Abstract
Nickel-rich layered oxides such as LiNixMnyCozO2 (NMC), LiNixCoyAlzO2 (NCA), and LiNixMnyCozAl(1–x–y–z)O2 (NMCA), where x ≥ 0.6, have emerged as key cathode materials in lithium-ion batteries due to their high operating voltage and superior energy density. These materials, characterized by low cobalt content, offer a promising path toward sustainable and cost-effective energy storage solutions. However, their electrochemical performance remains below theoretical expectations, primarily due to challenges related to structural instability, limited thermal safety, and suboptimal cycle life. Intensive research efforts have been devoted to addressing these issues, resulting in substantial performance improvements and enabling the development of next-generation lithium-ion batteries with higher nickel content and reduced cobalt dependency. In this review, we present recent advances in material design and engineering strategies to overcome the problems limiting their electrochemical performance (cation mixing, phase stability, oxygen release, microcracks during cycling). These strategies include synthesis methods to optimize the morphology (size of the particles, core–shell and gradient structures), surface modifications of the Ni-rich particles, and doping. A detailed comparison between these strategies and the synergetic effects of their combination is presented. We also highlight the synergistic role of compatible lithium salts and electrolytes in achieving state-of-the-art nickel-rich lithium-ion batteries.
1. Introduction
Lithium-ion batteries (LIBs) have been a major advance in the energy storage, and in the development of many devices, from portable phones and laptops to electric vehicles (EVs). For the latter application, LIBs fall short of meeting the demand for ever-increasing EV mileage, requiring LIBs with ever-increasing energy density. This energy density is determined by the cathode active material. The more redox potential of this material vs. Li+/Li, the more energy density; thus, a lot of attention has been devoted to the study of cathode materials with a redox potential > 4 V.
The initial commercial success of LiNi1/3Mn1/3Co1/3O2 (NMC333) has been the motivation for continuous research on NMC-based cathodes. In these materials, Ni is the active element, as it can cycle between Ni2+/3+ and Ni3+/4+ redox couples. Mn and Co stabilize the layered structure. Therefore, research has been focused on ways to increase the Ni concentration at the expense of Mn and Co without compromising the layered structure needed to the electrochemical performance. In this context, Ni-rich LiNi1–x–yCoxMyO2 (NMC, 1 − x − y ≥ 0.5, M = Mn or Al) are a promising choice for high-capacity and low-cost cathode materials [,]. They are already utilized in many electric vehicles [], and many efforts are being made to improve their performance [,,,,]. Another family of Ni-rich cathode elements is LiNixCoyAlzO2 with x ≥ 0.8 (NCA). NCA and NMC have related structures, very similar electrochemical behavior, and show similar performance. Both cells of the same size 21700 with NMC and NCA cathodes are available at a capacity of 5 Ah. The current commercial high-Ni cathode materials are LiNi0.8Mn0.1Co0.1O2 (NMC811) and LiNi0.8Co0.15Al0.05O2, i.e., materials with Ni concentration x = 0.8. However, compared to NCA, NMC811 possesses the advantages of lower capital and production cost (simple co-precipitation method without extra heat treatment process), higher energy density, and better thermal stability; thus, it has been the subject of particular attention [,].
Super Ni-rich cathode materials with higher Ni contents (x ≥ 0.85) are currently under study []. Higher Ni concentrations, however, mean aggravated electrode–electrolyte interphase deterioration [], microcrack formation [], and surface rock-salt phase (NiO) formation, which is already a problem when x = 0.8 [,].
The increase in Ni concentration did increase the capacity, but the correlated decrease in the stability of the structure associated with the decrease in Co and Mn concentrations resulted in a large increase in the capacity fading and growth of impedance [,]. In general, NMC-based cathodes with more Mn content tend to possess better cycling stability [,], whereas NMC-based cathodes with more Ni content are incapable of reaching high cutoff voltages due to the lack of Mn4+ as a structure stabilizer [].
The investigation of the thermal runaways of 18650 lithium-ion batteries shows that the safety issues of Ni-rich NMC and NCA are similar []. More precisely, NCA cells react faster than NMC cells above 1 °C min−1 and, thus, reach thermal runaway a little earlier, but react slower than NMC cells below 1 °C min−1 []. The high redox potential of Ni-rich cathode materials is also the cause for their poor thermal and structural stability. Ten years ago, Huggins already warned the community that the partial pressure of oxygen at equilibrium increases with voltage, which accelerates exothermic reactions and causes instability with oxygen release []. Therefore, the utilization of Ni-rich cathode elements is possible only if the material is modified to avoid oxygen release and improve safety, following processes that have been the subject of intensive research since then.
Many efforts have been made to find remedies to this drawback, which were reviewed [,,,,,], including cationic doping and coating of the particles [,,], gradient layers and core–shell structuration [], and electrolyte additives []. The studies have revealed that parasitic reactions [], cation mixing (leading to restructured surface regions) [], oxygen release to maintain the valence equilibrium upon surface transitioning from the layered to the spinel and finally the rock-salt structure of the surface layer [,,,,,,], active material dissolution [], the evolution of surface layers, and surface chemistries during cycling [,] are the primary factors responsible for cathode degradation. Higher energy density with Ni-rich cathodes issues from the higher operating voltage of the lithium battery. The gaseous oxygen released from the cathode will migrate to the anode side, where it will undertake exothermic reactions, eventually enhanced by thermal abuse, resulting in thermal runaway []. The main cause of thermal runaway is the chemical interactions between the cathode and both the anode and the liquid electrolyte []; thus, it is crucial to protect the surface of Ni-rich cathode elements, and/or utilize solid electrolytes. In addition, intergranular cracking can weaken connections between primary particles [,,], and accelerate transition-metal (TM) ion dissolution and migration []. Moreover, the surface chemistry and bulk microstructure engage in a complex interplay closely linked to the electrochemical properties []. Over the years, different reviews have reported the advances in the research on Ni-rich batteries. They include a discussion on the mitigation strategies and trade-offs, such as the selection of dopants or coating materials [], and the design []. An analysis of surface modification techniques, analysis of the manufacturing process, and cost of the surface modification methods has been published few years ago [].
In the present work, attention is focused on the progress that had been made recently to obtain batteries with Li-rich cathode materials with improved energy density and rate capability, without compromising safety, at the laboratory scale. For a report and discussion to bridge the technological gap between basic research on materials and industrial-scale fabrication, while controlling the cost-effectiveness in the process, we guide the reader to a recent review on the advances in multi-scale design and fabrications processes [].
2. Goals and Issues
2.1. Why Decrease the Co Concentration?
The first motivation to reduce the use of cobalt is its scarcity, and thus its high cost []. The Democratic Republic of the Congo (DRC) accounts for 69% of global cobalt production. Russia ranks number two, but its production is ten times smaller than that of DRC [,], and the DRC has the world’s largest reserves of cobalt ore. Refined cobalt products are centralized mainly in China, accounting for 67% of the world’s refined battery-grade cobalt sulfate (CoSO4) capacity in 2020. Other than China, only Finland has significant refining capacity for cobalt materials, accounting for only 10% of total supply in 2020 []. This concentration in a single country, both in production (DRC) and in refining (China), poses a political problem of energy independence to other countries. The United States has taken steps to increase domestic cobalt supplies with the announcement in 2022 of a USD 3 billion investment toward increasing domestic supplies of refined battery metals [,].
Another motivation to decrease the consumption of cobalt comes from its toxicity. Impacts of cobalt-bearing LIB chemistries on the environment are well-documented [,,,,,] and are greater than cobalt-free chemistries []. The results show that the refining process is the major contributor to global warming potential, freshwater ecotoxicity, and carcinogenic and noncarcinogenic emissions. This stems from the diesel fuel used in refining processes that also contributes to ozone depletion []. Moreover, mining contributes significant levels of particulate matter formation.
Due to the high cost and supply difficulties of cobalt, efforts have been made to reduce the concentration of cobalt in Ni-rich cathode materials [,]. Nevertheless, cobalt plays a critical role to ensure thermodynamic stability, so the continued use of cobalt in nickel-based EV batteries is predicted [,], even though continuous progress has been made to decrease its concentration [,,,,]. Liu et al. demonstrated that the chemical and structural stability of the deep delithiated NMC cathodes are significantly dominated by Co rather than the widely reported Mn []. Operando synchrotron X-ray characterization coupled with in situ mass spectrometry reveals that the Co4+ reduces prior to the reduction of Ni4+, which postpones the oxygen release and thermal failure. The results of these authors highlight the difficulty to guarantee the safe operation of Ni-rich/Co-free layered oxide cathode materials, even though Mn4+ can enhance the thermal/crystal stability in NMCs []. The role of Mn in the crystal stability, however, is controversial. Song et al. have recently revealed that the real origin of the stability of Co-free Ni-rich cathodes is Li/Ni intermixing rather than the traditional Mn stabilization mechanism [].
The Ni-rich materials crystallize in the Rm structure. In this structure, Li/Ni intermixing, a problem we will discuss in the next section, is favored by the strong 180° interplane super-exchange interaction [,,,,]. The introduction of the non-magnetic ion Co3+ in the TM layer reduces this super-exchange interaction, so that Co can efficiently reduce the Li/Ni exchange []. For example, Co is still needed in NCA, where it not only reduces the Li/Ni mixing, but also increases the energy density; Co-free LiNi0.95Al0.05O2 has an improved rate capability, but a smaller cycling ability [], the reason for the doping with Al []. LiNiO2 suffers from instability issues that plague the material. Some Co-free ultrahigh-Ni NMC cathodes including LiNi0.90Mn0.10O2 [], LiNi0.90Mn0.05Al0.05O2 [], and LiNi0.96Mg0.02Mn0.02O2 [], exhibit an improved cycle ability compared to the current commercial cathodes, but a lower capacity because the cutoff voltage is lowered. Due to the dissolution tendency of Mn, efforts are being made to obtain not only Co-free, but also Mn-free ultrahigh-Ni NMC cathodes, namely LiNixFeyAlzO2, [] LiNi0.96Mg0.02Ti0.02O2 [,], LiNi0.95Al0.05O2, and LiNi0.95Mg0.05O2 []. All of them, however, suffer from a capacity penalty. On the other hand, Cui et al. have synthesized a LiNi0.93Al0.05Ti0.01Mg0.01O2 (NATM) cathode that delivered a capacity of 221 mAh g−1 with a capacity retention of 82% after 800 deep cycles in pouch full cells []. This cathode exhibits superior electrochemical performance and improved thermal stability with respect to the Co-containing cathode LiNi0.94Co0.06O2 and the NMC LiNi0.90Mn0.05Co0.05O2 cathode. Noteworthy, despite the absence of Co, this NATM shows a negligible degree of Ni/Li mixing.
The strategies that have been implemented to overcome these difficulties with Co-free cathode materials, and open questions that remain to be addressed were reviewed by Bianchini et al. [], and more recently by Ge et al. [].
2.2. Problems Limiting the Electrochemical Performance
2.2.1. Cation Mixing
The radii of Ni2+ (0.69 Å) and Li+ (0.76 Å) are similar, so that Li+ and Ni2+ can exchange positions in a delithiated state through Ni2+ diffusion to the octahedral sites of Li+. This antisite defect can lower spacing between atomic layers in the lattice of the NMC-based cathodes, hinder Li+ movement, and reduce the amount of active Ni and Li. This antisite defect and its negative effect on the electrochemical performance have been extensively studied a long time ago for NMC333, and the problem is the same in the Ni-rich NMCs. In this later case, Cui et al. have shown that this defect is predominantly observed at the surface of the particles [], where it creates a Li+ diffusion bottleneck []. As a result, the rate capability is reduced. The antisite defect also presumably plays a role in the irreversibility in the first cycle, which is related to the irreversibility of the Ni charge state [] and irreversible O3/H1-3 phase transition []. In Ni-rich NMC, Mn4+ can partially be replaced by Ni, which increases the valence of Ni and therefore lowers the possibility of Ni atoms migrating to 3a sites []. Nevertheless, Ni2+ migration still occurs. The origin and the migration of Ni is well-known and well-documented []. The gradual growth of Li vacancies with increasing state of charge leads to easier migration. Moreover, in the high charge voltage, oxygen vacancies are generated, favoring a large Ni migration. Therefore, the tendency of Li/Ni mixing is more severe and frequent with increasing charge state and operating voltage. Using operando neutron diffraction measurements, Wu et al. showed that Li−O−Li configurations resulting from the Li−Ni antisite defect can increase anionic redox activity and promote Ni migrations, particularly at high voltages []. Nevertheless, the degradation of electrochemical performance of Ni-rich cathode materials under overcharging conditions primarily originates from the collapse of the cathode material, the precipitation of oxygen, which mainly occurs on the surface of the primary particles, and the formation of voids in the grain boundary region [].
2.2.2. Phase Stability
Cation mixing can also induce a transition to disordered spinel structure (Fd3m) when LIBs are charged to high voltages (4.8 V) [,,,]. Spinel phases can also be formed on charged cathodes stored at 90 °C for a week []. Investigations on NMC revealed that long term cycling can result in phase changes to an Fm3m structure (cubic rock-salt phase) consisting of Ni2+, Mn2+, and Co2+ [,], an effect also observed with NCA cathodes []. This phase transition is accompanied with a release of O2 into the cell, which can react with electrolytes to generate CO2 and increase the interfacial resistance of the cell []. NMC811 cathodes pretreated with ramping to 4.5 V limited the growth of the rock-salt layer in subsequent cycles by isolating the surface of NMC particles [].
2.2.3. Oxygen Release
Oxygen release is a significant cause of degradation of the Ni-rich cathodes [,]. The mechanisms of the oxygen release of the Ni-rich materials are well-documented []. This release essentially results from the change of the TM-O bond from ionic to more covalent character induced by Li+ extraction, due to the removal of electrons to maintain charge neutrality. In particular, this effect has been clearly evidenced in NCA []. This oxygen release results in a destabilization of the layered structure, which is aggravated in highly delithiated states, and increases with Ni concentration []. Therefore, in practice, the voltage of the batteries with pristine NMC811 is limited at 4.2 V []. Moreover, the oxygen release results in a thermal instability and safety concern []. In addition, the thermal stability is impacted by chemical reactions with the electrolyte. This is illustrated in Figure 1 for the case of NMC84 (LiNi0.84Co0.11Mn0.05O2) charged at 4.3 V in the commonly used carbonate electrolyte with LiPF6 salt (LB301).
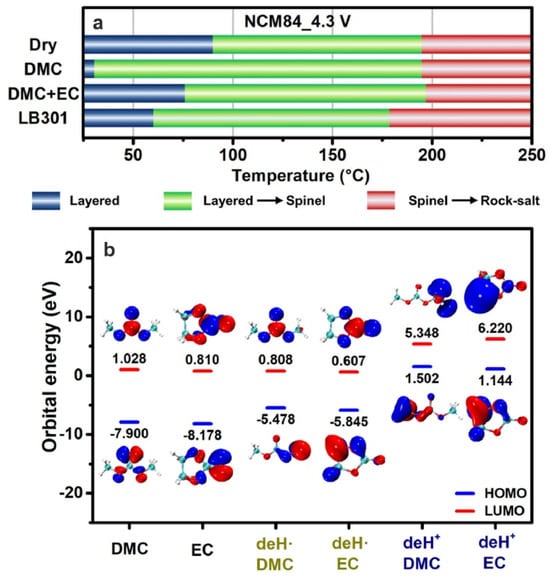
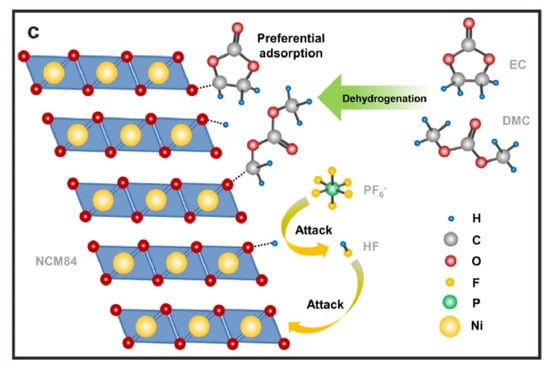
Figure 1.
Temperature regions of different thermal decomposition stages of NCM84_4.3 V samples in different electrolyte components (a). Molecular orbital energy levels of carbonate molecules and their dehydrogenation products (b). Schematic diagram of near-surface structural thermal stability modulated by electrolyte components (c). Reproduced from ref. []. Copyright 2022 Elsevier.
The onset temperature of thermal decomposition is already reduced by the carbonates, in particular dimethyl carbonate (DMC), and further reduced by the introduction of LiPF6. Furthermore, the outward migration of oxygen is accompanied with an outward migration of Ni. This process of Ni and O co-migration along (003) planes was observed, for example, in NMC811 []. This continuous advance of the surface degradation layer toward the particle interior results in an increase of the thickness of the surface inactive rock-salt phase layer, leading to a rapid degradation of the electrochemical properties []. In addition, during the delithiation process, the outward diffusion of Li+ generates a concentration gradient and vacancies in the layered structure []. The smaller variation of the lattice in the Li-rich (vacancy-poor) domains along the c-axis direction than in Li-poor (vacancy-rich) domains results in an anisotropic lattice contraction that may induce microcrack formation at grain boundaries [,].
2.2.4. Modifications During Cycling
NMC oxide possesses a Rm structure (rhombohedral symmetry) with a Li-layer on the 3a site, an NMC layer on the 3b site and an oxygen layer on the 6c site []. X-ray measurements and infrared spectroscopy reveal the deformation of the lattice during cycling [,,]. The c-axis parameter expands during charging, due to the repulsive force generated from MO6 slabs, which are positively charged, whereas the a-parameter is reduced because the radii of the transition metal ions are reduced by the loss of electrons [] and the increase of their valences []. The a-axis of the Ni-rich cathode material remains constant at potentials exceeding 4.3 V, and the steep drop of the c-axis occurs at potentials exceeding 4.2 V, becoming steeper as the Ni content increases [,,,]. This drop is due to the fact that the repulsion between O2− layers decreases with more covalent M–O bonding at higher delithiated states [,]. Two processes are involved in the delithiation of Ni-rich NMC-based cathodes. Until a certain degree of delithiation, Li can exit through oxygen dumbbell hopping, while at higher degree of delithiation, Li can exit through tetrahedral site hopping [,].
During cycling, Ni2+/Ni4+ mainly reacts in the low-voltage region and Co3+/Co4+ mainly reacts in the high-voltage region. As Li+ is removed from the Li slab during charging, the Ni-rich cathode undergoes the successive phase transitions H1-M-H2–H3, due to change in the repulsive force in O-Li-O caused by local structural distortion []. The H2–H3 phase transition is the most problematic for the electrochemical performance, because it is accompanied with an evolution of oxygen in the lattice and large volume changes. These changes can cause microcracks in the cathode active material, an additional exposure of the cathode active material surface to the electrolyte []. Both effects result in reduced cycle ability [], and rate capability [] (see Figure 2). This problem is particularly important in Ni-rich cathode materials as the onset potential corresponding to the H2–H3 phase transition decreases when the Ni concentration decreases, from 4.6 V vs. Li+/Li in NMC111, to 4.2 V in NMC811. While we have explained in a previous section (Section 2.1) the current efforts to reduce the cobalt concentration, it should also be mentioned that cobalt has also adverse effects. The addition of Co to Ni-rich layered structure can cause a more serious H2–H3 phase transition when charging and discharging between 2.8 and 4.5 V [,]. Moreover, the structural instability of the Ni-rich cathode materials is strongly correlated with the cell gas release in a charged state. That is why, even though adequate strategy (coating, doping) reviewed hereunder is efficient to reduce the instability, the practical voltage for charging is limited to 4.5 V [], inasmuch as at such a high potential, the formation of Ni/Li antisite is unavoidable. The main attention on the Ni-rich cathodes these recent years has thus been focused on the study of these degradation mechanics and the solutions to avoid them [,,,]. Otherwise, operando X-ray diffraction (XRD) and scanning electron microscopy (SEM) results indicated that particle fracture from increased structural distortions at >4.3 V was a contributor to capacity fade [].
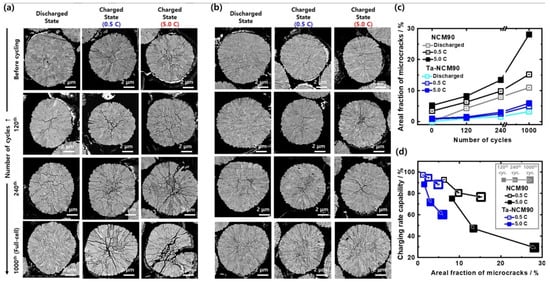
Figure 2.
Cross-sectional SEM images of the (a) NCM90 and (b) Ta-doped NCM90 cathodes at fully discharged (2.7 V) or charged state (4.3 V, at 0.5 or 5.0 C) depending on number of cycles. (c) Areal fraction of microcracks in cathodes plotted against number of cycles. (d) Charging rate capability of cathodes with respect to areal fraction of microcracks at 120th, 240th, and 1000th cycles. Reproduced from ref. []. Copyright 2024 Wiley.
2.2.5. Microcracks
The capacity degradation of Ni-rich NMC is not only due to surface chemical instability, but also to mechanical destruction in the form of microcracks [,,,,]. The H2 → H3 transition is not the only source of microcracks. As a matter of fact, different sources of cracking may affect Ni-rich cathode materials: intragranular cracking may occur under the effect of cation mixing [], while the H2–H3 phase transition is mainly responsible for intergranular cracking. In addition, the antisite defect can also be formed during cycling, and cause a structural transition from rom the layered phase to the spinel-like phase [], so that both the rate capability and the cycle ability are reduced. In addition, the cracking exposes fresh surfaces to the electrolyte, and thus reduces the efficacy of protective surface. It is thus crucial to avoid the cracking of the particles, which has been the subject of intensive research.
The cracking process is initiated by the anisotropic volume change in primary particles and lattice strain, which provokes the mechanical destruction of secondary particles [,,]. Differences in the lattice volume of domains with different Li+ concentrations inside a primary particle induce the localization of stress between the domains, which is likely to create intraparticle microcracks along (003) planes [,,,]. These effects explain that microcracks are generated by the inhomogeneous distribution of Li, so that their formation depends on the state of charge of the cathode []. Note that we consider here electrochemical crack formation that occurs mainly at high state-of-charge (SOC) levels. Another mechanism of chemical crack formation has been recently evidenced at low SOC levels, driven by chemical corrosion [], but this phenomenon is observed at high temperature (~60 °C). The inhomogeneity of the lithium distribution during cycling has been observed in NMCs by operando optical microscopy []. The evolution of the stress associated with the anisotropic Li distribution has been studied as a function of the state of charge in different works [,,]. Combined with lattice strain, the anisotropic volume change results in both intergranular and intragranular cracking [,,,,]. When the Ni concentration is ≤0.6, only one phase transition H1 ↔ M phase is observed upon cycling [,]. Unfortunately, the cracking effects are more important in Ni-rich NMC at Ni concentration x ≥ 0.8, since the structural phase transitions are more severe []. During the first charge–discharge cycle, a series of phase transition processes increase interlayer spacing, including H1–M and M–H2 transitions, followed by a shrinking of the interlayer spacing during the H2–H3 phase transition process, which is most damageable to the integrity of the particles [,,,]. The local stress distribution associated with the anisotropic lattice contraction responsible for the structural instability caused by the H2–H3 transition in the deeply charged state can be overcome by the synthesis of single-crystal Ni-rich cathode elements [,]. Consequently, monocrystalline NMCs demonstrate a significantly better cycling ability than polycrystalline NMCs []. Even the morphology of the precursors matters. Liang et al. demonstrated that the spheroidization of the precursor can enhance uniformity in structural evolution during solid-phase lithiation [] (see Figure 3). The resulting LiNi0.92Co0.04Mn0.04O2 cathode material delivered a capacity of 234.2 mAh g−1, in the range of 2.7–4.3 V, with capacity retention of 89.3% after 200 cycles at 0.5 C. Ni-rich cathode materials with different morphology structures obtained by adjusting the ratio of solvent and water in the solvothermal process []. This work illustrates the impact of different morphologies on the electrochemical properties. Coating is also a pertinent strategy, because the surface of Ni-rich materials free from electrolyte attack undergo limited structural degradation []. Morphologies of the primary particles of Co-containing Li[NixCoy(Al or Mn)1–x–y]O2 cathodes can be engineered to optimize the electrochemical properties [,]. For example, ultrafine-grained structures in the Li[Ni0.95Co0.04Mo0.01]O2 cathode provided paths deterring the propagation of intergranular cracks, allowing for high-capacity retention and fast-charging capability [].
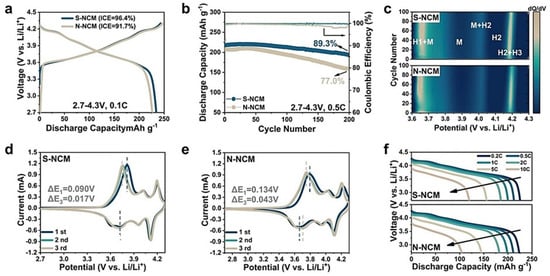
Figure 3.
Electrochemical characterization of coin-cells at 25 °C with LiNi0.92Co0.04Mn0.04O2 cathode materials derived from spherical precursors (S-NCM) and non-spherical precursors (N-NCM). (a) Initial charge–discharge voltage profiles of S-NCM and N-NCM. (b) Cycling stability of half-cells for S-NCM and N-NCM versus Li+/Li between 2.7 and 4.3 V at 0.5 C. (c) dQ/dV curves of S-NCM and N-NCM at charge process determined from every five cycles. CV profiles of the S-NCM (d) and the N-NCM (e) at the 1st, 2nd, and 3rd cycles. (f) Discharge profiles of S-NCM and N-NCM at different rates. Reproduced from ref. []. Copyright 2024 Wiley.
Wang et al. introduced a coherent perovskite phase into the layered structure functioning as a ‘rivet’, which significantly mitigates the pernicious structural evolutions by a pinning effect. The resulting enhanced mechanochemical stability was attributed to the presence of significant energy barriers for phase transitions within the rivet phase, similar to a pinning effect []. The gas release pattern and stress distribution of NCM particles can be governed by the microstructure engineering []. In particular, the strain between the grains in the NMC811 secondary particle can be dissipated by radially aligned grains near the particle surface. However, the mismatched strain between disoriented primary grains in the interior of the particle leads to the development of microcracks in the central region of the NMC811 secondary particle []. The electrolyte penetrates in the interior of the particles via these microcracks, resulting in the formation of NiO rock-salt phase that reduces the Li+ interfacial resistance []. On the other hand, the electrolyte infiltration shortens the Li+ diffusion distance []. As a result of the balance between these two effects, the Li+ diffusion kinetics has been found accelerated in polycrystalline cathodes with respect to single-crystal cathodes without grain boundaries []. In addition, typical structural defects of primary particles such as twin boundaries [], dislocations [], or even Li+/Ni2+ anti-site defects [] serve as preferred sites for the nucleation and propagation of intragranular microcracks. In single-crystallized particles, the tensile stress triggers gliding along the (003) plane. This effect has been observed by through aberration-corrected scanning transmission electron microscopy (STEM) and analyzes by theoretical calculations []. These planar gliding processes corresponds to the in-plane migration of Ni ions, the barrier energy of which is reduced in presence of oxygen vacancies []. Since these processes are not entirely reversible, they also lead to microcracking along the (003) plane in single-crystallized particles. However, Sun et al. showed that the destructive volume change and H3 phase formation presented in polycrystals are effectively suppressed in single-crystals when cycling at a high cut-off voltage of 4.6 V []. This feature clarifies the origin of high-voltage stability in single-crystal cathode, but also the improved cycle ability with respect to polycrystals.
3. Synthesis Methods
A review on the synthesis process has been recently published []. Different synthesis routes give good results, including hydrothermal process [,,], sol–gel process [,], and solid-sate process [,]. The sol–gel method offers some advantages. In particular, Zheng et al. successfully synthesized single-crystal Ni-rich cathode materials via a two-step solid-state method [], but the preparation and refinement of raw ingredients required is still difficult. That is why the co-precipitated method is widely used for the current commercial synthesis of NMC Ni-rich materials by stirring and mixing the stoichiometric ratio of transition metal salt solutions to form homogeneous precursors []. Among various synthetic methods for NMC materials mentioned above, the co-precipitation method is the recognized as one of the most effective processes for preparing Ni-rich cathode materials with the best electrochemical performance [,]. This method, however, is difficult to use for NCA because Al3+ ions have a propensity to form double hydroxides in solution. Therefore, alternative solutions have been adopted for NCA, namely the hydrothermal method, which is complicated and not cheap, or the solid-state method, which has been reviewed by Wang et al. []. However, a recent comparison of the electrochemical properties of LiNi0.8Mn0.15Al0.05O2 prepared by co-precipitation and sol–gel methods highlights the superior crystallinity, particle uniformity, and compositional accuracy of coprecipitation, making it more effective for enhancing electrochemical stability and efficiency, despite the presence of Al []. The common and commercialized method is to prepare precursors via co-precipitation first, and then subject it to high temperature calcination with lithium salt together. Efforts have been made to optimize the synthesis parameters [], including the calcination temperature [,], lithium source type [,], and calcination atmosphere [].
The influence of the precursors on the electrochemical performances of Ni-rich layered oxides has been discussed by several workers. Cui et al. [] investigated the effect of cationic uniformity in the precursors on Li/Ni mixing of Ni-rich layered cathodes. The cationic uniformity in precursors is regulated by the sol–gel and solvothermal method. All cations in the precursors obtained by the sol–gel method mix uniformly at a molecular scale, while the solvothermal method with a post-addition of Li salts causes a heterogeneous mixture between Li and transition-metal ions. Therefore, advanced Ni-rich layered cathodes should simultaneously possess low Li/Ni mixing and reversible H2–H3 transition.
The conventional precursor for the synthesis of NMC materials is Ni1–x–yCoxMy(OH)2 particles, prepared by co-precipitation in a mechanically stirred tank reactor, under conditions that lead to a homogeneous elemental distribution [,,,,]. This is considered as the most viable option for large-scale production [,,,]. The effects of the β-(Ni0.6Mn0.2Co0.2)(OH)2 content in hydroxide precursor on the structure and electrochemical performance of NCM622 cathode materials were investigated by Xu et al. []. The cathode material sintered from the mixed phase of α-(Ni0.6Mn0.2Co0.2)(OH)2 and β-(Ni0.6Mn0.2Co0.2)(OH)2 shows the highest discharge capacity of 192 mAh g−1 and best rate performance.
The microstructures of Ni1–x–yCoxMy(OH)2 particles are crucial to obtain Ni-rich cathodes with the best electrochemical properties, and many works have been devoted to optimize them, reviewed in []. These microstructures depend on various factors, including the pH value [,,], ammonia concentration [,] (see Figure 4), reaction temperature [], reaction time [], and stirring rate []. Depending on these different parameters, different morphologies of the precursor particles are obtained, including microspheres [,,], rods [,,], and hollow spheres []. Among them, the spherical Ni1–x–yCoxMy(OH)2 particles form spherical LiNi1–x–yCoxMyO2 after lithiation, a shape that form a more tightly packed structure during battery manufacturing. As a result, the volume energy density is increased, and the Li+ ion migration is facilitated [,].
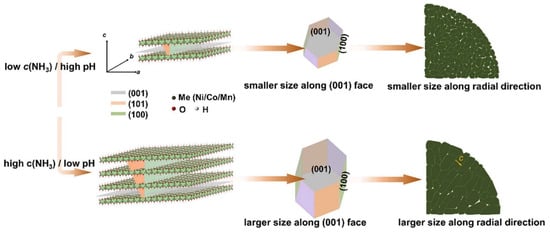
Figure 4.
Schematic illustration of growth of grains synthesized at different pH at different ammonia concentration in precipitation process. High ammonia concentration induces crystal growth along the direction perpendicular to [001] and the direction parallel to [001]. With the maximized face along the (001) crystal face and proper size along the (100) crystal face, a precursor with radially ordered structure is easily formed. Reproduced from ref. []. Copyright 2024 Elsevier.
These examples illustrate that the morphology of the particles is important. In particular, the role of ammonia concentration in determining the particle shape and size of Ni-rich cathode materials during co-precipitation has been recently deciphered by Zhang et al. Moreover, they showed that ammonia concentration affects the thickness-to-length ratio of the precursor’s primary particles, which in turn influences the morphology of the LiNi0.95Al0.05O2 cathode materials during lithiation []. Lower NH4OH/metal ratios produce more porous precursors, which enhance Li-source diffusion and promote uniform sintering []. Pristine NMC811 nano-cubic microstructure is successfully synthesized via a rapid precipitation assisted with hydrothermal treatment, which maintains a high capacity (188.2 mAh·g−1) with a retention of 87.2% at 1 C after 200 cycles, a remarkable result that shows how important the synthesis of nano-sized and single crystallized particles is []. We will return to morphology aspects later in this review, when they are monitored by doping, for example.
The lithiation process consists in the calcination of Ni1–x–yCoxMy(OH)2 with a lithium source (Li2CO3 or LiOH) at high temperature and in an oxygen atmosphere [,,,,]. New synthesis processes have recently emerged, such as the synthesis of Ni-rich gradient material by Taylor–Couette cylindrical reactor [], and the synthesis of single-crystallized samples by microfluidic co-precipitation []. The lithium salt used as a precursor is usually Li2CO3 or LiOH·H2O. Li2CO3 has lower corrosion, and it is suitable for NCM523 and NCM622. Unlike these, preparation of NMC811 should be calcined under the oxygen atmosphere and lower temperature, so the more reactive LiOH·H2O is the best option. Oxygen partial pressure during the calcination process is also an important parameter [,].
The sintering temperature is a crucial parameter. A too-small sintering temperature fails to provide enough energy for the decomposition of the precursors. The high sintering temperature is needed to obtain a good crystallinity, which is essential to the electrochemical performance of the cathode material. However, the high sintering temperature has two negative effects. First, as Li is volatile, some Li evaporates. This problem can be solved by the addition of excess Li during the high-temperature calcination process, which must be adjusted to obtain a stoichiometric final product [,]. The consequence is an excess of lithium at the surface. However, a dry cobalt hydroxide coating effective removes the residual lithium, as shown by Kim et al. in the particular case of NMC91 []. The second drawback is more challenging; this high temperature makes almost inevitable the cation mixing between Ni and Li [], which is detrimental to the electrochemical performance. In practice, above 780 °C, the cationic mixed degree increases []. Therefore, 750 °C is the best calcination temperature for NMC811 []. However, the synthesis temperature of single-crystal structures is usually about 100 °C higher than that of polycrystals [], implying more severe Li/Ni cationic disorder in single-crystal structures than in polycrystalline structures [,]. Post-process (i.e., heat-treatment after washing) is commonly used, as it improves the cycle life of Ni-rich materials [].
To minimize Ni2+ migration, researchers also reported that mitigation methods can be used to modify cathodes []. Luo et al. have recently reported an ion-exchange method to prepare Ni-rich layered oxide materials, consisting in the choice of sodium-based layered oxides as precursors, for ion exchange with Li+ ion in lithium molten salts []. The reaction temperature in this process is reduced to 300 °C. However, additional heat treatment at 700 °C was essential for obtaining well-ordered layered structure. Nevertheless, Ni-rich cathodes synthesized by sodium-to-lithium-ion exchange are devoid of Ni∙Li antisite defects []. In a different approach, Wang et al. reported that the Li/Ni disorder can be facilely tuned through post-synthesis annealing []. Recently, Mo et al. adopted a certain amount of H2O2 to treat the precursor of spherical Ni0.8Co0.1Mn0.1(OH)2. the H2O2 pre-oxidation reduced Li+/Ni2+ mixing to below 0.34%, extending the capacity retention of NMC811 to 98.5% over 100 cycles []. However, this does not exempt us from using the usual means to reinforce the structural and thermal stability of these materials, such as doping or coating.
These results illustrate the major influence of the precursors on the electrochemical properties. The shape, size distribution, and primary/secondary particle structure of the hydroxide precursors directly translate to the final cathode material, strongly affecting Li-ion diffusion and electrode kinetics. Co-precipitation parameters (pH, temperature, stirring, ammonia feed rate) are crucial—altering them changes nucleation and growth, which in turn impacts particle uniformity and tap density. Solid vs. polycrystalline vs. single-crystal morphologies in precursors yields cathodes with starkly different cycling stability and crack resistance. The precursor’s crystallinity and defect landscape steer the final oxide’s structural integrity. Poor crystallinity can promote lithium–nickel mixing, which undermines capacity and cycle life. As we shall see in the next section, incorporating dopants or surface modifiers during precursor formation—like tungsten (W), Al, or rare-earth ions—can significantly enhance cycle life, mitigate microcracking, and stabilize surface layers.
3.1. Single Crystals Versus Polycrystals
Many works demonstrated that polycrystals have a significantly weaker structural stability than single crystallized Ni-rich particles [,,]. This explains that many efforts have been made to synthesize single-crystallized Ni-rich particles, reviewed in []. In particular, Liu et al. reported that heavily cycled commercial-grade pouch cells with NMC811 cathodes showed very little microcracking when single particles are employed []. Through comparative experiments, Kong et al. found that single-crystal NMC811 particles have better storage performance than their polycrystalline counterparts []. They also demonstrated that the concentration of impurities generated on single-crystal materials is also significantly less, and the electrochemical performance can be better maintained. Lüther et al. studied the electrochemical properties of single- and poly-crystallized NMC811 synthesized with comparable bulk and surface characteristics from identical self-synthesized precursors. This study also demonstrated the superiority of single-crystallized particles []. Qian et al. showed that single-crystal NMC622 particles exhibited remarkable cycling stability, and retained ~94% of the initial capacity after 300 cycles at 1 C rate (from 161 to 151 mAh g−1), significantly higher than that of the polycrystalline NMC622 under the same condition (~52%, from 155 to 82 mAh g−1). This result was attributed to the limitation of the Li-ion transport across neighboring primary grains in poly-crystallized particles, as these grains are not perfectly aligned, while there is no such misalignment effect in single-crystal particles []. Fan et al. also reported that single-crystallization of LiNi0.83Co0.11Mn0.06O2 particles mitigate undesired side interactions and significantly prevent the generation of intergranular cracks, to achieve a capacity retention of 84.8% at 45 °C after 600 cycles at a current rate of 1 C []. For the same reason, in full cells between 3.00 V and 4.35 V, LiNi0.83Co0.10Mn0.07O2 delivers a reversible capacity of 167.0 mAh g−1 with capacity retention of 84.8% after 400 cycles at 0.5 C/25 °C, far larger than 107.7 mAh g−1 and 54.1% of conventional polycrystalline LiNi0.83Co0.10Mn0.07O2 []. Still, these results can be improved by appropriate interfacial modification. Examples with single-crystal NMC811 are the in situ planting of an electrolyte film-forming additive on a single-crystal NMC811 surface by consuming the residual lithium to develop a functional Al(Li)BOB nano-layer [], and surface spinel-phase modification []. On the other hand, Ge et al. reported sluggish kinetics in single-crystals governed by ionic transport at low state of charge, which, however, can be alleviated through synergistic interaction with polycrystals integrated into a same electrode [].
Therefore, the single crystallization is an important path to avoid cracking and ensure that high-nickel cathode materials have both high energy density and long cycling stability [,,]. This is clearly evidenced, for instance, in the work of Zhao et al. [] on NMC622. Within operando X-ray imaging, spectroscopic technologies, and diffraction methods, Zhang et al. observed that phase transitions from homogeneity to heterogeneity during cycling induce particle crack formations at the surface, and play a dominant role in the structural robustness of single crystals. They also demonstrated that using a lithium source to replenish lattice sites during re-calcination under oxygen flow and high-temperatures (denoted as t-NCM) can induce homogeneous phase distribution, enhancing the capacity retention []. In addition, Hu et al. found that at high potentials, the amount of oxygen gas released in single-crystalline Ni-rich cathode materials is significantly lower than in polycrystalline cathode materials []. With NMC cathodes, single crystallization avoids cracking during cycling, but only when the Ni content does not exceed 90% []. Also, the study of cobalt-free polycrystalline (PC) and single-crystalline (SC) LiNi0.9Mn0.1O2 showed that the PC material has lower Li+/Ni2+ mixing and a faster Li ion diffusion rate. As a consequence, intergranular cracking, surface pulverization, disorder phase transition, and interfacial side reactions were suppressed in the PC material but not in the SC material []. These two last results show that a Ni content greater than 90% is a bottleneck in improving the energy density and cycling performance of high-nickel single-crystal materials [,]. On the other hand, the study of single-crystallized and poly-crystallized LiNi0.76Mn0.14Co0.1O2 cathode materials evidenced that the gas evolution of single crystals is much lower than that of polycrystals even at high voltage potentials []. Therefore, the single crystallization of Ni-rich cathode materials is also important to solve the safety issues of these batteries. Single-crystal NMC811 demonstrated 98% capacity retention with no cracking after 1100 cycles []. Huang et al. [] prepared single-crystal Li(Ni0.9Co0.05Mn0.05)O2, in which an extra pulse high-temperature sintering at 1040 °C for 1 min is added in the traditional calcination process at 750 °C, which increased the tap density by 1/3 to 2.76 g cm−3 and suppressed the microcracks, improving both the cycling performance and thermal stability. High-temperature sintering is the most effective synthesis strategy for single-crystal materials, but a sintering temperature exceeding 830 °C can lead to undesirable outcomes, such as Li/Ni mixing and surface reconstruction of the cathode particles. The work of Huang et al. shows that this drawback can be avoided by reducing to the pulse-level the time during which higher sintering temperature is applied. In addition, high tap densities are obtained with small particles, more prone to failure during the cycling process. The work of Huang et al. also demonstrates a way to balance the tap density of the material with the selective failure of smaller particles, without the need of incorporating a mixture of polycrystalline and monocrystalline particles proposed by Li et al. []. In addition, the orientation of the crystals matters. For example, CeO2 with well-oriented (011) facets have a shorter linear Li+ migration path and higher electron conductivity, so that they perform better when coated on NMC811 than the (124) and (311) orientations []. Single-crystalline-based electrodes show significantly improved cycling ability because the smaller size of the particles result in a more uniform current distribution in the cathode, whereas in the case of polycrystalline electrodes, inert dead zones are caused by large microcracks []. The single-crystal architecture can effectively retard the layered to spinel and rock-salt phase transition and mitigate the crack formation []. The comparison of polycrystalline and single-crystal cathodes has been reviewed [,,], and demonstrates that the single-crystallization is one of the most prospective routes for the modification of Ni-rich layered cathodes, in particular when the synthesis parameters are chosen to obtained particles with optimum micro-size []. The main drawback is the lower redox kinetics of the single-crystal cathodes. The Ni3+ oxidizes to Ni4+ all over the course of delithiation in polycrystals, but only at the end of delithiation in single crystals []. The sluggish redox kinetics at low state of charge leads to an abrupt reduction in Li+ diffusivity, responsible for responsible for the low Coulombic efficiency and poor rate capability of single-crystal cathodes [,]. On the other hand, the single-crystal cathodes are more resistant to mechanical damage during calendar aging []. Since the oxygen release occurs mainly near the surface, the small surface area of single crystals significantly limits the activation of oxygen vacancies, which not only improves the electrochemical properties, but also enhances thermal stability. This has been evidenced in NMC811 [,]. Single crystals give bigger particles than polycrystals, which tends to give cathodes delivering a capacity slightly smaller than their polycrystalline counterparts, but this drawback can be easily overcome by doping [].
3.2. Synthesis of Single Crystals
Single crystals have a clear advantage to improve the electrochemical properties, but their synthesis is complex and expensive []. Recently, the difficulty has been circumvented by using a facile ball-milling method to transform pristine micron-sized polycrystalline NCM particles into submicron pseudo-single-crystal particles []. The co-precipitation method is widely used to prepare the precursors for both the polycrystals and the single crystals. In addition, the spray-drying method is of interest for the synthesis of the precursors for single crystals, because the porous precursor caused by solvent volatilization is conducive to the fusion and growth of grains, allowing for the synthesis of single crystals the single crystals at a lower operating temperature (e.g., 750 °C) []. In addition, pores inside Ni-rich cathode materials can effectively reduce stress accumulation caused by anisotropic lattice expansion and shrinkage, and decrease the generation of microcracks []. Indeed, the synergetic effects of porosity and radial structure significantly improve the electrochemical performance of Ni-rich cathode materials []. Precursors with larger specific surface area exhibit smaller pore size, ensuring the better wettability and enhanced capillarity for homogeneous lithiation reaction []. The generation of meso- and macropores can be alleviated by controlling the homogeneity of the synthetic reaction []. At present, the sintering processes for single-crystal cathode materials include continuous high-temperature sintering, pulse high-temperature sintering, and molten-salt sintering [,,] (see Figure 5). For high-temperature synthesis, higher calcinating temperature is required, bringing aggravated particle agglomeration and Li/Ni mixing. On the other hand, the low synthesis temperature used in the synthesis of polycrystals is damageable to the mechanical properties. As a consequence, the particles on the upper layer of the electrode may be damaged by the pressure applied during the manufacturing []. This destruction of the polycrystalline particles during the calendaring process in the manufacturing of the cathode was also observed by Raman spectroscopy measurements []. As an example of the performance achieved with the high-temperature synthesis, single-crystal LiNi0.83Co0.10Mn0.07O2 in full cells between 3.00 and 4.35 V delivers reversible capacity of 167 mAh g−1 and capacity retention of 84.8% after 400 cycles at 0.5 C []. With a low-temperature synthesis (sintered at 750 °C with 50% Li-excess), NMC811 exhibits a discharge capacity of 178.1 mAh g−1 with the capacity retention of 95.1% after 100 cycles at 1 C []. As for molten-salt synthesis, modifications on the conventional tunnel furnace to prevent an explosive spillover of hot liquid, additional salts as assisted solvent, and water-washing process to remove the salt residues are explored. However, the water-washing process itself may degrade the electrochemical properties, so additional treatments such as heating and coating are undertaken [,]. However, Hu et al. have shown that washing NCA with ammonium dihydrogen phosphate (NH4H2PO4) solution simultaneously enhances electrochemical performance and air stability, due to an in situ generated Li3PO4 coating layer []. Therefore, a lot of attention is devoted to the fabrication of single-crystallized Li-rich cathode elements []. The molten-salt-assisted method using low-melting-point carbonates was used to synthesize single-crystalline NMC622 delivering 174 mAh g−1 at 0.1 C, 3.0–4.3 V, with capacity retention of 87.5% after 300 cycles at 1 C []. In addition, this molten-salt-assisted method exhibits its effectiveness in directly regenerating NMC622 from the spent material. Single-crystal NMC811 prepared by molten-salt process still delivered a capacity of 80.7 mAh g−1 after 300 cycles at the high rate of 5 C, which corresponds to a capacity retention of 62.0% []. The infusion of molten salts to protect grain boundaries of LiNi0.5Mn1.5O4 increase the capacity of this cathode element from 89.5 to 133 mAh g−1 at 2 C, 50 °C []. Wu et al. have recently reported the important relationship between the precursors, lithium salts, sintering atmospheres, sintering temperature, and sintering procedure to be used to obtain such materials [], including LiNiO2 []. Insight on calcination and sintering of these materials can be found in [,]. The aspects of precursor orientational growth, crystal morphology optimization, and composite structure design can be found in [].
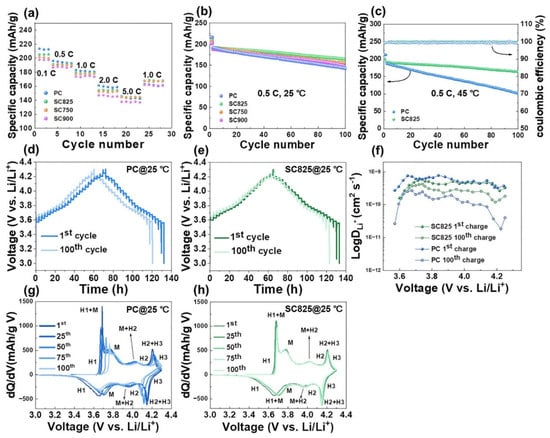
Figure 5.
Electrochemical properties of polycrystalline (PC), and single-crystal (SC) LiNi0.92Co0.04Mn0.04O2 synthesized via LiCl-NaCl molten-salt method at different temperatures (750, 825, and 900 °C). (a) Rate capabilities of PC, SC750, SC825, and SC900 cathodes (RT). (b) Cycling performance of PC, SC750, SC825, and SC900 cathodes at 0.5 C (RT). (c) Cycling performance of PC and SC825 at 0.5 C (45 °C). (d,e) GITT curves of PC and SC825 cathodes during charge/discharge process and (f) corresponding diffusion coefficient. (g,h) dQ/dV curves of PC and SC825 cathodes, respectively. Reproduced from ref. []. Copyright 2024 under Creative Commons CC BY license.
4. Coating
To improve the thermal stability and structural stability, surface modifications of the Ni-rich materials is a predominant strategy []. Surface coating is a common process to avoid the presence of residual alkalis, such as LiOH and Li2CO3 at the surface of the particles. These residual alkalis are one of the dominant reasons for the phase change and electrochemical degradation []. In addition, the coating also protects them against side-reactions with the electrolyte. It is difficult to compare the results obtained with different coating materials, because the efficiency of the coating depends not only on its chemical formula, but also on the synthesis method. Han et al. demonstrated this effect by comparing two different synthesis approaches, wet chemical (WC) coating and atomic layer deposition (ALD), for alumina coatings on LiNi0.5Mn0.3Co0.2O2 []. They showed that the ALD method leads to a better initial quality of the coating layers and is less influenced by the post-coating annealing process, while for WC method the high-temperature treatment is highly needed. Cheng et al. utilized ALD coating and annealing protocol to demonstrate the individual and coupling effects of surface coating and grain boundary engineering on cycling stability of NMC811. This engineering allowed them to achieve superior cycle stability even upon high voltage cycling (91% retention after 200 cycles at 2.7–4.7 V at C/3 rate) []. Moreover, the electrochemical performance of the materials depends on the synthesis process and synthesis parameters described above, and also on the morphology. That is why we prefer, when possible, to evaluate the performance by comparing the results reported for coated and uncoated samples in the same work, since the coating is the only parameter that makes a different in this case.
4.1. Li-Free Surface Layers
Carbon coating is commonly used for all kinds of active particles for cathodes of lithium batteries, and it has been also applied to Ni-rich cathode materials with success [,,]. Different forms of conductive carbon have been utilized, including composites such as graphene nanosheets with NMC811 [], carbon nanotubes with NMC532 [,], and interwoven carbon fibers with NMC622 []. Density functional theory calculations (DFT) play a crucial role in screening dopants (e.g., Al, Mg, Zr) that can stabilize the Ni-rich structure and reduce cation disordering. It also models interactions with protective coatings such as LiNbO3 and Al2O3, helping design surface modifications that enhance stability and minimize electrolyte decomposition. Ning et al. prepared uniform reduced graphene oxide (rGO)-wrapped NMC811 with the help of a semi-conductive perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) layer, ensuring the adhesion of graphene on the particle surface []. Poursalehi et al. employed the scalable electrophoretic deposition (EPD) procedure to make instantly a binder-free Li-ion battery cathode, consisting of LiNi0.8Mn0.1Co0.1O2 (NMC811), sulfonated reduced graphene oxide (SRGO) sheets, and carbon black (CB) particles []. The EPD process caused a squeezing force, which not only wrapped the SRGO sheets around the NMC811 nanoparticles, but also inserted the CB particles into the composite. The volume expansion of the active particles was mitigated during cycling due to wrapping graphene sheets, and the restacking of graphene sheets was inhibited by sulfonated groups and CB spacers. At the current density of 0.2 C, the half-cell with this electrode and 1M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1:1, v/v) electrolyte delivered discharge capacities of 170.3 and 143.7 mAh g−1 at the 1st and 200th cycle, respectively. The rate capability was also enhanced (42.3 mAh g−1 at 10 C). The method is also scalable, because it takes only 10 min of ultrasonication and EPD when mass-produced SRGO and commercial NMC811 and CB particles are used as the starting materials. Graphitic carbon nitride (g-C3N4)-coating can also improve the electrochemical properties of NMC811, provided that the thickness of the coat is thin enough (20 nm) to avoid the hindering of the lithium diffusion []. Park et al. used a dispersion of electrochemically exfoliated graphite nanosheets that are functionalized with an amphiphilic surfactant acting as glue for graphite coating on the surface of Ni-rich oxides []. As a result, the cathode (99 wt.% NCA; electrode density (ρ) ~4.3 g cm−3) exhibited a 38% increase of the areal capacity and 34% increase of the volumetric capacity at current rate of 0.2 C with respect to the bare commercial NCA cathodes. We shall return on the coating devoted to the protection of the particles against side reactions with the electrolyte in a section devoted to the formation of the cathode–electrolyte interface.
Li-free coatings have been extensively investigated, including metal oxides such as SiO2 [,], ZrO2 [,,], TiO2 [,,], CeO2 [], and Al2O3. In particular, Al2O3 coating of LiNi0.6Co0.2Mn0.2O2 improves coulombic efficiency, reduces impedance, and improves capacity retention []. Even better electrochemical properties for this material were found with uniform C-Al2O3 coating obtained by the pyrolysis of molecular layer deposited alucone in argon, due to the synergistic effect between the amorphous Al2O3 and conductive carbon composite coating []. For Ni-richer NMC811, the positive effect of Al2O3 coating is evidenced by the increase of the capacity retention from 500 cycles to 1100 cycles at 1 C rate []. In a comparison between LiAlO2 and Al2O3 coatings of NMC622, Liu et al. showed that LiAlO2 coating is superior to Al2O3 and significantly improves the capacity retention and rate capability []. Note, however, that the efficiency of the coating depends on the properties of the coat, so that direct comparison may be misleading. In particular, microporous Al2O3 coating [] and ultrathin Al2O3 coating [] gives remarkable electrochemical properties. Therefore, Al2O3 coating is still widely used today, inasmuch as it is now possible to control not only the uniformity but also the thickness of the Al2O3 coating, allowing for its optimization []. Other metal oxide coatings include ZnO [], MgO [], Mg-Al layered double oxide [], and Ta2O5 []. A Ta2O5 protective layer on the surface of NCA with Ta5+ entering the lattice gifts the cathode had a capacity retention of 94.46% at 1 C after 200 cycles (60.97% for the pristine NCA) []. B2O3 coating treatment is an effective approach to reinforce the air storage stability and cyclic stability of Ni-rich cathode materials. Even exposing to ambient air for 8 weeks, B2O3-coated NMC811 cathode still delivers a high initial capacity of 161 mAh g−1 and a satisfactory capacity retention of 75.6% after 200 cycles at 0.5 C []. B2O3-coated LiNi0.87Co0.10Al0.03O2 with 2.0 wt.% B2O3 delivered a capacity of 184 mAh g−1 with a capacity retention of 86% after 100 cycles at 0.2 C [].
Among metal oxides, Al2O3 coating has been most investigated, because of its efficiency. In particular, it introduces new energy levels that prevent the reduction of Ni ions by O2− [,,] (see Figure 6). It can also suppress the Li-Ni cation mixing. It also maintains stabler cathode–electrolyte interface []. Wang et al. reported a surface-targeted healing (TH) strategy through atomic layer deposition of Al2O3 []. The modified NMC811 cathodes exhibited 78.6% capacity retention after 400 cycles at 1 C and 55 °C compared to 70.6% after 200 cycles for the pristine material). Cheng et al. coated polycrystals of LiNi0.83Mn0.05Co0.12O2 with Al2O3 by ALD and a subsequent annealing method according to which the sample was placed at a high temperature of 750 °C and annealed for 2 h. With this process, the primary grains remain intact, and the Al is enriched at the grain boundaries only. After 300 cycles at 4.3 V and at rate C/3, this cathode maintained 143.8 mAh g−1 with 84.7% capacity retention, compared to 118.6 mAh g−1 in case of gradual heating annealing []. This improvement was attributed to the rapid heating process, which minimizes Li/O loss and prevents the formation of a disordered phase. It is worth noting that a rapid heating process (by microwave technics in this case) has recently been used to synthesize a Ni-rich Co-free LiNi0.9Fe0.05Al0.05O2 with also improved electrochemical properties with respect to gradual heating annealing [].
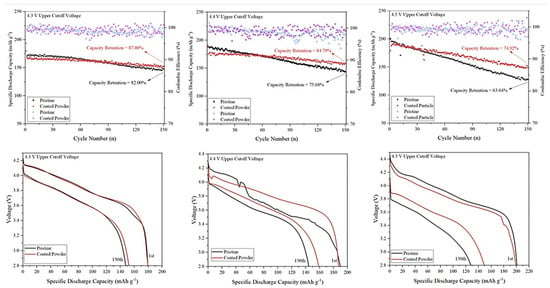
Figure 6.
Electrochemical properties of bare and Al2O3-coated NMC811 over 150 cycles (1 C) at 4.3, 4.4, and 4.5 upper cutoff voltages. Upper figures compare cycling stability of samples, while lower figures illustrate voltage fading after 150 cycles. Reproduced from ref. []. Copyright 2024 under the Creative Commons CC BY license.
The crystallization of amorphous TiO2 applied on NMC811 surfaces can result in Li2TiO3-coated NMC811, which can further inhibit phase transformation from H2 to H3 []. The capacity retention of Li2TiO3-coated NMC811 was raised at 90% (174.6 mAh g−1) after 200 cycles at 1 C with the voltage 2.70–4.55 V, owing to the additional gradient Ti-doping below the surface layer []. Sulfated ZrO2 coatings can incorporate sulfate groups forming –SO3−– to stabilize the interface of NMC particles with the electrolyte []. The synergetic effect of the ZrO2 coating in combination with a zirconium doping enhanced the cycling stability of NMC811 []. Defect-rich strontium titanate (SrTiO3–x) coating of NCA cathode exhibited a capacity of 170 mAh g−1 after 200 cycles under 1 C with capacity retention of 81.1% []. Al2(WO3)4 is a negative thermal expansion (NTE) material, having the unique properties of abnormal thermal expansion, and has been utilized to significantly suppress heat release and deformation of NMC811 []. When cycled at 1 C, NMC811 material modified with 7 wt.% Al2(WO3)4 retained 162.0 and 171.5 mAh g−1 after 100 cycles at 25 and 60 °C, respectively, about 13.8 and 25.4% higher than those of the pristine NMC811. Not only did Al2(WO4)3 reduce the side reactions between the cathode and electrolyte, but it also enhanced the thermal stability of the pristine NMC811.
A drawback of these oxide-based materials utilized to coat the Ni-rich cathode materials their small ionic conductivity. It is thus desirable to investigate more conductive coatings [,]. In particular, MoO3 is attractive, because its ionic conductivity is orders of magnitude larger, compared to Al2O3 or MgO. MoO3-coated NMCs demonstrated the superior capacity retention of 79.8% under the high 5 C []. Benefitting from a high ionic and electronic conductivity of NiFe2O4, a NiFe2O4-coated NMC622 cathode achieved a capacity retention of 81.72% after 100 cycles (at 60 °C@1 C) and an excellent rate capability (109.86 vs. 49.52 mAh·g−1 for the pristine material at 10 C) []. This coating was recently extended to NMC811 [] (see Figure 7). The NiFe2O4-coated NMC811 exhibited a superior rate capability of 112.7 mA h g−1 at 10 C, and high-temperature stability of 130 mA h g−1 after 100 cycles at 60 °C. Li2SiO3 is also employed, as it is a fast ionic conductor []. TiNb2O7 coating was found beneficial to the electrochemical performance of LiNi0.88Co0.06Mn0.06O2, by significantly improving the capacity retention of modified material after 200 cycles at 1 C from 59.8% of the pristine material to 87.2% []. A 0.3% Nb2O5-coated NMC811 cathode exhibits superior rate capability (146.4 mAh g−1@400 mA g−1) and remarkable rate cyclic stability (188.5 mA h g−1 after 100 cycles with capacity retention of 94.8% [].
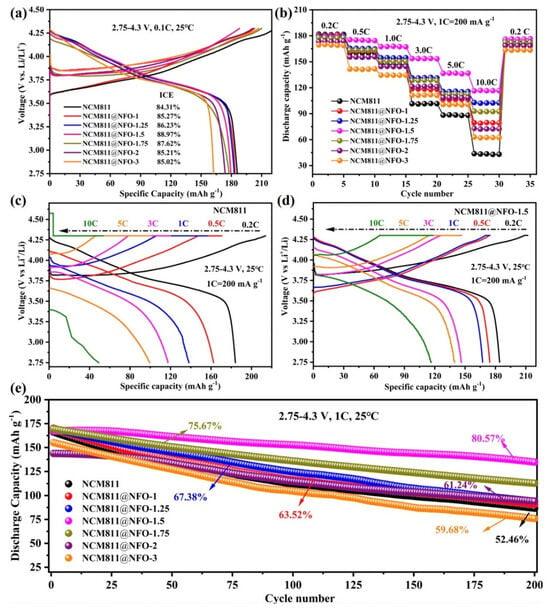
Figure 7.
Electrochemical properties of NMC811 uncoated and coated with different NiFe2O4 (NFO) weights (in wt.%). (a) Initial charge/discharge profiles of different cathodes at 0.1 C, (b) rate capability, (c,d) capacity–voltage curves at various current densities, and (e) long-term cycling performance at 1 C of prepared cathodes (within 2.75–4.3 V at 25 °C). Reproduced from ref. []. Copyright 2024 American Chemical Society.
Coatings of TiOx (TO) and LixTiyOz (LTO) deposited over NMC622 proved to be efficient to protect significantly the particles and suppress the crack formation during cycling []. The surficial Ti4+ doping induces the reduction of Ni3+ to Ni2+ at surface region, and the latter tends to migrate into Li-slabs, preforming a 5 nm-thick cation-mixing layer. This less-ordered layer makes the H2–H3 transition reversible []. This surficial Ti4+ substitution in LiNi0.9Co0.1O2 cathode material for the optimized Ti-concentration of 5 mol% enhanced the capacity retention from 69.7% to 97.9%. Coating with metal phosphates is also of interest because they are thermodynamically stable at the operation window of Ni-rich cathode materials []. Moreover, the strong covalent bonds between PO43− and the metal ions hinder the reactions of the Ni-rich material with the electrolyte. Examples include coating of NCA with Ni3(PO4)2 [] and NMC811 with Co3(PO4)2 [], FePO4 [,], AlPO4 [], LaPO4 [], Li3PO4 [,,,], and MnPO4 []. LiNi0.83Co0.12Mn0.05O2 was coated with CeP2O7 through a polyethylene glycol (PEG)-assisted water deposition method and obtained a cathode exhibiting a retention rate of 92.38% after 100 cycles under the conditions of 2 C and 4.3 V upper cut-off voltage []. In a study of the efficiency of 16 metal phosphate coatings on LiNi0.91Co0.06Mn0.03O2, Min et al. determined that Mn-, Co-, and Fe-phosphate materials improve the electrochemical properties; only TiPO4 failed []. Peng et al. constructed LiNiO2/Na1–xNi1–yPO4 surface hybrid coating layer and Na bulk doping of NMC811 simultaneously []. DFT calculations showed that the improvement of the electrochemical properties was attributable to the removal of surface oxygen vacancy and residual lithium. Zeng et al. coated NMC811 with dihexadecyl phosphate (DHP). The outer long-chain alkyl layer inhibited the adverse chemical reaction with H2O/CO2 in ambient air and improved the structural stability, while the phosphate-based inner coating accelerates Li+ transport [].
Huang et al. used alkyl phosphoric material with hydrophobic groups to coat LiNi0.83Mn0.11Co0.06O2 (N83) []. This coat effectively suppressed the surface residual lithium due to H+/Li+ ion exchange and enhanced the chemical stability of the cathode–air interface. In the case of LaPO4, the LiNi0.87Co0.09Al0.04O2 sample modified with the optimum 2 wt.% LaPO4 exhibited capacity retention rates of 96.0% and 85.1% after 100 cycles at 25 and 60 °C, respectively, against 87.1% and 74.2%, respectively, for the uncoated material. NMC622 was coated with Mn3(PO4)2 []. In the case of LaPO4, the LiNi0.87Co0.09Al0.04O2 sample modified with the optimum 2 wt.% LaPO4 exhibited capacity retention rates of 96.0% and 85.1% after 100 cycles at 25 and 60 °C, respectively, against 87.1% and 74.2%, respectively for the uncoated material.
4.2. Li-Based Surface Layers
These coatings with metal oxides or phosphides are efficient to reduce the side reactions with the electrolyte. However, as they do not contain Li in their composition, they increase the charge transfer resistance. To remedy this problem, the Ni-rich particles were also coated with Li-containing layers, such as NMC333 [], Li2ZrO3 [,,], LiAlO2 [], Li2SiO3 [], Li2MnO3 [,], LiNbO3 [,,,], Li2ZrO3 [], LiAlF4 [], Li2SO4 [], LiBO [], Li0.5La2Al0.5O4 [], LixWyOz [] including LixWO3 [], Li2MoO4 [], and LiTaO3 [,]. Sun et al. reported on a 2.5 nm-thick Li2ZrO3 layer coated onto LiNi0.90Co0.04Mn0.03Al0.03O2 and demonstrated that the coating layer lowers activation energy and polarization potential, thereby facilitating the Li+ motion. At 1 C, the capacity retention rate of the cathode material coated with 1 wt.% of the layer after 100 cycles was 90.2%, while it was only 74.6% for the bare material []. The comparison of coated NMCs shows the following trend for coatings to increase in rate performance: TiO2 < Al2O3 < Li4Zr3O8 < LiAlO2 < Li4Ti5O12 < ZrO2 []. The difference in the ranking between Li4Zr3O8 and ZrO2 comes from the reduced porosity of the Li4Zr3O8 coating, and high porosity is required to obtain a high-rate capability. On the other hand, a high coverage of the surface enhances long-term cycling stability and decrease the crack generation, which is best obtained with Al-containing coats. That is why long-term cycling stability with high capacity has been obtained for the NMC coated with LiAlO2 []. Tang et al. reported an effective etching-induced coating strategy to shield LiNi0.8Co0.1Mn0.1O2 electrode materials by LiAlO2 []. The 2.2 wt.% LiAlO2-coated NMC811 cathode delivers a high-rate capacity of 135.2 mAh g−1 at 10 C and capacity retention of 85.8% after 200 cycles at 0.5 C. With an optimized LiAlO2 coating amount of 3 wt.%, the capacity retention of Ni0.9Co0.07Mn0.01Al0.02O2 increased from 70.7% to 88.3% after 100 cycles at 1 C. It also delivered a capacity of 162 mAh g−1 at 5 C, while that of an uncoated sample is only 144 mAh g−1 []. This result illustrates the efficiency of LiAlO2 coating to improve the structural stability of Ni-rich cathode elements. Nevertheless, this coat is not sufficient to protect the electrode materials form HF etching, in contrast with fluoride coatings []. To circumvent this drawback, Wang et al. constructed a hybrid LiAlO2/LiF-AlF3 coating layer, combining the advantages of LiAlO2 and fluorinated LiF-AlF3. The hybrid layer modified NMC611 exhibited a discharge capacity of 166.8 mAh g−1 with capacity retention of 74.5% at 5 C after 300 cycles []. Zhang et al. benchmarked Al2O3, ZrO2, and LBO (Li2O-2B2O3) coatings on NMC811, and found that LBO is the best to improve charge–discharge specific capacity, Coulomb efficiency, water absorption stability, cycle characteristics, and resistance stability of the NMC811 cathode material []. Jamil et al. coated NCA with Li2O-BPO4 and obtained a cathode exhibiting a capacity retention of 92.3% at 1 C in 2.7–4.3 V and 76.2% at high voltage in 2.7–4.7 V []. Wu et al. coated LiNi0.82Co0.15Mn0.03O2 (Ni82) with the fast-ion conductor Li6.4La3Zr1.4Ta0.6O12 (LLZTO). This modified cathode delivered a discharge capacity from 168.3 to 121.2 mAh g−1 with the retention rate of 72% after 80 charge/discharge cycle at the very high voltage of 4.7 V [].
Lithium boron oxide (LiBO) coating of NCA increases the capacity retention to 93.59% after 100 cycles at 2 C []. Dot-like Nb12WO33 plus uniform LiBO coating of Li(Ni0.90Co0.06Mn0.04)0.995Al0.005O2 Nb12WO33 provided a rapid Li transmission channel, improving the rate capability with respect to the L-B-O coating alone, with a discharge capacity of 179.1 mAh g−1 at 5 C []. The LiBO-coated LiNi0.90Co0.06Mn0.04O2 material showed a discharge capacity of 222.0 mAh g−1 with 88.1% of capacity retention after 100 cycles at 1 C []. Qiao et al. synthesized Li2SiO3-coated NMC811 (NCM@LSO) by the precoating and solid-state lithiation methods. The capacity retention reached 97% after 500 cycles at 0.5 C, while that of bare NCM was limited to 79% after 450 cycles []. Xiao et al. synthesized LiNi0.8Co0.15Al0.05O2 using CoAl-layered double hydroxide (CoAl-LDH) nanosheet-coated Ni(OH)2 as the precursor, improving significantly the properties of NCA, compared with NCA synthesized from nickel–cobalt–aluminum hydroxide and Al(OH)3-coated nickel–cobalt hydroxide precursors. The improvement was attributed to the formation of a Li1–x(Co0.75Al0.25)1+xO2 mesophase as the buffer layer, reducing the Li+/Ni2+ cation mixing. In addition, the presence of Co on the surface promoted the diffusion of Al during the lithiation–calcination process, thus avoiding the formation of undesired Al-related phases. This cathode delivered a discharge capacity of 194.5 mAh·g−1 at 0.1 C and a capacity retention of 90.9% after 100 cycles at 0.2 C [].
Lithium phosphates have also been utilized as coating materials, including LiFePO4 to take advantage of the remarkable stability of this cathode element [,], Li3PO4 [,,,] (see Figure 8), LiH2PO4 [], LiZr2(PO4)3 [], and Li1.3Al0.3Ti1.7(PO4)3 []. Infusing Li3PO4 (LPO) in the grain boundaries of secondary particles to coat NMC particles is very promising, because it reinforces the microstructure by shielding off liquid electrolytes within secondary particles []. Due to the synergetic effects of Mg-doping and LiFePO4 coating NMC811, Zhang et al. obtained a cathode that delivered an initial capacity of 181.5 mAh g−1, with 90.8% capacity retention after 100 cycles at 0.2 C, demonstrating the increase of the structural stability due to the coating. The rate capability was also significantly increased, with a capacity still at 120.7 mAh g−1 at 5 C []. A still higher capacity at 5 C was obtained with LiH2PO4-coated NCA (147.8 mAh g−1). At 0.5 C, this LiH2PO4-coated NCA delivered a capacity of 180.2 mAh g−1, decreasing to 172.6 mAh g−1 after 100 cycles, which corresponds to a capacity retention of 95.8% [].
Figure 8.
Electrochemical performance of all-solid-state Li-ion cells with an Li-In anode, sulfide solid electrolyte, and NMC811 cathodes at 25 °C. (a) Charge/discharge curves under 0.1 and 0.5 C; (b) rate capabilities and (c) cycling performance of cells with two kinds of NMC811 cathodes: pristine, and modified by Li3PO4 coating. Reproduced from ref. []. Copyright 2025 Elsevier.
Coating fluorides on Ni-rich cathode materials can reduce charge transfer resistance and thus improve rate capability, but also increase the cycle life. Directly coating LiF on the surface of Li1.2Ni0.2Mn0.6O2 stabilized the cathode over 280 cycles and maintained a capacity of 110 mAh g−1 at 1 C []. Metal fluoride materials have better resistance to HF attack than metal oxides, and can thus be utilized to coat Ni-rich cathodes. In particular, AlF3 is known for its beneficial effects in Li-ion batteries []. In NMC-based cells, however, AlF3 coating has been used mainly in NMC333 []. On the other hand, NMC811 was coated with PrF3 []. This cathode exhibited a capacity retention of 86.3% after 100 cycles at 1 C.
The primary purpose of the coating is to prevent cathode surfaces containing highly reactive Ni4+ from contacting the electrolyte and therefore to reduce side reactions. But the coating can also avoid excessive Li on cathode surfaces from causing gas evolution due to its possible transformation into lithium carbonate and hydroxide as well as further side reactions with electrolytes []. In particular, metal oxides can allow for excessive Li on the surface of NMC particles to transform into LixMyOz coatings at certain temperatures. V2O5 coating is a good example. Ti-doped V2O5 (Ti0.05V1.96O5) on NMC811 effectively enhanced both the rate capability and the cycling stability []. DFT calculations demonstrated that the introduction of Ti into V2O5 introduced excessive free electrons, while reducing the energy barrier for Li+ diffusion. Li et al. proposed a hybrid coating strategy incorporating lithium ions conductor LixAlO2 with superconductor LixTi2O4 to resolve the issue of excessive Li []. With this coating, NMC811 exhibited an initial discharge capacity of 227 mAh g−1 at 4.4 V cutoff voltage with Coulombic efficiency of 87.3%, and reversible capacity of 200 mAh g−1 with 98% capacity retention after 100 cycles at a current density of 0.5 C. Let us recall that the investigation of oxygen release in NMC cathodes has evidenced that stable cycling of NMC is possible up to 4.5 V only for Ni compositions ≤ 0.6, and is possible only up to 4.0 V for pristine NMC811, because of the phase transition H2 → H3 []. Therefore, the result of Li et al. suggests that the coating utilized in this work prevents or at least softens this transition.
Recently, Qu et al. developed a lithium boron carbon oxide (LixByC1–yOz) coating using the sol–gel method to recover the air-exposed NMC811 from degradation []. The authors demonstrated that this coating is a promising approach to recovering the degraded surface structure and electrochemical performance of NMC cathodes.
4.3. Organic Layers
Organic compounds have also been considered as promising coating materials. Conductive polymers can also be utilized successfully. In particular, Hwang et al. [] synthesized a lithium-containing “BTJ-L” hybrid oligomer obtained through polymerization of bismaleimide by using a bismaleimide with a polyether monoamine, thiocyanic acid (TCA), and LiOH. NMC811 coated with this polymer demonstrated an improved capacity retention (86.1% vs. 72.9%) after 100 cycles at 1 C, attributed to the excellent wettability toward the electrolyte and the extra Li+ ions contributed by the hybrid BTJ-L oligomer additive. Coating with polypyrrole (PPy), another conductive polymer, is efficient to stabilize the Ni-rich particles [] (see Figure 9). Dry-coating PPy on NCA nanofibers increased the capacity retention from 73.7% to 83.1% after 50 cycles at 0.5 C []. Another polymer, polymethyl methacrylate (PMMA) was also successfully coated on NMC8111 []. The electron transfer from the interfacial Ni2+ to the ester group of PMMA anchors the Ni cations and reduced interfacial side reactions. The NMC811 with the PMMA nanolayer the electrode exhibited a discharge capacity of 181.1 mAh g−1 at 1 C rate, with capacity retention of 91.2% after 100 cycles. Gan et al. used polyvinylpyrrolidone (PVP) as an inductive agent to controllably coat a uniform conductive polyaniline (PANI) layer on NMC811 (NMC811@PANI–PVP) []. PANI-coated LiNi0.9Co0.085Mn0.015O2 also exhibited improved electrochemical properties compared to the pristine material []. He et al. coated LiNi0.95Mn0.05O2 (NM95) with polyaniline–polyethylene glycol (PANI-PEG) to obtain a cathode maintaining capacity retention of 94.7% (1 C, 100th cycle) and 79.0% (5 C, 200th cycle) under cut-off voltage of 4.3 V []. Huang et al. adopted an effective Mo/PANI co-modification strategy on a LiNi0.96Co0.02Mn0.02O2 cathode material []. This cathode delivered a capacity of 209.3 mAh g−1 with an upper cut-off voltage of 4.4 V at 1 C, and its capacity retention reached 95.7% after 100 cycles. Benefiting of the high conductivity of PANI, the rate capability was also significantly improved, with a capacity of 167.1 mAh g−1 at 10 C. Not only does PANI have a good electronic conductivity, but it also prohibits direct contact of the electrode with the electrolyte, so that the NMC811@PANI–PVP cathode exhibited good cycle ability (88.7% after 100 cycles at 200 mA g−1) and rate performance (152 mAh g−1 at 1000 mA g−1). Polyacrylonitrile (PAN) was employed by Huang et al. as a proof-of-concept organic coating with better electro-negativity on NMC81, because The C≡N functional groups in the PAN coating can adsorb Oα− (α < 2) and provide it with electrons for its reduction to stable O2−, thus inhibiting oxygen release. This modified NMC811 displayed a high-capacity retention of 86.3% after 200 cycles at 1 C, 75.1% after 350 cycles at 5 C, and 83.1% after 100 cycles at 1 C and 55 °C []. Li et al. coated NMC622 with a cross-linked epoxy natural rubber (CENR). The viscoelastic properties of CENR alleviated mechanical stresses during cycling. Moreover, the strong adhesion to the surface of Ni-rich cathodes suppressed detrimental interfacial reactions []. This coated NMC622 cathode material retained 95.9% of its capacity after 300 cycles at 0.5 C and 82.8% after 500 cycles at 1 C. LiNi0.83Co0.11Mn0.06O2 was coated with 7 nm-thick polysiloxane via in situ hydrolysis-polycondensation of tetraethyl orthosilicate. The corresponding cathode exhibited a capacity retention of 81.8% after 150 cycles at 1 C []. Lin et al. Coated NMC811 with poly (pyrrole-co-citral nitrile) (PPC). The discharge capacity of NMC811@PPC after 200 cycles at 1 C in the voltage range 3.0–4.5 V was 173.6 mAh g−1, which is even higher than the initial discharge capacity (102.66%). This little rebound was ascribed to the opening and broadening of the lithium ion channels [].
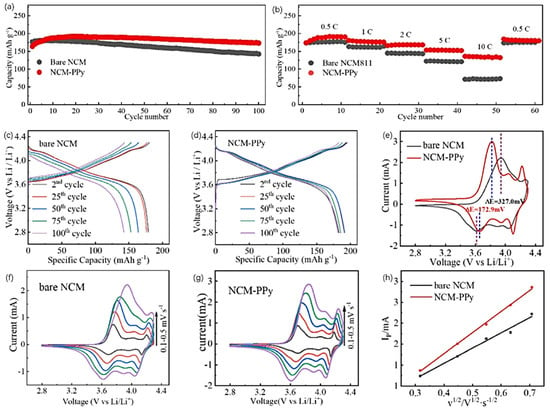
Figure 9.
NMC811 coated with PPy (a) Cycling performance at 2 C and (b) rate capability. (c,d) GCD curves at different cycles of NMC811@PPy and bare NMC811. (e) CV comparation between NMC811 and NMC811@PPy with rates of 0.5 mV s−1. CV curves of (f) NMC811 and (g) NMC811@PPy with scan rates increased from 0.1 to 0.5 mV s−1. (h) Plots of peak current density as a function of the square root of the scan rate. Reproduced from ref. []. Copyright 2023 Elsevier.
Yoon and Shin utilized a cyclopropenium cationic covalent organic framework (C-COF) as a coating material of NCM with 94% Ni content. In the C-COF skeleton, cation moieties attract PF6− anions in the Li salts of the electrolyte, suppressing the solvation between Li+ and PF6−. This coating increased the diffusion coefficient of the lithium by a factor 3, and increased the capacity retention from 60.17 to 71.31% after 200 cycles at 1 C (see Figure 10) []. A summary of the effect of different coatings on the cycle ability of Ni-rich cathodes is reported in Table 1.
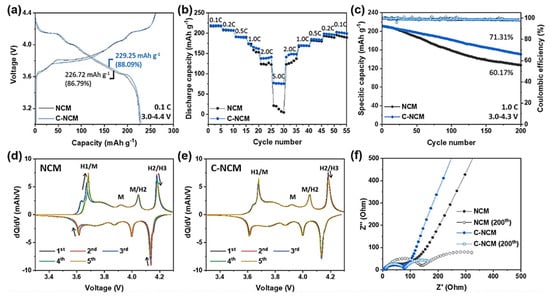
Figure 10.
Effect of COF-coating on LiNi0.948Co0.046Mn0.006O2 (C-NMC). (a) Initial charge–discharge performance of cells with NMC and C-NMC electrodes at a C-rate of 0.1 C. (b) Rate performance with various current densities from 0.1 to 5.0 C. (c) Long-term cycling performance during 200 cycles at 1.0 C. Differential capacity vs. voltage plots of (d) NCM and (e) C-NCM during initial five cycles. (f) Impedance spectra of EIS measurement. Reproduced from ref. []. Copyright 2024 American Chemical Society.

Table 1.
Effects of coating on cycling ability of Ni-rich cathode elements. Capacity densities are expressed in mAh g−1. Upper cut-off in cycles is 4.5 V for materials, followed by the symbol *. Otherwise, cut-off is 4.3 V. DHP = dihexadecyl phosphate; AP = alkyl phosphoric material (two hydrophobic coats intended to protect the Ni-rich element against moisture). BTJ-L = hybrid oligomer, obtained through polymerization of bismaleimide with a polyether monoamine (i.e., Jeffamine-M1000, JA), trithiocyanuric acid (TCA), and LiOH. CENR = cross-linked epoxy natural rubber.
4.4. Dual Coating
Dual-coating can also be performed to obtain a synergetic effect. For example, Zhao et al. coated NMC with Al2O3 and AlPO4 [], using a dry coating strategy based on the interaction between NMC811 and Al(OH)3 nanoparticles to obtain a dual LiAlO2 and chemical-inert Al2O3 coating. They obtained the optimal electrochemical performance when the coating strikes a balance of ion-conducting LiAlO2 and chemical-inert Al2O3 phases []. Bai et al. constructed a uniform LiAlO2+Li3PO4 protective layer with gradient Al doping (LAP modification) of single-crystalline LiNi0.90Co0.05Mn0.04Al0.01O2. This cathode exhibited a capacity retention of 74.4% at a high voltage of 4.5 V after 200 cycles at 1 C, while the capacity retention without this modification was only 63% after 100 cycles []. Similarly, Liu et al. combined La2Li0.5Al0.5O4 with gradient Al doping of NMC811 and obtained a cathode exhibiting a capacity retention of 90.9%, after 200 cycles at 1 C []. Mohsen et al. improved the structural stability and the rate capability of NCA by coating the particles with graphene and Y2O3 []. Poly (dimethyl diallyl ammonium chloride)/graphene oxide (PDDA/GO) dual-layer coating on the surface of single-crystal NMC811 exhibited a 77% capacity retention after 500 cycles at the current density of 50 mA g−1, against 28% for pristine NMC811 [].
Liu et al. reported LiNbO3+Li3BO3 dual-coating of single-crystal Li[Ni0.92Co0.06Mn0.02]O2) (SC-Ni0.92). The all-solid-state battery with this cathode Li6PS5Cl solid electrolyte and Li-In anode exhibited a capacity retention rate of 88.4% for 100 cycles at a current density of 1.5 mA cm−2 (1 C) and a discharge capacity of 150.1 mAh g−1 at a high current density of 7.49 mA cm−2 (5 C) []. This performance was attributed to the melting of Li3BO3 during hot sintering, which diffused into the crystalline LiNbO3 coating to form a dense coating. Yang et al. reported LiF+Li3BO3 dual-coating of NMC811. This cathode achieved a capacity of 205.4 mAh·g−1 within a voltage window of 2.8–4.5 V at 1 C. with a capacity retention of 70.6% after 500 cycles [].
Yang et al. prepared Li0.5La2Al0.5O4 (LLAO)-coated LiNi0.82Co0.14Al0.04O2 []. In situ coating LLAO is formed from the reaction among La2O3, Li2O, and Al2O3 at 800 °C. This reaction, however, will consume some Al atoms in the superficial phase of secondary particles. Therefore, stoichiometric Mn atoms are doped into the Al-deficient layer to form the LiNi0.82Co0.14Al0.04–xMnxO2 (LNCAM), which becomes a transition layer suppressing the lattice mismatch on the interface. As a result, the prepared material includes three layers which are the amorphous Li0.5La2Al0.5O4 in situ coating layer, the LNCAM transition layer, and the pristine LiNi0.82Co0.14Al0.04O2 (LNCA) phase from the surface to the inside, respectively. This cathode exhibited a capacity retention of 96.2% after 100 cycles in the voltage range of 3.0–4.4 V at 1 C (13% higher than that of the pristine material), and 80.8% at 1 C after 300 cycles. This performance is due to the synergetic effect of the Mn-doping, which suppressed internal strain, and improved the structural stability of NCA, while the coating effectively restrained cracking of the secondary particles. In addition, LLAO is a good ionic conductor, which enhanced the lithium mobility.
5. Doping
Doping is another strategy to minimize side reactions on Ni-rich NMC particle surfaces. Cation doping is of interest if the ionic radius of the dopant is large, increasing distances between atomic layers in the lattice structure for easier lithiation/delithiation. This expansion not only increases the lithium diffusion, but it also improves the structural stability. Moreover, the higher ionic radii enable dopants to reside on cathode particles, preserving the particle surface and refining particle morphology to suppress crack formation []. Doping with metal elements M are employed to expand pathways for Li+ diffusion, but also to oxidize Ni2+ to Ni3+ when doped into the material owing to its strong oxidation performance, thus reducing Li+/Ni2+ mixing. In addition, the strong M–O bonds can maintain the layered structure at high delithiation state.
5.1. Experimental Results
In situ operando analyses now give a comprehensive understanding of elemental doping of Ni-rich materials [,]. Fe3+ and Sn4+ with ionic radii larger than substituted Co3+ expand Ni-rich NMC crystal lattices horizontally [], while Ca2+ (with a radius larger than Ni2+) can expand lattices horizontally []. An extensive review on the results published on 46 dopant elements in NMCs through the years has been published by Ko et al. []. Hereunder, we focus attention on the best results obtained in recent years. Such doping elements include Mo6+ [,,,], W6+ [,,,,,,,,,,], Te6+ [], Ta5+ [,,,,,,,] Nb5+ [,,,,], Ce4+ [], Zr4+ [,,,], Ti4+ [,,,,,,,], Ge4+ [], Hf4+ [], Sn4+ [], Ce4+ [], Gd3+ [], and Al3+ [,]. Al-doping alleviates anisotropic lattice changes and volume changes during cycling. It also maintains a wider LiO6 inter-slab thickness without collapse at highly charged states, allowing Li-ions to be deintercalated/intercalated reversibly []. As a result, Al-doped LiNi0.7Co0.1Mn0.2O2 at a high cut-off voltage (4.4 V) delivered a capacity of 144.69 mAh g−1, with a capacity retention of 80.26% after 90 cycles at 1 C []. Due to the Al-doping, LiNi0.92Co0.03Mn0.03Al 0.02O2 demonstrates a capacity retention of 92% after 100 cycles at 0.5 C rate, against 69% with undoped LiNi0.94Co0.03Mn0.03O2 []. An important parameter is the concentration of Al, 2% in this case, while the optimized concentration is 3% after Kim et al. []. They reported that Li[Ni0.894Co0.041Mn0.034Al0.031]O2 cathode demonstrates excellent long-term cycling stability, retaining >90% of its initial capacity after 500 cycles, while the Li[Ni0.91Co0.04Mn0.04Al0.01]O2 cathode loses 38.3% of its initial capacity after 500 cycles []. Positive effects of Al-doping have also been observed with cobalt-free cathodes, such as LiNi0.90Mn0.06Al0.04O2 [], LiNi0.8Mn0.16Al0.04O2 [], LiNiO2 [], Al-doped NCM [], and LiNixMn1–xO2 [], although the electrochemical performance remains below that of Co-containing Ni-rich cathodes, even with single crystallized particles []. Other doping elements of Ni-rich elements include Gd3+ [], Cr3+ [,], Sc3+ [], Y3+ dopant of both NMC [] and NCA [], La3+ [], Mg2+ [,,,,,,], Ca2+ [], Sr2+ [,], Zn2+ [,], Ag+ [], and Na+ [,,].
All the transition metal doping elements we have mentioned form stronger bonds with oxygen and reduced energy loss, promote lithium migration, and reduce the Ni/Li mixing of NMCs. Nevertheless, among V, Nb, and Zr doping, V-doping is the most advantageous to improve the electrochemical properties of NMC811 by reducing significantly the lithium divacancy barrier []. This may not be true, however, for NCA cathodes, because this effect in NMC comes from the suppression of the lattice distortion, due to the radius of V5+ being close to the radius of Mn4+. Actually, 0.3 wt.% Nb-doped LiNi0.94Co0.02Al0.04O2 delivers 172 mAh g−1 after 300 charge–discharge (voltage window: 2.8–4.3 V) cycles at 2 C (see Figure 11) []. Recently, Li et al. investigated the relationship between the diffusion depth and distribution behavior of foreign elements within the cathode material and the composition of the Li-rich cathode material. They determined the diffusion coefficients between Zr4+ and transition metal elements TMn+ (TM = Ni, Co, Mn; n = 3, 4) []. As a result, these coefficients follow the trend: Zr4+/Mn4+ > Zr4+/Mn3+ > Zr4+/Co3+ > Zr4+/Ni3+, the Zr4+/Mn4+ diffusion couple having the smallest diffusion activation energy of only 0.43 eV. In particular, this work explains the reason for the formation of a Li2ZrO3 secondary phase coating layer on the surface of Ni-rich cathode material particles.
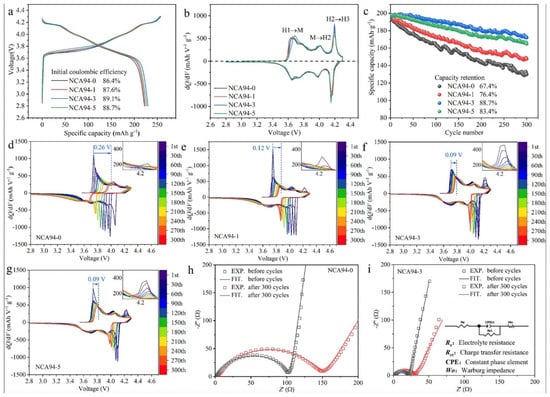
Figure 11.
Galvanostatic charge–discharge measurements and EIS results. (a) Initial charge–discharge curves at 0.1 C. (b) Differential capacity plots derived from 0.1 C charge–discharge curves. (c) Cycling performances at 2 C. (d–g) Differential capacity plots derived from 2 C charge–discharge curves at different cycles. (h,i) Nyquist plots of pristine LiNi0.94Co0.02Al0.04O2 (NCA94-0) and NCA94 doped with 3 wt.% Nb (NCA-3) after 300 cycles at 2 C. Reproduced from ref. []. Copyright 2023 Elsevier.
Al-doped LNO (LiNi1–xAlxO2) with x = 0.02 exhibits a very good cycle ability, with a capacity of 145 mAh g−1 after 400 cycles at 0.5 C []. The 1 mol% Cr-doped NMC622 exhibits a capacity of 100.6 mAh g−1 after 200 cycles at 10 C, corresponding to a retention of 93.1%, in the potential range of 2.7–4.3 V. Extending the potential range to 2.7–4.5 V, the cathode still delivered 166.8 mAh g−1 and keeps 75.0% of the initial value at the 200th cycle at 3 C and 50 °C []. At 5 C, the Cu-doped NCM622 exhibits a high discharge capacity of 140.8 mAh g−1 with an increased capacity retention of 77% as compared to that of the bare NCM622 (62%) after 350 cycles []. At 1 C, the capacity retention of Ta-doped LiNi0.88Co0.09Al0.03O2 is 93% (171 mAhg−1) after 100 cycles at 1 C, compared to 68.6% for the pristine material []. Park et al. conducted the study of the effects of Co3+, Al3+, Ti4+, Nb5+, Ta5+, W6+, and Mo6+ doping on the properties of LiNi0.9Mn0.1O2 [] and concluded that Mo-doping give the best results.
Non-metallic dopant elements include B3+ [,,,]. The introduction of high-bond-energy B-O covalent bonds can inhibit the change of 2p orbitals of oxygen atoms and the formation of particle microcracks during cycling. Sun et al. [] studied the effect of B doping on the microstructure of NCA. The study found that B-doping can reduce the surface energy of the (003) crystal plane, promote the preferential growth of the (003) crystal plane, and change the microstructure. LiNi0.878Co0.097Al0.015B0.01O2 cathode material can maintain 83% of its capacity retention after 1000 cycles at a discharge depth of 100%, while the normal undoped LiNi0.885Co0.1Al0.015O2 showed a capacity retention rate of 49%. LiNi0.83Co0.05Mn0.12O2 doped with 0.6 mol% B showed enhanced rate capability and excellent cycling stability. The retention after 500 cycles in the soft-pack full cell can reach 91.35%, and the capacity decay per cycle was only 0.0173% [].
Among Li-site dopants (Na, K, Rb, Ca), Na gives the best results. The substitution of Na+ for Li+ at 3a sites enlarges the distance between layers, benefitting from the larger radius of Na+. Additionally, Na doping suppresses the cation mixing [] and weakens Li–O bonds, thus enhancing the potential capacity. A Na-doped NCM622 cathode shows an initial discharge specific capacity of 175 mAh g−1 and a high-capacity retention of 90.8% after 100 cycles at 0.2 C, compared to an initial capacity of 160 mAh g−1 and retention of 67.5% for the pristine material []. Na+-doped Li0.98Na0.02Ni0.6Co0.05Mn0.35O2 exhibited a higher capacity retention rate (93.3% vs. 83.2%) after 100 cycles and a superior rate capacity (121 mAh g−1 vs. 93 mAh g−1) at 3 C current density compared to the pristine NMC under 4.5 V voltage []. On a general basis, Li-site doping ions with suitable ionic radii are beneficial to improve Li+ diffusion in the positive electrode materials, and thus improve the electrochemical properties. Nevertheless, the dopant must have an ionic radius that is not too big; otherwise, it can generate blocking effects and lattice distortion that are damageable to the cycle life. For example, among alkali metal elements, Na-doped NMC811 has significantly higher capacity and capacity retention than Rb- and K-doped NMC811 []. For the same reason, Na-doping has also positive effects in Li-rich, Mn-rich NMC [].
The bore is a O-dopant. It raises the capacity retention of LiNi0.95Co0.04Al0.01O2 to 88% after 100 cycles at 0.5 C []. Anion doping is mostly conducted using halogens, such as F− [,,,]. Other halogens doping elements include Br and Cl, which increased the capacity retention of NMC811 by 10%, due to the low-melting eutectic salt and the increase of the electrochemically active surface area [].
Positive effects of Sn-doping have been demonstrated both in NMC622 [,] and more recently in NMC811 [,]. In particular, the capacity for the optimized 2% Sn-doping of NMC811 remains at 183.9 mAh g−1 after 270 cycles at 0.5 C, corresponding to 93.7% of the initial discharge capacity. Sn-doped Li[Ni0.90Co0.05Mn0.05]O2 (Li[Ni0.897Co0.05Mn0.05Sn0.003]O2) delivers a discharge capacity of 224.3 mAh g−1 and exhibits a capacity retention of 92.9% after 100 cycles at 4.3 V and 82.9% at 4.4 V []. Like doping with other elements, Sn-doping stabilizes the structure, but in addition, it modifies the structure, as Sn-doped material has radially oriented thin primary particles, which is not the case for the undoped material, and this radial orientation is known to increase the electrochemical properties [,].
Mg2+ acts differently. It possesses a similar radius to Li+ and will enter the Li-layer rather than the transition metal layer on the surface region of Li-rich NMC particles []. The larger valence with respect to Li+ is responsible for a repulsion of Mg2+ to transition metal ions, allowing for the suppression of cation mixing, and delaying the surface transformation to a spinel structure. As for anionic doping, fluorine is most popular. Not only does it expand both lattice parameters by reducing transition metal ions (mainly Ni and Mn), but it also increases mean charging voltages due to increased bond energy of Li–F as compared with Li–O. In particular, 0.5% Mg-doping of LiNi0.9Co0.05Mn0.05O2 reduces the capacity loss at 1 C rate from 3.5% to 1.7% after 100 cycles [].
Kim et al. compared the electrochemical performance of Li[Ni0.92–xCo0.04Mn0.04Alx]O2 cathodes for different Al concentrations x. Primary particle elongation and radial alignment increase with increasing x, which dissipates strain resulting from the abrupt contraction of unit cells during the H2 → H3 transition. The best results were obtained for an Al content of 3 mol%. The Li[Ni0.894Co0.041Mn0.034Al0.031]O2 cathode demonstrated a capacity retention above 90% after 500 cycles, and an energy density of 740 Wh kg−1 []. For NCA, the optimum Al concentration is 5.6%. At C/3 rate, the capacity retention of LiNi0.85Co0.094Al0.056O2 is almost 100% after 100 cycles, [].
The cathode obtained by incorporating 2 mol% Zn2+ into LiNi0.94Co0.06O2 delivers an initial discharge capacity of 200 mA h g−1 at a C/2 rate, and retains 74% of the initial capacity after 500 cycles in a full cell assembled with a graphite anode (against 62% before Zn doping) []. Yi et al. synthesized a LiNiO2 co-doped with 1 mol% Mg and 2 mol% B []. This cathode in pouch full cell configurations with graphite anode exhibited a high initial 1 C discharge capacity of 210 mAh g−1 with a 20% capacity retention improvement over 500 cycles when benchmarked against pristineLiNiO2. The result was attributed to the stabilization of the CEI reducing TM dissolution.
All the dopants serve as pillars for enhancing the structural stability. In addition, high-valence dopants Nb5+, Ta5+, Sb5+, Mo6+, and W6+ modify the microstructure through lowering the energy of the (003) surface, which dramatically dissipates the strain arising from H2 → H3 phase transition [,]. The change in morphology, crystal structure, and electrochemical properties associated with such doping is illustrated in Figure 12 for the particular case of liNiO2. They are thus the subject of increasing interest, and their application as modifiers in Ni-rich cathodes []. W-doping showed remarkable results. At 1 C, the 1.0-mol% W-doped Li[Ni0.90Co0.05Mn0.05]O2 cathode in a pouch-type full cell using a graphite anode retained 89% of its initial capacity after 500 cycles []. Kim et al. constructed a W-doped Li[Ni0.8Co0.15Mn0.05]O2 cathode that showed nearly no drop in specific capacity even after 1000 cycles at full depth of discharge at 1 C []. Kim et al. obtained W-doped Li[Ni0.95Co0.04Al0.01]O2 cathode with columnar grains by introducing WS2. []. The study of W-doped LiNi0.88Co0.09Mn0.03O2 also demonstrated that the primary particles of the cathode become finer with the increase of W-doping amount []. As a result, at a high rate of 10 C, this W-modified cathode exhibited capacity retentions of 94.68% and 89.63% at 25 and 45 °C after 100 cycles, respectively. At full depth of discharge, Li[Ni0.90Co0.09Ta0.01]O2 exhibits 90% capacity retention after 2000 cycles, when Li[Ni0.90Co0.09Al0.01]O2 cathode loses ~40% of its initial capacity within 500 cycles []. Another example of high-valence doping is provided by Nb-doped Ni-rich cathode that consists of the elongated and radially aligned primary particles with increased oxygen stable (003) planes []. In this work, Nb-doped LiNi0.9Co0.1O2 cathodes in a pouch-type full cell exhibited a capacity retention of 91.9% at 1 C after 500 cycles and 80.5% at 5 C after 2000 cycles within 3.0–4.2 V. The Nb-doping is efficient to stabilize the structure, but coating may be desirable to increase the conductivity at the surface of the particles. For example, a dual-modification strategy of Nb doping and Li4SiO4 coating combined the two effects to obtain a NCA cathode exhibiting a specific capacity of 199.2 mA h g−1 at 1 C with a capacity retention of 93.3% after 100 cycles due to the doping, but it also delivered a high capacity of 160.0 mAh g−1 at 10 C, much higher than the pristine NCA (138.2 mAh g−1), due to the coating []. Note, however, that a simple dual-site Nb-doped NMC811 cathode delivered 202.8 mAh·g−1 with a capacity retention of 81% after 200 cycles at 2 C, and delivered a capacity of 176 mAh·g−1 at 10 C rate []. This result demonstrates that Nb doping is capable of effectively stabilizing the structure at a high voltage, suppressing the Li/Ni disordering, and improving the electrochemical performance of the NCM cathode. Zhang et al. demonstrated recently that the introduction of Co at a doping level (1.5%) into LiNi0.9Mn0.1O2 is sufficient to obtain a cathode exhibiting a discharge specific capacity of 167.1 mAh g−1 after 100 cycles at 1 C and 121.2 mAh g−1 after 200 cycles at 5 C, respectively [].
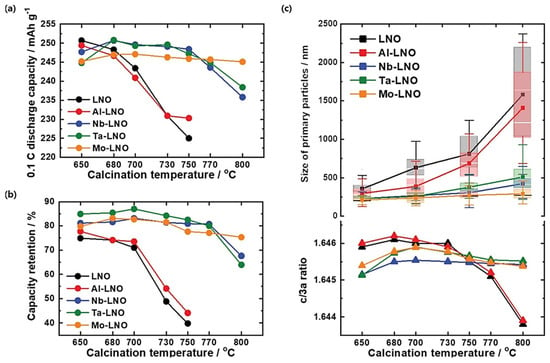
Figure 12.
Effect of doping on electrochemical performance of LiNiO2 (LNO) with high-valence elements. (a) Discharge capacity (at 0.1 C) and (b) capacity retention after 100 cycles (at 0.5 C in half cells) of LNO, Al-LNO, Nb-LNO, Ta-LNO, and Mo-LNO cathodes as a function of calcination temperature. (c) Fundamental physical properties (average particle size and c/3a ratio) of cathodes as a function of calcination temperature. Reproduced from ref. []. Copyright 2023 Wiley.
Machine learning techniques can be applied to efficiently and accurately screen appropriate dopants []. Recently, Kim et al. developed a machine-learning-based model for Ni-rich cathodes. They implemented 33 elements as potential dopants, which led to the identification of 101 Co-free compounds demonstrating their superior performance. Among them, the top ten Li0.85D′xD″(0.15–x)O2 compounds are obtained. Only one of them contains Mn: LiNi0.85Ti0.05Mn0.1O2, and one of them has only one dopant: LiNi0.85B0.15O2. The other ones are co-doped, with B, Cu, Al, Cr, Ti, and V as D′ and D″ dopants. Their calculated energy density is in the range 1160–1204 mWh g−1, calculated capacity in the range 277–287 mAh g−1, with a calculated volume change during cycling ≤ 2.6% []. For comparison with cathodes that are currently investigated, NMC811 has a theoretical capacity of 200 mAh g−1 [], average voltage 3.5 V when doped with Fe [], and volume change of 7.8% ± 1.5% []; NCA80 (LiNi0.80CoxAlyO2) has a theoretical capacity of 279 mAh g−1, average voltage 3.9 V at x = 0.15 and y = 0.05 [], and volume change of 4.2% at x = 0.16 and y = 0.04 []. This result illustrates how useful artificial intelligence (AI) is for helping to determine next-generation cathode candidates. A comparative study of the effects of doping on the cycling ability of Ni-rich cathode materials is summarized in Table 2.

Table 2.
Effects of single doping on cycling ability of Ni-rich cathode elements. Capacity densities are expressed in mAh g−1. Asterisk symbol after cathode element indicates that upper voltage cut-off for cycles is 4.5 V. Otherwise, unless specified, cut-off is 4.3 V.
5.2. Theoretical Calculations
Density functional theory calculations provide critical insights into Ni-rich cathode materials at the atomic and electronic levels, aiding in their optimization and performance enhancement. They enable the prediction of phase transitions and structural changes during battery operation. By analyzing lattice parameters and atomic interactions, DFT helps identify factors contributing to phase segregation and degradation, such as the migration of Ni ions into the Li layer. DFT is used to calculate the band structure and density of states (DOS), revealing how Ni concentration affects charge transport. Additionally, Li-ion diffusion barriers can be computed to optimize ionic conductivity and rate capability. These insights help fine-tune material compositions for better conductivity and fast-charging capabilities. By evaluating lithium chemical potential, DFT aids in understanding the stability of Ni-rich cathodes and their decomposition tendencies. This information is vital for designing materials with higher capacity retention and minimal voltage fade. DFT calculations allow for the investigation of oxygen evolution. By understanding these degradation pathways, researchers can develop strategies to mitigate these effects and enhance cathode longevity. DFT calculations determine vacancy formation energies and their impact on charge compensation mechanisms. By analyzing these defects, researchers can fine-tune material stoichiometry to achieve optimal electrochemical properties. That is why DFT calculations are very often included in the publications to support the experimental results and their interpretation. Another example of the contribution of DFT is the calculation on LiNi0.9Co0.1O2 providing insights into the performance-limiting factors of this cathode element, emphasizing the need for a higher concentration of cobalt doping to effectively stabilize the Ni-rich cathode [].
In the context of doping and coating strategies, DFT plays a crucial role in screening dopants that can stabilize the Ni-rich structure and reduce cation disordering. It also models interactions with protective coatings, helping design surface modifications that enhance stability and minimize electrolyte decomposition. For example, DFT calculations demonstrate that Mg2+ doping not only inhibits the H2–H3 phase, but also improves the number of free electrons near the fermi level from 15.66 for the pristine LiNi0.80Co0.05Mn0.15O2 (FNCM) to 17.30 for Mg-doped FNCM []. Bader atomic charge calculation found that Ga-doping brings an additional charge of 0.1864 to the oxygen atom in LiNiO2 []. DFT calculations also gave insight on the effects of oxidized dopants with more electrons near the fermi level, which reduces charge transfer resistance and improves electron conductivity [,,] (see Figure 13). DFT is also useful in determining the chemical stability of the coatings. For example, it shows that the coating with phosphates LiMPO4 (M = Ni, Co, Mg) has improved stability compared with sulfates, which is confirmed by experiments []. The calculation of binding energy between the Li-rich material and the coating layer also gives insight into the structural stability and improvement of the lithium diffusion [,,,]. Finally, DFT calculations facilitate the exploration and parameter optimization of new materials, and the coupling with machine learning will be of great value in selecting the most appropriate coating materials and dopants for Ni-rich cathodes []. A first example of this coupling has been recently reported [].
Figure 13.
Results of DFT calculations: the band structures of (a) LiNi0.94Co0.04Al0.02O2 (N94CA) and (b) W-doped N94CA; density of states of (c) N94CA and (d) W-N94CA; (e) integration of DOS from (c,d). DFT calculations show that W-doping results in an increase of Fermi level toward conduction band and an increase of total density of states (TDOS). as shown in the insets. Results are in agreement with the increase of electronic conductivity of powder samples in (f). Reproduced from ref. []. Copyright 2023 Elsevier.
5.3. Morphology Aspects
Doping has beneficial effects on the morphology of the particles. For example, we have already mentioned that Nb5+ doping significantly improves the electrochemical properties of Ni-rich materials. In addition, Nb5+ doping of LiNi0.88Co0.05Mn0.07O2 modifies the particle morphology, yielding radially distributed, elongated, rod-like structures. This cathode delivered an initial capacity of 200.3 mAh g−1 at 1 C, within a voltage range of 2.7–4.5 V, with a capacity retention of 92.9% after 100 cycles []. This rod-like morphology effectively mitigates the anisotropic volume changes during cycling and thus increases the cycle ability. Li et al. have shown that the one-dimensional morphology of the primary particles provides shorter electron/ion diffusion distances and high tolerance to corresponding stress changes, significantly improving the electrochemical properties []. Other dopants preserve the rod-like structure. In particular, excess Al dopant effectively preserves the needle-like morphology of compositionally partitioned precursors during lithiation [], and segregates at grain boundaries, inhibiting the growth of primary particles []. This segregation of high-oxidation-state dopants at grain boundaries is commonly observed because their ionic radii limit their solubility []. The amount of dopant also modifies the size and morphology of primary particles. B-dopant is an example. It lowers the surface energy of the (003) planes, thereby producing a strong crystallographic texture in B-doped cathodes []. In addition, as the bore dopant concentration increases, the primary particles elongate along the radial direction []. All these results show that the orientation of primary particles has been extensively controlled using high-valence dopants during calcination. Concentration gradients also favor the rod-like structure, as will be seen in the section devoted to them. Ta-doping increases the number of small primary particles and increases the degree of heterogeneity of particle orientation. This effectively mitigates the local strain accumulation during cycling due to the random orientation of primary particles [].
In addition, the role of the hydroxide precursor is also critical in synthesizing radially aligned Ni-rich cathodes. Through stepwise control of ammonia concentration and pH during precipitation, elongated primary particles with appropriate size along [001] are promoted, facilitating the formation of radially aligned particles in the precursor. This approach enabled Wang et al. to synthesize LiNi0.94Co0.02Mn0.04O2 cathodes with exceptional radially aligned microstructure []. This cathode material delivered a capacity of 230 mAh g−1 at 0.05 C and 186 mAh g−1 at 5 C, with capacity retention of 95.6% after 100 cycles at 1 C and 25 °C in half cell and 93.2% after 1000 cycles at 1 C and 30 °C in full cell. This is an illustration of the morphological relationship of cathode materials to their electrochemical performance, which has been reviewed by Meng et al. [].
Other morphologies are also of interest. Wu et al. successfully synthesized NMC811 cathode material, arranging the hexagonal nanosheets with exposed {104} facets by a co-precipitation process and a high-temperature lithiation reaction. The tightly adhered nanosheets on the (001) surface help to alleviate stress changes caused by the anisotropic structure and inhibit the fragmentation of secondary particles []. This cathode exhibited a discharge capacity of 203.8 mAh·g−1 at 0.1 C and stable cycling performance (89.3% capacity retention after 100 cycles at 1 C, 55.3% after 300 cycles at 5 C, and 59.6% after 300 cycles at 10 C).
5.4. Synergetic Effects of Co-Doping and-or Multiple Coating
Synergetic effects can be obtained by combing multiple dopants and/or multiple coatings [,].
5.4.1. Dual Doping
For example, as the Mg-doping is beneficial for stabilizing the structure, while Zr-doping improves the kinetics, the electrochemical properties of NMC811 are enhanced by Mg+Zr co-doping with the discharge capacity 199.7 mAh g−1 at cut-off potential of 4.7 V, along with a capacity retention of 74.4% after 100 cycles []. W+Mg co-doping improves the electrochemical performance, because of the reduced particle size induced by the W6+ and increased free electron induced by the Mg2+ [] (see Figure 14). This co-doping of LiNi0.9Co0.06Mn0.04O2 single-crystal raised the capacity retention to 86.7% after 150 cycles at 2 C current density. K+F co-doping of single-crystalline LiNi0.85Co0.05Mn0.10O2 (NCM85) endowed this cathode with a very high reversible capacity of 222.3 mAh g−1.
Figure 14.
Electrochemical performance comparison of pristine LiNi0.9Co0.06Mn0.04O2 (NCM) and NCM co-doped with W and Mg (NCM-W). (a) Rate capability of NCM-WM in 3.0–4.3 V. Cycling performance of NCM and NCM-WM at 25 °C between 3.0 and 4.3 V at (b) 0.5 C, (c) 1 C, and (d) 2 C. Reproduced from ref. []. Copyright 2025 Elsevier.
A superior capacity retention of 91.3% was obtained after 500 times at 1 C in pouch-type full cells, and a prediction value of 75.3% is given after cycling for 5000 h []. K+Ti-co-doped NMC811 delivered a capacity of 160.42 mAh g−1 and 91.19% capacity retention at 1 C after 200 cycles between 2.75 and 4.35 V due to the synergistic effect of K doping and Li2TiO3 coating associated with the introduction of Ti [].
K doping enlarges the interlayer space to accelerate Li+ transport, while Ti doping can strengthen Ni–O bonds to improve the structure stability. The Li2TiO3 coating protected the surface of the particles against side reactions with the electrolyte. Al+Fe co-doping into NMC622 combines the polarization effects of Fe with the lower chemical potential attributed to Al []. However, the performance was not as good as in NMC doped with Sn, studied in the same work. He et al. synthesized single-crystal LiNi0.92Co0.04Mn0.04O2 with cation Mo6+ and anion F− elements co-doped in the near-surface region. For this purpose, nano-MoO3 was additionally added at the stage of mixing precursor of the NMC synthesis. Then, NMC was powder was blended with NH4F via ball-milling []. Al+Na co-doping combines Al-doping that forms strong Al-O bonds and reduces the Li+/Ni2+ mixing, and Na-doping that widens the ion diffusion channels. As a result, the full concentration gradient Li1–yNayNi0.80Co0.05Mn0.15–xAlxO2 co-doped with 0.1 mol% Al and 1 mol% Na exhibited a capacity retention of 93% after 200 cycles at a rate of 1 C []. Mo6+ and F− constructed an appropriate Li+/Ni2+ antisite defects structure at the particle surface, boosting the interfacial stability and reducing the lattice mismatch. In addition, this surface modification maintained the low-defect Li+ layered channel inside the bulk simultaneously. The corresponding cathode delivered a high capacity of 204 mAh g−1 at 1 C. A full cell constructed with this cathode element exhibited a capacity retention of 91.9% after 400 cycles in 2.8–4.4 V, against 76.9% for the pristine cathode. Na+F co-doping of NMC622 also significantly improved the electrochemical properties []. Nb+B co-doping of NMC811 exhibits strong radial orientation and exposes a large number of active (003) lattice plane on the particle surface. As a cathode, this modified NMC811 maintained a capacity retention of 86.71% after 200 cycles at 1 C []. Li et al. synthesized a Ce+Gd dopant-concentrated layer single-crystal LiNi0.83Co0.07Mn0.10O2, including a Gd doped LiCeO2 (LCGO) shell and Ce/Gd dopant-concentrated layer []. The corresponding cathode capacity retention of 90.2% after 100 cycles at 1.0 C. In a different approach, Park and Kim introduced B and Ca in the cathode–electrolyte interface, where the B-functional group decreases the residual Li+ species, while Ca suppresses the microcracks []. With this CEI, the capacity retention after 100 cycles at 0.5 C was improved from 44.3% to 72.4%. Recently, Usman et al. synthesized V+Zn-co-doped LiNiO2 and found that LiNi0.52Zn0.16V0.32O2 has a discharge capacity of 48–246 mAhg−1 with an intercalation voltage of 5.77–3.35 V []. The Ti+Al co-substituted LiNi0.92Co0.08O2 cycled in a voltage range of 2.75−4.3 V at a rate of 0.2 C, delivered an initial capacity of 197.7 mAh g−1 with a capacity retention of 82.6% after 100 cycles []. Ti+F-co-doped NMC811 delivered a discharge capacity of 202.2 mA h g−1 at 1 C and 45 °C, with a capacity retention of 88.1% after 200 cycles (only 45.2% for the pristine NMC811). This performance was attributed not only to the bulk doping effect, but also the fact that Ti4+ and F− co-dopants induce the formation of an ultra-thin rock-salt phase on the cathode surface, which provides a protective layer []. Such a surface rock-salt nanolayer was also successfully developed on the LiNi0.9Co0.1O2 subsurface via heteroatom anchoring utilizing high-valence element molybdenum modification []. With this modification, the cathode exhibited a capacity of 245.4 mAh g−1 at 0.1 C, remarkable rate performance of 169.3 mAh g−1 at 10 C at 4.5 V, and a high capacity retention of 70.5% after 1000 cycles in full cells at a high cut-off voltage of 4.4 V. Fe+F-co-doped Ni-rich, Li-rich cathode elements have improved electrochemical properties, because the Fe dopant increases both the electronic and ionic conduction, while the F dopant can improve the cycling stability remarkably by lowering the O2p band top to suppress the lattice oxygen escape []. In addition, F doping within the oxygen sites with a larger ionic radius increases the distance between the (003) planes, and thus increases the lithium diffusivity. Moreover, the bonding energy between transition metals (TMs) and F (TM-F) is larger than that of TM-O, so that F can fix Ni2+ at the Ni site, thereby reducing the cation mixing []. In+Sn-co-doped LiNi0.85Mn0.09Al0.06O2 exhibited a capacity retention of ~100% and ~90% within the voltage range of 2.7–4.5 V at 30 °C and 45 °C, respectively, after 100 cycles at 0.4 C []. This result is due not only to the bulk doping, but also to in situ formed coating of LiInO2 effectively protecting the cathode. Na+ inserted into the Li site expands the Li slab, due to its large ionic radius, while Al3+ stabilizes the structure through a high Al-O bonding energy. Combing these two advantages, Na+Al-co-doped LiNi0.88Co0.08Mn0.04O2 cathode material exhibited a capacity retention of 84% after 50 cycles at 1 C [], higher compared with the pristine material doped with only Al or only Na. Peng et al. synthesized NMC811 modified by Mg+Ti not only in the bulk, but also by the formation of an Mg2TiO4 and Mg0.5–xTi2–y(PO4)3 outer layer with Mg and Ti vacancies []. The corresponding cathode cycled in the voltage range 2.7–4.5 V at 1 C rate exhibited a capacity retention of 89.3% after 200 cycles. Zhang et al. evaluated concentration-gradient Mg+Al-co-doped LiNi0.95Co0.03Al0.01Mg0.01O2. As a cathode, this material exhibited a high-rate capacity (172.9 mAh g−1 at 10 C rate), enhanced cyclability (95.6%, 100 cycles at 1 C) and less microcrack formation []. Ba+Al co-doping of LiNi0.85Co0.05Mn0.10O2 (NCM85) single-crystalline cathode increased the capacity up to 87.5% after 500 cycles at 1 C in a pouch-type full cell []. The strong Zr-O bond facilitates the lithium diffusion, while Ga doping can promote cation order and strengthen the TM-O bond energy, diminishing the oxygen loss. As a result, Zr+Ga-co-doped LiNi0.94Co0.03Mn0.03O2 exhibited a capacity retention of 91.9% at 0.5 C after 100 cycles, compared to 72.64% for the pristine material []. The optimal amount (0.5 mol.%) of each Na+ and Y3+ dopants increased the capacity retention of NCA to 92.34% of its capacity after 100 charge and discharge cycles at a rate of 0.5 C, compared to 67.11% for the pristine NCA []. In this case, the calculated Bader charge of oxygen in pristine and modified materials increased from −1.18 to −1.38 e in the fully lithiated structures, confirming the significant impact of doping on reducing the oxygen release rate.
Co-doping with high-valence dopants is also efficient. Li-ion pouch cells with Ta5+- and Mo6+-doped Li[Ni0.91Co0.09]O2 cathodes retain about 81.5% of their initial specific capacity after 3000 cycles at 200 mA g−1 []. Y3+ and W6+ co-doping of the single-crystal cobalt-free LiNi0.9Mn0.1O2 cathode exhibited a capacity retention of 82.1% at 1.0 C after 100 cycles (compared to 71.9% for the undoped cathode), and at 3 C the co-doped sample displayed a high specific capacity of 152.0 mAh g−1, while the value for the pristine is only 139.8 mAh g−1 []. More recently, Zhang et al. co-doped LiNi0.9Mn0.1O2 with Zr4+ and Mo6+ substituting transition metal element and Mg2+ replacing Li+ to obtain a cathode exhibiting 87.5% capacity retention after 210 cycles at 1 C and 85.5% after 150 cycles at 2 C [] (see Figure 15).
Figure 15.
Electrochemical performance comparison of pristine LiNi0.9Mn0.1O2 (NM) and NM doped with Zr4+, Mo6+ substituting transition metal element and Mg2+ replacing Li+ (NM-ZMM). Cycling performance of pristine NM and NM-ZMM electrode materials between 3.0 and 4.3 V at (a) 0.5 C, (b) 1 C, and (c) 2 C. Reproduced from ref. []. Copyright 2025 Elsevier.
The high-valence dopant W6+ and low-valence Na+ co-doping is also a good strategy, since the high-valence doping element improves the structural stability by forming strong metal–oxygen binding forces, while the low-valence doping element eliminates high cation mixing. A single-crystal Ni-rich LiNi0.83Co0.12Mn0.05O2 cathode material with this-co-doping exhibited a capacity retention of 87% after 1000 cycles (vs. graphite/Li+) in pouch-type full cells at a high temperature of 55 °C [].
Other co-doping improving the electrochemical and structural stability of NMC materials includes Na+F [,], Na+Al [], Al+Nb [,,], Ga+B [], Al+Y [], Zr+B [,], Zr+Mg [], Zr+Al [], Zr+La [], Ti+Ta [], Mo+F [], Mo+Ti [], Ti+Al [,], Cu+Ti [], B+Ti [], Mg+Ti [,], Mg+F [], Na+Y [], and Co+Ti []. Xiao et al. gave evidence of the important role of Co+Al doping in inhibiting cation disorder to increase the lithium-ion diffusion rate in LiNi0.84Mn0.10Co0.03Al0.03O2 []. Shi et al. selected Co+La co-coating. The single-crystal NMC811 prepared by Shi et al. with 2 wt.% La2Li0.5Co0.5O4 coating exhibited a capacity retention of 81.15% after 200 cycles at 1 C []. This is a 18% improvement with respect to the pristine NMC811 prepared by Shi et al., but smaller than that of the pristine nano-sized and single crystallized NMC811 particles of Lao et al. [], which emphasizes the important role of the morphology to optimize the electrochemical properties. Yoon et al. reported a room-temperature synthesis route to achieve a full surface coverage of secondary particles and facile infusion into grain boundaries, and thus offered a complete Co+B ‘coating-plus-infusion’ strategy, by applying cobalt boride metallic glass to NMC811 []. The effect of B+Al doping of LiNiO2 was investigated by Kim et al. []. They found that the low solubility of B into the host structure leads to a surface-confined distribution of B, inhibiting the growth of primary particles, whereas the highly soluble Al facilitates primary particle growth. As a consequence of this difference, boron-doping was found the most effective dopant strategy for microstructural engineering of the primary particles. The B-doped LiNiO2 cathode exhibited a capacity retention of 81% in full cells, after 300 cycles, significantly better compared with Al-doped LiNiO2. While B accumulates close to the surface, Ti is uniformly distributed in NMCs, so that the formation of strong Ti-O bond effectively alleviates the bulk phase structure degradation. As a result, Ti+B-modified NMC exhibited a capacity retention of 95.4% after 150 cycles between 2.7–4.3 V at 1 C and excellent rate performance of 137.7 mAh g−1 at 5 C []. Kuo et al. investigated the effect of B3+ and Ru5+ on the structure and electrochemical performance of NMC811 []. They demonstrated that B-doping leads to the formation of a boron oxide-containing surface coating, mainly on the outer surface of secondary particles, thus improving the cycle ability. On the other hand, Ru preferentially occupies incoherent grain boundary sites, resulting in smaller primary particle size. DFT calculations confirm that B-doping forms an enriched surface, and also find that Al-doping results in Al-rich bulk [].
Zhang et al. [] synthesized high-voltage single-crystal cathode material LiNi0.6Co0.1Mn0.3O2 with in situ doping of Zr4+ and Ti4+ by precursor co-precipitation. The synergistic effect of Zr/Ti co-doping on the transition metal (TM) sites in the SC-NCM material not only effectively improved the diffusion migration rate of Li+ but also reduced the stress concentration and cation disorder inside particles. The capacity retention rate of its soft-packed full battery was 80.6% after 4000 cycles.
Al+Zr-co-doped single-crystalline LiNi0.88Co0.09Mn0.03O2 delivered a discharge capacity of 121.2 mAh g−1 at 5.0 C (1000 mA g−1) with a cut-off voltage of 4.6 V after 350 cycles, while the pristine material falls to nearly zero after 200 cycles under the same conditions []. The authors attributed the effect of the Al/Zr doping to the reduction of the Li+ ion energy barrier from 500 meV in the pristine material to 70–130 meV, and the increase of the Ni atomic transfer barrier from the initial 2.07 eV to 2.37–2.68 eV, indicating that Ni can exist in a stable state. The Al3+ and PO43− co-doping of NMC811 inhibited the mixing of lithium and nickel, and maintained the good lamellar structure of the cathode material []. As a result, the capacity retention rate of the cathode with this modified NMC811 was 14.1% higher than that of the raw material after 100 cycles at 1 C. The rate capability was also increased, with a capacity of 155.8 mAh g−1 at 5 C. In the particular case of NCA, Al+Mn, Mg+Mn, Fe+Al, Ti+MgAl+Ti+Mg co-doping also proved useful to improve the electrochemical properties and structural stability, as reviewed in []. A comparative study of the dual-doping effect on Ni-rich cathodes is reported in Table 3.

Table 3.
Effects of dual-doping on cycling ability of Ni-rich cathode elements. Capacity densities are expressed in mAh g−1. Asterisk symbol after cathode element indicates that upper voltage cut-off for cycles is 4.5 V. Otherwise, unless specified, cut-off is 4.3 V.
5.4.2. Doping Plus Coating
Doping is often concomitant with surface coating to benefit from the synergistic effect []. When cycled at a discharge rate of 3 C for 500 cycles within 2.75–4.3 V, the cell with Ge-doped ((Ni+Mn):Ge = 100:0.5 in molar ratio) LiNi0.9Mn0.1O2 cathode and graphite anode exhibited a discharge capacity retention of 80.5%, whereas that of the cell without Ge is only 52.9% []. Note that the improvement does not come only from the Ge4+-doping, but also from the fact that the doping is accompanied with the coating with a Li4GeO4 layer. The doping stabilizes the ordered layered structure and limits the Ni-Li substitution, which increases the rate capability, while the coat performs as a barrier to reduce the parasitic reactions, which increases the cycle life. Park et al. have advanced in microstructure engineering by the formation of F-rich species on the surfaces of internal grains, combined with 2 mol% F− doping inside Li[Ni0.900Co0.047Mn0.048Mo0.005]O2 []. The full cell with this cathode material and graphite anode delivered a capacity of 177.2 mAh g−1 (90.4% of its initial capacity) after 800 cycles at 1 C and 45 °C. Synergetic effects are obtained by combining doping and coating. We have already mentioned the capacity retention of 93.7% obtained for 2% Sn-doped NMC811, after 270 cycles at 0.5 C. This capacity retention rises to 96.6% when the Sn-doped particles are also Li2SnO3-coated []. The positive effect of Sn-doping is mainly due to higher structural stability and reduction of the Li/Ni mixing, and lower polarization than those of the bare NMC811. The Li2SnO3-coating improves the interfacial stability. The same Li2SnO3-coating plus Sn4+ doping strategy has been recently utilized to modify LiNi0.8Mn0.2O2 [] (see Figure 16). This Co-free cathode with the optimized amount (2 wt.%) Li2SnO3 delivered an initial capacity of 173.20 mA h g−1 and a capacity retention of 90.54% after 300 cycles at 0.2 C, outperforming the results with pristine LiNi0.8Mn0.2O2 cathode.
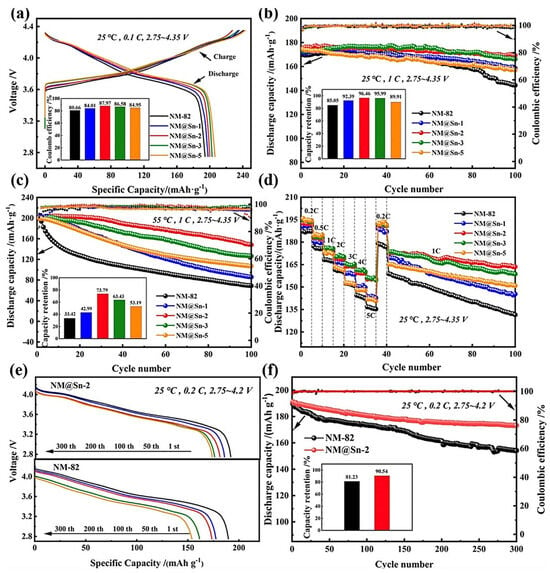
Figure 16.
Electrochemical performance of different Sn-doped LiNi0.8Mn0.2O2 materials coated with Li2SnO3 (NM@Sn-n) materials, where n is nominal mass fraction of SnO2 in wt.%. Half-cell tests: (a) Charge/discharge profiles for an initial cycle at 0.1 C, (b) cycle performance at 1.0 C at 25 °C, (c) cycle performance at 1.0 C at 55 °C, and (d) rate capability at 25 °C. Full-cell tests: (e) Charge/discharge profiles and (f) cycle performance of the NM-82 and NM@Sn-2 cathodes. Reproduced from ref. []. Copyright 2023 American Chemical Society.
The same strategy consisting in combining coating and doping was applied, choosing boron instead of the tin element. Lithium tetraborate Li2B4O7 coating and boron doping applied to the Co-less Ni-rich LiNi0.925Co0.03Mn0.045O2 cathode increased the capacity retention at 0.2 C from 59.65% for the pristine material to 72.86% after 100 cycles at 55 °C. At room temperature, the capacity retention of this B-modified cathode was raised to 76.29% after 150 cycles at 1 C []. Li et al. synthesized B-doped and La4NiLiO8-coated LiNi0.825Co0.115Mn0.06O2. This cathode demonstrated a capacity retention of 93.49% after 500 cycles at 1 C. []. This outstanding performance is due to the combination of the protecting role played by the La4NiLiO8 coat, and the boron doping that not only stabilized the structure by restraining the H2–H3 transition, but also adjusted the orientation of primary particles to a radial alignment, which is obstructive to the arise of microcracks. This result outperforms a previous study of the NMC811 with the same coating but Ti-doped instead of B-doped, which exhibited a capacity of 158.3 mAh g−1 after 200 cycles at 1 C, corresponding to a capacity retention of 90.6% []. Kim et al. synthesized a fast ionic conductor Li3BO3 coating and B3+-doped co-modified LiNi0.91Co0.06Mn0.03O2 []. This significantly improved the rate capability, with a discharge capacity of 88.6 mAh g−1 at 5 C while pristine delivered only 45.8 mAh g−1. It also improved the cycle ability, with the capacity retention of 79.4% as compared to 67.3% for the pristine material after 100 cycles 2 C. The same synergetic effect of Li3BO3 coating was used to modify single-crystal Zr-doped LiNi0.83Co0.11Mn0.06O2 (Zr-NCM83@B) []. The Zr-NCM83@B//graphite pouch-type full cell displayed excellent capacity retention of 83.5% over 1400 cycles at 1 C with high voltage range of 2.8–4.4 V.
Cao et al. produced a dual-modified coating of B2O3 and LiBO2 to the high-nickel material LiNi0.89Co0.08Mn0.03O2 []. With this protection, the cathode delivered a high capacity of 180.4mAh g−1 along with an excellent capacity retention of 90% after 100 cycles at 1 C in 2.75–4.35 V (59% for the pristine material). The same dual-modified coating was applied to NMC811 with the same success []. Li3BO3-coating was also associated with La-doping. When applied to NMC811, the capacity was raised to 230.6 mAh g−1 at 0.5 C under a high cutoff voltage of 4.8 V, with a 73.8% capacity retention after 100 cycles [].
NASICON is an acronym for sodium (Na) super ionic conductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3–xO12, 0 < x < 3. We use this term in the following in a broader sense, for similar compounds where Na, Zr and/or Si are replaced by isovalent elements. Tang et al. constructed a protective NASICON-type Li1.3Y0.3Zr1.7(PO4)3 (LYZP) coating with Zr gradient doping on the surface/subsurface of single-crystalline LiNi0.83Co0.11Mn0.06O2 (SC-NCM83) []. The full-cell LYZP-modified SC-NCM83//graphite demonstrated a capacity retention of 83.7% after 500 cycles at 0.5 C, a performance that highlights its potential for commercial applications. Another NASICON-type LiZr2(PO4)3 coating of NCA enhanced the capacity to 182 mAh g−1 at 1 C in 2.7–4.3 V, with the capacity retention of 84.6% after 100 cycles [].
The Li-free Nb oxide treatment removed surface impurities forming a LiNbO3/Li3NbO4 coating of Ni-rich cathode materials, to reduce the first capacity loss and to improve the rate performance. Nb substitution stabilized the structure, increasing the capacity retention to 93.2% after 250 cycles at C/3 rate in the voltage range 2.8–4.4 V []. Xin et al. performed Li-Nb-O coating and Nb substitution on NMC811, enhancing both the rate (158 vs. 135 mAh g−1 at 2 C) and capacity retention (89.6 vs. 81.6% after 60 cycles) performance []. Zhang et al. proposed dual-modification strategy, in which Sm was both doped into and coated on NMC811 []. With the optimum Sm content (0.5%), this modified NMC811 demonstrated a discharge specific capacity of 184.2 mAh g−1 at 1 C rate, a capacity retention of 94.19% after 100 cycles at 1 C, and a good rate performance (152.2 mAh g−1 at 5 C).
A thermochemical investigation shows that Zr preferentially form secondary phases rather than becoming doped in the NMC crystal; therefore, the equilibrium doping concentration of Zr in the NMC is very low and the material is a mixture of almost undoped NMC with a Li2ZrO3 secondary phase []. In the case of LiNi0.96Mn0.04O2, the introduction of Zr resulted in the Li2ZrO3 coating of the particles []. On the other hand, Zheng et al. successively processed NCA carbonate oxalate precursors with (NH4)2HPO4 and ZrOCl2 solutions to obtain an ultrathin Zr-contained gradient layer at the surface of NCA. Experimental characterization together with DFT calculations revealed that Zr at lithium site strengthens the adjacent (Ni,Co)-O bonds, inhibiting the oxygen evolution and stabilizing the surface. As a result, the cathode delivered a high capacity of 219.7 mA h·g−1, 23% higher than NCA at 0.1 C, and exhibits superior rate capability (128.2 mAh·g−1 capacity at 16 C) and cycling stability (capacity of 110.5 mAh g−1 after 300 cycles at 1 C) []. Dai et al. synthesized a highly-antioxidative BaZrO3 thermal barrier engineered LiNi0.8Co0.1Mn0.1O2 cathode. Analyses showed that the Zr element was penetrated uniformly into the interior region, so that the material benefited of the rigid Zr–O bonds, which improve the structural stability. On the other hand, the Ba element was only concentrated in the exterior region of secondary particles, impeding the fast heat exchange between electrode and electrolyte []. This cathode in a pouch cell delivered a specific energy density of 690 Wh kg−1 at active material level with an excellent capacity retention of 92.5% after 1400 cycles under 1 C at 25 °C. At high temperature of 55 °C, the pouch cell exhibited 88.7% in capacity retention after 1200 cycles. Here, Zr was effectively incorporated in the structure to form Zr-O bonds ensuring stability of lattice O upon cycling. As a result, the cell with this cathode and graphite anode exhibited a superior capacity retention of 92.5% after 1400 cycles at 25 °C, and a resultant 174.1 mAh g−1 with 88.7% in capacity retention after 1200 cycles at 55 °C. In situ Zr-doping achieved a gradient distribution of Zr inside the particles of full-concentration-gradient LiNi0.80Co0.05Mn0.15O2. The corresponding cathode delivered an initial discharge specific capacity of 181.7 mAh g−1 with 91.9% capacity retention at room temperature and 85.2% at 50 °C, after 200 cycles (1 C, 2.7–4.3 V) []. Instead of Zr, yttrium can be utilized []. Luo et al. utilized synergetic effects of Y3+ doping and Y2O3 coating of NMC to prolong the lifetime of Ni-rich Co-less material exposed to air []. The same synergetic effect has been observed in ultrahigh-Ni NMC cathodes, such as in situ synthesized Y-doped and yttrium orthophosphate (YPO4)-modified LiNi0.88Co0.09Al0.03O2 []. The enrichment of these functional elements Ti [], Zr, or Y at the particle surface is easily obtained in situ due to the slow kinetics of their diffusion.
Ti-doped and TiO2-coated NMC811 full cells cycled for 200 cycles between 2.8 and 4.4 V at C/3 improves from 86.1% for the pristine NMC811 to 89.4% and 91.5%, respectively []. TiO2 coating improves the cycling stability of NMC811 by minimizing contact between the active material and the electrolyte [] and reduces the air and moisture sensitivity of NMC622 []. Due to the synergistic effects of the Li3PO4 coating and Ti-doping, the dual-modified LiNi0.9Co0.09Mo0.01O2 exhibited a discharge capacity of 218.7 mAh g−1 at 0.1 C (1 C = 220 mA g−1) and a capacity retention of 93.7% at 0.5 C after 100 cycles []. Al2O3-coated/Al3+ and Zr4+ co-doped NMC811 delivered an initial capacity of 196 mAh g−1 and arrived at a high discharge capacity of 120 mAh g−1 after 200 cycles at 0.5 C, corresponding to a capacity retention of 98%, compared to 70.4% for the pristine material [].
Recently, Chen et al. modified LiNi0.84Co0.11Mn0.05O2 with Al3+ doped bulk lattice, W6+ doped subsurface region and hetero-epitaxially grown Li2WO4. The modified LiNi0.84Co0.11Mn0.05O2 showed a capacity retention rate of 88.98% after 200 cycles at 1 C rate in the potential range of 2.7–4.3 V and 82.41% after 200 cycles at 1 C with a high cut-off voltage of 4.5 V at 30 °C []. With Zr4+ doping plus Li6Zr2O7 coating LiNi0.83Co0.12Mn0.05O2 cycled at 1 C delivered a capacity of 183.7 mAh g−1 with a capacity retention of 94.6% after 200 cycles, compared to 129.9 mAh g−1 and 68.6%, respectively, for the pristine material []. W6+ doping plus WO3 coating improved the capacity retention of NMC above 90.2% after 200 cycles at 1 C []. Li et al. have recently evidenced the fastest inter-diffusivity of W6+/Mnn+ (n = 3 and 4) couple in Ni-rich cathodes. As a result, when Mn proportion is decreased to 10%, Li6WO6 coating of grain boundaries and bulk W-doping have been achieved in LiNi0.9Mn0.1O2 and LiNi0.8Co0.1Mn0.1O2 cathodes, so cyclic stabilities of W-modified LiNi0.9Mn0.1O2 have been prominently improved []. Recently, Li(Ni0.80Mn0.18Al0.02)1–xWxO2 with the optimized value x = 0.02 as a cathode delivered an initial discharge specific capacity of 212.9 mAh g−1 at 0.1 C in 2.7–4.5 V and the capacity retention reached 91.91% after 100 cycles at 1 C [], also emphasizing the effect of tungsten, which promotes Li+ migration and suppresses surface side reactions. WO3-coated and Mg2+-doped NMC811 exhibited a capacity retention of 73.5% after 100 cycles at 1 C rate against 53.6% for the pristine material [].
Xiao et al. coated K-doped LiNi0.83Co0.11Mn0.06O2 with Li3PO4. This cathode delivered a discharge specific capacity of 148.9 mAh·g−1 with a capacity retention of 84.1% after 200 cycles at 1.0 C []. PO43−-doping and Li3PO4-coating of dual modification of LiNiO2 significantly improved the structural stability, and thus the cycle ability of this electrode which exhibited a capacity retention of 81.6% (from 180.8 to 147.6 mAh g−1) after 200 cycles at 0.5 C, when cycled in the range 3–4.3V, and 51.0% capacity retention after 1000 cycles at 1 C []. The good cycle ability is due to the coating, while the improved rate capability (a capacity of 130.2 mAh g−1 at 5 C) is attributable to the enlargement in c-axis of the material crystal structure upon the PO43− doping. Li3PO4 coating was also associated with Nb5+ doping of LiNi0.83Co0.11Mn0.06O2 (NCM83) cathode powders []. The corresponding half-cell cell delivered an initial discharge specific capacity of 173.5 mAh g−1 at 1.0 C and 25 °C with a capacity retention ratio of 88.8% after 200 cycles. Li3PO4 coating was also associated with Mg2+ doping to modify LiNi0.95Mn0.05O2 to obtain a cathode with increased rate capability (130.6 mAh·g−1 at 10 C, 1 C = 189.6 mA·h·g−1) [].
Tang et al. introduced C6H9O6Sb with the precursors before calcination to synthesize LiNi0.92Co0.04Mn0.04O2 which was not only coated with Li3Ni2SbO6, but also doped with Sb3+ which has an ionic radius close to that of Ni2+ []. This Sb-modified NMC demonstrated a capacity at 5 C equal to 88.5% at 0.1 C and a capacity retention of 82.9% at 5 C after 125 cycles. Combining in situ Na+ doping with carbon coating single-crystal NMC811, Sun et al. obtained a cathode delivering a capacity of 140 mAh g−1 after 500 cycles at 5 C, which corresponds of a capacity retention of 83.1% []. In the case of Li3PO4 coating, the Ni-rich particles were not only coated, but also doped with Mg2+ [,]. In particular, Li3PO4-coated and Mg2+-doped single-crystal LiNi0.925Co0.03Mn0.045O2 cathode exhibited a retention of 89.36% after 100 cycles at 1 C (79.78% with the pristine NMC cathode) [].
Yu et al. combined in one-step Ta doping and CeO2 coating of NMC811 [] (see Figure 17). The strong Ta–O bond helped forming the elongated primary particles for inhibiting microcrack generation, and the CeO2 coating layer could effectively capture the remaining escaped oxygen. As a result, this cathode retained 90.9% of its initial capacity after 1000 cycles at 2.7–4.5 V and 1 C rate in a pouch-type full cell. LiNi0.92Co0.04Mn0.04O2 (NCM92) coated with a nano-level CeO2 buffer layer and simultaneously doped with Ce showed the discharge specific capacity of 141.4 mAh g−1 after 80 cycles at 0.2 C with 79.32% capacity retention at 60 °C []. Liu et al. prepared Mg/Ti/Sb-co-doped and CeO2-coated LiNi0.9Al0.1O2 cathode material. At a high voltage of 2.8–4.5 V and 1 C, the capacity retention of this cathode after 100 cycles reached 90.2% (from 200 mAh g−1 to 181 mAh g−1) [].
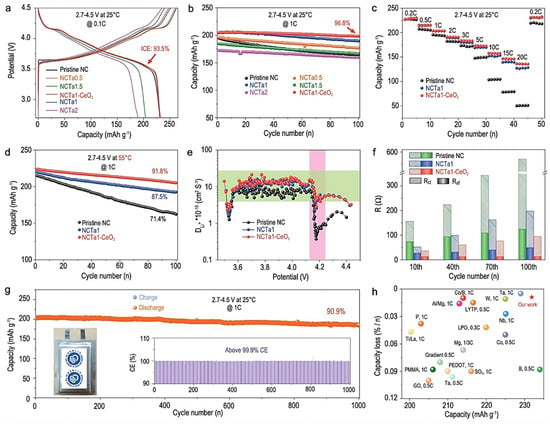
Figure 17.
Superior electrochemical performance and cycling stability in half cell and full cell with Ta-doped and CeO2 coated LiNi0.9Co0.1O2 (NCTa1–CeO2) cathodes with respect to pristine NCTa1. (a) Initial charge–discharge curves at 0.1 C and (b) cycle performance at 1 C within 2.7–4.5 V for all samples. (c) Specific capacities at 0.2–20 C, (d) cycle performance at 55 °C, (e) Li-ion diffusion coefficient based on GITT data and (f) comparison of Rsf and Rct at different cycles within 2.7–4.5 V for pristine NC, NCTa1, and NCTa1–CeO2. (g) Cycle stability at 1 C of NCTa1–CeO2//graphite full cells within 2.7–4.5 V. (h) Comparisons of 0.1 C discharge capacity and capacity loss per cycle with reported Ni-rich cathodes. Reproduced from ref. [].
Recently, Li4TeO5 surface coating along with bulk Te-gradient doping of single-crystalline Ni-rich LiNi0.90Co0.05Mn0.05O2 showed improved Li+ diffusion kinetics and thermodynamic stability, as well as an improved capacity retention of 95.83% and 82.12% after 200 cycles at the cut-off voltage of 4.3 and 4.5 V []. This illustrates the best performance that can be achieved when combining the synergetic effects of coating, doping, and single-crystal morphology. Wang et al. introduced an advanced surface reconstruction strategy combining a LiScF4 coating, Sc+F surface co-doping, and a cation-mixing layer, which improved the thermal stability, structural integrity, and electrochemical performance of Ni-rich cathodes []. A comparison between different results obtained on doped plus coated Ni-rich cathodes is reported in Table 4.

Table 4.
Effects of doping plus coating on the cycling ability of Ni-rich cathode elements. Capacity densities are expressed in mAh g−1. Unless specified, cut-off voltage is 4.3 V.
5.4.3. Multiple Coatings
Multiple coatings were achieved with PPY. In particular, dual-conductive layers composed of Li3PO4 and PPy were utilized to improve the performance of NMC811, due to the conversion of excessive Li into Li3PO4 []. Another dual-conductive coating utilized to coat NMC811 is PPy-LiAlO2. With the optimal amount of PPy coating (2 wt.%), the PPy-LiAlO2 coated NMC811delivered a capacity of 118 mAh g−1 at 10 C. The capacity retention was 90.9% after 100 loops at 1 C []. NMC811 was also enwrapped by a functional reduced graphene oxide (RGO)–KH560 polymer composite layer which consists of an inner high-flexibility epoxy-functionalized silane (KH560) layer and an outer RGO layer []. Here, the KH560 layer was especially critical in connecting the layer of outer RGO and the inner surface of the active material, while uniform coating with RGO alone is almost impossible, due to the 2D nature of RGO or graphite. The NMC811 cathode with RGO (0.5%)–KH560 (0.5%) coating exhibited a capacity retention of 95.2 after 150 cycles at 1 C.
Recently, Tan et al. designed a single-crystal LiNi0.9Co0.05Mn0.05O2 in which coherently grown MgO6 octahedra and BO4 tetrahedra were incorporated into the lattice, and a stabilizing Mg3(BO3)2 layer was concurrently formed on the particle surface []. These modifications reduced planar gliding and delamination cracking, even during prolonged cycling. A pouch-type full cell with this cathode and a graphite anode sustained a specific discharge capacity of 177 mAh g−1 after 500 cycles, with 90% capacity retention.
Cheng et al. coated NCA with a triple composite Li-ion conductor coating layer (Y(PO3)3-Li3PO4-YPO4) []. The metal phosphite Y(PO3)3 was here to optimize the surface residual alkaline lithium salt of NCA to expel H2O and CO2 in advance, which effectively reduced the electrolyte’s hydrolysis and gas generation. The electrode modified with 2 mol% Y(PO3)3 showed excellent rate performance (156.3 mAh·g−1 at 5 C) with a capacity retention of 88.3% after 200 cycles.
6. Core–Shell and Gradient Structures
6.1. Core–Shell Structures
The design of core–shell structures is a strategy commonly used to improve the electrochemical properties of Ni-rich cathode materials [,,,,,]. In such structures, the core is a Ni-rich material, and the shell is a Mn-rich component, which is structurally stronger and has thus the ability to protect the inner part from contact with the electrolyte. For example, Li et al. [] prepared a core–shell structure with the inner core composed of NMC811, while the outer layer named Li–Mn–O contains Mn4+, with higher concentration than that of the core. The corresponding cathode delivered the same capacity (118 mAh g−1) as that of pristine NMC811 at the rate of 10 C in the voltage range of 3.0–4.3 V, but the capacity decay was limited to 18.4% after 200 cycles compared to 27% decay with the pristine material. Cathode materials with the overall composition of NMC811 in which the nickel–rich composition (LiNi0.9Mn0.05Co0.05O2) is the core and the manganese–rich composition (LiNi0.33Mn0.33Co0.33O2) is the shell improved capacity retention of 76.6% compared to the commercial homogeneous NMC811 []. Bae et al. synthesized LiNi0.8Co0.1Mn0.1AlxO2 with an inner porous layer. This structure was obtained by the intermittent addition of Al salt during hydroxide precursor co-precipitation, exhibiting the spherical morphology with the structure of core (active material particle) and shell (the inner porous layer. This modified cathode material demonstrated a capacity retention of 98.2% after 100 cycles at 1 C and a cut-off voltage of 4.5 V []. In addition, this synthesis is scalable to commercial production due to its simple and facile procedure. Shi et al. redesigned Li[Ni0.7Co0.1Mn0.2]O2 into a core–shell structure with the core of Li[Ni0.8Co0.1Mn0.1]O2 delivering a high capacity and the shell of Li[Ni0.45Co0.1Mn0.45]O2 providing structurally stability []. The core–shell electrodes displayed enhanced cycling stability and thermal stability compared with the non-core–shell electrodes. Xia et al. proposed core–shell-structured Ni-rich cathodes composed of LiNi0.6Co0.2Mn0.2O2 (NCM622) shell and LiNi0.9Co0.05Mn0.05O2 (NCM9055) core. In addition, the NCM622 shell had radially oriented primary particles to reduce the surface side reaction with electrolyte and alleviate internal stress. The 40 wt.% NCM622 coating NCM9055 cathode exhibited a discharge capacity of 160.5 mAh g−1 with a capacity retention of 85.0% after 200 cycles at 0.5 C []. Ryu et al. synthesized a hybrid structure in which Li[Ni0.92Co0.04Mn0.03Al0.01]O2 in the interior of the particle is encapsulated by Li[Ni0.845Co0.067Mn0.078Al0.01]O2 that acts as a buffer against microstrain []. This hybrid cathode structure suppresses the formation of microcracks by creating a non-uniform spatial distribution of the microstrain within the cathode particle. As a result, this hybrid cathode material retained 84.7% of its initial capacity after 1500 cycles at 1 C, which is a 50% increase with respect to the retention obtained with the pristine Li[Ni0.9Co0.045Mn0.045Al0.01] bulk material. The presence of LixWyOz secondary phases in the grain boundaries eliminated the interdiffusion of Mn and Al from the shell to the core in these Ni-rich core–shell materials. Park et al. [] constructed a core–shell structure with the core consisting mainly of Li[Ni0.957Mn0.043]O2, while in the 3 µm-thick shell. the Ni concentration decreases to 83% and the Mn concentration increases to 17% at the secondary particle surface, resulting in the surface composition of Li[Ni0.834Mn0.166]O2. Therefore, the cathode material had the composition of Co-free Li[Ni0.9Mn0.1]O2. This cathode retains 78.5% of its initial capacity after 2000 cycles at 1 C charge and 0.8 C discharge rates, and retains 79.5% capacity after 1000 cycles under fast-charging conditions (3 C charge and 1 C discharge), demonstrating the effective dissipation of internal strain and chemical protection provided by the Mn-rich shell. This recent result outperforms the electrochemical properties of prior Co-free cathodes [,], and proves that both cycle life and rate capability can be reached even with Co-free Ni-rich cathodes. So far, such a performance was obtained only with presence of cobalt, because Co3+ alleviates magnetic frustration during cycling, thus reducing the disordered cation mixing of Li+ and Ni2+ [], and increases electronic conductivity through CoO6 octahedra with delocalized electronic states. The success of the cathode in [] also comes from the fact that Mn-based shells are less prone to interdiffusion, compared to Mg- and Al-based shells, while interdiffusion has been identified as a major challenge to overcome for the preservation of core–shell structure. Han et al. proposed a new approach that fully utilizes Co by forming a Co nanoshell on a Ni-rich [Ni0.90Co0.05Mn0.05](OH)2 precursor via a single coprecipitation process []. The Co nanoshell acts as a sintering inhibitor during calcination, thereby generating columnar structures that are not observed in the baseline Li[Ni0.90Co0.05Mn0.05]O2 cathode material. The Co nanoshell markedly improved the Li+ kinetics and cycling stabilities of Ni-rich cathode materials.
Xue et al. introduced a proton-rich (ammonium bicarbonate) shell on LiNi0.92Co0.06Mn0.02O2 single crystals []. Once released during the first charge, the proton is immediately captured by LiPF6, in situ electrochemically conversing to LiF and Li3PO4 sub-nano particle dense maskant. This surface modification enables 95% capacity retention after 100 cycles at a high voltage of 4.5 V in the half cell, and 83% capacity retention after 800 cycles in the full cell with graphite anode.
Tungsten oxide (WO3) coating on the surface of precursors leads to the formation of LixWyOz secondary phases during the heat treatment with LiOH·H2O. These LixWyOz phases infuse into the grain boundaries and prevent interdiffusion between the core and shell phases []. This process was proposed by Rathore et al. who constructed a cobalt-free core–shell cathode material composed of inner core Ni(OH)2 and outer shell NixMy(OH)2 [], enhancing the mechanical strength and electrochemical performance. This behavior is probably not unique to W, and it is expected that elements like Mo, Zr and Nb, which are also found in the grain boundaries, can be used to prevent the interdiffusion between shell and core, so that Co-free Ni-rich cathodes are very promising for the next generation of lithium batteries. Recently, Liu et al. used synergetic effects of Li2WO4 coating and W6 doping to obtain a modified single-crystal LiNi0.9Co0.05Mn0.05O2 (Ni90) cathode exhibiting 89.8% capacity retention after 300 cycles at 0.5 C []. Chu et al. coated single-crystal LiNi0.92Co0.04Mn0.04O2 with a layer composed of Li–B–O species and Li2WO4. The cycling stability of such a cathode at a high cut-off voltage of 4.5 V was improved from 71.8% to 87.1% at 1 C after 100 cycles []. Du et al. introduced W into LiNi0.9Co0.05Mn0.05O2 and discovered that, during high-temperature solid-state reactions, a Li4NiWO6 defect phase is formed. This phase, combined with the amorphous LixWyOz coating layer improved the electrochemical properties of this cathode, which exhibited a capacity retention of 97.31% (200.5 mAh g−1 to 195.1 mAh g−1) after 200 cycles in the voltage range 3–4.3 V at 0.5 C and 30 °C [].
The hollow multi-shell structure (HoMS) allows Ni-rich cathode materials to have sufficient pore space to buffer the structural instability caused by volume expansion in the cyclic process. In particular, Ding et al. chose micro- and nanostructured Co3O4 as a template of the HoMS because the annealed Co3O4 template has a unique HoMS layer structure, to prepare a NMC622cathode material that demonstrated a capacity of 148.5 mAh g−1 after 100 cycles at 0.5 C, corresponding to a capacity retention of 90.4% [].
6.2. Gradient Layers
Concentration gradient cathodes are a good strategy to concentrate high Ni-concentrations in the core region, and smaller Ni concentrations near the surface to improve the structural stability. The concentration gradient design originates from a simple core–shell structure in which the core is Ni-rich NMC while the shell has other NMC compositions (lower Ni content and higher Mn content) to stabilize the layer structure at the surface. Concentration gradients are an alternative to core–shell structure to confine the Ni-rich part of the particles in the core. Actually, the two procedures are related when some interdiffusion occurs between the shell and the core. However, concentration gradient concept has an advantage, as it can effectively solve the problem of the reduced Li diffusion caused by the contraction of the crystal cell volume between the high-nickel core and the gradient shell [,,,]. Moreover, the core–shell design is responsible for voids generated during cycling between the shell and the core during long-term cycling and mismatches of different lattice parameters. Gradient layers avoid this problem, and have been used constantly for a decade to synthesize Ni-rich cathode materials [].
The improved electrochemical properties of a gradient material with a high-nickel NMC811 as the core and a low-nickel LiNi0.46Co0.23Mn0.31O2 as the shell illustrate the benefit of the reduction of surface microcracks, and the rod-shaped morphology of the primary particles with radial growth orientation []. As a result, the capacity retention of 70% after 1000 cycles at a high temperature of 55 °C. The same morphology and same benefit were experienced with a material of average composition LiNi0.75Co0.10Mn0.15O2 with gradual composition with the inner core composed of high-nickel low-Mn LiNi0.86Co0.10Mn0.04O2, and the outer layer composed of low-nickel high-Mn LiNi0.70Co0.10Mn0.10O2 []. More recently, Wu et al. synthesized a Ni concentration gradient LiNi0.8Co0.15Al0.05O2 (NCG-NCA) cathode material using the characteristic reaction of Ni2+ and dimethylglyoxime []. Owing to the high nickel content in the core, and high cobalt content on the surface stabilizing the surface of the particles, the capacity retention was increased at 75% after 200 cycles at a current density of 10 C (1 C = 160 mA g−1) under a high cut-off voltage of 4.5 V, compared with 50% for the homogeneous NCA material. Park et al. synthesized a core–shell with concentration gradient Li[Ni0.90Co0.045Mn0.045Al0.01]O2 (CSG-NCMA90) consisting of fine, elongated primary particles that are radially aligned from the center of a spherical secondary particle []. This microstructure effectively suppressed the formation of cracks in the charged state, in agreement with the general trend that a spherical secondary cathode particle shape with uniform morphology can reduce the production of microcracks by effectively dispersing structural strain during cycling []. Moreover, these elongated particles largely featured (001) facets on their lateral sides, and were tolerant of electrolyte attack, thus suppressing surface degradation, enabling the CSG-NCMA90 cathode to retain 90.7% of its initial capacity after 1000 cycles at 100% depth of discharge (in the voltage range 3.0–4.2 V) at 1 C rate. The oriented growth of primary grains along (001) and parallel stacking of nanosheets can be achieved by optimizing the stable adsorption structures of metal-ammonia complexes on the precursor crystal facets under aqueous ammonia conditions [,]. Ryu et al. constructed a hybrid-structured Li[Ni0.9Co0.045Mn0.045Al0.01] (HS-NCMA90) cathode, in which Li[Ni0.92Co0.04Mn0.03Al0.01]O2 forms the interior of the cathode particle enclosed in a buffer layer of Li[Ni0.845Co0.067Mn0.078Al0.01]O2. This structure develops radially aligned primary particles, enabling the primary particles to contract uniformly, thereby effectively suppressing the propagation of microcracks toward the outer surface []. The pouch cell with this cathode and a graphite anode retained retaining 90.0% of its initial capacity after 1000 cycles at 1 C in the voltage range 3.0–4.2 V. Another example of the effectiveness of the nanorod configuration is provided by Liu et al. who designed a unique bayberry-like Li1.2Mn0.54Co0.13Ni0.13O2 cathode material, composed of a spherical core and the shell self-assembled by radially oriented nanorods [] (see Figure 18). The radial texturing of the nanorods in shell layer formed a natural protective interface constituted by thermodynamically stable (003) planes, so this cathode delivered remarkable high-rate long-term cycling stability with capacity retentions of 91.2% after 500 cycles at 1 C and of 81.3% after 1000 cycles at 5 C. Guo et al. constructed a concentration-gradient LiNi0.753Mn0.247O2 cathode that consists of the elongated and radially aligned primary particles through Mo+Ti+Mg doping []. With the synergetic effect of gradient concentrations and multi-doping, this Co-free Ni-rich cathode exhibited a capacity retention of 81.1% after 1000 cycles in a pouch full cell (commercial graphite anode) at 1 C within 2.7–4.5 V.
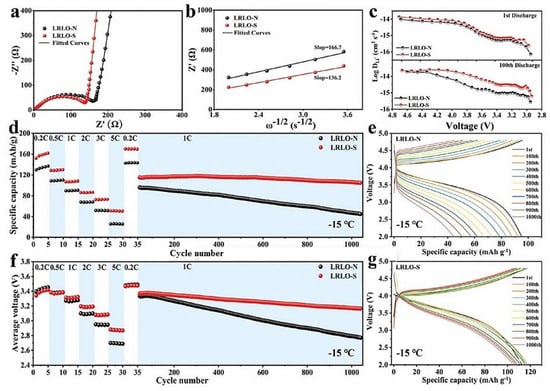
Figure 18.
Properties of bayberry-like Li1.2Mn0.54Co0.13Ni0.13O2 (LRLO-S) cathode material, composed of a spherical core and the shell self-assembled by radially oriented nanorods, in comparison with the same material with a different microstructure (LRLO-N). (a) Nyquist plots and (b) corresponding profiles of Z′ versus w−1/2 of LRLO-N and LRLO-S in half-cells. (c) Calculated Li+ diffusion coefficients of LRLO-N and LRLO-S during discharging process after 1st and 100th cycles at 1 C. (d) Rate performance and subsequent long-term cycling performance at −15 °C and (f) corresponding average voltage and low-temperature charge/discharge curves at 1 C for (e) LRLO-N and (g) LRLO-S. Reproduced from ref. []. Copyright 2024 Wiley.
These results illustrate that concentration gradient layers can reshape primary particles into a rod-like or even needle-shaped structure, which can promote Li+ movement by minimizing diffusion pathways []. Such cathode materials are usually prepared by calcining hydroxide precursors with Li salts at an optimum temperature, high enough to obtain a good crystallization, but small enough to keep the rod-like morphology of the precursors. Jeyakumar et al. synthesized a double concentration-gradient Li[Ni0.90Co0.04Mn0.03Al0.03]O2 with a Ni-rich-core, an Mn-rich shell, and Al on top surface []. This cathode exhibited a capacity retention of 91.5% after 100 cycles and 70.3% after 500 cycles at 1 C (against 83.4% and 47.6% for the material with uniform composition). While the concentration gradient concerns usually the concentration of nickel, Liu et al. synthesized a 0.5(LiNi0.8Mn0.2O2)bulk0.5(LiNi0.8Co0.2O2)surface sample (denoted as NM-NC82), where the Ni concentration is constant (x = 0.8) with a composition design where the central component is Ni/Mn/Co (8:2:0, molar ratio), then gradually changes to Ni/Mn/Co (8:0:2, molar ratio) toward the surface of the particle []. Benefiting from lower stiffness in Co-enriched surfaces, this cathode material showed negligible morphology damage, due to an improved stability of the H2–H3 phase transition. Maximizing the advantages of coating, doping, and concentration gradient, Park et al. constructed a core–shell structure in which the shell with Ni fraction of ≈84% uniformly surrounded the core part of Ta-doped NMC particles of composition Li[Ni0.935Co0.040Mn0.020Ta0.005]O2 with Ni fraction of ≈97% []. When cycled in pouch-type full cells using graphite anodes in the voltage range of 3.0–4.2 V, this cathode demonstrated a capacity retention of 92.6% after 1000 cycles when charged at 2 C (discharge current 1 C).
Wu et al. identified recently the effectiveness of using such an anionic surfactant (β-styryl phosphonic acid) to assist the preparation of Ni0.8Co0.1Mn0.1(OH)2 precursors in the coprecipitation []. With this configuration, the NMC811 exhibited capacity retention rates could reach 93% (164.5 mAh g−1) after 100 cycles and 84.3% (149.0 mAh g−1) after 300 cycles. Shang et al. synthesized NMC811 with dual Ni gradients on both primary and secondary particles by applying Ni-MOF-74 during the coprecipitation of precursors []. This cathode delivered a capacity of 203.5 mAh g−1 at 0.1 C. At C/3, and the capacity retention was 88.5% after 300 cycles with a coulombic efficiency larger than 99.86%. At 1 C, the capacity retention was 84.1% after 500 cycles.
Gradient NMC cathode materials with high stability and excellent performance can be synthesized by capitalizing on the unique reactivity of the NMC precursor with ammonia and KMnO4 []. According to this method, surficial Ni is dissolved from the NMC precursor through ammoniacal leaching and subsequently MnO2 is deposited on the surface by reducing KMnO4. This process allows for precise adjustment with reduced surficial Ni and enriched Mn. A 1.2 Ah pouch cell configurated with this modified NMC cathode and graphite anode demonstrates a lifespan of over 500 cycles with only 8% capacity loss.
NMC811 with a surface gradient layer and lithium phosphorus oxynitride (LIPON) coating showed improved capacity retention []. The formation of highly electronegative PO43− polyanions in the layered material after P-doping enables the expansion of the layer spacing and stabilization of the lattice structure. Liu et al. synthesized P-doped NMC811 by a co-precipitation process, to obtain a cathode that exhibited a capacity retention of 83.53% after 100 cycles at 1 C []. Choi et al. coated 1 wt.% lithium and cobalt acetate precursor on the inner and outer surfaces of commercial polycrystalline NMC811, which improved electrochemical properties in cells with liquid electrolytes. On the other hand, when the coating was mainly on the outer surface of the material, the performance was improved in cells with solid-state electrolytes, as the contact between the electrolyte and the active material is only at the surface of the polycrystalline NMC811 [].
Gradient layers can be extended into full concentration gradient (FCG) layers or multiple gradient layers with different slopes, to maximize the Ni content at the core as compared with single-sloped gradient layers. For instance, full-concentration-gradient Li[Ni0.78Co0.10Mn0.12]O2 obtained with the optimized calcination temperature of 790 °C exhibited remarkable cycling stability, retaining 86.3% of its initial capacity after 4000 cycles, and superior rate capability []. Wu et al. studied a series of full concentration gradient-tailored Li-rich layered oxides, and found that the gradient-tailored materials with medium-slope show the best electrochemical performance (capacity retention of 88.4% within 200 cycles at 200 mA g−1) []. Zhang et al. combined the full concentration strategy of Ni-rich material (overall composition: LiNi0.75Co0.15Mn0.1O2) with Ti pillar and Li2ZrO3 (LZO) coating modification []. The corresponding cathode exhibited 92.3% capacity retention at 1 C, and delivered a capacity of 137.9 mAh g−1 at 10 C rate. Radially oriented rod-shaped primary particles in a cathode, resulting from the concentration grading (or compositional partitioning) of the cathode, also showed markedly improved electrochemical and thermal properties. [,,]. In particular, a cathode with full-concentration-gradient Li[Ni0.78Co0.10Mn0.12]O2 active element retained 86.3% of its initial discharge capacity after 4000 cycles at 1 C in a full cell []. NMC811 with radially orientated primary particles resulting from Mn composition grading delivered ≈180.1 mAh g−1 at 1 C and retained 96.2% of its initial discharge capacity after 100 cycles []. This is also the capacity retention obtained by designing a concentration gradient core–shell structure of Li(Ni0.794Co0.11Mn0.096)O2 consisting of a nickel-rich core with a Ni:Co:Mn mole ratio of 9:0.5:0.5, two interlayers with increasing Mn content gradient, and a high-manganese thin shell []. Ni0.95Mn0.025Co0.025O2 with a Ni/Co gradient and radially oriented particles demonstrated greatly improved cycling stability (capacity fade per cycle of 0.04 ± 0.01% over 300 cycles at 1 C) []. Guo et al. reported the design and synthesis of a full concentration gradient LiNi0.75Mn0.20Co0.05O2 cathode with a Mn-rich Ni-poor surface, which has been realized by in situ forming a PO43− gradient distribution after Li0.1B0.967PO4 coating []. This cathode delivered a capacity of 212.6 mAh g−1 at 2.7–4.5 V with an energy density of >800 Wh kg−1cathode. In commercial-grade full cells with commercial graphite anodes, a superior cycle life of 80.5% capacity retention is achieved at 1 C within 2.7–4.5 V after 1700 cycles. This performance is comparable to the state-of-the-art cathodes with Ni content of 90% at 2.7–4.3 V, but is safer as the exothermic peak is at higher temperature (261.4 °C). The concentration gradient design is thus particularly effective in the fabrication of high-density lithium batteries.
Gradient structure refers usually to a Ni concentration gradient, but concentration-gradient doping element is also a good strategy to improve the electrochemical properties [,]. Mu et al. developed a gradient Zr element doping, the orientations of which were achieved via coprecipitation in a positive or negative correlation between the concentrations of Zr and Ni. They found that the material behaves better when the Zr content is abundant in the core. The gradient Zr-doped LiNi0.65Co0.20Mn0.15O2 delivered a higher capacity retention of 85.6% after 300 cycles at 1 C (70.7%, 121.4 mAh g−1 for the pristine sample) and an outstanding rate performance of 122.5 mAh g−1 at 20 C []. Zhang et al. improved the performance of LiNiO2 via concentration-gradient yttrium modification with LiYO2-Y2O3 coating layer []. Other doping elements with gradient concentrations also led to better electrochemical properties. In particular, concentration-gradient niobium-doping strategy was applied to modify single-crystal LiNi0.83Co0.12Mn0.05O2, with Nb concentration decreasing linearly from the surface to the core of the particle [].
7. Cells with Ni-Rich Cathodes
The general progress on the engineering of electrolytes for lithium batteries is out of the scope of the present work and have been reviewed elsewhere (for a recent review, see []). We only report in the following recent results obtained concerning batteries based on Ni-rich cathodes with Ni content > 0.5 of interest in the present work. The reason why we specify this point comes from the fact that electrolytes working for Ni concentrations ≤ 0.5 do not necessarily work at higher concentrations. For instance, for various additives, which can greatly enhance the performance of Li-ion batteries with LiNixCoyMn1–x–yO2 (x < 0.6) cathodes, there is no obvious improvement for Ni-richer cathode materials [].
7.1. Solid Electrolytes
All-solid-state lithium batteries (ASSLBs) with Ni-rich cathodes are the most promising solution to improve the safety and thermal stability limited by the conventional organic liquid electrolytes. In particular, sulfide-based solid electrolytes have been considered []. However, the interfacial resistance caused by poorly conductive side products, the contact loss, and the space charge layer effect have limited the application of Ni-rich oxide cathodes in sulfide-based ASSBs []. Again, coating has also been considered as a solution to overcome these difficulties. Payandeh et al. studied the effect of Li2CO3/LiNbO3 coating on the cycling performance of NCM-851005 (85% Ni content) in pellet-stack solid-state-battery with Li6PS5Cl as solid electrolyte and Li4Ti5O12 anode and found that 80% of the initial specific discharge capacity is retained after 200 cycles (~160 mAh·g−1, ~1.7 mAh·cm−2) at C/5 and 45 °C []. Walther et al. showed that the Li2CO3/LiNbO3 coating suppresses the interfacial reaction at the cathode active material/Li6PS5Cl interface, in particular, the formation of oxygenated phosphorous and sulfur compounds []. An ASSB with LiNbO3-coated NMC811, an electrolyte consisted of a polar poly(vinylidene fluoride–co–trifluoroethylene) (P(VDF–TrFE)) framework and Li6PS5Cl via an electrospinning–permeation–heating–pressing way Li6PS5Cl electrolyte, and Li-In anode showed 71% capacity retention after 2000 cycles at current density of 0.1 mA cm−2 []. Li3PO4 coating also fulfill the purpose, because this phosphate contains the same anion (O2−) and cation (P5+) species as those present in the cathode and sulfide electrolyte, respectively, preventing the exchange of S2− and O2− in Li6PS5Cl and NMC []. Atomic layer deposition of LiAl(PO3)4 onto LiNi0.88Co0.09Mn0.03O2 (NCM88) accelerated the charge transfer and mitigated the interfacial deterioration. The ASSB with this modified cathode, Li6PS5Cl electrolyte, and Li-In anode, with a high loading of 20.1 mg cm−2 exhibited a reversible capacity of 4.25 mAh/cm2 and a capacity retention of 98.3% after 440 cycles at 0.5 C [] (see Figure 19). Lin et al. constructed a LiNi0.83Co0.12Mn0.05O2 (NCM83125) cathode modified by Zr+F co-doping, plus cyclized polyacrylonitrile (cPAN) coating. This polymer was chosen because the pyridine-based structure with delocalized π bonds via thermal cyclization of PAN units endows cPAN with satisfactory electronic conductivity. ASSLBs utilizing this modified NCM83125 as a cathode, Li6PS5Cl electrolyte, and Li-In anode, exhibited a higher initial capacity (175 mAh g−1) and Coulombic efficiency (82.7%), a capacity retention of 95% after 300 cycles at a rate of 0.2 C, and an enhanced rate performance (109 mAh g−1 at 3 C) []. Via atomic layer deposition of Li3PO4 and subsequent in situ formation of a gradient Li3P1+xO4S4x coating, Liang et al. obtained a conformal covering for NMC811 particles []. Tested in combination with an indium metal negative electrode and a Li10GeP2S12 solid electrolyte, this modified NMC811 enabled the delivery of a specific discharge capacity of 128 mAh g−1 after almost 250 cycles at 0.178 mA cm−2 and 25 °C. using advanced molecular layer deposition (MLD) technique, Su et al. coated single-crystal NMC811 with an elastic and sticky alucone/glycol layer []. This coat enabled NMC to be in close contact with the sulfide electrolyte, so that the ASSB with a mass loading of 20.4 mg cm−2 at 60 °C exhibited the capacity retention of 80.0% after 1000 cycles at 1 C.
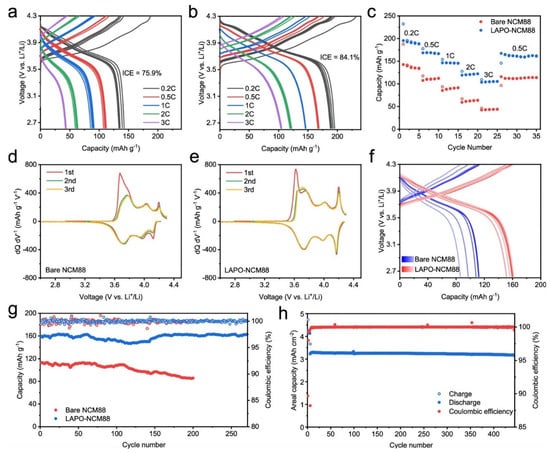
Figure 19.
Electrochemical tests of bare LiNi0.88Co0.09Mn0.03O2 (NCM88) and LiAl(PO3)4-coated NCM88 (LAPO-NCM88) between 2.62 and 4.3 V at 30 °C. Galvanostatic charge–discharge voltage profiles of ASSLBs based on (a) bare NCM88 and (b) LAPO-NCM88 at different rates. (c) Rate capability comparison between bare NCM88 and LAPO-NCM88-based ASSLBs. Mass-normalized dQ/dV curves of ASSLBs based on (d) bare NCM88 and (e) LAPO-NCM88 at 0.2 C. (f) Galvanostatic charge–discharge voltage profiles of bare NCM88 and LAPO-NCM88-based ASSLBs at 1st, 20th, 50th, 100th, 150th, and 200th cycle at 0.5 C. (g) Cycling stability and Coulombic efficiency of bare NCM88 and LAPO-NCM88-based ASSLBs at 0.5 C. (h) Cycling stability and Coulombic efficiency of LAPO-NCM88-based ASSLB at a LAPO-NCM88 areal loading of 20.1 mg cm−2. Reproduced from ref. []. Copyright 2024 American Chemical Society.
To overcome the limitation of the performance due to the low conductivity of metal oxides used as coating layers, Kim et al. coated Li[Ni0.88Co0.11Al0.01]O2 with Li3YCl6, the conductivity of which is 0.40 mS cm−1. The solid-state battery with this Li3YCl6-coated NCA, Li6PS5Cl as solid electrolyte, and Li-In anode exhibited a capacity of 134 mA h g−1 at 2 C (vs. 53 with the pristine NCA) []. Li6.25La3Zr2Al0.25O12 -coated Zr doped NMC811 exhibited a capacity retention of 70.1% after 1000 cycles at 1 C in a full ASSB cell with Li-In anode and Li6PS5Cl electrolyte, demonstrating that this coating layer enhanced the stability between the Ni-rich cathode material and the sulfide electrolyte []. Wang et al. proposed a new process, where LiNi0.88Co0.09Mn0.03O2 (NCM88) cathode is sulfidized via a time-/cost-efficient strategy in a mixed N2 and CS2 gas atmosphere, improving the interfacial compatibility with Li6PS5Cl. The corresponding ASSB with Li6PS5Cl electrolyte and Li4Ti5O12 anode delivered a capacity of 200.7 mAh g−1, 87% capacity retention after 500 cycles, and satisfactory rate capability (158.3 mAh g−1 at 1 C) []. Kitsche et al. prepared HfO2-coated LiNi0.85Co0.10Mn0.05O2 (NCM-851005) via atomic layer deposition (ALD) of tetrakis(ethylmethylamido)hafnium(IV) with O3 as the oxidant []. The test in a cell with this modified cathode, Li6PS5Cl electrolyte and Li4Ti5O12 anode exhibited a capacity retention of 82% after 60 cycles at 0.5 C and 45 °C. A drawback with the use of thiophosphate-based electrolytes is their limited stability toward the oxidizing potentials of Ni-rich cathodes, which limits the cycle ability of the ASSBs. Therefore, efforts are made to choose different solid electrolytes, such as chloride-based SEs, principally Li3InCl6. However, Li3InCl6 is unstable when in contact with the Li anode, so that Gao et al. introduced a layer of Li3InCl6 adjacent to the LiNi0.83Mn0.06Co0.11O2 cathode, and a Li6PS5Cl layer placed between this and the Li-In alloy acting as the anode []. Therefore, Li6PS5Cl could not be entirely suppressed. Nevertheless, the cell made of NMC, solid electrolyte, and carbon nanofiber mass ratios of 65, 30, and 5 wt.%, respectively, showed significantly improved electrochemical properties. When cycled at 1 mA cm−2 and 80 °C under 2 MPa stack pressure at a cutoff voltage of 4.2 V, the cell delivered a capacity of 118 mA h g−1total, with capacity retention of 94% ± 1.2% after 50 cycles. Note the stack pressure was needed to obtain this result by limiting the volume change of the cathode material to approximately 2.5% during cycling.
The coating of the particles proved useful to suppress the cracking of the particles and protect them from side reactions with the electrolyte. However, the process increases the cost of fabrication of the cathodes. Recently, solid-state electrolytes (SSE) were employed to prevent the cracking of the Ni-rich particles without the need of coating, provided that intimate contact between the cathode material and the solid electrolyte is maintained. For this purpose, sulfide solid electrolytes should be avoided with unprotected Ni-rich materials, because they decompose at a cathode voltage as low as 2.6 V []. Note, however, that Kim et al. constructed an all-solid-state battery utilizing the argyrodite-type sulfide electrolyte, Li6PS5Cl (LPSCl), as the solid electrolyte, with an NMC811 cathode material coated with lithium lanthanum titanate (LLTO). The authors employed a rapid technique known as flash-light sintering (FLS) to fabricate a uniform and pure perovskite protective layer without inducing cation mixing in NMC []. The Li-In/LPSCl/modified NMC811 cell cycled in the voltage range 2.5–4.25 V exhibited an initial capacity at 1 C of approximatively 100 mAh g−1 lower than that of bare NMC811 (167.96 mAh g−1), and a better cycle ability, with a capacity retention of 95%, but over 40 cycles only. Zhao et al. coated quasi single-crystalline LiNi0.83Co0.12Mn0.05O2 (SC83) particles to protect the cathode material from Li6PS5Cl solid electrolyte. The all-solid-state cell exhibited a capacity retention of 88% after 1000 cycles at the 1 C rate, compared to only 71% for the uncoated counterpart [] (see Figure 20).
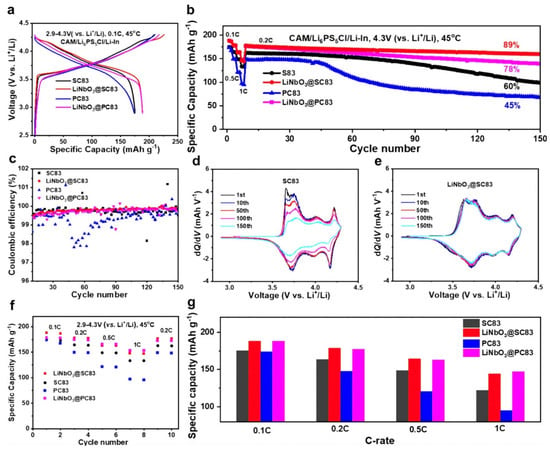
Figure 20.
Cathode performance of all-solid-state batteries using quasi single-crystalline LiNi0.83Co0.12Mn0.05O2 (SC83) and SC83 coated with LiNbO3 cathode materials, and Li6PS5Cl electrolyte. (a) First-cycle voltage profiles at 0.1 C and 45 °C, (b) long-term cycling stabilities at 0.2 C, and (c) corresponding Coulomb efficiencies. Differential capacity curves of 1st, 10th, 50th, 100th, and 150th cycles for (d) SC83 and (e) LiNbO3@SC83. (f) Rate capabilities and (g) corresponding specific capacities at C-rates ranging from 0.1 to 1 C. Reproduced from ref. []. Copyright 2025 American Chemical Society.
On the other hand, the employment of >4 V-class cathode materials is enabled without any protective coating with lithium metal halide solid-state electrolytes, due to their high electrochemical oxidation stability [,,,]. An all-solid-state Li battery with LiNi0.85Co0.1Mn0.05O2 cathode and Li2InxSc0.666–xCl4 (0 ≤ x ≤ 0.666) electrolyte exhibits a long life of >3000 cycles with 80% capacity retention at room temperature. High cathode loadings are also demonstrated in ASSBs with stable capacity retention of >4 mAh cm−2 (~190 mAh g−1) []. A remarkable performance was achieved in an all-solid-state cell composed of a single crystallized uncoated NMC811 as a cathode, a Li3YCl6 electrolyte, and a Li–In alloy anode: this ASSB delivered a discharge capacity of 170 mAh g−1 at C/5 and an impressive capacity retention of nearly 90% after 1000 cycles []. Liu et al. reported the development of free-standing-type composite cathodes containing NCM (Ni stoichiometries ≥ 0.83) active materials and Li3InCl6 (LIC) solid electrolyte. The strong interface was realized via a novel in situ recrystallization process of LIC. The resulting free-standing composite cathode containing LiNi0.88Co0.06Mn0.05Al0.01O2 (NCM88) and LIC sustained 1500 charge–discharge cycles under a low operating stacking pressure ~2 MPa [].
7.2. Liquid Electrolytes
With liquid electrolytes, the residual Li species present on the surfaces of Ni-rich particles promote the decomposition of electrolytes to produce corrosive HF. This enhances the dissolution of the TM ions in the electrolyte [], and it reduces the Li+ diffusion coefficient []. The migration of these TM ions to the anode side hinders the insertion of lithium inside the anode, which implies a loss of capacity and the deterioration of the battery []. Note that there is a difference between NMC and NCA materials concerning the dissolution of TM ions. The disproportionation of Mn ions (Mn3+ → Mn4+ + Mn2+) may lead to more severe dissolution of Mn than Ni and Co ions []. This is consistent with the fact that the capacity loss of batteries is closely related to the concentration of Mn ions in the cathode. Actually, a Ni-rich pristine NCA cathode exhibits much less dissolution of TM (where TM = Ni) than a Ni-rich pristine NMC (where TM = Ni + Mn), due to the stabilized effect of Al3+ [].
Highly delithiated (highly charged) states generate large amounts of Ni4+ that can react with electrolytes. This results in a thickening of the cathode–electrolyte interface (CEI), reducing the number of available Li+ and generating a resistive surface layer []. The high Ni4+ concentration also leads to a decay in the electrochemical reaction activity []. It will also result in a decrease of the thermal stability and thus an increase of the risk of thermal runaway of the battery [,]. Above 4.2 V, the evolution of CO2 gas and the generation of a heat flow from NMC cathodes [] give evidence of such a reaction of the particles with the electrolyte. In addition, remaining at a highly delithiated state can cause significant electrolyte oxidation resulting in other side reactions []. To remedy this drawback, additives are employed in the electrolytes. In particular, a mixture with 2 wt.% prop-1-ene-1,3-sultone (PES) + 2 wt.% tris-(trimethyl-silyl) phosphite (TTSPi) + 2 wt.% 1,3,2-dioxathiolane-2,2-dioxide (DTD) in the control electrolyte (1 M LiPF6 ethylene carbonate (EC): ethyl methyl carbonate (EMC) 3:7 w:w electrolyte), named PES211, can be used in Ni-rich cells to suppress the gas evolution []. In addition, rock-salt surface layers known to be formed with the use of VC are not formed with PES211. In practice, however, the Ni-rich cells should not be fully charged, which, however, implies a loss of energy density available with these batteries []. In addition, additives added to the electrolyte must help in the formation of the cathode–electrolyte interphase (CEI), but also of the solid–electrolyte interphase (SEI) on the anode side. In particular, in a full cell graphite/Ni-rich cathode, the dissolved Ni migrate to the graphite side and the strong correlation between Ni2+ and Li+ in the SEI is the main reason for the increase of SEI resistivity []. Many results are reported for full cells with graphite anode. However, other anodes can be chosen, in particular calcium tetrafluoroborate (Ca(BF4)2) as additive for the rational design of interfacial layers on both Li metal anode and Ni-rich cathode []. In this case, Li-Ca alloy suppressed the growth of Li dendrite by reducing the nucleation polarization. The preferential oxidation of BF4− in Ca(BF4)2 results in the formation of B-based CEI. For batteries with Ni-rich cathodes and Si-based anodes, we guide the reader to a recent review devoted to them []. In addition, we would like to mention a result too recent to be included in this review, dating from 2023. Huang et al. introduced tetravinylsilane (TVSi) as a multifunctional electrolyte additive into a commercial electrolyte to engineer tailored interphases simultaneously on Si anode and LiNi0.92Mn0.05Co0.03O2 cathode []. This full cell demonstrated a capacity retention of 83% after 200 cycles at 1 C. In addition, Zhang et al. proposed 3-Fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)picolinonitrile (FTDP), which decomposed to form B-contained and cyano (CN) group-rich CEI, as well as LiF-, Li3N-rich solid SEI, simultaneously, resulting in the integrity and stability of electrodes []. The 1.6 Ah NMC811/SiOx pouch cell with 0.2 wt.% of this additive delivered a high-capacity retention of 84.0% after 300 cycles at the current of 1.0 A.
7.2.1. Additives
The surface reactivity of the layered cathodes is related to the presence of surface-active oxygen resulting from TM-O bond covalency, which increases with increasing Ni content []. The consequence is a dehydrogenation of the ethylene carbonate (EC), which has been observed on NMC811 electrodes at a voltage of 3.8 V vs. Li+/Li []. This oxidation of the carbonate solvent is considered as the main route for the electrolyte decomposition [,]. This oxidative decomposition of the electrolyte generates a CEI that must be homogeneous to protect the particles from further degradation [], and thin enough to avoid a thick resistive CEI that would reduce the rate capability []. To construct such a CEI, an electrolyte additive is a good strategy [,]. The most popular additive for this purpose is fluoroethylene carbonate (FEC) [,,]. The reason for the use of fluorinated carbonates is their lower HOMO and LUMO energies compared to their nonfluorinated counterparts, due to the strong electronegativity of fluorine. Therefore, they have higher oxidation potentials and higher reduction potentials, so that they can be used in high-voltage cells. In addition, they are known to generate a SEI protecting the anode.
Organic additives can be sacrificed at high voltages (4.3–4.5 V) because their HOMOs are higher than the HOMOs of organic solvents, so that they are able to prevent the oxidative decomposition of the solvents []. The electro-polymerization of electrolyte additives can also provide electrons to counteract the decomposition of solvents []. Other solvents can be used to allow for the formation of more even CEIs, reducing contact between electrode surfaces and electrolytes [].
Replacing Mn and Co with Ni in layered LiNixMnyCo1–x–yO2 (NMC) positive electrodes promotes the dehydrogenation of carbonate-based electrolytes on the oxide surface, which generates protic species to decompose LiPF6 in the electrolyte. The stability of the surface of NMC can be enhanced by decreasing free-solvent activity in the electrolyte through controlling salt concentration and salt dissociation. In particular, NMC811 with capacities greater than 150 mAh g−1 (77% retention) after 500 cycles could be achieved by simply increasing the LiPF6 concentration []. Otherwise, protection of the Ni-rich particles is needed to avoid thermal runaway due to the reaction of NMC with the LiPF6 salt of the electrolyte. Forero-Saboya et al. used lithium hydridoaluminate as electrolyte additive, which acts as HF scavenger. Moreover, its oxidation results in the formation of an Al-rich protective layer on the cathode. With this additive in the electrolyte, the LiNi0.86Mn0.07Co0.07O2 exhibited a capacity retention of 66% at C/3 after 200 cycles with a graphite anode, raising at 87% with Li4Ti5O12 anode []. Hu et al. proposed the dual additives of tetrabutyl titanate (TBT) and lithium difluoroxalate borate (LiDFOB) to form a stable Ti-, B-, and F-rich CEI []. The LiDFOB additive is functionalized by fluorine (F)-rich functional groups, which leads to the formation of the LiF-based SEI layers via reductive decomposition. In addition, TBT stabilized the electrolyte by removing H2O/HF. The Li∣NMC811 cell with the dual additives exhibited a capacity retention of 86% after 200 cycles at 1 C and a high cut-off voltage of 4.5 V. This is also the capacity retention obtained with N,O-bis(trimethylsilyl)acetamide additive []. Li3(CB11H12)2(CB9H10) hydroborate electrolyte was successfully integrated in a NMC811 cell. With the Li-In anode, the cell had a discharge capacity at C/10 of ~145 mAh g−1 at room temperature. It retained 98% of the initial discharge capacity after 100 cycles at C/5 and 70% after 1000 cycles at C/2 []. Capacity retentions of 97% after 100 cycles at C/5 and 75% after 350 cycles at C/2 are also achieved with a graphite anode without any excess Li. The energy density per cathode composite weight of 460 Wh kg−1 is on par with the best solid-state batteries.
Hu et al. tuned the spatial linking structure of dipentacyclic anhydride (DPA) to obtain a new class of DPA-based additives to obtain robust CEI and SEI layers []. Among them, the best results were obtained with 1,2,3,4-cyclobutane-tetracarboxylic dianhydride (CBDA). The CBDA with a quaternary ring spatial linking structure changes Li+ coordination, which decreases the content of organic species in the CEI. In addition, CBDA makes more PF6− and FEC to transfer to both of the electrodes’ surfaces for decomposition owing to their stronger binding energies. As a result, a prototypical 216 Wh kg−1 (1.2 Ah) NMC811//graphite pouch cell with this additive in the electrolyte exhibited an ultrahigh capacity retention 94% over 800 cycles at a current density of 1 C. Moreover, prolonged stable cycling for 900 cycles (93% capacity retention at 1 C) in an NMC811//graphite pouch cell was enabled by CBDA, outperforming the best cycling performance of NMC811//Li-metal batteries incorporating monocyclic anhydride additives (83% after 200 cycles at 1 C) []. Jiang et al. employed glutaric anhydride (GA) as a multifunctional electrolyte additive to in situ construct a CEI, which significantly enhanced the cycling performance of Li/NMC811 cell []. Therefore, anhydride additives can be used with Ni-rich based batteries, although the functions and mechanisms of these additives are not well understood and need further investigations.
Qin et al. reported a Janus-faced CEI film constructed by succinonitrile (SN) and cyclohexylbenzene (CHB) additives []. SN is adsorbed on electrode particles, while CHB is electrochemically polymerized generating an outer layer subsequently. The Li/LiNi0.8Co0.1Mn0.1O2 cell with SN+CHB electrolyte presented excellent long-cycle performance with a capacity retention ~72.3% in 600 cycles at the rate of 0.5/1.0 C between 2.8–4.7 V.
VC is known to generate a poly(carbonate)-based SEI layer, but it usually hinders Li+ migration at the Ni-rich NCM interface [,]. A co-additive is thus recommended to compensate for the inferior properties of the VC additive. Kim et al. proposed the choice of LiDFOB as a co-additive of VC for Ni-rich NCM and SiOx electrode materials []. The LiNi0.83Co0.11Mn0.06O2 (NCM83)//SiOx cell with this modifies electrolyte exhibited a capacity retention of 72.7% after 100 cycles at 1 C. Dai et al. selected LiNO3 and LiBF4 as dual additives. With an electrolyte consisting in 1.0 M LiPF6 in SL/FEC/EMC with 0.5 wt.% LiBF4–LiNO3, NMC811-graphite cell retains 90.4% of the initial capacity after 200 cycles at 2 C (~6 mAh cm−2), owing to the formation of a uniform and LiF-inorganic (including B and S)-rich CEI and a dense, thin Li3N–LiF-rich SEI [].
Excessive Li is usually added in the preparation of NMC-based cathodes and tends to accumulate on the surface of particles and react with electrolytes to convert to lithium carbonate and hydroxide [,]. LiPF6 can react with excessive alkaline Li on the surfaces of cathode particles to produce chemically stable Li3PO4 and LiF, and significantly suppress parasitic reactions caused by excessive Li []. Phelan et al. showed that higher concentration of LiPF6 leads to formation of more LixPOyFz-/LiF-rich CEI that protects Ni-rich cathode materials []. Qiao et al. introduced Li2O as a preloaded sacrificial agent on a NMC811 cathode to provide an additional Li source to offset the irreversible loss of Li, and employed fluorinated ether additive to neutralize O2− species released through Li2O oxidation []. This led to the construction of a LiF-based layer at the CEI. The pouch cell constructed with this modified NMC811 cathode has a gravimetric energy density of 320 Wh kg−1, maintaining 80% capacity after 300 cycles. On the other hand, reactions between LiPF6 and trace amounts of water generate HF. To prevent such reactions, 3-IPTS can be used as an electrolyte additive: it can consume the trace amounts of water in electrolytes []. Zhang et al. demonstrated an effective approach to inhibit the dissolution of TM ions by using medium concentrated lithium bis(fluorosulfonyl)imide (LiFSI)-based electrolytes with an optimized fluoroethylene carbonate (FEC) content. In addition, this electrolyte creates the formation of a LiF-rich layer on the LiNi0.90Mn0.05Co0.05O2 cathode surface []. Triallyl cyanurate (TAC) additive forms robust CEI via oxidation decomposition and inhibits LiPF6 hydrolysis. It also modifies SEI on the anode through reduction decomposition. As a result, TAC substantially enhances the electrochemical performance of Ah-level pouch cells with various Ni-rich cathodes, achieving long cycle life at both room temperature and 45 °C, alongside improved high-temperature storage capabilities, with both graphite and graphite-SiOx anodes [] (see Figure 21). Zhuang et al. proposed bis(di-tert-butyl)-4-dimethylaminophenylphosphine (Bis-4TMPA) as electrolyte additive []. The phosphine group in Bis-4TMPA reacted with the oxygen species and formed a stable CEI, while the nitrogen-containing group reacted with HF to reduce the corrosion on the electrode. The LiNi0.8Co0.1Mn0.1O2 with the addition of 0.5 wt.% Bis-4TMPA exhibited a capacity retention rate of 76.3% after 400 cycles (1 C = 200 mAh g−1), against 69.1% for the pristine NMC811. Zou et al. utilized 4-fluoro-N, N-dimethylbenzenesulfonamide (FBSN) as an additive to refrain the dehydrogenation of EC []. This result is due to the formation of a CEI composed of LiF and benzene ring skeleton along with conductive S-rich and N-rich lithium species. Moreover, this CEI can be effectively self-repaired in time when damaged. As a result, the LiNi0.9Co0.05Mn0.05O2 (NCM90)//Li cells with 2% FBSN-containing electrolyte delivers a capacity retention of 90.2% after 200 cycles at 1 C, and 83.1% after 200 cycles even at higher voltage of 4.5 V With 2,4,6-triphenyl boroxine (TPBX) additive, the graphite/NMC811 maintained 84% of the initial discharged capacity after 100 cycles at 0.5 C (69% for the cells using the baseline electrolyte []. Zhang et al. proposed a diazacyclo type additive, 2-fluoropyrazine (2-FP). When adding 0.2% 2-FP to the electrolyte, the capacity retention of single-crystal LiNi0.90Co0.05Mn0.05O2 (NCM90) cathode was promoted from 72.3% to 82.1% after 200 cycles at 1 C []. This improvement is attributed to the formation of a CEI film through ring-opening electrochemical polymerization of 2-FP upon the NCM90 electrode particles; This film suppressed of the decomposition of LiPF6 and protected the cathode from side reactions with the electrolyte. Yang et al. fabricated a robust CEI through in situ polymerization of ethylene carbonate induced by aluminum isopropoxide []. This increased the capacity retention of the NMC811//Li cell at 1 C rate after the 3rd cycle from 80.8% to 97.8% with a highly reversible capacity of 176 mAh g−1 after 200 cycles at 1 C.
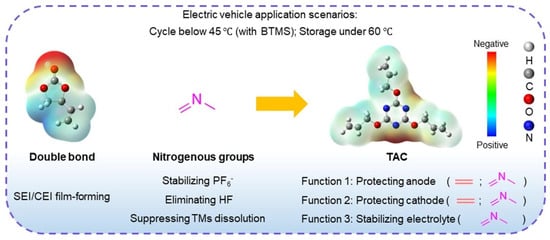
Figure 21.
Structure of triallyl cyanurate (TAC) and how it reflects the ‘multi-in-one’ design strategy. TAC, a triple-functional additive, extends battery life by forming a protective SEI on the anode, a CEI on cathode, and stabilizing the electrolyte against hydrolysis and corrosivity. It significantly improves cycle life and high-temperature storage for Ni-rich cathode Li-ion cells. Reproduced from ref. []. Copyright 2025 Elsevier.
Sulfone and sulfonate additives are used to stabilize both CEI of Ni-rich cathodes and the SEI at the anode side []. Kim et al. constructed a sulfonate-based CEI layer that selectively protect the surface of the Ni-rich NCMA cathode materials and control parasitic reactions. They selected 3-(N,N-dimethylpalmitylammonio)propane sulfonate (PAPS), which features amphiphilic characteristics due to its combination of hydrophilic (sulfonate) and hydrophobic (carbon) functional group. Above the melting point of PAPS (242 °C), the liquefied PAPS evenly attached to the Ni-rich NCMA material, and a further increase in temperature decomposed the PAPS precursor to form the sulfonate-based CEI layer []. The capacity retention of the PAPS-modified NCMA cathode after 100 cycles at 0.5 C improved at both 25 °C (92.7%) and at 45 °C (86.4%) compared with that of the pristine NCMA cathode (58.5% and 28.8%, respectively). Enhanced performance of the NMC811//graphite cell was achieved with 2-thiophene sulfonamide (2-TS) additive []. After 500 cycles in the voltage range of 2.80–4.20 V, the cell with 1% 2-TS lost 8.40% of the initial discharge capacity (23.59% without the additive). Li et al. proposed p-toluene sulfonylmethyl isocyanide (TOSMIC) additive. The high reduction activity of TOSMIC additive is mainly contributed by its sulfone group, while the high oxidation activity is primarily caused by its phenyl group. The graphite/LiNi0.8Co0.15Al0.05O2 cell with 3 wt.% TOSMIC exhibited a capacity retention of 67% after 100 cycles at 0.5 C []. The 3,3-diethylene di-sulfite (DES)-functionalized electrolyte regulates both the graphite anode and the NMC811 cathode, increasing the capacity retention of the cell to 82.53% after 150 cycles at 1 C when the cut-off voltage is raised to 4.5 V []. A noticeable result was obtained with 4-(allyloxy)phenyl fluorosulfate (APFS) additive. On one hand, the radical copolymerization of vinylene carbonate (VC) with APFS via electrochemical initiation creates a spatially deformable polymeric SEI on the SiG-C (30 wt.% graphite + 70 wt.% SiC composite) anode. On the other hand, it generates a thermally stable CEI containing S-O and S-F species on the NMC cathode material. The SiG-C/LiNi0.8Co0.1Mn0.1O2 full cell with the combined use of VC with APFS exhibited a capacity retention of 72.5% with a capacity of 143.5 mAh g−1 after 300 cycles at 45 °C []. The crucial role of VC polymerization in enhancing CEI stability and mitigating surface reconstruction on NMC cathodes has been explored recently []. Liu et al. proposed bis(4-fluorophenyl) sulfone (BFS) as an additive, which improved the capacity retention of NMC811 from 75.19% to 83.04% after 100 cycles at the rate of 1 C []. This additive led to a stable CEI, and can be oxidized at state of charge into two sulfonates, which prevent the carbonate electrolyte from decomposition. Another sulfone-based additive, phenyl trans-styryl sulfone (PTSS) increases the capacity retention of NMC811//graphite full cell from 48% to 63% after 100 cycles at 1 C []. This is attributed to the formation of the CEI, but in addition, the PTSS induced-SEI film enhances the interphasial stability of graphite anode. A sulfonate-based CEI was obtained by using ammonium sulfate [], increasing the capacity retention of NMC from 52.2% to 75.6% after 100 cycles at 0.5 C at 45 °C. Joung and Yim proposed N,N,N,N-tetraethylsulfamide to improve surface stability for Ni-rich oxides cathode []. This sulfone and amine group-based additive effectively constructed an effective CEI layer on NMC811 cathodes, inhibiting electrolyte decomposition. In addition, it lowered the fluoride concentration in the cell. Ahn et al. proposed allyl phenyl sulfone additive []. A sulfonate ester additive, 1,3-propane sultone, is effective to stabilize the CEI of NMC, and the SEI on the lithium anode by constructing a LiF/Li2SO3-rich interphase layer []. Like in the other sulfone-based additives already cited, the sulfone functional group effectively suppresses electrolyte decomposition during cycling. The allyl functional group promotes additional crosslinking reaction during CEI formation, which renders the CEI more durable. In addition, it scavenged fluoride (F−) species in the cell with NMC811. As a result, the capacity retention at a relatively high temperature of 45 °C where the standard electrolyte decomposition is accelerated, the capacity retention of the Li//NMC811 cell at 0.5 C raises from 64.3% with the pristine electrolyte, to 78.9% with 0.25 wt.% additive. A cell with SiOx/C anode, NCM90 (LiNi0.9Co0.05Mn0.05O2) cathode, in electrolyte with allyl phenyl sulfone additive still delivered a capacity of 777 mAh g−1 after 200 cycles at 0.5 C and 30 °C, corresponding to 79.3% capacity retention (57.8% capacity retention without the additive) []. Trimethylsilyl 2-(fluorosulfonyl) difluoroacetate (TMSFS) additive in the electrolyte enhanced the capacity retention of LiNi0.9Co0.05Mn0.05O2 (NCM90) from 73% to 85.1% at 1 C up to 200 cycles [], compared to 78% obtained with bis(vinylsulphonyl)methane instead of TMSFS []. Another scavenger of HF is 1-(trimethylsilyloxy)cyclohexene (TMSCH) with functional groups (C=C, Si-O) electrolyte additive, which in addition regulars both the CEI and the SEI with lithium metal anode []. The retention of the Li/NMC811 batteries at 1 C are enhanced from 68.2% to 84.9% at 30 °C and 64.2% to 85.8% at 45 °C. with this additive. Choi et al. chose a sultone additive: 1,4-butane sultone (BS), because its HOMO is similar with that of the carbonate-based solvents, and can thus form a stable CEI. The Li[Ni0.75Mn0.25]O2‖graphite full cell using the carbonate-based electrolyte with 1 wt.% BS additive exhibited a capacity retention of ~80% after 200 cycles at 200 mA g−1 (~68% in absence of BS) [].
Phosphorous containing additives can also participate in the formation of stable and robust CEI, and scavenge HF. Jo et al. introduced monobasic sodium phosphate (NaH2PO4) additive, which improved the thermal stability of NMC811 and enhanced the electrochemical performance of graphite//NMC811 cell at 60 °C []. On the other hand, lithium bis(trimethylsilyl) phosphate (LiTMSP) was incorporated as an electrolyte additive in graphite/NMC811 for improving the low-temperature performance (at −10 °C). Zhao et al. utilized carbonate-based electrolytes containing vinylene carbonate (VC) additive to stabilize graphite and phosphate compounds like triallyl phosphate (TPPC2) and tripropargyl phosphate (TPPC3) additives to stabilize the Ni-rich cathode. The artificial graphite/NMC811 with this modified electrolyte increased the capacity retention of NMC811/AG cells to 90.5% after 500 cycles at 1.0 C and 55 °C []. A better compromise between voltage drop, gas emission, and thermal stability in artificial graphite/NMC811 can be obtained with lithium difluoro(dioxalato)phosphate (LiDFDOP) additive, singly and in combination with co-additives fluoroethylene carbonate (FEC) and vinylene carbonate (VC) [].
Allyltrimethylsilane additive has the same functions owing to C=C, Si-C groups, enhancing the capacity retention of NMC811 to from 46.4% to 82.9% after 200 cycles at 1 C []. We have already mentioned the improvement of the electrochemical performance of Ni-rich cathodes by coating the particles with siloxane []. 1,3,5-Trimethyl-1,3,5-tris(3,3,3-trifluoropropyl) cyclotrisiloxane (D3F) as an electrolyte additive is also effective. 5 wt.% of this additive enhances the capacity retention of the graphite/NMC811cell from 38 to 76% after 300 cycles at 1 C []. Among siloxane additives, propargyloxytrimethylsilane (PMSL), allyloxytrimethylsilane (AMSL), and trimethylmethoxysilane (TMSL) have been tested by Chen et al. They concluded that the highest cycle ability at 60 °C is obtained with PMSL, the artificial graphite//NMC811 cell with this additive exhibiting a capacity retention of 85% after 300 cycles at 0.5 C in the voltage range 3.0–4.3 V []. Ren et al. introduced cyano-groups into a siloxane to synthesize a novel cyano-siloxane additive, namely 2,2,7,7-tetramethyl-3,6-dioxa-2,7-disilaoctane-4,4,5,5-tetracarbonitrile (TDSTCN). 0.5 wt.% TDSTCN additive enables ultrahigh nickel LiNi0.9Co0.05Mn0.05O2//graphite (NCM90//Gr) full cells with significantly increased cycle life, especially at an elevated temperature of 50 °C and a high charging cut-off voltage of 4.5 V []. Zhang et al. introduced lithium hexamethyldisilazide (LiHMDS) with a low oxidation potential, which improve the cycling stability of Li//NMC811 batteries at high-stress conditions such as high voltage (4.5 V) and high temperature (60 °C) [].
Fluorine-containing electrolyte additives have excellent kinetic reactivity, which can preferentially generate stable SEI films and uniform CEI []. Lithium difluoro(oxalato)borate (LiDFOB) additive is a film-forming additive protecting the surface of Ni-rich cathodes []. The same additive has been used with a fluorinated electrolyte composed of 1 M LiPF6 as a lithium salt in fluoroethylene carbonate (FEC)/ethyl methyl carbonate (EMC)/2,2,2-trifluoroethyl acetate (TFA) (1:3:1 by volume) []. The NMC811 cathode with this electrolyte and 0.2 M LiDFOB additive exhibited superior discharge capability (247.2 mAh g−1), cycling stability (81.4% retention at 200 cycles, 0.5 C) and rate performance (154.5 mAh g−1 at 5 C) at 4.6 V compared to commercial electrolytes (66.2% retention at 200 cycles, 108.0 mAh g−1 at 5 C) in Li//NMC811 half-cells. Ahn et al. proposed 2,4-difluorobiphenyl (FBP) as a fluorine-based additive to form a stable CEI, where the biphenyl provides an overcharge protection []. This additive successfully enhanced the high-voltage cycling stability of the Ni-rich NCM83 cathode. Chen et al. utilized LiDFOB and tris(trimethylsilyl)phosphate (TMSP) as dual additives, where TMSP was added to scavenge HF []. Owing to the synergistic effect of LiDFOB and TMSP, the Li/LiNi0.8Co0.1Mn0.1O2 half cells exhibited the capacity retention 76.3% after 500 cycles at a super high voltage of 4.7 V and 1 C rate. 2-aminoethyldiphenyl borate (AEDB) significantly improves long-term cycling of a graphite//LiNi0.85Co0.1Mn0.05O2 full cell, with a capacity retention of 88% after 100 cycles with cutoff voltage set to 4.35 V, by forming a stable CEI []. LiNi0.8Mn0.1Co0.1O2, or NMC811 is particularly attractive, as it can achieve a notably high specific capacity (200–220 mAh g−1 at a relatively high average discharge voltage of ~3.8 V vs. Li+/Li), stable cycling with an ultrahigh cut-off voltage of 4.8 V can be realized by using an appropriate amount of lithium difluorophosphate (LiPO2F2) in a common commercial electrolyte. The Li//LiNi0.76Mn0.14Co0.10O2 cell retains 97% of the initial capacity (235 mAh g−1) after 200 cycles []. The decomposition products (Li3PO4 and LiF) of LiPO2F2 generated a protective SEI. This result is even better than the performance of NMC811 in presence of sulfonamide-based electrolyte [].
Isocyanate additives also improve the electrochemical performance of Ni-rich cathodes []. Oh et al. proposed icosafluoro-15-crown-5-ether (IF-CE) as an additive []. Each molecule of this additive contains 20 fluorine atoms, which participate at the formation of SEI on the anode, and of CEI. A pouch-type full cell with 5 wt.% SiO-graphite anode and LiNi0.88Co0.08Mn0.04O2 cathode, and IF-CE + FEC additives in the electrolyte, demonstrates an initial capacity of 194 mAh g−1 at 1 C, with capacity retention of 78% after 300 cycles. Peng et al. selected fluorine- and nitrogen-containing methyl-2-nitro-4-(trifluoromethyl)benzoate (MNTB) regulated with FEC to generate a robust CEI film while capturing HF [] (see Figure 22 and Figure 23). The NMC811//Li batteries with 15% MNTB-containing electrolyte exhibited a specific capacity of 150.12 mA h g−1 with a capacity retention of 81.10% after 300 cycles with 0.5 C charging and 1 C discharging in the voltage range of 3.0–4.3 V. Lei et al. chose pentafluorophenyl trifluoroacetate (PFTF) to adapt the NMC811cell by forming LiF-rich CEI/SEI films and eliminate HF, extending the cycling stability of the symmetrical Li cell is expanded to 500 h []. Cyclopentyl isocyanate (CPI) with a single isocyanate (−NCO) functional group as a bifunctional electrolyte additive was used to improve the interfacial stability of Ni-rich cathode LiNi0.83Co0.12Mn0.05O2 []. Tan et al. utilized anhydride additives to improve the electrochemical performance of LiNi0.5Mn1.5O4 cathode []. Han et al. used 4-fluorophenyl isocyanate (4-FI) as an additive to improve the low-temperature performance of the NMC811//SiOx@G battery due to the optimization of F in the SEI membrane components. The capacity retention of the NMC811/SiOx@G pouch cell increased from 92.5% (without additive) to 94.2% (with 1% 4-FI) after 200 cycles at 0.5 C. At −20 °C, the cyclic stability of the NMC811//SiOx@G pouch cell increased from 83.2% (without additive) to 88.6% (with 1% 4-FI) after 100 cycles at 0.33 C []. 4-fluorobenzene isocyanate (4-FBC) electrolyte additive is also efficient to form a CEI film on Ni-rich cathodes. Tested in the NMC532//SiO@Graphite pouch full cells with electrolytes containing a mass fraction of 1% 4-FBC additives demonstrate improved capacity retention of 81.3% after 200 cycles at 1 C (39.1% without additive) []. 1,2,4-1H-Triazole (HTZ) was added to the electrolyte to improve the interfacial stability of LiNi0.9Co0.05Mn0.05O2, resulting in an increase of both rate capability and cycle ability up to 60 °C []. Owing to its −N=C=O group, isocyanoethyl methacrylate additive improves the performance of NMC811//graphite, increasing the capacity retention from 62.8% to 81.9% after 150 cycles at 1 C in the voltage range 2.75–4.4 V [].
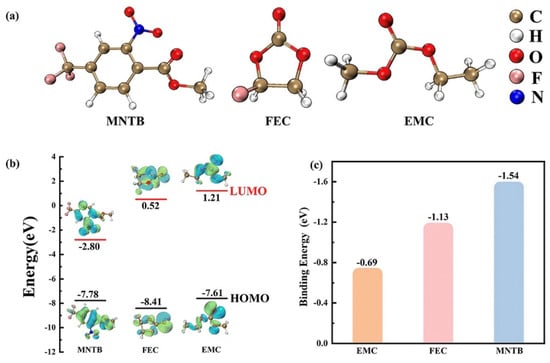
Figure 22.
(a) Molecular structures of methyl-2-nitro-4-(trifluoromethyl)benzoate (MNTB), fluoroethylene carbonate (FEC), and ethyl methyl carbonate (EMC). (b) HOMO and LUMO energy levels of MNTB, FEC, and EMC. (c) Binding energies between Li+ and solvents (binding energy = the energy of solvent after binding to Li+ – the energy of solvent before binding to Li+). The performance of NMC811 with MNTB/FEC electrolyte is reported in Figure 23. Reproduced from ref. []. Copyright 2024 American Chemical Society.
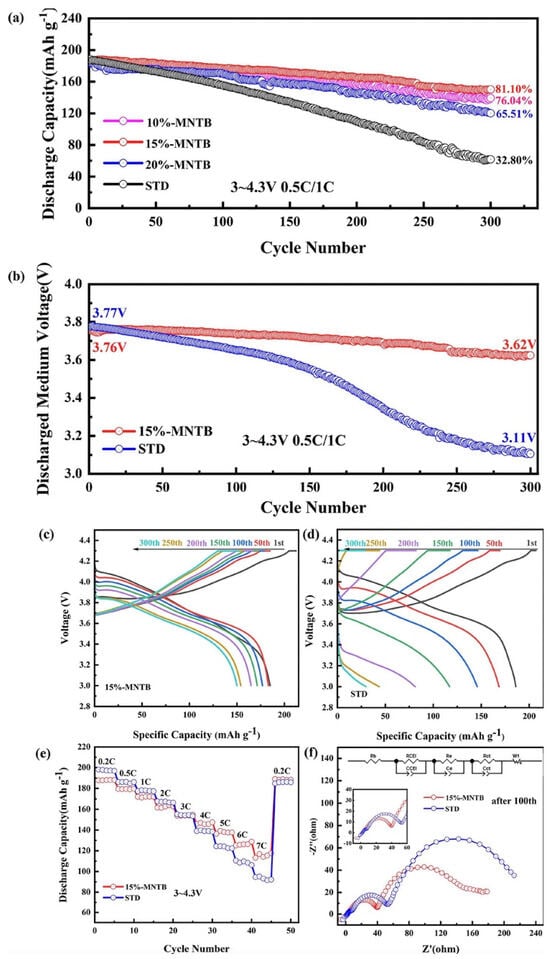
Figure 23.
Electrochemical performance of NMC811//Li cells. (a) cycling performance of standard electrolyte (STD) for reference, and different ratios of MNTB groups regulated with FEC after 300 cycles. (b) Discharged medium voltage. (c,d) Voltage–capacity graphs corresponding to different cycles. (e) Rate capability (0.2, 0.5, 1, 2, 3, 4, 5, 6, 7, and 0.2 C) diagrams. (f) EIS figure after 100 cycles. Reproduced from ref. []. Copyright 2024 American Chemical Society.
Other HF scavengers include silane derivatives []. In particular, with 3% silane-based electrolyte additive, vinyltrimethylsilane (VTMS) in the LiPF6-based electrolyte, the graphite//NMC811 cell maintained a discharge capacity of 131.9 mAh g−1 after 500 cycles in the voltage range of 2.8–4.3 V at 1 C []. Cheng et al. reported that a dual additive containing lithium bis(oxalate)borate and dopamine produces a uniform inorganic/polymer CEI that eliminates undesirable interfacial side reactions []. Guan et al. synthesized niobium-doped strontium titanate (Nb-STO) via a facile solvothermal method and used it as a surface modification layer onto the NMC811 cathode. []. Not only did Nb-STO prevent the dissolution of the transition metals, it also alleviated the internal resistance growth from uncontrollable CEI evolution. With this protection, the capacity of NMC811 was ~190 mAh g−1 after 100 cycles under 1 C with capacity retention over 84% in the voltage range of 3.0–4.5 V (61% with pristine NMC811). Zhang et al. proposed an electrolyte additive, cis-1,2,3,6-tetrahydrophthalic anhydride (THPA), to enhance the interfacial stability of the Ni-rich LiNi0.90Co0.05Mn0.05O2 (NCM90) cathode []. This additive not only hinders the oxidation of carbonate solvents, but also generates a conductive protective CEI film. The capacity retention of NCM90 with an electrolyte containing 2.0% THPA reached 93.2% after 120 cycles at 1 C (1 C = 180 mA g−1) at 30 °C, against 80.0% in absence of this additive. Zou et al. proposed a diboron electrolyte additive 5,5,5′,5′-tetramethyl-2,2′-bi-1,3,2-dioxaborinane (TBDB) to construct a stable LiNi0.9Co0.05Mn0.05O2 (NCM90)/electrolyte interface []. With 0.3% TBDB-containing electrolyte, the NCM90 cathode shows a capacity retention of 84.3% after 200 cycles at 1 C at a charge cutoff of 4.4 V (70.1% with the baseline electrolyte). Zeng et al. employed Tolylene-2,4-diisocyanate (TDI) additive containing lone-pair electrons. With this additive in the electrolyte, the graphite//NMC811 cell exhibited a capacity retention of 94% at 1 C 1000 cycles at 25 °C (78% in absence of this additive) []. The authors attributed this significant increase of the cycle ability to the isocyanate group playing a crucial role in inhibiting the generation of H2O and HF, which leads to the formation of a thin and dense CEI. Lim et al. synthesized tetrakis(methacryloyloxyethyl)pyrophosphate additive to form a stable CEI, which increased the capacity retention of graphite/NMC811 cell to 82.2% after 300 cycles at a 1.0 C rate [].
The oxidative decomposition of nitrile leads to the formation of a stable CEI responsible for the high-voltage stability of nitrile-containing electrolytes. Li et al. utilized lithium difluoro(oxalato)borate (LiDFOB) as the lithium salt and adiponitrile (C6H8N2) as the additive. The LiNi0.5Mn1.5O4//Li half-cell with this electrolyte delivered a capacity of 100.9 mAh g−1 at 0.5 C, with a capacity retention of 81.2% after 50 cycles, significantly better than the result obtained in absence of the adiponitrile additive []. The additive adiponitrile complexed with Ni4+ ions on the surface of the cathode electrode inhibited the dissolution of Ni4+ and contributed to the formation of a CEI protective and conductive passivation film. The authors also calculated that the Ni4+ ion with a strong cathode potential and the cyanide group with a strong anode potential participated in charge transfer to stabilize the cathode material. In addition, adiponitrile also stabilizes the lithium anode, so that using 1 wt.% adiponitrile in 0.8 m LiTFSI + 0.2 M LiDFOB + 0.05 M LiPF6 dissolved in EMC/FEC = 3:1 electrolyte, the Li/full concentration gradient Li[Ni0.73Co0.10Mn0.15Al0.02]O2 battery achieves a cycle retention of 75% over 830 cycles under high-capacity loading of 1.8 mAh cm−2 and fast charge–discharge time of 2 h []. Another nitrile additive, 1,3,6-hexanetricarbonitrile was used to raise the capacity retention of LiNi0.83Co0.07Mn0.1O2 to 81.42% at 1 C after 300 cycles at a cutoff voltage of 4.5 V []. Note, however, that nitrile has a poor reductive stability. Therefore, it should not be used with lithium anode, unless another additive is added to form a SEI protecting the lithium or graphite anode.
Qiu et al. designed 1-methyl-1-cyanopropylpyrrolidine bisfluoromethanesulfonimide salt (PYR1(4CN)TFSI) to reduce the interface impedance on the NMC811 cathode side and enhance the compatibility between the electrolytes and the lithium metal anode. With the addition of 0.5 wt.% PYR1(4CN)TFSI in the electrolyte, the Li//LiNi0.8Co0.1Mn0.1O2 cells delivered 107 mAh g−1 after 200 cycles at 1 C []. The result is attributed to the combination of TFSI− with pyrrole, which promotes the migration of Li+, while the cyano group can coordinate with oxygen atom electron pairs on TFSI− to participate in the SEI film on the lithium anode. Park et al. utilized 5-methyl-4-((trifluoromethoxy)methyl)-1,3-dioxol-2-one and 5-methyl-4-((trimethylsilyloxy)methyl)-1,3-dioxol-2-one to provide spatial flexibility to the vinylene carbonate-derived SEI via polymeric propagation with the vinyl group of vinylene carbonate. This electrolyte was compatible with Si-C anode, and with high-voltage cathode, so the Si-C/NMC cell exhibited 81.5% capacity retention after 400 cycles at 1 C and fast-charging capability (1.9% capacity fading after 100 cycles at 3 C) []. Zhuang et al. introduced lithium tetrafluoro (1,2-dihydroxyethane-1,1,2,2-tetracarbonitrile) phosphate (LiTFTCP) additive, adding the effects of cyano-groups (−CN) into lithium fluorinated phosphate. NMC811//SiOx-graphite full cells (cathode loading 17.2 mg cm−2, 3.5 mAh cm−2) using 0.5 wt.% LiTFTCP additive deliver a capacity retention of 90.24% (172.8 mAh g−1/191.5 mAh g−1) after 600 cycles at 1 C, 30 °C, in the voltage range 2.7–4.25 V, with an average Coulombic efficiency (ACE) of 99.91% []. This remarkable result is due to cyano-enriched CEI layer and the stable LiF-enriched SEI layer.
Song et al. reported a systematic study of electrolyte additives in single-crystal and bimodal artificial graphite/NMC811, including vinylene carbonate (VC), 1,3,2-dioxathiolane-2,2-dioxide (ethylene sulfate or DTD), fluoroethylene carbonate (FEC), lithium difluorophosphate (LFO), and lithium bis(oxalate) borate (LiBOB) []. They concluded that 2VC + 1DTD cells possess the best or close to the best performance on long-term cycling at 40 °C and 55 °C, but their high charge transfer resistance (Rct) leads to relatively poor performance in 20 °C 1 C long-term cycling. 2FEC + 1LFO cells are also the top performer on formation gas and Rct and possess lower Rct and ∆Rct than 2VC + 1DTD cells in cycling and storage tests, but perform slightly worse in capacity retention during cycling at 40 and 55 °C.
Innovative electrolyte solvent technologies are also a good strategy. We have already mentioned fluorinated solvents [,]. We can also cite fluoroacetonitrile (FAN) solvent enabling 4.5-V graphite//NMC811 pouch cells (1.2 Ah, 2.85 mAh cm−2) to achieve high reversibility (0.62 Ah) when the cells are charged and discharged even at −65 °C [], fluorinated cyclic ether allowing Li (50 µm)//NMC811 (4 mAh cm−2) cells to maintain 80% capacity after 568 and 218 cycles at room temperature and 60 °C, respectively [], and 1,2-difluorobenzene enabling highly stable cycling of Li//NMC811 cells (4.4 V) at C/3 charge and 1 C discharge (1 C = 2 mA cm−2) for 500 cycles with a capacity retention of 93% []. By dissolving fluorinated electrolytes into highly fluorinated non-polar solvents, the LiNi0.8Co0.15Al0.05O2//Li battery can still deliver ~50% of its room-temperature capacity at −85 °C []. With an all-fluorinated electrolyte containing fluoroethylene carbonate (FEC), and ethyl (2,2,2-trifluoroethyl) carbonate (ETFEC), the Li//NMC811 cell showed a capacity retention of 72.3% with an average CE of 99.8% at high voltage (up to 4.6 V) after 225 cycles at 1 C []. β-fluorinated sulfone-based electrolytes enable the very stable long-term cycling of graphite//LiNi0.6Co0.2Mn0.2O2 full cells [].
10% sulfone plus 0.02 M Lithium difluoro(oxalato)borate (LiDFOB) enabling ~400 Wh kg−1 NMC811//Li cells at 4.7 V to demonstrate an 82% capacity retention after 200 cycles []. Chen et al. chose ethyl butyrate (EB) as a solvent to be fluorinated because of its low melting point. In particular, ethyl heptafluorobutyrate (EHFB) enabled 4.5 V graphite//NMC811 pouch cells (1 Ah) to perform stably over 200 cycles at −10 °C with only 2% capacity loss []. Xiao et al. enhanced the solubility of LiNO3 in ester-based electrolytes by employing triethyl phosphate (TEP) as a co-solvent. As a result, NO3− is preferentially adsorbed on the electrode surface to derive a CEI layer with the homogeneous oxynitridation, while NO3− dominated weakly dissociated solvation clusters (NSC) suppress the dendrite formation on the lithium metal anode []. With this design, the Li//NMC811 full cell achieves 80% capacity retention after 300 cycles of 0.5 C. Another solubilizer, tris (2,2,2-trifluoroethyl) borate (TTFEB), utilizes its electron-deficient B atom to snatch electron-rich NO3− anion of insoluble LiNO3 and thus forms a unique TTFEB-LiNO3 solvation structure in carbonate electrolytes []. The full cell, featuring a thin Li anode (50 µm) and a high-loading NMC811 cathode (4.04 mAh cm−2) in the dual-additive electrolyte (FEC+TTFEB), demonstrated a capacity retention of 81.5% after 140 cycles at 1 C.
7.2.2. Electrolyte Formulations
An et al. designed a fire-resistant liquid electrolyte formulation consisting of propylene carbonate and 2,2,2-trifluoroethyl group-containing linear ester solvents paired with 1 mol L−1 LiPF6 salt and FEC additive. The replacement of linear carbonate with fluorinated linear ester yielded a fire-resistant and outperforming electrolyte under harsh condition. The fire-resistant electrolyte-incorporated industrial 730 mAh graphite//NMC811 Li-ion pouch battery achieved 82% retention after 400 cycles under 4.3 V charge voltage, 45 °C and 1 C []. Huang et al. designed an electrolyte consisting in1 M LiPF6 in FEC/acetonitrile (FEC/AN, 7/3 by vol.). Combining the FEC-dominated solvation structure and the AN-rich environment, the designed electrolyte triples the capacity of the 1 Ah graphite//NMC811 pouch cells at 8 C in comparison with the commercial electrolyte [].
Other electrolytes have been formulated to improve the performance of batteries with Ni-rich cathodes. In particular, fluorinated linear carboxylate esters with low freezing point and low viscosity as co-solvents enhance the performance of such batteries at subzero temperatures [,], while perfluorinated electrolytes improve their thermal stability [,], and EC-free electrolytes are studied to enhance the high-voltage cycle life and safety [,,,,].
Another class of electrolytes is obtained with substitution of LiPF6 salt by other salts (LiTFSI, LiFSI, and LiDFOB). In particular, dual-salt LiDFOB-LiBF4 in a sulfolane-based electrolyte with fluorobenzene additive to restrain the decomposition of sulfonate endowed graphite//NMC811cell with capacity retentions of 83% after 500 cycles at 25 °C and 82% after 400 cycles at 60 °C []. Even better results were reported with 1.6 m LiFSI and (2-cyanoethyl)triethoxysilane (TEOSCN), which enabled long-term cycling mesocarbon microbead (MCMB)//NMC811 for 1000 cycles with an ultrahigh-capacity retention of 91% at 25 °C and for 500 cycles with a retention of 81% at 60 °C []. Xu et al. chose LiTFSI as the salt, and selected solvents with low donor number (DN < 10) and high dielectric constant to minimize Li+ desolvation energy, and thus the interfacial resistance, while still dissociating LiTFSI. These properties were fulfilled with an electrolyte consisting in 1 M LiTFSI is dissolved into methyl difluoroacetate (MDFA), methyl 2,2-difluoro-2 (fluorosulfonyl)acetate (MDFSA), and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (TTE). In addition, the fluorination endowed the electrolyte with non-flammability characteristics []. NMC811//graphite pouch cells with this lean electrolyte (2.5 grams per ampere hour) achieve stable cycling with an average Coulombic efficiency of more than 99.9 per cent at −30 °C. Su et al. developed a dual-anion regulated electrolyte using LiTFSI and lithium difluoro(bisoxalato)phosphate (LiDFBOP) as anion regulators. The incorporation of TFSI− in the solvation sheath reduces the desolvation energy of Li+, and DFBOP− promotes the formation of the CEI. As a result, Li//LiNi0.83Co0.11Mn0.06O2 pouch cells exhibited 84.6% capacity retention at 0.1 C charge and 0.5 C discharge rates [].
Chen et al. also utilized LiFSI as the primary salt and TTE as the dilutant. Moreover, trimethyl phosphate (TMP) provided the basis for the introduction of lithium nitrate (LiNO3) as the secondary lithium salt. LiNO3 regulated the solvation structure and successfully prepared the phosphate ester-based localized high-concentration electrolyte with an apparent Li+ concentration of 1.15 M. Meanwhile, NO3− generated nitride-dominated electrode/electrolyte interfaces on the lithium anode and nickel-rich cathode surfaces. As a result, the Li//NMC811 battery with this electrolyte maintained 81.7% capacity retention after 800 cycles at 4.3 V, 1 C, and 93.3% capacity retention after 200 cycles at a high voltage of 4.5 V []. On the other hand, Yang et al. kept a carbonate electrolyte, and used double salts synergistic effect (LiPF6+LiFSI) to construct a super concentrated (DSSC) electrolyte of 1 M LiPF6 + 8 M LiFSI in EC:DMC (LiPF6: LiFSI = 1:8, EC:DMC = 1:1 by weight) The LiNi0.90Co0.05Mn0.05O2 (NCM9055)//Li cells with this electrolyte exhibited the capacity retention of 93.04% and reversible capacity of 173.8 mAh g−1 after 100 cycles at 1 C rate. This result was attributed to the formation of a stable and uniform 2–3 nm rock-salt phase protection layer on the surface of NCM9055, induced by the electrolyte []. Zhang et al. proposed an electrolyte consisting of 1.9 m LiFSI in a mixture of 2,2,2-trifluoroethyl trifluoromethanesulfonate (TTMS) and 2,2,2-trifluoroethyl methanesulfonate (TM) (1:2 by volume). These two solvents integrate the properties of sulfonates and fluorination forming a Li-rich CEI and low impedance SEI layer containing S species on graphite, so that this electrolyte endows 4.6 V NMC811//graphite (1 Ah) with a high-capacity retention of 83% after 1000 cycles []. Zhang et al. fabricated an advanced ether-based localized high-concentration electrolyte (LHCE) consisting of LiFSI, 1,2-dimethoxyethane (DME) and TTE with a molar ratio of 1:1.2:3 []. The LiNi0.94Co0.06O2 (NC94) in this advanced ether-based LHCE exhibited capacity retentions of 81.4% after 500 cycles at 25 °C and 91.6% after 100 cycles at 60 °C in the voltage range of 2.8–4.4 V in Li//NC94 cells at 1 C cycling rate.
Note, however, that the LiFSI corrodes aluminum of the current collector at voltage ≥ 4.3 V [], which is thus the limit of the upper potential range to be used, even if NMC is coated. Moreover, LiFSI and LiTFSI salts corrode the Ni-rich cathode materials [], because the nickel ions induce the breakage of C-F bond of these fluor-lithium salts driven by voltage. To avoid these drawbacks, Qiao et al. designed a non-corrosive sulfonimide salt lithium((difluoromethanesulfonyl) (trifluoromethanesulfonyl)imide (LiDFTFSI)) []. The improvement of the electrochemical properties by the replacement of LiPF6 or LiTFSI by this salt demonstrates the decisive role of the salt anion. Another demonstration is the outstanding improvement of the rate capability of Li//NMC811 pouch cell with an asymmetric Li salt, lithium 1,1,1-trifuoro-N-[2-[2-(2-methoxyethoxy)ethoxy)]ethyl] methanesulfonamide (LiFEA) []. This pouch cell with LiFEA maintained 81% capacity after 100 cycles under fast-cycling conditions (charging: 1.46 mA cm−2, discharging: 3.66 mA cm−2). A highly fluorinated (8-CF3) aluminum-centered lithium salt of lithium perfluoropinacolatoaluminate (LiFPA) facilitates the formation of a passivating CEI layer (see Figure 24) []. A Li//NMC622 cell in a high loading of 3.5 mAh cm−2 with LiFPA-EC/DMC electrolyte can perform 100 stable cycles delivered a capacity of 3.43 mAh cm−2 after 100 cycles at 0.2 C, corresponding to a capacity retention rate of 97.7%. Wu et al. utilized a dual-anion ionic liquid, 0.8Pyr14FSI-0.2LiTFSI. This salt allows for stable interfaces at both Li-metal and Ni-rich electrodes. With this specific electrolyte, the LiNi0.88Co0.09Mn0.03O2 (NCM88)//Li cell achieved an initial specific capacity of 214 mAh g−1 and outstanding capacity retention of 88% over 1000 cycles at 0.3C, and an average Coulombic efficiency of 99.94% [].
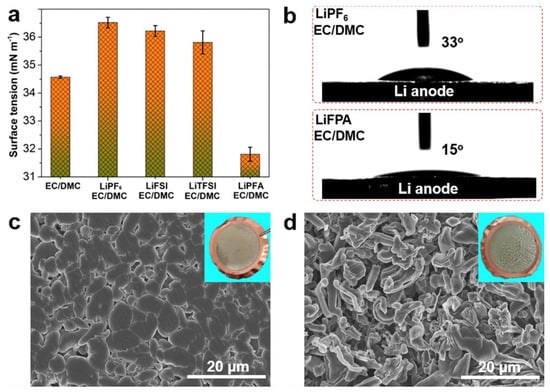
Figure 24.
Effect of a highly fluorinated (8-CF3) aluminum (Al)-centered lithium salt of lithium perfluoropinacolatoaluminate (LiFPA) on electrolyte wettability and Li deposition. (a) Surface tension of varied electrolytes. (b) Contact angle of 1 M LiPF6-EC/DMC and 1 M LiFPA-EC/DMC to Li anode. Morphology of deposited Li on Cu foil using (c) 1 M LiFPA-EC/DMC and (d) 1 M LiPF6-EC/DMC, at a current density of 0.5 mA cm−2 for 16 h. Insets in (c,d) are optical images of deposited Li on Cu foil. Reproduced from ref. []. Copyright 2022 American Chemical Society.
Since Li-derived impurities at the surface of the particles degrade the electrochemical performance, attempts have been made to remove them. Exposition of the Ni-rich layered materials in air results in the presence of LiOH and Li2CO3 impurities at the surface, which can react with the electrolyte to generate CO2. Rinsing with water is a way to remove them []. Unfortunately, the water molecules induce the extraction of Li+ from the surface due to Li+/H+ exchange [], triggering surface degradation and increasing the CEI []. We have mentioned earlier that the best strategy to remove the Li-derived impurities is coating. In addition, coating of NMC avoids the dehydrogenation of EC solvent, observed at voltage as low as 3.8 V on the surface of pristine NMC811 []. Wang et al. replaced the highly flammable organic solvents EC in conventional electrolytes with dimethyl methyl phosphonate (DMMP) and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (HFE) to obtain an electrolyte having a wide potential window beyond 5.0 V, and forming an effective CEI. The Li//NMC622 battery with this electrolyte showed no capacity loss over 100 cycles, and an average CE of 99.6% at a high cutoff voltage of 4.5 V [].
The interaction between the electrolytes and the Ni-rich materials is not yet well understood. A step has been made, however, by a recent work of Wang et al., who proposed a sp2-induction mechanism to address coordination deficiency through the coupling of interfacial orbitals between molecules and the cathode surface []. Among the sp2-hybrid high-fluorinated olefins in this work, (perfluorobutyl)ethylene (PFBE) is identified as an optimal inducing molecule. The Li//LiNi0.8Mn0.1Co0.1O2 (30 μm Li, high load 3.7 mAh cm−2 NMC811) cell with PFBE electrolyte achieving 80% capacity retention over 320 cycles (voltage range 2.8–4.4 V, charge/discharge 0.2/0.3 C), compared to 175 cycles with a PFBE-absent electrolyte. The electrolyte was composed of LiFSI salt and methoxytrimethylsilane (MOTMS) solvent, chosen because PFBE cannot dissolve salt (molar ratio LiFSI:MOTMS 1.2:2).
7.3. Recycling Ni-Rich Batteries
Lithium, cobalt, and nickel resources are limited [,]. To overcome this criticality and the energy costs and environmental hazards associated with the extraction of these metals, large-scale recycling of Ni-rich-based batteries is mandatory [,]. At present, only cobalt is recycled at a low price, taking into account the high cost of cobalt. Advancements in recycling technologies to mitigate environmental impact and recover valuable materials is thus desirable [,]. The industrial recycling processes of positive electrode materials comprise pyrometallurgy and hydrometallurgy. They are energy-intensive due to their reliance on high temperatures, and potentially damaging due to toxic chemicals. In hydrometallurgical processes, acidic leaching in the presence of a reducing agent is commonly used, including the use of H2O2 as a reducing agent for Co3+ []. To limit the extensive amount of chemicals used in hydrometallurgy, an alternative approach has emerged consisting of solid-state reactions to convert oxide-based materials into salts that are readily soluble in aqueous media. This approach, hereafter denoted salt conversion, has already been demonstrated for different salts including sulfates [,,]. The fundamental understanding of the reactivity of layered compounds with these media has been investigated in this paper. The authors established the conditions to convert layered lithiated transition metal compounds into sulfate-based products targeting the stabilization of the metal into the langbeinite structure K2M2(SO4)3. This conversion enables subsequent metal recovery using only water as medium, which therefore prevents the classical use of highly acidic solutions usually used in hydrometallurgy to destabilize the oxidic compounds. To illustrate the possibility of recovering metals using salt conversion, the authors highlighted LiCoO2 as an example. However, the langbeinite-type structure can accommodate different cations such as M = Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Zn2+, and Cd2+ [] Therefore, the extension of the salt conversion to NMC and NCA compounds supports the versatility of this method for different types of electrode chemistry of LIBs, opening practical outcomes to the vital question of battery recycling. Moreover, Wang et al. published a synthesis method of new cathodes, using as raw materials NMCs obtained from spent lithium-ion batteries recycling system []. Hamitouche et al. proposed a method to chemically convert lithiated transition metal oxides into readily water-soluble sulfate products []. This method comprises a temperature-driven solid-state reaction between the electrode material and potassium hydrogenosulfate molten salt yielding K2M2(SO4)3 and potassium/lithium sulfates.
Recently, Ji et al. [] proposed a greener and more economic effective pathway, centered on “repair” to regenerate spent Ni-rich cathode materials, following a process already proposed to regenerate LiFePO4 cathode by using a multifunctional organic lithium salt []. Ji et al. introduced a closed-loop upcycling strategy that leverages the detritus of the cathode and anode current collectors produced during dismantling process, involving two dopants (Al and Cu) in a ternary eutectic molten salt system (NaCl-KCl-LiOH). The two dopants work synergistically, as Al3+ tends to fill TM layer vacancies, strengthening bonding and reducing antisite defects, while Cu2+ tends to fill Li layer vacancies. The process was applied to highly degraded polycrystalline LiNi0.83Co0.12Mn0.05O2 cathode. The upcycled material achieved a first-cycle discharge capacity of over 215 mAh g−1 and maintained 93.3% capacity retention after 100 cycles at 1 C, and a fast-charging capability of 85 mAh g−1 at 15 C. The 1.2 Ah pouch cell using this upcycled cathode material retained 91.1% capacity retention after 200 cycles.
It is crucial to address the non-uniform lithium distribution among the repeatedly reacted cathode particles. The solution can be achieved by chemical relithiation by using a lithiation agent in the regeneration process [,,,,,,]. The problem is that these reagents can cause overlithiation and structural damage. In particular, the introduction of redox mediators (RMs), such as quinone-based and organic electron-donating materials avoid this problem and has proven more effective in the restoration of NMC cathode materials [,]. Recently, Kim et al. achieved 100% efficiency of chemical relithiation of NMC622 assisted by the redox mediator 3,5-di-tert-butyl-o-benzoquinone (DTBQ) []. This result offers a facile and sustainable solution for Ni-rich battery recycling.
Zheng et al. proposed a different process. They investigated selective leaching of lithium from spent NCM using advanced oxidant ammonium persulfate (NH4)2S2O8, resulting in the recovery of lithium carbonate Li2CO3. Then, the recovered Li2CO3 is combined with the selective leaching residue to synthesize fully-regenerated NCM via high-temperature solid-phase synthesis []
7.4. Safety Issues
Ni-rich lithium batteries suffer from severe thermal runaway under abuse conditions [,,]. The release of oxygen by the cathode material is part of the problem, and cross-talking effects between cathode and anode play a critical role. The cross-talk of cathode-released oxygen to fully lithiated graphite anode [,], as well as the reaction between oxygen species and the electrolyte [], can trigger the thermal runaway. It has also been suggested that the recombination of atomic hydrogen accumulated in graphite anode plays an important role []. LiH induced exothermic reactions at the anode side and H2 generation and migration to the cathode side are also at the origin of thermal runaway [,,,]. The conclusion of these different works is that the thermal-induced cross-talking effects between the cathode and anode triggers the thermal runaway of the Ni-rich lithium batteries. This also implies that the stability of the CEI and SEI is more essential than that of the electrolyte components [].
8. Conclusions
Progress in recent years on Ni-rich cathode elements have been made along different issues. Structural and chemical stability have been enhanced. Single-crystallized materials show better mechanical integrity compared to polycrystalline materials, reducing cracking and degradation. Efforts in research should then focus on this morphology. Introducing doping elements can stabilize the crystal structure, mitigate cation mixing, and improve thermal stability, and surface coating can suppress side reactions with electrolytes and reduce microcracking.
Progress has also been achieved to mitigate oxygen loss and electrolyte decomposition. High-voltage stable electrolytes with additives help reduce electrolyte decomposition at high voltages. Replacing liquid electrolytes with ceramic solid electrolytes can enhance stability and lifespan. Designing gradient Ni concentration across cathode particles can minimize oxygen release and improve cycle life.
Mechanical and microstructural improvements include crack-resistant design preventing the performance loss. Combining Ni-rich cathodes with other materials (e.g., Li-rich layered oxides) can balance energy density and stability.
Improved manufactory and scalability results from lower cobalt content, which reduces cobalt dependence, cuts costs, and improves sustainability.
An optimized sintering process has been achieved, due to advanced synthesis techniques like spray drying and atomic layer deposition, which can refine particle morphology and enhance material uniformity.
However, additional research is needed, and the future outlook depends on following research along the following lines:
- The interaction between the electrolytes and the Ni-rich materials and the related CEI needs further investigation. Newly-developed experimental set-ups, such as cryogenic transmission electron microscopy revealing atomic-resolution CEI structures, should help for this purpose. An example is the dynamic evolution of over cycling and its impact on the performance of NMC811, revealed by the ultrafine images of CEI obtained by this process in presence of FEC-based electrolytes [].
- Safety issues with high-voltage cathode materials would plead in favor of the use of solid electrolytes. As shown in this review, some attempts are promising, but the research should continue in the next years. The space charge layer between the cathode and a ceramic solid electrolyte may result in high polarization and capacity degradation. Another difficulty comes from the deterioration of the contact between the cathode and solid electrolyte upon cycling, due to the rigid ceramic nature of the solid electrolyte and the change in the lattice parameters of the cathode material, in particular at high charge. The columnar shape of the Ni-rich particles or actually any elongated form will help [], pointing to the need for further investigation on the correlations between morphology, synthesis, and electrochemical performance Elongated polymer electrolytes have already made possible the commercialization of LiFePO4-based batteries, due to the low voltage (3.5 V) of the cells. With higher voltage cells, the same polymers cannot be used, because of their poor antioxidative ability. Further investigations on polymer electrolytes with wide electrochemical window are thus needed, and under studies []. Further research is needed to pair Ni-rich cathodes with lithium metal or silicon anodes. The reaction of the electrolytes with the Ni-rich cathode and the anode materials is not well understood, and further studies on the additives able to generate both a SEI and a CEI protecting the electrodes against side reactions with an electrolyte stable at high voltage are still needed.
- The application of AI to the research on Li-rich batteries is needed and most promising. It is possible today to predict material degradation pathways according to operating environments, which will help to optimize the design and composition of electrode materials for enhanced performance and durability of the Ni-rich cathode materials. Machine learning (ML) can accelerate the design of new Ni-rich cathode chemistries, and can also be used to build next-generation battery architectures. Many trials have been made to dope Ni-rich cathode materials with different single and multiple doping elements. ML models can predict dopants that improve stability without expensive trial-and-error experiments. In particular, deep learning models trained on large materials databases (e.g., Materials Project) predict stable Ni-rich structures with minimal degradation. ML will also help designing single-crystal vs. polycrystalline Ni-rich cathodes to reduce cracking. Computer vision models will be used to analyze SEM/TEM images to detect defects and suggest improvements in synthesis processes.
Ni-rich lithium-based cathode materials, particularly those in the layered LiNixCoyMnzO2 (NCM) and LiNixCoyAlzO2 (NCA) families, have emerged as frontrunners in the pursuit of high-energy-density lithium-ion batteries. Their increased nickel content directly contributes to elevated specific capacities, making them attractive for applications ranging from electric vehicles to grid storage. Over the past two decades, the field has experienced remarkable advancements in material synthesis, structural stabilization, and electrochemical performance optimization. This review has comprehensively explored the multifaceted aspects of Ni-rich LIBs, spanning from fundamental crystal chemistry and degradation mechanisms to cutting-edge approaches in coating, doping, and electrolyte engineering. Despite these advancements, the commercialization and long-term viability of Ni-rich cathodes are still hindered by persistent challenges. Chief among these are structural degradations during cycling, irreversible phase transitions, surface instability, gas evolution (notably O2 and CO2), transition metal dissolution, and thermal runaway risks at high states of charge. These degradation pathways are exacerbated by the high reactivity of Ni4+ and the increased lattice strain that arises during deep lithiation/delithiation cycles. Addressing these issues requires a deep understanding of bulk–surface–interface interactions under both normal and abusive conditions.
Innovations in material engineering, such as gradient compositions, core–shell morphologies, single-crystal structures, and atomic-scale doping, have demonstrated notable progress in mitigating degradation while improving thermal stability and capacity retention. Furthermore, the strategic development of advanced electrolytes, including localized high-concentration electrolytes (LHCEs), fluorinated solvents, and hybrid solid–liquid systems, has opened new frontiers in interfacial stability. Coupled with surface coatings based on metal oxides, phosphates, or ion-conductive polymers, these approaches form a synergistic framework to extend cycle life and enhance safety.
At the device level, progress in electrode architecture, electrode–electrolyte interface design, and formation protocols have collectively contributed to more robust, scalable battery systems. However, translating these laboratory-scale improvements into commercial viability necessitates further breakthroughs in synthesis cost reduction, scalable coating technologies, and recyclability strategies.
The field also stands to benefit from deeper integration of advanced characterization techniques—such as operando X-ray diffraction (XRD), transmission electron microscopy (TEM), neutron scattering, and synchrotron-based spectroscopy—alongside computational modeling and machine learning. These tools are critical for real-time insight into degradation pathways and for predictive materials discovery. Data-driven optimization and digital twin modeling may accelerate the design of next-generation Ni-rich cathode systems that are both high-performing and durable.
Looking forward, Ni-rich cathode chemistries will likely remain central to LIB technology for the foreseeable future, especially as efforts to reduce or eliminate cobalt become more urgent for ethical and economic reasons. Sustainable sourcing of nickel, closed-loop recycling systems, and lifecycle assessments will be crucial components of this ecosystem. Simultaneously, a holistic approach that incorporates safety engineering, environmental considerations, and total cost of ownership must guide the development and deployment of these advanced materials.
To our view, a breakdown of the best, most effective strategies-ranked by impact and practicality are as follows:
- Surface coating to prevent electrolyte attack, suppress transition metal (TM) dissolution, and stabilize the cathode–electrolyte interface. The benefits are the reduction of surface reactivity, minimization of HF attack, and improvement of the cycling stability. To fulfill this goal, however, the coating must be uniform, thin (<10 nm), and ionically conductive to avoid impeding Li+ transport.
- Doping, to enhance structural stability, suppress phase transitions, and reduce oxygen release. The cation doping stabilizes the layered structure and suppresses Li/Ni disorder, while anion doping, in particular F− (fluorination), strengthens TM–O bonds and suppresses oxygen evolution. The benefits are improved structural integrity, better capacity retention, and reduced gas generation.
- Single-crystal morphology minimizes grain boundaries and microcracks, which are common initiation sites for degradation. The benefits are improved mechanical integrity, longer cycle life, and better thermal stability. However, there are drawbacks: this morphology is more difficult and costly to synthesize. It may require higher sintering temperatures.
- Gradient composition or core–shell design combines a Ni-rich core for capacity with a stable outer shell (lower-Ni or doped) to buffer against side reactions. These architectures enhance thermal and chemical stability and delay impedance rise.
- Optimized electrolyte formulations reduce parasitic reactions, stabilize CEI (cathode–electrolyte interface), and suppress gas generation.
- Particle size and morphology engineering is useful to reduce mechanical stress and optimize Li+ diffusion. Spherical secondary particles with controlled porosity or dense, crack-resistant structures are preferred, resulting in improved structural stability and rate capability.
- Thermal and pressure management during cycling, to reduce gas generation and mechanical damage. The strategy for this purpose is to use stack pressure or thermal management in cell design.
- Advanced synthesis techniques, to achieve controlled doping, precise morphology, and homogeneous element distribution.
The efforts in the next years should then be focused on the integrated design combining the above approaches, such as the synthesis of a Ni-rich cathode with gradient composition, surface coating (e.g., LiNbO3), W-doping, and used with a fluorinated LHCE, which will significantly outperform a conventional NMC811 cell in both energy density and cycle life, even at high voltages (>4.3 V).
In conclusion, while Ni-rich lithium-ion batteries have achieved a high level of maturity, their full potential will only be realized through continued interdisciplinary collaboration across materials science, electrochemistry, manufacturing, and systems engineering (see Table S1). The challenges are complex, but the rewards—in terms of decarbonizing transportation, stabilizing renewable grids, and powering the next generation of electronic devices—are immense. With sustained innovation and strategic investment, Ni-rich cathode technologies are well poised to drive the next wave of energy storage breakthroughs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries11070254/s1, Table S1: Highlights of top-performing Ni-rich cathodes [,,,].
Author Contributions
Conceptualization, A.M.; writing—original draft preparation, A.M.; writing—review and editing, C.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALD | atomic layer deposition |
| ASSLBs | all-solid-state lithium batteries |
| CB | carbon black |
| CBDA | cyclobutane-tetracarboxylic dianhydride |
| CEI | cathode electrolyte interphase |
| COF | covalent organic framework |
| DFT | density functional theory |
| DMC | dimethyl carbonate |
| DTBQ | di-tert-butyl-o-benzoquinone |
| EPD | electrophoretic deposition |
| EV | electric vehicle |
| HOMO | Highest Occupied Molecular Orbital |
| HoMS | hollow multi-shell structure |
| LIBs | Lithium-ion batteries |
| LIPON | lithium phosphorus oxynitride |
| LLAO | Li0.5La2Al0.5O4 |
| LUMO | Lowest Unoccupied Molecular Orbital |
| NASICON | sodium (Na) superionic conductor |
| NATM | LiNi0.93Al0.05Ti0.01Mg0.01O2 |
| NCA | LiNixCoyAlzO2 |
| NMC | LiNi1–x–yMxCoyxO2 |
| NMCA | LiNixMnyCozAl(1–x–y–z)O2 |
| NMC811 | LiNi0.8Mn0.1Co0.1O2 |
| NMC333 | LiNi1/3Mn1/3Co1/3O2 |
| NMC622 | LiNi0.6Mn0.2Co0.2O2 |
| PANI | polyaniline |
| PEG | polyethylene glycol |
| PPC | pyrrole-co-citral nitrile |
| PPy | polypyrrole |
| SEI | solid–electrolyte interphase |
| SEM | scanning electron microscopy |
| SOC | state-of-charge |
| TM | transition metal |
| VC | vinylene carbonate |
| XRD | X-ray diffraction |
References
- You, L.Z.; Chu, B.B.; Li, G.X.; Huang, T.; Yu, A.S. H3BO3 washed LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance and storage characteristics. J. Power Sources 2021, 482, 228940. [Google Scholar] [CrossRef]
- Yu, H.F.; Cao, Y.Q.; Chen, L.; Hu, Y.J.; Duan, X.Z.; Dai, S.; Jiang, H. Surface enrichment and diffusion enabling gradient-doping and coating of Ni-rich cathode toward Li ion batteries. Nat. Commun. 2021, 12, 4564. [Google Scholar] [CrossRef] [PubMed]
- Rajkamal, A.; Sharma, A.; Pullagura, B.K.; Thapa, R.; Kim, H. Engineering lithium nickel cobalt manganese oxides cathodes: A computational and experimental approach to bridging gaps. Chem. Eng. J. 2024, 481, 148223. [Google Scholar] [CrossRef]
- Ding, H.; Wang, X.; Wang, J.; Zhang, H.; Liu, G.; Yu, W.; Wang, J. Morphology-controllable synthesis and excellent electrochemical performance of Ni-rich layered NCM622 as cathode materials for lithium-ion batteries via glycerin-assisted solvothermal method. J. Power Sources 2023, 553, 232307. [Google Scholar] [CrossRef]
- Kim, J.; Lee, I.; Kim, Y.-H.; Bae, J.H.; Hwang, K.; Kang, H.; Yoon, S. Ni-rich cathode material with isolated porous layer hindering crack propagation under 4.5 V high cut-off voltage cycling. Chem. Eng. J. 2023, 455, 140578. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhu, Z.Y.; Yan, Q.Z.; Yu, S.C.; He, X.; Chen, Y.; Liu, P. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63–67. [Google Scholar] [CrossRef]
- Xie, H.; Peng, H.; Jiang, D.; Xiao, Z.; Liu, X.; Liang, H.; Wu, M.; Liu, D.; Li, Y.; Sun, Y.; et al. Structures, issues, and optimization strategies of Ni-rich and Co-low cathode materials for lithium-ion battery. Chem. Eng. J. 2023, 470, 144051. [Google Scholar] [CrossRef]
- Tian, X.; Guo, R.; Bai, Y.; Li, N.; Wang, X.; Wang, J.; Wu, C. High-performance high-nickel multi-element cathode materials for lithium-ion batteries. Batteries 2023, 9, 319. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Namkoong, B.; Kim, J.H.; Belharouak, I.; Yoon, C.S.; Sun, Y.K. Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes. ACS Energy Lett. 2021, 6, 2726–2734. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, R.; Wang, L.; Cheng, K.; Zhao, Z.; Mu, D.; Wu, B. A short review on layered LiNi0.8Mn0.1Co0.1O2 positive electrode material for lithium-ion batteries. Energy Proc. 2017, 105, 2941–2952. [Google Scholar] [CrossRef]
- Mubarac, S.; Silva, M.N.; Silva, G.T.; Freitas, B.; Gonçalves, J.M.; Zanin, H. Super Ni-rich and Co-poor LiNixCoyMn1-x-yO2, LiNixCoyAl1-x-yO2, and LiNixCoyMnzAl(1-x-y-z)O2 (x ≥ 0.85) based cathodes for lithium-ion batteries: A review on emerging trends, recent developments, and future perspectives. J. Energy Storage 2024, 96, 112612. [Google Scholar] [CrossRef]
- Li, J.; Manthiram, A. A comprehensive analysis of the interfacial and structural evolution over long-term cycling of ultrahigh-nickel cathodes in lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1902731. [Google Scholar] [CrossRef]
- Cui, Z.; Xie, Q.; Manthiram, A. Zinc-doped high-nickel, low-cobalt layered oxide cathodes for high-energy-density lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 15324–15332. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Xia, J.; Cameron, A.R.; Nie, M.; Botton, G.A.; Dahn, J.R. The impact of electrolyte additives and upper cut-off voltage on the formation of a rocksalt surface layer in LiNi0.8Mn0.1Co0.1O2 electrodes. J. Electrochem. Soc. 2017, 164, A655–A665. [Google Scholar] [CrossRef]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Mehdi, B.L.; et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 84–92. [Google Scholar] [CrossRef]
- Li, J.; Downie, L.E.; Ma, L.; Qiu, W.; Dahn, J.R. Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 Cathode material for lithium ion batteries. J. Electrochem. Soc. 2015, 162, A1401–A1408. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, electrochemical, and thermal properties of nickel-rich LiNixMnyCozO2 materials. Chem. Mater. 2018, 30, 8852–8860. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review: Recent advances and remaining challenges for lithium-ion battery cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Kim, M.; Zhu, J.; Li, L.; Wang, C.; Chen, G. Understanding reactivities of Ni-rich Li[NixMnyCo1-x-y]O2 single-crystal cathode materials. ACS Appl. Energy Mater. 2020, 3, 12238–12245. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. A 2017, 5, 874–901. [Google Scholar] [CrossRef]
- Duh, Y.S.; Sun, Y.; Lin, X.; Zheng, J.; Wang, M.; Wang, Y.; Lin, X.; Jiang, X.; Zheng, Z.; Zheng, S.; et al. Characterization on thermal runaway of commercial 18650 lithium-ion batteries used in electric vehicles: A review. J. Energy Storage 2021, 41, 102888. [Google Scholar] [CrossRef]
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Zizebert, C. Thermal and mechanical safety assessment of type 21700 lithium-ion batteries with NMC, NCA and LFP cathodes–investigation of cell abuse by means of accelerating rate calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar] [CrossRef]
- Huggins, R.A. Energy Storage: Fundamentals, Materials and Applications, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 181–208. [Google Scholar]
- Manthiram, A. An outlook on lithium-ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Devnani, H.; Sharma, A.; Lim, W.C.; Dhyani, A.; Chae, K.H.; Lee, S. Challenges and opportunities using Ni-rich layered oxide cathodes in Li-ion rechargeable batteries: The case of nickel cobalt manganese oxides. Energy Adv. 2024, 3, 1869–1893. [Google Scholar] [CrossRef]
- Ahangari, M.; Szalai, B.; Lujan, J.; Zhou, M.; Luo, H. Advancements and challenges in high-capacity Ni-rich cathode materials for lithium-ion batteries. Materials 2024, 17, 801. [Google Scholar] [CrossRef]
- Butt, A.; Ali, G.; Kubra, K.T.; Sharif, R.; Salman, A.; Bashir, M.; Jamil, S. Recent advances in enhanced performance of Ni-rich cathode materials for Li-ion batteries: A review. Energy Technol. 2022, 10, 2100775. [Google Scholar] [CrossRef]
- Jamil, S.; Wang, G.; Fasehullah, M.; Xu, M. Challenges and prospects of nickel-rich layered oxide cathode material. J. Alloys Compd. 2022, 909, 164727. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Britala, L.; Marinaro, M.; Kucenskis, G. A review of the degradation mechanisms of NCM cathodes and corresponding mitigation strategies. J. Energy Storage 2023, 73A, 108875. [Google Scholar] [CrossRef]
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Hou, P.Y.; Yin, J.M.; Ding, M.; Huang, J.; Xu, X. Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: Advances and perspectives. Small 2017, 13, 1701802. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2(NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Strehle, B.; Kleiner, K.; Jung, R.; Chesneau, F.; Mendez, M.; Gasteiger, H.A.; Piana, M. The role of oxygen release from Li- and Mn-rich layered oxides during the first cycles investigated by on-line electrochemical mass spectrometry. J. Electrochem. Soc. 2017, 164, A400–A406. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Pan, Q.-L.; Wei, H.-X.; Huang, Y.-D.; Tang, L.-B.; Wang, Z.-Y.; He, Z.-J.; Yan, C.; Mao, J.; Dai, K.-H.; et al. Towards Ni-rich layered oxides cathodes with low Li/Ni intermixing by mild molten-salt ion exchange for lithium-ion batteries. Nano Energy 2022, 102, 107626. [Google Scholar] [CrossRef]
- Xiao, P.; Li, W.-H.; Chen, S.; Li, G.; Dai, Z.-J.; Feng, M.-D.; Chen, X.; Yang, W.-S. Effects of Oxygen pressurization on Li+/Ni2+ cation mixing and the oxygen vacancies of LiNi0.8Co0.15Al0.05O2 cathode materials. ACS Appl. Mater. Interfaces 2022, 14, 31851–31861. [Google Scholar] [CrossRef]
- Chen, J.-N.; Yang, Y.; Tang, Y.-S.; Wang, Y.-F.; Li, H.; Xiao, X.-H.; Wang, S.-N.; Mariyam, S.D.D.; Etter, M.; Missyul, A.; et al. Constructing a thin disordered self-protective layer on the LiNiO2 primary particles against oxygen release. Adv. Funct. Mater. 2022, 33, 2211515. [Google Scholar] [CrossRef]
- Li, X.-H.; Wang, Q.; Guo, H.-Y.; Artrith, N.; Urban, A. Understanding the onset of surface degradation in LiNiO2 cathodes. ACS Appl. Energy Mater. 2022, 5, 5730–5741. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, S.P.; Zhang, L.; Guo, L.; Shi, F.N. Review on oxygen release mechanism and modification strategy of nickel-rich NCM cathode materials for lithium-ion batteries: Recent advances and future directions. Energy Fuel 2024, 38, 5607–5631. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Piana, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC impedance. J. Electrochem. Soc. 2015, 162, A2737–A2746. [Google Scholar] [CrossRef]
- Komagata, S.; Itou, Y.; Kondo, H. Impact of surface layer formation during cycling on the thermal stability of the LiNi0.8Co0.1Mn0.1O2 cathode. ACS Appl. Mater. Interfaces 2022, 14, 8931–8937. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, P.; Zhou, X.; Wu, W.; Liu, X.; Liu, Y.; Feng, G.; Zhang, B.; Xing, W.; Zuo, M.; et al. Slightly Li-enriched chemistry enabling super stable LiNi0.5Mn0.5O2 cathodes under extreme conditions. Chem. Sci. 2024, 15, 14415–14424. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, Y.; Wang, L.; Fu, N.; Li, Y.; Luo, Y.; Wang, J.; Xiao, X.; Wang, X.; Yang, X.; et al. Challenges of thermal stability of high-energy layered oxide cathode materials for lithium-ion batteries: A review. Mater. Today 2023, 69, 236–261. [Google Scholar] [CrossRef]
- Lim, J.M.; Hwang, T.; Kim, D.; Park, M.-S.; Cho, K.; Cho, M. Intrinsic origins of crack generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 2017, 7, 39669. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Li, W.; Dolocan, A.; Celio, H.; Park, H.; Warner, J.H.; Manthiram, A. In-depth analysis of the degradation mechanisms of high-nickel, low/no-cobalt layered oxide cathodes for lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2100858. [Google Scholar] [CrossRef]
- Laakso, E.; Efimova, S.; Colalongo, M.; Kauranen, P.; Lahtinen, K.; Napolitano, E.; Ruiz, V.; Moskon, J.; Gaberscek, M.; Park, J.; et al. Aging mechanisms of NMC811/Si-Graphite Li-ion batteries. J. Power Sources 2024, 599, 234159. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Yuan, F.; Lyu, M.; Yang, P.; Zhang, L.; Zhou, M.; Wang, L.; Zhang, S.; Wang, L. Ni-rich cathode materials for stable high-energy lithium-ion batteries. Nano Energy 2024, 126, 109620. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, D. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, X.; Zhang, J.; Manthiram, A.; Meng, Y.S.; Xu, W. A Review on the stability and surface modification of layered transition-metal oxide cathodes. Mater. Today 2021, 46, 155–182. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.; Yuan, W.; Zhang, G.; Zheng, H.; Chen, Z. Advances in multi-scale design and fabrication processes for thick electrodes in lithium-ion batteries. Energy Rev. 2024, 3, 100066. [Google Scholar] [CrossRef]
- Das, J.; Kleiman, A.; Rehman, A.U.; Verma, R.; Young, M.H. The cobalt supply chain and environmental life cycle impacts of lithium-ion battery energy storage systems. Sustainability 2024, 16, 1910. [Google Scholar] [CrossRef]
- The Organisation for Economic Co-Operation and Development (OECD). Interconnected Supply Chains: A Comprehensive Look at Due Diligence Challenges and Opportunities Sourcing Cobalt and Copper from the Democratic Republic of the Congo. In Responsible Business Conduction; OECD: Paris, France, 2019; Available online: https://mneguidelines.oecd.org/Interconnected-supply-chains-a-comprehensive-look-at-due-diligence-challenges-and-opportunities-sourcing-cobalt-and-copper-from-the-DRC.pdf (accessed on 1 January 2019).
- U.S. Geological Survey (USGS). Mineral Commodity Summaries 2022-Cobalt; U.S. Geological Survey: Reston, VA, USA, 2022. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-cobalt.pdf (accessed on 25 May 2023).
- Cobalt Institute. State of Cobalt Market Report. 2021. Available online: https://www.cobaltinstitute.org/wp-content/uploads/2021/09/Cobalt-Institute-State-of-the-Cobalt-Market-Report_2020.pdf (accessed on 25 May 2023).
- U.S. Department of Energy. Energy Storage Grand Challenge: Energy Storage Market Report; U.S. Department of Energy: Washington, DC, USA, 2020. Available online: https://www.energy.gov/sites/prod/files/2020/12/f81/Energy%20Storage%20Market%20Report%202020_0.pdf (accessed on 1 December 2020).
- The White House. FACT SHEET: Securing a Made in America Supply Chain for Critical Minerals. 2022. Available online: https://bidenwhitehouse.archives.gov/briefing-room/statements-releases/2022/02/22/fact-sheet-securing-a-made-in-america-supply-chain-for-critical-minerals/ (accessed on 22 February 2022).
- Nriagu, J.O. Toxic metal pollution in Africa. Sci. Total. Environ. 1992, 121, 1–37. [Google Scholar] [CrossRef]
- Banza, C.L.N.; Nawrot, T.S.; Haufroid, V.; Decrée, S.; De Putter, T.; Smolders, E.; Kabyla, B.I.; Luboya, O.N.; Ilunga, A.N.; Mutombo, A.M.; et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009, 109, 745–752. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Water Issues in the Democratic Republic of Congo: Challenges and Opportunities—Technical Report. 2011. Available online: https://www.ircwash.org/sites/default/files/Partow-2011-Water.pdf (accessed on 1 January 2011).
- Slack, J.F.; Kimball, B.E.; Shedd, K.B. Cobalt Report 1802F. Professional Paper; USGS Publications Warehouse: Reston, VA, USA, 2017. Available online: https://pubs.er.usgs.gov/publication/pp1802F (accessed on 25 May 2023).
- Shengo, M.; Kime, M.-B.; Mambwe, M.; Nyembo, T. A review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J. Sustain. Min. 2019, 18, 226–246. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A.; Nkulu, C.B.L.; Mwitwa, J.; Kampemba, F.M.; Nabuyanda, M.M.; Haufroid, V.; Smolders, E.; Nemery, B. Contamination of water and food crops by trace elements in the African Copperbelt: A collaborative cross-border study in Zambia and the Democratic Republic of Congo. Environ. Adv. 2021, 6, 100103. [Google Scholar] [CrossRef]
- Schmidt, T.; Buchert, M.; Schebek, L. Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour. Conserv. Recycl. 2016, 112, 107–122. [Google Scholar] [CrossRef]
- Voronina, N.; Sun, Y.-K.; Myung, S.-T. Co-free layered cathode materials for high energy density lithium-ion batteries. ACS Energy Lett. 2020, 5, 1814–1824. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Sun, H.H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energy Environ. Sci. 2021, 14, 844–852. [Google Scholar] [CrossRef]
- Gent, W.E.; Busse, G.M.; House, K.Z. The predicted persistence of cobalt in lithium-ion batteries. Nat. Energy 2022, 7, 1132. [Google Scholar] [CrossRef]
- Chu, B.; Guo, Y.-J.; Shi, J.-L.; Yin, Y.-X.; Huang, T.; Su, H.; Yu, A.; Guo, Y.-G. Cobalt in high-energy-density layered cathode materials for lithium-ion batteries. J. Power Sources 2022, 544, 231873. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Liu, J.; Lu, J.; Bi, X.; Dai, A.; Li, M.; Li, M.; Hu, Z.; Ma, L.; et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 2021, 6, 277–286. [Google Scholar] [CrossRef]
- Ge, H.; Shen, Z.; Wang, Y.; Sun, Z.; Cao, X.; Wang, C.; Fan, X.; Bai, J.; Li, R.; Yang, T.; et al. Design of high-performance and sustainable Co-free Ni-rich cathodes for next-generation lithium-ion batteries. SusMat 2024, 4, 48–71. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Fan, Y.; Yang, W.; Zhan, C.; Liu, G. Controversy on necessity of cobalt in nickel-rich cathode materials for lithium-ion batteries. J. Ind. Eng. Chem. 2022, 110, 120–130. [Google Scholar] [CrossRef]
- Mallick, S.; Patel, A.; Sun, X.-G.; Paranthaman, M.P.; Mou, M.; Mugumya, J.H.; Jiang, M.; Rasche, M.L.; Lopez, H.; Gupta, R.B. Low-cobalt active cathode materials for high-performance lithium-ion batteries: Synthesis and performance enhancement methods. J. Mater. Chem. A 2023, 11, 3789–3821. [Google Scholar] [CrossRef]
- Hussain, S.K.; Bang, J.H. Recent progress in Co-free, Ni-rich cathode materials for lithium-ion batteries. Bull. Korean Chem. Soc. 2024, 45, 4–15. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.-L.; Yin, L.; Hwang, I.; Li, Y.; Lu, L.; Xu, W.; Zhang, X.; Chen, Y.; Ren, Y.; et al. Probing the thermal-driven structural and chemical degradation of Ni-rich layered cathodes by Co/Mn exchange. J. Am. Chem. Soc. 2020, 142, 19745. [Google Scholar] [CrossRef]
- Choi, J.U.; Voronina, N.; Sun, Y.-K.; Myung, S.-T. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: Yesterday, today, and tomorrow. Adv. Energy Mater. 2020, 10, 2002027. [Google Scholar] [CrossRef]
- Song, Y.; Cui, Y.; Geng, L.; Li, B.; Ge, L.; Zhou, L.; Qiu, Z.; Nan, J.; Wu, W.; Xu, H.; et al. Li/Ni intermixing: The real origin of lattice oxygen stability in Co-free Ni-rich cathode materials. Adv. Energy Mater. 2024, 14, 2303207. [Google Scholar] [CrossRef]
- Kim, Y.; Seong, W.M.; Manthiram, A. Cobalt-free, high-nickel layered oxide cathodes for lithium-ion batteries: Progress, challenges, and perspectives. Energy Storage Mater. 2021, 34, 250–259. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, T.; Liu, J.; He, L.; Chen, J.; Zhang, J.; Chen, H. Insight into the origin of lithium/nickel ions exchange in layered Li(NixMnyCoz)O2 cathode materials. Nano Energy 2018, 49, 77–85. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, Y.; Liu, T.; Xiao, Y.; Wang, F.; Pan, F. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control. Acc. Chem. Res. 2019, 52, 2201–2209. [Google Scholar] [CrossRef]
- Wang, D.; Xin, C.; Zhang, M.; Bai, J.; Zheng, J.; Kou, R.; Ko, J.Y.P.; Huq, A.; Zhong, G.; Sun, C.-J.; et al. Intrinsic role of cationic substitution in tuning Li/Ni mixing in high-Ni layered oxides. Chem. Mater. 2019, 31, 2731–2740. [Google Scholar] [CrossRef]
- Ben Kamel, K.; Amdouni, N.; Abdel-Ghany, A.; Zaghib, K.; Mauger, A.; Gendron, F.; Julien, C. Local structure and electrochemistry of LiNiyMnyCo1-2yO2 electrode materials for Li-ion batteries. Ionics 2008, 14, 89–97. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Yang, X.; Liu, Y.; Wu, Z.; Li, H.; Indris, S.; Ehrenberg, H.; Hua, W. Unravelling the peculiar role of Co and Al in highly Ni-rich layered oxide cathode materials. Chem. Eng. J. 2024, 484, 149599. [Google Scholar] [CrossRef]
- Lin, J.; Lian, X.; Wang, X.; Ma, Y.; Fang, L.; Suo, X. Effects of Al content on the electrochemical performance and thermal stability of Li(Ni0.9Co0.06Mn0.04)1–xAlxO2 cathode materials for Li-ion batteries. Ionics 2023, 29, 2153–2162. [Google Scholar] [CrossRef]
- Aishova, A.; Park, G.; Yoon, C.S.; Sun, Y. Cobalt-free high-capacity Ni-rich layered Li[Ni0.9Mn0.1]O2 cathode. Adv. Energy Mater. 2020, 10, 1903179. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-nickel NMA: A cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Kan, W.H.; Kuai, C.; Yang, Z.; Li, L.; Sun, C.-J.; Sainio, S.; Avdeev, M.; Nordlund, D.; Lin, F. Structural and electrochemical impacts of Mg/Mn dual dopants on the LiNiO2 cathode in Li-metal batteries. ACS Appl. Mater. Interfaces 2020, 12, 12874–12882. [Google Scholar] [CrossRef]
- Muralidharan, N.; Essehli, R.; Hermann, R.P.; Parejiya, A.; Amin, R.; Bai, Y.; Du, Z.; Belharouak, I. LiNixFeyAlzO2, a new cobalt-free layered cathode material for advanced Li-ion batteries. J. Power Sources 2020, 471, 228389. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, R.; Kan, W.H.; Zhang, Y.; Li, L.; Kuai, C.; Zydlewski, B.; Rahman, M.M.; Sun, C.-J.; Sainio, S.; et al. Dopant distribution in Co-free high-energy layered cathode materials. Chem. Mater. 2019, 31, 9769–9776. [Google Scholar] [CrossRef]
- Yang, Z.; Mu, L.; Hou, D.; Rahman, M.M.; Xu, Z.; Liu, J.; Nordlund, D.; Sun, C.; Xiao, X.; Lin, F. Probing dopant redistribution, phase propagation, and local chemical changes in the synthesis of layered oxide battery cathodes. Adv. Energy Mater. 2020, 11, 2002719. [Google Scholar] [CrossRef]
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is cobalt needed in Ni-rich positive electrode materials for lithium ion batteries? J. Electrochem. Soc. 2019, 166, A429–A439. [Google Scholar] [CrossRef]
- Cui, Z.; Xie, Q.; Manthiram, A. A cobalt- and manganese-free high-nickel layered oxide cathode for long-life, safer lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2102421. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and back again—The journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 2019, 58, 10434–10458. [Google Scholar] [CrossRef]
- Cui, J.; Ding, X.; Luo, D.; Xie, H.; Zhang, Z.; Zhang, B.; Tan, F.; Liu, C.; Lin, Z. Effect of cationic uniformity in precursors on Li/Ni mixing of Ni-rich layered cathodes. Energy Fuels 2021, 35, 1842–1850. [Google Scholar] [CrossRef]
- Wang, S.; Hua, W.; Missyul, A.; Darma, M.S.D.; Tayal, A.; Indris, S.; Ehrenberg, H.; Liu, L.; Knapp, M. Kinetic control of long-range cationic ordering in the synthesis of layered Ni-rich oxides. Adv. Funct. Mater. 2021, 31, 2009949. [Google Scholar] [CrossRef]
- Paidi, A.K.; Lee, A.T.; Paidi, V.K.; Ahn, H.; Lim, J.; Lee, K.-S.; Lee, S.; Ahn, D. Atomic-level insights into the first cycle irreversible capacity loss of Ni-rich layered cathodes for Li-ion batteries. J. Mater. Chem. A 2023, 11, 12002–12012. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.Z.; Ren, D.S.; Liao, J.Y.; Song, Y.Z.; Liang, H.M.; Wang, A.P.; He, Y.F.; Wang, L.; Chen, Z.H.; et al. Revisiting the initial irreversible capacity loss of LiNi0.6Co0.2Mn0.2O2 cathode material batteries. Energy Storage Mater. 2022, 50, 373–379. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Lu, Y.; Xie, W.; Yan, Z.; Chen, J. Insights into cation migration and intermixing in advanced cathode materials for lithium-ion batteries. Adv. Energy Mater. 2024, 14, 2402068. [Google Scholar] [CrossRef]
- Wu, K.; Ran, P.; Yin, W.; He, L.; Wang, B.; Wang, F.; Zhao, E.; Zhao, J. Dynamic evolution of antisite defect and coupling anionic redox in high-voltage ultrahigh-Ni cathode. Angew. Chem. Int. Ed. 2024, 63, e202410326. [Google Scholar]
- Shi, C.-G.; Peng, X.-X.; Dai, P.; Xiao, P.H.; Zheng, W.-C.; Li, H.-Y.; Li, H.; Indris, S.; Mangold, S.; Hong, Y.-H.; et al. Investigation and suppression of oxygen release by LiNi0.8Co0.1Mn0.1O2 cathode under overcharge conditions. Adv. Energy Mater. 2022, 12, 2200569. [Google Scholar] [CrossRef]
- Gabrisch, H.; Yi, T.H.; Yazami, R. Transmission electron microscope studies of LiNi1/3Mn1/3Co1/3O2 before and after long-term aging at 70 °C. Electrochem. Solid State Lett. 2008, 11, A119–A124. [Google Scholar] [CrossRef]
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2 Mn0.3O2 cathode material in lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Robert, R.; Villevieille, C.; Novák, P. Enhancement of the high potential specific charge in layered electrode materials for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 8589–8598. [Google Scholar] [CrossRef]
- Konishi, H.; Yoshikawa, M.; Hirano, T. The effect of thermal stability for high-Ni-content layer-structured cathode materials, LiNi0.8Mn0.1–xCo0.1MoxO2 (x = 0, 0.02, 0.04). J. Power Sources 2013, 244, 23–28. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.-H.; Wang, Z.X.; Guo, H.; Huang, Z.; Kong, L.; Ru, J. Improved high voltage electrochemical performance of Li2ZrO3-coated LiNi0.5Co0.2Mn0.3O2 cathode material. J. Alloys Compd. 2015, 647, 612–619. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Hwang, S.; Chang, W.; Kim, S.M.; Su, D.; Kim, D.Y.; Lee, J.Y.; Chung, K.Y.; Stach, E.A. Investigation of changes in the surface structure of LixNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem. Mater. 2014, 26, 1084–1092. [Google Scholar] [CrossRef]
- Yang, J.; Hou, M.Y.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. Improving the cycling performance of the layered Ni-rich oxide cathode by introducing low content Li2MnO3. Electrochim. Acta 2016, 189, 101–110. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.Y. Enhancement on the cycling stability of the layered Ni-rich oxide cathode by in situ fabricating nano-thickness cation-mixing layers. J. Electrochem. Soc. 2016, 163, A2665–A2672. [Google Scholar] [CrossRef]
- Liu, J.; Du, Z.; Wang, X.; Tan, S.; Wu, X.; Geng, L.; Song, B.; Chien, P.-H.; Everett, S.M.; Hu, E. Anionic redox induced anomalous structural transition in Ni-rich cathodes. Energy Environ. Sci. 2021, 14, 6441–6454. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, J.; Zhang, Q.; Wong, D.; Yin, W.; Zhang, N.; Chen, Z.; Gu, L.; Hu, Z.; Liu, X. Oxygen anion redox chemistry correlated with spin state in Ni-rich layered cathodes. Adv. Sci. 2023, 10, 2206442. [Google Scholar] [CrossRef]
- Chu, Y.; Mu, Y.; Zou, L.; Wu, F.; Yang, L.; Feng, Y.; Zeng, L. Oxygen release in Ni-rich layered cathode for lithium-ion batteries: Mechanisms and mitigating strategies. ChemElectroChem 2024, 11, e20230065. [Google Scholar] [CrossRef]
- Flores, E.; Vonrüti, N.; Novák, P.; Aschauer, U.; Berg, E.J. Elucidation of LixNi0.8Co0.15Al0.05O2 redox chemistry by operando raman spectroscopy. Chem. Mater. 2018, 30, 4694–4703. [Google Scholar] [CrossRef]
- Li, J.; Hua, H.; Kong, X.; Yang, H.; Dai, P.; Zeng, J.; Zhao, J. In-situ probing the near-surface structural thermal stability of high-nickel layered cathode materials. Energy Storage Mater. 2022, 46, 90–99. [Google Scholar] [CrossRef]
- Lee, E.; Muhammad, S.; Kim, T.; Kim, H.; Lee, W.; Yoon, W.S. Tracking the influence of thermal expansion and oxygen vacancies on the thermal stability of Ni-rich layered cathode materials. Adv. Sci. 2020, 7, 1902413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, A.; Tang, Z.; Meng, F.; Shang, T.; Guo, S.; Ding, J.; Luo, Y.; Xiao, D.; Wang, X.; et al. Robust surface reconstruction induced by subsurface Ni/Li antisites in Ni-rich cathodes. Adv. Funct. Mater. 2021, 31, 2010291. [Google Scholar] [CrossRef]
- Chae, B.G.; Park, S.Y.; Song, J.H.; Lee, E.; Jeon, W.S. Evolution and expansion of Li concentration gradient during charge-discharge cycling. Nat. Commun. 2021, 12, 3814. [Google Scholar] [CrossRef]
- Yin, S.; Deng, W.; Chen, J.; Gao, X.; Zou, G.; Hou, H.; Ji, X. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 2021, 83, 105854. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, S.M.; Bak, S.-M.; Chung, K.Y.; Chang, W. Investigating the reversibility of structural modifications of LixNiyMnzCo1–y–zO2 cathode materials during initial charge/discharge, at multiple length scales. Chem. Mater. 2015, 27, 6044–6052. [Google Scholar] [CrossRef]
- Ghanty, C.; Markovsky, B.; Erickson, E.M.; Talianker, M.; Haik, O.; Tal-Yossef, Y.; Mor, A.; Aurbach, D.; Lampert, J.; Volkov, A.; et al. Li+-ion extraction/insertion of Ni-rich Li1+x(NiyCozMnz)wO2 (0.005 < x < 0.03; y:z = 8:1, w ≈ 1) electrodes: In situ XRD and Raman spectroscopy study. ChemElectroChem 2015, 2, 1479–1486. [Google Scholar]
- Wang, X.L.; An, K.; Cai, L.; Feng, Z.; Nagler, S.E.; Daniel, C.; Rhodes, K.J.; Stoica, A.D.; Skorpenske, H.D.; Liang, C.; et al. Visualizing the chemistry and structure dynamics in lithium-ion batteries by in situ neutron diffraction. Sci. Rep. 2012, 2, 747. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.Y. Suppressing the phase transition of the layered Ni-rich oxide cathode during high-voltage cycling by introducing low-content Li2MnO3. ACS Appl. Mater. Interfaces 2016, 8, 1297–1308. [Google Scholar] [CrossRef]
- Li, J.; Shunmugasundaram, R.; Doig, R. In situ X-ray diffraction study of layered Li–Ni–Mn–Co oxides: Effect of particle size and structural stability of core–shell materials. Chem. Mater. 2016, 28, 162–171. [Google Scholar] [CrossRef]
- Yoon, S.; Park, H.G.; Koo, S.; Hwang, J.; Lee, Y.; Park, K.; Kim, D. An in-depth understanding of chemomechanics in Ni-rich layered cathodes for lithium-ion batteries. J. Alloys Compd. 2023, 939, 168531. [Google Scholar] [CrossRef]
- Laubach, S.; Laubach, S.; Schmidt, P.; Ensling, D.; Schmid, S.; Jaegermann, W.; Thissen, A.; Nikolowski, K.; Ehrenberg, H. Changes in the crystal and electronic structure of LiCoO2 and LiNiO2 upon Li intercalation and de-intercalation. Phys. Chem. Chem. Phys. 2009, 11, 3278–3289. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Makimura, Y.; Ohzuku, T. Solid-state chemistry and electrochemistry of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Electrochem. Soc. 2007, 154, A314–A321. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yan, M.Y.; Li, Y.; Vinado, C.; Yang, J. Separating electronic and ionic conductivity in mix-conducting layered lithium transition-metal oxides. J. Power Sources 2018, 393, 75–82. [Google Scholar] [CrossRef]
- Cui, S.; Wei, Y.; Liu, T.; Deng, W.; Hu, Z.; Su, Y.; Li, H.; Li, M.; Guo, H.; Duan, Y.; et al. Optimized temperature effect of Li-ion diffusion with layer distance in Li(NixMnyCoz)O2 cathode materials for high performance Li-ion battery. Adv. Energy Mater. 2016, 6, 1501309. [Google Scholar] [CrossRef]
- Wu, F.; Liu, N.; Chen, L.; Su, Y.; Tan, G.; Bao, L.; Zhang, Q.; Lu, Y.; Wang, J.; Chen, S.; et al. Improving the reversibility of the H2–H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy 2019, 59, 50–57. [Google Scholar] [CrossRef]
- Yoon, C.S.; Ryu, H.H.; Park, G.T.; Kim, J.H.; Kim, K.H.; Sun, Y.K. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries. J. Mater. Chem. A 2018, 6, 4126–4132. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, H.; Park, J.; Roh, Y.; Byun, S.; Lim, J.; Jung, S.; Kim, N.; Lee, K.-T.; Lee, Y.-M. A microcrack propagation-based life prediction model for lithium-ion batteries with Ni-rich cathode materials. J. Energy Storage 2023, 58, 106420. [Google Scholar] [CrossRef]
- Park, N.-Y.; Kim, M.-C.; Han, S.-M.; Park, G.-T.; Kim, D.-H.; Kim, M.-S.; Sun, Y.-K. Mechanism behind the loss of fast charging capability in nickel-rich cathode materials. Angew. Chem. Int. Ed. 2024, 63, e202319707. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, L.; Lu, J.; Zhou, T.; Huang, X.; Cai, Z.; Dai, A.; Gim, J.; Ren, Y.; Xiao, X.; et al. Rational design of mechanically robust Ni-rich cathode materials via concentration gradient strategy. Nat Commun. 2021, 12, 6024. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Ryu, H.H.; Park, N.Y.; Yoon, C.S.; Sun, Y.K. Tungsten doping for stabilization of Li[Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage. J. Power Sources 2019, 442, 227242. [Google Scholar] [CrossRef]
- Lv, C.; Li, Z.; Ren, X.; Li, K.; Ma, J.; Duan, X. Revealing the degradation mechanism of Ni-rich cathode materials after ambient storage and related regeneration method. J. Mater. Chem. A 2021, 9, 3995–4006. [Google Scholar] [CrossRef]
- Su, M.; Chen, Y.; Liu, H.; Li, J.; Fu, K.; Zhou, Y.; Dou, A.; Liu, Y. Storage degradation mechanism of layered Ni-rich oxide cathode material LiNi0.8Co0.1Mn0.1O2. Electrochim. Acta 2022, 422, 140559. [Google Scholar] [CrossRef]
- Yang, J.; Liang, X.; Ryu, H.-H.; Yoon, C.S.; Sun, Y.-K. Ni-rich layered cathodes for lithium-ion batteries: From challenges to the future. Energy Storage Mater. 2023, 63, 102969. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Zhao, Y.; Ray, S.; Lu, X.; Hou, Y.; Zhang, J. A review of nickel-rich layered oxide cathodes: Synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects. Appl. Energy 2022, 305, 117849. [Google Scholar] [CrossRef]
- Quilty, C.-D.; West, P.-J.; Li, W.-Z.; Dunkin, M.-R.; Wheeler, G.-P.; Ehrlich, S.; Ma, L.; Jaye, C.; Fischer, D.-A.; Takeuchi, E.-S.; et al. Multimodal electrochemistry coupled microcalorimetric and X-ray probing of the capacity fade mechanisms of Nickel rich NMC–progress and outlook. Phys. Chem. Chem. Phys. 2022, 24, 11471–11485. [Google Scholar] [CrossRef]
- Sun, H.-H.; Manthiram, A. Impact of microcrack generation and surface degradation on a nickel-rich layered Li[Ni0.9Co0.05Mn0.05]O2 cathode for lithium-ion batteries. Chem. Mater. 2017, 29, 8486–8493. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; Sui, T.; Song, B. Explicit formulae for the internal stress in spherical particles of active material within lithium-ion battery cathodes during charging and discharging. Mater. Des. 2015, 69, 247–252. [Google Scholar] [CrossRef]
- Brandt, L.R.; Marie, J.-J.; Moxham, T.; Förstermann, D.P.; Salvati, E.; Besnard, C.; Papadaki, C.; Wang, Z.; Bruce, P.G.; Korsunsky, A.M. Synchrotron X-ray quantitative evaluation of transient deformation and damage phenomena in a single nickel-rich cathode particle. Energy Environ. Sci. 2020, 13, 3556–3566. [Google Scholar] [CrossRef]
- Park, N.-Y.; Park, G.-T.; Kim, S.-B.; Jung, W.; Park, B.-C.; Sun, Y.-K. Degradation mechanism of Ni-rich cathode materials: Focusing on particle interior. ACS Energy Lett. 2022, 7, 2362–2369. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, J.; Guo, H.; Li, X.; Gui, W.; Chen, N.; Yan, G. Anchoring K+ in Li+ sites of LiNi0.8Co0.15Al0.05O2 cathode material to suppress its structural degradation during high-voltage cycling. Energy Technol. 2018, 6, 2358–2366. [Google Scholar] [CrossRef]
- Lee, E.J.; Chen, Z.; Noh, H.-J.; Nam, S.C.; Kang, S.; Kim, D.H.; Amine, K.; Sun, Y.-K. Development of microstrain in aged lithium transition metal oxides. Nano Lett. 2014, 14, 4873–4880. [Google Scholar] [CrossRef]
- Xu, G.-L.; Liu, Q.; Lau, K.K.S.; Liu, Y.; Liu, X.; Gao, H.; Zhou, X.; Zhuang, M.; Ren, Y.; Li, J.; et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 2019, 4, 484–494. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Hu, E.; Chae, S.; Jia, H.; Liu, T.; Wu, B.; Bi, J.; Amine, K.; Wang, C.; et al. Mesoscale-architecture-based crack evolution dictating cycling stability of advanced lithium-ion batteries. Nano Energy 2020, 79, 105420. [Google Scholar] [CrossRef]
- Bi, Y.J.; Tao, J.H.; Wu, Y.Q.; Li, L.Z.; Xu, Y.B.; Hu, E.Y.; Wu, B.B.; Hu, J.T.; Wang, C.M.; Zhang, J.G.; et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 2020, 370, 1313–1317. [Google Scholar] [CrossRef]
- Tian, H.; Gao, L.T.; Guo, Z.-S. Microstructural adjusting crack Evolution of polycrystalline NCM particle during charge/discharge cycle. J. Electrochem. Soc. 2022, 169, 090513. [Google Scholar] [CrossRef]
- Han, G.-M.; Kim, Y.-S.; Ryu, H.-H.; Sun, Y.-K.; Yoon, C.S. Structural stability of single-crystalline Ni-rich layered cathode upon delithiation. ACS Energy Lett. 2022, 7, 2919–2926. [Google Scholar] [CrossRef]
- Su, Y.F.; Zhang, Q.Y.; Chen, L.; Bao, L.Y.; Lu, Y.; Chen, S.; Wu, F. Stress accumulation in Ni-rich layered oxide cathodes: Origin, impact, and resolution. J. Energy Chem. 2022, 65, 236–253. [Google Scholar] [CrossRef]
- Koo, J.K.; Ran, W.T.A.; Yun, Y.; Seo, J.K.; Kim, M.; Lee, J.; Lee, S.; Kim, J.; Hwang, S.M.; Kim, Y.-J. Probing intraparticle heterogeneity in Ni-rich layered cathodes with different carbon black contents using scanning probe microscopy. J. Energy Storage 2022, 51, 104395. [Google Scholar] [CrossRef]
- Lee, S.; Song, G.; Yun, B.; Kim, T.; Choi, S.H.; Kim, H.; Doo, S.W.; Lee, K.T. Revealing the nanoscopic corrosive degradation mechanism of nickel-rich layered oxide cathodes at low state-of-charge levels: Corrosion cracking and pitting. ACS Nano 2024, 18, 10566–10581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Vasconcelos, L.S.; Hassan, S.; Zhao, K. Asynchronous-to-synchronous transition of li reactions in solid-solution cathodes. Nano Lett. 2022, 22, 5883–5890. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Cao, T.; Wu, R.; Wang, M.; Liu, H.; Liu, X.; Lu, J.; Zhang, Y. Real-time observation of chemomechanical breakdown in a layered nickel-rich oxide cathode realized by in situ scanning electron microscopy. ACS Energy Lett. 2021, 6, 1703–1710. [Google Scholar] [CrossRef]
- Mu, L.; Lin, R.; Xu, R.; Han, L.; Xia, S.; Sokaras, D.; Steiner, J.D.; Weng, T.C.; Nordlund, D.; Doeff, M.M.; et al. Oxygen release induced chemomechanical breakdown of layered cathode materials. Nano Lett. 2018, 18, 3241–3249. [Google Scholar] [CrossRef]
- Kim, U.H.; Ryu, H.H.; Kim, J.H.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles. Adv. Energy Mater. 2019, 9, 1803902. [Google Scholar] [CrossRef]
- Morzy, J.K.; Dose, W.M.; Vullum, P.E.; Lai, M.C.; Mahadevegowda, A.; De Volder, M.F.L.; Ducati, C. Origins and importance of intragranular cracking in layered lithium transition metal oxide cathodes. ACS Appl. Energy Mater. 2024, 7, 3945–3956. [Google Scholar] [CrossRef]
- Park, K.-J.; Hwang, J.-Y.; Ryu, H.-H.; Maglia, F.; Kim, S.-J.; Lamp, P.; Yoon, C.S.; Sun, Y.-K. Degradation mechanism of Ni-enriched NCA cathode for lithium batteries: Are microcracks really critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef]
- Li, W.; Asl, H.-Y.; Xie, Q.; Manthiram, A. Collapse of LiNi1-x-yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 2019, 141, 5097–5101. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Bi, X.; Chen, Z.; Amine, K.; Zhong, C.; Lu, J. Cationic and anionic redox in lithium-ion based batteries. Chem. Soc. Rev. 2020, 49, 1688–1705. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich Li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, G.T.; Yoon, C.S.; Sun, Y.K. Microstructural degradation of Ni-rich Li[NixCoyMn1-x-y]O2 cathodes during accelerated calendar aging. Small 2018, 14, 1803179. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Zhong, S.; Li, Z.; Zeng, M.; Fan, F.; Yan, T. Extebded fast-charging life of ultrahigh-Ni layered oxides by adjusting the current density during the H2 -> H3 phase transition. Energy Storage Mater. 2022, 50, 757–759. [Google Scholar]
- Dai, P.P.; Kong, X.B.; Yang, H.Y.; Li, J.Y.; Zeng, J.; Zhao, J.B. Single-crystal Ni-rich layered LiNi0.9Mn0.1O2 enables superior performance of Co-free cathodes for lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 4381–4390. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lee, S.B.; Yoon, C.S.; Sun, Y.K. Morphology-dependent battery performance of Ni-rich layered cathodes: Single-crystal versus refined polycrystal. ACS Energy Lett. 2022, 7, 3072–3079. [Google Scholar] [CrossRef]
- Niu, S.; Xu, J.; Wu, K.; Liang, C.; Zhu, G.; Qu, Q.; Zheng, H. Different mechanical and electrochemical behavior between the two major Ni-rich cathode materials in Li-ion batteries. Mater. Chem. Phys. 2021, 260, 124046. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Shi, L.; Wang, Z.; Yuan, S. Spheroidization: The impact of precursor morphology on solid-state lithiation process for high-quality ultrahigh-nickel oxide cathodes. Angew. Chem. Int. Ed. 2024, 63, e202407477. [Google Scholar] [CrossRef]
- Zou, L.; Zhao, W.; Jia, H.; Zheng, J.; Li, L.; Abraham, D.P.; Chen, G.; Croy, J.R.; Zhang, J.-G.; Wang, X.C. The role of secondary particle structures in surface phase transitions of Ni-rich cathodes. Chem. Mater. 2020, 32, 2884–2892. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, J.-H.; Aishova, A.; Ribas, R.M.; Monteiro, R.S.; Griffith, K.J.; Yoon, C.S.; Sun, Y.-K. Microstructure engineered Ni-rich layered cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 2100884. [Google Scholar] [CrossRef]
- Park, G.-T.; Yoon, D.R.; Kim, U.-H.; Namkoong, B.; Lee, J.; Wang, M.M.; Lee, A.C.; Gu, X.W.; Chueh, W.C.; Yoon, C.S.; et al. Ultrafine-grained Ni-rich layered cathode for advanced Li-ion batteries. Energy Environ. Sci. 2021, 14, 6616–6626. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wu, T.; Lu, J. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 2022, 611, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xu, Z.; Yang, Z.; Kuai, C.; Du, Z.; Sun, C.-J.; Ren, Y.; Liu, J.; Xiao, X.; Lin, F. Effect of the grain arrangements on the thermal stability of polycrystalline nickel-rich lithium-based battery cathodes. Nat. Commun. 2022, 13, 3437. [Google Scholar] [CrossRef]
- Namkoong, B.; Park, N.-Y.; Park, G.-T.; Shin, J.-Y.; Beierling, T.; Yoon, C.S.; Sun, Y.-K. High-energy Ni-rich cathode materials for long-range and long-life electric vehicles. Adv. Energy Mater. 2022, 12, 2200615. [Google Scholar] [CrossRef]
- Trevisanello, E.; Ruess, R.; Conforto, G.; Richter, F.H.; Janek, J. Polycrystalline and single crystalline NCM cathode materials—Quantifying particle cracking, active surface area, and lithium diffusion. Adv. Energy Mater. 2021, 11, 2003400. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, R.; Zhou, K.; Gao, Z.; He, W.; Zhang, L.; Han, C.; Kang, F.; Li, B. A comparative investigation of single crystal and polycrystalline Ni-rich NCMs as cathodes for lithium-ion batteries. Energy Environ. Mater. 2022, 6, e12331. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Ge, M.; Xin, H.L. Accelerated degradation in a quasi-single-crystalline layered oxide cathode for lithium-ion batteries caused by residual grain boundaries. Nano Lett. 2022, 22, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yao, Z.; Zheng, J.; Fu, M.; Cen, J.; Hwang, S.; Jin, H.; Orlov, A.; Gu, L.; Wang, S.; et al. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode. Angew. Chem. Int. Ed. 2020, 59, 22092–22099. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, W.; Zhou, J.; Meng, J.; Huang, W.; Chen, T.; Gao, Q.; Wei, X.; Zeng, Y.; Li, J.; et al. Ni–Li anti-site defect induced intragranular cracking in Ni-rich layer-structured cathode. Nano Energy 2020, 76, 105021. [Google Scholar] [CrossRef]
- Meng, X.H.; Lin, T.; Mao, H.; Shi, J.L.; Sheng, H.; Zou, Y.G.; Fan, M.; Jiang, K.; Xiao, R.J.; Xiao, D.; et al. Kinetic origin of planar gliding in single-crystalline Ni-rich cathodes. J. Am. Chem. Soc. 2022, 144, 11338–11347. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhang, R.; Lei, T.; Kisslinger, K.; Xin, H.L. Resolving complex intralayer transition motifs in high-Ni-content layered cathode materials for lithium-ion batteries. Nat. Mater. 2023, 22, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cao, X.; Yang, H.; He, P.; Dato, M.A.; Cabana, J.; Zhou, H. The origin of high-voltage stability in single-crystal layered Ni-rich cathode materials. Angew. Chem. 2022, 134, e202207225. [Google Scholar] [CrossRef]
- Saaid, F.I.; Kasim, M.F.; Winie, T.; Elong, K.A.; Azahidi, A.; Basri, N.D.; Yaakob, M.K.; Mastuli, M.S.; Shaffee, S.N.A.; Yaakob, M.K.; et al. Ni-rich lithium nickel manganese cobalt oxide cathode materials: A review on the synthesis methods and their electrochemical performances. Heliyon 2024, 10, e23968. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Du, K.; Cao, Y.B.; Lu, Y.; Peng, Z.D.; Fan, J.; Hu, G. Hydrothermal preparing agglomerate LiNi0.8Co0.1Mn0.1O2 cathode material with submicron primary particle for alleviating microcracks. J. Power Sources 2020, 477, 228701. [Google Scholar] [CrossRef]
- Li, Y.; He, J.J.; Luo, L.; Li, X.; Chen, Z.; Zhang, Y.B.; Deng, L.; Dong, P.; Yang, S.; Wu, K.; et al. Highly dispersed micrometer nickel-rich single-crystal construction: Benefits of supercritical reconstruction during hydrothermal synthesis. ACS Appl. Energy Mater. 2022, 5, 6302–6312. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Wang, Z.; Tong, J.; Qi, N.; Han, G.; Zhang, M. Self-assembled porous LiNi0.8Co0.1Mn0.1O2 cathode materials with micro/nano-layered hollow morphologies for high-power lithium-ion batteries. Appl. Surf. Sci. 2021, 539, 148034. [Google Scholar] [CrossRef]
- Li, S.Y.; Liang, W.B.; Xie, J.; Wei, Y.; Cui, X.L. Synthesis of hollow microspheres LiNi0.5Mn1.5O4 coated with Al2O3 and characterization of the electrochemical capabilities. J. Electrochem. Energy Convers. Storage 2020, 17, 031007. [Google Scholar]
- Guo, J.; Li, W. Synthesis of single-crystal LiNi0.7Co0.15Mn0.15O2 materials for Li-ion batteries by a sol-gel method. ACS Appl. Energy Mater. 2022, 5, 397–406. [Google Scholar] [CrossRef]
- Bai, X.; Wei, A.J.; He, R.; Li, W.; Li, X.H.; Zhang, L.H.; Liu, Z. The structural and electrochemical performance of Mg-doped LiNi0.85Co0.10Al0.05O2 prepared by a solid-state method. J. Electroanal. Chem. 2020, 858, 113771. [Google Scholar] [CrossRef]
- Zheng, L.; Bennett, J.C.; Obrovac, M.N. All-dry synthesis of single crystal NMC cathode materials for Li-ion batteries. J. Electrochem. Soc. 2020, 167, 130536. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Xu, C.; Cheng, J.; Zhao, J.; Huang, Q.; Yang, C. Advances in reactive co-precipitation technology for preparing high-performance cathodes. Green Carbon 2023, 1, 193–209. [Google Scholar] [CrossRef]
- He, F.R.; Tian, Z.Q.; Xiang, W.; Yang, W.; Zheng, B.P.; Cai, J.Y.; Guo, X.D. Insight into the surface reconstruction-induced structure and electrochemical performance evolution for Ni-rich cathodes with postannealing after washing. ACS Appl. Mater. Interfaces 2023, 15, 9160–9170. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Shi, L.; Wang, Z.; Wang, Y.; Zhang, M.; Yuan, S. Advances of high-performance LiNi1-x-yCoxMyO2 cathode materials and their precursor particles via co-precipitation process. Particuology 2024, 86, 67–85. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Q.; Zhan, C.; Liu, G. One-step solid-state synthesis of Ni-rich cathode materials for lithium-ion batteries. Materials 2023, 16, 3079. [Google Scholar] [CrossRef]
- El Kouihen, F.; Chakir, M.; Faik, A. The effect of synthesis methods on cation mixing degree in cobalt-free, nickel-rich NMA materials. Chem. Pap. 2025, 79, 2411–2420. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Wang, S.; Wang, M.; Jiang, F.; Liu, Y.; Huang, Y.; Zheng, J. Optimal synthetic conditions for a novel and high-performance Ni-rich cathode material of LiNi0.68Co0.10Mn0.22O2. Sustain. Energy Fuels 2018, 2, 1772–1780. [Google Scholar]
- Ying, B.X.; Fitzpatrick, J.R.; Teng, Z.J.; Chen, T.X.; Lo, T.W.B.; Siozios, V.; Murray, C.A.; Brand, H.E.A.; Day, S.; Tang, C.C.; et al. Monitoring the formation of nickel-poor and nickel-rich oxide cathode materials for lithium-ion batteries with synchrotron radiation. Chem. Mater. 2023, 35, 1514–1526. [Google Scholar] [CrossRef]
- Han, Q.; Cai, L.L.; Yang, Z.F.; Hu, Y.J.; Jiang, H.; Li, C.Z. New insights into the pre-lithiation kinetics of single-crystalline Ni-rich cathodes for long-life Li-ion batteries. Green Energy Environ. 2024, 9, 556–564. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, L.; Li, J.T.; Zhang, B.; Hu, Q.; Liu, J.L.; Wu, Y.Q.; Gao, J.H.; Chen, Y.B.; Song, S.L.; et al. Rational synthesis of high-performance Ni-rich layered oxide cathode enabled via probing solid-state lithiation evolution. Nano Energy 2023, 113, 108528. [Google Scholar] [CrossRef]
- Liu, J.J.; Yuan, Y.F.; Zheng, J.H.; Wang, L.G.; Ji, J.; Zhang, Q.; Yang, L.; Bai, Z.Y.; Lu, J. Understanding the synthesis kinetics of single-crystal Co-free Ni-rich cathodes. Angew. Chem. Int. Ed. 2023, 62, e202302547. [Google Scholar] [CrossRef] [PubMed]
- Wolfman, M.; Wang, X.P.; Garcia, J.C.; Barai, P.; Stubbs, J.E.; Eng, P.J.; Kahvecioglu, O.; Kinnibrugh, T.L.; Madsen, K.E.; Iddir, H.; et al. Spheroidization: The impact of precursor morphology on solid-state lithiation process for high-quality ultrahigh-nickel oxide cathodes. Adv. Energy Mater. 2022, 12, 2102951. [Google Scholar] [CrossRef]
- Xu, Z.R.; Jiang, Z.S.; Kuai, C.G.; Xu, R.; Qin, C.D.; Zhang, Y.; Rahman, M.M.; Wei, C.; Nordlund, D.; Sun, C.-J.; et al. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials. Nat. Commun. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Polierer, S.; Guse, D.; Wild, S.; Delgado, K.H.; Otto, T.N.; Zevaco, T.A.; Kind, M.; Sauer, J.; Studt, F.; Pitter, S. Enhanced direct dimethyl ether synthesis from CO2 rich syngas with Cu/ZnO/ZrO2 catalysts prepared by continuous co-precipitation. Catalysts 2020, 10, 816. [Google Scholar] [CrossRef]
- Ahmed, R.R.; Mubarak, T.H.; Mohamed, I.H. A study of structural and chemical properties of Ni1-xZnxFe2O4 ferrite powder prepared by co-precipitation method. Digest J. Nanomater. Biostruct. 2022, 17, 741–748. [Google Scholar] [CrossRef]
- Entwistle, T.; Sanchez-Perez, E.; Murray, G.J.; Anthonisamy, N.; Cussen, S.A. Co-precipitation synthesis of nickel-rich cathodes for Li-ion batteries. Energy Rep. 2022, 8, 67–73. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, K.Y.; Choi, B.K.; Yoo, H.D. A kinetic descriptor to optimize Co-precipitation of Nickel-rich cathode precursors for lithium-ion batteries. J. Electroanal. Chem. 2022, 924, 116828. [Google Scholar] [CrossRef]
- Heo, K.; Lee, J.S.; Kim, H.S.; Kim, M.Y.; Jeong, H.; Kim, J.; Lim, J. Ionic conductor-LiNi0.8Co0.1Mn0.1O2 composite synthesized by simultaneous co-precipitation for use in lithium-ion batteries. J. Electrochem. Soc. 2018, 165, A2955–A2960. [Google Scholar] [CrossRef]
- Xu, L.P.; Zhou, F.; Kong, J.Z.; Zhou, H.B.; Zhang, Q.C.; Wang, Q.Z.; Yan, G. Influence of precursor phase on the structure and electrochemical properties of LiNi0.6Mn0.2Co0.2O2 cathode materials. Solid State Ion. 2018, 324, 49–58. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, G.R.; Peng, Z.D.; Cao, Y.B.; Li, L.Y.; Tan, C.P.; Du, K. Synthesis and characterization of mono-dispersion LiNi0.8Co0.1Mn0.1O2 micrometer particles for lithium-ion batteries. Ceram. Int. 2021, 47, 25680–25688. [Google Scholar] [CrossRef]
- Pollen, H.N.; Tolchard, J.R.; Svensson, A.M.; Wagner, N.P. A single-pot co-precipitation synthesis route for Ni-rich layered oxide materials with high cycling stability. ChemElectroChem 2022, 9, e202200859. [Google Scholar] [CrossRef]
- Para, M.L.; Alidoost, M.; Shiea, M.; Boccardo, G.; Buffo, A.; Barresi, A.A.; Marchisio, D. A modelling and experimental study on the co-precipitation of Ni0.8Mn0.1Co0.1(OH)2 as precursor for battery cathodes. Chem. Eng. Sci. 2022, 254, 117634. [Google Scholar] [CrossRef]
- Zhang, H.W.; Cen, T.; Tian, Y.H.; Zhang, X.J. Synthesis of high-performance single-crystal LiNi0.8Co0.1Mn0.1O2 cathode materials by controlling solution super-saturation. J. Power Sources 2022, 532, 231037. [Google Scholar] [CrossRef]
- Kumar, D.; Kurian, E.; Ramesha, K. Synthesis of quasi-spherical LiNi0.86Mn0.1Co0.04O2 cathode particles via hydroxide coprecipitation: Influence of pH on precursor particle size in enhancing capacity and stability of Ni-rich cathode for lithium-ion batteries. Energy Technol. 2025, 2402418. [Google Scholar] [CrossRef]
- Duan, J.H.; Zhang, R.C.; Zhu, Q.H.; Xiao, H.; Huang, Q.S. The effect of controlling strategies of pH and ammonia concentration on preparing full concentration gradient Ni0.8Co0.1Mn0.1(OH)2 via co-precipitation in a pilot-scale reactor. Energy Technol. 2020, 8, 1901437. [Google Scholar] [CrossRef]
- Park, B.H.; Kim, T.; Park, H.; Sohn, Y.; Shin, J.; Kang, M. Electrochemical performance of layer-structured Ni0.8Co0.1Mn0.1O2 cathode active materials synthesized by carbonate co-precipitation. Nanomaterials 2022, 12, 3610. [Google Scholar] [CrossRef]
- Shen, W.-Z.; Ma, Y.; Yao, C.; Liang, F. Controlling the precursor morphology of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode for lithium-ion battery. Nano 2019, 14, 100–108. [Google Scholar] [CrossRef]
- Hu, K.H.; He, Y.Y.; Zhu, C.Q.; Zhou, K.; Chen, Q.; Yang, Z.M.; Wan, B.-R.; Yang, E.-Q.; Zhang, T.-T.; Qin, Y.-D. Insight into the evolution of precursor and electrochemical performance of Ni-rich cathode modulated by ammonia during hydroxide precipitation. J. Alloys Compd. 2019, 803, 538–545. [Google Scholar] [CrossRef]
- Liang, H.L.; Yuan, S.; Shi, L.Y.; Zhao, Y.; Wang, Z.Y.; Zhu, J.F. Highly-ordered microstructure and well performance of LiNi0.6Mn0.2Co0.2O2 cathode material via the continuous microfluidic synthesis. Chem. Eng. J. 2020, 394, 124846. [Google Scholar] [CrossRef]
- Zou, L.; Li, J.; Liu, Z.; Wang, G.; Manthiram, A.; Wang, C. Lattice doping regulated interfacial reactions in cathode for enhanced cycling stability. Nat. Commun. 2019, 10, 3447. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Hwang, J.U.; Im, J.S.; Lee, J.D. Electrochemical properties of LiNi0.9Co0.1O2 cathode material prepared by co-precipitation using an eco-friendly chelating agent. J. Solid State Electrochem. 2022, 26, 1567–1576. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Shen, J.; Xu, X.; Zeng, L.; Zhang, B.; Zhu, H.; Liu, Q.; Liu, J.; Zhu, M. A nanorod-like Ni-rich layered cathode with enhanced Li+ diffusion pathways for high-performance lithium-ion batteries. J. Mater. Chem. 2021, 9, 2830–2839. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Ren, L.; Wu, W.; Zuo, M.; Xing, W.; Zhang, B.; Fan, W.; He, Z.; Yu, Z.; et al. Micro-structure tuning and evolution of hydroxide precursor with radially oriented grains during industrial-scale continuous precipitation process. J. Alloys Compd. 2024, 977, 173458. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, F.; Wang, S.; Zhao, T.; Peng, J.; Zhang, B.; Fan, W.; Xing, W.; Zuo, M.; Zhang, P.; et al. Precision engineering of high-performance Ni-rich layered cathodes with radially aligned microstructure through architectural regulation of precursors. eScience 2024, 4, 100276. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Xiao, H.; Zhang, R.C.; Geng, S.J.; Huang, Q.S. Effect of impeller type on preparing spherical and dense Ni1-x-yCoxMny(OH)2 precursor via continuous co-precipitation in pilot scale: A case of Ni0.6Co0.2Mn0.2(OH)2. Electrochim. Acta 2019, 318, 1–13. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Liu, Y.; Ye, Y.; Yang, Y. Facile synthesis and electrochemical performance of lithium-rich layered oxides with stable hierarchical structure through HEPES-assisted co-precipitation method. Electrochim. Acta 2022, 401, 139485. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, X.; Zhao, T.; Yang, X.; Wang, Q.; Chen, Z.; Chen, M.-C.; Ma, J.-J.; Lu, Y.-R.; Hung, S.-F.; et al. What impact does ammonia have on the microstructure of the precursor and the electrochemical performance of Ni-rich layered oxides? J. Mater. Chem. A 2025, 13, 1181–1190. [Google Scholar] [CrossRef]
- Kim, E.; Cho, Y.H.; Shin, J.H.; Eun, H.J.; Song, H.I.; Lee, S.-H.; Suk, J.; Moon, S. Unlocking the superior performance of Ni-rich layered materials through precise precursor engineering. J. Eur. Ceram. Soc. 2025, 45, 117280. [Google Scholar] [CrossRef]
- Lao, J.; Lu, Y. Synthesis of nano cubic microstructure LiNi0.8Co0.1Mn0.1O2 cathode materials via rapid precipitation assisted with hydrothermal treatment. Heliyon 2024, 10, e40201. [Google Scholar] [CrossRef]
- Chen, A.; Wang, K.; Li, J.; Mao, Q.; Xiao, Z.; Zhu, D.; Wang, G.; Liao, P.; He, J.; You, Y.; et al. The formation, detriment and solution of residual lithium compounds on Ni-rich layered oxides in lithium-ion batteries. Front. Energy Res. 2020, 8, 593009. [Google Scholar] [CrossRef]
- Li, W.D.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Dai, A.; De Andrade, V.; Ren, Y.; Xu, W.; Lee, S.; Zhang, Q.; Gu, L.; Wang, S.; et al. Reaction inhomogeneity coupling with metal rearrangement triggers electrochemical degradation in lithium-rich layered cathode. Nat. Commun. 2021, 12, 5370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, X.; Zhang, Y.; Zhou, J.; Wang, J.; Xu, S. Grain size regulation for balancing cycle performance and rate capability of LiNi0.9Co0.055Mn0.045O2 single crystal nickel-rich cathode materials. J. Energy Chem. 2022, 65, 681–687. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Seenivasan, M.; Wu, Y.-S.; Wu, S.-H.; Chang, J.-K.; Jose, R.; Yang, C.-C. Preparation of long-term cycling stable Ni-rich concentration-gradient NCMA cathode materials for li-ion batteries. J. Colloid Interface Sci. 2023, 639, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Jin, F.; Zhao, Y.; Shi, L.; Liu, Q.; Wang, Z.; Wang, Y.; Zhang, M.; Zhu, J.; Yuan, S. Synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 materials for Li-ion batteries by microfluidic technology. Chem. Eng. J. 2023, 464, 142656. [Google Scholar] [CrossRef]
- Riewald, F.; Kurzhals, P.; Bianchini, M.; Sommer, H.; Janek, J.; Gasteiger, H.A. The LiNiO2 cathode active material: A comprehensive study of calcination conditions and their correlation with physicochemical properties part II. Morphology. J. Electrochem. Soc. 2022, 169, 020529. [Google Scholar] [CrossRef]
- Yu, Z.; Qu, X.; Wan, T.; Dou, A.; Zhou, Y.; Peng, X.; Su, M.; Liu, Y.; Chu, D. Synthesis and mechanism of high structural stability of nickel-rich cathode materials by adjusting Li-excess. ACS Appl. Mater. Interfaces 2020, 12, 40393–40403. [Google Scholar] [CrossRef]
- Kan, H.; Yang, Z.; Meng, Q.; Dong, P.; Zhang, Y. Preparation and electrochemical performance of the Ni-rich Co-free cathode material LiNi0.94Mn0.04Al0.02O2. Energy Fuels 2024, 38, 6420–6426. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Warner, J.H.; Manthiram, A. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Lett. 2021, 6, 941–948. [Google Scholar] [CrossRef]
- Li, J.; Liang, G.; Zheng, W.; Zhang, S.; Davey, K.; Pang, W.K.; Guo, Z. Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review. Nano Mater. Sci. 2023, 5, 404–420. [Google Scholar] [CrossRef]
- Li, X.Q.; Xiong, X.; Wang, Z.; Chen, Q. Effect of sintering temperature on cycling performance and rate performance of LiNi0.8Co0.1Mn0.1O2. Trans. Nonferr. Met. Soc. China 2014, 24, 4023–4029. [Google Scholar] [CrossRef]
- Ding, Y.; Mu, D.; Wu, B.; Wang, R.; Zhao, Z.; Wu, F. Recent progresses on nickel-rich layered oxide positive electrode materials used in lithium-ion batteries for electric vehicles. Appl. Energy 2017, 195, 586–599. [Google Scholar] [CrossRef]
- Wang, L.; Lei, X.; Liu, T.; Dai, A.; Su, D.; Amine, K.; Lu, J.; Wu, T. Regulation of surface defect chemistry toward stable Ni-rich cathodes. Adv. Mater. 2022, 34, 2200744. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Tan, X.; Guo, H.; Peng, W.; Li, X.; Wang, J.; Yan, G. Correlating morphological and structural evolution with the electrochemical performance of nickel-rich cathode materials: From polycrystal to single crystal. J. Electrochem. Soc. 2022, 169, 090520. [Google Scholar] [CrossRef]
- Allen, E.; Shin, Y.; Judge, W.; Wolfman, M.; De Andrade, V.; Cologna, S.M.; Cabana, J. 3D quantification of elemental gradients within heterostructured particles of battery cathodes. ACS Energy Lett. 2023, 8, 1371–1378. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.; Kim, Y.; Choi, W.; Yoon, W.-S. Enhancing the structural durability of Ni-rich layered materials by post-process: Washing and heat-treatment. J. Mater. Chem. A 2020, 8, 10206–10216. [Google Scholar] [CrossRef]
- Karger, L.; Korneychuk, S.; Sicolo, S.; Li, H.; van der Bergh, W.; Zhang, R.; Indris, S.; Kondrakov, A.; Janek, J.; Brezesinski, T. Decoupling substitution effects from point defects in layered Ni-rich oxide cathode materials for lithium-ion batteries. Adv. Funct. Mater. 2024, 34, 2402444. [Google Scholar] [CrossRef]
- Wang, T.; Ren, K.; Xiao, W.; Dong, W.; Qiao, H.; Duan, A.; Pan, H.; Yang, Y.; Wang, H. Tuning the Li/Ni disorder of the NMC811 cathode by thermally driven competition between lattice ordering and structure decomposition. J. Phys. Chem. C 2020, 124, 5600–5607. [Google Scholar] [CrossRef]
- Mo, Y.; Liu, S.; Yuan, G.; Li, Z.; Zhang, M.; Guo, L. Enhancing the reversibility of the chemical evolution of the Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode via a simple pre-oxidation process. RSC Adv. 2024, 14, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huo, H.; Jian, J.-Y.; Wang, L.-G.; Zhu, H.; Xu, S.; He, X.-S.; Yin, G.-P.; Du, C.-Y.; Sun, X.-L. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1803963. [Google Scholar] [CrossRef]
- Wang, T.; Ren, K.; He, M.; Dong, W.; Xiao, W.; Pan, H.; Yang, J.; Yang, Y.; Liu, P.; Cao, Z.; et al. Synthesis and manipulation of single-crystalline lithium nickel manganese cobalt oxide cathodes: A review of growth mechanism. Front. Chem. 2020, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Harlow, J.; Dahn, J. Microstructural observations of “single crystal” positive electrode materials before and after long term cycling by cross-section scanning electron microscopy. J. Electrochem. Soc. 2020, 167, 020512. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Peng, S.; Zeng, J.; Zhao, J. Superiority of single crystal to polycrystalline LiNixCoyMn1-x-yO2 cathode materials in storage behaviors for lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 14938–14948. [Google Scholar] [CrossRef]
- Lüther, M.J.; Jiang, S.-K.; Lange, M.A.; Buchmann, J.; Martin, A.G.; Schmuch, R.; Placke, T.; Hwang, B.J.; Winter, M.; Kasnatscheew, J. Systematic “apple-to-apple” comparison of single-crystal and polycrystalline Ni-rich cathode active materials: From comparable synthesis to comparable electrochemical conditions. Small 2024, 5, 2400119. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Chen, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Fan, X.-M.; Hu, G.-R.; Zhang, B.; Ou, X.; Zhang, J.-F.; Zhao, W.-G.; Jia, H.-P.; Zou, L.F.; Li, P.; Yang, Y. Crack-free single crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim. Acta 2021, 365, 137380. [Google Scholar] [CrossRef]
- Han, Y.; Xu, J.; Wang, W.; Long, F.; Qu, Q.; Wang, Y.; Zheng, H. Implanting an electrolyte additive on a single crystal Ni-rich cathode surface for improved cycle ability and safety. J. Mater. Chem. A 2020, 8, 24579–24589. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Fan, C.; Han, S. A highly stabilized single crystalline nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode through a novel surface spinel-phase modification. Electrochim. Acta 2020, 341, 136075. [Google Scholar] [CrossRef]
- Ge, M.; Wi, S.; Liu, X.; Bai, J.; Ehrlich, S.; Lu, D.; Lee, W.K.; Chen, Z.; Wang, F. Kinetic limitations in single-crystal high-nickel cathodes. Angew. Chem. Int. Ed. 2021, 60, 17350–17355. [Google Scholar] [CrossRef]
- You, B.; Wang, Z.; Shen, F.; Chang, Y.; Peng, W.; Li, X.; Guo, H.; Hu, Q.; Deng, C.; Yang, S.; et al. Research progress of single-crystal nickel-rich cathode materials for lithium-ion batteries. Small Methods 2021, 5, 2100234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, E.; Zhang, X.; Yu, H. High-voltage “single-crystal” cathode materials for lithium-ion batteries. Energy Fuels 2021, 35, 918–932. [Google Scholar] [CrossRef]
- Han, Y.; Lei, Y.; Ni, J.; Zhang, Y.; Geng, Z.; Ming, P.; Zhang, C.; Tian, X.; Shi, J.-L.; Guo, Y.; et al. Single-crystalline cathodes for advanced Li-ion batteries: Progress and challenges. Small 2022, 18, 2107048. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, L.; Zhang, L.; Fan, X.; Zhang, H.; Pagani, F.; Brack, E.; Seidl, L.; Ou, X.; Egorov, K.; et al. Assessing long-term cycling stability of single-crystal versus polycrystalline nickel-rich NCM in pouch cells with 6 mAh cm−2 electrodes. Small 2022, 18, 2107357. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, S.; Li, S.; Yu, Z.; Liu, Q.; Dai, A.; Cao, C.; Toney, M.F.; Ge, M.; Xiao, X.; et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 2020, 11, 3050. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Bi, Y.; Tao, J.; Lochala, J.; Liu, D.; Wu, B.; Cao, X.; Chae, S.; Wang, C.; et al. Locking oxygen in lattice: A quantifiable comparison of gas generation in polycrystalline and single crystal Ni-rich cathodes. Energy Storage Mater. 2022, 47, 195–202. [Google Scholar] [CrossRef]
- Ge, J.; Xie, M.; Zhao, Q.; Zhang, S.; Sun, H. Advances in Co-free layered cathode materials for Li-ion batteries. Int. J. Electrochem. Sci. 2023, 18, 100292. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Tian, H.; Yan, J.; Tong, J.; Liu, X.; Zhang, H.; Huang, H.; Hao, S.; Gao, J.; et al. Pulse high temperature sintering to prepare single-crystal high nickel oxide cathodes with enhanced electrochemical performance. Adv. Energy Mater. 2023, 13, 2203188. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Song, Y.; Zhang, Z.; Du, A.; Tang, Y.; Wang, J.; He, X. Why the synthesis affects performance of layered transition metal oxide cathode materials for Li-ion batteries. Adv. Mater. 2024, 36, 2312292. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lv, F.; Wang, Z.; Feng, J.; Wu, M.; Liu, Z.; Chen, L.; Gu, Y. Open-channel [011] facet CeO2 with a shorter pathway of Li+ migration as a modification material for LiNi0.8Co0.1Mn0.1O2 toward high-rate lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 8795–8802. [Google Scholar] [CrossRef]
- Liu, H.-W.; Parthasarathi, S.-K.; Thi, S.; Weng, Y.-T.; Bolloju, S.; Chen, C.C.; Jeng, R.-J.; Wu, N.-L. A comparative study of polycrystal/single-crystal LiNi0.8Co0.1Mn0.1O2 in all-solid-state Li-ion batteries with halide-based electrolyte under low stacking pressure. Energy Technol. 2023, 11, 2201439. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Deng, Q.; Chu, Y.; Dong, P.; Chen, C.; Wang, Z.; Xia, Z.; Yang, C. In situ and real-time monitoring the chemical and thermal evolution of lithium-ion batteries with single-crystalline Ni-rich layered oxide cathode. Angew. Chem. Int. Ed. 2024, 63, e202401716. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Zhao, J.; Zhao, W.; Liang, Z.; Su, Y.; Xie, C.; Zhou, K.; Xiang, Y.; Zhu, J.; et al. Electrochemo-mechanical effects on structural integrity of Ni-rich cathodes with different microstructures in all solid-state batteries. Adv. Energy Mater. 2021, 11, 2003583. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, Y.; Wiaderek, K.M.; Borkiewicz, O.J.; Ren, Y.; Zhang, J.; Ren, J.; Fan, L.; Li, C.C.; Li, D.; et al. Spontaneous strain buffer enables superior cycling stability in single-crystal nickel-rich NCM cathode. Nano Lett. 2021, 21, 9997–10005. [Google Scholar] [CrossRef]
- Ran, A.; Chen, S.; Cheng, M.; Liang, Z.; Li, B.; Zhou, G.; Kang, F.; Zhang, X.; Wei, G. A single-crystal nickel-rich material as a highly stable cathode for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 19680–19689. [Google Scholar] [CrossRef]
- Han, Q.; Yu, H.; Cai, L.; Jiang, H. Unique insights into the design of low-strain single-crystalline Ni-rich cathodes with superior cycling stability. Proc. Natl. Acad. Sci. USA 2024, 121, e2317282121. [Google Scholar] [CrossRef]
- Cha, H.; Kim, J.; Lee, H.; Kim, N.; Hwang, J.; Sung, J.; Yoon, M.; Kim, K.; Cho, J. Boosting reaction homogeneity in high-energy lithium-ion battery cathode materials. Adv. Mater. 2020, 32, 2003040. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Li, J.; Yang, H.; Dai, P.; Zeng, J.; Zhao, J. Single-crystal structure helps enhance the thermal performance of Ni-rich layered cathode materials for lithium-ion batteries. Chem. Eng. J. 2022, 434, 134638. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Siu, C.; Ge, M.; Kisslinger, K.; Shin, Y.; Xin, H.L. Chemomechanically stable ultrahigh-Ni single-crystalline cathodes with improved oxygen retention and delayed phase degradations. Nano Lett. 2021, 21, 9797–9804. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, W.; Karger, L.; Murugan, S.; Janek, J.; Kondrakov, A.; Brezesinski, T. Single crystal layered oxide cathodes: The relationship between particle size, rate capability, and stability. ChemElectroChem 2023, 10, e202300165. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, S.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. A comparative investigation of single crystal and polycrystalline Ni-rich layered cathodes. Energy Environ. Mater. 2022, 12, 2201510. [Google Scholar]
- Zhou, X.; Wang, X.; Xu, Z.; Wang, Q.; Lu, C.; Ma, R.; Zheng, Y.; Ruan, M.; Mao, Q.; Xu, J.; et al. Facile ball-milling synthesis of pseudo-single-crystalline nickel-rich layered oxide cathodes towards long-lifespan lithium-ion batteries. J. Electron. Mater. 2024, 53, 7355–7366. [Google Scholar] [CrossRef]
- Leng, J.; Wang, J.; Peng, W.; Tang, Z.; Xu, S.; Liu, Y.; Wang, J. Highly-dispersed submicrometer single-crystal nickel-rich layered cathode: Spray synthesis and accelerated lithium-ion transport. Small 2021, 17, e2006869. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, M.; Guo, Z.; Cao, L.; Xiao, Y.; Chen, L.; Cheng, Y.-J.; Xia, Y. Simultaneous enhancement of ordered layered structure and inhibition of micro-cracks via porous structure to improve stability for Ni-rich cathode. J. Energy Storage 2024, 76, 109936. [Google Scholar] [CrossRef]
- Chen, J.; Feng, S.; Deng, J.; Zhou, Y. Insights into the porosity and radial structure for precursor of Ni-rich LiNi0.9Co0.05Mn0.05O2 cathode materials. Chem. Engin. Sci. 2025, 304, 121092. [Google Scholar] [CrossRef]
- Zou, K.; Xie, S.; Jiang, M.; Wang, P.; Ning, T.; Tan, L.; Li, H.; Zhou, Y.; Wang, W.; Li, L. Insights into the precursor specific surface area for engineering Co-free Ni-rich cathodes with tailorable properties. Chem. Eng. J. 2024, 483, 149189. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, H.S.; Hong, S.; Jeon, H.; Lim, J.; Jung, Y.H.; Ahn, H.; Jang, J.D.; Kim, M.-H.; Seo, J.H.; et al. Toward a nanoscale-defect-free Ni-rich layered oxide Cathode through Regulated pore evolution for long-lifespan Li rechargeable batteries. Adv. Func. Mater. 2024, 34, 2306654. [Google Scholar] [CrossRef]
- Lv, F.; Zhang, Y.; Wu, M.; Gu, Y. A molten-salt method to synthesize ultrahigh-nickel single-crystalline LiNi0.92Co0.06Mn0.02O2 with superior electrochemical performance as cathode material for lithium-ion batteries. Small 2022, 18, 2201946. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Liu, X.; Gao, J.; Hao, S.; Yang, Y.; Qiu, J. Perspective on the preparation methods of single crystalline high nickel oxide cathode materials. Adv. Energy Mater. 2023, 13, 2300378. [Google Scholar] [CrossRef]
- Yoo, Y.-W.; Kang, C.-Y.; Kim, H.-K.; Lee, J.-K.; Kumar, R.V.; Kim, K.-N.; Yoon, J.-R.; Lee, S.-H. Enhanced structure/interfacial properties of single-crystal Ni-rich LiNi0.92Co0.04Mn0.04O2 cathodes synthesized via LiCl-NaCl molten-salt method. Energy Environ. Mater. 2025, 8, e12778. [Google Scholar] [CrossRef]
- Kim, S.; Park, K. Electrode design to mitigate the kinetic issue of cathodes in high energy lithium-ion batteries. J. Power Sources 2022, 547, 231916. [Google Scholar] [CrossRef]
- Guo, F.; Xie, Y.; Zhang, Y. Low-temperature strategy to synthesize single-crystal LiNi0.8Co0.1Mn00.1O2 with enhanced cycling performances as cathode material for lithium-ion batteries. Nano Res. 2022, 15, 2052–2059. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.; Lee, E.; Choi, M.; Thangavel, R.; Lee, Y.; Yoon, W.-S. Destabilization of the surface structure of Ni-rich layered materials by water-washing process. Energy Storage Mater. 2022, 44, 441–451. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Lim, H.-W.; Lee, S.G.; Sun, Y.-K. Near-surface reconstruction in Ni-rich layered cathodes for high-performance lithium-ion batteries. Nat. Energy 2024, 9, 47–56. [Google Scholar] [CrossRef]
- Hu, B.-R.; Yuan, Y.-Y.; Wang, Y.-C.; Xiong, X.-H. Simultaneously enhanced electrochemical performance and air stability of Ni-rich cathode with a modified washing process. Rare Met. 2023, 43, 87–97. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Wang, F.; Ding, J.; An, B.; Rua, J.; Sun, D.; Fang, F.; Wang, F. One-step molten-salt-assisted approach for direct preparation and regeneration of LiNi0.6Co0.2Mn0.2O2 cathode. Small 2024, 20, 2400762. [Google Scholar] [CrossRef]
- Guo, Z.; Jian, Z.; Zhang, S.; Feng, Y.; Kou, W.; Ji, H.; Yang, G. The effect of Ni oxidation state on the crystal structure and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material for highly reversible lithium storage. J. Alloys Compd. 2021, 882, 160642. [Google Scholar] [CrossRef]
- Gao, C.; Liu, H.; Zhang, J.; Guo, Z.; Huang, H. Simplified crystal grain boundary engineering of solid electrolyte-infused LiNi0.5Mn1.5O4 cathodes for high cycling stability lithium-ion batteries. J. Power Sources 2023, 582, 233434. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Deng, J.; Han, Z.; Xiao, X.; Wang, L.; Chen, Z.; Deng, Y.; He, X. Insight of synthesis of single crystal Ni-rich LiNi1–x–yCoxMnyO2 cathodes. Adv. Energy Mater. 2024, 14, 2303758. [Google Scholar] [CrossRef]
- Tayal, A.; Barai, P.; Zhong, H.; Kahvecioglu, O.; Wang, X.; Pupek, K.Z.; Ma, L.; Ehrlich, N.; Srinivasan, V.; Qu, X.; et al. In situ insights into cathode calcination for predictive synthesis: Kinetic crystallization of LiNiO2 from hydroxides. Adv. Mater. 2024, 36, 2312027. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, H.; Gao, J.; Wang, J.; Shao, M.; Zhou, W. Grain-growth inhibitor with Three-section-sintering for highly dispersed single-crystal NCM90 cubes. Angew. Chem. Int. Ed. 2024, 136, e202314457. [Google Scholar] [CrossRef]
- Choi, J.H.; Embleton, T.J.; Ko, K.; Yang, H.; Son, Y.; Park, J.; Lee, S.; Oh, P. A perspective on the requirements of Ni-rich cathode surface modifications for application in lithium-ion batteries and all-solid-state lithium-ion batteries. ChemElectroChem 2024, 11, e202300705. [Google Scholar] [CrossRef]
- Yu, H.; Pei, D. A review of the origin, adverse influence and modification method of residual surface lithium in Ni-rich cathodes. Int. J. Electrochem. Sci. 2024, 19, 100391. [Google Scholar] [CrossRef]
- Han, B.; Key, B.; Lipton, A.S.; Vaughey, J.T.; Hughes, B.; Trevey, J.; Dogan, F. Influence of coating protocols on alumina-coated cathode material: Atomic layer deposition versus wet-chemical coating. J. Electrochem. Soc. 2019, 166, A3679–A3684. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, J.; Lu, J.; Li, Y.; Yan, P.; Zhang, Y. Realizing superior cycling stability of Ni-rich layered cathode by combination of grain boundary engineering and surface coating. Nano Energy 2019, 62, 30–37. [Google Scholar] [CrossRef]
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. 2020, 10, 11114. [Google Scholar] [CrossRef]
- Chen, G.; Peng, B.; Han, R.; Chen, N.; Wang, Z.; Wang, Q. A robust carbon coating strategy toward Ni-rich lithium cathodes. Ceram. Int. 2020, 46, 20985. [Google Scholar] [CrossRef]
- Qiu, Y.; Wei, X.; Liu, N.; Song, Y.; Bi, L.; Long, X.; Chen, Z.; Wang, S.; Liao, J. Plasma-induced amorphous N-nano carbon shell for improving structural stability of LiNi0.8Co0.1Mn0.1O2 cathode. Electrochim. Acta 2022, 428, 140973. [Google Scholar] [CrossRef]
- Jan, S.S.; Nurgul, S.; Shi, X.; Xia, H.; Pang, H. Improvement of chemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by graphene nanosheets modification. Electrochim. Acta 2014, 149, 86–93. [Google Scholar] [CrossRef]
- Du, Z.; Li, J.; Wood, M.; Mao, C.; Daniel, C.; Wood, D.L. Three-dimensional conductive network formed by carbon nanotubes in aqueous processed NMC electrode. Electrochim. Acta 2018, 270, 54–61. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Zheng, J.; Wei, Y.; Qiao, R.; Shen, F.; Dai, J.; Hu, L.; Xu, K.; Lin, Y.; et al. Depolarized and fully active cathode based on Li(Ni0.5Co0.2Mn0.3)O2 embedded in carbon nanotube network for advanced batteries. Nano Lett. 2014, 14, 4700–4706. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Pham, H.Q.; Kang, D.-H.; Park, H.-Y.; Song, S.-W. Improved rate capability of highly loaded carbon fiber-interwoven LiNi0.6Co0.2Mn0.2O2 cathode material for high-power Li-ion batteries. J. Alloys Compd. 2016, 657, 464–471. [Google Scholar] [CrossRef]
- Ning, R.; Yuan, K.; Zhang, K.; Shen, C.; Xie, K. A scalable snowballing strategy to construct uniform rGO-wrapped LiNi0.8Co0.1Mn0.1O2 with enhanced processability and electrochemical performance. Appl. Surf. Sci. 2021, 542, 148663. [Google Scholar] [CrossRef]
- Poursalehi, F.; Javanbakht, M.; Daryakenari, A.A.; Gao, B. Preparing a binder-free cathode composed of NMC811/sulfonated reduced graphene oxide sheets/carbon black via in-situ electrophoretic deposition to enhance cycleability of Li-ion batteries. J. Electroanal. Chem. 2024, 971, 118593. [Google Scholar] [CrossRef]
- She, S.; Zhou, Y.; Hong, Z.; Huang, Y.; Wu, Y. Surface coating of NCM-811 cathode materials with g-C3N4 for enhanced electrochemical performance. ACS Omega. 2022, 7, 24851–24857. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.-H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.-J. Graphene collage on Ni-rich layered oxide cathodes for advanced lithium-ion batteries. Nat. Commun. 2021, 12, 2145. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lim, H.-W.; Kang, G.-C.; Park, N.-Y.; Sun, Y.-K. Long-lasting Ni-rich NCMA cathodes via simultaneous microstructural refinement and surface modification. ACS Energy Lett. 2023, 8, 1354–1361. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.-M.; Song, J.-H.; Yim, T.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Han, B.; Xu, S.; Zhao, S.; Lin, G.-X.; Feng, Y.-Z.; Chen, L.-B.; Ivey, G.-D.; Wang, P.; Wei, W.-F. Enhancing the structural stability of Ni-rich layered oxide cathodes with a preformed Zr-concentrated defective nanolayer. ACS Appl. Mater. Interfaces 2018, 10, 39599–39607. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liang, F.; Jin, J.; Chowdari, B.V.R.; Yang, J.; Wen, Z. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via in-situ ZrO2 coating for high energy density lithium-ion batteries. Chem. Eng. J. 2020, 389, 124403. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Hemmelmann, H.; Walther, F.; Bianchini, M.; Kondrakov, A.; Janek, J.; Brezesinski, T. Atomic layer deposition derived zirconia coatings on Ni-rich cathodes in solid-state batteries: Correlation between surface constitution and cycling performance. Small Sci. 2023, 3, 2200073. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, B.; Wang, Z.; Lu, C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sources 2014, 256, 20–27. [Google Scholar] [CrossRef]
- Li, W.-W.; Zhang, X.-J.; Si, J.-J.; Yang, J.; Sun, X.-Y. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met. 2021, 40, 1719–1726. [Google Scholar] [CrossRef]
- Wang, W.-C.; Lee, C.-H.; Yu, D.; Kondo, Y.; Miyahara, Y.; Abe, T.; Miyazaki, K. Effects of a solid solution outer layer of TiO2 on the surface and electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathodes for lithium-ion batteries through the use of thin-film electrodes. ACS Appl. Energy Mater. 2022, 5, 5117–5126. [Google Scholar] [CrossRef]
- Su, S.; Zheng, W.; Qin, Y.; Wu, J.; Ke, J.; Sun, Y.; Lin, X. Controllable construction of CeO2 shells with a nanoscale thickness to enhance the high-voltage electrochemical performance of Ni-rich cathodes. ACS Appl. Nano Mater. 2025, 8, 3039–3049. [Google Scholar] [CrossRef]
- Arumugam, R.S.; Ma, L.; Li, J.; Xia, X.; Paulsen, J.M.; Dahn, J.R. Special synergy between electrolyte additives and positive electrode surface coating to enhance the performance of Li[Ni0.6Mn0.2Co0.2]O2/graphite cells. J. Electrochem. Soc. 2016, 163, A2531–A2538. [Google Scholar] [CrossRef]
- Kong, J.Z.; Chen, Y.; Cao, Y.Q.; Wang, Q.-Z.; Li, A.-D.; Li, H.; Zhou, F. Enhanced electrochemical performance of Ni-rich LiNi0.6Co0.2Mn0.2O2 coate;d by molecular layer deposition derived dual-functional C-Al2O3 composite coating. J. Alloys Compd. 2019, 799, 89–98. [Google Scholar] [CrossRef]
- Neudeck, S.; Strauss, F.; Garcia, G.; Wolf, H.; Janek, J.; Hartmann, P.; Brezesinski, T. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material. Chem. Commun. 2019, 55, 2174–2177. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.F.; Xiong, D.B.; Hao, Y.C.; Li, J.W.; Kou, H.R.; Yan, B.; Li, D.J.; Lu, S.G.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium-ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Li, M.L.; Wahyudi, W.; Sheng, G.; Miao, X.H.; Anthopoulos, T.D.; Huang, K.W.; Li, Y.X.; Lai, Z.P. Performance and stability improvement of layered NCM lithium-ion batteries at high voltage by a microporous Al2O3 sol–gel coating. ACS Omega 2019, 4, 13972–13980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycle ability at extended voltage ranges. Coatings 2019, 9, 92. [Google Scholar] [CrossRef]
- Hu, D.; Du, F.; Cao, H.; Zhou, Q.; Sun, P.; Xu, T.; Mei, C.; Hao, Q.; Fan, Z.; Zheng, J. An effective strategy to control thickness of Al2O3 coating layer on nickel-rich cathode materials. J. Electroanal. Chem. 2021, 880, 114910. [Google Scholar] [CrossRef]
- Anh, V.T.; Vu, H.-A.T.; Nguyen, V.; Nang, H.X.; Do, L.T.; Dao, V.-D.; Vu, N.H. Enhancing electrochemical performance of Ni-rich cathodes for Li-ion batteries through spatial atomic layer deposition of ZnO coatings. Mater. Chem. Phys. 2025, 336, 130544. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Wei, G.; Qiu, S.; Qu, J. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via wet-chemical coating of MgO. J. Solid State Electrochem. 2019, 23, 2213–2224. [Google Scholar] [CrossRef]
- Liu, W.; Gao, F.; Zang, Y.; Qu, J.; Xu, J.; Ji, S.; Huo, Y.; Qiu, J. Boosting cycle stability of NMC811 cathode material via 2D Mg-Al-LDO nanosheet coating for lithium-ion battery. J. Alloys Compd. 2021, 867, 159079. [Google Scholar] [CrossRef]
- Mohanty, D.; Dahlberg, K.; King, D.-M.; David, L.-A.; Sefat, A.-S.; Wood, D.-L.; Daniel, C.; Dhar, S.; Mahajan, V.; Lee, M.; et al. Modification of Ni-rich FCG NMC and NCA cathodes by atomic layer deposition: Preventing surface phase transitions for high-voltage lithium-ion batteries. Sci. Rep. 2016, 6, 26532. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Zeng, T.; Yu, Z.; Qu, X.; Peng, X.; Zhou, Y.; Duan, X.; Dou, A.; Su, M.; et al. Improving the structure stability of LiNi0.8Co0.15Al0.05O2 by double modification of tantalum surface coating and doping. ACS Appl. Energy Mater. 2021, 4, 8641–8652. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, A.; Wang, K.; Mao, Q.; Huang, H.; Zhang, J.; He, X.; Gan, Y.; Xiao, Z.; Zhang, W. Industrial modification comparison of Ni-Rich cathode materials towards enhanced surface chemical stability against ambient air for advanced lithium-ion batteries. Chem. Eng. J. 2022, 450, 138382. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.L.; Jin, E.M.; Moon, S.-G.; Jeong, S.M. Optimization of B2O3 coating process for NCA cathodes to achieve long-term stability for application in lithium-ion batteries. Energy 2021, 222, 119913. [Google Scholar] [CrossRef]
- Cui, X.; Ai, L.; Mao, L.; Xie, Y.; Liang, Y.; Zhang, N.; Feng, Y.; Wang, S.; Li, S. Enhanced electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathode material by the diffusional Al2O3 coating layer. Ionics 2019, 25, 411–419. [Google Scholar] [CrossRef]
- Negi, R.S.; Elm, M.T. Reproducible long-term cycling data of Al2O3 coated LiNi0.70Co0.15Mn0.15O2 cathodes for lithium-ion batteries. Sci. Data 2022, 9, 127. [Google Scholar] [CrossRef]
- Ahangari, M.; Xia, F.; Szalai, B.; Zhou, M.; Luo, H. Advancing lithium-ion batteries’ electrochemical performance: Ultrathin alumina coating on Li(Ni0.8Co0.1Mn0.1)O2 cathode materials. Micromachines 2024, 15, 894. [Google Scholar] [CrossRef]
- Woo, S.G.; Han, J.H.; Kim, K.J.; Kim, J.-H.; Yu, J.-S.; Kim, Y.-J. Surface modification by sulfated zirconia on high-capacity nickel-based cathode materials for Li-ion batteries. Electrochim. Acta 2015, 153, 115–121. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, D.; Li, Z.; Huan, Y.; Cheng, G.; Ren, Z.; Liu, X.; Wang, K.; Chen, L.; Gu, H.; et al. Breaking the cycle of heterogeneous degradation: Surface-targeted protection for Ni-rich cathodes in practical high-energy batteries. ACS Energy Lett. 2025, 10, 1457–1465. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, T.; Qin, D.; Leng, L.; Tian, J.; Sun, T.; Lu, J.; Liu, X.; Zhang, Y. Optimizing annealing strategy to achieve effective grain boundary modification with aluminum oxide for stable cycling Ni-rich cathodes. ACS Appl. Mater. Interfaces 2025, 17, 13988–13996. [Google Scholar] [CrossRef]
- Mitra, S.; Mudiyanselage, T.R.; Wang, X.; Lohar, G.; Dubal, D. Bolstering the rate performance of Co-free Ni-rich layered oxide cathode through a rapid heating method. Batter. Supercaps 2025, e202400782. [Google Scholar] [CrossRef]
- Meng, K.; Wang, Z.; Guo, H.; Li, X.; Wang, D. Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3. Electrochim. Acta 2016, 211, 822–831. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Mu, D.; Li, L.; Wu, F.; Chen, R. Surface reconstruction reduces internal stress of Ni-rich cathode particles. Small 2025, 21, 2406495. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-Z.; Wang, S.-S.; Tai, G.-A.; Zhu, L.; Wang, L.-G.; Zhai, H.-F.; Wu, D.; Li, A.-D.; Li, H. Enhanced electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material by ultrathin ZrO2 coating. J. Alloys Compd. 2016, 657, 593–600. [Google Scholar] [CrossRef]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From Surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
- Guan, P.; Min, J.; Chen, F.; Zhang, S.; Hu, L.; Ma, Z.; Han, Z.; Zhou, L.; Jia, H.; Liu, Y.; et al. Enhancing the electrochemical properties of nickel-rich cathode by surface coating with defect-rich strontium titanate. ACS Appl. Mater. Interfaces 2023, 15, 29308–29320. [Google Scholar] [CrossRef]
- Lou, F.; Xie, Q.; Luo, X.; Xie, Y.; Wang, M.; Hao, H.; Wang, Z.; Yang, L.; Wang, G.; Chen, J.; et al. Preventing structural collapse and thermal runaway to improve the electrochemical performance and safety of LiNi0.8Co0.1Mn0.1O2 by a negative-thermal-expansion material of Al2(WO3)4. Ind. Eng. Chem. Res. 2022, 61, 4588–4600. [Google Scholar] [CrossRef]
- Wustrow, A.; Hancock, J.C.; Incorvati, J.T.; Vaughey, J.T.; Poeppelmeier, K.R. Effect of fluoride doping on lithium diffusivity in layered molybdenum oxide. ACS Appl. Energy Mater. 2019, 2, 2080–2086. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Bin Sapari, M.A.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Park, S.-B.; Kang, C.-Y.; Hwang, D.-Y.; Yoo, Y.-W.; Park, H.-J.; Park, J.-H.; Kim, H.-S.; Lee, S.-H. Effect of MoO3 surface coating on electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode. Current Appl. Phys. 2022, 44, 1–5. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Sun, Q.; Nie, W.; Chang, L.; Chen, S.; Cheng, H. Architecting versatile NiFe2O4 coating for enhancing structural stability and rate capability of layered Ni-rich cathodes. Chem. Eng. J. 2023, 470, 144210. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Chang, L.; Liu, Y.; Zhang, J.; Yuan, Y.; Lu, X.; Cheng, H. Promoting durability of Ni-rich layered oxide via inverse spinel NiFe2O4 interface layer for high-voltage lithium-ion batteries. ACS Appl. Energy Mater. 2024, 7, 6236–6247. [Google Scholar] [CrossRef]
- Wang, L.; Mu, D.; Wu, B.; Yang, G.; Gai, L.; Liu, Q.; Fan, Y.; Peng, Y.; Wu, F. Enhanced electrochemical performance of lithium metasilicate-coated LiNi0.6Co0.2Mn0.2O2 Ni-rich cathode for Li-ion batteries at high cutoff voltage. Electrochim. Acta 2016, 222, 806–813. [Google Scholar] [CrossRef]
- Qu, X.; Huang, H.; Wan, T.; Hu, L.; Yu, Z.; Liu, Y.; Dou, A.; Zhou, Y.; Su, M.; Peng, X.; et al. An integrated surface coating strategy to enhance the electrochemical performance of nickel-rich layered cathodes. Nano Energy 2022, 91, 16665. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, A.; Lu, C.; Ma, R.; Fang, R.; Gan, Y.; Wang, G.; Xu, J.; Mao, Q.; Lu, X.; et al. Ultra-low-dose Nb2O5 coating promotes electrochemical kinetics and rate capability of Ni-rich oxide cathode. J. Solid State Electrochem. 2024, 1–10. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Miikkulainen, V.; Mäntimäki, M.; Mousavihashemi, S.; Lahtinen, J.; Lide, Y.; Yang, H.; Mizohata, K.; Kankaanpää, T.; Kallio, T. Understanding the stabilizing effects of nanoscale metal oxide and Li-metal oxide coatings on lithium-ion battery positive electrode materials. ACS Appl. Mater. Interfaces 2021, 13, 42773–42790. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Ryu, H.-H.; Kim, U.-H.; Weeks, J.A.; Heller, A.; Sun, Y.-K.; Mullins, C.B. Beyond doping and coating: Prospective strategies for stable high-capacity layered Ni-rich cathodes. ACS Energy Lett. 2020, 5, 1136–1146. [Google Scholar] [CrossRef]
- Lee, D.-J.; Scrosati, B.; Sun, Y.-K. Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55 °C. J. Power Sources 2011, 196, 7742–7746. [Google Scholar] [CrossRef]
- Kim, T.; Goh, M.S.; Moon, H.; Shin, H.; Lee, J.; Jeong, H.; Joo, S.W.; Kim, Y.S.; Im, Y.; Kang, M. Enhanced battery capacity and cycle life due to suppressed side reactions on the surface of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials coated with Co3(PO4)2. J. Colloid Int. Sci. 2024, 670, 729–741. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Wang, Z.; Guo, H. A facile process for coating amorphous FePO4 onto LiNi0.8Co0.15Al0.05O2 and the effects on its electrochemical properties. Mater. Lett. 2014, 131, 210–213. [Google Scholar] [CrossRef]
- Zha, G.; Luo, Y.; Hu, N.; Ouyang, C.; Hou, H. Surface modification of the LiNi0.8Co0.1Mn0.1O2 cathode material by coating with FePO4 with a yolk–shell structure for improved electrochemical performance. ACS Appl. Mater. Interfaces. 2020, 12, 36046–36053. [Google Scholar] [CrossRef]
- Qi, R.; Shi, J.; Zhang, X.; Zeng, X. Improving the stability of LiNi0.80Co0.15Al0.05O2 by AlPO4 nanocoating for lithium-ion batteries. Sci. China Chem. 2017, 60, 1230–1235. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, W.; Hao, S.; Li, L.; Ran, Q.; Liu, L.; Ji, Y.; Huo, J.; Liu, X. Utilizing dual functions of LaPO4 to enhance the electrochemical performance of LiNi0.87Co0.09Al0.04O2 cathode material. Nanotechnology 2023, 34, 075706. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Lin, Z.; Fan, M.; Wang, F.; Liu, Y.; Xiong, X. Facile and efficient fabrication of Li3PO4-coated Ni-rich cathode for high-performance lithium-ion battery. Appl. Surf. Sci. 2020, 504, 144506. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Li, X.; Luo, J.; Deng, S.; Zhao, Y.; Sun, Y.; Wu, D.; Hu, Y.; Li, W.; et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. X. Sun. Nat. Commun. 2023, 14, 146. [Google Scholar] [CrossRef]
- Park, H.G.; Kwon, D.; Cho, W.; Yoon, S.; Kim, D.; Park, K. Correlation between grain boundary coating and chemomechanics in Ni-rich layered Li cathodes. Chem. Eng. J. 2023, 452, 139442. [Google Scholar] [CrossRef]
- Luo, Z.; Ding, C.; Wang, W.; Hu, G.; Du, K.; Cao, Y.; Peng, Z. Improving storage performance and lattice oxygen stability of single crystal LiNi0.925Co0.03Mn0.045O2 by integrating utilization of surface residual lithium and lattice modulation. Appl. Surf. Sci. 2024, 665, 160238. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.T.; Guang, Y.; Bresser, D.; Diemant, Y.; Huang, M.; Copley, R.; Behm, J.; Passerini, S.; Shen, Z. Manganese phosphate coated Li[Ni0.6Co0.2Mn0.2]O2 cathode material: Towards superior cycling stability at elevated temperature and high voltage. J. Power Sources 2018, 402, 263–271. [Google Scholar] [CrossRef]
- Peng, Z.-D.; Huang, M.; Wang, W.-G.; Du, K.; Guan, D.-C.; Hu, G.; Cao, Y.-B. Enhancing the structure and interface stability of LiNi0.83Co0.12Mn0.05O2 cathode material for Li-ion batteries via facile CeP2O7 coating. ACS Sustain. Chem. Eng. 2022, 10, 4881–4893. [Google Scholar] [CrossRef]
- Min, K.; Park, K.; Park, S.Y.; Seo, S.W.; Choi, B.; Cho, E. Improved electrochemical properties of LiNi0.91Co0.06Mn0.03O2 cathode material via Li-reactive coating with metal phosphates. Sci. Rep. 2017, 7, 7151. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, L.; Yang, G.; Li, Y.; Pan, Q.; Li, Y.; Hu, S.; Zheng, F.; Wang, H.; Li, Q. Nanoscale surface modification to suppress capacity fade of Ni-rich layered oxide material at high cut-off voltage. Chem. Eng. J. 2023, 451, 138911. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, K.; Qiu, B.; Liang, H.; Li, J.; Zhao, W.; Li, S.; Zhang, W.; Liu, Z.; Liu, Q. Hydrophobic surface coating against chemical environmental instability for Ni-rich layered oxide cathode materials. Chem. Eng. J. 2022, 437, 135276. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.-Y.; Wei, H.-X.; Luo, Y.-H.; Tang, L.-B.; Chen, H.-Z.; Zhang, X.; Zheng, J. Suppress moisture sensitivity of Ni-rich cathode materials by bioinspired self-assembly hydrophobic layer. Chem. Eng. J. 2023, 477, 146850. [Google Scholar] [CrossRef]
- Jo, M.; Oh, P.; Kim, J.; Choi, J.-H.; Kim, S.; Ha, S.; Son, Y. Electrochemical lithium storage performance at high voltage and temperature of LiNi0.6Co0.2Mn0.2O2 cathode for Lithium-ion batteries by facile Mn3(PO4)2 dry coating. Appl. Surf. Sci. 2013, 613, 156018. [Google Scholar] [CrossRef]
- Yang, C.; Shao, R.; Mi, Y.; Shen, L.; Zhao, B.; Wang, Q.; Wu, K.; Lui, W.; Gao, P.; Zhou, H. Stable interstitial layer to alleviate fatigue fracture of high nickel cathode for lithium-ion batteries. J. Power Sources 2018, 376, 200–206. [Google Scholar] [CrossRef]
- Ahn, J.; Jang, E.K.; Yoon, S.; Lee, S.-J.; Sung, S.-J.; Kim, D.-H.; Cho, K.Y. Ultrathin ZrO2 on LiNi0.5Mn0.3Co0.2O2 electrode surface via atomic layer deposition for high-voltage operation in lithium-ion batteries. Appl. Surf. Sci. 2019, 484, 701–709. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Ma, J.; Yu, X.; Zhang, S.; Zhou, X.; Cui, G. A bifunctional chemomechanics strategy to suppress electrochemo-mechanical failure of Ni-rich cathodes for all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2022, 14, 17674–17681. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Wu, Y.; Wu, S.; Jose, R.; Yang, C. Surface-modified quaternary layered Ni-rich cathode materials by Li2ZrO3 for improved electrochemical performance for high-power Li-ion batteries. ACS Appl. Energy Mater. 2022, 5, 4796–4807. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, F.; Zhu, C.; Qiu, L.; Zhou, J.; Deng, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Effective and low-cost in situ surface engineering strategy to enhance the interface stability of an ultrahigh Ni-rich NCMA cathode. ACS Appl. Mater. Interfaces 2022, 14, 51835–51845. [Google Scholar] [CrossRef]
- Qiao, Y.; Hao, R.; Shi, X.; Li, Y.; Wang, Y.; Zhang, Y.; Tang, C.; Li, G.; Wang, G.; Liu, J.; et al. Improving the cycling stability of LiNi0.8Co0.1Mn0.1O2 by enhancing the structural integrity via synchronous Li2SiO3 coating. ACS Appl. Energy Mater. 2022, 5, 4885–4892. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, W.; Yao, J.; Bu, L.; Li, X.; Tian, K.; Lu, H.; Quan, C.; Xu, S.; Xu, K.; et al. Suppressing structural degradation of Ni-rich cathode materials towards improved cycling stability enabled by a Li2MnO3 coating. J. Mater. Chem. A 2020, 8, 17429–17441. [Google Scholar] [CrossRef]
- Dong, L.; Guan, X.; Zhou, Y.; Tang, S.; Chen, F. Supplying active lithium to single-crystal Li(Ni0.90Co0.05Mn0.05)0.98Ta0.02O2 with Li2MnO3 coating served as cathode for Li-ion batteries. Particuology 2024, 95, 303–318. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Strauss, F.; Bartsch, T.; Teo, J.-H.; Janek, J.; Brezesinski, T. Effect of surface carbonates on the cyclability of LiNbO3-coated NCM622 in all-solid-state batteries with lithium thiophosphate electrolytes. Sci. Rep. 2021, 11, 5367. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, S.; Hu, Y.; He, G.; Quo, L.; Parkin, B.P.; Iang, H. Lithium-conductive LiNbO3 coated high-voltage LiNi0.5Co0.2Mn0.3O2 cathode with enhanced rate and cyclability. Green Energy Environ. 2022, 7, 266–274. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Xie, Y.; Luo, S.; Wang, Z.; Zhu, L.; Zhang, X. Insights into capacity fading mechanism and coating modification of high-nickel cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 55491–55502. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, J.; Duan, R.; Bai, Y.; Xu, K.; Zhang, K.; Wang, J.; Zhang, K.; Yang, Z.; Li, M.; et al. Grain binding derived reinforced interfacial mechanical behavior of Ni-rich layered cathode materials. Nano Energy 2024, 121, 109214. [Google Scholar] [CrossRef]
- Xie, J.; Sendek, A.-D.; Cubuk, E.-D.; Zhang, X.; Lu, Z.; Gong, Y.; Wu, T.; Shi, F.; Liu, W.; Reed, E.-J.; et al. Atomic layer deposition of stable LiAlF4 lithium-ion conductive interfacial layer for stable cathode cycling. ACS Nano 2017, 11, 7019–7027. [Google Scholar] [CrossRef] [PubMed]
- Susai, F.A.; Sclar, H.; Maiti, S.; Burstein, L.; Perkal, O.; Grinblat, J.; Talianker, M.; Ruthstein, S.; Erk, C.; Hartmann, P.; et al. Stabilized behavior of LiNi0.85Co0.10Mn0.05O2 cathode materials induced by their treatment with SO2. ACS Appl. Energy Mater. 2020, 3, 3609–3618. [Google Scholar] [CrossRef]
- Tan, X.; Peng, W.; Duan, H.; Wang, Z.; Guo, H.; Luo, G.; Yuan, R.; Li, X.; Wang, J. A scalable dry chemical method for lithium borate coating to improve the performance of LiNi0.90Co0.06Mn0.04O2 cathode material. Solid State Ion. 2022, 28, 2073–2082. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Xi, X.; Zheng, J.; Yu, J.; He, Z. Suppressed internal intrinsic stress engineering in high-performance Ni-Rich cathode via multilayered in situ coating structure. Energy Environ. Mater. 2022, 24, 12574. [Google Scholar] [CrossRef]
- Llanos, S.; Ahaliabadeh, Z.; Miikkulainen, V.; Kong, X.; Obrezkov, F.; Lahtinen, J.; Yao, L.; Jiang, H.; Lassi, U.; Kallio, T. Structural and interfacial stability of a coated Ni-rich layered oxide cathode at high-voltage operation. Mater. Today Energy 2025, 50, 101862. [Google Scholar] [CrossRef]
- Byeon, Y.S.; Lee, H.B.; Hong, Y.; Kim, H.; Cho, W.; Park, M.-S. Cation-deficient LixWO3 surface coating on Ni-rich cathodes materials for lithium-ion batteries. ACS Appl. Interfaces 2025, 17, 9322–9331. [Google Scholar] [CrossRef]
- Yu, J.; Peng, J.; Huang, W.; Wang, L.; Wei, Y.; Yang, N.; Li, L. Inhibition of excessive SEI-forming and improvement of structure stability for LiNi0.8Co0.1Mn0.1O2 by Li2MoO4. Ionics 2021, 27, 2867–2876. [Google Scholar] [CrossRef]
- You, M.J.; Jung, J.; Byeon, Y.S.; Jung, J.Y.; Hong, Y.; Park, M.-S. Controlled crystallinity of LiTaO3 surface layer for single-crystalline Ni-rich cathodes for lithium-ion batteries and all-solid-state batteries. Chem. Engin. J. 2024, 483, 149199. [Google Scholar] [CrossRef]
- Herzog, M.J.; Gauquelin, N.; Esken, D.; Verbeeck, J.; Janek, J. Increased performance improvement of lithium-ion batteries by dry powder coating of high-nickel NMC with nanostructured fumed ternary lithium metal oxides. ACS Appl. Energy Mater. 2021, 4, 8832–8848. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Xiong, F.; Chen, F.; Huang, C.; Gao, Q.; Wang, T.; Yang, Z.; Zhang, W. An effective etching-induced coating strategy to shield LiNi0.8Co0.1Mn0.1O2 electrode materials by LiAlO2. J. Power Sources 2019, 412, 246–254. [Google Scholar] [CrossRef]
- Ran, Q.; Zhao, H.; Hu, Y.; Shen, Q.; Liu, W.; Liu, J.; Shu, X.; Zhang, M.; Liu, S.; Tan, M.; et al. Enhanced electrochemical performance of dual-conductive layers coated Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode for Li-ion batteries at high cut-off voltage. Electrochim. Acta 2018, 289, 82–93. [Google Scholar] [CrossRef]
- Wang, H.; Chu, Y.; Pan, Q.; Yang, G.; Lai, A.; Liu, Z.; Zheng, F.; Hu, S.; Huang, Y.; Li, Q. Enhanced interfacial reaction interface stability of Ni-rich cathode materials by fabricating dual-modified layer coating for lithium-ion batteries. Electrochim. Acta 2021, 366, 137476. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Zhang, J. Surface-coated LiNi0.8Co0.1Mn0.1O2 (NMC811) cathode materials by Al2O3, ZrO2, and Li2O-2B2O3 thin-layers for improving the performance of lithium-ion batteries. Front. Mater. 2019, 6, 309. [Google Scholar] [CrossRef]
- Jamil, S.; Ran, Q.; Yang, L.; Huang, Y.; Cao, S.; Yang, X.; Wang, X. Improved high-voltage performance of LiNi0.87Co0.1Al0.03O2 by Li+-conductor coating. Chem. Eng. J. 2021, 407, 126442. [Google Scholar] [CrossRef]
- Wu, K.; Li, Z.; Chen, X. Designing a corrosion inhibiting layer to enhance cycling stability of 4.7 V Ni-rich cathode. Adv. Func. Mater. 2024, 34, 15327. [Google Scholar] [CrossRef]
- Du, F.; Sun, P.; Zhou, Q.; Zeng, D.; Hu, D.; Fan, Z.; Hao, Q.; Mei, C.; Xu, T.; Zheng, J. Interlinking primary grains with lithium boron oxide to enhance the stability of LiNi0.8Co0.15Al0.05O2. ACS Appl. Mater. Interfaces 2020, 12, 56963–56973. [Google Scholar] [CrossRef]
- Lin, J.; Guo, H.; Sun, K.; Fang, L. A nickel-rich oxide, Li(Ni0.90Co0.06Mn0.04)0.995Al0.005O2, with a dual conductive coating. J. Alloys Compd. 2023, 940, 168870. [Google Scholar] [CrossRef]
- Xiao, P.; Cao, Y.; Li, W.; Li, G.; Yu, Y.; Dai, Z.; Du, Z.; Chen, X.; Sun, J.; Yang, W. Simple strategy for synthesizing LiNi0.8Co0.15Al0.05O2 using Co Al-LDH nanosheet-coated Ni(OH)2 as the precursor: Dual effects of the buffer layer and synergistic diffusion. ACS Appl. Mater. Interfaces 2021, 13, 29714–29725. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, L.; Jia, D.; Jiang, X.; Wu, Y.; Hao, Q.; Xia, X.; Ouyang, Y.; Peng, L.; Tang, W.; et al. LiNi0.8Co0.15Al0.05O2 cathodes exhibiting improved capacity retention and thermal stability due to a lithium iron phosphate coating. Electrochim. Acta 2019, 312, 179–187. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, Z.; Xi, R.; Li, Y.; Zhang, C. The cycle stability and rate performance of LiNi0.8Mn0.1Co0.1O2 enhanced by Mg doping and LiFePO4 coating. ChemElectroChem 2022, 9, e202101654. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Liu, J.; Wang, B.; Cheng, X.; Zhang, Y.; Sun, C.; Wang, X.; Zhang, J. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600–605. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Wang, Z.; Li, Z.; Zhu, Y.; Li, Y.; Gu, S.; Yuan, H.; Luo, W.; Lu, L.; et al. Revealing mechanism of Li3PO4 coating suppressed surface oxygen release for commercial Ni-rich layered cathodes. ACS Appl. Energy Mater. 2020, 3, 7445–7455. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.; Jin, B.; Kim, H. Dual function Li-reactive coating from residual lithium on Ni-rich NCM cathode material for Lithium-ion batteries. Sci. Rep. 2021, 11, 18590. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, J.; Zhou, X.; Chen, J.; Shi, Y.; Kalimudina, G.; Wang, F.; Belgibayeva, A.; Kong, L. Basicity regulation of Ni-rich layered oxide cathodes for all-solid-state Li-ion batteries. J. Energy Chem. 2025, 105, 454–460. [Google Scholar] [CrossRef]
- Cheng, W.; Hao, S.; Ji, Y.; Li, L.; Liu, L.; Xiao, Y.; Wu, Y.; Huo, J.; Tang, F.; Liu, X. Optimizing surface residual alkali and enhancing electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode by LiH2PO4. Nanotechnology 2022, 33, 045404. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Xu, C.; Wu, K.; Zou, F.; Hu, X.; Hu, Z. LiZr2(PO4)3 surface coating towards stable layer structure Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials with long cycle performance. Nano Res. 2023, 16, 2373–2382. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, X.; Dai, W.; Yao, C.; Qian, J.; Chen, Z.; Liu, C. Optimized Ni-rich LiNi0.83Co0.06Mn0.06Al0.05O2 cathode material with a Li1.3Al0.3Ti1.7(PO4)3 fast ion conductor coating for Lithium-ion batteries. J. Alloys Compd. 2022, 923, 166277. [Google Scholar] [CrossRef]
- Lv, Z.C.; Wang, F.F.; Wang, J.C.; Wang, P.F.; Yi, T.F. Durable lithium-ion insertion/extraction and migration behavior of LiF-encapsulated cobalt-free lithium-rich manganese-based layered oxide cathode. J. Colloid Interface Sci. 2023, 649, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A. Functional behavior of AlF3 coatings for high-performance cathode materials for lithium-ion batteries. AIMS Mater. Sci. 2019, 6, 406–440. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Lu, X.; Jiang, H.; Zhang, Q.; Wang, B.; Lai, C. Enhancing high-potential stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode with PrF3 coating. Ceram. Int. 2021, 47, 6341–6351. [Google Scholar] [CrossRef]
- Kaufman, L.A.; McCloskey, B.D. Surface lithium carbonate influences electrolyte degradation via reactive oxygen attack in lithium-excess cathode materials. Chem. Mater. 2021, 33, 4170–4176. [Google Scholar] [CrossRef]
- Kang, W.; Jiang, A.; Chen, S.; Cao, X.; Yu, X.; Luo, Y.; Deng, W. High-performance Ti-doped V2O5 coating on the Ni-rich layered cathode: Construction and theoretical calculation. J. Alloys Compd. 2024, 970, 172700. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Yao, Q.; Chen, Z.; Song, L.; Zhang, Z.; Gao, C.; Wang, P.; Yu, Z.; Lai, Y. Alleviating surface degradation of nickel-rich layered oxide cathode material by encapsulating with nanoscale Li-ions/electrons superionic conductors hybrid membrane for advanced Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 30879–30889. [Google Scholar] [CrossRef]
- Qu, J.; Sadighi, Z.; Yuan, H.; Zuin, L.; Goward, G.R.; Botton, G.A. Effective recovery of the air-exposed Ni-rich lithium transition metal oxide cathodes with a coating-stabilized surface. J. Power Sources 2024, 614, 234983. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Pham, Q.-T.; Yang, C.-C.; Chern, C.-S.; Babulal, L.M.; Seenivasan, M.; Jeyakumar, J.; Mengesha, T.H.; Placke, T.; Brunklaus, G.; et al. Coating of a novel lithium-containing hybrid oligomer additive on nickel-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for high-stability and high-safety lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 7394–7408. [Google Scholar] [CrossRef]
- Nie, H.; Shang, C.; Hu, P.; Li, Y. In-situ electrochemical polymerization of polypyrrole on LiNi0.8Co0.1Mn0.1O2 cathode with improved performance for lithium-ion batteries. Mater. Lett. 2023, 335, 133768. [Google Scholar] [CrossRef]
- Kim, H.I.; Jang, H.J.; Park, E.J.; Kim, S.-J.; Son, J.-T. Dramatic enhancement of the electrochemical property of nano-fiber Li[Ni0.9Co0.05Al0.05]O2 as a lithium-ion battery cathode material using a polypyrrole coating. J. Korean Phys. Soc. 2021, 79, 567–572. [Google Scholar] [CrossRef]
- Han, Y.; Heng, S.; Wang, Y.; Qu, Q.; Zheng, H. Anchoring interfacial nickel cations on single-crystal LiNi0.8Co0.1Mn0.1O2 cathode surface via controllable electron transfer. ACS Energy Lett. 2020, 5, 2421–2433. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Zhu, Y.; Huang, Z.; Zhang, F.; Gu, S.; Xie, J.; Zhang, K.; Lu, L.; Lu, Z. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-rich. LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 12594–12604. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, S.; Shin, M.W. Fundamental understanding of the effect of a polyaniline coating layer on cation mixing and chemical states of LiNi0.9Co0.085Mn0.015O2 for Li-ion batteries. ACS Appl. Polym. Mater. 2023, 5, 1344–1353. [Google Scholar] [CrossRef]
- He, P.; Zhang, M.; Wang, S.; Wan, M.; Wang, D.Y.; Wang, Y.; Yan, D.; Zhang, X. Sun. Enhanced high-rate and cyclic performance of Co-free and Ni-rich LiNi0.95Mn0.05O2 cathodes by coating electronic/Li+ conductive PANI-PEG layer. J. Solid State Electrochem. 2024, 28, 4259–4272. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Tang, H.; Huang, S.; Tang, Y.; Ma, J.; Huang, B.; Li, Y.; Sun, Y.; Xiao, S.; et al. A Mo/PANI co-modified ultra-high nickel NCM9622 cathode composite with excellent cycle stability and high-rate performance for power batteries. J. Mater. Chem. A 2024, 12, 21412–21424. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, M.; Mo, L.; Zhang, L.; Su, D.; Jiang, J.; Pan, Q.; Hu, S.; Wang, H.; Li, Q.; et al. Organic coating strategy with oxidized oxygen anion capture to suppress lattice oxygen evolution of Ni-rich cathode materials at high voltage. Chem. Eng. J. 2024, 493, 152525. [Google Scholar] [CrossRef]
- Li, Z.-W.; Lin, F.; Zhang, X.-D.; Zhang, X.-S.; Wen, R.; Li, X.; Zheng, Z.-J. Stress-relieving protective elastomeric interphase for stable Ni-rich cathodes. Adv. Func. Mater. 2025, 35, 2415035. [Google Scholar] [CrossRef]
- Liu, C.; Liao, C.M.; Li, Z.; Nie, S.; Yi, Z.; Xiao, W. Revealing the origin of enhanced structural stability for nickel-rich LiNi0.83Co0.11Mn0.06O2 cathodes in situ modified with multifunctional polysiloxane. Chem. Eng. J. 2024, 491, 152078. [Google Scholar] [CrossRef]
- Lin, K.; Yang, S.; Shi, Z.; Fan, Q.; Liu, Z.; Liu, L. Knitting a sweater with UV-induced in situ polymerization of poly(pyrrole-co-citral nitrile) on Ni-rich layer oxide cathode materials for lithium ion batteries. J. Power Sources 2022, 520, 230768. [Google Scholar] [CrossRef]
- Yoon, Y.; Shin, M.W. Cationic covalent organic framework coating of the Ni-rich cathode surface for the significant improvement of the interfacial Li+ transport and structural stability. ACS Appl. Energy Mater. 2024, 7, 5244–5252. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode properties at high operating voltage by double coating layer of Al2O3 and AlPO4. J. Alloys Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Wu, F.; Shi, Q.; Chen, L.; Dong, J.; Zhao, J.; Wang, H.; Gao, F.; Liu, J.; Zhang, H.; Li, N.; et al. New insights into dry-coating-processed surface engineering enabling structurally and thermally stable high-performance Ni-rich cathode materials for lithium-ion batteries. Chem. Eng. J. 2023, 470, 144045. [Google Scholar] [CrossRef]
- Bai, H.; Yuan, K.; Zhang, C.; Zhang, W.; Tang, X.; Jiang, S.; Jin, T.; Ma, Y.; Kou, L.; Shen, C.; et al. Advantageous surface engineering to boost single-crystal quaternary cathodes for high-energy-density lithium-ion batteries. Energy Storage Mater. 2023, 61, 102879. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, T.; Li, G.; Wan, T.; Li, M.; Zhang, X.; Li, M.; Su, M.; Dou, A.; Zeng, W.; et al. The surface double-coupling on single-crystal LiNi0.8Co0.1Mn0.1O2 for inhibiting the formation of intragranular cracks and oxygen vacancies. Energy Storage Mater. 2022, 52, 534–546. [Google Scholar] [CrossRef]
- Mohsen, M.; Rahim, L.; Hossein, E.; Manesha, M. Preparation and characteristics of graphene/Y2O3/LiNi0.8Co0.15Al0.05O2 composite for the cathode of lithium-ion battery. J. Electroanal. Chem. 2020, 862, 113971. [Google Scholar]
- Yua, J.; Zhang, J.; Zhang, Y.; Wang, Z.; Chen, Z.; Gao, A.; Zhang, J.; Wang, Y.; Zhao, R. Electrostatic adsorption facilitating dual-layer coating for stabilized cathode-electrolyte interphases and boosted lithium intercalation thereof in LiNi0.8Co0.1Mn0.1O2 cathode. Appl. Surf. Sci. 2022, 577, 151716. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Zeng, L.; Lin, J.; Zheng, B.; Liu, H.; Chen, L.; Wu, F. Single-crystal Ni-rich layered oxide cathodes with LiNbO3-Li3BO3 coating for sulfide all-solid-state batteries. Nano Energy 2025, 137, 110798. [Google Scholar] [CrossRef]
- Yang, G.; Liu, B.; Lai, F.; Xue, K.; Zhang, X.; Wang, H.; Xie, M.; Wang, C. Low-temperature synthesis of amorphous LiF/Li3BO3 iterfaces with F, B co-doped subsurface for long-cycling and high-rate Ni-rich cathodes. Nano Energy 2025, 140, 111009. [Google Scholar] [CrossRef]
- Rambukwella, I.; Ponnuru, H.; Yan, C. The role of dopants in mitigating the chemo-mechanical degradation of Ni-rich cathode: A critical review. EcoEnergy 2025, 3, 321–353. [Google Scholar] [CrossRef]
- Dang, R.; Qu, Y.; Ma, Z.; Yu, L.; Duan, L.; Lü, W. The effect of elemental doping on nickel-rich NCM cathode materials of lithium ion batteries. J. Phys. Chem. C 2022, 126, 151–159. [Google Scholar] [CrossRef]
- Byeon, Y.S.; Lee, W.; Park, S.; Kim, D.; Jung, J.; Park, M.-S.; Yoon, W.-S. Comprehensive understanding of elemental doping and substitution of Ni-rich cathode materials for lithium-ion batteries via in situ operando analyses. Small Sci. 2024, 4, 2400165. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, U.-H.; Yoon, C.S.; Sun, Y.-K. Enhanced cycling stability of Sn-doped Li[Ni0.90Co0.05Mn0.05]O2 via optimization of particle shape and orientation. Chem. Eng. J. 2021, 405, 126887. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, E.Y.; Chen, D.F.; Wu, M.; Han, S.; Huang, Q.; Yang, L.; Xiao, X.; Hu, Z. Decreasing Li/Ni disorder and improving the electrochemical performances of Ni-rich LiNi0.8Co0.1Mn0.1O2 by Ca doping. Inorg. Chem. 2017, 56, 8355–8362. [Google Scholar] [CrossRef]
- Ko, G.; Jeong, S.; Park, S.; Lee, J.; Kim, S.; Shin, Y.; Kim, W.; Kwo, K.J. Doping strategies for enhancing the performance of lithium nickel manganese cobalt oxide cathode materials in lithium-ion batteries. Energy Storage Mater. 2023, 60, 102840. [Google Scholar] [CrossRef]
- Susai, F.A.; Kovacheva, D.; Kravchuk, T.; Ravikumar, R.; Talianker, M.; Grinblat, J.; Burstein, L.; Kauffmann, Y.; Major, D.T.; Markovski, B.; et al. Improving performance of LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries by doping with molybdenum-ions: Theoretical and experimental studies. ACS Appl. Energy Mater. 2019, 2, 4521–4534. [Google Scholar] [CrossRef]
- Sattar, T.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Influence of Mo addition on the structural and electrochemical performance of Ni-rich cathode material for lithium-ion batteries. Sci. Rep. 2020, 10, 8562. [Google Scholar] [CrossRef]
- Park, G.-T.; Namkoong, B.; Kim, S.-B.; Liu, J.; Yoon, C.-S.; Sun, Y.-K. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 2022, 7, 946–954. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Lim, H.-W.; Lee, S.G.; Sun, Y.-K. Optimization of molybdenum-doped Ni-rich layered cathodes for long-term cycling. Energy Storage Mater. 2023, 59, 102771. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, G.-T.; Yoon, C.-S.; Sun, Y.-K. Suppressing detrimental phase transitions via tungsten doping of LiNiO2 cathode for next generation lithium-ion batteries. J. Mater. Chem. A 2019, 7, 18580–18588. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, K.-J.; Yoon, D.-R.; Aishova, A.; Yoon, C.-S.; Sun, Y.-K. Li[Ni0.9Co0.09W0.01]O2: A new type of layered oxide cathode with high cycling stability. Adv. Energy Mater. 2019, 9, 1902698. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, N.-Y.; Park, G.-T.; Kim, H.; Yoon, C.-S.; Sun, Y.-K. High-energy W-doped Li[Ni0.95Co0.04Al0.01]O2 cathodes for next-generation electric vehicles. Energy Storage Mater. 2020, 33, 399–407. [Google Scholar] [CrossRef]
- Wang, J.-L.; Liu, C.-J.; Wang, Q.; Xu, G.-L.; Miao, C.; Xu, M.-B.; Wang, C.-J.; Xiao, W. Investigation of W6+-doped in high-nickel LiNi0.83Co0.11Mn0.06O2 cathode materials for high-performance lithium-ion batteries. J. Colloid Interface Sci. 2022, 628, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Leng, Q.; Yang, X.; Jiao, T.; Gong, Z.; Wang, M.-S.; Yang, Y. Boosting electrochemical performance of Co-free Ni-rich cathodes by combination of Al and high-valence elements. Chem. Eng. J. 2023, 474, 145869. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Jiang, M.; Ning, T.; Tan, L.; Zhang, X.; Zheng, J.; Wang, J.; Wu, Q.; Ji, X.; et al. Uncovering mechanism behind tungsten bulk/grain-boundary modification of Ni-rich cathode. Energy Storage Mater. 2025, 75, 104016. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Yu, R.; Cao, S.; Pei, Y.; Luo, Z.; Zhao, Q.; Chang, B.; Wang, Y.; Wang, X. Tellurium surface doping to enhance the structural stability and electrochemical performance of layered Ni-rich cathodes. ACS Appl. Mater. Interfaces 2019, 11, 40022–40033. [Google Scholar] [CrossRef]
- Jamil, S.; Yu, R.; Wang, Q.; Fasehullah, M.; Huang, Y.; Yang, Z.; Yang, X.; Wang, X. Enhanced cycling stability of nickel-rich layered oxide by tantalum doping. J. Power Sources 2020, 473, 228597. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, G.-T.; Son, B.-K.; Nam, G.-W.; Liu, J.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat. Energy 2020, 5, 860–869. [Google Scholar] [CrossRef]
- Chu, B.; Liu, S.; You, L.; Liu, D.; Huang, T.; Li, Y.; Yu, A. Enhancing the cycling stability of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode at a high cutoff voltage with Ta doping. ACS Sustain. Chem. Eng. 2020, 8, 3082–3090. [Google Scholar] [CrossRef]
- Sun, H.-H.; Kim, U.-H.; Park, J.-H.; Park, S.-W.; Seo, D.-H.; Heller, A.; Mullins, C.-B.; Yoon, C.-S.; Sun, Y.-K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef]
- Li, X.; Ge, W.; Zhang, K.; Zhang, K.; Peng, G.; Fu, Y.; Ma, X. Comprehensive study of tantalum doping on morphology, structure, and electrochemical performance of Ni-rich cathode materials. Electrochem. Acta 2022, 403, 139653. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zeng, T.; Dou, A.; Zhang, P.; Su, M.; Zhou, Y.; Liu, Y. Conversion of residual lithium into fast ionic conductor coating to achieve one-step double modification strategy in LiNi0.8Co0.15Al0.05O2. J. Alloys Compd. 2023, 931, 167638. [Google Scholar] [CrossRef]
- Monsees, F.; Misiewicz, C.; Dalkilic, M.; Diddens, D.; Heuer, A. Enhancing the stability and performance of Ni-rich cathode materials through Ta doping: A combined theoretical and experimental study. Phys. Chem. Chem. Phys. 2025, 27, 834–843. [Google Scholar] [CrossRef]
- Fan, F.; Zheng, R.; Zeng, C.; Zeng, C.; Xu, H.; Wang, X.; Tian, G.; Wang, S.; Liu, P.; Shu, C. Synergistically dissipating the local strain and restraining lattice oxygen escape by fine-tuning of microstructure enabling Ni-rich cathodes with superior cyclabilities. J. Energy Chem. 2025, 105, 24–34. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Liu, Z.; Monteiro, R.S.; Ribas, R.M.; Gao, P.; Zhu, Y.; Yu, H.; Ben, L.; Huang, X. Investigation of structure and cycling performance of Nb5+ doped high-nickel ternary cathode materials. Solid State Ion. 2021, 359, 115520. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Zhang, D.; Yan, Y.; Li, Z. An effective doping strategy to improve the cyclic stability and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode. Chem. Eng. J. 2020, 402, 126195. [Google Scholar] [CrossRef]
- Liu, L.-H.; Li, M.-C.; Chu, L.-H.; Jiang, B.; Lin, R.-X.; Zhu, X.-P.; Cao, G.-Z. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar]
- Kim, U.-H.; Lee, S.-B.; Park, N.-Y.; Kim, S.-J.; Yoon, C.-S.; Sun, Y.-K. High-energy-density Li-ion battery reaching full charge in 12 min. ACS Energy Lett. 2022, 7, 3880–3888. [Google Scholar] [CrossRef]
- Wang, J.-L.; Yi, Z.-C.; Liu, C.-J.; He, M.-Y.; Miao, C.; Li, J.-Q.; Xu, G.-L.; Xiao, W. Revealing the effect of Nb5+ on the electrochemical performance of nickel-rich layered LiNi0.83Co0.11Mn0.06O2 oxide cathode for lithium-ion batteries. J. Colloid Interface Sci. 2023, 635, 295–304. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Chen, L.; Lu, Y.; Su, Y.; Bao, L.; Chen, R.; Chen, S. Use of Ce to reinforce the interface of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries under high operating voltage. ChemSusChem 2019, 12, 935–943. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Wu, S.; Wang, Z.; Liu, X.; Li, W.; Li, N.; Wang, J.; Zhuang, W. Utilizing diverse functions of zirconium to enhance the electrochemical performance of Ni-rich layered cathode materials. ACS Appl. Energy Mater. 2020, 3, 11741–11751. [Google Scholar] [CrossRef]
- Che, W.; Wan, X.-W.; Zhang, D.-Y.; Chang, C.-K. Stabilized performance of LiNi0.90Co0.07Al0.03O2 cathodes via Zr4+ doping upon 4.5 V application due to the suppression of H2–H3 phase transitions. ACS Sustain. Chem. Eng. 2021, 9, 5536–5545. [Google Scholar] [CrossRef]
- Ni, J.-X.; Tan, Y.-Y.; Xu, K.; Jiang, Y.-J.; Chang, W.-Y.; Lai, C.-Y.; Liu, H. Cobalt-free nickel-rich layered LiNi0.9Al0.1-xZrxO2 cathode for high energy density and stable lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 136, 104421. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Z.; Wang, W.; Wen, Y.; Zhang, S.; Chen, X.; Zhang, Z.; Yin, Z.; Yang, T.; Li, T.; et al. Superior fast-charging Ni-rich cathode via promoted kinetic-mechanical performance. Nano Energy 2024, 128, 109908. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, B.; Yi, M.-Y.; Fan, X.-M.; Zhang, Z.; Huang, X.-B.; Lai, Y.-Q. In situ co-doping strategy for achieving long-term cycle stability of single-crystal Ni-rich cathodes at high voltage. Chem. Eng. J. 2022, 445, 136825. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, W.-J.; Kim, Y.-C.; Jung, J.Y.; Rhee, D.Y.; Song, J.H.; Cho, W.; Park, M.-S. Incorporation of titanium into Ni-rich layered cathode materials for lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 12204–12211. [Google Scholar] [CrossRef]
- Yang, R.-K.; Wu, Z.-G.; Li, Y.-C.; Li, R.; Qiu, L.; Wang, D.; Yang, L.; Guo, X.-D. Optimization of the electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material by titanium doping. Ionics 2020, 26, 3223–3230. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, Y.; Yao, J.; Zhao, M.; Xu, Y. Improved cycling stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by optimizing Ti doping. Funct. Mater. Lett. 2021, 14, 2150002. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, X.; Wang, C.; Zhao, B.; Huang, B. Enhancing high cycle stability of Ni-rich LiNi0.94Co0.04Al0.02O2 layered cathode material. Ionics 2021, 27, 4619–4628. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, C.; Miao, C.; Wang, Z.; Wang, J.; Xin, Y.; Xiao, W. In-situ Ti4+-doped modification of layer-structured Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials for high-energy lithium-ion batteries. J. Colloid Interface Sci. 2025, 677, 91–100. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Luo, W.; Cao, Y.; Zhao, C.; Dong, P.; Duan, J.; Wang, D.; Wang, X.; Zhou, Z.; et al. Enhanced reversibility of single crystal Ni-rich cathode materials via Ti doping. Electrochim. Acta 2024, 479, 143855. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Cao, H.; Peng, Y.; Ding, G.; Hua, Y.; Zhao, J.; Cui, C.; Huang, S. Enhancing the cycling stability of Ni-rich cathodes via dual-site modification induced by slight Ti-rich doping. J. Power Sources 2025, 628, 235913. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Wang, Z.-H.; Zhu, Y.-H.; Jiang, H.; Li, C.-Z. Enhancing surface-to-bulk stability of layered Co-free Ni-rich cathodes for long-life Li-ion batteries. Battery Energy 2022, 2, 20220048. [Google Scholar] [CrossRef]
- Wang, B.; Cai, F.; Chu, C.; Fu, B.; Swierczek, K.; Li, L.; Zhao, H. Modification of the Ni-rich layered cathode material by Hf addition: Synergistic microstructural engineering and surface stabilization. ACS Appl. Mater. Interfaces 2024, 16, 12599–12611. [Google Scholar] [CrossRef]
- Jeyakumar, J.; Seenivasan, M.; Wu, Y.-S.; Kuo, L.-Y.; Wu, S.-H.; Chang, J.-K.; Yang, C.-C. Sn-doped/coated Ni-rich LiNi0.90Co0.04Mn0.03Al0.03O2 cathode materials for improved electrochemical performance of Li-ion batteries. ACS Appl. Energy Mater. 2024, 7, 4919–4934. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Yang, Z.; Zhang, M.; Zhang, D.; Yan, Y. Synergistic effect of Ce4+ modification on the electrochemical performance of LiNi0⋅6Co0⋅2Mn0⋅2O2 cathode materials at high cut-off voltage. Ceram. Int. 2021, 47, 1268–1276. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, G.; Li, Y.; Zhu, J.; Zheng, J.; Tan, Z.; Xi, X.; Yang, J. The synergistic Effect of Gd modification on improving the electrochemical performance of LiNi0.88Co0.09Al0.03O2 cathode materials. J. Electrochem. Soc. 2021, 168, 0305510. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.-J.; Lee, E.; Yoon, W.-S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, B.; Su, S.L.; Ming, L.; Zhao, Y.; Tan, X.X. Al-doping enables high stability of single-crystalline LiNi0.7Co0.1Mn0.2O2 lithium-ion cathodes at high voltage. RSC Adv. 2020, 11, 124–128. [Google Scholar] [CrossRef]
- Zhang, C.; Wan, J.; Li, Y.; Zheng, S.; Zhou, K.; Wang, D.; Wang, D.; Hong, C.; Gong, Z.; Yang, Y.; et al. Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping. J. Mater. Chem. 2020, 8, 6893–6901. [Google Scholar] [CrossRef]
- Kim, U.-H.; Lee, S.-B.; Ryu, J.-H.; Yoon, C.-S.; Sun, Y.-G. Optimization of Ni-rich Li[Ni0.92–xCo0.04Mn0.04Alx]O2 cathodes for high energy density lithium-ion batteries. J. Power Sources 2023, 564, 232850. [Google Scholar] [CrossRef]
- Xi, R.-H.; Zhang, J.-N.; Lan, Z.-W.; Yuan, Y.-X.; Kang, J.-L.; Li, Y.-Y.; Wang, J.-T.; Zhang, C.-H.; Hou, X.-Y. High-nickel and cobalt-free layered LiNi0.90Mn0.06Al0.04O2 cathode for lithium-ion batteries. Ceram. Int. 2022, 48, 36690–36696. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Z.; Sun, J.-Y.; Xu, R.-M.; Li, X.-W.; Yu, X.; Liu, Y. Facile synthesis of crack-free single-crystalline Al-doped Co-free Ni-rich cathode material for lithium-ion batteries. Electrochim. Acta 2023, 437, 141473. [Google Scholar] [CrossRef]
- Kong, X.; Li, D.; Fedorovskaya, E.O.; Kallio, T.; Ren, X. New insights in Al-doping effects on the LiNiO2 positive electrode material by a sol-gel method. Int. J. Energy Res. 2021, 45, 10489–10499. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Su, W.; Zhu, X.; Hao, L.; Wang, X.; Wu, S.; Chen, L.; Cao, D.; Su, Y.; et al. Aluminium doping in single-crystal nickel-rich cathodes: Insights into electrochemical degradation and enhancement. J. Mater. Chem. A 2024, 12, 20910–20920. [Google Scholar] [CrossRef]
- Yan, H.; Ha, Y.; Ye, T.; Gao, X.; Yue, X.; Li, Z. High performance of Co-free LiNixMn1-xO2 cathodes realized by nonmagnetic ion substitution for Li-ion batteries. Chem. Eng. J. 2023, 465, 142926. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.-H.; Zheng, J.-C.; Zhang, D.-W.; Yang, J.-C.; Chen, Y.-X.; Guo, J.; Zhu, J.; Xiong, Y.; Li, W. Dual-modification of Gd2O3 on the high-voltage electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode materials via the solid-state method. J. Solid State Electrochem. 2020, 24, 863–872. [Google Scholar] [CrossRef]
- Sallard, S.; Billaud, J.; Sheptyakov, D.; Novak, P.; Villevieille, C. Cr-doped Li-rich nickel cobalt manganese oxide as a positive electrode material in Li-ion batteries to enhance cycling stability. ACS Appl. Energy Mater. 2020, 3, 8646–8657. [Google Scholar] [CrossRef]
- Xu, Y.-D.; Zhang, J.; Wu, Z.-G.; Xu, C.-L.; Li, Y.-C.; Xiang, W.; Wang, Y.; Zhong, Y.-J.; Guo, X.-D.; Chen, H. Stabilizing the structure of nickel-rich lithiated oxides via Cr doping as cathode with boosted high-voltage/temperature cycling performance for Li-ion battery. Energy Technol. 2020, 8, 1900498. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Su, Y.; Dong, J.; Yan, K.; Liu, J.; Lu, Y.; Wang, H.; Wang, C.; Wu, F.; et al. Rare-earth doping induced pinning effect with enhanced chemo-mechanical stability in Ni-rich cathodes for lithium-ion batteries. Chin. J. Chem. 2025, 43, 889–896. [Google Scholar] [CrossRef]
- Luo, Z.; Li, H.; Wang, W.; Fang, Z.; Zhao, B.; Hu, G.; Peng, Z.; Du, K.; Cao, Y. Mitigating irreversible phase transition of Y-doped LiNi0.925Co0.03Mn0.045O2 by lattice engineering. Ceram. Int. 2024, 50, 9535–9547. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, S.; Xie, X.; Wu, C.; Jamil, S.; Zhao, Q.L.; Chang, B.B.; Wang, Y.; Wang, X.Y. Improving the structure and cycling stability of Ni-rich layered cathodes by dual modification of yttrium doping and surface coating. ACS Appl. Mater. Interfaces 2020, 12, 19483–19494. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Z.; Chen, Y.; Gao, X. La-doped ultrahigh-nickel layered oxide cathode with enhanced cycle stability for Li-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 35043–35051. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, X.; Qiang, W.; Huang, B. Improved electrochemical performances of Ni-rich LiNi0.83Co0.12Mn0.05O2 by Mg-doping. J. Power Sources 2020, 450, 227718. [Google Scholar] [CrossRef]
- Weber, R.; Li, H.-Y.; Chen, W.-F.; Kim, C.-Y.; Plucknett, K.; Dahn, J.-R. In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials. J. Electrochem. Soc. 2020, 167, 100501. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-free Ni-rich single crystal positive electrode materials for lithium-ion batteries: Part I. Two-step lithiation method for Al or Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 040531. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-Free Ni-rich single crystal positive electrode materials for lithium-ion batteries: Part II. One-step lithiation method of Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 050506. [Google Scholar] [CrossRef]
- Yu, H.-F.; Zhu, H.-W.; Yang, Z.-F.; Liu, M.-M.; Jiang, H.; Li, C.-Z. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes. Chem. Eng. J. 2021, 412, 128625. [Google Scholar] [CrossRef]
- Xiao, W.; Nie, Y.; Miao, C.; Wang, J.; Tan, Y.; Wen, M. Structural design of high-performance Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode materials enhanced by Mg2+ doping and Li3PO4 coating for lithium-ion battery. J. Colloid Interface Sci. 2022, 607, 1071–1082. [Google Scholar] [CrossRef]
- Lin, Y.; Abram, C.M.; Shi, X.; McKendry, I.G.; Wang, Z.; Zhong, H.; Zhao, H.; Yang, X.; Koel, B.E.; Yan, C.; et al. Enhanced thermal stability of aerosol-synthesized Ni-rich Li-ion battery cathode materials via concentration-gradient Ca doping. ACS Appl. Energy Mater. 2022, 5, 10751–10757. [Google Scholar] [CrossRef]
- Wang, L.-C.; Chu, Y.-Q.; Nong, Y.-T.; Zheng, F.-H.; Li, Y.; Huang, Y.-Z.; Li, Y.-H.; Pan, Q.-C.; Wang, H.; Li, Q. Sr-based sub/surface integrated layer and bulk doping to enhance high-voltage cycling of a Ni-rich cathode material. ACS Sustain. Chem. Eng. 2022, 10, 7883–7895. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zheng, M.; Cai, F. Strontium doping promotes low-temperature growth of single-crystalline Ni-rich cathodes with enhanced electrochemical performance. Materials 2025, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Park, S.; Park, J.; Beak, M.; Lee, J.; Kwon, E.E.; Kwon, K. The enhancement of cyclability of Ni-rich LiNi0.9Co0.05–xMn0.05Znx cathode materials by the substitution of Zn for Co. Int. J. Energy Res. 2022, 46, 19177. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, Y.; Zhao, L.; Zhang, Y.; Tang, H.; Xu, Y.; Tan, Y.; Hou, X.; Wang, J. Improving the electrochemical performance of Ag-doped Ni-rich Li(Ni0.88Co0.09Al0.03)1–xO2 layered cathode material. Appl. Phys. A 2025, 131, 177. [Google Scholar] [CrossRef]
- Hu, Y.; Qin, Z.; Pei, J.; Cong, B.; Yang, X.; Chen, G. Reduced lithium/nickel disorder degree of sodium-doped lithium-rich layered oxides for cathode materials: Experiments and calculations. ChemElectroChem 2020, 7, 246–251. [Google Scholar] [CrossRef]
- Li, S.-C.; Liu, Y.-Y.; Zhang, Y.-G.; He, W.; Zheng, H.-F.; Guo, W.-B.; Wu, H.-L.; Gao, G.-Y.; Sa, B.-S.; Wang, L.-S.; et al. Interfacial oxygen coordination environment regulation towards high-performance Li-rich layered oxide cathode. Chem. Eng. J. 2023, 462, 142194. [Google Scholar] [CrossRef]
- Li, C.-F.; Chen, L.-D.; Wu, L.; Liu, Y.; Hu, Z.-Y.; Cui, W.-J.; Dong, W.-D.; Liu, X.; Yu, W.-B.; Li, Y.; et al. Directly revealing the structure-property correlation in Na+-doped cathode materials. Appl. Surf. Sci. 2023, 612, 155810. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, J.; Li, Y.; Zhang, Y.F.; Huang, S.P.; Lin, W.; Chen, W.K. Effects of doping high-valence transition metal (V, Nb and Zr) ions on the structure and electrochemical performance of LIB cathode material LiNi0.8Co0.1Mn0.1O2. Phys. Chem. Chem. Phys. 2021, 23, 11528–11537. [Google Scholar] [CrossRef]
- Li, X.; Lai, T.; Sheng, A.; Yang, J.; Li, Y.; Xiao, S.; Li, W.; Tan, H.; Huang, B. Effects of Nb-doping on cycle life, self-discharge and crack resistance of Ni-rich LiNi0.94Co0.02Al0.04O2 cathode for Li-ion batteries. J. Energy Storage 2023, 72, 108262. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Liu, Z.; Zhao, N.; Liang, H.; Zhou, M.; Zou, K.; Tan, L.; Ning, T. Revealing the mechanism of Zr coating modification on Ni-rich cathode materials through solid-phase diffusion. Chin. Chem. Lett. 2025, 111079. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Selent, M.; Tynjälä, P.; Lassi, U. Correlation of aluminum doping and lithiation temperature with electrochemical performance of LiNi1-xAlxO2 cathode material. J. Solid State Electrochem. 2023, 27, 641–654. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, H.; Mo, Y.; Qu, Y.; Du, B.; Chen, Y. Synthesis and characterization of Cu-doped LiNi0.6Co0.2Mn0.2O2 materials for Li-ion batteries. J. Alloys Compd. 2020, 844, 156180. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Seo, J.-H.; Yu, Y.-S.; Sharma, M.; Mucke, R.; Kaghazchi, P.; Yoon, C.-S.; Sun, Y.-K. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries. Mater. Today 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Wu, C.Y.; Bao, Q.; Tsai, Y.T.; Duh, J.G. Tuning (003) interplanar space by boric acid co-sintering to enhance Li+ storage and transfer in Li(Ni0.8Co0.1Mn0.1)O2 cathode. J. Alloys Compd. 2021, 865, 158806. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.-M.; Luo, B.; Zhao, Z.-W.; Shen, J.-X.; Liu, Z.-H.; Xiao, Z.-M.; Zhang, B.; Zhang, J.-F.; Ming, L. Understanding the enhancement effect of boron doping on the electrochemical performance of single-crystalline Ni-rich cathode materials. J. Colloid Interface Sci. 2021, 604, 776–784. [Google Scholar] [CrossRef]
- Roitzheim, C.; Kuo, L.-Y.; Sohn, Y.J.; Finsterbusch, M.; Möller, S.; Sebold, D.; Valencia, H.; Meledina, M.; Mayer, J.; Breuer, U.; et al. Boron in Ni-rich NMC811 cathode material: Impact on atomic and microscale properties. ACS Appl. Energy Mater. 2022, 5, 524–538. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, Z.; Liang, J.; Lu, D.; Liang, C.; Guan, X.; Gao, A.; Chen, H. Understanding the role of Na-doping on Ni-rich layered oxide LiNi0.5Co0.2Mn0.3O2. J. Alloys Compd. 2016, 689, 318–325. [Google Scholar] [CrossRef]
- Shen, Y.; Yao, X.; Zhang, J.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Zhang, Y.; Hu, J.; Cheng, Y.; et al. Sodium doping derived electromagnetic center of lithium layered oxide cathode materials with enhanced lithium storage. Nano Energy 2022, 94, 106900. [Google Scholar] [CrossRef]
- He, T.; Chen, L.; Su, Y.; Lu, Y.; Bao, L.; Chen, G.; Zhang, Q.; Chen, S.; Wu, F. The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni-Rich cathode materials. J. Power Sources 2019, 441, 22719. [Google Scholar] [CrossRef]
- Vanaphuti, P.; Chen, J.; Cao, J.; Bigham, K.; Chen, B.; Yang, L.; Chen, H.; Wang, Y. Enhanced electrochemical performance of the lithium-manganese-rich cathode for Li-ion batteries with Na and F co-doping. ACS Appl. Mater. Interfaces 2019, 11, 37842–37849. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.H.; Sun, Y.K.; Yoon, C.S. Evolution of a radially aligned microstructure in boron-doped LiNi0.95Co0.04Al0.01O2 cathode particles. ACS Appl. Mater. Interfaces 2022, 14, 17500–17508. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-B.; Park, D.-H.; Park, K.-W. Fluorine-doped LiNi0.8Mn0.1Co0.1O2 cathode for high-performance lithium-ion batteries. Energies 2020, 13, 4808. [Google Scholar] [CrossRef]
- Qiu, Q.-Q.; Yuan, S.-S.; Bao, J.; Wang, Q.-C.; Yue, X.-Y.; Li, X.-L.; Wu, X.-J.; Zhou, Y.-N. Suppressing irreversible phase transition and enhancing electrochemical performance of Ni-rich layered cathode LiNi0.9Co0.05Mn0.05O2 by fluorine substitution. J. Energy Chem. 2021, 61, 574–581. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Xu, G.; Miao, C.; Wen, M.; Xu, M.; Wang, C.; Xiao, W. Strengthened the structural stability of in-situ F− doping Ni-rich LiNi0.8Co0.15Al0.05O2 cathode materials for lithium-ion batteries. Chem. Eng. J. 2022, 438, 135537. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Park, D.-H.; Kim, J.-H.; Jang, J.-S.; Kim, W.-C.; Lee, G.-I.; Lim, J.-W.; Hong, J.-M.; Park, K.-W. F-doped Co-free LiNixMn1-xO2 (0.7 ≤ x ≤ 0.9) cathodes for ameliorating electrochemical performance of Li-ion batteries. Mater. Today Energy 2024, 41, 101520. [Google Scholar] [CrossRef]
- Azhari, L.; Sousa, B.; Ahmed, R.; Wang, R.; Yang, Z.; Gao, G.; Han, Y.; Wang, Y. Stability enhancement and microstructural modification of Ni-rich cathodes via halide doping. ACS Appl. Mater. Interfaces 2022, 14, 46523–46536. [Google Scholar] [CrossRef]
- Eilers-Rethwisch, M.; Winter, M.; Schappacher, F.M. Synthesis, electrochemical investigation and structural analysis of doped Li[Ni0.6Mn0.2Co0.2-xMx]O2 (x = 0, 0.05; M = Al, Fe, Sn) cathode materials. J. Power Sources 2018, 387, 101–107. [Google Scholar] [CrossRef]
- Eilers-Rethwisch, M.; Hildebrand, S.; Evertz, M.; Ibing, L.; Dagger, T.; Winter, M.; Schappacher, F.M. Comparative study of Sn-doped Li[Ni0.6Mn0.2Co0.2-xSnx]O2 cathode active materials (x = 0 − 0.5) for lithium ion batteries regarding electrochemical performance and structural stability. J. Power Sources 2018, 397, 68–78. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Lv, Y.; Qi, L.; Ma, Y.; Zhang, H.; Song, D.; Shi, X.; Zhang, L. Investigation on the structure and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 modified with Sn. Electrochim. Acta 2021, 400, 139468. [Google Scholar] [CrossRef]
- Angellinnov, F.; Subhan, A.; Drew, A.J.; Syahrial, A.Z. Synthesis of Sn doped and rice husk derived activated carbon surface coating NMC 811 through solution combustion method. Heliyon 2024, 10, e23199. [Google Scholar] [CrossRef]
- Huang, Z.J.; Wang, Z.X.; Zheng, X.B.; Guo, H.; Li, X.; Jing, Q.; Yang, Z. Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Electrochim. Acta 2015, 182, 795–802. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B.; Manthiram, A. An in-depth understanding of the effect of aluminum doping in high-nickel cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Yi, M.; Dolocan, A.; Manthiram, A. Stabilizing the interphase in cobalt-free, ultrahigh-nickel cathodes for lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2213164. [Google Scholar] [CrossRef]
- Park, N.; Kim, S.; Kim, M.; Han, S.; Kim, D.; Kim, M.; Sun, Y. Mechanism of doping with high-valence elements for developing Ni-rich cathode materials. Adv. Energy Mater. 2023, 13, 2301530. [Google Scholar] [CrossRef]
- Zybert, M.; Rondura, H.; Rarog-Pilecka, W.; Wieczorek, W. Application of rare earth elements as modifiers for Ni-rich cathode materials for Li-ion batteries: A mini review. Front. Energy Res. 2023, 11, 1248641. [Google Scholar] [CrossRef]
- Kim, U.H.; Jun, D.-W.; Park, K.-J.; Zhang, Q.; Kaghazchi, P.; Aurbach, D.; Major, D.T.; Goobes, G.; Dixit, M.; Leifer, N.; et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries. Energy Environ. Sci. 2018, 11, 1271–1279. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Y.; Huang, B.; Zho, Z.; Li, Y.; Li, W. Effects of W-doping on precursor growth of LiNi0.88Co0.09Mn0.03O2 and its electrochemical performance. Trans. Nonferr. Met. Soc. China 2024, 34, 1251–1262. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Chen, L.; Hu, Y.; Jiang, H.; Li, C. Strain engineering of Ni-rich cathode enables exceptional cyclability in pouch-type full cells. Adv. Mater. 2023, 35, 2209357. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, H.; Yu, H.; Wang, Z.; Jiang, H.; Li, C. Enhancing surface and crystal stability of the Ni-high NCA cathode for high-energy and durable lithium-ion batteries. Ind. Eng. Chem. Res. 2022, 61, 2817–2824. [Google Scholar] [CrossRef]
- Chu, M.; Huang, Z.; Zhang, T.; Wang, R.; Shao, T.; Wang, C.; Zhu, W.; He, L.; Chen, J.; Zhao, W.; et al. Enhancing the electrochemical performance and structural stability of Ni-rich layered cathode materials via dual-site doping. ACS Appl. Mater. Interfaces 2021, 13, 19950–19958. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Huang, J.; Peng, J.; Xie, H.; Huang, B.; Li, Y.; Sun, Y.; Xiao, S.; Wang, R. Effective reinforcement on the structural stability and electrochemical performances of ultra-high nickel cobalt free cathode material by co-doping. J. Energy Storage 2024, 78, 110073. [Google Scholar] [CrossRef]
- Yao, Z.; Lum, Y.; Johnston, A.; Mejia-Mendoza, L.M.; Zhou, X.; Wen, Y.; Aspuru-Guzik, A.; Sargent, E.H.; She, Z.W. Machine learning for a sustainable energy future. Nat. Rev. Mater. 2022, 8, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kang, S.; Park, H.G.; Park, K.; Min, K. Maximizing the energy density and stability of Ni-rich layered cathode materials with multivalent dopants via machine learning. Chem. Eng. J. 2023, 452, 139254. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0·8Co0·1Mn0·1O2 (NMC811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Zha, G.; Hu, W.; Agarwal, S.; Ouyang, C.; Hu, N.; Hou, H. High performance layered LiNi0.8Co0.07Fe0.03Mn0.1O2 cathode materials for Li-ion battery. Chem. Eng. J. 2021, 409, 128343. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic lattice strain and mechanical degradation of high- and low-nickel NCM cathode materials for Li-ion batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
- Jo, E.; Park, J.-H.; Park, J.; Hwang, J.; Chung, K.Y.; Nam, K.-W.; Kim, S.M.; Chang, W. Different thermal degradation mechanisms: Role of aluminum in Ni-rich layered cathode materials. Nano Energy 2020, 78, 105367. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, U.-H.; Kim, J.-H.; Jun, S.; Yoon, C.S.; Jung, Y.S.; Sun, Y.-K. Ni-rich layered cathode materials with electrochemo-mechanically compliant microstructures for all-solid-state Li batteries. Adv. Energy Mater. 2020, 10, 1903360. [Google Scholar] [CrossRef]
- Ikuerowo, T.O.; Tomomewo, O.; Akande, S.O. Unlocking the potential of Ni-rich LiNi0.9Co0.1O2 cathodes: A DFT investigation of performance-limiting factors. Phys. Chem. Chem. Phys. 2025, 27, 1494–1502. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Y.; Yi, Z.; Yang, C.; Liu, F.; Wang, K.; Cao, J.; Chen, Z. Regulating the internal structure by magnesium doping to enhance cycle stability of full-concentration-gradient Ni-rich layered cathodes. Chem. Eng. J. 2023, 474, 145554. [Google Scholar] [CrossRef]
- Jamil, S.; Yue, L.; Li, C.; Fasehullah, M.; Aizaz Uddin, M.; Yang, W.; Bao, S.; Xu, M. Significance of gallium doping for high Ni, low Co/Mn layered oxide cathode material. Chem. Eng. J. 2022, 441, 135821. [Google Scholar] [CrossRef]
- Bunyanidhi, P.; Phattharasupakun, N.; Duangdangchote, S.; Prempluem, S.; Joraleechanchai, N.; Sawangphruk, M. Exploring the impact of metal oxide coating and metal atom doping on the electrochemical performance of Ni-rich cathode materials. J. Mater. Chem. A 2023, 11, 23223–23227. [Google Scholar] [CrossRef]
- Gao, D.; Che, W.; Xu, Q.; Zhao, X.; Huang, Y.; Chang, C. W doping improves the electrochemical performance of LiNi0.94Co0.04Al0.02O2 cathode through dual enhanced electronic and ionic conductivities: A study from DFT calculations and experimental results. Scr. Mater. 2023, 234, 115586. [Google Scholar] [CrossRef]
- Yang, J.; Gao, D.; Zhang, D.; Chang, C. Effect of dual improved electronic and cationic conductivity via W doping on cyclability and rate performance of LiNi0.90Co0.04Mn0.03Al0.03O2 cathode for rechargeable LiBs. J. Energy Storage 2023, 63, 107088. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Yang, J.; Wang, D.; Ye, C.; Wang, D.; Avdeev, M.; Shi, S.; Yang, J.; Zhang, W. Ab initio thermodynamic optimization of Ni-rich Ni–Co–Mn oxide cathode coatings. J. Power Sources 2020, 450, 227693. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, R.; Sun, J.; Chen, Y.; Chen, J.; He, J.; Zhang, Y.; Han, B.; Liao, G.; Wu, J.S.; et al. Precursor-oriented ultrathin Zr-based gradient coating on Ni-riched cathodes. Nano Energy 2023, 105, 108000. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Lu, Y.; Yang, L.; Xiong, L.; Zhao, S.; Bai, L.; Huang, C.; Zhou, C.; Zhu, J.; et al. An epitaxial coating with preferred orientation stabilizing High-Energy Ni-Rich NCA cathodes. Appl. Surf. Sci. 2022, 579, 152183. [Google Scholar] [CrossRef]
- Lee, H.B.; Hoang, T.D.; Byeon, Y.S.; Jung, H.; Moon, J.; Park, M.S. Surface stabilization of Ni-rich layered cathode materials via surface engineering with LiTaO3 for lithium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Q.; Ren, Z.; Sun, C.; Zhang, J.; Wang, R.; Qian, M.; Shi, Q.; Shao, R.; Mu, D.; et al. Thermochemical cyclization constructs bridged dual-coating of Ni-rich layered oxide cathodes for high-energy Li-ion batteries. Nano Lett. 2022, 22, 5221–5229. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Kim, M.; An, H.; Min, K.; Park, K. A review of problems and solutions in Ni-rich cathode-based Li-ion batteries from two research aspects: Experimental studies and computational insights. J. Power Sources 2024, 597, 234132. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Ji, W.; Wu, Y.; Zhao, S.; Li, Y.; Zhou, N. Insights into the doping rules of heteroatom on Ni-rich ternary cathode stability by integrating high throughput calculation and machine learning. J. Energy Chem. 2025, 106, 161–172. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Wang, B.; Wang, J.; Wang, Y.; Meng, W.; Cai, F. A Study on the microstructure regulation effect of niobium doping on LiNi0.88Co0.05Mn0.07O2 and the electrochemical performance of the composite material under high voltage. Materials 2024, 17, 2127. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-T.; Park, N.-Y.; Noh, T.-C.; Namkoong, B.; Ryu, H.-H.; Shin, J.-Y.; Beierling, T.; Yoon, C.S.; Sun, Y.-K. High-performance Ni-rich Li[Ni0.9–xCo0.1Alx]O2 cathodes via multi-stage microstructural tailoring from hydroxide precursor to the lithiated oxide. Energy Environ. Sci. 2021, 14, 5084–5095. [Google Scholar] [CrossRef]
- Park, N.-Y.; Ryu, H.-H.; Park, G.-T.; Noh, T.-C.; Sun, Y.-K. Optimized Ni-rich NCMA cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 2003767. [Google Scholar] [CrossRef]
- Park, N.-Y.; Ryu, H.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. High-energy cathodes via precision microstructure tailoring for next-generation electric vehicles. ACS Energy Lett. 2021, 6, 4195–4202. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Yoon, D.R.; Kim, U.-H.; Yoon, C.S.; Sun, Y.-K. New class of Ni-rich cathode materials Li[NixCoyB1–x–y]O2 for next lithium batteries. Adv. Energy Mater. 2020, 10, 2000495. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Zhao, T.; Peng, J.; Zhang, B.; Xing, W.; Zuo, M.; Zhang, P.; Fan, W.; Lv, G.; et al. Precise regulation of particle orientation for Ni-rich cathodes with ultra-long cycle life. Nano Energy 2024, 129, 110008. [Google Scholar] [CrossRef]
- Meng, Z.; Ma, X.; Azhari, L.; Hou, J.; Wang, Y. Morphology controlled performance of ternary layered oxide cathodes. Commun. Mater. 2023, 4, 90. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Zhou, Y.; Hai, C.-X.; Zeng, J.-B.; Ren, X.-F.; Sun, Y.-X.; Shen, Y.; Li, X.; Dong, S.-D.; Zhang, G.-T. Improving electrochemical performance of NMC811 cathodes for lithium-ion batteries via consistently arranging the hexagonal nanosheets with exposed {104} facets. Ceram. Int. 2022, 48, 17279–17288. [Google Scholar] [CrossRef]
- Shang, M.; Han, E.; Tian, Y.; He, Y.; Sun, L.; Zhu, L. The effects of multiple metals (K, Cu, Al) substitution on LiNi0.66Co0.20Mn0.14O2 for lithium-ion batteries. Ionics 2020, 26, 2699–2713. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Kong, X.; Fedorovskaya, E.; Kallio, T. Extensive comparison of doping and coating strategies for Ni-rich positive electrode materials. J. Power Sources 2022, 540, 231633. [Google Scholar] [CrossRef]
- Darjazi, H.; Gonzalo, E.; Acebedo, B.; Cid, R.; Zarrabeitia, M.; Bonilla, F.; Munoz-Marquez, M.A.; Nobili, F. Improving high-voltage cycling performance of nickel-rich NMC layered oxide cathodes for rechargeable lithium–ion batteries by Mg and Zr co-doping. Mater. Today Sustain. 2022, 20, 100236. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, C.; Xiao, Z.; Xian, K.; Wen, H.; Lu, N.; He, X.; Ye, L.; Wang, J.; Ou, X.; et al. Synergistic doping chemistry enable the cycling properties of single-crystal Ni-rich cathode for lithium-ion batteries. Appl. Surface Sci. 2025, 684, 161839. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.; Chen, L.; Demir, M.; Cheng, Q.; Jiang, H. Limiting cationic mixing and lattice oxygen loss of single-crystalline Ni-rich Co-poor cathodes for high-voltage Li-ion batteries. Green Energy Environ. 2025. [Google Scholar] [CrossRef]
- Yao, W.; Liu, Y.; Li, D.; Zhang, Q.; Zhong, S.; Cheng, H.; Yan, Z. Synergistically enhanced electrochemical performance of Ni-rich cathode materials for lithium-ion batteries by K and Ti Co-modification. J. Phys. Chem. C 2020, 124, 2346–2356. [Google Scholar] [CrossRef]
- He, X.; Shen, J.; Zhang, B.; Xiao, Z.; Ye, L.; Mao, Q.; Zhong, Q.; Ou, X. Surface Li+/Ni2+ antisite defects construction for achieving high-voltage stable single-crystal Ni-rich cathode by anion/cation co-doping. Adv. Func. Mater. 2024, 34, 2401300. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Z.; Yang, C.; Shi, W.; Yang, Y.; Yin, C.; Yi, Y.; Cao, J. Aluminium–sodium targeted co-doping to boost the electrochemical stability of full concentration gradient Ni-rich LiNi0.80Co0.05Mn0.15O2 cathodes. J. Colloid Interface Sci. 2025, 683, 335–346. [Google Scholar] [CrossRef]
- Xiang, W.; Zhu, C.-Q.; Zhang, J.; Shi, H.; Liang, Y.T.; Yu, M.H.; Zhu, X.-M.; He, F.-R.; Lv, G.-P.; Guo, X.-D. Synergistic coupling effect of sodium and fluorine co-substitution on enhancing rate capability and cycling performance of Ni-rich cathode for lithium-ion battery. J. Alloys Compd. 2019, 786, 56–64. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Shi, H.; Wang, C.; Li, W.; Wang, Z.; Zhang, W.; Xiong, Z.; Gao, Y.; Yan, X. Synergistic effect of B-Nb co-modified to achieve crystal structure and interface tuning for Ni-rich cathode. Electrochem. Acta 2024, 474, 143475. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Deng, Q.; Li, W.; Zhang, Q.; Zhang, Z.; Chu, Y.; Yang, C. Stabilizing Ni-rich Single-crystalline LiNi0.83Co0.07Mn0.10O2 Cathodes using Ce/Gd Co-doped High-entropy Composite Surfaces. Angew. Chem. Int. Ed. 2024, 63, e202318042. [Google Scholar] [CrossRef]
- Park, J.; Yim, T. Boron- and calcium-interspersed cathode-electrolyte interphases on Ni-rich layered oxide cathodes for advanced performance lithium-ion batteries. Appl. Surface Sci. 2024, 663, 160195. [Google Scholar] [CrossRef]
- Usman, A.; Murtaza, G.; Ayyaz, A.; Al-Muhimeed, T.; Farid, G. Synthesis, characterization, and evaluation of improved electrochemical performance of vanadium and zinc co-doped Ni-rich oxide cathode materials: Experimental and first-principles study. Ionics 2024, 30, 5989–6001. [Google Scholar] [CrossRef]
- Wu, F.; Liu, N.; Chen, L.; Li, N.; Lu, Y.; Cao, D.; Xu, M.; Wang, Z.; Su, Y. A universal method for enhancing the structural stability of Ni-rich cathodes via the synergistic effect of dual-element cosubstitution. ACS Appl. Mater. Interfaces 2021, 13, 24925–24936. [Google Scholar] [CrossRef]
- Si, Z.; Shi, B.; Huang, J.; Yu, Y.; Han, Y.; Zhang, J.; Li, W. Titanium and fluorine synergetic modification improves the electrochemical performance of Li(Ni0.8Co0.1Mn0.1)O2. J. Mater. Chem. A 2021, 9, 9354–9363. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Q.; Sun, X.-G.; Li, C.; Huang, Y.; Mu, W.; Tan, J.; Li, J.; Liu, K.; Zheng, S.; et al. An epitaxial surface heterostructure anchoring approach for high-performance Ni-rich layered cathodes. J. Energy Chem. 2025, 105, 158–169. [Google Scholar] [CrossRef]
- Mao, D.; Tan, X.; Fan, Z.; Song, L.; Zhang, Y.; Zhang, P.; Su, S.; Liu, G.; Wang, H.; Chu, W. Unveiling the roles of trace Fe and F Co-doped into high-Ni Li-rich layered oxides in performance improvement. ACS Appl. Mater. Interfaces 2023, 15, 10774–10784. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Lu, S.; Jia, T.; Liu, Y.; Ding, W.; Yu, X.; Kang, F.; Zhang, J.; Cao, Y. Engineering of cobalt-free Ni-rich cathode material by dual-element modification to enable 4.5 V-class high-energy-density lithium-ion batteries. Chem. Eng. J. 2023, 455, 140652. [Google Scholar] [CrossRef]
- Park, H.G.; Min, K.; Park, K. A synergistic effect of Na+ and Al3+ Dual doping on electrochemical performance and structural stability of LiNi0.88Co0.08Mn0.04O2 cathodes for Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 5168–5176. [Google Scholar] [CrossRef]
- Peng, F.; Chu, Y.; Li, Y.; Pan, Q.; Yang, G.; Zhang, L.; Hu, S.; Zheng, F.; Wang, H.; Li, Q. Mg,Ti-base surface integrated layer and bulk doping to suppress lattice oxygen evolution of Ni-rich cathode material at a high cut-off voltage. J. Energy Chem. 2022, 71, 434–444. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Liu, J.D.; Xu, W.C.; Lu, Y.; Ma, H.; Cheng, F.Y.; Chen, J. Gradient doping Mg and Al to stabilize Ni-rich cathode materials for rechargeable lithium-ion batteries. J. Power Sources 2022, 535, 231445. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, L.; Sedlacik, M.; Saha, P.; Cheng, Q.; Yu, H.; Jiang, H. Enhanced Li-ion intercalation kinetics and lattice oxygen stability in single-crystalline Ni-rich Co-poor layered cathodes. J. Mater. Chem. A 2024, 12, 3682–3688. [Google Scholar] [CrossRef]
- Jamil, S.; Bin Yousaf, A.; Yoon, S.H.; Han, D.S.; Yang, L.; Kasak, P.; Wang, X. Dual cationic modified high Ni-low co layered oxide cathode with a heteroepitaxial interface for high energy-density lithium-ion batteries. Chem. Eng. J. 2021, 416, 129118. [Google Scholar] [CrossRef]
- Ziraki, S.; Kanani, M.; Hashemi, B.; Loghavi, M.M. Effect of sodium and yttrium co-doping on electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries: Computational and experimental investigation. J. Energy Storage 2023, 70, 107983. [Google Scholar] [CrossRef]
- Feng, H.; Xu, Y.; Zhou, Y.; Song, J.; Yang, J.; Tan, Q. The Y3+ and W6+ co-doping into Ni-rich Co-free single-crystal cathode LiNi0.9Mn0.1O2 for achieving high electrochemical properties in lithium-ion batteries. J. Alloys Compd. 2024, 976, 173043. [Google Scholar] [CrossRef]
- Zhang, B.; Wen, H.; Xian, K.; Lu, N.; Zheng, C.; Xiao, Z.; He, X.; Ye, L.; Wang, J.; Ming, L.; et al. Synergistically regulating the structural tolerance of Co-free Ni-rich cathode materials at high-rate through multi-heteroatoms substitution. J. Colloid Interface Sci. 2025, 682, 589–598. [Google Scholar] [CrossRef]
- Shen, J.; Li, H.; Qi, H.; Lin, Z.; Li, Z.; Zheng, C.; Du, W.; Chen, H.; Zhang, S. Enhancing thermodynamic stability of single-crystal Ni-rich cathode material via a synergistic dual-substitution strategy. J. Energy Chem. 2024, 88, 428–436. [Google Scholar] [CrossRef]
- Vanaphuti, P.; Bai, J.; Ma, L.; Ehrlich, S.; Kisslinger, K.; Wang, F.; Wang, Y. Unraveling Na and F coupling effects in stabilizing Li, Mn-rich layered oxide cathodes via local ordering modification. Energy Storage. Mater. 2020, 31, 459–469. [Google Scholar] [CrossRef]
- Ko, G.; Park, S.; Kim, W.; Kwon, K. Synergistic effect of Na and Al co-doping on the electrochemical properties of Li[Ni0.8Mn0.1Co0.1]O2 cathode materials for Li-ion batteries. J. Alloys Compd. 2022, 925, 166678. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Wang, F.; Wang, W.-N.; Liu, S.; Gao, X.-P. Enhancing structural rigidity of ultrahigh-Ni oxide through Al and Nb dual-bulk-doping for high-voltage lithium-ion batteries. Small Methods 2024, 8, 2400224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Park, N.-Y.; Park, G.-T.; Kim, U.-H.; Sohn, S.-J.; Kang, M.-S.; Ribas, R.M.; Monteiro, R.S.; Sun, Y.-K. Doping strategy in developing Ni-rich cathodes for high-performance lithium-ion batteries. ACS Energy Lett. 2024, 9, 740–747. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Huang, K.; Lian, C.; Su, H.; Liu, H. Al- and Nb-comodified Ni-rich NCM cathode for high-performance lithium-ion batteries. Ind. Eng. Chem. Res. 2024, 63, 2740–2749. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, U.-H.; Sun, Y.-K.; Yoon, C.-S. Multi-doped (Ga, B) Li[Ni0.885Co0.100Al0.015]O2 cathode. J. Electrochem. Soc. 2020, 167, 100557. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, S.; Li, G.; Zhou, Z.; Xu, N.; Wu, M.; Gao, X. Building the stable oxygen framework in high-Ni layered oxide cathode for high-energy-density Li-ion batteries. Energy Environ. Mater. 2022, 5, 1260–1269. [Google Scholar] [CrossRef]
- Feng, Z.; Rajagopalan, R.; Zhang, S.; Sun, D.; Tang, Y.-G.; Ren, Y.; Wang, H.-Y. A three in one strategy to achieve zirconium doping, boron doping, and interfacial coating for stable LiNi0.8Co0.1Mn0.1O2 cathode. Adv. Sci. 2020, 8, 2001809. [Google Scholar] [CrossRef]
- Choi, C.-M.; Park, J.-H.; Sun, Y.-K.; Yoon, C.-S. Ultra-stable cycling of multi-doped (Zr,B) Li[Ni0.885Co0.100Al0.015] O2 cathode. J. Power Sources 2021, 513, 230548. [Google Scholar] [CrossRef]
- Ming, Y.; Xiang, W.; Qiu, L.; Hua, W.-B.; Li, R.; Wu, Z.-G.; Xu, C.-L.; Li, Y.-C.; Wang, D.; Chen, Y.-X.; et al. Dual elements coupling effect induced modification from the surface into the bulk lattice for Ni-rich cathodes with suppressed capacity and voltage decay. ACS Appl. Mater. Interfaces 2020, 12, 8146–8156. [Google Scholar] [CrossRef]
- Cheng, X.; Zhong, F.; Wang, T.; Cao, X.; Liang, M.; Liu, Y.; Wu, B.; Li, J. The dual modification of La and Zr of Ni-rich cathodes for improved cycling stability at elevated voltage and temperature. J. Power Sources 2024, 612, 234801. [Google Scholar] [CrossRef]
- Kumar, D.; Ramesha, K. Comprehensive study of Ti and Ta co-doping in Ni-rich cathode material LiNi0.8Mn0.1Co0.1O2 towards improving the electrochemical performance. ChemPhysChem 2024, 25, e202400064. [Google Scholar] [CrossRef]
- Guan, S.; Tao, L.; Tang, P.; Fang, R.; Wu, H.; Piao, N.; Yang, H.; Hu, G.; Geng, X.; Li, L.; et al. Regulating interfacial chemistry and kinetic behaviors of F/Mo co-doping Ni-rich layered oxide cathode for long-cycling lithium-ion batteries over −20 °C–60 °C. J. Energy Chem. 2024, 94, 449–457. [Google Scholar] [CrossRef]
- Rambukwella, I.; Firestein, K.L.; Xu, Y.; Sun, Z.; Zhang, S.; Yan, C. Unveiling the effect of molybdenum and titanium co-doping on degradation and electrochemical performance in Ni-rich cathodes. Mater. Rep. Energy 2025, 5, 100314. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Chen, X.; Yang, Q.; Zhang, F.; Mu, D.; Li, L.; Wu, F.; Chen, R. Al & Ti Synergy enhancing ionic diffusion and stabilizing lattice oxygen for the high voltage single crystal Ni-rich layered oxide cathode materials. J. Power Sources 2024, 603, 234439. [Google Scholar]
- Han, E.; Du, X.; Yang, P.; Han, Y. The effects of copper and titanium co-substitution on LiNi0.6Co0.15Mn0.25O2 for lithium-ion batteries. Ionics 2018, 24, 393–401. [Google Scholar] [CrossRef]
- Zhu, F.; Shi, Y.; Hu, G.; Peng, Z.; Cao, Y.; Sun, Q.; Xue, Z.; Zhang, Y.; Du, K. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 via titanium and boron co-doping. Ceram. Int. 2021, 47, 3070–3078. [Google Scholar]
- He, Z.; Zhang, M.; Zhou, K.; Cheng, Y.; Luo, M.; Su, Y.; Hao, J.; Sun, Y.; Li, Y.; Yang, Y. Enabling excellent thermal stability of an ultrahigh nickel-rich cathode (LiNi0.90Co0.05Mn0.05O2) by a magnesium and titanium codoping strategy. ACS Appl. Energy Mater. 2023, 6, 3422–3431. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, F.; Zhang, Y. Multifunctional Ti-Mg co-doping strategy to enhance long-term cycling performance for Ni-rich cathode materials. J. Alloys Compd. 2024, 981, 173592. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Wang, Y.; Wan, T.; Guan, P.; Zhou, X.; Wang, X.; Chen, Y.; Shi, H.; Dou, A.; et al. Relieving stress concentration through anion–cation codoping toward highly stable nickel-rich cathode. ACS Nano 2023, 17, 20621–20633. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Qiu, Y.-G.; Cheng, F.-Y.; Wei, P.; Li, Y.-Y.; Liu, Y.; Sun, S.-X.; Xu, Y.; Li, Q.; Fang, C.; et al. Realization of a high-voltage and high-rate nickel-rich NCM cathode material for LIBs by Co and Ti dual modification. ACS Appl. Mater. Interfaces 2021, 13, 17707–17716. [Google Scholar] [CrossRef]
- Xiao, Z.M.; Zhang, B.; He, X.Y.; Ou, X. Cobalt/aluminum co-substitution in a LiNi0.9Mn0.1O2 layered cathode for improving kinetics. Chem. Commun. 2023, 59, 7935–7938. [Google Scholar] [CrossRef]
- Shi, H.; Zeng, T.; Zhang, H.; Zhou, Y.; Su, M.; Li, X.; Zhang, P.; Dou, A.; Naveed, A.; Liu, Y. Enhanced structure and electrochemical stability of single crystal nickel-rich cathode material by La2Li0.5Co0.5O4 surface coating. Ceram. Int. 2022, 48, 17548–17555. [Google Scholar] [CrossRef]
- Yoon, M.; Dong, Y.-H.; Hwang, J.; Sung, J.; Cha, H.; Ahn, K.; Huang, Y.; Kang, S.-J.; Cho, J. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 2021, 6, 362–371. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Shin, W.; Jo, E.; Manthiram, A. Insights into the microstructural engineering of cobalt-free, high-nickel cathodes based on surface energy for lithium-ion batteries. Adv. Energy Mater. 2023, 13, 2204054. [Google Scholar] [CrossRef]
- Zeng, C.; Zheng, R.; Fan, F.; Wang, X.; Tian, G.; Liu, S.; Liu, P.; Wang, C.; Wang, S.; Shu, C. Phase compatible surface engineering to boost the cycling stability of single-crystalline Ni-rich cathode for high energy density lithium-ion batteries. Energy Storage Mater. 2024, 72, 103788. [Google Scholar] [CrossRef]
- Kuo, L.-Y.; Roitzheim, C.; Valencia, H.; Mayer, J.; Möller, S.; Myung, S.-T.; Finsterbusch, M.; Guillon, O.; Fattakhova-Rohlfing, D.; Kaghazch, P. Doping-induced surface and grain boundary effects in Ni-rich layered cathode materials. Small 2024, 20, 2307678. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Zhang, C.-H.; Xin, S.; Shi, J.-L.; Wang, W.-P.; Fan, M.; Chang, Y.-X.; He, W.-H.; Wang, E.; Zou, Y.-G.; et al. Competitive doping chemistry for nickel-rich layered oxide cathode materials. Angew. Chem. Int. Ed. 2022, 61, e202116865. [Google Scholar] [CrossRef]
- Ou, X.; Liu, T.; Zhong, W.; Fan, X.; Guo, X.; Huang, X.; Cao, L.; Hu, J.; Zhang, B.; Chu, Y.S.; et al. Enabling high energy lithium metal batteries via single-crystal Ni-rich cathode material co-doping strategy. Nat. Commun. 2022, 13, 2319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, X.; Xiao, L.; Xiao, Z. Influence of Al3+ and PO43− co-doping on structure and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials. J. Solid State Electrochem. 2023, 27, 1185–1194. [Google Scholar] [CrossRef]
- Reissig, F.; Lange, M.A.; Haneke, L.; Placke, T.; Zeier, W.G.; Winter, M.; Schmuch, R.; Gomez-Martin, A. Synergistic effects of surface coating and bulk doping in Ni-rich lithium nickel cobalt manganese oxide cathode materials for high-energy lithium-ion batteries. ChemSusChem 2022, 15, e202102220. [Google Scholar] [CrossRef]
- Park, G.-T.; Yoon, J.I.; Kim, G.-H.; Park, N.-Y.; Park, B.-C.; Sun, Y.-K. Ni-rich cathode materials enabled by cracked-surface protection strategy for high-energy lithium batteries. Mater. Sci. Eng. R Rep. 2025, 164, 100945. [Google Scholar] [CrossRef]
- Gong, M.; Fu, Y.; Yao, W.; Rao, X.; Zhang, Q.; Zhong, S.; Sun, Q.; Cheng, H. Profiting the Co-modifications of Li2SnO3 coating and Sn4+ doping in Co-free Ni-rich cathode particles for lithium-ion batteries. ACS Appl. Energy Mater. 2023, 6, 1248–1258. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, G.; Wang, W.; Du, K.; Peng, Z.; Zeng, J.; Li, L.; Cao, Y. Enhancing the electrochemical performance of Co-less Ni-rich LiNi0.925Co0.03Mn0.045O2 cathode material via Co-modification with Li2B4O7 coating and B3+ doping. J. Power Sources 2022, 548, 232092. [Google Scholar] [CrossRef]
- Li, L.; Fu, L.; Li, M.; Wang, C.; Zhao, Z.; Xie, S.; Lin, H.; Wu, X.; Liu, H.; Zhang, L.; et al. B-doped and La4NiLiO8-coated LiNi0.825Co0.115Mn0.06O2 cathode with enhanced structural and interfacial stability for lithium-ion batteries. J. Energy Chem. 2022, 71, 588–594. [Google Scholar]
- Yang, H.; Wu, H.H.; Ge, M.; Li, L.; Yuan, Y.; Yao, Q.; Chen, J.; Xia, L.; Zheng, J.; Chen, Z.; et al. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv. Funct. Mater. 2019, 29, 1808825. [Google Scholar] [CrossRef]
- Sattar, T.; Sim, S.-J.; Doo, S.-G.; Jin, B.-S.; Kim, H.-S. A synergetic modification approach toward high-capacity Ni-rich cathode materials for next generation lithium-ion batteries. Solid State Ion. 2022, 387, 116053. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, B.; He, X.; Xiao, B.; Xiao, Z.; Li, X.; Ou, X. Synergistic regulation of kinetic reaction pathway and surface structure degradation in single-crystal high-nickel cathodes. J. Colloid Interface Sci. 2023, 629, 388–398. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.-F.; Lu, S.-R.; Ding, W.-B.; Yu, X.-L.; Liang, G.-M.; Zou, J.-S.; Kang, F.-Y.; Zhang, J.-J.; Cao, Y.-D. B2O3/LiBO2 dual-modification layer stabilized Ni-rich cathode for lithium-ion battery. J. Power Sources 2022, 536, 231510. [Google Scholar] [CrossRef]
- Su, Y.; Li, L.; Chen, L.; Wang, L.; Lu, Y.; Zhang, Q.; Bao, L.; Wu, F. Enhanced electrochemical performance of Ni-rich cathode materials with an in situ-formed LiBO2/B2O3 hybrid coating layer. ACS Appl. Energy Mater. 2022, 5, 2231–2241. [Google Scholar] [CrossRef]
- Xu, M.; Lu, J.; Sun, Z.; Yang, M.; Sheng, B.; Chen, M.; Chen, J.; Zhang, Q.; Han, X. Lanthanum doping and surface Li3BO3 passivating layer enabling 4.8 V nickel-rich layered oxide cathodes toward high energy lithium-ion batteries. J. Colloid Interface Sci. 2024, 673, 386–394. [Google Scholar] [CrossRef]
- Tang, W.; Shu, Z.; Li, A.; Huang, X.; Li, W. Enhancing structural integrity and long-term cycling stability of high-voltage single-crystalline Ni-rich cathodes via surface/subsurface dual-functional modification engineering. Energy Storage Mater. 2025, 77, 104185. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Ou, X.; Wang, C.; Peng, C.; Zhang, B. Enhancing high-voltage performance of Ni-rich cathode by surface modification of self-assembled NASICON fast ionic conductor LiZr2(PO4)3. ACS Appl. Mater. Interfaces 2019, 11, 15507–15516. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, H.; Zong, Y.; Zuba, M.; Chen, Y.; Chernova, N.A.; Bai, J.; Pei, B.; Goel, A.; Rana, J.; et al. What is the role of Nb in nickel-rich layered oxide cathodes for lithium-ion batteries? ACS Energy Lett. 2021, 6, 1377–1382. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, H.; Chen, X.; Zuba, M.; Chernova, N.; Zhou, G.; Whittingham, M. Li–Nb–O coating/substitution enhances the electrochemical performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) cathode. ACS Appl. Mater. Interfaces 2019, 11, 34889–34894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, M.; Zhang, D.; Yan, Y.; Wang, Y.; Li, J.; Li, Z. Enhanced electrochemical properties of NMC811 cathode material due to synergistic modification with Sm as doping and coating agent. J. Alloys Compd. 2022, 909, 164712. [Google Scholar] [CrossRef]
- Kim, Y. Thermochemical investigation of Zr doping in LiNi8/12Co2/12Mn2/12O2 based on phase equilibria simulation. Int. J. Quant. Chem. 2019, 119, e26028. [Google Scholar] [CrossRef]
- Tan, X.; Peng, W.; Wang, M.; Luo, G.; Wang, Z.; Yan, G.; Guo, H.; Li, Q.; Wang, J. Al, Zr dual-doped cobalt-free nickel-rich cathode materials for lithium-ion batteries. Prog. Nat. Sci. Mater. Int. 2023, 33, 108–115. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Zhao, H.; Bai, Y. Ultra-high temperature operated Ni-rich cathode stabilized by thermal barrier for high-energy lithium-ion batteries. Adv. Mater. 2024, 36, 2313500. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Y.; Hu, G.; Yang, C.; Liu, F.; Yin, C.; Cao, J.; Chen, Z. Enhancing the cycling stability of full-concentration-gradient Ni-rich layered cathodes via in-situ Zr doping. Chem. Eng. J. 2024, 493, 152872. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Gao, M.-Y.; Liu, S.; Li, G.-R.; Gao, X.-P. Yttrium surface gradient doping for enhancing structure and thermal stability of high-Ni layered oxide as cathode for Li–ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 7343–7354. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, G.; Wang, W.; Zhao, B.; Li, H.; Peng, Z.; Du, K.; Cao, Y. Ni-rich Co-less cathode materials with LiYO2 and Y3+ modification toward improved storage performance against ambient air for lithium-ion batteries. ACS Sustain. Chem. Eng. 2024, 12, 24–36. [Google Scholar] [CrossRef]
- Bizzotto, F.; Dachraoui, W.; Grissa, R.; Zhao, W.; Pagani, F.; Querel, E.; Khünel, R.-S.; Battaglia, C. Modification of NMC811 with titanium for enhanced cycling and high-voltage stability. Electrochim. Acta 2023, 462, 142758. [Google Scholar] [CrossRef]
- Fan, Q.; Lin, K.; Yang, S.; Guan, S.; Chen, J.; Feng, S.; Liu, J.; Liu, L.; Li, J.; Shi, Z. Constructing effective TiO2 nano-coating for high-voltage Ni-rich cathode materials for lithium-ion batteries by precise kinetic control. J. Power Sources 2020, 477, 228745. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Z.; Huang, K.; Jiang, J.; Chen, Z.; Qi, X.; Dou, H.; Zhang, X. In situ tuning residual lithium compounds and constructing TiO2 coating for surface modification of a nickel-rich cathode toward high-energy lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 12423–12432. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, W.; Zhao, G.; Liu, Q.; Duan, L.; Wang, S.; An, Q.; Wang, H.; Yang, Y.; Zhang, C.; et al. LiNi0.9Co0.09Mo0.01O2 cathode with Li3PO4 coating and Ti doping for next-generation lithium-ion batteries. ACS Energy Lett. 2023, 8, 1629–1638. [Google Scholar] [CrossRef]
- Hou, Y.; Ren, Y.; Shi, T.; Li, J.; Li, H.; Zhang, L.; Liu, Y.; Wan, Y.; Zhang, S.; Liu, Y.; et al. The surface Al2O3 coating and bulk Zr doping drastically improve the voltage fade and cycling stability of Li(Ni0.8Mn0.1Co0.1)O2 cathode materials. J. Alloys Compd. 2023, 939, 168778. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, X.; Wang, S.; Zhang, P.; Zu, W.; Liu, X.; Feng, G.; Zhang, B.; Xing, W.; Zuo, M.; et al. High-performance single crystal Ni-rich cathode with regulated lattice and interface constructed by separated lithiation and crystallization calcination. Green Chem. Eng. 2024. [CrossRef]
- Shi, H.; Zheng, J.; Wan, T.; Wang, H.; Wen, Z.; Zheng, F.; Su, M.; Dou, A.; Zhou, Y.; Naveed, A.; et al. Constructing electrochemically stable single crystal Ni-rich cathode material via modification with high valence metal oxides. J. Energy Chem. 2025, 101, 392–401. [Google Scholar] [CrossRef]
- Song, W.; Gao, A.; Liu, Y.; Liu, A.; Peng, H.; Li, M.; Yang, R.; Wang, F. Tungsten-doped Ni-rich LiNi0.8Mn0.18Al0.02O2 cathode with fast kinetics and stable surface structure. J. Alloys Compd. 2025, 1010, 177611. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, C.; Song, L.; Cao, Z.; Jiang, P. Dual-modification of WO3-coating and Mg-doping on LiNi0.8Co0.1Mn0.1O2 cathodes for enhanced electrochemical performance at high voltage. Ionics 2021, 27, 1909–1917. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.-L.; Yi, Z.-C.; Liu, C.-J.; Miao, C.; Xin, Y.; Nie, S.-Q. Dual modification of LiNi0.83Co0.11Mn0.06O2 cathode materials by K+ doping and Li3PO4 coating for lithium ions batteries. Rare Met. 2024, 43, 3007–3018. [Google Scholar] [CrossRef]
- Shen, L.; Gu, Y.; Xu, T.; Zhou, Q.; Peng, P.; Chan, Y.; Du, F.; Zheng, J. Dual modification of phosphate toward improving electrochemical performance of LiNiO2 cathode materials. J. Colloid Interface Sci. 2024, 662, 505–515. [Google Scholar] [CrossRef]
- Liu, C.; Yi, Z.; Wan, J.; Miao, C.; Wang, Z.; Wang, J.; Xiao, W. Nb5+ ions doping and Li3PO4 artificial layer synergetic strategy to strengthen structure, stabilize interface, and promote ions migration for high-performance Ni-rich cathode. Chem. Eng. J. 2025, 503, 158633. [Google Scholar] [CrossRef]
- Lin, H.; Shen, Y.; Wei, L.; Song, R.; Wu, F.; Wei, P.; Yang, Z.; Ren, Y.; Qu, K.; Ding, Z. Enhancing structural stability and electrochemical performance of ultra-high Ni-rich Co-free cathode via MgHPO4 dual-functional modification. Chem. Res. Chin. Univ. 2025, 41, 333–342. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Z.; Lin, F.; Zhu, H.; Wen, J.; Wang, Y.; Bai, M.; Duan, J. Synergistic role of Sb doping and surface modification in high performance ultrahigh-nickel layered oxides cathode materials. J. Alloys Compd. 2023, 959, 170552. [Google Scholar] [CrossRef]
- Sun, G.; Zhuang, S.; Jiang, S.; Ren, Y.; Sun, Y.; Pan, X.; Wen, Y.; Li, X.; Tu, F. Elevating cycle stability of Ni-rich NMC811 cathode via single-crystallization integrating dual-modification strategy for lithium-ion batteries. J. Alloys Compd. 2024, 976, 173097. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, H.; Jiang, H.; Su, X.; Hu, Y.; Jiang, H.; Li, C. Restraining the escape of lattice oxygen enables superior cyclic performance towards high-voltage Ni-rich cathodes. Natl. Sci. Rev. 2023, 10, nwac166. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Lv, K.; Liu, J.; Bayaguud, A.; Li, X. Surface-to-bulk synergistic modification enables stable interface of Ni-rich oxide cathode for all-solid-state lithium batteries. Electrochim. Acta 2024, 497, 144595. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Shan, L.; Jiang, G.; Zhang, Y.; Meng, Q.; Dong, P. Improved electrochemical performance of Co-free Ni-rich cathode material for lithium-ion battery through multi-element composite modification. Electrochim. Acta 2024, 477, 143804. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Li, Z.; Wu, L.; Chen, J.; Liu, X.; Hu, H.; Ding, H.; Cao, S.; Wei, Q.; et al. Improving structure stability of single-crystalline Ni-rich cathode at high voltage by element gradient doping and interfacial modification. J. Energy Chem. 2025, 101, 630–640. [Google Scholar] [CrossRef]
- Wang, H.; Dong, J.; Zhang, H.; Liu, J.; Lu, Y.; Liu, Y.; Wang, X.; Li, N.; Huang, Q.; Wu, F.; et al. Enhancing structural and thermal stability of ultrahigh-Ni cathodes via anion-cation codoping induced surface reconstruction strategy. J. Energy Chem. 2025, 106, 9–19. [Google Scholar] [CrossRef]
- Chen, S.; He, T.; Su, Y.; Lu, Y.; Chen, L.; Zhang, Q.; Wang, J.; Chen, R.; Wu, F. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high-performance cathode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Zhang, J.; Liu, R.; Wang, Y.; Xiao, H.; Huang, Y.; Yuan, G. Improving electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode for Li-ion batteries by dual-conductive coating layer of PPy and LiAlO2. J. Alloys Compd. 2020, 848, 156387. [Google Scholar] [CrossRef]
- Yang, H.; Wu, K.; Hu, G.; Peng, Z.; Cao, Y.; Du, K. Design and synthesis of double-functional polymer composite layer coating to enhance the electrochemical performance of the Ni-rich cathode at the upper cutoff voltage. ACS Appl. Mater. Interfaces 2019, 11, 8556–8566. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, X.; Lin, J.; Huang, Y.; Cheng, W.; Liu, Q.; Zhang, H.; Ren, F.; Huang, Y.; Liu, Z.; et al. Restraining planar gliding in single-crystalline LiNi0.9Co0.05Mn0.05O2 cathodes by combining bulk and surface modification strategies. Angew. Chem. Inter. Ed. 2025, 64, e202419903. [Google Scholar] [CrossRef]
- Cheng, W.-D.; Li, L.; Hao, S.-L.; Wu, Y.-X.; Huo, J.-S.; Ji, Y.-Y.; Liu, X.-Q. Face-lifting the surface of LiNi0.8Co0.15Al0.05O2 cathode via Y(PO3)3 to form an in situ triple composite Li-ion conductor coating layer with the enhanced electrochemical performance. Nanotechnology 2022, 33, 375701. [Google Scholar] [CrossRef]
- Li, Q.; Dang, R.; Chen, M.; Lee, Y.; Hu, Z.-B.; Xiao, X.-L. A synthesis method for long cycle life lithium-ion cathode material: Ni-rich core-shell LiNi0.8Co0.1Mn0.1O2. ACS Appl. Mater. Interfaces 2018, 10, 17850–17860. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-H.; Park, N.-Y.; Noh, T.-C.; Kang, G.-C.; Maglia, F.; Kim, S.-J.; Yoon, C.-S.; Sun, Y.-K. Microstrain alleviation in high-energy Ni-rich NCMA cathode for long battery life. ACS Energy Lett. 2021, 6, 216–223. [Google Scholar] [CrossRef]
- Bi, Y.-J.; Liu, M.; Xiao, B.-W.; Jiang, Y.; Lin, H.; Zhang, Z.-G.; Chen, G.-X.; Sun, Q.; He, H.-Y.; Huang, F.; et al. Highly stable Ni-rich layered oxide cathode enabled by a thick protective layer with bio-tissue structure. Energy Storage Mater. 2020, 24, 291–296. [Google Scholar] [CrossRef]
- Maeng, S.; Chung, Y.; Min, S.; Shin, Y. Enhanced mechanical strength and electrochemical performance of core–shell structured high–nickel cathode material. J. Power Sources 2020, 448, 227395. [Google Scholar] [CrossRef]
- Park, G.-T.; Sun, H.H.; Noh, T.-C.; Maglia, F.; Kim, S.-J.; Lamp, P.; Sun, Y.-K. Nanostructured Co-free layered oxide cathode that affords fast-charging lithium-ion batteries for electric vehicles. Adv. Energy Mater. 2022, 12, 2202719. [Google Scholar] [CrossRef]
- Rathore, D.; Garayt, M.; Liu, Y.-L.; Geng, C.-X.; Johnson, M.; Dahn, J.-R.; Yang, C.-Y. Preventing interdiffusion during synthesis of Ni-rich core-shell cathode materials. ACS Energy Lett. 2022, 7, 2189–2195. [Google Scholar] [CrossRef]
- Bae, J.H.; Hwang, K.; Kim, J.; Kang, H.; Lee, I.; Park, C.W.; Sohn, H.; Yoon, S. Enhanced electrochemical performance of Ni-rich cathode for lithium-ion batteries under elevated cut-off voltage (4.5 V): Elongated cycle stability of cathode by relaxed mechanical stress in the presence of internal porous layer. J. Electroanal. Chem. 2024, 957, 118123. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Hou, P.; Zhou, E.; Guo, J.; Zhang, J.; Wang, D.; Guo, F.; Song, D.; Shi, X.; et al. Core–shell structured Li[(Ni0.8Co0.1Mn0.1)0.7(Ni0.45Co0.1Mn0.45)0.3]O2 cathode material for high-energy lithium ion batteries. J. Alloys Compd. 2024, 587, 710–716. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, A.; Wang, K.; Xiao, Z.; Mao, Q.; Lu, X.; Wang, G.; Lu, C.; Zhang, J.; Huang, H.; et al. Binary-compositional core-shell structure Ni-rich cathode material with radially oriented primary particles in shell for long cycling lifespan lithium-ion batteries. Mater. Today Energy 2023, 34, 101292. [Google Scholar] [CrossRef]
- Ye, T.; Li, Z.M.; Yan, H.L.; Ha, Y.; Zhang, D.Y.; Zhou, C.Y.; Wang, L.J.; Li, L.; Xiang, Q.X. Magnetic frustration effect on the rate performance of LiNi0.6Co0.4-xMnxO2 cathodes for lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2201556. [Google Scholar] [CrossRef]
- Han, S.-M.; Park, G.-T.; Kim, D.-H.; Seo, M.-G.; Park, N.-Y.; Sun, Y.-K. Introducing Co nanoshells onto Ni-rich cathode materials for high long-life Li-ion batteries. ACS Energy Lett. 2024, 9, 5859–5868. [Google Scholar] [CrossRef]
- Xue, H.; Liang, Y.; Huang, Y.; Ji, Y.; Xu, Z.; Chen, X.; Wang, H.; Liu, J.; Amine, K.; Liu, T.; et al. In situ conversion of artificial proton-rich shell to inorganic maskant toward stable single-crystal Ni-rich cathode. Adv. Mater. 2025, 37, 2415860. [Google Scholar] [CrossRef]
- Song, G.; Zhong, H.; Dai, Y.; Zhou, X.; Yang, J. WO3 membrane-encapsulated layered LiNi0.6Co0.2Mn0.2O2 cathode material for advanced Li-ion batteries. Ceram. Int. 2019, 45, 6774–6781. [Google Scholar] [CrossRef]
- Liu, J.-K.; Yang, X.-R.; Wang, C.-W.; Yin, Z.-W.; Hu, Y.-Y.; Deng, L.; Wang, Z.; Zhou, Y.; Li, J.-T. Surface-to-bulk engineering with high-valence W6+ enabling stabilized single-crystal LiNi0.9Co0.05Mn0.05O2 cathode. J. Energy Chem. 2024, 98, 67–76. [Google Scholar] [CrossRef]
- Chu, B.; Xu, R.; Li, G.; Chen, J.; Xu, Z.; Huang, T.; Wang, B.; Yu, A. Simultaneous B/W dual coating on ultra-high nickel single crystal cathode material for lithium-ion batteries. J. Power Sources 2023, 577, 233260. [Google Scholar] [CrossRef]
- Du, F.; Qu, H.; Yu, X.; Ding, L.; Zhang, P.; Wang, Y.; Chen, Y.; Wang, Y.; Zhang, X.; Zheng, J. Pre-introducing Li4NiWO6 defect phase by tungsten modification enables highly stabilized Ni-rich cathode. Chem. Eng. J. 2024, 492, 152357. [Google Scholar] [CrossRef]
- Ding, H.; Wang, X.; Zhao, J.; Wang, X.; Zhang, H.; Liu, G.; Yu, W.; Dong, X.; Wang, J. Construction of hollow multi-shell structural NCM622 as cathode to improve the cycling stability of its Li-ion batteries. J. Energy Storage 2025, 107, 114835. [Google Scholar] [CrossRef]
- Yoon, C.-S.; Sun, Y.K.; Kim, U.-H.; Park, K.-J.; Ryu, H.-H.; Kim, H.-S.; Sun, Y.-K. Microstructure evolution of concentration gradient Li[Ni0.75Co0.10Mn0.15]O2 cathode for lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1802090. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.-Y.; Li, Q.; Yang, Y.-Q.; Deng, X.; Dang, R.-B.; Wu, M.-M.; Wu, Z.-J.; Xiao, X.-L.; Yu, X.-Q. In situ synthesis of nickel concentration gradient structure promising superior electrochemical properties of Ni-rich LiNi0.8Co0.15Al0.05O2 at high cut-off voltage. Nanoscale 2020, 12, 11182–11191. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.-W.; Chen, X.; Niu, J.-J. Nickel-rich layered LiNi0.8Mn0.1Co0.1O2 with dual gradients on both primary and secondary particles in lithium-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100767. [Google Scholar] [CrossRef]
- Yerkinbekova, Y.; Kumarov, A.; Tatykayev, B.; Mentbayeva, A.; Repo, E.; Laasko, E. Ni-rich cathode materials with concentration gradients for high-energy and safe lithium-ion batteries: A comprehensive review. J. Power Sources 2025, 626, 235686. [Google Scholar] [CrossRef]
- Noh, H.-J.; Chen, Z.-H.; Yoon, C.-S.; Lu, J.; Amine, K.; Sun, Y.-K. Cathode material with nanorod structure—An application for advanced high-energy and safe lithium batteries. Chem. Mater. 2013, 25, 2109–2115. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Liu, X.; Feng, G.; Wang, S.; Zhang, B.; Zhang, P.; Zuo, M.; Xing, W.; Fan, W.; et al. Surface reactivity versus microcracks in Ni-rich layered oxide cathodes: Which is critical for long cycle life? Chem. Eng. J. 2024, 488, 150795. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Y.; Hai, C.; Zeng, J.; Sun, Y.; Ren, X.; Shen, Y.; Li, X.; Zhang, G. Analysis of the growth mechanism of hierarchical structure Ni0.8Co0.1Mn0.1(OH)2 agglomerates as precursors of LiNi0.8Co0.1Mn0.1O2 in the presence of aqueous ammonia. Appl. Surf. Sci. 2023, 619, 156379. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Y.; Zeng, J.; Hai, C.; Sun, Y.; Ren, X.; Shen, Y.; Li, X. Investigating the effect of pH on the growth of coprecipitated Ni0.8Co0.1Mn0.1(OH)2 agglomerates as precursors of cathode materials for Li-ion batteries. Ceram. Int. 2023, 49, 15851–15864. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Lin, L.; Ai, X.; Gui, S.; Guo, W.; Li, S.; Wang, L.; Yang, H.; Peng, D.-L.; et al. Intrinsic highly conductive and mechanically robust Li-rich cathode materials enabled by microstructure engineering for enhanced electrochemical properties. Adv. Funct. Mater. 2023, 34, 2308494. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Z.; Yu, H.; Demir, M.; Cheng, Q.; Jiang, H. Robust concentration gradient Co-free Ni-rich cathodes enable long-life and safe operations in high-voltage Li-ion batteries. Energy Fuels 2025, 39, 4507–4514. [Google Scholar] [CrossRef]
- He, X.; Su, S.; Zhang, B.; Xiao, Z.; Zhang, Z.; Ou, X. Alleviating the anisotropic microstructural change and boosting the lithium ions diffusion by grain orientation regulation for Ni-rich cathode materials. J. Energy Chem. 2024, 88, 213–222. [Google Scholar] [CrossRef]
- Park, N.Y.; Cho, G.; Kim, S.B.; Sun, Y.K. Multifunctional doping strategy to develop high-performance Ni-rich cathode material. Adv. Energy Mater. 2023, 13, 2204291. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Hai, C.; Ren, X.; Zeng, J.; Shen, Y.; Zhou, Y. Anionic-surfactant-assisted synthesis of Ni0.8Co0.1Mn0.1(OH)2 as precursors of high-performance cathode materials for lithium-ion batteries. J. Power Sources 2024, 615, 235092. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Dai, Z.; Feng, S.; Zheng, M.; Zheng, C.; Jin, J.; Wu, M.; Wu, X.; Lu, J.; et al. Surface gradient Ni-rich cathode for Li-ion batteries. Adv. Mater. 2024, 36, 2401052. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Du, C.Y.; Xu, X.; He, X.; Yin, G.; Ma, Y.; Zuo, P.; Cheng, X.; Gao, Y. Lithium phosphorus oxynitride coated concentration gradient Li[Ni0.73Co0.12Mn0.15]O2 cathode material with enhanced electrochemical properties. Electrochim. Acta 2016, 192, 340–345. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, S.; Zhou, Y.; Tian, X. Enhancing the electrochemical performance of single-crystal LiNi0.8Co0.1Mn0.1O2 cathode material by phosphorus doping. Chem. Eng. Sci. 2024, 285, 119627. [Google Scholar] [CrossRef]
- Choi, J.H.; Hwang, J.; Embleton, T.J.; Ko, K.; Jo, M.; Lee, C.; Yun, J.; Park, S.; Son, Y.; Oh, P. Selective outer surface modification of polycrystalline Ni-rich cathode for sulfide all-solid-state lithium-ion battery. Korean J. Chem. Eng. 2023, 40, 548–554. [Google Scholar] [CrossRef]
- Park, G.-T.; Ryu, H.-H.; Noh, T.-C.; Kang, G.-C.; Sun, Y.-K. Microstructure-optimized concentration-gradient NCM cathode for long-life Li-ion batteries. Mater. Today 2022, 52, 9–18. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Zhang, X.; Lu, Y.; Wang, B.; Deng, Q.; Yang, Y.; Wang, E.; Lyu, Z.; Li, Y.; et al. Full concentration gradient-tailored Li-rich layered oxides for high-energy lithium-ion batteries. Adv. Mater. 2021, 33, 2001358. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Xue, B.; Wu, X.; Li, L.; Guo, Y.; Zhang, L. Synergistic modification of Ni-rich full concentration gradient materials with enhanced thermal stability. Chem. Eng. J. 2023, 451, 138518. [Google Scholar] [CrossRef]
- Sun, H.-H.; Weeks, J.A.; Heller, A.; Mullins, C.B. Nanorod gradient cathode: Preventing electrolyte penetration into cathode particles. ACS Appl. Energy Mater. 2019, 2, 6002–6011. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, J.; Liu, Z.; Wang, W.; Zhang, Z.; Tang, Y.; Zhang, Z.; Yang, T.; Wang, X.; Li, X.; et al. Achieving long-life Ni-rich cathodes with improved mechanical-chemical properties via concentration gradient structure. Adv. Func. Mater. 2024, 34, 2400956. [Google Scholar] [CrossRef]
- Song, L.; Xiao, M.; Du, J.; Li, L.; Zhao, T.; Xia, Y.; Xiang, Y.; Chen, T.; Jiang, L.; Xiao, Z.; et al. An effective gradient coating strategy to improve the structure stability of Ni-rich nickel-cobalt-manganese ternary layer oxide cathode. J. Energy Storage 2024, 98, 113123. [Google Scholar] [CrossRef]
- Sitnikova, L.A.; Savina, A.; Morozov, A.V.; Golubnichiy, A.A.; Dolzhikova, E.A.; Moiseev, I.A.; Luchkin, S.Y.; Abakumov, A.M. Improving electrochemical performance of Ni-rich layered cathode material with combining Co-enriched compositional gradient and radial microstructure. J. Power Sources 2024, 602, 234302. [Google Scholar] [CrossRef]
- Guo, W.; Yu, H.; Wang, M.; Wu, M.; Chen, L.; Jiang, H.; Li, C. Compositional gradient design of Ni-rich Co-poor cathodes enhanced cyclability and safety in high-voltage Li-ion batteries. ACS Nano 2025, 19, 8371–8380. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, J.; Zhang, J.; Cheng, F.; Chen, J. LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries. J. Mater. Chem. A 2019, 7, 20958–20964. [Google Scholar] [CrossRef]
- Tao, J.; Mu, A.; Geng, S.; Xiao, H.; Zhang, L.; Huang, Q. Influences of direction and magnitude of Mg2+ doping concentration gradient on the performance of full concentration gradient cathode material. J. Solid State Electrochem. 2021, 25, 1959–1974. [Google Scholar] [CrossRef]
- Mu, Y.; Chen, X.; Ming, H.; Zhang, S.; Zhu, X.; Qiu, J. Oriented gradient doping of zirconium in Ni-rich cathode to achieve ultrahigh stability and rate capability. ACS Appl. Mater. Interfaces 2023, 15, 49289–49298. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, J.; Liu, J.; Ma, H.; Cheng, F. Enhancing LiNiO2 cathode materials by concentration-gradient yttrium modification for rechargeable lithium-ion batteries. J. Energy Chem. 2021, 63, 312–319. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Yang, C.; Xu, D.; Zhu, Y.-H.; Zhou, T.; Xin, S.; You, Y. Concentration-gradient Nb-doping in a single-crystal LiNi0.83Co0.12Mn0.05O2 cathode for high-rate and long-cycle lithium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 18828–18835. [Google Scholar] [CrossRef]
- Yang, S.; Yang, K.; Mi, J.; Guo, S.; An, X.; Su, H.; He, Y. Electrolyte engineering and interphase chemistry toward high-performance nickel-rich cathodes: Progress and perspectives. Mater. Rep. Energy 2025, 5, 100317. [Google Scholar] [CrossRef]
- Maleki Kheimeh Sari, H.; Li, X. Controllable cathode–electrolyte interface of Li[Ni0.8Co0.1Mn0.1]O2 for lithium ion batteries: A review. Adv. Energy Mater. 2019, 9, 1901597. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Wang, Z.; Li, X.; Liu, J.; Bayaguud, A.; Zhang, L. Interface problems, modification strategies and prospects of Ni–rich layered oxide cathode materials in all–solid–state lithium batteries with sulfide electrolytes. J. Power Sources 2023, 571, 233079. [Google Scholar] [CrossRef]
- Wu, J.H.; Shen, L.; Zhang, Z.H.; Liu, G.Z.; Wang, Z.Y.; Zhou, D.; Wan, H.L.; Xu, X.X.; Yao, X.Y. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes. Electro. Energy. Rev. 2020, 4, 411. [Google Scholar] [CrossRef]
- Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. Energy 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Walther, F.; Strauss, F.; Wu, X.; Mogwitz, B.; Hertle, J.; Sann, J.; Rohnke, M.; Brezesinski, T.; Janek, J. The working principle of a Li2CO3/LiNbO3 coating on NCM for thiophosphate-based all-solid-state batteries. Chem. Mater. 2021, 33, 2110–2125. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Han, J.; Wen, K.; Guan, S.; Xue, C.; Zhang, Z.; Xu, B.; Lin, Y.; Shen, Y.; et al. Super long-cycling all-solid-state battery with thin Li6PS5Cl-based electrolyte. Adv. Energy Mater. 2022, 12, 2200660. [Google Scholar] [CrossRef]
- Lee, J.Y.; Noh, S.; Seong, J.Y.; Lee, S.; Park, Y.J. Suppressing unfavorable interfacial reactions using polyanionic oxides as efficient buffer layers: Low-cost Li3PO4 coatings for sulfide-electrolyte-based all-solid-state batteries. ACS Appl. Mater. Interfaces 2023, 15, 12998–13011. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, L.; Zhang, K.; Zhang, Z.; Zhang, L.; Li, Z.; Kong, T.; Xie, Y.; Wang, Y. High-performance sulfide all-solid-state batteries enabled by high-voltage Ni-rich cathode with a conformal and conductive protective layer. ACS Appl. Energy Mater. 2024, 7, 2524–2532. [Google Scholar] [CrossRef]
- Lin, C.; Liu, Y.; Su, H.; Zhong, Y.; Wang, X.; Gu, C.; Tu, J. Elevating cycle stability and reaction kinetics in Ni-rich cathodes through tailored bulk and interface chemistry for sulfide-based all-solid-state lithium batteries. Adv. Funct. Mater. 2024, 34, 2311564. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Yan, H.; Zhao, J.; Cheng, Y.; Luo, Y.; Gu, J.; Zhong, H.; Fu, A.; Wang, K.; et al. Assembly of an elastic & sticky interfacial layer for sulfide-based all-solid-state batteries. Nano Energy 2023, 113, 108572. [Google Scholar]
- Kim, J.S.; Jung, S.; Kwak, H.; Han, Y.; Kim, S.; Lim, J.; Lee, Y.M.; Jung, Y.S. Synergistic halide-sulfide hybrid solid electrolytes for Ni-rich cathodes design guided by digital twin for all-solid-State Li batteries. Energy Storage Mater. 2023, 55, 193–204. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zeng, Z.; Xu, X.; Cheng, J.; Zhang, H.; Li, J.; Rao, Y.; Deng, Y.; Ci, L.; et al. Surface to bulk design empowering Ni-rich layered oxide cathode in sulfide-based All-Solid-State batteries. Chem. Eng. J. 2024, 498, 155029. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wu, D.; Niu, Q.; Lu, P.; Ma, T.; Su, Y.; Chen, L.; Li, H.; Wu, F. Stable Ni-rich layered oxide cathode for sulfide-based all-solid-state lithium battery. eScience 2022, 2, 537–545. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Ma, Y.; Goonetilleke, D.; Sann, J.; Walther, F.; Bianchini, M.; Janek, J.; Brezesinski, T. High performance all-solid-state batteries with a Ni-rich NCM cathode coated by atomic layer deposition and lithium thiophosphate solid electrolyte. ACS Appl. Energy Mater. 2021, 4, 7338–7345. [Google Scholar] [CrossRef]
- Gao, X.; Liu, B.; Hu, B.; Ning, Z.; Jolly, D.S.; Zhang, S.; Perera, J.; Bu, J.; Liu, J.; Doerrer, C.; et al. Solid-state lithium battery cathodes operating at low pressures. Joule 2022, 6, 636–646. [Google Scholar] [CrossRef]
- Zheng, H.; Peng, S.; Liang, S.; Yang, W.; Chen, C.; Wang, C.; Yu, R. Progress and challenges of Ni-rich layered cathodes for all-solid-state lithium batteries. Adv. Funct. Mater. 2025, 35, 2418274. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Ku, M.; Park, J.; Lee, J.; Kim, Y.-B. Coating robust layers on Ni-rich cathode active materials while suppressing cation mixing for all-solid-state lithium-ion batteries. ACS Nano 2024, 18, 25096–25106. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Ren, F.; Karger, L.; Dreyer, S.L.; Lin, J.; Ma, Y.; Cheng, Y.; Pal, A.S.; Velazquez-Rizo, M.; et al. Protective coating of single-crystalline Ni-rich cathode enables fast charging in all-solid-state batteries. ACS Nano 2025, 19, 8595–8607. [Google Scholar] [CrossRef]
- Asano, T.; Sakai, A.; Ouchi, S.; Sakaida, M.; Miyazaki, A.; Hasegawa, S. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state-batteries. Adv. Mater. 2018, 30, 1803075. [Google Scholar] [CrossRef]
- Park, K.-H.; Kaup, K.; Assoud, A.; Zhang, Q.; Wu, X.; Nazar, L.F. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 2020, 5, 533–539. [Google Scholar] [CrossRef]
- Zhou, L.; Kwok, C.Y.; Shyamsunder, A.; Zhang, Q.; Wu, X.; Nazar, L.F. A new halospinel superionic conductor for high-voltage all solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 2056–2063. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kaup, K.; Park, K.-H.; Assoud, A.; Zhou, L.; Liu, J.; Wu, X.; Nazar, L.F. Lithium ytterbium-based halide solid electrolytes for high voltage all-solid-state batteries. ACS Mater. Lett. 2021, 3, 930–938. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, T.-T.; Kwok, C.Y.; Kim, S.Y.; Assoud, A.; Zhang, Q.; Janek, J.; Nazar, L.F. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 2022, 7, 83–93. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cha, H.; Kostecki, R.; Chen, G.-Y. Composite cathode design for high-energy all-solid-state lithium batteries with long cycle life. ACS Energy Lett. 2023, 8, 521–528. [Google Scholar] [CrossRef]
- Liu, H.-W.; Chen, H.; Thi, S.; Yu, P.-J.; Chen, J.-L.; Pao, C.-W.; Chang, P.-Y.; Haw, S.-C.; Liao, Y.-F.; Shao, Y.-C.; et al. High-capacity Ni-rich composite cathodes having chemically fused interface with Li3InCl6 electrolyte towards low-pressure operating all-solid-state Li-ion batteries. Compos. Part B Eng. 2025, 293, 112133. [Google Scholar] [CrossRef]
- Seong, W.M.; Cho, K.H.; Park, J.W.; Park, H.; Eum, D.; Lee, M.H.; Kim, I.S.; Lim, J.; Kang, K. Controlling residual lithium in high-nickel (>90%) lithium layered oxides for cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 18662–18669. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Huang, H.; Tan, L.; Song, L.; Wu, H.H.; Wang, C.; Zhao, Z.; Yi, H.; Duan, J.; et al. Role of residual Li and oxygen vacancies in Ni-rich cathode materials. ACS Appl. Mater. Interfaces 2021, 13, 42554–42563. [Google Scholar] [CrossRef]
- Klein, S.; Bärmann, P.; Fromm, O.; Borzutzki, K.; Reiter, J.; Fan, Q.; Winter, M.; Placke, T.; Kasnatscheew, J. Prospects and limitations of single-crystal cathode materials to overcome cross-talk phenomena in high-voltage lithium ion cells. J. Mater. Chem. A 2021, 9, 7546–7555. [Google Scholar] [CrossRef]
- Hou, X.; Liu, X.; Wang, H.; Zhang, X.; Zhou, J.; Wang, M. Specific countermeasures to intrinsic capacity decline issues and future direction of LiMn2O4 cathode. Energy Storage Mater. 2023, 57, 577–606. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.-L.; Zhou, X.A.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.B.; Zhang, N.S.; Li, S.Y. Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 2021, 9, 13540–13551. [Google Scholar] [CrossRef]
- Wang, Z.P.; Yuan, J.; Zhu, X.Q.; Wang, H.; Huang, W.; Wang, Y.; Xu, S. Overcharge-to-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Xiong, D.J.; Hynes, T.; Ellis, L.D.; Dahn, J.R. Effects of surface coating on gas evolution and impedance growth at LiNixMnyCo1–x–yO2 Positive electrodes in Li-ion cells. J. Electrochem. Soc. 2017, 164, A3174–A3181. [Google Scholar] [CrossRef]
- Wang, X.X.; Ding, Y.L.; Deng, Y.P.; Chen, Z.W. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: Promises and challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Liu, T.; Han, C.; Guo, C.; Zhao, H.; Li, Q.; Lu, J.; Amine, K.; Qiu, X. Impacts of dissolved Ni2+ on the solid electrolyte interphase on a graphite anode. Angew. Chem. Int. Ed. 2022, 61, e202202894. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, J.I.; Park, B.K.; Han, J.W.; Lee, J.-W.; Kim, H.; Kim, K.J. Two-in-one strategy: Discretely functionalized electrolyte additive for stable lithium metal batteries coupled with Ni-rich cathode. Chem. Eng. J. 2024, 486, 150354. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Jo, E.; Manthiram, A. Inhibition of transition-metal dissolution with advanced electrolytes in batteries with silicon-graphite anodes and high-nickel cathodes. Energy Storage Mater. 2023, 56, 562–571. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, Y.; Zheng, G.; Cao, H.; Xue, H.; Yao, X.; Wang, L.; Chen, S.; Yin, Z.; Pan, F.; et al. Tailoredi construction for enhanced Si anode and Ni-rich cathode performance in lithium-ion batteries. Chin. Chem. Soc. Chem. 2025, 7, 429–439. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Wang, X.; Gong, H.; Cao, Y.; Ma, K.; Zhang, S.; Wang, S.; Yang, W.; Wang, L.; et al. Trace multifunctional additive enhancing 4.8 V ultra-high voltage performance of Ni-rich cathode and SiOx anode battery. Adv. Energy Mater. 2025, 15, 2403751. [Google Scholar] [CrossRef]
- Yu, Y.; Karayaylali, P.; Katayama, Y.; Giordano, L.; Gauthier, M.; Maglia, F.; Jung, R.; Lund, I.; Shao-Horn, Y. Coupled LiPF6 decomposition and carbonate dehydrogenation enhanced by highly covalent metal oxides in high-energy Li-ion batteries. J. Phys. Chem. C 2018, 122, 27368–27382. [Google Scholar] [CrossRef]
- Zhang, Y.; Katayama, Y.; Tatara, R.; Giordano, L.; Yu, Y.; Fraggedakis, D.; Sun, J.G.; Maglia, F.; Jung, R.; Bazant, M.Z.; et al. Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy. Energy Environ. Sci. 2020, 13, 183–199. [Google Scholar] [CrossRef]
- Dose, W.M.; Temprano, I.; Allen, J.P.; Bjorklund, E.; O’Keefe, C.A.; Li, W.; Mehdi, B.L.; Weatherup, R.S.; De Volder, M.F.L.; Grey, C.P. Electrolyte reactivity at the charged Ni-rich cathode interface and degradation in Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 13206–13222. [Google Scholar] [CrossRef]
- Rinkel, B.L.D.; Vivek, J.P.; Garcia-Araez, N.; Grey, C.P. Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries. Energy Environ. Sci. 2022, 15, 3416–3438. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wu, F.; Zhao, J.; Shi, Q.; Lu, Y.; Li, N.; Cao, D.; Li, W.; Hao, J.; Yang, X.; et al. Multifunctional self-reconstructive cathode/electrolyte interphase layer for cobalt-free Li-rich layered oxide cathode. Energy Storage Mater. 2023, 60, 102798. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Y.; Zhang, X.; Zhang, Z.; Wang, E.; He, S.; Wang, B.; Han, Z.; Lu, J.; Amine, K.; et al. In situ construction of uniform and robust cathode–electrolyte interphase for Li-rich layered oxides. Adv. Funct. Mater. 2020, 31, 2009192. [Google Scholar] [CrossRef]
- Wu, F.; Dong, J.; Chen, L.; Bao, L.; Li, N.; Cao, D.; Lu, Y.; Xue, R.; Liu, N.; Wei, L.; et al. High-voltage and high-safety nickel-rich layered cathode enabled by a self-reconstructive cathode/electrolyte interphase layer. Energy Storage Mater. 2021, 41, 495–504. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, S.; Ren, Z.; Huang, L.; Xu, G.; Liu, T.; Wang, S.; Cui, G. Electrolyte engineering toward high performance high nickel (Ni ≥ 80%) lithium-ion batteries. Adv. Sci. 2024, 11, 2305753. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, S.L.; Zhang, Z.Y.; Fan, J.; Qi, W.; Chen, S. Fluoroethylene carbonate as an electrolyte additive for improving interfacial stability of high-voltage LiNi0.6Co0.2Mn0.2O2 cathode. Ionics 2019, 25, 1035–1043. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Zhao, K.; Li, J.; Yang, Y.; Gia, G. Improving the rate performance and stability LiNi0.6Co0.2Mn0.2O2 in high voltage lithium-ion battery by using fluoroethylene carbonate as electrolyte additive. Ionics 2018, 24, 3337–3346. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, J.; Wang, M.; Tang, X.; Cao, Z.; Wang, C.; Shi, Q.; Qian, Y.; Deng, Y. Multifunctional fluoroethylene carbonate for improving high-temperature performance of LiNi0.8Mn0.1Co0.1O2||SiOx@graphite lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 9989–10000. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Gao, W.; Liu, L.; Zhang, S. A new additive 3-isocyanatopropyltriethoxysilane to improve electrochemical performance of Li/NCM622 half-cell at high voltage. J. Power Sources 2019, 423, 90–97. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Liu, J.; Yu, X.; Zhang, Y.; Jiang, X.; Dong, P.; Zhang, Y. 4-bromoanisole (4BA) as additive for overcharge protection of lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 4571–4579. [Google Scholar] [CrossRef]
- Cao, Z.; Hashinokuchi, M.; Doi, T.; Inaba, M. Improved cycle performance of LiNi0.8Co0.1Mn0.1O2 positive electrode material in highly concentrated LiBF4/DMC. J. Electrochem. Soc. 2019, 166, A82–A88. [Google Scholar] [CrossRef]
- Tatara, R.; Yu, Y.; Karayaylali, P.; Chan, A.K.; Zhang, Y.; Jung, R.; Maglia, F.; Giordano, L.; Shao-Horn, Y. Enhanced cycling performance of Ni-rich positive electrodes (NMC) in Li-ion batteries by reducing electrolyte free-solvent activity. ACS Appl. Mater. Interfaces 2019, 11, 34973–34988. [Google Scholar] [CrossRef]
- Forero-Saboya, J.; Moiseev, I.A.; Vlara, M.-L.; Foix, D.; Deschamps, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. A Hydridoaluminate additive producing a protective coating on Ni-rich cathode materials in lithium-ion batteries. Adv. Energy Mater. 2024, 14, 2402051. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, F.; Fang, C.; Han, J. High-voltage electrolyte design for a Ni-rich layered oxide cathode for lithium-ion batteries. Sci. China Mater. 2023, 66, 3046–3053. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, N.; Chen, S.; Liao, Y.; Zhong, G.; Zhang, Z.; Yang, Y. Construction of a stable LiNi0.8Co0.1Mn0.1O2 (NMC811) cathode interface by a multifunctional organosilicon electrolyte additive. ACS Appl. Energy Mater. 2020, 3, 2837–2845. [Google Scholar] [CrossRef]
- Braun, H.; Asakura, R.; Remhof, A.; Battaglia, C. Hydroborate solid-state lithium battery with high-voltage NMC811 cathode. ACS Energy Lett. 2024, 9, 707–714. [Google Scholar] [CrossRef]
- Hu, R.; Mao, C.; Zhuo, H.; Dai, X.; Yang, L.; Shu, X.; Li, Y.; Li, Z.; Ruan, W.; Yang, F.; et al. Molecular space linkage of dipentacyclic anhydride additives for long-lifespan Li-metal batteries with Ni-rich cathode. Energy Storage Mater. 2025, 75, 104033. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Lin, X.; Mo, C.; Zhou, X.; Quan, L.; Zhou, K.; Li, S.; Wang, H.; Li, W. Highly improved cyclic stability of Ni-rich/Li batteries with succinic anhydride as electrolyte additive and underlying mechanism. J. Energy Chem. 2023, 78, 80–90. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Zhu, X.; Wei, L.; Xi, K.; Zhang, L.; Lan, Y.; Wu, H.; Gao, Y. Tailoring electrode-electrolyte interphases to enable the cycling stability of lithium metal batteries with LiNi0.8Co0.1Mn0.1O2 cathode. J. Power Sources 2022, 529, 231195. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, H.; Zhou, J.; Liu, M.; Ding, X.; Li, Y.; Huang, Y.; Chen, Z.; Shen, C.; Wang, D.; et al. A tough Janus-faced CEI film for high voltage layered oxide cathodes beyond 4.6 V. Energy Storage Mater. 2023, 57, 411–420. [Google Scholar] [CrossRef]
- He, Y.; Liu, N.; Kohl, P.A. High conductivity, lithium ion conducting polymer electrolyte based on hydrocarbon backbone with pendent carbonate. J. Electrochem. Soc. 2020, 167, 100517. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced liquid electrolytes for rechargeable Li metal batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, S.Y.; Ahn, J.; Yim, T. Simultaneous realization of multilayer interphases on a Ni-rich NCM cathode and a SiOx anode by the combination of vinylene carbonate with lithium difluoro(oxalato)borate. ACS Appl. Mater. Interfaces 2024, 16, 14940–14953. [Google Scholar] [CrossRef]
- Dai, P.; Kong, X.; Yang, H.; Kuang, S.; Zeng, J.; Zhao, J. Synergistic effect of dual-anion additives promotes the fast dynamics and high-voltage performance of Ni-rich lithium-ion batteries by regulating the electrode/electrolyte interface. ACS Appl. Mater. Interfaces 2022, 14, 39927–39938. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, X. Surface and subsurface reactions of lithium transition metal oxide cathode materials: An overview of the fundamental origins and remedying approaches. Adv. Energy Mater. 2018, 8, 1802057. [Google Scholar] [CrossRef]
- Zhang, S.S.; Fan, X.L.; Wang, C.S. Enhanced electrochemical performance of Ni-rich layered cathode materials by using LiPF6 as a cathode additive. ChemElectroChem 2019, 6, 1536–1541. [Google Scholar] [CrossRef]
- Phelan, C.M.E.; Björklund, E.; Singh, J.; Fraser, M.; Didwal, P.N.; Rees, G.J.; Ruff, Z.; Ferrer, P.; Grinter, D.C.; Grey, C.P.; et al. Role of salt concentration in stabilizing charged Ni-rich cathode interfaces in Li-ion batteries. Chem. Mater. 2024, 36, 3334–3344. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, H.; Chang, Z.; Deng, H.; Li, X.; Zhou, H. A high-energy-density and long-life initial-anode-free lithium battery enabled by a Li2O sacrificial agent. Nat. Energy 2021, 6, 653–662. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, M.; Li, J.; Wang, J.; Zhang, G.; Wang, J.; Zuo, J.; Cao, G.; Duan, R.; Hao, Y.; et al. LiF-rich cathode electrolyte interphases homogenizing Li+ fluxes toward stable interface in Ni-rich Mn-based cathodes. Adv. Mater. 2025, 37, 2417620. [Google Scholar] [CrossRef]
- Min, X.; Wang, L.; Shen, M.; Ma, G.; He, X. A multifunctional additive extending the calendar life of Ni-rich cathode-based lithium-ion batteries for electric vehicles. Mater. Today 2025, 83, 157–165. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, Y.; Bao, Y.; Zhang, W.; Guan, M. Bis(di-tert-butyl)-4-dimethylaminophenylphosphine as electrolyte additive to improve the electrochemical performance of LiNi0.8Co0.1Mn0.1O2. Electrochim. Acta 2023, 441, 141745. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, Q.; Tang, Y.; Yan, Y.; Zhou, S.; Liao, H.-G.; Qiao, Y.; Bao, J.; Sun, S.-G. Self-repaired cathode electrolyte interphase to stabilize the high-nickel cathode interface by a sustained-release multifunctional electrolyte additive. Chem. Eng. J. 2024, 488, 151153. [Google Scholar] [CrossRef]
- Li, G.; Liao, Y.; Li, Z.; Xu, N.; Lu, Y.; Lan, G.; Sun, G.; Li, W. Constructing a low-impedance interface on a high-voltage LiNi0.8Co0.1Mn0.1O2 cathode with 2,4,6-triphenyl boroxine as a film-forming electrolyte additive for Li-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 37013–37026. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, G.; Zhou, K.; Cheng, Y.; Jiao, T.; Huang, L.; Zou, Y.; Wang, M.S.; Yang, Y.; Zheng, J. Dictating the interfacial stability of nickel-rich LiNi0.90Co0.05Mn0.05O2 via a diazacyclo electrolyte additive—2-Fluoropyrazine. J. Colloid Interface Sci. 2022, 618, 431–441. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Wang, Y.; Zhou, X.; Weng, L.; Liu, Y.; Ren, Y.; Zhao, C.; Dahbi, M.; Alami, J.; et al. Electrolytes polymerization-induced cathode-electrolyte-interphase for high voltage lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2101956. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Zhang, Y.; Zeng, J.; Zhao, J. An effective electrolyte design to improve the high-voltage performance of high-capacity NMC811/ SiOx-Gr batteries. Electrochim. Acta 2020, 349, 136356. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.; Yoon, S.C.; Yim, T. Amphiphilic surfactant-assisted cathode-electrolyte interphases for prolonged cycling performance in Ni-rich NCMA cathode materials of lithium-ion batteries. J. Power Sources 2025, 633, 236447. [Google Scholar] [CrossRef]
- Li, S.; Yang, T.; Wang, W.; Lu, J.; Zhao, X.; Fan, W.; Zuo, X.; Nan, J. 2-Thiophene sulfonamide (2-TS)-contained multi-functional electrolyte matching high-voltage LiNi0.8Mn0.1Co0.1O2/graphite batteries with enhanced performances. Electrochim. Acta 2020, 352, 136492. [Google Scholar] [CrossRef]
- Li, Z.; Lin, X.; Zhou, H.; Xing, L.; Lan, G.; Zhang, W.; Chen, J.; Liu, M.; Huang, Q.; Li, W. Stabilizing the interphasial layer of Ni-rich cathode and graphite anode for lithium-ion battery with multifunctional additive. J. Power Sources 2020, 467, 228343. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yang, T.; Wang, W.; Lu, J.; Fan, W.; Zhao, X.; Zuo, X.; Tie, S.; Nan, J. 3,3-diethylene di-sulfite (DES) as a high-voltage electrolyte additive for 4.5 V LiNi0.8Co0.1Mn0.1O2/graphite batteries with enhanced performances. ChemElectroChem 2021, 8, 745–754. [Google Scholar] [CrossRef]
- Moon, H.; Nam, H.; Kim, M.P.; Lee, S.M.; Kim, H.; Jeon, M.H.; Lee, Y.-S.; Kim, K.; Chun, J.-H.; Kwak, S.K.; et al. Elastic interfacial layer enabled the high-temperature performance of lithium-ion batteries via utilization of synthetic fluorosulfate additive. Adv. Func. Mater. 2023, 33, 2303029. [Google Scholar] [CrossRef]
- Dai, H.; Gomes, L.; Maxwell, D.; Zamani, S.; Yang, K.; Atienza, D.; Dale, N.; Mukerjee, S. Exploring the role of an electrolyte additive in suppressing surface Reconstruction of a Ni-rich NMC cathode at ultrahigh Voltage via enhanced in situ and operando characterization methods. ACS Appl. Mater. Interfaces 2024, 16, 8639–8654. [Google Scholar] [CrossRef]
- Liu, L.; Gao, W.; Cui, Y.; Chen, S. A bifunctional additive bi(4-flurorophenyl) sulfone for enhancing the stability and safety of nickel-rich cathode-based cells. J. Alloys Compd. 2020, 820, 153069. [Google Scholar] [CrossRef]
- Lan, G.; Xing, L.; Bedrov, D.; Chen, J.; Guo, R.; Che, Y.; Li, Z.; Zhou, H.; Li, W. Enhanced cyclic stability of Ni-rich lithium-ion battery with electrolyte film-forming additive. J. Alloys Compd. 2020, 821, 153236. [Google Scholar] [CrossRef]
- Song, H.J.; Jang, S.H.; Choi, K.; Nam, S.C.; Mun, J.; Yim, T. Sulfonate-based artificial cathode-electrolyte interface to enhance electrochemical performance of Ni-rich layered oxide cathode materials. ACS Sustain. Chem. Eng. 2020, 8, 7316–7323. [Google Scholar] [CrossRef]
- Jung, K.; Yim, T. High-performance lithium-ion batteries combined with bi-functionalized electrolyte additive and nickel-rich layered oxides. J. Alloys Compd. 2020, 834, 155155. [Google Scholar] [CrossRef]
- Ahn, J.; Yim, T. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide functionalized by allyl phenyl sulfone as high-performance cathode material for lithium-ion batteries. J. Alloys Compd. 2021, 867, 159153. [Google Scholar] [CrossRef]
- Zheng, W.-C.; Shi, C.-G.; Dai, P.; Huang, Z.; Lin, J.-X.; Chen, H.; Sun, M.-L.; Shen, C.-H.; Luo, C.-X.; Wang, Q.; et al. A functional electrolyte additive enabling robust interphases in high-voltage Li//LiNi0.8Co0.1Mn0.1O2 batteries at elevated temperatures. J. Mater. Chem. A 2022, 10, 21912–21922. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Xia, M.; Cheng, Y.; Wang, M.; Hong, W.; Yang, Y.; Zheng, J. Strengthening the interfacial stability of the silicon-based electrode via an electrolyte additive—Allyl phenyl sulfone. ACS Appl. Mater. Interfaces 2022, 14, 38281. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Liu, G.; Zou, Y.; Yang, X.; Zhang, X.; Fu, A.; Zheng, J.; Yang, Y. A novel trimethylsilyl 2-(fluorosulfonyl)difluoroacetate additive for stabilizing the Ni-rich LiNi0.9Co0.05Mn0.05O2/electrolyte interface. J. Power Sources 2021, 515, 230618. [Google Scholar] [CrossRef]
- Jiao, T.; Liu, G.; Huang, L.; Zou, Y.; Zhang, X.; Zheng, J.; Yang, Y. Tuning interface stability of nickel-rich LiNi0.9Co0.05Mn0.05O2 cathode via a novel bis(vinylsulphonyl)methane additive. J. Power Sources 2022, 521, 230917. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Wu, H.; Zhu, X.; Guan, H.; Wei, L.; Xi, K.; Lan, Y.; Zhang, L.; et al. Regulating electrode-electrolyte interphases and eliminating hydrogen fluoride to boost electrochemical performances of Li/NMC811 batteries. Chem. Eng. J. 2023, 451, 138359. [Google Scholar] [CrossRef]
- Choi, M.; Choi, H.; Park, S.; Seong, W.M.; Lee, Y.; Ko, W.; Cho, M.; Ahn, J.; Kong, Y.; Kim, J. Stabilized high-voltage operation of Co-free NMX cathode via CEI-controlling. Energy Storage Mater. 2024, 67, 103291. [Google Scholar] [CrossRef]
- Jo, M.; Park, S.-H.; Lee, H. NaH2PO4 as an electrolyte additive for enhanced thermal stability of LiNi0.8Co0.1Mn0.1O2/graphite batteries. J. Electrochem. Soc. 2020, 167, 130502. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, Y.; Luo, G.; Chang, J.; Wang, C.; Wang, J.; Shao, H.; Deng, Y. Cathode-anode reaction products interplay enabling high performance of LiNi0.8Co0.1Mn0.1O2/artificial graphite pouch batteries at elevated temperature. J. Power Sources 2021, 514, 230583. [Google Scholar] [CrossRef]
- Song, W.; Gauthier, R.; Taskovic, T.; Ouyang, D.; Ingham, H.A.; Eldesoky, A.; Azam, S.M.; Zsoldos, E.S.; Deng, Z.; Heino, D.H.; et al. Lithium difluoro(dioxalato) phosphate as an electrolyte additive for NMC811/graphite Li-ion pouch cells. J. Electrochem. Soc. 2022, 169, 110513. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, X.; Yin, J.; Lei, Y.; Wu, X.; Gao, Y. Interfacial regulation and hydrogen fluoride capture enabled by allyltrimethylsilane as multifunctional electrolyte additive for Li/LiNi0.8Co0.1Mn0.1O2 batteries. ACS Appl. Energy Mater. 2022, 5, 13501–13510. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Lin, X.; Li, G.; Zhang, W.; Li, W. Insight into the improved performances of Ni-rich/graphite cells by 1,3,5-Trimethyl-1,3,5-tris(3,3,3-trifluoropropyl) cyclotrisiloxane as an electrolyte additive. ACS Appl. Energy Mater. 2022, 5, 11684–11693. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Mo, Y.; Zhou, W.; Hao, Y.; Piao, N.; Liu, C.; Xiao, P.; Liu, H.; Li, B.; et al. Engineering an insoluble cathode electrolyte interphase enabling high performance NMC811//graphite pouch cell at 60 °C. Adv. Energy Mater. 2022, 12, 2201631. [Google Scholar] [CrossRef]
- Ren, Z.; Qiu, H.; Fan, C.; Zhang, S.; Zhang, Q.; Ma, Y.; Qiao, L.; Wang, S.; Xu, G.; Cui, Z.; et al. Delicately designed cyano-siloxane as multifunctional additive enabling high voltage LiNi0.9Co0.05Mn0.05O2/graphite full cell with long cycle life at 50 °C. Adv. Funct. Mater. 2023, 33, 302411. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, M.; Ma, J.; Yang, K.; Chen, Z.; Li, K.; Zhang, C.; Wei, Y.; Zhou, M.; Wang, P.; et al. Lithium hexamethyldisilazide as electrolyte additive for efficient cycling of high-voltage non-aqueous lithium metal batteries. Nat Commun. 2022, 13, 6966. [Google Scholar] [CrossRef]
- Xu, N.; Shi, J.; Liu, G.; Yang, X.; Zheng, J.; Zhang, Z.; Yang, Y. Research progress of fluorine-containing electrolyte additives for lithium ion batteries. J. Power Sources Adv. 2021, 7, 100043. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, F.; Cheng, Z.; Mao, Y.; Huang, R.; Li, F.; Dong, H.; Zhang, Q.; Li, W.; Chen, H.; et al. Insights into the dual role of lithium difluoro(oxalato)borate additive in Improving the electrochemical performance of NMC811//graphite cells. ACS Appl. Energy Mater. 2020, 3, 695–704. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Y.; Yang, Z.; Song, D.; Sun, X.; Wang, C.; Yang, L.; Zhang, Y.; Gao, J.; Ohsaka, T.; et al. A fluorinated electrolyte stabilizing high-voltage graphite/NMC811 batteries with an inorganic-rich electrode-electrolyte interface. Chem. Eng. J. 2022, 440, 135939. [Google Scholar] [CrossRef]
- Ahn, J.; Im, J.; Seo, H.; Yoon, S.; Cho, K.Y. Enhancing the cycling stability of Ni-rich LiNi0.83Co0.11Mn0.06O2 cathode at 4.5 V via 2,4-difluorobiphenyl additive. J. Power Sources 2021, 512, 230513. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Wei, P.; Sun, S.; Xu, Y.; Li, Q.; Fang, C.; Han, J.; Huang, Y. Tailoring electrolyte enables high-voltage Ni-rich NCM cathode against aggressive cathode chemistries for Li-ion batteries. Sci. Bull. 2022, 67, 2225–2234. [Google Scholar] [CrossRef]
- Pham, H.Q.; Nguyen, M.T.; Tarik, M.; El Kazzi, M.; Trabesinger, S. Cross-talk-suppressing electrolyte additive enabling high voltage performance of Ni-rich layered oxides in Li-ion batteries. ChemSusChem 2021, 14, 2461–2474. [Google Scholar] [CrossRef]
- Tan, S.; Shadike, Z.; Li, J.; Wang, X.; Yang, Y.; Lin, R.; Cresce, A.; Hu, J.; Hunt, A.; Waluyo, I.; et al. Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V. Nat. Energy 2022, 7, 484–494. [Google Scholar] [CrossRef]
- Xue, W.; Huang, M.; Li, Y.; Zhu, Y.G.; Gao, R.; Xiao, X.; Zhang, W.; Li, S.; Xu, G.; Yu, Y.; et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 2021, 6, 495–505. [Google Scholar]
- Oh, M.G.; Kwak, S.; An, K.; Tran, Y.H.T.; Kang, D.G.; Park, S.J.; Lim, G.; Kim, K.; Lee, Y.S.; Song, S.W. Perfluoro macrocyclic ether as an ambifunctional additive for high-performance SiO and nickel 88%-based high-energy Li-ion battery. Adv. Funct. Mater. 2023, 33, 2212890. [Google Scholar] [CrossRef]
- Peng, X.; Shen, H.; Su, K.; Wang, W.; Weng, S.; Tang, C.; Xue, Z.; Xiang, Y. Stable and fast ion transport electrolyte interfaces modified with novel fluorine- and nitrogen-containing solvents for Ni-rich cathode materials. ACS Appl. Mater. Interfaces 2024, 16, 34281–34293. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Xu, X.; Yin, J.; Jiang, S.; Xi, K.; Wei, L.; Gao, Y. LiF-rich interfaces and HF elimination achieved by a multifunctional additive enable high-performance Li/LiNi0.8Co0.1Mn0.1O2 batteries. ACS Appl. Mater. Interfaces 2023, 15, 11777–11786. [Google Scholar] [CrossRef]
- Liu, G.; Xu, N.; Zou, Y.; Zhou, K.; Yang, X.; Jiao, T.; Yang, W.; Yang, Y.; Zheng, J. Stabilizing Ni-rich LiNi0.83Co0.12Mn0.05O2 with cyclopentyl isocyanate as a novel electrolyte additive. ACS Appl. Mater. Interfaces 2021, 13, 12069–12078. [Google Scholar] [CrossRef]
- Tan, C.; Yang, J.; Pan, Q.; Li, Y.; Li, Y.; Cui, L.; Fan, X.; Zheng, F.; Wang, H.; Li, Q. Optimizing interphase structure to enhance electrochemical performance of high voltage LiNi0.5Mn1.5O4 cathode via anhydride additives. Chem. Eng. J. 2021, 410, 128422. [Google Scholar] [CrossRef]
- Han, F.; Cang, Z.; Wang, R.; Yun, F.; Wang, J.; Ma, C.; Zhang, Y.; Tang, L.; Ding, H.; Lu, S. Isocyanate additives improve the low-temperature performance of LiNi0.8Mn0.1Co0.1O2||SiOx@graphite lithium-ion batteries. ACS Appl. Mater. Interfaces 2023, 15, 20966–20976. [Google Scholar] [CrossRef]
- Zheng, J.; Qiu, Y.; Liao, S.; Yue, Z.; Fang, S.; Zhou, N.; Li, Y.; Jiang, Y. A novel 4-fluorophenyl isocyanate additive constructing solid cathodes-electrolyte interface for high-performance lithium-ion batteries. Small 2024, 20, 2405853. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, K.; Liu, G.; Xu, N.; Zhang, X.; Yang, Y.; Zhang, J.; Zheng, J. Enhanced cycle life and rate capability of single-crystal, Ni-rich LiNi0.9Co0.05Mn0.05O2 enabled by 1,2,4-1H-triazole additive. ACS Appl. Mater. Interfaces 2021, 13, 16427–16436. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Jiang, L.; Yang, T.; Fan, W.; Wang, W.; Zhao, X.; Zuo, X.; Nan, J. Isocyanoethyl methacrylate (IMA) as a bifunctional electrolyte additive for LiNi0.8Co0.1Mn0.1O2/graphite batteries with enhanced performance. ChemElectroChem 2021, 8, 3716–3725. [Google Scholar] [CrossRef]
- Pham, H.Q.; Mirolo, M.; Tarik, M.; El-Kazzi, M.; Trabesinger, S. Multifunctional electrolyte additive for improved interfacial stability in Ni-rich layered oxide full-cells. Energy Storage Mater. 2020, 33, 216–229. [Google Scholar] [CrossRef]
- Li, Y.; Qu, Q.; Lv, L.; Shao, J.; Zheng, H. A multifunctional additive capable of electrolyte stabilization and structure/interphases regulation of high-energy Li-ion batteries. Adv. Funct. Mater. 2024, 34, 2314100. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Qiu, Y.; Zhang, J.; Liu, Y.; Wei, P.; Ou, M.; Sun, S.; Xu, Y.; Li, Q.; et al. Tailoring electrolyte to enable high-rate and super-stable Ni-rich NCM cathode materials for Li-ion batteries. Nano Energy 2021, 88, 106301. [Google Scholar] [CrossRef]
- Guan, P.; Min, J.; Zhang, S.; Lu, Y.; Liang, T.; Meng, L.; Yuan, Y.; Zhou, Y.; Chen, F.; Zhou, L.; et al. Stabilizing high-voltage performance of nickel-rich cathodes via facile solvothermally synthesized niobium-doped strontium titanate. ACS Appl. Mater. Interfaces 2024, 16, 26167–26181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Jiao, T.; Cheng, Y.; Zou, Y.; Wang, M.-S.; Yang, Y.; Zheng, J. Electrolyte additive cis-1,2,3,6-tetrahydrophthalic anhydride enhanced the cycle life of nickel-rich LiNi0.9Co0.05Mn0.05O2. ACS Appl. Energy Mater. 2021, 4, 12275–12284. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, G.; Zhou, K.; Zhang, J.; Jiao, T.; Zhang, X.; Yang, Y.; Zheng, J. Enhanced interfacial stability of a LiNi0.9Co0.05Mn0.05O2 cathode by a diboron additive. ACS Appl. Energy Mater. 2021, 4, 11051–11061. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, X.; Zhou, P.; Li, H.; He, X.; Fan, W.; Fan, C.; Yang, T.; Ma, Z.; Zhao, X.; et al. Eliminating H2O/HF and regulating interphase with bifunctional tolylene-2, 4-diisocyanate (TDI) additive for long life Li-ion battery. J. Energy Chem. 2024, 95, 519–528. [Google Scholar] [CrossRef]
- Lim, D.-A.; Shin, Y.-K.; Seok, J.-H.; Hong, D.; Ahn, K.H.; Lee, C.H.; Kim, D.-W. Cathode electrolyte interphase-forming additive for improving cycling performance and thermal stability of Ni-rich LiNixCoyMn1-x-yO2 cathode materials. ACS Appl. Mater. Interfaces 2022, 14, 54688–54697. [Google Scholar] [CrossRef]
- Li, S.; Zhao, D.; Wang, P.; Cui, X.; Tang, F. Electrochemical effect and mechanism of adiponitrile additive for high-voltage electrolyte. Electrochim. Acta 2016, 222, 668–677. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.Y.; Park, S.J.; Park, G.T.; Sun, Y.K. Adiponitrile (C6H8N2): A new Bi functional additive for high performance Li metal batteries. Adv. Funct. Mater. 2019, 29, 1902496. [Google Scholar] [CrossRef]
- Li, X.; Han, X.; Li, G.; Du, J.; Cao, Y.; Gong, H.; Wang, H.; Zhang, Y.; Liu, S.; Zhang, B.; et al. Nonsacrificial nitrile additive for armoring high-voltage LiNi0.83Co0.07Mn0.1O2 cathode with reliable electrode-electrolyte interface toward durable battery. Small 2022, 18, 2202989. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, Z.; Pan, J.; Hong, Y.; Li, J.; Lin, Y.; Shi, K.; Liu, Q. Designing stable electrode interfaces from a pyrrolidine-based electrolyte for improving LiNi0.8Co0.1Mn0.1O2 batteries. Ind. Eng. Chem. Res. 2022, 61, 14173–14180. [Google Scholar] [CrossRef]
- Park, S.; Jeong, S.Y.; Lee, T.K.; Park, M.W.; Lim, H.Y.; Sung, J.; Cho, J.; Kwak, S.K.; Hong, S.Y.; Choi, N.-S. Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat. Commun. 2021, 12, 838. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, S.; Cui, Z.; Cui, Z.; Xie, B.; Gong, T.; Zhang, X.; Li, J.; Wu, R.; Wang, S.; et al. Interphase regulation by multifunctional additive empowering high energy lithium-ion batteries with enhanced cycle life and thermal safety. Angew. Chem. Int. Ed. 2024, 63, e202315710. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Harlow, J.; Logan, E.; Hebecker, H.; Coon, M.; Molino, L.; Johnson, M.; Dahn, J.; Metzger, M. A systematic study of electrolyte additives in single crystal and bimodal LiNi0.8Mn0.1Co0.1O2/graphite pouch cells. J. Electrochem. Soc. 2021, 168, 090503. [Google Scholar] [CrossRef]
- Lu, D.; Li, R.; Rahman, M.M.; Yu, P.; Lv, L.; Yang, S.; Huang, Y.; Sun, C.; Zhang, S.; Zhang, H.; et al. Ligand-channel-enabled ultrafast Li-ion conduction. Nature 2024, 627, 101–107. [Google Scholar] [CrossRef]
- Li, G.-X.; Koverga, V.; Nguyen, A.; Kou, R.; Ncube, M.; Jiang, H.; Wang, K.; Liao, M.; Guo, H.; Chen, J.; et al. Enhancing lithium-metal battery longevity through minimized coordinating diluent. Nat. Energy 2024, 9, 817–827. [Google Scholar] [CrossRef]
- Liu, X.; Mariani, A.; Diemant, T.; Pietro, M.E.D.; Dong, X.; Kuenzel, M.; Mele, A.; Passerini, S. Difluorobenzene-based locally concentrated ionic liquid electrolyte enabling stable cycling of lithium metal batteries with nickel-rich cathode. Adv. Energy Mater. 2022, 12, 2200862. [Google Scholar] [CrossRef]
- Fan, X.; Ji, X.; Chen, L.; Chen, J.; Deng, T.; Han, F.; Yue, J.; Piao, N.; Wang, R.; Zhou, X.; et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 2019, 4, 882–890. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, T.; Liao, X.; Song, Y.; Zhao, Y. All-fluorinated electrolyte directly tuned Li+ solvation sheath enabling high-quality passivated interfaces for robust Li metal battery under high voltage operation. Energy Storage Mater. 2023, 57, 249–259. [Google Scholar] [CrossRef]
- Su, C.-C.; He, M.; Shi, J.; Amine, R.; Yu, Z.; Cheng, L.; Guo, J.; Amine, K. Principle in developing novel fluorinated sulfone electrolyte for high voltage lithium-ion batteries. Energy Environ. Sci. 2021, 14, 3029–3034. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Wang, A.; Zhang, D.; Li, B.; He, X.; Fan, X.; Liu, J. Cosolvent occupied solvation tuned anti-oxidation therapy toward highly safe 4.7 V-class NMC811 batteries. Energy Environ. Sci. 2024, 17, 6113–6126. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Zhao, Y.; Zhou, W.; Xiao, P.; Gao, P.; Tavajohi, N.; Tu, J.; Li, B.; He, X.; et al. Breaking solvation dominance of ethylene carbonate via molecular charge engineering enables lower temperature battery. Nat. Commun. 2023, 14, 8326. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, W.; Dong, W.; Yang, K.; Chao, Y.; Xi, C.; Li, M.; Zhang, Q.; Liu, Z.; Du, P.; et al. Enhancing the cathode/electrolyte interface in Ni-rich lithium-ion batteries through homogeneous oxynitridation enabled by NO3− dominated clusters. Chem. Eng. J. 2024, 494, 153001. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Ahmad, W.; Zhao, J.; Li, H.; Wang, L.; Wan, Z.; Jiang, W.; Li, S.; Yang, F.; et al. Synergistic dual-additive regulated carbonate electrolyte stabilizes bidirectional interface for aggressive Ni-rich Li-metal full batteries. Nano Energy 2024, 128, 109862. [Google Scholar] [CrossRef]
- An, K.; Tran, Y.H.T.; Kwak, S.; Han, J.; Song, S.-W. Design of fire-resistant liquid electrolyte formulation for safe and long-cycled lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2106102. [Google Scholar] [CrossRef]
- Huang, X.; Li, R.; Sun, C.; Zhang, H.; Zhang, S.; Lv, L.; Huang, Y.; Fan, L.; Chen, L.; Noked, M.; et al. Solvent-assisted hopping mechanism enables ultrafast charging of lithium-ion batteries. ACS Energy Lett. 2022, 7, 3947–3957. [Google Scholar] [CrossRef]
- Li, Z.; Yao, N.; Yu, L.; Yao, Y.-X.; Jin, C.-B.; Yang, Y.; Xiao, Y.; Yue, X.-Y.; Cai, W.-L.; Xu, L.; et al. Inhibiting gas generation to achieve ultralong-lifespan lithium-ion batteries at low temperatures. Matter 2023, 6, 2274–2292. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, Z.; Liu, G.; Li, Q.; Yin, D.; Shi, X.; Cao, Z.; Tian, Z.; Kim, H.; Guo, Y.; et al. Non-flammable electrolyte enables high-voltage and wide-temperature lithium-ion batteries with fast charging. Angew. Chem. Int. Ed. 2023, 62, e202216189. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Yang, X.; Xin, T.; Chen, S.; Yang, M.; Peng, Y.; Xu, H.; Yin, Y.; Deng, T.; et al. Thermal runaway suppression of high-energy lithium-ion batteries by designing the stable interphase. J. Electrochem. Soc. 2021, 168, 090563. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, X.; Liu, X.; Wang, X.; Ren, D.; Wang, L.; Yang, M.; Wang, Y.; Zhang, W.; Li, Y.; et al. In-built ultraconformal interphases enable high-safety practical lithium batteries. Energy Storage Mater. 2021, 43, 248–257. [Google Scholar] [CrossRef]
- Pan, R.; Cui, Z.; Yi, M.; Xie, Q.; Manthiram, A. Ethylene carbonate-free electrolytes for stable, safer high-nickel lithium-ion batteries. Adv. Energy Mater. 2022, 12, 2103806. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, D.; Liu, X.; Xu, G.-L.; Feng, X.; Zheng, Y.; Li, Y.; Yang, M.; Peng, Y.; Han, X.; et al. High-voltage and high-safety practical lithium batteries with ethylene carbonate-free electrolyte. Adv. Energy Mater. 2021, 11, 2102299. [Google Scholar] [CrossRef]
- Li, W.; Dolocan, A.; Li, J.; Xie, Q.; Manthiram, A. Ethylene carbonate-free electrolytes for high-nickel layered oxide cathodes in lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1901152. [Google Scholar] [CrossRef]
- Klein, S.; van Wickeren, S.; Röser, S.; Bärmann, P.; Borzeutzki, K.; Heidrich, B.; Börner, M.; Winter, M.; Placke, T.; Kasnatscheew, J. Understanding the outstanding high-voltage performance of NCM523||graphite lithium ion cells after elimination of ethylene carbonate solvent from conventional electrolyte. Adv. Energy Mater. 2021, 11, 2003738. [Google Scholar] [CrossRef]
- Xu, C.; Liang, Y.; Zhang, R.; Cheng, J.; Yun, H. Ethylene carbonate-free electrolytes for high voltage lithium-ion batteries: Progress and perspectives. Energy Fuels 2024, 38, 18208–18226. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, H.; Lu, D.; Yu, Y.; Qi, J.; Zhang, J.; Zhang, S.; Li, R.; Deng, T.; Chen, L.; et al. A low-concentration sulfone electrolyte enables high-voltage chemistry of lithium-ion batteries. Energy Mater. 2022, 2, 200030. [Google Scholar] [CrossRef]
- Lu, D.; Lei, X.; Weng, S.; Li, R.; Li, J.; Lv, L.; Zhang, H.; Huang, Y.; Zhang, J.; Zhang, S.; et al. A self-purifying electrolyte enables high energy Li ion batteries. Energy Environ. Sci. 2022, 15, 3331–3342. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, H.; Hu, E.; et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 2023, 614, 694. [Google Scholar] [CrossRef]
- Su, H.; Chen, Z.; Li, M.; Bai, P.; Li, Y.; Ji, X.; Liu, Z.; Sun, J.; Ding, J.; Yang, M.; et al. Achieving practical high-energy-density lithium-metal batteries by a dual-anion regulated electrolyte. Adv. Mater. 2023, 35, 2301171. [Google Scholar] [CrossRef]
- Chen, P.-P.; Zhang, B.-H.; Li, Z.-A.; Lei, J.-T.; Chen, J.-Z.; Hou, Y.-L.; Zhao, D.-L. Regulate the solvation structure and interface by nitrate in phosphate-based electrolytes for 4.5 V-class Ni-rich batteries. Small 2024, 20, 2403079. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Tan, G.; Li, L.; Chen, R.; Wu, F. Double-salts super concentrated carbonate electrolyte boosting electrochemical performance of Ni-rich LiNi0.90Co0.05Mn0.05O2 lithium metal batteries. Small 2024, 20, 2311650. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Weng, S.; Li, R.; Lu, D.; Deng, T.; Zhang, S.; Lv, L.; Qi, J.; Xiao, X.; et al. Multifunctional solvent molecule design enables high-voltage Li-ion batteries. Nat. Commun. 2023, 14, 2211. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Cui, Z.; Jia, H.; Engelhard, M.H.; Matthews, B.E.; Cao, X.; Xie, Q.; Wang, C.; Manthiram, A.; et al. Stabilizing ultrahigh-nickel layered oxide cathodes for high-voltage lithium metal batteries. Mater Today 2021, 44, 15–24. [Google Scholar] [CrossRef]
- Zhang, S.S. Unveiling the mystery of lithium bis(fluorosulfonyl)imide as a single salt in low-to-moderate concentration electrolytes of lithium metal and lithium-ion batteries. J. Electrochem. Soc. 2022, 169, 110515. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, J.; Li, H.; Guan, S.; Zhao, H.; Han, W.; Zhang, H.; Jiang, G.; Li, L.; An, B.; et al. Self-induced corrosion of Ni-rich cathode materials by fluor-lithium salts. Energy Storage Mater. 2025, 74, 103953. [Google Scholar] [CrossRef]
- Qiao, L.; Oteo, U.; Martinez-Ibañez, M.; Santiago, A.; Cid, R.; Sanchez-Diez, E.; Lobato, E.; Meabe, L.; Armand, M.; Zhang, H. Stable non-corrosive sulfonimide salt for 4-V-class lithium metal batteries. Nat. Mater. 2022, 21, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, P.; Kong, X.; Tian, J.; Zhang, W.; Yan, S.; Hou, W.-H.; Zhou, H.-Y.; Dong, H.; Chen, X.; et al. Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries. Nat. Energy 2023, 8, 934–945. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Zhang, S.; Dong, S.; Wang, S.; Cui, Z.; Du, X.; Wang, C.; Xie, B.; Du, J.; et al. Highly fluorinated Al-centered lithium salt boosting the interfacial compatibility of Li-metal batteries. ACS Energy Lett. 2022, 7, 591–598. [Google Scholar] [CrossRef]
- Wu, F.; Fang, S.; Kuenzel, M.; Mullaliu, A.; Kim, J.-K.; Gao, X.; Diemant, T.; Kim, G.-T.; Passerini, S. Dual-anion ionic liquid electrolyte enables stable Ni-rich cathodes in lithium-metal batteries. Joule 2021, 5, P2177–P2194. [Google Scholar] [CrossRef]
- Kato, Y.; Nagahara, A.; Gerile, N.; Fujinaka, S.; Hamamoto, N.; Nishimura, H.; Nakai, H. Investigation of water-washing effect on electrochemical properties of Ni-Rich NCA cathode material for lithium-ion batteries. J. Electrochem. Soc. 2022, 169, 060543. [Google Scholar] [CrossRef]
- Hartmann, L.; Pritzl, D.; Beyer, H.; Gasteiger, H.A. Evidence for Li+/H+ exchange during ambient storage of Ni-rich cathode active materials. J. Electrochem. Soc. 2021, 168, 070507. [Google Scholar] [CrossRef]
- Wang, X.; He, W.; Xue, H.; Zhang, D.; Wang, J.; Wang, L.; Li, J. A nonflammable phosphate-based localized high-concentration electrolyte for safe and high-voltage lithium metal batteries. Sustain. Energy Fuels 2022, 6, 1281–1288. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Li, R.; Chen, L.; Zhang, H.; Ma, B.; Jiang, S.; Zhou, T.; Huang, J.; Zhu, H.; et al. Surface chemical coordination stabilizes Ni-rich cathodes for high-energy Li-metal batteries. J. Am. Chem. Soc. 2025, 147, 9396–9406. [Google Scholar] [CrossRef] [PubMed]
- Pillot, C. Lithium-Ion Battery Raw Material Supply and Demand 2016–2025. Available online: http://cii-resource.com/cet/AABE-03-17/Presentations/BRMT/Pillot_Christophe.pdf (accessed on 1 February 2024).
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Meng, Y.S.; Chen, Z. Ambient-pressure relithiation of degraded LixNi0.5Co0.2Mn0.3O2 (0 < x < 1) via eutectic solutions for direct regeneration of lithium-ion battery cathodes. Adv. Energy Mater. 2019, 9, 1900454. [Google Scholar]
- Wang, T.; Luo, H.M.; Bai, Y.C.; Li, J.L.; Belharouak, I.; Dai, S. Direct recycling of spent NCM cathodes through ionothermal lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Ji, H.; Wei, X.; Zhou, G.; Cheng, H.-M. A review of direct recycling methods for spent lithium-ion batteries. Energy Storage Mater. 2024, 70, 103475. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef]
- Mao, J.; Ye, C.; Zhang, S.; Xie, F.; Zeng, R.; Davey, K.; Guo, Z.; Qiao, S. Toward practical lithium-ion battery recycling: Adding value, tackling circularity and recycling-oriented design. Energy Environ. Sci. 2022, 15, 2732–2752. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Chen, H.; Sun, J. Separation of Li and Co from the active mass of spent Li-ion batteries by selective sulfating roasting with sodium bisulfate and water leaching. Miner. Eng. 2018, 126, 28–35. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, X.; Zhang, B.; Zhao, Y.; Xie, H.; Zhao, J.; Ning, Z.; Xing, P.; Yin, H. Recycling of spent lithium nickel cobalt manganese oxides via a low-temperature ammonium sulfation roasting approach. J. Clean. Prod. 2021, 279, 123633. [Google Scholar]
- Lander, L.; Rousse, G.; Batuk, D.; Colin, C.V.; Corte, D.A.D.; Tarascon, J.-M. Synthesis, structure, and electrochemical properties of K-based sulfates K2M2(SO4)3 with M = Fe and Cu. Inorg. Chem. 2017, 56, 2013–2021. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, C.; Zhang, L.; Wen, J.; Wang, C.; Huang, G. Synthesis of high-nickel and high-performance ternary cathode materials through spent lithium-ion batteries recycling system. Sustain. Chem. Pharm. 2023, 31, 100959. [Google Scholar] [CrossRef]
- Hamitouche, L.; Robert, C.; Rollet, A.-L.; Briois, V.; Selmane, M.; Gutierrez, A.G.P.; Leclerc, S.; Krulic, D.; Fatouros, N.; Sirieix-Plénet, J.; et al. Elucidating salt conversion mechanisms of lithiated transition metal oxides for lithium-ion battery recycling. Chem. Mater. 2024, 36, 2544–2553. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Qu, H.; Li, J.; Ji, W.; Qiu, X.; Zhu, Y.; Ren, H.; Shi, R.; Li, G.; et al. Closed-loop direct upcycling of spent Ni-rich layered cathodes into high-voltage cathode materials. Adv. Mater. 2024, 36, 2407029. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Wang, J.; Liang, Z.; Jia, K.; Ma, J.; Zhuang, Z.; Zhou, G.; Cheng, H.-M. Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt. Nat. Commun. 2023, 14, 584. [Google Scholar] [CrossRef]
- Xu, M.L.; Wu, C.; Zhang, F.X.; Zhang, Y.H.; Ren, J.X.; Zhang, C.Y.; Wang, X.Z.; Xiao, L.; Fontaine, O.; Qian, J.F. Potential regulation strategy enables ferrocene as p-type redox mediator for direct regeneration of spent LiFePO4 cathode. Energy Storage Mater. 2024, 71, 103611. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Zhang, C.; Ye, M.X.; Li, H.M.; Fu, Z.; Zhang, H.M.; Wang, G.Z.; Zhang, Y.X. Closed-loop regeneration of a spent LiFePO4 cathode by integrating oxidative leaching and electrochemical relithiation. ACS Appl. Energy. Mater. 2022, 5, 14323–14334. [Google Scholar] [CrossRef]
- Wu, C.; Hu, J.M.; Ye, L.; Su, Z.P.; Fang, X.L.; Zhu, X.L.; Zhuang, L.; Ai, X.P.; Yang, H.X.; Qian, J.F. Direct regeneration of spent Li-ion battery cathodes via chemical relithiation reaction. ACS Sustain. Chem. Eng. 2021, 9, 16384–16393. [Google Scholar] [CrossRef]
- Wu, C.; Xu, M.L.; Zhang, C.Y.; Ye, L.; Zhang, K.L.; Cong, H.J.; Zhuang, L.; Ai, X.P.; Yang, H.X.; Qian, J.F. Cost-effective recycling of spent LiMn2O4 cathode via a chemical lithiation strategy. Energy Storage Mater. 2023, 55, 154–165. [Google Scholar] [CrossRef]
- Park, K.; Yu, J.L.; Coyle, J.; Dai, Q.; Frisco, S.; Zhou, M.; Burrell, A. Direct cathode recycling of end-of-life Li-ion batteries enabled by redox mediation. ACS Sustain. Chem. Eng. 2021, 9, 8214–8221. [Google Scholar] [CrossRef]
- Ko, S.; Choi, J.; Hong, J.; Kim, C.; Hwang, U.; Kwon, M.; Lim, G.; Sohn, S.S.; Jang, J.; Lee, U.; et al. Thermodynamically controlled chemical regeneration of spent battery cathodes using recyclable electron donors under ambient conditions. Energy Environ. Sci. 2024, 17, 4064. [Google Scholar] [CrossRef]
- Kim, S.; Shin, U.; Yoon, H.J.; Song, J.; Ma, J.; Woo, J.-J.; Nam, K.-W.; Seo, D.-H.; Ryu, W.-H. Unraveling redox mediator-assisted chemical relithiation mechanism for direct recycling of spent Ni-rich layered cathode materials. Adv. Sci. 2025, 12, 2417094. [Google Scholar] [CrossRef]
- Zheng, Y.; Pu, J.; Yang, Q.; You, L.; Wang, W.; Liu, J. Efficient selective leaching and direct regeneration of spent Ni-rich cathodes via advanced oxidation. Sep. Purif. Technol. 2025, 361, 131366. [Google Scholar] [CrossRef]
- Xu, G.; Huang, L.; Lu, C.; Zhou, X.; Cui, G. Revealing the multilevel thermal safety of lithium batteries. Energy Storage Mater. 2020, 31, 72–86. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Huang, W.; He, X.; Wang, L.; Ouyang, M. Challenges and opportunities to mitigate the catastrophic thermal runaway of high-energy batteries. Adv. Energy Mater. 2023, 13, 2203841. [Google Scholar] [CrossRef]
- Cui, Z.; Manthiram, A. Thermal stability and outgassing behaviors of high-nickel cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202307243. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Hou, J.; Feng, X.; Wang, L.; Liu, X.; Ohma, A.; Lu, L.; Ren, D.; Huang, W.; Li, Y.; Yi, M.; et al. Unlocking the self-supported thermal runaway of high-energy lithium-ion batteries. Energy Storage Mater. 2021, 39, 395–402. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Wang, L.; Feng, X.; Ren, D.; Wu, Y.; Xu, G.; Lu, L.; Hou, J.; Zhang, W.; et al. Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials. Nano Energy 2021, 85, 105878. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Mechanism of thermal runaway in lithium-ion cells. J. Electrochem. Soc. 2018, 165, A1303–A1308. [Google Scholar] [CrossRef]
- Huang, L.; Xu, G.; Du, X.; Li, J.; Xie, B.; Liu, H.; Han, P.; Dong, S.; Cui, G.; Chen, L. Uncovering LiH triggered thermal runaway mechanism of a high-energy LiNi0.5Co0.2Mn0.3O2/graphite pouch cell. Adv. Sci. 2021, 8, 2100676. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z.; et al. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Wang, C.; Du, X.; Lu, D.; Xie, B.; Wang, X.; Lu, C.; Liu, H.; Dong, S.; et al. The formation/decomposition equilibrium of LiH and its contribution on anode failure in practical lithium metal batteries. Angew. Chem. Int. Ed. 2021, 60, 7770–7776. [Google Scholar] [CrossRef]
- Xu, G.; Du, X.; Zhang, S.; Li, J.; Dong, S.; Hu, Z.; Cui, G. Revealing the importance of suppressing formation of lithium hydride and hydrogen in Li anode protection. Interdiscip. Mater. 2023, 2, 337–347. [Google Scholar] [CrossRef]
- Soto, F.A.; Ma, Y.; Martinez De La Hoz, J.M.; Seminario, J.M.; Balbuena, P.B. Formation and growth mechanisms of solid-electrolyte interphase layers in rechargeable batteries. Chem. Mater. 2015, 27, 7990–8000. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Wei, X.; Zhu, Y.; Sun, X.; Gu, M.D. Cathode–electrolyte interphase of Ni-rich layered oxides: Evolving structure and implication on stability. Nano Lett. 2025, 25, 2769–2776. [Google Scholar] [CrossRef]
- Park, N.-Y.; Lee, H.-H.; Kim, H.; Park, S.-M.; Jung, H.-G.; Jung, Y.-C.; Sun, Y.-K. High-energy, long-life Ni-rich cathode materials with columnar structures for all-solid-state batteries. Nature Energy 2025, 10, 479–489. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Hu, Z.; Cui, G.; Chen, L. Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials. Chem. Mater. 2019, 31, 6033–6065. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, G.-T.; Conlin, P.; Ashburn, N.; Cho, K.; Yu, Y.-S.; Shapiro, D.A.; Maglia, F.; Kim, S.J.; Lamp, P.; et al. Cation ordered Ni-rich layered cathode for ultra-long battery life. F1-GC80: Fluorine doped compositionally graded cathode, with an average concentration of Li[Ni0.80Co0.05Mn0.15]O2. Energy Environ. Sci. 2021, 14, 1573–1583. [Google Scholar] [CrossRef]
- Wang, S.; Dai, A.; Cao, Y.; Yang, H.; Khalil, A.; Lu, J.; Li, H.; Ai, X. Enabling stable and high-rate cycling of a Ni-rich layered oxide cathode for lithium-ion batteries by modification with an artificial Li+-conducting cathode-electrolyte interphase. NMC811 with artificial Li+-conducting cathode-electrolyte interphase (ALCEI). J. Mater. Chem. A 2021, 9, 11623–11631. [Google Scholar]
- Ma, J.; Sun, Y.; Wu, D.; Wang, C.; Yu, R.; Duan, H.; Zheng, M.; Li, R.; Gu, D.; Zhao, Y.; et al. Highly stabilized Ni-rich cathodes enabled by artificially reversing naturally-formed interface. Adv. Energy Mater. 2025, 15, 2403150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).