Breaking of Odd Chirality in Magnetoelectrodeposition
Abstract
:1. Introduction
2. Materials and Methods
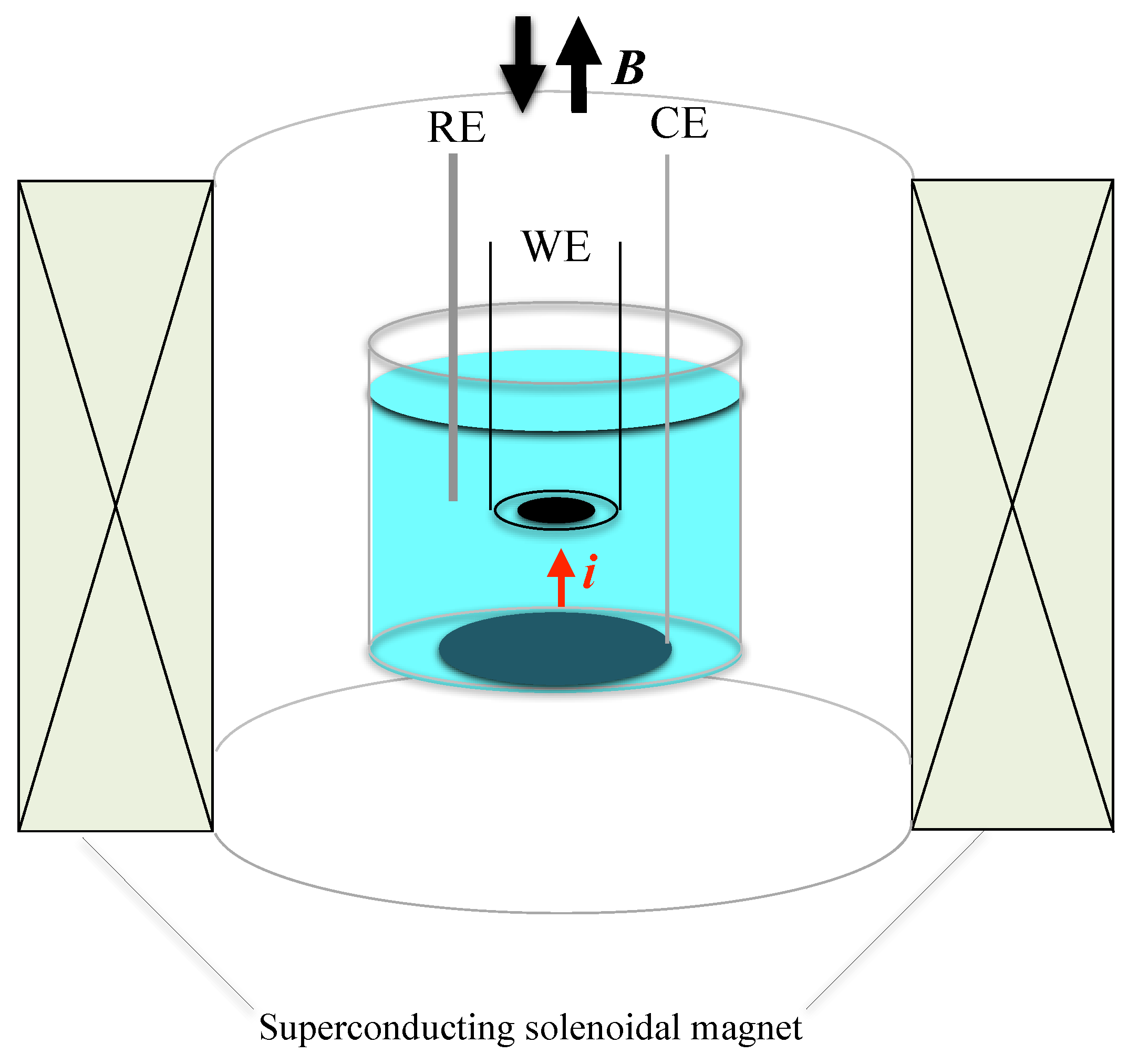
2.1. Experimental Methods of MED
2.2. Experimental Methods of Rotational MED (RMED)
2.3. Estimation of Surface Chirality
3. Results and Discussion
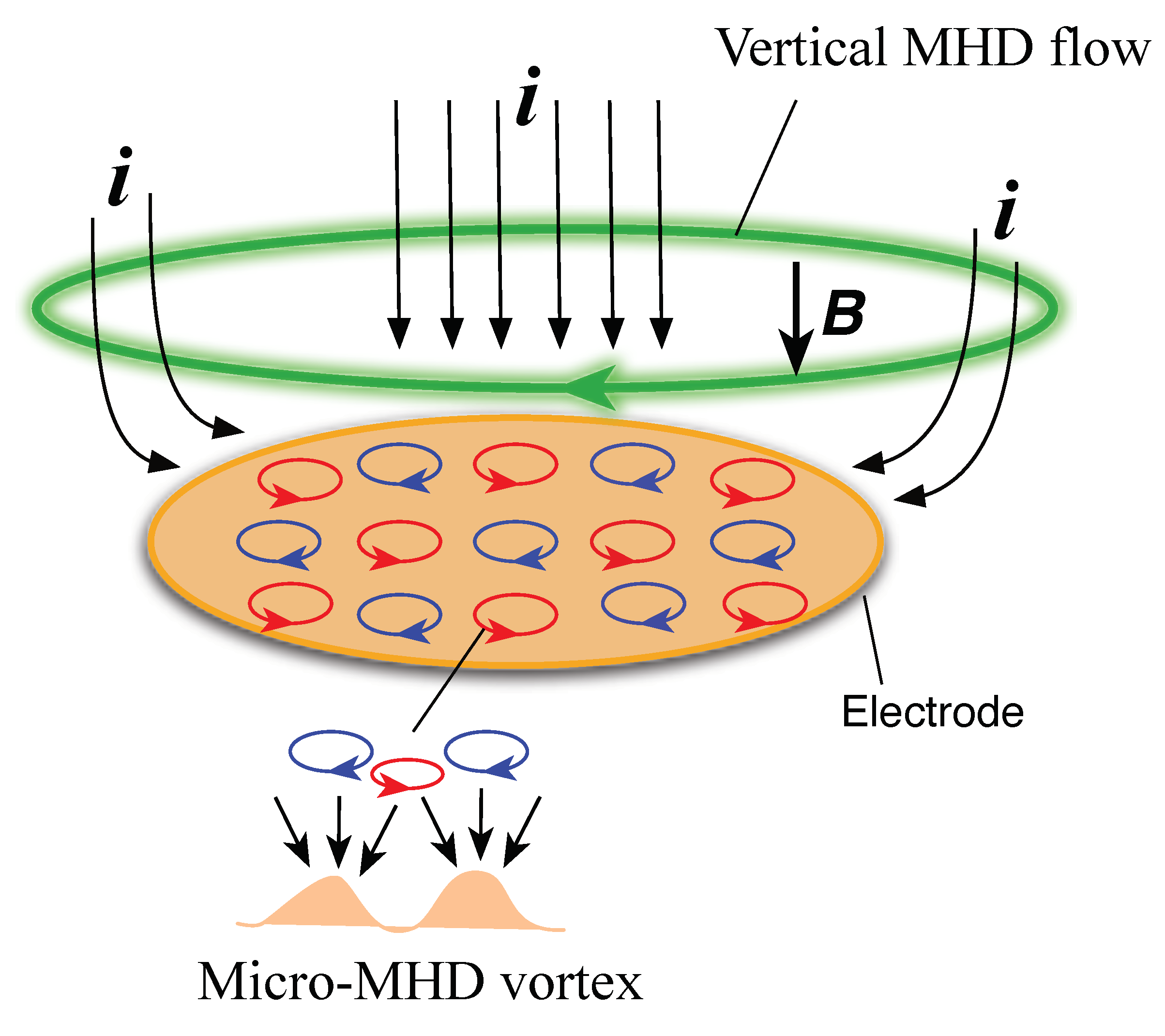
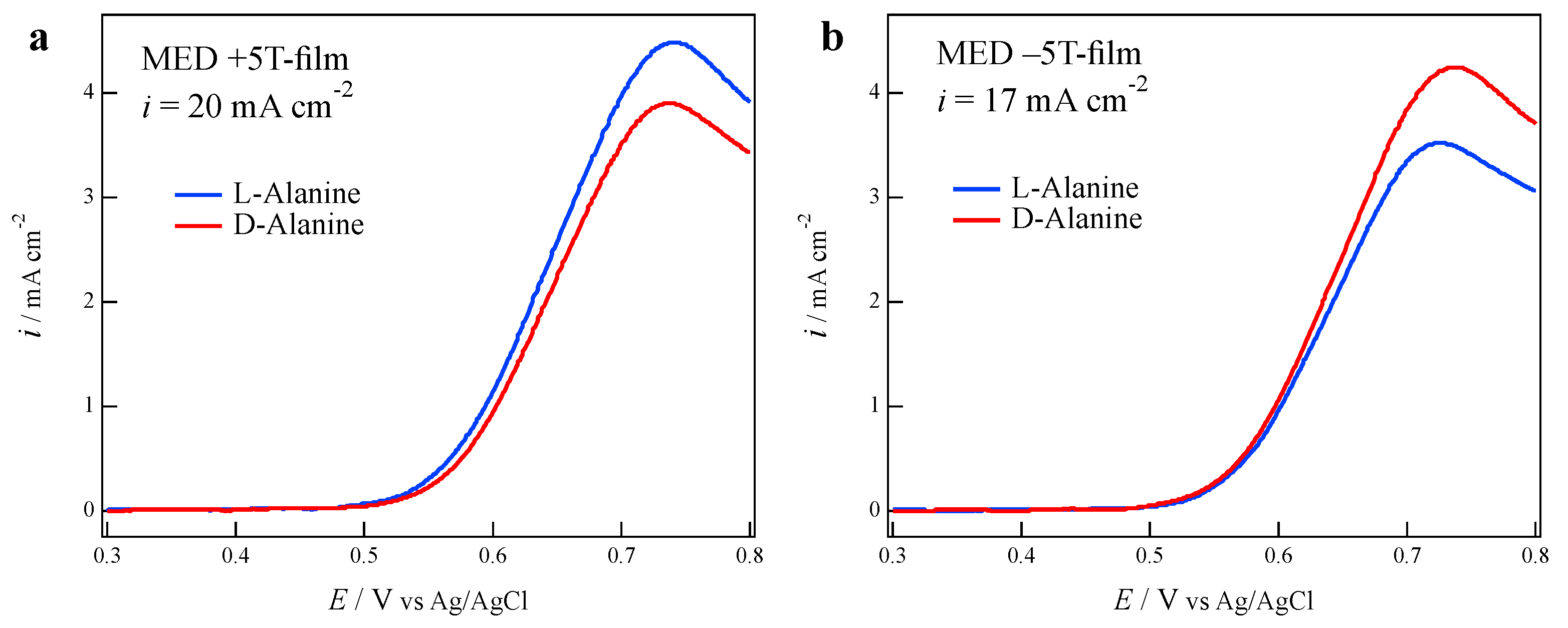
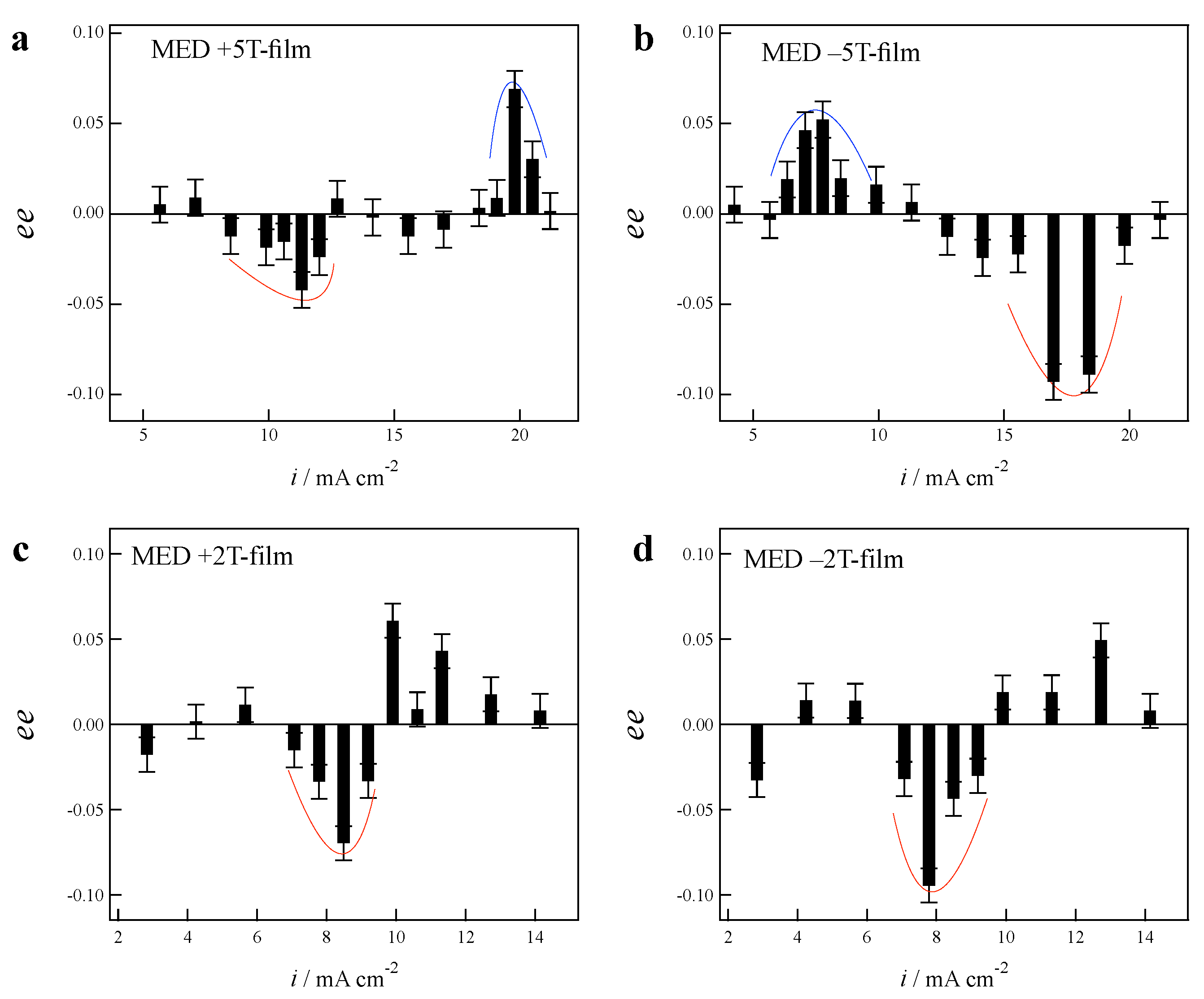
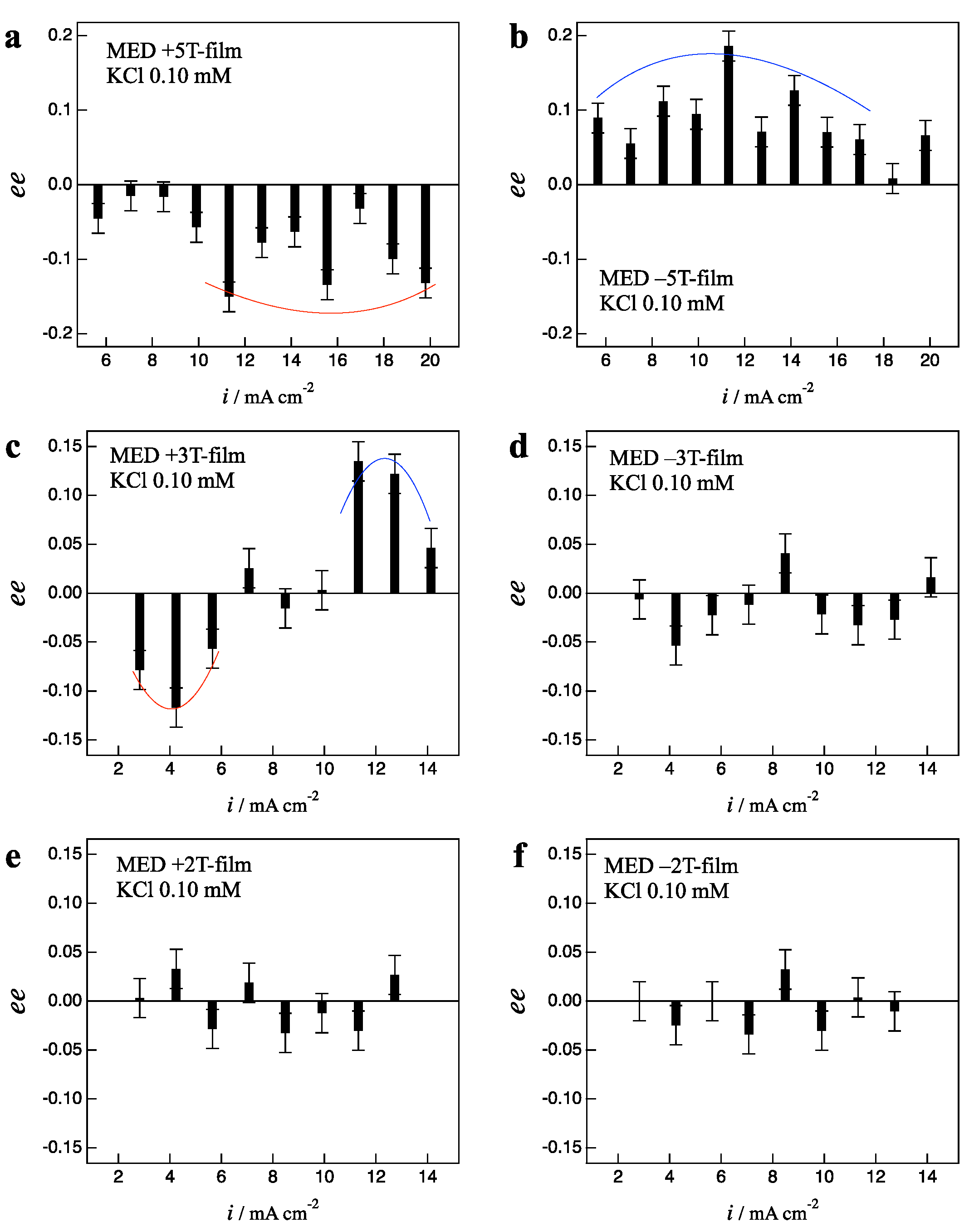
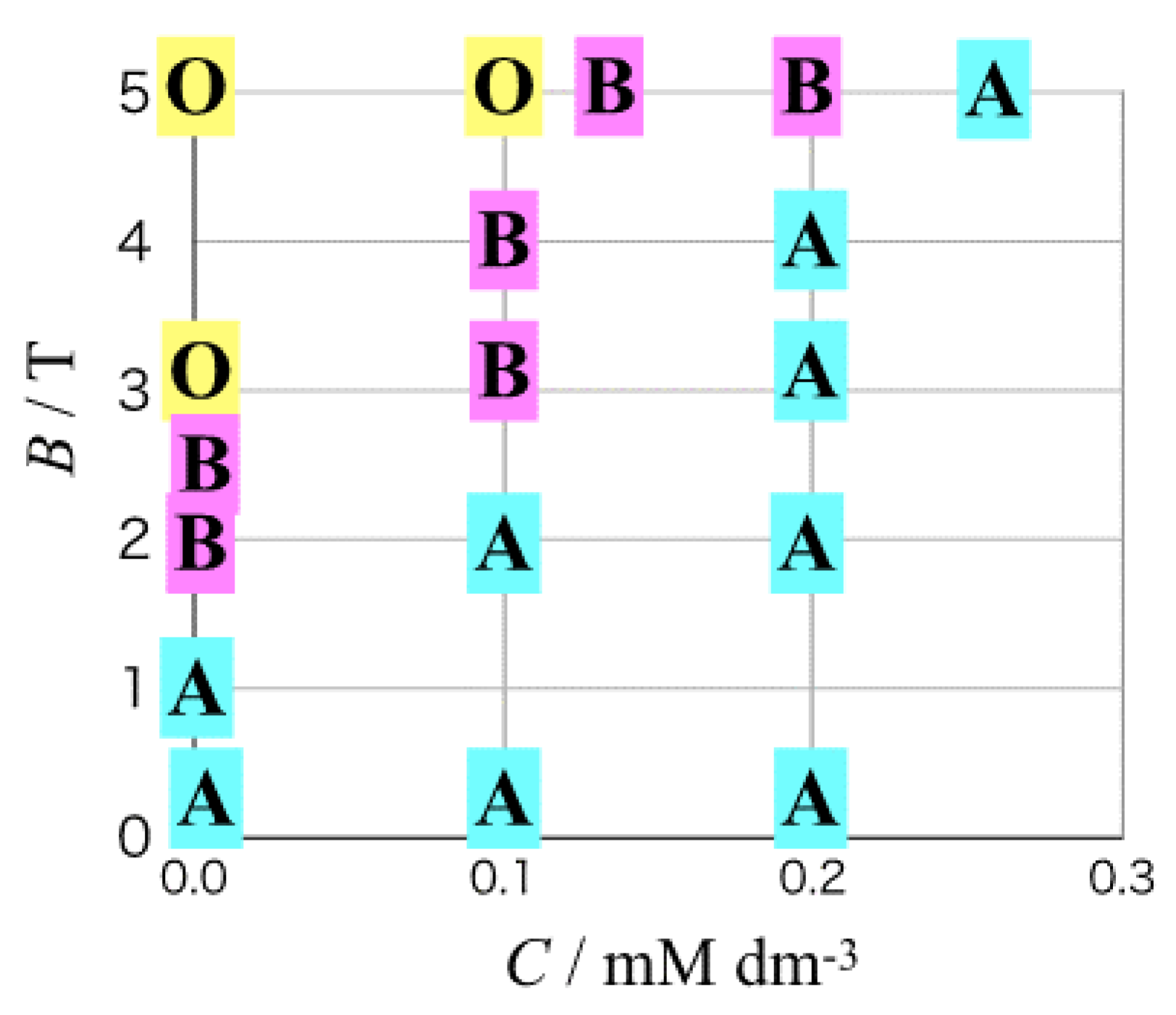
3.1. Odd Chirality and Effects of Low Magnetic Fields
3.2. Effects of Chloride Additives
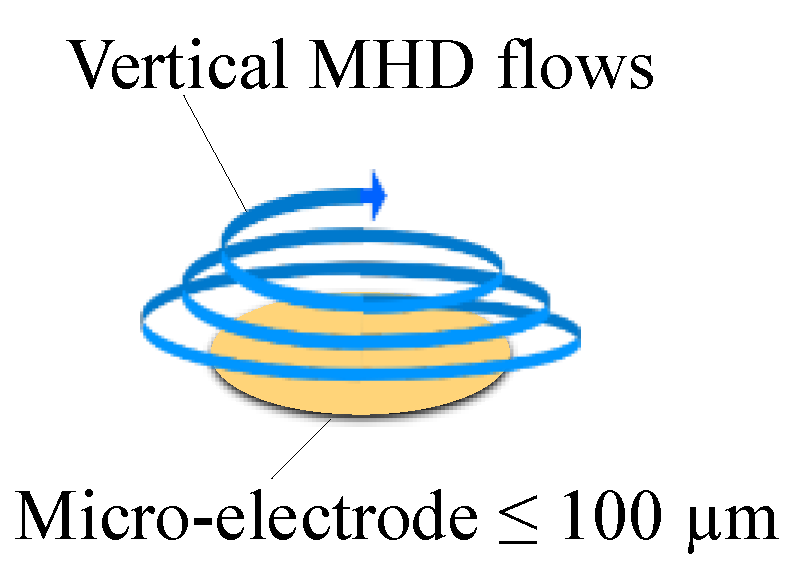
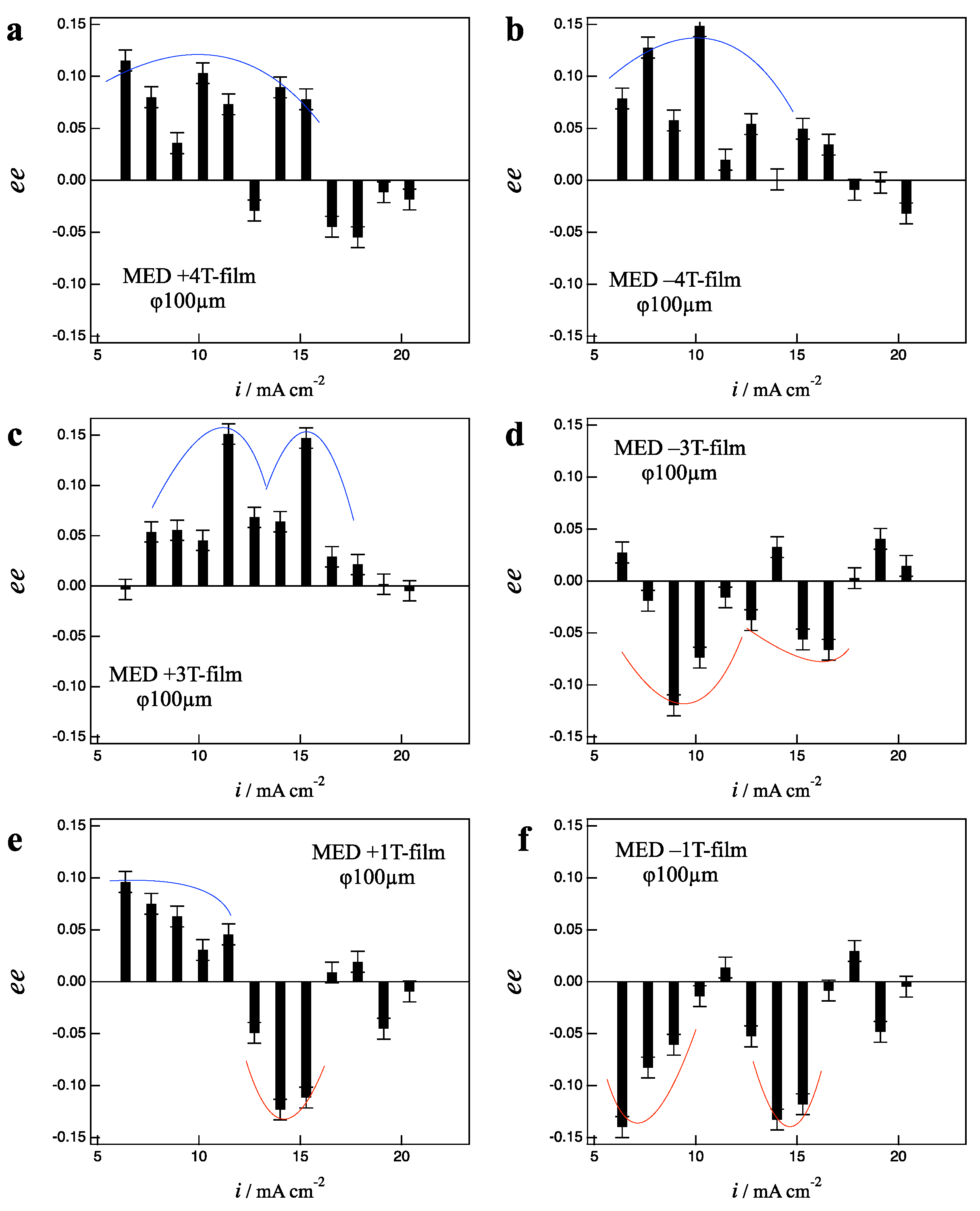
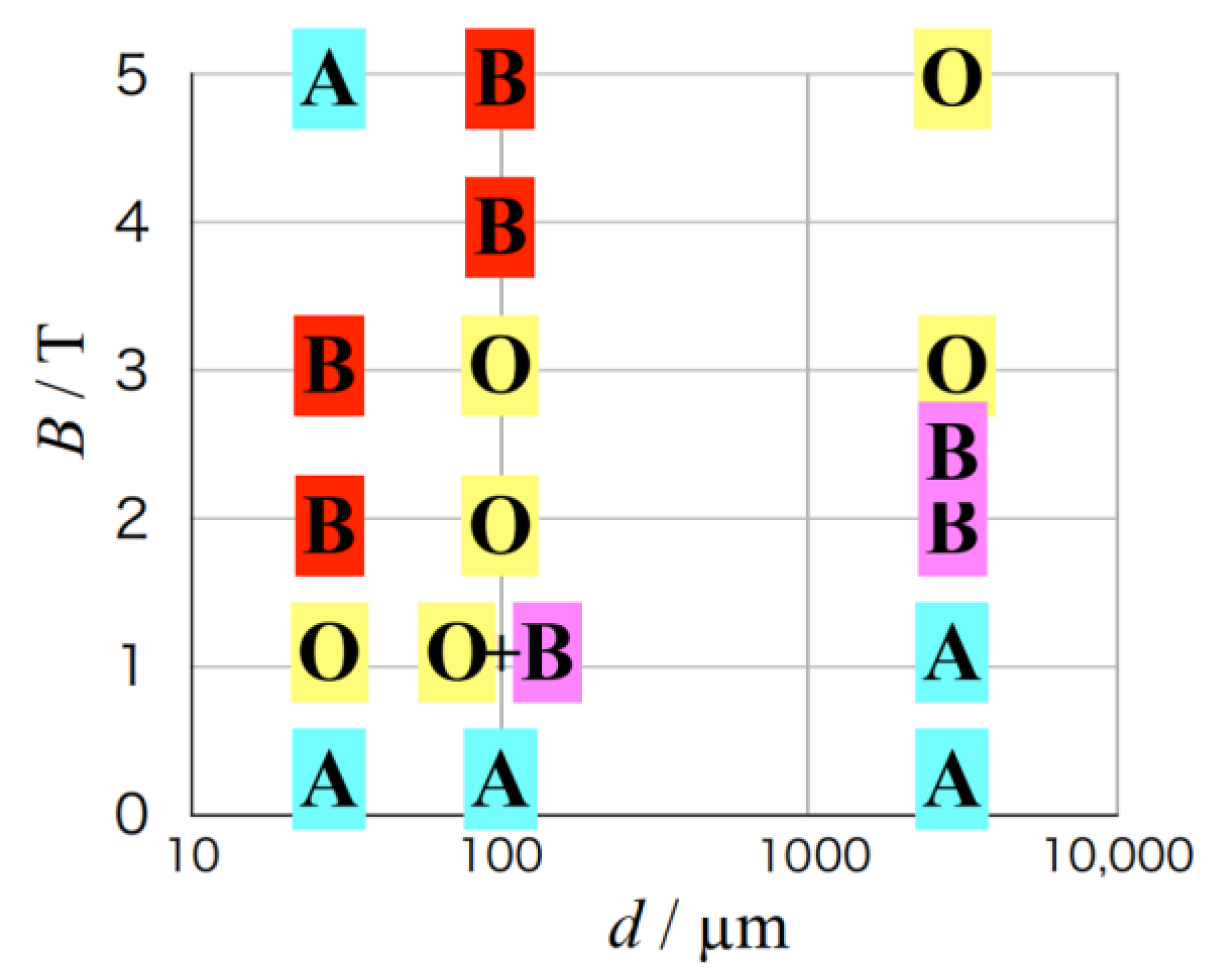
3.3. Effects of Micro-Electrode
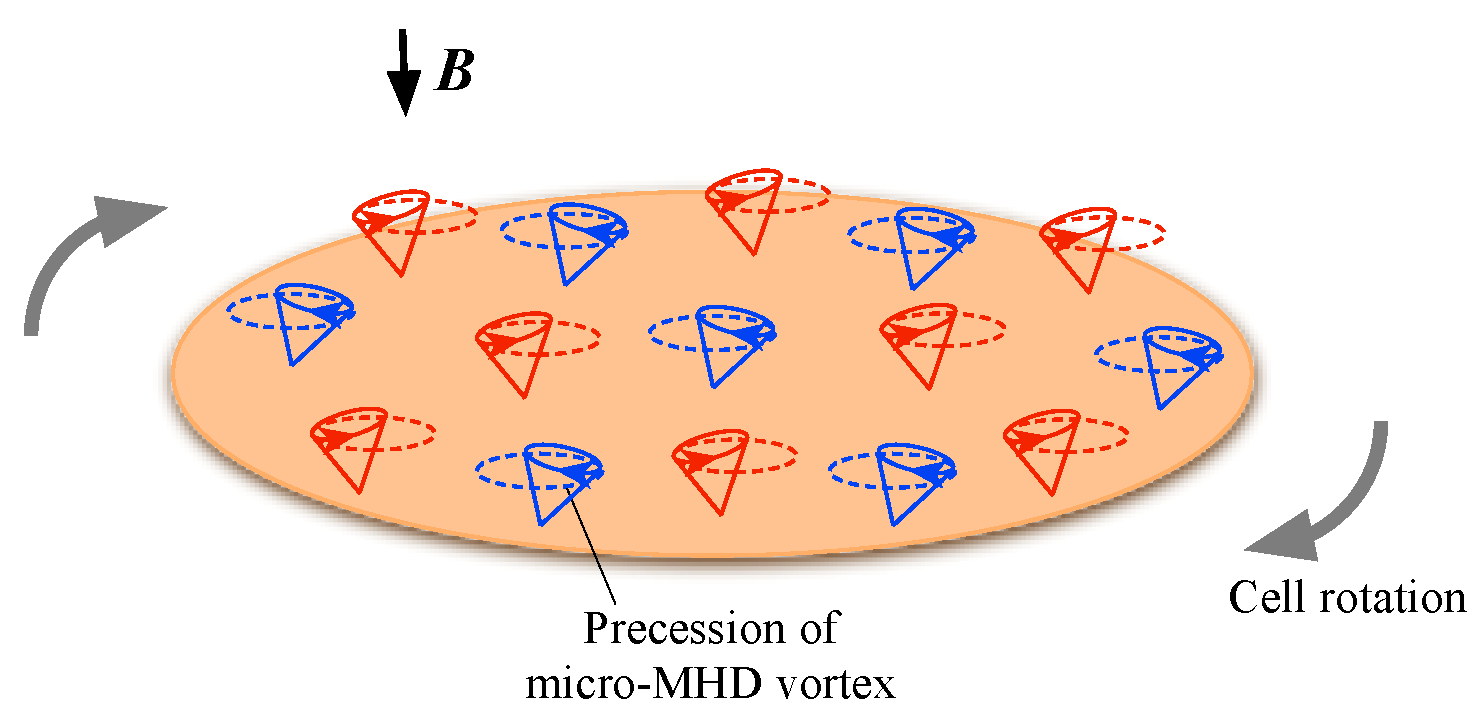
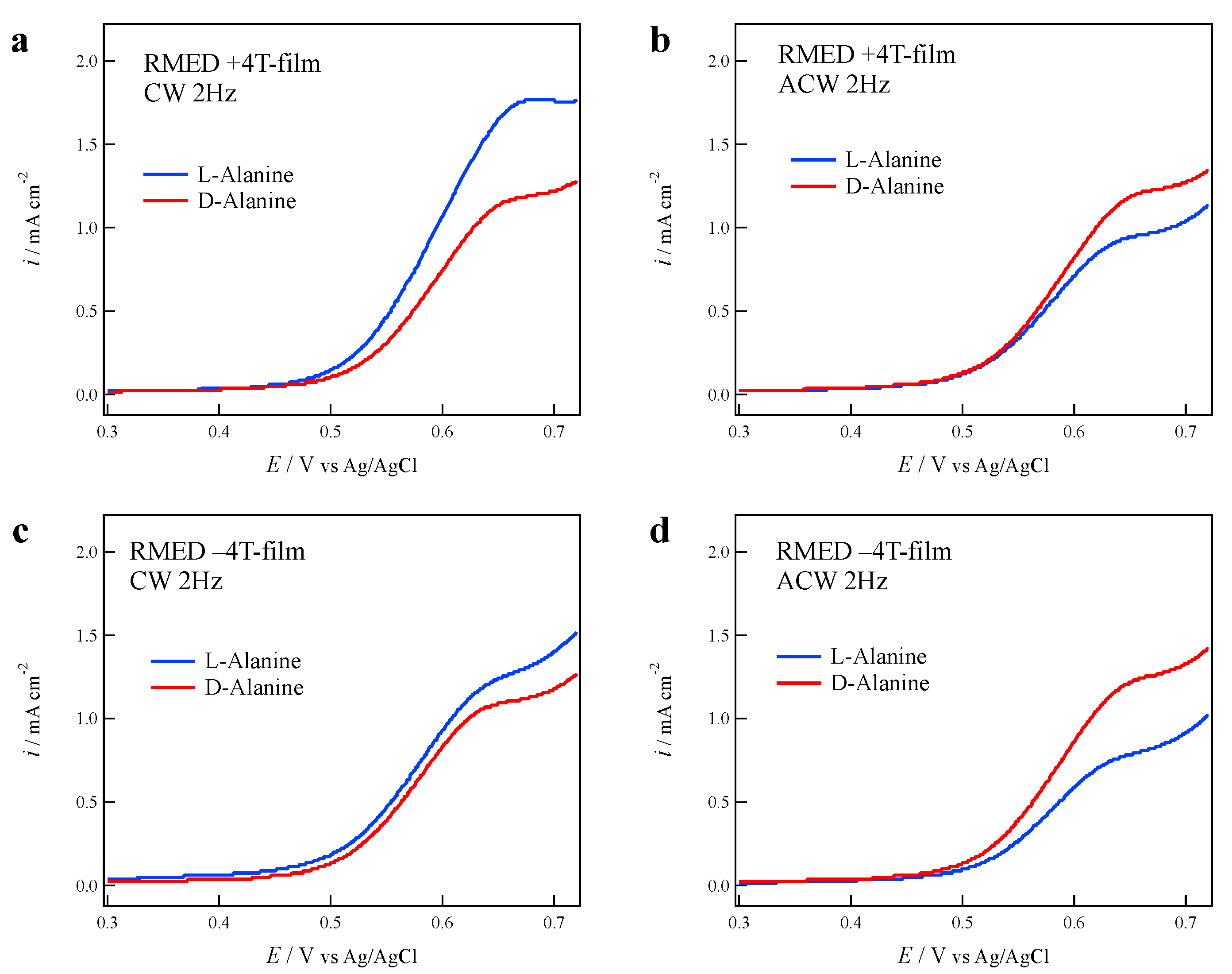
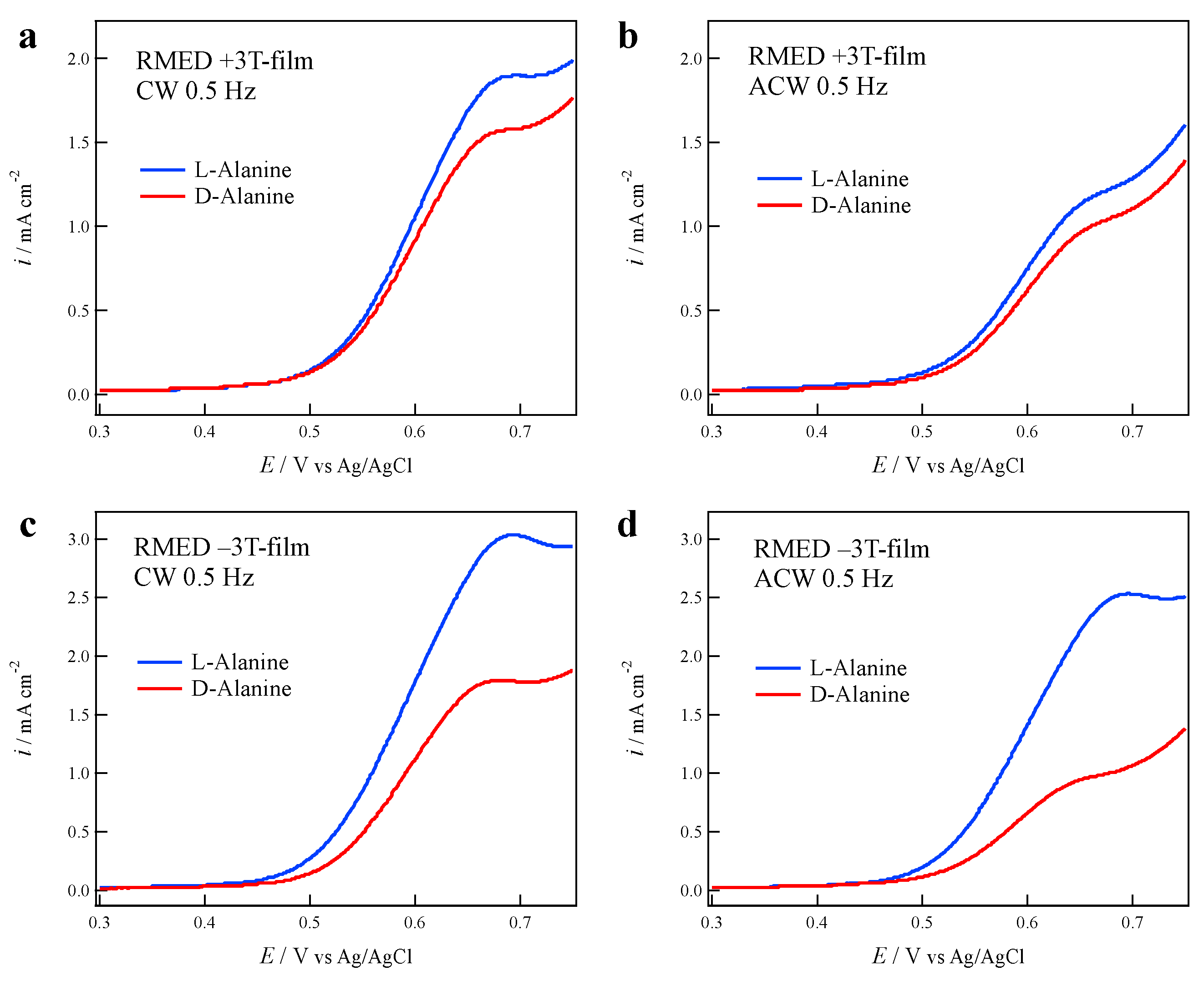
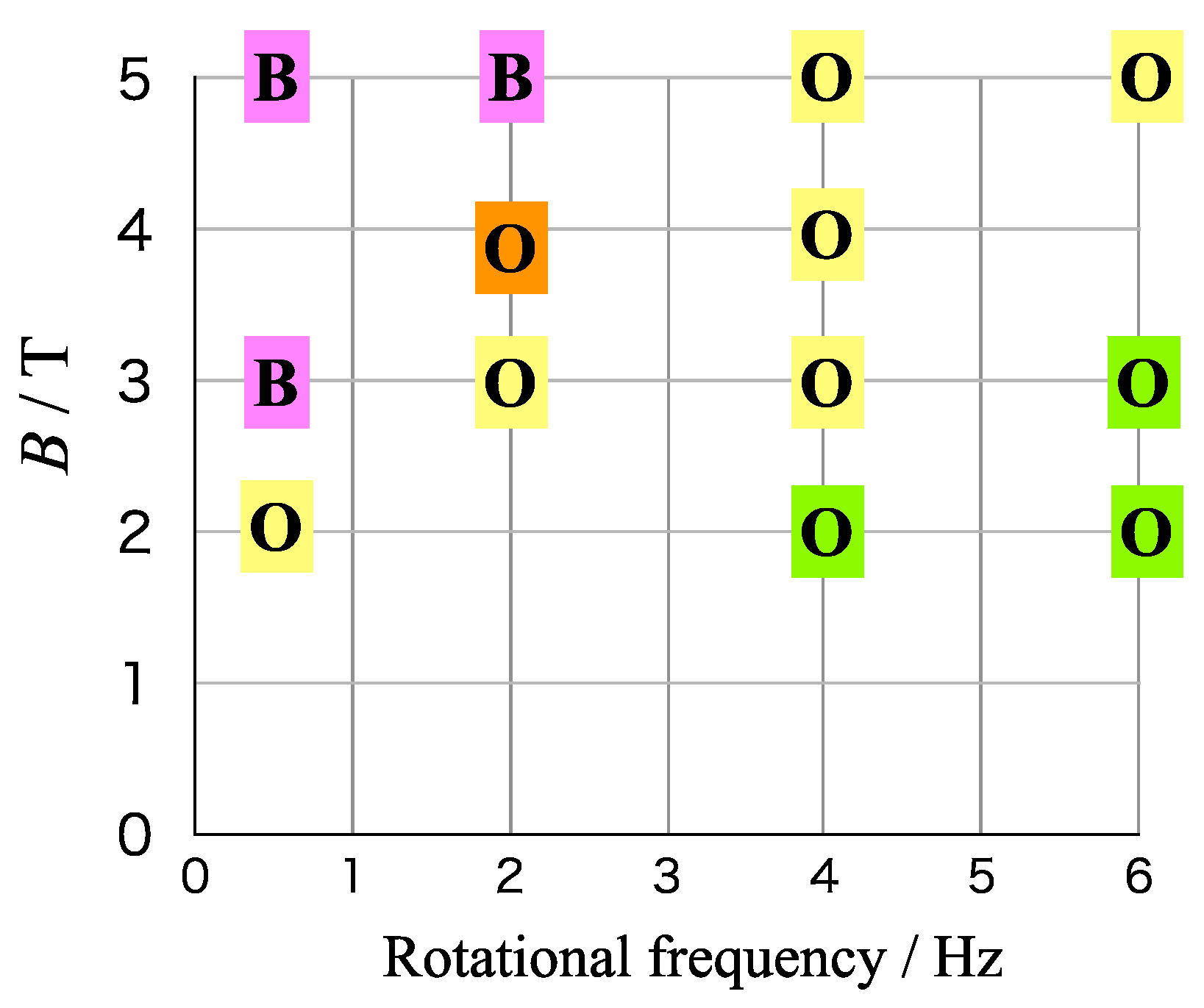
3.4. Effects of Cell Rotation
3.5. Breaking of Odd Chirality in Magnetoelectrochemical Etching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fahidy, T.Z. Magnetoelectrolysis. J. Appl. Electrochem. 1983, 13, 553–563. [Google Scholar] [CrossRef]
- Fahidy, T.Z. Hydrodynamic Models in Magnetoelectrolysis. Electrochim. Acta 1973, 18, 607–614. [Google Scholar] [CrossRef]
- Mohanta, S.; Fahidy, T.Z. Mass Transfer in a Magnetoelectrolyric Flow Cell. Electrochim. Acta 1974, 189, 835–840. [Google Scholar] [CrossRef]
- Aogaki, R.; Fueki, K.; Mukaibo, T. Application of Magnetohydrodynamic Effect to the Analysis of Electrochemical Reaction. Electrochemistry 1975, 43, 504–514. [Google Scholar]
- Monzon, L.M.A.; Coey, L.M.D. Magnetic Fields in Electrochemistry: The Lorentz Force. Electrochem. Commun. 2014, 42, 38–41. [Google Scholar] [CrossRef]
- Tacken, R.A.; Janssen, L.J.J. Applications of Magnetoelectrolysis. J. Appl. Electrochem. 1995, 25, 1–5. [Google Scholar] [CrossRef]
- Iwakura, C.; Edamoto, T.; Tamura, H. Effect of a Relatively Weak Magnetic Field on Electrochemical Reactions. J. Electrochem. Soc. Jpn. 1984, 52, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Mohanta, S.; Fahidy, T.Z. The Effect of a Uniform Magnetic Field on Mass Transfer in Electrolysis. Can. J. Chem. Eng. 1972, 50, 248–253. [Google Scholar] [CrossRef]
- Dash, J.; King, W.W. Electrothinning and Electrodeposition of Metals in Magnetic Fields. J. Electrochem. Soc. 1972, 119, 51–56. [Google Scholar] [CrossRef]
- O’Brien, R.N.; Santhanam, K.S.V. Magnetic Field Effects on the Growth of the Diffusion Layer at Vertical Electrodes during Electrodeposition. J. Electrochem. Soc. 1982, 129, 1266–1268. [Google Scholar] [CrossRef]
- Olivier, A.; Chopart, J.P.; Douglade, J.; Gabrielli, C. Investigation of Magnetic Effects on Mass Transport at the Electrode/Electrolyte Interface by Impedance Techniques. J. Electroanal. Chem. Interfacial Electrochem. 1987, 217, 443–452. [Google Scholar] [CrossRef]
- Hinds, G.; Coey, J.D.M.; Lyons, M.E.G. Magnetoelectrolysis of Copper. J. Appl. Phys. 1998, 83, 6447–6449. [Google Scholar] [CrossRef]
- Mogi, I.; Okubo, S.; Nakagawa, Y. Dense Radial Growth of Silver Metal Leaves in a High Magnetic Field. J. Phys. Soc. Jpn. 1991, 60, 3200–3202. [Google Scholar] [CrossRef]
- Mogi, I.; Kamiko, M.; Okubo, S.; Kido, G. Pattern Formation of Electrodeposit of Zinc in Magnetic Fields. Phys. B Condens. Matter 1994, 201, 606–610. [Google Scholar] [CrossRef]
- Ni Mhiochain, T.R.; Coey, J.M.D. Chirality of Electrodeposits Grown in a Magnetic Field. Phys. Rev. E 2004, 69, 061404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coey, J.M.D.; Hinds, G.; Lyons, M.E.G. Magnetic-Field Effects on Fractal Electrodeposits. Europhys. Lett. 1999, 47, 267–272. [Google Scholar] [CrossRef]
- Heresanu, V.; Ballou, R.; Molho, P. Electrochemical Growth of Zn and Fe Arborescences under Normal Magnetic Field. Magnetohydrodynamics 2003, 39, 461–468. [Google Scholar]
- Heresanu, V.; Ballou, R.; Molho, P. Electrochemical Deposition of Iron in a Thin Cell under an In-Plane Magnetic Field. Magnetohydrodynamics 2006, 42, 403–408. [Google Scholar]
- Hirota, N.; Hara, S.; Uetake, H.; Nakamura, H.; Kitazawa, K. In Situ Microscopic Observations of an Electroless Silver Deposition Process under High Magnetic Fields. J. Cryst. Growth 2006, 286, 465–469. [Google Scholar] [CrossRef]
- Bodea, S.; Vignon, L.; Ballou, R.; Molho, P. Electrochemical Growth of Iron Arborescences under In-Plane Magnetic Field: Morphology Symmetry Breaking. Phys. Rev. Lett. 1999, 83, 2612–2615. [Google Scholar] [CrossRef]
- Katsuki, A.; Watanabe, S.; Tokunaga, R.; Tanimoto, Y. The Effects of High Magnetic Field on the Deposition of Silver. Chem. Lett. 1996, 25, 219–220. [Google Scholar] [CrossRef]
- Katsuki, A.; Tanimoto, Y. Precession of Silver Dendrites in a Magnetic Field Due to MHD Induced Convection. Chem. Lett. 2005, 34, 726–727. [Google Scholar] [CrossRef]
- Fahidy, T.Z. Characteristics of Surfaces Produced via Magnetoelectrolytic Deposition. Prog. Surf. Sci. 2001, 68, 155–188. [Google Scholar] [CrossRef]
- Duan, W.; Kitamura, S.; Uechi, I.; Katsuki, A.; Tanimoto, Y. Three-dimensional morphological chirality induction using high magnetic fields in membrane tubes prepared by a silicate garden reaction. J. Phys. Chem. B 2005, 109, 13445–13450. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Kuroda, N.; Kakudate, Y. Magnetic field induced helical structure in freestanding metal silicate tubes. J. Appl. Phys. 2005, 97, 10R513. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Kamiko, M. Pattern Formation in Magneto-electropolymerization of pyrrole. J. Electrochem. Soc. Jpn. 1996, 64, 842–844. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Shinyama, A.; Omote, K. Three-Dimensional Morphological Chirality Induction in Polythiophene Polymer Deposit Using a Magnetic Field. Bull. Chem. Soc. Jpn. 2009, 82, 695–697. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Folling, J.; Wyder, P. Electrical Magnetochiral Anisotropy. Phys. Rev. Lett. 2001, 87, 236602. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Chirality of Magnetoelectropolymerized Polyaniline Electrodes. Jpn. J. Appl. Phys. 2005, 44, L199–L201. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Electrocatalytic Chirality on Magneto-Electropolymerized Polyaniline electrodes. J. Solid State Electrochem. 2007, 11, 751–756. [Google Scholar] [CrossRef]
- Kumar, A.; Mondal, P.C.; Fontanesi, C. Chiral Magneto-Electrochemistry. Magnetochemistry 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Gazzotti, M.; Arnaboldi, S.; Grecchi, S.; Giovanardi, R.; Cannio, M.; Pasquali, L.; Giacomino, A.; Abollino, O.; Fontanesi, C. Spin-Dependent Electrochemistry: Enantio-Selectivity Driven by Chiral-Induced Spin Selectivity Effect. Electrochim. Acta 2018, 286, 271–278. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Chiral electrode behavior of magneto-electrodeposited silver films. ISIJ Int. 2007, 47, 585–587. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Watanabe, K. Magnetoelectrochemical Chirality in Ag Electrodeposition. J. Chem. Chem. Eng. 2010, 4, 16–22. [Google Scholar]
- Mogi, I.; Watanabe, K. Chiral recognition of amino acids by magnetoelectrodeposited Cu film electrodes. Int. J. Electrochem. 2011, 2011, 239637. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Watanabe, K. Chirality of magnetoelectrodeposited Cu films. Magnetohydrodynamics 2012, 48, 251–259. [Google Scholar]
- Mogi, I.; Watanabe, K. Enantioselective recognition of tartaric acid on magnetoelectrodeposited copper film electrodes. Chem. Lett. 2012, 41, 1439–1441. [Google Scholar] [CrossRef]
- Aogaki, R. Micro-MHD effect on electrodeposition in the vertical magnetic field. Magnetohydrodynamics 2003, 4, 453–460. [Google Scholar]
- Aogaki, R.; Morimoto, R. Nonequilibrium Fluctuations in Micro-MHD Effects on Electrodeposition. In Heat and Mass Transfer: Modeling and Simulation; Hossain, M., Ed.; InTech: London, UK, 2011; pp. 189–216. [Google Scholar]
- Mogi, I.; Iwasaka, K.; Aogaki, R.; Takahashi, K. Communication—Visualization of Magnetohydrodynamic Micro-Vortices with Guanine Micro-Crystals. J. Electrochem. Soc. 2017, 164, H584–H586. [Google Scholar] [CrossRef]
- Daltin, A.L.; Chopart, J.P. Magnetic Field Effect on Electrodeposition of Cu2O Crystals. Magnetohydrodynamics 2009, 45, 267–273. [Google Scholar] [CrossRef]
- Sommeria, J.; Meyers, S.D.; Swinney, H.L. Laboratory Simulation of Jupiter’s Great Red Spot. Nature 1988, 331, 689–693. [Google Scholar] [CrossRef]
- Marcus, S.M. Numerical Simulation of Jupiter’s Great Red Spot. Nature 1988, 331, 693–696. [Google Scholar] [CrossRef]
- Yanson, Y.I.; Rost, M.J. Structural Accelerating Effect of Chloride on Copper Electrodeposition. Angew. Chem. Int. Ed. 2013, 52, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Mogi, I.; Aogaki, R.; Watanabe, K. Tailoring of Surface Chirality by Micro-Vortices and Specific Adsorption in Magnetoelectrodeposition. Bull. Chem. Soc. Jpn. 2015, 88, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Aogaki, R.; Takahashi, K. Fluctuation Effects of Magnetohydrodynamic Micro-Vortices on Odd Chirality in Magnetoelectrolysis. Magnetochemistry 2020, 6, 43. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Chiral Surface Formation in Magnetoelectrolysis on Micro-Electrodes. Magnetohydrodynamics 2017, 53, 321–328. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Breaking of Odd Chirality in Magnetoelectrodeposition of Copper Films on Micro-Electrodes. Magnetochemistry 2021, 7, 142. [Google Scholar] [CrossRef]
- Mogi, I.; Morimoto, R.; Aogaki, R. Surface Chirality Effects Induced by Magnetic Fields. Curr. Opin. Electrochem. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Takahashi, K. Surface Chirality in Rotational Magnetoelectrodeposition of Copper Films. Magnetochemistry 2019, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Wachterchauser, G. Before Enzyme and Templates: Theory of Surface Metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar] [CrossRef]
- Switzer, J.A.; Kothari, H.M.; Poizot, P.; Nakanishi, S.; Bohannan, E.W. Enantiospecific Electrodeposition of a Chiral Catalyst. Nature 2003, 425, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.A.; Harris, C.; Herrero, E.; Feliu, J. The Influence of Anions and Kink Structure on the Enantioselective Electro-Oxidation of Glucose. Faraday Discuss. 2002, 121, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.A.; Ahmadi, A.; Jenkins, D.J.; Hazzazi, O.A.; Wells, P.B.; Griffin, K.G.; Johnston, P.; Gillies, J.E. The Characterisation of Supported Platinum Nanoparticles on Carbon Used for Enantioselective Hydrogenation: A Combined Electrochemical-STM Approach. ChemPhysChem 2003, 4, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Gellman, A.J.; Horvath, J.D.; Buelow, M.T. Chiral Single Crystal Surface Chemistry. J. Mol. Catal. Chem. 2001, 167, 3–11. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, F.; Baldwin, R.P. Constant Potential Amperometric Detection of Underivatized Amino Acids and Peptides at A Copper Electrode. Anal. Chem. 1991, 63, 1702–1707. [Google Scholar] [CrossRef]
- Shao, W.; Pattanaik, G.; Zangari, G. Influence of Chloride Anions on Mechanism of Copper Electrodeposition from Acidic Sulfate Electrolytes. J. Electrochem. Soc. 2007, 154, D201–D207. [Google Scholar] [CrossRef]
- Kao, Y.L.; Li, K.C.; Tu, G.C.; Huang, C.A. Microstructual Study of the Effect of Chloride Ion on Electroplating of Copper in Cupric Sulfate-Sulfuric Acid Bath. J. Electrochem. Soc. 2005, 152, C605–C611. [Google Scholar] [CrossRef]
- Dow, W.P.; Huang, H.S. Roles of Chloride Ion in Microvia Filling by Copper Electrodeposition. J. Electrochem. Soc. 2005, 152, C67–C76. [Google Scholar] [CrossRef]
- Nagy, Z.; Blaudeau, J.P.; Hung, N.C.; Curtiss, L.A.; Zurawski, D.J. Chloride Ion Catalysis of the Copper Deposition Reaction. J. Electrochem. Soc. 1995, 142, L87–L89. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Watanabe, K. Chiral Surface Formation of Copper Films by Magnetoelectrochemical Etching. Magnetohydrodynamics 2015, 51, 361–368. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Chiral Symmetry Breaking in Magnetoelectrochemical Etching with Chloride Additives. Molecules 2018, 23, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogi, I.; Morimoto, R.; Aogaki, R.; Takahashi, K. Breaking of Odd Chirality in Magnetoelectrodeposition. Magnetochemistry 2022, 8, 67. https://doi.org/10.3390/magnetochemistry8070067
Mogi I, Morimoto R, Aogaki R, Takahashi K. Breaking of Odd Chirality in Magnetoelectrodeposition. Magnetochemistry. 2022; 8(7):67. https://doi.org/10.3390/magnetochemistry8070067
Chicago/Turabian StyleMogi, Iwao, Ryoichi Morimoto, Ryoichi Aogaki, and Kohki Takahashi. 2022. "Breaking of Odd Chirality in Magnetoelectrodeposition" Magnetochemistry 8, no. 7: 67. https://doi.org/10.3390/magnetochemistry8070067
APA StyleMogi, I., Morimoto, R., Aogaki, R., & Takahashi, K. (2022). Breaking of Odd Chirality in Magnetoelectrodeposition. Magnetochemistry, 8(7), 67. https://doi.org/10.3390/magnetochemistry8070067





