Abstract
As an emerging secondary battery system, aqueous zinc-ion batteries (AZIBs) show a broad application prospect in the fields of large-scale energy storage and wearable devices. Manganese-based cathode materials have been widely investigated by many researchers due to their high natural abundance, low toxicity, and multiple variable valence states. However, limited active sites, insufficient solvation, and reactivity kinetics of Mn2+ lead to the attenuation of their electrochemical performance. Herein, we introduce appropriate oxygen vacancies into the δ-MnO2 structure by modulating the annealing temperature. The obtained δ-MnO2-400 electrode provided 503 mAh/g capacity at 0.2 A/g and 99% capacity retention after 3000 times cycling at 1 A/g.
1. Introduction
Efficient and environmentally friendly energy storage technologies are becoming an important support for the development of modern society to address the problems of energy depletion and climate change [1,2]. Electrochemical energy storage shows tremendous potential for development, as the global energy transition and carbon reduction accelerate [3,4]. Lithium-ion batteries are widely used in electric vehicles, portable electronic devices, and other fields due to their high energy density, long life, environmental protection, and high performance [5]. Their use promotes green traveling, energy conservation, and emission reduction [6]. But they are at risk of thermal runaway when overcharged, over discharged, or subjected to physical damage, which can cause fires or even explosions. At the same time, the scarcity of lithium resources limits the sustainability of its large-scale application [7,8]. AZIBs have been a hot research topic in recent years and solve the existing problems at the present stage owing to their numerous advantages such as superior energy density, low cost and high safety [9,10]. The cathode materials are one of the major criteria for the performance evaluation of the battery [11,12]. They mainly include vanadium-based oxides [13], manganese-based oxides [14,15,16], Prussian blue analogs [17,18], polyanionic compounds [19], and quinone analogs [20]. In contrast, MnO2 materials are favored for their high discharge platform, excellent electrochemical reactivity, and ease of preparation [21]. Also, their various polycrystalline structures, such as α, β, γ, ε, δ, λ, and other crystalline phase configurations confer different physical and chemical properties [22,23]. Among them, the δ-MnO2 material possesses a unique layered structure, which favors the formation of oxygen vacancies and electron transfer [24]. However, the actual specific capacity is inferior to the theoretical value and the capacity retention is poor at high current densities. This may be attributed to the fact that the repeated embedding/de-embedding of Zn2+ leads to the deformation or collapse of the electrode structure, which results in a rapid decrease in cycling stability. Moreover, the partial dissolution of Mn2+ increases the solution internal resistance and reduces the cell efficiency and lifetime. Therefore, the intrinsic capacity of the MnO2 cathode is improved by expanding the layer spacing [25], constructing composites with conductive frameworks [26,27], and modifying the polycrystalline structure [28,29]. Despite some improvement, these advances have gradually reached a bottleneck and the products prepared still cannot address the demand for high-performance AZIBs [30].
The introduction of defect engineering provides new approaches to improve the electrochemical properties of transition metal oxides (TMOs) [31,32]. In different types of defects, oxygen vacancies play an essential role for local charge redistribution in TMOs. They can act as a shallow donor, changing the surface and geometry of the material, modulating the band gap and electron density to radically improve conductivity [33,34]. They also reduce the embedding energy barrier of Zn2+ in the cathode material and accelerate the charge transfer [35,36]. Furthermore, the generated vacancies form unsaturated coordination sites on the surface or inside the material. This contributes to the full contact between the active material and the electrolyte, effectively increasing the reaction rate and enhancing the charge storage capacity of the electrode [37,38]. For example, Cao et al. synthesized epsilon-MnO2 nanosheets rich in oxygen vacancies, and the assembled battery delivered a capacity of 337 mAh/g at a current density of 0.1 A/g. The capacity retention rate is 85.9% after 1000 cycles [39]. Xu and co-workers et al. designed a Zn//V-O-MnO2@CNF battery that achieved a capacity of 135 mA h/g at a 1 A/g for 740 cycles [40]. Thus, oxygen vacancies contribute to the improvement of the reaction/diffusion kinetics of ions, providing excellent electrochemical properties for ZIBs.
In this work, we designed δ-MnO2-400 nanoflower structures using hydrothermal and annealing treatment. Appropriate oxygen vacancy concentrations were obtained by controlling different annealing temperatures. Compared to typical birnessite MnO2 materials (with a specific capacity of 250–280 mA h/g, and a theoretical value of 308 mA h/g), we assembled Zn//δ-MnO2-400 cells offer a specific capacity of 503 mA h/g at 0.2 A/g. The capacity retention is 99% for 3000 cycles at 1 A/g current density. The introduction of oxygen defects in MnO2 cathode materials provides an effective strategy for the development of high-performance AZIBs.
2. Experimental Section
2.1. Preparation of Materials
The chemicals used in the experiment were all of analytical grade (AR) and used directly without any purification. In total, 25 mL of 0.025 mol/L MnSO4 and 25 mL of 0.15 mol/L KMnO4 were mixed and stirred at room temperature for 30 min. Then, the mixture was transferred into an 80 mL Teflon-lined autoclave and kept at 160 °C for 12 h. The synthesized brown powder was washed with anhydrous ethanol and deionized (DI) water three times, respectively, and dried at 60 °C overnight. Finally, it was annealed in a muffle furnace at 2°/min for 2 h at 300 °C, 400 °C, and 500 °C. The final obtained samples were named δ-MnO2, δ-MnO2-300, δ-MnO2-400, and δ-MnO2-500, respectively.
2.2. Morphology and Structure Characterization
The morphologies of the samples were observed using a scanning electron microscope (SEM, Tescan mira3Zeiss sigma500) and a high-resolution transmission electron microscope (HRTEM, FEI Tecnai G2 F30JEOL-2100F). The crystal structure and chemical bonding were characterized using X-ray diffraction (XRD, Shimadzu-7000, Cu Kα radiation, k = 0.1541 nm), an X-ray Photoelectron Spectrometer (XPS, Thermo Scientific Escalab 250 Xi) and Electron Paramagnetic Resonance (EPR, Bruker EMX plus). Specific surface area, pore size, and elemental distribution were determined using the N2 adsorption–desorption isotherm method (BET, JWGB-T200) and energy dispersive spectroscopy (EDS), respectively.
2.3. Electrochemical Characterization
The active substance, super p, and polyfluoroethylene were ground with a mass ratio (7:2:1) and an appropriate amount of N-methyl-2 pyrrolidone (NMP) was added to form a slurry. They were then evenly coated on carbon paper and kept in a vacuum oven at 60 °C for 12 h. Type 2032 cells were assembled at room temperature by the obtained samples, glass fiber separator, electrolyte (2 M ZnSO4 + 0.2 M MnSO4), and zinc foil. The average mass of the cathode material was 1.05–1.5 mg. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were tested in an electrochemical workstation (CHI660E). Cycling, multiplier performance, and the galvanostatic charge/discharge (GCD) curves were tested on the Neweare (CT-4008 T) system.
3. Results and Discussion
We firstly characterized the crystal structure of the samples using XRD. It is noteworthy from Figure 1a that the annealing temperature can affect the crystallographic orientation of MnO2 materials. The diffraction peaks of the δ-MnO2-300 and δ-MnO2-400 powders at 12.1, 24.6, 36.9, and 66.1° correspond to the (001), (002), (11-1), and (114) planes, respectively. They can be indexed as MnO2 phases with a birnessite structure (JCPDs no. 13-0105), and no other peaks are found, which proves their high purity. The peaks at the (001) and (11-1) crystal planes gradually broaden with increasing temperature, indicating that the generated vacancy defects induced lattice distortion [41,42]. In addition, the structure is destroyed due to its poor thermal stability and a phase transition occurs at 500 o. Its diffraction peaks match well with the MnO2 phase (JCPDs no. 72-1982). Figure 1b shows the N2 isothermal curves and pore size distributions of the four samples. The specific surface area of δ-MnO2-400 is 63 m2/g, which is larger than the other three samples (δ-MnO2: 23 m2/g; δ-MnO2-300: 53 m2/g; and δ-MnO2-500: 17 m2/g). This can be attributed to the introduction of oxygen vacancies. This mitigates the volume change during the redox process while providing sufficient active sites. Their pore sizes are 4.6 nm, 4.3 nm, 3.2 nm, and 2.8 nm, respectively. The mesoporous structure facilitates the penetration of the electrolyte and effectively accelerates the rapid migration of Zn2+, thus allowing the active material to be fully utilized. However, the appearance of a new phase may occupy the space that originally belongs to the main material, leading to the reduction in the voids between the nanosheets.
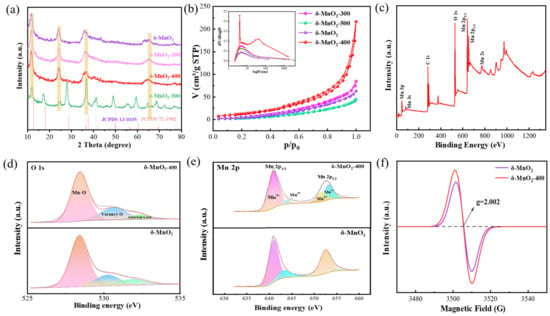
Figure 1.
Structure characterization: (a) XRD patterns of four samples; (b) N2 isotherms and pore size; (c–e) XPS survey spectra of O 1s and Mn 2p; (f) EPR.
Then, XPS was used to study the chemical composition and valence distribution of the δ-MnO2 and δ-MnO2-400 samples. The signal peaks of Mn 2p, Mn 3p, Mn 3s, Mn 2s, and O 1s can be found in the survey spectra (Figure 1c). In Figure 1d, the peaks located at 528.28 eV, 530.98 eV, and 532.43 eV can be assigned to Mn-O bonds, oxygen vacancies, and adsorbed oxygen [43]. It can be obviously observed that the peak intensity of the δ-MnO2-400 sample is stronger than that of the pristine sample. The results confirm an increase in the vacancy concentration of the samples after annealing treatment, which is in agreement with previous reports [44]. From Figure 1e, Mn 2p3/2 and Mn 2p1/3 are located at 640.88 eV and 652.98 eV, with a spin difference of 12.1 eV, which is consistent with the characteristics of the MnO2 material [45]. In the Mn2p1/2 orbital, the integral area of Mn3+/Mn4+ gradually changes in order to balance the produced oxygen vacancies. In addition, EPR can further confirm the increase in the concentration of vacancies, as shown in Figure 1f. Because the electrons are bound by vacancy, there is an electron spin resonance (ESR) signal at g = 2.002 [46]. The ESR intensity of the δ-MnO2-400 sample is higher than that of δ-MnO2, indicating sufficient oxygen vacancy, which is consistent with the XPS results.
The morphology of the four samples was observed using SEM. They are all microspheres consisting of smooth and intersecting nanosheets (Figure 2a–d). This unique structure ensures that the nanosheets possess sufficient gaps between them to provide channels for the transport of ions and accelerate the reaction rate. It can be observed that some nanorods are interspersed between the microspheres owing to the high annealing temperature. They may not only clog the pores of the matter, but also promote the agglomeration phenomenon. This is the main reason for the small specific surface area of the δ-MnO2-500 material. At the same time, the creation of new phases changes the chemistry of the material and affects its electrochemical properties. This will be discussed in detail in the next section. In addition, the microstructures of the δ-MnO2-400 and δ-MnO2-500 powders were investigated using HRTEM, respectively, as shown in Figure 2e–h. The interplanar spacings of 0.262 nm and 0.244 nm can be indexed to the (11-1) and (510) crystal planes, respectively, and can be calculated from line profiles. As seen the EDS (Figure 2i), the Mn and O elements are uniformly distributed on the surface.
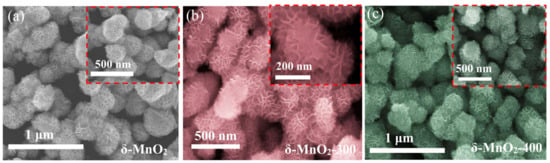
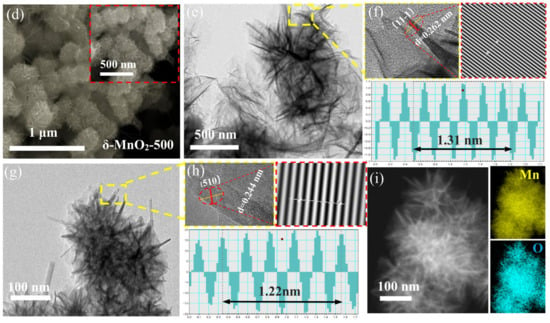
Figure 2.
Morphology characterization: (a–d) SEM images of four powders; (e–h) TEM and HRTEM images of δ-MnO2-400 and δ-MnO2-500; (i) EDS.
In Figure 3a, some button cells are assembled at room temperature in order to investigate the effect of annealing temperatures on the electrochemical properties of the δ-MnO2 electrode. Figure 3b shows the CV of the δ-MnO2-400 cathode from the first to third circles at 0.2 mV/s in the voltage range of 0.8–2 V. The two pairs of redox peaks prove that the reaction process is a two-step process, which refers to the insertion/extraction of H+ and Zn2+ ions. Also, compared with other electrodes, δ-MnO2-400 possesses higher current intensity and lower polarization, demonstrating higher chemical activity and superior electrochemical performance. Figure 3c shows the CV of the δ-MnO2-400 cathode from the first to third circles. The overlap of the curve shapes indicates that the reaction is highly reversible. Meanwhile, it can be observed that there are two discharge platforms located at 1.39 V and 1.22 V in the GCD curves. The rate performance is an important criterion for evaluating ZIBs. As can be seen from Figure 3d–f, the specific capacity of the δ-MnO2-400 batteries can reach 500, 308, 154, 83, 51, and 29 mAh/g at 0.2, 0.5, 1, 2, 3, and 5 A/g, respectively. When the current density is restored to 0.2 A/g, the capacity retention rate is 81.7%. The GCD curve shows that the capacity decreases with the increase in current density. In addition, the cell retains a capacity of 417 mAh/g after 100 cycles at 0.2 A/g (Figure 3g). Significantly, it maintains 99% capacity retention and 100% coulombic efficiency after 3000 cycles, as shown in Figure 3h. Table 1 lists the various data of different types of batteries [15,47,48,49,50,51,52,53,54,55,56,57]. The above results demonstrate the excellent cycling stability of the prepared δ-MnO2-400 cathodes.
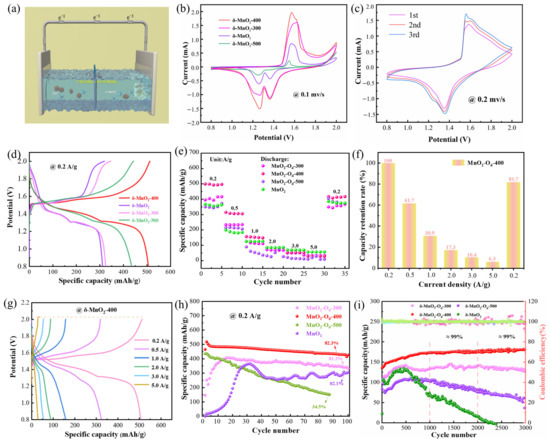
Figure 3.
Electrochemical performance: (a) The ZIB structure of δ-MnO2-X; (b) CV curves of δ-MnO2-X; (c) CV of δ-MnO2-400 at 0.2 mV/s; (d) GCD curves of four electrodes; (e) rate capability; (f) capacity retention rate of δ-MnO2-400; (g) GCD curves of δ-MnO2-400; (h,i) cycling performance at 1.0 A/g.

Table 1.
Initial capacity of various cathode materials.
We further investigated δ-MnO2-400’s electrochemical kinetics and energy storage mechanism by utilizing CV, GITT, and EIS. Figure 4a shows the CV curves at 0.1–0.5 mV/s, with the redox peak shift with the sweep speed increasing. The peak intensities located at 1.21 V and 1.67 V are gradually smooth. This is due to the inability of reactants on the electrode surface to diffuse in time or the formation of intermediates becoming less obvious, resulting in peak merging. In addition, the peak current (i) and sweep rate (v) can be defined by the following equation [58]:
where a and b are adjustable parameters, where the value of b can quantify the contribution of surface and diffusion control, as shown in Figure 4b,c. After fitting, the four b values of the δ-MnO2-400 electrode are 0.63 (peak 1), 0.69 (peak 2), 0.75 (peak 3), and 0.77 (peak 4). This suggests that capacitance control and diffusion synergistically dominate the charge storage [59]. In addition, the contribution of the pseudo-capacitance (k1v) and the diffusion component (k2v1/2) can be expressed by equation [60]:
where i is the current at a fixed potential. Capacitive contribution increases from the original 44.4% to 72.6% with sweep speed, demonstrating its excellent electrochemical kinetics. The Zn2+ diffusion coefficients (DZn2+) of the samples are then estimated using the galvanostatic intermittent titration technique (GITT) (Figure 4d). It is calculated using the following formula [61]:
where τ is the relaxation time; ∆Es is the pulse-induced voltage change and ∆Et is the voltage change for constant current charging (discharging). The discharge process is divided into two regions; the first region (platform I) corresponds mainly to the embedding of H+, while the second region (platform II) is dominated by the embedding of Zn2+. The ionic diffusion coefficients of platform I are between 10−8 and 10−6 cm2/s, whereas those of platform II are about 10−10 cm2/s. This is attributed to the large difference in the radii of H+ and Zn2+, with the H+ ionic diffusion coefficient being two orders of magnitude higher than that of Zn2+. This is in accordance with the above studies on the kinetics of redox reactions at the MnO2 electrode. The DZn2+ of δ-MnO2-400 (10−6–10−8 cm2/s) is significantly higher than that of the other obtained cathodes, demonstrating its excellent kinetics. Compared to the other three electrodes, δ-MnO2-400 shows the longest discharge time, representing more time for Zn2+ to diffuse inside the electrode material during the relaxation phase (the phase where no current passes through). This helps the ions to achieve a more uniform distribution within the active material, thus reducing the phenomenon of concentrated polarization. Meanwhile, the proper oxygen vacancies play an important role in facilitating the transport of Zn2+.
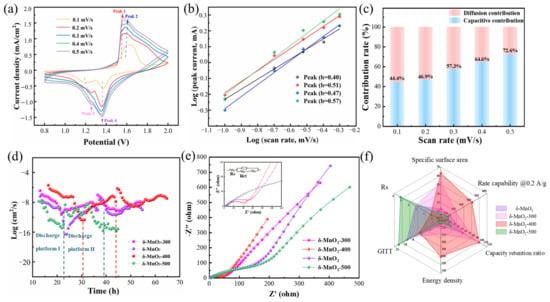
Figure 4.
Reaction kinetics: (a) CV curves of δ-MnO2-400 at 0.1–0.5 mV/s; (b) log(i) versus log(v); (c) capacitive contribution ratios; (d) GITT; (e) EIS; (f) radar chart.
As shown in Figure 4e, we further studied the EIS of the Zn//δ-MnO2-X cells in the range of 100 kHz–0.01 Hz. In the high-frequency region, the diameter of the semicircle in the curve is the charge transfer resistance (Rct) between the electrode and the electrolyte. Its size is proportional to the resistance. In the low-frequency region, the slope of the straight line (Zw) represents the degree of ion diffusion. In addition, the intercept between the straight line and the X-axis is the series resistance (Rs) (the solution internal resistance). In contrast, the δ-MnO2-400 electrode show smaller Rs (δ-MnO2: 3.248; δ-MnO2-300: 3.267; δ-MnO2-400: 1.292; δ-MnO2-500: 5.215) and Rct (98; 42; 29; 104) and higher slopes. This indicates a rapid and effective ion transport diffusion process. For the electrode materials, the energy density and power density are calculated using the following equations (Equations (4) and (5)):
where E (W h/kg) is the energy density and P is the power density (kW/kg). Q (A h), V (V), i (A), and m (kg) denote the discharge capacity of cathode, the operating potential, the discharge current, and the mass load. The energy density of the Zn//δ-MnO2-400 cell is calculated to be 300 Wh/kg at a power density of 60 W/kg. We used radar charts in order to compare the performance of different electrodes (Figure 4f). Taken together, the δ-MnO2-400 cathode possesses excellent reaction kinetics and rate performance.
E = QU/2m
P = iU/2m
In order to further explore the energy storage mechanism, we studied the structure of δ-MnO2-400 samples at specific charging/discharging states using ex situ XRD and XPS. Figure 5a,b show the XRD patterns in different states. During the first cycle of the discharge, the (001) plane shifts 0.5° from 12.16° to the left, suggesting that Zn2+ is successfully inserted into the host structure. In the subsequent reaction process, some additional peaks are generated at 11.58° and 38.94°, which can be indexed to the zinc sulfate hydroxide hydrate (Zn4SO4(OH)6·4H2O, ZSH, PDF: 44-0673) phases [62]. Also, the ZnMn2O4 (ZMO, PDF: 77-0470) phase is detected. When charged to 2.0 V, the (001) crystalline peak returns to 12.14°, which can be attributed to the shuttling effect of Zn2+. In addition, the new peaks gradually disappear as the reaction proceeds again, demonstrating the high reversibility of the δ-MnO2-400 sample. Then, we studied the XPS at different voltages of the electrodes in order to verify the phase transition process. The Mn 2p spectra are convoluted into three peaks, situated at 638.78, 641.08, and 643.68 eV, corresponding to Mn2+, Mn3+, and Mn4+, respectively (Figure 5c). The presence of Mn2+ may be caused by the embedding of Zn2+ resulting in the reduction in part of the Mn3+ to Mn2+ [63]. It also can be observed that the intensity of the Mn3+ and Mn4+ peaks diminishes at complete discharge to 0.8 V. Figure 5d shows the Zn 2p spectra with characteristic peaks at 1020.98 eV and 1044.08 eV. The decrease in peak intensity compared to the discharge state is due to the precipitation of Zn2+ and the decomposition of ZSH.
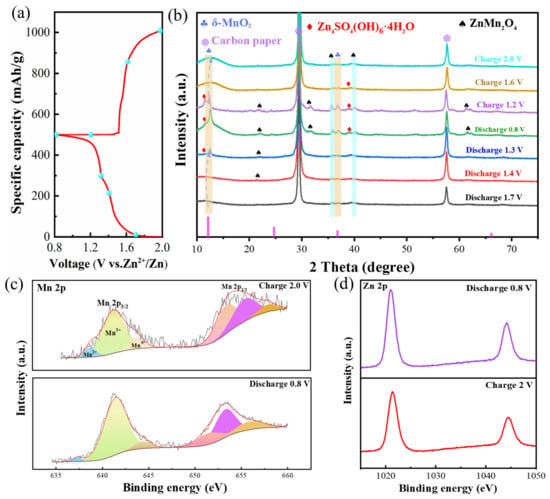
Figure 5.
(a,b) ex situ XRD patterns; (c) Mn 2p; (d) Zn 2p.
4. Conclusions
In summary, we prepared several Mn-based electrode materials for zinc-ion batteries. The introduction of appropriate oxygen vacancies accelerated the transport rates of Zn2+ and H+ and promoted the electrochemical reactions. At the same time, it improved the charge transfer and structural stability of the material. These advantages lead to a high specific capacity, long cycle life, and high energy density of the assembled Zn//δ-MnO2-400 cells. This study provides potential high-performance cathode materials for next-generation ZIBs. Also, it puts forward an effective vacancy modulation strategy to design future electrode materials for portable micro-/nano-devices.
Author Contributions
Conceptualization, S.L. and X.W.; Software, S.L.; Validation, S.L.; Formal analysis, S.L. and X.W.; Investigation, S.L.; Resources, X.W.; Data curation, S.L. and X.W.; Writing—original draft, S.L.; Writing—eview & editing, X.W.; Project administration, X.W.; Funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, J.N.; Xiao, J.F.; Chen, X.F.; Liang, X.M.; Fan, L.Y.; Ye, D.Q. Allowance and allocation of industrial volatile organic compounds emission in China for year 2020 and 2030. J. Environ. Sci. 2018, 69, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. A facile carbon modification avenue to construct highly stable V2O5 electrode for aqueous zinc ion batteries. ACS Sustain. Chem. Eng. 2023, 11, 13298–13305. [Google Scholar] [CrossRef]
- Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of aqueous-and non-aqueous-based binder polymers and the mixing ratios for Zn//MnO2 batteries with mildly acidic aqueous electrolytes. Batteries 2021, 7, 40. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.H.; Fu, W.Q.; Cui, Y.W.; Wang, J.Q.; Zhao, D.G.; Yang, C.; Wang, X.T.; Cao, B.Q. Plasma-induced ε-MnO2 based aqueous zinc-ion batteries and their dissolution-deposition mechanism. J. Mater. Sci. Technol. 2022, 127, 206–213. [Google Scholar] [CrossRef]
- Li, S.L.; Zhao, M.; Zhang, D.D.; Wu, X. High-capacity aqueous Zn/MnO2 batteries: A clue of K ion pre-intercalation. Cryst. Growth Des. 2023, 23, 8156–8162. [Google Scholar] [CrossRef]
- Wang, T.T.; Li, C.P.; Xie, X.S.; Lu, B.G.; He, Z.X.; Liang, S.Q.; Zhou, J. Anode materials for aqueous zinc ion batteries: Mechanisms, properties, and perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef]
- Liu, Y.; Umar, A.; Wu, X. Metal-organic framework derived porous cathode materials for hybrid zinc ion capacitor. Rare Met. 2022, 41, 2985–2991. [Google Scholar] [CrossRef]
- Durena, R.; Zukuls, A. A Short Review: Comparison of Zinc-Manganese dioxide batteries with different pH aqueous electrolytes. Batteries 2023, 9, 311. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of vanadium-based electrode materials for rechargeable aqueous zinc ion batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Chen, L.N.; An, Q.Y.; Mai, L.Q. Recent advances and prospects of cathode materials for rechargeable aqueous zinc-ion batteries. Adv. Mater. Interfaces 2019, 6, 1900387. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wu, X. Dual-ion carrier storage through Mg2+ addition for high-energy and long-life zinc-ion hybrid capacitor. Int. J. Min. Met. Mater. 2024, 31, 179–185. [Google Scholar] [CrossRef]
- Yan, M.Y.; He, P.; Chen, Y.; Wang, S.Y.; Wei, Q.L.; Zhao, K.N.; Xu, X.; An, Q.Y.; Shuang, Y.; Shao, Y.Y.; et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, X. Defect engineering of vanadium-based electrode materials for zinc ion battery. Chinese Chem. Lett. 2023, 34, 107839. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.Y.; Liu, Y.C.; Zhao, Q.; Lei, K.X.; Chen, C.C.; Liu, X.S.; Chen, J. Cation-deficient spinel ZnMn2O4 cathode in Zn (CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef]
- Pan, H.L.; Shao, Y.Y.; Yan, P.F.; Cheng, Y.W.; Han, K.S.; Nie, Z.M.; Wang, C.M.; Yang, J.H.; Li, X.L.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.Y.; Yang, C.Y.; Fan, X.L.; Ma, Z.H.; Han, F.D.; Hu, R.D.; Zhu, M.; Wang, C.S. Zn/MnO2 battery chemistry with H+ and Zn2+ co-insertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, L.; Zhou, X.F.; Liu, Z.P. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Trócoli, R.; Mantia, F. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef]
- Wang, K.H.; Shangguan, M.L.; Zhao, Y.B.; Tian, H.R.; Wang, F.; Yuan, J.L.; Lan, X. Flexible and stable n-isopropylacrylamide/sodium alginate gel electrolytes for aqueous Zn-MnO2 batteries. Batteries 2023, 9, 426. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, W.W.; Luo, Z.Q.; Liu, L.J.; Lu, Y.; Li, Y.X.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, 1761. [Google Scholar] [CrossRef]
- Wen, S.; Gu, X.; Ding, X.W.; Dai, P.C.; Zhang, D.J.; Li, L.J.; Liu, D.D.; Zhao, X.B.; Yang, J. Boosting fast and stable alkali metal ion storage by synergistic engineering of oxygen vacancy and amorphous structure. Adv. Funct. Mater. 2021, 32, 2106751. [Google Scholar] [CrossRef]
- Xie, H.X.; Cui, J.X.; Yao, Z.; Ding, X.K.; Zhang, Z.H.; Luo, D.; Lin, Z. Revealing the role of spinel phase on Li-rich layered oxides: A review. Chem. Eng. J. 2022, 427, 131978. [Google Scholar] [CrossRef]
- Luo, H.; Wang, B.; Jian, J.H.; Wu, F.D.; Peng, L.; Wang, D.L. Stress-release design for high-capacity and long-time lifespan aqueous zinc-ion batteries. Mater. Today Energy 2021, 21, 100799. [Google Scholar] [CrossRef]
- Yadav, P.; Putro, D.; Kim, J.; Rai, A.K. Pom-pom flower-like morphology of δ-MnO2 with superior electrochemical performances for rechargeable aqueous zinc ion batteries. Batteries 2023, 9, 133. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. Enhanced electrochemical performance of Zn/VOx batteries by a carbon-encapsulation strategy. ACS Appl. Mater. Interfaces 2022, 14, 11654–11662. [Google Scholar] [CrossRef]
- Cao, Z.W.; Zhang, H.; Song, B.; Xiong, D.Y.; Tao, S.S.; Deng, W.T.; Hu, J.G.; Hou, H.S.; Zou, G.Q.; Ji, X.B. Angstromlevel ionic sieve 2D-MOF membrane for high power aqueous zinc anode. Adv. Funct. Mater. 2023, 33, 2300339. [Google Scholar] [CrossRef]
- Liu, C.; Deng, L.J.; Li, X.Z.; Wu, T.; Zhang, W.J.; Cui, H.S.; Yang, H. Metal–organic frameworks for solid-state electrolytes: A mini review. Electrochem. Commun. 2023, 150, 107491. [Google Scholar] [CrossRef]
- Wei, C.G.; Xu, C.J.; Li, B.H.; Du, H.D.; Kang, F.Y. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage. J. Phys. Chem. Solids 2012, 73, 1487–1491. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Putro, D.Y.; Mathew, V.; Kim, S.; Jo, J.; Kim, S.; Sun, Y.K.; Kim, K.; Kim, J. Structural transformation and electrochemical study of layered MnO2 in rechargeable aqueous zinc-ion battery. Electrochim. Acta 2018, 276, 1–11. [Google Scholar] [CrossRef]
- Huang, J.H.; Wang, Z.; Hou, M.Y.; Dong, X.L.; Liu, Y.; Wang, Y.G.; Xia, Y.Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, T.; Lu, J.P.; Sun, L.T.; Ni, Z.H. Defect engineering in 2D materials: Precise manipulation and improved functionalities. Research 2019, 2019, 4641739. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yan, D.F.; Chen, W.; Zou, Y.Q.; Chen, R.; Zang, S.Q.; Wang, Y.Y.; Yao, X.D.; Wang, Y.Y.; Yao, X.D.; et al. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, H.; Zhang, Z.H.; Liu, Y.; Song, J.N.; Liu, T.; He, Y.N.; Meng, A.L.; Sun, C.L.; Hu, M.; et al. The semicoherent interface and vacancy engineering for constructing Ni(Co)Se2@Co(Ni)Se2 heterojunction as ultrahigh-rate battery-type supercapacitor cathode. Adv. Funct. Mater. 2022, 32, 2202063. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Yao, Z.G.; Shadike, Z.; Lei, M.; Liu, J.J.; Li, C.L. Defect concentration-mediated T-Nb2O5 anodes for durable and fast charging Li-ion batteries. Adv. Funct. Mater. 2022, 32, 2107060. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Chang, C.; Teng, F.; Zhao, Y.F.; Chen, G.B.; Shi, R.; Waterhouse, G.; Huang, W.F.; Zhang, T.R. Defect-engineered ultrathin δ-MnO2 nanosheet arrays as bifunctional electrodes for efficient overall water splitting. Adv. Energy Mater. 2017, 7, 1700005. [Google Scholar] [CrossRef]
- Pan, X.Y.; Yang, M.Q.; Fu, X.Z.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Li, Y.; Qian, J.; Zhang, M.H.; Wang, S.; Wang, Z.H.; Li, M.S.; Bai, Y.; An, Q.Y.; Xu, H.J.; Wu, F. Co-construction of sulfur vacancies and heterojunctions in tungsten disulfide to induce fast electronic/ionic diffusion kinetics for sodium-ion batteries. Adv. Mater. 2020, 32, 2005802. [Google Scholar] [CrossRef]
- Yao, W.Q.; Tian, C.H.; Yang, C.; Xu, J.; Meng, Y.F.; Manke, I.; Chen, N.; Wu, Z.L.; Zhan, L.; Wang, Y.L.; et al. P-doped NiTe2 with Te-vacancies in lithium-sulfur batteries prevents shuttling and promotes polysulfide conversion. Adv. Mater. 2022, 34, 2106370. [Google Scholar] [CrossRef]
- Zhao, M.; Li, S.L.; Wu, X.; Luo, S.H. Gallium ion pre-insertion protocol to (NH4)2V10O25·8H2O cathode materials for reversible aqueous Zn battery. Adv Mater. Technol. 2024, 9, 2400125. [Google Scholar] [CrossRef]
- Liu, W.; Su, Q.M.; Zhu, R.R.; Shi, W.H.; Zhang, F.; Du, G.H.; Zhao, W.Q.; Zhao, M.; Xu, B.S. Chemical lithiation-induced oxygen vacancies in MnO2 at room temperature for aqueous zinc-ion batteries. ACS Appl. Energy Mater. 2023, 6, 6689–6699. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.L.; Cao, D.X.; Yan, J. Creating oxygen-vacancies in MoO3−x nanobelts toward high volumetric energy-density asymmetric supercapacitors with long lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, P.; Wang, Q.Y.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.L.; Cao, D.X.; Yan, J.; Zhang, Q. High capacity and kinetically accelerated lithium storage in MoO3 enabled by oxygen vacancies and heterostructure. Adv. Energy Mater. 2021, 11, 2101712. [Google Scholar] [CrossRef]
- Zhai, X.Z.; Qu, J.; Hao, S.M.; Jing, Y.Q.; Chang, W.; Wang, J.; Li, W.; Abdelkrim, Y.; Yuan, H.F.; Yu, Z.Z. Layered birnessite cathode with a displacement/intercalation mechanism for high-performance aqueous zinc-ion batteries. Nano-Micro Lett. 2020, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Q.; Deng, H.; Pan, T.T.; Zhang, C.B.; He, H. Thermal annealing induced surface oxygen vacancy clusters in α-MnO2 nanowires for catalytic ozonation of VOCs at ambient temperature. ACS Appl. Mater. Interfaces 2023, 15, 9362–9372. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Zhang, W.; Sheng, J.Z.; Amiinu, I.S.; Kou, Z.K.; Yang, J.L.; Mai, L.Q.; Mu, S.C. Na Mn-O nanocrystals as a high capacity and long-life anode material for Li-ion batteries. Adv. Energy Mater. 2017, 7, 1602092. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; He, W.; Shao, Y.; Li, Y. Oxygen vacancy modulation of bimetallic oxynitride anodes toward advanced Li-ion capacitors. Adv. Funct. Mater. 2020, 30, 2000350. [Google Scholar] [CrossRef]
- Zhang, Z.C.Y.; Xi, B.J.; Wang, X.; Ma, X.J.; Chen, W.H.; Feng, J.K.; Xiong, S.L. Oxygen defects engineering of VO2·xH2O nanosheets via in situ polypyrrole polymerization for efficient aqueous zinc ion storage. Adv. Funct. Mater. 2021, 31, 2103070. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Q.H.; Liu, H.M.; Tian, S.; Chen, B.L.; Zhao, J.; Li, J.F. A case study of β-and δ-MnO2 with different crystallographic forms on ion-storage in rechargeable aqueous zinc ion battery. Electrochem. Acta 2019, 324, 134867. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Hydrogen and sodium ions Co-intercalated vanadium dioxide electrode materials with enhanced zinc ion storage capacity. Nano Energy 2021, 86, 106124. [Google Scholar] [CrossRef]
- Bai, Y.C.; Zhang, H.; Xiang, B.; Yao, Q.; Dou, L.; Dong, G.Y. Engineering porous structure in Bi-component-active ZnO quantum dots anchored vanadium nitride boost’s reaction kinetics for zinc storage. Nano Energy 2021, 89, 106386. [Google Scholar] [CrossRef]
- Xiong, T.; Yu, Z.G.; Wu, H.J.; Du, Y.H.; Xie, Q.D.; Chen, J.S.; Xue, J.M. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Li, N.; Hou, Z.D.; Liang, S.Y.; Cao, Y.J.; Liu, H.Y.; Hua, W.; Wei, C.G.; Kang, F.Y.; Wang, J.G. Highly flexible MnO2@polyaniline core-shell nanowire film toward substantially expedited zinc energy storage. Chem. Eng. J. 2023, 452, 139408. [Google Scholar] [CrossRef]
- Xie, Q.X.; Cheng, G.; Xue, T.; Huang, L.H.; Chen, S.H.; Sun, Y.; Sun, M.; Wang, H.Z.; Yu, L. Alkali ions pre-intercalation of δ-MnO2 nanosheets for high-capacity and stable Zn-ion battery. Mater. Today Energy 2022, 24, 100934. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhang, P.J.; Liang, J.R.; Xia, X.Y.; Ren, L.T.; Li, S.; Liu, W.; Sun, X.M. Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Storage Mater. 2022, 47, 424–433. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Kim, J. Ambient redox synthesis of vanadium-doped manganese dioxide nanoparticles and their enhanced zinc storage properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Tang, H.; Chen, W.H.; Li, N.; Hu, Z.L.; Xiao, L.; Xie, Y.J.; Xi, L.J.; Ni, L.; Zhu, Y.R. Layered MnO2 nanodots as high-rate and stable cathode materials for aqueous zinc-ion storage. Energy Storage Mater. 2022, 48, 335–343. [Google Scholar] [CrossRef]
- Zhao, C.R.; Liu, Y.; Li, S.L.; Wu, X.; Liu, J.H. PVP decorated H3.78V6O13 microspheres assembled by nanosheets for aqueous zinc ion batteries at variable work temperature. Chinese Chem. Lett. 2024, 2024, 110185. [Google Scholar] [CrossRef]
- Liu, S.C.; Zhu, H.; Zhang, B.H.; Zhu, H.K.; Ren, Y.; Geng, H.B.; Li, Q.; Li, C.C. Tuning the kinetics of zinc-ion insertion/extraction in V2O5 by in situ polyaniline intercalation enables improved aqueous zinc-ion storage performance. Adv. Mater. 2020, 32, 2001113. [Google Scholar] [CrossRef]
- Yang, X.; Deng, W.Z.; Chen, M.; Wang, Y.B.; Sun, C.F. Mass-producible, quasi-zero-strain, lattice-water-rich inorganic open frameworks for ultrafast-charging and long-cycling zinc-ion batteries. Adv. Mater. 2020, 32, 2003592. [Google Scholar] [CrossRef]
- Yang, S.H.; Zhang, L.; Luo, M.J.; Cui, Y.W.; Wang, J.Q.; Zhao, D.G.; Yang, C.; Wang, X.T.; Cao, B.Q. Synergistic combination of a Co-doped σ-MnO2 cathode with an electrolyte additive for a high-performance aqueous zinc-ion battery. ChemPhysMater 2023, 2, 77–82. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.X.; Fang, G.Z.; Wang, Z.Q.; Cai, Y.S.; Tang, B.Y.; Zhou, J.; Liang, S.Q. V2O5 nanospheres with mixed vanadium valences as high electrochemically active aqueous zinc-ion battery cathode. Nano-Micro Lett. 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Gao, Q.L.; Xia, Y.M.; Lin, X.S.; Liu, W.L.; Ren, M.M.; Kong, F.G.; Wang, S.J.; Lin, C. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).