Corrosion Behavior of ZnMn Coatings Magnetoelectrodeposited
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Elaboration
2.2. Corrosion Behavior
3. Results and Discussions
3.1. Coating Elaboration
3.2. Corrosion Behavior
3.2.1. Long Immersion Time
- -
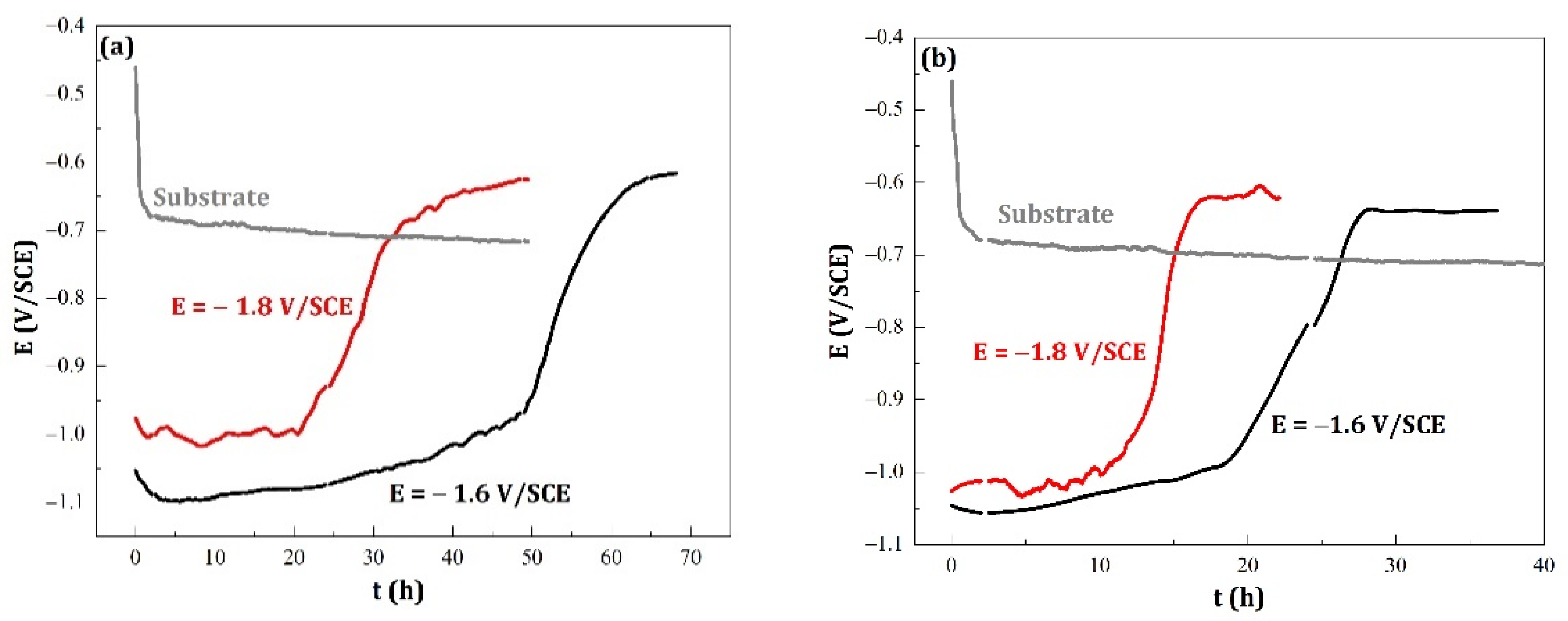
- For the same conditions of imposed potential during the electrodeposition, the superimposition of the magnetic field equal to 0.3 T caused a decrease of tlim (tlim is the time for which the corrosion potential (Ecorr) reaches its limit value).
- -
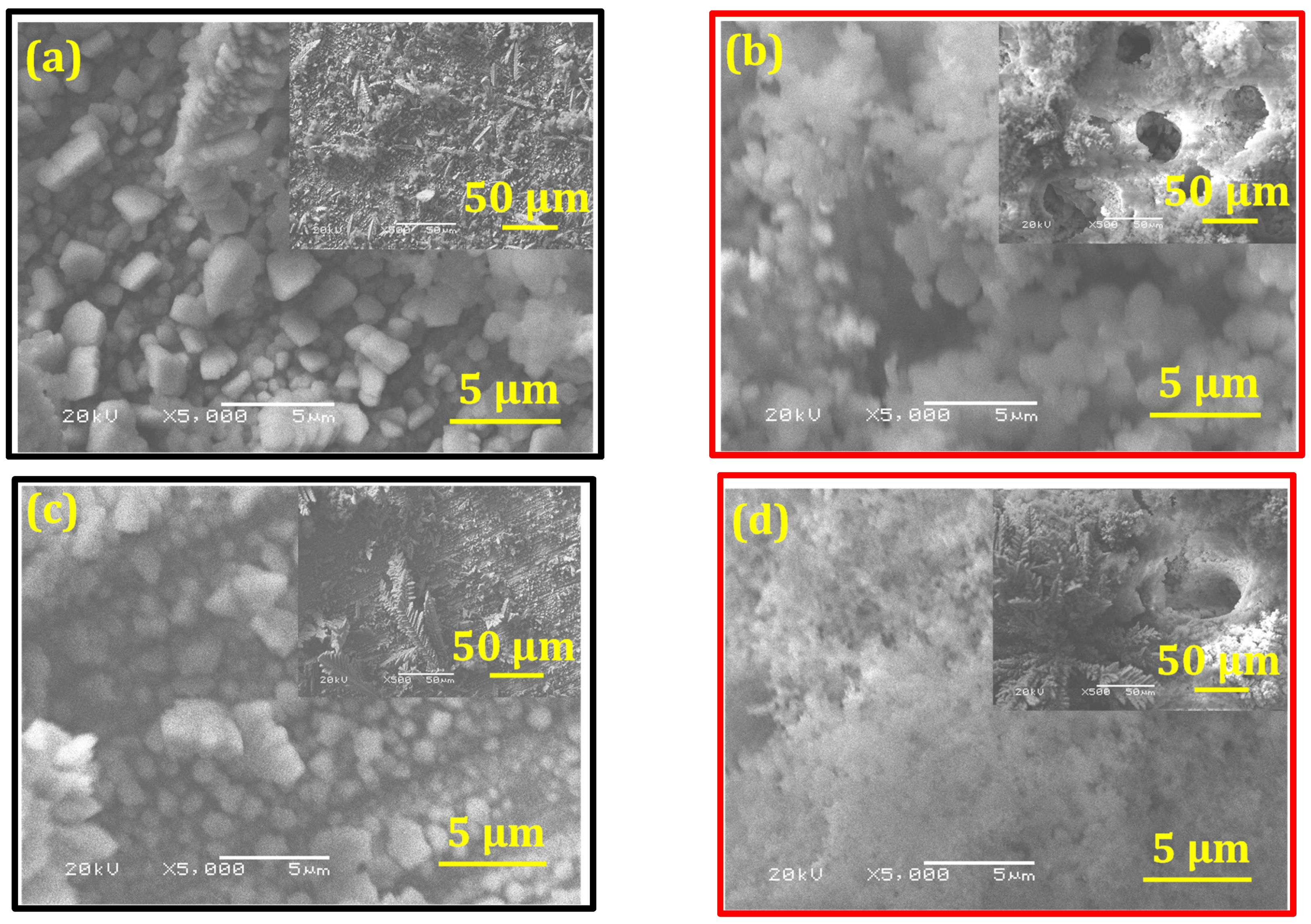
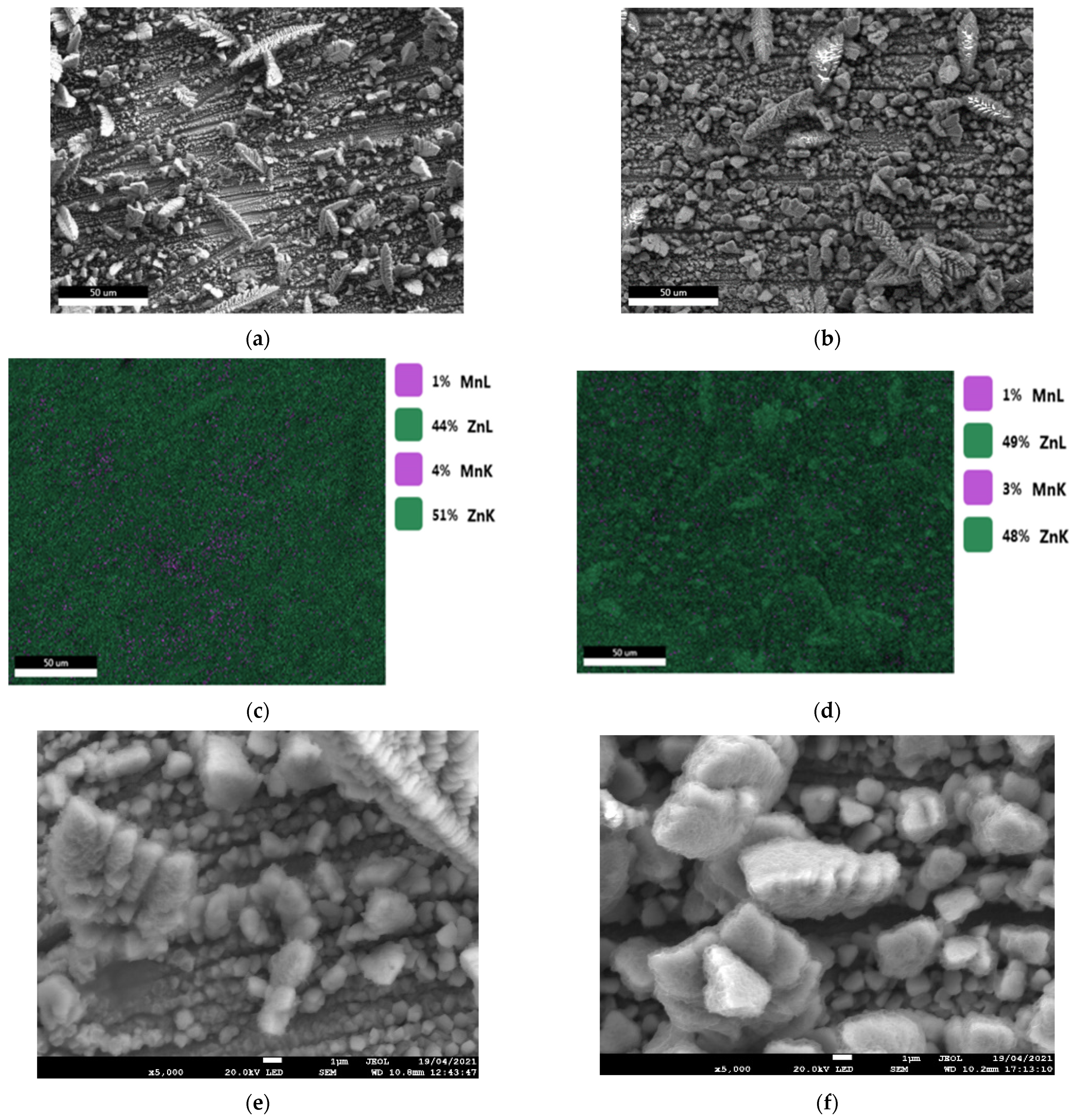
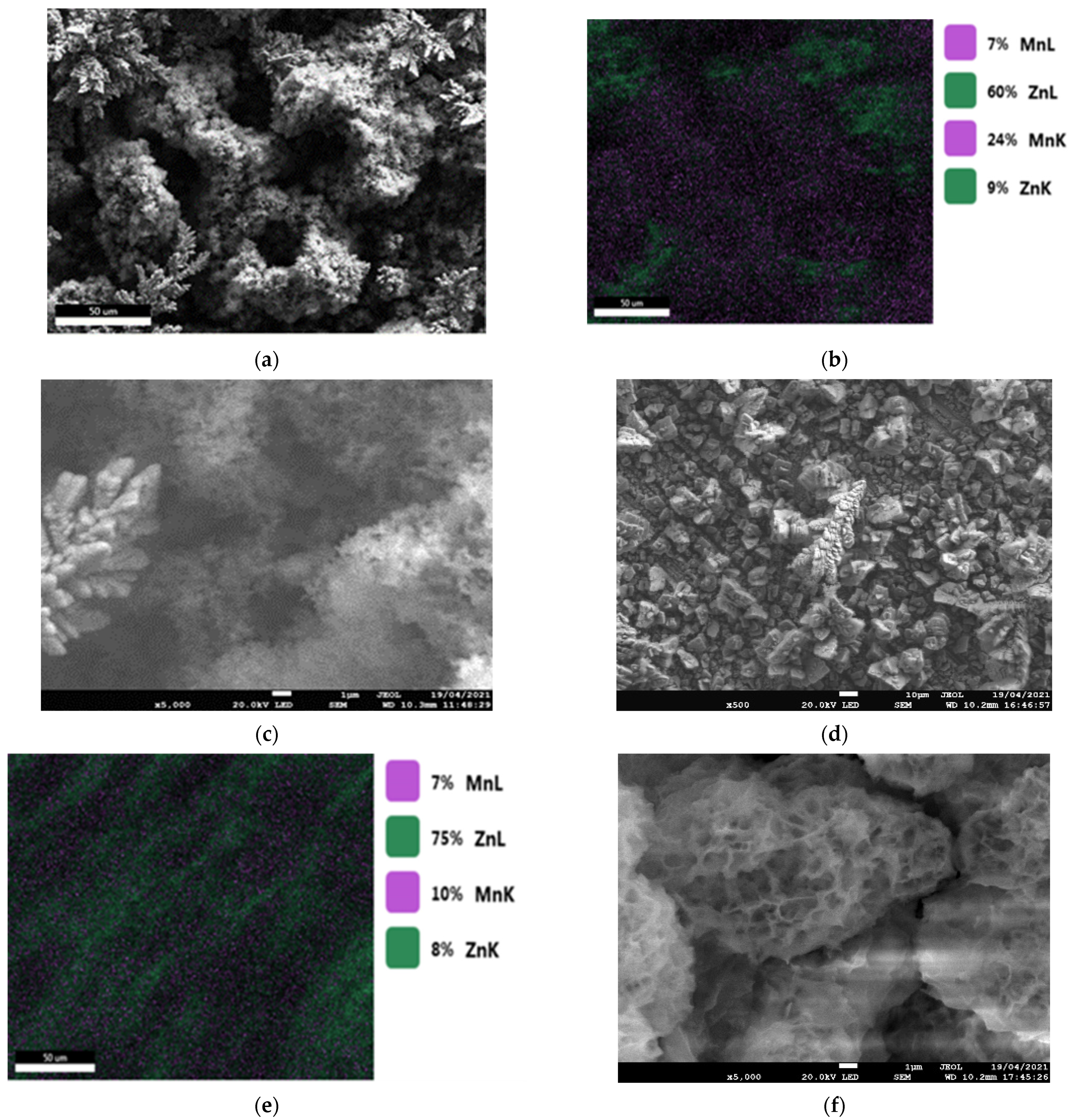
- The results of the corrosion behavior of deposits developed without a magnetic field are in good agreement with the literature. Indeed, Ganesan et al. [28] and Bučko et al. [12] reported that alloy coatings elaborated at E = −1.6 V/SCE (low current density) showed better corrosion resistance than those at E = −1.8 V/SCE (high current density). This can also be related to the morphology of the deposits (Figure 3) and the presence of open pores, accelerated by the coating dissolution. These characteristics are related to lower corrosion resistance [12,13,14,16].
- -
- Regarding the corrosion potential at immersion, there are two slightly different values, but differences are reproducible. For the coatings rich in zinc, and having the η phase in the majority, the Ecorr value is of the order of −1.05 V/SCE, while for the coatings rich with MnZn3 phase (therefore, richer in manganese), the Ecorr value is higher with an average value equal to −1.00 V/SCE. This result is similar to those found in the literature [10,29].
- -
- Whatever the magnetic field amplitude, t1 is greatly decreased when the cathodic potential increased.
- -
- Whatever the imposed potential, the superimposition of the magnetic field causes a 50% decrease of t1 while the quantities of electric charge are either constant (for E = −1.8 V/SCE) or reduced by only about 20% (for E = −1.6 V/SCE) (see Figure 1);
- -
- The rate of variation of the corrosion potential in the increasing section is almost constant for deposits elaborated without superimposition of magnetic field; on the other hand, it is greatly increased when a magnetic field is superimposed for B = 0.3 T during deposition and when the cathodic potential is equal to −1.8 V/SCE; and
- -
- The term value (E1 − E2) shows the same variations as those observed in the experimental curves (Figure 4), namely, a lower value for deposits rich in zinc (E = −1.6 V/SCE).
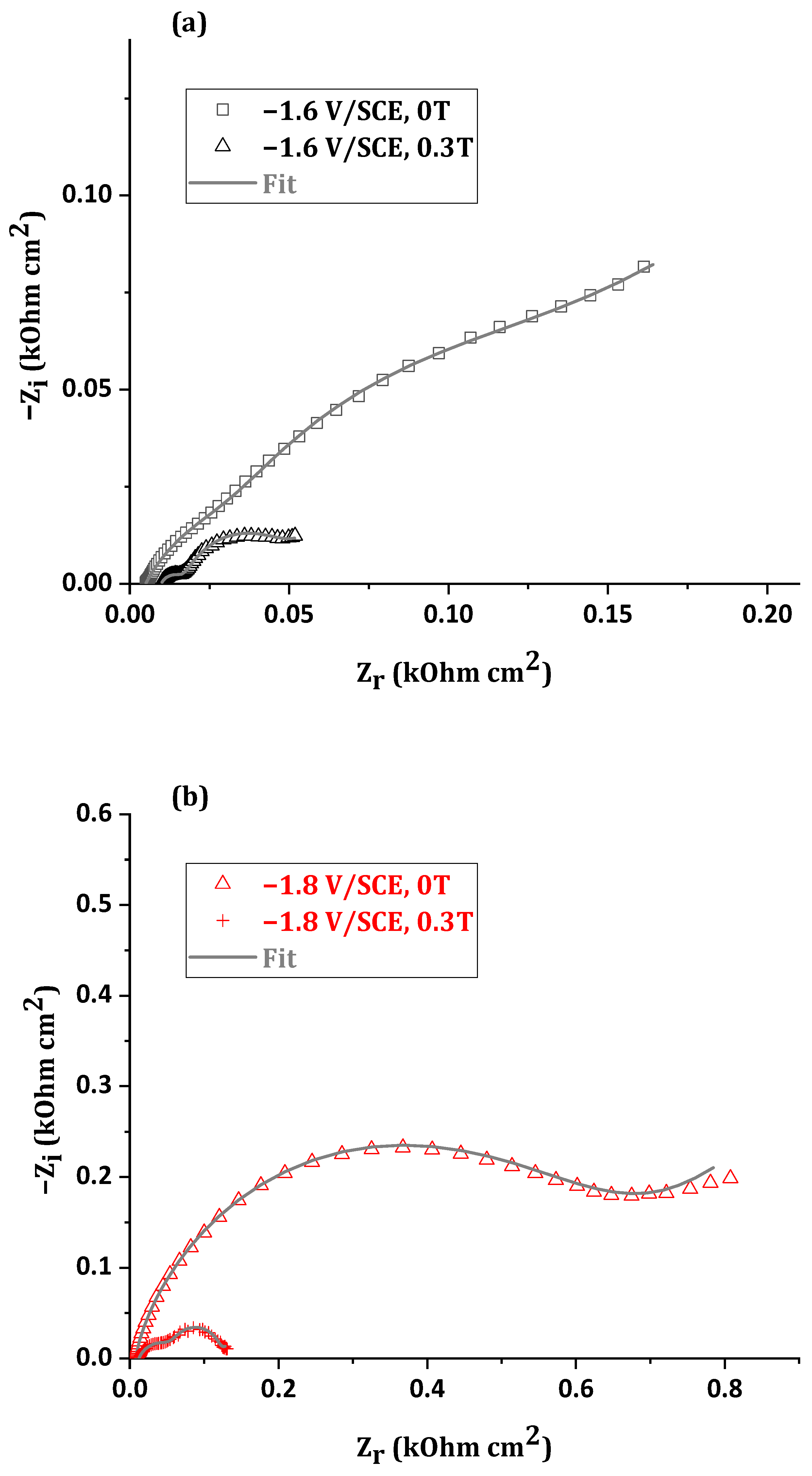
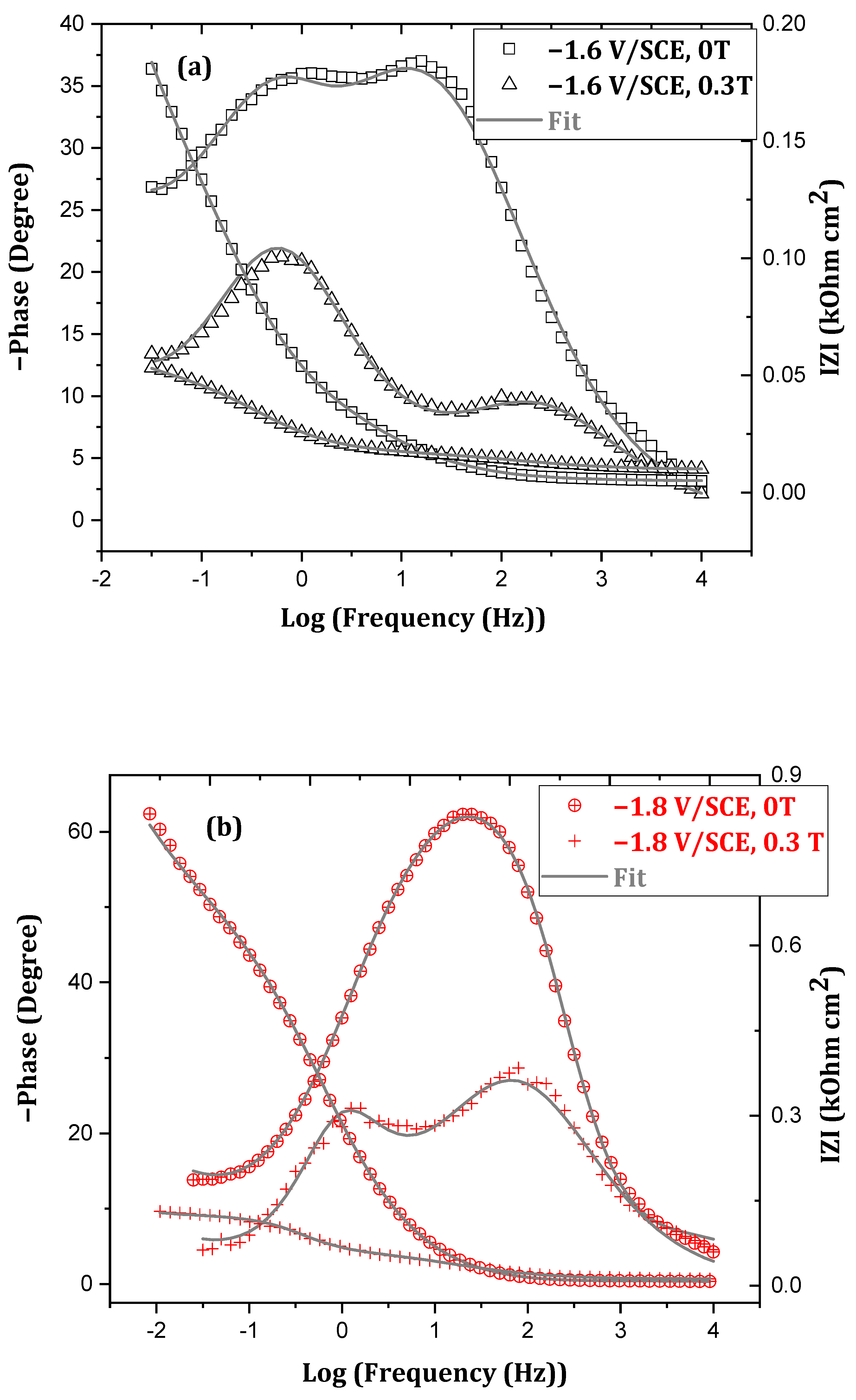
3.2.2. Short Immersion Time
- Rs is the solution resistance between the reference electrode (SCE) and the working electrode (CE).
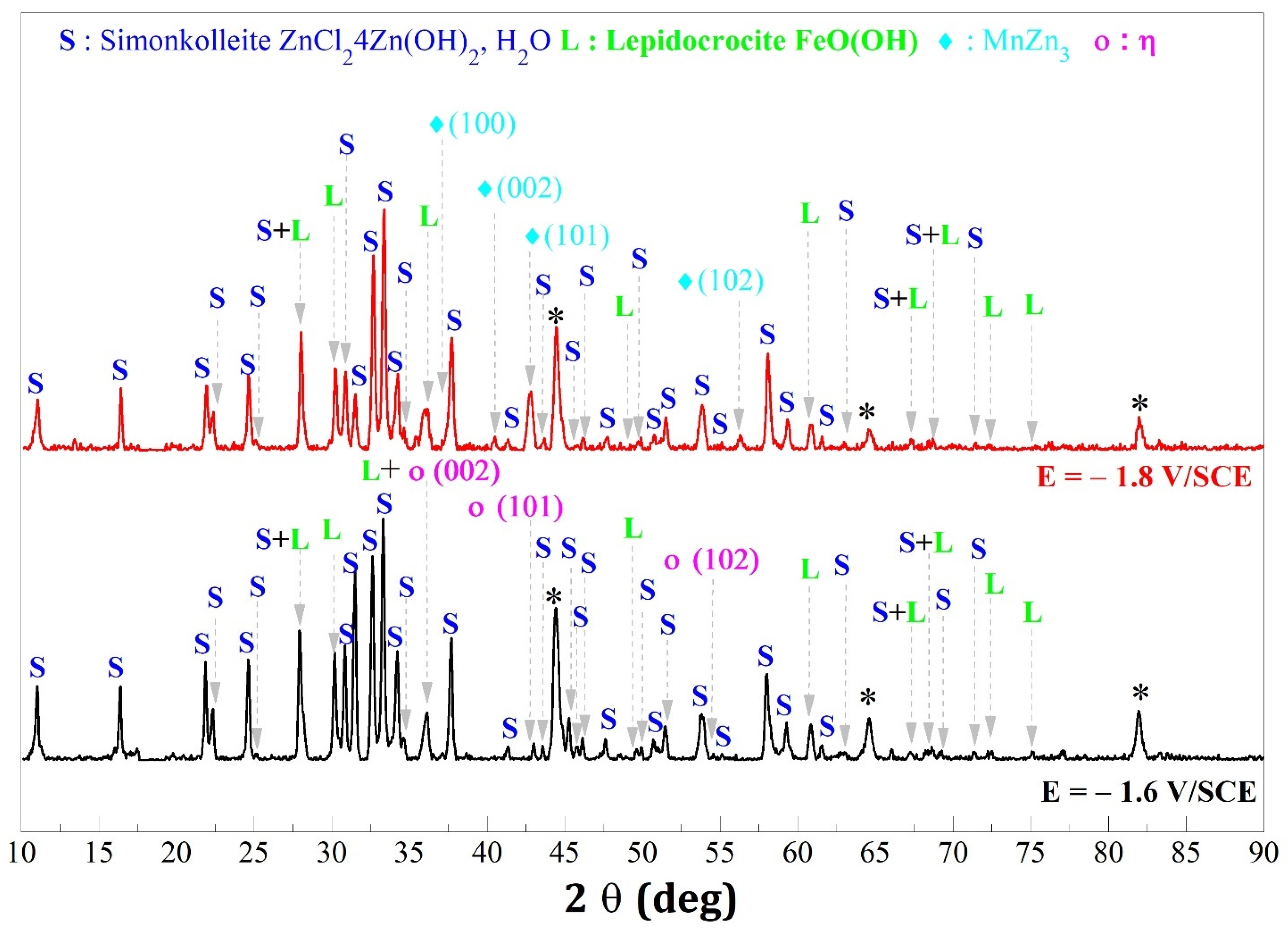
- Rf represents the resistance of the layer containing the corrosion products formed on the surface (according to simonkolleite in Figure 6).
- Rt is attributed to the charge transfer resistance of the corrosion mechanism.
- Qf is the constant phase element (CPE) relative to the capacity of the corroded ZnMn alloy coating.
- Qdl is the CPE element relating to the electrochemical double layer capacity.
- W is the Warburg impedance characteristic of the oxygen diffusion phenomenon.
- -
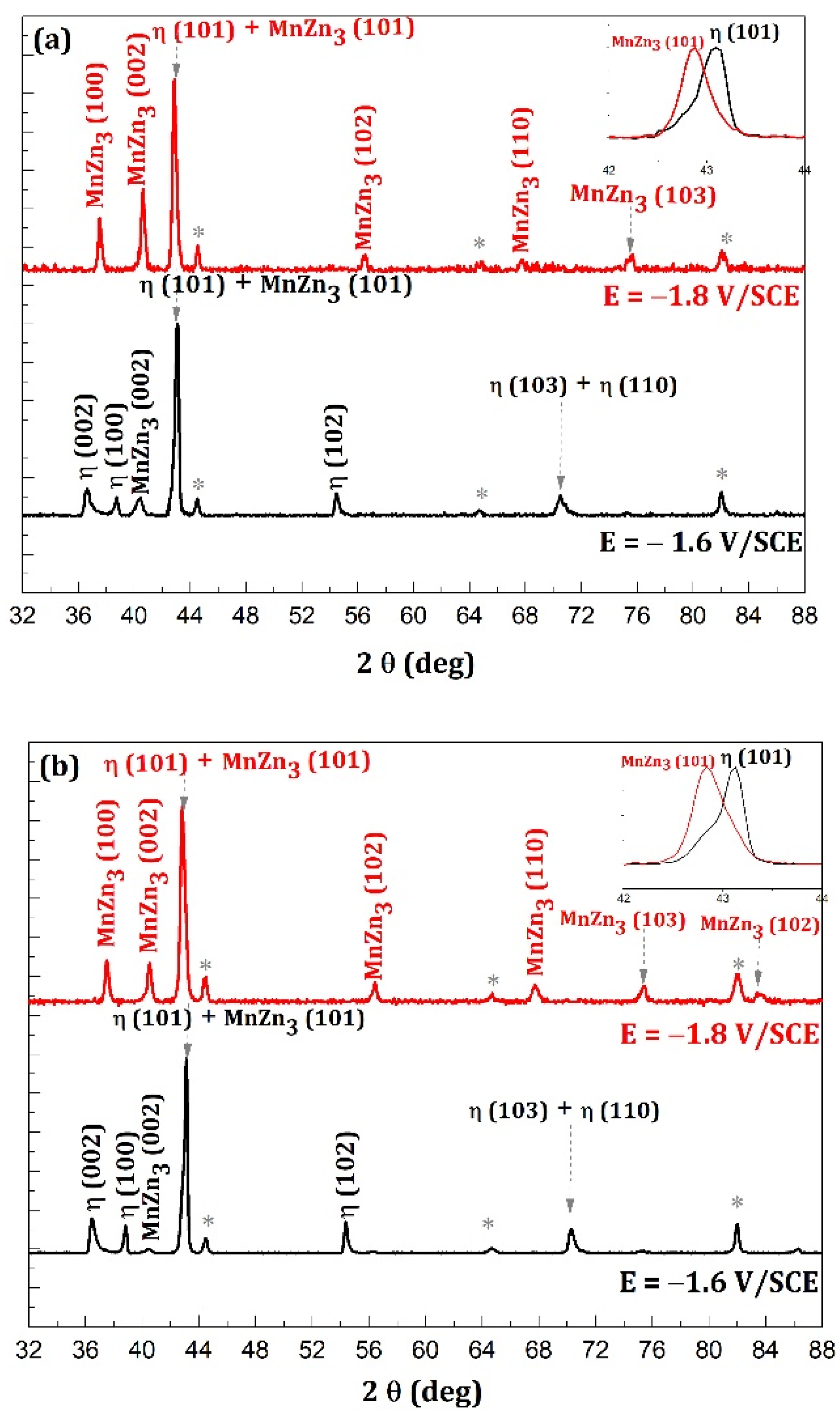
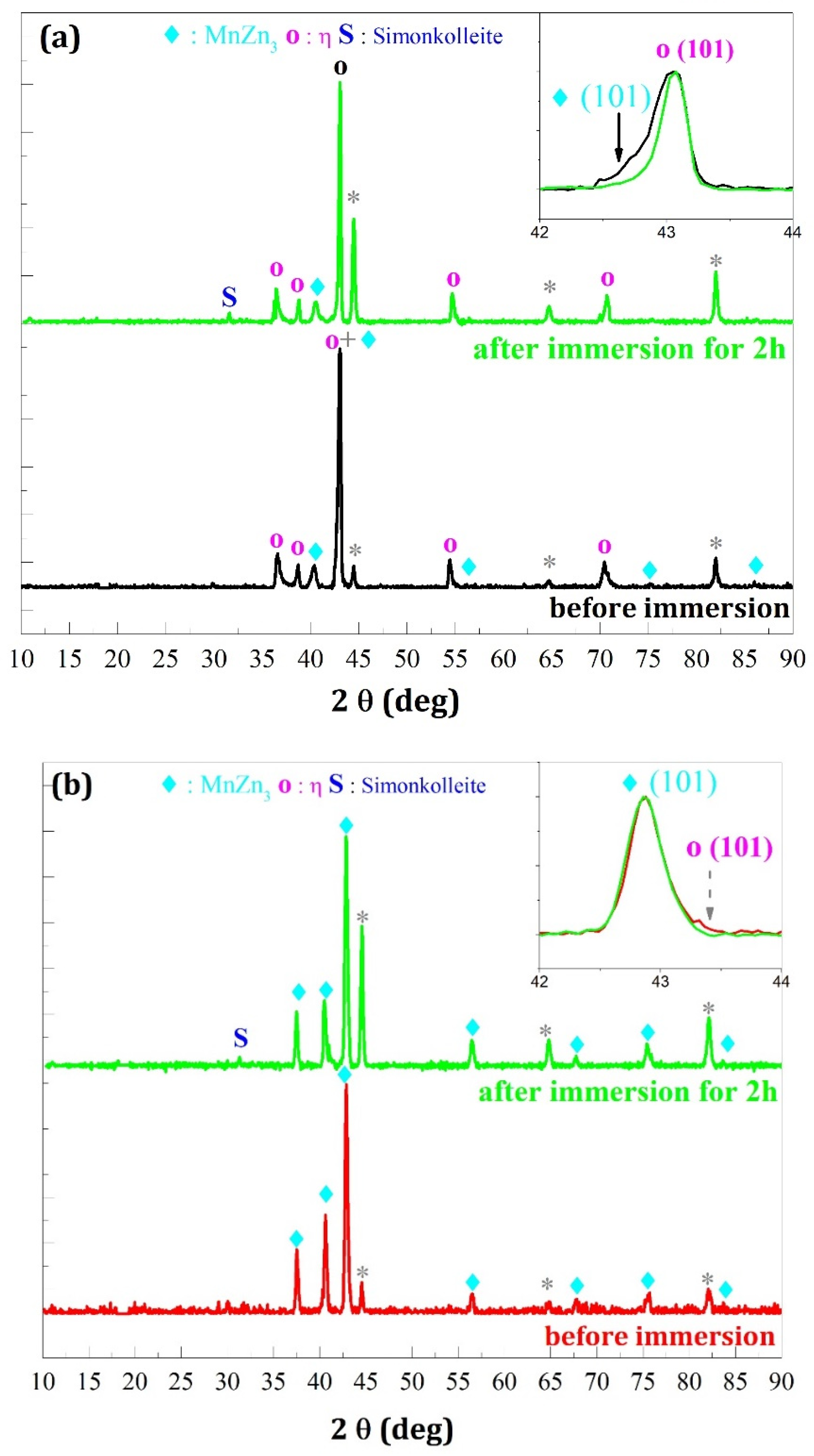
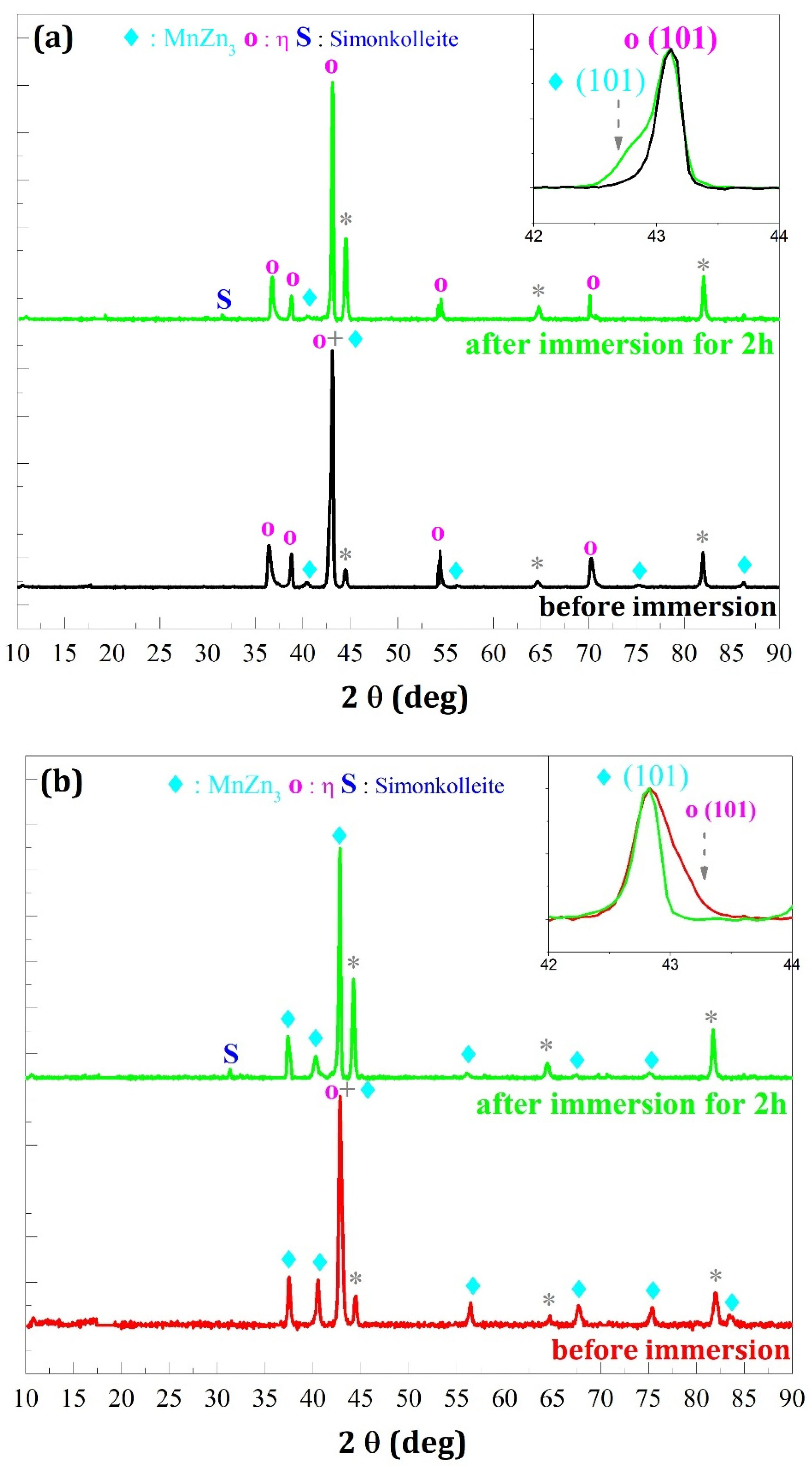
- In the case where the η phase is majority (cathodic potential deposition equal to −1.6 V/SCE) (Figure 14a and Figure 15a) the relative intensities of the η phase peaks are not modified with respect to each other, whereas for the minority phase MnZn3 we observe a disappearance of the characteristic line (101) and a decrease of the line (002) which are more important for the deposition carried out under magnetic field (Figure 15a).
- -
- For the deposits carried out at a potential of −1.8 V/SCE (Figure 14b and Figure 15b), the same observation on the selectivity of the corrosive attack can be made. Indeed, for the deposit carried out without magnetic field (Figure 14b), the relative intensity of the line (002) of the MnZn3 phase decreases strongly whereas for all the other lines the intensities are appreciably identical before and after corrosion. Finally, for the deposit elaborated under magnetic field (B = 0.3 T), we can observe the disappearance of the (101) line of the η phase leading to a monophasic deposit with a more textured MnZn3 phase after corrosion since all the relative intensities of these phases, except (100) and (101), have strongly decreased or even have disappeared. These structural modifications are to be put in parallel with the previous observations obtained by EDS which show differences in the rate of disappearance of the element manganese according to the electrodeposition process.
4. Conclusions
- For deposits obtained for substantially equal current densities, mainly presenting majority the η phase (deposits made at E = −1.60 V/SCE) with a percentage of zinc greater than 90% and identical XRD diffractograms, the behavior against corrosion depends on the electrodeposition process (superimposition, or not, of the magnetic field);
- For identical chemical compositions of coatings, the superimposition of a magnetic field greatly decreases their corrosion resistance; and
- The same observation can be noted for Zn–Mn coatings having a high proportion of the MnZn3 phase with zinc proportions less than 90% where, in addition to the corrosion-resistance decrease, a significant increase in the speed of the corrosion potential transition stage has been observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira, E.M.; Carlos, I.A. Study of the effect of mannitol on ZnNi alloy electrodeposition from acid baths and on the morphology, composition, and structure of the deposit. J. Appl. Electrochem. 2009, 39, 1849–1856. [Google Scholar] [CrossRef]
- Ye, M.; Ding, T.; Zhou, H.; He, F. Nucleation and growth mechanism of electrodeposited Ni−W alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 1842–1852. [Google Scholar] [CrossRef]
- Gabe, D.R. Protective layered electrodeposits. Electrochim. Acta 1993, 39, 1115–1121. [Google Scholar] [CrossRef]
- Loukil, N.; Feki, M. Review—Zn–Mn electrodeposition: A literature review. J. Electrochem. Soc. 2020, 167, 022503. [Google Scholar] [CrossRef]
- Bučko, M.; Rogan, J.; Stevanović, S.I.; Stanković, S.; Bajat, J.B. The influence of anion type in electrolyte on the properties of electrodeposited ZnMn alloy coatings. Surf. Coat. Technol. 2013, 228, 221–228. [Google Scholar] [CrossRef]
- Eyraud, M.; Garnier, A.; Mazeron, F.; Crousier, J. Morphology and composition of electrodeposited zinc-manganese alloys. Plat. Surf. Finish. 1995, 82, 63–70. [Google Scholar]
- Bozzini, B.; Griskonis, E.; Fanigliulo, A.; Sulcius, A. Electrodeposition of Zn–Mn alloys in the presence of thiocarbamide. Surf. Coat. Technol. 2002, 154, 294–303. [Google Scholar] [CrossRef]
- Boshkov, N. Galvanic Zn–Mn alloys—Electrodeposition, phase composition, corrosion behaviour and protective ability. Surf. Coat. Technol. 2003, 172, 217–226. [Google Scholar] [CrossRef]
- Ortiz, Z.I.; Díaz-Arista, P.; Meas, Y.; Ortega-Borges, R.; Trejo, G. Characterization of the corrosion products of electrodeposited Zn, Zn–Co and Zn–Mn alloys coatings. Corros. Sci. 2009, 51, 2703–2715. [Google Scholar] [CrossRef]
- Tomić, M.V.; Bučko, M.M.; Pavlović, M.G.; Bajat, J.B. Corrosion stability of lectrochemically deposited Zn-Mn alloy coatings. Contemp. Mater. 2010, I, 87–93. [Google Scholar] [CrossRef]
- Sylla, D.; Savall, C.; Gadouleau, M.; Rebere, C.; Creus, J.; Refait, P. Electrodeposition of Zn–Mn alloys on steel using an alkaline pyrophosphate-based electrolytic bath. Surf. Coat. Technol. 2005, 200, 2137–2145. [Google Scholar] [CrossRef]
- Bučko, M.; Rogan, J.; Stevanović, S.I.; Perić-Grujić, A.; Bajat, J.B. Initial corrosion protection of Zn–Mn alloys electrodeposited from alkaline solution. Corros. Sci. 2011, 53, 2861–2871. [Google Scholar] [CrossRef]
- Bučko, M.; Rogan, J.; Jokić, B.; Mitrić, M.; Lačnjevac, U.; Bajat, J.B. Electrodeposition of Zn–Mn alloys at high current densities from chloride electrolyte. J. Solid State Electrochem. 2013, 17, 1409–1419. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Zhang, J.L.; Zheng, H.; Wang, X.L.; Tu, J.P. Electrodeposition, Morphology, composition, and corrosion performance of Zn-Mn coatings from a deep eutectic solvent. J. Mater. Eng. Perform. 2015, 24, 434–444. [Google Scholar] [CrossRef]
- Ramanauskas, R. Structural factor in Zn alloy electrodeposit corrosion. Appl. Surf. Sci. 1999, 153, 53–64. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Gudavičiūtė, L.; Juškėnas, R.; Ščit, O. Structural and corrosion characterization of pulse plated nanocrystalline zinc coatings. Electrochim. Acta 2007, 53, 1801–1810. [Google Scholar] [CrossRef]
- Müller, C.; Sarret, M.; Andreu, T. Electrodeposition of Zn-Mn alloys at low current densities. J. Electrochem. Soc. 2002, 149, C600–C606. [Google Scholar] [CrossRef]
- Touazi, S.; Bučko, M.; Makhloufi, L.; Legat, A.; Bajat, J.B. The electrochemical behavior of Zn–Mn alloy coating in carbonated concrete solution. Surf. Rev. Lett. 2016, 23, 1650030-1–1650030-10. [Google Scholar] [CrossRef]
- Marchandise, D. Les Alliages de Zinc et La Problématique de La Supression Des Cr VI. In Proceedings of the 1st Int Surface Tretaments Institute of Franche-Comté Conference; 2003; pp. 17–28. [Google Scholar]
- Ballote, L.D.; Ramanauskas, R.; Bartolo-Perez, P. Mn oxide film as corrosion inhibitor of Zn-Mn coatings. Corros. Rev. 2000, 18, 41–52. [Google Scholar] [CrossRef]
- Bashkov, N.; Vitkova, S.; Petrov, K. Corrosion products of zinc-manganese coatings: Part I—Investigations using microprobe analysis and X-ray diffraction. Met. Finish 2001, 99, 56–60. [Google Scholar] [CrossRef]
- Bashkov, N.; Petrov, K.; Vitkova, S. Corrosion products of zinc-manganese coatings—Part III: Double-protective action of manganese. Met. Finish 2002, 100, 98–102. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Vitkova, S.; Raichevsky, G. Galvanic alloys Zn–Mn—Composition of the corrosion products and their protective ability in sulfate containing medium. Surf. Coat. Technol. 2005, 194, 276–282. [Google Scholar] [CrossRef]
- Chouchane, S.; Levesque, A.; Zabinski, P.; Rehamnia, R.; Chopart, J.-P. Electrochemical corrosion behavior in NaCl medium of zinc–nickel alloys electrodeposited under applied magnetic field. J. Alloy. Compd. 2010, 506, 575–580. [Google Scholar] [CrossRef]
- Shetty, A.R.; Hegde, A.C. Magnetoelectrodeposition of Ni-Mo-Cd alloy coating for improved corrosion resistance. Chem. Data Collect. 2021, 32, 100639. [Google Scholar] [CrossRef]
- Allam, L.; Lazar, F.; Benfedda, B.; Chopart, J.P. Zn-Mn alloy coating elaboration by magnetoelectrodeposition. J. Solid State Electrochem. 2021, 25, 2041–2053. [Google Scholar] [CrossRef]
- Ichino, R.; Cachet, C.; Wiart, R. Mechanism of zinc electrodeposition in acidic sulfate electrolytes containing Pb2+ ions. Electrochim. Acta 1996, 41, 1031–1039. [Google Scholar] [CrossRef]
- Ganesan, S.; Prabhu, G.; Popov, B.N. Electrodeposition and characterization of Zn-Mn coatings for corrosion protection. Surf. Coat. Technol. 2014, 238, 143–151. [Google Scholar] [CrossRef]
- Müller, C.; Sarret, M.; Andreu, T. Corrosion Behaviour of Zinc-Manganese Coatings. In Proceedings of the European Corrosion Conference: Long Term Prediction and Modelling of Corrosion, Nice, France, 12–16 September 2004. [Google Scholar]
- Bučko, M.; Bajat, J.; Jokić, B.; Gačić, S.B. Interesting surface morphology of Zn-Mn alloy coatings rich in manganese. In Proceedings of the 5th International Scientific Conference on Defensive Technologies, Belgrade, Serbia, 18 September 2012; pp. 576–581. [Google Scholar]





| E (V/SCE) | B = 0 T | B = 0.3 T |
|---|---|---|
| −1.6 | Zn = 95% Mn = 5% | Zn = 94% Mn = 6% |
| −1.8 | Zn = 70% Mn = 30% | Zn = 81% Mn = 19% |
| Elaboration Parameters | Modeling Parameters | ||||||
|---|---|---|---|---|---|---|---|
| B (T) | E (V/SCE) | E1 (V/SCE) | E2 (V/SCE) | t1 (h) | t2 (h) | 105 · MSE | |
| 0 | −1.6 | −0.827 | 0.247 | 53.0 | 11.6 | 0.024 | 35 |
| −1.8 | −0.822 | 0.181 | 28.3 | 6.9 | 0.030 | 7.4 | |
| 0.3 | −1.6 | −0.834 | 0.202 | 22.9 | 5.4 | 0.042 | 17.9 |
| −1.8 | −0.814 | 0.199 | 14.1 | 2.2 | 0.104 | 12 | |
| E (V/SCE) | B (T) | Rs (Ω·cm2) | Qf | Rf (Ω·cm2) | Qdl | Rt (Ω·cm2) | 102 W (Ω·s−0.5 cm−2) | 103 𝝌² | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 103 Yf (Ssnf cm−2) | nf | 103 Ydl (Ssndl cm−2) | ndl | |||||||
| −1.6 | 0 | 5.0 | 3.1 | 0.7 | 65.6 | 4.50 | 0.9 | 101 | 2.62 | 0.30 |
| 0.3 | 9.7 | 2.2 | 0.6 | 8.73 | 14.5 | 0.8 | 30.6 | 19.6 | 0.14 | |
| −1.8 | 0 | 7.7 | 0.28 | 0.8 | 491 | 1.53 | 0.8 | 197 | 1.10 | 3.0 |
| 0.3 | 12 | 0.57 | 0.7 | 50.0 | 3.48 | 0.9 | 67.5 | 26.0 | 0.74 | |
| Elaboration Parameters | E = −1.6 V/SCE | E = −1.8 V/SCE | ||
|---|---|---|---|---|
| B = 0 T | B = 0.3 T | B = 0 T | B = 0.3 T | |
| Rms before immersion (µm) | 7.9 ± 0.3 | 8 ± 0.3 | 10.3 ± 0.1 | 16.1 ± 0.6 |
| Rms after 2 h immersion (µm) | 8.4 ± 0.3 | 19.3 ± 0.2 | 14.4 ± 0.2 | 20.8 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allam, L.; Lazar, F.S.; Chopart, J.-P. Corrosion Behavior of ZnMn Coatings Magnetoelectrodeposited. Magnetochemistry 2022, 8, 69. https://doi.org/10.3390/magnetochemistry8070069
Allam L, Lazar FS, Chopart J-P. Corrosion Behavior of ZnMn Coatings Magnetoelectrodeposited. Magnetochemistry. 2022; 8(7):69. https://doi.org/10.3390/magnetochemistry8070069
Chicago/Turabian StyleAllam, Lamia, Florica S. Lazar, and Jean-Paul Chopart. 2022. "Corrosion Behavior of ZnMn Coatings Magnetoelectrodeposited" Magnetochemistry 8, no. 7: 69. https://doi.org/10.3390/magnetochemistry8070069
APA StyleAllam, L., Lazar, F. S., & Chopart, J.-P. (2022). Corrosion Behavior of ZnMn Coatings Magnetoelectrodeposited. Magnetochemistry, 8(7), 69. https://doi.org/10.3390/magnetochemistry8070069






