PAN—Composite Electrospun-Fibers Decorated with Magnetite Nanoparticles
Abstract
1. Introduction
2. Experimental Part
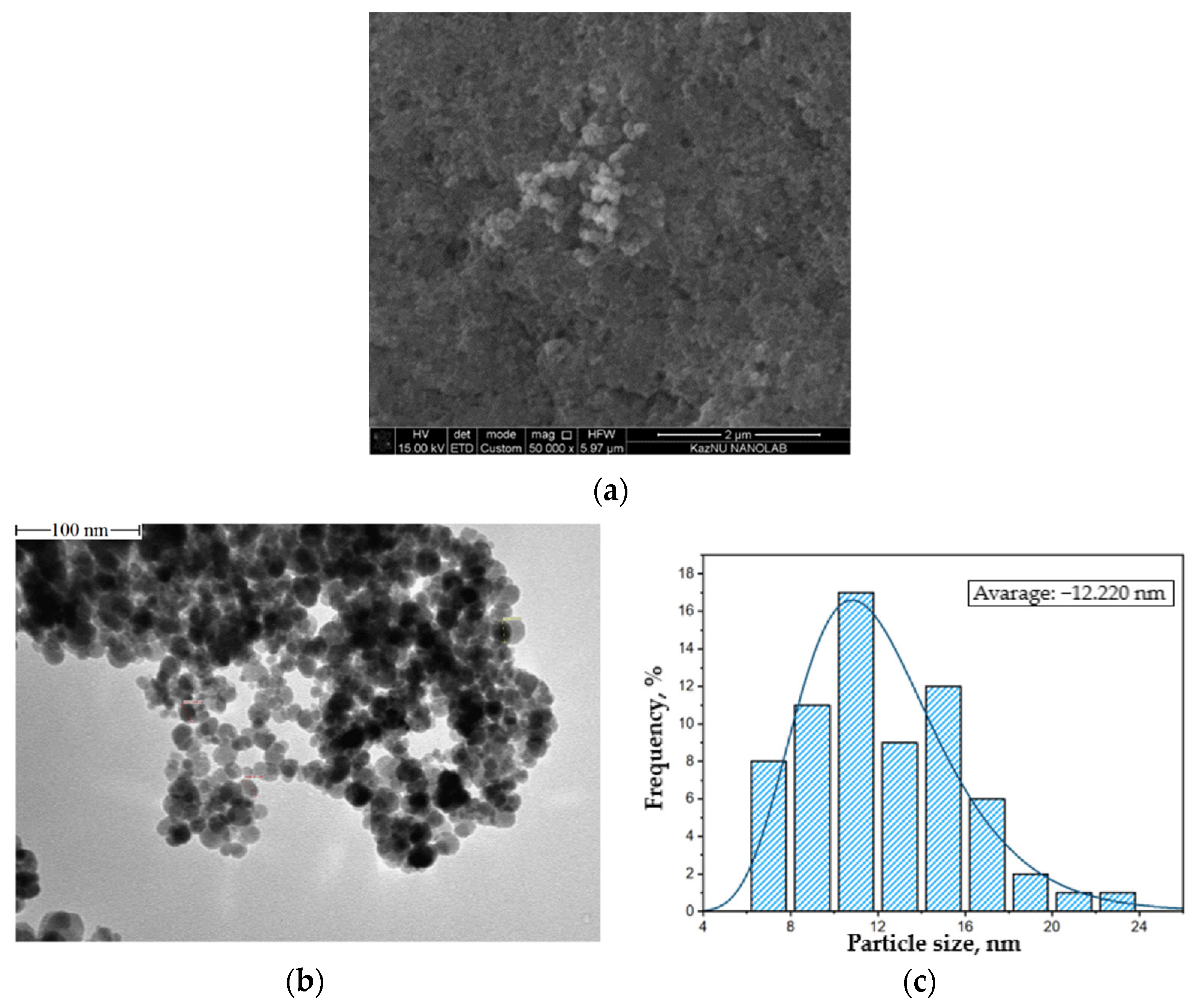
2.1. Synthesis of Fe3O4 Magnetite Nanoparticles
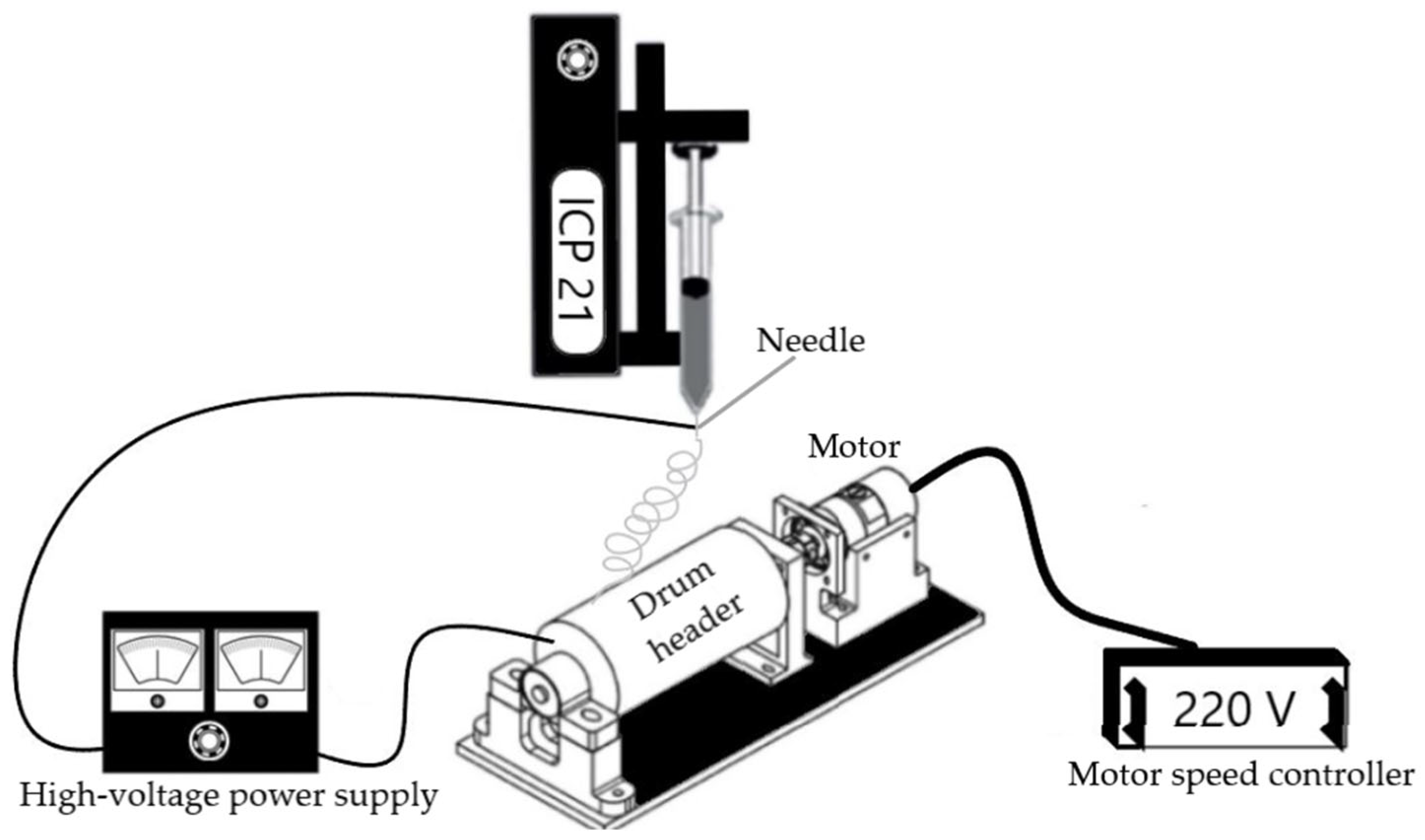
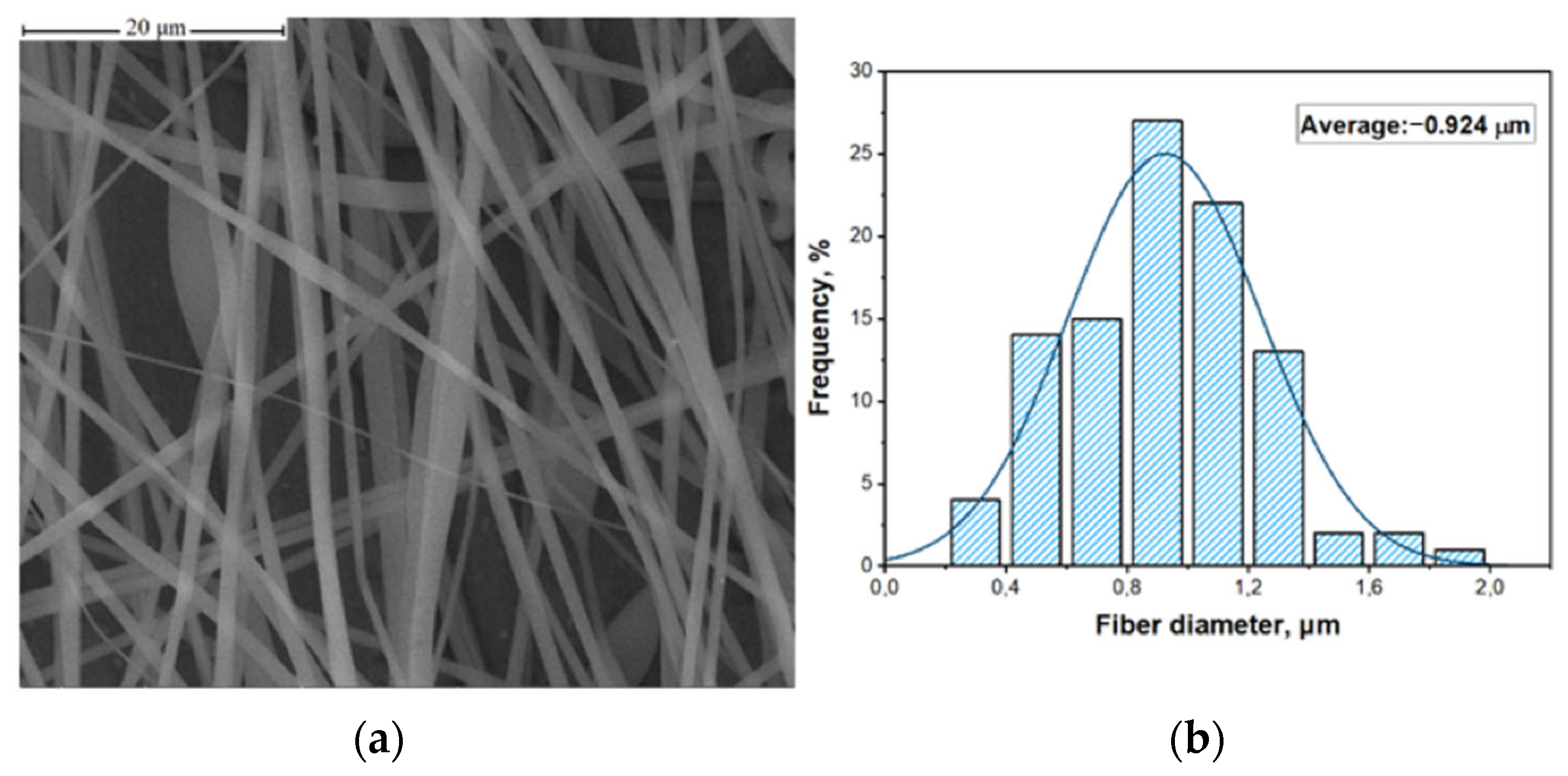
2.2. Preparation of PAN/Fe3O4 Solution and Obtaining of Composite Fibers
2.3. Analytical Methods of Investigation for Fe3O4 Magnetite Nanoparticles and Composite Fibers
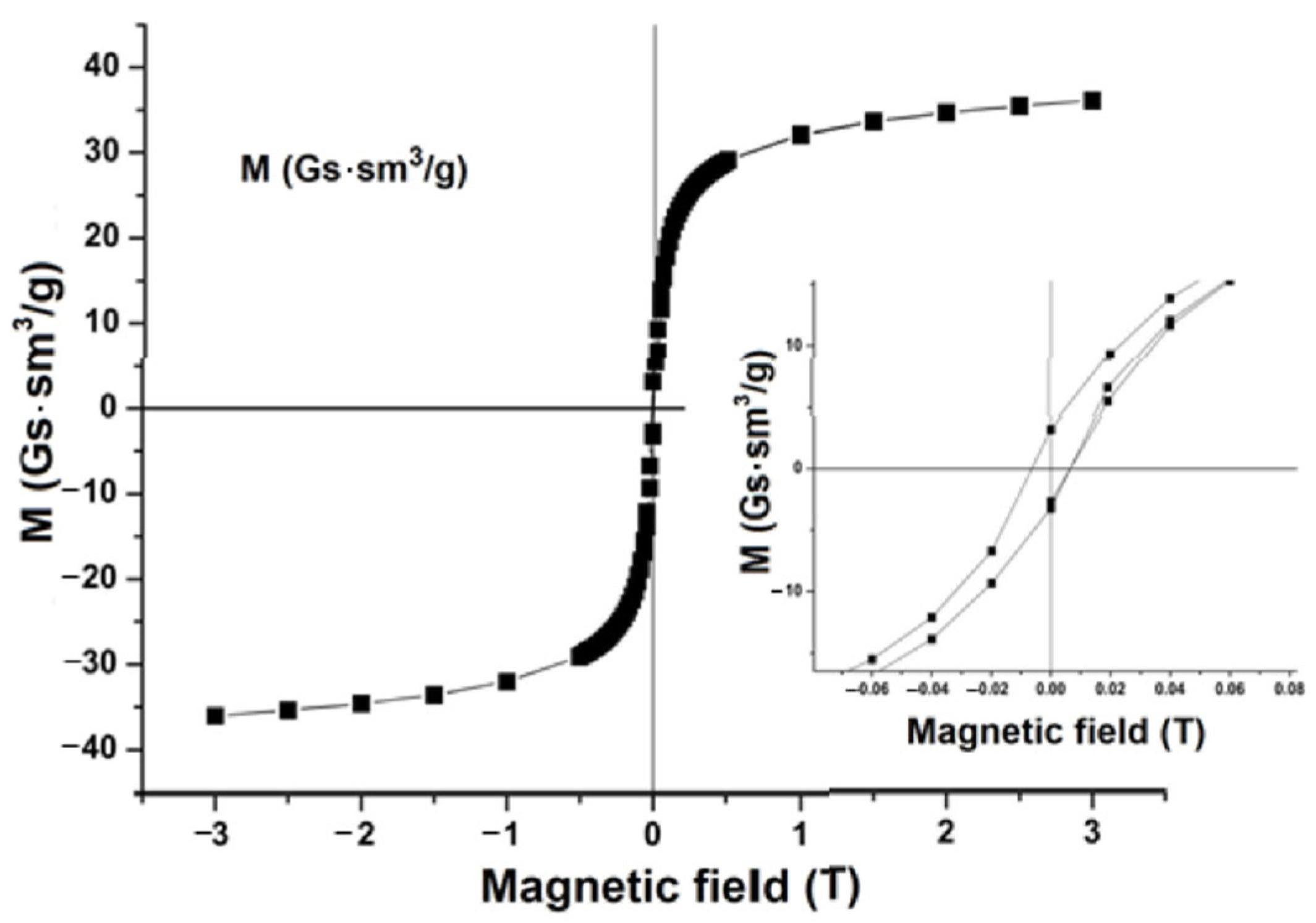
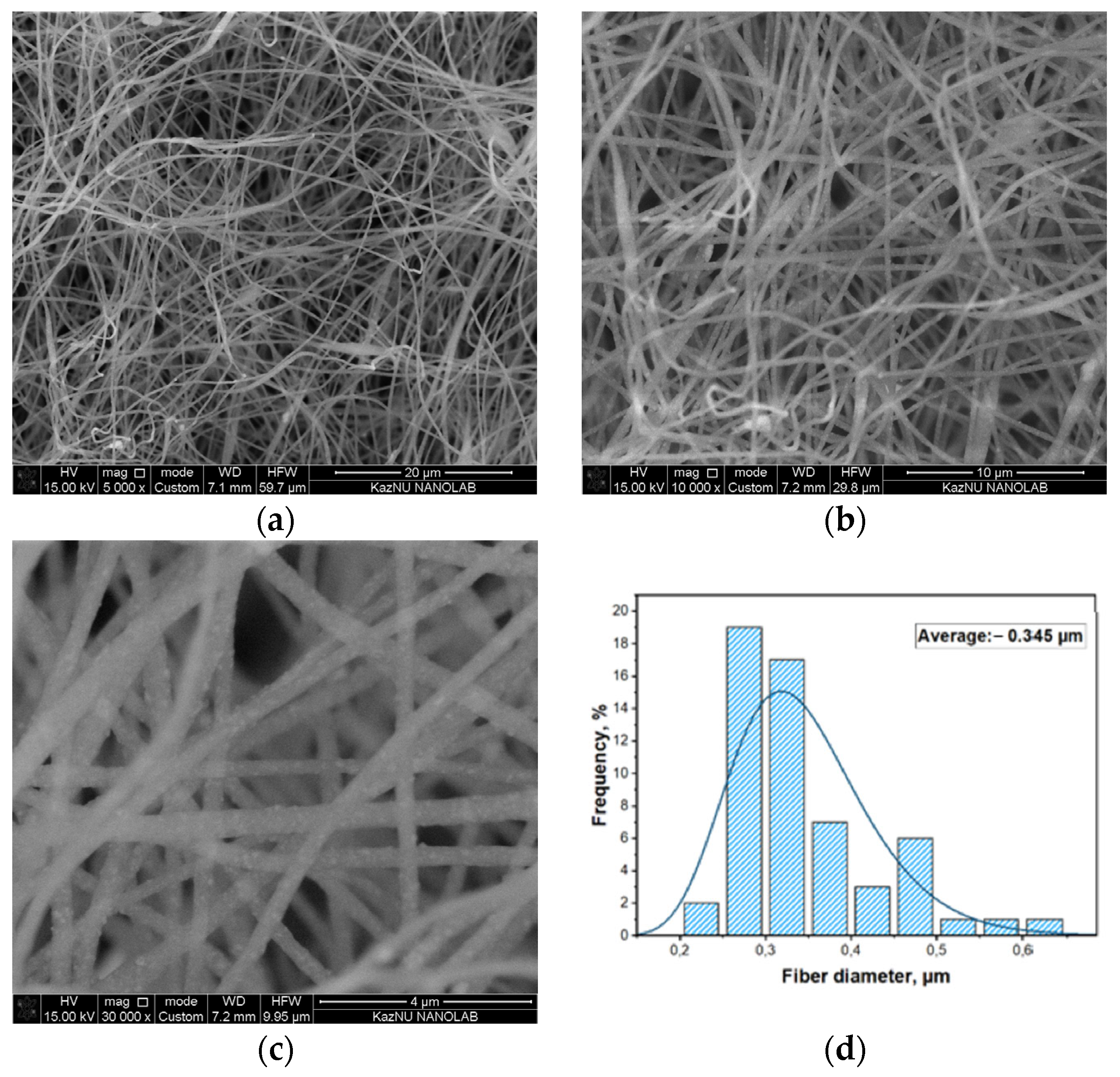
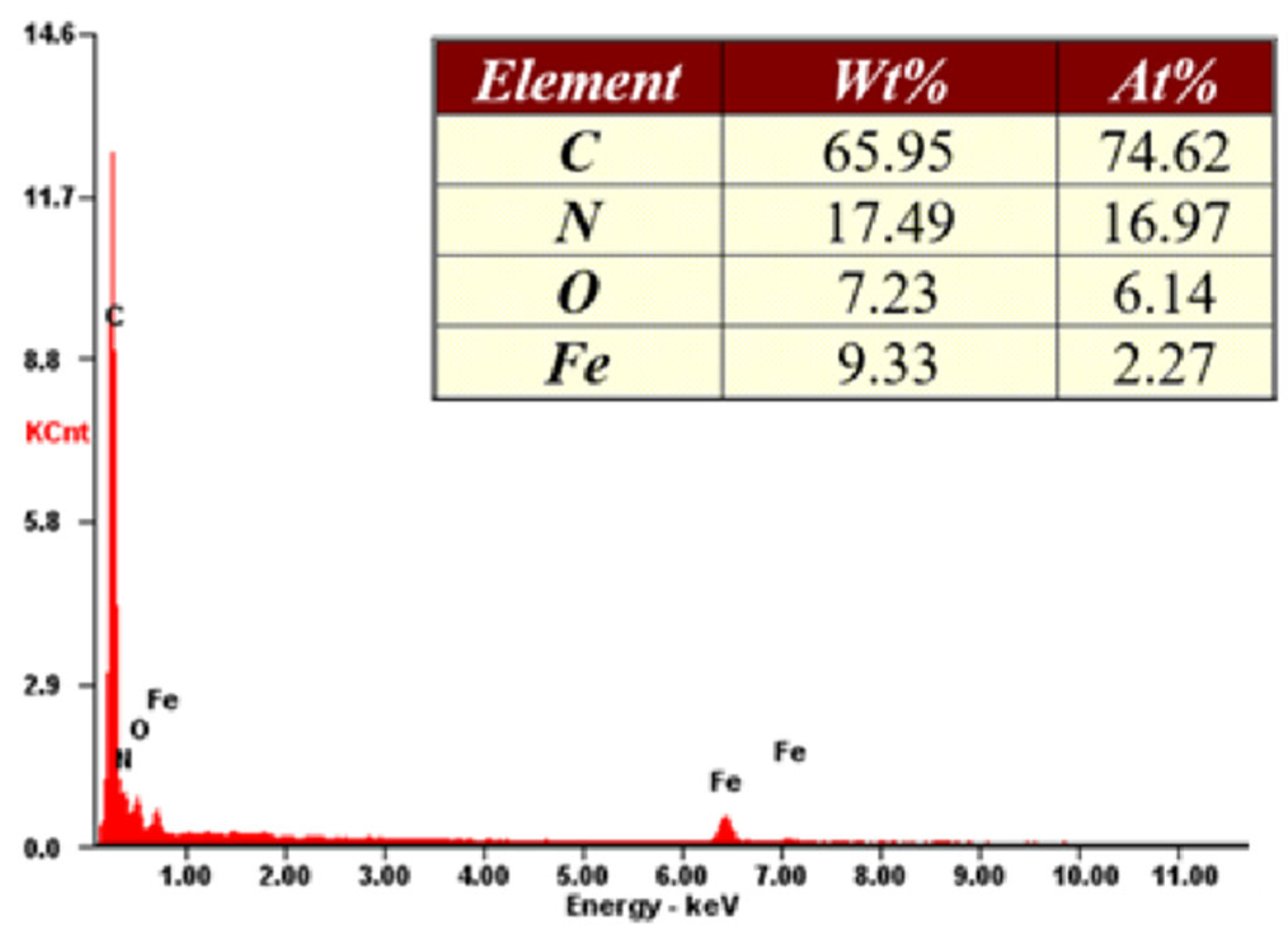
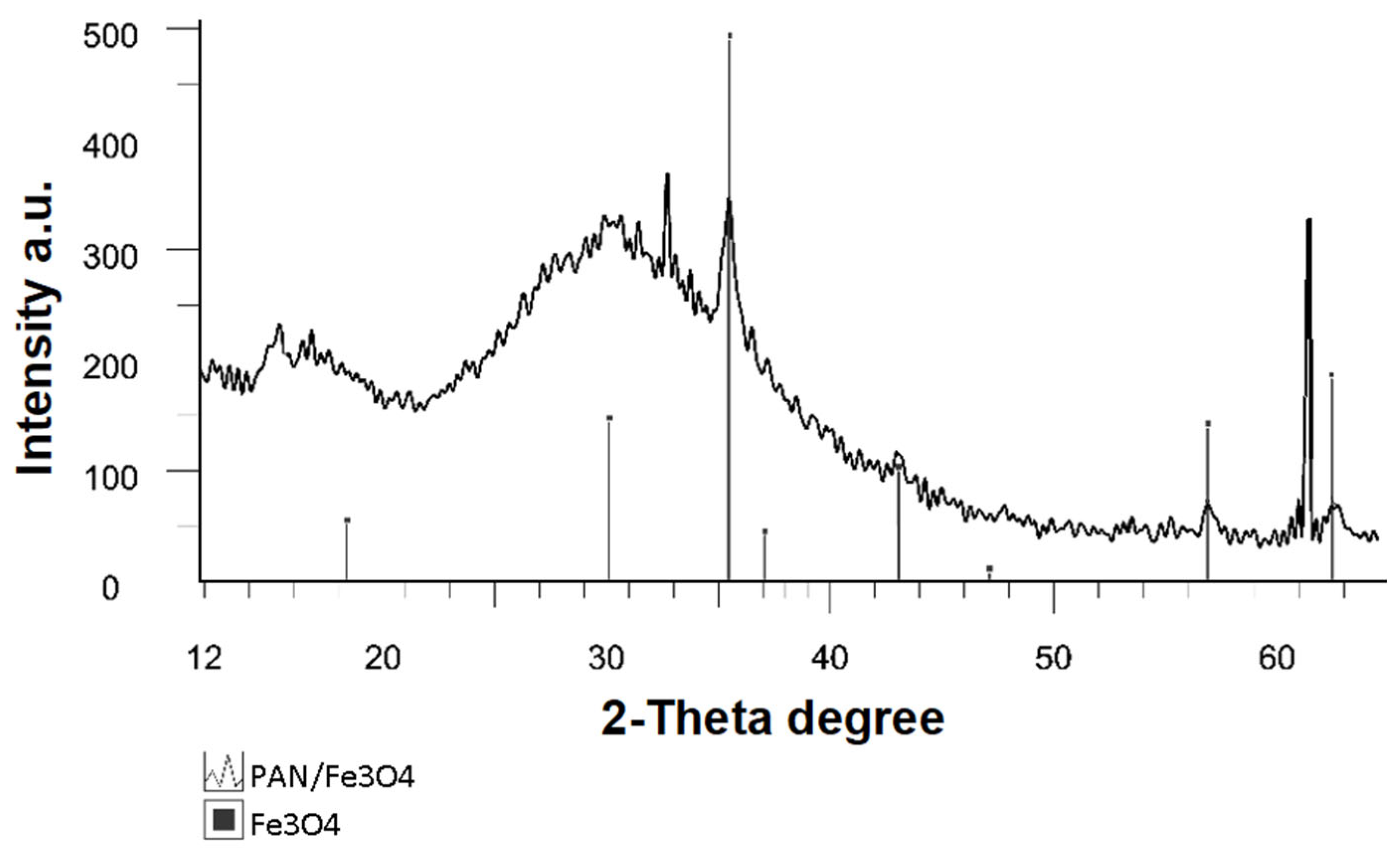
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the Application of Magnetic Nanoparticles for Sensing. Adv. Mater. 2019, 31, e1904385. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.S.; Meenalochini, P.; Sathiya, S.S.B.; Prakash, G.R. A review on selection of soft magnetic materials for industrial drives. Mater. Today Proc. 2021, 45, 1591–1596. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Fan, Z. A mini review: Application progress of magnetic graphene three-dimensional materials for water purification. Front. Chem. 2020, 8, 595643. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Qin, B.; Li, B.; Duan, X. Synthesis, characterization, properties and applications of two-dimensional magnetic materials. Nano Today 2022, 42, 101338. [Google Scholar] [CrossRef]
- Kaur, B.; Kaushal, G.; Rana, S.; Kumar, P.; Khanra, P.; Dhiman, M. Magnetic Ferrites: A Brief Review About Substation on Electric and Magnetic Properties. ECS Trans. 2022, 107, 9093. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, X.; Liu, J.; Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020, 188, 106943. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, W.; Khan, A.; Chong, X.; Feng, J. The investigation on structural, electronic, elastic, adsorptive, catalytic and magnetic properties of precious metal materials via first-principles calculations based on density functional theory—A review. J. Micromechanics Mol. Phys. 2020, 5, 2030001. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef]
- Mikhailov, Y.M.; Aleshin, V.V.; Zhemchugova, L.V.; Kovalev, D.Y. Transformations of Iron (III) Precursors in a Wave of Flameless RDX Combustion. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 162–166. [Google Scholar] [CrossRef]
- Arévalo-Cid, P.; Isasi, J.; Martín-Hernández, F. Comparative Study of Core-Shell Nanostructures Based on Amino-Functionalized Fe3O4@SiO2 and CoFe2O4@SiO2 Nanocomposites. J. Alloys Compd. 2018, 766, 609–618. [Google Scholar] [CrossRef]
- Katz, E. Synthesis, Properties and Applications of Magnetic Nanoparticles and Nanowires—A Brief Introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef]
- Evdokimov, S.I.; Pan’shin, A.M.; Kanashvili, M.Z. Magnetic Fluid: New Technology. Russ. J. Non-Ferr. Met. 2008, 49, 78–81. [Google Scholar] [CrossRef]
- Han, C.; Cai, W.; Tang, W.; Wang, G.; Liang, C. Protein Assisted Hydrothermal Synthesis of Ultrafine Magnetite Nanoparticle Built-Porous Oriented Fibers and Their Structurally Enhanced Adsorption to Toxic Chemicals in Solution. J. Mater. Chem. 2011, 21, 11188. [Google Scholar] [CrossRef]
- Mamun, A.; Sabantina, L.; Klöcker, M.; Heide, A.; Blachowicz, T.; Ehrmann, A. Electrospinning Nanofiber Mats with Magnetite Nanoparticles Using Various Needle-Based Techniques. Polymers 2022, 14, 533. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Bakry, A.; Kolek, O.; Martinová, L.; Stibor, I. Electrospun functionalized magnetic polyamide 6 composite nanofiber: Fabrication and stabilization. Polym. Compos. 2019, 40, 296–303. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Wang, H. Preparation and Properties of Electrospun PAN/Fe3O4 Magnetic Nanofibers. J. Appl. Polym. Sci. 2010, 115, 1781–1786. [Google Scholar] [CrossRef]
- Fokin, N.; Grothe, T.; Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Döpke, C.; Blachowicz, T.; Hütten, A.; Ehrmann, A. Magnetic Properties of Electrospun Magnetic Nanofiber Mats after Stabilization and Carbonization. Materials 2020, 13, 1552. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Rutman, D.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. Magnetic Polyacrylonitrile-Fe@FeO Nanocomposite Fibers—Electrospinning, Stabilization and Carbonization. Polymer 2011, 52, 2947–2955. [Google Scholar] [CrossRef]
- Storck, J.L.; Grothe, T.; Mamun, A.; Sabantina, L.; Klöcker, M.; Blachowicz, T.; Ehrmann, A. Orientation of Electrospun Magnetic Nanofibers Near Conductive Areas. Materials 2019, 13, 47. [Google Scholar] [CrossRef]
- Liu, G.-L.; Zhang, Y.-M.; Tian, D.; Zhou, B.-Z.; Lu, Z.-Q.; Wang, C.-X. Last Patents on Bubble Electrospinning. Recent Pat. Nanotechnol. 2020, 14, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Shakil, U.A.; Bin Abu Hassan, S.; Yahya, M.Y.; Rejab, M.R.M. A focused review of short electrospun nanofiber preparation techniques for composite reinforcement. Nanotechnol. Rev. 2022, 11, 1991–2014. [Google Scholar] [CrossRef]
- Furlan, D.M.; Morgado, D.L.; de Oliveira, A.J.A.; Faceto, Â.D.; de Moraes, D.A.; Varanda, L.C.; Frollini, E. Sisal Cellulose and Magnetite Nanoparticles: Formation and Properties of Magnetic Hybrid Films. J. Mater. Res. Technol. 2019, 8, 2170–2179. [Google Scholar] [CrossRef]
- Xia, S.; Song, L.; Hohn, N.; Wang, K.; Grott, S.; Opel, M.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P. Spray-Coating Magnetic Thin Hybrid Films of PS-b-PNIPAM and Magnetite Nanoparticles. Adv. Funct. Mater. 2019, 29, 1808427. [Google Scholar] [CrossRef]
- Ganonyan, N.; He, J.; Temkin, A.; Felner, I.; Gvishi, R.; Avnir, D. Ultralight Monolithic Magnetite Aerogel. Appl. Mater. Today 2021, 22, 100955. [Google Scholar] [CrossRef]
- Raju, R.R.; Liebig, F.; Klemke, B.; Koetz, J. Ultralight Magnetic Aerogels from Janus Emulsions. RSC Adv. 2020, 10, 7492–7499. [Google Scholar] [CrossRef]
- Kostishyn, V.G.; Ostafiychuk, B.K.; Moklyak, V.V.; Nuriev, A.V. Mossbauer Investigation of Magnetic Polymer Nanocomposites Based on Magnetite And Polyvinyl Alcohol. Izv. Vyss. Uchebnykh Zaved. Mater. Elektron. Tekhniki Mater. Electron. Eng. 2015, 4, 22. [Google Scholar] [CrossRef]
- Lesbayev, A.; Elouadi, B.; Borbotko, T.; Manakov, S.; Smagulova, G.; Boiprav, O.; Prikhodko, N. Influence of Magnetite Nanoparticles on Mechanical and Shielding Properties of Concrete. Eurasian Chem.-Technol. J. 2017, 19, 223–229. [Google Scholar] [CrossRef]
- Lesbayev, A.B.; Elouadi, B.; Lesbayev, B.T.; Manakov, S.M.; Smagulova, G.T.; Prikhodko, N.G. Obtaining of Magnetic Polymeric Fibers with Additives of Magnetite Nanoparticle. Procedia Manuf. 2017, 12, 28–32. [Google Scholar] [CrossRef]
- Mansurov, Z.A. Recent Achievements and Future Challenges in Nanoscience and Nanotechnology. Eurasian Chem.-Technol. J. 2020, 22, 241. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Bakry, A.; Al-Harbi, L.M.; Khowdiary, M.M.; El-Henawy, A.A.; Yoon, J. Core/shell PA6@ Fe3O4 nanofibers: Magnetic and shielding behavior. J. Dispers. Sci. Technol. 2020, 11, 1711–1719. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.; Ooi, B.S.; Ahmad, A.L. Agglomeration, colloidal stability, and magnetic separation of magnetic nanoparticles: Collective influences on environmental engineering applications. J. Nanoparticle Res. 2017, 19, 368. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data. JCPDS Card 03–065–3107. International Centre for Diffraction Data: Newtown Square, PA, USA. Available online: https://www.icdd.com/ (accessed on 5 October 2022).
- Taufiq, A.; Ainun Jannah, M.; Hidayat, A.; Hidayat, N.; Mufti, N.; Munasir; Susanto, H. Structural and Magnetic Behaviours of Magnetite/Polyvinyl Alcohol Composite Nanofibers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012081. [Google Scholar] [CrossRef]
- Sabzroo, N.; Rohani Bastami, T.; Karimi, M.; Heidari, T. Optimization of electrospinning conditions for magnetic poly (acrylonitrile-co-acrylic acid) nanofibers. J. Nanostructures 2019, 9, 301–315. [Google Scholar] [CrossRef]
- Zhan, Y.; Long, Z.; Wan, X.; Zhang, J.; He, S.; He, Y. 3D carbon fiber mats/nano-Fe3O4 hybrid material with high electromagnetic shielding performance. Appl. Surf. Sci. 2018, 444, 710–720. [Google Scholar] [CrossRef]
- Apler, A.; Snowball, I.; Frogner-Kockum, P.; Josefsson, S. Distribution and dispersal of metals in contaminated fibrous sediments of industrial origin. Chemosphere 2019, 215, 470–481. [Google Scholar] [CrossRef]
- Gan, Y.; Yu, C.; Panahi, N.; Gan, J.; Cheng, W. Processing Iron Oxide Nanoparticle-Loaded Composite Carbon Fiber and the Photosensitivity Characterization. Fibers 2019, 7, 25. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Most Recent Developments in Electrospun Magnetic Nanofibers: A Review. J. Eng. Fibers Fabr. 2020, 15, 155892501990084. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Li, C.; Li, X.; Wu, Y.; Chen, X.; Wu, J.; Li, X.; Chen, Y. Electrospun carbon nanofibers for lithium metal anodes: Progress and perspectives. Chin. Chem. Lett. 2021, 33, 141–152. [Google Scholar] [CrossRef]
- Matos, R.J.R.; Chaparro, C.I.P.; Silva, J.C.; Valente, M.A.; Borges, J.P.; Soares, P.I.P. Electrospun composite cellulose acetate/iron oxide nanoparticles non-woven membranes for magnetic hyperthermia applications. Carbohydr. Polym. 2018, 198, 9–16. [Google Scholar] [CrossRef]
- Hu, Q.-L.; Wang, L.-S.; Yu, N.-N.; Zhang, Z.-F.; Zheng, X.; Hu, X.-M. Preparation of Fe3O4@C@TiO2 and its application for oxytetracycline hydrochloride adsorption. Rare Met. 2020, 39, 1333–1340. [Google Scholar] [CrossRef]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in electrospun TiO2 nanofibers: Design, construction, and applications. Chem. Eng. J. 2021, 431, 134343. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansurov, Z.; Smagulova, G.; Kaidar, B.; Imash, A.; Lesbayev, A. PAN—Composite Electrospun-Fibers Decorated with Magnetite Nanoparticles. Magnetochemistry 2022, 8, 160. https://doi.org/10.3390/magnetochemistry8110160
Mansurov Z, Smagulova G, Kaidar B, Imash A, Lesbayev A. PAN—Composite Electrospun-Fibers Decorated with Magnetite Nanoparticles. Magnetochemistry. 2022; 8(11):160. https://doi.org/10.3390/magnetochemistry8110160
Chicago/Turabian StyleMansurov, Zulkhair, Gaukhar Smagulova, Bayan Kaidar, Aigerim Imash, and Aidos Lesbayev. 2022. "PAN—Composite Electrospun-Fibers Decorated with Magnetite Nanoparticles" Magnetochemistry 8, no. 11: 160. https://doi.org/10.3390/magnetochemistry8110160
APA StyleMansurov, Z., Smagulova, G., Kaidar, B., Imash, A., & Lesbayev, A. (2022). PAN—Composite Electrospun-Fibers Decorated with Magnetite Nanoparticles. Magnetochemistry, 8(11), 160. https://doi.org/10.3390/magnetochemistry8110160






