Synthesis and Characterization of Composites with Y-Hexaferrites for Electromagnetic Interference Shielding Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of PPY-DBSA
2.2. Synthesis of Ferrite
2.3. Ferrites Polymer Composite
3. Results and Discussion
3.1. Electrical Properties
3.2. Dielectric Properties
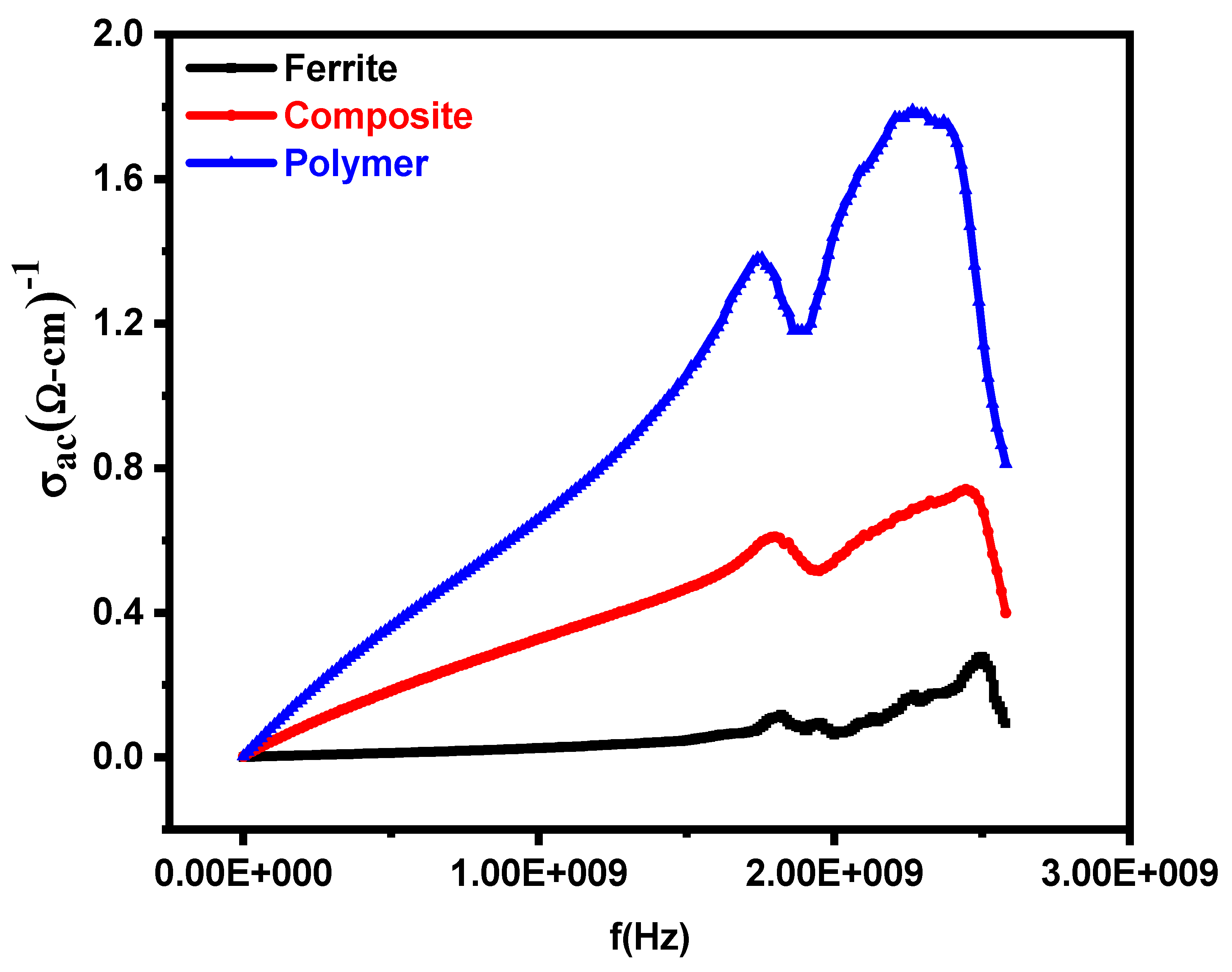
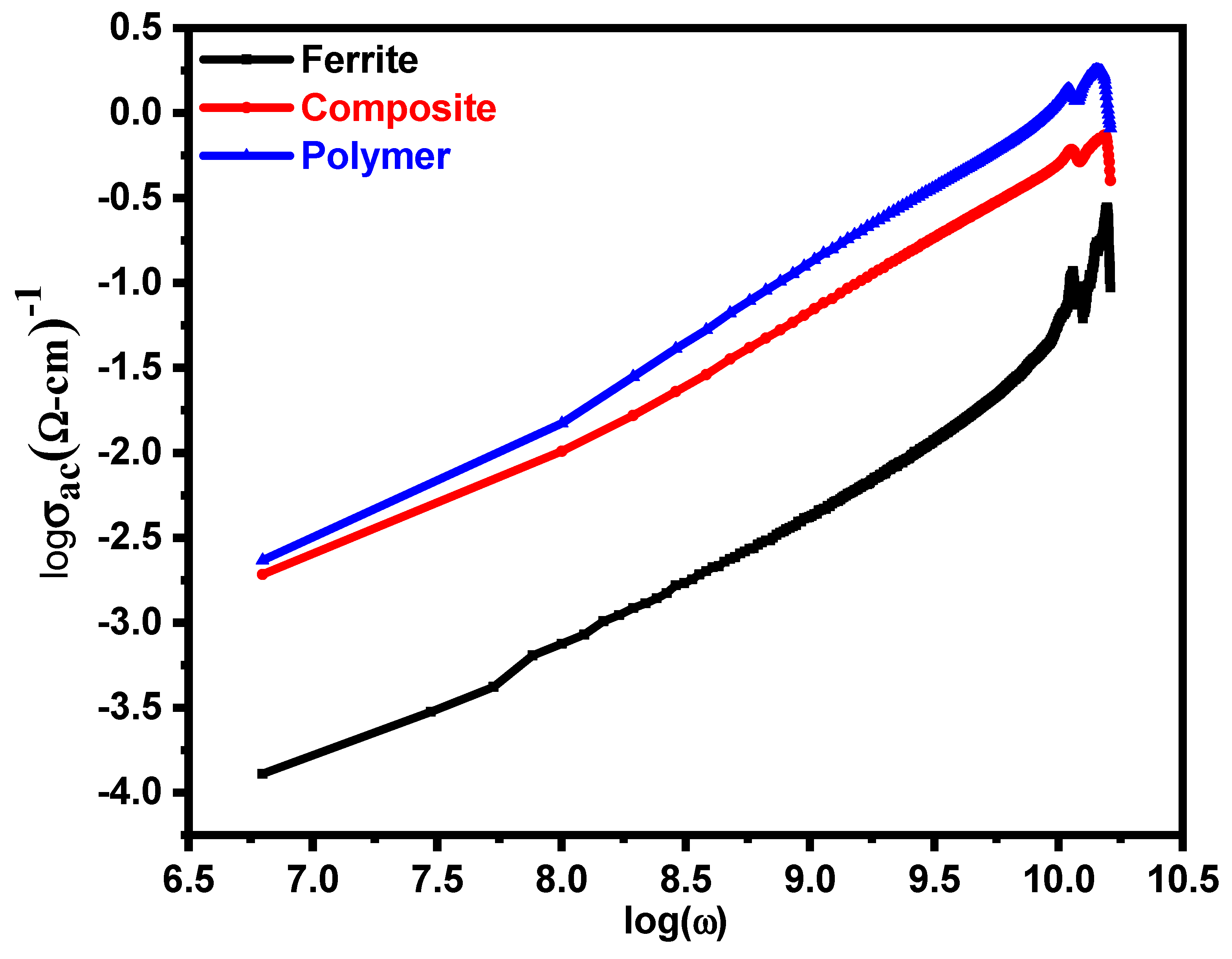
3.3. AC Conductivity
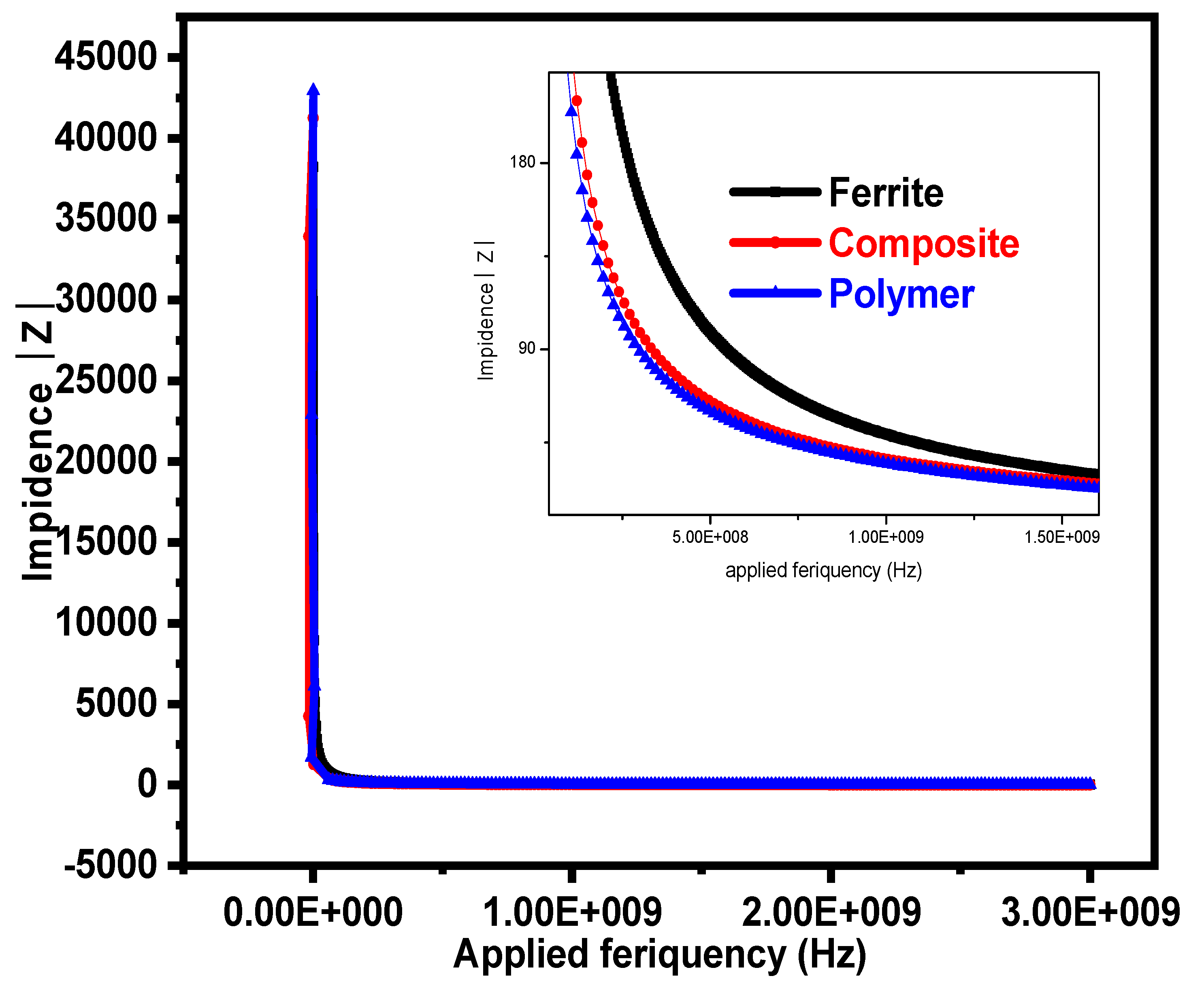
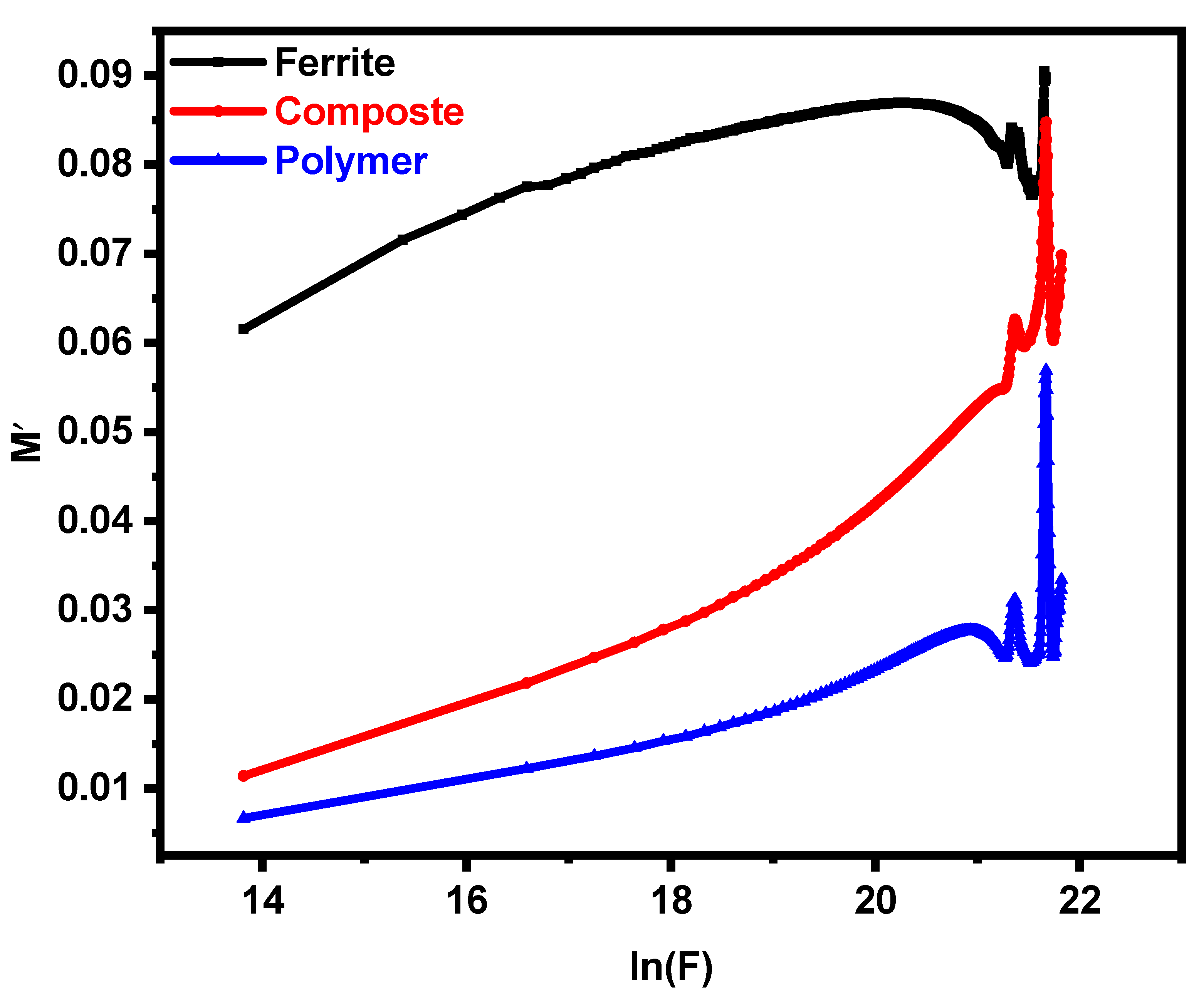
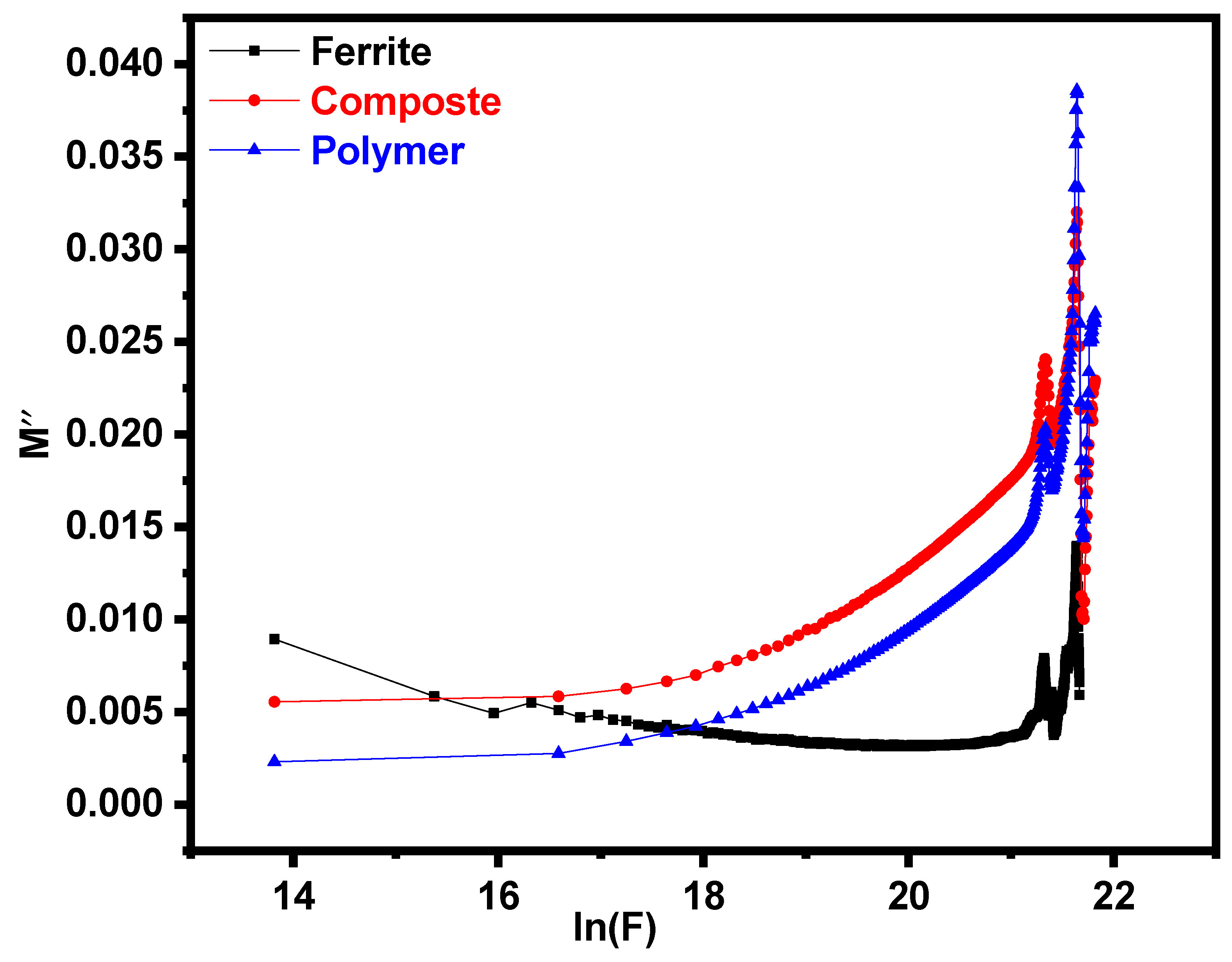
3.4. Impedance Analysis
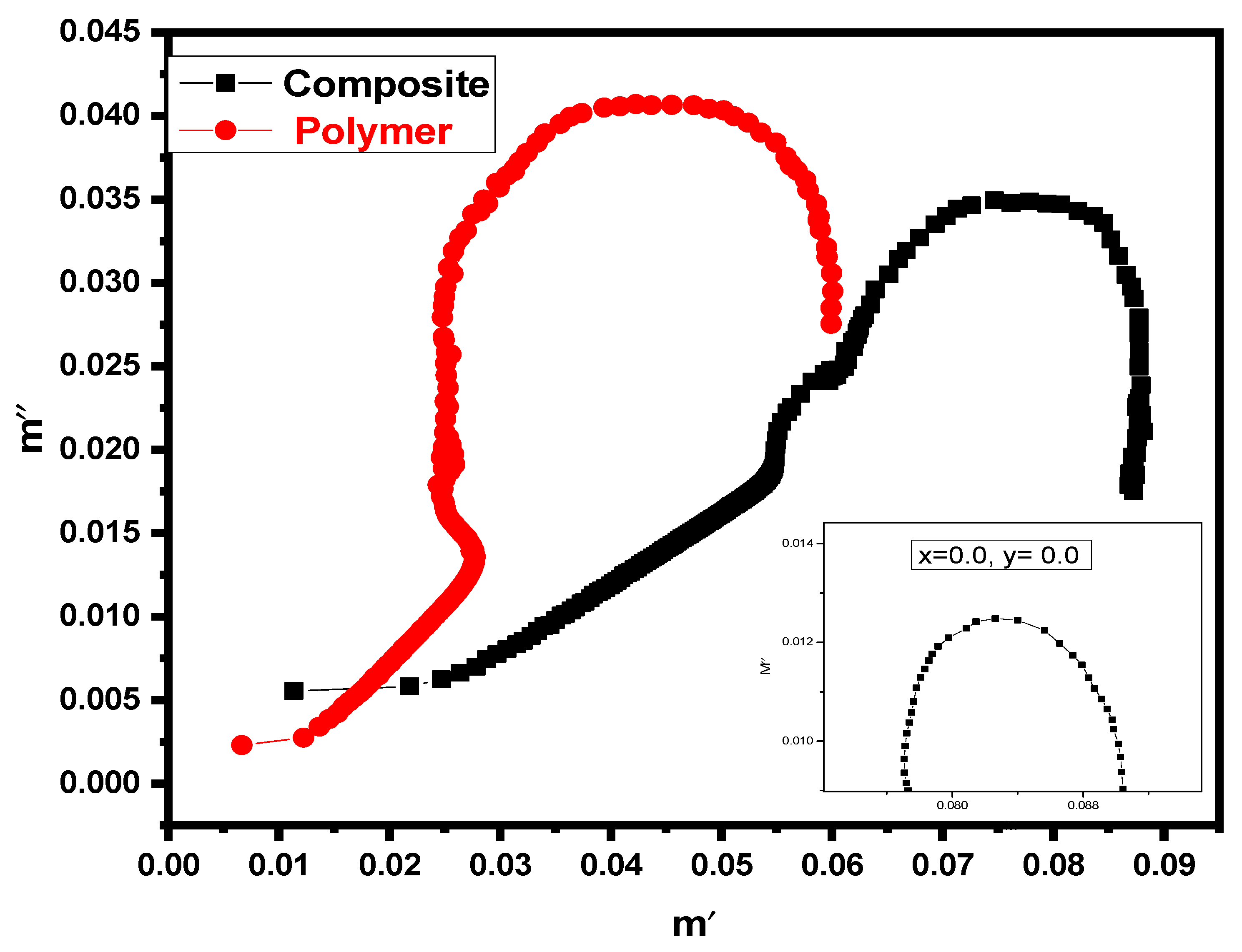
3.5. Nyquist Plot (Cole-Cole Plot)
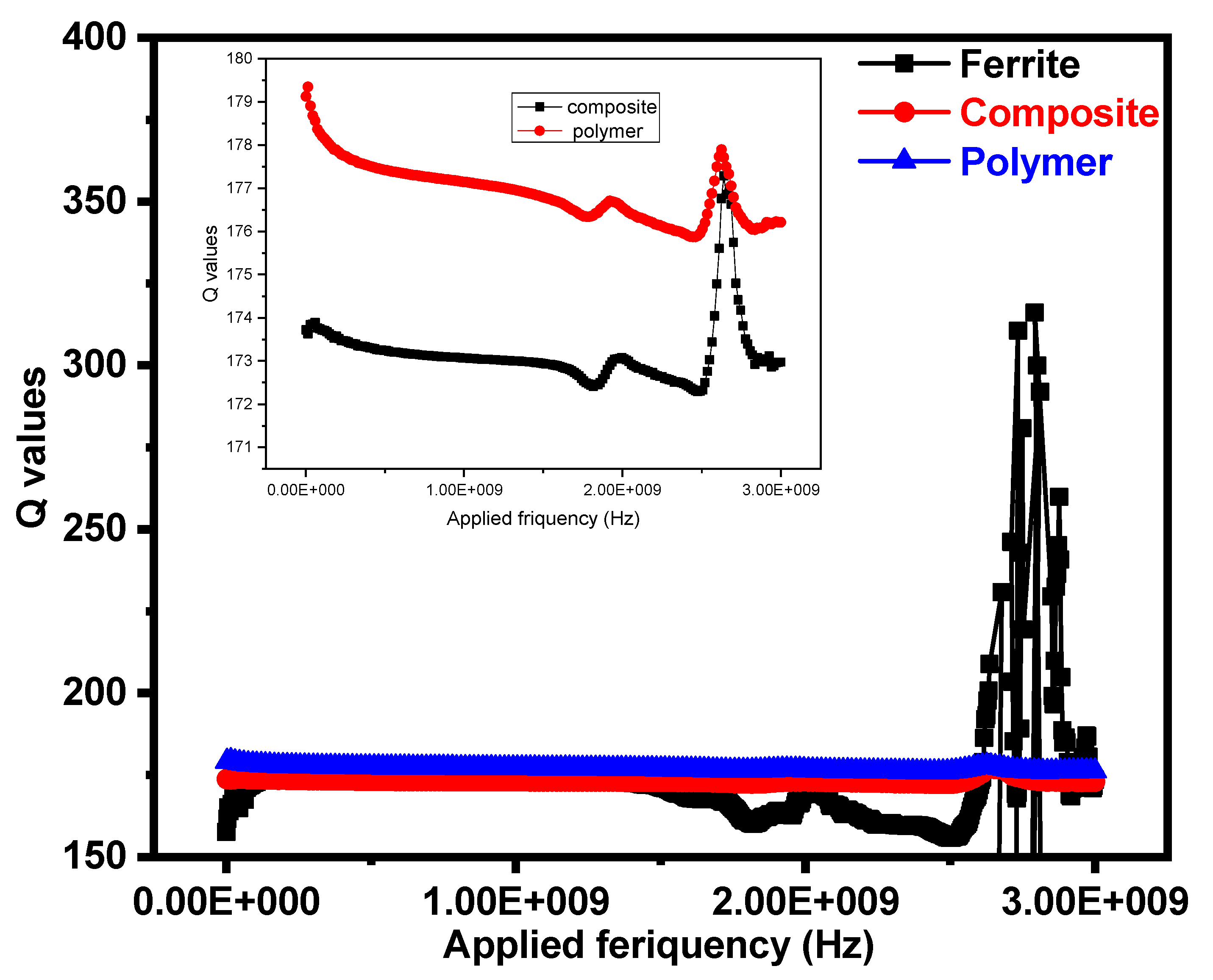
3.6. Quality Factor
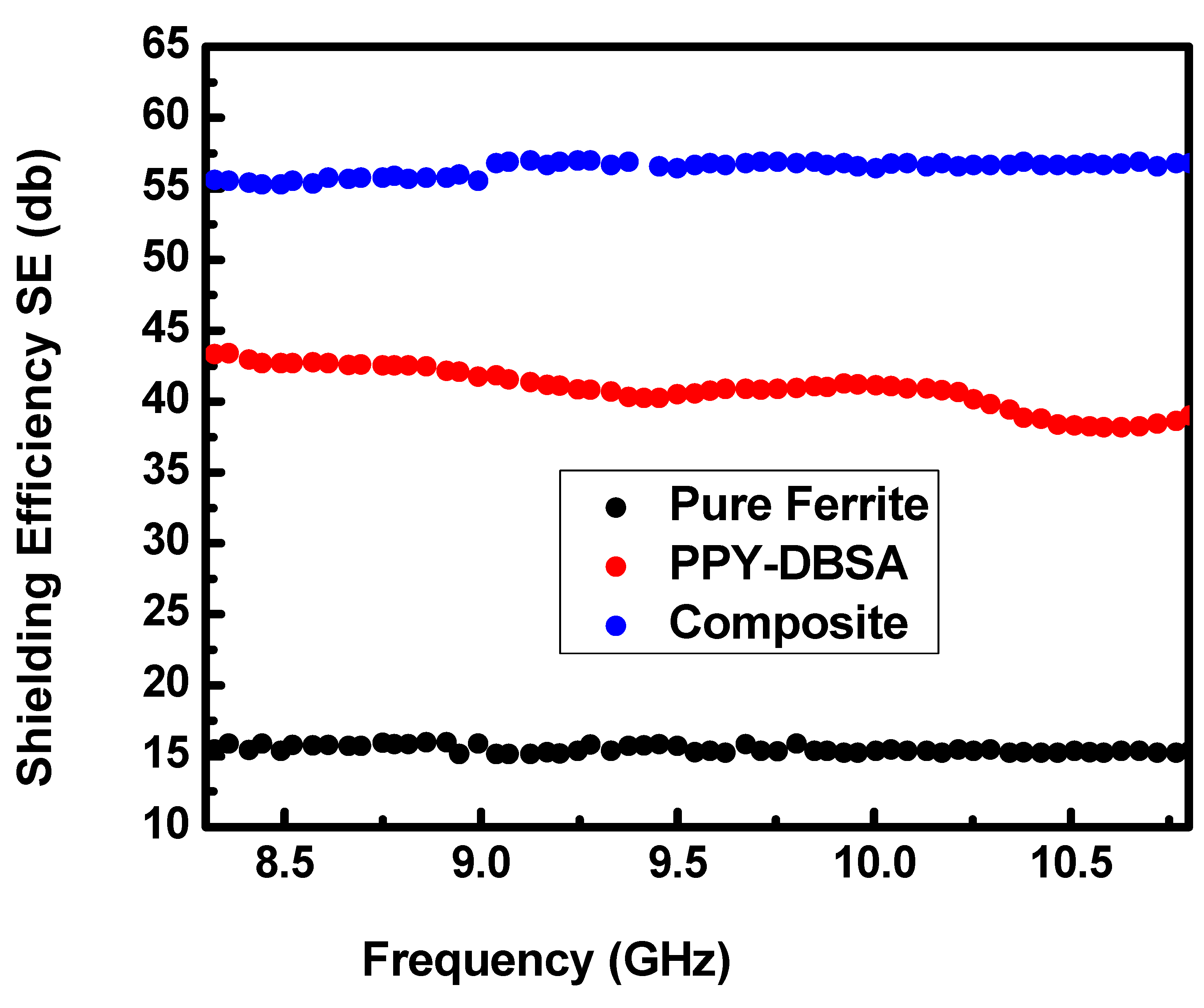
3.7. EMI Shielding Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gubbala, S.; Nathani, H.; Koizol, K.; Misra, R.D.K. Magnetic properties of nanocrystalline Ni–Zn, Zn–Mn, and Ni–Mn ferrites synthesized by reverse micelle technique. Phys. B Condens. Matter 2004, 348, 317–328. [Google Scholar] [CrossRef]
- Gul, I.H.; Ahmed, W.; Maqsood, A. Electrical and magnetic characterization of nanocrystalline Ni–Zn ferrite synthesis by co-precipitation route. J. Magn. Magn. Mater. 2008, 320, 270–275. [Google Scholar] [CrossRef]
- Farid, H.M.T.; Ahmad, I.; Bhatti, K.A.; Ali, I.; Ramay, S.M.; Mahmood, A. The effect of praseodymium on Cobalt-Zinc spinel ferrites. Ceram. Int. 2017, 43, 7253–7260. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Pasnicu, C.; Craus, M.L. Effects of the rare-earth ions on some properties of a nickel-zinc ferrite. J. Phys. Condens. Matter 1994, 6, 5707. [Google Scholar] [CrossRef]
- Gul, I.H.; Maqsood, A. Structural, magnetic and electrical properties of cobalt ferrites prepared by the sol–gel route. J. Alloys Compd. 2008, 465, 227–231. [Google Scholar] [CrossRef]
- Saafan, S.A.; Assar, S.T.; Moharram, B.M.; El Nimr, K. Comparison study of some structural and magnetic properties of nano-structured and bulk Li–Ni–Zn ferrite samples. J. Magn. Magn. Mater. 2010, 322, 628–632. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Ishaque, M.; Khan, H.M.; Ashiq, M.N.; Rana, M.U. Structural and magnetic properties of holmium substituted cobalt ferrites synthesized by chemical co-precipitation method. J. Magn. Magn. Mater. 2012, 324, 3773–3777. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Z.; Jiang, L.; Chen, S.; Ma, J.; Zhou, F. Flexible and conductive meta-aramid fiber paper with high thermal and chemical stability for electromagnetic interference shielding. Appl. Surf. Sci. 2020, 533, 147431. [Google Scholar] [CrossRef]
- Gong, S.; Sheng, X.; Li, X.; Sheng, M.; Wu, H.; Lu, X.; Qu, J. A multifunctional flexible composite film with excellent multi-source driven thermal management, electromagnetic interference shielding, and fire safety performance, inspired by a “Brick-Mortar” sandwich structure. Adv. Funct. Mater. 2022, 32, 2200570. [Google Scholar] [CrossRef]
- Hashim, M.; Alimuddin, K.S.; Shirsath, S.E.; Kotnala, R.K.; Shah, J.; Kumar, R. Synthesis and characterizations of Ni2+ substituted cobalt ferrite nanoparticles. Mater. Chem. Phys. 2013, 139, 364–374. [Google Scholar] [CrossRef]
- Turchenko, V.A.; Trukhanov, S.V.; Kostishin, V.G.; Damay, F.; Porcher, F.; Klygach, D.S.; Vakhitov, M.G.; Matzui, L.Y.; Yakovenko, O.S.; Bozzo, B.; et al. Impact of In3+cations on structure and electromagnetic state of M-type hexaferrites. J. Energy Chem. 2022, 69, 667–676. [Google Scholar] [CrossRef]
- Bamzai, K.K.; Kour, G.; Kaur, B.; Arora, M.; Pant, R.P. Infrared spectroscopic and electron paramagnetic resonance studies on Dy substituted magnesium ferrite. J. Magn. Magn. Mater. 2014, 345, 255–260. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Paterson, R.; Bhatti, I.A.; Asi, M.R. Survey of aflatoxins in chillies from Pakistan produced in rural, semirural and urban environments. Food Addit. Contam. 2010, 3, 268–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bai, Y.; Zhou, J.; Gui, Z.; Li, L. Electrical properties of nonstoichiometric Y-type hexagonal ferrite. J. Magn. Magn. Mater. 2004, 278, 208–213. [Google Scholar] [CrossRef]
- Xu, W.; Yang, J.; Bai, W.; Zhang, Y.; Tang, K.; Duan, C.-G.; Tang, X.; Chu, J. Effects of aluminum substitution on the crystal structure and magnetic properties in Zn2Y-type hexaferrites. J. Appl. Phys. 2015, 117, 17D909. [Google Scholar] [CrossRef]
- Mirzaee, O.; Mohamady, R.; Ghasemi, A.; Farzin, Y.A. Study of the magnetic and structural properties of Al–Cr codoped Y-type hexaferrite prepared via sol–gel auto-combustion method. Int. J. Mod. Phys. B 2015, 29, 1550090. [Google Scholar] [CrossRef]
- Nikzad, A.; Ghasemi, A.; Tehrani, M.K.; Gordani, G.R. Y-type strontium hexaferrite: The role of Al substitution, structural, and magnetic consequence. J. Supercond. Nov. Magn. 2015, 28, 3579–3586. [Google Scholar] [CrossRef]
- Farzin, M.R.; Kahreh, M.S.; Hesan, M.; Khalouei, A. A survey of critical success factors for strategic knowledge management implementation: Applications for service sector. Proc. Soc. Behav. Sci. 2014, 109, 595–599. [Google Scholar] [CrossRef][Green Version]
- Ali, I.; Islam, M.U.; Ashiq, M.N.; AsifIqbal, M.; Karamat, N.; Awan, M.S.; Naseem, S. Role of Tb–Mn substitution on the magnetic properties of Y-type hexaferrites. J. Alloys Compd. 2014, 599, 131–138. [Google Scholar] [CrossRef]
- Matsushita, N.; Chinose, M.I.; Nagakawa, S.; Naoe, M. Preparation and characteristics of Co-Zn ferrite rigid disks without protective layers for high density recording. IEEE Trans. Magn. 1998, 4, 1639–1641. [Google Scholar] [CrossRef]
- Chen, Y.; Kryder, M.H. Temperature dependent magnetic properties of barium-ferrite thin-film recording media. IEEE Trans. Magn. 1998, 4, 729–742. [Google Scholar] [CrossRef]
- Chen, Y.; Laughlin, D.E.; Ma, X.; Kryder, M.H. Influence of Ba content on grain size and dynamics of crystallization in barium ferrite thin films. J. Appl. Phys. 1997, 81, 4380–4382. [Google Scholar] [CrossRef]
- Morisako, A.; Liu, X.; Matsumoto, M. The effect of underlayer for Ba-ferrite sputtered films on c-axis orientation. J. Appl. Phys. 1997, 81, 4374–4376. [Google Scholar] [CrossRef]
- Samikannu, K.; Sinnappan, J.; Mannarswamy, S.; Cinnasamy, T.; Thirunavukarasu, K. Synthesis and magnetic properties of conventional and microwave calcined strontium hexaferrite powder. Mater. Sci. Appl. 2011, 2, 638–642. [Google Scholar] [CrossRef][Green Version]
- Iqbal, M.J.; Ashiq, M.N. Physical and electrical properties of Zr–Cu substituted strontium hexaferrite nanoparticles synthesized by co-precipitation method. Chem. Eng. J. 2008, 136, 383–389. [Google Scholar] [CrossRef]
- Leccabue, F.; Muzio, O.A.; Kany, M.S.E.; Calestani, G.; Albanese, G. Magnetic properties and phase formation of SrMn2Fe16O27 (SrMn2-W) hexaferrite prepared by the coprecipitation method. J. Magn. Magn. Mater. 1987, 68, 201–212. [Google Scholar] [CrossRef]
- Zhong, W.; Ding, W.; Zhang, N.; Hong, J.; Yan, Q.; Du, Y. Key step in synthesis of ultrafine BaFe12O19 by sol–gel technique. J. Magn. Magn. Mater. 1997, 168, 196–202. [Google Scholar] [CrossRef]
- Rezlescu, L.; Rezlescu, E.; Popa, P.D.; Rezlescu, N. Fine barium hexaferrite powder prepared by the crystallisation of glass. J. Magn. Magn. Mater. 1999, 193, 288–290. [Google Scholar] [CrossRef]
- Karimi, Z.; Mohammadifar, Y.; Shokrollahi, H.; KhamenehAsl, S.; Yousefi, G.; Karimi, L. Magnetic and structural properties of nano sized Dy-doped cobalt ferrite synthesized by co-precipitation. J. Magn. Magn. Mater. 2014, 361, 150–156. [Google Scholar] [CrossRef]
- Farid, M.T.; Ahmad, I.; Kanwal, M.; Murtaza, G.; Ali, I.; Ashiq, M.N.; Khan, S.A. Synthesis, electrical and magnetic properties of Pr-substituted Mn ferrites for high-frequency applications. J. Electron. Mater. 2017, 46, 1826–1835. [Google Scholar] [CrossRef]
- Barde, W.S.; Pakade, S.V.; Yawale, S.P. Ionic conductivity in polypyrrole–poly (vinyl acetate) films synthesized by chemical oxidative polymerization method. J. Non-Cryst. Solids 2007, 353, 1460–1465. [Google Scholar] [CrossRef]
- Suri, K.; Annapoorni, S.; Tandon, R.P. AC conduction in nanocomposites of polypyrrole. J. Non-Cryst. Solids 2003, 332, 279–285. [Google Scholar] [CrossRef]
- Yakovenko, O.; Lazarenko, O.; Matzui, L.; Vovchenko, L.; Borovoy, M.; Tesel’ko, P.; Lozitsky, O.; Astapovich, K.; Trukhanov, A.; Trukhanov, S. Effect of Ga content on magnetic properties of BaFe12-xGaxO19/epoxy composites. J. Mater. Sci. 2020, 55, 9385–9395. [Google Scholar] [CrossRef]
- Abdeen, A.M. Electric conduction in Ni–Zn ferrites. J. Magn. Magn. Mater. 1998, 185, 199–206. [Google Scholar] [CrossRef]
- Kumar, A.M.; Varma, M.C.; Dube, C.L.; Rao, K.H.; Kashyap, S.C. Development of Ni–Zn nanoferrite core material with improved saturation magnetization and DC resistivity. J. Magn. Magn. Mater. 2008, 320, 1995–2000. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Ali, S.; Baykal, A.; Balasamy, R.J.; Guner, S.; Auwal, İ.A.; Trukhanov, A.V.; Trukhanov, S.V.; Manikandan, A. Impact of Ga3+ ions on the structure, magnetic, and optical features of Co-Ni nanostructured spinel ferrite microspheres. Nanomaterials 2022, 12, 2872. [Google Scholar] [CrossRef]
- Batoo, K.M.; Kumar, S.; Lee, C.G.; Alimuddin. Finite size effect and influence of temperature on electrical properties of nanocrystalline Ni–Cd ferrites. Curr. Appl. Phys. 2009, 9, 1072–1078. [Google Scholar] [CrossRef]
- El-Shahaway, M.A. Polymethyl methacrylate mixtures with some organic laser dyes: II. Electric conduction. Polym. Test. 2000, 19, 821–829. [Google Scholar] [CrossRef]
- Abo El Ata, A.M.; Attia, S.M. Dielectric dispersion of Y-type hexaferrites at low frequencies. J. Magn. Magn. Mater. 2003, 257, 165–174. [Google Scholar] [CrossRef]
- Khan, H.M.; Islam, M.U.; Xu, Y.; Ashiq, M.N.; Ali, I.; Iqbal, M.A.; Ishaque, M. Structural and magnetic properties of Pr-Ni substituted Ca0.5Ba0.5Fe12O19 hexaferrite nanoparticles. Ceram. Int. 2014, 40, 6487–6493. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Islam, M.U.; Ali, I.; Khan, H.M.; Mustafa, G.; Ali, I. Study of electrical transport properties of Eu+3substituted MnZn-ferrites synthesized by co-precipitation technique. Ceram. Int. 2013, 39, 1539–1545. [Google Scholar] [CrossRef]
- Farid, M.T.; Ahmad, I.; Kanwal, M.; Murtaza, G.; Ali, I.; Khan, S.A. The role of praseodymium substituted ions on electrical and magnetic properties of Mg spinel ferrites. J. Magn. Magn. Mater. 2017, 428, 136–143. [Google Scholar] [CrossRef]
- Watawe, S.C.; Sarwede, B.D.; Bellad, S.S.; Sutar, B.D.; Chougule, B.K. Microstructure, frequency and temperature-dependent dielectric properties of cobalt-substituted lithium ferrites. J. Magn. Magn. Mater. 2000, 214, 55–60. [Google Scholar] [CrossRef]
- Bellad, S.S.; Chougule, B.K. Composition and frequency dependent dielectric properties of Li–Mg–Ti ferrites. Mater. Chem. Phys. 2000, 66, 58–63. [Google Scholar] [CrossRef]
- Singh, A.K.; Goel, T.C.; Mendiratta, R.G.; Thakur, O.P.; Prakash, C. Dielectric properties of Mn-substituted Ni–Zn ferrites. J. Appl. Phys. 2002, 91, 6626–6629. [Google Scholar] [CrossRef]
- Khan, H.M.; Islam, M.U.; Xu, Y.; Iqbal, M.A.; Ali, I.; Ishaque, M.M.; Khan, A. Structural, magnetic, and microwave properties of NdZn-substituted Ca0.5Ba0.5Fe12O19 hexaferrites. J. Sol-Gel Sci. Technol. 2015, 75, 305–312. [Google Scholar] [CrossRef]
- El-Shater, R.E.; El Shimy, H.; Saafan, S.A.; Darwish, M.A.; Zhou, D.; Trukhanov, A.V.; Trukhanov, S.V.; Fakhry, F. Synthesis, characterization, and magnetic properties of Mn nanoferrites. J. Alloys Compd. 2022, 928, 166954. [Google Scholar] [CrossRef]
- Habasaki, J.; Ngai, K.L. Molecular dynamics simulation of ion dynamics in glassy ionic conductors: Evidence of the primitive ion hopping process. J. Non-Cryst. Solids 2006, 352, 5170–5177. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.A.; Díaz-Guillén, M.R.; Padmasree, K.P.; Fuentes, A.F.; Santamaría, J.; León, C. High ionic conductivity in the pyrochlore-type Gd2−yLayZr2O7 solid solution (0 ≤ y ≤ 1). Solid State Ionics 2008, 179, 2160–2164. [Google Scholar] [CrossRef]
- Farid, H.M.T.; Ahmad, I.; Ali, I.; Ramay, S.M.; Mahmood, A.; Murtaza, G. Dielectric and impedance study of praseodymium substituted Mg-based spinel ferrites. J. Magn. Magn. Mater. 2017, 434, 143–150. [Google Scholar] [CrossRef]
- Rodrigues, H.O.; PiresJunior, G.F.M.; Sales, A.J.M.; Silva, P.M.O.; Costa, B.F.O.; AlcantaraJr, P.; Moreira, S.G.C.; Sombra, A.S.B. BiFeO3 ceramic matrix with Bi2O3 or PbO added: Mössbauer, Raman and dielectric spectroscopy studies. Phys. B Condens. Matter 2011, 406, 2532–2539. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Chourashiya, M.G.; Patil, J.Y.; Pawar, S.H.; Jadhav, L.D. Studies on structural, morphological and electrical properties of Ce1−xGdxO2−(x/2). Mater. Chem. Phys. 2008, 109, 39–44. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Cheng, P.-W.; Yen, F.-S. Low temperature firing of Co2Y–NiCuZn ferrite composites. Ceram. Int. 2012, 38, 4915–4921. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Saini, P.; Dhawan, S.K. Poly (3,4-ethylenedioxythiophene) γ-Fe2O3 polymer composite–super paramagnetic behavior and variable range hopping 1D conduction mechanism–synthesis and characterization. Polym. Adv. Technol. 2008, 19, 229. [Google Scholar] [CrossRef]
- Gholampoor, M.; Movassagh, F.; Salimkhani, H. Fabrication of nano-Fe3O4 3D structure on carbon fibers as a microwave absorber and EMI shielding composite by modified EPD method. Solid State Sci. 2017, 64, 51–61. [Google Scholar] [CrossRef]
- Tuleushev, A.Z.; Harrison, F.E.; Kozlovskiy, A.L.; Zdorovets, M.V. Evolution of the absorption edge of PET films irradiated with Kr ions after thermal annealing and ageing. Optical Mater. 2021, 119, 111348. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L.; Borgekov, D.B.; Shlimas, D.I. Influence of irradiation with heavy Kr15+ ions on the structural, optical and strength properties of BeO ceramic. J. Mater. Sci. Mater. Electron. 2021, 32, 15375–15385. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Alina, A.; Zdorovets, M.V. Study of the effect of ion irradiation on increasing the photocatalytic activity of WO3 microparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 3863–3877. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Zdorovets, M.V. Study of hydrogenation processes in radiation-resistant nitride ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 11227–11237. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Zhumanazar, N.; Gorin, Y.G.; Yeszhanov, A.B.; Zdorovets, M.V. Enhancement of electrochemical detection of Pb2+ by sensor based on track-etched membranes modified with interpolyelectrolyte complexes. J. Mater. Sci. Mater. Electron. 2020, 31, 20368–20377. [Google Scholar] [CrossRef]
- Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V. Study of the formation effect of the cubic phase of LiTiO2 on the structural, optical, and mechanical properties of Li2±xTi1±xO3 ceramics with different contents of the X component. J. Mater. Sci. Mater. Electron. 2021, 32, 7410–7422. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Shlimas, D.I.; Zdorovets, M.V. Synthesis, structural properties and shielding efficiency of glasses based on TeO2-(1−x)ZnO-xSm2O3. J. Mater. Sci. Mater. Electron. 2021, 32, 12111–12120. [Google Scholar] [CrossRef]
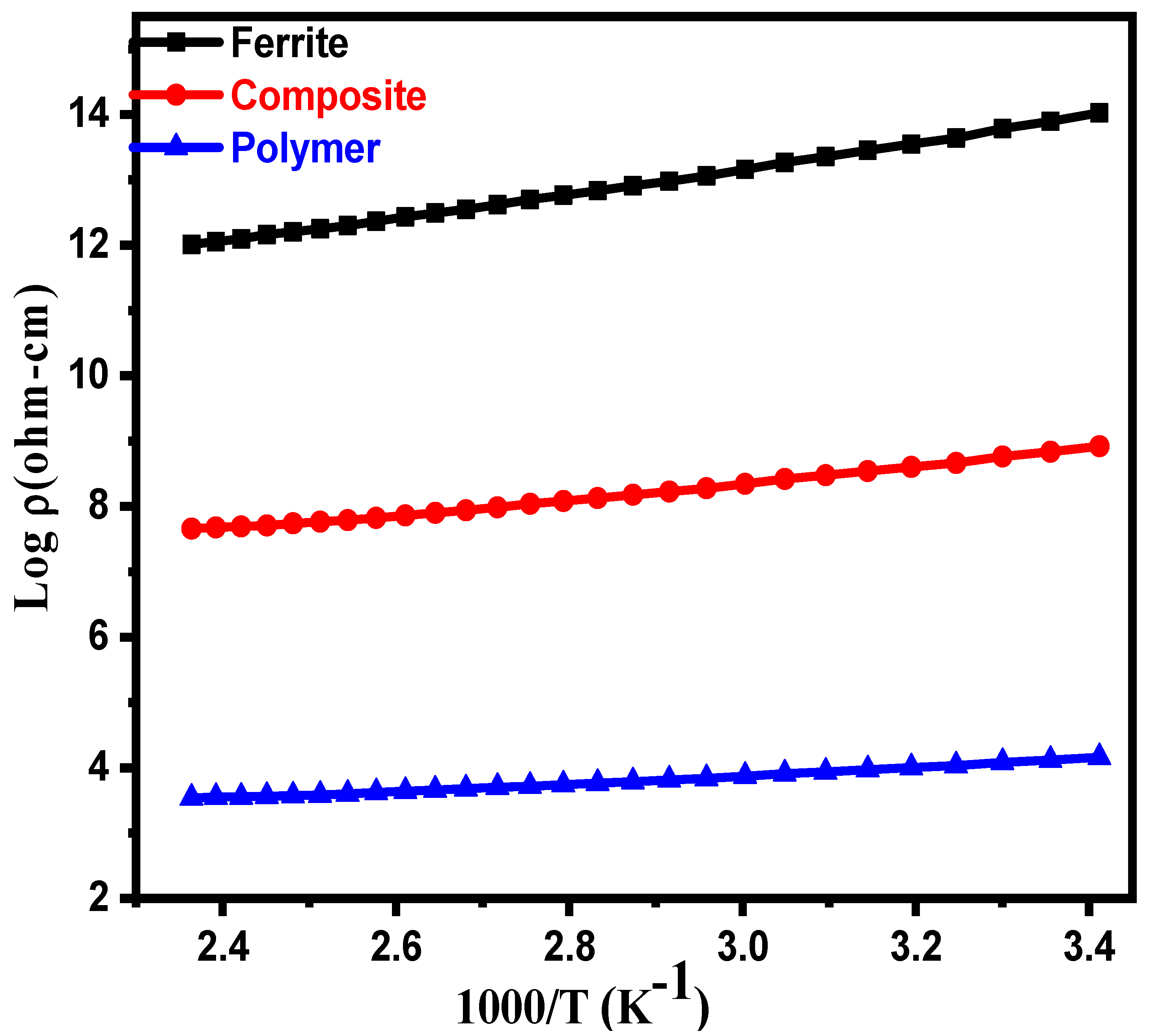
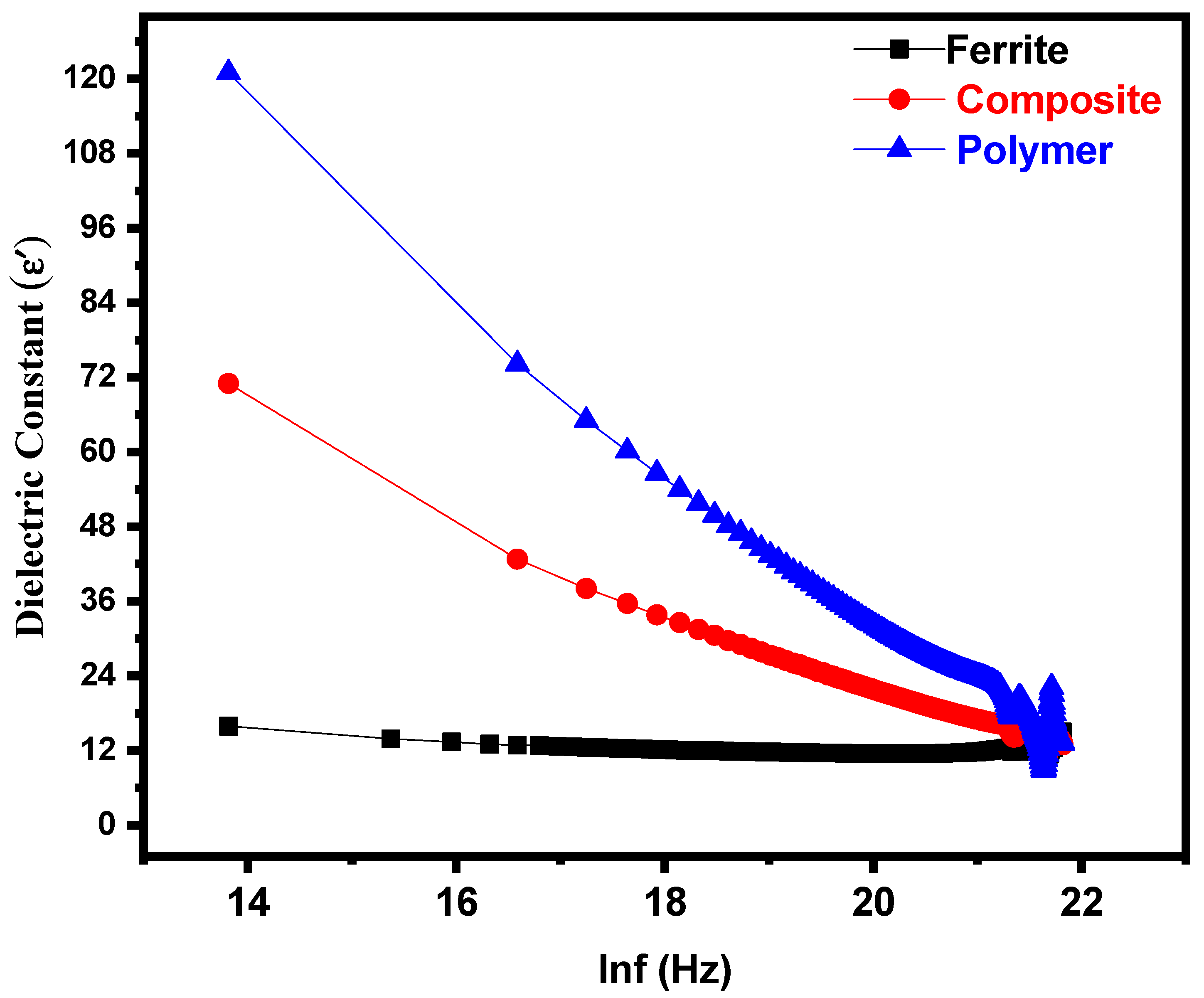
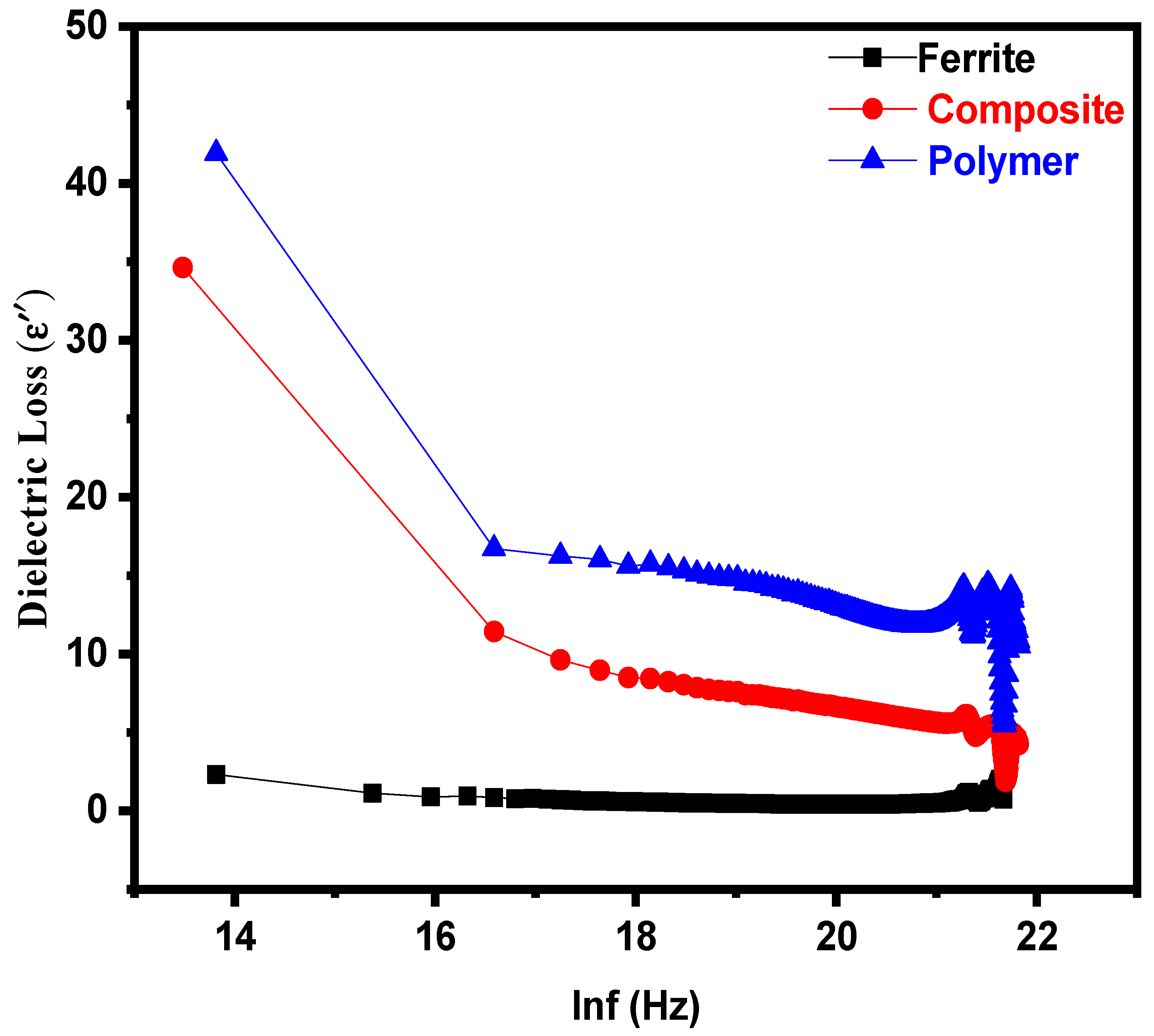
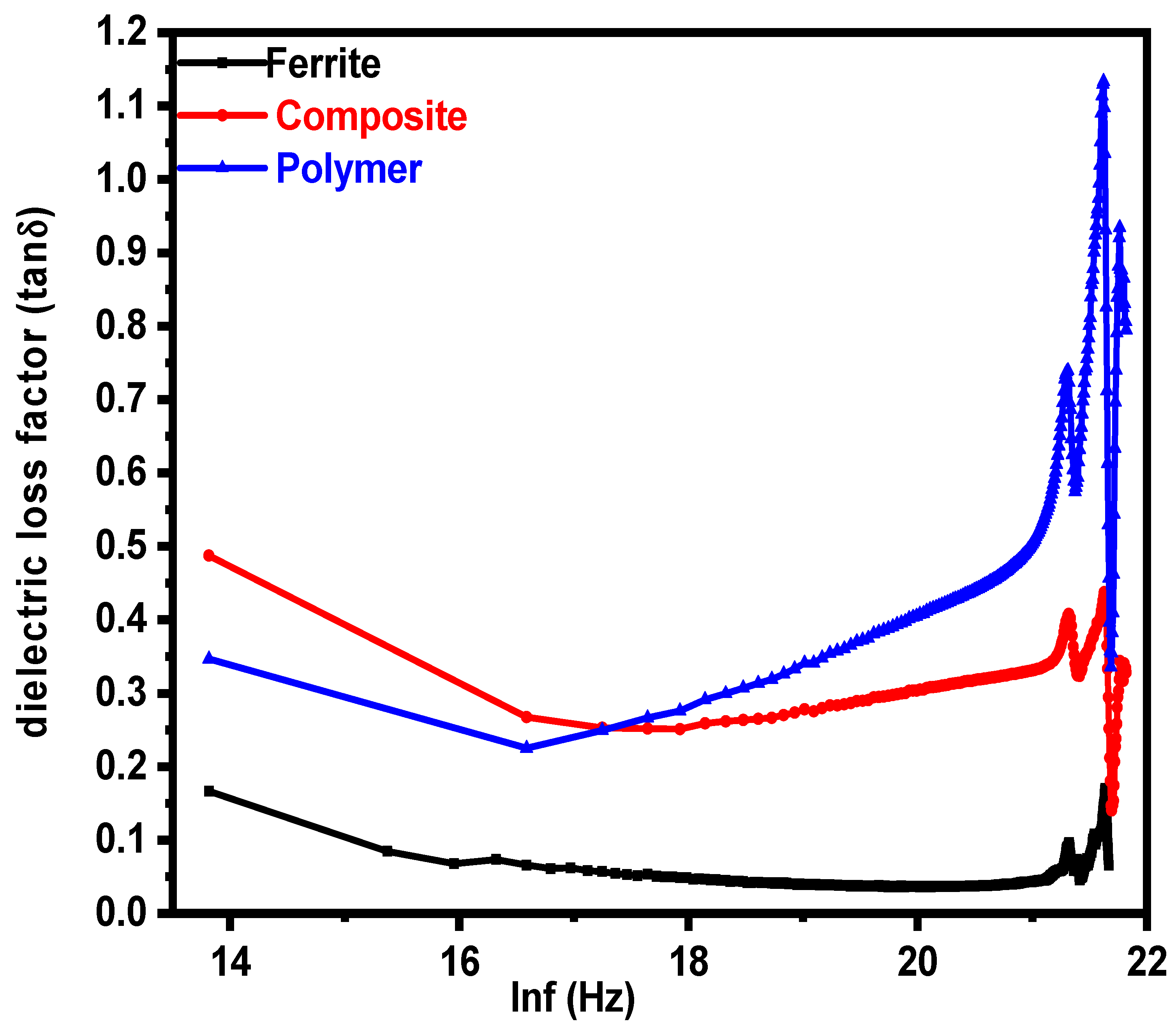
| Parameters | Ferrite | Composite | Polymer |
|---|---|---|---|
| Dielectric constant(ε′) | 15.910 | 71.020 | 120.990 |
| Dielectric loss(ε″) | 2.310 | 34.630 | 41.910 |
| Tangent loss(ε″/ε′) | 0.160 | 0.480 | 0.340 |
| AC conductivity(σAC) | 1.29 × 10−4 | 1.92 × 10−3 | 2.33 × 10−3 |
| Impedance |Z| (Ω) | 38,218 | 41,247 | 42,933 |
| Real part of electric modulus (M′) | 0.061 | 0.011 | 0.006 |
| Imaginary part of electric modulus (M″) | 0.008 | 0.005 | 0.002 |
| Exponential Factor n (±0.011) | 0.810 | 0.820 | 0.900 |
| Estimated activation energy (EAC) | 0.067 | 0.043 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.A.; Ali, I.; Hussain, A.; Javed, H.M.A.; Turchenko, V.A.; Trukhanov, A.V.; Trukhanov, S.V. Synthesis and Characterization of Composites with Y-Hexaferrites for Electromagnetic Interference Shielding Applications. Magnetochemistry 2022, 8, 186. https://doi.org/10.3390/magnetochemistry8120186
Khan SA, Ali I, Hussain A, Javed HMA, Turchenko VA, Trukhanov AV, Trukhanov SV. Synthesis and Characterization of Composites with Y-Hexaferrites for Electromagnetic Interference Shielding Applications. Magnetochemistry. 2022; 8(12):186. https://doi.org/10.3390/magnetochemistry8120186
Chicago/Turabian StyleKhan, Sajjad Ahmad, Irshad Ali, Abid Hussain, Hafiz Muhammad Asif Javed, Vitalii A. Turchenko, Alex V. Trukhanov, and Sergei V. Trukhanov. 2022. "Synthesis and Characterization of Composites with Y-Hexaferrites for Electromagnetic Interference Shielding Applications" Magnetochemistry 8, no. 12: 186. https://doi.org/10.3390/magnetochemistry8120186
APA StyleKhan, S. A., Ali, I., Hussain, A., Javed, H. M. A., Turchenko, V. A., Trukhanov, A. V., & Trukhanov, S. V. (2022). Synthesis and Characterization of Composites with Y-Hexaferrites for Electromagnetic Interference Shielding Applications. Magnetochemistry, 8(12), 186. https://doi.org/10.3390/magnetochemistry8120186








