Synthesis of Cathode Material Li2FeTiO4 for Lithium-Ion Batteries by Sol–Gel Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results
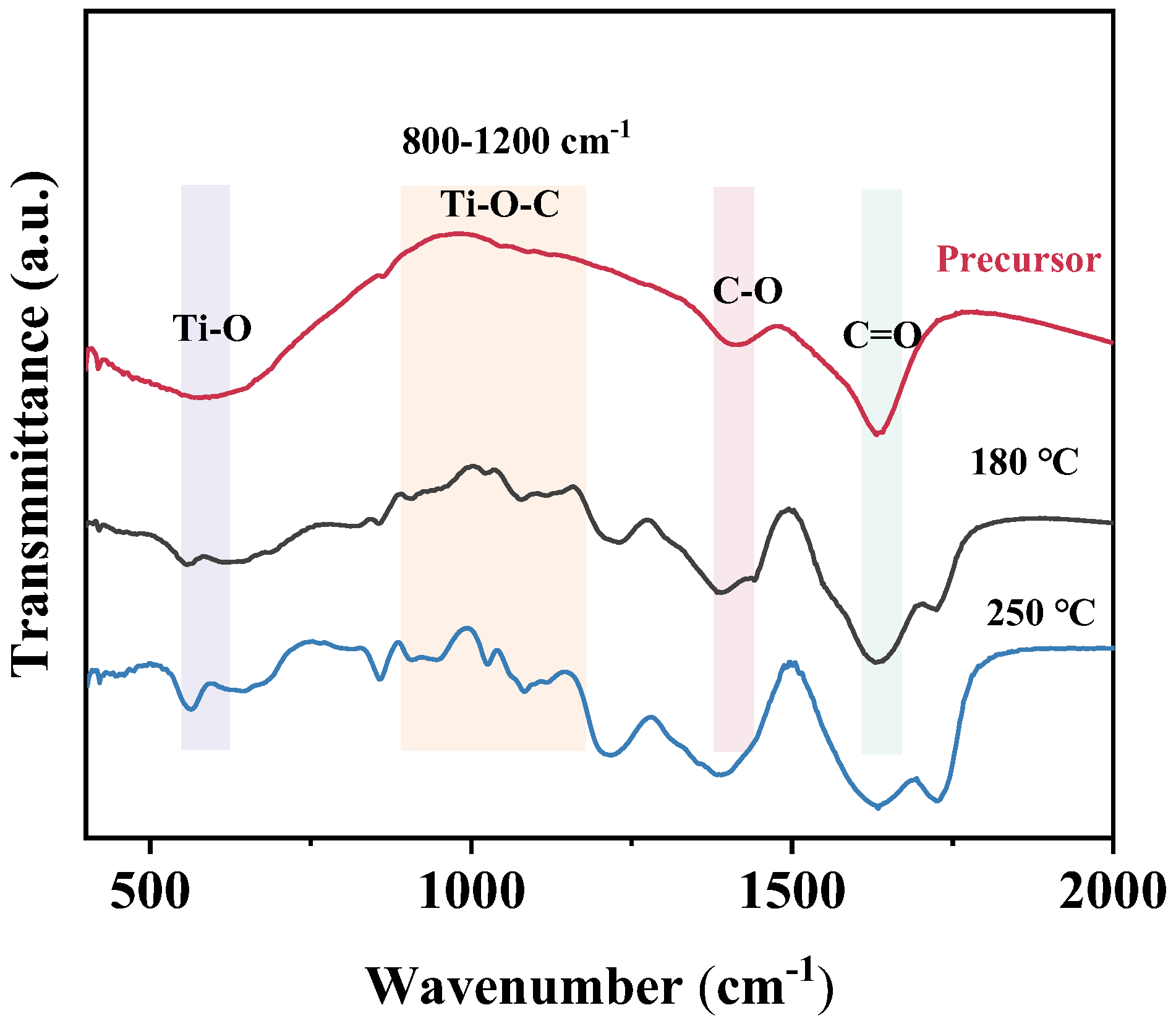
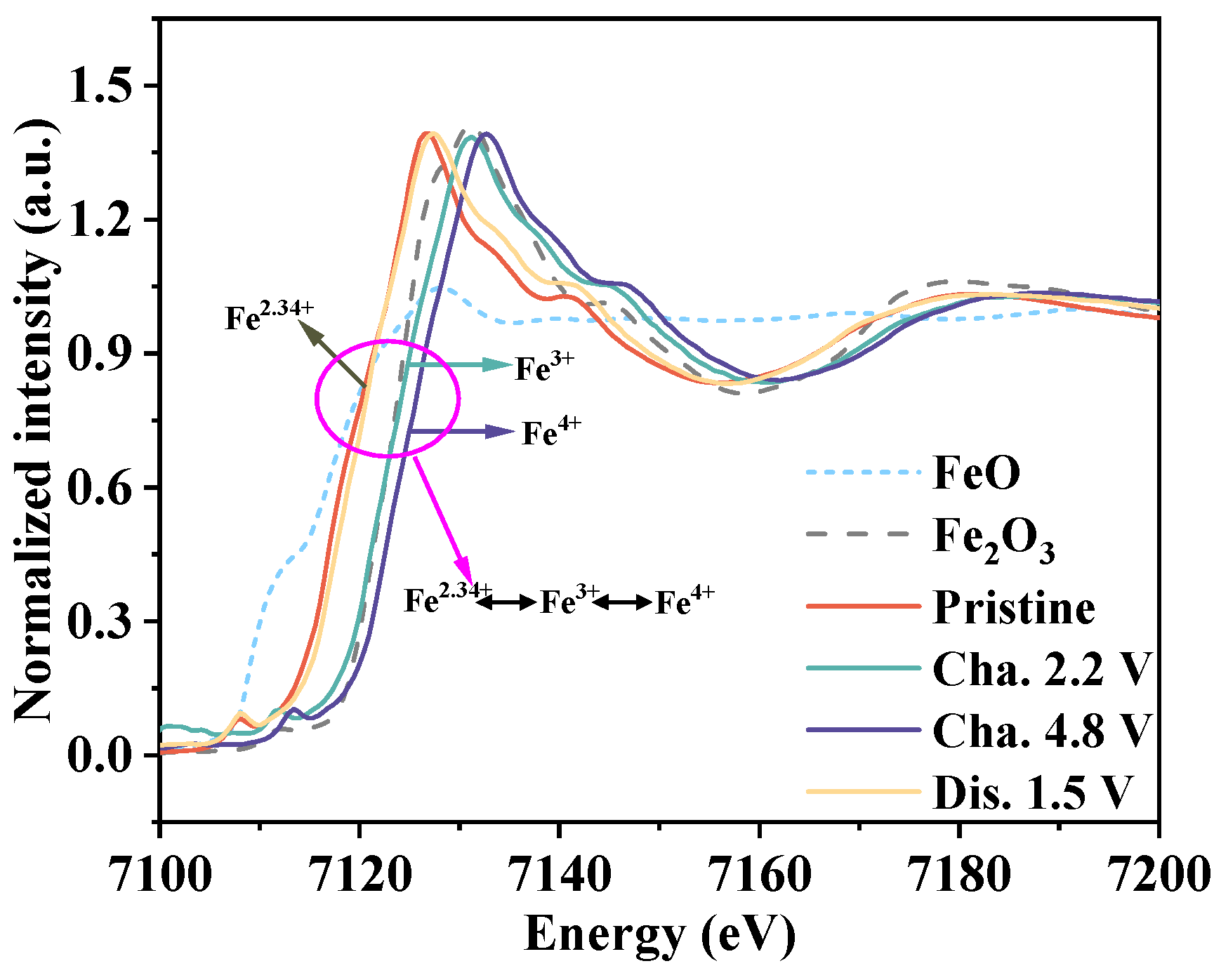
3.1. Research on the Mechanism of Reaction
3.2. Research on Reduction Reaction
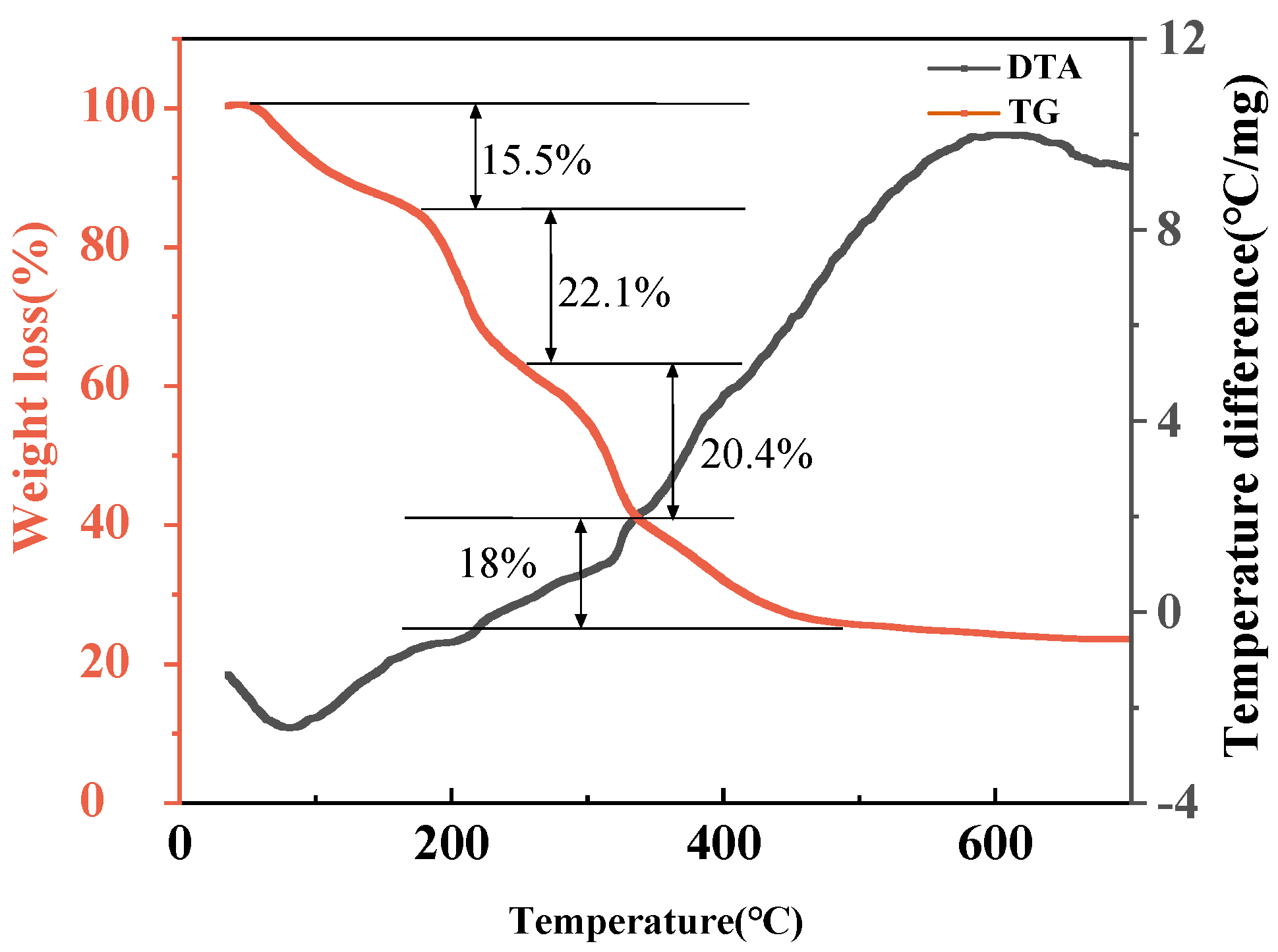
3.3. Thermogravimetry-Differential Thermal Analysis Study on Precursors
- (1)
- Room temperature ~180 °C stage: This stage mainly refers to the weight loss reaction of raw materials losing crystalline water, accompanied by the gradual rupture of complex coordination bonds.
- (2)
- 180–250 °C stage: During this stage, the thermal decomposition of citric acid (C6H8O7) occurs, and the product is mainly organic matter. Aldehydes (such as formaldehyde, acetaldehyde, etc.) and CO will serve as reducing agents to achieve the reduction of Fe3+ in the system.
- (3)
- 250–330 °C stage: This stage mainly involves the thermal decomposition of metal salts (such as FeCl2, Ti(OC4H9)4, CH3COOLi, etc.), resulting in Li2O, TiO2, Fe, among which Fe3O4 is the intermediate phase obtained from insufficient reduction of trivalent iron.
- (4)
- 330–470 °C stage: The reactions that occur during this stage include further reduction of Fe3O4 and the initial formation of Li2FeTiO4 crystals.
- (5)
- Stage above 470 °C: This stage is the further formation of Li2FeTiO4 crystals and changes in their crystallization properties and microstructure.
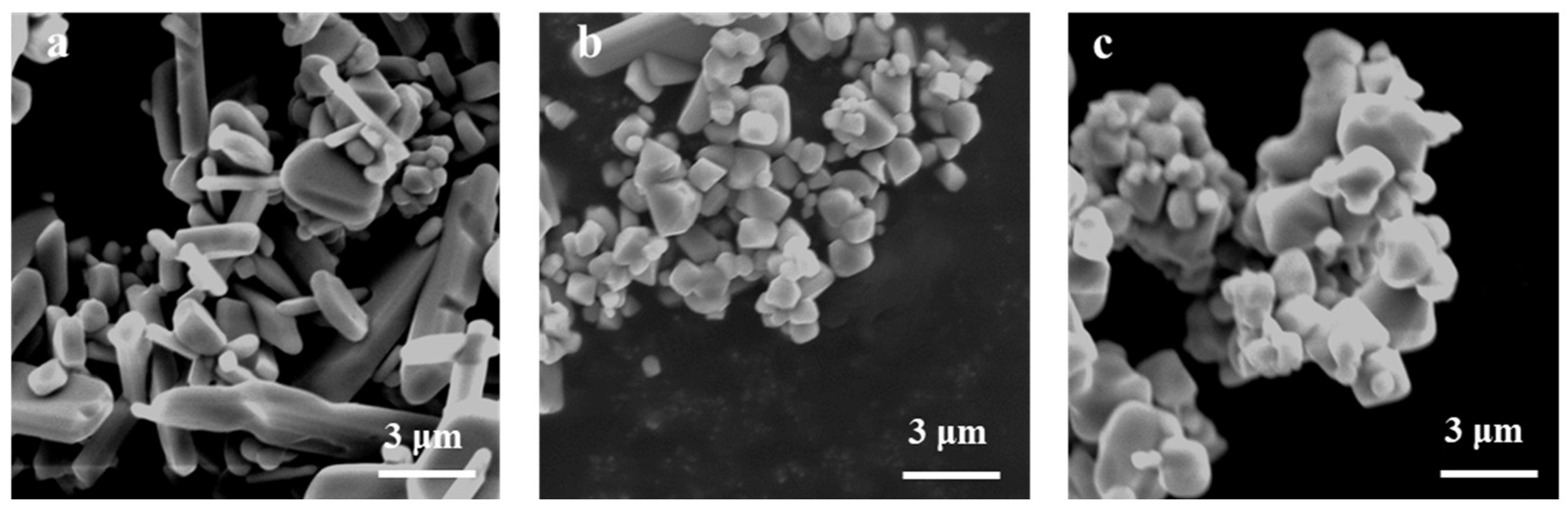
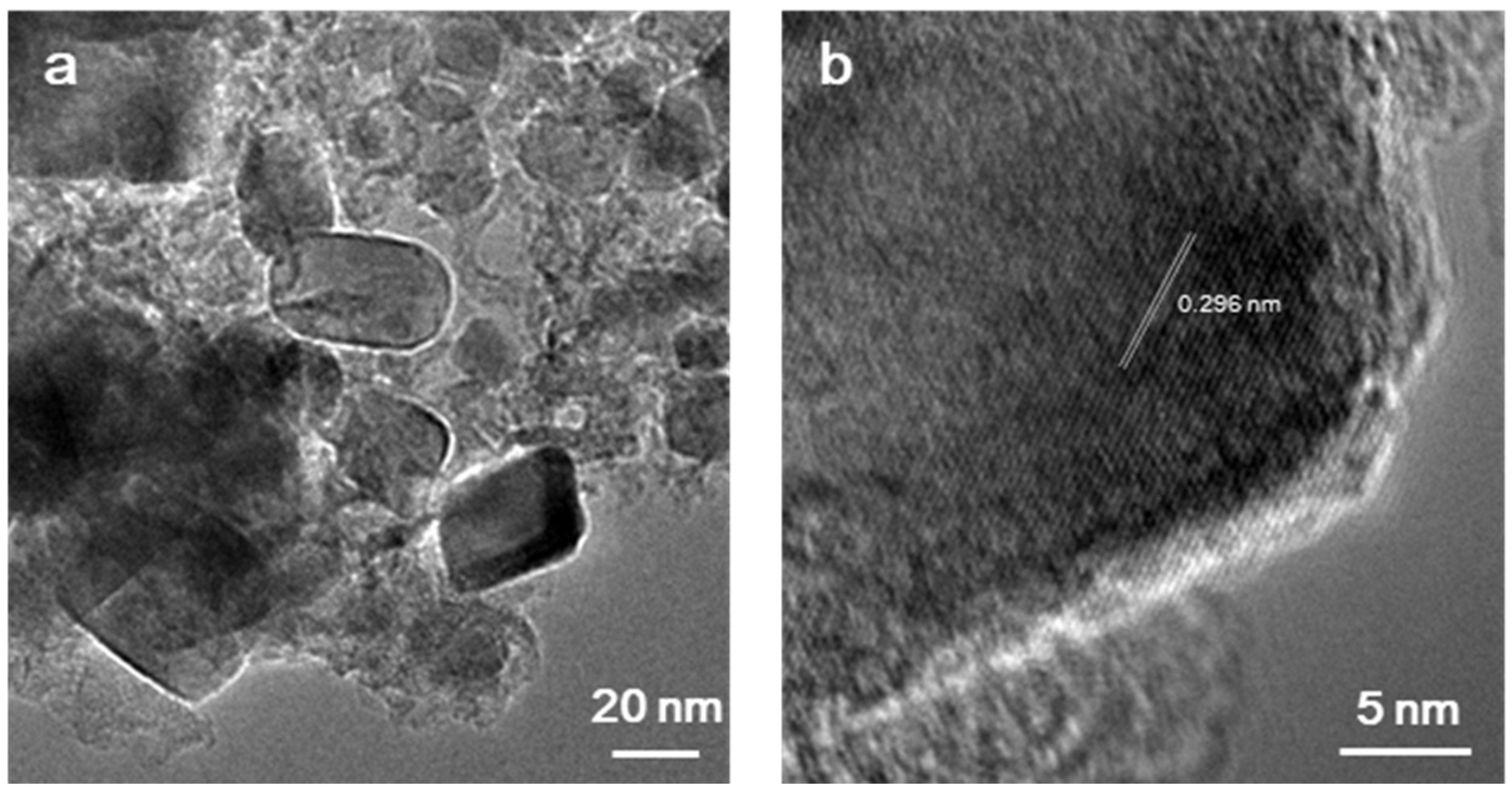
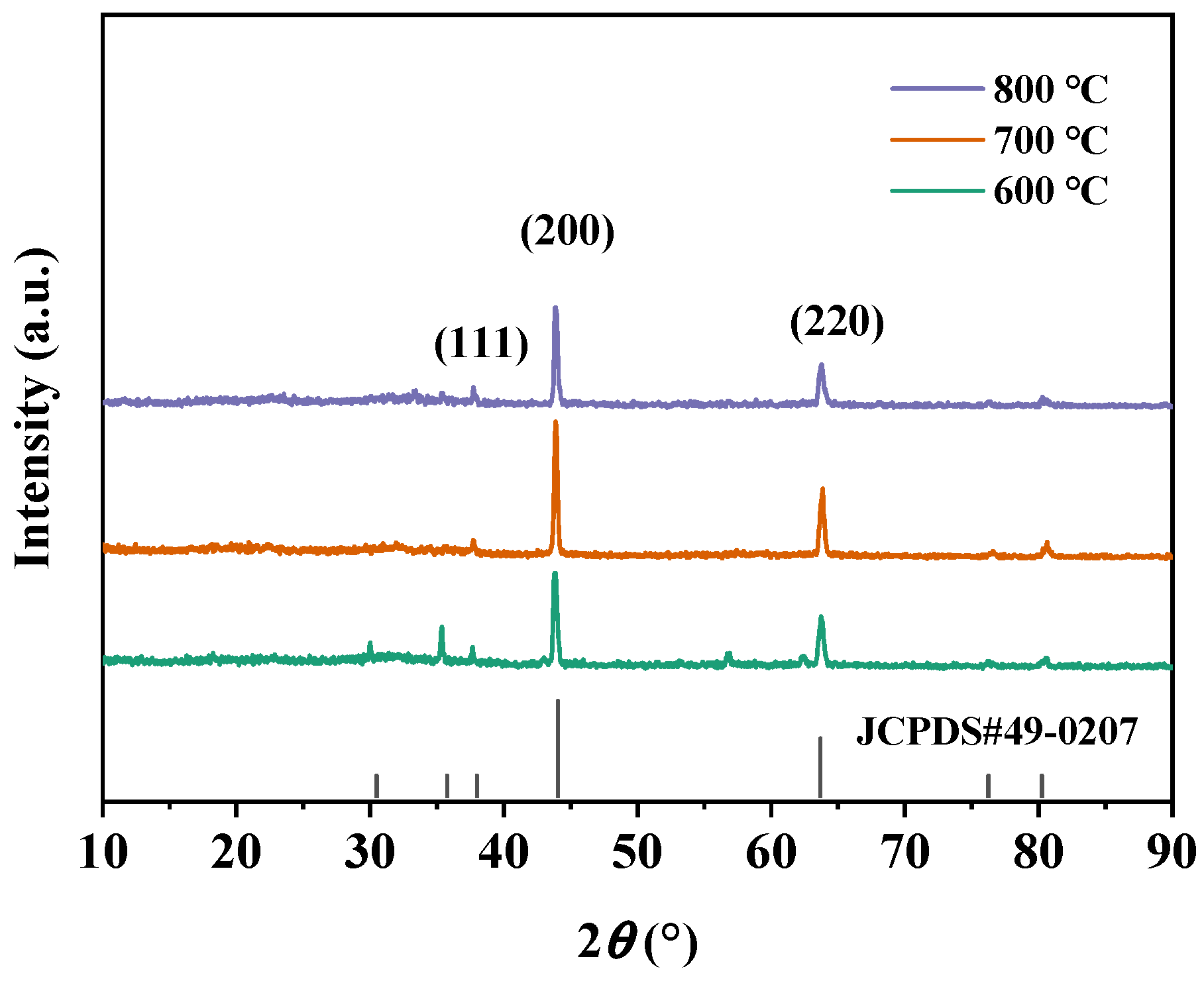
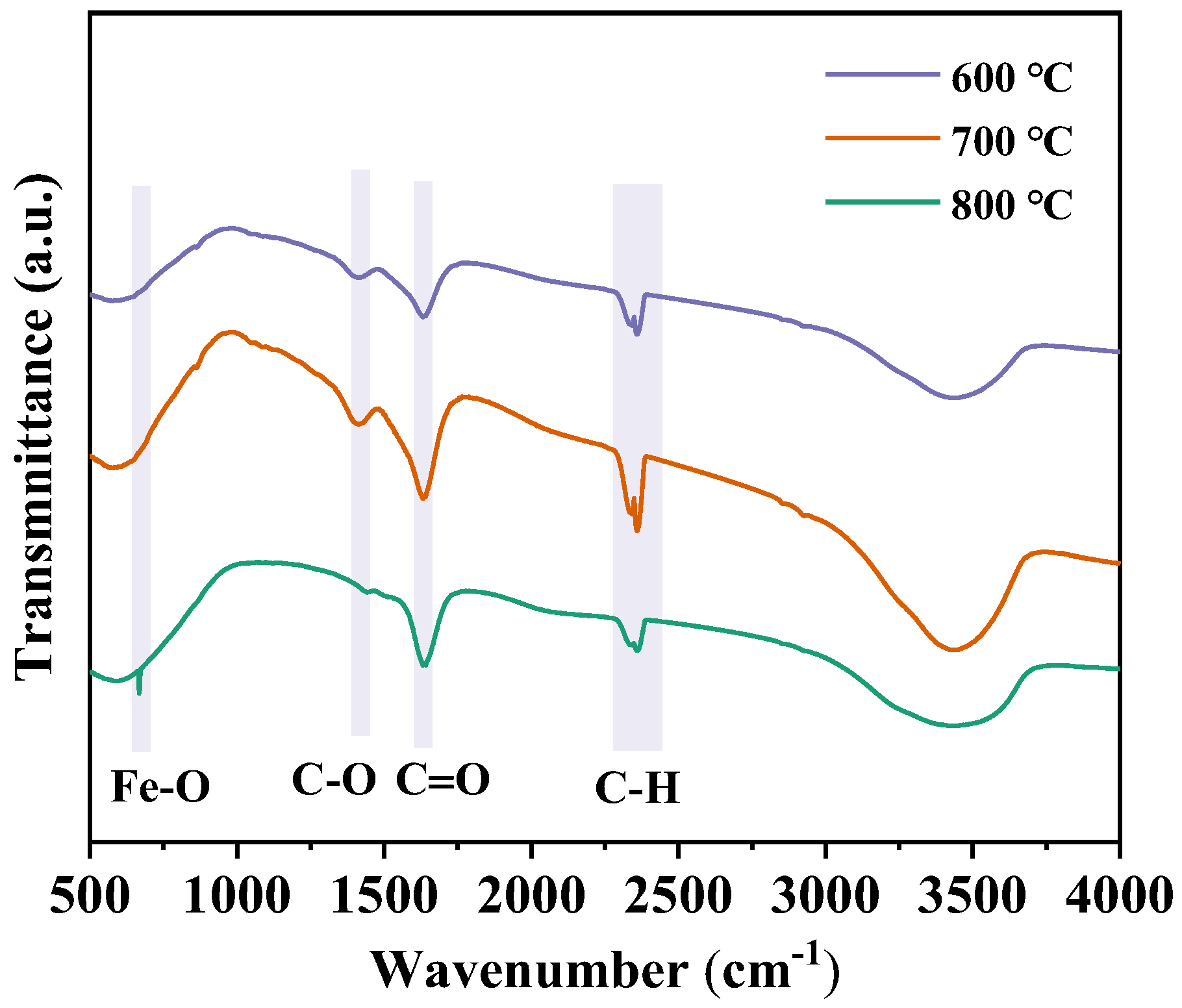
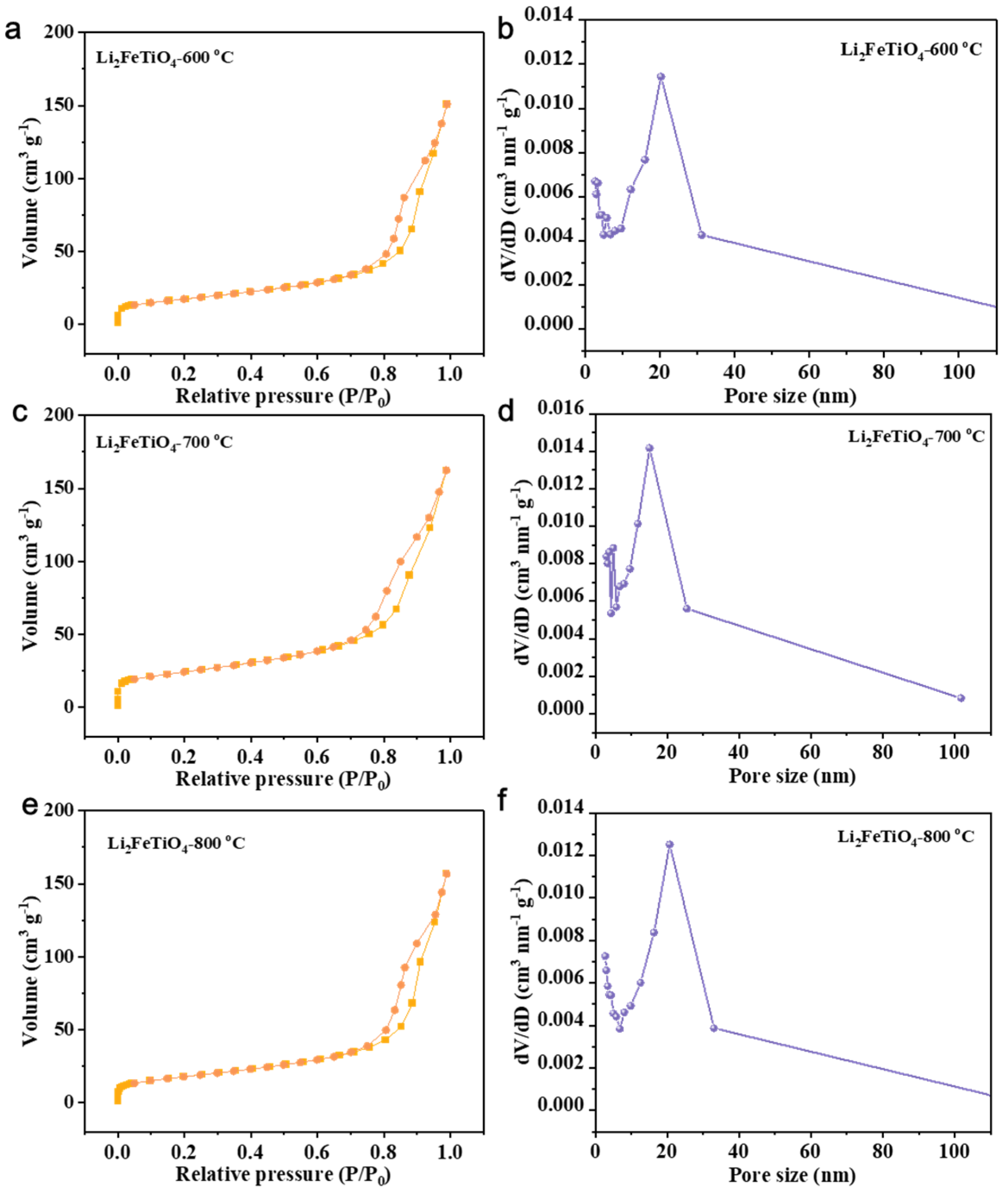
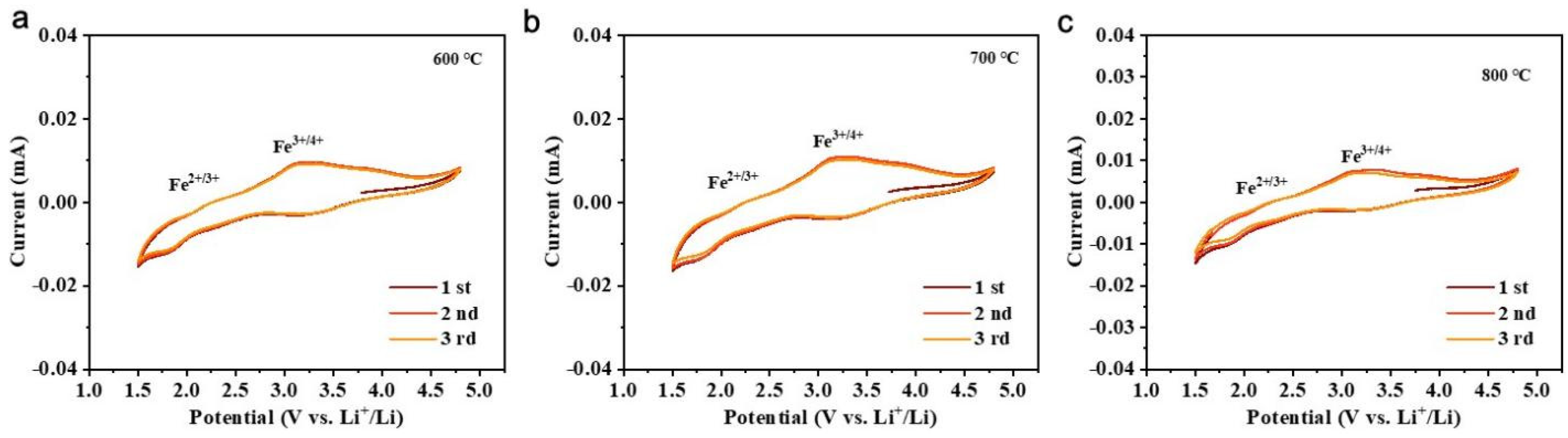
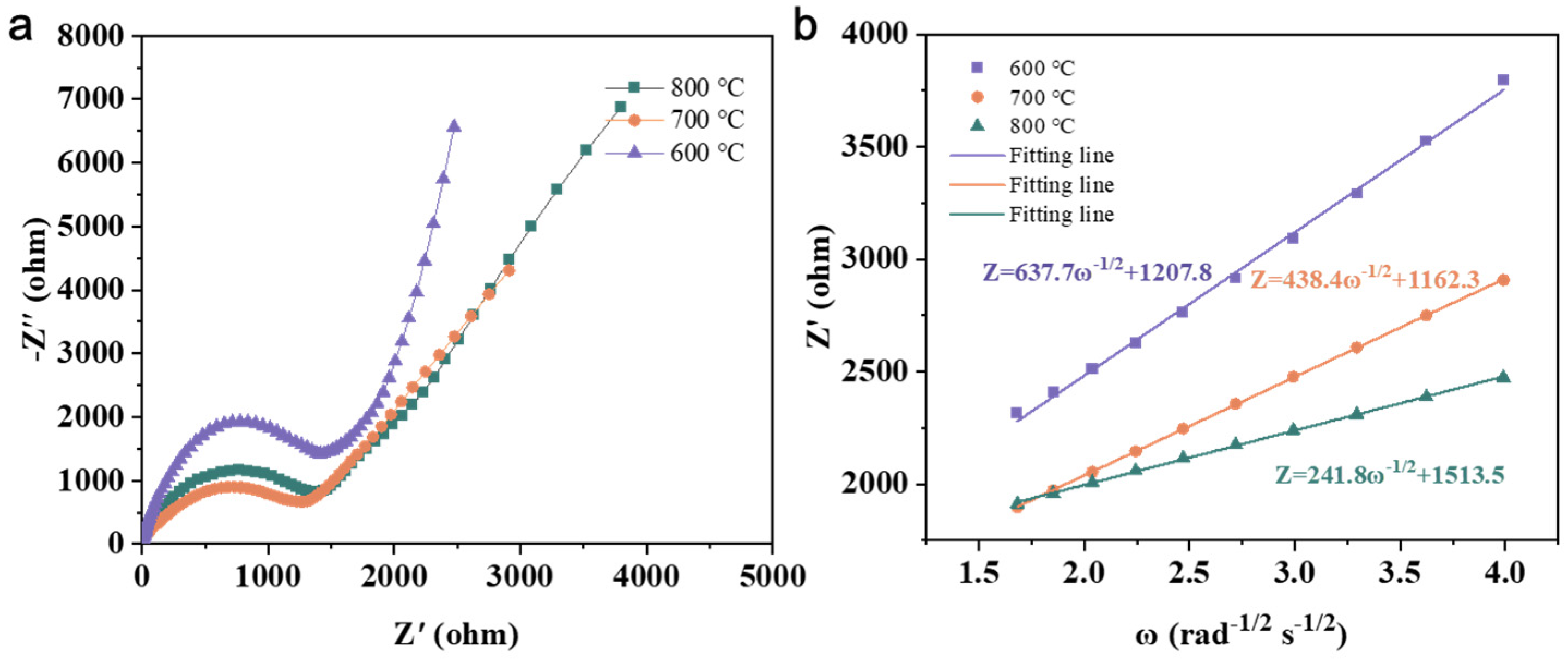
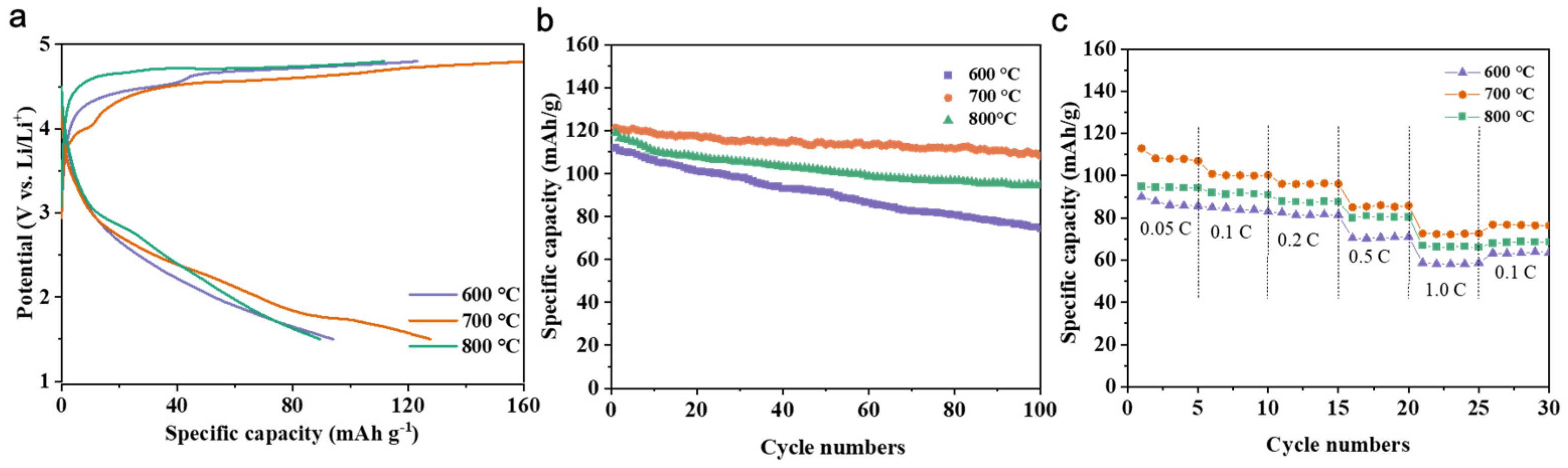
3.4. Effect of Calcination Firing Temperature on Li2FeTiO4 Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oboh, R.K.; Huang, K.; Pan, L. Overcoming heterocoagulation challenge in selective flotation separation between lithium cobalt oxide and lithium manganese oxide. Sep. Purif. Technol. 2025, 364, 132480. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, B.; Lu, Y. Progress and perspective of high-voltage lithium cobalt oxide in lithium-ion batteries. J. Energy Chem. 2022, 74, 283–308. [Google Scholar] [CrossRef]
- Zhao, T.; Li, W.; Traversy, M.; Choi, Y.; Ghahreman, A.; Zhao, Z.; Zhang, C.; Zhao, W.; Song, Y. A review on the recycling of spent lithium iron phosphate batteries. J. Environ. Manag. 2024, 351, 119670. [Google Scholar] [CrossRef] [PubMed]
- Bin Abu Sofian, A.D.A.; Imaduddin, I.S.; Majid, S.R.; Kurniawan, T.A.; Chew, K.W.; Lay, C.-H.; Show, P.L. Nickel-rich nickel–cobalt–manganese and nickel–cobalt–aluminum cathodes in lithium-ion batteries: Pathways for performance optimization. J. Clean. Prod. 2024, 435, 140324. [Google Scholar] [CrossRef]
- Cao, J.; Hu, Y.; Zhu, Y.; Cao, H.; Fan, M.; Huang, C.; Shu, K.; He, M.; Chen, H.C. Synthesis of mesoporous nickel-cobalt-manganese sulfides as electroactive materials for hybrid supercapacitors. Chem. Eng. J. 2021, 405, 126928. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Yao, H.; Lin, G.; Lee, J.; Chen, Y.; Chen, Q. Research Progress in Lithium-Excess Disordered Rock-Salt Oxides Cathode. Energy Environ. Mater. 2022, 5, 1139–1154. [Google Scholar] [CrossRef]
- Kesavan, T.; Senthil, C.; Sasidharan, M. Solvothermally synthesized Ti-rich LiMnTiO4 as cathode material for high Li storage. J. Mater. Sci. 2018, 53, 4406–4416. [Google Scholar] [CrossRef]
- Yamauchi, K.; Momida, H.; Oguchi, T. First-Principles Study on Cathode Properties of Li2MTiO4 and Na2MTiO4 (M = V, Cr, Mn, Fe, Co, Ni). J. Phys. Soc. Jpn 2022, 91, 034704. [Google Scholar] [CrossRef]
- Kitajou, A.; Tanaka, K.; Miki, H.; Koga, H.; Okajima, T.; Okada, S. Improvement of Cathode Properties by Lithium Excess in Disordered Rocksalt Li2+2xMn1−xTi1−xO4. Electrochemistry 2016, 84, 597–600. [Google Scholar] [CrossRef]
- Li, J.; Luo, S.-h.; Wang, Q.; Yan, S.; Feng, J.; Liu, H.; Ding, X.; He, P. Facile synthesis of carbon-LiMnPO4 nanorods with hierarchical architecture as a cathode for high-performance Li-ion batteries. Electrochim. Acta 2018, 289, 415–421. [Google Scholar] [CrossRef]
- Chen, Z.; Qiu, S.; Cao, Y.; Qian, J.; Ai, X.; Xie, K.; Hong, X.; Yang, H. Hierarchical porous Li2FeSiO4/C composite with 2 Li storage capacity and long cycle stability for advanced Li-ion batteries. J. Mater. Chem. A 2013, 1, 4988–4992. [Google Scholar] [CrossRef]
- Saracibar, A.; Van der Ven, A.; Arroyo-de Dompablo, M.E. Crystal Structure, Energetics, And Electrochemistry of Li2FeSiO4 Polymorphs from First Principles Calculations. Chem. Mater. 2012, 24, 495–503. [Google Scholar] [CrossRef]
- Delmas, C.; Carlier, D.; Guignard, M. The Layered Oxides in Lithium and Sodium-Ion Batteries: A Solid-State Chemistry Approach. Adv. Energy Mater. 2021, 11, 2001201. [Google Scholar] [CrossRef]
- Han, J.; Kim, J.C. A solid-state route to stabilize cubic Li7La3Zr2O12 at low temperature for all-solid-state-battery applications. Chem. Commun. 2020, 56, 15197–15200. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, S.Z.; Feng, Z.H.; Qi, H.B.; Fu, H.; Liu, R.P. Improving vacuum gas nitriding of a Ti-based alloy via surface solid phase transformation. Vacuum 2022, 197, 110860. [Google Scholar] [CrossRef]
- Li, Y.; Zan, M.; Chen, P.; Huang, Y.; Xu, X.; Zhang, C.; Cai, Z.; Yu, X.; Li, H. Facile Solid-State Synthesis to In Situ Generate a Composite Coating Layer Composed of Spinel-Structural Compounds and Li3PO4 for Stable Cycling of LiCoO2 at 4.6 V. ACS Appl. Mater. Interfaces 2023, 15, 51262–51273. [Google Scholar] [CrossRef]
- Li, Y.; Xia, D.; Tao, L.; Xu, Z.; Yu, D.; Jin, Q.; Lin, F.; Huang, H. Hydrothermally Assisted Conversion of Switchgrass into Hard Carbon as Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 28461–28472. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, X. Preparation of CuBi2O4 nanosheets as a high-capacity and cycle-stable anode material for Li recharge batteries. J. Energy Stor. 2024, 93, 112217. [Google Scholar] [CrossRef]
- Amedzo-Adore, M.; Han, J.I. Chemically lithiated layered VOPO4 by a microwave-assisted hydrothermal method and its electrochemical properties in rechargeable Li-ion batteries and supercapacitor applications. J. Alloys Compd. 2022, 911, 165067. [Google Scholar] [CrossRef]
- Yao, L.; Xi, Y.; Han, H.; Li, W.; Wang, C.; Feng, Y. LiMn2O4 prepared from waste lithium ion batteries through sol-gel process. J. Alloys Compd. 2021, 868, 159222. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, S.-H.; Sui, L.-L.; Ren, Q.-X.; Qin, Y.; Zhang, D.-J. Preparation of neodymium-doped LiMnPO/C cathode by sol-gel method with excellent electrochemical performance for lithium-ion batteries. Int. J. Energy Res. 2021, 45, 10590–10598. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Vasylechko, L.; Selvan, R.K. Li-ion diffusional insights and temperature dependent electrical & dielectric properties of LiNi1/3Mn1/3Co1/3PO4 electrodes. J. Phys. Chem. Solids 2025, 197, 112420. [Google Scholar] [CrossRef]
- Alsamet, M.A.M.M.; Burgaz, E. Synthesis and characterization of nano-sized LiFePO4 by using consecutive combination of sol-gel and hydrothermal methods. Electrochim. Acta 2021, 367, 137530. [Google Scholar] [CrossRef]
- Küzma, M.; Dominko, R.; Meden, A.; Makovec, D.; Bele, M.; Jamnik, J.; Gaberšček, M. Electrochemical activity of Li2FeTiO4 and Li2MnTiO4 as potential active materials for Li ion batteries: A comparison with Li2NiTiO4. J. Power Sources 2009, 189, 81–88. [Google Scholar] [CrossRef]
- Xu, D.; Wan, J.; Xu, D.; Luo, T.; Zhong, Y.; Yang, X.; Yang, L.; Zhang, Z.; Wang, X. Chelation of metal ions with citric acid in the ammoniation process of wet-process phosphoric acid. Can. J. Chem. Eng. 2020, 98, 665–675. [Google Scholar] [CrossRef]
- Rodrigo, M.M.; Ribeiro, A.C.F.; Verissimo, L.M.P.; Esteso, M.A.; Leaist, D.G. Coupled diffusion in aqueous citric acid + calcium citrate solutions. J. Chem. Thermodyn. 2019, 131, 314–321. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, F.; Zhang, C.; Zou, X.; Bi, X.; Wang, D.; Li, H.; Zhang, S. Photodegradation of Malachite Green Catalyzed by Aqueous Iron(III)-Citrate Complex: Roles of Iron(II) and Hydrogen Peroxide. ChemistrySelect 2019, 4, 2089–2094. [Google Scholar] [CrossRef]
- Nejad, M.I.; Price, N.E.; Haldar, T.; Lewis, C.; Wang, Y.; Gates, K.S. Interstrand DNA Cross-Links Derived from Reaction of a 2-Aminopurine Residue with an Abasic Site. ACS Chem. Biol. 2019, 14, 1481–1489. [Google Scholar] [CrossRef]
- Hong, C.; Brookes, V.J.; Zadoks, R.N.; Webb, C.E. Citric Acid and Sodium Bicarbonate as an Alternative Carbon Dioxide Source for Mosquito Surveillance. Insects 2025, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, D.; Jin, H.; Zeng, X.; Qi, J.; Yang, Z.; You, F.; Yuan, F. Urchin-like band-matched Fe2O3@In2S3 hybrid as an efficient photocatalyst for CO2 reduction. J. Colloid Interface Sci. 2023, 648, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, J.; Cao, Z. CH4 Carbonylation to Acetic Acid Using H2O as an Oxidant on a Rh-Functionalized UiO-67 Combined with Oriented External Electric Fields: Selectivity and Mechanistic Insights from DFT Calculations. Inorg. Chem. 2024, 63, 21110–21120. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Luo, S.; Li, P.; Sun, Q.; Sun, K.; Zhao, W.; Wang, J.; Yan, S. Enhanced structural stability of P2-Na0.75Ni0.25Mn0.75−xTixO2 cathodes with high-voltage non-phase transitions property by Mn-sites manipulation strategy. J. Alloys Compd. 2024, 1005, 176168. [Google Scholar] [CrossRef]
- Luo, M.; Yu, H.; Lan, H.; Yan, L.; Qian, S.; Ye, W.; Shui, M.; Long, N.; Shu, J. Synthesis and electrochemical characteristics of isostructural LiMTiO4 (M = Mn, Fe, Co). Ceram. Int. 2017, 43, 5728–5733. [Google Scholar] [CrossRef]
- Hawley, W.B.; Du, Z.; Kukay, A.J.; Dudney, N.J.; Westover, A.S.; Li, J. Deconvoluting sources of failure in lithium metal batteries containing NMC and PEO-based electrolytes. Electrochim. Acta 2022, 404, 139579. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, G.; Li, J.; Liu, J.; Huang, S.; Cheng, F. Heterogeneous doping-induced surface reconstruction toward high-performance LiNiO2 cathode materials for lithium-ion batteries. Sci. China Mater. 2023, 66, 2582–2590. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Szczęsna, A.; Trzeciak, T.; Ostrowski, A.; Wieciński, P.; Wieczorek, W.; Raróg-Pilecka, W.; Marcinek, M. Addition of yttrium oxide as an effective way to enhance the cycling stability of LiCoO2 cathode material for Li-ion batteries. Solid State Ion. 2020, 355, 115426. [Google Scholar] [CrossRef]
- Hou, P.-Q.; Qu, Y.; Huang, R.; Tian, X.; Li, G.; Luo, S. Facile design and synthesis of Li2FeTiO4 as advanced cathode material for lithium-ion batteries. J. Solid State Electrochem. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, P.; Sun, Q.; Yan, S.; Li, G.; Qu, Y.; Luo, S. Synthesis of Cathode Material Li2FeTiO4 for Lithium-Ion Batteries by Sol–Gel Method. Batteries 2025, 11, 142. https://doi.org/10.3390/batteries11040142
Hou P, Sun Q, Yan S, Li G, Qu Y, Luo S. Synthesis of Cathode Material Li2FeTiO4 for Lithium-Ion Batteries by Sol–Gel Method. Batteries. 2025; 11(4):142. https://doi.org/10.3390/batteries11040142
Chicago/Turabian StyleHou, Pengqing, Qi Sun, Shengxue Yan, Guanglong Li, Yingdong Qu, and Shaohua Luo. 2025. "Synthesis of Cathode Material Li2FeTiO4 for Lithium-Ion Batteries by Sol–Gel Method" Batteries 11, no. 4: 142. https://doi.org/10.3390/batteries11040142
APA StyleHou, P., Sun, Q., Yan, S., Li, G., Qu, Y., & Luo, S. (2025). Synthesis of Cathode Material Li2FeTiO4 for Lithium-Ion Batteries by Sol–Gel Method. Batteries, 11(4), 142. https://doi.org/10.3390/batteries11040142





