Use of Time Domain Nuclear Magnetic Resonance Relaxometry to Monitor the Effect of Magnetic Field on the Copper Corrosion Rate in Real Time
Abstract
:1. Introduction
2. Materials and Methods
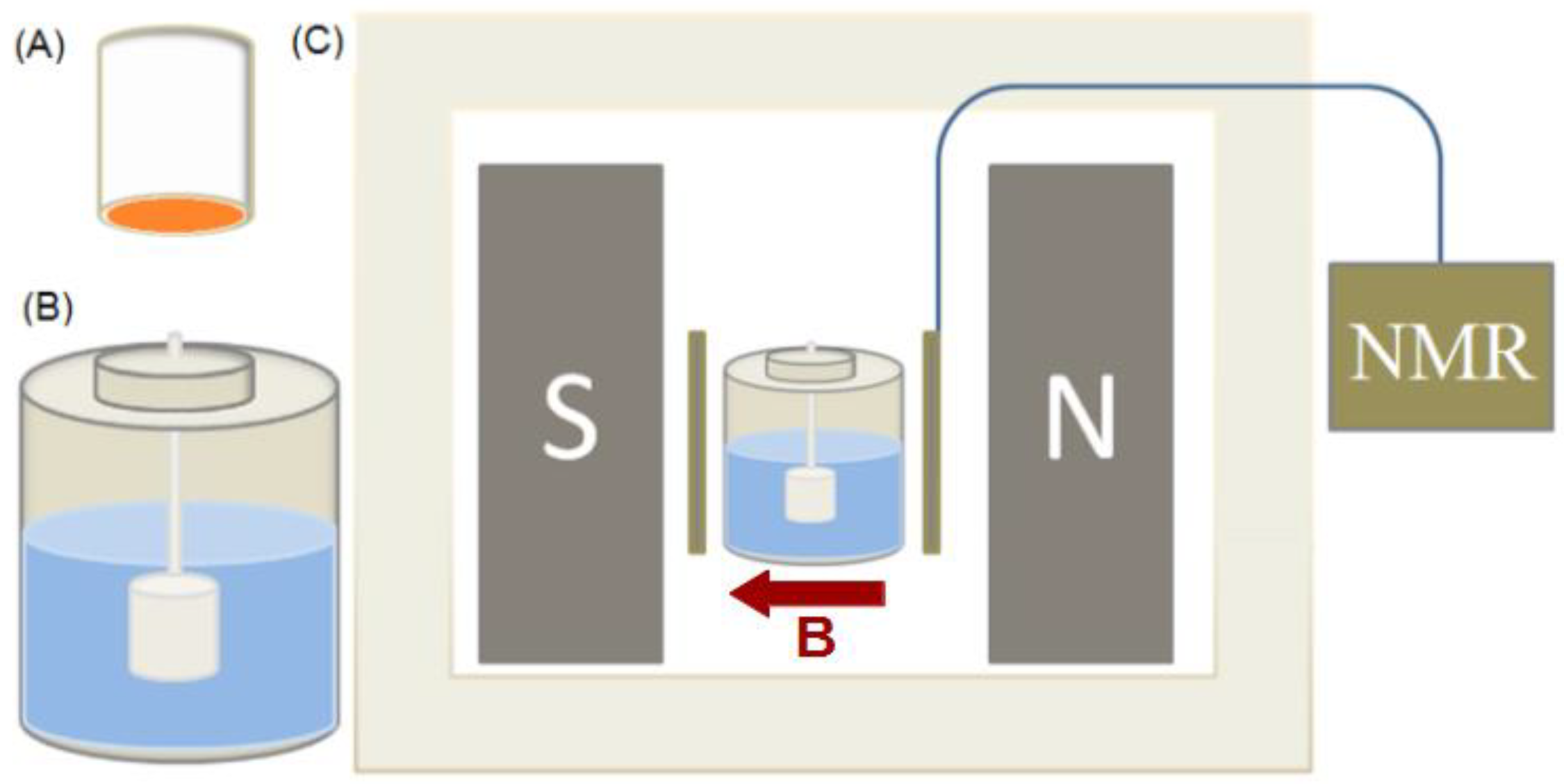
2.1. Corrosion Cell
2.2. TD-NMR Measurements
2.3. Atomic Absorption Analysis
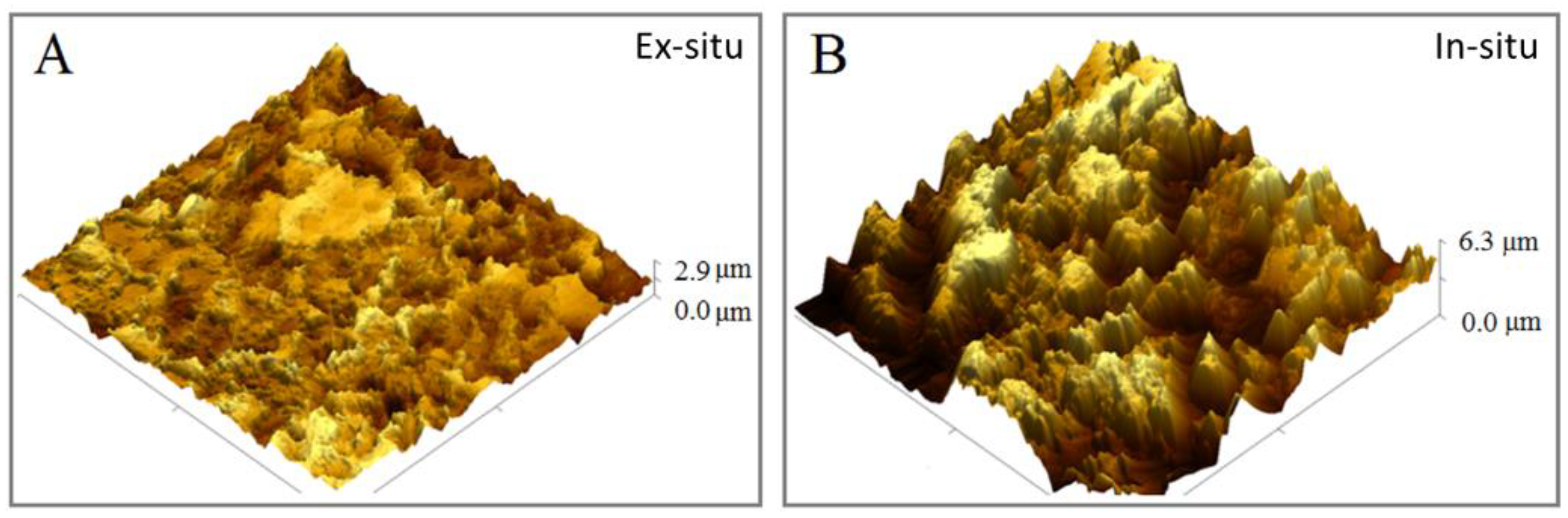
2.4. Atomic Force Microscopy (AFM) Characterization
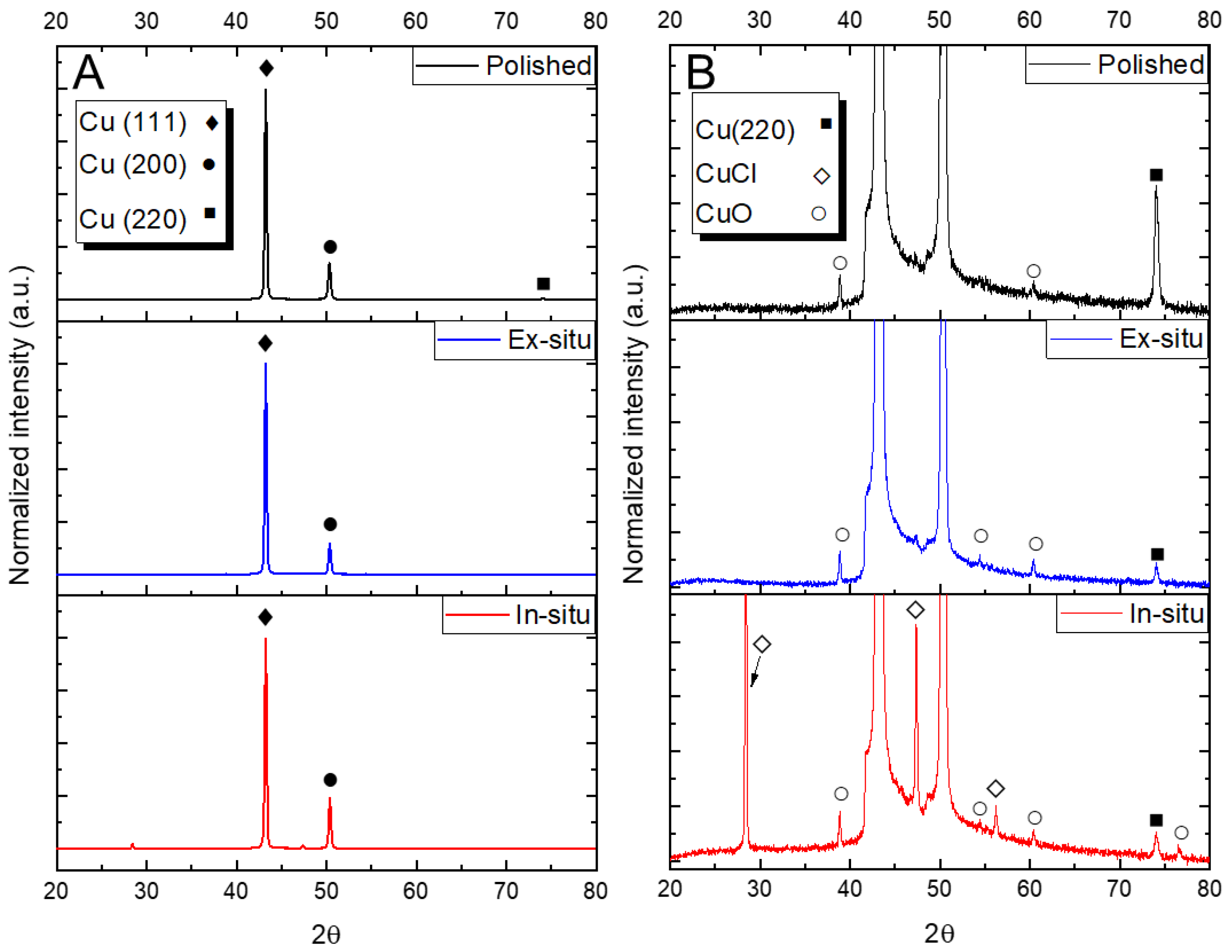
2.5. X-ray Diffraction
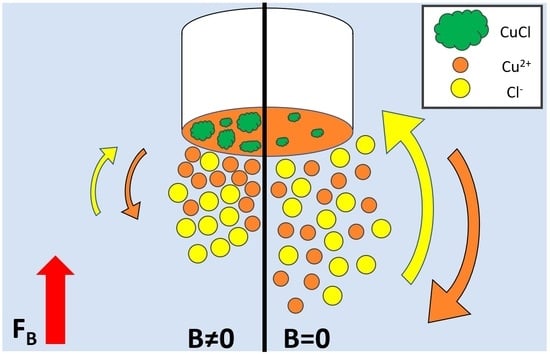
3. Results
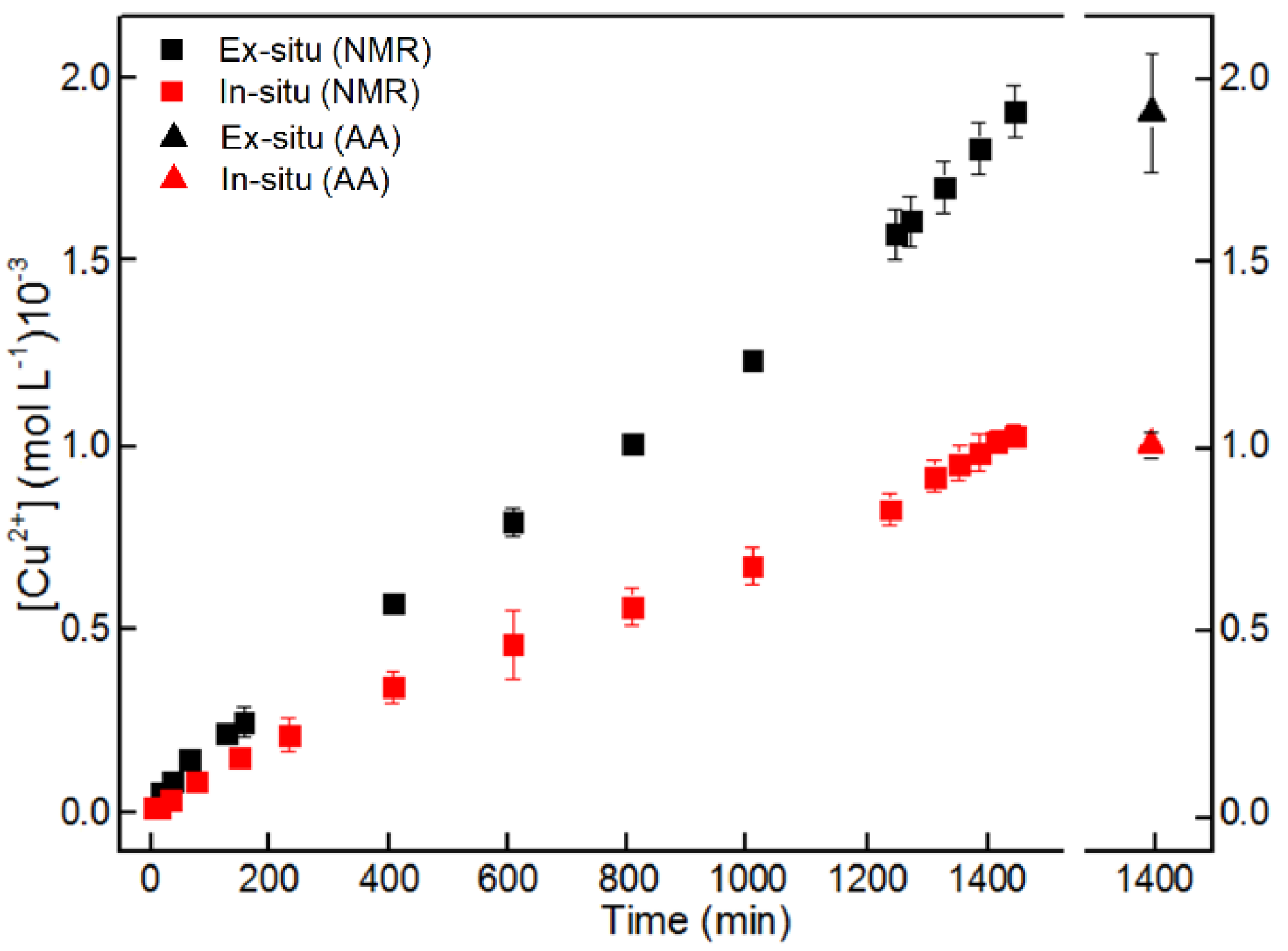
3.1. NMR and AA Measurements
3.2. Surface Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Philip, A.; Schweitzer, P.E. Fundamentals of Corrosion Mechanisms, Causes, and Preventative Methods; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Hansson, C.M. The Impact of Corrosion on Society. Metall. Mater. Trans. A 2011, 42, 2952–2962. [Google Scholar] [CrossRef]
- Koch, G. Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Boston, MA, USA, 2017; pp. 3–30. [Google Scholar]
- Palanisamy, G. Corrosion Inhibitors; Singh, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Formulation of corrosion rate of magnesium alloys using microstructural parameters. J. Magnes. Alloy 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on-site. Constr. Build Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Schmitt, R.J.; Christian, R.R. Monitoring Corrosion. Ind. Eng. Chem. Res. 1960, 52, 71A–74A. [Google Scholar] [CrossRef]
- Usman, A.D.; Victoria, A.F.; Okoro, L.N. Weight Loss Corrosion Study of Some Metals in Acid Medium. J. Adv. Chem. 2016, 11, 3434–3440. [Google Scholar] [CrossRef] [Green Version]
- Oparaodu, K.O.; Okpokwasili, G.C. Comparison of Percentage Weight Loss and Corrosion Rate Trends in Different Metal Coupons from two Soil Environments. Int. J. Environ. Bioremediat. Biodegrad. 2022, 2, 243–249. [Google Scholar] [CrossRef]
- Zou, F.; Cegla, F.B. On quantitative corrosion rate monitoring with ultrasound. J. Electroanal. Chem. 2018, 812, 115–121. [Google Scholar] [CrossRef]
- Grachev, V.A.; Rozen, A.E.; Perelygin, Y.P.; Kireev, S.Y.; Los, I.S.; Rozen, A.A. Measuring corrosion rate and protector effectiveness of advanced multilayer metallic materials by newly developed methods. Heliyon 2018, 4, e00731. [Google Scholar] [CrossRef] [Green Version]
- Vukmirovic, M.B.; Adzic, R.R.; Akolkar, R. Copper Electrodeposition from Deep Eutectic Solvents—Voltammetric Studies Providing Insights into the Role of Substrate: Platinum vs Glassy Carbon. J. Phys. Chem. B 2020, 124, 5465–5475. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. Corrosion of copper in aerated acidic pickling solutions and its inhibition by 3-amino-1,2,4-triazole-5-thiol. J. Colloid Interface Sci. 2007, 306, 96–104. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Habashi, F. Kinetics of corrosion of metals. J. Chem. Educ. 1965, 42, 318. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Shinohara, K.; Aogaki, R. Magnetic Field Effect on Copper Corrosion in Nitric Acid. Electrochemistry 1999, 67, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Kountouras, D.T.; Vogiatzis, C.A.; Tsouknidas, A.; Skolianos, S. Preventing or accelerating galvanic corrosion through the application of a proper external magnetic field. Corros. Eng. Sci. Technol. 2014, 49, 603–607. [Google Scholar] [CrossRef]
- Sagawa, M. Effect of a Local Magnetic Field on the Dissolution of Copper and Iron in Nitric Acid Solution. Trans. Jpn. Inst. Met. 1982, 23, 38–40. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Huang, D.; Yang, W.; Congleton, J. Effects of an applied magnetic field on the dissolution and passivation of iron in sulphuric acid. Corros. Sci. 2003, 45, 2233–2249. [Google Scholar] [CrossRef]
- Sueptitz, R.; Tschulik, K.; Uhlemann, M.; Gebert, A.; Schultz, L. Impact of magnetic field gradients on the free corrosion of iron. Electrochim. Acta 2010, 55, 5200–5203. [Google Scholar] [CrossRef]
- Nasher, L.S.H.; Shalash, L.A.B.T. Study the effect of magnetic field on the corrosion of steel in sodium chloride solution (NaCl). Misan J. Acad. Stud. 2010, 9, 30–38. [Google Scholar]
- Ang, L.Y.; Othman, N.K.; Jalar, A.; Ismail, I. The Effect of Magnetic Field on Copper in Various Corrosive Medium, in 14th Post-Graduate Colloquium of Faculty-of-Science-and-Technology of Universiti-Kebangsaan-Malaysia, Univ Kebangsaan Malaysia, Fac Sci & Technol, Selangor, Malaysia, 1614. AIP Conf. Proc. 2014, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Mitre, C.I.N.; Tosin, G.; Colnago, L.A. In-operando analysis of the corrosion patterns and rates under magnetic fields using metallic film. NPJ Mater. Degrad. 2022, 6, 24. [Google Scholar] [CrossRef]
- Iwai, M.; Majima, H.; Awakura, Y. Dissolution of copper in hydrochloric acid solutions with dissolved molecular oxygen. Hydrometallurgy 1988, 20, 87–95. [Google Scholar] [CrossRef]
- Sisso, O.; Dor, S.; Eliyahu, D.; Sabatani, E.; Eliaz, N. Corrosion inhibition of copper in ferric chloride solutions with organic inhibitors. NPJ Mater. Degrad. 2020, 4, 38. [Google Scholar] [CrossRef]
- Gomes, B.F.; Nunes, L.M.S.; Lobo, C.M.S.; Cabeca, L.F.; Colnago, L.A. In Situ Study of the Magnetoelectrolysis Phenomenon during Copper Electrodeposition Using Time Domain NMR Relaxometry. Anal. Chem. 2014, 86, 9391–9393. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.F.; Nunes, L.M.S.; Lobo, C.M.S.; Carvalho, A.S.; Cabeca, L.F.; Colnago, L.A. In situ analysis of copper electrodeposition reaction using unilateral NMR sensor. J. Magn. Reson. 2015, 261, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.M.S.; Gomes, B.F.; Bouzouma, H.; Danieli, E.; Blümich, B.; Colnago, L.A. Improving in operando low field NMR copper electrodeposition analyses using inductively coupled coils. Electrochim. Acta 2019, 298, 844–851. [Google Scholar] [CrossRef]
- Nunes, L.M.S.; Cobra, P.F.; Cabeca, L.F.; Barbosa, L.L.; Colnago, L.A. In Situ Quantification of Cu(II) during an Electrodeposition Reaction Using Time-Domain NMR Relaxometry. Anal. Chem. 2012, 84, 6351–6354. [Google Scholar] [CrossRef]
- Gomes, B.F.; Burato, J.; Lobo, C.M.S.; Colnago, L.A. Use of the Relaxometry Technique for Quantification of Paramagnetic Ions in Aqueous Solutions and a Comparison with Other Analytical Methods. Int. J. Anal. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bloch, F.; Hansen, W.W.; Packard, M. Nuclear Induction. Phys. Rev. 1946, 69, 127. [Google Scholar] [CrossRef] [Green Version]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Kock, F.V.C.; Machado, M.P.; Athayde, G.P.B.; Colnago, L.A.; Barbosa, L.L. Quantification of paramagnetic ions in solution using time domain NMR. Pros and Cons to optical emission spectrometry method. Microchem. J. 2018, 137, 204–207. [Google Scholar] [CrossRef]
- Gomes, B.F.; Lobo, C.M.; Colnago, L.A. Monitoring Electrochemical Reactions in Situ with Low Field NMR: A Mini-Review. Appl. Sci. 2019, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Cobra, P.F.; Gomes, B.F.; Mitre, C.I.N.; Barbosa, L.L.; Marconcini, L.V.; Colnago, L.A. Measuring the solubility product constant of paramagnetic cations using time-domain nuclear magnetic resonance relaxometry. Microchem. J. 2015, 121, 14–17. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Montrazi, E.T.; Monaretto, T.; Bonagamba, T.J.; Colnago, L.A. New and rapid pulse sequences for two-dimensional D-T1 correlation measurements. J. Magn. Reson. 2020, 315, 106749. [Google Scholar] [CrossRef]
- Neto, R.P.C.; Rodrigues, E.J.d.R.; Tavares, M.I.B. Single-shot measurement of solids and liquids T1 values by a small-angle flip-flop pulse sequence. Magn. Reason. Chem. 2019, 57, 395–403. [Google Scholar] [CrossRef]
- Weston, M.C.; Gerner, M.D.; Fritsch, I. Magnetic Fields for Fluid Motion. Anal. Chem. 2010, 82, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, I.; Sharma, A. Stochastic resetting of active Brownian particles with Lorentz force. Soft Matter 2021, 17, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Jiles, D. Introduction to Magnetism and Magnetic Materials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igreja Nascimento Mitre, C.; Ferreira Gomes, B.; Paris, E.; Silva Lobo, C.M.; Roth, C.; Colnago, L.A. Use of Time Domain Nuclear Magnetic Resonance Relaxometry to Monitor the Effect of Magnetic Field on the Copper Corrosion Rate in Real Time. Magnetochemistry 2022, 8, 40. https://doi.org/10.3390/magnetochemistry8040040
Igreja Nascimento Mitre C, Ferreira Gomes B, Paris E, Silva Lobo CM, Roth C, Colnago LA. Use of Time Domain Nuclear Magnetic Resonance Relaxometry to Monitor the Effect of Magnetic Field on the Copper Corrosion Rate in Real Time. Magnetochemistry. 2022; 8(4):40. https://doi.org/10.3390/magnetochemistry8040040
Chicago/Turabian StyleIgreja Nascimento Mitre, Cirlei, Bruna Ferreira Gomes, Elaine Paris, Carlos Manuel Silva Lobo, Christina Roth, and Luiz Alberto Colnago. 2022. "Use of Time Domain Nuclear Magnetic Resonance Relaxometry to Monitor the Effect of Magnetic Field on the Copper Corrosion Rate in Real Time" Magnetochemistry 8, no. 4: 40. https://doi.org/10.3390/magnetochemistry8040040
APA StyleIgreja Nascimento Mitre, C., Ferreira Gomes, B., Paris, E., Silva Lobo, C. M., Roth, C., & Colnago, L. A. (2022). Use of Time Domain Nuclear Magnetic Resonance Relaxometry to Monitor the Effect of Magnetic Field on the Copper Corrosion Rate in Real Time. Magnetochemistry, 8(4), 40. https://doi.org/10.3390/magnetochemistry8040040