Influence of a Constant Magnetic Field on the Mechanism of Adrenaline Oxidation
Abstract
1. Introduction
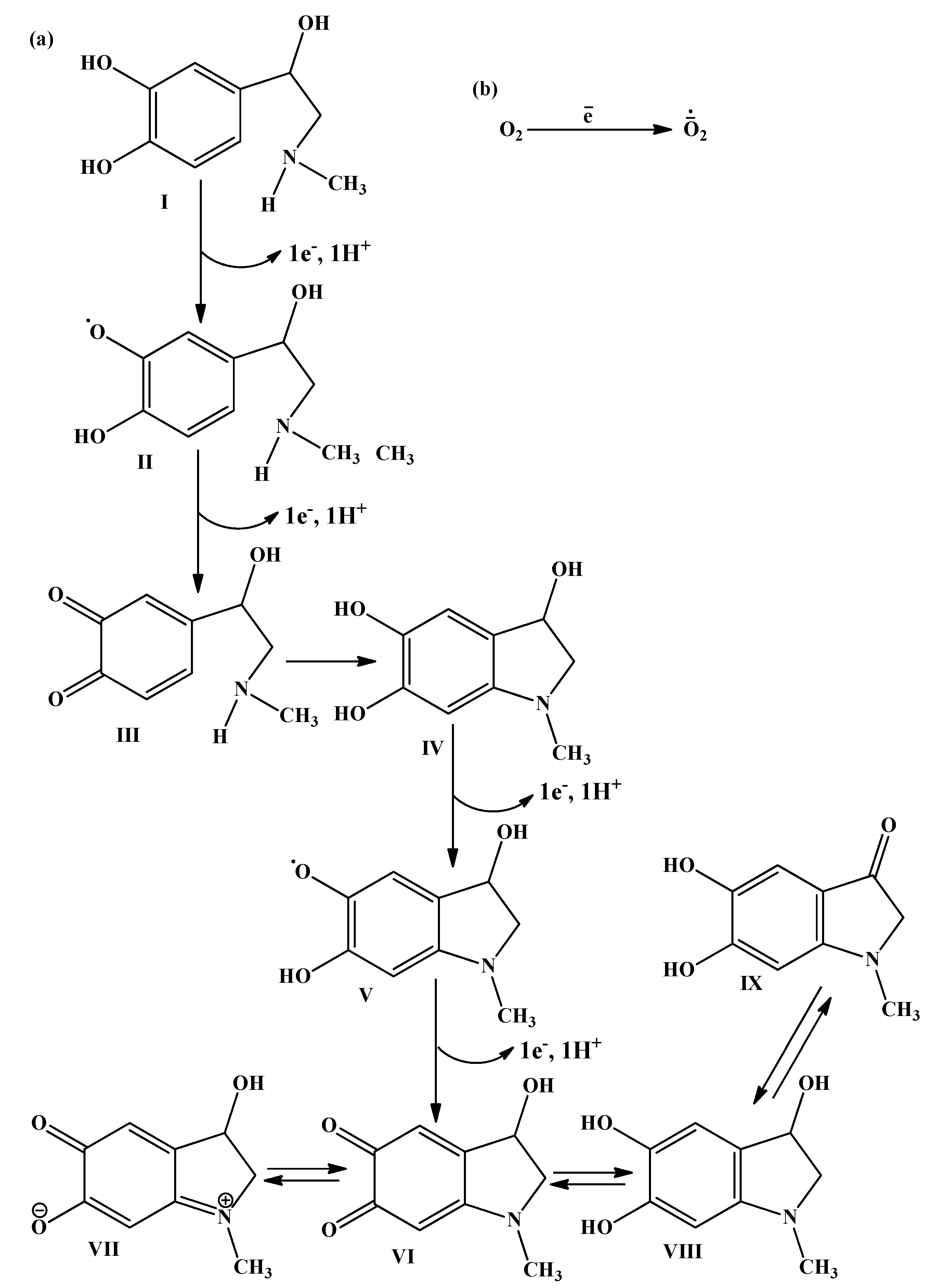
1.1. The Base Mechanisms
1.2. Purpose of the Work and Research Methodology
2. Experimental
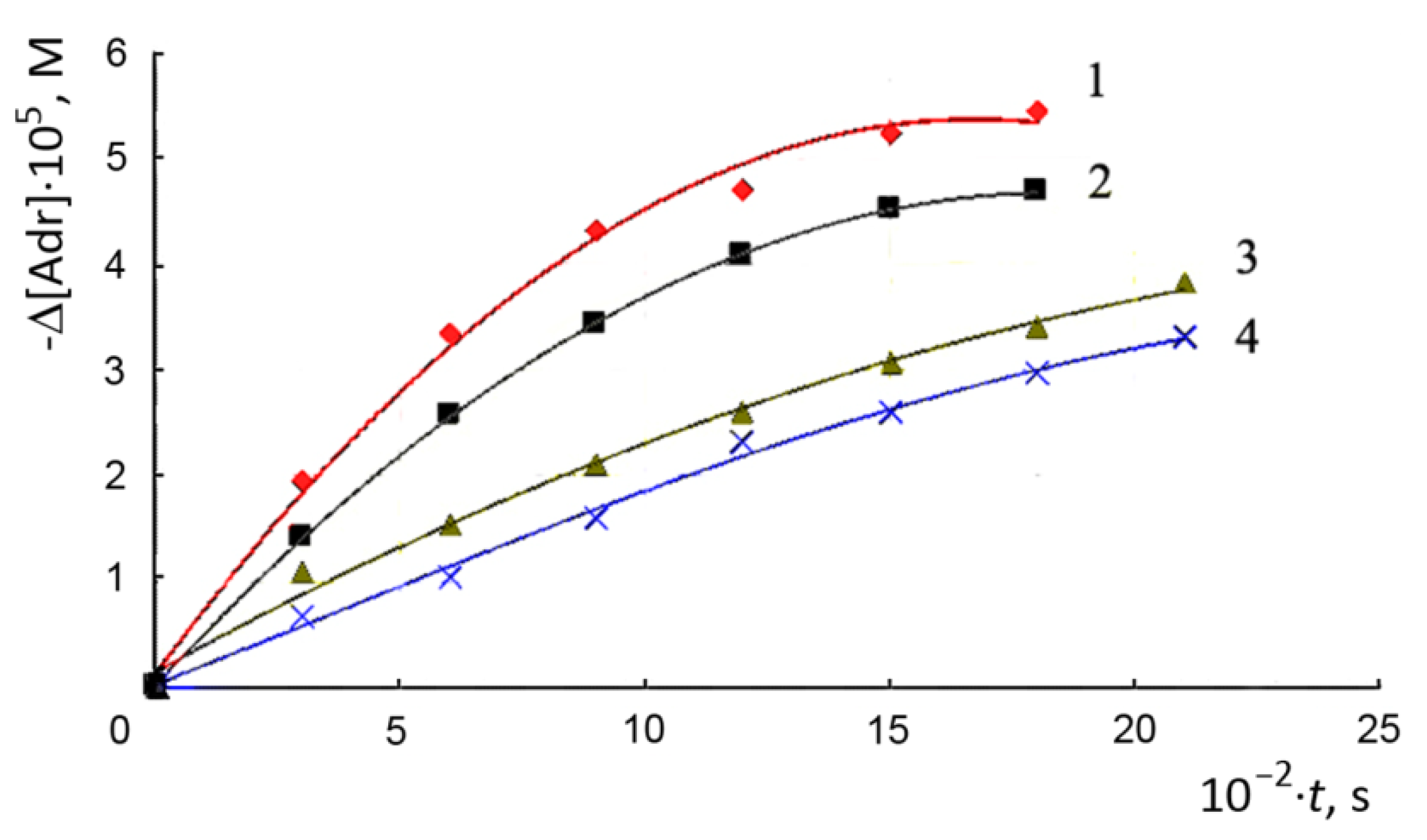
3. Results and Discussion
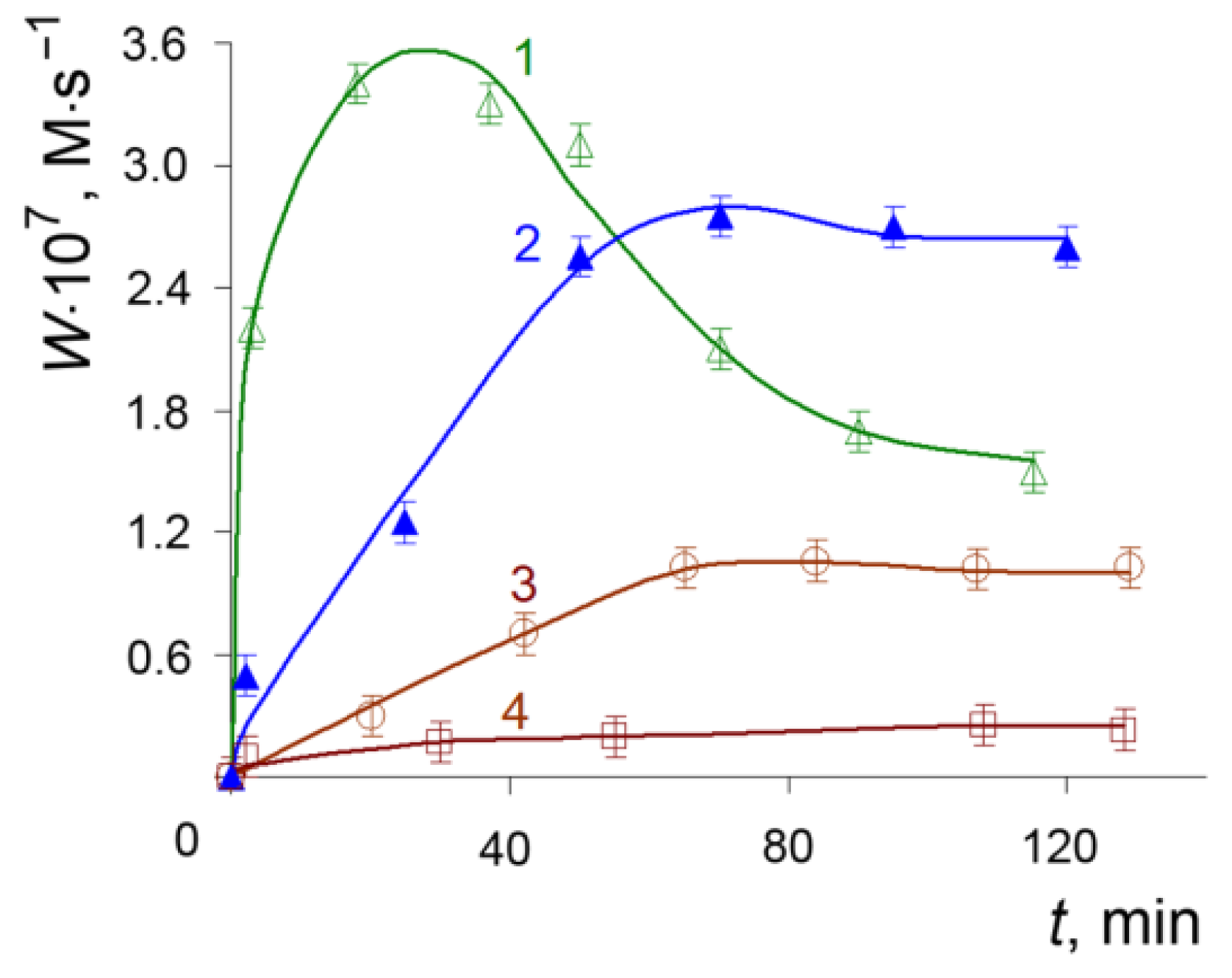
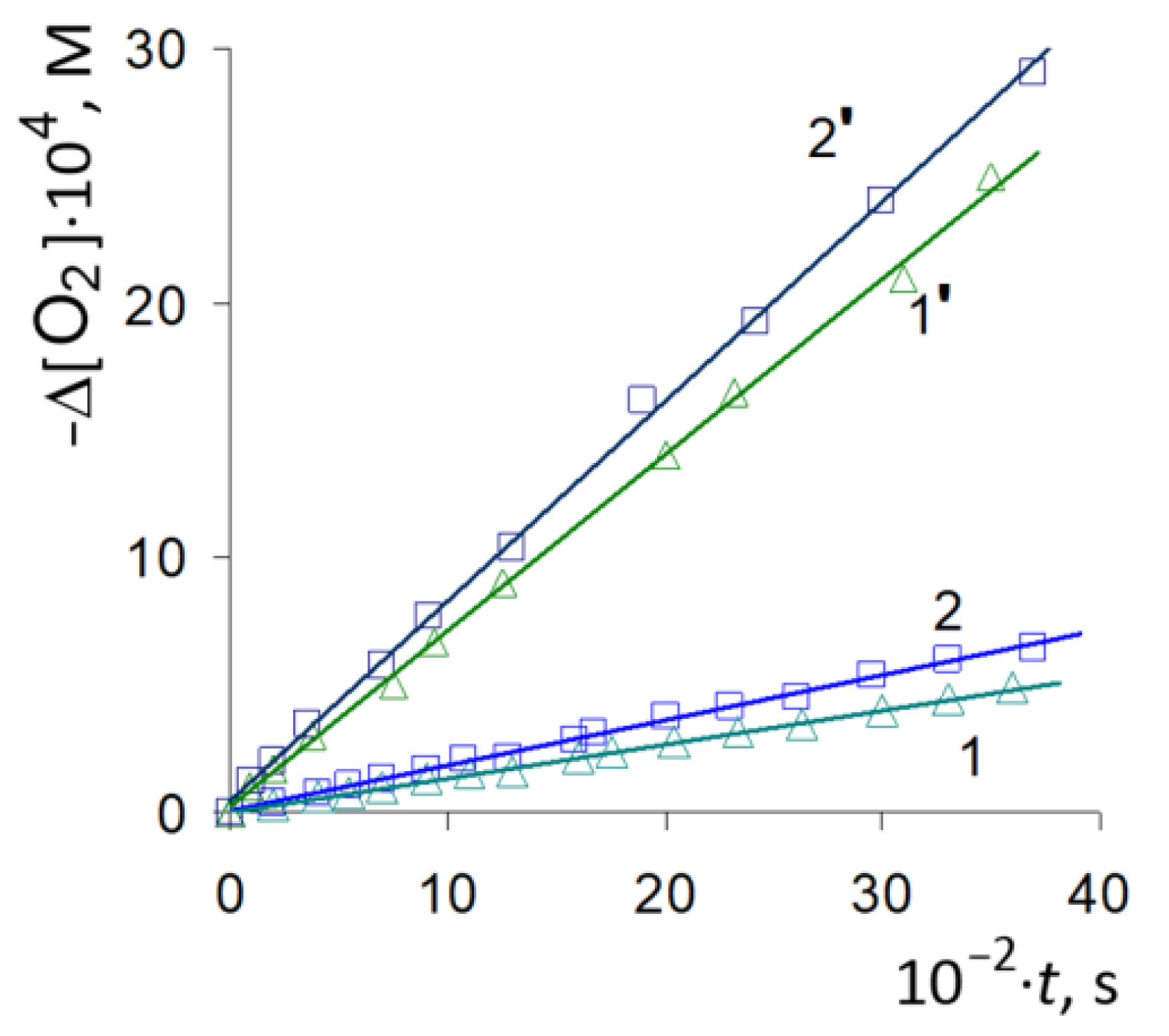
3.1. Autooxidation of Adrenaline
3.2. Radical-Chain Oxidation of Adrenaline
4. Conclusions
- The quinoid process proceeds at high rates only in carbonate buffer at high pH values. Radical-chain oxidation proceeds at a measurable rate in both carbonate and phosphate buffers. However, the considered mechanism is valid for physiological values of pH = 7.4. Therefore, each process must be analyzed separately, although their superposition is not excluded;
- The effect of SOD on the rate of autoxidation (measured by the rate of adrenaline consumption) is significantly higher than on the rate of oxygen consumption;
- One should take into account the multiphase nature of the processes, the analysis of which still requires proving the eligibility of using liquid-phase kinetic models.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayashi, H. Introduction to Dynamic Spin Chemistry: Magnetic Field Effects on Chemical and Biochemical Reactions; World Scientific Printers (S) Pte Ltd.: Singapore, 2004; 236p. [Google Scholar]
- Buchachenko, L. Magneto-Biology and Medicine; Nova Science: Hauppauge, NY, USA, 2014; 248p. [Google Scholar]
- Kokhan, V.; Matveeva, M.; Mukhametov, A.; Shtemberg, A. Risk of defeats in the central nervous system during deep space missions. Neurosci. Biobehav. Rev. 2016, 71, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.; Prato, F. Biological effects of the hypomagnetic field: An analytical review of eexperiments a and theories. PLoS ONE 2017, 12, e179340. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, L. Reactive oxygen species: Potential regulatory molecules in response to hypomagnetic field exposure. Bioelectromagnetics 2020, 41, 573–580. [Google Scholar] [CrossRef]
- Ghirri, A.; Candini, A.; Affonte, M. Molecular Spins in the Context of Quantum Technologies. Magnetochemistry 2017, 3, 12. [Google Scholar] [CrossRef]
- Funk, R. Endogenous Bioelectric Phenomena and Interfaces for Exogenous Effects. In Bioengineering and Biophysical Aspects of Electromagnetic Fields, 4th ed.; Greenebaum, B., Barnes, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton FL, USA, 2019. [Google Scholar] [CrossRef]
- Sampson, C.; Keens, R.; Kattnig, D. On the magnetosensitivity of lipid peroxidation: Two- versus three-radical dynamics. Phys. Chem. Chem. Phys. 2019, 21, 13526–13538. [Google Scholar] [CrossRef] [PubMed]
- Lukzen, N.; Ivanov, K.; Sadovsky, V.; Sagdeev, R. Magnetic field effect on recombination of radicals diffusing on a two-dimensional plane. J. Chem. Phys. 2020, 152, 34103. [Google Scholar] [CrossRef] [PubMed]
- Kasaikina, O.; Pisarenko, L. Magnetic effects in hydroperoxide decomposition in mixed micelles with cationic surfactants. Rus. Chem. Bull. 2015, 10, 2319–2324. [Google Scholar] [CrossRef]
- Pliss, E.; Grobov, A.; Kuzaev, A.; Buchachenko, A.L. Magnetic field effect on the oxidation of organic substances by molecular oxygen. J. Phys. Org. Chem. 2019, 32, e3915. [Google Scholar] [CrossRef]
- Kazin, V.; Makaryin, V.; Guzov, E.; Moshareva, V.; Kovchiy, K. Spectrophotometric study of the effect of a magnetic field on human blood components. J. Appl. Spectr. 2016, 83, 406–411. [Google Scholar] [CrossRef]
- Kazin, V.; Guzov, E.; Pliss, E.; Moshareva, V.; Makaryin, V.; Levshin, N.; Baranov, A. The Effect of a Constant Magnetic Field on Components of Protein Structures in Human Blood. Biophysics 2017, 62, 821–828. [Google Scholar] [CrossRef]
- Bindoli, A.; Deeble, D.J.; Rigobello, M.P.; Galzigna, L. Direct and respiratory chain-mediated redox cycling of adrenochrome. Biochim. Biophys. Acta 1990, 1016, 349–356. [Google Scholar] [CrossRef]
- Sirota, T. Involvement of carbonate/bicarbonate ions in the superoxide-generating reaction of adrenaline autoxidation. Biomed. Chem. 2015, 61, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sirota, T. Standardization and regulation of the rate of the superoxide-generating adrenaline autoxidation reaction used for evaluation of pro/antioxidant properties of various materials. Biomed. Chem. 2016, 62, 650–655. [Google Scholar] [CrossRef][Green Version]
- Sirota, T. Effect of the sulfur-containing compounds on the quinoid process of adrenaline autoxidation; potential neuroprotectors. Biomed. Chem. 2019, 65, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Sirota, T. A chain reaction of adrenaline autoxidation is a model of quinoid oxidation of catecholamines. Biophysics 2020, 65, 548–556. [Google Scholar] [CrossRef]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine Oxidation and Autophagy. Parkinsons Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef] [PubMed]
- Bielski, H.; Cabelli, D.; Arudi, R.; Ross, A. Reactivity of HO2/O−2 radicals in aqueous solution. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Denisov, E.; Afanas’ev, I. Oxidation and Antioxidantsin Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005; 992p. [Google Scholar] [CrossRef]
- Pliss, E.; Safiulin, R.; Zlotsky, S. Inhibited Oxidation of Unsaturated Compounds—Kinetics, Mechanism, Correlation of Structure with Reactionary Ability; LAP LAMBERT Academic Publishing: Saarbruchen, Germany, 2012; 130p. [Google Scholar]
- Roginsky, V. Chain-breaking antioxidant activity of natural polyphenols as determined during the chain oxidation of methyl linoleate in Triton X-100 micelles. Arch. Biochem. Biophys. 2003, 414, 261–270. [Google Scholar] [CrossRef]
- Pliss, E.; Grobov, A.; Kuzaev, A.; Buchachenko, A. Magnetic field effect on the oxidation of hydrocarbons by molecular oxygen. Mendeleev Commun. 2017, 27, 246–247. [Google Scholar] [CrossRef]
- Pliss, E.; Grobov, A.; Kuzaev, A.; Buchachenko, A. Magnetic field as a means to identify initiating reaction in oxidation of organic substances by molecular oxygen. Mendeleev Commun. 2020, 30, 433–435. [Google Scholar] [CrossRef]
- Pliss, E.; Grobov, A.; Kuzaev, A.; Buchachenko, A. Magnetic field effects on the initiation of chain oxidation. Mendeleev Commun. 2021, 3, 341–343. [Google Scholar] [CrossRef]
- Pliss, E.; Soloviev, M. Magnetic Field Effect on the Oxidation of Unsaturated Compounds by Molecular Oxygen. Magnetochemistry 2022, 8, 44. [Google Scholar] [CrossRef]
- Guzov, E.; Kazin, V.; Moshareva, V.; Zhukova, A. Application of electronic spectroscopy and quantum-chemical modeling for analysis of products of autoxidation of adrenaline. J. Appl. Spectr. 2019, 85, 1107–1113. [Google Scholar] [CrossRef]
- Sokolov, A.; Popov, S.; Pliss, E.; Loshadkin, V. Computer Program “Kinetics of 2012—A Program to Calculate the Kinetic Parameters of Chemical and Biochemical Processes. In Official Bulletin of the Federal Service for Intellectual Property; Computer Programs, Database, Topographies of Integrated Circuits 3; Federal Service for Intellectual Property: Moscow, Russia, 2013. [Google Scholar]
- Vladimirov, G.; Vikulina, A.; Volodkin, D.; Vladimirov, Y. Structure of the complex of cytochrome c with cardiolipin in non-polar environment. Chem. Phys. Lipids 2018, 214, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Pliss, E.; Soloviev, M.; Loshadkin, D.; Molodochkina, S.; Kasaikina, O. Kinetic model of polyunsaturated fatty acids oxidation in micelles. Chem. Phys. Lipids 2021, 237, 105089. [Google Scholar] [CrossRef]
- Lukzen, N.; Ivanov, K.; Sadovsky, V.; Kaptein, R.; Sagdeev, R. Magnetic field and spin effects on the recombination of radicals on two-dimensional surfaces. Dokl. Phys. Chem. 2013, 449, 44–47. [Google Scholar] [CrossRef]
- Ivanov, K.; Lukzen, N.; Doktorov, A. The integral encounter theory of multistage reactions containing association-dissociation reaction stages. III. Taking account of quantum states of reactants. J. Chem. Phys. 2004, 15, 5115–5124. [Google Scholar] [CrossRef]
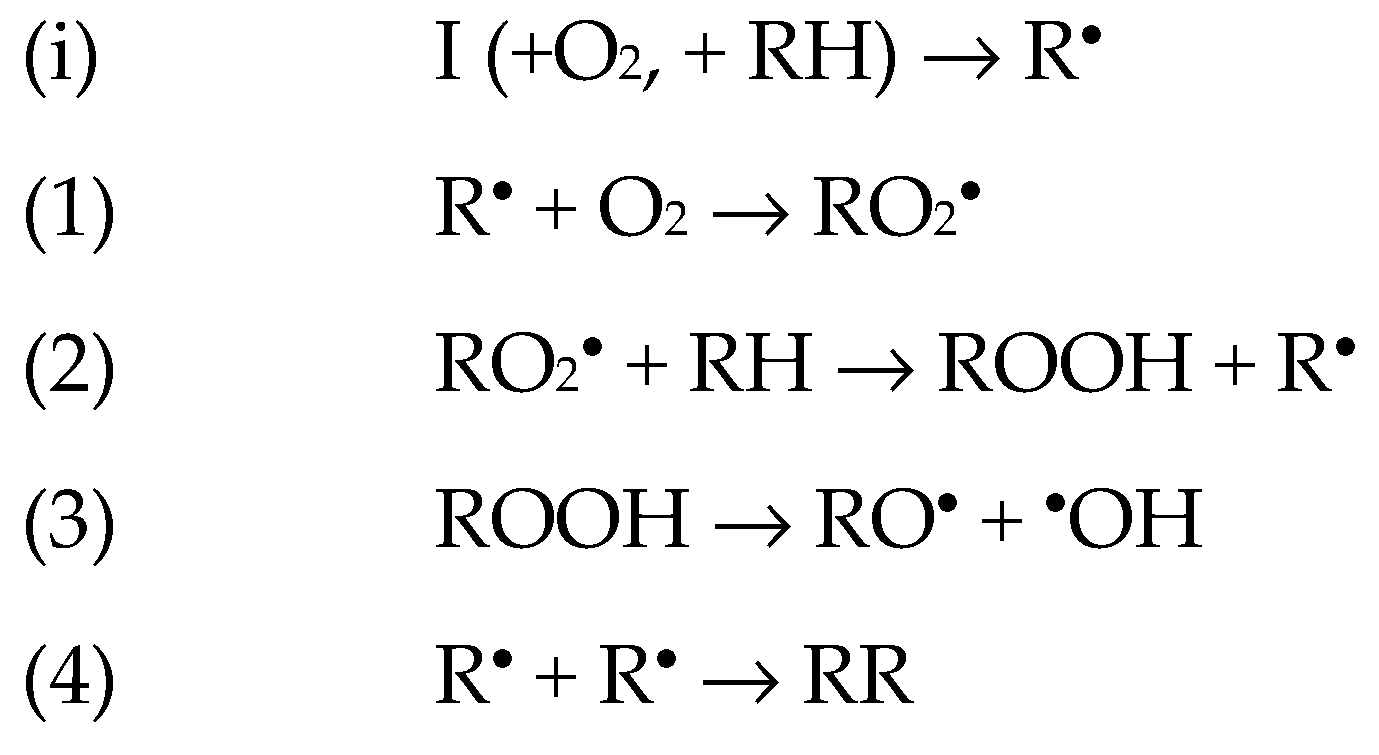
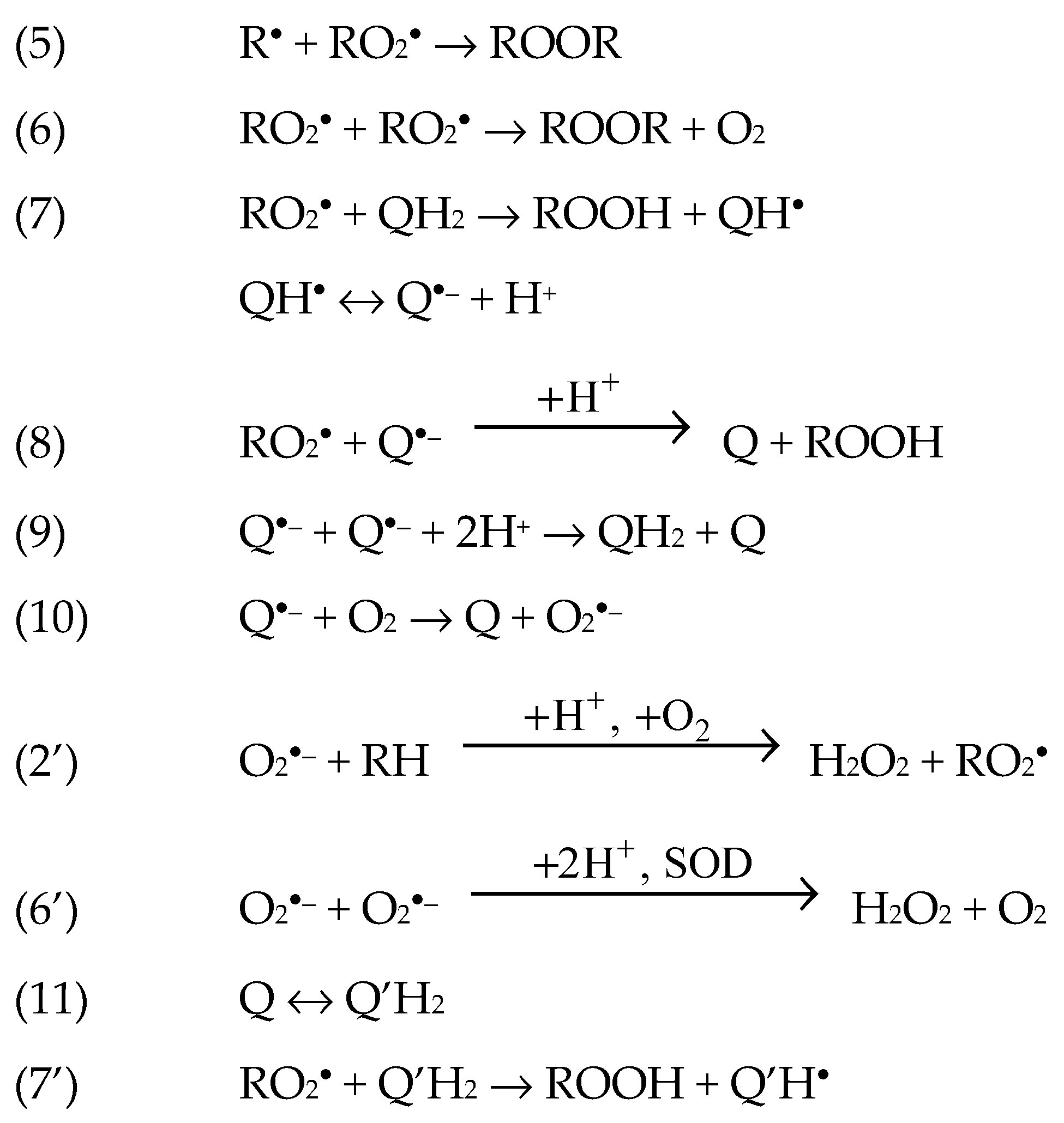

| pH | 10.40 | 10.60 | 10.70 |
|---|---|---|---|
| W·103, M·s−1 | 3.8 | 6.5 | 10.2 |
| [SOD]0, U/mL | 0 | 30 | 60 | 90 |
|---|---|---|---|---|
| W·103, M·s−1 | 6.5 | 5.0 | 2.8 | 1.5 |
| B, μT | 0 | 75 | 90 | 125 |
|---|---|---|---|---|
| W·103, M·s−1 | 6.5 | 4.9 | 4.7 | 3.2 |
| MFE ± 0.02 | 1 | 0.77 | 0.72 | 0.53 |
| [SOD], U/mL | 0 | 30 | 60 | 90 |
|---|---|---|---|---|
| W·103, M·s−1 | ||||
| B = 0 B = 90 μT | 6.5 4.7 | 4.9 3.9 | 2.8 2.3 | 1.5 1.2 |
| MFE ± 0.1 | 0.72 | 0.61 | 0.33 | 0.18 |
| Wi∙109, M s−1 | W0(20)∙108, M s−1 | W0(70)∙108, M s−1 | W0(100)∙108, M s−1 | W0(150)∙108, M s−1 |
|---|---|---|---|---|
| 1.0 | 0.82 | 0.81 | 0.80 | 0.78 |
| 4.0 | 1.74 | 1.66 | 1.62 | 1.55 |
| ni ± 0.02 | 0.54 | 0.52 | 0.51 | 0.50 |
| B, T | 0.3 | 0.4 | 0.5 | 0.6 |
|---|---|---|---|---|
| [SOD], U/mL | MFE ± 0.02 | |||
| 0 | 1.11 | 1.31 | 1.52 | 1.72 |
| 100 | 1.14 | 1.35 | 1.56 | 1.74 |
| 200 | 1.17 | 1.38 | 1.58 | 1.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazin, V.N.; Guzov, E.A.; Moshareva, V.A.; Pliss, E.M. Influence of a Constant Magnetic Field on the Mechanism of Adrenaline Oxidation. Magnetochemistry 2022, 8, 70. https://doi.org/10.3390/magnetochemistry8070070
Kazin VN, Guzov EA, Moshareva VA, Pliss EM. Influence of a Constant Magnetic Field on the Mechanism of Adrenaline Oxidation. Magnetochemistry. 2022; 8(7):70. https://doi.org/10.3390/magnetochemistry8070070
Chicago/Turabian StyleKazin, Vyacheslav N., Evgenii A. Guzov, Valentina A. Moshareva, and Evgenii M. Pliss. 2022. "Influence of a Constant Magnetic Field on the Mechanism of Adrenaline Oxidation" Magnetochemistry 8, no. 7: 70. https://doi.org/10.3390/magnetochemistry8070070
APA StyleKazin, V. N., Guzov, E. A., Moshareva, V. A., & Pliss, E. M. (2022). Influence of a Constant Magnetic Field on the Mechanism of Adrenaline Oxidation. Magnetochemistry, 8(7), 70. https://doi.org/10.3390/magnetochemistry8070070






