The Structure of Biologically Active Functionalized Azoles: NMR Spectroscopy and Quantum Chemistry
Abstract
:1. Introduction
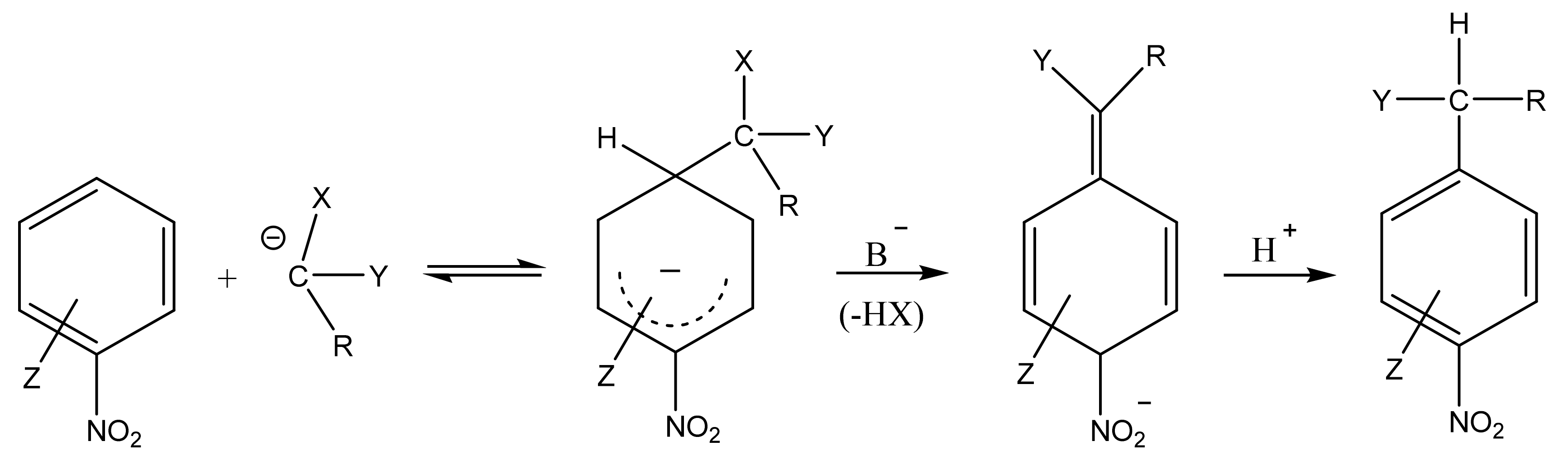
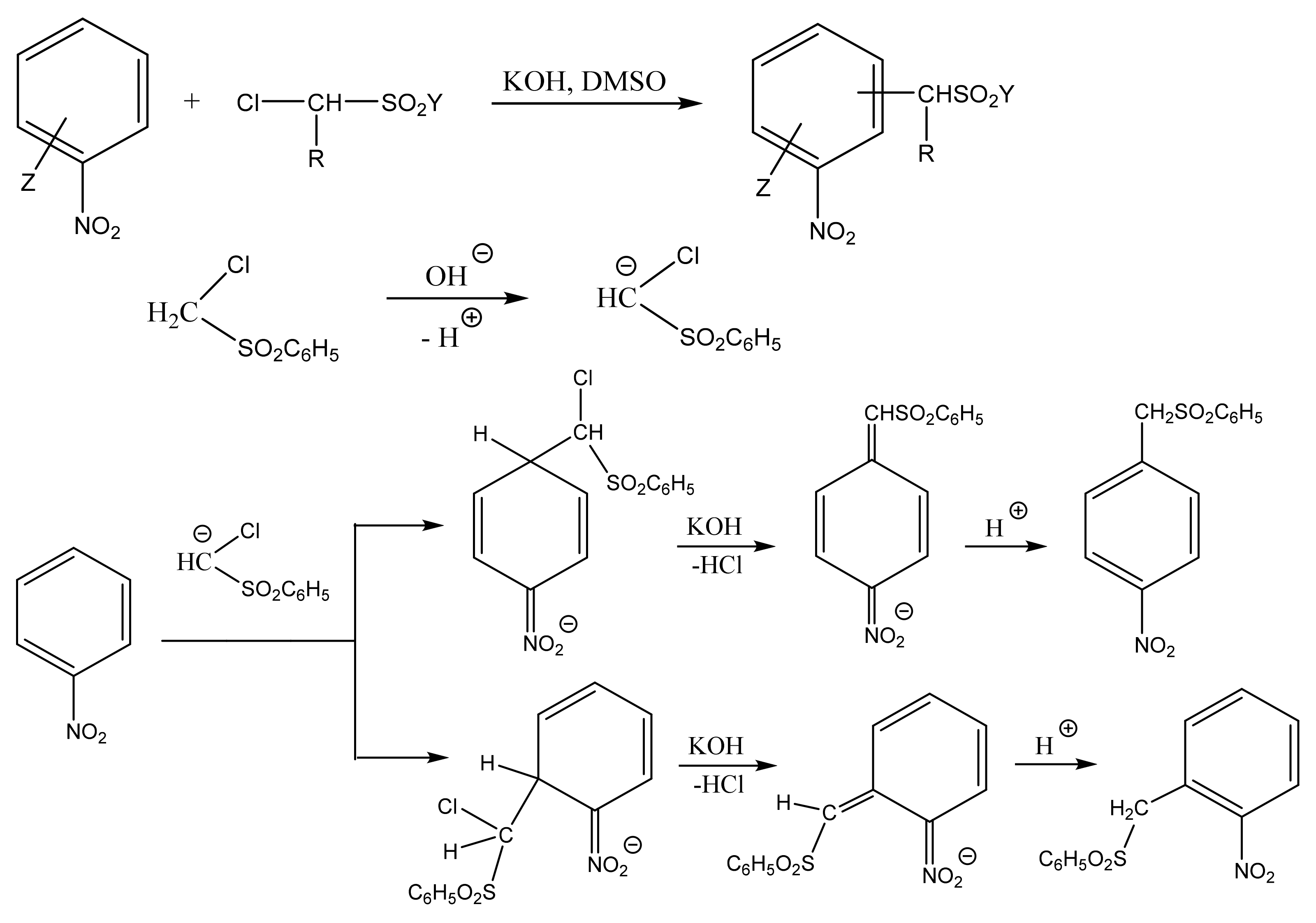
2. The Products of the Vicarious Nucleophilic Substitution of Hydrogen in N-organyl-Substituted Nitroazoles
- the high activity of the carbanion precursor as alkylating agent;
- the instability of the carbanion or its low nucleophilicity, when the X, Y and R groups effectively delocalize the negative charge.
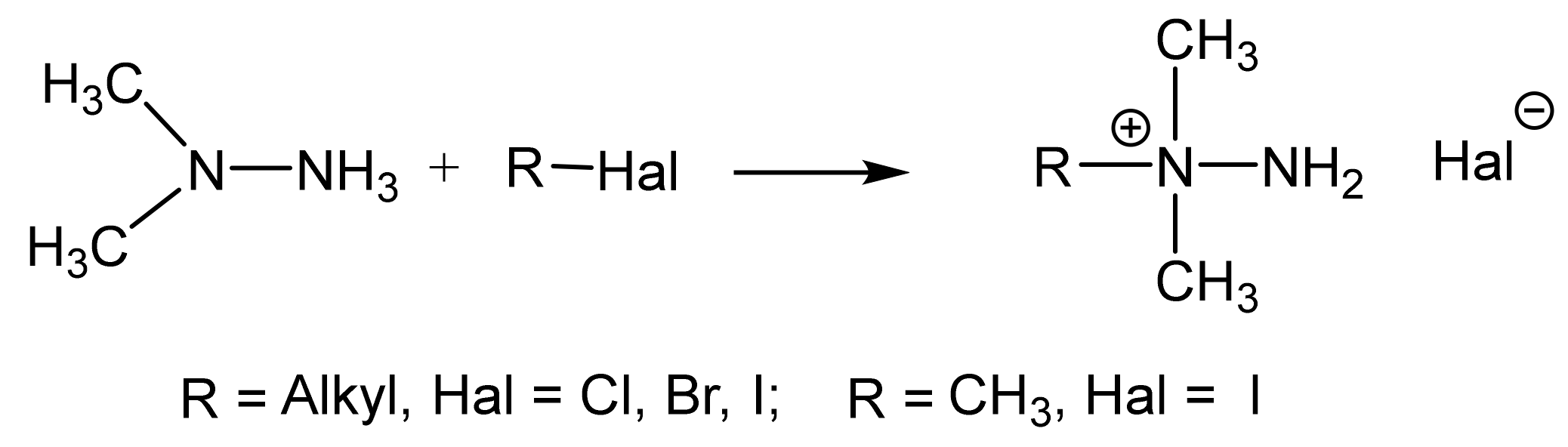
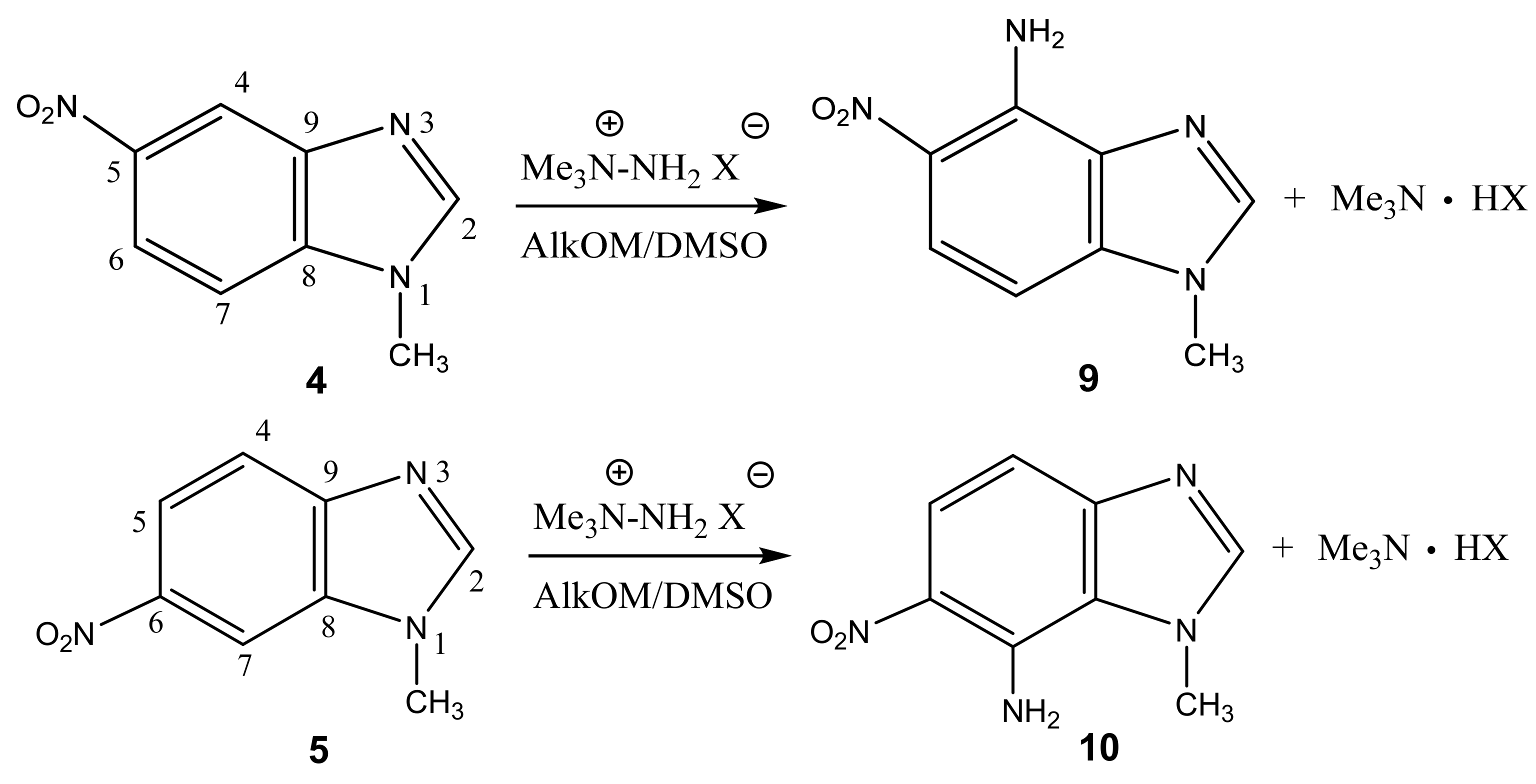
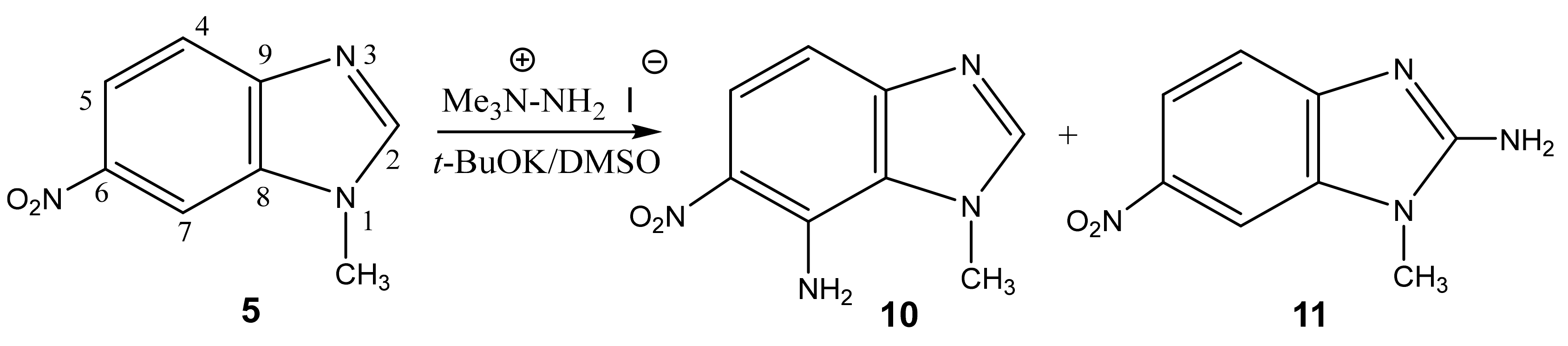
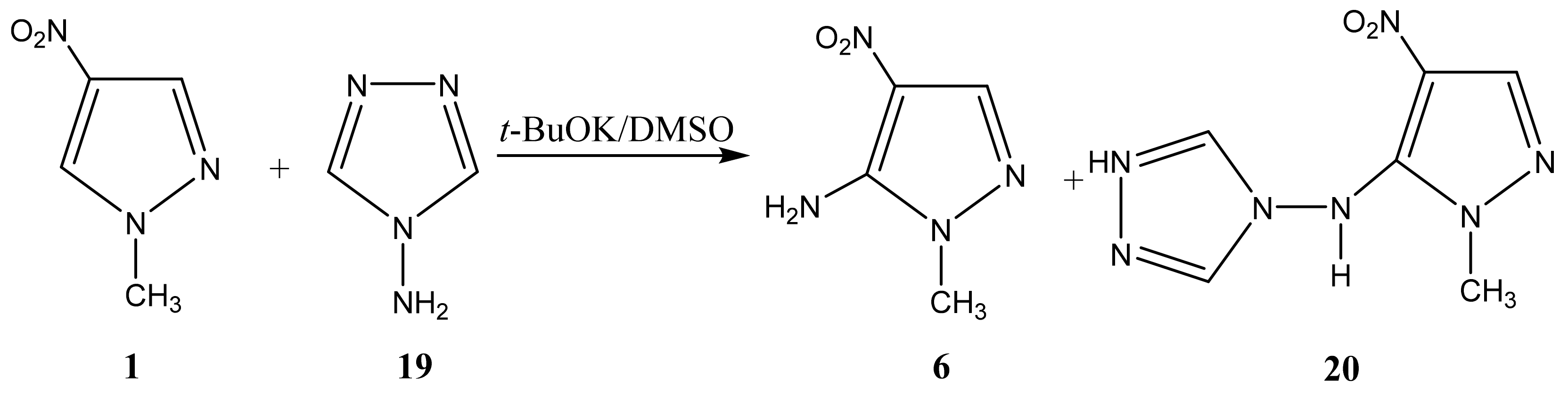
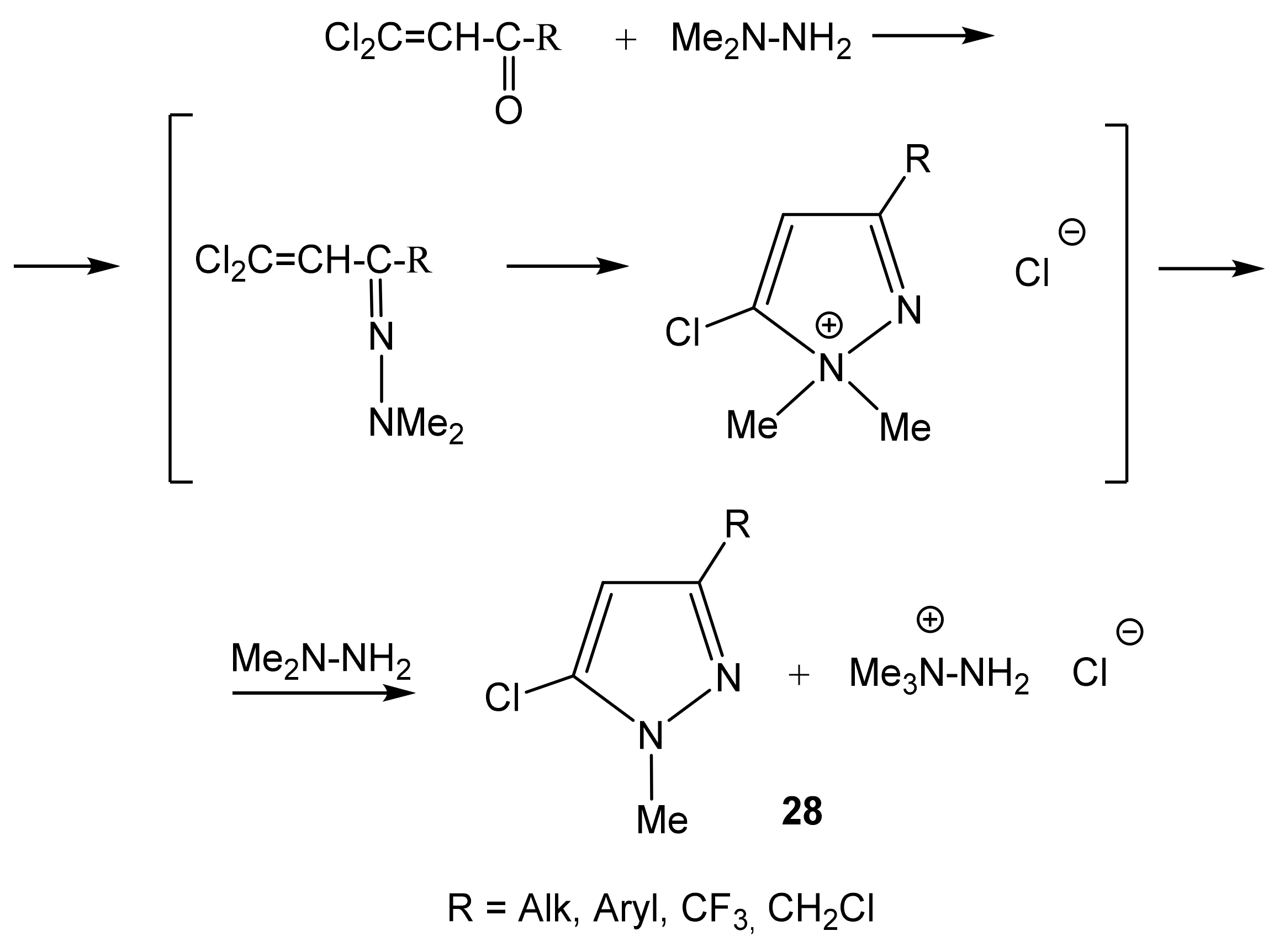
2.1. Structure of the C-Amination Products of N-Substituted Nitroazoles with 1,1,1-trimethylhydrazinium Halides
| Compound | δ1H | δ13C | δ15N | |
|---|---|---|---|---|
| 1 | 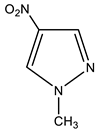 | 3.91 CH3 8.22 s H-3 8.83 s H-5 | 39.71 CH3 130.94 C-5 135.43 C-3 - C-4 | −18.3 NO2 −69.9 N-2 −172.0 N-1 |
| 2 | 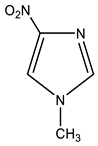 | 3.76 CH3 7.80 s H-2 8.35 s H-5 | 34.18 CH3 122.49 C-5 138.00 C-2 - C-4 | −18.1 NO2 −127.7 N-3 −208.5 N-1 |
| 3 | 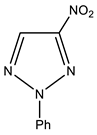 | 7.58 p-Ph 7.66 m-Ph 8.08 o-Ph 9.01 s H-5 | 119.37 o-Ph 129.81 p-Ph 130.05 m-Ph 132.99 C-5 138.22 ipso-Ph 154.22 C-4 | −27.0 NO2 −50.8 N-1 −65.5 N-3 −122.8 N-2 |
| 4 | 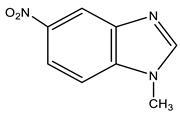 | 3.92 s CH3 7.80 d H-7 3J 9.0 Hz 8.18 d H-6 3J 9.0 Hz 8.49 s H-2 8.53 s H-4 | 31.20 CH3 110.94 C-7 115.51 C-4 117.84 C-6 138.94 C-8 142.39 C-9 142.73 C-5 148.75 C-2 | −11.4 NO2 −125.0 N-3 −220.9 N-1 |
| 5 | 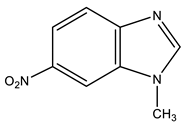 | 3.96 s CH3 7.53 d H-4 3J 8.9 Hz 7.80 d H-6 3J 8.9 Hz 8.34 s H-7 8.49 s H-2 | 33.29 CH3 107.64 C-7 117.00 C-5 119.67 C-4 134.44 C-8 142.89 C-6 147.83 C-9 149.85 C-2 | −9.8 NO2 −123.4 N-3 −217.1 N-1 |
| 6 | 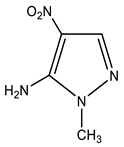 | 3.56 s CH3 7.38 br NH2 7.84 s H-3 | 35.04 CH3 117.84 C-4 134.37 C-5 146.01 C-3 | −18.5 NO2 −92.1 N-2 −207.2 N-1 −316.9 NH2 |
| 7 | 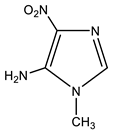 | 3.43 CH3 7.23 s H-2 7.51 br NH2 | 30.71 CH3 124.0 br C-4 132.46 C-2 143.97 C-5 | −18.8 NO2 −124.7 N-3 −228.5 N-1 −306.7 NH2 |
| 8 | 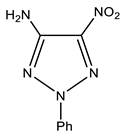 | 4.50 br NH2 7.25 p-Ph 7.46 m-Ph 7.54 o-Ph | 108.67 C-5 - C-4 117.58 o-Ph 126.48 p-Ph 129.70 m-Ph 142.75 ipso-Ph | −27.0 NO2 −50.8 N-1 −65.5 N-3 −122.8 N-2 −309.5 NH2 |
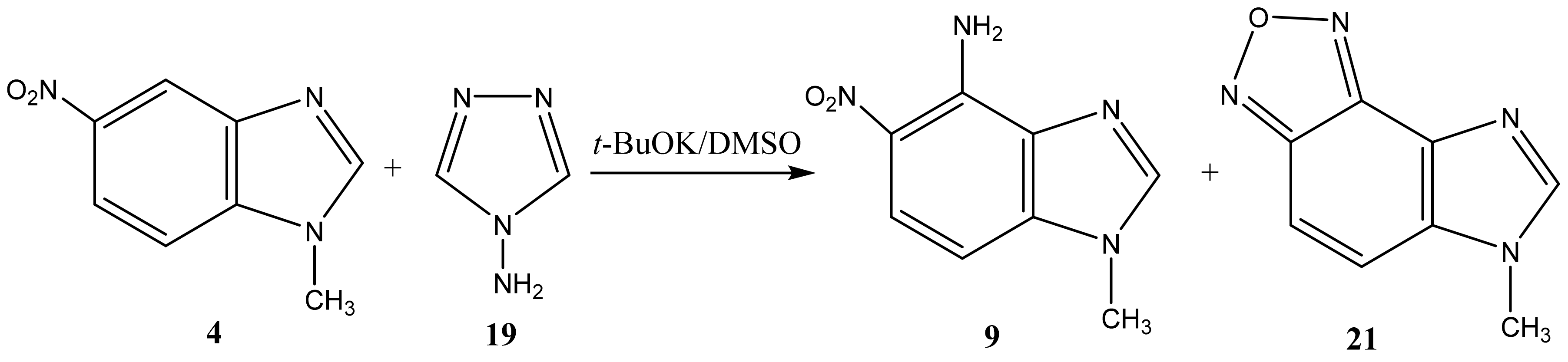
| 9 | 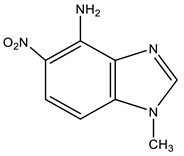 | 3.82 CH3 6.88 d H-7 3J 9.3 Hz 7.65 br NH2 7.90 d H-6 3J 9.3 Hz 8.18 s H-2 | 31.05 CH3 99.92 C-7 120.38 C-6 124.90 C-5 131.67 C-9 137.46 C-8 140.50 C-4 143.80 C-2 | −3.3 NO2 −131.8 N-3 −222.9 N-1 −307.6 NH2 |
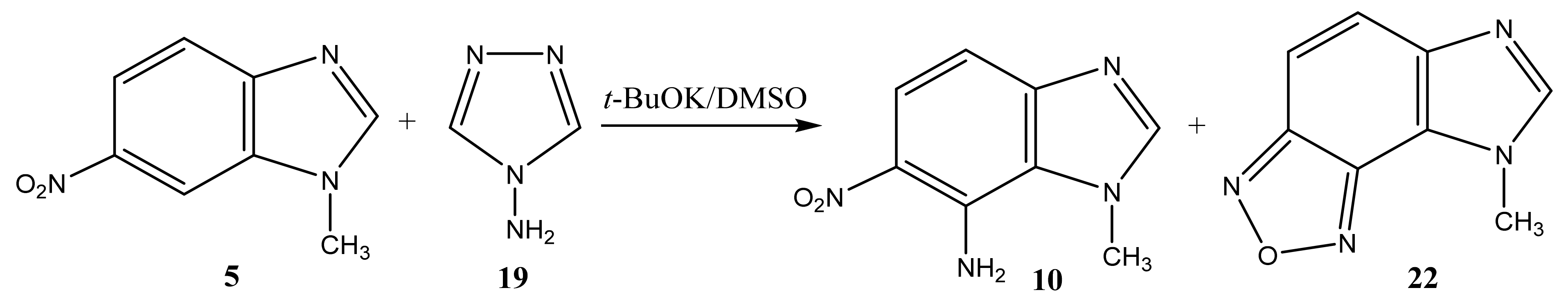
| 10 | 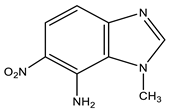 | 4.17 CH3 6.92 d H-4 3J 9.2 Hz 7.46 br NH2 7.84 d H-5 3J 9.2 Hz 8.21 s H-2 | 34.24 CH3 109.37 C-4 119.98 C-5 122.56 C-8 126.57 C-6 137.10 C-7 148.44 C-2 148.76 C-9 | −3.2 NO2 −129.0 N-3 −222.5 N-1 −305.6 NH2 |
| 11 | 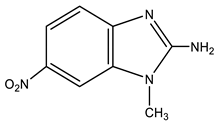 | 3.58 CH3 7.18 d H-4 3J 9.1 Hz 7.21 br NH2 7.93 d H-5 3J 9.1 Hz 8.06 s H-7 | 28.84 CH3 103.64 C-9 113.48 C-4 117.91 C-5 134.67 C-8 138.89 C-6 149.74 C-7 159.71 C-2 | −4.8 NO2 −132.4 N-3 −224.5 N-1 −311.4 NH2 |
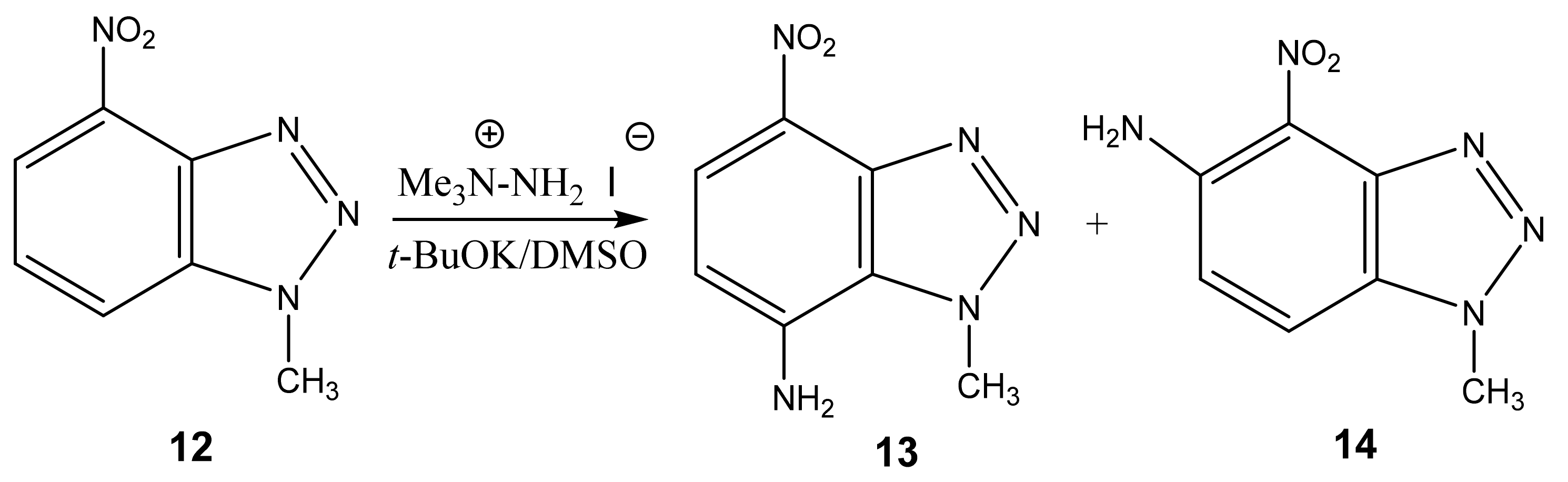
| 12 This work | 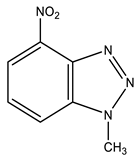 | 4.42 s CH3 7.79 dd H-6 3J 8.3 Hz 3J 7.7 Hz 8.32 d H-5 3J 7.7 Hz 8.39 d H-7 3J 8.3 Hz | 34.98 CH3 118.83 C-5 121.52 C-7 126.78 C-6 135.86 C-8 137.41 C-9 137.86 br C-4 | 8.3 N-2 −10.0 NO2 −42.3 N-3 −153.7 N-1 |
| 13 This work | 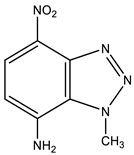 | 4.51 s CH3 6.59 d H-6 3J 8.8 Hz 7.21 NH2 8.12 d H-5 3J 8.8 Hz | 38.12 CH3 109.80 C-6 123.94 C-8 126.82 C-5 135.86 C-4 141.21 C-7 142.60 C-9 | 8.9 N-2 −9.1 NO2 −44.3 N-3 −159.5 N-1 −312.40 NH2 |
| 14 This work | 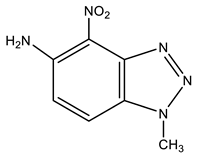 | 4.22 s CH3 6.21 d H-6 3J 9.0 Hz 7.92 d H-7 3J 9.0 Hz 8.25 br NH2 | 35.30 CH3 99.08 C-7 125.43 C-5 126.09 C-6 137.39 br C-4 137.70 C-8 141.32 C-9 | 9.3 N-2 −11.5 NO2 −49.0 N-3 −157.9 N-1 −309.1 NH2 |
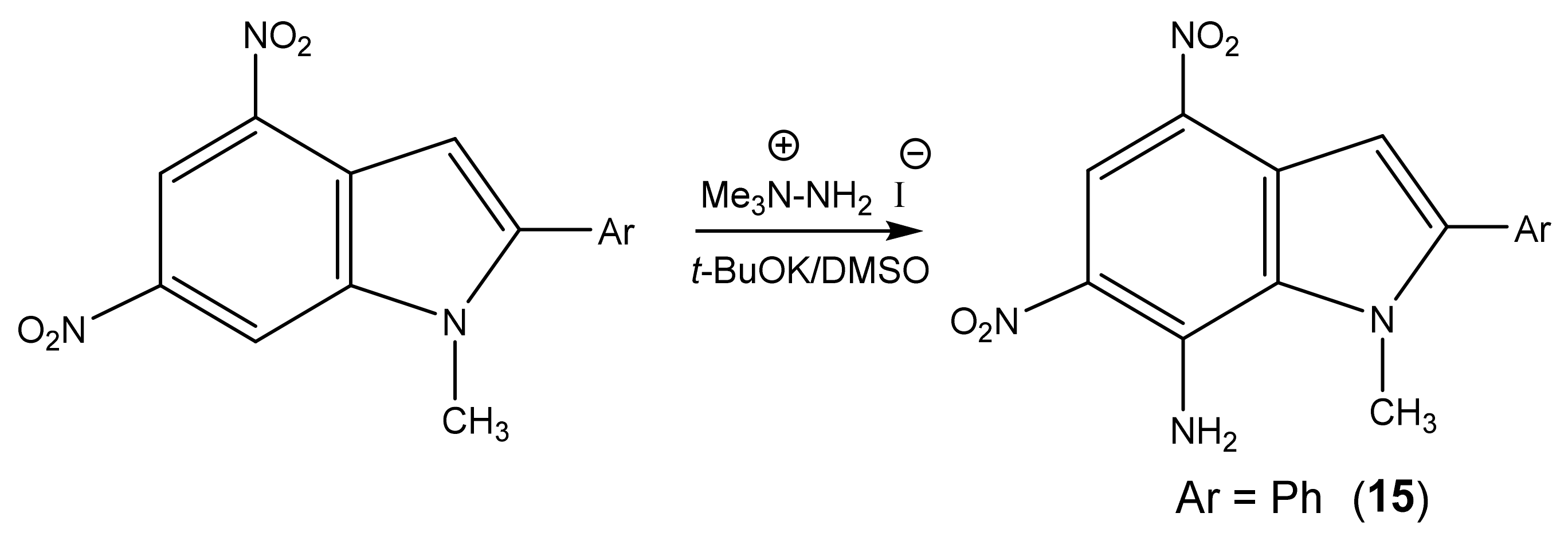
| 15 [95] | 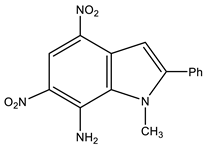 | 4.03 s CH3 7.13 s H-3 7.58 p Ph 7.66 m Ph 8.08 o Ph 8.21 br NH2 8.79 s H-5 | −8.1 4-NO2 −9.8 6-NO2 −215.5 N-3 −318.8 N-2 This work | |
| 18a This work | 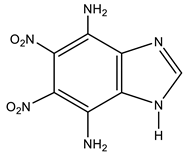 | 7.46 br 7NH2 7.65 br 4NH2 8.06 s H-2 | 102.24 C-7 125.15 C-4 125.64 C-8 130.18 C-9 138.34 C-6 142.05 C-2 149.23 C-5 | −7.3 NO2 −192.4 N-1.3 −301.4 NH2 |
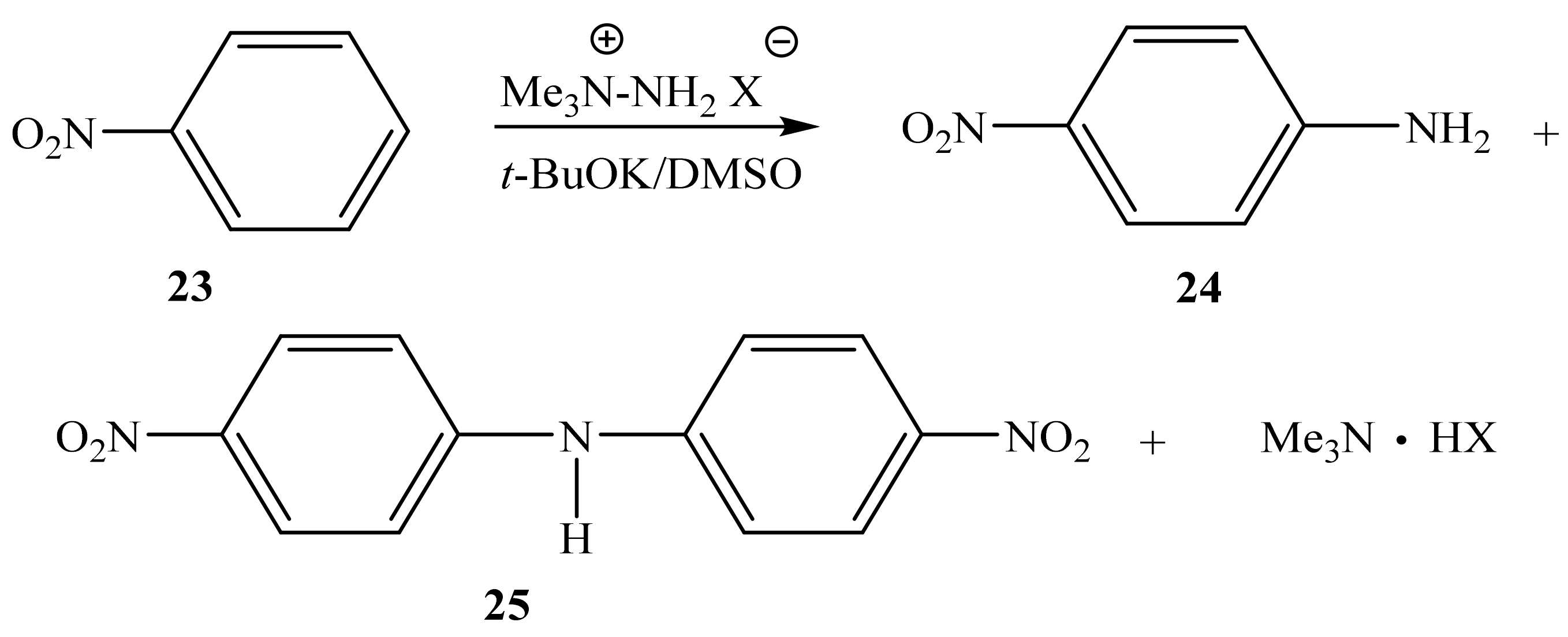
| 19 |  | 6.36 br NH2 8.47 s H-3,5 | 144.51 C-3,5 | −66.1 N-1,2 −197.2 N-4 −314.3 NH2 |
| 20 | 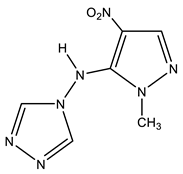 | 3.46 s CH3 7.12 s H-3 8.51 s H-3′,5′ 14.1 NH | 34.02 CH3 128.39 C-3 146.20 C-4 155.04 C-5 | −41.0 NO2 −64.3N-1′,2′ −117.0 N-2 −180.8 N-4′ −220.5 N-1 −305.5 NH |
| 21 | 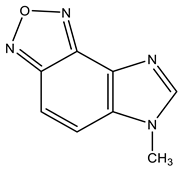 | 3.98 s CH3 7.81 d H-6 3J 8.6 Hz 8.00 d H-7 3J 8.6 Hz 8.35 s H-2 | 31.78 CH3 110.18 C-6 121.26 C-7 127.10 C-9 133.92 C-8 143.92 C-4 144.02 C-2 149.11 C-5 | +29.9 NO +30.6 NO −130.6 N-3 −220.9 N-1 |
| 22 | 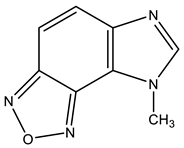 | 3.58 s CH3 7.18 d H-4 3J 8.7 Hz 7.93 d H-5 3J 8.7 Hz 8.30 s H-2 | 28.84 CH3 103.64 C-9 113.48 C-4 117.91 C-5 134.67 C-8 138.89 C-6 149.74 C-7 159.71 C-2 | +30.7 NO +31.9 NO −135.6 N-3 −224.7 N-1 |
2.2. The Products of the Interaction of N-Substituted Nitroazoles with 4-amino-1,2,4-triazole
2.3. The Structure of the Reaction Products of Nitrobenzene with 1,1,1 trimethylhydrazinium Halides, and 4-amino-1,2,4-triazole
3. Organylpyrazole Derivatives
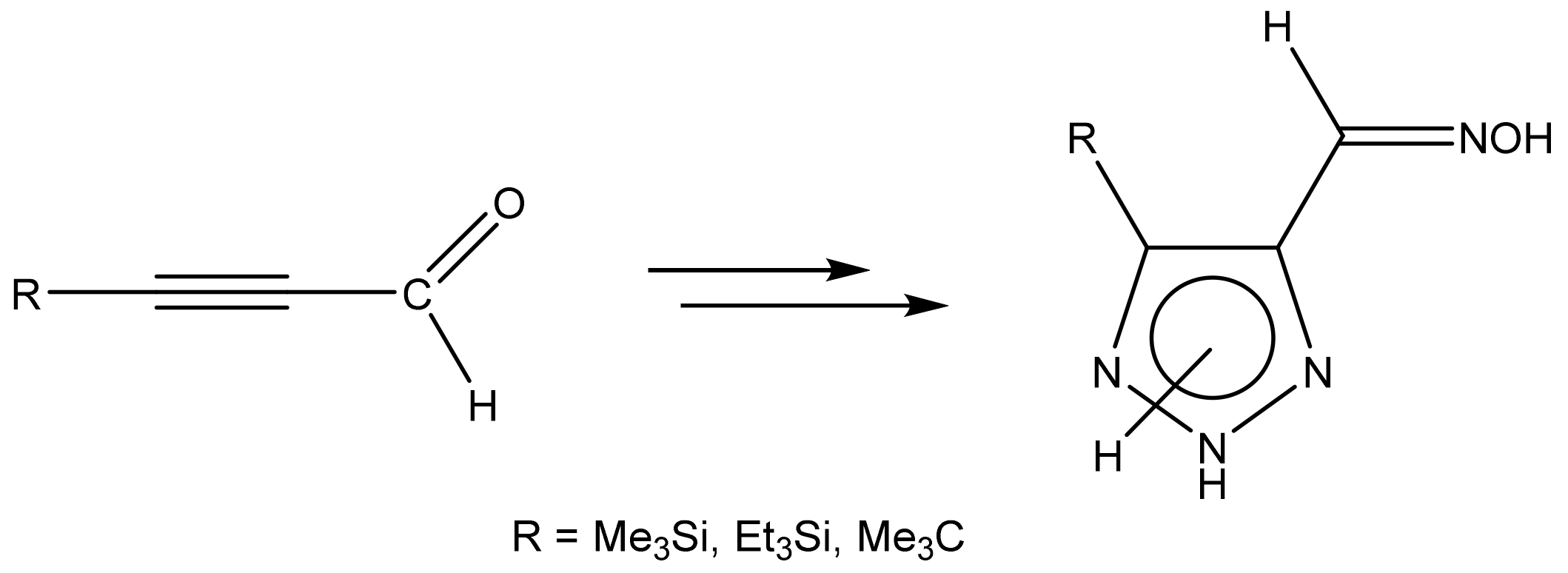
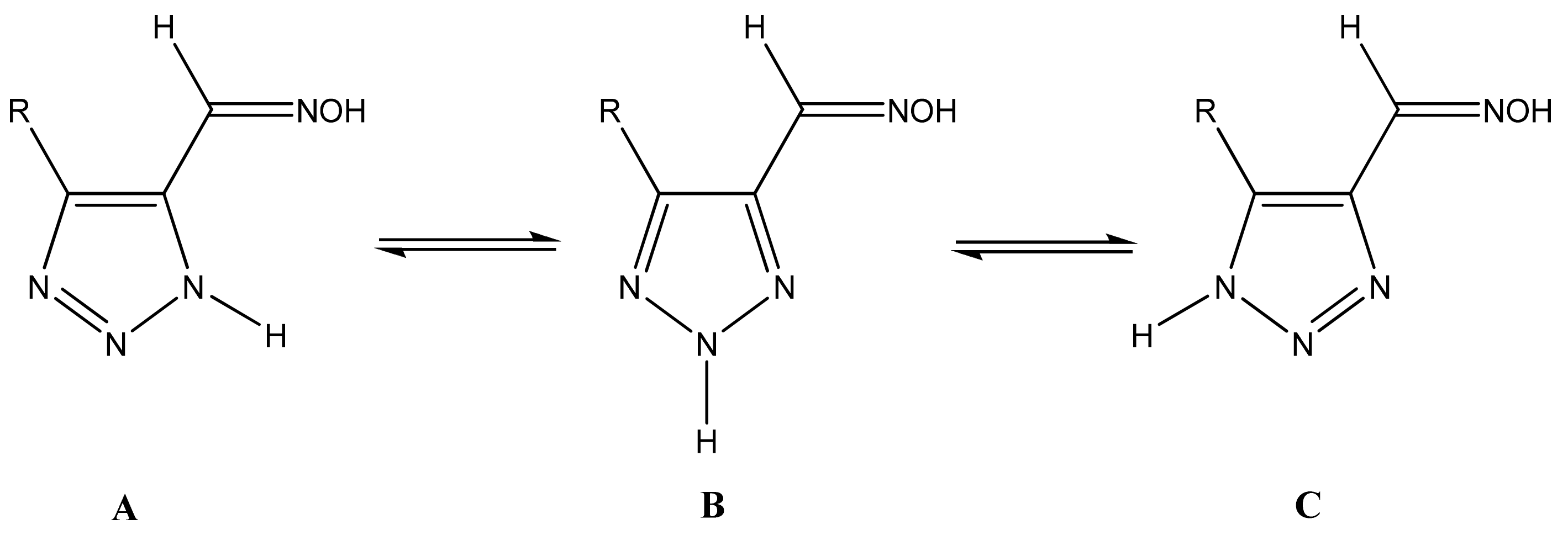
4. Functional 1,2,3-Triazole Derivatives
5. Functional Thiazole Derivatives
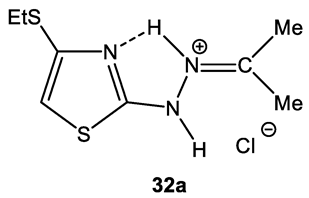
5.1. The Structure of 2,4-disubstituted Thiazoles
- (1)
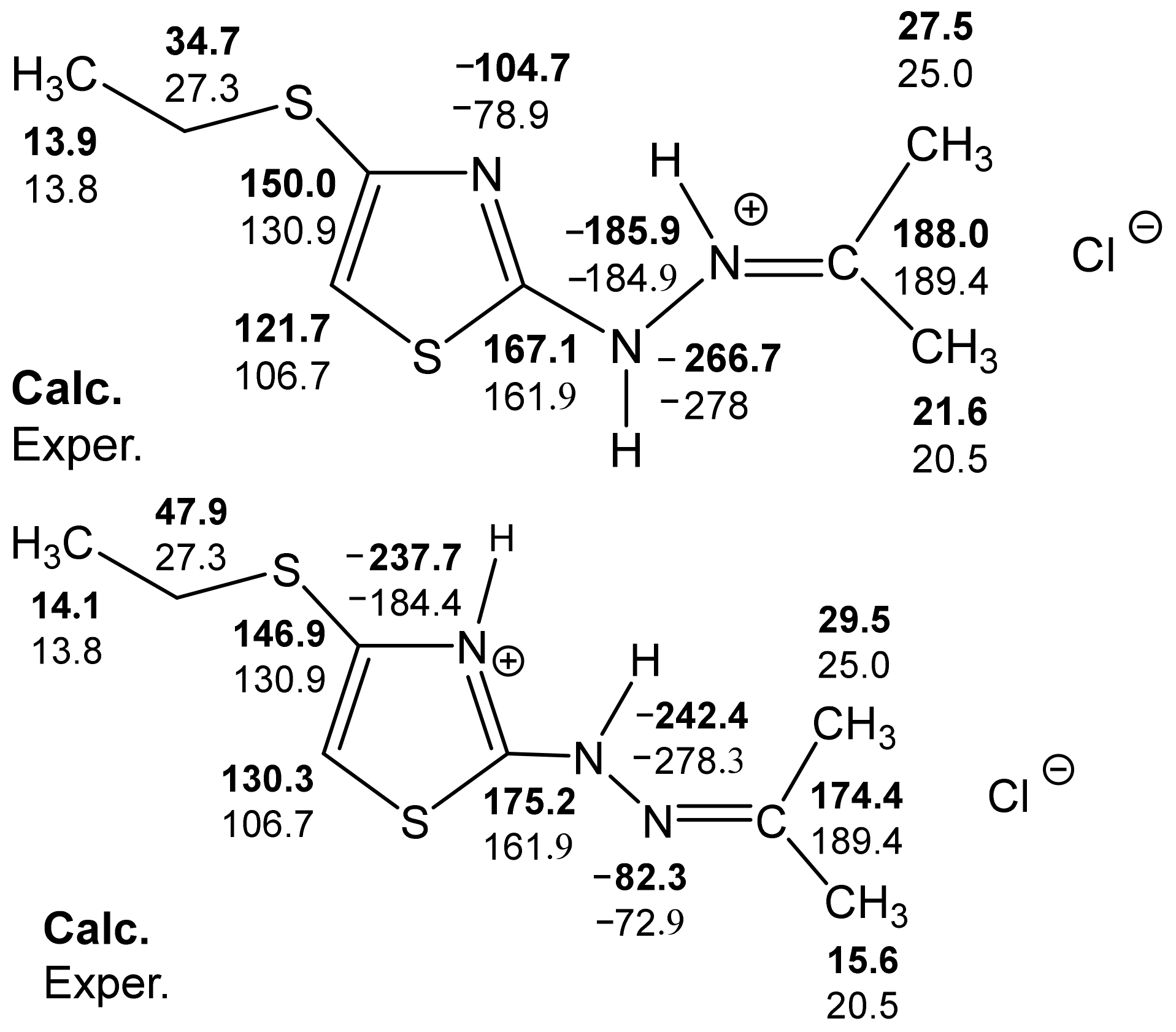
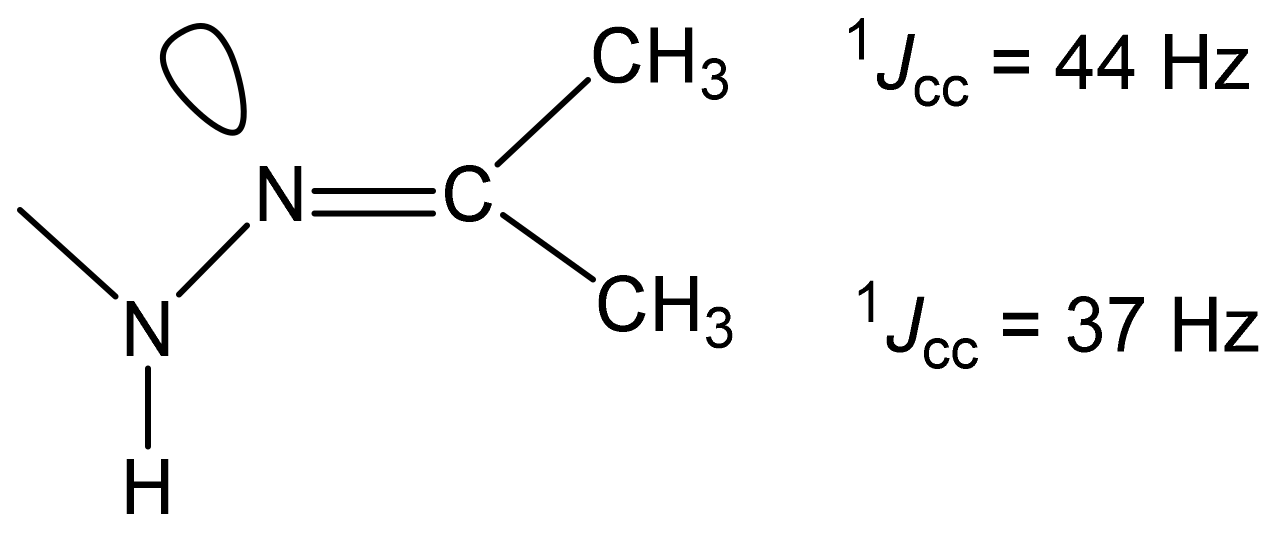
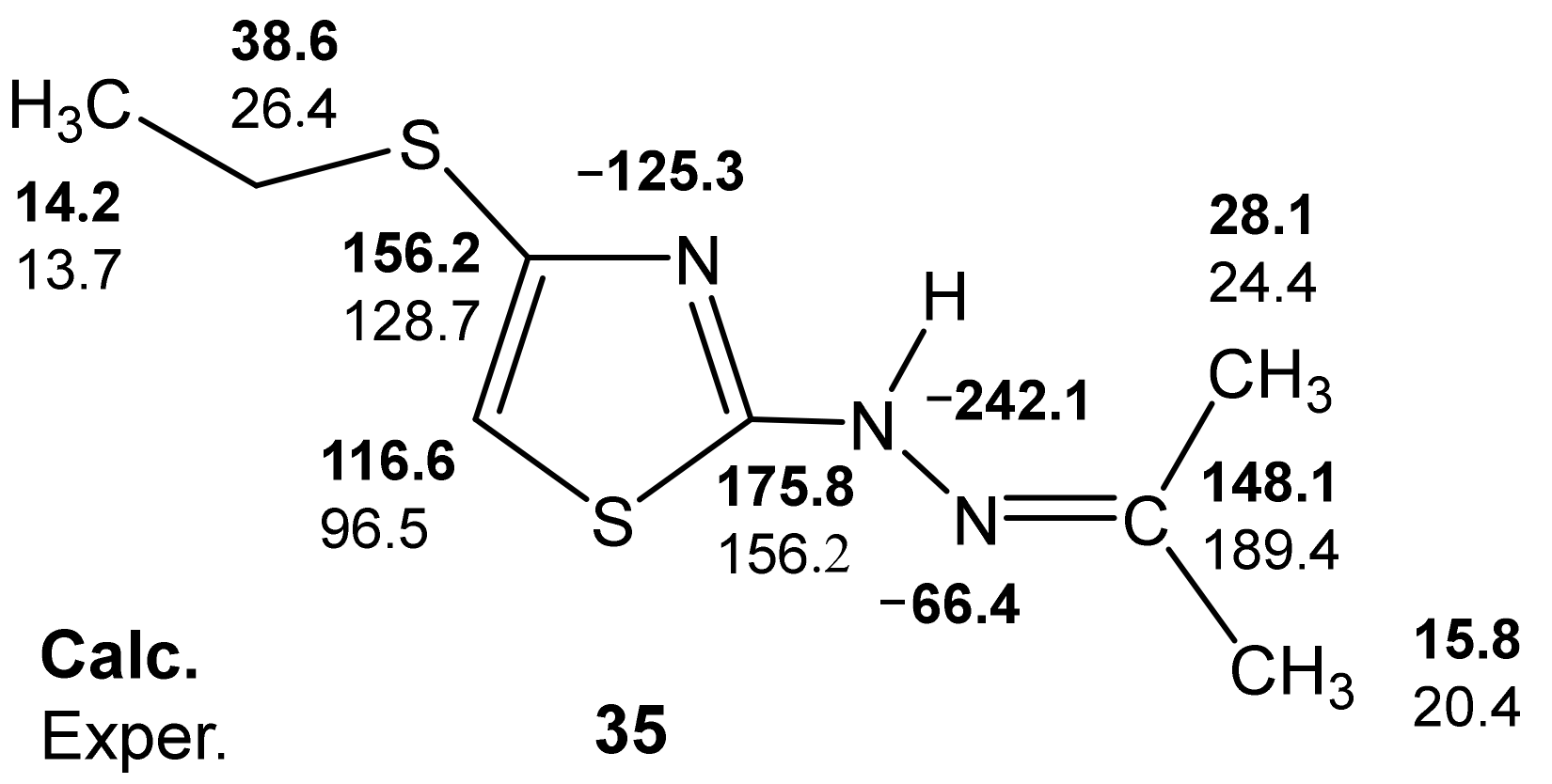
- the presence of a possible intramolecular hydrogen bond (32a), which is not fully taken into account by the used basis;
- (2)
- the need to use a slightly different basis in the calculations of molecules containing sulfur.
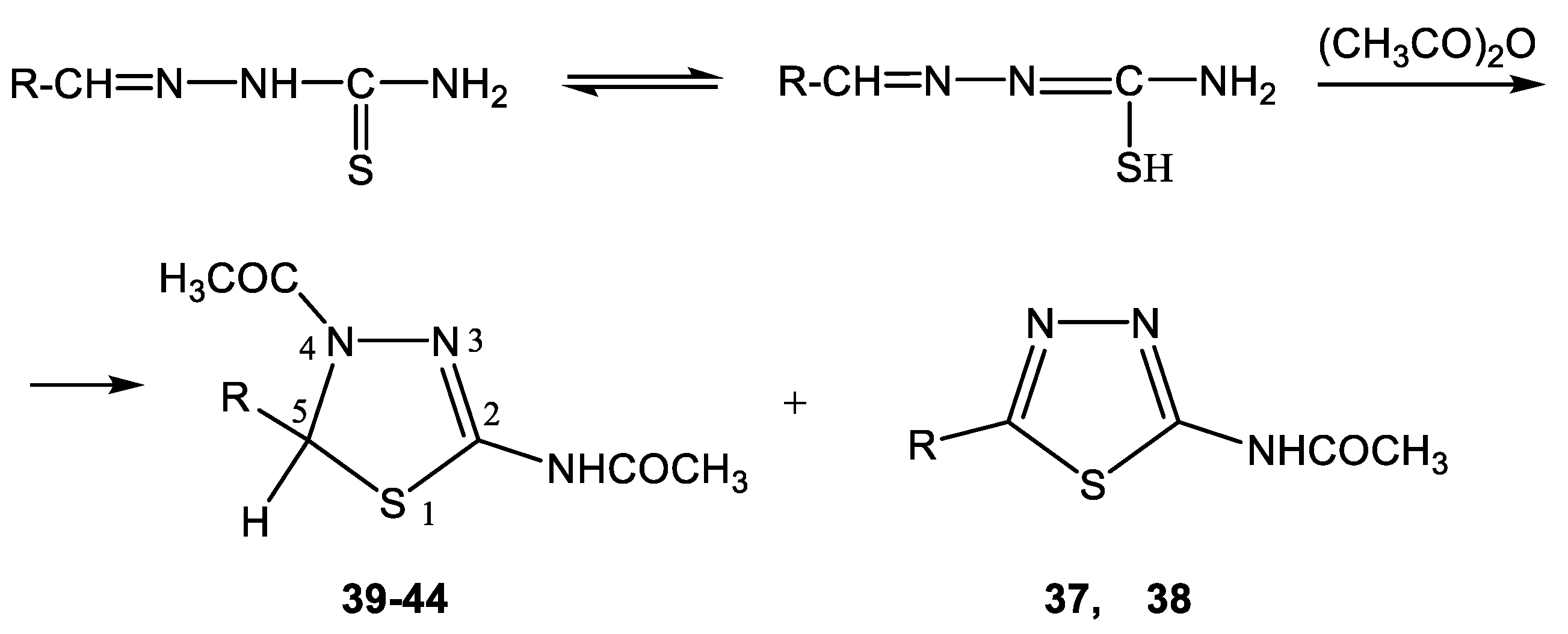
5.2. The Structure of 1,3,4-thiadiazoles and 1,3,4-thiadiazolines
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, R.N. Green synthetic approach to 5-substituted-1H-tetrazoles via recycle and reuse of tributyltin chloride. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Ress, W., Scriven, E.F.V., Eds.; Elsevier: New York, NY, USA, 1996; Volume 4, p. 621. [Google Scholar]
- Walczak, K.; Gondela, A.; Suwinski, J. Synthesis and anti-tuberculosis activity of N-aryl-C-nitroazoles. Eur. J. Med. Chem. 2004, 39, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, J.P.; Hodgson, R.D. Energetic compounds: N-Heterocycles. In Organic Chemistry of Explosives; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Curtis, A.D.M.; Jennings, N.A. Review of the Literature 1995–2007. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, p. 160. [Google Scholar]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Handbook of Heterocyclic Chemistry, 3rd ed.; Elsevier: New York, NY, USA, 2010; p. 1010. [Google Scholar]
- Pozharskii, A.F.; Soldatenko, A.T.; Katritzky, A.R. Why nature prefers heterocycles. In Heterocycles in life and society: An Introduction to Heterocyclic Chemistry and Biochemistry, Medicine and Agriculture, 2nd ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2011. [Google Scholar]
- Najafi, M.A.; Samangani, K. Non-isothermal kinetic study of the thermal decomposition of melamine 3-nitro-1,2,4-triazol-5-one salt. Propellants Explos. Pyrotech. 2011, 36, 487–492. [Google Scholar] [CrossRef]
- Das, D.; Sikdar, P.; Bairagi, M. Recent developments of 2-aminothiazoles in medicinal chemistry. Eur. J. Med. Chem. 2016, 109, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ayatia, A.; Emamib, S.; Foroumadia, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef]
- Zhanga, T.Y. The Evolving landscape of heterocycles in drugs and drug candidates. Adv. Heterocycl. Chem. 2017, 121, 1–12. [Google Scholar] [CrossRef]
- Yin, P.; Shreeve, J.M. Nitrogen-rich azoles as high density energy materials: Reviewing the energetic footprints of heterocycles. Adv. Heterocycl. Chem. 2017, 121, 89–131. [Google Scholar] [CrossRef]
- Meanwell, N.A.; Sistla, R.A. A survey of applications of tetrahydropyrrolo-3,4-azoles and tetrahydropyrrolo-2,3-azoles in medicinal chemistry. Adv. Heterocycl. Chem. 2021, 134, 31–100. [Google Scholar] [CrossRef]
- Serafini, M.; Pirali, T.; Tron, G.C. Click 1,2,3-triazoles in drug discovery and development: From the flask to the clinic? Adv. Heterocycl. Chem. 2021, 134, 101–148. [Google Scholar] [CrossRef]
- Rotella, D. Heterocycles in drug discovery: Properties and preparation. Adv. Heterocycl. Chem. 2021, 134, 149–183. [Google Scholar] [CrossRef]
- Ulu, O.D.; Ozdemir, I. Direct arylation of heteroaromatic compounds by Pd(OAc)2/tetrakis(N- benzimidazoliummethyl)benzene salt system. Arkivoc 2021, viii, 286–295. [Google Scholar] [CrossRef]
- Al-Soud, V.A.; Alhelal, K.A.S.; Saeed, B.A.; Abu-Qatouseh, L.; Al-Soud, H.H.; Al-Ahmad, A.H.; Al-Masoudi, N.A.; Qawasmeh, R.A. Synthesis, anticancer activity and molecular docking studies of new 4-nitroimidazole derivatives. Arkivoc 2021, viii, 296–309. [Google Scholar] [CrossRef]
- Singh, J.; Staples, R.J.; Shrivee, J.M. Pushing the limit of nitro groups on a pyrazole ring with energy-stability balance. Appl. Mater. Interfaces 2021, 13, 61357–61364. [Google Scholar] [CrossRef]
- Elguero, J.; Katritzky, A.R.; Denisko, O.V. Ptototropic tautomerism of heterocycles: Heteroaromatic tautomerism—general overview and methodology. Adv. Heterocycl. Chem. 2000, 76, 1–86. [Google Scholar]
- Minkin, V.I.; Garnovskii, A.D.; Elguero, J.; Katritzky, A.R.; Denisko, O.V. Tautomerism of heterocycles: Five-membered rings with two or more heteroatoms. Adv. Heterocycl. Chem. 2000, 76, 159–323. [Google Scholar]
- Elguero, J.; Goya, P.; Jagerovic, N.; Siva, A.M.S. Pyrazoles as drugs: Facts and fantasies. In Targets in Heterocyclic Systems—Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society Chemistry: Rome, Italy, 2002; Volume 6, pp. 52–98. [Google Scholar]
- Katritzky, A.R.; Scriven, E.F.V.; Majumder, S.; Akhmedova, R.G.; Akhmedov, N.G.; Vakulenko, A.V. Direct nitration of five-membered heterocycles. Arkivoc 2005, iii, 179–191. [Google Scholar] [CrossRef]
- Ramsden, C.A. The influence of aza-substitution on azole aromaticity. Tetrahedron 2010, 66, 2695–2699. [Google Scholar] [CrossRef]
- Alvares-Builla, J.; Vaqiero, J.J.; Barluenga, J. (Eds.) Modern Heterocyclic Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Golobokova, T.V.; Vereshchagin, L.I.; Zhitov, R.G.; Kizhnyaev, V.N. Uncondensed Vicinal Triazoles; Irkutsk State University: Irkutsk, Russia, 2012; ISBN 978-5-9624-0666-4. [Google Scholar]
- Perez-Fernandez, R.; Goya, P.; Elguero, J. A review of recent progress (2002–2012) on the biological activities of pyrazoles. Arkivoc 2014, ii, 233–293. [Google Scholar] [CrossRef]
- Brown, A.W. Recent developments in the chemistry of pyrazoles. Adv. Heterocycl. Chem. 2018, 126, 55–107. [Google Scholar] [CrossRef]
- Sukhanov, G.T.; Istoshina, V.A.; Krupnova, I.A.; Filippova, Y.V.; Sukhanova, A.G.; Bosov, K.K. Heterylation of 4-methyl-3-nitro-1,2,4-triazole with azolide anions. South-Sib. Sci. Bull. 2018, 24, 333–337. [Google Scholar] [CrossRef]
- Sapegin, A.; Krasavin, M. Ring-opening reactions of 2-imidazolines and their applications. Adv. Heterocycl. Chem. 2020, 130, 195–250. [Google Scholar] [CrossRef]
- Sainas, S.; Pippione, A.C.; Boschi, D.; Loll, M.L. Hydroxyazoles as acid isosteres and their drug design applications—Part 1: Monocyclic systems. Adv. Heterocycl. Chem. 2021, 134, 185–272. [Google Scholar] [CrossRef]
- Pippione, A.C.; Sainas, S.; Boschi, D.; Lolli, M.L. Hydroxyazoles as acid isosteres and their drug design applications—Part 2: Bicyclic systems. Adv. Heterocycl. Chem. 2021, 134, 273–311. [Google Scholar] [CrossRef]
- Fischer, G. 1,2,4-Triazolo[5,1-b]- and -[1,5-a]quinazolines and their hydro derivatives. Adv. Heterocycl. Chem. 2021, 135, 1–55. [Google Scholar] [CrossRef]
- Abdelhamid, I.A.; Hawass, M.A.E.; Sanad, S.M.H.; Elmahy, A.H.M. Synthesis of various pyrazole-fused heterocyclic systems using pyrazole-4-carbaldehydes as versatile precursors. Arkivoc 2021, ix, 42–74. [Google Scholar] [CrossRef]
- Hoz, A.; Claramunt, R.M.; Elguero, J.; Alkorta, I. 3-Pyrazolines (2,3-dihydro-1H-pyrazoles). Arkivoc 2021, ix, 75–129. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Malkina, A.G. Cyanoacetylenic alcohols: Molecules of interstellar relevance in the synthesis of essential heterocycles, amino acids, nucleobases and nucleosides. Synthesis 2021, 53, 2740–2767. [Google Scholar] [CrossRef]
- Larina, L.I.; Lopyrev, V.A. Nitroazoles: Synthesis, Structure and Applications; Springer: New York, NY, USA, 2009; 441p. [Google Scholar]
- Lopyrev, V.A.; Larina, L.I.; Voronkov, M.G. Nitration of Azoles. Rev. Heteroatom Chem. 1994, 11, 27–64. [Google Scholar]
- Larina, L.I.; Lopyrev, V.A.; Voronkov, M.G. Methods of nitroazoles synthesis. Russ. J. Org. Chem. 1994, 30, 1141–1179. [Google Scholar]
- Larina, L.I.; Lopyrev, V.A. Synthesis of nitrobenzazoles. Part 1. In Targets in Heterocyclic Systems—Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society Chemistry: Rome, Italy, 2005; Volume 9, pp. 327–365. [Google Scholar]
- Larina, L.I.; Titiva, I.A.; Lopyrev, V.A. Synthesis of nitrobenzazoles. Part 2. In Targets in Heterocyclic Systems—Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society Chemistry: Rome, Italy, 2006; Volume 10, pp. 321–359. [Google Scholar]
- Lopyrev, V.A.; Larina, L.I.; Voronkov, M.G. Trimethylsilylazoles chemistry. Russ. J. Org. Chem. 2001, 37, 149–193. [Google Scholar] [CrossRef]
- Larina, L.I.; Lopyrev, V.A. Nuclear Magnetic Resonance of Nitroazoles. In Topics in Heterocyclic Systems—Synthesis, Reactions and Properties; Attanasi, O.A., Spinelli, D., Eds.; Research Signpost: Trivandrum, India, 1996; Volume 1, pp. 187–237. [Google Scholar]
- Larina, L.I.; Lopyrev, V.A.; Klyba, L.V.; Bochkarev, V.N. Mass spectrometry of nitroazoles. In Targets in Heterocyclic Systems. Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Italian Society Chemistry: Rome, Italy, 1998; Volume 2, pp. 443–470. [Google Scholar]
- Larina, L.I.; Lopyrev, V.A.; Vakulskaya, T.I. Quantitative estimation of electronic substituent effects in five membered, nitrogen-containing aromatic heterocycles. Russ. Chem. Rev. 1986, 55, 411–425. [Google Scholar]
- Larina, L.I. NMR Spectroscopy and Structure of Substituted Azoles. Ph.D. Thesis, Irkutsk Institute of Chemistry, Russian Academy of Science, Irkutsk, Russia, 2003; 385p. (In Russian). [Google Scholar]
- Larina, L.I. Tautomerism and Structure of azoles: Nuclear Magnetic Resonance Spectroscopy. Adv. Heterocycl. Chem. 2018, 124, 233–321. [Google Scholar] [CrossRef]
- Larina, L.I. Nuclear Quadrupole Resonance Spectroscopy: Tautomerism and structure of functional azoles. Crystals 2019, 9, 366. [Google Scholar] [CrossRef]
- Larina, L.I. Organosilicon azoles: Structure, silylotropy and NMR spectroscopy. Adv. Heterocycl. Chem. 2021, 133, 1–63. [Google Scholar] [CrossRef]
- Pozharskii, A.F. The concept of π-redundancy in the chemistry of heterocyclic compounds. Chem. Heterocycl. Compd. 1977, 13, 583–590. [Google Scholar] [CrossRef]
- Boyer, J.H. Nitroazoles. The C-Nitro Derivatives of Five- Membered N- and N,O-Heterocycles; VCH Publishers: Deerfield Beach, FL, USA, 1986; 368p. [Google Scholar]
- Stanovnik, B. New developments in heterocyclic tautomerism: Desmotropes, carbenes and betaines. Adv. Heterocyc. Chem. 2016, 119, 209–239. [Google Scholar]
- Hall, C.D.; Panda, S.S. The benzotriazole story. Adv. Heterocyc. Chem. 2016, 119, 1–23. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Hall, C.D.; El-Gendy, B.E.-D.M.; Draghici, B. Tautomerism in drug discovery. J. Comput. Aided Mol. Des. 2010, 24, 475–499. [Google Scholar] [CrossRef]
- Kaur, R.; Dwivedi, A.R.; Kumar, B.; Kumar, V. Recent developments on 1,2,4-triazole nucleus in anticancer compounds: A review. Anti-Cancer Agents Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef]
- Braga, R.C.; Alves, V.M.; Silva, A.C.; Nascimento, M.N.; Silva, F.; Liao, L.M.; Andrade, C.H. Virtual screening strategies in medicinal chemistry: The state of the art and current challenges. Curr. Top. Med. Chem. 2014, 14, 1899–1912. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Paluch, M. Tautomerism in drug delivery. In Disordered Pharmaceutical Materials; Descamps, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Rams-Baron, M.; Paluch, M. Current research trends in dielectric relaxation studies of amorphous pharmaceuticals: Physical stability, tautomerism, and the role of hydrogen bonding. Trends Anal. Chem. 2020, 134, 116097. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Lee, G.S.; Mitchell, A.R.; Schmidt, R.D.A. Review of Energetic Materials Synthesis. Thermochim. Acta 2002, 384, 187–204. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, C.; Zhang, L.; Wang, Y.; Li, S.; Zhao, F.; Pang, S. Amination of nitroazoles—A comparative study of structural and energetic properties. Molecules 2014, 19, 896–910. [Google Scholar] [CrossRef]
- Golinski, J.; Makosza, M. Vicarious nucleophilic substitution of hydrogen in aromatic nitro compounds. Tetrahedron Lett. 1978, 19, 3495–3498. [Google Scholar] [CrossRef]
- Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley-VCH: Weinheim, Germany, 2013; 472p. [Google Scholar]
- Mąkosza, M. Reactions of nucleophiles with nitroarenes: Multifacial and versatile electrophiles. Chem. A Eur. J. 2014, 20, 5536–5545. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N. Recent advances in the field of nucleophilic aromatic substitution of hydrogen. Tetrahedron Lett. 2016, 57, 2665–2672. [Google Scholar] [CrossRef]
- Sample, H.C.; Senge, M.O. Nucleophilic aromatic substitution (SNAr) and related reactions of porphyrinoids: Mechanistic and regiochemical aspects. Eur. J. Org. Chem. 2020, 2021, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Donskaya, O.V.; Dolgushin, G.V.; Lopyrev, V.A. Vicarious nucleophilic substitution of hydrogen in nitro-substituted pyrroles, azoles, and benzannelated systems based on them (Review). Chem. Heterocycl. Comp. 2002, 38, 371–386. [Google Scholar] [CrossRef]
- Mąkosza, M. Nucleophilic substitution of hydrogen in electron-deficient arenes, a general process of great practical value. Chem. Soc. Rev. 2010, 39, 2855–2868. [Google Scholar] [CrossRef]
- Ehlers, D.; Klapctke, T.M.; Pfliger, C. Investigations of the vicarious C-aminations of 5,7-dinitrobenzotriazole and 4,6-dinitrobenzotriazol-3-ium-1-oxide and their energetic properties. Chem. Eur. J. 2015, 21, 16073–16082. [Google Scholar] [CrossRef]
- Meneses, L.; Morocho, S.; Castellanos, A.; Cuesta, S. Computational study of vicarious nucleophilic substitution reactions. J. Mol. Modeling 2021, 23, 301–310. [Google Scholar] [CrossRef]
- Bernard, M.K.; Makosza, M.; Szafran, B.; Wrzeciono, U. Azoles. 26. Vicarious nucleophilic substitution of hydrogen in nitropyrazole derivatives. Liebigs Ann. Chem. 1989, 6, 545–549. [Google Scholar] [CrossRef]
- Makosza, M. Vicarious Nucleophilic Substitution of Hydrogen. Russ. Chem. Rev. 1989, 58, 747–765. [Google Scholar] [CrossRef]
- Makosza, M. Vicarious Nucleophilic Substitution of Hydrogen in the Chemistry of Heterocyclic. Synthesis 1991, 1991, 103–111. [Google Scholar] [CrossRef]
- Suwinski, J.; Swierczek, K. Nucleophilic amination and transformation in 2-methyl-4-nitro-1-phenylimidazole. Tetrahedron Lett. 1992, 33, 7941–7944. [Google Scholar] [CrossRef]
- Suwinski, J.; Swierczek, K. Nitroimidazoles XVII. Nucleophilic amination or ring transformation in reactions of 1-aryl-4-nitroimidazoles with 4-amino-1,2,4-triazole or hydroxylamine. Tetrahedron 1993, 49, 5339–5350. [Google Scholar] [CrossRef]
- Bernard, M.K. Azoles. 40. Vicarious nucleophilic substitution of hydrogen in benzimidazoles. Polish J. Chem. 1995, 1120–1125. [Google Scholar]
- Makosza, M.; Kwast, E. Vicarious Nucleophilic Substitution of Hydrogen in Nitroderivatives of Fife-Membered Heteroaromatic Compounds. Tetrahedron 1995, 51, 8339–8354. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Mitchell, A.R.; Schmidt, R.D. 1,1,1-Trimethylhydrazinium Iodide: A Novel, highly reactive reagent for aromatic amination via vicarious nucleophilic substitution of hydrogen. J. Org. Chem. 1996, 61, 2934–2935. [Google Scholar] [CrossRef]
- Makosza, M. Arene Chemistry: Reaction Mecanisms and Methods for Aromatic Compounds; Mortier, J., Ed.; John Wiley and Sons, Inc: Hoboken, NJ, USA, 2016; p. 269. [Google Scholar]
- Makosza, M.; Bialeski, M. Nitroarylamines via the vicarious nucleophilic substitution of hydrogen: Amination, alkylamination, and arylamination of nitroarenes with sulfenamides. J. Org. Chem. 1998, 63, 4878–4888. [Google Scholar] [CrossRef]
- Dolgushin, G.V.; Levkovskaya, G.G.; Titova, I.A.; Bozhenkov, G.V.; Larina, L.I.; Abramova, E.V.; Komarova, T.N.; Vakulskaya, T.I.; Lopyrev, V.A. 1,1-Dimetylhydrazine and its derivatives in the synthesis of substituted nitrogen containing heterocycles. In Proceedings of the 7th International seminar Scientific Advances in Chemistry: Heterocycles, Catalysis and Polymers as Driving Forces, Ekaterinburg, Russia, 2–4 November 2004. [Google Scholar]
- Blaziak, K.; Danikiewicz, W.; Makosza, M. How Does Nucleophilic Aromatic Substitution Really Proceed in Nitroarenes? Computational Prediction and Experimental Verification. J. Am. Chem. Soc. 2016, 138, 7276–7281. [Google Scholar] [CrossRef]
- Błaziak, K.; Danikiewicz, W.; Mąkosza, M. How do aromatic nitro compounds react with nucleophiles? Theoretical description using aromaticity, nucleophilicity and electrophilicity indices. Molecules 2020, 25, 4819. [Google Scholar] [CrossRef] [PubMed]
- Larina, L.I.; Lopyrev, V.A.; Elokhina, V.N.; Krylova, O.V.; Nakhmanovich, A.S.; Vokin, A.I. Study of vicarious amination of C-nitroazoles. In Proceedings of the 17th International Congress of Heterocyclic Chemistry, Vienna, Austria, Book of Abstracts. 1–4 August 1999. PO–297. [Google Scholar]
- Lopyrev, V.A.; Elokhina, V.N.; Krylova, O.V.; Nakhmanovich, A.S.; Larina, L.I.; Sorokin, M.S.; Vokin, A.I. Vicarious C-Amination of 1-methyl-4-nitropyrazole. Chem. Heterocycl. Comp. 1999, 35, 1109–1110. [Google Scholar] [CrossRef]
- Elokhina, V.N.; Krylova, O.V.; Larina, L.I.; Nakhmanovich, A.S.; Sorokin, M.S.; Volkova, K.A.; Lopyrev, V.A. Interaction of 1-methyl-4-nitropyrazole with 4-amino-1,2,4-triazole. Chem. Heterocycl. Comp. 2000, 36, 5476–5477. [Google Scholar] [CrossRef]
- Schmidt, R.D.; Lee, G.S.; Pagoria, P.F.; Mitchell, A.R.; Giraldi, R. Synthesis of 4-amino-3,5-dinitro-1H-pyrazole using vicarious nucleophilic substitution of hydrogen. J. Heterocyc. Chem. 2002, 38, 1227–1230. [Google Scholar] [CrossRef]
- Krylova, O.V.; Elokhina, V.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A. Vicarious C-amination of nitrobenzene. Russ. J. Org. Chem. 2001, 31, 933–934. [Google Scholar]
- Donskaya, O.V.; Elokhina, V.N.; Nakhmanovich, A.S.; Vakulskaya, T.I.; Larina, L.I.; Vokin, A.I.; Albanov, A.I.; Lopyrev, V.A. Vicarious C-amination of 1-methyl-4-nitroimidazole. Tetrahedron Lett. 2002, 43, 1613–1616. [Google Scholar] [CrossRef]
- Larina, L.I.; Vakulskaya, T.I.; Titova, I.A.; Volkov, V.A.; Vereshchagin, L.I.; Lopyrev, V.A.; Dolgushin, G.V. Vicarious C-amination of Nitroazoles. In Proceedings of the 7th IUPAC International Conference on Heteroatom Chemistry, Shanghai, China, 20–25 August 2004. [Google Scholar]
- Titova, I.A.; Vakulskaya, T.I.; Larina, L.I.; Mizandrontsev, M.I.; Volkov, V.A.; Dolgushin, G.V.; Lopyrev, V.A. Vicarious nucleophilic C-amination of nitrobenzene and 1-methylderivatives 5- and 6-nitrobenzimidazoles. Russ. J. Org. Chem. 2005, 41, 1306–1315. [Google Scholar] [CrossRef]
- Vakulskaya, T.I.; Titova, I.A.; Larina, L.I.; Verkhozina, O.N.; Dolgushin, G.V.; Lopyrev, V.A. Anion-radicals in reactions of vicarious C-amination of N-substituted nitrotriazoles. Chem. Heterocycl. Comp. 2006, 42, 1427–1434. [Google Scholar] [CrossRef]
- Larina, L.I.; Titova, I.A.; Mizandrontsev, M.I.; Volkov, V.A.; Lopyrev, V.A. Unexpected reaction of 1-methyl-5-nitrobenzimidazole with 4-amino-1,2,4-triazole in DMSO/t-BuOK medium. In Proceedings of the 18th Mendeleev Congress, Moscow, Russia, 24–20 September 2007; Volume 5, p. 481. [Google Scholar]
- Larina, L.I.; Titova, I.A.; Mizandrontsev, M.I.; Lopyrev, V.A.; Amosova, S.V. Novel products of vicarious C-amination of nitroazoles by 4-amino-1,2,4-triazole in the presence of metal salts. In Proceedings of the XVII EuCheMS Conference on Organometallic Chemistry, Sofia, Bulgary, 1–6 September 2007; p. 165. [Google Scholar]
- Vakulskaya, T.I.; Larina, L.I.; Protsyk, N.I.; Lopyrev, V.A. Tautomerism of 3-nitro-1,2,4-triazole-5-one radical anions. Magn. Reson. Chem. 2009, 47, 716–719. [Google Scholar] [CrossRef]
- Ugrak, B.I.; Manaev, Y.A.; Perevalov, V.P.; Shevelev, S.A. Parameters of 15N NMR spectra of N-methylpyrazole derivatives. Russ. Chem. Bull. 1992, 41, 2554–2560. [Google Scholar] [CrossRef]
- Makhova, N.N.; Blinnikov, A.N.; Khmelnitskii, L.I. The Schmidt rearrangement of methyl furoxanyl ketones and furoxancarboxylic acids: A new synthetic route to aminofuroxans. Mendeleev Commun. 1995, 2, 56–58. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Starosotnikov, A.M.; Shakhnes, A.K.; Shevelev, S.A. Functionalization of 4,6-dinitro-2-phenylindole at position 7. Russ. Chem. Bull. 2000, 57, 1539–1542. [Google Scholar] [CrossRef]
- Dudzinska-Uzarewicz, J.; Wrzeciono, U.; Frankiewicz, A.; Linkowska, E.; Kohler, T.; Nuhn, P. Azoles. 18. Sulfonylindazole derivatives. Pharmazie 1988, 43, 611–613. [Google Scholar]
- Gzella, A.; Wrzeciono, U.; Dudzinska-Uzarewicz, J.; Borowiak, T. Azoles. 19. Structure of 4-nitro-7-phenylsulfonylmethylindazole. Acta Crystallogr. 1989, 45, 642–644. [Google Scholar] [CrossRef]
- Strelenko, Y.A.; Godovikova, T.I.; Ignateva, E.L. 14N and 15N NMR Spectroscopy of 2-methyl-4,5-dinitro-1,2,3-triazole and 2-methyl-4(5)-nitro-1,2,3-triazole-1-oxide substituted. Chem. Heterocycl. Comp. 2002, 38, 547–552. [Google Scholar] [CrossRef]
- Witanowski, M.; Stefaniak, L.; Webb, G.A. Nitrogen NMR Spectroscopy. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: London, UK, 1981; Volume 118, 502p. [Google Scholar]
- Butler, D.E.; De Ward, H.A. New general methods for substitution of 5-chloropyrazoles. The synthesis of 1,3-dialkyl-5-chloropyrazol-4-yl aryl ketones and new 1,3-dialkyl-2-pyrazolin-5-ones. J. Org. Chem. 1971, 36, 2542–2547. [Google Scholar] [CrossRef]
- Nazarinia, M.; Sharifian, A.; Shafiee, A. Syntheses of substituted 1-(2-phenethyl)pyrazoles. J. Heterocycl. Chem. 1995, 32, 223–225. [Google Scholar] [CrossRef]
- Levkovskaya, G.G.; Bozhenkov, G.V.; Larina, L.I.; Evstafieva, I.T.; Mirskova, A.N. Synthesis and properties of trifluoromethyl-2,2-dichlorovinyl ketone. Russ. J. Org. Chem. 2001, 31, 644–648. [Google Scholar] [CrossRef]
- Levkovskaya, G.G.; Bozhenkov, G.V.; Larina, L.I.; Mirskova, A.N. New synthesis and properties of 3-alkyl-, 3-chloroalkyl-, 3-perfluoroalkyl-, and 3-aryl-1-methyl-5-(halo)-pyrazoles from chloro(bromo)vinyl ketones and N,N-dimethylhydrazine. Russ. J. Org. Chem. 2002, 38, 1501–1506. [Google Scholar] [CrossRef]
- Popov, A.V.; Rudyakova, E.V.; Larina, L.I.; Kobelevskaya, V.A.; Levkovskaya, G.G. The ratio of 1,3- and 1,5-dialkylsubstituted pyrazoles obtained from chlorovinylalkyl ketones and alkylhydrazines, 3(5)-pyrazoles and bromoalkanes. Russ. J. Org. Chem. 2014, 50, 1650–1662. [Google Scholar] [CrossRef]
- Kobelevskaya, V.A.; Larina, L.I.; Popov, A.V.; Rudyakova, E.V.; Levkovskaya, G.G. Synthesis, structure of 1-tert-butyl-substituted 3(5)-alkylpyrazoles from 2-chlorovinyl ketones. Russ. J. Org. Chem. 2015, 51, 231–239. [Google Scholar] [CrossRef]
- Popov, A.V.; Kobelevskaya, V.A.; Larina, L.I.; Levkovskaya, G.G. Synthesis of 3-(5-chloropyrazol-3-yl) propenals. Mendeleev Commun. 2017, 27, 178–183. [Google Scholar] [CrossRef]
- Levkovskaya, G.G.; Rudyakova, E.V.; Kobelevskaya, V.A.; Popov, A.V.; Rozentsveig, I.B. Novel directed synthesis of functionalized pyrazole derivatives via regioselective solvent-free thiylation of 3-alkenylpyrazoles with arenethiols. Arkivoc 2016, iii, 82–89. [Google Scholar] [CrossRef]
- Kobelevskaya, V.A.; Popov, A.V.; Levkovskaya, G.G. Directed synthesis of 3-(2,2-dichlorocyclopropyl)pyrazoles. Russ. J. Org. Chem. 2017, 53, 144–146. [Google Scholar] [CrossRef]
- Kobelevskaya, V.A.; Popov, A.V.; Levkovskaya, G.G.; Rudyakova, E.V.; Rozentsveig, I.B. Regioselective Synthesis of 3-[2-(Alkylsulfanyl)ethyl]pyrazoles by Reaction of Alkanethiols with 3-Alkenylpyrazoles. Russ. J. Org. Chem. 2018, 54, 1505–1508. [Google Scholar] [CrossRef]
- Popov, A.V.; Kobelevskaya, V.A.; Larina, L.I.; Rozentsveig, I.B. Synthesis of poly-functionalized pyrazoles under Vilsmeier-Haack reaction conditions (19-10934ZP). Arkivoc 2019, vi, 1–14. [Google Scholar] [CrossRef]
- Popov, A.V.; Kobelevskaya, V.A.; Titov, I.D.; Larina, L.I.; Rozentsveig, I.B. Synthesis of 5-chloroisoxazoles derived from 2,2-dichlorovinyl ketones. Russ. J. Org. Chem. 2020, 56, 1958–1962. [Google Scholar] [CrossRef]
- Kobelevskaya, V.A.; Dyachkova, S.G.; Popov, A.V.; Levkovskaya, G.G. Sulfonation of unsymmetrically substituted 5-chloropyrazoles. Russ. J. Org. Chem. 2016, 52, 911–913. [Google Scholar] [CrossRef]
- Semenov, A.V.; Larina, L.I.; Demina, M.M. Stereochemistry and Tautomerism of Silicon-Containing 1,2,3-triazole: Ab initio and NMR Study. Struct. Chem. 2020, 31, 1927–1933. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Demina, M.M.; Konkova, T.V.; Vu, T.D.; Larina, L.I. Synthesis of 4-trialkyl(germyl)-1H- 1,2,3-triazolecarbaldehyde oximes. Chem. Heterocycl. Comp. 2014, 50, 967–971. [Google Scholar] [CrossRef]
- Medvedeva, A.S.; Demina, M.M.; Vu, T.D.; Andreev, M.V.; Shaglaeva, N.S.; Larina, L.I. β-Cyclodextrin-catalyzed three-component synthesis of 4,5-disubstituted 1,2,3-(NH)-triazoles from propynals, trimethylsilyl azide and malononitrile in water. Mendeleev Commun. 2016, 26, 326–328. [Google Scholar] [CrossRef]
- Demina, M.M.; Medvedeva, A.S.; Nguyen, T.L.H.; Vu, C.Z.; Larina, L.I. One-pot three-component green synthesis of [1H-(1,2,3-triazol-5-yl)methylidene] heterocycles based on element-substituted propynals. Russ. Chem. Bull. 2017, 66, 2253–2257. [Google Scholar] [CrossRef]
- Vu, T.D.; Demina, M.M.; Shaglaeva, N.S.; Medvedeva, A.S. One-pot, three component synthesis of Si-, Ge-, C-substituted 1H-1,2,3-triazole carbaldehyde oximes. In Proceedings of the Siberian Winter Conference: Current of Topics in Organic Chemistry, Sheregesh, Russia, 21–27 March 2015; p. 207. [Google Scholar]
- Demina, M.M.; Medvedeva, A.S.; Vu, T.D.; Larina, L.I.; Mitroshina, I.V.; Shemyakina, O.A. Catalyst-free three-component synthesis of hydroxyalkyltriazolylmethylidene barbiturates. Mendeleev Commun. 2019, 29, 655–657. [Google Scholar] [CrossRef]
- Barone, V.; Peralta, J.E.; Contreras, R.H.; Sosnin, A.V.; Krivdin, L.B. Natural J coupling (NJC) analysis of the electron lone pair effect on NMR couplings: Part 1. The lone pair orientation effect of an α-nitrogen atom on 1J(C,C) couplings. Magn. Reson. Chem. 2001, 39, 600–606. [Google Scholar] [CrossRef]
- Chernyshev, K.A.; Krivdin, L.B.; Larina, L.I.; Konkova, T.V.; Demina, M.M.; Medvedeva, A.S. Configurational assignment of carbon, silicon and germanium containing propynal oximes by means of 13C-1H, 13C-13C and 15N-1H spin–spin coupling constants. Magn. Reson. Chem. 2007, 45, 661–668. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. Theochem 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Harding, M.E.; Szalay, P.G.; Auer, A.A.; Bartlett, R.J.; Benedikt, U.; Berger, C.; Bernholdt, D.E.; Bomble, Y.J. CFOUR, a Quantum Chemical Program Package. Available online: http://www.cfour.de (accessed on 25 March 2020).
- Jensen, F. Basis Set Convergence of Nuclear Magnetic Shielding Constants Calculated by Density Functional Methods. J. Chem. Theory Comput. 2008, 4, 719–727. [Google Scholar] [CrossRef]
- Mirskova, A.N.; Seredkina, S.G.; Kalikhman, I.D.; Voronkov, M.G. Reactions of alkylthiochloroacetylenes with O,N- and S,N-containing bifunctional nucleophiles. Russ. Chem. Bull. 1988, 37, 518–520. [Google Scholar] [CrossRef]
- Dyachkova, S.G.; Gusarova, N.K.; Nikitina, E.A.; Larina, L.I.; Sinegovskaya, L.M.; Abramov, A.V.; Trofimov, B.A. Organylthio-chloroacetylenes. IV. Reaction with thiourea. Russ. J. Gen. Chem. 2001, 71, 1721–1725. [Google Scholar] [CrossRef]
- Dyachkova, S.G.; Gusarova, N.K.; Beskrylaya, E.A.; Larina, L.I.; Trofimov, B.A. Reaction of (alkylthio)chloroacetylenes with thiourea. Russ. Chem. Bull. 2000, 49, 1319–1321. [Google Scholar] [CrossRef]
- Dyachkova, S.G.; Nikitina, E.A.; Larina, L.I.; Ushakov, P.E.; Gusarova, N.K.; Sinegovskaya, L.S.; Trofimov, B.A. Reaction of (alkylthio)-chloroacetylenes with thiosemicarbazones: A route to functionalized thiazoles. Synthesis 2002, 916–920. [Google Scholar] [CrossRef]
- Larina, L.I.; Dyachkova, S.N.; Gusarova, N.K.; Nikitina, E.A.; Trofimov, B.A. Structure of new 2-alkanon-N-[4-organylthio)-1,3-thiazol-2-yl]hydrazones. In Proceedings of the 20th International Symposium on the Organic Chemistry of Sulfur, Flagstaff, AZ, USA, 14–19 July 2002. [Google Scholar]
- Larina, L.I.; Karnaukhova, R.V.; Nakhmanovich, A.S.; Shagun, V.A.; Ushakov, P.E.; Lopyrev, V.A. Structure of N,N-Dimethylhydrazone Alkylation Products. J. Mol. Structure. Theochem 2002, 604, 165–176. [Google Scholar] [CrossRef]
- Rozentsveig, I.B.; Levkovskaya, G.G.; Rozentsveig, G.N.; Mirskova, A.N.; Krivdin, L.B.; Larina, L.I.; Albanov, A.I. Amidine derivatives of α-arylglycines from N-(1-aryl-2,2,2-trichloroethyl)amides of arenesulfonic acids and secondary amines. Tetrahedron Lett. 2005, 46, 8889–8893. [Google Scholar] [CrossRef]
- Krivdin, L.B.; Larina, L.I.; Chernyshev, K.A.; Rozentsveig, I.B. Non-empirical calculations of NMR indirect spin–spin coupling constants. Part 13. Configurational assignment of aminosulfonylamidines. Magn. Reson. Chem. 2005, 43, 937–942. [Google Scholar] [CrossRef]
- Elokhina, V.N.; Karnaukhova, V.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A. Reactions of heterocyclic thiosemicarbazones with acetic anhydride. Russ. J. Org. Chem. 2002, 38, 318–320. [Google Scholar] [CrossRef]
- Elokhina, V.N.; Karnaukhova, V.N.; Nakhmanovich, A.S.; Larina, L.I. Spiro[2-acetylamino-4-acetyl-1,3,4 -thiadiazole-5,3′-N-acetylindole-2-on]. In Nitrogen Heterocycles and Alkaloids; Iridium-Press: Moscow, Russia, 2001; p. 393. [Google Scholar]
- Elokhina, V.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A.; Desmurs, J.-R. Synthesis of Substituted 1,3,4-Thiadiazolines and 1,3,4-Thiadiazoles. In Proceedings of the 8th Blue Danude Symposium on Heterocyclic Chemistry, Bled, Slovenia, 24–27 September 2000; p. 73. [Google Scholar]
- Larina, L.I.; Nizovtseva, T.V.; Elokhina, V.N.; Komarova, T.N.; Karnaukhova, R.V.; Nakhmanovich, A.S.; Ushakov, P.E.; Lopyrev, V.A.; Desmurs, J.-R. Synthesis and Structure of Novel 5- and 6-Membered S,N-containing Heterocycles. In Proceedings of the 20th International Symposium on the Organic Chemistry of Sulfur, Flagstaff, AZ, USA, 14–19 July 2002. [Google Scholar]
- Tretyakov, A.V.; Rudaya, L.I.; Eltsov, A.V.; Larin, M.F.; Lopyrev, V.A. Carbon-13 NMR spectra of isomeric 2-arylimidazopyridines. Magn. Reson. Chem. 1985, 23, 7–9. [Google Scholar] [CrossRef]
- Zhao, T.; Li, L.; Dong, Z.; Zhang, Y.; Zhang, G.; Huang, M.; Li, H. Research Progress on the Synthesis of Energetic Nitroazoles. Chin. J. Org. Chem. 2014, 34, 304–315. [Google Scholar] [CrossRef]
- Jin, X.; Xiao, M.; Zhou, J.; Zhou, G.; Hu, B. Design of energetic materials based on asymmetric oxadiazole. ChemistryOpen 2019, 8, 692–700. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Lian, Z.; Li, H.; Zhang, J. Computational design and screening of promising energetic materials: The coplanar family of novel heterocycle-based explosives. Quantum Chem. 2021, 121, e26788. [Google Scholar] [CrossRef]
- Cao, W.; Dong, W.; Lu, Z.; Bi, Y.; Hu, Y.; Wang, T.; Zhang, C.; Li, Z.; Yu, Q.; Zhang, J. Construction of Coplanar Bicyclic Backbones for 1,2,4-Triazole-1,2,4-Oxadiazole-Derived Energetic Materials. Chem. A Eur. J. 2021, 27, 13807–13818. [Google Scholar] [CrossRef]
- Grishchenko, L.A.; Parshina, L.N.; Larina, L.I.; Belovezhets, L.A.; Klimenkov, I.V.; Ustinov, A.Y.; Trofimov, B.A. Arabinogalactan propargyl ethers: Au-catalysed hydroamination by imidazols. Carbohydr. Polym. 2020, 246, 116638. [Google Scholar] [CrossRef] [PubMed]



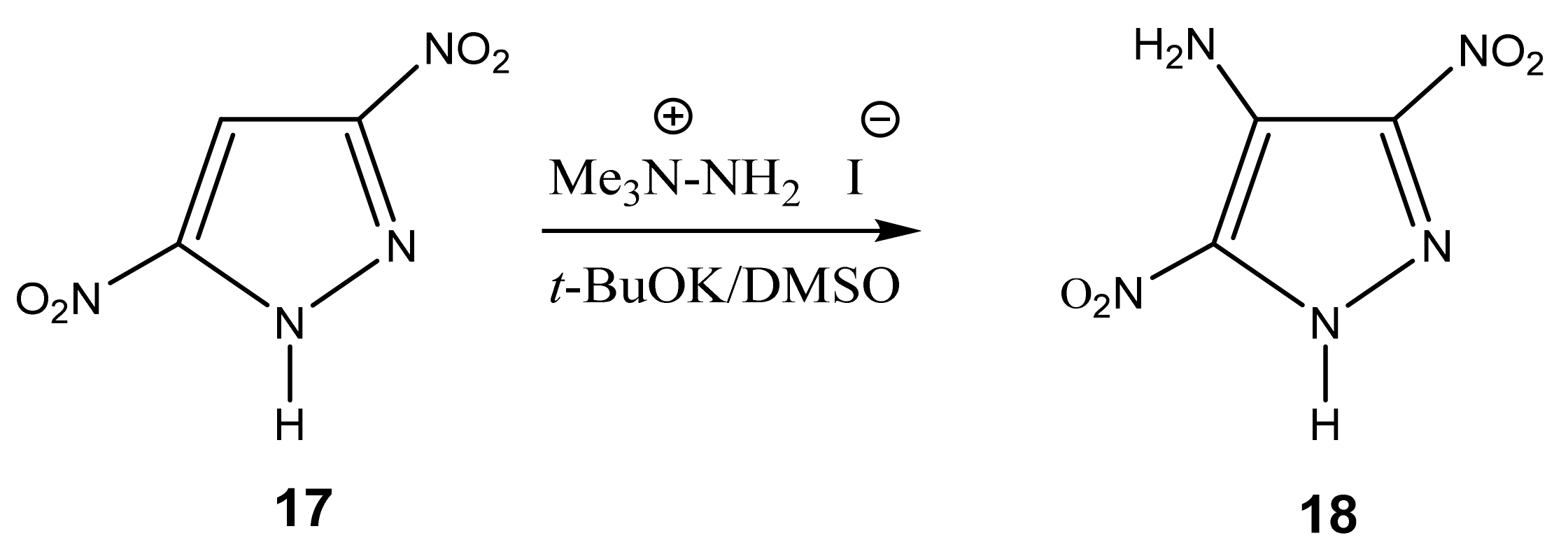



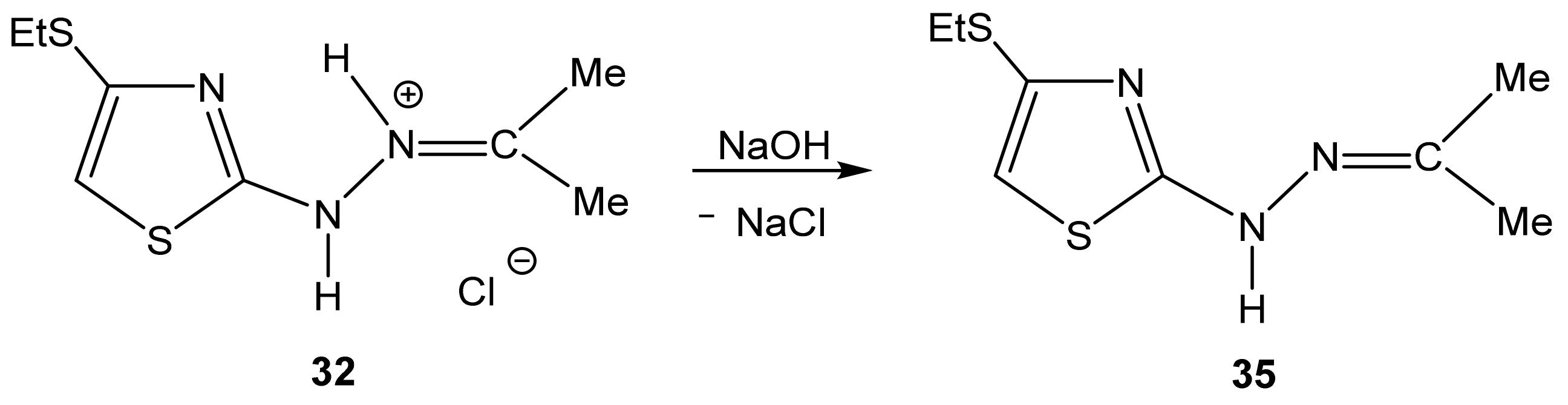
| Compound | δ 1H | δ 13C | |
|---|---|---|---|
| 24 |  | 6.61 d H-3,5 3J = 8.3 Hz 8.05 d H-2,6 3J = 8.3 Hz 6.70 br NH2 | 111.45 C-3,5 126.44 C-2,6 136.00 C-1 156.00 C-4 |
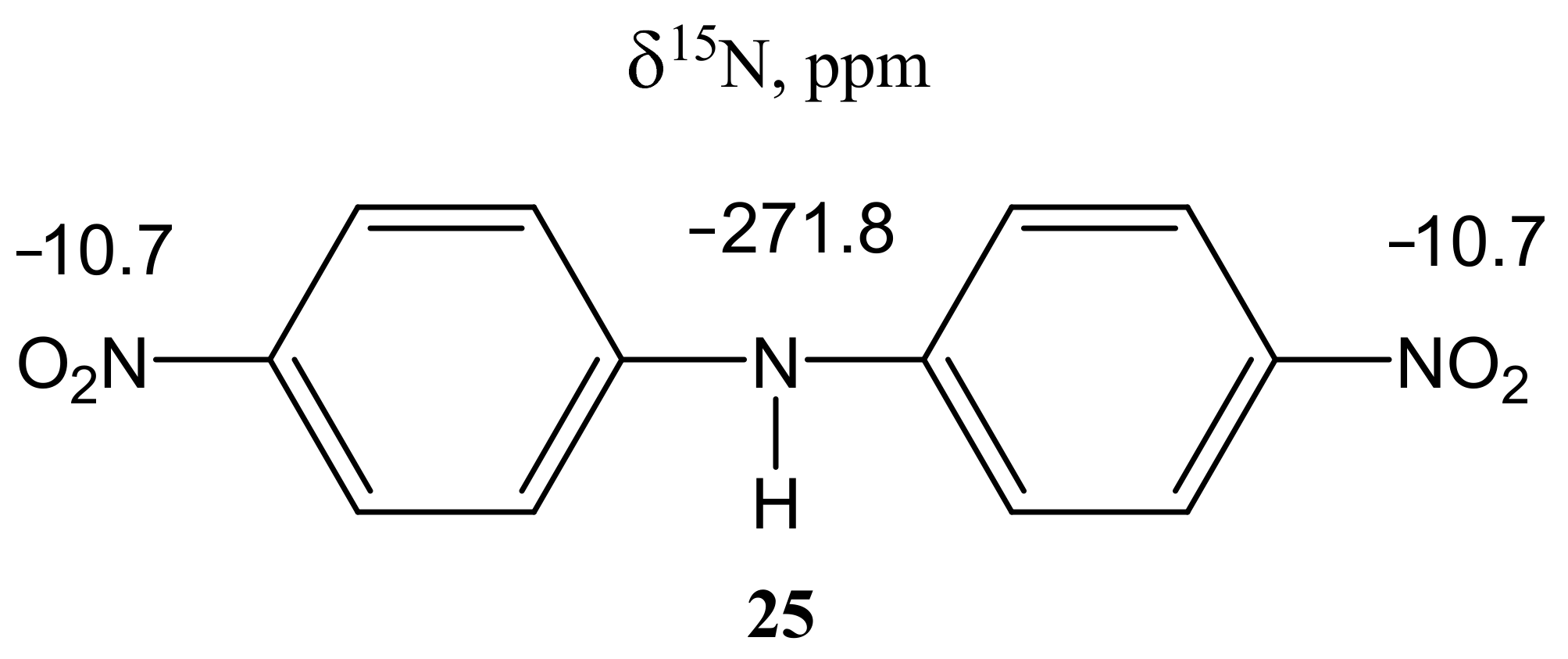
| 25 | 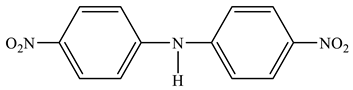 | 7.35 d H-3,5 3J = 9.5 Hz 8.20 d H-2,6 3J = 9.5 Hz | 117.04 C-3,5 125.77 C-2,6 140.56 C-1 147.60 C-4 |
| 26 | 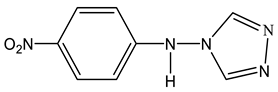 | 6.59 d H-3,5 3J = 9.2 Hz 8.16 d H-2,6 3J = 9.2 Hz 8.87 s H-3′,5′ 10.52 br NH | 111.70 C-3,5 126.13 C-2,6 140.05 C-1 144.08 C-3′,5′ 156.00 C-4 |
| 27 | 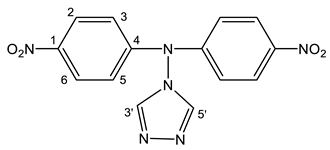 | 6.79 d H-3,5 3J = 9.0 Hz 7.44 d H-2,6 3J = 9.0 Hz 8.61 s H-3′,5′ | 113.22 C-3,5 129.42 C-2,6 140.95 C-1 142.67 C-3′,5′ 158.08 C-4 |
| Compd | R | δ 1H | δ 13C | δ 15N | |||||
|---|---|---|---|---|---|---|---|---|---|
| H-4 | CH3 | C-3 | C-4 | C-5 | CH3 | N-1 | N-2 | ||
| 28a | CH3 | 5.94 | 3.72 | 148.00 | 103.77 | 127.03 | 35.53 | ||
| 28b | C2H5 | 5.62 | 3.54 | ||||||
| 28c | C3H7 | 5.97 | 3.75 | 152.95 | 103.10 | 127.10 | 35.84 | −188.2 | −78.4 |
| 28d | i-C3H7 | 5.99 | 3.73 | ||||||
| 28e | CH2Cl | 6.24 | 3.78 | 118.40 | 103.97 | 127.86 | 38.61 | −183.8 | −74.4 |
| 28f a | CF3 | 6.43 | 3.84 | 141.80 | 103.40 | 128.89 | 36.78 | −178.5 | −74.3 |
| 28g | C6H5 | 6.44 | 3.81 | 101.84 | 128.00 | ||||
| 28h | 4-CH3C6H4 | 6.44 | 3.85 | 150.96 | 101.63 | 128.09 | 36.30 | −184.8 | −81.6 |
| 28i | 4-CH3OC6H4 | 6.39 | 3.79 | 150.75 | 101.30 | 128.05 | 36.19 | −185.3 | −82.8 |
| 28j | 4-BrC6H4 | 6.44 | 3.85 | 149.54 | 101.65 | 128.25 | 36.24 | −183.2 | −80.3 |
| 28k | 4-ClC6H4 | 6.39 | 3.81 | 149.81 | 101.86 | 128.00 | 36.42 | −184.8 | −81.6 |
| 28l b | 4-NO2C6H4 | 6.58 | 3.90 | 148.45 | 102.83 | 129.11 | 36.66 | −180.0 | −76.8 |
| 28m c | 3-NO2C6H4 | 6.58 | 3.90 | 148.21 | 102.01 | 129.43 | 36.33 | −181.3 | −78.0 |
| Structure | 1H | 13C | 15N * | |
|---|---|---|---|---|
| 29 | 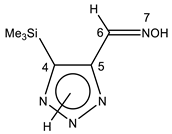 | 0.39 SiMe3 8.21 CH=N 11.30 br OH 15.1 br NH | -0.50 SiMe3 132.7 C-4 142.5 CH=N 1JCH = 164.0 Hz 147.3 C-5 | −5.6 N-7 −27.9 N-1 |
| 30 | 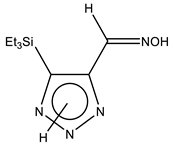 | 0.87 Me3 0.97 SiCH2 8.20 CH=N 11.24 br OH 15.08 br NH | 3.84 SiCH2 7.06 Me3 129.03 C-4 141.33 CH=N 1JCH = 164.7 Hz 146.68 C-5 | −5.8 N-7 −7.7 N-2 −120.0 N-3 1JNH = 101.6 Hz |
| 31 | 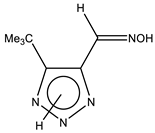 | 1.35 Me3 8.25 CH=N 11.40 br OH 14.75 br NH | 28.98 CH3 59.74 C- CH3 136.93 C-5 141.68 CH=N 1JC-H = 165.8 Hz 153.09 C-4 | −3.1 N-7 −48.5 N-1 −132.0 N-3 1JNH = 114.2 Hz |
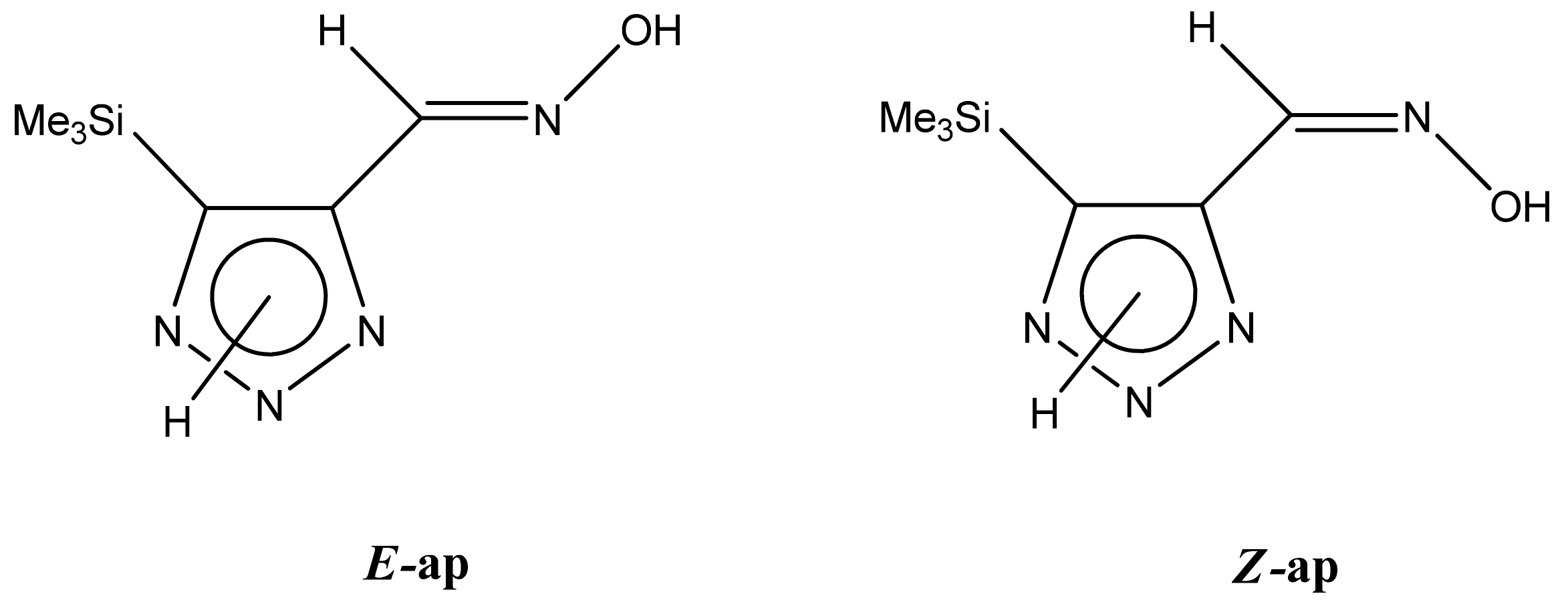
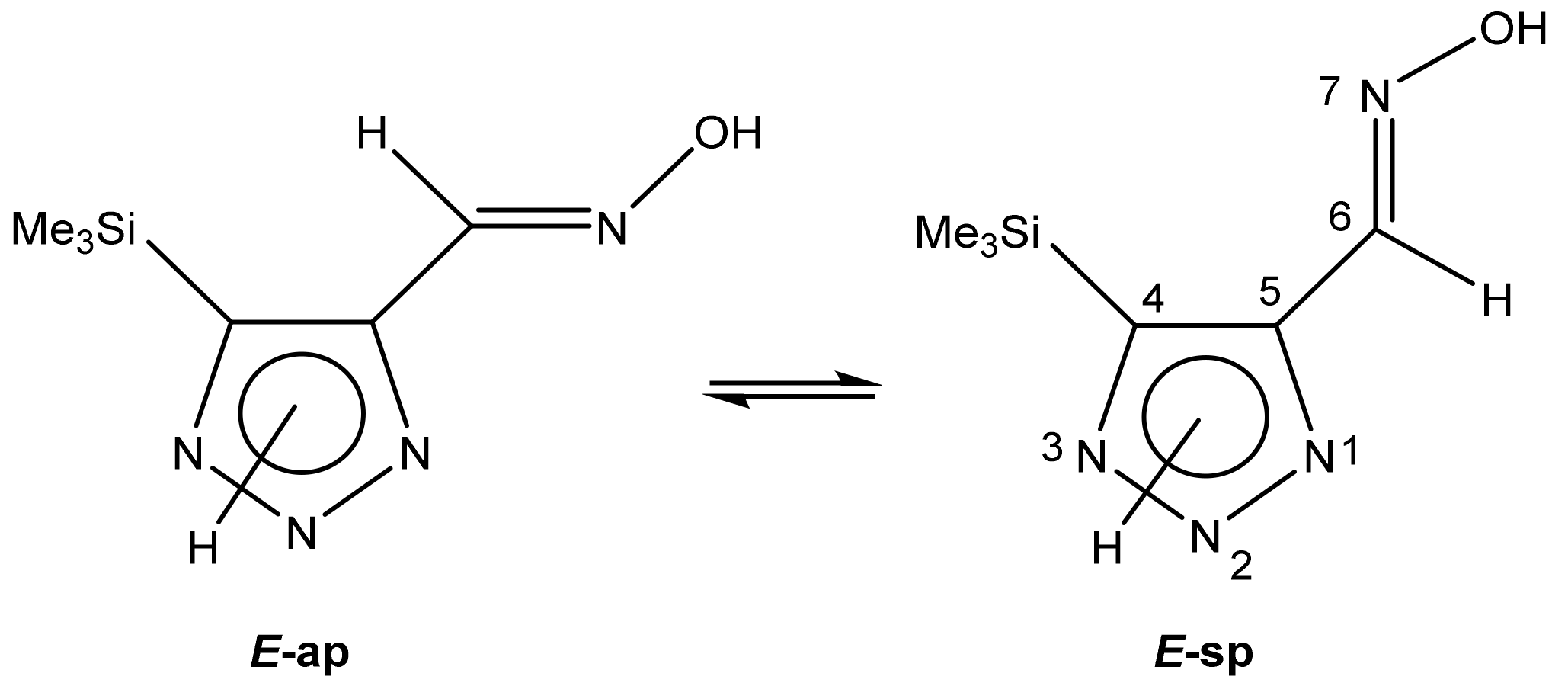
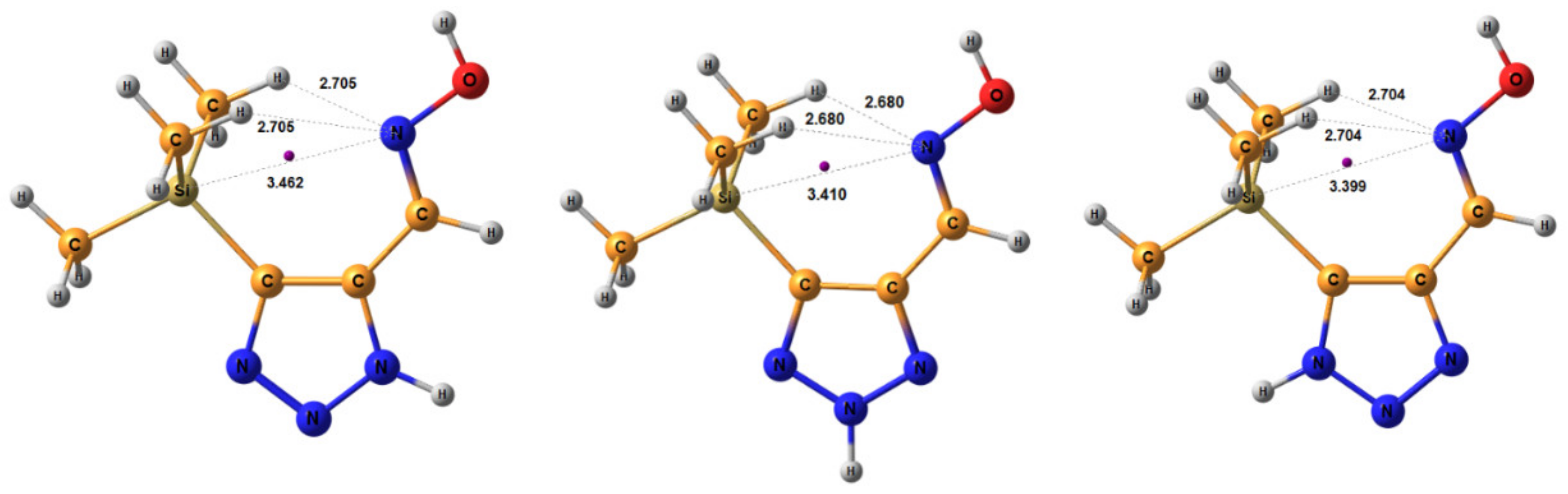
| Conformer | Structure | E, Hartree | ΔE, kcal/mol * |
|---|---|---|---|
| E-ap | 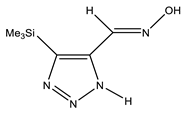 | −818.1342766 | 4.71 |
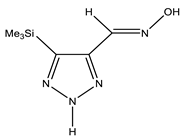 | −818.1355085 | 3.91 | |
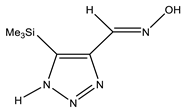 | −818.1316053 | 6.45 | |
| Z-ap | 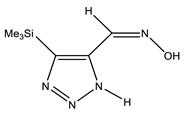 | −818.1375023 | 2.61 |
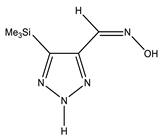 | −818.1376553 | 2.51 | |
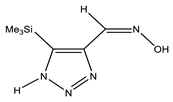 | −818.1368360 | 3.05 | |
| E-sp | 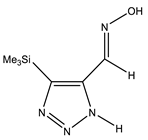 | −818.1317761 | 6.34 |
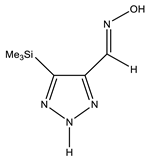 | −818.1415116 | 0 | |
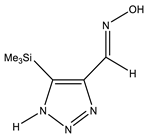 | −818.1395942 | 1.25 | |
| Z-sp | 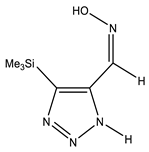 | −818.1278248 | 8.91 |
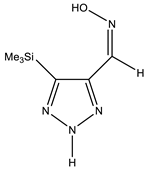 | −818.1362968 | 3.40 | |
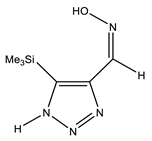 | −818.1363759 | 3.34 |
| Structure | 1H | 13C | 15N | ||||
|---|---|---|---|---|---|---|---|
| A | 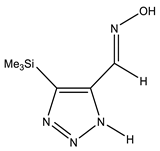 | NH OH N=CH CH3 | 11.37 7.84 7.12 −0.70 | CH3 C-4 C-5 N=CH | 13.5 147.3 141.7 139.1 | N-1 N-2 N-3 N-7 | −149.2 13.4 18.9 38.3 |
| B | 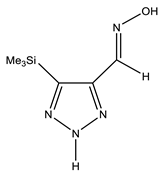 | NH OH N=CH CH3 | 11.48 7.06 8.23 0.30 | CH3 C-4 C-5 N=CH | 0.2 150.9 151.1 141.1 | N-1 N-2 N-3 N-7 | −48.8 −130.0 −31.9 −3.4 |
| C | 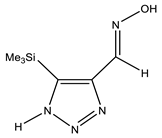 | NH OH N=CH CH3 | 11.15 7.05 8.17 0.30 | CH3 C-4 C-5 N=CH | −0.3 135.3 150.3 140.3 | N-1 N-2 N-3 N-7 | −8.6 6.8 −143.2 −5.4 |
| Compound | 1H | 13C | |
|---|---|---|---|
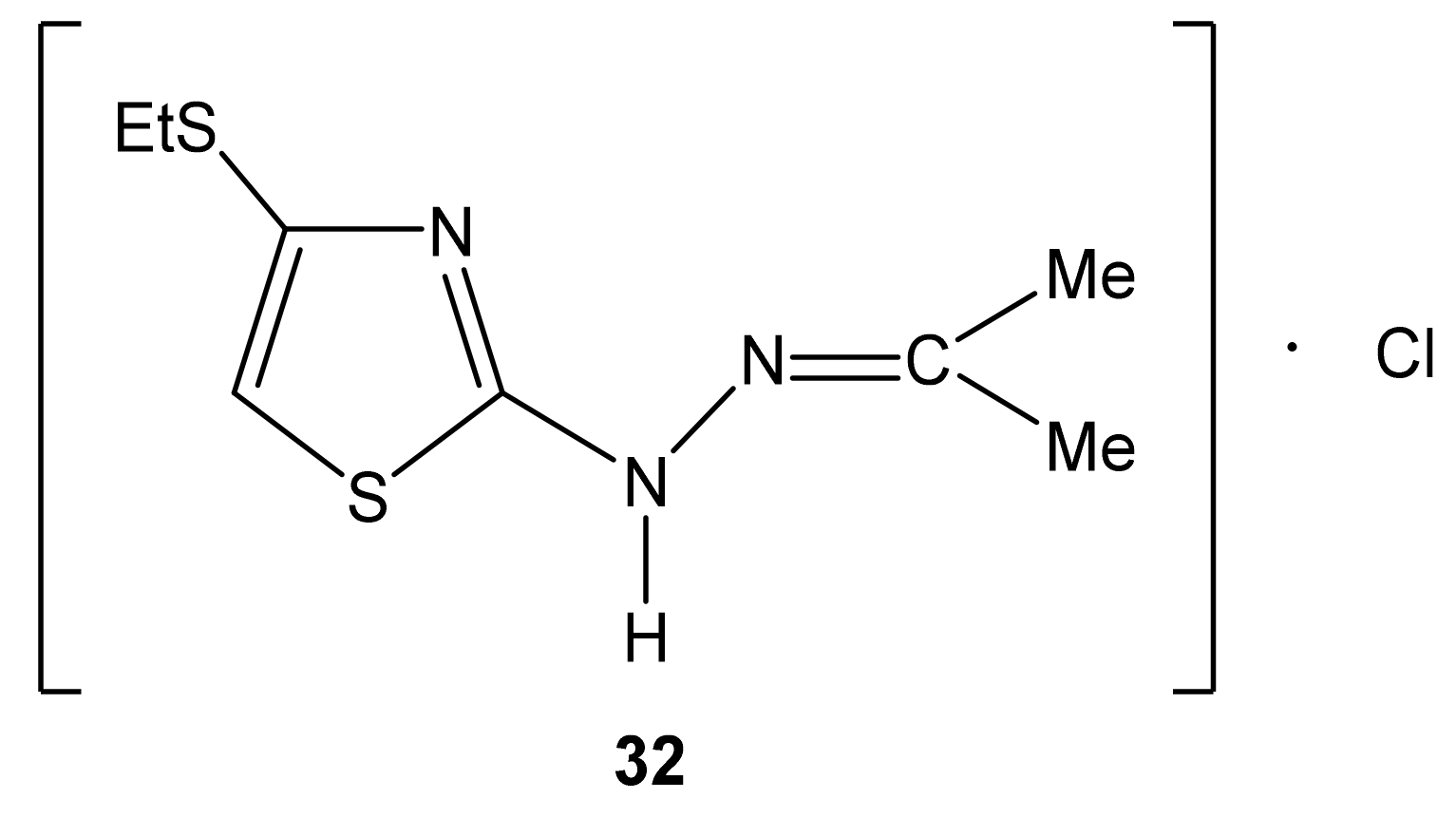
| 32 | 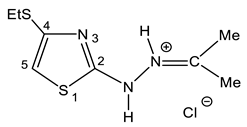 | 1.20 t (3H, Me 1.92 s (3H, Me) 2.29 s (3H, Me) 2.88 q (2H, CH2S) 7.14 s (1H, H-5) 9.93 br (2H, NH) | 13.74 (S-Me) 20.46 (Me) 24.97 (Me) 27.38 (SCH2) 106.51 C-5 130.12 C-4 161.10 C-2 189.43 C=N |
| 33 | 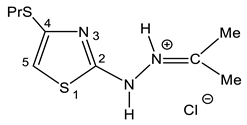 | 0.93 t (3H, Me) 1.18 t (3H, Me 1.55 m (2H, CH2) 1.89 s (3H, Me 2.64 q (2H, CH2) 2.84 q (2H, SCH2) 7.14 s (1H, H-5) 9.95 br (2H, NH) | 13.74 20.46 (Me) 27.38 (Et) 24.97 (Me) 107.05 C-5 130.23 C-4 161.03 C-2 192.42 C=N |
| 34 | 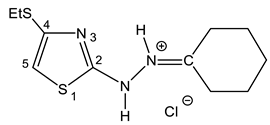 | 1.21 t (3H, Me) 1.51-1.59 m 2.11 t, 2.50-2.60 cycle 2.81 q (SCH2) 7.05 s (1H, H-5) 9.85 br (2 NH) | 13.70, 27.39 (Et) 24.33, 26.33, 27.39, 30.23, 34.81(cycle) 106.5 C-5 130.3 C-4 161.27 C-2 193.1 C=N |
| 35 |  | 1.21 t (Me) 1.92 s (3H, Me) 2.31 s (3H, Me) 2.87 q (SCH2) 7.11 s (1H, H-5) 9.88 br (NH) | 20.38, 24.36 (Me) 13.73, 26.45 Et 96.0 C-5 128.7 C-4 156.25 C-2 179.2 C=N |
| Compound | δ1H | δ13C/nJ(CH) | δ15N |
|---|---|---|---|
 36 | 2.19 s CH3 9.13 s H-5 12.6 br NH | 22.49 q CH3 1J = 129.0 148.54 d C-5 1J = 212.0 158.56 C-2 2J = 4.0 168.70 C=O 2J = 6.4 | −19.9 N-4 −55.5 N-3 −242.3 NH |
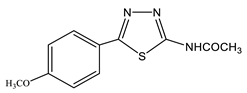 37 | 2.21 s CH3 3.83 s OCH3 7.05 d H-3′,5′ 3J = 8.8 7.81 d H-2′,6′ 3J = 8.8 12.9 br NH | 22.40 d CH3 1J = 129.4 55.73 d CH3O 1J = 144.6 115.13 C-3′,5′ 1J = 161.6, 2J = 4.7 123.68 C-1′ 1J = 161.0, 2J = 7.2 128.68 C-2′,6′ 158.04 C-2 161.52 C-4′ 162.08 C-5 168.83 C=O | |
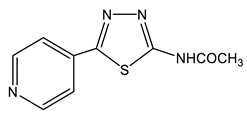 38 | 2.34 s CH3 7.89 d H-2′,6′ 3J = 5.8 8.72 d H-3′,5′ 3J = 5.8 11.8 br NH | 22.32 CH3 120.69 C-2′,6′ 137.14 C-1′ 150.61 C-3′,5′ 159.48 C-5 159.53 C-2 168.81 C=O | −19.9 N-4 −55.5 N-3 −162.5 Npyr −242.3 NH |
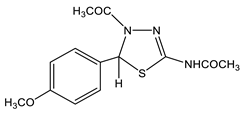 39 | 2.03 s CH3 2.17 s CH3(NH) 3.72 s OCH3 6.77 s H-5 6.88 d H-2′,6′ 3J = 8.5 7.17 d H-3′,5′ 3J = 8.5 11.7 br NH | 21.94 q CH3 1J = 129.0 22.45 q CH3(NH) 1J = 129.0 55.22 q OCH3 1J = 144.2 65.68 C-5 dt 1J = 159.5, 3J = 4.0 114.05 dd C-3′,5′ 1J = 160.6, 3J = 4.8 126.70 ddd C-2′,6′ 1J = 158.2, 2,3J = 6.8,3.6 133.56 dd C-1′ 2J = 8.4, 2J = 7.2 146.06 d C-2 2J = 4.1, 159.12 d C-4′ 2J = 8.8 167.33 q C=O 2J = 6.3 169.39 qd C=O(NH) 2J = 6.5, 3J = 2.3 | −113.5 N-3 −194.8 N-4 −243.8 NH |
 40 | 2.04 s CH3 2.24 s CH3(NH) 6.85 s H-5 7.26 d H-2′,6′ 3J = 8.7 8.56 d H-3′,5′ 3J = 8.7 11.8 br NH | 21.67 q CH3 1J = 129.0 22.43 q CH3(NH) 1J = 129.8 64.56 dt C-5 1J = 161.4 3J = 4.4 119.87 d C-2′,6′ 1J = 163.6 145.80 d C-2 2J = 4.8 149.45 t C-1′ 2J = 6.0 150.06 dd C-3′,5′ 1J = 179.8, 2J = 11.2 167.66 q C=O 2J = 6.4 169.52 q C=O (NH) 2J = 6.5 | −113.6 N-3 −172.4 Npyr −198.8 N-4 −243.6 NH |
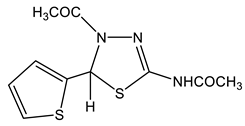 41 | 2.06 CH3 2.16 CH3 (NH) 6.94 H-4′ 7.07 H-3′ 7.11 H-5 7.44 H-3′ 11.7 br NH | 21.71, 22.48 61.46 C-5 125.23 C-5′ 126.11 C-4′ 126.74 C-3′ 144.54 C-2′ 146.12 C-2 167.22, 169.43 C=O | −115.1 N-3 −199.2 N-4 −243.4 NH |
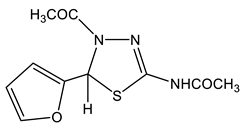 42 | 2.08 CH3 2.21 CH3 (NH) 6.31 H-3′ 6.39 H-4′ 6.90 H-5 7.59 H-5′ 11.6 br NH | 21.86 q CH3 1J = 129.4 22.56 q CH3 (NH) 1J = 129.4 59.38 q C-5 1J = 159.8 107.19 dt C-4′ 1J = 176.6, 2J = 3.2 110.69 ddd C-3′ 1J = 176.1, 2,3J = 13.6, 4.0 143.17 ddd C-5′ 1J = 204.5, 2,3J = 11.2, 7.6 145.87 d C-2 2J = 4.8 151.60 dt C-2′ 2J = 17.2, 2J = 7.2 167.42 q C=O 2J = 6.4 169.55 C=O (NH) 2J = 6.4 | −117.1 N-3 −196.2 N-4 −246.4 NH |
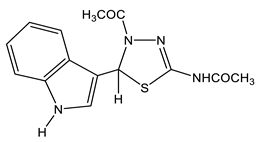 43 | 2.07, 2.15 CH3 7.00 H-6′ 7.11 H-5′ 7.15 H 5 7.30 H-2 7.38 H-4′ 7.49 H-7′ 11.09 NH-indol 11.7 br NH | 22.02 q CH3 (N-4) 1J = 129.0 22.69 q CH3 (NH) 1J = 129.4 60.99 d C-5 1J = 158.2 111.93 dd C-7′ 1J = 159.4, 2J = 7.6 114.94 C-3′ 2J = 8.4, 2J = 6.0 118.80 dd C-5′ 1J = 158.2, 2J = 7.6 119.15 dd C-6′ 1J = 158.6, 2J = 7.2 121.49 dd C-4′ 1J = 158.2, 2J = 7.6 123.63 dd C-2′ 1J = 182.6, 2J = 5.2 123.99 m C-9′ 136.82 dd C-8′ 2J = 9.2, 2J = 3.2 146.76 C-2 2J = 4.9 167.23 q C=O 2J = 6.4 169.32 q C=O (NH) 2J = 6.8 | −116.6 N-3 −192.3 N-4 −243.2 NH −245.4 NH |
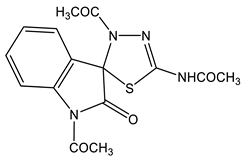 44 | 2.10 CH3(N-4) 2.15 CH3(NH) 2.56 CH3 7.27 dd H-6′ 3J = 8.1, 3J = 7.3 7.41 dd H-5′ 3J = 7.3, 3J = 7.2 7.47 d H-4′ 3J = 7.2 8.08 d H-7′ 3J = 8.2 12.0 br NH | 21.86 q CH3 (N-4) 1J = 129.8 22.33 q CH3 (NH) 1J = 129.0 26.05 q CH3 (N-1′) 1J = 130.6 75.13 d C-5 3J = 3.2 115.72 dd C-7′ 1J = 169.4, 2J = 7.2 123.96 dd C-6′ 1J = 164.6, 2J = 8.8 125.88 dd C-5′ | −115.9 N-3 −192.4 N-4 −200.6 N-1′ −245.5 NH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larina, L.I. The Structure of Biologically Active Functionalized Azoles: NMR Spectroscopy and Quantum Chemistry. Magnetochemistry 2022, 8, 52. https://doi.org/10.3390/magnetochemistry8050052
Larina LI. The Structure of Biologically Active Functionalized Azoles: NMR Spectroscopy and Quantum Chemistry. Magnetochemistry. 2022; 8(5):52. https://doi.org/10.3390/magnetochemistry8050052
Chicago/Turabian StyleLarina, Lyudmila I. 2022. "The Structure of Biologically Active Functionalized Azoles: NMR Spectroscopy and Quantum Chemistry" Magnetochemistry 8, no. 5: 52. https://doi.org/10.3390/magnetochemistry8050052
APA StyleLarina, L. I. (2022). The Structure of Biologically Active Functionalized Azoles: NMR Spectroscopy and Quantum Chemistry. Magnetochemistry, 8(5), 52. https://doi.org/10.3390/magnetochemistry8050052








