Cell Behavioral Changes after the Application of Magneto-Mechanical Activation to Normal and Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
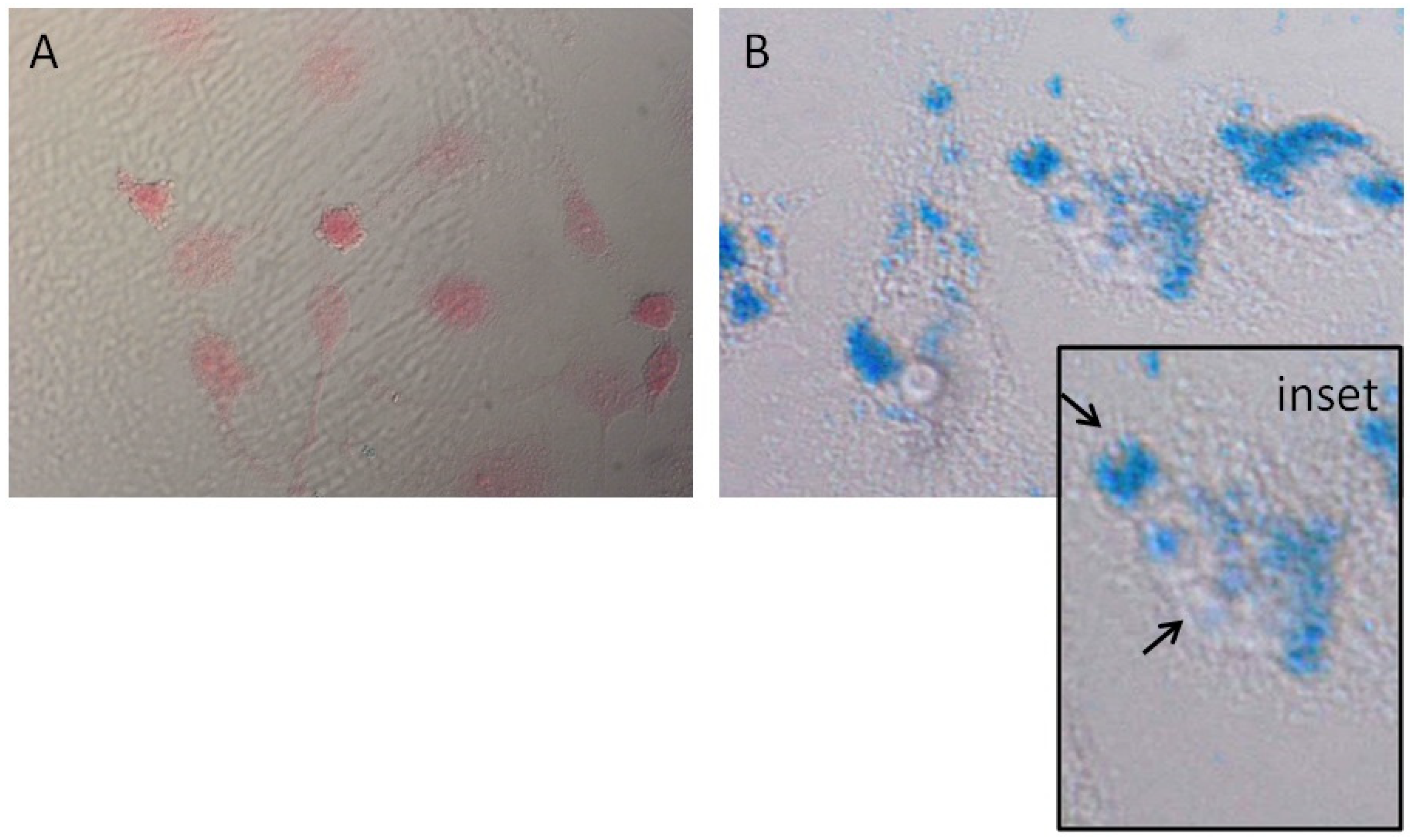
2.3. Prussian Blue Staining
2.4. Magneto-Mechanical Treatment of Cells
2.5. MTT Test
2.6. Double Staining for F-Actin and Cell Nuclei
3. Results and Discussion
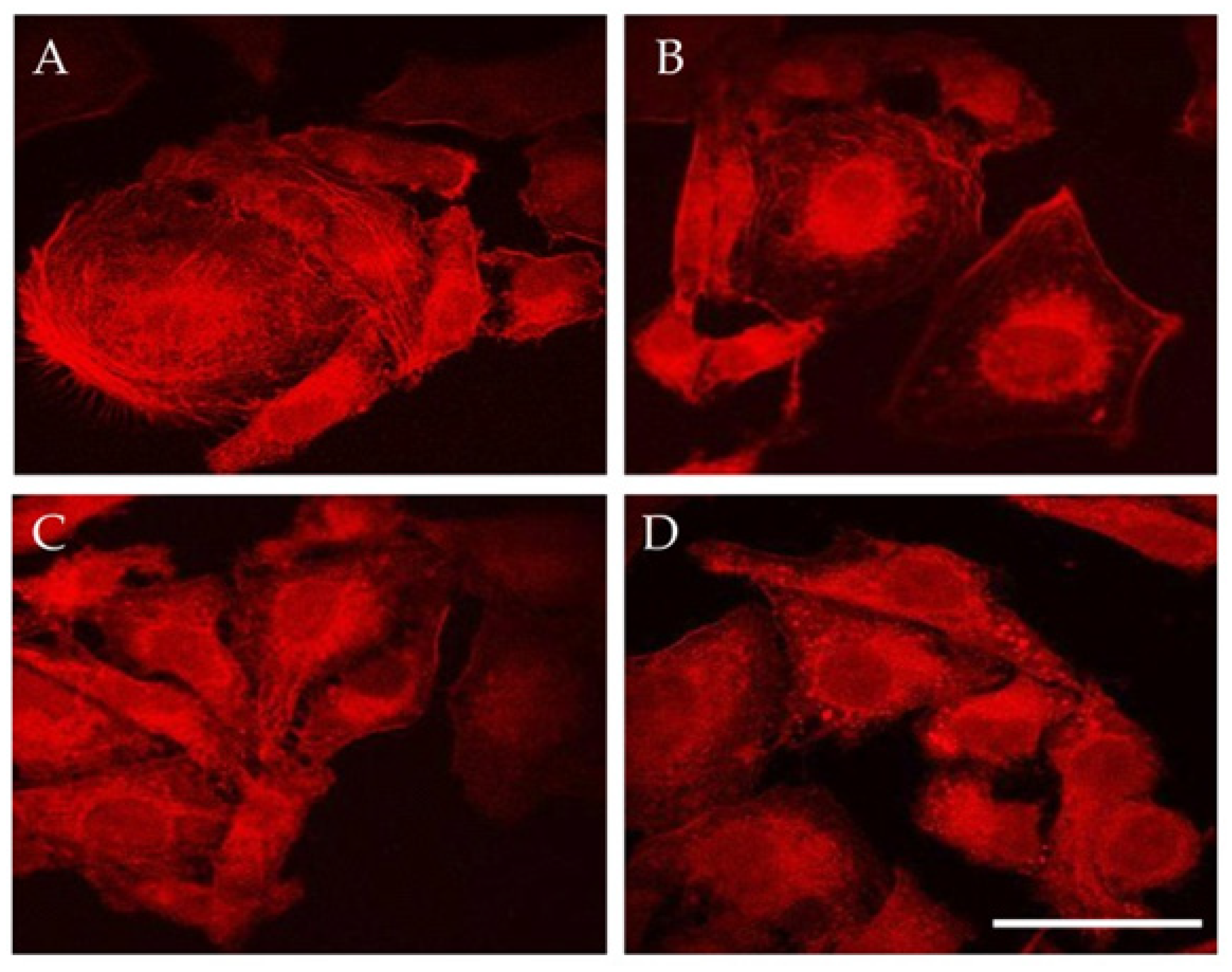
3.1. MNPs Internalization
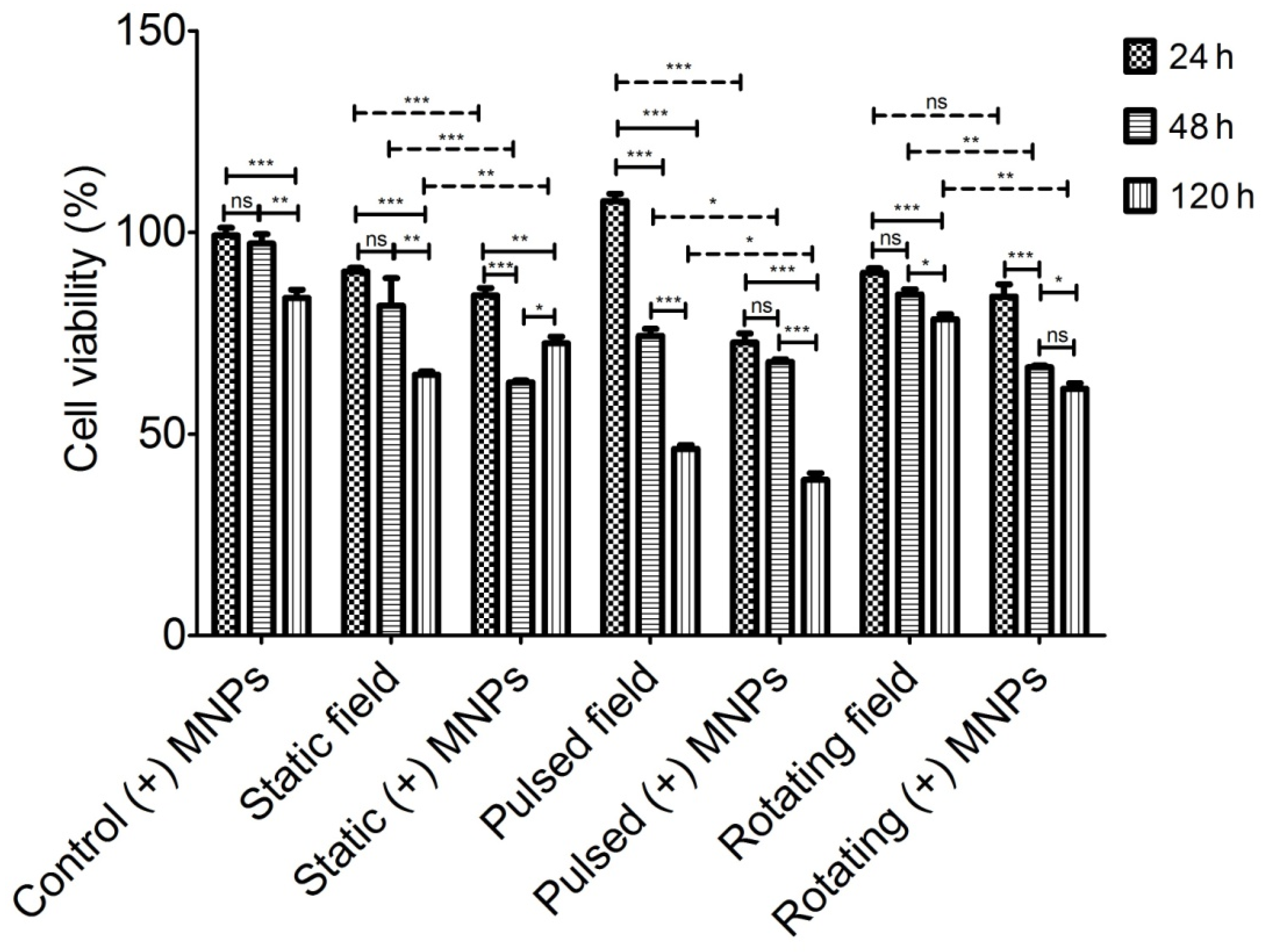
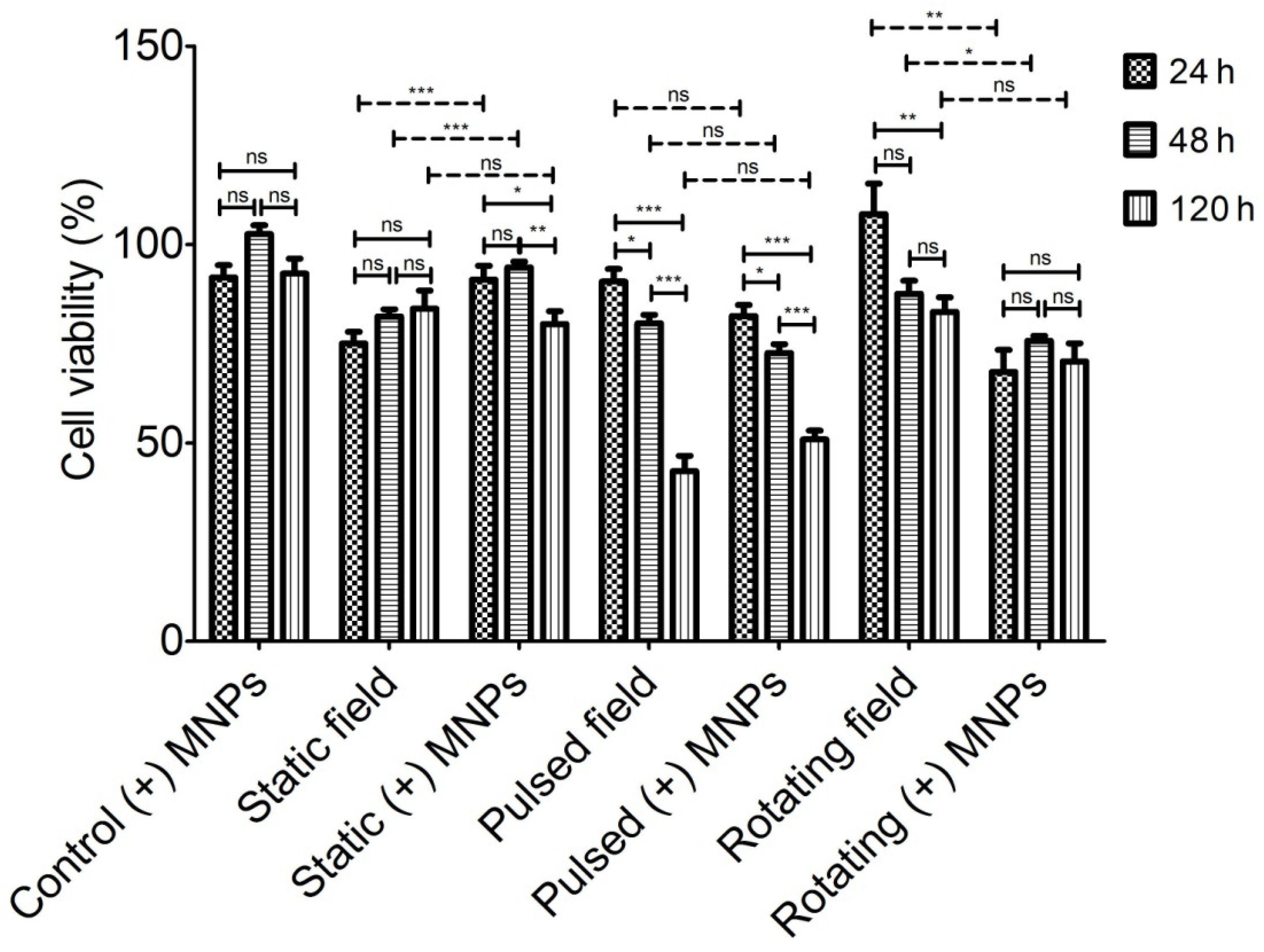
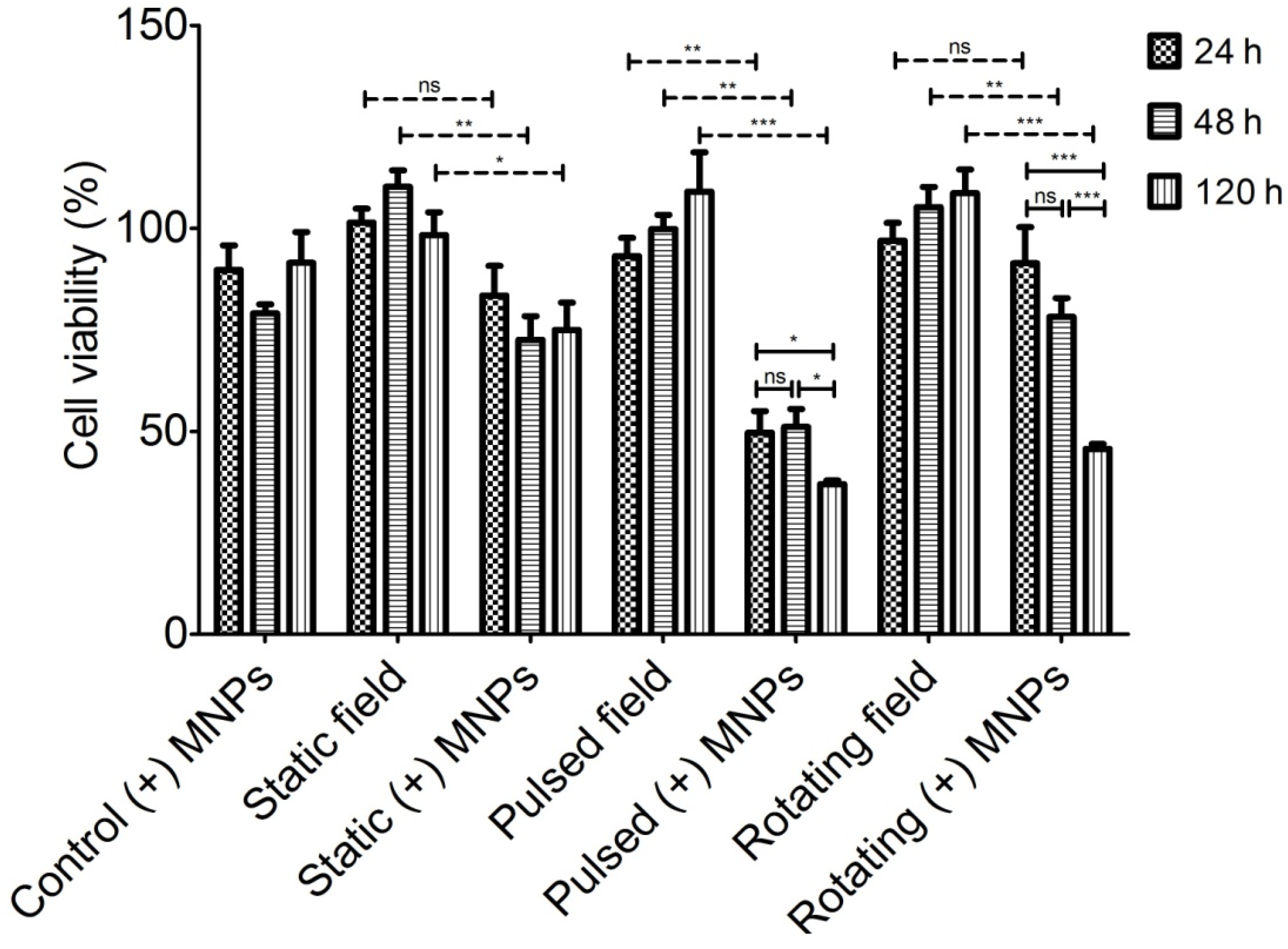
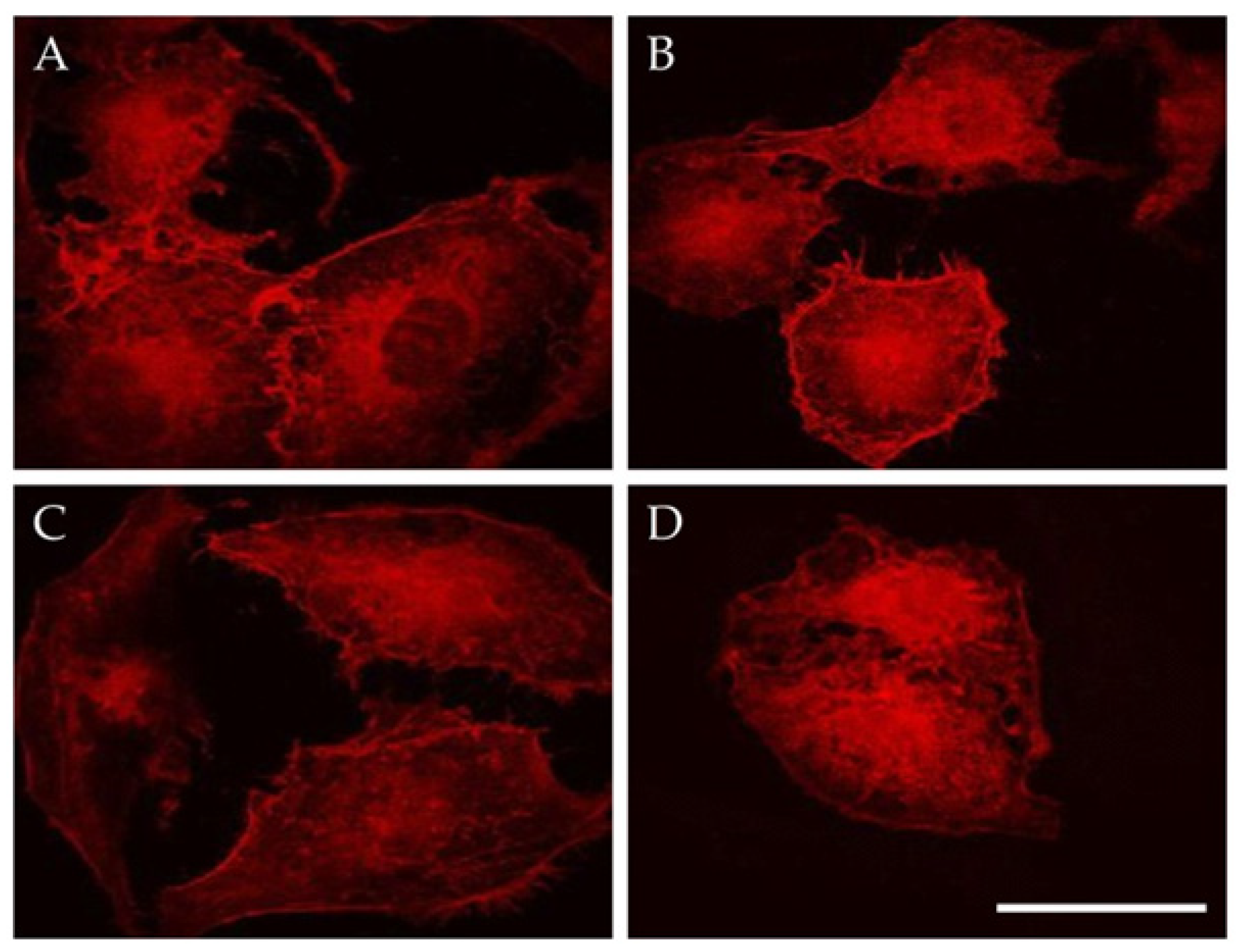
3.2. Magneto-Mechanical Activation
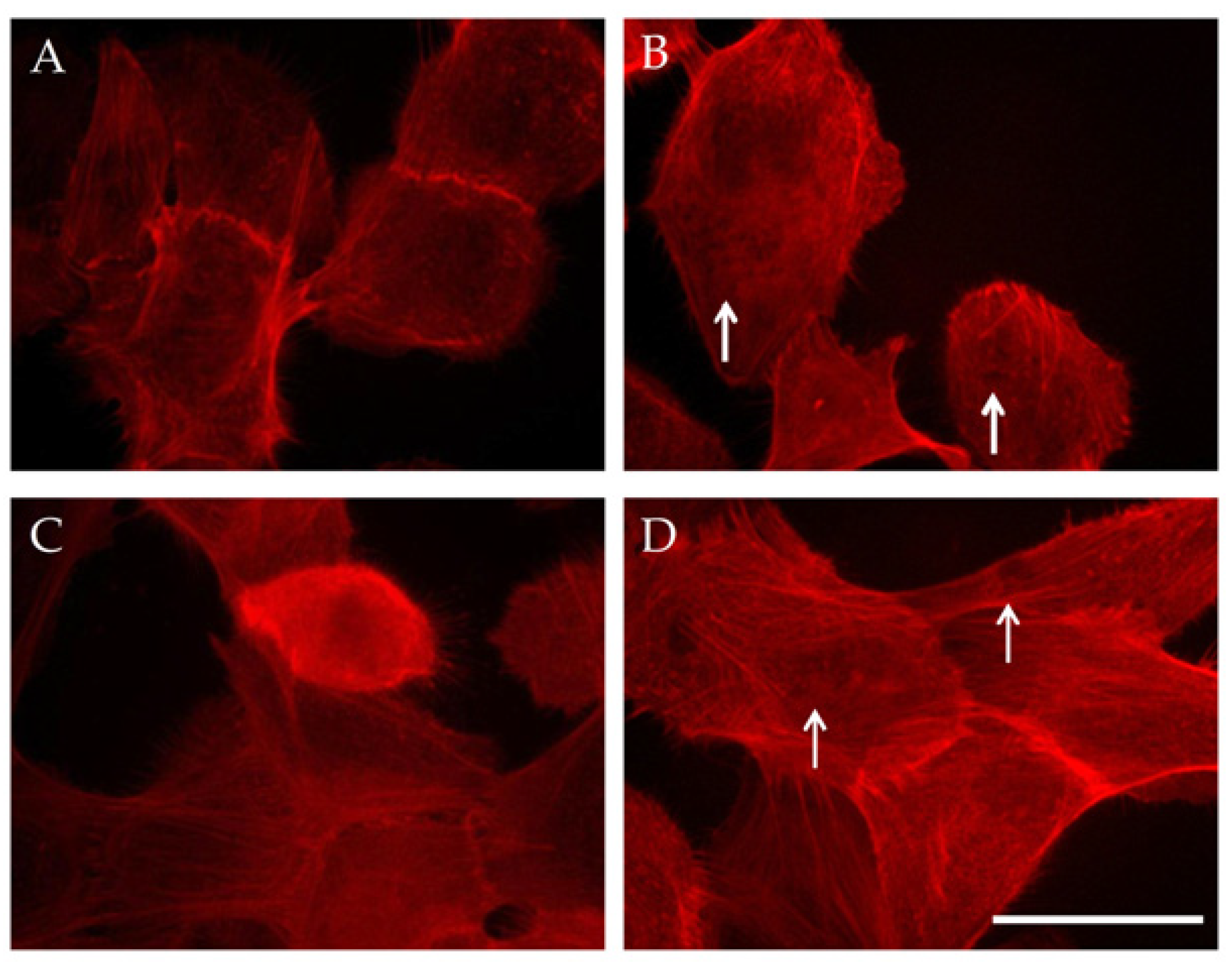
3.3. Combined Effect of PMF, RMF, and MNPs on Actin Cytoskeleton and Cell Nuclei
3.3.1. Actin Cytoskeleton
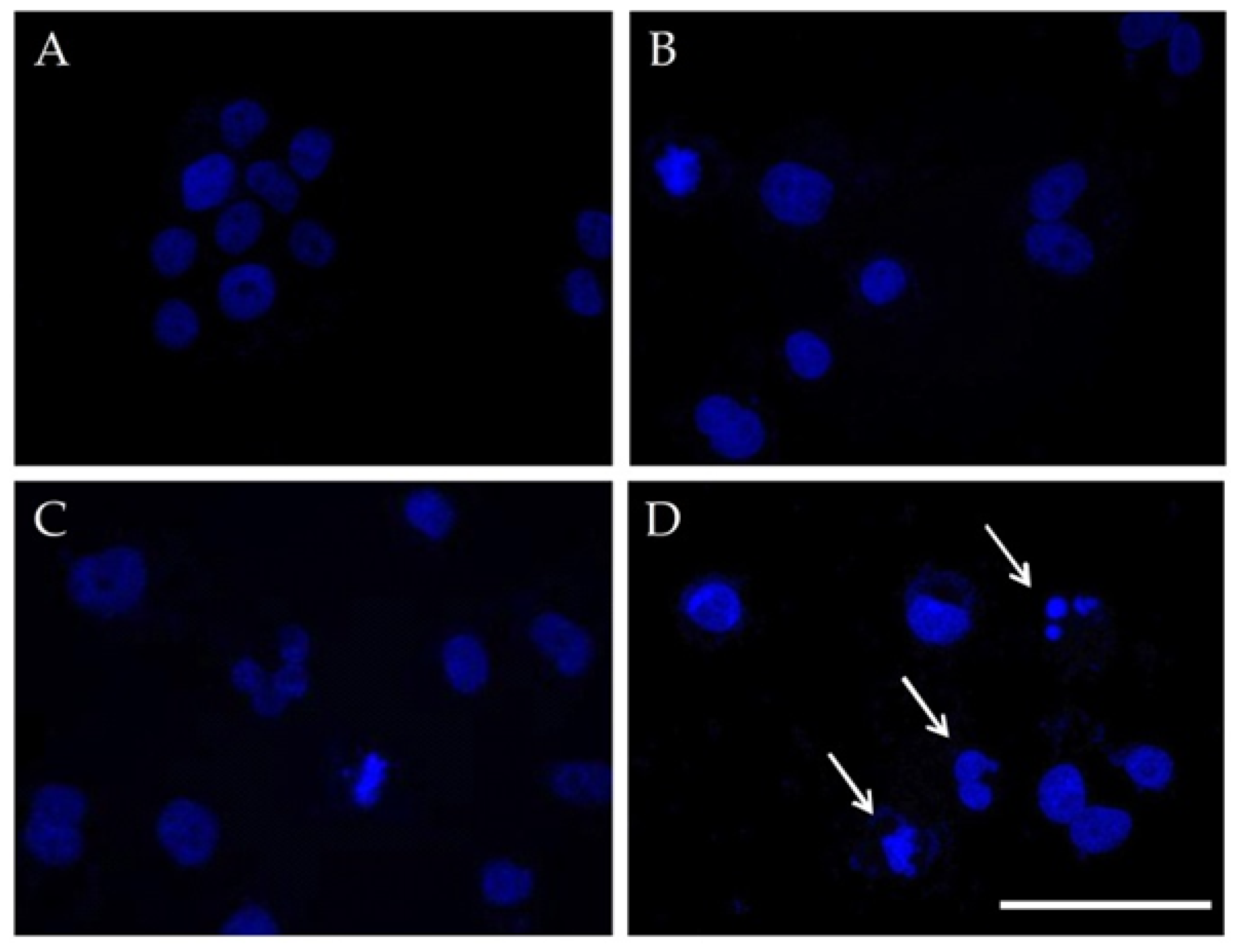
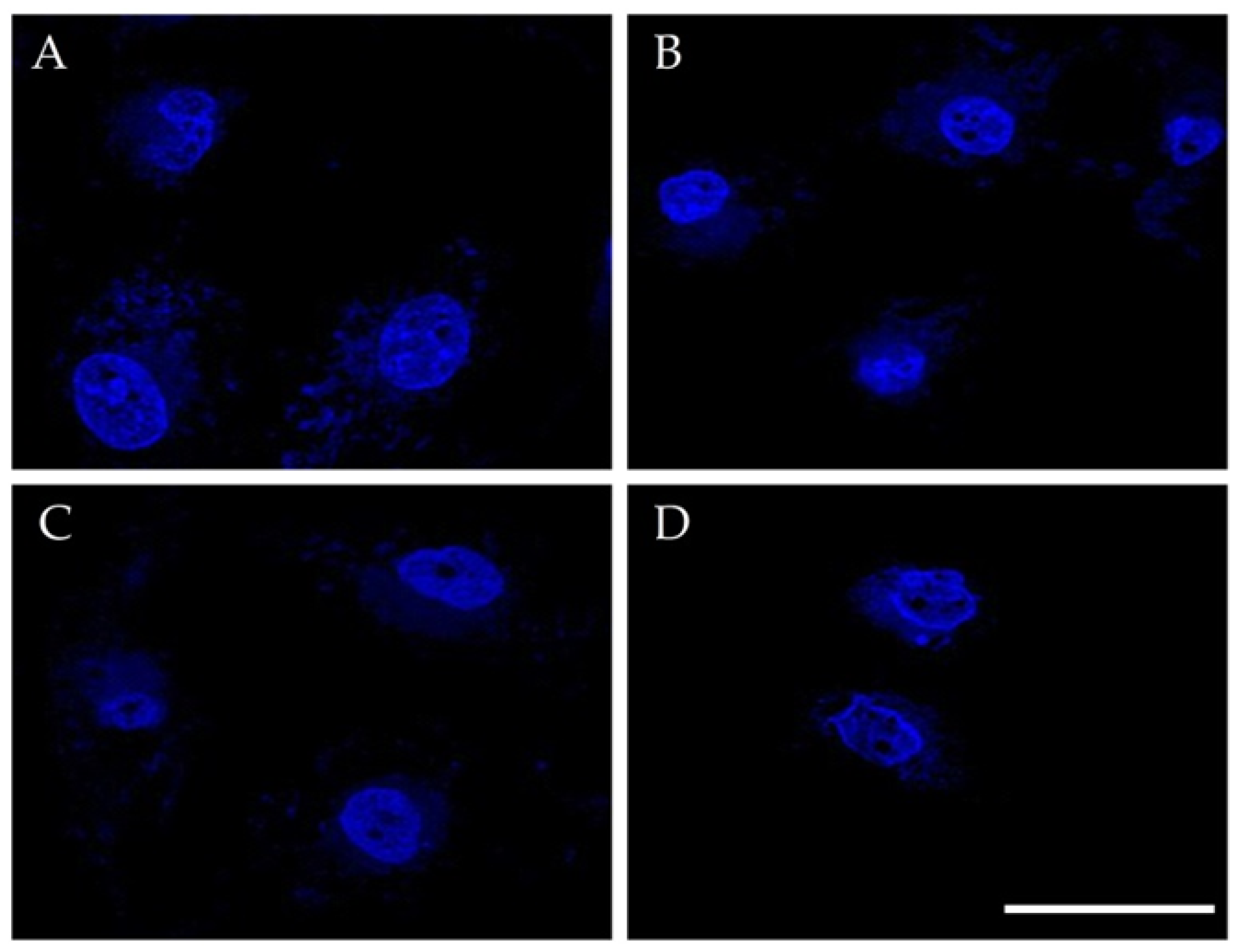
3.3.2. Cell Nuclei Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hose, O.; Tertis, M.; Cristea, C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, J.; Zhang, Y.; Xu, W.; Xiao, J.; Suo, A. Growth of MCF-7 breast cancer cells and efficacy of anti-angiogenic agents in a hydroxyethyl chitosan/glycidyl methacrylate hydrogel. Cancer Cell Int. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola-Leyva, A.; Jabalera, Y.; Chico-Lozano, M.A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Reactive oxygen species (ROS) production in HepG2 cancer cell line through the application of localized alternating magnetic field. J. Mater. Chem. B 2020, 8, 7667–7676. [Google Scholar] [CrossRef] [PubMed]
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.; Malatesta, M.; Calderan, L.; et al. Improving the Cellular Uptake of Biomimetic Magnetic Nanoparticles. Nanomaterials 2021, 11, 766. [Google Scholar] [CrossRef]
- Hepel, M. Magnetic Nanoparticles for Nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Sarella, A.; Torti, A.; Donolato, M.; Pancaldi, M.; Vavassori, P. Two-dimensional programmable manipulation of magnetic nanoparticles on-chip. Adv. Mater. 2014, 26, 2384–2390. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Wang, P.; Song, T. Mechanisms of Cellular Effects Directly Induced by Magnetic Nanoparticles under Magnetic Fields. J. Nanomater. 2017, 2017, 1564634. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.; Miaskowski, A.; Jenkins, S.I.; Lim, J.; Dobson, J. Remote manipulation of magnetic nanoparticles using magnetic field gradient to promote cancer cell death. Appl. Phys. A 2019, 125, 226. [Google Scholar] [CrossRef] [Green Version]
- Maniotis, N.; Makridis, A.; Myrovali, E.; Theopoulos, A.; Samaras, T.; Angelakeris, M. Magneto-mechanical action of multimodal field configurations on magnetic nanoparticle environments. J. Magn. Magn. Mater. 2019, 470, 6–11. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Makridis, A.; Maniotis, N.; Karypidou, N.; Myrovali, E.; Samaras, T.; Angelakeris, M.; Chlichlia, A.; Kalogirou, O. Effect of low frequency magnetic fields on the growth of MNP-treated HT29 colon cancer cells. Nanotechnology 2018, 29, 175101. [Google Scholar] [CrossRef]
- Goiriena-Goikoetxea, M.; Muñoz, D.; Orue, I.; Fernández-Gubieda, M.L.; Bokor, J.; Muela, A.; García-Arribas, A. Disk-shaped magnetic particles for cancer therapy. Appl. Phys. Rev. 2020, 7, 011306. [Google Scholar] [CrossRef]
- Zhang, E.; Kircher, M.F.; Koch, M.; Eliasson, L.; Goldberg, S.N.; Renström, E. Dynamic magnetic fields remote-control apoptosis via nanoparticle rotation. ACS Nano 2014, 8, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rozhkova, E.A.; Ulasov, I.V.; Bader, S.D.; Rajh, T.; Lesniak, M.S.; Novosad, V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2010, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Golovin, Y.I.; Golovin, D.Y.; Vlasova, K.Y.; Veselov, M.M.; Usvaliev, A.D.; Kabanov, A.V.; Klyachko, N.L. Non-Heating Alternating Magnetic Field Nanomechanical Stimulation of Biomolecule Structures via Magnetic Nanoparticles as the Basis for Future Low-Toxic Biomedical Applications. Nanomaterials 2021, 11, 2255. [Google Scholar] [CrossRef] [PubMed]
- Golovin, Y.I.; Gribanovsky, S.L.; Golovin, D.Y.; Klyachko, N.L.; Majouga, A.G.; Master, А.M.; Sokolsky, M.; Kabanov, A.V. Towards nanomedicines of the future: Remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J. Control. Release 2015, 219, 43–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://www.chemicell.com/products/protocols/fluidd/index.html (accessed on 28 December 2021).
- Makridis, A.; Tziomaki, M.; Topouridou, K.; Yavropoulou, M.P.; Yovos, J.G.; Kalogirou, O.; Samaras, T. A novel strategy combining magnetic particle hyperthermia pulses with enhanced performance binary ferrite carriers for effective in vitro manipulation of primary human osteogenic sarcoma cells. Int. J. Hyperthermia 2016, 32, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Uzunova, V.; Tsiapla, A.R.; Stoyanova, T.; Myrovali, E.; Momchilova, A.; Kalogirou, O.; Tzoneva, R. Biocompatibility of iron oxide nanoparticles. J. Chem. Technol. Metall. 2021, 56, 1187–1191. [Google Scholar]
- Creixell, M.; Bohorquez, A.C.; Torres-Lugo, M.; Rinaldi, C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011, 5, 7124–7129. [Google Scholar] [CrossRef]
- Wang, M.H.; Chen, K.W.; Ni, D.X.; Fang, H.-J.; Jang, L.-S.; Chen, C.-H. Effect of extremely low frequency electromagnetic field parameters on the proliferation of human breast cancer. Electromagn. Biol. Med. 2021, 40, 384–392. [Google Scholar] [CrossRef]
- Ispanixtlahuatl-Meráz, O.; Schins, R.P.F.; Chirino, Y.I. Cell type specific cytoskeleton disruption induced by engineered nanoparticles. Environ. Sci. Nano 2018, 5, 228–245. [Google Scholar] [CrossRef]
- Tzoneva, R. Influence of electric field on cell behavior. Electrotreatment of cells for biomedical applications. Asian J. Phys. 2014, 23, 789–814. [Google Scholar]
- Pehlivanova, V.; Tsoneva, I.; Tzoneva, R. Multiple effects of electroporation on the adhesive behavior of breast cancer cells and fibroblasts. Cancer Cell Int. 2012, 9, 9144. [Google Scholar]
- Tzoneva, R.; Uzunova, V.; Apostolova, S.; Krüger-Genge, A.; Neffe, A.T.; Jung, F.; Lendlein, A. Angiogenic potential of endothelial and tumor cells seeded on gelatin–based hydrogels in response to electrical stimulations. Clin. Hemorheol. Microcirc. 2017, 64, 941–949. [Google Scholar] [CrossRef]
- Master, A.; Williams, P.; Pothayee, N.; Zhang, R.; Vishwasrao, H.; Golovin, Y.I.; Riffle, J.S.; Sokolsky, M.; Kabanov, A.V. Remote Actuation of Magnetic Nanoparticles for Cancer Cell Selective Treatment through Cytoskeletal Disruption. Sci. Rep. 2016, 6, 33560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, X.M.; Yang, X.X.; Zhang, X. Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 2017, 8, 13126–13141. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Hu, P.; Xiang, L.; Liu, Y.; He, R.; Lu, T. A Static Magnetic Field Inhibits the Migration and Telomerase Function of Mouse Breast Cancer Cells. Biomed Res. Int. 2020, 2020, 7472618. [Google Scholar] [CrossRef]
- Lin, T.; Wan, L.; Qi, X.; Shi, W.; Lin, J. A moderate static magnetic field enhances TRAIL-induced apoptosis by the inhibition of Cdc2 and subsequent downregulation of survivin in human breast carcinoma cells. Bioelectromagnetics 2014, 35, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Aljarrah, K.; Mhaidat, N.M.; Al-Akhras, M.A.H.; Aldaher, A.N.; Albiss, B.A.; Aledealat, K.; Alsheyab, F.M. Magnetic nanoparticles sensitize MCF-7 breast cancer cells to doxorubicin-induced apoptosis. World J. Surg. Oncol. 2012, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Ji, X.; Liu, J.; Li, Z.; Wang, W.; Chen, W.; Wang, J.; Liu, Q.; Zhang, X. Moderate intensity static magnetic fields affect mitotic spindles and increase the antitumor efficacy of 5-FU and Taxol. Bioelectrochemistry 2016, 109, 31–40. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiapla, A.-R.; Uzunova, V.; Oreshkova, T.; Angelakeris, M.; Samaras, T.; Kalogirou, O.; Tzoneva, R. Cell Behavioral Changes after the Application of Magneto-Mechanical Activation to Normal and Cancer Cells. Magnetochemistry 2022, 8, 21. https://doi.org/10.3390/magnetochemistry8020021
Tsiapla A-R, Uzunova V, Oreshkova T, Angelakeris M, Samaras T, Kalogirou O, Tzoneva R. Cell Behavioral Changes after the Application of Magneto-Mechanical Activation to Normal and Cancer Cells. Magnetochemistry. 2022; 8(2):21. https://doi.org/10.3390/magnetochemistry8020021
Chicago/Turabian StyleTsiapla, Aikaterini-Rafailia, Veselina Uzunova, Tsvetelina Oreshkova, Makis Angelakeris, Theodoros Samaras, Orestis Kalogirou, and Rumiana Tzoneva. 2022. "Cell Behavioral Changes after the Application of Magneto-Mechanical Activation to Normal and Cancer Cells" Magnetochemistry 8, no. 2: 21. https://doi.org/10.3390/magnetochemistry8020021
APA StyleTsiapla, A.-R., Uzunova, V., Oreshkova, T., Angelakeris, M., Samaras, T., Kalogirou, O., & Tzoneva, R. (2022). Cell Behavioral Changes after the Application of Magneto-Mechanical Activation to Normal and Cancer Cells. Magnetochemistry, 8(2), 21. https://doi.org/10.3390/magnetochemistry8020021







