Investigating the Thermal Runaway Characteristics of the Prismatic Lithium Iron Phosphate Battery Under a Coupled Charge Rate and Ambient Temperature
Abstract
1. Introduction
1.1. Related Work
1.2. Innovative Contributions
1.3. Organization
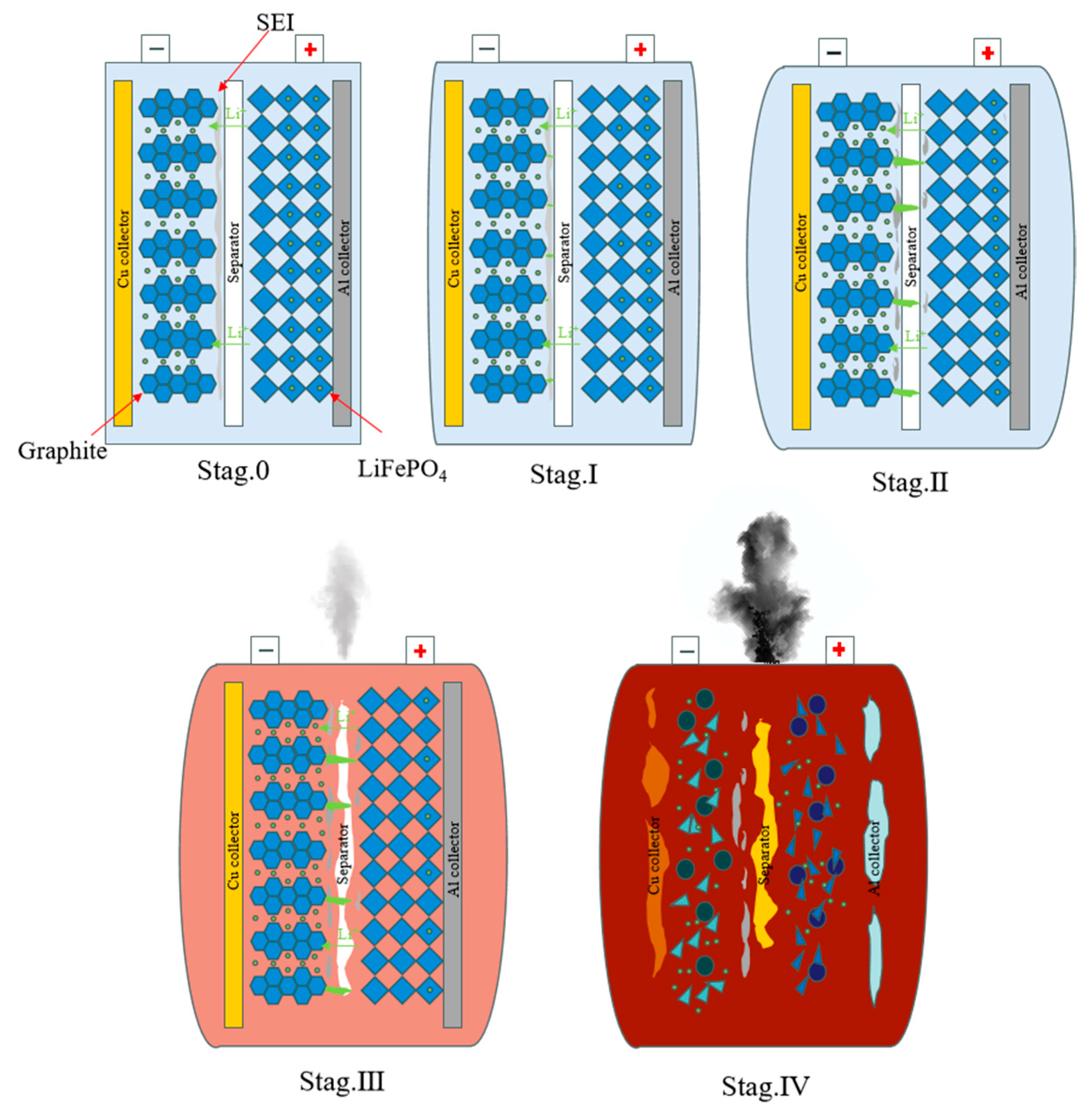
2. Theoretical Analysis of Overcharge-Induced Thermal Runaway in LFP Batteries
2.1. Stage I: Incipient Thermal Accumulation (Initially–80 °C)
2.2. Stage II: Electrochemical Interface Degradation (90–120 °C)
2.3. Stage III: Thermal Escalation (120–150 °C)
2.4. Stage IV: Critical Thermal Runaway (150–300 °C)
3. Experimental Design
3.1. Battery Sample Parameters
3.2. Experimental Device
3.3. Experimental Plan
4. Experimental Analysis and Discussion
4.1. Analysis of Battery State Changes
4.1.1. Changes in Shape and Surface
- Early-stage overcharging-induced deformation showed no ambient temperature dependence.
- Higher C-rates accelerated deformation onset, though deformation alone did not necessarily trigger severe thermal runaway.
- With the exception of Group 1.5C/5 °C (where thermal runaway may have been suppressed by low-temperature effects, as analyzed later), the severity of thermal runaway showed a positive correlation with ambient temperature across experimental groups.
4.1.2. Changes in Battery Mass
4.1.3. Analysis of Battery Thermal Propagation
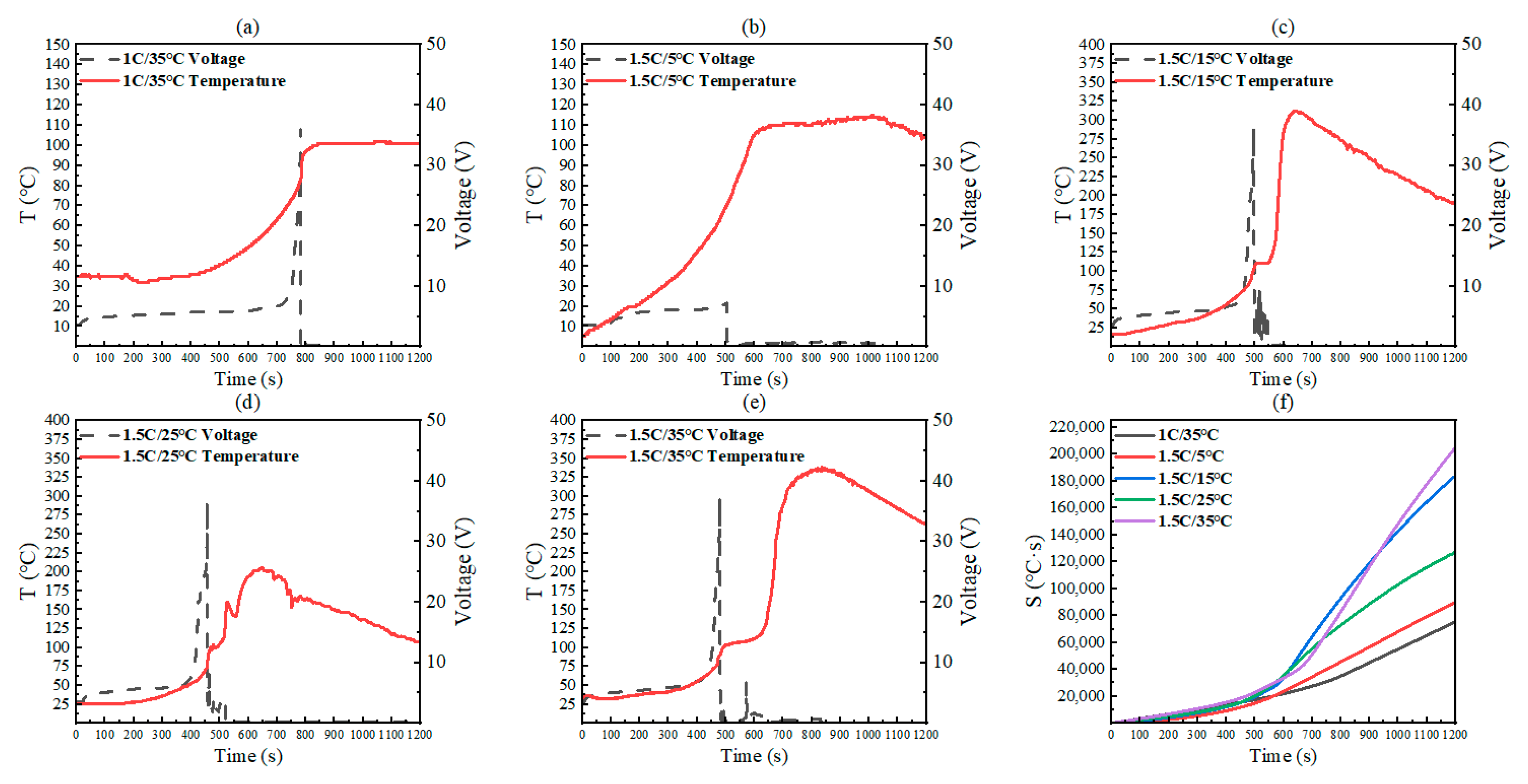
4.2. Analysis of Temperature and Voltage
4.2.1. Changes in Complete Thermal Runaway Group
4.2.2. Changes in Thermal Runaway Interruption Group
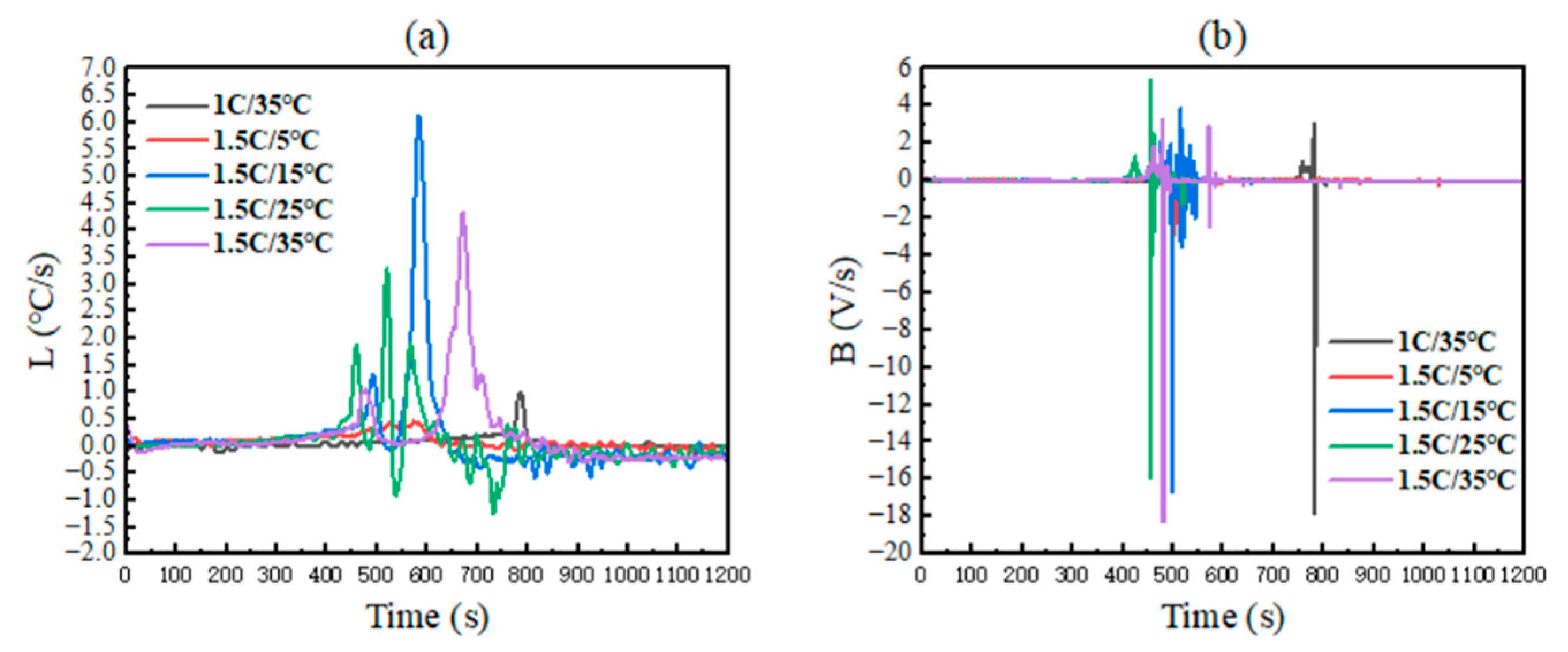
4.2.3. Change Rates of Temperature and Voltage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; He, X.; Ouyang, M.; Wang, L.; Lu, L.; Ren, D.; Santhanagopalan, S. A Coupled Electrochemical-Thermal Failure Model for Predicting the Thermal Runaway Behavior of Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3748. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A Critical Review of Thermal Management Models and Solutions of Lithium-Ion Batteries for the Development of Pure Electric Vehicles. Renew. Sustain. Energy Rev. 2016, 64, 106–128. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Abdulrahman, G.A.Q. A Recent Comprehensive Review of Fuel Cells: History, Types, and Applications. Int. J. Energy Res. 2024, 2024, 7271748. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse Behavior of High-Power, Lithium-Ion Cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.-E. Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1611. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge Investigation of Large Format Lithium-Ion Pouch Cells with Li(Ni0.6Co0.2Mn0.2)O2 Cathode for Electric Vehicles: Thermal Runaway Features and Safety Management Method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, H.-J. Recent Advances in Early Warning Methods and Prediction of Thermal Runaway Events in Li-Ion Batteries. J. Ind. Eng. Chem. 2025, 145, 63–74. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Leising, R.A.; Palazzo, M.J.; Takeuchi, E.S.; Takeuchi, K.J. Abuse Testing of Lithium-Ion Batteries: Characterization of the Overcharge Reaction of LiCoO2/Graphite Cells. J. Electrochem. Soc. 2001, 148, A838. [Google Scholar] [CrossRef]
- Saito, Y.; Takano, K.; Negishi, A. Thermal Behaviors of Lithium-Ion Cells during Overcharge. J. Power Sources 2001, 97–98, 693–696. [Google Scholar] [CrossRef]
- Kang, R.; Jia, C.; Zhao, J.; Zhao, L.; Zhang, J. Effects of Capacity on the Thermal Runaway and Gas Venting Behaviors of Large-Format Lithium Iron Phosphate Batteries Induced by Overcharge. J. Energy Storage 2024, 87, 111523. [Google Scholar] [CrossRef]
- Meng, D.; Wang, X.; Chen, M.; Wang, J. Effects of Environmental Temperature on the Thermal Runaway of Lithium-Ion Batteries during Charging Process. J. Loss Prev. Process Ind. 2023, 83, 105084. [Google Scholar] [CrossRef]
- An, C.; Zhai, J.; Luo, Z.; Lei, Z. Numerical Study on Thermal Runaway of LFP Batteries Triggered by Low Temperature Heating. Fire Technol. 2024, 60, 3927–3947. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Ramadass, P.; Zhang, J. (Zhengming) Analysis of Internal Short-Circuit in a Lithium Ion Cell. J. Power Sources 2009, 194, 550–557. [Google Scholar] [CrossRef]
- Ouyang, D.; Chung, Y.-H.; Liu, J.; Bai, J.; Zhou, Y.; Chen, S.; Wang, Z.; Shu, C.-M. Characteristics and Mechanisms of as Well as Evaluation Methods and Countermeasures for Thermal Runaway Propagation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2025, 108, 101209. [Google Scholar] [CrossRef]
- Ding, S.; Dong, C.; Zhao, T.; Koh, L.; Bai, X.; Luo, J. A Meta-Learning Based Multimodal Neural Network for Multistep Ahead Battery Thermal Runaway Forecasting. IEEE Trans. Ind. Inform. 2021, 17, 4503–4511. [Google Scholar] [CrossRef]
- Feng, X.; Wong, S.K.; Chen, T.; Ouyang, M. An Automatic Identification Method of Thermal Physical Parameter for Lithium-Ion Batteries Suffering from Thermal Runaway. J. Energy Storage 2024, 83, 110358. [Google Scholar] [CrossRef]
- Ren, D.; Liu, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. Model-Based Thermal Runaway Prediction of Lithium-Ion Batteries from Kinetics Analysis of Cell Components. Appl. Energy 2018, 228, 633–644. [Google Scholar] [CrossRef]
- Tang, Z.; Ji, Y.; Yu, P.; Cheng, J. Investigation on the Thermal Management Performance of a Non-Contact Flow Boiling Cooling System for Prismatic Batteries. J. Energy Storage 2023, 66, 107499. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; Ouyang, M.; Zheng, S.; Li, J.; He, X. An Electrochemical-Thermal Coupled Overcharge-to-Thermal-Runaway Model for Lithium Ion Battery. J. Power Sources 2017, 364, 328–340. [Google Scholar] [CrossRef]
- Rao, H.; Huang, Z.; Zhang, H.; Xiao, S. Study of Fire Tests and Fire Safety Measures on Lithiumion Battery Used on Ships. In Proceedings of the 2015 International Conference on Transportation Information and Safety (ICTIS), Wuhan, China, 25–28 June 2015; pp. 865–870. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, G.; Duan, Q.; Chen, M.; Li, Y.; Wu, K.; Liu, B.; Peng, P.; Sun, J. The Efficiency of Heptafluoropropane Fire Extinguishing Agent on Suppressing the Lithium Titanate Battery Fire. Fire Technol. 2016, 52, 387–396. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Chen, H.; Lu, W.; Wang, Q. Experimental Study on the Efficiency of Dodecafluoro-2-Methylpentan-3-One on Suppressing Lithium-Ion Battery Fires. RSC Adv. 2018, 8, 42223–42232. [Google Scholar] [CrossRef] [PubMed]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal Runaway of Commercial 18650 Li-Ion Batteries with LFP and NCA Cathodes—Impact of State of Charge and Overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Mao, N.; Zhang, T.; Wang, Z.; Gadkari, S.; Wang, J.; He, T.; Gao, T.; Cai, Q. Revealing the Thermal Stability and Component Heat Contribution Ratio of Overcharged Lithium-Ion Batteries during Thermal Runaway. Energy 2023, 263, 125786. [Google Scholar] [CrossRef]
- Jie, D.; Baohui, C.; Jiazheng, L.; Tiannian, Z.; Chuanping, W. Thermal Runaway and Combustion Characteristics, Risk and Hazard Evaluation of Lithium-iron Phosphate Battery under Different Thermal Runaway Triggering Modes. Appl. Energy 2024, 368, 123451. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, D.; Wang, Z.; Mou, L.; Ou, M.; Fan, T.; Bi, S.; Zhang, X.; Yu, Z.; Fang, Y. Thermal Runaway Early Warning and Risk Estimation Based on Gas Production Characteristics of Different Types of Lithium-Ion Batteries. Batteries 2023, 9, 438. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.-E. Toxic Fluoride Gas Emissions from Lithium-Ion Battery Fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Chang, C.; Zhang, J.; Ouyang, D.; Qian, X. Charging Rate Effect on Overcharge-Induced Thermal Runaway Characteristics and Gas Venting Behaviors for Commercial Lithium Iron Phosphate Batteries. J. Clean. Prod. 2024, 434, 139992. [Google Scholar] [CrossRef]
- Knott, L.M.; Long, E.; Garner, C.P.; Fly, A.; Reid, B.; Atkins, A. Insights Into Lithium-Ion Battery Cell Temperature and State of Charge Using Dynamic Electrochemical Impedance Spectroscopy. Int. J. Energy Res. 2024, 2024, 9657360. [Google Scholar] [CrossRef]
- Wu, T.; Chen, H.; Wang, Q.; Sun, J. Comparison Analysis on the Thermal Runaway of Lithium-Ion Battery under Two Heating Modes. J. Hazard. Mater. 2018, 344, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Chalise, D.; Jain, A. Experimental and Theoretical Analysis of a Method to Predict Thermal Runaway in Li-Ion Cells. J. Power Sources 2016, 330, 167–174. [Google Scholar] [CrossRef]
- Li, H.; Duan, Q.; Zhao, C.; Huang, Z.; Wang, Q. Experimental Investigation on the Thermal Runaway and Its Propagation in the Large Format Battery Module with Li(Ni1/3Co1/3Mn1/3)O2 as Cathode. J. Hazard. Mater. 2019, 375, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental Study on Thermal Runaway Behavior of Lithium-Ion Battery and Analysis of Combustible Limit of Gas Production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of Multi Factors on Thermal Runaway Induced by Overcharging of Lithium-Ion Battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Wang, D.; Peng, K.; Fu, Y.; Zhu, C.; Yang, Y. Kinetics of Lithium Dendrite Growth in Garnet-Type Solid Electrolyte. J. Power Sources 2021, 487, 229421. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Zhao, S.; Jin, T.; Zhong, M.; Cen, Z.; Gao, P.; Yan, W.; Ling, M. Room Temperature Resistive Hydrogen Sensor for Early Safety Warning of Li-Ion Batteries. Chemosensors 2023, 11, 344. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Z.; Wei, D.; Jiang, X.; Lu, H.; Sun, L.; Tao, F.; Guo, D.; Liu, Y.; Gao, J.; et al. Detection of Micro-Scale Li Dendrite via H2 Gas Capture for Early Safety Warning. Joule 2020, 4, 1714–1729. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Mei, W.; Jiang, L.; Jia, Z.; Cheng, Z.; Sun, J.; Wang, Q. Understanding of Thermal Runaway Mechanism of LiFePO4 Battery In-Depth by Three-Level Analysis. Appl. Energy 2023, 336, 120695. [Google Scholar] [CrossRef]
- Kim, H.; Jung, T.; Jung, J.; Noh, Y.; Lee, B. Accurate Prediction of Electrochemical Degradation Trajectory for Lithium-Ion Battery Using Self-Discharge. Int. J. Energy Res. 2024, 2024, 1758578. [Google Scholar] [CrossRef]
- Chun, H.; Choi, H.; Jun, Y.; Lee, H. Comprehensive Study on Thermal Characteristics of Lithium-Ion Battery With Entropic Heat. Int. J. Energy Res. 2024, 2024, 8815580. [Google Scholar] [CrossRef]



| Name | Parameter |
|---|---|
| Positive electrode | Lithium iron phosphate (LiFePO4) |
| Negative electrode | Graphite |
| Dimensions (length × width × height) | 100 mm × 21 mm × 140 mm |
| Capacity | 25 Ah |
| Mass | 630 g |
| Nominal voltage | 3.2 V |
| Operating voltage | 2.3–3.65 V |
| Discharge temperature | −30–55 °C |
| AC impedance | 0.6–1.5 mΩ |
| Specific heat capacity | 830 J kg−1 K−1 [20] |
| Condition Abbreviation | C-Rate (C) | Ambient Temperature (°C) | Initial State of Charge (SOC) |
|---|---|---|---|
| 100-1.0-35 | 1.0 | 35 | 100 |
| 100-1.5-05 | 1.5 | 05 | 100 |
| 100-1.5-15 | 1.5 | 15 | 100 |
| 100-1.5-25 | 1.5 | 25 | 100 |
| 100-1.5-35 | 1.5 | 35 | 100 |
| Initial | 1C/35 °C | 1.5C/5 °C | 1.5C/15 °C | 1.5C/25 °C | 1.5C/35 °C | |
|---|---|---|---|---|---|---|
| mass | 630.0 g ± 5.0 g | 574.3 g | 580.8 g | 506.1 g | 404.2 g | 506.4 g |
| thickness | 21.0 mm | 58.4 mm | 54.7 mm | 61.1 mm | Explosion | 54.2 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Wang, Z.; Kong, L.; Xu, F.; Dong, X.; Shen, J. Investigating the Thermal Runaway Characteristics of the Prismatic Lithium Iron Phosphate Battery Under a Coupled Charge Rate and Ambient Temperature. Batteries 2025, 11, 253. https://doi.org/10.3390/batteries11070253
Tian J, Wang Z, Kong L, Xu F, Dong X, Shen J. Investigating the Thermal Runaway Characteristics of the Prismatic Lithium Iron Phosphate Battery Under a Coupled Charge Rate and Ambient Temperature. Batteries. 2025; 11(7):253. https://doi.org/10.3390/batteries11070253
Chicago/Turabian StyleTian, Jikai, Zhenxiong Wang, Lingrui Kong, Fengyang Xu, Xin Dong, and Jun Shen. 2025. "Investigating the Thermal Runaway Characteristics of the Prismatic Lithium Iron Phosphate Battery Under a Coupled Charge Rate and Ambient Temperature" Batteries 11, no. 7: 253. https://doi.org/10.3390/batteries11070253
APA StyleTian, J., Wang, Z., Kong, L., Xu, F., Dong, X., & Shen, J. (2025). Investigating the Thermal Runaway Characteristics of the Prismatic Lithium Iron Phosphate Battery Under a Coupled Charge Rate and Ambient Temperature. Batteries, 11(7), 253. https://doi.org/10.3390/batteries11070253






