Abstract
Magnetic nanoparticles (MNPs) have great potential in biochemistry and medical science. In particular, iron oxide nanoparticles have demonstrated a promising effect in various biomedical applications due to their high magnetic properties, large surface area, stability, and easy functionalization. However, colloidal stability, biocompatibility, and potential toxicity of MNPs in physiological environments are crucial for their in vivo application. In this context, many research articles focused on the possible procedures for MNPs coating to improve their physic-chemical and biological properties. This review highlights one viable fabrication strategy of biocompatible iron oxide nanoparticles using human serum albumin (HSA). HSA is mainly a transport protein with many functions in various fundamental processes. As it is one of the most abundant plasma proteins, not a single drug in the blood passes without its strength test. It influences the stability, pharmacokinetics, and biodistribution of different drug-delivery systems by binding or forming its protein corona on the surface. The development of albumin-based drug carriers is gaining increasing importance in the targeted delivery of cancer therapy. Considering this, HSA is a highly potential candidate for nanoparticles coating and theranostics area and can provide biocompatibility, prolonged blood circulation, and possibly resolve the drug-resistance cancer problem.
1. Introduction
Magnetic nanoparticles (MNPs) open a wide range of applications, including contrast agents area for magnetic resonance imaging (MRI), material science, magnetic delivery, magnetic fluid hyperthermia, structural biology, drug and gene delivery, theranostics [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Iron oxide MNPs are promising tags due to their high stability, cost-effectivity, and optimal MRI and hyperthermia characteristics [18]. Manipulation with an external magnetic field provides easy separation of MNPs from any liquids and desired location. Moreover, combining approaches of induction local heating in the tumor region, anticancer drugs, and effective monitoring by MRI has a great potential in targeted drug delivery and theranostics area (therapy + diagnostics) [7,11,12,15]. One of the most perspectives ferromagnetic MNPs is magnetite, Fe3O4. However, Fe3O4 is not stable upon oxidation and possesses high surface energy, leading to aggregation. Therefore, surface functionalization is required for such MNPs. The wrong coating leads to instability in the bloodstream and acute or delayed toxicity due to the highly reactive oxygen species (ROS) formation in cell lines and animal models [2,11,19,20,21,22,23,24]. Protein coating usually possesses biocompatibility, biodegradability, less immunogenicity, and lower cytotoxicity of MNPs [25,26,27,28]. Recently, biotechnological applications of human serum albumin (HSA), including bioinspired materials and nanoparticles coating, were reported [29,30,31,32]. Instead, the above-mentioned protein coating features, albumin, one of the major human plasma proteins, reduces unwanted adsorption of blood components and increases the efficiency of tissue and cell targeting [29,30,31,33]. Albumin-constructions transcytosis in the cells is provided by gp60, g30, gp18, and FcRn receptors binding. Moreover, accumulation in a tumor is facilitated by binding to the SPARC receptor and the enhanced permeation and retention effect (EPR) [34,35,36,37,38,39,40,41]. The albumin structure contains many drugs or natural ligand binding sites, which can be used for therapeutics loading.
Herein, we combined human serum albumin features for magnetic nanoparticles coating. For the first time, this review summarizes the present possibilities and provides ideas about the potential future technologies. In Section 2, we focused on the albumin structure, drug binding sites, and its passive and targeted delivery, representing the biocompatibility and versatility of albumin. Section 3 presented the possible albumin modification with reporter groups, drugs, imaging residues that can be used to further MNPs coating. Such procedures are suitable for multimodal imaging or theranostics smart platforms productions based on MNPs core. Section 4 shows the advantages of MNPs coating by albumin as water solution and biosystems stability, low toxicity, targeted delivery in vivo, and some improvements in physical properties. Studying MNPs stability and coating procedures opens a doorway for multifunctional and bioinspired material, probes, and devices.
2. Role of Albumin in Humans
Human serum albumin (HSA) is a well-known major protein in human plasma [42,43,44]. Its general concentration varies between 0.2 and 0.7 mM in plasma. In lower concentrations but the higher amount, it can be found in the extravascular spaces of tissues, the cerebrospinal fluid, etc. [43,44,45,46]. During the long circulation time, the protein undergoes several posttranslational modifications: S-homocysteinylation, S-cysteinylation, S-glutathionylation, nitrosylation, N-homocysteinylation, glycosylation, etc. [44,47,48,49]. Albumin is synthesized in liver hepatocytes but can distribute to the various tissues. However, there are many «blank spots» in the protein distribution mechanism and its different concentrations. Moreover, HSA is the primary carrier of endogenous and exogenous compounds and displays an extraordinary binding capacity to «fatty» ligands. Indeed, HSA represents the carrier for fatty acids, heme, nucleic acids, cholesterol, nitric oxide, bile pigments, hormones, and metals ions (ex. calcium (II), zinc (II), nickel (II)), and renders potential toxins harmless [44,50,51]. As one of the most abundant plasma proteins, not a single drug in the blood will pass without its «strength test». It influences the stability, pharmacokinetics, biodistribution of drug-delivery systems by binding or forming its protein corona on the surface. Albumin has various (pseudo-)enzymatic properties such as esterase, RNA-hydrolyzing, enolase, lipid peroxidase, etc., activities [44,50,51]. These weak activities are essential considering the large amount of HSA in the body [43,52]. HSA level in the blood is a valuable biomarker of different pathologies, including ischemia, cancer, rheumatoid arthritis, etc. [44,53,54]. The free SH-group of Cys34 leads to high antioxidant properties of albumin. For this reason, HSA level and its oxidated forms are associated with predicting severe coronavirus disease 2019 (COVID-19) and mortality [55,56,57,58,59]. However, the precise mechanism of such prediction is openly debated [55,59].
2.1. Albumin Structure
HSA is a three-domain allosteric macromolecule with molecular weight 66.5 kDa and half-life ~20 days responsible for plasma oncotic pressure and fluid distribution between body compartments [44,51]. HSA consists of 65–68% α-helices with some turns, extended loops, and little β-sheets content (~1–3%). The whole structure of HSA contains the following three homologous α-helical domains, I (residues 1–195), II (196–383), and III (384–585), which are divided into two subdomains (Figure 1). It has 585 amino acids but no attached sugars or carbohydrates. It contains a high number of Cys, Leu, Glu, Arg, and Lys residues, an average amount of aromatic amino acids, a low number of Met, Gly, and Ile residues, and only one Trp. HSA comprises 35 cysteine residues involved in 17 disulfide bridges. Only one Cys34 exposes the free thiol group. However, the Cys34 thiol group has a vital role in the redox status of tissues and protection against reactive oxygen and nitrogen species [42,43]. In healthy individuals, up to 70–90% of the Cys34 has a free thiol called mercaptoalbumin (HMA). The other 20–30% is bound in a disulfide bond with low molecular weight thiols, precisely cysteine, glutathione, and homocysteine, called nonmercaptoalbumin (HNA1). Less than 10% converts to the higher oxidation forms as sulfenic, sulfinic, or sulfonic acid (HNA2). Under pathologies, the amount of HNA increases up to ~50–80%, which can be used as several diseases biomarker [43,44,54,60,61].
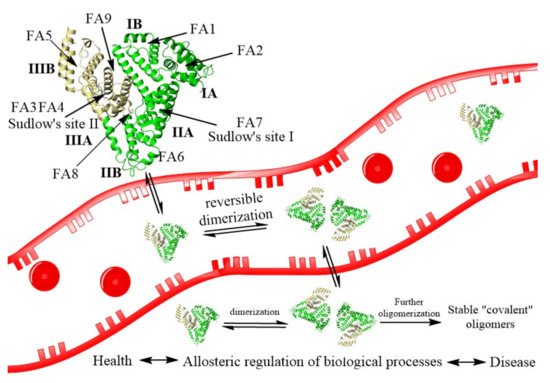
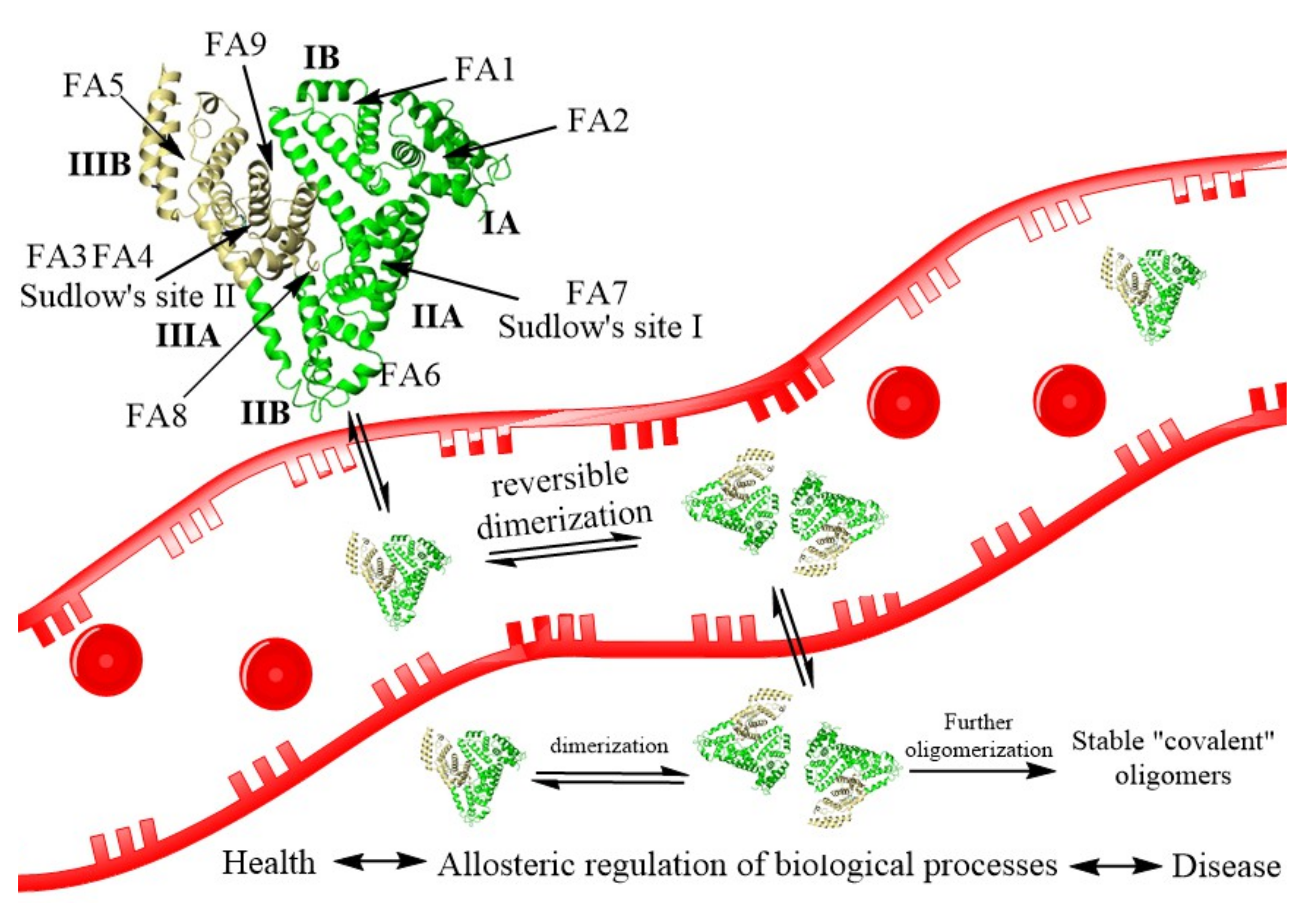
Figure 1.
The process of HSA reversible dimerization with further formation of stable covalent dimers. The structure of HSA (PDB ID: 1AO6) is shown schematically as a heart-like structure of helical ribbons. FA—fatty acids binding sites, Sudlow’ sites I and II—multi ligand huge binding sites.
HSA can form stable dimers, trimers, and highly polymeric structures whose mechanism formation is unknown. Some covalent dimers have a disulfide bond linking their Cys34 [60]. The other covalent dimers have an unknown nature dimer with a free SH-group in Cys34. Recently, the formation of reversible noncovalent dimers was shown to be the possible first step of the larger structures formation (Figure 1) [62]. The equilibrium between monomer and dimer form will alter processes involving HSA and the detection and treatment of diseases. For example, the dimer is formed by protein domains I. The Cys34, in subdomain IA, acts as an antioxidant by attacking free radical species. Conversely, the rate of oxidized albumin to the total albumin can be enhanced in multiple diseases such as cancer, COVID-19, etc. [59]. HSA dimers formation by the allosteric effect affects the albumin ligand binding, transport, enzymatic activities, modifications, body distribution by the molecular weight, and receptor binding, which reregulate many processes.
HSA has two major binding sites called Sudlow’s site I and II and is spotted on subdomain IIA and IIIA of the protein molecule, respectively. Site I binds bulky heterocyclic anions. Site II prefers aromatic carboxylates with an extended conformation [44]. Besides these multi-binding sites, albumin has nine fatty acid-binding sites (FA, Figure 1), two metal ions sites, and some other interdomain hydrophobic or hydrophobic/electrostatic cavities. Sudlow’s sites are capable of attaching with a wide range of drugs [63]. Therefore, they are well recognized and thoroughly investigated [43,44,50,54,63]. For example, the crystal structures of HSA with bilirubin, fatty acids, heme, thyroxine, and various drugs diazepam, ibuprofen, warfarin, aspirin, phenylbutazone are known [63]. Sudlow’s site I is huge and roughly divided into three nonoverlapping subsites with positively charged, hydrophobic, and aromatic cavities. Sudlow’s site II is much smaller and accommodates the binding of hydrophobic drugs. Besides Sudlow’s sites, the most relevant drug binding sites are FA1, FA3-FA4, and FA7 [50,63]. It should be noted that fatty acids binding to HSA play a crucial role in regulating its antioxidant and binding properties. Upon binding, HSA reveals conformational changes in all protein domains (allosteric effect), which influence drugs binding to the protein [50]. During the pathologies, the concentration of native albumin ligand can be changed, altering the affinity of the drugs and having important consequences for pharmacokinetics, pharmacodynamics, and drug interactions [43,48,49,50].
2.2. Binding of Receptors to Albumin
Many drugs and therapeutic constructions with a smaller size than the renal filtration threshold are rapidly excreted from the body. Improving the drugs’ therapeutic index is necessary to find a suitable carrier that provides targeted delivery and release in the tissue. This bottleneck may be overcome by using albumin as a versatile drug carrier system with an extremely long half-life (~20 days) in the bloodstream. Association, conjugation, or fusion of drugs to albumin is a well-accepted and established half-life extension technology. Understanding the mechanism of such long albumin circulation time is necessary to improve drugs pharmacokinetics and pharmacodynamics.
Such extraordinarily albumin lifetime is employed to protein size and interaction with the neonatal Fc receptor (FcRn) mediated recycling pathway. FcRn is an intracellular receptor widely distributed in many tissues and cells (Table 1). It rescues both IgG and albumin from degradation and renal clearance. The FcRn protects albumin from proteolysis by binding at low pH in the acid endosome and diverting them from a lysosomal pathway. At physiological pH, albumin is released, thereby prolonging circulation [64,65,66] (Figure 2). It is essential to mention that albumin nanoparticles show good epithelial transcytosis via the FcRn receptor [67]. This fact shows high perspectives of albumin constructions for oral drug delivery or the possibility of the enhancement efficiency drug delivery of the drugs with poor intestinal permeability.
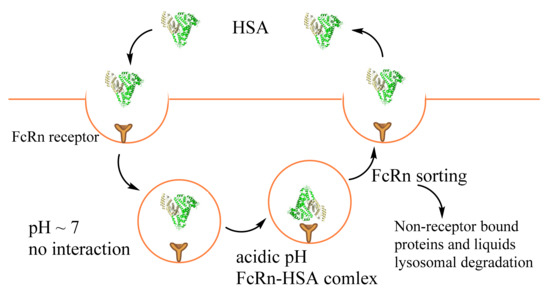
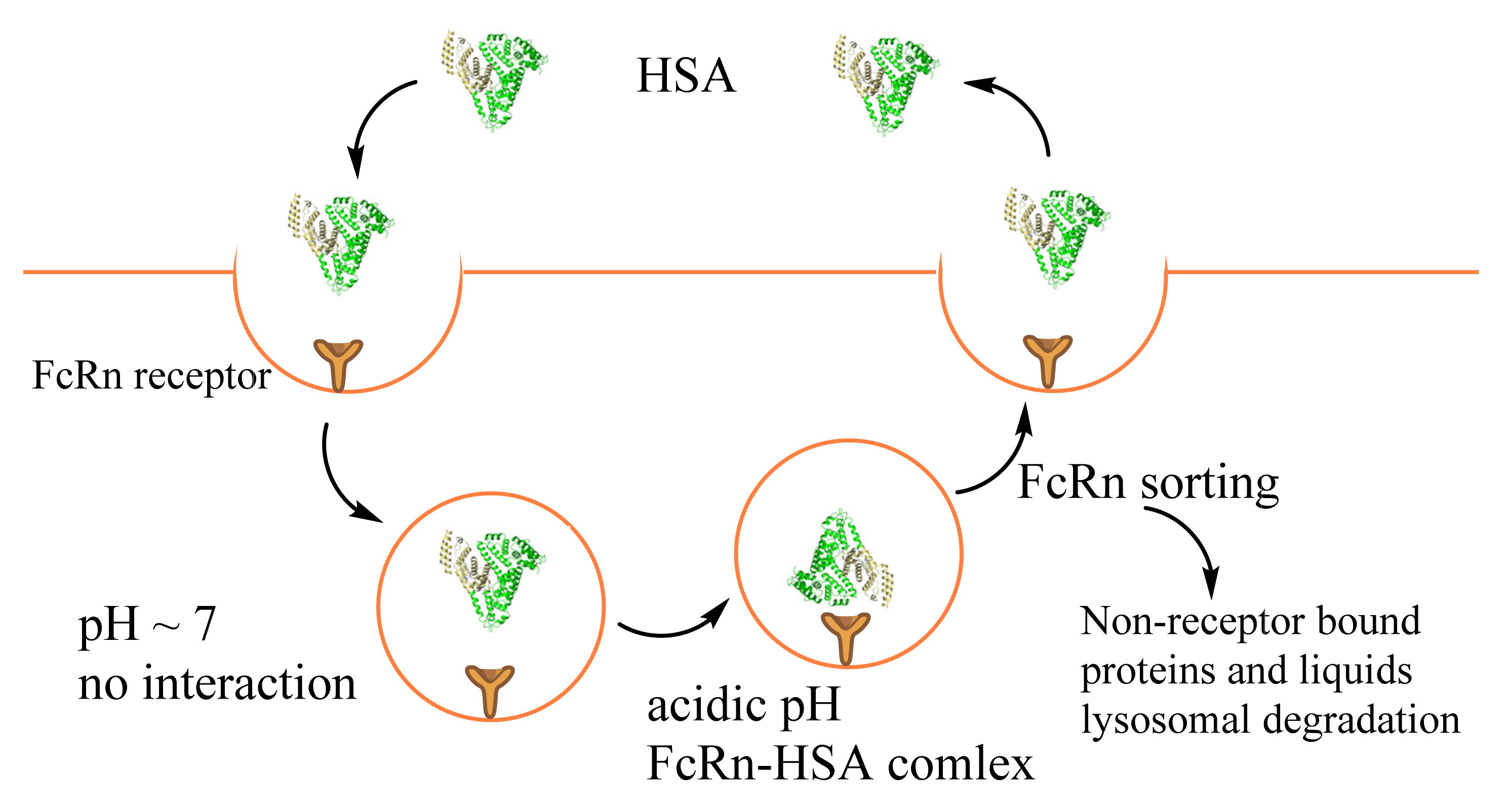
Figure 2.
The process of FcRn-mediated albumin recycling or proteolysis.
In kidneys, albumin passes through the glomerular basement membrane and accumulates before the slit diaphragm by transcytosis. FcRn binds albumin and excretes it into the glomerular capsular space. The albumin then passes into the proximal tubule and is reabsorbed by the renal tubular apparatus’s proximal tubule epithelial cells [64,65]. The processes are required FcRn, megalin, and cubilin receptors (Table 1). In healthy individuals, less than 1% filtered by kidneys albumin appears in the urine [39]. Cubilin is responsible for capturing normally filtered levels of albumin. In contrast, megalin has a lower affinity to HSA. Its expression is essential for enabling the recovery of high albumin concentrations in the fluid phase [68]. Moreover, a primary function of megalin is to maintain cubilin-dependent uptake under normal conditions and to enable fluid-phase uptake of albumin in nephrotic states. Interestingly, megalin binds to cubilin with high affinity, and it was proposed that megalin contributes to the internalization of cubilin complexes as a co-receptor [35,68].

Table 1.
Albumin binding receptors [35,65,67,69,70,71,72,73].
Table 1.
Albumin binding receptors [35,65,67,69,70,71,72,73].
| Receptor/Protein | Tissue/Cells |
|---|---|
| FcRn | Renal, brain and intestinal endothelium, vascular and antigen-presenting cells, gut, kidneys, liver, lungs, and the blood–brain barrier (endothelium and choroid plexus) |
| Cubulin | Kidney proximal tubule, absorptive intestinal, placenta, and visceral yolk-sac |
| Megalin | Kidney proximal tubule, absorptive intestinal, placenta, visceral yolk-sac, choroid plexus, thyrocytes, ciliary epithelium, lungs, parathyroid, endometrium, oviduct, inner ear |
| gp60 (Albonding) | Continuous endothelium excluding the brain, alveolar epithelium |
| gp18 *, gp30 * | Endothelium, macrophages, fibroblasts, and breast cancer |
| SPARC | Endothelial, vascular smooth muscle, skeletal muscle, fibroblasts, testicular, ovarian, pancreatic, and a range of tumor cells |
* Only for modified albumin.
Dysregulation of FcRn cellular recycling may account for enhanced intracellular albumin, endogenous HSA binding drugs, and HSA drug conjugates uptake associated with metabolic reprogramming of cancers (Figure 2). Furthermore, downregulation of FcRn expression in individuals was obtained in non-small cell lung carcinoma, breast, prostate, and colorectal cancer [73]. The low FcRn expression was associated with poor patient survival. On the contrary, high FcRn expression in both cancerous was associated with a favorable prognosis [73]. Cancer cells acquire sufficient amino acids for growth by albumin proteolytic degradation in lysosomes [65,69]. The active internalization of HSA by tumor cells was recognized long before its interaction with FcRn was discovered. The interaction of albumin with FcRn passes through the C-terminal domain III (DIII) and N-terminal domain I (DI) [74]. Precisely, the loops I and II of DI and DIII nearby Lys466 are in proximity with the receptor. Therefore, the chemical or posttranslational modification in such sites and ligand binding possibly leads to conformation changes, lower FcRn binding, and better albumin degradation in lysosomes; for example, Cys34 (DI domain) oxidation, cysteinylation, or cysteinylglycinylation act negatively on HSA–FcRn interaction [75]. Moreover, one of the major albumin modifications, glycosylation, leads to reduced affinity with FcRn. This, in turn, reduces transcytosis and increases intracellular catabolism, resulting in the excretion of albumin fragments and rapid vascular clearance [75,76]. It should be noted, among the investigated modifications (Lys525, Lys195, and Lys233), only Lys525, which alters the conformation changes in domain III, decreases HSA–FcRn binding. These data show that significant structural changes occurring to albumin with modification, particularly in the FcRn-binding region, could be used in the drug design for cancer treatment.
Besides FcRn, a number of the receptors can bind albumin (Table 1) and participate in its transport between compartments, degradation, etc. [35,39,70]. HSA binds to glycoprotein receptors (gp60, gp30, gp18), which results in the activation of caveolin-1 and transcytosis through endothelial cells. Gp60, also called albondin, is a glycoprotein that binds albumin and facilitates its internalization and subsequent transcytosis. Caveolin-1 induces invagination of the surface membrane with gp60-albumin complex and compounds bound or associated with albumin and forms vesicle or caveolae with a large volume of the surrounding liquid. Such a process does not lead to degradation by the endosome–lysosome system. It should be noted that gp60 can bind only native albumin or its complexes. On the contrary, gp18 and gp30 have a 1000-fold higher affinity for denatured or modified albumin (ex. oxidated, non-enzymatic glycation, albumin–gold nanoparticles, chemically modified, etc.). These receptors are perhaps part of the organism’s protective pathway to select and remove old, damaged, altered, or potentially toxic albumin species [35,39]. However, only ~50% of albumin leaves the capillary lumen via albondin, with the remainder traversing this barrier through intercellular junctions and/or fluid-phase mechanisms. Moreover, solid tumors usually have an immature, highly permeable vasculature. They disrupt the lymphatic system, which leads to the accumulation of macromolecules or nanoconstructions (>40 kDa) within the tumor interstitium and does not facilitate the reentry into the circulation. This effect is known as the enhanced permeation and retention effect (EPR). The long albumin time of albumin is increasing such effect. It is still unclear whether the precise mechanism makes the greatest contribution to albumin tumor delivery. It perhaps depends on the type of tumor and requires a detailed study [35,39].
Another protein that can bind albumin is SPARC (secreted protein acidic and rich in cysteine) [35,77,78]. SPARC is also known as highly expressed in various cancers. SPARC specifically interacts with native albumin similarly to gp60, sharing the same binding domains [35]. Recently, SPARC mediates active targeting of HSA in glioma tumors was shown [77]. Therefore, the SPARC pathway has excellent potential for HSA-based drug delivery.
3. Albumin Modification for Further MNPs Coating as a Versatile Tool for Theranostics Production
Nanomaterials have been used widely for drug delivery. However, when designing universal delivery systems, there are a number of fundamental problems associated with the requirements for starting nanomaterials. One of the most important is that nanomaterials must be non-toxic and biocompatible. Protein–nanoparticle corona has gained importance due to stability, biocompatibility, reduced toxic side effects, etc. Preformed albumin coating has decreased non-specific association with blood proteins and liver association and reduced the body’s clearance due to specific receptor interactions (see Section 2.2). Modifying the surface of nanomaterials by albumin provides a target tumor delivery, drug or ligands binding, and improves the solubility of hydrophobic drugs, possible surface modification. Functionalization with a specific address or reporter groups increases theranostics efficiency and allows molecular probe imaging. Herein we present two main strategies for albumin drug/probes/reporter groups loading or modification, which can be perspective for the further MNPs coating. One of them is albumin therapeutics, in which the molecules are covalently bound to the protein. The second one is preformed albumin binding or in situ binders [78].
3.1. Covalent Strategy
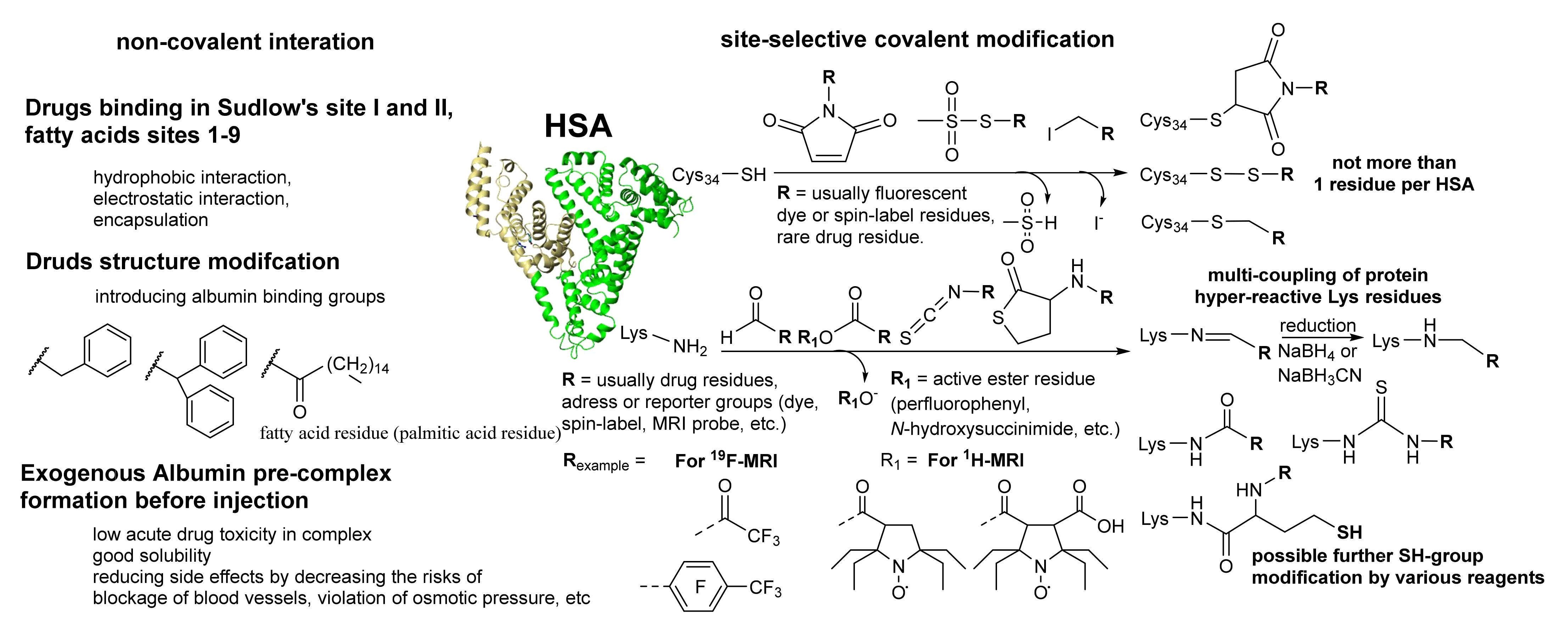
HSA is a negatively charged at neutral pH very stable protein in various conditions: (1) under heating, (2) in a wide pH range from 4 to 10, (3) and soluble in an organic solvent such as 40% ethanol or DMSO. Covalent attachment of therapeutic molecules to proteins is a feature. Under the non-specific modification, most proteins can lose their biological function and form oligomers or aggregate with further precipitation. Therefore, site-specific well-proved methods are required [79,80,81]. One of the possible proven procedures is the modification of only one cysteine No. 34 residue with a free SH-group. As mentioned above, the thiol group can be in various oxidation states (R-SH, R-S-S-R, R-S-OH, R-SO3H). Commercial HSA usually has not more than 30% SH-group. Therefore, the thiol enrichment method was proposed by reducing disulfides with dithiothreitol (DTT) before reacting to thiol-coupling reagents [82]. Such a mild condition does not lead to the reduction in proteins disulfide bridges. Three site-selective types of the active reagents are used for SH-group labeling: (1) maleimide, (2) methanethiosulfonate, (3) and alkylating (Figure 3). Methanethiosulfonate derivatives are considered to be the most selective at labeling a wide range of biomolecules. Maleimide and alkylating reagents can be not so specific in the case of using high excess of the reagent or too high pH > 8. However, the Cys34 has unexpectedly low pKa ~ 5 [83], which leads to a high rate and good efficiency of the reaction using low excess of the reagents. However, this method introduces the albumin one residue per molecule. Usually, the procedure is used for fluorescence dye for the cell experiments [84], fluorescence imaging [38,85] applications, or spin-label conjugation for the protein complexes investigation [62,86,87,88]. For drug delivery, another method is required for better therapeutic effect, which possesses multi-conjugation.
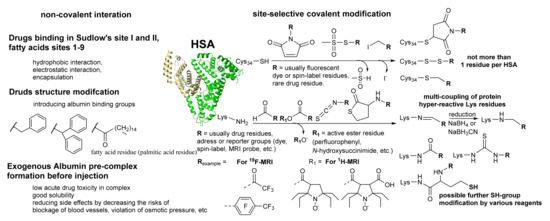
Figure 3.
Covalent and noncovalent strategy features and examples of albumin modification. The most common, easy-synthesized, cheap, and commercially available reagents for the covalent procedure are presented.
HSA harbors 59 lysines, which also can be used for covalent modification. However, conjugation to lysine tends to result in non-specific conjugation of albumin due to the multiple amino acid residues available. In Figure 3, the most common acylation reagents are presented. A balance should be observed between the degree of modification, the reagent activity, and its excess. The highly active reagents such as anhydrides possess protein precipitation. Protein over-labeling usually leads to significant changes in protein conformation and oligomerization. For artificial polymer synthesis, thiolactone chemistry was recently used [89]. This approach is suitable for the albumin lysine residues modification under physiological conditions [84,90,91]. The reaction proceeds site-specifically and involves only “high-reactive” lysine residues [85,91]. The main conjugation site is Lys525, one of the lowest pKa among the albumin residues. Moreover, the lysine residues, known for the natural N-homocysteinylation modification, are susceptible to the reaction with N-substituted homocysteine thiolactone [91].
The thiolactone ring-opening results in releasing a free sulfhydryl group, which can be used for further modification (Figure 3). Therefore, this reaction can introduce two different residues into the polymer chain [92]. Using N-substituted HTL derivatives, several probes for the glioma 19F-MRI [84,90] and 1H-MRI based [91] on HSA were synthesized. The standard toxicity test confirmed the safety of HSA conjugates [84]. Using maleimide derivatives of anticancer drugs, further modification of the new SH-groups leads to theranostic constructions for imaging and chemotherapy [85,93]. Initial animal experiments have shown the efficiency of the construction for glioma tumor imaging by 19F MRI [84] and fluorescence [85]. N-homocysteinylation of HSA by natural homocysteine thiolactone causes protein damage and increases the radical formation and oligomer formation with subsequent amyloid transformation [92,94]. On the contrary, the reaction with N-substituted thiolactone derivatives leads only to slight changes in α-helical and β-sheet content and inhibits aggregation and radical formation [84,92]. The unique properties of the thiolactone tool open the area of theranostics production based on artificial or natural polymers, involving proteins, in mild conditions.
3.2. Noncovalent Strategy
Another approach to promote interaction with albumin is to alter targeted delivery [7,95,96,97,98]. Several technologies with albumin are possible to encapsulate the drugs in the nanoparticles. They are self-assembly, nab-technology, double emulsification, desolvation, thermal gelation, nano-spray drying, emulsification, etc. [99,100,101]. Most of the presented methods lead to high-sized albumin oligomers or nanoparticles. Therefore, these structures after MNPs coating will lead to nanoparticles with a higher than appropriate «biological size limit» of about 100 nm. Nanoparticles ranging between 10 and 100 nm in diameter tend to represent optimal properties. However, several works on drug-loaded albumin-coated MNPs preparation via desolvation and chemical co-precipitation method are known [102,103,104]. In this method, a desolvation agent such as acetone was added to the albumin and iron salts solution. Some therapeutic reagents can be added to obtain the theranostic construction. After obtaining the optimal size of nanoparticles, a cross-linker reagent has to be added to stabilize the nanoparticles. However, looked perspective method is not typical for protein-coated MNPs. Therefore, we focused on the albumin drug loading suitable for further MNPs coating.
Some natural ligands and drugs can form a stable complex with HSA (see Section 2.1) [63]. In order to improve the binding effect, some natural or some fatty and aromatic residues non-natural residues can be used [63,97,98,105]. For example, some of the albumin-bound gadolinium chelates such as Multihance, Vasovist, or Primovist have the group presented in Figure 3. The medical advantages of such gadolinium complexes can be found elsewhere. One more feature is the relaxivity increase of gadolinium chelates after binding with albumin, which also highly increases the sensitivity in the MRI method.
The reversible and multivalent affinity to albumin significantly increases the prolonged circulation and tumor-targeting efficiency in vivo [96]. The comparative study on the covalent approach and albumin-binding molecules approach [96] for targeted tumor delivery showed that both assays have good results in SCC7 tumor-bearing mice after intravenous injection. Moreover, the noncovalent assay using palmitic acid residue shows higher tumor-targeting efficiency. This fatty acid-based albumin prodrug demonstrated the potential of in vivo albumin-binding prodrugs for safe and efficient anticancer therapy with reversible and multiple binding capabilities, in vitro and in vivo. Besides the increased drug half-life and targeted delivery, such an approach possesses better drug solubility, decreased toxicity, and enhanced stability.
One other possibility is to combine the covalent and noncovalent albumin binding in one construct to improve the properties of the two assays. Moreover, usually for the molecules with a fatty tail, such as in the noncovalent assay precomplex with albumin, is suitable to provide better solubility and relatively high dose injection. This means that before the organism’s injection, a solid-state or solution albumin–drug complex forms in a tube in vitro. Such precomplex approach possesses less toxicity and removes some possible adverse effects related to the low solubility in water.
4. Albumin-Coated Magnetic Nanoparticles Properties
Nanocarriers provide new perspectives in the delivery of anticancer drugs and imaging probes. In particular, MNPs have various applications such as MRI, hyperthermia, controlled drug delivery, etc. (Figure 4). However, several disadvantages have to mention. Low biostability, possible toxicity, and low tissue specificity are among them. Recently, novel strategies such as bioinspired surface coating, coating functionalization with address molecules, and reporter groups have emerged [13]. This study presents the mechanism of the below-presented problems and their possible solutions due to albumin protein coating with further surface functionalization. Albumin is a good candidate for the biosensor, bioimaging, and theranostics carrier [53,101,106]. Due to its unique properties, albumin coating has several advantages mentioned in the previous sections. Possible surface modification leads to various smart systems with address groups, imaging probes, drug complexes, and conjugates (Figure 3 and Figure 4, Section 3). Another advantage is a passive (EPR-effect) and targeted delivery to cancer tissue due to the albumin receptor interactions (see Section 2).

Figure 4.
Applications of albumin-coated MNPs.
4.1. Albumin Coating Effect on MNPs Water Solution Stability and Biostability
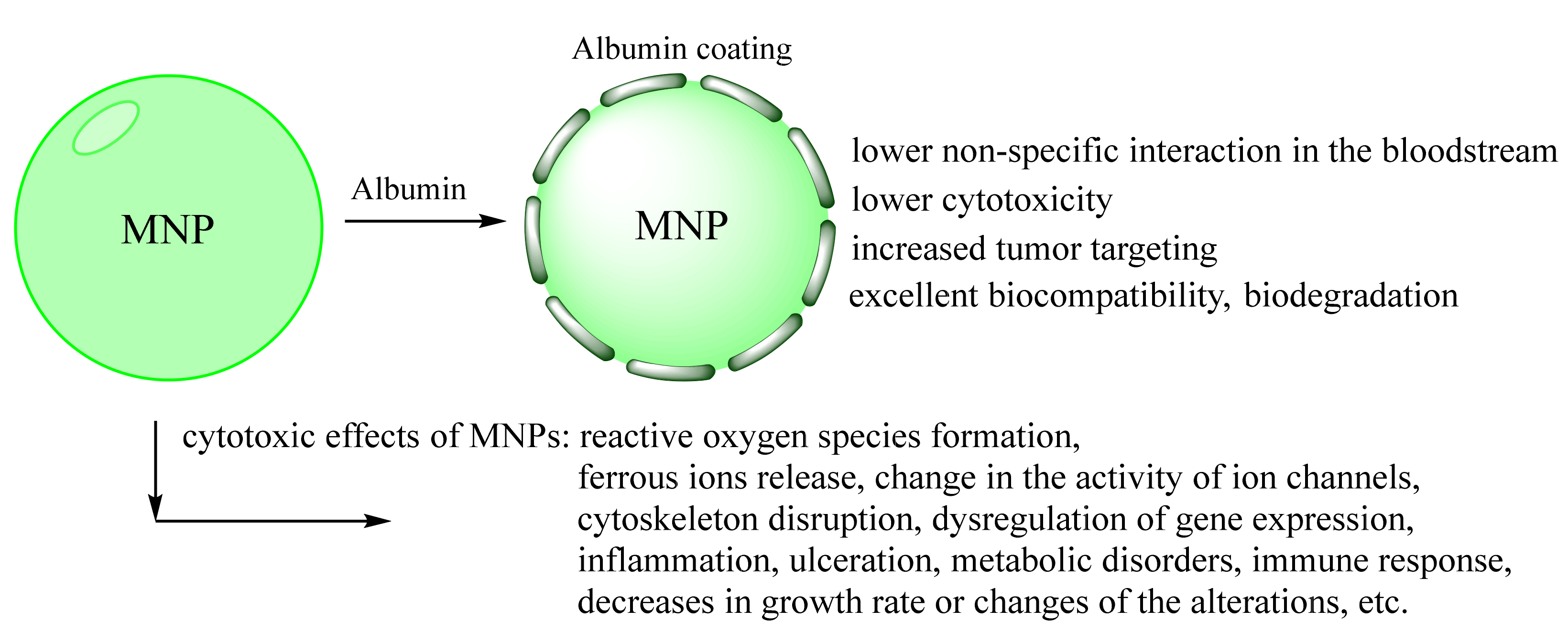
The main issue of MNPs is long-term inherent instability. MNPs tend to agglomerate due to the high surface energy and the strong magnetic attraction between particles. Moreover, simple physiological-like high salt concentrations strongly affect the colloidal stability of MNPs. For the Fe3O4 MNPs, magnetism loss occurs under oxygen oxidation. These two main routes can be handled by surface functionalization [12,13,19,33]. Coated MNPs have various advantages over bare MNPs. One more thing is once MNPs enter the blood, biological molecules, especially proteins, cover their surface. So-called protein corona is one reason for the rapid clearance of nanoparticles from the bloodstream after intravenous injection [33]. Since preventing such irregular coating is complex, forming stable pre-coating with optimal characteristics before the injection is required. Organic polymers and low-molecular-weight surfactant coating are among the most popular procedures [10,12,19,107].
A modern approach is using biomolecules coating for improved biocompatibility [3,12]. Albumin adsorption prevents nucleation and the aggregation of MNPs, increases colloidal stability, and is optimal for the in vivo use of nanoparticles [26,107,108,109,110,111,112,113,114,115,116]. For example, BSA-coated MNP remained excellent colloidal stability at 0.15 M sodium chloride concentration for more than one week. In comparison, tannic acid-coated MNPs already formed aggregates at 0.05 M and higher sodium chloride concentration [107]. The albumin-coated nanoparticles size did not alter for a long time in various pH and bioreagents temperature storage range and under 37°C [26,109,111]. There were no significant changes in blood serum/plasma [26]. Albumin preformed MNPs corona is good protection of non-specific interactions with blood components, immune response, and extended half-life (see Section 2) [26,33,107,108,115,116]. Some approaches use tannic, carboxylic acid (lauric, myristic, or oleic), hyaluronic acid, etc., for ferrofluid colloidal stabilization and optimal nucleus size formation with further albumin coating for biostability [114,117,118,119,120,121].
4.2. Preventing Toxicity and Targeted Delivery In Vivo of Albumin-Coated MNPs
The toxicological research of MNPs is a significant step for in vivo application. The main mechanisms of the cytotoxic effects of MNPs are reactive oxygen species (ROS) formation, ferrous ions release, change in the activity of ion channels, cytoskeleton disruption, and dysregulation of gene expression [13,24]. The possible concepts of toxicity are summarized in Figure 5. Besides the mentioned advantages, most of the works are related only to the simple toxicity test such as MTT cell assay [122,123]. However, cancer cells usually have better activated survival systems than normal cells. MTT test has not shown non-specific interaction with blood components, tissue-specific toxicity, chronic toxicity, etc. Only some of the MNPs demonstrated acute toxic effects. However, most of them have chronic toxicity or can cause disorders such as inflammation, ulceration, metabolic disorders, immune response, decreases in growth rate, or changes in animal models [20,21,24]. MNPs accumulation in some organs may interfere with the physiological iron metabolism after the degradation with further mitochondria, membrane, and nucleic acid damage (somatic or inherited mutation). It is oblivious that the extended toxicity experiment is a laborious work of many researchers [24]. However, the simplest tests combinations as plasma stability, ROS formation, and several cell types (for MTT assay) are required for any research, which claims that their MNPs can be used for further clinical investigations.
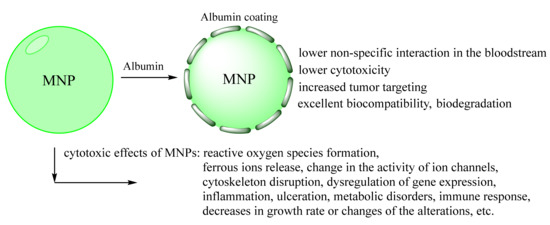
Figure 5.
Schematic representation of possible biological responses to MNPs [2,21,124]. The preformed albumin coating inhibits MNPs sub effects.
Albumin coating usually results in very moderate particle uptake and low ROS production cytotoxicity, as many works on in vitro and cell experiments [23,37,102,103,112,120,125,126,127,128,129,130,131]. It should be noted that albumin coating has to be enough for the surface of the nanoparticles. If the nanoparticles’ Zeta potential has not changed to the negative or, perhaps, stayed positive, it is not such an incredible effect of protection can be obtained [132]. Albumin-coating improves the in vitro therapeutic outcome of drug-loaded MNPs, highlighting the potential for success in vivo studies [102,112,114,125]. Moreover, albumin coating could prevent cardiac effects of MNPs [133]. No changes in central hemodynamics, microcirculation, and endothelial integrity factors were detected [134]. The presented results show that albumin coating provides a stable and biocompatible shell and prevents cytotoxicity of magnetite core.
As mentioned above (see Section 2.2), albumin can bind with various receptors, which possess the targeted delivery of albumin-coated MNPs [135]. However, the possibility of surface functionalization provides the feasibility to accumulate or increase the accumulation at specific locations and organs using specific receptor-mediated targeting [13,29,136,137]. Some of the possible albumin surface functionalization chemistry is presented in Section 3.1 (Figure 3). For albumin surface modification, vitamin or vitamin-like derivatives (biotin, folate), carbohydrates (glucose, galactose, lactose, and mannose), and peptides (RGD or cell-penetrating peptides) are widely used [29,136,137]. For example, due to the interaction with specific receptors biotin modified HSA targets breast and cervical cancer [136]. Folic acid conjugated albumin–MNPs are effective for cell targeting and brain tumor MRI imaging [136,138,139]. The rare possibility is to conjugate the albumin–MNPs with antibodies (anti-EGFR and VEGF) [128,140]. However, anti-EGFR and VEGF antibody-conjugated HSA–MNPs effectively targeted breast tumor and brain glioma delivery in a mice model, respectively [128,140].
4.3. Albumin-Coated MNPs for MRI
MRI is a great non-invasive diagnostic tool. Various contrast agents can improve anatomic resolution and diseased tissue region. The contrast agents are usually divided into longitudinal T1 and transverse T2 contrast agents. Using T1 agents MRI image becomes brighter and T2 darker. The contrast ability of a contrast agent can be quantitatively characterized by relaxivity (r1 and r2), which is a proportionality coefficient between relaxation time T and contrast agent concentration. MNPs usually are T2 contrast agents with high r2. MNPs coating influences both T1 and T2 relaxation processes due to changes in the availability of water molecules near the magnetic core [141]. However, the universal recipe to obtain the best MRI agent is unknown [141]. The thin coating usually highly decreases r1 but does not influence r2 values due to the different relaxation mechanisms. Some layer is required to have relevant stability and biocompatibility effect. As expected, albumin absorption of the MNPs surface decreases r1 from 12 to 6 mM−1 s−1 and increases r2 from 480 to 600 mM−1 s−1 (magnetic field 1.5 T) [107]. Interestingly, the coated MNPs have about six times higher r2 value than the contrast agent Resovist® (coated Fe3O4), which is recommended to use only in low doses due to the sub effects [107]. Another example is that 30 nm HSA-coated MNPs have an r2 of 314 mM−1 s−1, 2.5 times higher than Feridex [37,142]. Some other works show the same tendencies on slightly or high enhancement of the r2 relaxivity, which is the feature of their magnetic nucleus formation procedure and size [26,37,107,109,125,140,142,143,144,145]. Enhanced r2 values and potential for MRI of albumin-coated iron oxide nanoparticles were previously reported in a number of in vitro and in vivo studies [37,107,128,135,140,142,144,145,146].
4.4. Albumin-Coated Multimodal Imaging or Theranostics MNPs
Recently, albumin-coated MNPs were actively used for the multimodal imaging or theranostics production [102,103,112,114,117,118,119,121,127,130,135,138,139,145,147,148,149,150]. The albumin-coated MNPs surface was labeled by 64Cu-DOTA complex (for positron emission tomography, PET) and fluorescence dye (Cy5.5) to assess the possibility of multimodal imaging. Triple-imaging (PET, near-infrared fluorescence imaging, and MRI) was successfully tested on the glioma mouse model [142]. Another possibility is tumor-targeted folate delivery with simultaneous bimodal imaging by fluorescence and MRI [138]. A possible albumin surface labeling technique by the fluorescence reporter group is widely useful for simple cell uptake experiments or in vivo fluorescence imaging using NIR fluorescence dye [138,142,150].
An even more difficult goal is to obtain theranostic constructions based on albumin-coated MNPs [102,103,114,117,121,125,139,145,148,149]. Theranostics MNPs offer great potential in drug-resistance cancer treatment. However, the progress in the area is limited and has been raised in the last several years. Some potential for MRI and drug release was conducted with paclitaxel anticancer drug loading on albumin-coated MNPs [114]. The significant results were obtained using albumin-coated MNPs loaded with doxorubicin [149], methotrexate [121], curcumin [102,103,145], or synergistic delivery of curcumin with 5-fluorouracil [139], which was shown on the cell line model. An excellent therapeutic effect on rat models with gliosarcoma tumors was obtained [121]. Integrating hyperthermia and chemotherapy was shown in vitro on cell lines using paclitaxel [125] and etoposide (topoisomerase-II inhibitor) [148]. These results highlight the great potential of simultaneous imaging and therapy on one nanoparticle species. Several therapy strategies are preferable to solve the problem of the drug-resistance cancer problem.
5. Conclusions
Nanoparticles are a promising platform for creating new drugs for simultaneous therapy and diagnostics (theranostics). Such systems can take an important place in creating new generation drugs due to the possibility of manipulating their physicochemical and biological properties, targeted regulation of the composition, size, and surface functionalization. MNPs are a promising nucleus of theranostics due to possible MRI diagnostics, external field guiding, and hyperthermia effect. The main problem of the MNPs coating is to possess the biostability and targeted delivery to the tumor. It is vital to save and/or improve physical properties under the functionalization process. The biochemical and biophysical properties of albumin make it an ideal candidate for MNPs’ coating. Its excellent biocompatibility, biodegradability, and outstanding cancer tissue accumulation, due to the enhanced permeation and retention effect and specific receptor binding, have great proven potential. The possibility of albumin surface modification with reporter or address groups can simultaneously possess better tissue targeting and imaging procedures. Furthermore, the diversity in the preparation of covalent or binding an albumin-based drug delivery system gives numerous opportunities to include a wide range of therapeutic or theranostic agents. Combining the effects of MNPs, albumin coating, and albumin modification provides the resulting system with outstanding properties.
Funding
This research was funded by Russian Science Foundation (grant no. 21-74-00120); the albumin covalent modification section was supported by young scientist president scholarship project CΠ-4330.2021.4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Sharma, S.; Parul; Verma, A.K.; Roy, I.; Sen, T. Iron oxide-based magneto-optical nanocomposites for in vivo biomedical applications. Biomedicines 2021, 9, 288. [Google Scholar] [CrossRef]
- Sharma, B.; Pervushin, K. Magnetic nanoparticles as in vivo tracers for alzheimer’s disease. Magnetochemistry 2020, 6, 13. [Google Scholar] [CrossRef]
- Katz, E. Synthesis, properties and applications of magnetic nanoparticles and nanowires—A brief introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef]
- Bruschi, M.L.; de Toledo, L.D.A.S. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef]
- Creţu, B.E.B.; Dodi, G.; Shavandi, A.; Gardikiotis, I.; Şerban, I.L.; Balan, V. Imaging constructs: The rise of iron oxide nanoparticles. Molecules 2021, 26, 3437. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Bobrikova, E.; Chubarov, A.; Dmitrienko, E. The Effect of pH and Buffer on Oligonucleotide Affinity for Iron Oxide Nanoparticles. Magnetochemistry 2021, 7, 128. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Chouhan, R.S.; Horvat, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Gandhi, S. Magnetic nanoparticles—A multifunctional potential agent for diagnosis and therapy. Cancers 2021, 13, 2213. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Hepel, M. Magnetic nanoparticles for nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Goud, K.Y. Magnetic Particle Bioconjugates: A Versatile Sensor Approach. Magnetochemistry 2019, 5, 64. [Google Scholar] [CrossRef]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic nanoparticle systems for nanomedicine—A materials science perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 nanoparticles in targeted drug/gene delivery systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflore, O.B.; Ger, T.R.; Hsiao, C. Der Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Nelson, N.; Port, J.; Pandey, M. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranost. 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021, 15, 11341–11357. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef]
- Samanta, B.; Yan, H.; Fischer, N.O.; Shi, J.; Jerry, D.J.; Rotello, V.M. Protein-passivated Fe3O4 nanoparticles: Low toxicity and rapid heating for thermal therapy. J. Mater. Chem. 2008, 18, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic nanoclusters coated with albumin, casein, and gelatin: Size tuning, relaxivity, stability, protein corona, and application in nuclear magnetic resonance immunoassay. Nanomaterials 2019, 9, 1345. [Google Scholar] [CrossRef]
- Bychkova, A.V.; Sorokina, O.N.; Pronkin, P.G.; Tatikolov, A.S.; Kovarski, A.L.; Rosenfeld, M.A. Protein-Coated Magnetic Nanoparticles: Creation and Investigation. In Proceedings of the International Conference Nanomaterials: Applications and Properties, Alushta, the Crimea, Ukraine, 16–21 September 2013; Volume 2, pp. 1–5. [Google Scholar]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Salaklang, J.; Hofmann, H. Protein corona composition of superparamagnetic iron oxide nanoparticles with various physico-Chemical properties and coatings. Sci. Rep. 2014, 4, 5020. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Srivastava, A.; Prajapati, A. Albumin and functionalized albumin nanoparticles: Production strategies, characterization, and target indications. Asian Biomed. 2020, 14, 217–242. [Google Scholar] [CrossRef]
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, 14, 6387–6406. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Pyshnaya, I.A.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Poletaeva, J.; Zavjalov, E.L.; Silnikov, V.N.; Ryabchikova, E.I.; Godovikova, T.S. Rational Design of Albumin Theranostic Conjugates for Gold Nanoparticles Anticancer Drugs: Where the Seed Meets the Soil? Biomedicines 2021, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Mariam, J.; Sivakami, S.; Dongre, P.M. Albumin corona on nanoparticles—A strategic approach in drug delivery. Drug Deliv. 2016, 23, 2668–2676. [Google Scholar] [CrossRef]
- Kratz, F.; Elsadek, B. Clinical impact of serum proteins on drug delivery. J. Control. Release 2012, 161, 429–445. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Damascelli, B.; Soon-Shiong, P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl. Oncol. 2009, 2, 59–64. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Niu, G.; Huang, J.; Chen, K.; Li, X.; Chen, X. Human serum albumin coated iron oxide nanoparticles for efficient cell labeling. Chem. Commun. 2010, 46, 433–435. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery-new applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta 2013, 1830, 5526–5534. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Oh, P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 1994, 269, 6072–6082. [Google Scholar] [CrossRef]
- Bern, M.; Sand, K.M.K.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release 2015, 211, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Human Serum Albumin in Blood Detoxification Treatment. In Albumin in Medicine; Springer: Singapore, 2016; pp. 209–225. [Google Scholar]
- Kragh-hansen, U. Human Serum Albumin: A Multifunctional Protein. In Albumin in Medicine; Springer: Singapore, 2016; pp. 1–24. ISBN 978-981-10-2115-2. [Google Scholar]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Reiber, H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta 2001, 310, 173–186. [Google Scholar] [CrossRef]
- Otagiri, M.; Chuang, V.T.G. Pharmaceutically Important Pre- and Posttranslational Modifications on Human Serum Albumin. Biol. Pharm. Bull. 2009, 32, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Anguizola, J.; Matsuda, R.; Barnaby, O.S.; Hoy, K.S.; Wa, C.; DeBolt, E.; Koke, M.; Hage, D.S. Review: Glycation of human serum albumin. Clin. Chim. Acta 2013, 425, 64–76. [Google Scholar] [CrossRef]
- Lee, P.; Wu, X. Review: Modifications of Human Serum Albumin and their Binding Effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef]
- De Simone, G.; Masi, A.; Ascenzi, P.; Scienze, D.; Roma, S.; Marconi, V. Serum Albumin: A Multifaced Enzyme. Int. J. Mol. Sci. 2021, 221, 86. [Google Scholar] [CrossRef]
- Zeeshan, F.; Madheswaran, T.; Panneerselvam, J.; Taliyan, R.; Kesharwani, P. Human Serum Albumin as Multifunctional Nanocarrier for Cancer Therapy. J. Pharm. Sci. 2021, 110, 3111–3117. [Google Scholar] [CrossRef]
- Kragh-Hansen, U. Molecular and practical aspects of the enzymatic properties of human serum albumin and of albumin-ligand complexes. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 5535–5544. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Kumar, P.; Gautam, A.K.; Singh, N.; Bera, H.; Sarkar, S.; Saraf, S.A.; Saha, S. Albumin-based nanomaterials in drug delivery and biomedical applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 407–426. ISBN 9780128208748. [Google Scholar]
- Watanabe, H.; Maruyama, T. Albumin as a Biomarker. In Albumin in Medicine; Springer: Singapore, 2016; pp. 51–69. [Google Scholar]
- Rizo-téllez, S.A.; Méndez-garcía, L.A.; Rivera-rugeles, A.C.; Miranda-garcía, M.; Manjarrez-reyna, A.N.; Viurcos-sanabria, R.; Solleiro-villavicencio, H.; Becerril-villanueva, E.; Carrillo-ruíz, J.D.; Cota-arce, J.M.; et al. The Combined Use of Cytokine Serum Values with Laboratory Parameters Improves Mortality Prediction of COVID-19 Patients: The Interleukin-15-to-Albumin Ratio. Microorganisms 2021, 9, 2159. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Fatima, R.; Lee-Smith, W.; Assaly, R. The association of low serum albumin level with severe COVID-19: A systematic review and meta-analysis. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Ahn, N.S. Review: Roles of human serum albumin in prediction, diagnoses and treatment of COVID-19. Int. J. Biol. Macromol. 2021, 193, 948–955. [Google Scholar] [CrossRef]
- Violi, F.; Cangemi, R.; Romiti, G.F.; Ceccarelli, G.; Oliva, A.; Alessandri, F.; Pirro, M.; Pignatelli, P.; Lichtner, M.; Carraro, A.; et al. Is Albumin Predictor of Mortality in COVID-19? Antioxidants Redox Signal. 2021, 35, 139–142. [Google Scholar] [CrossRef]
- Rahmani-Kukia, N.; Abbasi, A.; Pakravan, N.; Hassan, Z.M. Measurement of oxidized albumin: An opportunity for diagnoses or treatment of COVID-19. Bioorg. Chem. 2020, 105, 104429. [Google Scholar] [CrossRef]
- Watanabe, H.; Imafuku, T.; Otagiri, M.; Maruyama, T. Clinical Implications Associated with the Posttranslational Modification–Induced Functional Impairment of Albumin in Oxidative Stress–Related Diseases. J. Pharm. Sci. 2017, 106, 2195–2203. [Google Scholar] [CrossRef]
- Oettl, K.; Marsche, G. Redox State of Human Serum Albumin in Terms of Cysteine-34 in Health and Disease. In Methods Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 474, pp. 181–195. [Google Scholar]
- Chubarov, A.; Spitsyna, A.; Krumkacheva, O.; Mitin, D.; Suvorov, D.; Tormyshev, V.; Fedin, M.; Bowman, M.K.; Bagryanskaya, E. Reversible Dimerization of Human Serum Albumin. Molecules 2021, 26, 108. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef]
- Knudsen Sand, K.M.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the interaction between FcRn and albumin: Opportunities for design of albumin-based therapeutics. Front. Immunol. 2015, 6, 682. [Google Scholar] [CrossRef]
- Schmidt, E.G.W.; Hvam, M.L.; Antunes, F.; Cameron, J.; Viuff, D.; Andersen, B.; Kristensen, N.N.; Howard, K.A. Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin. J. Biol. Chem. 2017, 292, 13312–13322. [Google Scholar] [CrossRef]
- Hashem, L.; Swedrowska, M.; Vllasaliu, D. Intestinal uptake and transport of albumin nanoparticles: Potential for oral delivery. Nanomedicine 2018, 13, 1255–1265. [Google Scholar] [CrossRef]
- Ren, Q.; Weyer, K.; Rbaibi, Y.; Long, K.R.; Tan, X.R.J.; Nielsen, R.; Christensen, E.I.; Baty, C.J.; Kashlan, O.B.; Weisz, O.A.; et al. Distinct functions of megalin and cubilin receptors in recovery of normal and nephrotic levels of filtered albumin. Am. J. Physiol. Ren. Physiol. 2020, 318, 1284–1294. [Google Scholar] [CrossRef]
- Swiercz, R.; Mo, M.; Khare, P.; Schneider, Z.; Ober, R.J.; Ward, E.S. Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget 2017, 8, 3528–3541. [Google Scholar] [CrossRef]
- Otagiri, M.; Giam Chuang, V.T. Albumin in Medicine: Pathological and Clinical Applications; Springer: Singapore, 2016; pp. 1–277. [Google Scholar] [CrossRef]
- Qi, T.; Cao, Y. In translation: Fcrn across the therapeutic spectrum. Int. J. Mol. Sci. 2021, 22, 3048. [Google Scholar] [CrossRef]
- Kuo, T.T.; Baker, K.; Yoshida, M.; Qiao, S.W.; Aveson, V.G.; Lencer, W.I.; Blumberg, R.S. Neonatal Fc receptor: From immunity to therapeutics. J. Clin. Immunol. 2010, 30, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Sand, K.M.K.; Bern, M.; Nilsen, J.; Dalhus, B.; Gunnarsen, K.S.; Cameron, J.; Grevys, A.; Bunting, K.; Sandlie, I.; Andersen, J.T. Interaction with both domain I and III of albumin is required for optimal pH-dependent binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 2014, 289, 34583–34594. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, Y.; Berger, M.; Seifert, A.; Bihoreau, N.; Chevreux, G. Human serum albumin presents isoform variants with altered neonatal Fc receptor interactions. Protein Sci. 2019, 28, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.C.; Myslinski, J.; Pratap, S.; Flores, B.; Rhodes, G.; Campos-bilderback, S.B.; Sandoval, R.M.; Kumar, S.; Patel, M.; Molitoris, B.A.; et al. Mechanism of increased clearance of glycated albumin by proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2016, 310, F1089–F1102. [Google Scholar] [CrossRef]
- Park, C.R.; Jo, J.H.; Song, M.G.; Park, J.Y.; Kim, Y.H.; Youn, H.; Paek, S.H.; Chung, J.K.; Jeong, J.M.; Lee, Y.S.; et al. Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models. Theranostics 2019, 9, 7447–7457. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, L.; Di Stasi, R.; Romanelli, A.; D’andrea, L.D. Exploiting protein n-terminus for site-specific bioconjugation. Molecules 2021, 26, 3521. [Google Scholar] [CrossRef] [PubMed]
- Biosci, I.J.; Ndayisenga, F.; Lin, P.; Li, R.; Hameed, A.; Zhang, Y. Site-specific modification of proteins by chemical/enzymatic strategies. Int. J. Biosci. 2020, 6655, 12–50. [Google Scholar]
- Shadish, J.A.; DeForest, C.A. Site-Selective Protein Modification: From Functionalized Proteins to Functional Biomaterials. Matter 2020, 2, 50–77. [Google Scholar] [CrossRef]
- Funk, W.E.; Li, H.; Iavarone, A.T.; Williams, E.R.; Riby, J.; Rappaport, S.M. Enrichment of cysteinyl adducts of human serum albumin. Anal. Biochem. 2010, 400, 61–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sengupta, S.; Chen, H.; Togawa, T.; DiBello, P.M.; Majors, A.K.; Büdy, B.; Ketterer, M.E.; Jacobsen, D.W. Albumin Thiolate Anion Is an Intermediate in the Formation of Albumin-S-S-Homocysteine. J. Biol. Chem. 2001, 276, 30111–30117. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Zakharova, O.D.; Koval, O.A.; Romaschenko, A.V.; Akulov, A.E.; Zavjalov, E.L.; Razumov, I.A.; Koptyug, I.V.; Knorre, D.G.; Godovikova, T.S. Design of protein homocystamides with enhanced tumor uptake properties for 19F magnetic resonance imaging. Bioorg. Med. Chem. 2015, 23, 6943–6954. [Google Scholar] [CrossRef]
- Lisitskiy, V.A.; Khan, H.; Popova, T.V.; Chubarov, A.S.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Koptyug, I.V.; Moshkin, M.P.; et al. Multifunctional human serum albumin-therapeutic nucleotide conjugate with redox and pH-sensitive drug release mechanism for cancer theranostics. Bioorg. Med. Chem. Lett. 2017, 27, 3925–3930. [Google Scholar] [CrossRef]
- Tormyshev, V.; Chubarov, A.; Krumkacheva, O.; Trukhin, D.; Rogozhnikova, O.; Spitsina, A.; Kuzhelev, A.; Koval, V.; Fedin, M.; Bowman, M.; et al. A Methanethiosulfonate Derivative of OX063 Trityl: A Promising and Efficient Reagent for SDSL of Proteins. Chemistry 2020, 26, 1–9. [Google Scholar] [CrossRef]
- Krumkacheva, O.A.; Timofeev, I.O.; Politanskaya, L.V.; Polienko, Y.F.; Tretyakov, E.V.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Chubarov, A.S.; Bagryanskaya, E.G.; et al. Triplet Fullerenes as Prospective Spin Labels for Nanoscale Distance Measurements by Pulsed Dipolar EPR. Angew. Chem. Int. Ed. 2019, 58, 13271–13275. [Google Scholar] [CrossRef] [PubMed]
- Sannikova, N.E.; Timofeev, I.O.; Chubarov, A.S.; Lebedeva, N.S.; Semeikin, A.S.; Kirilyuk, I.A.; Tsentalovich, Y.P.; Fedin, M.V.; Bagryanskaya, E.G.; Krumkacheva, O.A. Application of EPR to porphyrin-protein agents for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 211, 112008. [Google Scholar] [CrossRef] [PubMed]
- Espeel, P.; Du Prez, F.E. One-pot multi-step reactions based on thiolactone chemistry: A powerful synthetic tool in polymer science. Eur. Polym. J. 2015, 62, 247–272. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Shakirov, M.M.; Koptyug, I.V.; Sagdeev, R.Z.; Knorre, D.G.; Godovikova, T.S. Synthesis and characterization of fluorinated homocysteine derivatives as potential molecular probes for 19F magnetic resonance spectroscopy and imaging. Bioorg. Med. Chem. Lett. 2011, 21, 4050–4053. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, S.; Kutseikin, S.; Morozov, D.; Krumkacheva, O.; Spitsyna, A.; Gatilov, Y.; Silnikov, V.; Angelovski, G.; Bowman, M.K.; Kirilyuk, I.; et al. Human Serum Albumin Labelled with Sterically-Hindered Nitroxides as Potential MRI Contrast Agents. Molecules 2020, 25, 1709. [Google Scholar] [CrossRef]
- Chubarov, A.S. Homocysteine Thiolactone: Biology and Chemistry. Encyclopedia 2021, 1, 445–459. [Google Scholar] [CrossRef]
- Popova, T.V.; Khan, H.; Chubarov, A.S.; Lisitskiy, V.A.; Antonova, N.M.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Silnikov, V.N.; Ahmad, S.; et al. Biotin-decorated anti-cancer nucleotide theranostic conjugate of human serum albumin: Where the seed meets the soil? Bioorg. Med. Chem. Lett. 2018, 28, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Tao, H.; Wang, R.; Sheng, W.; Zhen, Y. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int. J. Biol. Macromol. 2021, 187, 24–34. [Google Scholar] [CrossRef]
- Um, W.; Park, J.; Youn, A.; Cho, H.; Lim, S.; Lee, J.W.; Yoon, H.Y.; Lim, D.K.; Park, J.H.; Kim, K. A Comparative Study on Albumin-Binding Molecules for Targeted Tumor Delivery through Covalent and Noncovalent Approach. Bioconjug. Chem. 2019, 30, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Linciano, S.; Angelini, A. Non-covalent albumin-binding ligands for extending the circulating half-life of small biotherapeutics. MedChemComm 2019, 10, 1068–1081. [Google Scholar] [CrossRef]
- Loureiro, A.; Azoia, N.G.; Gomes, A.C.; Cavaco-Paulo, A. Albumin-Based Nanodevices as Drug Carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Manjili, H.K.; Danafar, H.; Davaran, S. Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. Int. J. Biol. Macromol. 2018, 108, 909–915. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, B.; Dai, X.; Wang, X.; Gao, F.; Zhang, X.; Tang, J. Glutaraldehyde mediated conjugation of amino-coated magnetic nanoparticles with albumin protein for nanothermotherapy. J. Nanosci. Nanotechnol. 2010, 10, 7117–7120. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A. Development of an Albumin-Binding Ligand for Prolonging the Plasma Half-Life of Peptide Therapeutics; EPFL: Lausanne, Switzerland, 2017; Volume 7728. [Google Scholar]
- Chen, Q.; Liu, Z. Albumin carriers for cancer theranostics: A conventional platform with new promise. Adv. Mater. 2016, 28, 10557–10566. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-coated single-core iron oxide nanoparticles for enhanced molecular magnetic imaging (Mri/mpi). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The Protein Corona and its Effects on Nanoparticle-Based Drug Delivery Systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Moya, C.; Escudero, R.; Malaspina, D.C.; De La Mata, M.; Hernández-Saz, J.; Faraudo, J.; Roig, A. Insights into Preformed Human Serum Albumin Corona on Iron Oxide Nanoparticles: Structure, Effect of Particle Size, Impact on MRI Efficiency, and Metabolization. ACS Appl. Bio Mater. 2019, 2, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Mazario, E.; Forget, A.; Belkahla, H.; Lomas, J.S.; Decorse, P.; Chevillot-Biraud, A.; Verbeke, P.; Wilhelm, C.; Ammar, S.; El Hage Chahine, J.M.; et al. Functionalization of Iron Oxide Nanoparticles With HSA Protein for Thermal Therapy. IEEE Trans. Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Rahdar, S.; Rahdar, A.; Ahmadi, S.; Trant, J.F. Adsorption of bovine serum albumin (BSA) by bare magnetite nanoparticles with surface oxidative impurities that prevent aggregation. Can. J. Chem. 2019, 97, 577–583. [Google Scholar] [CrossRef]
- Aires, A.; Ocampo, S.M.; Cabrera, D.; La Cueva, L.D.; Salas, G.; Teran, F.J.; Cortajarena, A.L. BSA-coated magnetic nanoparticles for improved therapeutic properties. J. Mater. Chem. B 2015, 3, 6239–6247. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Borowska, M. Magnetic nanoparticles coated with aminated starch for HSA immobilization- simple and fast polymer surface functionalization. Int. J. Biol. Macromol. 2019, 136, 106–114. [Google Scholar] [CrossRef]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.G.; et al. Albumin and hyaluronic acid-coated superparamagnetic iron oxide nanoparticles loaded with paclitaxel for biomedical applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef]
- Yu, S.M.; Laromaine, A.; Roig, A. Enhanced stability of superparamagnetic iron oxide nanoparticles in biological media using a pH adjusted-BSA adsorption protocol. J. Nanopart. Res. 2014, 16, 2484. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.M.; Carenza, E.; Laromaine, A.; Roig, A. Protective Effects of Bovine Serum Albumin on Superparamagnetic Iron Oxide Nanoparticles Evaluated in the Nematode Caenorhabditis elegans. ACS Biomater. Sci. Eng. 2015, 1, 1129–1138. [Google Scholar] [CrossRef]
- Zaloga, J.; Feoktystov, A.; Garamus, V.M.; Karawacka, W.; Ioffe, A.; Brückel, T.; Tietze, R.; Alexiou, C.; Lyer, S. Studies on the Adsorption and Desorption of Mitoxantrone to Lauric Acid / Albumin Coated Iron Oxide Nanoparticles. Colloids Surf. B Biointerfaces 2018, 161, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, J.; Pöttler, M.; Leitinger, G.; Friedrich, R.P.; Almer, G.; Lyer, S.; Baum, E.; Tietze, R.; Heimke-Brinck, R.; Mangge, H.; et al. Pharmaceutical formulation of HSA hybrid coated iron oxide nanoparticles for magnetic drug targeting. Eur. J. Pharm. Biopharm. 2016, 101, 152–162. [Google Scholar] [CrossRef]
- Zaloga, J.; Stapf, M.; Nowak, J.; Pöttler, M.; Friedrich, R.P.; Tietze, R.; Lyer, S.; Lee, G.; Odenbach, S.; Hilger, I.; et al. Tangential flow ultrafiltration allows purification and concentration of lauric acid-/albumin-coated particles for improved magnetic treatment. Int. J. Mol. Sci. 2015, 16, 19291–19307. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [PubMed]
- Corem-Salkmon, E.; Ram, Z.; Daniels, D.; Perlstein, B.; Last, D.; Salomon, S.; Tamar, G.; Shneor, R.; Guez, D.; Margel, S.; et al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int. J. Nanomed. 2011, 6, 1595–1602. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays. Methods in Molecular Biology; Springer: Berlin, Germany, 2017; pp. 1–17. [Google Scholar]
- Geppert, M.; Himly, M. Iron Oxide Nanoparticles in Bioimaging—An Immune Perspective. Front. Immunol. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- An, L.; Yan, C.; Mu, X.; Tao, C.; Tian, Q.; Lin, J.; Yang, S. Paclitaxel-Induced Ultrasmall Gallic Acid-Fe@BSA Self-Assembly with Enhanced MRI Performance and Tumor Accumulation for Cancer Theranostics. ACS Appl. Mater. Interfaces 2018, 10, 28483–28493. [Google Scholar] [CrossRef]
- Balk, M.; Haus, T.; Band, J.; Unterweger, H.; Schreiber, E.; Friedrich, R.P.; Alexiou, C.; Gostian, A.O. Cellular spion uptake and toxicity in various head and neck cancer cell lines. Nanomaterials 2021, 11, 726. [Google Scholar] [CrossRef]
- Poller, J.M.; Zaloga, J.; Schreiber, E.; Unterweger, H.; Janko, C.; Radon, P.; Eberbeck, D.; Trahms, L.; Alexiou, C.; Friedrich, R.P. Selection of potential iron oxide nanoparticles for breast cancer treatment based on in vitro cytotoxicity and cellular uptake. Int. J. Nanomed. 2017, 12, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Erdal, E.; Demirbilek, M.; Yeh, Y.; Akbal, Ö.; Ruff, L.; Bozkurt, D.; Cabuk, A.; Senel, Y.; Gumuskaya, B.; Algın, O.; et al. A Comparative Study of Receptor-Targeted Magnetosome and HSA-Coated Iron Oxide Nanoparticles as MRI Contrast-Enhancing Agent in Animal Cancer Model. Appl. Biochem. Biotechnol. 2018, 185, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Pöttler, M.; Hofmann, S.; Dürr, S.; Unterweger, H.; Wiest, I.; Zaloga, J.; Alexiou, C.; Jeschke, U.; Janko, C. Effect of BSA-coated superparamagnetic iron oxide nanoparticles on granulosa cells. Anticancer Res. 2016, 36, 3147–3154. [Google Scholar]
- Hai, J.; Piraux, H.; Mazarío, E.; Volatron, J.; Ha-Duong, N.T.; Decorse, P.; Lomas, J.S.; Verbeke, P.; Ammar, S.; Wilhelm, C.; et al. Maghemite nanoparticles coated with human serum albumin: Combining targeting by the iron-acquisition pathway and potential in photothermal therapies. J. Mater. Chem. B 2017, 5, 3154–3162. [Google Scholar] [CrossRef]
- Gou, Y.; Miao, D.; Zhou, M.; Wang, L.; Zhou, H.; Su, G. Bio-Inspired Protein-Based Nanoformulations for Cancer Theranostics. Front. Pharmacol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Toropova, Y.G.; Motorina, D.S.; Zelinskaya, I.; Korolev, D.V.; Schulmeister, G.; Skorik, Y. Generation of Reactive Oxygen Species by Human Whole Blood Cells Exposed to Iron Oxide Magnetic Nanoparticles Coated with Different Shells. Bull. Exp. Biol. Med. 2021, 171, 77–80. [Google Scholar] [CrossRef]
- Nunes, A.D.C.; Gomes-Silva, L.A.; Zufelato, N.; Prospero, A.G.; Quini, C.C.; Matos, R.V.R.; Miranda, J.R.A.; Bakuzis, A.F.; Castro, C.H. Albumin Coating Prevents Cardiac Effect of the Magnetic Nanoparticles. IEEE Trans. Nanobiosci. 2019, 18, 640–650. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Zelinskaya, I.A.; Gorshkova, M.N.; Motorina, D.S.; Korolev, D.V.; Velikonivtsev, F.S.; Gareev, K.G. Albumin covering maintains endothelial function upon magnetic iron oxide nanoparticles intravenous injection in rats. J. Biomed. Mater. Res. Part A 2021, 109, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkhov, P.; Semkina, A.; Naumenko, V.; Plotnikova, E.; Yakubovskaya, R.; Vodopyanov, S.; Abakumov, A.; Majouga, A.; Grin, M.; Chekhonin, V.; et al. HSA—Coated magnetic nanoparticles for mri-guided photodynamic cancer therapy. Pharmaceutics 2018, 10, 284. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Albumin based versatile multifunctional nanocarriers for cancer therapy: Fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater. Sci. Eng. C 2017, 81, 607–626. [Google Scholar] [CrossRef]
- Lamichhane, S.; Lee, S. Albumin nanoscience: Homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharm. Res. 2020, 43, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Tian, B.; Yi, Y.; Wei, Z.Z.; Wei, F. Synthesis of tumor-targeted folate conjugated fluorescent magnetic albumin nanoparticles for enhanced intracellular dual-modal imaging into human brain tumor cells. Anal. Biochem. 2016, 512, 8–17. [Google Scholar] [CrossRef]
- Hiremath, C.G.; Kariduraganavar, M.Y.; Hiremath, M.B. Synergistic delivery of 5-fluorouracil and curcumin using human serum albumin-coated iron oxide nanoparticles by folic acid targeting. Prog. Biomater. 2018, 7, 297–306. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Nukolova, N.V.; Sokolsky-Papkov, M.; Shein, S.A.; Sandalova, T.O.; Vishwasrao, H.M.; Grinenko, N.F.; Gubsky, I.L.; Abakumov, A.M.; Kabanov, A.V.; et al. VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Kostevšek, N. A review on the optimal design of magnetic nanoparticle-based t2 mri contrast agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Shamsutdinova, N.; Zairov, R.; Nizameev, I.; Gubaidullin, A.; Mukhametshina, A.; Podyachev, S.; Ismayev, I.; Kadirov, M.; Voloshina, A.; Mukhametzyanov, T.; et al. Tuning magnetic relaxation properties of “hard cores” in core-shell colloids by modification of “soft shell”. Colloids Surf. B Biointerfaces 2018, 162, 52–59. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, G.T.; Kim, H.K.; Sung, B.; Lee, Y.; Kim, M.; Chang, Y.; Seo, J.H. Surface Design of Eu-Doped Iron Oxide Nanoparticles for Tuning the Magnetic Relaxivity. ACS Appl. Mater. Interfaces 2018, 10, 25080–25089. [Google Scholar] [CrossRef]
- Maboudi, S.A.; Shojaosadati, S.A.; Aliakbari, F.; Arpanaei, A. Theranostic magnetite cluster@silica@albumin double-shell particles as suitable carriers for water-insoluble drugs and enhanced T2 MR imaging contrast agents. Mater. Sci. Eng. C 2019, 99, 1485–1492. [Google Scholar] [CrossRef]
- Tzameret, A.; Ketter-Katz, H.; Edelshtain, V.; Sher, I.; Corem-Salkmon, E.; Levy, I.; Last, D.; Guez, D.; Mardor, Y.; Margel, S.; et al. In vivo MRI assessment of bioactive magnetic iron oxide/human serum albumin nanoparticle delivery into the posterior segment of the eye in a rat model of retinal degeneration. J. Nanobiotechnol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Ostroverkhov, P.; Semkina, A.; Nikitin, A.; Smirnov, A.; Vedenyapina, D.; Vlasova, K.; Kireev, I.; Grin, M.; Chekhonin, V.; Majouga, A.; et al. Human serum albumin as an effective coating for hydrophobic photosensitizes immobilization on magnetic nanoparticles. J. Magn. Magn. Mater. 2019, 475, 108–114. [Google Scholar] [CrossRef]
- Babincová, M.; Vrbovská, H.; Sourivong, P.; Babinec, P.; Durdík, Š. Application of albumin-embedded magnetic nanoheaters for release of etoposide in integrated chemotherapy and hyperthermia of U87-MG glioma cells. Anticancer Res. 2018, 38, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Sodium alginate–polyvinyl alcohol–bovin serum albumin coated Fe3O4 nanoparticles as anticancer drug delivery vehicle: Doxorubicin loading and in vitro release study and cytotoxicity to HepG2 and L02 cells. Mater. Sci. Eng. C 2017, 79, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Sher, I.; Corem-Salkmon, E.; Ziv-Polat, O.; Meir, A.; Treves, A.J.; Nagler, A.; Kalter-Leibovici, O.; Margel, S.; Rotenstreich, Y. Bioactive magnetic near Infra-Red fluorescent core-shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J. Nanobiotechnol. 2015, 13, 34. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).