Utilizing diverse functions of zirconium to enhance the electrochemical performance of Ni-rich layered cathode materials. ACS Appl. Energy Mater. 2020, 3, 11741–11751. [Google Scholar] [CrossRef]
- Che, W.; Wan, X.-W.; Zhang, D.-Y.; Chang, C.-K. Stabilized performance of LiNi0.90Co0.07Al0.03O2 cathodes via Zr4+ doping upon 4.5 V application due to the suppression of H2–H3 phase transitions. ACS Sustain. Chem. Eng. 2021, 9, 5536–5545. [Google Scholar] [CrossRef]
- Ni, J.-X.; Tan, Y.-Y.; Xu, K.; Jiang, Y.-J.; Chang, W.-Y.; Lai, C.-Y.; Liu, H. Cobalt-free nickel-rich layered LiNi0.9Al0.1-xZrxO2 cathode for high energy density and stable lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 136, 104421. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Z.; Wang, W.; Wen, Y.; Zhang, S.; Chen, X.; Zhang, Z.; Yin, Z.; Yang, T.; Li, T.; et al. Superior fast-charging Ni-rich cathode via promoted kinetic-mechanical performance. Nano Energy 2024, 128, 109908. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, B.; Yi, M.-Y.; Fan, X.-M.; Zhang, Z.; Huang, X.-B.; Lai, Y.-Q. In situ co-doping strategy for achieving long-term cycle stability of single-crystal Ni-rich cathodes at high voltage. Chem. Eng. J. 2022, 445, 136825. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, W.-J.; Kim, Y.-C.; Jung, J.Y.; Rhee, D.Y.; Song, J.H.; Cho, W.; Park, M.-S. Incorporation of titanium into Ni-rich layered cathode materials for lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 12204–12211. [Google Scholar] [CrossRef]
- Yang, R.-K.; Wu, Z.-G.; Li, Y.-C.; Li, R.; Qiu, L.; Wang, D.; Yang, L.; Guo, X.-D. Optimization of the electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material by titanium doping. Ionics 2020, 26, 3223–3230. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, Y.; Yao, J.; Zhao, M.; Xu, Y. Improved cycling stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by optimizing Ti doping. Funct. Mater. Lett. 2021, 14, 2150002. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, X.; Wang, C.; Zhao, B.; Huang, B. Enhancing high cycle stability of Ni-rich LiNi0.94Co0.04Al0.02O2 layered cathode material. Ionics 2021, 27, 4619–4628. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, C.; Miao, C.; Wang, Z.; Wang, J.; Xin, Y.; Xiao, W. In-situ Ti4+-doped modification of layer-structured Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials for high-energy lithium-ion batteries. J. Colloid Interface Sci. 2025, 677, 91–100. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Luo, W.; Cao, Y.; Zhao, C.; Dong, P.; Duan, J.; Wang, D.; Wang, X.; Zhou, Z.; et al. Enhanced reversibility of single crystal Ni-rich cathode materials via Ti doping. Electrochim. Acta 2024, 479, 143855. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Cao, H.; Peng, Y.; Ding, G.; Hua, Y.; Zhao, J.; Cui, C.; Huang, S. Enhancing the cycling stability of Ni-rich cathodes via dual-site modification induced by slight Ti-rich doping. J. Power Sources 2025, 628, 235913. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Wang, Z.-H.; Zhu, Y.-H.; Jiang, H.; Li, C.-Z. Enhancing surface-to-bulk stability of layered Co-free Ni-rich cathodes for long-life Li-ion batteries. Battery Energy 2022, 2, 20220048. [Google Scholar] [CrossRef]
- Wang, B.; Cai, F.; Chu, C.; Fu, B.; Swierczek, K.; Li, L.; Zhao, H. Modification of the Ni-rich layered cathode material by Hf addition: Synergistic microstructural engineering and surface stabilization. ACS Appl. Mater. Interfaces 2024, 16, 12599–12611. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Seenivasan, M.; Wu, Y.-S.; Kuo, L.-Y.; Wu, S.-H.; Chang, J.-K.; Yang, C.-C. Sn-doped/coated Ni-rich LiNi0.90Co0.04Mn0.03Al0.03O2 cathode materials for improved electrochemical performance of Li-ion batteries. ACS Appl. Energy Mater. 2024, 7, 4919–4934. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Yang, Z.; Zhang, M.; Zhang, D.; Yan, Y. Synergistic effect of Ce4+ modification on the electrochemical performance of LiNi0⋅6Co0⋅2Mn0⋅2O2 cathode materials at high cut-off voltage. Ceram. Int. 2021, 47, 1268–1276. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, G.; Li, Y.; Zhu, J.; Zheng, J.; Tan, Z.; Xi, X.; Yang, J. The synergistic Effect of Gd modification on improving the electrochemical performance of LiNi0.88Co0.09Al0.03O2 cathode materials. J. Electrochem. Soc. 2021, 168, 0305510. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.-J.; Lee, E.; Yoon, W.-S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, B.; Su, S.L.; Ming, L.; Zhao, Y.; Tan, X.X. Al-doping enables high stability of single-crystalline LiNi0.7Co0.1Mn0.2O2 lithium-ion cathodes at high voltage. RSC Adv. 2020, 11, 124–128. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, J.; Li, Y.; Zheng, S.; Zhou, K.; Wang, D.; Wang, D.; Hong, C.; Gong, Z.; Yang, Y.; et al. Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping. J. Mater. Chem. 2020, 8, 6893–6901. [Google Scholar] [CrossRef]
- Kim, U.-H.; Lee, S.-B.; Ryu, J.-H.; Yoon, C.-S.; Sun, Y.-G. Optimization of Ni-rich Li[Ni0.92–xCo0.04Mn0.04Alx]O2 cathodes for high energy density lithium-ion batteries. J. Power Sources 2023, 564, 232850. [Google Scholar] [CrossRef]
- Xi, R.-H.; Zhang, J.-N.; Lan, Z.-W.; Yuan, Y.-X.; Kang, J.-L.; Li, Y.-Y.; Wang, J.-T.; Zhang, C.-H.; Hou, X.-Y. High-nickel and cobalt-free layered LiNi0.90Mn0.06Al0.04O2 cathode for lithium-ion batteries. Ceram. Int. 2022, 48, 36690–36696. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Z.; Sun, J.-Y.; Xu, R.-M.; Li, X.-W.; Yu, X.; Liu, Y. Facile synthesis of crack-free single-crystalline Al-doped Co-free Ni-rich cathode material for lithium-ion batteries. Electrochim. Acta 2023, 437, 141473. [Google Scholar] [CrossRef]
- Kong, X.; Li, D.; Fedorovskaya, E.O.; Kallio, T.; Ren, X. New insights in Al-doping effects on the LiNiO2 positive electrode material by a sol-gel method. Int. J. Energy Res. 2021, 45, 10489–10499. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Su, W.; Zhu, X.; Hao, L.; Wang, X.; Wu, S.; Chen, L.; Cao, D.; Su, Y.; et al. Aluminium doping in single-crystal nickel-rich cathodes: Insights into electrochemical degradation and enhancement. J. Mater. Chem. A 2024, 12, 20910–20920. [Google Scholar] [CrossRef]
- Yan, H.; Ha, Y.; Ye, T.; Gao, X.; Yue, X.; Li, Z. High performance of Co-free LiNixMn1-xO2 cathodes realized by nonmagnetic ion substitution for Li-ion batteries. Chem. Eng. J. 2023, 465, 142926. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.-H.; Zheng, J.-C.; Zhang, D.-W.; Yang, J.-C.; Chen, Y.-X.; Guo, J.; Zhu, J.; Xiong, Y.; Li, W. Dual-modification of Gd2O3 on the high-voltage electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode materials via the solid-state method. J. Solid State Electrochem. 2020, 24, 863–872. [Google Scholar] [CrossRef]
- Sallard, S.; Billaud, J.; Sheptyakov, D.; Novak, P.; Villevieille, C. Cr-doped Li-rich nickel cobalt manganese oxide as a positive electrode material in Li-ion batteries to enhance cycling stability. ACS Appl. Energy Mater. 2020, 3, 8646–8657. [Google Scholar] [CrossRef]
- Xu, Y.-D.; Zhang, J.; Wu, Z.-G.; Xu, C.-L.; Li, Y.-C.; Xiang, W.; Wang, Y.; Zhong, Y.-J.; Guo, X.-D.; Chen, H. Stabilizing the structure of nickel-rich lithiated oxides via Cr doping as cathode with boosted high-voltage/temperature cycling performance for Li-ion battery. Energy Technol. 2020, 8, 1900498. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Su, Y.; Dong, J.; Yan, K.; Liu, J.; Lu, Y.; Wang, H.; Wang, C.; Wu, F.; et al. Rare-earth doping induced pinning effect with enhanced chemo-mechanical stability in Ni-rich cathodes for lithium-ion batteries. Chin. J. Chem. 2025, 43, 889–896. [Google Scholar] [CrossRef]
- Luo, Z.; Li, H.; Wang, W.; Fang, Z.; Zhao, B.; Hu, G.; Peng, Z.; Du, K.; Cao, Y. Mitigating irreversible phase transition of Y-doped LiNi0.925Co0.03Mn0.045O2 by lattice engineering. Ceram. Int. 2024, 50, 9535–9547. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, S.; Xie, X.; Wu, C.; Jamil, S.; Zhao, Q.L.; Chang, B.B.; Wang, Y.; Wang, X.Y. Improving the structure and cycling stability of Ni-rich layered cathodes by dual modification of yttrium doping and surface coating. ACS Appl. Mater. Interfaces 2020, 12, 19483–19494. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Z.; Chen, Y.; Gao, X. La-doped ultrahigh-nickel layered oxide cathode with enhanced cycle stability for Li-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 35043–35051. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, X.; Qiang, W.; Huang, B. Improved electrochemical performances of Ni-rich LiNi0.83Co0.12Mn0.05O2 by Mg-doping. J. Power Sources 2020, 450, 227718. [Google Scholar] [CrossRef]
- Weber, R.; Li, H.-Y.; Chen, W.-F.; Kim, C.-Y.; Plucknett, K.; Dahn, J.-R. In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials. J. Electrochem. Soc. 2020, 167, 100501. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-free Ni-rich single crystal positive electrode materials for lithium-ion batteries: Part I. Two-step lithiation method for Al or Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 040531. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-Free Ni-rich single crystal positive electrode materials for lithium-ion batteries: Part II. One-step lithiation method of Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 050506. [Google Scholar] [CrossRef]
- Yu, H.-F.; Zhu, H.-W.; Yang, Z.-F.; Liu, M.-M.; Jiang, H.; Li, C.-Z. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes. Chem. Eng. J. 2021, 412, 128625. [Google Scholar] [CrossRef]
- Xiao, W.; Nie, Y.; Miao, C.; Wang, J.; Tan, Y.; Wen, M. Structural design of high-performance Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials enhanced by Mg2+ doping and Li3PO4 coating for lithium-ion battery. J. Colloid Interface Sci. 2022, 607, 1071–1082. [Google Scholar] [CrossRef]
- Lin, Y.; Abram, C.M.; Shi, X.; McKendry, I.G.; Wang, Z.; Zhong, H.; Zhao, H.; Yang, X.; Koel, B.E.; Yan, C.; et al. Enhanced thermal stability of aerosol-synthesized Ni-rich Li-ion battery cathode materials via concentration-gradient Ca doping. ACS Appl. Energy Mater. 2022, 5, 10751–10757. [Google Scholar] [CrossRef]
- Wang, L.-C.; Chu, Y.-Q.; Nong, Y.-T.; Zheng, F.-H.; Li, Y.; Huang, Y.-Z.; Li, Y.-H.; Pan, Q.-C.; Wang, H.; Li, Q. Sr-based sub/surface integrated layer and bulk doping to enhance high-voltage cycling of a Ni-rich cathode material. ACS Sustain. Chem. Eng. 2022, 10, 7883–7895. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zheng, M.; Cai, F. Strontium doping promotes low-temperature growth of single-crystalline Ni-rich cathodes with enhanced electrochemical performance. Materials 2025, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Park, S.; Park, J.; Beak, M.; Lee, J.; Kwon, E.E.; Kwon, K. The enhancement of cyclability of Ni-rich LiNi0.9Co0.05–xMn0.05Znx cathode materials by the substitution of Zn for Co. Int. J. Energy Res. 2022, 46, 19177. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, Y.; Zhao, L.; Zhang, Y.; Tang, H.; Xu, Y.; Tan, Y.; Hou, X.; Wang, J. Improving the electrochemical performance of Ag-doped Ni-rich Li(Ni0.88Co0.09Al0.03)1–xO2 layered cathode material. Appl. Phys. A 2025, 131, 177. [Google Scholar] [CrossRef]
- Hu, Y.; Qin, Z.; Pei, J.; Cong, B.; Yang, X.; Chen, G. Reduced lithium/nickel disorder degree of sodium-doped lithium-rich layered oxides for cathode materials: Experiments and calculations. ChemElectroChem 2020, 7, 246–251. [Google Scholar] [CrossRef]
- Li, S.-C.; Liu, Y.-Y.; Zhang, Y.-G.; He, W.; Zheng, H.-F.; Guo, W.-B.; Wu, H.-L.; Gao, G.-Y.; Sa, B.-S.; Wang, L.-S.; et al. Interfacial oxygen coordination environment regulation towards high-performance Li-rich layered oxide cathode. Chem. Eng. J. 2023, 462, 142194. [Google Scholar] [CrossRef]
- Li, C.-F.; Chen, L.-D.; Wu, L.; Liu, Y.; Hu, Z.-Y.; Cui, W.-J.; Dong, W.-D.; Liu, X.; Yu, W.-B.; Li, Y.; et al. Directly revealing the structure-property correlation in Na+-doped cathode materials. Appl. Surf. Sci. 2023, 612, 155810. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, J.; Li, Y.; Zhang, Y.F.; Huang, S.P.; Lin, W.; Chen, W.K. Effects of doping high-valence transition metal (V, Nb and Zr) ions on the structure and electrochemical performance of LIB cathode material LiNi0.8Co0.1Mn0.1O2. Phys. Chem. Chem. Phys. 2021, 23, 11528–11537. [Google Scholar] [CrossRef]
- Li, X.; Lai, T.; Sheng, A.; Yang, J.; Li, Y.; Xiao, S.; Li, W.; Tan, H.; Huang, B. Effects of Nb-doping on cycle life, self-discharge and crack resistance of Ni-rich LiNi0.94Co0.02Al0.04O2 cathode for Li-ion batteries. J. Energy Storage 2023, 72, 108262. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Liu, Z.; Zhao, N.; Liang, H.; Zhou, M.; Zou, K.; Tan, L.; Ning, T. Revealing the mechanism of Zr coating modification on Ni-rich cathode materials through solid-phase diffusion. Chin. Chem. Lett. 2025, 111079. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Selent, M.; Tynjälä, P.; Lassi, U. Correlation of aluminum doping and lithiation temperature with electrochemical performance of LiNi1-xAlxO2 cathode material. J. Solid State Electrochem. 2023, 27, 641–654. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, H.; Mo, Y.; Qu, Y.; Du, B.; Chen, Y. Synthesis and characterization of Cu-doped LiNi0.6Co0.2Mn0.2O2 materials for Li-ion batteries. J. Alloys Compd. 2020, 844, 156180. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Seo, J.-H.; Yu, Y.-S.; Sharma, M.; Mucke, R.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries. Mater. Today 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Wu, C.Y.; Bao, Q.; Tsai, Y.T.; Duh, J.G. Tuning (003) interplanar space by boric acid co-sintering to enhance Li+ storage and transfer in Li(Ni0.8Co0.1Mn0.1)O2 cathode. J. Alloys Compd. 2021, 865, 158806. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.-M.; Luo, B.; Zhao, Z.-W.; Shen, J.-X.; Liu, Z.-H.; Xiao, Z.-M.; Zhang, B.; Zhang, J.-F.; Ming, L. Understanding the enhancement effect of boron doping on the electrochemical performance of single-crystalline Ni-rich cathode materials. J. Colloid Interface Sci. 2021, 604, 776–784. [Google Scholar] [CrossRef]
- Roitzheim, C.; Kuo, L.-Y.; Sohn, Y.J.; Finsterbusch, M.; Möller, S.; Sebold, D.; Valencia, H.; Meledina, M.; Mayer, J.; Breuer, U.; et al. Boron in Ni-rich NMC811 cathode material: Impact on atomic and microscale properties. ACS Appl. Energy Mater. 2022, 5, 524–538. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, Z.; Liang, J.; Lu, D.; Liang, C.; Guan, X.; Gao, A.; Chen, H. Understanding the role of Na-doping on Ni-rich layered oxide LiNi0.5Co0.2Mn0.3O2. J. Alloys Compd. 2016, 689, 318–325. [Google Scholar] [CrossRef]
- Shen, Y.; Yao, X.; Zhang, J.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Zhang, Y.; Hu, J.; Cheng, Y.; et al. Sodium doping derived electromagnetic center of lithium layered oxide cathode materials with enhanced lithium storage. Nano Energy 2022, 94, 106900. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J. Power Sources 2019, 441, 22719. [Google Scholar] [CrossRef]
- Vanaphuti, P.; Chen, J.; Cao, J.; Bigham, K.; Chen, B.; Yang, L.; Chen, H.; Wang, Y. Enhanced electrochemical performance of the lithium-manganese-rich cathode for Li-ion batteries with Na and F co-doping. ACS Appl. Mater. Interfaces 2019, 11, 37842–37849. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.H.; Sun, Y.K.; Yoon, C.S. Evolution of a radially aligned microstructure in boron-doped LiNi0.95Co0.04Al0.01O2 cathode particles. ACS Appl. Mater. Interfaces 2022, 14, 17500–17508. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-B.; Park, D.-H.; Park, K.-W. Fluorine-doped LiNi0.8Mn0.1Co0.1O2 cathode for high-performance lithium-ion batteries. Energies 2020, 13, 4808. [Google Scholar] [CrossRef]
- Qiu, Q.-Q.; Yuan, S.-S.; Bao, J.; Wang, Q.-C.; Yue, X.-Y.; Li, X.-L.; Wu, X.-J.; Zhou, Y.-N. Suppressing irreversible phase transition and enhancing electrochemical performance of Ni-rich layered cathode LiNi0.9Co0.05Mn0.05O2 by fluorine substitution. J. Energy Chem. 2021, 61, 574–581. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Xu, G.; Miao, C.; Wen, M.; Xu, M.; Wang, C.; Xiao, W. Strengthened the structural stability of in-situ F− doping Ni-rich LiNi0.8Co0.15Al0.05O2 cathode materials for lithium-ion batteries. Chem. Eng. J. 2022, 438, 135537. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Park, D.-H.; Kim, J.-H.; Jang, J.-S.; Kim, W.-C.; Lee, G.-I.; Lim, J.-W.; Hong, J.-M.; Park, K.-W. F-doped Co-free LiNixMn1-xO2 (0.7 ≤ x ≤ 0.9) cathodes for ameliorating electrochemical performance of Li-ion batteries. Mater. Today Energy 2024, 41, 101520. [Google Scholar] [CrossRef]
- Azhari, L.; Sousa, B.; Ahmed, R.; Wang, R.; Yang, Z.; Gao, G.; Han, Y.; Wang, Y. Stability enhancement and microstructural modification of Ni-rich cathodes via halide doping. ACS Appl. Mater. Interfaces 2022, 14, 46523–46536. [Google Scholar] [CrossRef]
- Eilers-Rethwisch, M.; Winter, M.; Schappacher, F.M. Synthesis, electrochemical investigation and structural analysis of doped Li[Ni0.6Mn0.2Co0.2-xMx]O2 (x = 0, 0.05; M = Al, Fe, Sn) cathode materials. J. Power Sources 2018, 387, 101–107. [Google Scholar] [CrossRef]
- Eilers-Rethwisch, M.; Hildebrand, S.; Evertz, M.; Ibing, L.; Dagger, T.; Winter, M.; Schappacher, F.M. Comparative study of Sn-doped Li[Ni0.6Mn0.2Co0.2-xSnx]O2 cathode active materials (x = 0 − 0.5) for lithium ion batteries regarding electrochemical performance and structural stability. J. Power Sources 2018, 397, 68–78. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Lv, Y.; Qi, L.; Ma, Y.; Zhang, H.; Song, D.; Shi, X.; Zhang, L. Investigation on the structure and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 modified with Sn. Electrochim. Acta 2021, 400, 139468. [Google Scholar] [CrossRef]
- Angellinnov, F.; Subhan, A.; Drew, A.J.; Syahrial, A.Z. Synthesis of Sn doped and rice husk derived activated carbon surface coating NMC 811 through solution combustion method. Heliyon 2024, 10, e23199. [Google Scholar] [CrossRef]
- Huang, Z.J.; Wang, Z.X.; Zheng, X.B.; Guo, H.; Li, X.; Jing, Q.; Yang, Z. Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Electrochim. Acta 2015, 182, 795–802. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B.; Manthiram, A. An in-depth understanding of the effect of aluminum doping in high-nickel cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Yi, M.; Dolocan, A.; Manthiram, A. Stabilizing the interphase in cobalt-free, ultrahigh-nickel cathodes for lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2213164. [Google Scholar] [CrossRef]
- Park, N.; Kim, S.; Kim, M.; Han, S.; Kim, D.; Kim, M.; Sun, Y. Mechanism of doping with high-valence elements for developing Ni-rich cathode materials. Adv. Energy Mater. 2023, 13, 2301530. [Google Scholar] [CrossRef]
- Zybert, M.; Rondura, H.; Rarog-Pilecka, W.; Wieczorek, W. Application of rare earth elements as modifiers for Ni-rich cathode materials for Li-ion batteries: A mini review. Front. Energy Res. 2023, 11, 1248641. [Google Scholar] [CrossRef]
- Kim, U.H.; Jun, D.-W.; Park, K.-J.; Zhang, Q.; Kaghazchi, P.; Aurbach, D.; Major, D.T.; Goobes, G.; Dixit, M.; Leifer, N.; et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries. Energy Environ. Sci. 2018, 11, 1271–1279. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Y.; Huang, B.; Zho, Z.; Li, Y.; Li, W. Effects of W-doping on precursor growth of LiNi0.88Co0.09Mn0.03O2 and its electrochemical performance. Trans. Nonferr. Met. Soc. China 2024, 34, 1251–1262. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Chen, L.; Hu, Y.; Jiang, H.; Li, C. Strain engineering of Ni-rich cathode enables exceptional cyclability in pouch-type full cells. Adv. Mater. 2023, 35, 2209357. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, H.; Yu, H.; Wang, Z.; Jiang, H.; Li, C. Enhancing surface and crystal stability of the Ni-high NCA cathode for high-energy and durable lithium-ion batteries. Ind. Eng. Chem. Res. 2022, 61, 2817–2824. [Google Scholar] [CrossRef]
- Chu, M.; Huang, Z.; Zhang, T.; Wang, R.; Shao, T.; Wang, C.; Zhu, W.; He, L.; Chen, J.; Zhao, W.; et al. Enhancing the electrochemical performance and structural stability of Ni-rich layered cathode materials via dual-site doping. ACS Appl. Mater. Interfaces 2021, 13, 19950–19958. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Huang, J.; Peng, J.; Xie, H.; Huang, B.; Li, Y.; Sun, Y.; Xiao, S.; Wang, R. Effective reinforcement on the structural stability and electrochemical performances of ultra-high nickel cobalt free cathode material by co-doping. J. Energy Storage 2024, 78, 110073. [Google Scholar] [CrossRef]
- Yao, Z.; Lum, Y.; Johnston, A.; Mejia-Mendoza, L.M.; Zhou, X.; Wen, Y.; Aspuru-Guzik, A.; Sargent, E.H.; She, Z.W. Machine learning for a sustainable energy future. Nat. Rev. Mater. 2022, 8, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kang, S.; Park, H.G.; Park, K.; Min, K. Maximizing the energy density and stability of Ni-rich layered cathode materials with multivalent dopants via machine learning. Chem. Eng. J. 2023, 452, 139254. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0·8Co0·1Mn0·1O2 (NMC811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Zha, G.; Hu, W.; Agarwal, S.; Ouyang, C.; Hu, N.; Hou, H. High performance layered LiNi0.8Co0.07Fe0.03Mn0.1O2 cathode materials for Li-ion battery. Chem. Eng. J. 2021, 409, 128343. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic lattice strain and mechanical degradation of high- and low-nickel NCM cathode materials for Li-ion batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Jo, E.; Park, J.-H.; Park, J.; Hwang, J.; Chung, K.Y.; Nam, K.-W.; Kim, S.M.; Chang, W. Different thermal degradation mechanisms: Role of aluminum in Ni-rich layered cathode materials. Nano Energy 2020, 78, 105367. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, U.-H.; Kim, J.-H.; Jun, S.; Yoon, C.S.; Jung, Y.S.; Sun, Y.-K. Ni-rich layered cathode materials with electrochemo-mechanically compliant microstructures for all-solid-state Li batteries. Adv. Energy Mater. 2020, 10, 1903360. [Google Scholar] [CrossRef]
- Ikuerowo, T.O.; Tomomewo, O.; Akande, S.O. Unlocking the potential of Ni-rich LiNi0.9Co0.1O2 cathodes: A DFT investigation of performance-limiting factors. Phys. Chem. Chem. Phys. 2025, 27, 1494–1502. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Y.; Yi, Z.; Yang, C.; Liu, F.; Wang, K.; Cao, J.; Chen, Z. Regulating the internal structure by magnesium doping to enhance cycle stability of full-concentration-gradient Ni-rich layered cathodes. Chem. Eng. J. 2023, 474, 145554. [Google Scholar] [CrossRef]
- Jamil, S.; Yue, L.; Li, C.; Fasehullah, M.; Aizaz Uddin, M.; Yang, W.; Bao, S.; Xu, M. Significance of gallium doping for high Ni, low Co/Mn layered oxide cathode material. Chem. Eng. J. 2022, 441, 135821. [Google Scholar] [CrossRef]
- Bunyanidhi, P.; Phattharasupakun, N.; Duangdangchote, S.; Prempluem, S.; Joraleechanchai, N.; Sawangphruk, M. Exploring the impact of metal oxide coating and metal atom doping on the electrochemical performance of Ni-rich cathode materials. J. Mater. Chem. A 2023, 11, 23223–23227. [Google Scholar] [CrossRef]
- Gao, D.; Che, W.; Xu, Q.; Zhao, X.; Huang, Y.; Chang, C. W doping improves the electrochemical performance of LiNi0.94Co0.04Al0.02O2 cathode through dual enhanced electronic and ionic conductivities: A study from DFT calculations and experimental results. Scr. Mater. 2023, 234, 115586. [Google Scholar] [CrossRef]
- Yang, J.; Gao, D.; Zhang, D.; Chang, C. Effect of dual improved electronic and cationic conductivity via W doping on cyclability and rate performance of LiNi0.90Co0.04Mn0.03Al0.03O2 cathode for rechargeable LiBs. J. Energy Storage 2023, 63, 107088. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Yang, J.; Wang, D.; Ye, C.; Wang, D.; Avdeev, M.; Shi, S.; Yang, J.; Zhang, W. Ab initio thermodynamic optimization of Ni-rich Ni–Co–Mn oxide cathode coatings. J. Power Sources 2020, 450, 227693. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, R.; Sun, J.; Chen, Y.; Chen, J.; He, J.; Zhang, Y.; Han, B.; Liao, G.; Wu, J.S.; et al. Precursor-oriented ultrathin Zr-based gradient coating on Ni-riched cathodes. Nano Energy 2023, 105, 108000. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Lu, Y.; Yang, L.; Xiong, L.; Zhao, S.; Bai, L.; Huang, C.; Zhou, C.; Zhu, J.; et al. An epitaxial coating with preferred orientation stabilizing High-Energy Ni-Rich NCA cathodes. Appl. Surf. Sci. 2022, 579, 152183. [Google Scholar] [CrossRef]
- Lee, H.B.; Hoang, T.D.; Byeon, Y.S.; Jung, H.; Moon, J.; Park, M.S. Surface stabilization of Ni-rich layered cathode materials via surface engineering with LiTaO3 for lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Q.; Ren, Z.; Sun, C.; Zhang, J.; Wang, R.; Qian, M.; Shi, Q.; Shao, R.; Mu, D.; et al. Thermochemical cyclization constructs bridged dual-coating of Ni-rich layered oxide cathodes for high-energy Li-ion batteries. Nano Lett. 2022, 22, 5221–5229. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Kim, M.; An, H.; Min, K.; Park, K. A review of problems and solutions in Ni-rich cathode-based Li-ion batteries from two research aspects: Experimental studies and computational insights. J. Power Sources 2024, 597, 234132. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Ji, W.; Wu, Y.; Zhao, S.; Li, Y.; Zhou, N. Insights into the doping rules of heteroatom on Ni-rich ternary cathode stability by integrating high throughput calculation and machine learning. J. Energy Chem. 2025, 106, 161–172. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Wang, B.; Wang, J.; Wang, Y.; Meng, W.; Cai, F. A Study on the microstructure regulation effect of niobium doping on LiNi0.88Co0.05Mn0.07O2 and the electrochemical performance of the composite material under high voltage. Materials 2024, 17, 2127. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-T.; Park, N.-Y.; Noh, T.-C.; Namkoong, B.; Ryu, H.-H.; Shin, J.-Y.; Beierling, T.; Yoon, C.S.; Sun, Y.-K. High-performance Ni-rich Li[Ni0.9–xCo0.1Alx]O2 cathodes via multi-stage microstructural tailoring from hydroxide precursor to the lithiated oxide. Energy Environ. Sci. 2021, 14, 5084–5095. [Google Scholar] [CrossRef]
- Park, N.-Y.; Ryu, H.-H.; Park, G.-T.; Noh, T.-C.; Sun, Y.-K. Optimized Ni-rich NCMA cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 2003767. [Google Scholar] [CrossRef]
- Park, N.-Y.; Ryu, H.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. High-energy cathodes via precision microstructure tailoring for next-generation electric vehicles. ACS Energy Lett. 2021, 6, 4195–4202. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Yoon, D.R.; Kim, U.-H.; Yoon, C.S.; Sun, Y.-K. New class of Ni-rich cathode materials Li[NixCoyB1–x–y]O2 for next lithium batteries. Adv. Energy Mater. 2020, 10, 2000495. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Zhao, T.; Peng, J.; Zhang, B.; Xing, W.; Zuo, M.; Zhang, P.; Fan, W.; Lv, G.; et al. Precise regulation of particle orientation for Ni-rich cathodes with ultra-long cycle life. Nano Energy 2024, 129, 110008. [Google Scholar] [CrossRef]
- Meng, Z.; Ma, X.; Azhari, L.; Hou, J.; Wang, Y. Morphology controlled performance of ternary layered oxide cathodes. Commun. Mater. 2023, 4, 90. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Zhou, Y.; Hai, C.-X.; Zeng, J.-B.; Ren, X.-F.; Sun, Y.-X.; Shen, Y.; Li, X.; Dong, S.-D.; Zhang, G.-T. Improving electrochemical performance of NMC811 cathodes for lithium-ion batteries via consistently arranging the hexagonal nanosheets with exposed {104} facets. Ceram. Int. 2022, 48, 17279–17288. [Google Scholar] [CrossRef]
- Shang, M.; Han, E.; Tian, Y.; He, Y.; Sun, L.; Zhu, L. The effects of multiple metals (K, Cu, Al) substitution on LiNi0.66Co0.20Mn0.14O2 for lithium-ion batteries. Ionics 2020, 26, 2699–2713. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Kong, X.; Fedorovskaya, E.; Kallio, T. Extensive comparison of doping and coating strategies for Ni-rich positive electrode materials. J. Power Sources 2022, 540, 231633. [Google Scholar] [CrossRef]
- Darjazi, H.; Gonzalo, E.; Acebedo, B.; Cid, R.; Zarrabeitia, M.; Bonilla, F.; Munoz-Marquez, M.A.; Nobili, F. Improving high-voltage cycling performance of nickel-rich NMC layered oxide cathodes for rechargeable lithium–ion batteries by Mg and Zr co-doping. Mater. Today Sustain. 2022, 20, 100236. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, C.; Xiao, Z.; Xian, K.; Wen, H.; Lu, N.; He, X.; Ye, L.; Wang, J.; Ou, X.; et al. Synergistic doping chemistry enable the cycling properties of single-crystal Ni-rich cathode for lithium-ion batteries. Appl. Surface Sci. 2025, 684, 161839. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.; Chen, L.; Demir, M.; Cheng, Q.; Jiang, H. Limiting cationic mixing and lattice oxygen loss of single-crystalline Ni-rich Co-poor cathodes for high-voltage Li-ion batteries. Green Energy Environ. 2025. [Google Scholar] [CrossRef]
- Yao, W.; Liu, Y.; Li, D.; Zhang, Q.; Zhong, S.; Cheng, H.; Yan, Z. Synergistically enhanced electrochemical performance of Ni-rich cathode materials for lithium-ion batteries by K and Ti Co-modification. J. Phys. Chem. C 2020, 124, 2346–2356. [Google Scholar] [CrossRef]
- He, X.; Shen, J.; Zhang, B.; Xiao, Z.; Ye, L.; Mao, Q.; Zhong, Q.; Ou, X. Surface Li+/Ni2+ antisite defects construction for achieving high-voltage stable single-crystal Ni-rich cathode by anion/cation co-doping. Adv. Func. Mater. 2024, 34, 2401300. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Z.; Yang, C.; Shi, W.; Yang, Y.; Yin, C.; Yi, Y.; Cao, J. Aluminium–sodium targeted co-doping to boost the electrochemical stability of full concentration gradient Ni-rich LiNi0.80Co0.05Mn0.15O2 cathodes. J. Colloid Interface Sci. 2025, 683, 335–346. [Google Scholar] [CrossRef]
- Xiang, W.; Zhu, C.-Q.; Zhang, J.; Shi, H.; Liang, Y.T.; Yu, M.H.; Zhu, X.-M.; He, F.-R.; Lv, G.-P.; Guo, X.-D. Synergistic coupling effect of sodium and fluorine co-substitution on enhancing rate capability and cycling performance of Ni-rich cathode for lithium-ion battery. J. Alloys Compd. 2019, 786, 56–64. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Shi, H.; Wang, C.; Li, W.; Wang, Z.; Zhang, W.; Xiong, Z.; Gao, Y.; Yan, X. Synergistic effect of B-Nb co-modified to achieve crystal structure and interface tuning for Ni-rich cathode. Electrochem. Acta 2024, 474, 143475. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Deng, Q.; Li, W.; Zhang, Q.; Zhang, Z.; Chu, Y.; Yang, C. Stabilizing Ni-rich Single-crystalline LiNi0.83Co0.07Mn0.10O2 Cathodes using Ce/Gd Co-doped High-entropy Composite Surfaces. Angew. Chem. Int. Ed. 2024, 63, e202318042. [Google Scholar] [CrossRef]
- Park, J.; Yim, T. Boron- and calcium-interspersed cathode-electrolyte interphases on Ni-rich layered oxide cathodes for advanced performance lithium-ion batteries. Appl. Surface Sci. 2024, 663, 160195. [Google Scholar] [CrossRef]
- Usman, A.; Murtaza, G.; Ayyaz, A.; Al-Muhimeed, T.; Farid, G. Synthesis, characterization, and evaluation of improved electrochemical performance of vanadium and zinc co-doped Ni-rich oxide cathode materials: Experimental and first-principles study. Ionics 2024, 30, 5989–6001. [Google Scholar] [CrossRef]
- Wu, F.; Liu, N.; Chen, L.; Li, N.; Lu, Y.; Cao, D.; Xu, M.; Wang, Z.; Su, Y. A universal method for enhancing the structural stability of Ni-rich cathodes via the synergistic effect of dual-element cosubstitution. ACS Appl. Mater. Interfaces 2021, 13, 24925–24936. [Google Scholar] [CrossRef]
- Si, Z.; Shi, B.; Huang, J.; Yu, Y.; Han, Y.; Zhang, J.; Li, W. Titanium and fluorine synergetic modification improves the electrochemical performance of Li(Ni0.8Co0.1Mn0.1)O2. J. Mater. Chem. A 2021, 9, 9354–9363. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Q.; Sun, X.-G.; Li, C.; Huang, Y.; Mu, W.; Tan, J.; Li, J.; Liu, K.; Zheng, S.; et al. An epitaxial surface heterostructure anchoring approach for high-performance Ni-rich layered cathodes. J. Energy Chem. 2025, 105, 158–169. [Google Scholar] [CrossRef]
- Mao, D.; Tan, X.; Fan, Z.; Song, L.; Zhang, Y.; Zhang, P.; Su, S.; Liu, G.; Wang, H.; Chu, W. Unveiling the roles of trace Fe and F Co-doped into high-Ni Li-rich layered oxides in performance improvement. ACS Appl. Mater. Interfaces 2023, 15, 10774–10784. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Lu, S.; Jia, T.; Liu, Y.; Ding, W.; Yu, X.; Kang, F.; Zhang, J.; Cao, Y. Engineering of cobalt-free Ni-rich cathode material by dual-element modification to enable 4.5 V-class high-energy-density lithium-ion batteries. Chem. Eng. J. 2023, 455, 140652. [Google Scholar] [CrossRef]
- Park, H.G.; Min, K.; Park, K. A synergistic effect of Na+ and Al3+ Dual doping on electrochemical performance and structural stability of LiNi0.88Co0.08Mn0.04O2 cathodes for Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 5168–5176. [Google Scholar] [CrossRef]
- Peng, F.; Chu, Y.; Li, Y.; Pan, Q.; Yang, G.; Zhang, L.; Hu, S.; Zheng, F.; Wang, H.; Li, Q. Mg,Ti-base surface integrated layer and bulk doping to suppress lattice oxygen evolution of Ni-rich cathode material at a high cut-off voltage. J. Energy Chem. 2022, 71, 434–444. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Liu, J.D.; Xu, W.C.; Lu, Y.; Ma, H.; Cheng, F.Y.; Chen, J. Gradient doping Mg and Al to stabilize Ni-rich cathode materials for rechargeable lithium-ion batteries. J. Power Sources 2022, 535, 231445. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, L.; Sedlacik, M.; Saha, P.; Cheng, Q.; Yu, H.; Jiang, H. Enhanced Li-ion intercalation kinetics and lattice oxygen stability in single-crystalline Ni-rich Co-poor layered cathodes. J. Mater. Chem. A 2024, 12, 3682–3688. [Google Scholar] [CrossRef]
- Jamil, S.; Bin Yousaf, A.; Yoon, S.H.; Han, D.S.; Yang, L.; Kasak, P.; Wang, X. Dual cationic modified high Ni-low co layered oxide cathode with a heteroepitaxial interface for high energy-density lithium-ion batteries. Chem. Eng. J. 2021, 416, 129118. [Google Scholar] [CrossRef]
- Ziraki, S.; Kanani, M.; Hashemi, B.; Loghavi, M.M. Effect of sodium and yttrium co-doping on electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries: Computational and experimental investigation. J. Energy Storage 2023, 70, 107983. [Google Scholar] [CrossRef]
- Feng, H.; Xu, Y.; Zhou, Y.; Song, J.; Yang, J.; Tan, Q. The Y3+ and W6+ co-doping into Ni-rich Co-free single-crystal cathode LiNi0.9Mn0.1O2 for achieving high electrochemical properties in lithium-ion batteries. J. Alloys Compd. 2024, 976, 173043. [Google Scholar] [CrossRef]
- Zhang, B.; Wen, H.; Xian, K.; Lu, N.; Zheng, C.; Xiao, Z.; He, X.; Ye, L.; Wang, J.; Ming, L.; et al. Synergistically regulating the structural tolerance of Co-free Ni-rich cathode materials at high-rate through multi-heteroatoms substitution. J. Colloid Interface Sci. 2025, 682, 589–598. [Google Scholar] [CrossRef]
- Shen, J.; Li, H.; Qi, H.; Lin, Z.; Li, Z.; Zheng, C.; Du, W.; Chen, H.; Zhang, S. Enhancing thermodynamic stability of single-crystal Ni-rich cathode material via a synergistic dual-substitution strategy. J. Energy Chem. 2024, 88, 428–436. [Google Scholar] [CrossRef]
- Vanaphuti, P.; Bai, J.; Ma, L.; Ehrlich, S.; Kisslinger, K.; Wang, F.; Wang, Y. Unraveling Na and F coupling effects in stabilizing Li, Mn-rich layered oxide cathodes via local ordering modification. Energy Storage. Mater. 2020, 31, 459–469. [Google Scholar] [CrossRef]
- Ko, G.; Park, S.; Kim, W.; Kwon, K. Synergistic effect of Na and Al co-doping on the electrochemical properties of Li[Ni0.8Mn0.1Co0.1]O2 cathode materials for Li-ion batteries. J. Alloys Compd. 2022, 925, 166678. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Wang, F.; Wang, W.-N.; Liu, S.; Gao, X.-P. Enhancing structural rigidity of ultrahigh-Ni oxide through Al and Nb dual-bulk-doping for high-voltage lithium-ion batteries. Small Methods 2024, 8, 2400224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Park, N.-Y.; Park, G.-T.; Kim, U.-H.; Sohn, S.-J.; Kang, M.-S.; Ribas, R.M.; Monteiro, R.S.; Sun, Y.-K. Doping strategy in developing Ni-rich cathodes for high-performance lithium-ion batteries. ACS Energy Lett. 2024, 9, 740–747. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Huang, K.; Lian, C.; Su, H.; Liu, H. Al- and Nb-comodified Ni-rich NCM cathode for high-performance lithium-ion batteries. Ind. Eng. Chem. Res. 2024, 63, 2740–2749. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, U.-H.; Sun, Y.-K.; Yoon, C.-S. Multi-doped (Ga, B) Li[Ni0.885Co0.100Al0.015]O2 cathode. J. Electrochem. Soc. 2020, 167, 100557. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, S.; Li, G.; Zhou, Z.; Xu, N.; Wu, M.; Gao, X. Building the stable oxygen framework in high-Ni layered oxide cathode for high-energy-density Li-ion batteries. Energy Environ. Mater. 2022, 5, 1260–1269. [Google Scholar] [CrossRef]
- Feng, Z.; Rajagopalan, R.; Zhang, S.; Sun, D.; Tang, Y.-G.; Ren, Y.; Wang, H.-Y. A three in one strategy to achieve zirconium doping, boron doping, and interfacial coating for stable LiNi0.8Co0.1Mn0.1O2 cathode. Adv. Sci. 2020, 8, 2001809. [Google Scholar] [CrossRef]
- Choi, C.-M.; Park, J.-H.; Sun, Y.-K.; Yoon, C.-S. Ultra-stable cycling of multi-doped (Zr,B) Li[Ni0.885Co0.100Al0.015] O2 cathode. J. Power Sources 2021, 513, 230548. [Google Scholar] [CrossRef]
- Ming, Y.; Xiang, W.; Qiu, L.; Hua, W.-B.; Li, R.; Wu, Z.-G.; Xu, C.-L.; Li, Y.-C.; Wang, D.; Chen, Y.-X.; et al. Dual elements coupling effect induced modification from the surface into the bulk lattice for Ni-rich cathodes with suppressed capacity and voltage decay. ACS Appl. Mater. Interfaces 2020, 12, 8146–8156. [Google Scholar] [CrossRef]
- Cheng, X.; Zhong, F.; Wang, T.; Cao, X.; Liang, M.; Liu, Y.; Wu, B.; Li, J. The dual modification of La and Zr of Ni-rich cathodes for improved cycling stability at elevated voltage and temperature. J. Power Sources 2024, 612, 234801. [Google Scholar] [CrossRef]
- Kumar, D.; Ramesha, K. Comprehensive study of Ti and Ta co-doping in Ni-rich cathode material LiNi0.8Mn0.1Co0.1O2 towards improving the electrochemical performance. ChemPhysChem 2024, 25, e202400064. [Google Scholar] [CrossRef]
- Guan, S.; Tao, L.; Tang, P.; Fang, R.; Wu, H.; Piao, N.; Yang, H.; Hu, G.; Geng, X.; Li, L.; et al. Regulating interfacial chemistry and kinetic behaviors of F/Mo co-doping Ni-rich layered oxide cathode for long-cycling lithium-ion batteries over −20 °C–60 °C. J. Energy Chem. 2024, 94, 449–457. [Google Scholar] [CrossRef]
- Rambukwella, I.; Firestein, K.L.; Xu, Y.; Sun, Z.; Zhang, S.; Yan, C. Unveiling the effect of molybdenum and titanium co-doping on degradation and electrochemical performance in Ni-rich cathodes. Mater. Rep. Energy 2025, 5, 100314. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Chen, X.; Yang, Q.; Zhang, F.; Mu, D.; Li, L.; Wu, F.; Chen, R. Al & Ti Synergy enhancing ionic diffusion and stabilizing lattice oxygen for the high voltage single crystal Ni-rich layered oxide cathode materials. J. Power Sources 2024, 603, 234439. [Google Scholar]
- Han, E.; Du, X.; Yang, P.; Han, Y. The effects of copper and titanium co-substitution on LiNi0.6Co0.15Mn0.25O2 for lithium-ion batteries. Ionics 2018, 24, 393–401. [Google Scholar] [CrossRef]
- Zhu, F.; Shi, Y.; Hu, G.; Peng, Z.; Cao, Y.; Sun, Q.; Xue, Z.; Zhang, Y.; Du, K. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 via titanium and boron co-doping. Ceram. Int. 2021, 47, 3070–3078. [Google Scholar]
- He, Z.; Zhang, M.; Zhou, K.; Cheng, Y.; Luo, M.; Su, Y.; Hao, J.; Sun, Y.; Li, Y.; Yang, Y. Enabling excellent thermal stability of an ultrahigh nickel-rich cathode (LiNi0.90Co0.05Mn0.05O2) by a magnesium and titanium codoping strategy. ACS Appl. Energy Mater. 2023, 6, 3422–3431. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, F.; Zhang, Y. Multifunctional Ti-Mg co-doping strategy to enhance long-term cycling performance for Ni-rich cathode materials. J. Alloys Compd. 2024, 981, 173592. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Wang, Y.; Wan, T.; Guan, P.; Zhou, X.; Wang, X.; Chen, Y.; Shi, H.; Dou, A.; et al. Relieving stress concentration through anion–cation codoping toward highly stable nickel-rich cathode. ACS Nano 2023, 17, 20621–20633. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Qiu, Y.-G.; Cheng, F.-Y.; Wei, P.; Li, Y.-Y.; Liu, Y.; Sun, S.-X.; Xu, Y.; Li, Q.; Fang, C.; et al. Realization of a high-voltage and high-rate nickel-rich NCM cathode material for LIBs by Co and Ti dual modification. ACS Appl. Mater. Interfaces 2021, 13, 17707–17716. [Google Scholar] [CrossRef]
- Xiao, Z.M.; Zhang, B.; He, X.Y.; Ou, X. Cobalt/aluminum co-substitution in a LiNi0.9Mn0.1O2 layered cathode for improving kinetics. Chem. Commun. 2023, 59, 7935–7938. [Google Scholar] [CrossRef]
- Shi, H.; Zeng, T.; Zhang, H.; Zhou, Y.; Su, M.; Li, X.; Zhang, P.; Dou, A.; Naveed, A.; Liu, Y. Enhanced structure and electrochemical stability of single crystal nickel-rich cathode material by La2Li0.5Co0.5O4 surface coating. Ceram. Int. 2022, 48, 17548–17555. [Google Scholar] [CrossRef]
- Yoon, M.; Dong, Y.-H.; Hwang, J.; Sung, J.; Cha, H.; Ahn, K.; Huang, Y.; Kang, S.-J.; Cho, J. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 2021, 6, 362–371. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Shin, W.; Jo, E.; Manthiram, A. Insights into the microstructural engineering of cobalt-free, high-nickel cathodes based on surface energy for lithium-ion batteries. Adv. Energy Mater. 2023, 13, 2204054. [Google Scholar] [CrossRef]
- Zeng, C.; Zheng, R.; Fan, F.; Wang, X.; Tian, G.; Liu, S.; Liu, P.; Wang, C.; Wang, S.; Shu, C. Phase compatible surface engineering to boost the cycling stability of single-crystalline Ni-rich cathode for high energy density lithium-ion batteries. Energy Storage Mater. 2024, 72, 103788. [Google Scholar] [CrossRef]
- Kuo, L.-Y.; Roitzheim, C.; Valencia, H.; Mayer, J.; Möller, S.; Myung, S.-T.; Finsterbusch, M.; Guillon, O.; Fattakhova-Rohlfing, D.; Kaghazch, P. Doping-induced surface and grain boundary effects in Ni-rich layered cathode materials. Small 2024, 20, 2307678. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Zhang, C.-H.; Xin, S.; Shi, J.-L.; Wang, W.-P.; Fan, M.; Chang, Y.-X.; He, W.-H.; Wang, E.; Zou, Y.-G.; et al. Competitive doping chemistry for nickel-rich layered oxide cathode materials. Angew. Chem. Int. Ed. 2022, 61, e202116865. [Google Scholar] [CrossRef]
- Ou, X.; Liu, T.; Zhong, W.; Fan, X.; Guo, X.; Huang, X.; Cao, L.; Hu, J.; Zhang, B.; Chu, Y.S.; et al. Enabling high energy lithium metal batteries via single-crystal Ni-rich cathode material co-doping strategy. Nat. Commun. 2022, 13, 2319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, X.; Xiao, L.; Xiao, Z. Influence of Al3+ and PO43− co-doping on structure and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials. J. Solid State Electrochem. 2023, 27, 1185–1194. [Google Scholar] [CrossRef]
- Reissig, F.; Lange, M.A.; Haneke, L.; Placke, T.; Zeier, W.G.; Winter, M.; Schmuch, R.; Gomez-Martin, A. Synergistic effects of surface coating and bulk doping in Ni-rich lithium nickel cobalt manganese oxide cathode materials for high-energy lithium-ion batteries. ChemSusChem 2022, 15, e202102220. [Google Scholar] [CrossRef]
- Park, G.-T.; Yoon, J.I.; Kim, G.-H.; Park, N.-Y.; Park, B.-C.; Sun, Y.-K. Ni-rich cathode materials enabled by cracked-surface protection strategy for high-energy lithium batteries. Mater. Sci. Eng. R Rep. 2025, 164, 100945. [Google Scholar] [CrossRef]
- Gong, M.; Fu, Y.; Yao, W.; Rao, X.; Zhang, Q.; Zhong, S.; Sun, Q.; Cheng, H. Profiting the Co-modifications of Li2SnO3 coating and Sn4+ doping in Co-free Ni-rich cathode particles for lithium-ion batteries. ACS Appl. Energy Mater. 2023, 6, 1248–1258. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, G.; Wang, W.; Du, K.; Peng, Z.; Zeng, J.; Li, L.; Cao, Y. Enhancing the electrochemical performance of Co-less Ni-rich LiNi0.925Co0.03Mn0.045O2 cathode material via Co-modification with Li2B4O7 coating and B3+ doping. J. Power Sources 2022, 548, 232092. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Li, M.; Wang, C.; Zhao, Z.; Xie, S.; Lin, H.; Wu, X.; Liu, H.; Zhang, L.; et al. B-doped and La4NiLiO8-coated LiNi0.825Co0.115Mn0.06O2 cathode with enhanced structural and interfacial stability for lithium-ion batteries. J. Energy Chem. 2022, 71, 588–594. [Google Scholar]
- Yang, H.; Wu, H.H.; Ge, M.; Li, L.; Yuan, Y.; Yao, Q.; Chen, J.; Xia, L.; Zheng, J.; Chen, Z.; et al. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv. Funct. Mater. 2019, 29, 1808825. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.-J.; Doo, S.-G.; Jin, B.-S.; Kim, H.-S. A synergetic modification approach toward high-capacity Ni-rich cathode materials for next generation lithium-ion batteries. Solid State Ion. 2022, 387, 116053. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, B.; He, X.; Xiao, B.; Xiao, Z.; Li, X.; Ou, X. Synergistic regulation of kinetic reaction pathway and surface structure degradation in single-crystal high-nickel cathodes. J. Colloid Interface Sci. 2023, 629, 388–398. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.-F.; Lu, S.-R.; Ding, W.-B.; Yu, X.-L.; Liang, G.-M.; Zou, J.-S.; Kang, F.-Y.; Zhang, J.-J.; Cao, Y.-D. B2O3/LiBO2 dual-modification layer stabilized Ni-rich cathode for lithium-ion battery. J. Power Sources 2022, 536, 231510. [Google Scholar] [CrossRef]
- Su, Y.; Li, L.; Chen, L.; Wang, L.; Lu, Y.; Zhang, Q.; Bao, L.; Wu, F. Enhanced electrochemical performance of Ni-rich cathode materials with an in situ-formed LiBO2/B2O3 hybrid coating layer. ACS Appl. Energy Mater. 2022, 5, 2231–2241. [Google Scholar] [CrossRef]
- Xu, M.; Lu, J.; Sun, Z.; Yang, M.; Sheng, B.; Chen, M.; Chen, J.; Zhang, Q.; Han, X. Lanthanum doping and surface Li3BO3 passivating layer enabling 4.8 V nickel-rich layered oxide cathodes toward high energy lithium-ion batteries. J. Colloid Interface Sci. 2024, 673, 386–394. [Google Scholar] [CrossRef]
- Tang, W.; Shu, Z.; Li, A.; Huang, X.; Li, W. Enhancing structural integrity and long-term cycling stability of high-voltage single-crystalline Ni-rich cathodes via surface/subsurface dual-functional modification engineering. Energy Storage Mater. 2025, 77, 104185. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Ou, X.; Wang, C.; Peng, C.; Zhang, B. Enhancing high-voltage performance of Ni-rich cathode by surface modification of self-assembled NASICON fast ionic conductor LiZr2(PO4)3. ACS Appl. Mater. Interfaces 2019, 11, 15507–15516. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, H.; Zong, Y.; Zuba, M.; Chen, Y.; Chernova, N.A.; Bai, J.; Pei, B.; Goel, A.; Rana, J.; et al. What is the role of Nb in nickel-rich layered oxide cathodes for lithium-ion batteries? ACS Energy Lett. 2021, 6, 1377–1382. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, H.; Chen, X.; Zuba, M.; Chernova, N.; Zhou, G.; Whittingham, M. Li–Nb–O coating/substitution enhances the electrochemical performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) cathode. ACS Appl. Mater. Interfaces 2019, 11, 34889–34894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, M.; Zhang, D.; Yan, Y.; Wang, Y.; Li, J.; Li, Z. Enhanced electrochemical properties of NMC811 cathode material due to synergistic modification with Sm as doping and coating agent. J. Alloys Compd. 2022, 909, 164712. [Google Scholar] [CrossRef]
- Kim, Y. Thermochemical investigation of Zr doping in LiNi8/12Co2/12Mn2/12O2 based on phase equilibria simulation. Int. J. Quant. Chem. 2019, 119, e26028. [Google Scholar] [CrossRef]
- Tan, X.; Peng, W.; Wang, M.; Luo, G.; Wang, Z.; Yan, G.; Guo, H.; Li, Q.; Wang, J. Al, Zr dual-doped cobalt-free nickel-rich cathode materials for lithium-ion batteries. Prog. Nat. Sci. Mater. Int. 2023, 33, 108–115. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Zhao, H.; Bai, Y. Ultra-high temperature operated Ni-rich cathode stabilized by thermal barrier for high-energy lithium-ion batteries. Adv. Mater. 2024, 36, 2313500. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Y.; Hu, G.; Yang, C.; Liu, F.; Yin, C.; Cao, J.; Chen, Z. Enhancing the cycling stability of full-concentration-gradient Ni-rich layered cathodes via in-situ Zr doping. Chem. Eng. J. 2024, 493, 152872. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Gao, M.-Y.; Liu, S.; Li, G.-R.; Gao, X.-P. Yttrium surface gradient doping for enhancing structure and thermal stability of high-Ni layered oxide as cathode for Li–ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 7343–7354. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, G.; Wang, W.; Zhao, B.; Li, H.; Peng, Z.; Du, K.; Cao, Y. Ni-rich Co-less cathode materials with LiYO2 and Y3+ modification toward improved storage performance against ambient air for lithium-ion batteries. ACS Sustain. Chem. Eng. 2024, 12, 24–36. [Google Scholar] [CrossRef]
- Bizzotto, F.; Dachraoui, W.; Grissa, R.; Zhao, W.; Pagani, F.; Querel, E.; Khünel, R.-S.; Battaglia, C. Modification of NMC811 with titanium for enhanced cycling and high-voltage stability. Electrochim. Acta 2023, 462, 142758. [Google Scholar] [CrossRef]
- Fan, Q.; Lin, K.; Yang, S.; Guan, S.; Chen, J.; Feng, S.; Liu, J.; Liu, L.; Li, J.; Shi, Z. Constructing effective TiO2 nano-coating for high-voltage Ni-rich cathode materials for lithium-ion batteries by precise kinetic control. J. Power Sources 2020, 477, 228745. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Z.; Huang, K.; Jiang, J.; Chen, Z.; Qi, X.; Dou, H.; Zhang, X. In situ tuning residual lithium compounds and constructing TiO2 coating for surface modification of a nickel-rich cathode toward high-energy lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 12423–12432. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, W.; Zhao, G.; Liu, Q.; Duan, L.; Wang, S.; An, Q.; Wang, H.; Yang, Y.; Zhang, C.; et al. LiNi0.9Co0.09Mo0.01O2 cathode with Li3PO4 coating and Ti doping for next-generation lithium-ion batteries. ACS Energy Lett. 2023, 8, 1629–1638. [Google Scholar] [CrossRef]
- Hou, Y.; Ren, Y.; Shi, T.; Li, J.; Li, H.; Zhang, L.; Liu, Y.; Wan, Y.; Zhang, S.; Liu, Y.; et al. The surface Al2O3 coating and bulk Zr doping drastically improve the voltage fade and cycling stability of Li(Ni0.8Mn0.1Co0.1)O2 cathode materials. J. Alloys Compd. 2023, 939, 168778. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, X.; Wang, S.; Zhang, P.; Zu, W.; Liu, X.; Feng, G.; Zhang, B.; Xing, W.; Zuo, M.; et al. High-performance single crystal Ni-rich cathode with regulated lattice and interface constructed by separated lithiation and crystallization calcination. Green Chem. Eng. 2024. [CrossRef]
- Shi, H.; Zheng, J.; Wan, T.; Wang, H.; Wen, Z.; Zheng, F.; Su, M.; Dou, A.; Zhou, Y.; Naveed, A.; et al. Constructing electrochemically stable single crystal Ni-rich cathode material via modification with high valence metal oxides. J. Energy Chem. 2025, 101, 392–401. [Google Scholar] [CrossRef]
- Song, W.; Gao, A.; Liu, Y.; Liu, A.; Peng, H.; Li, M.; Yang, R.; Wang, F. Tungsten-doped Ni-rich LiNi0.8Mn0.18Al0.02O2 cathode with fast kinetics and stable surface structure. J. Alloys Compd. 2025, 1010, 177611. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, C.; Song, L.; Cao, Z.; Jiang, P. Dual-modification of WO3-coating and Mg-doping on LiNi0.8Co0.1Mn0.1O2 cathodes for enhanced electrochemical performance at high voltage. Ionics 2021, 27, 1909–1917. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.-L.; Yi, Z.-C.; Liu, C.-J.; Miao, C.; Xin, Y.; Nie, S.-Q. Dual modification of LiNi0.83Co0.11Mn0.06O2 cathode materials by K+ doping and Li3PO4 coating for lithium ions batteries. Rare Met. 2024, 43, 3007–3018. [Google Scholar] [CrossRef]
- Shen, L.; Gu, Y.; Xu, T.; Zhou, Q.; Peng, P.; Chan, Y.; Du, F.; Zheng, J. Dual modification of phosphate toward improving electrochemical performance of LiNiO2 cathode materials. J. Colloid Interface Sci. 2024, 662, 505–515. [Google Scholar] [CrossRef]
- Liu, C.; Yi, Z.; Wan, J.; Miao, C.; Wang, Z.; Wang, J.; Xiao, W. Nb5+ ions doping and Li3PO4 artificial layer synergetic strategy to strengthen structure, stabilize interface, and promote ions migration for high-performance Ni-rich cathode. Chem. Eng. J. 2025, 503, 158633. [Google Scholar] [CrossRef]
- Lin, H.; Shen, Y.; Wei, L.; Song, R.; Wu, F.; Wei, P.; Yang, Z.; Ren, Y.; Qu, K.; Ding, Z. Enhancing structural stability and electrochemical performance of ultra-high Ni-rich Co-free cathode via MgHPO4 dual-functional modification. Chem. Res. Chin. Univ. 2025, 41, 333–342. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Z.; Lin, F.; Zhu, H.; Wen, J.; Wang, Y.; Bai, M.; Duan, J. Synergistic role of Sb doping and surface modification in high performance ultrahigh-nickel layered oxides cathode materials. J. Alloys Compd. 2023, 959, 170552. [Google Scholar] [CrossRef]
- Sun, G.; Zhuang, S.; Jiang, S.; Ren, Y.; Sun, Y.; Pan, X.; Wen, Y.; Li, X.; Tu, F. Elevating cycle stability of Ni-rich NMC811 cathode via single-crystallization integrating dual-modification strategy for lithium-ion batteries. J. Alloys Compd. 2024, 976, 173097. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, H.; Jiang, H.; Su, X.; Hu, Y.; Jiang, H.; Li, C. Restraining the escape of lattice oxygen enables superior cyclic performance towards high-voltage Ni-rich cathodes. Natl. Sci. Rev. 2023, 10, nwac166. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Lv, K.; Liu, J.; Bayaguud, A.; Li, X. Surface-to-bulk synergistic modification enables stable interface of Ni-rich oxide cathode for all-solid-state lithium batteries. Electrochim. Acta 2024, 497, 144595. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Shan, L.; Jiang, G.; Zhang, Y.; Meng, Q.; Dong, P. Improved electrochemical performance of Co-free Ni-rich cathode material for lithium-ion battery through multi-element composite modification. Electrochim. Acta 2024, 477, 143804. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Li, Z.; Wu, L.; Chen, J.; Liu, X.; Hu, H.; Ding, H.; Cao, S.; Wei, Q.; et al. Improving structure stability of single-crystalline Ni-rich cathode at high voltage by element gradient doping and interfacial modification. J. Energy Chem. 2025, 101, 630–640. [Google Scholar] [CrossRef]
- Wang, H.; Dong, J.; Zhang, H.; Liu, J.; Lu, Y.; Liu, Y.; Wang, X.; Li, N.; Huang, Q.; Wu, F.; et al. Enhancing structural and thermal stability of ultrahigh-Ni cathodes via anion-cation codoping induced surface reconstruction strategy. J. Energy Chem. 2025, 106, 9–19. [Google Scholar] [CrossRef]
- Chen, S.; He, T.; Su, Y.; Lu, Y.; Chen, L.; Zhang, Q.; Wang, J.; Chen, R.; Wu, F. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high-performance cathode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Zhang, J.; Liu, R.; Wang, Y.; Xiao, H.; Huang, Y.; Yuan, G. Improving electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode for Li-ion batteries by dual-conductive coating layer of PPy and LiAlO2. J. Alloys Compd. 2020, 848, 156387. [Google Scholar] [CrossRef]
- Yang, H.; Wu, K.; Hu, G.; Peng, Z.; Cao, Y.; Du, K. Design and synthesis of double-functional polymer composite layer coating to enhance the electrochemical performance of the Ni-rich cathode at the upper cutoff voltage. ACS Appl. Mater. Interfaces 2019, 11, 8556–8566. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, X.; Lin, J.; Huang, Y.; Cheng, W.; Liu, Q.; Zhang, H.; Ren, F.; Huang, Y.; Liu, Z.; et al. Restraining planar gliding in single-crystalline LiNi0.9Co0.05Mn0.05O2 cathodes by combining bulk and surface modification strategies. Angew. Chem. Inter. Ed. 2025, 64, e202419903. [Google Scholar] [CrossRef]
- Cheng, W.-D.; Li, L.; Hao, S.-L.; Wu, Y.-X.; Huo, J.-S.; Ji, Y.-Y.; Liu, X.-Q. Face-lifting the surface of LiNi0.8Co0.15Al0.05O2 cathode via Y(PO3)3 to form an in situ triple composite Li-ion conductor coating layer with the enhanced electrochemical performance. Nanotechnology 2022, 33, 375701. [Google Scholar] [CrossRef]
- Li, Q.; Dang, R.; Chen, M.; Lee, Y.; Hu, Z.-B.; Xiao, X.-L. A synthesis method for long cycle life lithium-ion cathode material: Ni-rich core-shell LiNi0.8Co0.1Mn0.1O2. ACS Appl. Mater. Interfaces 2018, 10, 17850–17860. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-H.; Park, N.-Y.; Noh, T.-C.; Kang, G.-C.; Maglia, F.; Kim, S.-J.; Yoon, C.-S.; Sun, Y.-K. Microstrain alleviation in high-energy Ni-rich NCMA cathode for long battery life. ACS Energy Lett. 2021, 6, 216–223. [Google Scholar] [CrossRef]
- Bi, Y.-J.; Liu, M.; Xiao, B.-W.; Jiang, Y.; Lin, H.; Zhang, Z.-G.; Chen, G.-X.; Sun, Q.; He, H.-Y.; Huang, F.; et al. Highly stable Ni-rich layered oxide cathode enabled by a thick protective layer with bio-tissue structure. Energy Storage Mater. 2020, 24, 291–296. [Google Scholar] [CrossRef]
- Maeng, S.; Chung, Y.; Min, S.; Shin, Y. Enhanced mechanical strength and electrochemical performance of core–shell structured high–nickel cathode material. J. Power Sources 2020, 448, 227395. [Google Scholar] [CrossRef]
- Park, G.-T.; Sun, H.H.; Noh, T.-C.; Maglia, F.; Kim, S.-J.; Lamp, P.; Sun, Y.-K. Nanostructured Co-free layered oxide cathode that affords fast-charging lithium-ion batteries for electric vehicles. Adv. Energy Mater. 2022, 12, 2202719. [Google Scholar] [CrossRef]
- Rathore, D.; Garayt, M.; Liu, Y.-L.; Geng, C.-X.; Johnson, M.; Dahn, J.-R.; Yang, C.-Y. Preventing interdiffusion during synthesis of Ni-rich core-shell cathode materials. ACS Energy Lett. 2022, 7, 2189–2195. [Google Scholar] [CrossRef]
- Bae, J.H.; Hwang, K.; Kim, J.; Kang, H.; Lee, I.; Park, C.W.; Sohn, H.; Yoon, S. Enhanced electrochemical performance of Ni-rich cathode for lithium-ion batteries under elevated cut-off voltage (4.5 V): Elongated cycle stability of cathode by relaxed mechanical stress in the presence of internal porous layer. J. Electroanal. Chem. 2024, 957, 118123. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Hou, P.; Zhou, E.; Guo, J.; Zhang, J.; Wang, D.; Guo, F.; Song, D.; Shi, X.; et al. Core–shell structured Li[(Ni0.8Co0.1Mn0.1)0.7(Ni0.45Co0.1Mn0.45)0.3]O2 cathode material for high-energy lithium ion batteries. J. Alloys Compd. 2024, 587, 710–716. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, A.; Wang, K.; Xiao, Z.; Mao, Q.; Lu, X.; Wang, G.; Lu, C.; Zhang, J.; Huang, H.; et al. Binary-compositional core-shell structure Ni-rich cathode material with radially oriented primary particles in shell for long cycling lifespan lithium-ion batteries. Mater. Today Energy 2023, 34, 101292. [Google Scholar] [CrossRef]
- Ye, T.; Li, Z.M.; Yan, H.L.; Ha, Y.; Zhang, D.Y.; Zhou, C.Y.; Wang, L.J.; Li, L.; Xiang, Q.X. Magnetic frustration effect on the rate performance of LiNi0.6Co0.4-xMnxO2 cathodes for lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2201556. [Google Scholar] [CrossRef]
- Han, S.-M.; Park, G.-T.; Kim, D.-H.; Seo, M.-G.; Park, N.-Y.; Sun, Y.-K. Introducing Co nanoshells onto Ni-rich cathode materials for high long-life Li-ion batteries. ACS Energy Lett. 2024, 9, 5859–5868. [Google Scholar] [CrossRef]
- Xue, H.; Liang, Y.; Huang, Y.; Ji, Y.; Xu, Z.; Chen, X.; Wang, H.; Liu, J.; Amine, K.; Liu, T.; et al. In situ conversion of artificial proton-rich shell to inorganic maskant toward stable single-crystal Ni-rich cathode. Adv. Mater. 2025, 37, 2415860. [Google Scholar] [CrossRef]
- Song, G.; Zhong, H.; Dai, Y.; Zhou, X.; Yang, J. WO3 membrane-encapsulated layered LiNi0.6Co0.2Mn0.2O2 cathode material for advanced Li-ion batteries. Ceram. Int. 2019, 45, 6774–6781. [Google Scholar] [CrossRef]
- Liu, J.-K.; Yang, X.-R.; Wang, C.-W.; Yin, Z.-W.; Hu, Y.-Y.; Deng, L.; Wang, Z.; Zhou, Y.; Li, J.-T. Surface-to-bulk engineering with high-valence W6+ enabling stabilized single-crystal LiNi0.9Co0.05Mn0.05O2 cathode. J. Energy Chem. 2024, 98, 67–76. [Google Scholar] [CrossRef]
- Chu, B.; Xu, R.; Li, G.; Chen, J.; Xu, Z.; Huang, T.; Wang, B.; Yu, A. Simultaneous B/W dual coating on ultra-high nickel single crystal cathode material for lithium-ion batteries. J. Power Sources 2023, 577, 233260. [Google Scholar] [CrossRef]
- Du, F.; Qu, H.; Yu, X.; Ding, L.; Zhang, P.; Wang, Y.; Chen, Y.; Wang, Y.; Zhang, X.; Zheng, J. Pre-introducing Li4NiWO6 defect phase by tungsten modification enables highly stabilized Ni-rich cathode. Chem. Eng. J. 2024, 492, 152357. [Google Scholar] [CrossRef]
- Ding, H.; Wang, X.; Zhao, J.; Wang, X.; Zhang, H.; Liu, G.; Yu, W.; Dong, X.; Wang, J. Construction of hollow multi-shell structural NCM622 as cathode to improve the cycling stability of its Li-ion batteries. J. Energy Storage 2025, 107, 114835. [Google Scholar] [CrossRef]
- Yoon, C.-S.; Sun, Y.K.; Kim, U.-H.; Park, K.-J.; Ryu, H.-H.; Kim, H.-S.; Sun, Y.-K. Microstructure evolution of concentration gradient Li[Ni0.75Co0.10Mn0.15]O2 cathode for lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1802090. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.-Y.; Li, Q.; Yang, Y.-Q.; Deng, X.; Dang, R.-B.; Wu, M.-M.; Wu, Z.-J.; Xiao, X.-L.; Yu, X.-Q. In situ synthesis of nickel concentration gradient structure promising superior electrochemical properties of Ni-rich LiNi0.8Co0.15Al0.05O2 at high cut-off voltage. Nanoscale 2020, 12, 11182–11191. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.-W.; Chen, X.; Niu, J.-J. Nickel-rich layered LiNi0.8Mn0.1Co0.1O2 with dual gradients on both primary and secondary particles in lithium-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100767. [Google Scholar] [CrossRef]
- Yerkinbekova, Y.; Kumarov, A.; Tatykayev, B.; Mentbayeva, A.; Repo, E.; Laasko, E. Ni-rich cathode materials with concentration gradients for high-energy and safe lithium-ion batteries: A comprehensive review. J. Power Sources 2025, 626, 235686. [Google Scholar] [CrossRef]
- Noh, H.-J.; Chen, Z.-H.; Yoon, C.-S.; Lu, J.; Amine, K.; Sun, Y.-K. Cathode material with nanorod structure—An application for advanced high-energy and safe lithium batteries. Chem. Mater. 2013, 25, 2109–2115. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Liu, X.; Feng, G.; Wang, S.; Zhang, B.; Zhang, P.; Zuo, M.; Xing, W.; Fan, W.; et al. Surface reactivity versus microcracks in Ni-rich layered oxide cathodes: Which is critical for long cycle life? Chem. Eng. J. 2024, 488, 150795. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Y.; Hai, C.; Zeng, J.; Sun, Y.; Ren, X.; Shen, Y.; Li, X.; Zhang, G. Analysis of the growth mechanism of hierarchical structure Ni0.8Co0.1Mn0.1(OH)2 agglomerates as precursors of LiNi0.8Co0.1Mn0.1O2 in the presence of aqueous ammonia. Appl. Surf. Sci. 2023, 619, 156379. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Y.; Zeng, J.; Hai, C.; Sun, Y.; Ren, X.; Shen, Y.; Li, X. Investigating the effect of pH on the growth of coprecipitated Ni0.8Co0.1Mn0.1(OH)2 agglomerates as precursors of cathode materials for Li-ion batteries. Ceram. Int. 2023, 49, 15851–15864. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Lin, L.; Ai, X.; Gui, S.; Guo, W.; Li, S.; Wang, L.; Yang, H.; Peng, D.-L.; et al. Intrinsic highly conductive and mechanically robust Li-rich cathode materials enabled by microstructure engineering for enhanced electrochemical properties. Adv. Funct. Mater. 2023, 34, 2308494. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Z.; Yu, H.; Demir, M.; Cheng, Q.; Jiang, H. Robust concentration gradient Co-free Ni-rich cathodes enable long-life and safe operations in high-voltage Li-ion batteries. Energy Fuels 2025, 39, 4507–4514. [Google Scholar] [CrossRef]
- He, X.; Su, S.; Zhang, B.; Xiao, Z.; Zhang, Z.; Ou, X. Alleviating the anisotropic microstructural change and boosting the lithium ions diffusion by grain orientation regulation for Ni-rich cathode materials. J. Energy Chem. 2024, 88, 213–222. [Google Scholar] [CrossRef]
- Park, N.Y.; Cho, G.; Kim, S.B.; Sun, Y.K. Multifunctional doping strategy to develop high-performance Ni-rich cathode material. Adv. Energy Mater. 2023, 13, 2204291. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Hai, C.; Ren, X.; Zeng, J.; Shen, Y.; Zhou, Y. Anionic-surfactant-assisted synthesis of Ni0.8Co0.1Mn0.1(OH)2 as precursors of high-performance cathode materials for lithium-ion batteries. J. Power Sources 2024, 615, 235092. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Dai, Z.; Feng, S.; Zheng, M.; Zheng, C.; Jin, J.; Wu, M.; Wu, X.; Lu, J.; et al. Surface gradient Ni-rich cathode for Li-ion batteries. Adv. Mater. 2024, 36, 2401052. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Du, C.Y.; Xu, X.; He, X.; Yin, G.; Ma, Y.; Zuo, P.; Cheng, X.; Gao, Y. Lithium phosphorus oxynitride coated concentration gradient Li[Ni0.73Co0.12Mn0.15]O2 cathode material with enhanced electrochemical properties. Electrochim. Acta 2016, 192, 340–345. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, S.; Zhou, Y.; Tian, X. Enhancing the electrochemical performance of single-crystal LiNi0.8Co0.1Mn0.1O2 cathode material by phosphorus doping. Chem. Eng. Sci. 2024, 285, 119627. [Google Scholar] [CrossRef]
- Choi, J.H.; Hwang, J.; Embleton, T.J.; Ko, K.; Jo, M.; Lee, C.; Yun, J.; Park, S.; Son, Y.; Oh, P. Selective outer surface modification of polycrystalline Ni-rich cathode for sulfide all-solid-state lithium-ion battery. Korean J. Chem. Eng. 2023, 40, 548–554. [Google Scholar] [CrossRef]
- Park, G.-T.; Ryu, H.-H.; Noh, T.-C.; Kang, G.-C.; Sun, Y.-K. Microstructure-optimized concentration-gradient NCM cathode for long-life Li-ion batteries. Mater. Today 2022, 52, 9–18. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Zhang, X.; Lu, Y.; Wang, B.; Deng, Q.; Yang, Y.; Wang, E.; Lyu, Z.; Li, Y.; et al. Full concentration gradient-tailored Li-rich layered oxides for high-energy lithium-ion batteries. Adv. Mater. 2021, 33, 2001358. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Xue, B.; Wu, X.; Li, L.; Guo, Y.; Zhang, L. Synergistic modification of Ni-rich full concentration gradient materials with enhanced thermal stability. Chem. Eng. J. 2023, 451, 138518. [Google Scholar] [CrossRef]
- Sun, H.-H.; Weeks, J.A.; Heller, A.; Mullins, C.B. Nanorod gradient cathode: Preventing electrolyte penetration into cathode particles. ACS Appl. Energy Mater. 2019, 2, 6002–6011. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, J.; Liu, Z.; Wang, W.; Zhang, Z.; Tang, Y.; Zhang, Z.; Yang, T.; Wang, X.; Li, X.; et al. Achieving long-life Ni-rich cathodes with improved mechanical-chemical properties via concentration gradient structure. Adv. Func. Mater. 2024, 34, 2400956. [Google Scholar] [CrossRef]
- Song, L.; Xiao, M.; Du, J.; Li, L.; Zhao, T.; Xia, Y.; Xiang, Y.; Chen, T.; Jiang, L.; Xiao, Z.; et al. An effective gradient coating strategy to improve the structure stability of Ni-rich nickel-cobalt-manganese ternary layer oxide cathode. J. Energy Storage 2024, 98, 113123. [Google Scholar] [CrossRef]
- Sitnikova, L.A.; Savina, A.; Morozov, A.V.; Golubnichiy, A.A.; Dolzhikova, E.A.; Moiseev, I.A.; Luchkin, S.Y.; Abakumov, A.M. Improving electrochemical performance of Ni-rich layered cathode material with combining Co-enriched compositional gradient and radial microstructure. J. Power Sources 2024, 602, 234302. [Google Scholar] [CrossRef]
- Guo, W.; Yu, H.; Wang, M.; Wu, M.; Chen, L.; Jiang, H.; Li, C. Compositional gradient design of Ni-rich Co-poor cathodes enhanced cyclability and safety in high-voltage Li-ion batteries. ACS Nano 2025, 19, 8371–8380. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, J.; Zhang, J.; Cheng, F.; Chen, J. LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries. J. Mater. Chem. A 2019, 7, 20958–20964. [Google Scholar] [CrossRef]
- Tao, J.; Mu, A.; Geng, S.; Xiao, H.; Zhang, L.; Huang, Q. Influences of direction and magnitude of Mg2+ doping concentration gradient on the performance of full concentration gradient cathode material. J. Solid State Electrochem. 2021, 25, 1959–1974. [Google Scholar] [CrossRef]
- Mu, Y.; Chen, X.; Ming, H.; Zhang, S.; Zhu, X.; Qiu, J. Oriented gradient doping of zirconium in Ni-rich cathode to achieve ultrahigh stability and rate capability. ACS Appl. Mater. Interfaces 2023, 15, 49289–49298. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, J.; Liu, J.; Ma, H.; Cheng, F. Enhancing LiNiO2 cathode materials by concentration-gradient yttrium modification for rechargeable lithium-ion batteries. J. Energy Chem. 2021, 63, 312–319. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Yang, C.; Xu, D.; Zhu, Y.-H.; Zhou, T.; Xin, S.; You, Y. Concentration-gradient Nb-doping in a single-crystal LiNi0.83Co0.12Mn0.05O2 cathode for high-rate and long-cycle lithium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 18828–18835. [Google Scholar] [CrossRef]
- Yang, S.; Yang, K.; Mi, J.; Guo, S.; An, X.; Su, H.; He, Y. Electrolyte engineering and interphase chemistry toward high-performance nickel-rich cathodes: Progress and perspectives. Mater. Rep. Energy 2025, 5, 100317. [Google Scholar] [CrossRef]
- Maleki Kheimeh Sari, H.; Li, X. Controllable cathode–electrolyte interface of Li[Ni0.8Co0.1Mn0.1]O2 for lithium ion batteries: A review. Adv. Energy Mater. 2019, 9, 1901597. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Wang, Z.; Li, X.; Liu, J.; Bayaguud, A.; Zhang, L. Interface problems, modification strategies and prospects of Ni–rich layered oxide cathode materials in all–solid–state lithium batteries with sulfide electrolytes. J. Power Sources 2023, 571, 233079. [Google Scholar] [CrossRef]
- Wu, J.H.; Shen, L.; Zhang, Z.H.; Liu, G.Z.; Wang, Z.Y.; Zhou, D.; Wan, H.L.; Xu, X.X.; Yao, X.Y. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes. Electro. Energy. Rev. 2020, 4, 411. [Google Scholar] [CrossRef]
- Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. Energy 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Walther, F.; Strauss, F.; Wu, X.; Mogwitz, B.; Hertle, J.; Sann, J.; Rohnke, M.; Brezesinski, T.; Janek, J. The working principle of a Li2CO3/LiNbO3 coating on NCM for thiophosphate-based all-solid-state batteries. Chem. Mater. 2021, 33, 2110–2125. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Han, J.; Wen, K.; Guan, S.; Xue, C.; Zhang, Z.; Xu, B.; Lin, Y.; Shen, Y.; et al. Super long-cycling all-solid-state battery with thin Li6PS5Cl-based electrolyte. Adv. Energy Mater. 2022, 12, 2200660. [Google Scholar] [CrossRef]
- Lee, J.Y.; Noh, S.; Seong, J.Y.; Lee, S.; Park, Y.J. Suppressing unfavorable interfacial reactions using polyanionic oxides as efficient buffer layers: Low-cost Li3PO4 coatings for sulfide-electrolyte-based all-solid-state batteries. ACS Appl. Mater. Interfaces 2023, 15, 12998–13011. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, L.; Zhang, K.; Zhang, Z.; Zhang, L.; Li, Z.; Kong, T.; Xie, Y.; Wang, Y. High-performance sulfide all-solid-state batteries enabled by high-voltage Ni-rich cathode with a conformal and conductive protective layer. ACS Appl. Energy Mater. 2024, 7, 2524–2532. [Google Scholar] [CrossRef]
- Lin, C.; Liu, Y.; Su, H.; Zhong, Y.; Wang, X.; Gu, C.; Tu, J. Elevating cycle stability and reaction kinetics in Ni-rich cathodes through tailored bulk and interface chemistry for sulfide-based all-solid-state lithium batteries. Adv. Funct. Mater. 2024, 34, 2311564. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Yan, H.; Zhao, J.; Cheng, Y.; Luo, Y.; Gu, J.; Zhong, H.; Fu, A.; Wang, K.; et al. Assembly of an elastic & sticky interfacial layer for sulfide-based all-solid-state batteries. Nano Energy 2023, 113, 108572. [Google Scholar]
- Kim, J.S.; Jung, S.; Kwak, H.; Han, Y.; Kim, S.; Lim, J.; Lee, Y.M.; Jung, Y.S. Synergistic halide-sulfide hybrid solid electrolytes for Ni-rich cathodes design guided by digital twin for all-solid-State Li batteries. Energy Storage Mater. 2023, 55, 193–204. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zeng, Z.; Xu, X.; Cheng, J.; Zhang, H.; Li, J.; Rao, Y.; Deng, Y.; Ci, L.; et al. Surface to bulk design empowering Ni-rich layered oxide cathode in sulfide-based All-Solid-State batteries. Chem. Eng. J. 2024, 498, 155029. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wu, D.; Niu, Q.; Lu, P.; Ma, T.; Su, Y.; Chen, L.; Li, H.; Wu, F. Stable Ni-rich layered oxide cathode for sulfide-based all-solid-state lithium battery. eScience 2022, 2, 537–545. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Ma, Y.; Goonetilleke, D.; Sann, J.; Walther, F.; Bianchini, M.; Janek, J.; Brezesinski, T. High performance all-solid-state batteries with a Ni-rich NCM cathode coated by atomic layer deposition and lithium thiophosphate solid electrolyte. ACS Appl. Energy Mater. 2021, 4, 7338–7345. [Google Scholar] [CrossRef]
- Gao, X.; Liu, B.; Hu, B.; Ning, Z.; Jolly, D.S.; Zhang, S.; Perera, J.; Bu, J.; Liu, J.; Doerrer, C.; et al. Solid-state lithium battery cathodes operating at low pressures. Joule 2022, 6, 636–646. [Google Scholar] [CrossRef]
- Zheng, H.; Peng, S.; Liang, S.; Yang, W.; Chen, C.; Wang, C.; Yu, R. Progress and challenges of Ni-rich layered cathodes for all-solid-state lithium batteries. Adv. Funct. Mater. 2025, 35, 2418274. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Ku, M.; Park, J.; Lee, J.; Kim, Y.-B. Coating robust layers on Ni-rich cathode active materials while suppressing cation mixing for all-solid-state lithium-ion batteries. ACS Nano 2024, 18, 25096–25106. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Ren, F.; Karger, L.; Dreyer, S.L.; Lin, J.; Ma, Y.; Cheng, Y.; Pal, A.S.; Velazquez-Rizo, M.; et al. Protective coating of single-crystalline Ni-rich cathode enables fast charging in all-solid-state batteries. ACS Nano 2025, 19, 8595–8607. [Google Scholar] [CrossRef]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state-batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef]
- Park, K.-H.; Kaup, K.; Assoud, A.; Zhang, Q.; Wu, X.; Nazar, L.F. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 2020, 5, 533–539. [Google Scholar] [CrossRef]
- Zhou, L.; Kwok, C.Y.; Shyamsunder, A.; Zhang, Q.; Wu, X.; Nazar, L.F. A new halospinel superionic conductor for high-voltage all solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 2056–2063. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kaup, K.; Park, K.-H.; Assoud, A.; Zhou, L.; Liu, J.; Wu, X.; Nazar, L.F. Lithium ytterbium-based halide solid electrolytes for high voltage all-solid-state batteries. ACS Mater. Lett. 2021, 3, 930–938. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, T.-T.; Kwok, C.Y.; Kim, S.Y.; Assoud, A.; Zhang, Q.; Janek, J.; Nazar, L.F. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 2022, 7, 83–93. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cha, H.; Kostecki, R.; Chen, G.-Y. Composite cathode design for high-energy all-solid-state lithium batteries with long cycle life. ACS Energy Lett. 2023, 8, 521–528. [Google Scholar] [CrossRef]
- Liu, H.-W.; Chen, H.; Thi, S.; Yu, P.-J.; Chen, J.-L.; Pao, C.-W.; Chang, P.-Y.; Haw, S.-C.; Liao, Y.-F.; Shao, Y.-C.; et al. High-capacity Ni-rich composite cathodes having chemically fused interface with Li3InCl6 electrolyte towards low-pressure operating all-solid-state Li-ion batteries. Compos. Part B Eng. 2025, 293, 112133. [Google Scholar] [CrossRef]
- Seong, W.M.; Cho, K.H.; Park, J.W.; Park, H.; Eum, D.; Lee, M.H.; Kim, I.S.; Lim, J.; Kang, K. Controlling residual lithium in high-nickel (>90%) lithium layered oxides for cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 18662–18669. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Huang, H.; Tan, L.; Song, L.; Wu, H.H.; Wang, C.; Zhao, Z.; Yi, H.; Duan, J.; et al. Role of residual Li and oxygen vacancies in Ni-rich cathode materials. ACS Appl. Mater. Interfaces 2021, 13, 42554–42563. [Google Scholar] [CrossRef]
- Klein, S.; Bärmann, P.; Fromm, O.; Borzutzki, K.; Reiter, J.; Fan, Q.; Winter, M.; Placke, T.; Kasnatscheew, J. Prospects and limitations of single-crystal cathode materials to overcome cross-talk phenomena in high-voltage lithium ion cells. J. Mater. Chem. A 2021, 9, 7546–7555. [Google Scholar] [CrossRef]
- Hou, X.; Liu, X.; Wang, H.; Zhang, X.; Zhou, J.; Wang, M. Specific countermeasures to intrinsic capacity decline issues and future direction of LiMn2O4 cathode. Energy Storage Mater. 2023, 57, 577–606. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.-L.; Zhou, X.A.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.B.; Zhang, N.S.; Li, S.Y. Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Wang, Z.P.; Yuan, J.; Zhu, X.Q.; Wang, H.; Huang, W.; Wang, Y.; Xu, S. Overcharge-to-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Xiong, D.J.; Hynes, T.; Ellis, L.D.; Dahn, J.R. Effects of surface coating on gas evolution and impedance growth at LiNixMnyCo1–x–yO2 Positive electrodes in Li-ion cells. J. Electrochem. Soc. 2017, 164, A3174–A3181. [Google Scholar] [CrossRef]
- Wang, X.X.; Ding, Y.L.; Deng, Y.P.; Chen, Z.W. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: Promises and challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Liu, T.; Han, C.; Guo, C.; Zhao, H.; Li, Q.; Lu, J.; Amine, K.; Qiu, X. Impacts of dissolved Ni2+ on the solid electrolyte interphase on a graphite anode. Angew. Chem. Int. Ed. 2022, 61, e202202894. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, J.I.; Park, B.K.; Han, J.W.; Lee, J.-W.; Kim, H.; Kim, K.J. Two-in-one strategy: Discretely functionalized electrolyte additive for stable lithium metal batteries coupled with Ni-rich cathode. Chem. Eng. J. 2024, 486, 150354. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Jo, E.; Manthiram, A. Inhibition of transition-metal dissolution with advanced electrolytes in batteries with silicon-graphite anodes and high-nickel cathodes. Energy Storage Mater. 2023, 56, 562–571. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, Y.; Zheng, G.; Cao, H.; Xue, H.; Yao, X.; Wang, L.; Chen, S.; Yin, Z.; Pan, F.; et al. Tailoredi construction for enhanced Si anode and Ni-rich cathode performance in lithium-ion batteries. Chin. Chem. Soc. Chem. 2025, 7, 429–439. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Wang, X.; Gong, H.; Cao, Y.; Ma, K.; Zhang, S.; Wang, S.; Yang, W.; Wang, L.; et al. Trace multifunctional additive enhancing 4.8 V ultra-high voltage performance of Ni-rich cathode and SiOx anode battery. Adv. Energy Mater. 2025, 15, 2403751. [Google Scholar] [CrossRef]
- Yu, Y.; Karayaylali, P.; Katayama, Y.; Giordano, L.; Gauthier, M.; Maglia, F.; Jung, R.; Lund, I.; Shao-Horn, Y. Coupled LiPF6 decomposition and carbonate dehydrogenation enhanced by highly covalent metal oxides in high-energy Li-ion batteries. J. Phys. Chem. C 2018, 122, 27368–27382. [Google Scholar] [CrossRef]
- Zhang, Y.; Katayama, Y.; Tatara, R.; Giordano, L.; Yu, Y.; Fraggedakis, D.; Sun, J.G.; Maglia, F.; Jung, R.; Bazant, M.Z.; et al. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy. Energy Environ. Sci. 2020, 13, 183–199. [Google Scholar] [CrossRef]
- Dose, W.M.; Temprano, I.; Allen, J.P.; Bjorklund, E.; O’Keefe, C.A.; Li, W.; Mehdi, B.L.; Weatherup, R.S.; De Volder, M.F.L.; Grey, C.P. Electrolyte reactivity at the charged Ni-rich cathode interface and degradation in Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 13206–13222. [Google Scholar] [CrossRef]
- Rinkel, B.L.D.; Vivek, J.P.; Garcia-Araez, N.; Grey, C.P. Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries. Energy Environ. Sci. 2022, 15, 3416–3438. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wu, F.; Zhao, J.; Shi, Q.; Lu, Y.; Li, N.; Cao, D.; Li, W.; Hao, J.; Yang, X.; et al. Multifunctional self-reconstructive cathode/electrolyte interphase layer for cobalt-free Li-rich layered oxide cathode. Energy Storage Mater. 2023, 60, 102798. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Y.; Zhang, X.; Zhang, Z.; Wang, E.; He, S.; Wang, B.; Han, Z.; Lu, J.; Amine, K.; et al. In situ construction of uniform and robust cathode–electrolyte interphase for Li-rich layered oxides. Adv. Funct. Mater. 2020, 31, 2009192. [Google Scholar] [CrossRef]
- Wu, F.; Dong, J.; Chen, L.; Bao, L.; Li, N.; Cao, D.; Lu, Y.; Xue, R.; Liu, N.; Wei, L.; et al. High-voltage and high-safety nickel-rich layered cathode enabled by a self-reconstructive cathode/electrolyte interphase layer. Energy Storage Mater. 2021, 41, 495–504. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, S.; Ren, Z.; Huang, L.; Xu, G.; Liu, T.; Wang, S.; Cui, G. Electrolyte engineering toward high performance high nickel (Ni ≥ 80%) lithium-ion batteries. Adv. Sci. 2024, 11, 2305753. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, S.L.; Zhang, Z.Y.; Fan, J.; Qi, W.; Chen, S. Fluoroethylene carbonate as an electrolyte additive for improving interfacial stability of high-voltage LiNi0.6Co0.2Mn0.2O2 cathode. Ionics 2019, 25, 1035–1043. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Zhao, K.; Li, J.; Yang, Y.; Gia, G. Improving the rate performance and stability LiNi0.6Co0.2Mn0.2O2 in high voltage lithium-ion battery by using fluoroethylene carbonate as electrolyte additive. Ionics 2018, 24, 3337–3346. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, J.; Wang, M.; Tang, X.; Cao, Z.; Wang, C.; Shi, Q.; Qian, Y.; Deng, Y. Multifunctional fluoroethylene carbonate for improving high-temperature performance of LiNi0.8Mn0.1Co0.1O2||SiOx@graphite lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 9989–10000. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Gao, W.; Liu, L.; Zhang, S. A new additive 3-isocyanatopropyltriethoxysilane to improve electrochemical performance of Li/NCM622 half-cell at high voltage. J. Power Sources 2019, 423, 90–97. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Liu, J.; Yu, X.; Zhang, Y.; Jiang, X.; Dong, P.; Zhang, Y. 4-bromoanisole (4BA) as additive for overcharge protection of lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 4571–4579. [Google Scholar] [CrossRef]
- Cao, Z.; Hashinokuchi, M.; Doi, T.; Inaba, M. Improved cycle performance of LiNi0.8Co0.1Mn0.1O2 positive electrode material in highly concentrated LiBF4/DMC. J. Electrochem. Soc. 2019, 166, A82–A88. [Google Scholar] [CrossRef]
- Tatara, R.; Yu, Y.; Karayaylali, P.; Chan, A.K.; Zhang, Y.; Jung, R.; Maglia, F.; Giordano, L.; Shao-Horn, Y. Enhanced cycling performance of Ni-rich positive electrodes (NMC) in Li-ion batteries by reducing electrolyte free-solvent activity. ACS Appl. Mater. Interfaces 2019, 11, 34973–34988. [Google Scholar] [CrossRef]
- Forero-Saboya, J.; Moiseev, I.A.; Vlara, M.-L.; Foix, D.; Deschamps, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. A Hydridoaluminate additive producing a protective coating on Ni-rich cathode materials in lithium-ion batteries. Adv. Energy Mater. 2024, 14, 2402051. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, F.; Fang, C.; Han, J. High-voltage electrolyte design for a Ni-rich layered oxide cathode for lithium-ion batteries. Sci. China Mater. 2023, 66, 3046–3053. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, N.; Chen, S.; Liao, Y.; Zhong, G.; Zhang, Z.; Yang, Y. Construction of a stable LiNi0.8Co0.1Mn0.1O2 (NMC811) cathode interface by a multifunctional organosilicon electrolyte additive. ACS Appl. Energy Mater. 2020, 3, 2837–2845. [Google Scholar] [CrossRef]
- Braun, H.; Asakura, R.; Remhof, A.; Battaglia, C. Hydroborate solid-state lithium battery with high-voltage NMC811 cathode. ACS Energy Lett. 2024, 9, 707–714. [Google Scholar] [CrossRef]
- Hu, R.; Mao, C.; Zhuo, H.; Dai, X.; Yang, L.; Shu, X.; Li, Y.; Li, Z.; Ruan, W.; Yang, F.; et al. Molecular space linkage of dipentacyclic anhydride additives for long-lifespan Li-metal batteries with Ni-rich cathode. Energy Storage Mater. 2025, 75, 104033. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Lin, X.; Mo, C.; Zhou, X.; Quan, L.; Zhou, K.; Li, S.; Wang, H.; Li, W. Highly improved cyclic stability of Ni-rich/Li batteries with succinic anhydride as electrolyte additive and underlying mechanism. J. Energy Chem. 2023, 78, 80–90. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Zhu, X.; Wei, L.; Xi, K.; Zhang, L.; Lan, Y.; Wu, H.; Gao, Y. Tailoring electrode-electrolyte interphases to enable the cycling stability of lithium metal batteries with LiNi0.8Co0.1Mn0.1O2 cathode. J. Power Sources 2022, 529, 231195. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, H.; Zhou, J.; Liu, M.; Ding, X.; Li, Y.; Huang, Y.; Chen, Z.; Shen, C.; Wang, D.; et al. A tough Janus-faced CEI film for high voltage layered oxide cathodes beyond 4.6 V. Energy Storage Mater. 2023, 57, 411–420. [Google Scholar] [CrossRef]
- He, Y.; Liu, N.; Kohl, P.A. High conductivity, lithium ion conducting polymer electrolyte based on hydrocarbon backbone with pendent carbonate. J. Electrochem. Soc. 2020, 167, 100517. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced liquid electrolytes for rechargeable Li metal batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, S.Y.; Ahn, J.; Yim, T. Simultaneous realization of multilayer interphases on a Ni-rich NCM cathode and a SiOx anode by the combination of vinylene carbonate with lithium difluoro(oxalato)borate. ACS Appl. Mater. Interfaces 2024, 16, 14940–14953. [Google Scholar] [CrossRef]
- Dai, P.; Kong, X.; Yang, H.; Kuang, S.; Zeng, J.; Zhao, J. Synergistic effect of dual-anion additives promotes the fast dynamics and high-voltage performance of Ni-rich lithium-ion batteries by regulating the electrode/electrolyte interface. ACS Appl. Mater. Interfaces 2022, 14, 39927–39938. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, X. Surface and subsurface reactions of lithium transition metal oxide cathode materials: An overview of the fundamental origins and remedying approaches. Adv. Energy Mater. 2018, 8, 1802057. [Google Scholar] [CrossRef]
- Zhang, S.S.; Fan, X.L.; Wang, C.S. Enhanced electrochemical performance of Ni-rich layered cathode materials by using LiPF6 as a cathode additive. ChemElectroChem 2019, 6, 1536–1541. [Google Scholar] [CrossRef]
- Phelan, C.M.E.; Björklund, E.; Singh, J.; Fraser, M.; Didwal, P.N.; Rees, G.J.; Ruff, Z.; Ferrer, P.; Grinter, D.C.; Grey, C.P.; et al. Role of salt concentration in stabilizing charged Ni-rich cathode interfaces in Li-ion batteries. Chem. Mater. 2024, 36, 3334–3344. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, H.; Chang, Z.; Deng, H.; Li, X.; Zhou, H. A high-energy-density and long-life initial-anode-free lithium battery enabled by a Li2O sacrificial agent. Nat. Energy 2021, 6, 653–662. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, M.; Li, J.; Wang, J.; Zhang, G.; Wang, J.; Zuo, J.; Cao, G.; Duan, R.; Hao, Y.; et al. LiF-rich cathode electrolyte interphases homogenizing Li+ fluxes toward stable interface in Ni-rich Mn-based cathodes. Adv. Mater. 2025, 37, 2417620. [Google Scholar] [CrossRef]
- Min, X.; Wang, L.; Shen, M.; Ma, G.; He, X. A multifunctional additive extending the calendar life of Ni-rich cathode-based lithium-ion batteries for electric vehicles. Mater. Today 2025, 83, 157–165. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, Y.; Bao, Y.; Zhang, W.; Guan, M. Bis(di-tert-butyl)-4-dimethylaminophenylphosphine as electrolyte additive to improve the electrochemical performance of LiNi0.8Co0.1Mn0.1O2. Electrochim. Acta 2023, 441, 141745. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, Q.; Tang, Y.; Yan, Y.; Zhou, S.; Liao, H.-G.; Qiao, Y.; Bao, J.; Sun, S.-G. Self-repaired cathode electrolyte interphase to stabilize the high-nickel cathode interface by a sustained-release multifunctional electrolyte additive. Chem. Eng. J. 2024, 488, 151153. [Google Scholar] [CrossRef]
- Li, G.; Liao, Y.; Li, Z.; Xu, N.; Lu, Y.; Lan, G.; Sun, G.; Li, W. Constructing a low-impedance interface on a high-voltage LiNi0.8Co0.1Mn0.1O2 cathode with 2,4,6-triphenyl boroxine as a film-forming electrolyte additive for Li-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 37013–37026. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, G.; Zhou, K.; Cheng, Y.; Jiao, T.; Huang, L.; Zou, Y.; Wang, M.S.; Yang, Y.; Zheng, J. Dictating the interfacial stability of nickel-rich LiNi0.90Co0.05Mn0.05O2 via a diazacyclo electrolyte additive—2-Fluoropyrazine. J. Colloid Interface Sci. 2022, 618, 431–441. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Wang, Y.; Zhou, X.; Weng, L.; Liu, Y.; Ren, Y.; Zhao, C.; Dahbi, M.; Alami, J.; et al. Electrolytes polymerization-induced cathode-electrolyte-interphase for high voltage lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2101956. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Zhang, Y.; Zeng, J.; Zhao, J. An effective electrolyte design to improve the high-voltage performance of high-capacity NMC811/ SiOx-Gr batteries. Electrochim. Acta 2020, 349, 136356. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.; Yoon, S.C.; Yim, T. Amphiphilic surfactant-assisted cathode-electrolyte interphases for prolonged cycling performance in Ni-rich NCMA cathode materials of lithium-ion batteries. J. Power Sources 2025, 633, 236447. [Google Scholar] [CrossRef]
- Li, S.; Yang, T.; Wang, W.; Lu, J.; Zhao, X.; Fan, W.; Zuo, X.; Nan, J. 2-Thiophene sulfonamide (2-TS)-contained multi-functional electrolyte matching high-voltage LiNi0.8Mn0.1Co0.1O2/graphite batteries with enhanced performances. Electrochim. Acta 2020, 352, 136492. [Google Scholar] [CrossRef]
- Li, Z.; Lin, X.; Zhou, H.; Xing, L.; Lan, G.; Zhang, W.; Chen, J.; Liu, M.; Huang, Q.; Li, W. Stabilizing the interphasial layer of Ni-rich cathode and graphite anode for lithium-ion battery with multifunctional additive. J. Power Sources 2020, 467, 228343. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yang, T.; Wang, W.; Lu, J.; Fan, W.; Zhao, X.; Zuo, X.; Tie, S.; Nan, J. 3,3-diethylene di-sulfite (DES) as a high-voltage electrolyte additive for 4.5 V LiNi0.8Co0.1Mn0.1O2/graphite batteries with enhanced performances. ChemElectroChem 2021, 8, 745–754. [Google Scholar] [CrossRef]
- Moon, H.; Nam, H.; Kim, M.P.; Lee, S.M.; Kim, H.; Jeon, M.H.; Lee, Y.-S.; Kim, K.; Chun, J.-H.; Kwak, S.K.; et al. Elastic interfacial layer enabled the high-temperature performance of lithium-ion batteries via utilization of synthetic fluorosulfate additive. Adv. Func. Mater. 2023, 33, 2303029. [Google Scholar] [CrossRef]
- Dai, H.; Gomes, L.; Maxwell, D.; Zamani, S.; Yang, K.; Atienza, D.; Dale, N.; Mukerjee, S. Exploring the role of an electrolyte additive in suppressing surface Reconstruction of a Ni-rich NMC cathode at ultrahigh Voltage via enhanced in situ and operando characterization methods. ACS Appl. Mater. Interfaces 2024, 16, 8639–8654. [Google Scholar] [CrossRef]
- Liu, L.; Gao, W.; Cui, Y.; Chen, S. A bifunctional additive bi(4-flurorophenyl) sulfone for enhancing the stability and safety of nickel-rich cathode-based cells. J. Alloys Compd. 2020, 820, 153069. [Google Scholar] [CrossRef]
- Lan, G.; Xing, L.; Bedrov, D.; Chen, J.; Guo, R.; Che, Y.; Li, Z.; Zhou, H.; Li, W. Enhanced cyclic stability of Ni-rich lithium-ion battery with electrolyte film-forming additive. J. Alloys Compd. 2020, 821, 153236. [Google Scholar] [CrossRef]
- Song, H.J.; Jang, S.H.; Choi, K.; Nam, S.C.; Mun, J.; Yim, T. Sulfonate-based artificial cathode-electrolyte interface to enhance electrochemical performance of Ni-rich layered oxide cathode materials. ACS Sustain. Chem. Eng. 2020, 8, 7316–7323. [Google Scholar] [CrossRef]
- Jung, K.; Yim, T. High-performance lithium-ion batteries combined with bi-functionalized electrolyte additive and nickel-rich layered oxides. J. Alloys Compd. 2020, 834, 155155. [Google Scholar] [CrossRef]
- Ahn, J.; Yim, T. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide functionalized by allyl phenyl sulfone as high-performance cathode material for lithium-ion batteries. J. Alloys Compd. 2021, 867, 159153. [Google Scholar] [CrossRef]
- Zheng, W.-C.; Shi, C.-G.; Dai, P.; Huang, Z.; Lin, J.-X.; Chen, H.; Sun, M.-L.; Shen, C.-H.; Luo, C.-X.; Wang, Q.; et al. A functional electrolyte additive enabling robust interphases in high-voltage Li//LiNi0.8Co0.1Mn0.1O2 batteries at elevated temperatures. J. Mater. Chem. A 2022, 10, 21912–21922. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Xia, M.; Cheng, Y.; Wang, M.; Hong, W.; Yang, Y.; Zheng, J. Strengthening the interfacial stability of the silicon-based electrode via an electrolyte additive—Allyl phenyl sulfone. ACS Appl. Mater. Interfaces 2022, 14, 38281. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Liu, G.; Zou, Y.; Yang, X.; Zhang, X.; Fu, A.; Zheng, J.; Yang, Y. A novel trimethylsilyl 2-(fluorosulfonyl)difluoroacetate additive for stabilizing the Ni-rich LiNi0.9Co0.05Mn0.05O2/electrolyte interface. J. Power Sources 2021, 515, 230618. [Google Scholar] [CrossRef]
- Jiao, T.; Liu, G.; Huang, L.; Zou, Y.; Zhang, X.; Zheng, J.; Yang, Y. Tuning interface stability of nickel-rich LiNi0.9Co0.05Mn0.05O2 cathode via a novel bis(vinylsulphonyl)methane additive. J. Power Sources 2022, 521, 230917. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Wu, H.; Zhu, X.; Guan, H.; Wei, L.; Xi, K.; Lan, Y.; Zhang, L.; et al. Regulating electrode-electrolyte interphases and eliminating hydrogen fluoride to boost electrochemical performances of Li/NMC811 batteries. Chem. Eng. J. 2023, 451, 138359. [Google Scholar] [CrossRef]
- Choi, M.; Choi, H.; Park, S.; Seong, W.M.; Lee, Y.; Ko, W.; Cho, M.; Ahn, J.; Kong, Y.; Kim, J. Stabilized high-voltage operation of Co-free NMX cathode via CEI-controlling. Energy Storage Mater. 2024, 67, 103291. [Google Scholar] [CrossRef]
- Jo, M.; Park, S.-H.; Lee, H. NaH2PO4 as an electrolyte additive for enhanced thermal stability of LiNi0.8Co0.1Mn0.1O2/graphite batteries. J. Electrochem. Soc. 2020, 167, 130502. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, Y.; Luo, G.; Chang, J.; Wang, C.; Wang, J.; Shao, H.; Deng, Y. Cathode-anode reaction products interplay enabling high performance of LiNi0.8Co0.1Mn0.1O2/artificial graphite pouch batteries at elevated temperature. J. Power Sources 2021, 514, 230583. [Google Scholar] [CrossRef]
- Song, W.; Gauthier, R.; Taskovic, T.; Ouyang, D.; Ingham, H.A.; Eldesoky, A.; Azam, S.M.; Zsoldos, E.S.; Deng, Z.; Heino, D.H.; et al. Lithium difluoro(dioxalato) phosphate as an electrolyte additive for NMC811/graphite Li-ion pouch cells. J. Electrochem. Soc. 2022, 169, 110513. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Lei, Y.; Wu, X.; Gao, Y. Interfacial regulation and hydrogen fluoride capture enabled by allyltrimethylsilane as multifunctional electrolyte additive for Li/LiNi0.8Co0.1Mn0.1O2 batteries. ACS Appl. Energy Mater. 2022, 5, 13501–13510. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Lin, X.; Li, G.; Zhang, W.; Li, W. Insight into the improved performances of Ni-rich/graphite cells by 1,3,5-Trimethyl-1,3,5-tris(3,3,3-trifluoropropyl) cyclotrisiloxane as an electrolyte additive. ACS Appl. Energy Mater. 2022, 5, 11684–11693. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Mo, Y.; Zhou, W.; Hao, Y.; Piao, N.; Liu, C.; Xiao, P.; Liu, H.; Li, B.; et al. Engineering an insoluble cathode electrolyte interphase enabling high performance NMC811//graphite pouch cell at 60 °C. Adv. Energy Mater. 2022, 12, 2201631. [Google Scholar] [CrossRef]
- Ren, Z.; Qiu, H.; Fan, C.; Zhang, S.; Zhang, Q.; Ma, Y.; Qiao, L.; Wang, S.; Xu, G.; Cui, Z.; et al. Delicately designed cyano-siloxane as multifunctional additive enabling high voltage LiNi0.9Co0.05Mn0.05O2/graphite full cell with long cycle life at 50 °C. Adv. Funct. Mater. 2023, 33, 302411. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, M.; Ma, J.; Yang, K.; Chen, Z.; Li, K.; Zhang, C.; Wei, Y.; Zhou, M.; Wang, P.; et al. Lithium hexamethyldisilazide as electrolyte additive for efficient cycling of high-voltage non-aqueous lithium metal batteries. Nat Commun. 2022, 13, 6966. [Google Scholar] [CrossRef]
- Xu, N.; Shi, J.; Liu, G.; Yang, X.; Zheng, J.; Zhang, Z.; Yang, Y. Research progress of fluorine-containing electrolyte additives for lithium ion batteries. J. Power Sources Adv. 2021, 7, 100043. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, F.; Cheng, Z.; Mao, Y.; Huang, R.; Li, F.; Dong, H.; Zhang, Q.; Li, W.; Chen, H.; et al. Insights into the dual role of lithium difluoro(oxalato)borate additive in Improving the electrochemical performance of NMC811//graphite cells. ACS Appl. Energy Mater. 2020, 3, 695–704. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Y.; Yang, Z.; Song, D.; Sun, X.; Wang, C.; Yang, L.; Zhang, Y.; Gao, J.; Ohsaka, T.; et al. A fluorinated electrolyte stabilizing high-voltage graphite/NMC811 batteries with an inorganic-rich electrode-electrolyte interface. Chem. Eng. J. 2022, 440, 135939. [Google Scholar] [CrossRef]
- Ahn, J.; Im, J.; Seo, H.; Yoon, S.; Cho, K.Y. Enhancing the cycling stability of Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode at 4.5 V via 2,4-difluorobiphenyl additive. J. Power Sources 2021, 512, 230513. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Wei, P.; Sun, S.; Xu, Y.; Li, Q.; Fang, C.; Han, J.; Huang, Y. Tailoring electrolyte enables high-voltage Ni-rich NCM cathode against aggressive cathode chemistries for Li-ion batteries. Sci. Bull. 2022, 67, 2225–2234. [Google Scholar] [CrossRef]
- Pham, H.Q.; Nguyen, M.T.; Tarik, M.; El Kazzi, M.; Trabesinger, S. Cross-talk-suppressing electrolyte additive enabling high voltage performance of Ni-rich layered oxides in Li-ion batteries. ChemSusChem 2021, 14, 2461–2474. [Google Scholar] [CrossRef]
- Tan, S.; Shadike, Z.; Li, J.; Wang, X.; Yang, Y.; Lin, R.; Cresce, A.; Hu, J.; Hunt, A.; Waluyo, I.; et al. Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V. Nat. Energy 2022, 7, 484–494. [Google Scholar] [CrossRef]
- Xue, W.; Huang, M.; Li, Y.; Zhu, Y.G.; Gao, R.; Xiao, X.; Zhang, W.; Li, S.; Xu, G.; Yu, Y.; et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 2021, 6, 495–505. [Google Scholar]
- Oh, M.G.; Kwak, S.; An, K.; Tran, Y.H.T.; Kang, D.G.; Park, S.J.; Lim, G.; Kim, K.; Lee, Y.S.; Song, S.W. Perfluoro macrocyclic ether as an ambifunctional additive for high-performance SiO and nickel 88%-based high-energy Li-ion battery. Adv. Funct. Mater. 2023, 33, 2212890. [Google Scholar] [CrossRef]
- Peng, X.; Shen, H.; Su, K.; Wang, W.; Weng, S.; Tang, C.; Xue, Z.; Xiang, Y. Stable and fast ion transport electrolyte interfaces modified with novel fluorine- and nitrogen-containing solvents for Ni-rich cathode materials. ACS Appl. Mater. Interfaces 2024, 16, 34281–34293. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xu, X.; Yin, J.; Jiang, S.; Xi, K.; Wei, L.; Gao, Y. LiF-rich interfaces and HF elimination achieved by a multifunctional additive enable high-performance Li/LiNi0.8Co0.1Mn0.1O2 batteries. ACS Appl. Mater. Interfaces 2023, 15, 11777–11786. [Google Scholar] [CrossRef]
- Liu, G.; Xu, N.; Zou, Y.; Zhou, K.; Yang, X.; Jiao, T.; Yang, W.; Yang, Y.; Zheng, J. Stabilizing Ni-rich LiNi0.83Co0.12Mn0.05O2 with cyclopentyl isocyanate as a novel electrolyte additive. ACS Appl. Mater. Interfaces 2021, 13, 12069–12078. [Google Scholar] [CrossRef]
- Tan, C.; Yang, J.; Pan, Q.; Li, Y.; Li, Y.; Cui, L.; Fan, X.; Zheng, F.; Wang, H.; Li, Q. Optimizing interphase structure to enhance electrochemical performance of high voltage LiNi0.5Mn1.5O4 cathode via anhydride additives. Chem. Eng. J. 2021, 410, 128422. [Google Scholar] [CrossRef]
- Han, F.; Cang, Z.; Wang, R.; Yun, F.; Wang, J.; Ma, C.; Zhang, Y.; Tang, L.; Ding, H.; Lu, S. Isocyanate additives improve the low-temperature performance of LiNi0.8Mn0.1Co0.1O2||SiOx@graphite lithium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 20966–20976. [Google Scholar] [CrossRef]
- Zheng, J.; Qiu, Y.; Liao, S.; Yue, Z.; Fang, S.; Zhou, N.; Li, Y.; Jiang, Y. A novel 4-fluorophenyl isocyanate additive constructing solid cathodes-electrolyte interface for high-performance lithium-ion batteries. Small 2024, 20, 2405853. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, K.; Liu, G.; Xu, N.; Zhang, X.; Yang, Y.; Zhang, J.; Zheng, J. Enhanced cycle life and rate capability of single-crystal, Ni-rich LiNi0.9Co0.05Mn0.05O2 enabled by 1,2,4-1H-triazole additive. ACS Appl. Mater. Interfaces 2021, 13, 16427–16436. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Jiang, L.; Yang, T.; Fan, W.; Wang, W.; Zhao, X.; Zuo, X.; Nan, J. Isocyanoethyl methacrylate (IMA) as a bifunctional electrolyte additive for LiNi0.8Co0.1Mn0.1O2/graphite batteries with enhanced performance. ChemElectroChem 2021, 8, 3716–3725. [Google Scholar] [CrossRef]
- Pham, H.Q.; Mirolo, M.; Tarik, M.; El-Kazzi, M.; Trabesinger, S. Multifunctional electrolyte additive for improved interfacial stability in Ni-rich layered oxide full-cells. Energy Storage Mater. 2020, 33, 216–229. [Google Scholar] [CrossRef]
- Li, Y.; Qu, Q.; Lv, L.; Shao, J.; Zheng, H. A multifunctional additive capable of electrolyte stabilization and structure/interphases regulation of high-energy Li-ion batteries. Adv. Funct. Mater. 2024, 34, 2314100. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Qiu, Y.; Zhang, J.; Liu, Y.; Wei, P.; Ou, M.; Sun, S.; Xu, Y.; Li, Q.; et al. Tailoring electrolyte to enable high-rate and super-stable Ni-rich NCM cathode materials for Li-ion batteries. Nano Energy 2021, 88, 106301. [Google Scholar] [CrossRef]
- Guan, P.; Min, J.; Zhang, S.; Lu, Y.; Liang, T.; Meng, L.; Yuan, Y.; Zhou, Y.; Chen, F.; Zhou, L.; et al. Stabilizing high-voltage performance of nickel-rich cathodes via facile solvothermally synthesized niobium-doped strontium titanate. ACS Appl. Mater. Interfaces 2024, 16, 26167–26181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Jiao, T.; Cheng, Y.; Zou, Y.; Wang, M.-S.; Yang, Y.; Zheng, J. Electrolyte additive cis-1,2,3,6-tetrahydrophthalic anhydride enhanced the cycle life of nickel-rich LiNi0.9Co0.05Mn0.05O2. ACS Appl. Energy Mater. 2021, 4, 12275–12284. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, G.; Zhou, K.; Zhang, J.; Jiao, T.; Zhang, X.; Yang, Y.; Zheng, J. Enhanced interfacial stability of a LiNi0.9Co0.05Mn0.05O2 cathode by a diboron additive. ACS Appl. Energy Mater. 2021, 4, 11051–11061. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, X.; Zhou, P.; Li, H.; He, X.; Fan, W.; Fan, C.; Yang, T.; Ma, Z.; Zhao, X.; et al. Eliminating H2O/HF and regulating interphase with bifunctional tolylene-2, 4-diisocyanate (TDI) additive for long life Li-ion battery. J. Energy Chem. 2024, 95, 519–528. [Google Scholar] [CrossRef]
- Lim, D.-A.; Shin, Y.-K.; Seok, J.-H.; Hong, D.; Ahn, K.H.; Lee, C.H.; Kim, D.-W. Cathode electrolyte interphase-forming additive for improving cycling performance and thermal stability of Ni-rich LiNixCoyMn1-x-yO2 cathode materials. ACS Appl. Mater. Interfaces 2022, 14, 54688–54697. [Google Scholar] [CrossRef]
- Li, S.; Zhao, D.; Wang, P.; Cui, X.; Tang, F. Electrochemical effect and mechanism of adiponitrile additive for high-voltage electrolyte. Electrochim. Acta 2016, 222, 668–677. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.Y.; Park, S.J.; Park, G.T.; Sun, Y.K. Adiponitrile (C6H8N2): A new Bi functional additive for high performance Li metal batteries. Adv. Funct. Mater. 2019, 29, 1902496. [Google Scholar] [CrossRef]
- Li, X.; Han, X.; Li, G.; Du, J.; Cao, Y.; Gong, H.; Wang, H.; Zhang, Y.; Liu, S.; Zhang, B.; et al. Nonsacrificial nitrile additive for armoring high-voltage LiNi0.83Co0.07Mn0.1O2 cathode with reliable electrode-electrolyte interface toward durable battery. Small 2022, 18, 2202989. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, Z.; Pan, J.; Hong, Y.; Li, J.; Lin, Y.; Shi, K.; Liu, Q. Designing stable electrode interfaces from a pyrrolidine-based electrolyte for improving LiNi0.8Co0.1Mn0.1O2 batteries. Ind. Eng. Chem. Res. 2022, 61, 14173–14180. [Google Scholar] [CrossRef]
- Park, S.; Jeong, S.Y.; Lee, T.K.; Park, M.W.; Lim, H.Y.; Sung, J.; Cho, J.; Kwak, S.K.; Hong, S.Y.; Choi, N.-S. Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat. Commun. 2021, 12, 838. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, S.; Cui, Z.; Cui, Z.; Xie, B.; Gong, T.; Zhang, X.; Li, J.; Wu, R.; Wang, S.; et al. Interphase regulation by multifunctional additive empowering high energy lithium-ion batteries with enhanced cycle life and thermal safety. Angew. Chem. Int. Ed. 2024, 63, e202315710. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Harlow, J.; Logan, E.; Hebecker, H.; Coon, M.; Molino, L.; Johnson, M.; Dahn, J.; Metzger, M. A systematic study of electrolyte additives in single crystal and bimodal LiNi0.8Mn0.1Co0.1O2/graphite pouch cells. J. Electrochem. Soc. 2021, 168, 090503. [Google Scholar] [CrossRef]
- Lu, D.; Li, R.; Rahman, M.M.; Yu, P.; Lv, L.; Yang, S.; Huang, Y.; Sun, C.; Zhang, S.; Zhang, H.; et al. Ligand-channel-enabled ultrafast Li-ion conduction. Nature 2024, 627, 101–107. [Google Scholar] [CrossRef]
- Li, G.-X.; Koverga, V.; Nguyen, A.; Kou, R.; Ncube, M.; Jiang, H.; Wang, K.; Liao, M.; Guo, H.; Chen, J.; et al. Enhancing lithium-metal battery longevity through minimized coordinating diluent. Nat. Energy 2024, 9, 817–827. [Google Scholar] [CrossRef]
- Liu, X.; Mariani, A.; Diemant, T.; Pietro, M.E.D.; Dong, X.; Kuenzel, M.; Mele, A.; Passerini, S. Difluorobenzene-based locally concentrated ionic liquid electrolyte enabling stable cycling of lithium metal batteries with nickel-rich cathode. Adv. Energy Mater. 2022, 12, 2200862. [Google Scholar] [CrossRef]
- Fan, X.; Ji, X.; Chen, L.; Chen, J.; Deng, T.; Han, F.; Yue, J.; Piao, N.; Wang, R.; Zhou, X.; et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 2019, 4, 882–890. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, T.; Liao, X.; Song, Y.; Zhao, Y. All-fluorinated electrolyte directly tuned Li+ solvation sheath enabling high-quality passivated interfaces for robust Li metal battery under high voltage operation. Energy Storage Mater. 2023, 57, 249–259. [Google Scholar] [CrossRef]
- Su, C.-C.; He, M.; Shi, J.; Amine, R.; Yu, Z.; Cheng, L.; Guo, J.; Amine, K. Principle in developing novel fluorinated sulfone electrolyte for high voltage lithium-ion batteries. Energy Environ. Sci. 2021, 14, 3029–3034. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Wang, A.; Zhang, D.; Li, B.; He, X.; Fan, X.; Liu, J. Cosolvent occupied solvation tuned anti-oxidation therapy toward highly safe 4.7 V-class NMC811 batteries. Energy Environ. Sci. 2024, 17, 6113–6126. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Zhao, Y.; Zhou, W.; Xiao, P.; Gao, P.; Tavajohi, N.; Tu, J.; Li, B.; He, X.; et al. Breaking solvation dominance of ethylene carbonate via molecular charge engineering enables lower temperature battery. Nat. Commun. 2023, 14, 8326. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, W.; Dong, W.; Yang, K.; Chao, Y.; Xi, C.; Li, M.; Zhang, Q.; Liu, Z.; Du, P.; et al. Enhancing the cathode/electrolyte interface in Ni-rich lithium-ion batteries through homogeneous oxynitridation enabled by NO3− dominated clusters. Chem. Eng. J. 2024, 494, 153001. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Ahmad, W.; Zhao, J.; Li, H.; Wang, L.; Wan, Z.; Jiang, W.; Li, S.; Yang, F.; et al. Synergistic dual-additive regulated carbonate electrolyte stabilizes bidirectional interface for aggressive Ni-rich Li-metal full batteries. Nano Energy 2024, 128, 109862. [Google Scholar] [CrossRef]
- An, K.; Tran, Y.H.T.; Kwak, S.; Han, J.; Song, S.-W. Design of fire-resistant liquid electrolyte formulation for safe and long-cycled lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2106102. [Google Scholar] [CrossRef]
- Huang, X.; Li, R.; Sun, C.; Zhang, H.; Zhang, S.; Lv, L.; Huang, Y.; Fan, L.; Chen, L.; Noked, M.; et al. Solvent-assisted hopping mechanism enables ultrafast charging of lithium-ion batteries. ACS Energy Lett. 2022, 7, 3947–3957. [Google Scholar] [CrossRef]
- Li, Z.; Yao, N.; Yu, L.; Yao, Y.-X.; Jin, C.-B.; Yang, Y.; Xiao, Y.; Yue, X.-Y.; Cai, W.-L.; Xu, L.; et al. Inhibiting gas generation to achieve ultralong-lifespan lithium-ion batteries at low temperatures. Matter 2023, 6, 2274–2292. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, Z.; Liu, G.; Li, Q.; Yin, D.; Shi, X.; Cao, Z.; Tian, Z.; Kim, H.; Guo, Y.; et al. Non-flammable electrolyte enables high-voltage and wide-temperature lithium-ion batteries with fast charging. Angew. Chem. Int. Ed. 2023, 62, e202216189. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Yang, X.; Xin, T.; Chen, S.; Yang, M.; Peng, Y.; Xu, H.; Yin, Y.; Deng, T.; et al. Thermal runaway suppression of high-energy lithium-ion batteries by designing the stable interphase. J. Electrochem. Soc. 2021, 168, 090563. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, X.; Liu, X.; Wang, X.; Ren, D.; Wang, L.; Yang, M.; Wang, Y.; Zhang, W.; Li, Y.; et al. In-built ultraconformal interphases enable high-safety practical lithium batteries. Energy Storage Mater. 2021, 43, 248–257. [Google Scholar] [CrossRef]
- Pan, R.; Cui, Z.; Yi, M.; Xie, Q.; Manthiram, A. Ethylene carbonate-free electrolytes for stable, safer high-nickel lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2103806. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, D.; Liu, X.; Xu, G.-L.; Feng, X.; Zheng, Y.; Li, Y.; Yang, M.; Peng, Y.; Han, X.; et al. High-voltage and high-safety practical lithium batteries with ethylene carbonate-free electrolyte. Adv. Energy Mater. 2021, 11, 2102299. [Google Scholar] [CrossRef]
- Li, W.; Dolocan, A.; Li, J.; Xie, Q.; Manthiram, A. Ethylene carbonate-free electrolytes for high-nickel layered oxide cathodes in lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1901152. [Google Scholar] [CrossRef]
- Klein, S.; van Wickeren, S.; Röser, S.; Bärmann, P.; Borzeutzki, K.; Heidrich, B.; Börner, M.; Winter, M.; Placke, T.; Kasnatscheew, J. Understanding the outstanding high-voltage performance of NCM523||graphite lithium ion cells after elimination of ethylene carbonate solvent from conventional electrolyte. Adv. Energy Mater. 2021, 11, 2003738. [Google Scholar] [CrossRef]
- Xu, C.; Liang, Y.; Zhang, R.; Cheng, J.; Yun, H. Ethylene carbonate-free electrolytes for high voltage lithium-ion batteries: Progress and perspectives. Energy Fuels 2024, 38, 18208–18226. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, H.; Lu, D.; Yu, Y.; Qi, J.; Zhang, J.; Zhang, S.; Li, R.; Deng, T.; Chen, L.; et al. A low-concentration sulfone electrolyte enables high-voltage chemistry of lithium-ion batteries. Energy Mater. 2022, 2, 200030. [Google Scholar] [CrossRef]
- Lu, D.; Lei, X.; Weng, S.; Li, R.; Li, J.; Lv, L.; Zhang, H.; Huang, Y.; Zhang, J.; Zhang, S.; et al. A self-purifying electrolyte enables high energy Li ion batteries. Energy Environ. Sci. 2022, 15, 3331–3342. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, H.; Hu, E.; et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 2023, 614, 694. [Google Scholar] [CrossRef]
- Su, H.; Chen, Z.; Li, M.; Bai, P.; Li, Y.; Ji, X.; Liu, Z.; Sun, J.; Ding, J.; Yang, M.; et al. Achieving practical high-energy-density lithium-metal batteries by a dual-anion regulated electrolyte. Adv. Mater. 2023, 35, 2301171. [Google Scholar] [CrossRef]
- Chen, P.-P.; Zhang, B.-H.; Li, Z.-A.; Lei, J.-T.; Chen, J.-Z.; Hou, Y.-L.; Zhao, D.-L. Regulate the solvation structure and interface by nitrate in phosphate-based electrolytes for 4.5 V-class Ni-rich batteries. Small 2024, 20, 2403079. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Tan, G.; Li, L.; Chen, R.; Wu, F. Double-salts super concentrated carbonate electrolyte boosting electrochemical performance of Ni-rich LiNi0.90Co0.05Mn0.05O2 lithium metal batteries. Small 2024, 20, 2311650. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Weng, S.; Li, R.; Lu, D.; Deng, T.; Zhang, S.; Lv, L.; Qi, J.; Xiao, X.; et al. Multifunctional solvent molecule design enables high-voltage Li-ion batteries. Nat. Commun. 2023, 14, 2211. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Cui, Z.; Jia, H.; Engelhard, M.H.; Matthews, B.E.; Cao, X.; Xie, Q.; Wang, C.; Manthiram, A.; et al. Stabilizing ultrahigh-nickel layered oxide cathodes for high-voltage lithium metal batteries. Mater Today 2021, 44, 15–24. [Google Scholar] [CrossRef]
- Zhang, S.S. Unveiling the mystery of lithium bis(fluorosulfonyl)imide as a single salt in low-to-moderate concentration electrolytes of lithium metal and lithium-ion batteries. J. Electrochem. Soc. 2022, 169, 110515. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, J.; Li, H.; Guan, S.; Zhao, H.; Han, W.; Zhang, H.; Jiang, G.; Li, L.; An, B.; et al. Self-induced corrosion of Ni-rich cathode materials by fluor-lithium salts. Energy Storage Mater. 2025, 74, 103953. [Google Scholar] [CrossRef]
- Qiao, L.; Oteo, U.; Martinez-Ibañez, M.; Santiago, A.; Cid, R.; Sanchez-Diez, E.; Lobato, E.; Meabe, L.; Armand, M.; Zhang, H. Stable non-corrosive sulfonimide salt for 4-V-class lithium metal batteries. Nat. Mater. 2022, 21, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, P.; Kong, X.; Tian, J.; Zhang, W.; Yan, S.; Hou, W.-H.; Zhou, H.-Y.; Dong, H.; Chen, X.; et al. Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries. Nat. Energy 2023, 8, 934–945. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Zhang, S.; Dong, S.; Wang, S.; Cui, Z.; Du, X.; Wang, C.; Xie, B.; Du, J.; et al. Highly fluorinated Al-centered lithium salt boosting the interfacial compatibility of Li-metal batteries. ACS Energy Lett. 2022, 7, 591–598. [Google Scholar] [CrossRef]
- Wu, F.; Fang, S.; Kuenzel, M.; Mullaliu, A.; Kim, J.-K.; Gao, X.; Diemant, T.; Kim, G.-T.; Passerini, S. Dual-anion ionic liquid electrolyte enables stable Ni-rich cathodes in lithium-metal batteries. Joule 2021, 5, P2177–P2194. [Google Scholar] [CrossRef]
- Kato, Y.; Nagahara, A.; Gerile, N.; Fujinaka, S.; Hamamoto, N.; Nishimura, H.; Nakai, H. Investigation of water-washing effect on electrochemical properties of Ni-Rich NCA cathode material for lithium-ion batteries. J. Electrochem. Soc. 2022, 169, 060543. [Google Scholar] [CrossRef]
- Hartmann, L.; Pritzl, D.; Beyer, H.; Gasteiger, H.A. Evidence for Li+/H+ exchange during ambient storage of Ni-rich cathode active materials. J. Electrochem. Soc. 2021, 168, 070507. [Google Scholar] [CrossRef]
- Wang, X.; He, W.; Xue, H.; Zhang, D.; Wang, J.; Wang, L.; Li, J. A nonflammable phosphate-based localized high-concentration electrolyte for safe and high-voltage lithium metal batteries. Sustain. Energy Fuels 2022, 6, 1281–1288. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Li, R.; Chen, L.; Zhang, H.; Ma, B.; Jiang, S.; Zhou, T.; Huang, J.; Zhu, H.; et al. Surface chemical coordination stabilizes Ni-rich cathodes for high-energy Li-metal batteries. J. Am. Chem. Soc. 2025, 147, 9396–9406. [Google Scholar] [CrossRef] [PubMed]
- Pillot, C. Lithium-Ion Battery Raw Material Supply and Demand 2016–2025. Available online: http://cii-resource.com/cet/AABE-03-17/Presentations/BRMT/Pillot_Christophe.pdf (accessed on 1 February 2024).
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Meng, Y.S.; Chen, Z. Ambient-pressure relithiation of degraded LixNi0.5Co0.2Mn0.3O2 (0 < x < 1) via eutectic solutions for direct regeneration of lithium-ion battery cathodes. Adv. Energy Mater. 2019, 9, 1900454. [Google Scholar]
- Wang, T.; Luo, H.M.; Bai, Y.C.; Li, J.L.; Belharouak, I.; Dai, S. Direct recycling of spent NCM cathodes through ionothermal lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Ji, H.; Wei, X.; Zhou, G.; Cheng, H.-M. A review of direct recycling methods for spent lithium-ion batteries. Energy Storage Mater. 2024, 70, 103475. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef]
- Mao, J.; Ye, C.; Zhang, S.; Xie, F.; Zeng, R.; Davey, K.; Guo, Z.; Qiao, S. Toward practical lithium-ion battery recycling: Adding value, tackling circularity and recycling-oriented design. Energy Environ. Sci. 2022, 15, 2732–2752. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Chen, H.; Sun, J. Separation of Li and Co from the active mass of spent Li-ion batteries by selective sulfating roasting with sodium bisulfate and water leaching. Miner. Eng. 2018, 126, 28–35. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, X.; Zhang, B.; Zhao, Y.; Xie, H.; Zhao, J.; Ning, Z.; Xing, P.; Yin, H. Recycling of spent lithium nickel cobalt manganese oxides via a low-temperature ammonium sulfation roasting approach. J. Clean. Prod. 2021, 279, 123633. [Google Scholar]
- Lander, L.; Rousse, G.; Batuk, D.; Colin, C.V.; Corte, D.A.D.; Tarascon, J.-M. Synthesis, structure, and electrochemical properties of K-based sulfates K2M2(SO4)3 with M = Fe and Cu. Inorg. Chem. 2017, 56, 2013–2021. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, C.; Zhang, L.; Wen, J.; Wang, C.; Huang, G. Synthesis of high-nickel and high-performance ternary cathode materials through spent lithium-ion batteries recycling system. Sustain. Chem. Pharm. 2023, 31, 100959. [Google Scholar] [CrossRef]
- Hamitouche, L.; Robert, C.; Rollet, A.-L.; Briois, V.; Selmane, M.; Gutierrez, A.G.P.; Leclerc, S.; Krulic, D.; Fatouros, N.; Sirieix-Plénet, J.; et al. Elucidating salt conversion mechanisms of lithiated transition metal oxides for lithium-ion battery recycling. Chem. Mater. 2024, 36, 2544–2553. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Qu, H.; Li, J.; Ji, W.; Qiu, X.; Zhu, Y.; Ren, H.; Shi, R.; Li, G.; et al. Closed-loop direct upcycling of spent Ni-rich layered cathodes into high-voltage cathode materials. Adv. Mater. 2024, 36, 2407029. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Wang, J.; Liang, Z.; Jia, K.; Ma, J.; Zhuang, Z.; Zhou, G.; Cheng, H.-M. Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt. Nat. Commun. 2023, 14, 584. [Google Scholar] [CrossRef]
- Xu, M.L.; Wu, C.; Zhang, F.X.; Zhang, Y.H.; Ren, J.X.; Zhang, C.Y.; Wang, X.Z.; Xiao, L.; Fontaine, O.; Qian, J.F. Potential regulation strategy enables ferrocene as p-type redox mediator for direct regeneration of spent LiFePO4 cathode. Energy Storage Mater. 2024, 71, 103611. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Zhang, C.; Ye, M.X.; Li, H.M.; Fu, Z.; Zhang, H.M.; Wang, G.Z.; Zhang, Y.X. Closed-loop regeneration of a spent LiFePO4 cathode by integrating oxidative leaching and electrochemical relithiation. ACS Appl. Energy. Mater. 2022, 5, 14323–14334. [Google Scholar] [CrossRef]
- Wu, C.; Hu, J.M.; Ye, L.; Su, Z.P.; Fang, X.L.; Zhu, X.L.; Zhuang, L.; Ai, X.P.; Yang, H.X.; Qian, J.F. Direct regeneration of spent Li-ion battery cathodes via chemical relithiation reaction. ACS Sustain. Chem. Eng. 2021, 9, 16384–16393. [Google Scholar] [CrossRef]
- Wu, C.; Xu, M.L.; Zhang, C.Y.; Ye, L.; Zhang, K.L.; Cong, H.J.; Zhuang, L.; Ai, X.P.; Yang, H.X.; Qian, J.F. Cost-effective recycling of spent LiMn2O4 cathode via a chemical lithiation strategy. Energy Storage Mater. 2023, 55, 154–165. [Google Scholar] [CrossRef]
- Park, K.; Yu, J.L.; Coyle, J.; Dai, Q.; Frisco, S.; Zhou, M.; Burrell, A. Direct cathode recycling of end-of-life Li-ion batteries enabled by redox mediation. ACS Sustain. Chem. Eng. 2021, 9, 8214–8221. [Google Scholar] [CrossRef]
- Ko, S.; Choi, J.; Hong, J.; Kim, C.; Hwang, U.; Kwon, M.; Lim, G.; Sohn, S.S.; Jang, J.; Lee, U.; et al. Thermodynamically controlled chemical regeneration of spent battery cathodes using recyclable electron donors under ambient conditions. Energy Environ. Sci. 2024, 17, 4064. [Google Scholar] [CrossRef]
- Kim, S.; Shin, U.; Yoon, H.J.; Song, J.; Ma, J.; Woo, J.-J.; Nam, K.-W.; Seo, D.-H.; Ryu, W.-H. Unraveling redox mediator-assisted chemical relithiation mechanism for direct recycling of spent Ni-rich layered cathode materials. Adv. Sci. 2025, 12, 2417094. [Google Scholar] [CrossRef]
- Zheng, Y.; Pu, J.; Yang, Q.; You, L.; Wang, W.; Liu, J. Efficient selective leaching and direct regeneration of spent Ni-rich cathodes via advanced oxidation. Sep. Purif. Technol. 2025, 361, 131366. [Google Scholar] [CrossRef]
- Xu, G.; Huang, L.; Lu, C.; Zhou, X.; Cui, G. Revealing the multilevel thermal safety of lithium batteries. Energy Storage Mater. 2020, 31, 72–86. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Huang, W.; He, X.; Wang, L.; Ouyang, M. Challenges and opportunities to mitigate the catastrophic thermal runaway of high-energy batteries. Adv. Energy Mater. 2023, 13, 2203841. [Google Scholar] [CrossRef]
- Cui, Z.; Manthiram, A. Thermal stability and outgassing behaviors of high-nickel cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202307243. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Hou, J.; Feng, X.; Wang, L.; Liu, X.; Ohma, A.; Lu, L.; Ren, D.; Huang, W.; Li, Y.; Yi, M.; et al. Unlocking the self-supported thermal runaway of high-energy lithium-ion batteries. Energy Storage Mater. 2021, 39, 395–402. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.; Ren, D.; Wu, Y.; Xu, G.; Lu, L.; Hou, J.; Zhang, W.; et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Mechanism of thermal runaway in lithium-ion cells. J. Electrochem. Soc. 2018, 165, A1303–A1308. [Google Scholar] [CrossRef]
- Huang, L.; Xu, G.; Du, X.; Li, J.; Xie, B.; Liu, H.; Han, P.; Dong, S.; Cui, G.; Chen, L. Uncovering LiH triggered thermal runaway mechanism of a high-energy LiNi0.5Co0.2Mn0.3O2/graphite pouch cell. Adv. Sci. 2021, 8, 2100676. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z.; et al. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Wang, C.; Du, X.; Lu, D.; Xie, B.; Wang, X.; Lu, C.; Liu, H.; Dong, S.; et al. The formation/decomposition equilibrium of LiH and its contribution on anode failure in practical lithium metal batteries. Angew. Chem. Int. Ed. 2021, 60, 7770–7776. [Google Scholar] [CrossRef]
- Xu, G.; Du, X.; Zhang, S.; Li, J.; Dong, S.; Hu, Z.; Cui, G. Revealing the importance of suppressing formation of lithium hydride and hydrogen in Li anode protection. Interdiscip. Mater. 2023, 2, 337–347. [Google Scholar] [CrossRef]
- Soto, F.A.; Ma, Y.; Martinez De La Hoz, J.M.; Seminario, J.M.; Balbuena, P.B. Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries. Chem. Mater. 2015, 27, 7990–8000. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Wei, X.; Zhu, Y.; Sun, X.; Gu, M.D. Cathode–electrolyte interphase of Ni-rich layered oxides: Evolving structure and implication on stability. Nano Lett. 2025, 25, 2769–2776. [Google Scholar] [CrossRef]
- Park, N.-Y.; Lee, H.-H.; Kim, H.; Park, S.-M.; Jung, H.-G.; Jung, Y.-C.; Sun, Y.-K. High-energy, long-life Ni-rich cathode materials with columnar structures for all-solid-state batteries. Nature Energy 2025, 10, 479–489. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Hu, Z.; Cui, G.; Chen, L. Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials. Chem. Mater. 2019, 31, 6033–6065. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, G.-T.; Conlin, P.; Ashburn, N.; Cho, K.; Yu, Y.-S.; Shapiro, D.A.; Maglia, F.; Kim, S.J.; Lamp, P.; et al. Cation ordered Ni-rich layered cathode for ultra-long battery life. F1-GC80: Fluorine doped compositionally graded cathode, with an average concentration of Li[Ni0.80Co0.05Mn0.15]O2. Energy Environ. Sci. 2021, 14, 1573–1583. [Google Scholar] [CrossRef]
- Wang, S.; Dai, A.; Cao, Y.; Yang, H.; Khalil, A.; Lu, J.; Li, H.; Ai, X. Enabling stable and high-rate cycling of a Ni-rich layered oxide cathode for lithium-ion batteries by modification with an artificial Li+-conducting cathode-electrolyte interphase. NMC811 with artificial Li+-conducting cathode-electrolyte interphase (ALCEI). J. Mater. Chem. A 2021, 9, 11623–11631. [Google Scholar]
- Ma, J.; Sun, Y.; Wu, D.; Wang, C.; Yu, R.; Duan, H.; Zheng, M.; Li, R.; Gu, D.; Zhao, Y.; et al. Highly stabilized Ni-rich cathodes enabled by artificially reversing naturally-formed interface. Adv. Energy Mater. 2025, 15, 2403150. [Google Scholar] [CrossRef]

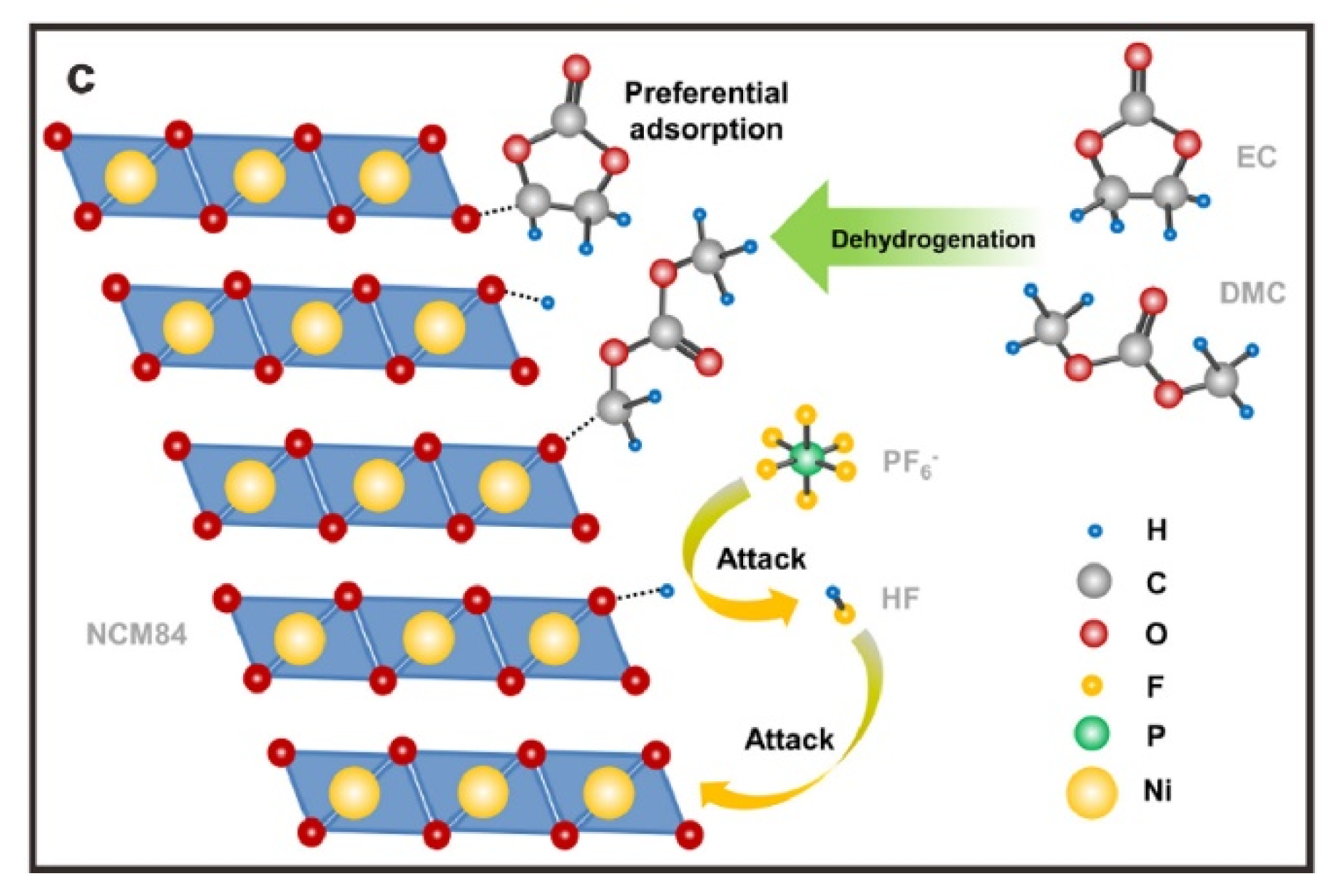

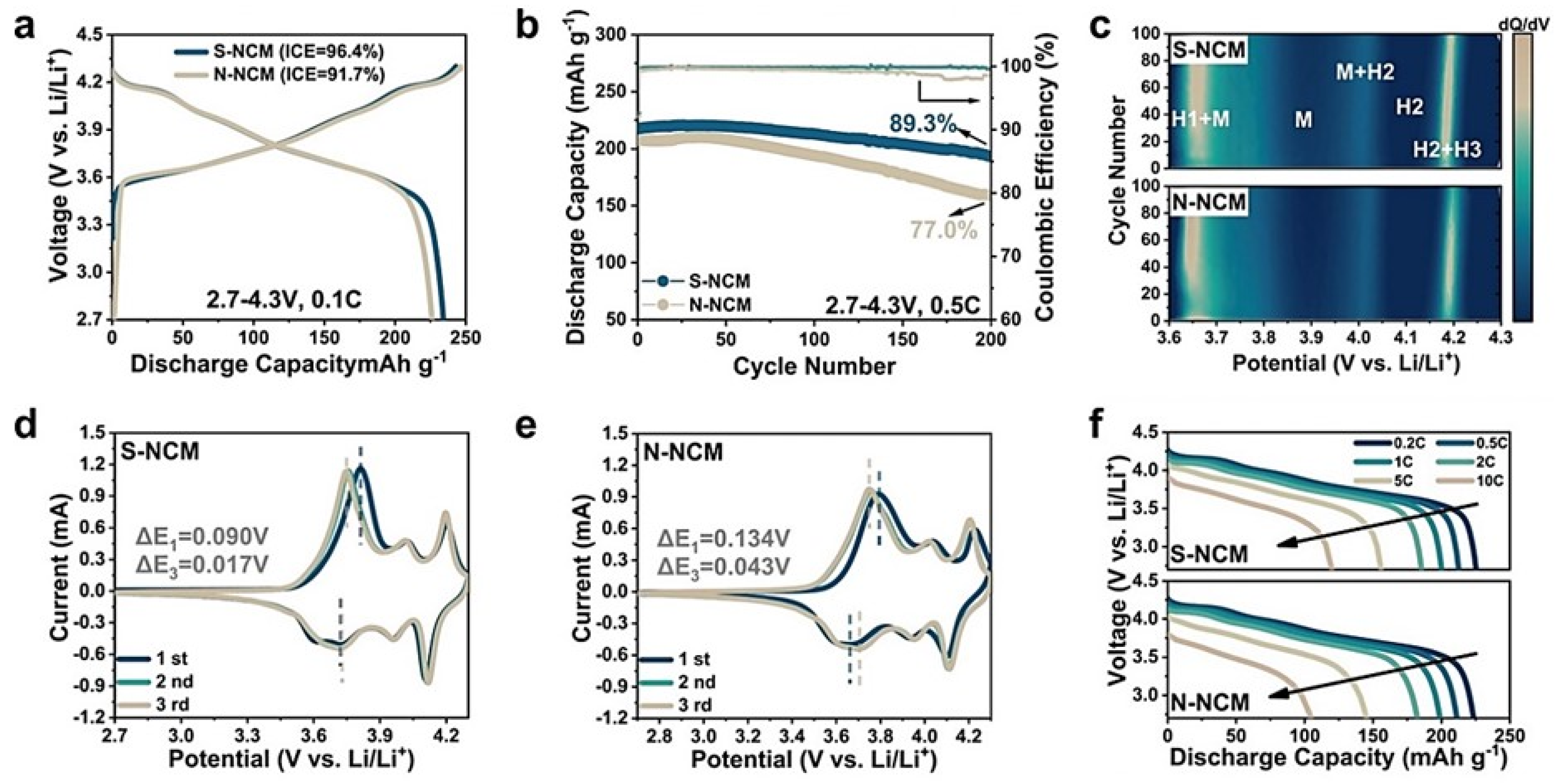

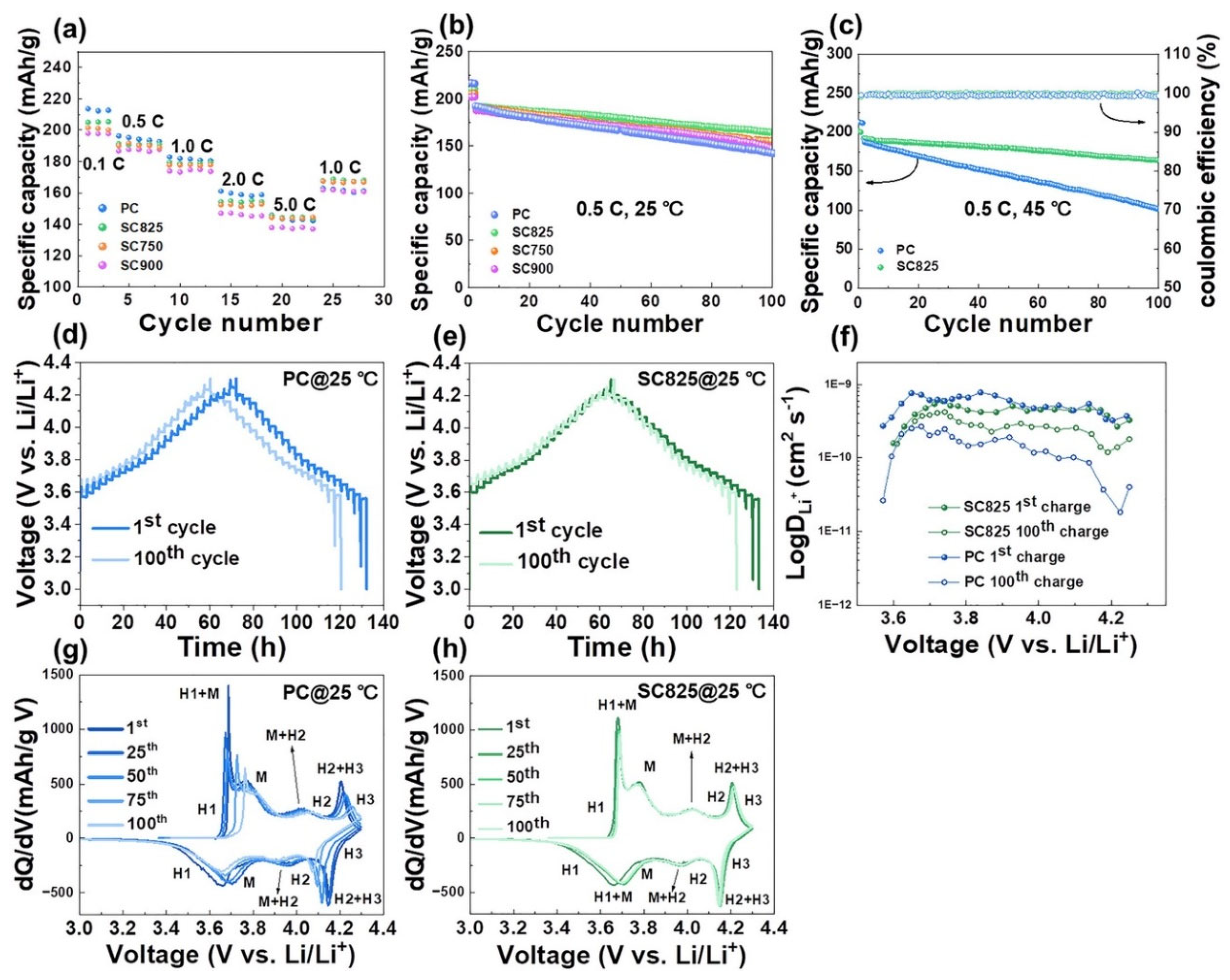
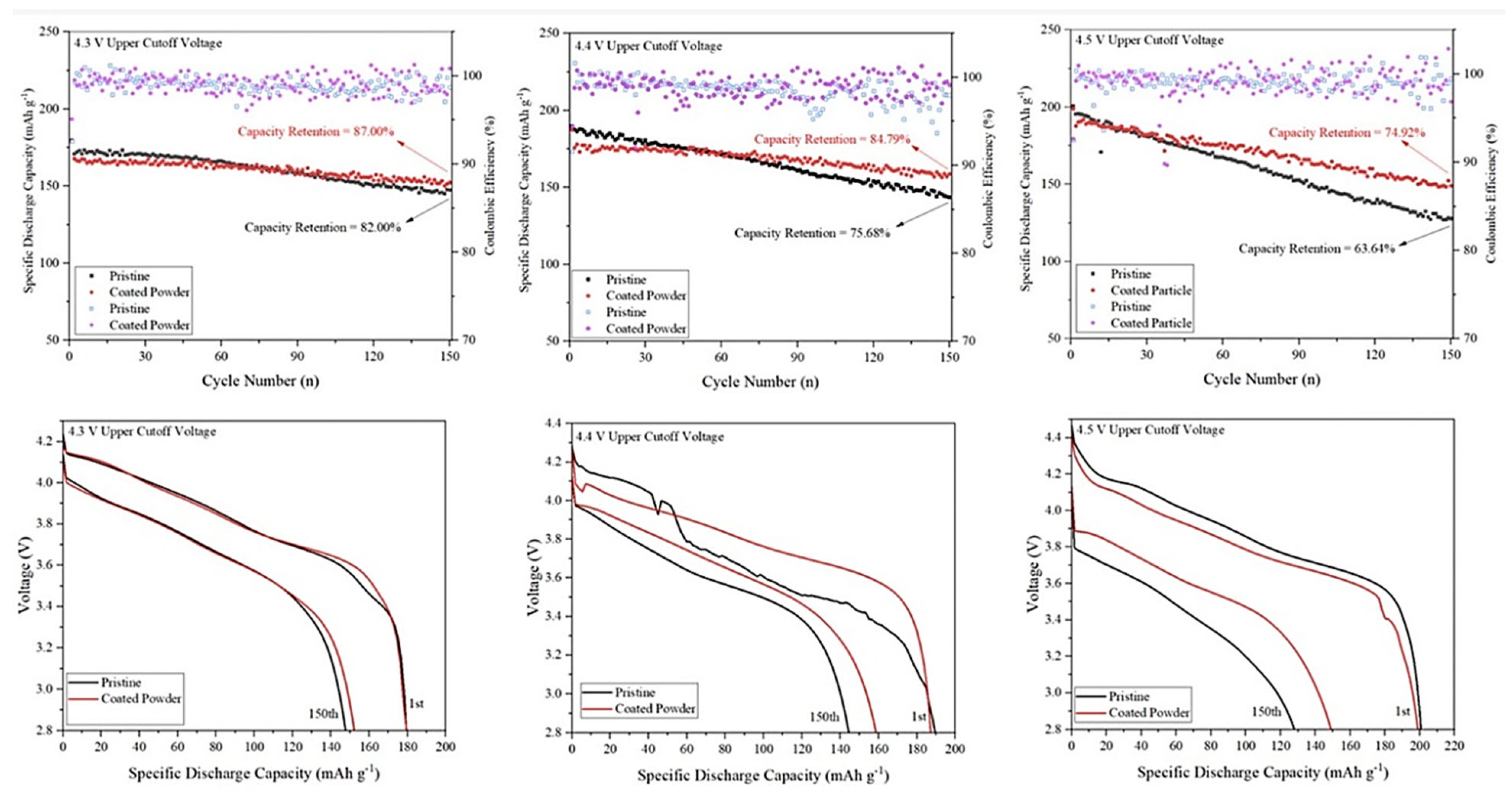

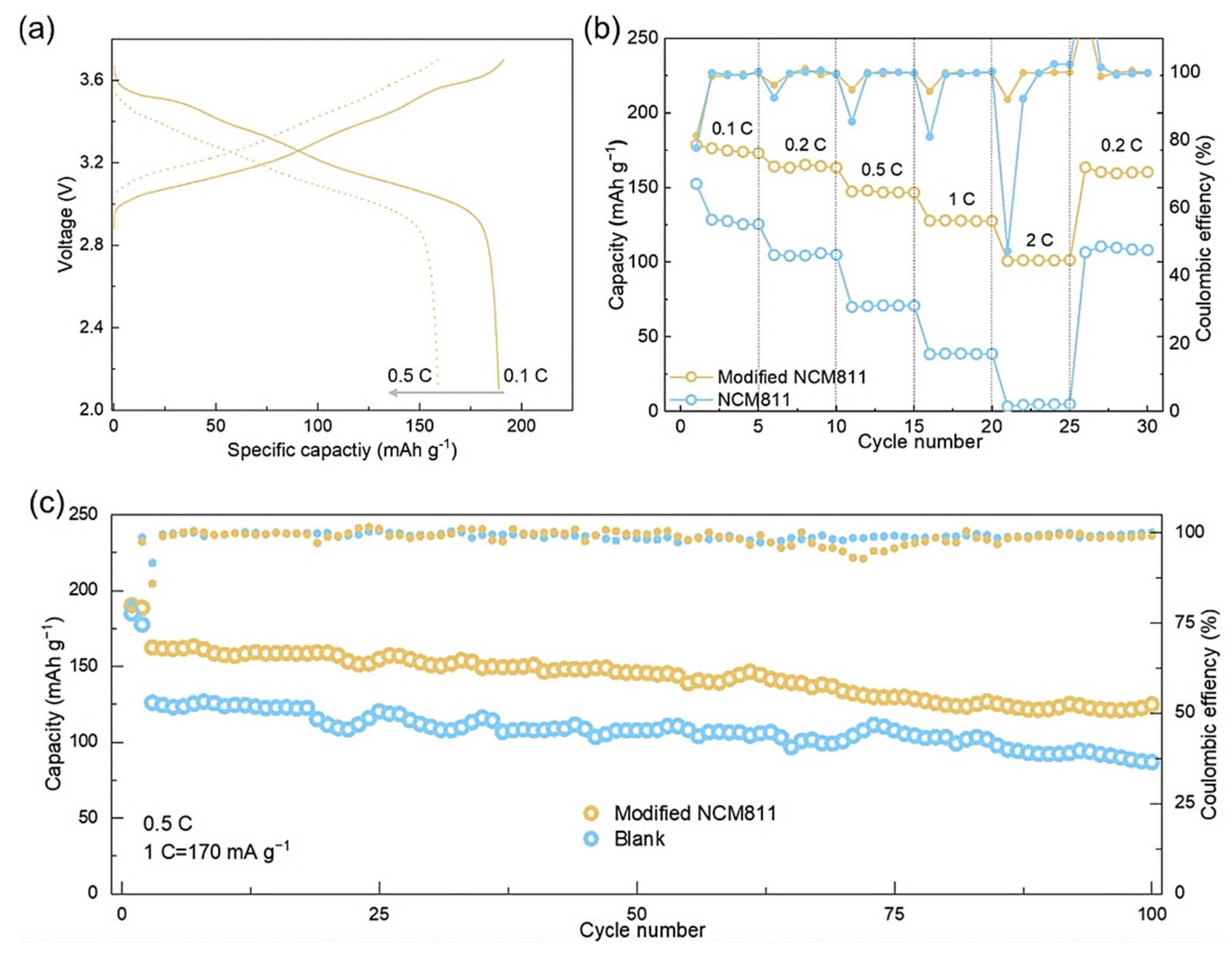
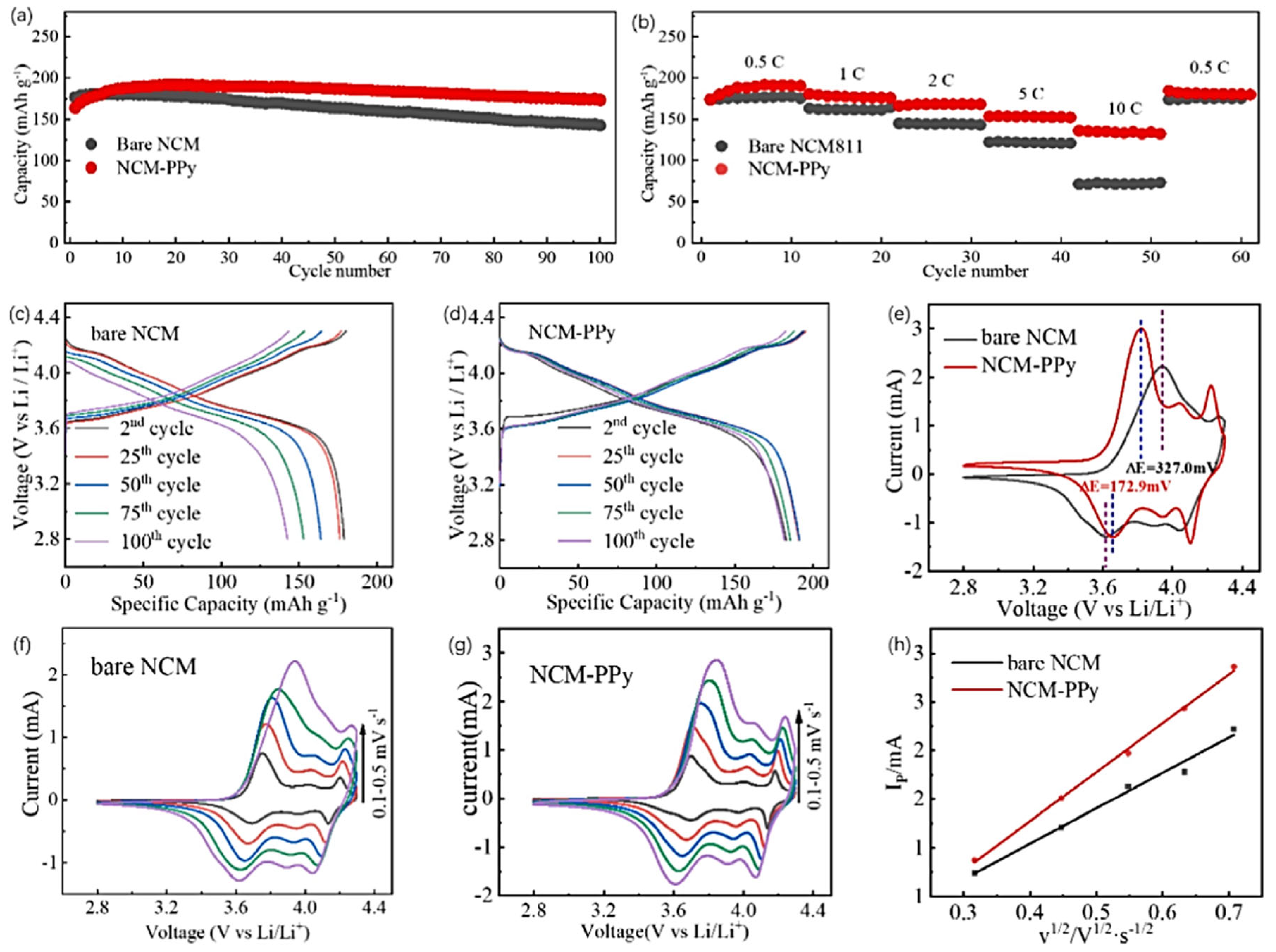
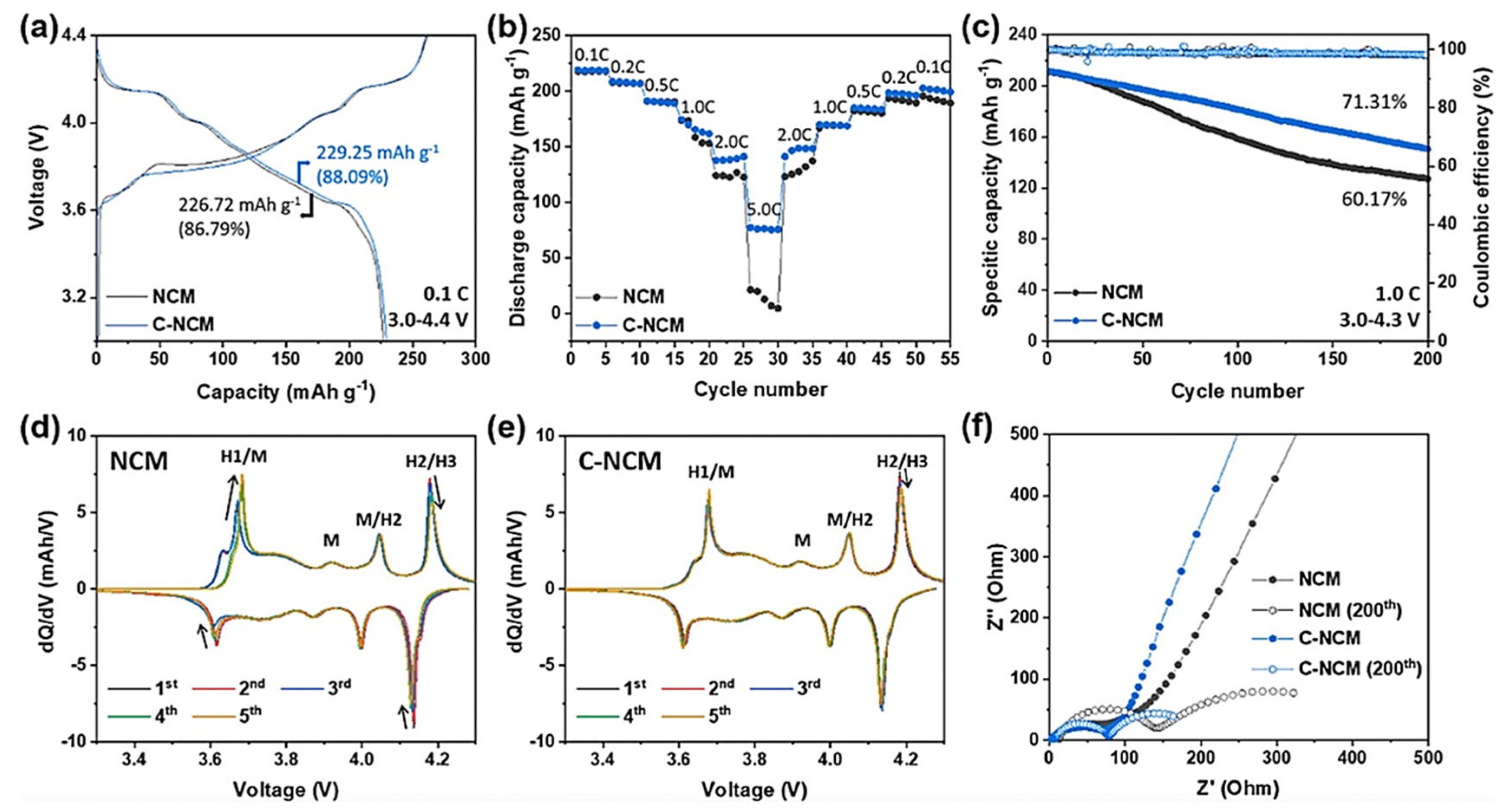
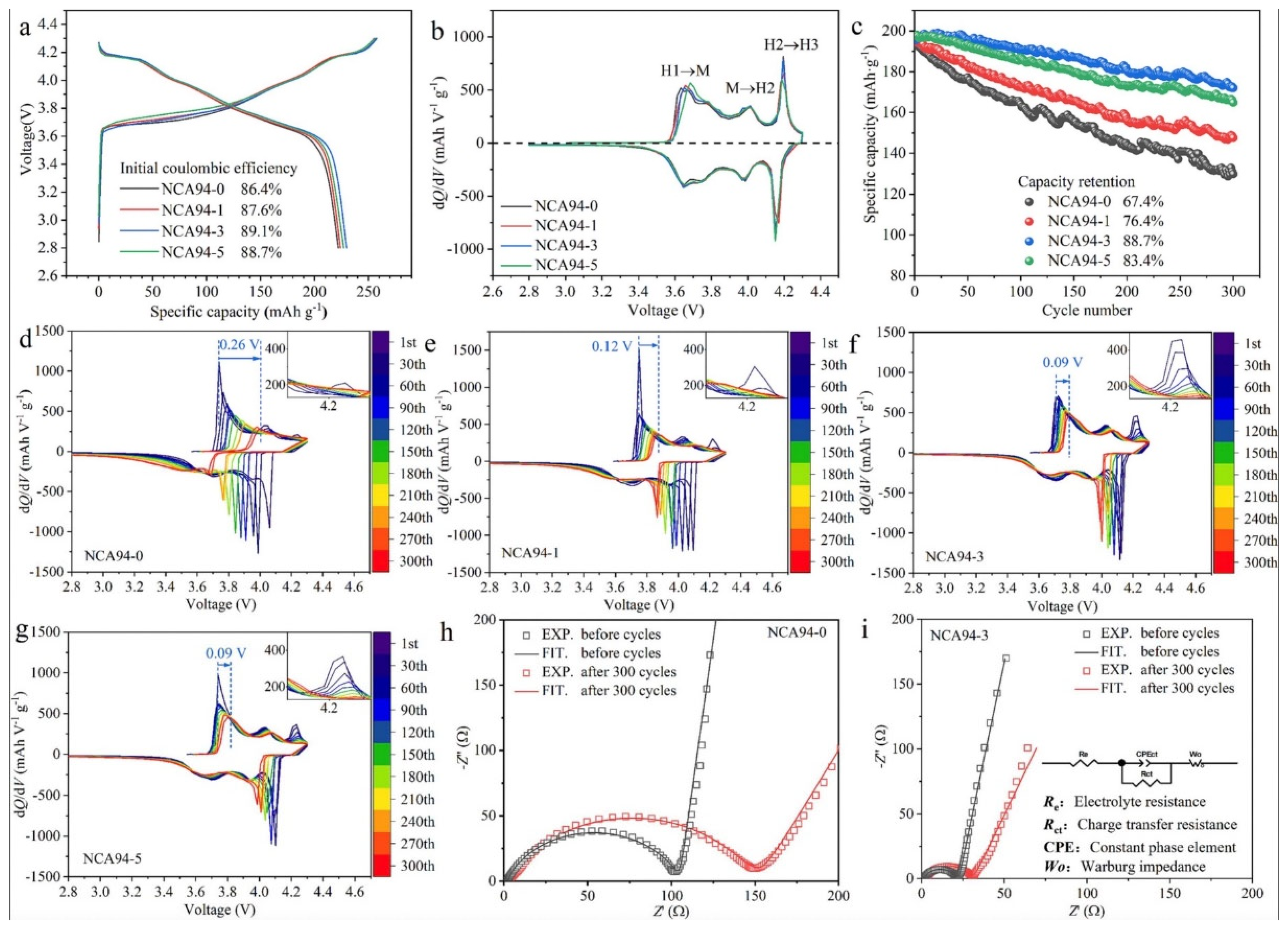

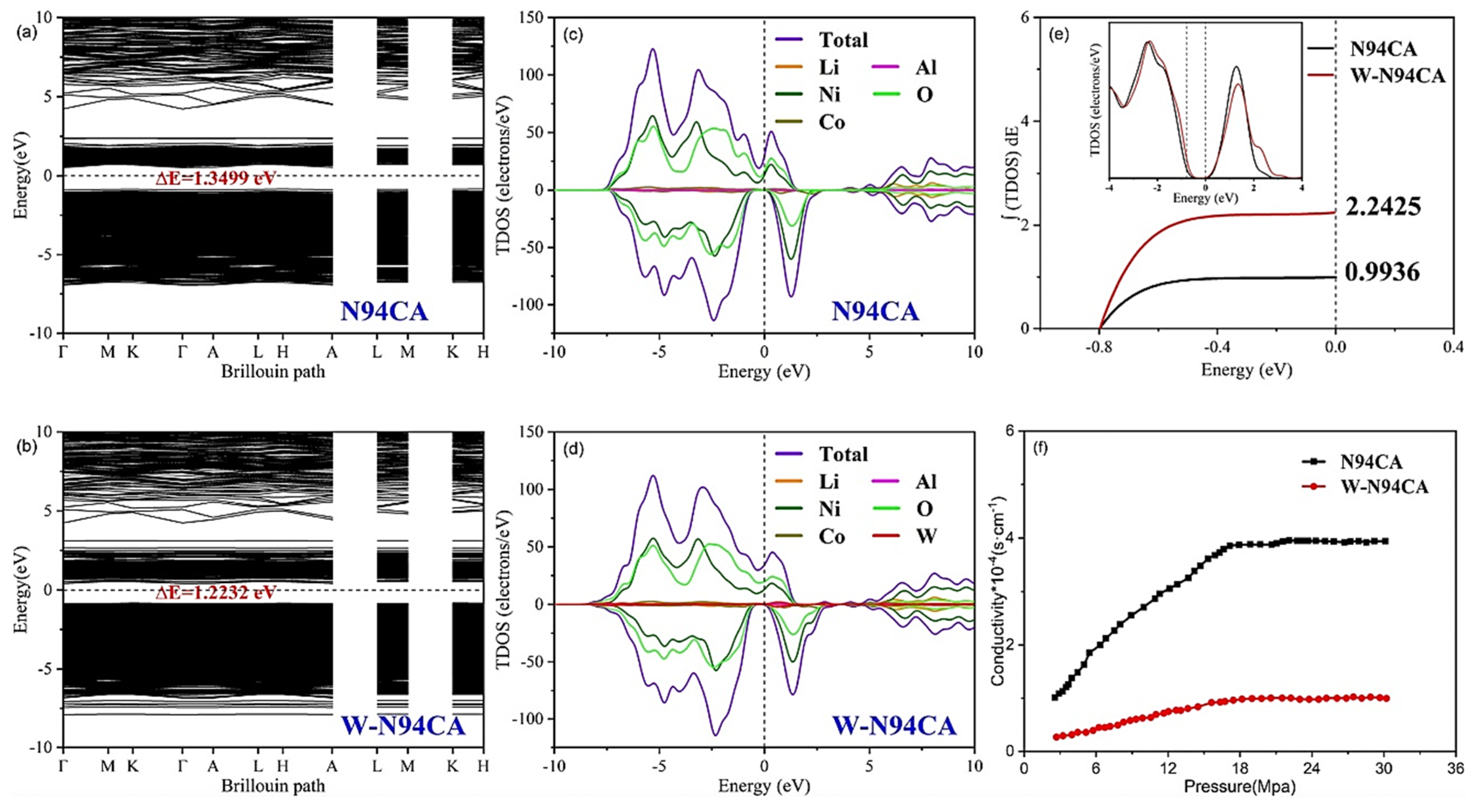
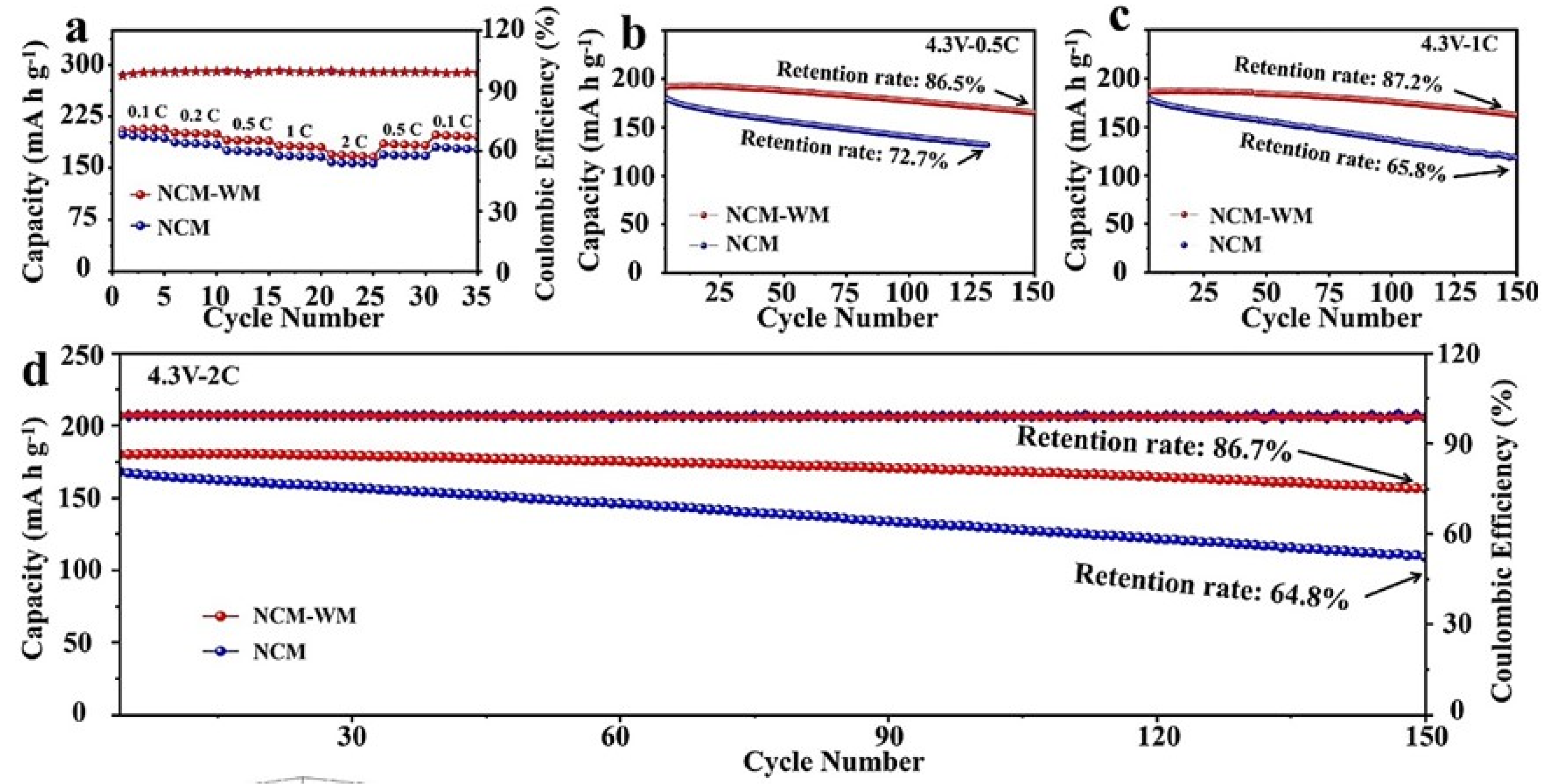

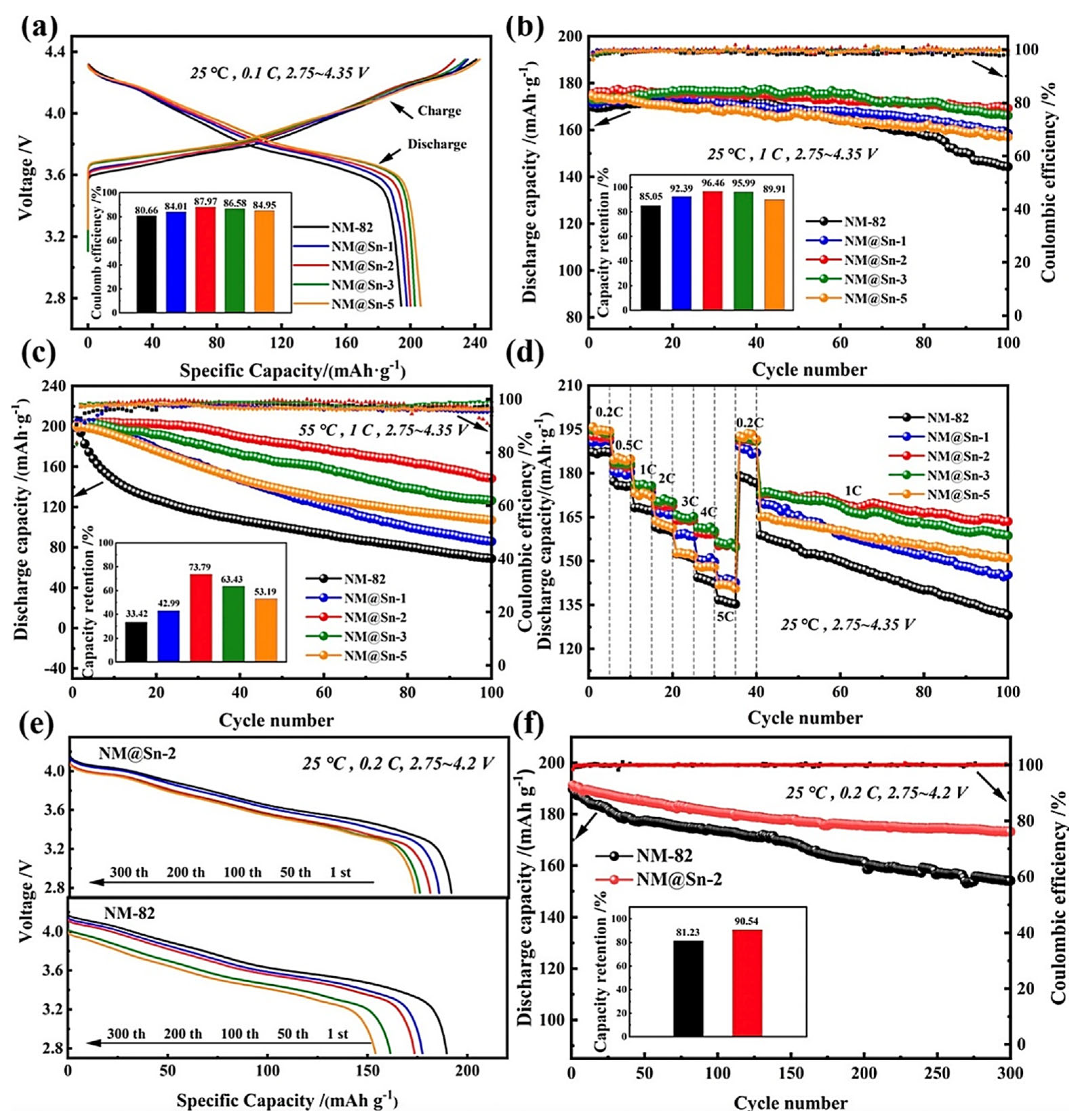
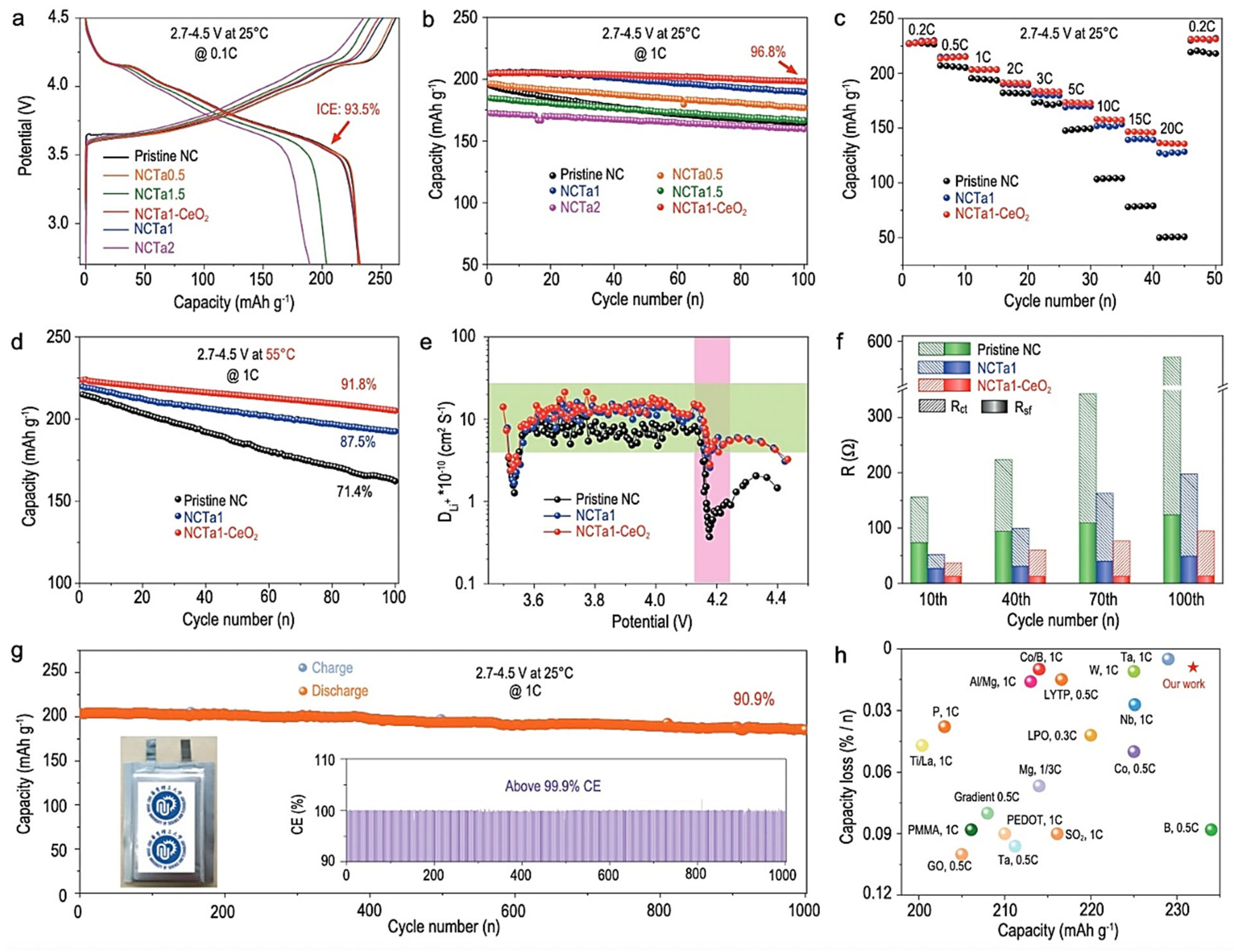

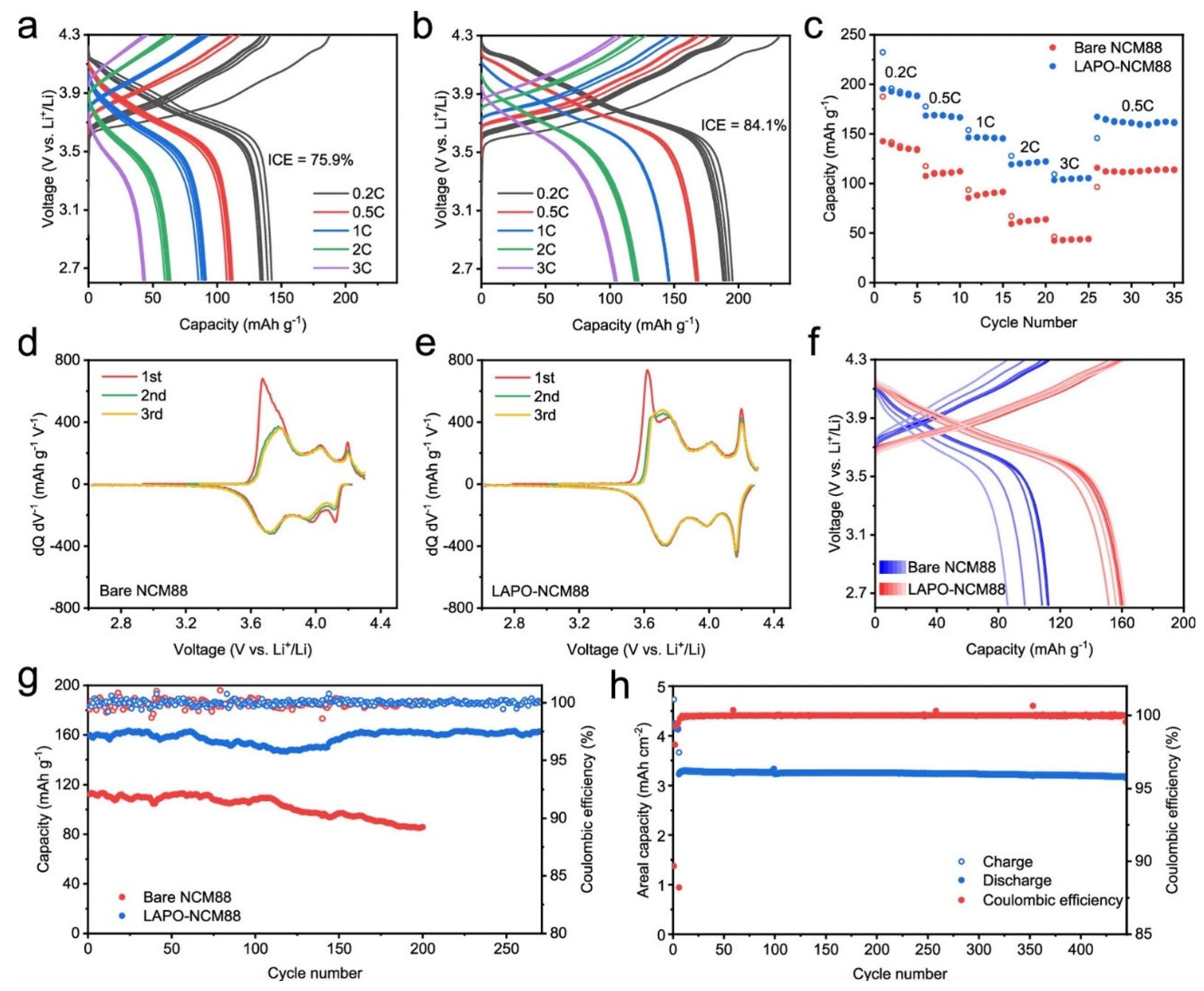
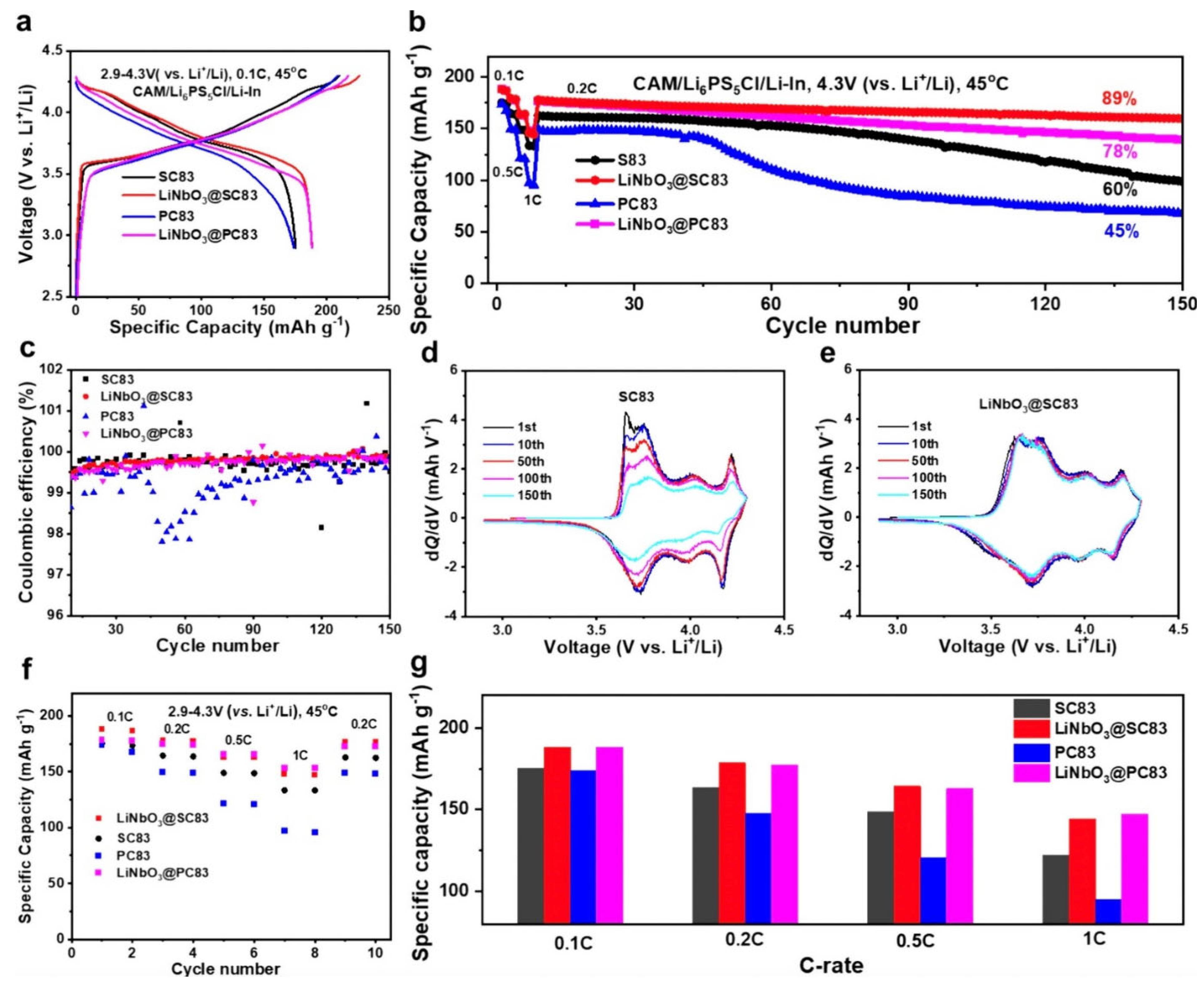
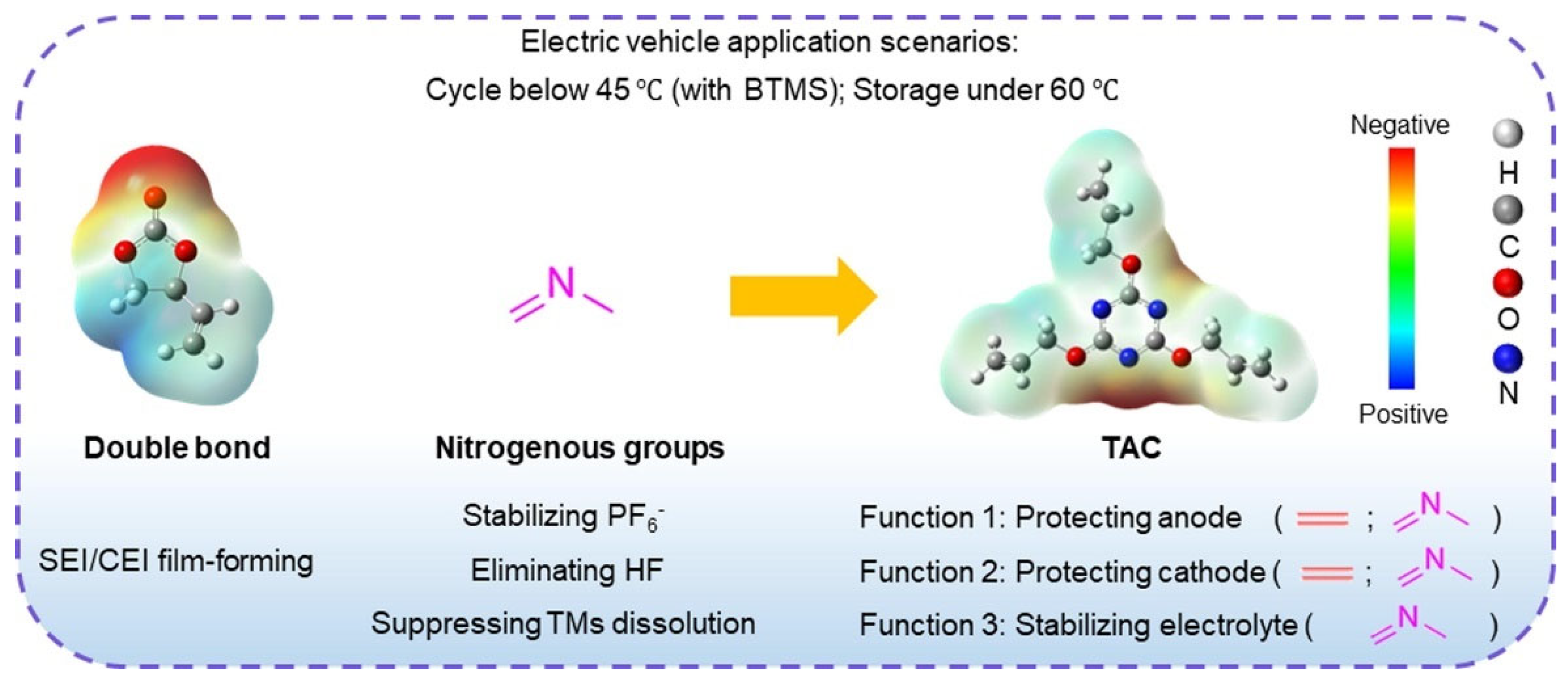
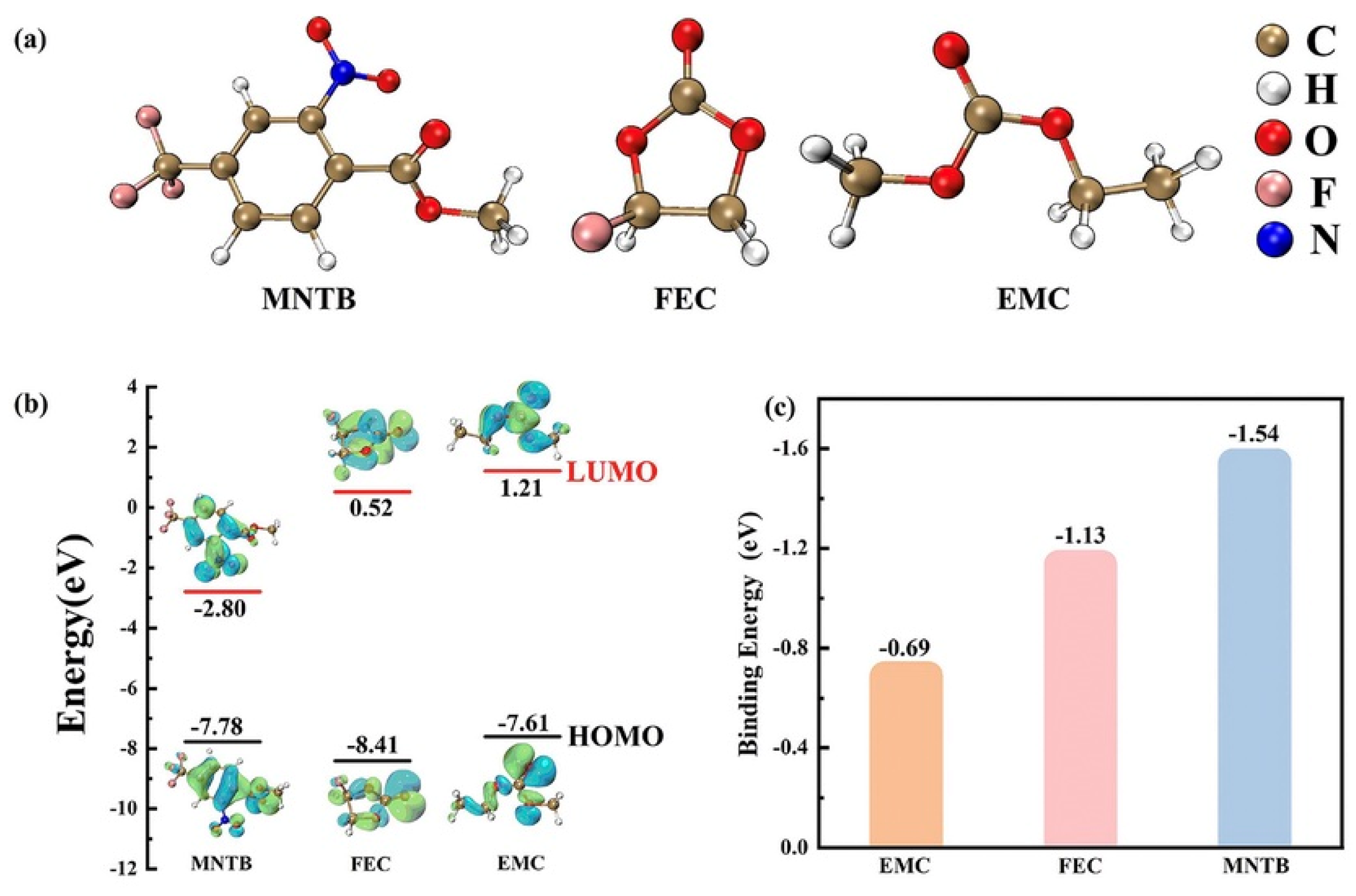
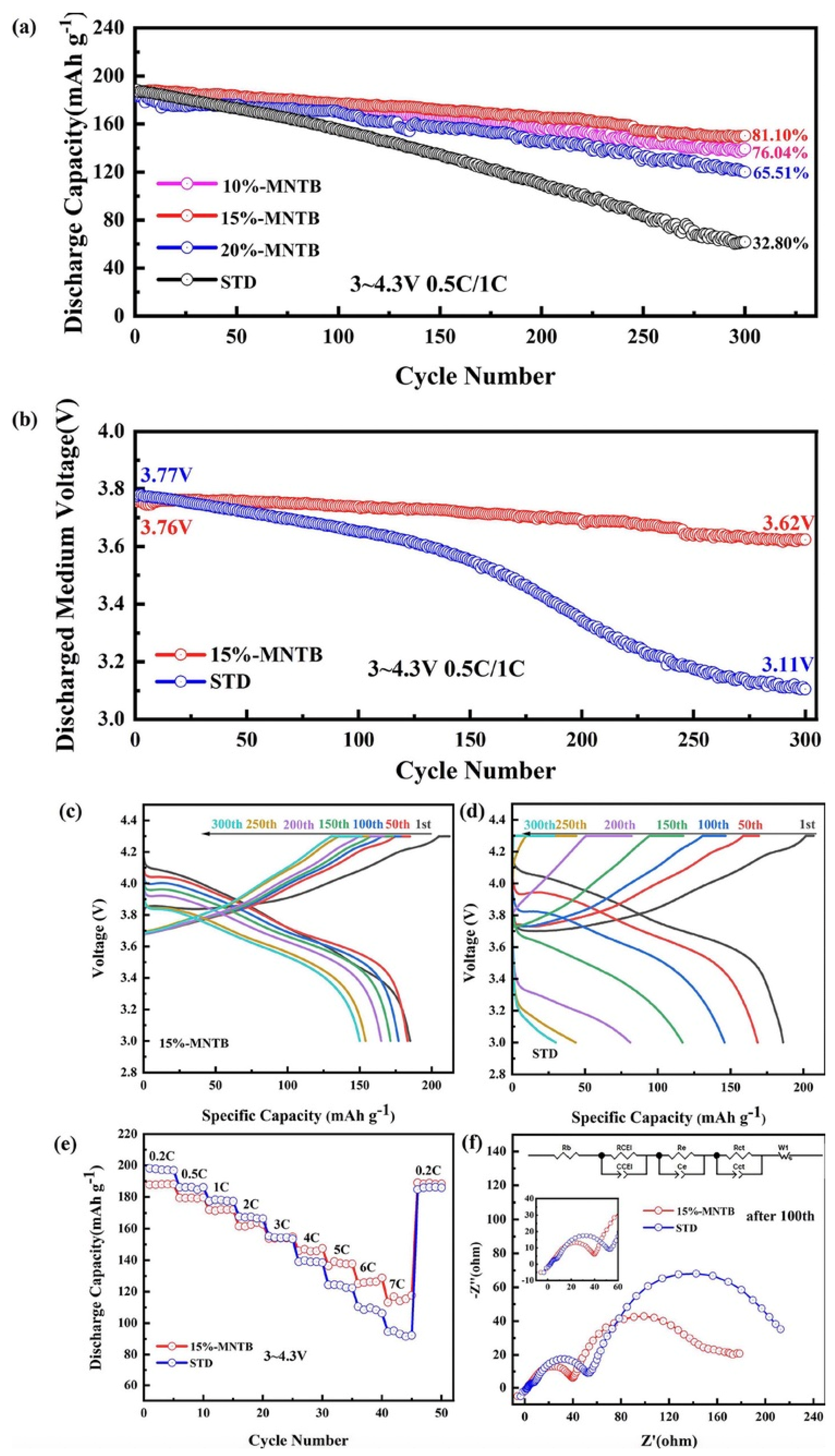

| Cathode Element | Coating | Cycles @C Rate | Pristine | Coated | Ref. | ||
|---|---|---|---|---|---|---|---|
| Capacity (mAh g−1) | Retention (%) | Capacity (mAh g−1) | Retention (%) | ||||
| NMC811 | g-C3N4 | 225@1 C | 170 | 76.4 | 170 | 82.3 | [316] |
| Sb-NCMA93 | LiF | 1000@1 C 4000@1 C | 195 | 89.6 | 195 | 93.7 79.2 | [318] |
| Zr-modified [Ni0.8Co0.1Mn0.1]CO3 * | Zr-rich | 100@2 C | 198 | 85.8 | 195.2 | 92.2 | [320] |
| NMC622 * | ZrO2 | 100@0.5 C | 190 | 52.4 | 187.6 | 82.5 | [321] |
| LiNi0.85Co0.10Mn0.05O2 | ZrO2 | 200@0.5 C | 165 | 67.9 | 170 | 77.6 | [322] |
| NMC532 | ZrO2 | - | 155 | 61.6 | 160 | 77.4 | [382] |
| NMC622 * | TiO2 | 50@1 C | 175.1 | 78.1 | 178 | 88.7 | [323] |
| LiNi0.9Co0.08Al0.02O2 | TiO2 | 100@2 C | 178 | 63.3 | 181 | 77.0 | [324] |
| LiNi0.83Co0.11Mn0.06O2 * | CeO2 | 200@1 C | 190 | 64.2 | 185 | 77.0 | [326] |
| NMC811 | Al2O3 | 200@1 C | 180.7 | 82.7 | 170 | 95.42 | [333] |
| NMC811 (at 50 °C) | ZnO | 100@1 C | 175 | 51.72 | 182 | 77.47 | [334] |
| NMC811 | Mg-Al oxides | 100@0.5 C | 200 | 65.3 | 176 | 83.3 | [336] |
| NMC811 | B2O3 | 200@0.5 C | 147 | 58.4 | 121.7 | 75.6 | [339] |
| LiNi0.87Co0.10Al0.03O2 | B2O3 | 100@0.2 C | 190 | 45 | 184 | 86 | [340] |
| LiNi0.70Co0.15Mn0.15O2 | Al2O3 | 130@C/2 | 152 | 60 | 155 | 90 | [343] |
| NMC811 | Al2O3 | 400@1 C | 800 (mAh) | failed | 780 (mAh) | 78.6 | [345] |
| LiNi0.83Mn0.05Co0.12O2 | Al2O3 | 300@C/3 | 193 | 61.5 | 170 | 84.7% | [346] |
| NMC811 | Ti-based | 200@1 C | 189 | 44 | 189 | 90.0 | [349] |
| LiNi0.8Co0.15Al0.05O2 | SrTiO3–x | 200@1 C | 205 | 72.1 | 210 | 81.1 | [352] |
| NMC811 | Al2(WO3)4 | 100@1 C | 190.0 | 73.0 | 186.5 | 86.8 | [353] |
| NMC811 | MoO3 | 100 100 | 182.5 | 85.8 | 186.1 160 | 92.5 79.8 | [356] |
| NMC622 | NiFe2O4 | 200@C/2 | 152 | 67.8 | 145 | 81.25 | [357] |
| NMC811 | NiFe2O4 | 200@1 C | 162 | 52.4 | 162 | 80.57 | [358] |
| NMC622 | Li2SiO3 | 100@1 C | 199 | 76.4 | 196 | 85.5 | [359] |
| NMC811 | Li2SiO3 | 450 | 170 | 79 | 166 | 98.0 | [386] |
| Li1.06(Ni0.88Co0.06Mn0.06)0.94O2 | TiNb2O7 | 200@1 C | 187 | 59.8 | 184 | 87.9 | [360] |
| NMC811 | Co3(PO4)2 | 100@1 C | 145 | 81.3 | 165 | 89.5 | [365] |
| NMC811 * | FePO4 | 400@C/5 | 195 | 63 | 197 | 86.0 | [367] |
| NMC811 * | Li3PO4 | 100@1 C | 196 | 75.4 | 192 | 88.4 | [370] |
| LiNi0.83Co0.12Mn0.05O2 | CeP2O7 | 100@1 C | 179.2 | 82.8 | 176.6 | 92.3 | [375] |
| NMC811 | DHP | 300@2 C | 172 | 40.7 | 188 | 64 | [378] |
| LiNi0.83Mn0.11Co0.06O2 | AP | 300 | 190 | 57.2 | 190 | 92.6 | [379] |
| NMC622 | Li3PO4 | 200@1 C | 189.3 | 89.6 | 189.8 | 80.9 | [380] |
| NMC622 | LiZr2(PO4)3 | 100@C/5 | 125.6 | 65.9 | 136.2 | 86.2 | [383] |
| LiNi0.90Co0.04Mn0.03Al0.03O2 | Li2ZrO3 | 100@1 C | 178.4 | 74.6 | 181.7 | 90.2 | [384] |
| LiNi0.9Co0.07Mn0.01Al0.02O2 | LiAlO2 | 100@1 C | 190 | 70.7 | 190 | 88.3 | [385] |
| NMC811 | Li2MnO3 | 500@1 C | 177 | 63.1 | 177 | 80.4 | [387] |
| NMC532 | LiNbO3 | 100@1 C | 174.6 | 73 | 190 | 92 | [390] |
| NMC811 | LiNbO3 | 500@1 C | 186 | 19 | 191 | 70 | [391] |
| LiNi0.90Co0.05Mn0.05O2 * | LiNbO3 | 200@1 C | 199.45 | 63.0 | 193.27 | 75.6 | [392] |
| LiNi0.85Co0.10Mn0.05O2 | Li2SO4 | 200@1 C | 190 | 73.3 | 189 | 81.5 | [394] |
| NMC811 (4.6 V) | LixWyOz | 100@1 C | 183 | 80 | 183 | 85 | [397] |
| NMC811 | LixWO3 | 100@C/2 | 180 | 48 | 182 | 93 | [398] |
| NMC811 | Li2MoO4 | 100@1 C | 177.7 | 64.6 | 177.7 | 90.5 | [399] |
| LiNi0.82Co0.12Mn0.06O2 | LiTaO3 | 100@C/2 | 175 | 55.2 | 180 | 92.3 | [400] |
| LiNi0.7Mn0.15Co0.15O2 | LiAlO2 | 100@C/2 | 170 | 85 | 180 | 88 | [401] |
| LiNi0.6Co0.2Mn0.2O2 | LiAlO2/LiF-AlF3 | 300@5 C | 160 | 4.6 | 166.8 | 74.5 | [404] |
| LiNi0.87Co0.1Al0.03O2 | Li2O-BPO4 | 100@1 C | 200 | 75.7 | 200 | 92.3 | [406] |
| LiNi0.8Co0.15Al0.05O2 | Li-B-O | 100@2 C | 170.7 | 72 | 159.7 | 94.9 | [408] |
| Li(Ni0.90Co0.06Mn0.04)0.995 Al0.005O2 (at 60 °C) | Nb12WO33 (dots) + Li-B-O | 100@3C | 217 | 76.91 | 227 | 84.7 | [409] |
| LiNi0.8Co0.15Al0.05O2 * | LiFePO4 | 100@C/2 | 204 | 82.5 | 210 | 95 | [411] |
| LiNi0.76Mn0.14Co0.10O2 * | Li3PO4 | 200@C/3 | 170 | 79 | 180 | 91.6 | [413] |
| NMC811 | Li3PO4 | 200@C/2 | 200 | 59.3 | 200 | 84.6 | [414] |
| LiNi0.91Co0.06Mn0.03O2 | Li3PO4 | 60@1 C | 192.3 | 60 | 180 | 81.8 | [415] |
| NMC811 | Li3PO4 | 100@C/2 | 125 | 60 | 162 | 77 | [416] |
| LiNi0.8Co0.15Al0.05O2 | LiH2PO4 | 200@2 C | 160.1 | 73.1 | 166.4 | 90.1 | [417] |
| LiNi0.83Co0.06Mn0.06Al0.05O2 | LATP | 300@1 C | 163.1 | 83.3 | 189.6 | 91.5 | [419] |
| NMC811 | PrF3 | 100@1 C | 197.4 | 73.8 | 186 | 86.3 | [422] |
| NMC811 | Ti0.05V1.96O5 | 100@1 C | 165.3 | 66.8 | 170.4 | 78.7 | [424] |
| NMC811 | BTJ-L | 100@1 C | 171 | 72.9 | 173 | 86.1 | [427] |
| NMC811 | PPy | 100@1 C | 175 | 84.5 | 175.3 | 90.7 | [428] |
| NMC811 | PMMA | 200@1 C | 177 | 80.1 | 181.1 | 91.2 | [430] |
| NMC811 | PAN | 200@1 C | 190 | 57.5 | 210 | 86.3 | [435] |
| NMC811 | PANI | 100@1 C | 198 | 80.1 | 200 | 88.7 | [431] |
| LiNi0.95Mn0.05O2 | PANI-PEG | 100@1 C | 203.7 | 33.3 | 219.4 | 94.7 | [433] |
| NMC622 | CENR | 500@1 C | 150 | 40.9 | 150 | 82.8 | [436] |
| LiNi0.83Co0.11Mn0.06O2 | polysiloxane | 150@1 C | 170 | 75.8 | 180 | 85.4 | [437] |
| LiNi0.948Co0.046Mn0.006O2 | COF | 200 | 210 | 60.1 | 210 | 71.3 | [439] |
| Cathode | Dopant | Cycles at C Rate | Pristine | Doped | Ref. | ||
|---|---|---|---|---|---|---|---|
| Capacity (mAh g−1) | Retention (%) | Capacity (mAh g−1) | Retention (%) | ||||
| NMC811 | Mo | 100@C/3 | 150 | 78 | 180 | 81.6 | [454] |
| LiNi0.84Co0.11Mn0.05O2 | Mo | 80@C/10 80@C/2 | 195 191 | 46.5 - | 205 191 | - 87.2 | [455] |
| NCMA94 | Mo | 100@C/2 | 238.3 | 87 | 236.8 | 93.1 | [457] |
| LiNiO2 | W | 100@C/2 | 230 | 83.7 | 228 | 90.3 | [458] |
| NCA95 | W | 100@C/2 | 220 | 81 | 230 | 90.3 | [460] |
| LiNi0.83Co0.11Mn0.06O2 | W | 500@2 C | 140 | 53.5 | 165 | 69.9 | [461] |
| NMC811 | W | 200@1 C | 170 | 81.3 | 170 | 92.3 | [463] |
| LiNi0.88Co0.09Mn0.03O2 | W | 100@1 C | 192 | 62.21 | 188 | 93.51 | [555] |
| LiNi0.9Mn0.1O2 | W | 200@1 C | 170 | 77.1 | 170 | 95.3 | [463] |
| LiNi0.88Co0.09Al0.03O2 | Ta | 100@1 C | 208 | 68 | 198 | 93 | [465] |
| NMC622 * | Ta | 100@1 C | 143.4 | 80.1 | 148.1 | 83.6 | [467] |
| Li[Ni0.91Co0.09]O2 | Ti Ta Mo | 100@C/2 | 227–230 | 78.8 | 227–230 | 94 97 94.9 | [468] |
| LiNi0.865Co0.095Al0.04O2 | Ta | 200@1 C | 180 | 97.87 | 188 | 79 | [469] |
| LiNi0.83Co0.11Mn0.06O2 | Ta | 200@1 C | 91.4 | 50 | 160.3 | 97.5 | [472] |
| NMC8111 | Nb | 300@1 C | 184.9 | 79.8 | 180.2 | 96.9 | [473] |
| NMC8111 | Nb | 100@1 C | 181.5 | 67.6 | 181.6 | 94.55 | [474] |
| LiNi0.83Co0.11Mn0.06O2 | Nb | 200@1 C | 181 | 61.2 | 181 | 86.6 | [477] |
| LiNi0.94Co0.02Al0.04O2 | Nb | 300@2 C | 192 | 67.4 | 194 | 88.7 | [527] |
| NMC811 * | Ce | 100@5 C | 129.6 | 70.7 | 161 | 86.9 | [478] |
| LiNi0.90Co0.07Al0.03O2 * | Zr | 100@C/2 | 205.9 | 62.02 | 191.7 | 84.86 | [480] |
| LiNi0.9Al0.1O2 | Zr | 100@1 C | 105 | 81.04 | 177.5 | 92.45 | [481] |
| LiNi0.83Co0.12Mn0.05O2 | Zr | 100@5 C | 178 | 86.5 | 180 | 97.6 | [482] |
| NMC811 | Ti | 50@C/2 | 180 | 91.1 | 180 | 96.7 | [484] |
| NMC811 | Ti | 100@C/10 | 116.5 | 64.1 | 180.6 | 96.9 | [486] |
| LiNi0.83Co0.11Mn0.06O2 | Ti | 100@2 C | 168.5 | 76.3 | 164 | 95.4 | [488] |
| LiNi0.88Co0.06Mn0.06O2 | Ti | 100@1 C | 181.5 | 78.9 | 193.8 | 93.6 | [489] |
| LiNi0.90Co0.05Mn0.05O2 | Ti | 150@1 C | 195 | 72.6 | 170 | 94.4 | [490] |
| LiNi0.9Co0.08Al0.02O2 | Hf | 100@1 C | 200 | 82.0 | 200 | 95.3 | [492] |
| NMC622 * | Ce | 100@1 C | 167.2 | 63.63 | 188.7 | 74.66 | [494] |
| LiNi0.88Co0.09Al0.03O2 | Gd | 100@1 C | 151 | 77 | 176 | 89.3 | [495] |
| NMC90 | Sn | 100@C/2 | 230 | 87.1 | 230 | 92.9 | [451] |
| NMC811 | Sn | 270@C/2 | 192 | 61.6 | 190.3 | 96.6 | [547] |
| LiNi0.90Co0.04Mn0.03Al0.03O2 | Sn | 200@1 C | 185 | 67 | 185 | 83 | [493] |
| LiNi0.80Co0.15Mn0.05O2 | Al | 100@C/10 | 214.1 | 92.4 | 208.1 | 97.2 | [496] |
| NMC811 (4.4 V) | Al | 90@1 C | 180 | 70 | 180 | 80 | [497] |
| LiNi0.92Co0.03Mn0.03Al0.02O2 | Al | 100@C/2 | 210 | 69 | 190 | 92 | [498] |
| Li[Ni0.92Co0.04Mn0.04]O2 (4.2 V) | Al | 1000@1 C | 193 | 53.4 | 186 | 88.3 | [499] |
| LiNi0.90Mn0.06Al0.04O2 LiNi0.90Mn0.06Co0.04O2 | Al | 200@1 C 200@1 C | - 170 | - 78.3 | 165 - | 82.9% - | [500] |
| LiNi0.8Mn0.16Al0.04O2 | Al | 200@1 C | 161 | 69.4 | 178 | 82.2 | [501] |
| LiNi0.6Mn0.4O2 * | Al | 100@1 C | 136.8 | 70.4 | 179 | 87.6 | [504] |
| LiNiO2 | Al | 600@C/2 | 187 | 66.8 | 175 | 81.7 | [529] |
| LiNi0.6Co0.2Mn0.2O2 | Cr | 200@1 C | 137.2 | 84.9 | 149.8 | 91.0 | [507] |
| NMC811 | Sc | 150@1 C | 185 | 77.9 | 185 | 81.7 | [508] |
| LiNi0.925Co0.03Mn0.045O2 | Y | 150@1 C | 197.7 | 65.39 | 196 | 82.7 | [509] |
| LiNi0.88Co0.09Al0.03O2 | Y | 100@1 C | 190.8 | 60.1 | 192.7 | 88.9 | [510] |
| LiNi0.9Co0.1O2 | La | 300@C/2 | 205 | 62.2 | 209 | 75.2 | [511] |
| LiNi0.88Co0.05Mn0.07O2 | Sr | 100@1 C | 191.4 | 88.48 | 189.1 | 96.61 | [520] |
| LiNi0.83Co0.12Mn0.05O2 * | Mg | 200@1 C | 201.8 | 74.0 | 199.7 | 87.2 | [512] |
| LiNi0.6Co0.2Mn0.2O2 | Mg | 100@1 C | 162.6 | 79.33 | 180 | 90.02 | [549] |
| LiNi0.9Co0.05Mn0.05O2 | Zn | 80@C/2 | 190 | 69.8 | 190 | 91.7 | [521] |
| NMC622 | Na | 100@C/5 | 160 | 67.5 | 175 | 90.8 | [525] |
| LiNi0.6Co0.05Mn0.35O2 (4.45 V) | Na | 150@C/2 | 160 | 80.23 | 160 | 84.4 | [536] |
| NMC811 | B | 100@C/2 | 178 | 93 | 180 | 97 | [532] |
| LiNi0.83Co0.05Mn0.12O2 | B | 500@C/2 | 1.73 Ah | 66.95 | 1.78 Ah | 91.35 | [533] |
| NMC811 | B | 120@C/2 | 170 | 53 | 170 | 87 | [534] |
| NMC811 | F | 100@C/2 | 170 | 58 | 175 | 93 | [540] |
| LiNi0.9Co0.05Mn0.05O2 | F | 100@2 C | 176.7 | 85.3 | 181.1 | 95.5 | [541] |
| LiNi0.8Co0.15Al0.05O2 | F | 100@2 C | 183 | 77.6 | 157.8 | 98.3 | [542] |
| LiNi0.82Mn0.18O2 * | F | 100@C/2 | 170 | 47 | 180 | 70 | [543] |
| NMC811 * | Cl Br | 100@C/2 100@C/2 | 197 197 | 81.8 81.8 | 202 197 | 96.1 95.3 | [544] |
| Cathode Element | Dual Doping | Cycle at C-Rate | Pristine | Doped | Ref. | ||
|---|---|---|---|---|---|---|---|
| Capacity (mAh g−1) | Retention (%) | Capacity (mAh g−1) | Retention (%) | ||||
| NMC811 (cut-off at 4.8 V) | Mg+Zr | 100@C/10 | 201.8 | 66 | 232.2 | 70.5 | [590] |
| LiNi0.90Co0.06Mn0.04O2 | Mg+W | 150@2 C | 170 | 64.8 | 180 | 86.7 | [591] |
| LiNi0.85Co0.05Mn0.10O2 * | K+F | 500@1 C | 185 | 75 | 190 | 91.5 | [592] |
| NMC811 | K+Ti | 200@1 C | 182 | 59.4 | 175 | 91.2 | [593] |
| LiNi0.92Co0.04Mn0.04O2 * | Mo+F | 200@1 C | 200 | 0 | 204 | 87.1 | [594] |
| LiNi0.80Co0.05Mn0.15O2 * | Al+Na | 200@1 C | 196 | 70 | 205 | 84 | [595] |
| NMC811 | B+Nb | 200@1 C | 175 | 76.1 | 175 | 86.7 | [597] |
| LiNi0.83Co0.07Mn0.10O2 | Ce+Gd | 100@1 C | 192.6 | 82.5 | 194.6 | 90.2 | [598] |
| LiNi0.83Co0.11Mn0.06O2 | B+Ca | 100@C/2 | 190 | 44.3 | 190 | 72.4 | [599] |
| LiNi0.92Co0.08O2 | Ti+Al | 100@C/5 | 208.7 | 76.5 | 197.7 | 93.8 | [601] |
| LiNi0.85Mn0.09Al0.06O2 | In+Sn | 100@C/2 | 160 | 86.6 | 162 | 100 | [605] |
| LiNi0.88Co0.08Mn0.04O2 | Na+Al | 50@1 C | 183.91 | 78.6 | 192.49 | 83.7 | [606] |
| LiNi0.95Co0.03Al0.01Mg0.01O2 | Mg+Al | 100@1 C | 190 | 60.8 | 200 | 95.6 | [608] |
| LiNi0.8Co0.15Al0.05O2 | Na+Y | 100@C/2 | 172 | 67.1 | 175 | 92.3 | [611] |
| LiNi0.9Mn0.1O2 | W+Y | 100@1 C | 178 | 71.9 | 180 | 82.1 | [612] |
| LiNi0.9Mn0.1O2 | Zr+Mo+Mg | 150@2 C | 180 | 63.2 | 160 | 85.5 | [613] |
| LiNi0.83Co0.12Mn0.05O2 at 55 °C | Na+W | 1000@1 C 500@1 C | 185 | 0 80 | 197.5 | 87 | [614] |
| NMC811 | Na+Al | 80@1 C | 175 | 84.5 | 175 | 93 | [616] |
| LiNi0.96Co0.04O2 (cut-off 4.4 V) | Al+Nb | 300 | 200 | 48.5 | 200 | 77.8 | [617] |
| Li[Ni0.92Co0.04Mn0.04]O2 | Al+Nb | 500@1 C | 195 | 0 | 183 | 75.6 | [618] |
| LiNi0.83Co0.12Mn0.05O2 | Al+Nb | 100@1 C | 172 | 75 | 172 | 95.1 | [619] |
| LiNi0.94Co0.06O2 | Al+Y | 500@1 C | 200 | 54.6 | 200 | 80.2 | [621] |
| Li[Ni0.885Co0.100Al0.015]O2 | Zr+B | 1000@1 C | 190 | 48 | 178 | 95 | [623] |
| LiNi0.83Co0.12Mn0.05O2 | Zr+Al | 300@5 C | 150 | 26.1 | 157 | 80.5 | [624] |
| LiNi0·83Co0·11Mn0·06O2 * | Zr+La | 200@1 C | 200.9 | 66.2 | 189.0 | 86.1 | [625] |
| NMC811 | Ti+Ta | 250@1 C | 155.4 | 59.8 | 161.1 | 87.1 | [626] |
| NMC811 | Mo+F | 500@1 C | 182 | 67.3 | 181 | 87.1 | [627] |
| Ni0.9Co0.05Mn0.05O2 | Mo+Ti | 100@C/2 | 96.09 | 53 | 98.85 | 69 | [628] |
| Li(Ni0·83Co0·12Mn0.05)O2 * | Ti+Al | 500@1 C | 170 | 35.1 | 180 | 71.8 | [629] |
| NMC811 | Ti+B | 100@1 C | 186.8 | 71.1 | 173.4 | 94.7% | [631] |
| Li[Ni0.90Co0.05Mn0.05]O2 | Ti+Mg | 100@1 C | 157 | 86.6 | 158 | 98.9 | [632] |
| LiNi0.89Co0.11O2 | Ti+Mg | 200@1 C | 195 | 74.3 | 190 | 86.5 | [633] |
| LiNi0.8Co0.15Al0.015O2 | Mg+F | 200@1 C | 175 | 55.7 | 175 | 82.6 | [634] |
| NMC811 | Co+Ti | 400@1 C | 175 | 89.9 | 170 | 97.1 | [635] |
| Cathode Element | Dopant + Coating | Cycles at C-Rate | Pristine | Coated | Ref. | ||
|---|---|---|---|---|---|---|---|
| Capacity (mAh g−1) | Retention (%) | Capacity (mAh g−1) | Retention (%) | ||||
| NMC811 | Mg + LiFePO4 | 100 | 183.48 | 81.7 | 181.5 | 90.8 | [412] |
| LiNi0.8Co0.15Al0.05O2 | Ta + Ta2O5 | 200@1 C | 168 | 60.97 | 180 | 94.46 | [338] |
| LiNi0.90Co0.05Mn0.05O2 | Ta + Li2MnO3 | 300@1 C | 201.9 | 85.8 | 204.2 | 91.6 | [388] |
| LiNi0.82Co0.14Al0.04O2 | Mn + Li0.5La2Al0.5O4 | 100@1 C | 181 | 83.2 | 182 | 96.2 | [396] |
| Zr + WO3/Li2WO4 | @C/3 | 173 | 85 | 180 | 93 | [645] | |
| NMC811 | Na + LiNiO2 + Na1–xNi1–yPO4 | 200@1 C | 200 | 59 | 202 | 88 | [377] |
| NMC811 | Sn + Li2SnO3 | 280@C/2 | 192 | 61.6 | 190.3 | 96.6 | [547] |
| LiNi0.8Mn0.2O2 | Sn + Li2SnO3 | 300@C/5 | 189.70 | 81.23 | 191.3 | 90.54 | [647] |
| LiNi0.925Co0.03Mn0.045O2 At 55 °C | B + Li2B4O7 | 100 | 210 | 59.65 | 210 | 72.86 | [648] |
| LiNi0.825Co0.115Mn0.06O2 | B + La4NiLiO8 | 500@1 C | 165 | 73 | 165 | 93.49 | [649] |
| LiNi0.91Co0.06Mn0.03O2 | B + Li3BO3 | 100@2 C | 170 | 67.3 | 175 | 79.4 | [651] |
| LiNi0.89Co0.08Mn0.03O2 | B + B2O3 & LiBO2 | 100@1 C | 195 | 59 | 195 | 90 | [653] |
| LiNi0.83Co0.11Mn0.06O2 (cut-off 4.4 V) | Zr + Li3BO3 | 1400@1 C | 700 Wh/kg | 71.4 | 700 Wh/kg | 83.5 | [652] |
| NMC811 | B + LiBO2/B2O3 | 140@1 C | 170 | 91 | 170 | >99 | [654] |
| NMC811 cut-off 4.6 V | La + Li3BO3 | 300@C/2 | 176.9 | 27.0 | 201.4 | 63.7 | [655] |
| LiNi0.83Co0.11Mn0.06O2 (full cell) | Zr + Li1.3Y0.3Zr1.7(PO4)3 | 500@C/2 | 19.87 mAh | 70.6 | 23.48 mAh | 83.7 | [656] |
| NMC811 | Sm dopant + coat | 100@1 C | 184 | 57.6 | 184.2 | 94.2 | [660] |
| LiNi0.96Mn0.04O2 | Al+Zr+ Li2ZrO3 | 100@1 C | 187 | 75 | 195 | 81.2 | [662] |
| NMC811 | Zr + BaZrO3 | 1400@1 C | 190 | 92.3 | 190 | 92.5 | [663] |
| NMC811 (cut-off 4.4 V) | Ti + TiO2 | 200@C/3 | 193 | 86.1 | 192 | 90.2 | [667] |
| NMC811 (cut-off 4.3 V) | Ti + TiO2 | 1000@1 C | 175 | 24.3 | 160 | 63.4 | [668] |
| LiNi0.9Co0.09Mo0.01O2 | Ti + Li3PO4 | 100@1 C | 156.1 | 75.1 | 189.8 | 92.1 | [670] |
| NMC811 | Zr + Al2O3 | 100@C/5 | 180 | 82.7 | 186 | 92 | [671] |
| LiNi0.84Co0.11Mn0.05O2 | Al + Li2WO4 | 200@1 C | 172 | 72.9 | 178 | 88.9 | [672] |
| NMC811 | W + WO3 | 200@1 C | 170.21 | 74.5 | 184.21 | 90.2 | [673] |
| LiNiO2 | PO43− + Li3PO4 | 1000@1 C | 200 | 23.3 | 175 | 51.0 | [677] |
| LiNi0.83Co0.11Mn0.06O2 | Nb + Li3PO4 | 200@1 C | 178 | 59.5 | 173.5 | 88.8 | [678] |
| LiNi0.92Co0.04Mn0.04O2 | Sb + Li3Ni2SbO6 | 200@5 C | 185.7 | 39.2 | 149.4 | 75.7 | [680] |
| NMC811 | Na + carbon | 500@5 C | 125 | 41.1 | 165 | 83.1 | [681] |
| LiNiO2 (cut-off 4.5 V) | Ta + CeO2 | 100@1 C | 195 | 85 | 205 | 96.8 | [682] |
| NMC92 at 60 °C | Ce + CeO2 | 80@C/5 | 91.8 | 49.25% | 141.5 | 79.32% | [683] |
| LiNi0.9Al0.1O2 (4.5 V) | Mg/Ti/Sb + CeO2 | 100@1 C | 192 | 60.1% | 200 | 90.2% | [684] |
| LiNi0.90Co0.05Mn0.05O2 | Te + Li4TeO5 | 200@1 C | 198 | 82.05% | 198 | 95.83% | [685] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, A.; Julien, C.M. Design Principles and Engineering Strategies for Stabilizing Ni-Rich Layered Oxides in Lithium-Ion Batteries. Batteries 2025, 11, 254. https://doi.org/10.3390/batteries11070254
Mauger A, Julien CM. Design Principles and Engineering Strategies for Stabilizing Ni-Rich Layered Oxides in Lithium-Ion Batteries. Batteries. 2025; 11(7):254. https://doi.org/10.3390/batteries11070254
Chicago/Turabian StyleMauger, Alain, and Christian M. Julien. 2025. "Design Principles and Engineering Strategies for Stabilizing Ni-Rich Layered Oxides in Lithium-Ion Batteries" Batteries 11, no. 7: 254. https://doi.org/10.3390/batteries11070254
APA StyleMauger, A., & Julien, C. M. (2025). Design Principles and Engineering Strategies for Stabilizing Ni-Rich Layered Oxides in Lithium-Ion Batteries. Batteries, 11(7), 254. https://doi.org/10.3390/batteries11070254







