Synergistic Effect of Combined Treatment with Magnetic Hyperthermia and Magneto-Mechanical Stress of Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Cultivation Conditions
2.3. Magnetic Hyperthermia (MHT) Set-Up and Treatment
2.4. Magneto-Mechanical (MM) Set-Up and Treatment
2.5. Combined Treatment of Cells with MHT and MM
2.6. Cell Viability Assay
2.7. Cell Morphology Analysis
2.8. Statistical Analysis
3. Results and Discussion
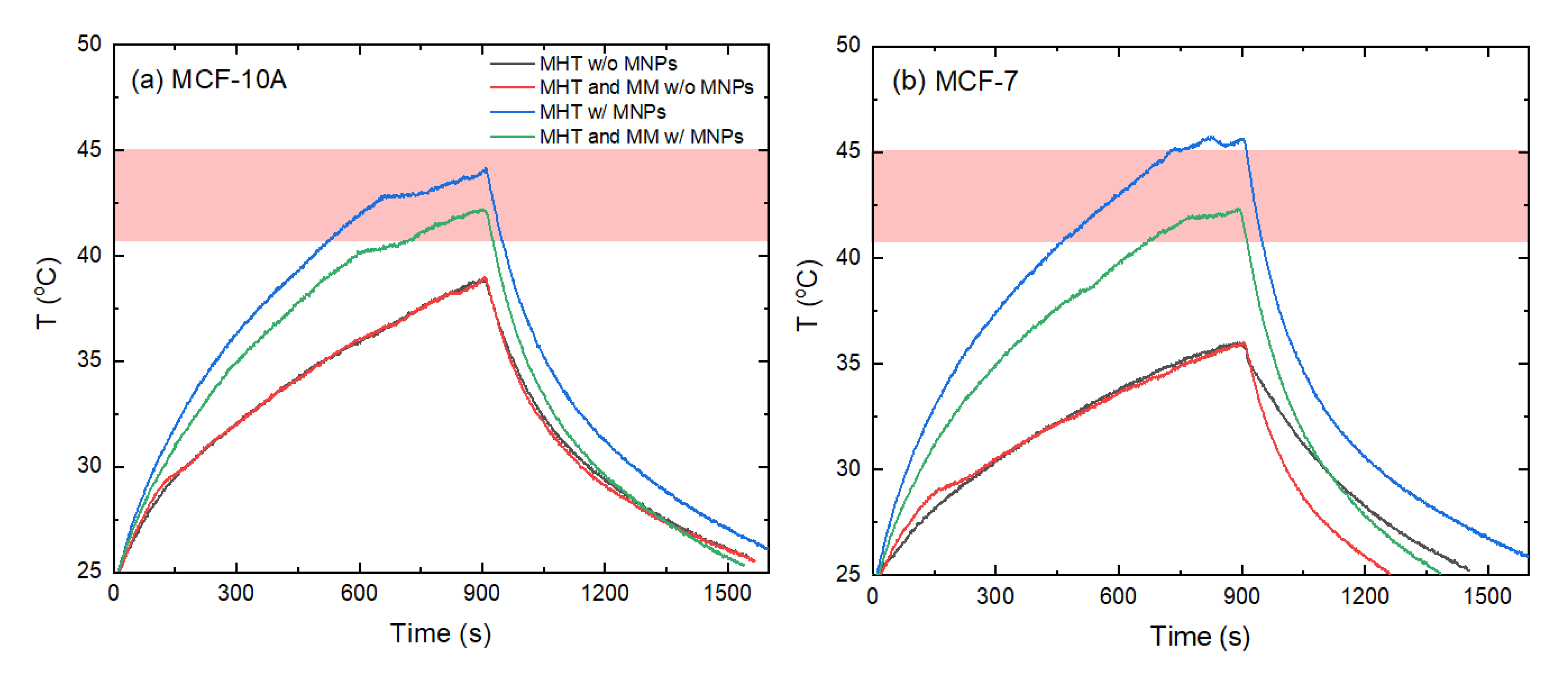
3.1. Impact of Exposure of Cells to MH and MM
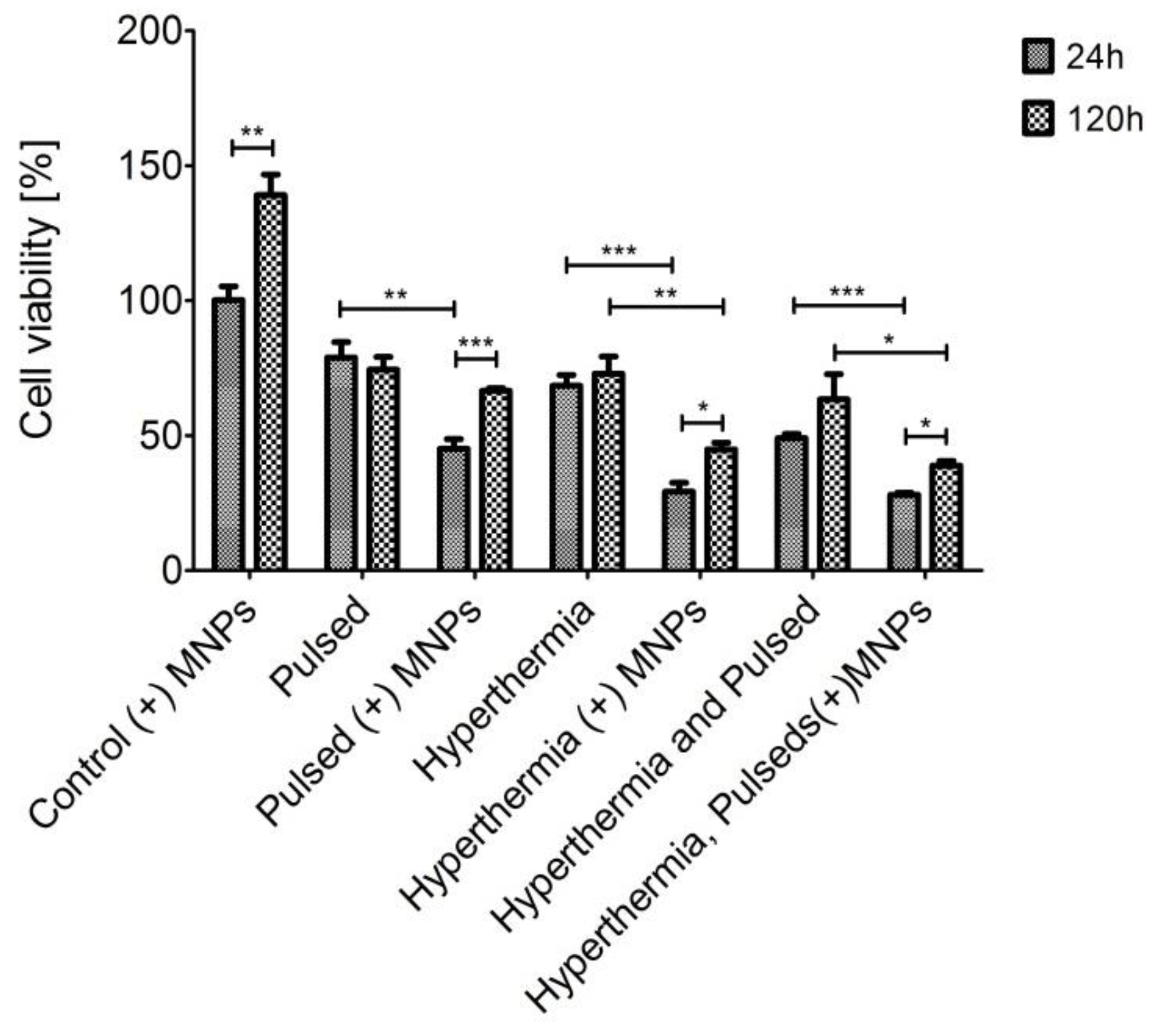
3.2. Cell Viability
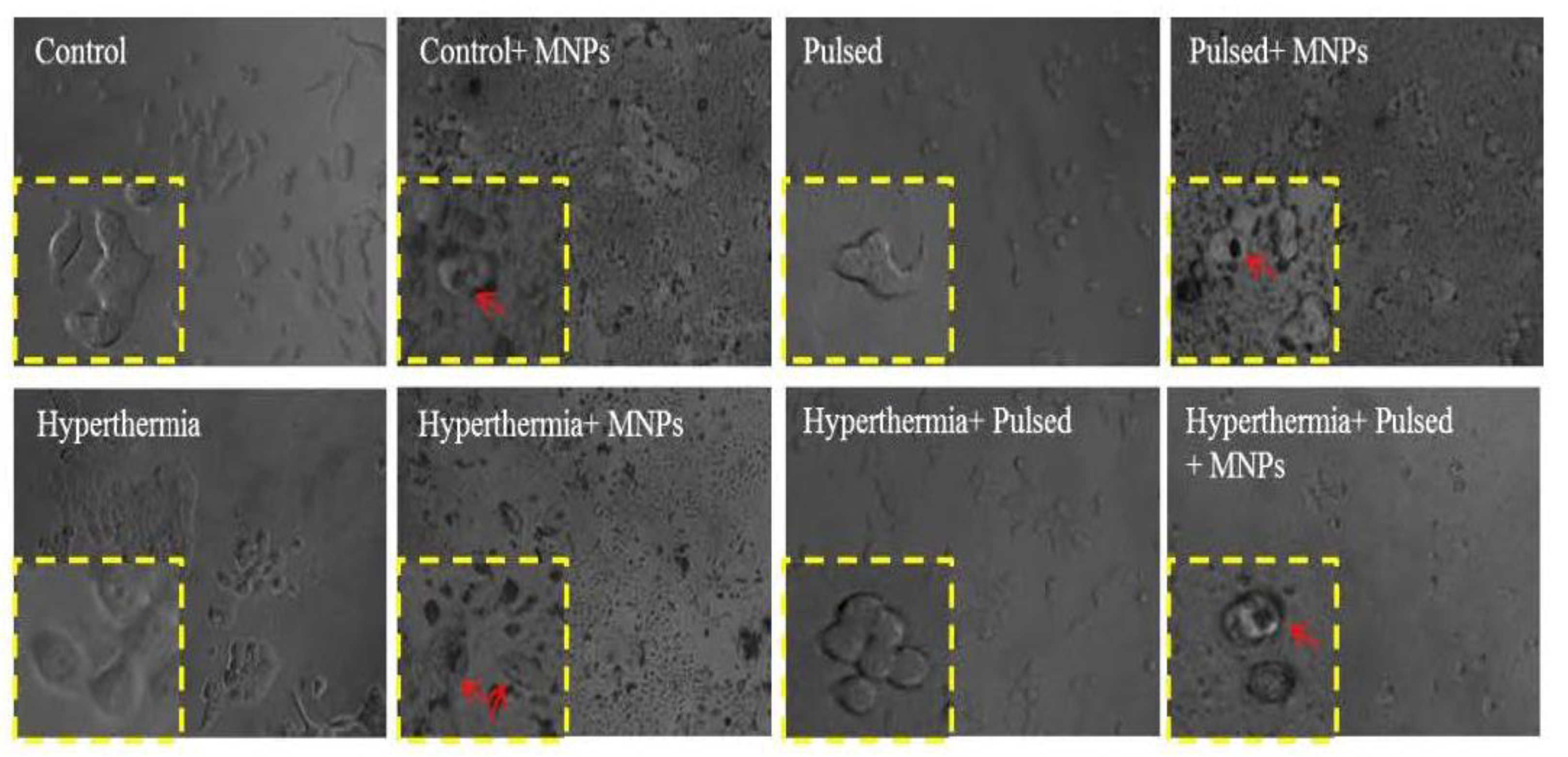
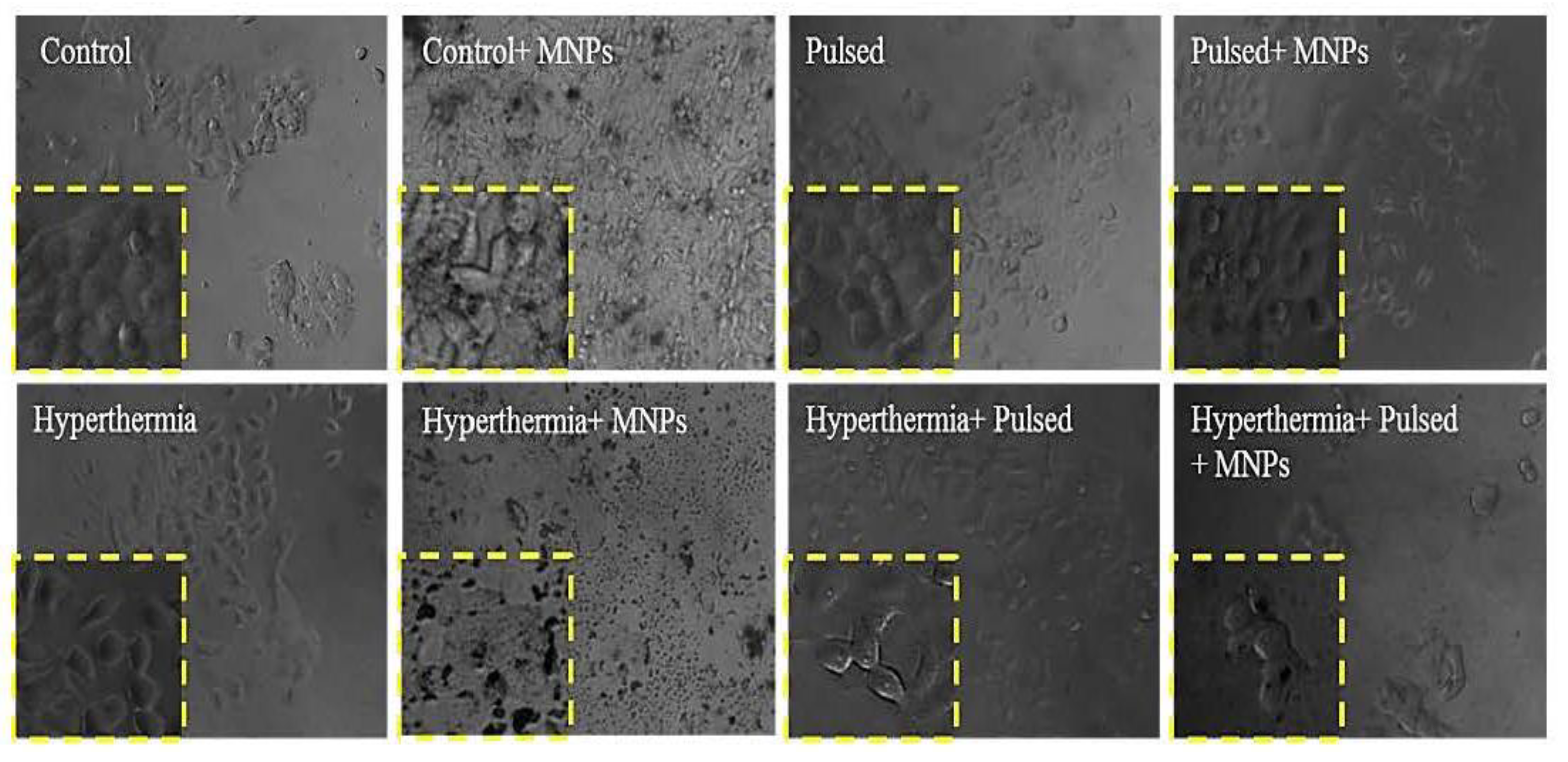
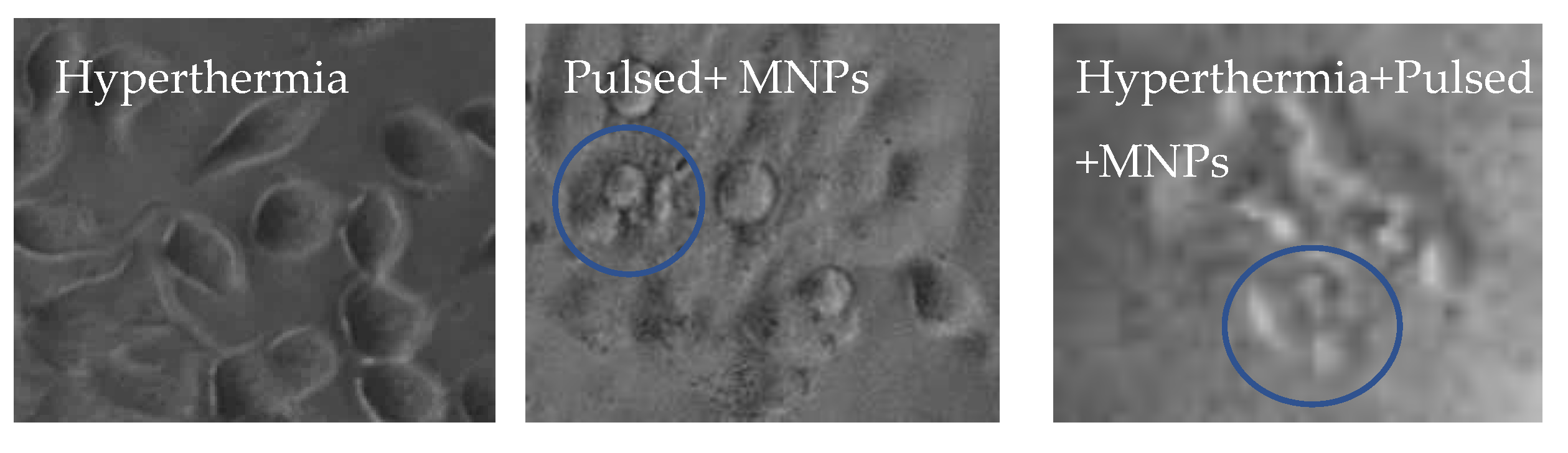
3.3. Cell Morphology Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosu, O.; Tertis, M.; Cristea, C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Zhang, Y.; Xu, W.; Xiao, J.; Suo, A. Growth of MCF-7 breast cancer cells and efficacy of anti-angiogenic agents in a hydroxyethyl chitosan/glycidyl methacrylate hydrogel. Cancer Cell Int. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Makridis, A.; Curto, S.; van Rhoon, G.C.; Samaras, T.; Angelakeris, M.A. standardisation protocol for accurate evaluation of specific loss power in magnetic hyperthermia. J. Phys. D Appl. Phys. 2019, 52, 255001. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Gordon, R.T.; Hines, J.R.; Gordon, D. Intracellular hyperthermia. A biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med. Hypotheses 1979, 5, 83–102. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef]
- Kirschning, A.; Kupracz, L.; Hartwig, J. New synthetic opportunities in miniaturized flow reactors with inductive heating. Chem. Lett. 2012, 41, 562–570. [Google Scholar] [CrossRef]
- Kalamida, D.; Karagounis, I.V.; Mitrakas, A.; Kalamida, S.; Giatromanolaki, A.; Koukourakis, M.I. Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PLoS ONE 2015, 10, e0116021. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Manganese-Doped Magnetic Nanoclusters for Hyperthermia and Photothermal Glioblastoma Therapy. ACS Appl. Nano Mater. 2020, 3, 2026–2037. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of Magnetic Hyperthermia for Glioblastoma Multiforme Therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, L.F.; Ding, W.J.; Wang, H.; Zhou, J.M.; Jin, H.K.; Su, S.F.; Ouyang, W.W. Clinical trials of magnetic induction hyperthermia for treatment of tumours. OA Cancer 2014, 18, 2. [Google Scholar]
- Maniotis, N.; Makridis, A.; Myrovali, E.; Theopoulos, A.; Samaras, T.; Angelakeris, M. Magneto-mechanical action of multimodal field configurations on magnetic nanoparticle environments. J. Magn. Magn. Mater. 2019, 470, 6–11. [Google Scholar] [CrossRef]
- Cheng, Y.; Muroski, M.E.; Petit, D.C.M.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.; et al. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J. Control. Release 2016, 223, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Spyridopoulou, K.; Makridis, A.; Maniotis, N.; Karypidou, N.; Myrovali, E.; Samaras, T.; Angelakeris, M.; Chlichlia, A.; Kalogirou. Effect of low frequency magnetic fields on the growth of MNP-treated HT29 colon cancer cells. Nanotechnology 2018, 29, 175101. [Google Scholar] [CrossRef]
- Zhang, E.; Kircher, M.F.; Koch, M.; Eliasson, L.; Goldberg, S.N.; Renström, E. Dynamic magnetic fields remote-control apoptosis via nanoparticle rotation. ACS Nano 2014, 8, 192–201. [Google Scholar] [CrossRef]
- Leulmi, S.; Chauchet, X.; Morcrette, M.; Ortiz, G.; Joisten, H.; Sabon, P.; Livache, T.; Hou, Y.; Carrière, M.; Lequien, S.; et al. Triggering the apoptosis of targeted human renal cancer cells by the vibration of anisotropic magnetic particles attached to the cell membrane. Nanoscale 2015, 7, 15904–15914. [Google Scholar] [CrossRef] [PubMed]
- Tsiapla, A.R.; Uzunova, V.; Oreshkova, T.; Angelakeris, M.; Samaras, T.; Kalogirou, O.; Tzoneva, R. Cell Behavioral Changes after the Application of Magneto-Mechanical Activation to Normal and Cancer Cells. Magnetochemistry 2022, 8, 21. [Google Scholar] [CrossRef]
- Sakellari, D.; Mathioudaki, S.; Kalpaxidou, Z.; Simeonidis, K.; Angelakeris, M. Exploring multifunctional potential of commercial ferrofluids by magnetic particle hyperthermia. J. Magn. Magn. Mater. 2015, 380, 360–364. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, Á.; Sanz, B.; San Román, J.; Goya, G.F.; Mijangos, C. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: From preparation to in vitro studies. Carbohydr. Polym. 2017, 157, 361–370. [Google Scholar] [CrossRef] [PubMed]
- De Paula, L.B.; Primo, F.L.; Pinto, M.R.; Morais, P.C.; Tedesco, A.C. Evaluation of a chloroaluminium phthalocyanine-loaded magnetic nanoemulsion as a drug delivery device to treat glioblastoma using hyperthermia and photodynamic therapy. RSC Adv. 2017, 7, 9115–9122. [Google Scholar] [CrossRef]
- Angelakeris, M.; Li, Z.A.; Sakellari, D.; Simeonidis, K.; Spasova, M.; Farle, M. Can commercial ferrofluids be exploited in AC magnetic hyperthermia treatment to address diverse biomedical aspects? EDP Sci. EPJ Web Conf. 2014, 75, 08002. [Google Scholar] [CrossRef]
- Uzunova, V.; Tsiapla, A.R.; Stoyanova, T.; Myrovali, E.; Momchilova, A.; Kalogirou, O.; Tzoneva, R. Biocompatibility of iron oxide nanoparticles. J. Chem. Technol. Metall. 2021, 56, 1187–1191. [Google Scholar]
- Tsiapla, A.R.; Kalimeri, A.A.; Maniotis, N.; Myrovali, E.; Samaras, T.; Angelakeris, M.; Kalogirou, O. Mitigation of magnetic particle hyperthermia side effects by magnetic field controls. Int. J. Hyperth. 2021, 38, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.C. Addressing the Dynamical Magnetic Response of Magnetic Nanoparticles after Interacting with Biological Entities. Doctoral Dissertation, Universidad Autónoma de Madrid, Madrid, Spain, 2018. [Google Scholar]
- Golovin, Y.I.; Gribanovsky, S.L.; Golovin, D.Y.; Klyachko, N.L.; Majouga, A.G.; Master, A.M.; Sokolsky, M.; Kabanov, A.V. Towards nanomedicines of the future: Remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J. Control. Release 2015, 219, 43–60. [Google Scholar] [CrossRef]
- Master, A.M.; Williams, P.N.; Pothayee, N.; Pothayee, N.; Zhang, R.; Vishwasrao, H.M.; Golovin, Y.I.; Riffle, J.S.; Sokolsky, M.; Kabanov, A.V. Remote actuation of magnetic nanoparticles for cancer cell selective treatment through cytoskeletal disruption. Sci. Rep. 2016, 6, 33560. [Google Scholar] [CrossRef]
- Wong, W.; Gan, W.L.; Teo, Y.K.; Lew, W.S. Interplay of cell death signaling pathways mediated by alternating magnetic field gradient. Cell Death Discov. 2018, 4, 49. [Google Scholar] [CrossRef]



| Circularity | Roundness | AR (μm) | Solidity | |||||
|---|---|---|---|---|---|---|---|---|
| Type of treatment | 24 h | 120 h | 24 h | 120 h | 24 h | 120 h | 24 h | 120 h |
| Control | 0.6 | 0.5 | 0.35 | 0.35 | 2.4 | 2.5 | 0.65 | 0.6 |
| Control (+) MNPs | 0.9 *** | 0.6 ** | 0.5 | 0.5 | 1.5 | 1.8 | 0.8 *** | 0.7 |
| Pulsed | 0.7 * | 0.55 | 0.55 | 0.4 | 1.45 *** | 2.2 | 0.7 ** | 0.7 |
| Pulsed (+) MNPs | 0.9 *** | 0.6 * | 0.45 | 0.45 | 2.5 | 2.4 | 0.9 *** | 0.7 |
| Hyperthermia | 0.5 *** | 0.7 *** | 0.4 | 0.45 | 2.55 | 2.4 | 0.65 | 0.85 *** |
| Hyperthermia (+) MNPs | 0.95 *** | 0.8 *** | 0.6 ** | 0.65 *** | 1.92 *** | 1.6* | 0.9 *** | 0.8 ** |
| Hyperthermia and Pulsed | 1 *** | 0.8 *** | 0.65 *** | 0.45 | 1.4 *** | 2.5 | 0.85 *** | 0.75 |
| Hyperthermia, pulsed (+) MNPs | 1 *** | 0.95 *** | 0.58 ** | 0.7 *** | 1.55 ** | 1.5 ** | 0.9 *** | 0.8 *** |
| Circularity | Roundness | AR (μm) | Solidity | |||||
|---|---|---|---|---|---|---|---|---|
| Type of treatment | 24 h | 120 h | 24 h | 120 h | 24 h | 120 h | 24 h | 120 h |
| Control | 0.3 | 0.2 | 0.4 | 0.35 | 1.9 | 2.2 | 0.55 | 0.5 |
| Control (+) MNPs | 0.45 | 0.25 | 0.7 | 0.4 | 1.5 | 1.5 | 0.6 *** | 0.55 |
| Pulsed | 0.35 | 0.25 | 0.3 | 0.45 | 2.7 | 2 | 0.55 ** | 0.55 |
| Pulsed (+) MNPs | 0.6 *** | 0.4 *** | 0.45 | 0.37 | 2.2 | 2.4 | 0.7 *** | 0.5 |
| Hyperthermia | 0.4 * | 0.35 *** | 0.5 | 0.5 | 2.4 | 1.8 | 0.55 | 0.55 |
| Hyperthermia (+) MNPs | 0.45 *** | 0.4 *** | 0.35 | 0.35 | 2 | 2.6 | 0.6 *** | 0.53 |
| Hyperthermia and pulsed | 0.35 * | 0.25 * | 0.4 | 0.3 | 2.3 | 2.3 | 0.5 *** | 0.5 |
| Hyperthermia, pulsed (+) MNPs | 0.75 *** | 0.55 *** | 0.55 * | 0.55 * | 1.6 | 1.7 | 0.8 *** | 0.65 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzoneva, R.; Tsiapla, A.-R.; Uzunova, V.; Stoyanova, T.; Samaras, T.; Angelakeris, M.; Kalogirou, O. Synergistic Effect of Combined Treatment with Magnetic Hyperthermia and Magneto-Mechanical Stress of Breast Cancer Cells. Magnetochemistry 2022, 8, 117. https://doi.org/10.3390/magnetochemistry8100117
Tzoneva R, Tsiapla A-R, Uzunova V, Stoyanova T, Samaras T, Angelakeris M, Kalogirou O. Synergistic Effect of Combined Treatment with Magnetic Hyperthermia and Magneto-Mechanical Stress of Breast Cancer Cells. Magnetochemistry. 2022; 8(10):117. https://doi.org/10.3390/magnetochemistry8100117
Chicago/Turabian StyleTzoneva, Rumiana, Aikaterini-Rafailia Tsiapla, Veselina Uzunova, Tihomira Stoyanova, Theodoros Samaras, Makis Angelakeris, and Orestis Kalogirou. 2022. "Synergistic Effect of Combined Treatment with Magnetic Hyperthermia and Magneto-Mechanical Stress of Breast Cancer Cells" Magnetochemistry 8, no. 10: 117. https://doi.org/10.3390/magnetochemistry8100117
APA StyleTzoneva, R., Tsiapla, A.-R., Uzunova, V., Stoyanova, T., Samaras, T., Angelakeris, M., & Kalogirou, O. (2022). Synergistic Effect of Combined Treatment with Magnetic Hyperthermia and Magneto-Mechanical Stress of Breast Cancer Cells. Magnetochemistry, 8(10), 117. https://doi.org/10.3390/magnetochemistry8100117








