Studying the Effect of Electrode Material and Magnetic Field on Hydrogen Production Efficiency
Abstract
:1. Introduction
2. Experimental Setup and Methods
3. Results and Discussion
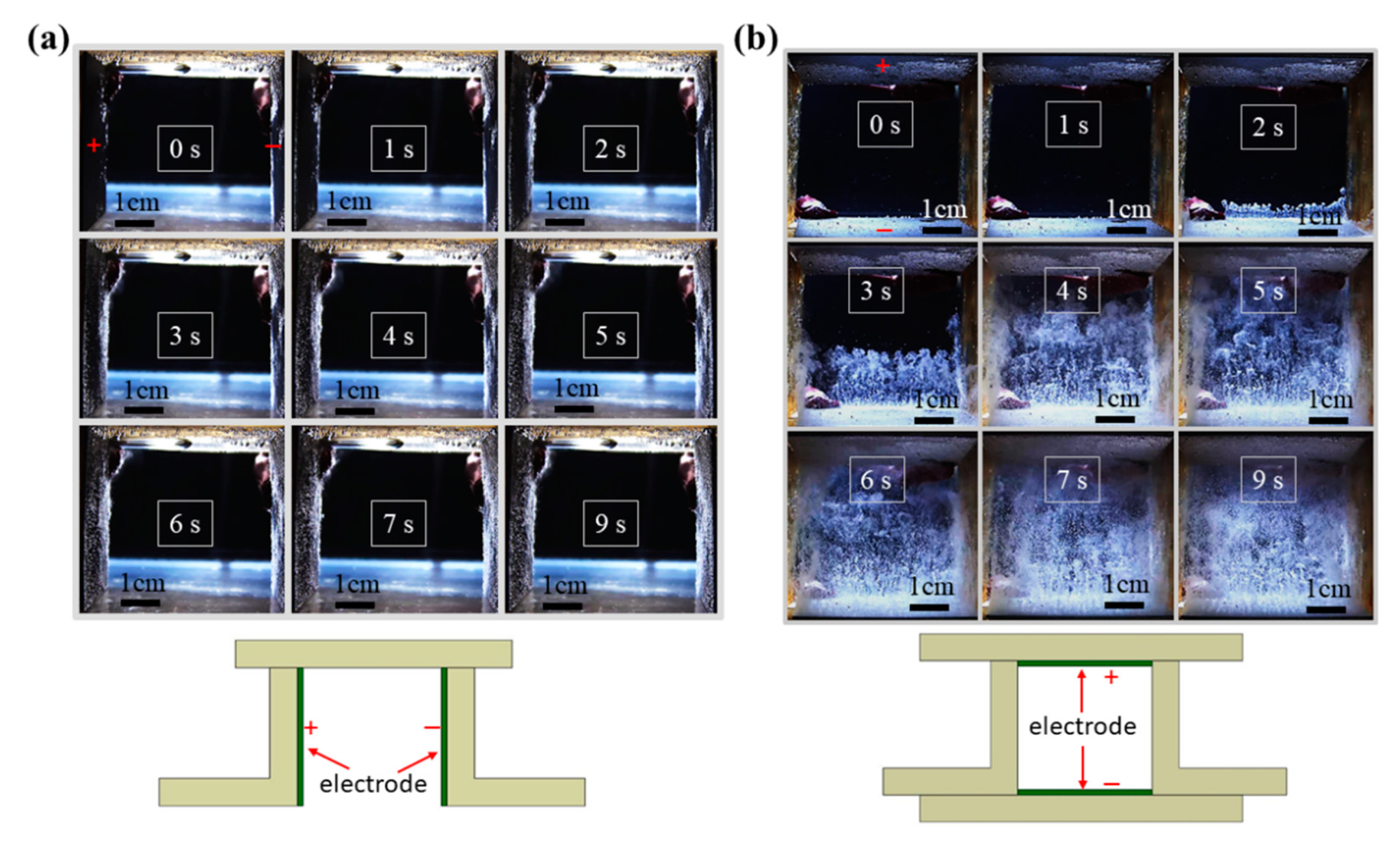
3.1. Influence of Electrode Layouts on the Detachment of Gas Bubbles
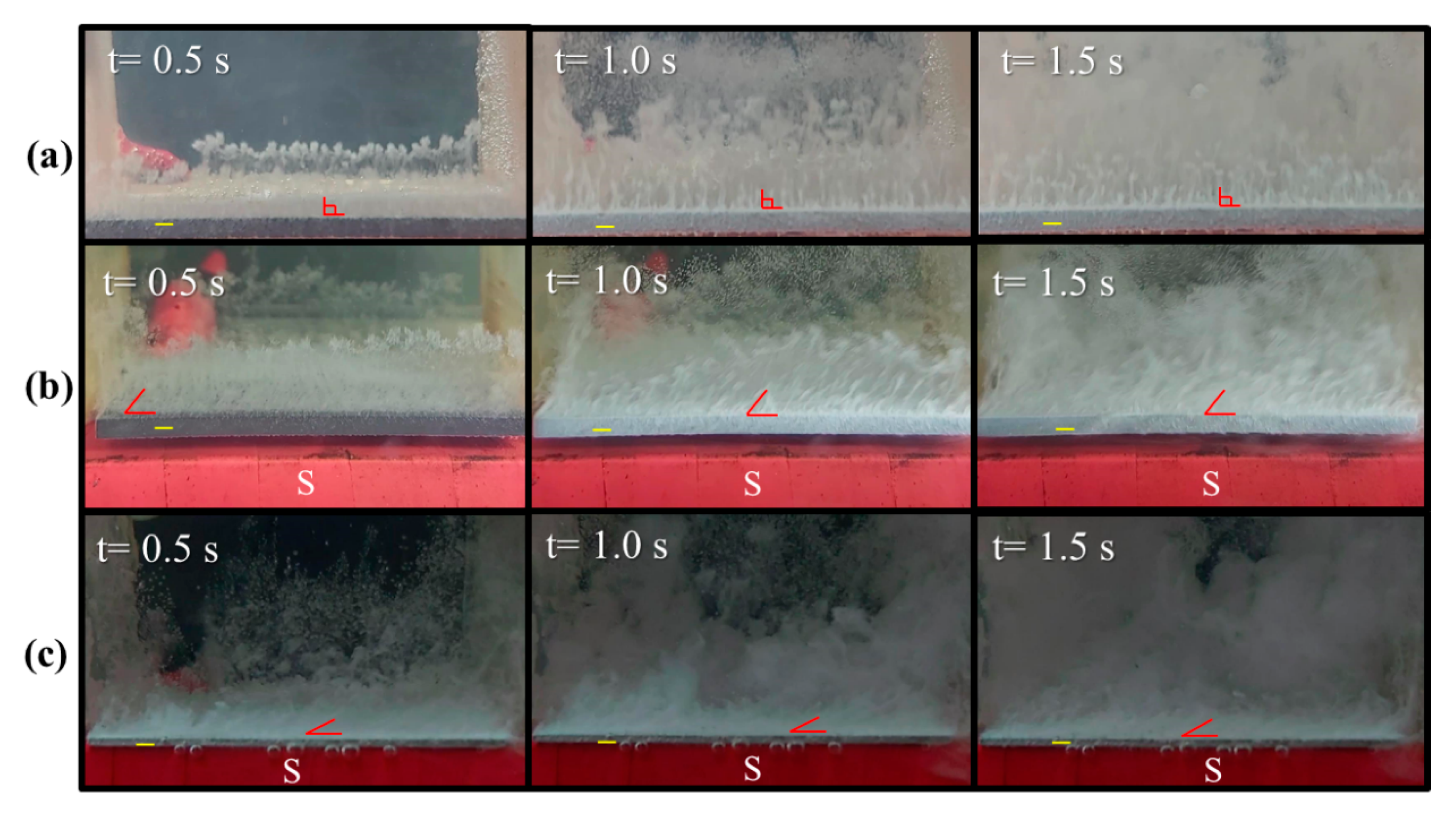
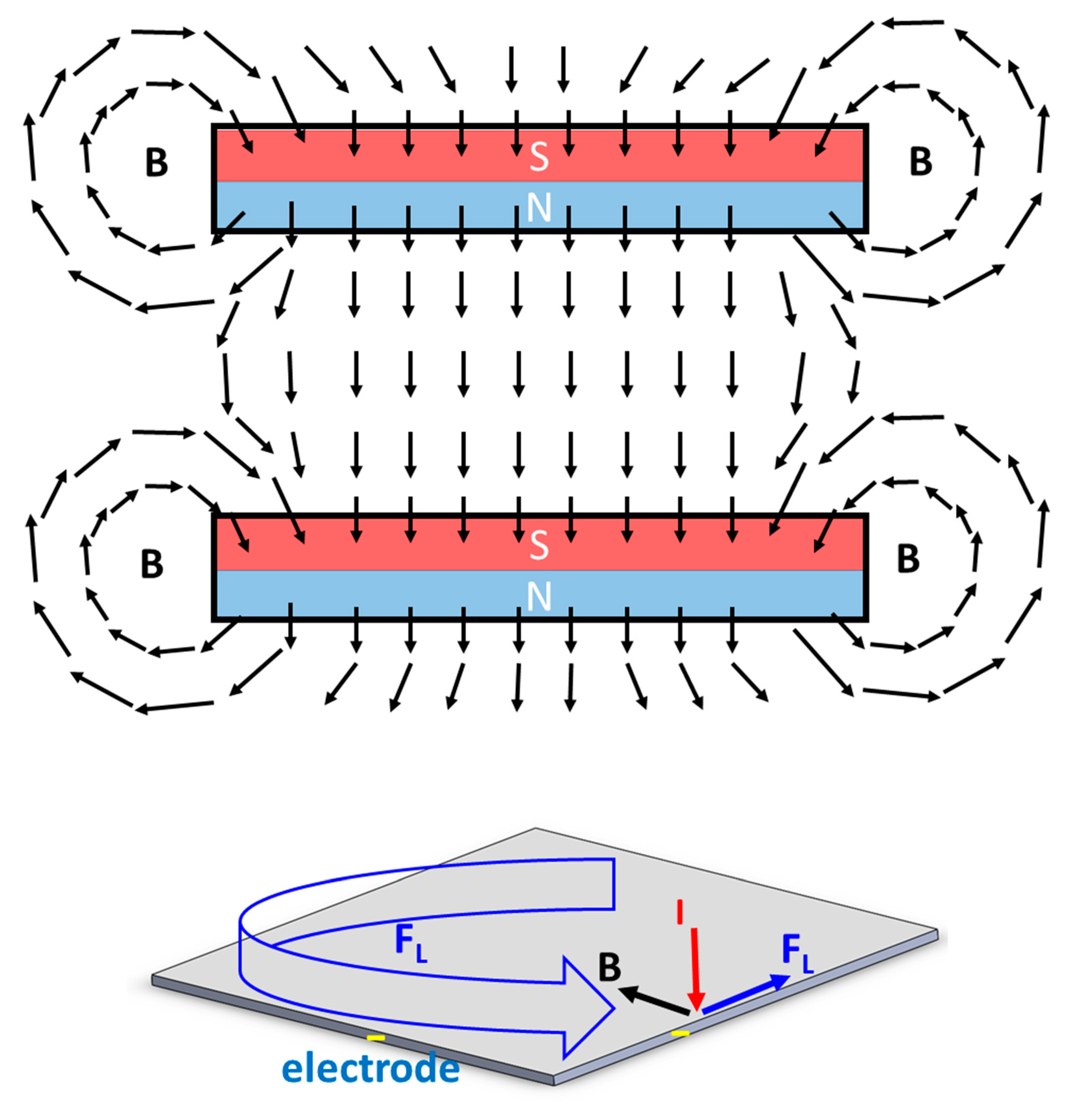
3.2. Effect of Magnetism on the Movement of Gas Bubbles
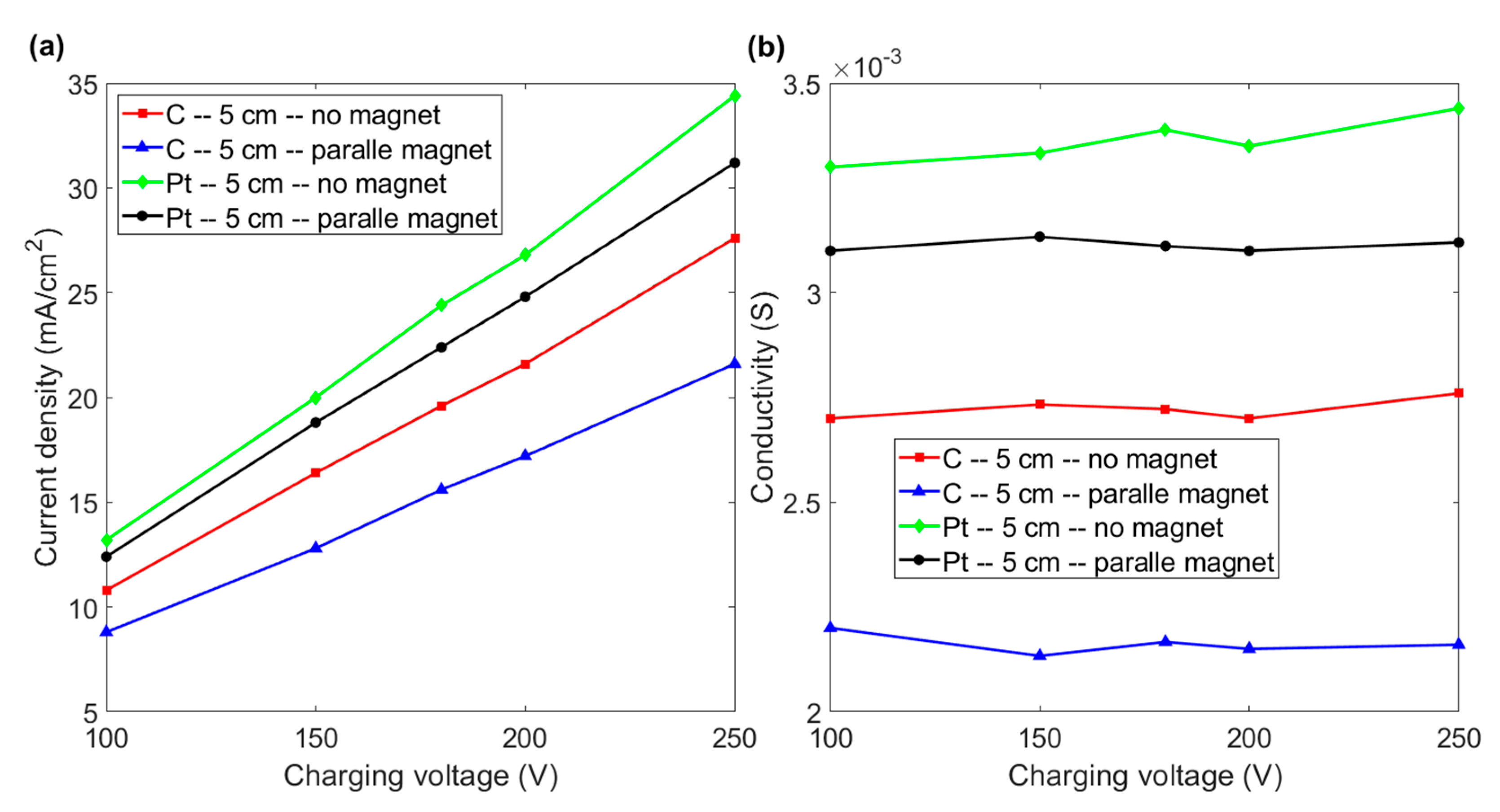
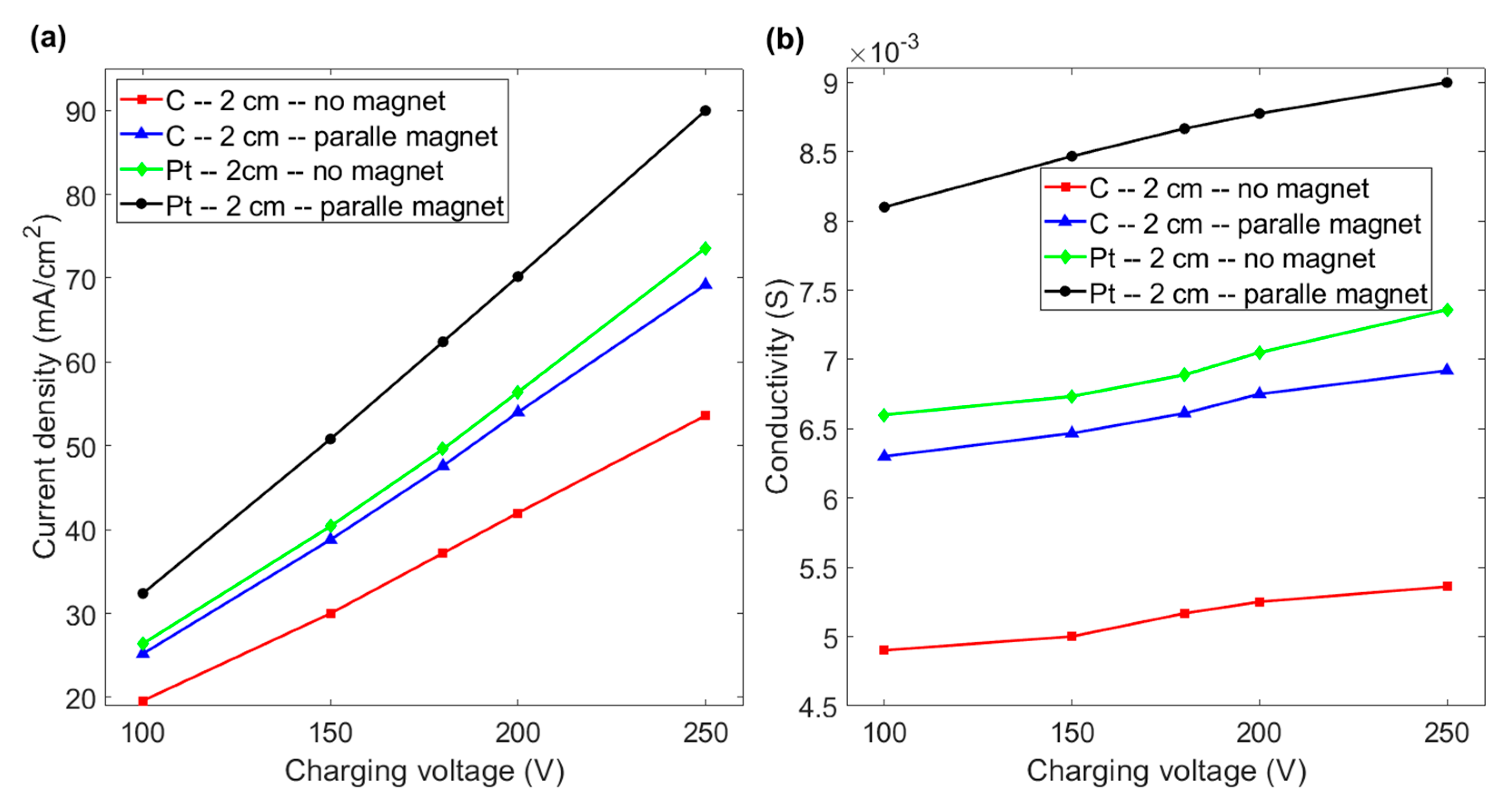
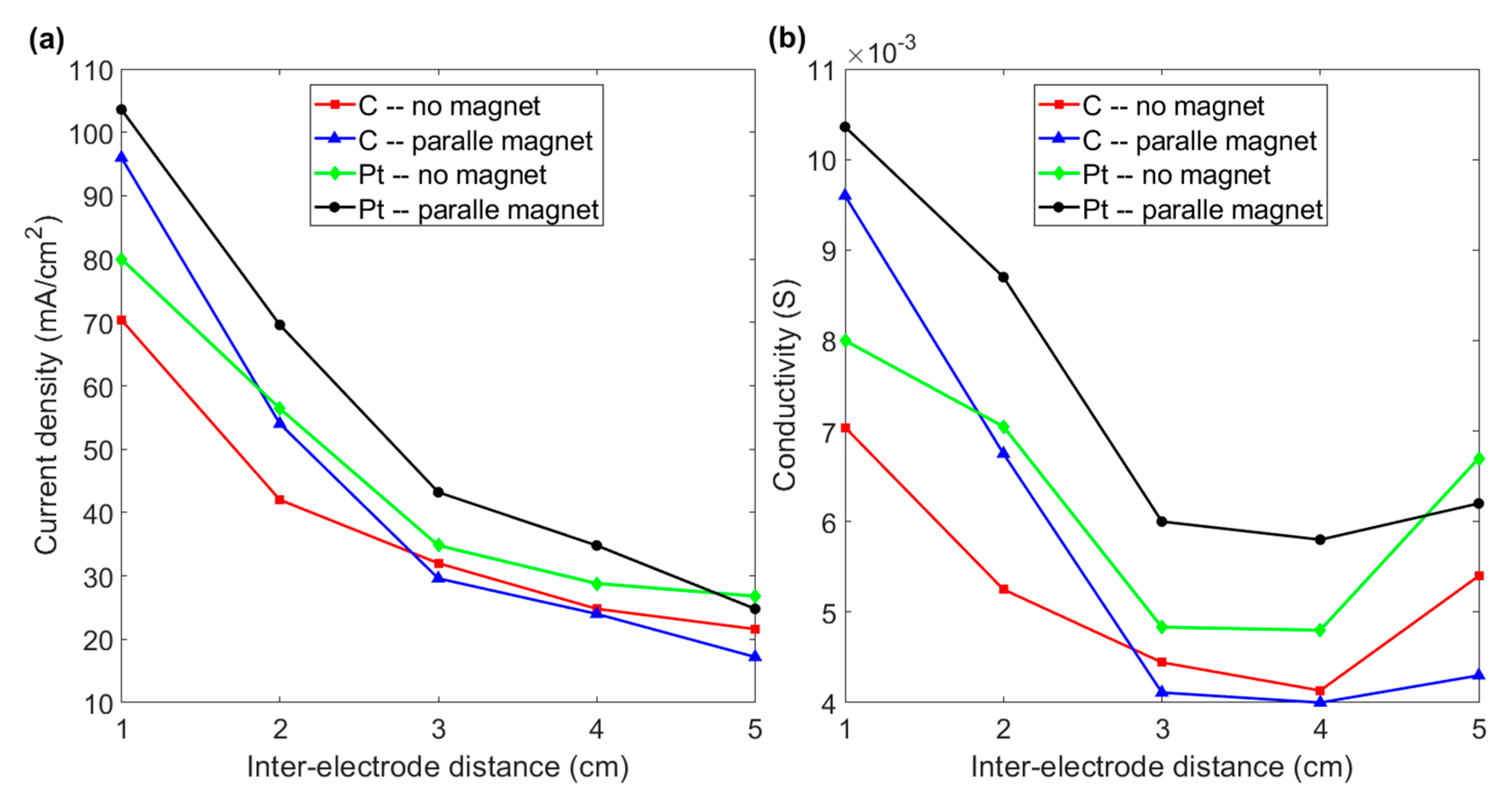
3.3. Effect of the Distance between Electrodes
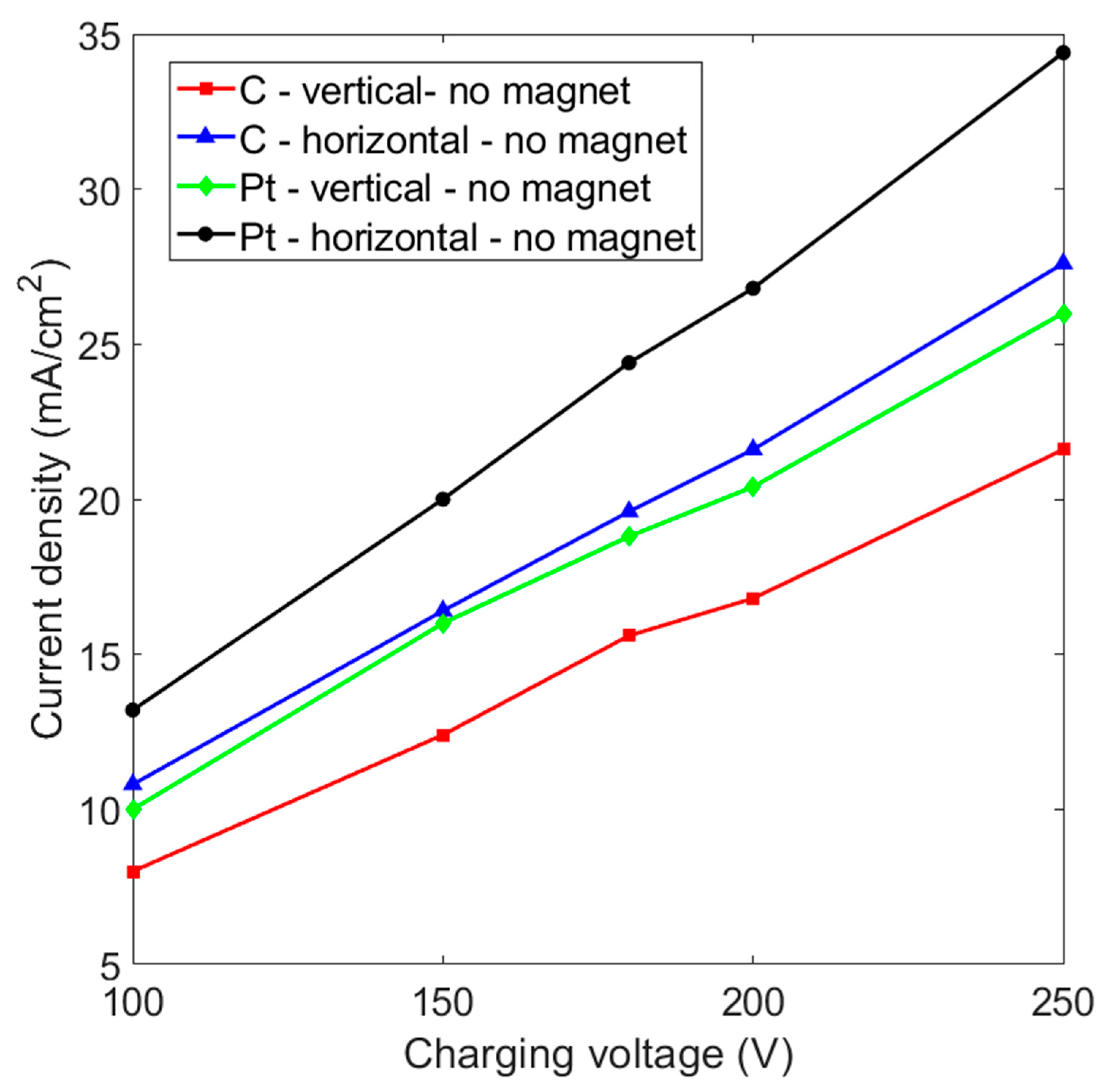
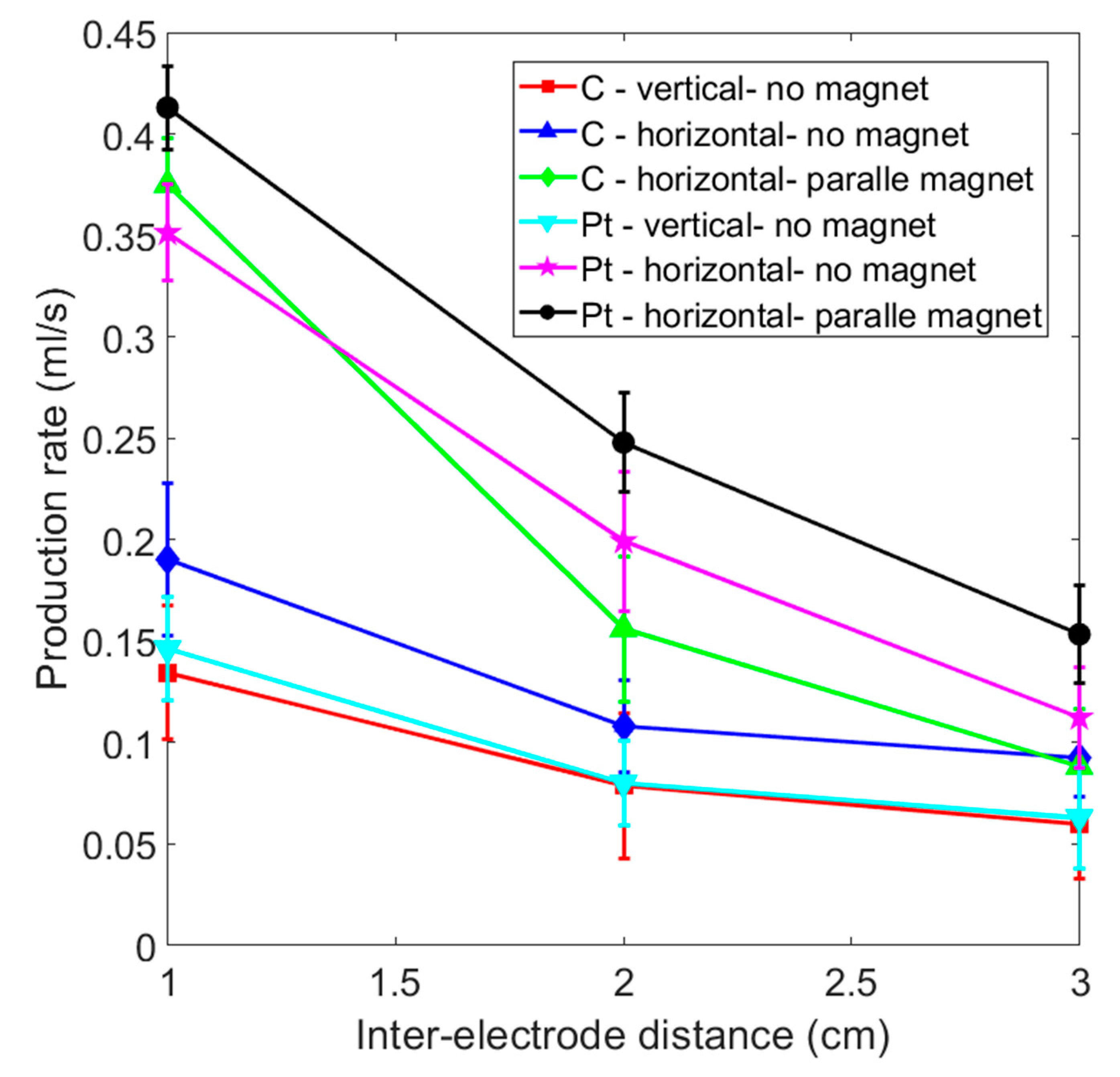
3.4. Comparison of the Gas Production Rate for Various Experimental Layouts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ball, M.; Wietschel, M. The future of hydrogen—Opportunities and challenges. Int. J. Hydrogen Energy 2009, 34, 615–662. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Arevalo, R.L.; Aspera, S.M.; Escano, M.C.S.; Nakanishi, H.; Kasai, H. Tuning methane decomposition on stepped Ni surface: The role of subsurface atoms in catalyst design. Sci. Rep. 2017, 7, 13963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembsky, V.I.; Fateev, V.N. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Aricò, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer electrolyte membrane water electrolysis: Status of technologies and potential applications in combination with renewable power sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Badwal, S.P.S.; Giddey, S.; Munnings, C. Hydrogen production via solid electrolytic routes. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 473–487. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Tasic, G.S.; Maksic, A.D.; Saponjic, D.P.; Miulovic, S.M.; Marceta Kaninski, M.P. Raising efficiency of hydrogen generation from alkaline water electrolysis—Energy saving. Int. J. Hydrogen. Energy 2010, 35, 12369–12373. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Quim. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Lao-Atiman, W.; Bumroongsil, K.; Arpornwichanop, A.; Bumroongsakulsawat, P.; Olaru, S.; Kheawhom, S. Model-based analysis of an integrated zinc-air flow battery/zinc electrolyzer system. Front. Energy Res. 2019, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Velusamy, D.B.; Sengodan, S.; Nagaraju, G.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc–air battery. Appl. Catal. 2022, 300, 120752. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhou, Z.; Pan, Y.; Yu, Z.; Pei, Z.; Chen, Y. Rechargeable zinc-air batteries with neutral electrolytes: Recent advances, challenges, and prospects. J. Energy Chem. 2021, 3, 100055. [Google Scholar] [CrossRef]
- Koza, J.A.; Mühlenhoff, S.; Żabiński, P.; Nikrityuk, P.A.; Eckert, K.; Uhlemann, M.; Gebert, A.; Weier, T.; Schultz, L.; Odenbach, S. Hydrogen evolution under the influence of a magnetic field. Electrochimica. Acta 2011, 56, 2665–2675. [Google Scholar] [CrossRef]
- Koza, J.A.; Uhlemann, M.; Gebert, A.; Schultz, L. Desorption of hydrogen from the electrode surface under influence of an external magnetic field. Electrochem. Commun. 2008, 10, 1330–1333. [Google Scholar] [CrossRef]
- Angulo, A.; van der Linde, P.; Gardeniers, H.; Modestino, M.; Rivas, D.F. Influence of bubbles on the energy conversion efficiency of electrochemical reactors. Joule 2020, 4, 555–579. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.Y.; Hourng, L.W.; Kuo, C.W. The effect of magnetic on hydrogen production efficiency in water electrolysis. Int. J. Hydrogen. Energy 2012, 37, 1311–1320. [Google Scholar] [CrossRef]
- Mühlenhoff, S.; Mutschke, G.; Koschichow, D.; Yang, X. Lorentz-force-driven con-vection during copper magnetoelectrolysis in the presence of a supportingbuoyancy force. Electrochim. Acta 2012, 69, 209–219. [Google Scholar] [CrossRef]
- Matsushima, H.; Iida, T.; Fukunaka, Y. Gas bubble evolution on transparent electrode during water electrolysis in a magnetic field. Electrochim. Acta 2013, 100, 261–264. [Google Scholar] [CrossRef]
- Li, Y.H.; Chen, Y.J. The effect of magnetic field on the dynamics of gas bubbles in water electrolysis. Sci. Rep. 2021, 11, 9346. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Magnetic water treatment–A review of the latest approaches. Chemosphere 2018, 203, 54–67. [Google Scholar] [CrossRef]
- Sueptitz, R.; Tschulik, K.; Uhlemann, M.; Schultz, L.; Gebert, A. Effect of high gradient magnetic fields on the anodic behaviour and localized corrosion of iron in sulphuric acid solutions. Corros. Sci. 2011, 53, 3222–3230. [Google Scholar] [CrossRef]
- Sueptitz, R.; Tschulik, K.; Uhlemann, M.; Gebert, A.; Schultz, L. Impact of magnetic field gradients on the free corrosion of iron. Electrochim. Acta 2010, 55, 5200–5203. [Google Scholar] [CrossRef]
- Zhan, S.; Huang, Y.; Zhang, W.; Li, B.; Jiang, M.; Wang, Z.; Wang, J. Experimental investigation on bubble growth and detachment characteristics on vertical microelectrode surface under electrode-normal magnetic field in water electrolysis. Int. J. Hydrogen Energy 2021, 46, 36640–36651. [Google Scholar] [CrossRef]
- Kaninski, M.P.M.; Miulovic, S.M.; Tasic, G.S.; Maksic, A.D.; Nikolic, V.M. A study on the Co–W activated Ni electrodes for the hydrogen production from alkaline water electrolysis–Energy saving. Int. J. Hydrogen Energy 2011, 36, 5227–5235. [Google Scholar] [CrossRef]
- Abbas, M.A.; Bang, J.H. Rising again: Opportunities and challenges for platinum-free electrocatalysts. Chem. Mat. 2015, 27, 7218–7235. [Google Scholar] [CrossRef]
- Kozlova, S.G.; Ryzhikov, M.R.; Kompankov, N.B.; Zavakhina, M.S. Influence of magnetic field on the mobility of aromatic chiral molecules. J. Phys. Chem. B 2016, 120, 7517–7521. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragsdale, S.R.; Grant, K.M.; White, S.H. Electrochemically generated magnetic forces. Enhanced transport of a paramagnetic redox species in large, nonuniform magnetic fields. J. Am. Chem. Soc. 1998, 120, 13461–13468. [Google Scholar] [CrossRef]
- Monzon, L.M.; Coey, J.M.D. Magnetic fields in electrochemistry: The Lorentz force. A mini-review. Electrochem. Commun. 2014, 42, 38–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, C.; Wu, J.; Liu, H.; Zhang, B.; Jiang, Z.; Xu, P. Recent advances in magnetic field-enhanced electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 10303–10316. [Google Scholar] [CrossRef]
| Electrode Conditions | Average Time (min) | Efficiency | ||
|---|---|---|---|---|
| Distance (cm) | Material | Layout | ||
| 5 | Graphite | Vertical | 25.1 | |
| Horizontal | 14.25 | + | ||
| Horizontal + Magnet | 16.18 | |||
| Platinum | Vertical | 23.5 | ||
| Horizontal | 7.5 | ++ | ||
| Horizontal + Magnet | 8.35 | |||
| 4 | Graphite | Vertical | 10.6 | |
| Horizontal | 9.25 | + | ||
| Horizontal + Magnet | 9.9 | |||
| Platinum | Vertical | 9.1 | ||
| Horizontal | 6.7 | |||
| Horizontal + Magnet | 5.2 | ++ | ||
| 3 | Graphite | Vertical | 8.37 | |
| Horizontal | 5.4 | + | ||
| Horizontal + Magnet | 5.7 | |||
| Platinum | Vertical | 7.9 | ||
| Horizontal | 4.45 | |||
| Horizontal + Magnet | 3.26 | ++ | ||
| 2 | Graphite | Vertical | 6.35 | |
| Horizontal | 4.62 | |||
| Horizontal + Magnet | 3.2 | + | ||
| Platinum | Vertical | 6.25 | ||
| Horizontal | 2.51 | |||
| Horizontal + Magnet | 2.02 | ++ | ||
| 1 | Graphite | Vertical | 3.71 | |
| Horizontal | 2.62 | |||
| Horizontal + Magnet | 1.33 | + | ||
| Platinum | Vertical | 3.4 | ||
| Horizontal | 1.42 | |||
| Horizontal + Magnet | 1.21 | ++ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Li, Y.-H.; Chen, C.-Y. Studying the Effect of Electrode Material and Magnetic Field on Hydrogen Production Efficiency. Magnetochemistry 2022, 8, 53. https://doi.org/10.3390/magnetochemistry8050053
Chen Y-J, Li Y-H, Chen C-Y. Studying the Effect of Electrode Material and Magnetic Field on Hydrogen Production Efficiency. Magnetochemistry. 2022; 8(5):53. https://doi.org/10.3390/magnetochemistry8050053
Chicago/Turabian StyleChen, Yen-Ju, Yan-Hom Li, and Ching-Yao Chen. 2022. "Studying the Effect of Electrode Material and Magnetic Field on Hydrogen Production Efficiency" Magnetochemistry 8, no. 5: 53. https://doi.org/10.3390/magnetochemistry8050053
APA StyleChen, Y.-J., Li, Y.-H., & Chen, C.-Y. (2022). Studying the Effect of Electrode Material and Magnetic Field on Hydrogen Production Efficiency. Magnetochemistry, 8(5), 53. https://doi.org/10.3390/magnetochemistry8050053






