Abstract
In this paper, (Tb0.7Lu0.3)2O3 magneto-optical transparent ceramics with different ZrO2 doping levels (0~5 at%) were prepared by hydrogen sintering and sequential HIP technique using ZrO2 as a sintering aid. The effect of ZrO2 doping content on the microstructure and optical properties of (Tb0.7Lu0.3)2O3 ceramics was analyzed. We found that the optimal doping content of ZrO2 was 3 at%. The transmittance of 3 at% ZrO2-doped (Tb0.7Lu0.3)2O3 ceramics at the wavelength of 1064 nm was 74.84 %, and the Verdet constant was approximately 275.28 rad·T−1·m−1 at the wavelength of 650 nm.
1. Introduction
A magneto-optical element (MOE) is one of the crucial components of Faraday rotators and isolators and can eliminate reflected light to ensure the stability of laser transmission in the laser system [1,2,3,4]. Usually, magneto-optical materials with high Verdet constants are chose for MOEs because small magneto-optical materials are required if the Verdet constant high according to the Faraday effect formula of , where V is the Verdet constant, θ is the rotation angle of the light vector and L is the length of the magneto-optical material.
At present, a TGG (Terbium gallium garnet) single crystal with a Verdet constant of approximately −134 rad·T−1·m−1 is the most widely used magneto-optical material [5,6,7]. However, owing to the size limitation of the crystal material and the relatively low Verdet constant, sesquioxide materials with higher Verdet constants, such as holmium oxide, terbium oxide, etc. [8,9,10], have attracted considerable attention. Among the sesquioxides, Tb2O3 has many excellent properties, such as high angular momentum [11] and only one absorption peak at 483 nm [12]. However, terbium sesquioxide easily oxidized into Tb4O7 without magneto-optical properties, which considerably affect the performance of this kind of material [13]. Furthermore, terbium oxide undergoes a reversible phase transition from the C-type cubic phase to the B-type monoclinic phase when the temperature exceeds 1600 °C. [14,15].
In order to prevent oxidation of terbium oxide, commercial Tb2O3 powders were used as original powder and sintered in an oxygen-free environment [16]. Some researchers attempted to deoxidize commercial Tb4O7 powder to Tb2O3 powder by hydrogen sintering [17] to reduce the sintering temperature. In the view of the phase change of terbium oxide during sintering, researchers have proposed a solution of doping rare earth elements [18,19,20]. Moreover, sintering additives including ZrO2, La2O3, MgO, etc., were added to reduce the sintering temperature [21,22]. All these strategies represent promising techniques.
In 2017, Ning et al. [23] used low-level (0.5 wt%) ZrO2-MgO as a double-sintering aid to prepare highly transparent Yb:Y2O3 ceramics. The addition of 0.5 wt% ZrO2 was found to effectively increase the density of the ceramics and promote pore elimination. In 2019, Hu et al. [24] prepared Dy2O3 transparent ceramics by vacuum sintering of nanopowders. A Dy2O3 phase appeared at 600 °C during the decomposition period of the precursor, and the in-line transmittance values of the optimal ceramic sample with 1.0 mm thickness are 75.3% at 2000 nm and 67.9% at 633 nm. Despite many studies on Yb2O3, Dy2O3 and other sesquioxide materials, few studies have investigated the effect of sintering additives on the sintering properties of Tb2O3 ceramics.
In this work, (Tb0.7Lu0.3)2O3 transparent ceramics were prepared by hydrogen pre-sintering combined with hot isostatic pressing sintering. We added varying amounts of ZrO2 as sintering aids to promote ceramic densification. The effect of ZrO2 content on the microstructure and sintering properties of these samples were investigated.
2. Experiment
2.1. Preparation Processes
High-purity Tb2O3 (99.99%), Lu2O3 (99.99%) and ZrO2(99.99%) powders were used as raw materials. The raw materials were weighted according to the chemical formula of (Tb0.7Lu0.3)2O3 and 0~5 at% ZrO2 and mixed by ball milling for 24 h at a speed of 200 r/min. After drying by rotary evaporator, the dried powders were sieved through 100 meshes. The green ceramic samples were dry-pressed into a disk with a diameter of 20 mm at 20 MPa and further cold isostatically pressed at 200 MPa for 120s to increase the density. Before hydrogen sintering, the samples were calcined at 1000 °C for 10 h to remove organic impurities and residual carbon. The green bodies were sintered between 1550 °C and 1700 °C for 4 h in a hydrogen atmosphere. All the presintered samples were hot isostatically pressed at 1575 °C in an argon atmosphere to eliminate closed pores to improving performance of the ceramics. Finally, transparent (Tb0.7Lu0.3)2O3 ceramics were obtained by mirror polishing to 2.0 mm thickness for measurement.
2.2. Characterization
The phase compositions of the ceramics were determined by X-ray diffraction (XRD; DX-1000CSC, Tongda Co. Ltd., Dandong, Liaoning, China) using Cu Kα radiation with a scan speed of 10°/min and a step size of 0.03° in the range of 2θ = 10°–70°. The linear shrinkage rate of the ceramics was calculated according to the following formula:
where △L is the linear shrinkage of the sample after sintering, L0 is the size of the green body before sintering and L1 is the size of the samples after sintering. Microstructures of the fracture surfaces were examined using field emission scanning electron microscopy (SEM, Inspect F, FEI, Hillsborocity, OR, USA). The in-line transmittance of ceramics was measured by a UV-VIS-NIR spectrometer (Lambda950, PerkinElmer, Waltham, MA, USA) over the wavelength region from 200 nm to 1600 nm. Verdet constants were recorded on a Faraday effect experimental instrument (FD-FZ-C, Shanghai Fudan Tianxin Scientific & Educational Instruments Co., Ltd., Shanghai, China), with a laser wavelength of 650 nm.
3. Result and Discussion
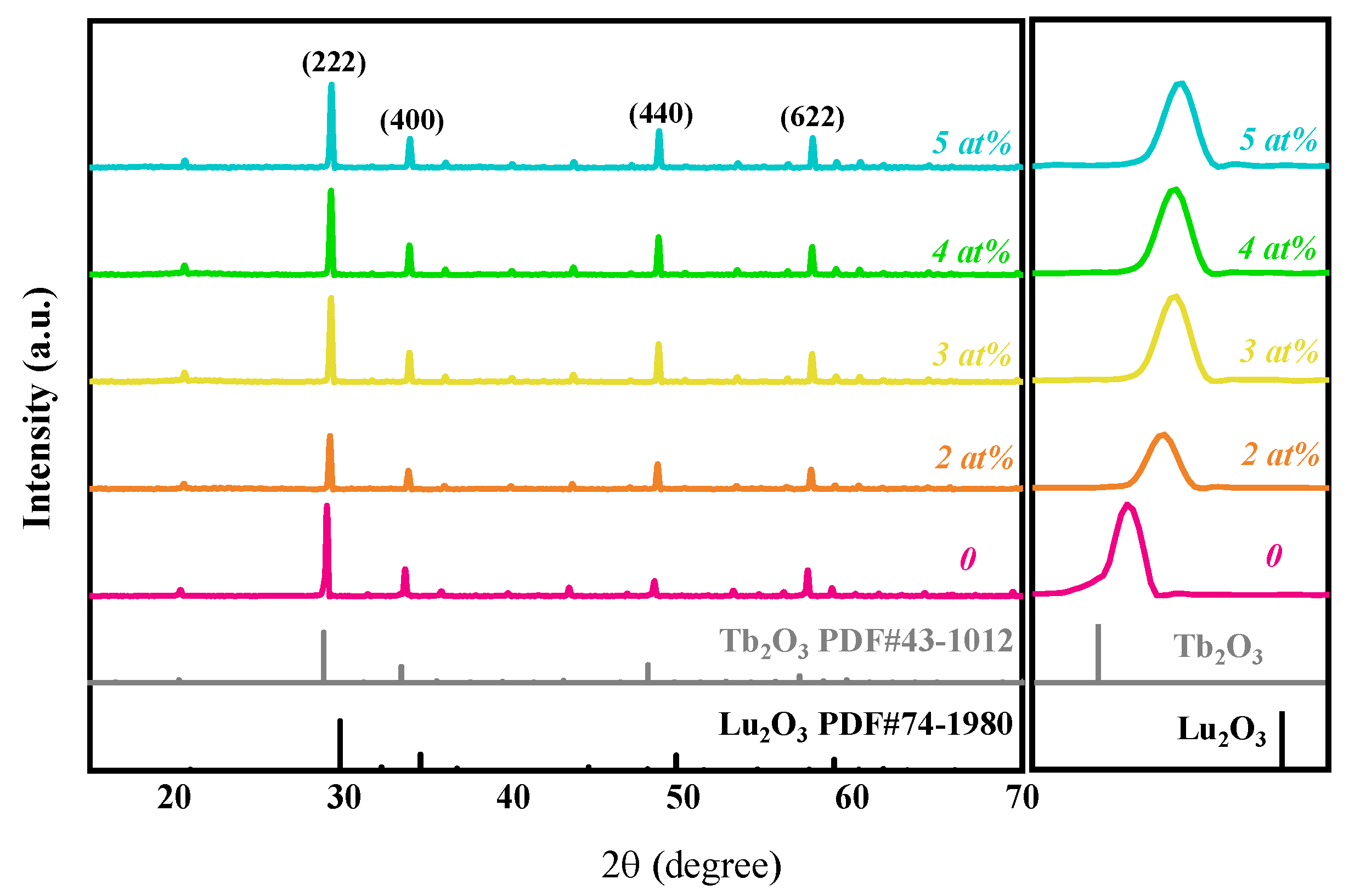
Figure 1 presents XRD patterns of the (Tb0.7Lu0.3)2O3 ceramic samples with varying Zr4+ concentrations after hot isostatic pressing sintering. Compared with the standard XRD spectra of Lu2O3 (PDF#74-1980) and Tb2O3 (PDF#43-1012), there is no other impurity phase, and all the samples are approximately consistent with the characteristic peak of Tb2O3. The four diffraction peaks at 29°, 33.6°, 48.5° and 57.64° in the figure correspond to the crystal face of the cubic phases Tb2O3 (222), (400), (440) and (622), respectively [11]. The diffraction patterns located between the diffraction patterns of Tb2O3 and Lu2O3 indicate that the Lu3+ successfully entered the Tb2O3 lattice and replaced the Tb3+ sites to form a (Tb0.7Lu0.3)2O3 solid solution.
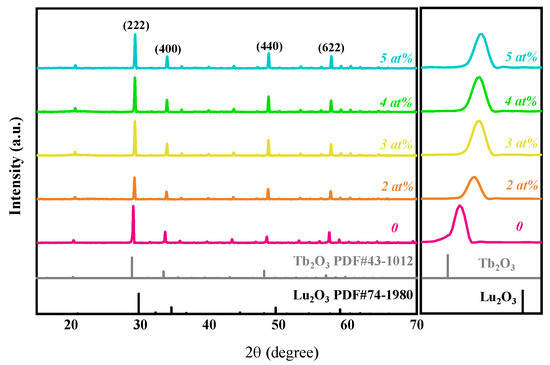
Figure 1.
XRD patterns of (Tb0.7Lu0.3)2O3 ceramics with 0~5 at% ZrO2 as sintering aids after hot isostatic pressing sintering.
With the increase in ZrO2 content, the characteristic peak shifts towards higher angles, which can be explained by the formulae (2) and (3), for which the standard notations of Kröger and Vink were used [25]. According to previous research [23], when ZrO2 is added as a sintering aid, Zr4+ replaces the position of Tb3+/Lu3+, and the following reactions occur to generate cation vacancies, which reduce the cell volume.
In addition, the radius of Zr4+ (0.072 nm) is smaller than that of Tb3+ (0.0923 nm) and Lu3+ (0.0861 nm), which may also decrease the lattice cell constant.
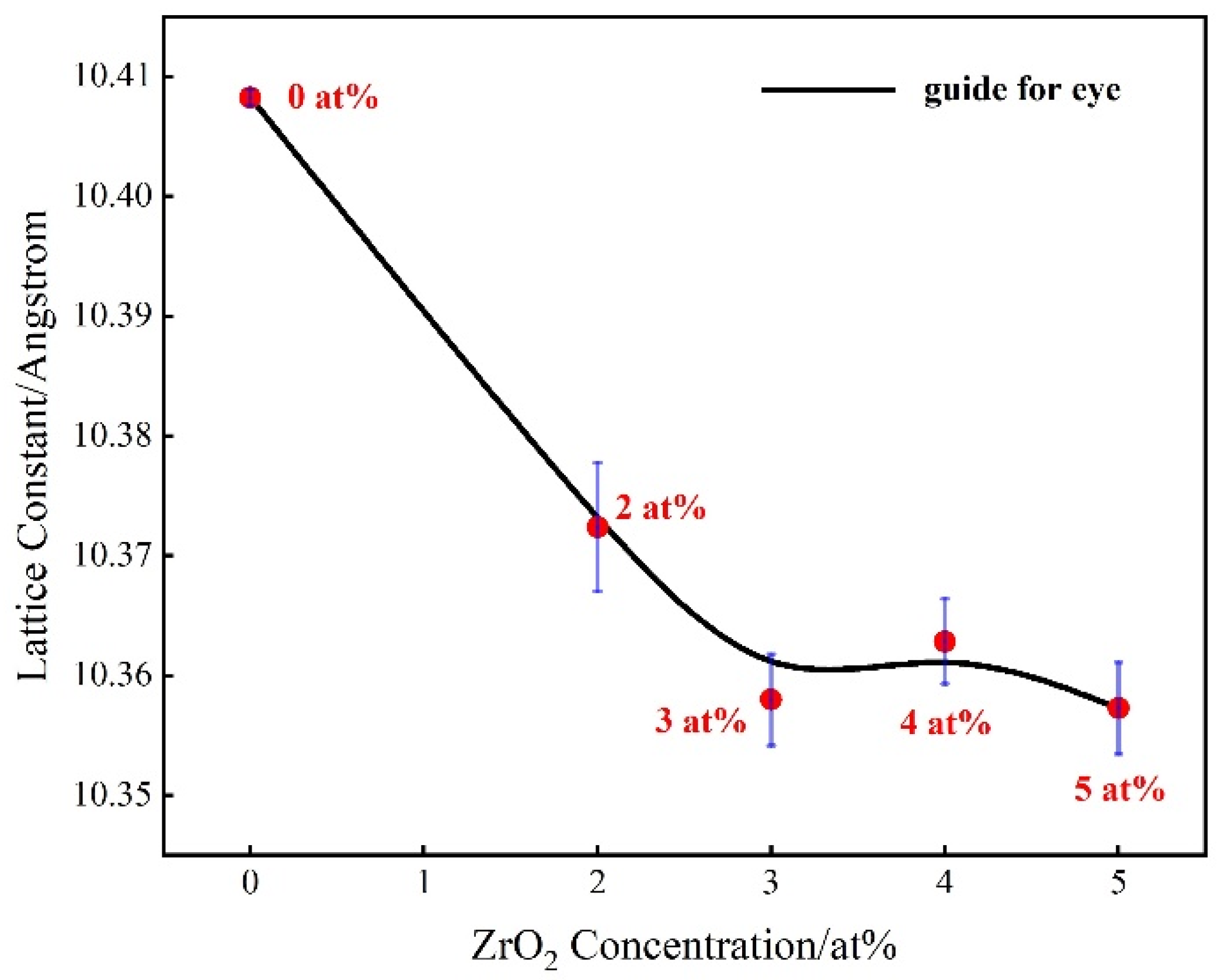
Figure 2 shows the changes in lattice constant calculated by the XRD patterns. The cell size of the undoped (Tb0.7Lu0.3)2O3 ceramic is approximately 10.4 angstrom. When zirconia is added from 2 at% to 3 at%, the cell size decreases from 10.37 Å to 10.35 Å. However, the lattice constant does not change linearly with the zirconia content. When the zirconia content exceeds 4 at%, the change in lattice constant is not obviously (approximately 0.01 Å), which could be ascribed to the zirconia solid solubility limit. The excess zirconia cannot enter the lattice, with an insignificant effect on the lattice constant. When the zirconia content is 5 at%, the lattice constant is 10.35 Å.
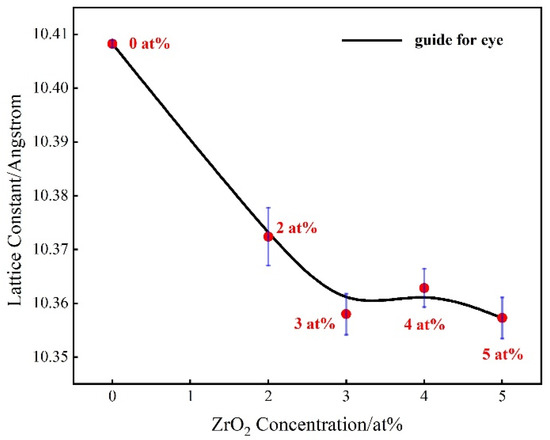
Figure 2.
Change in lattice constants with varying Zr concentrations.
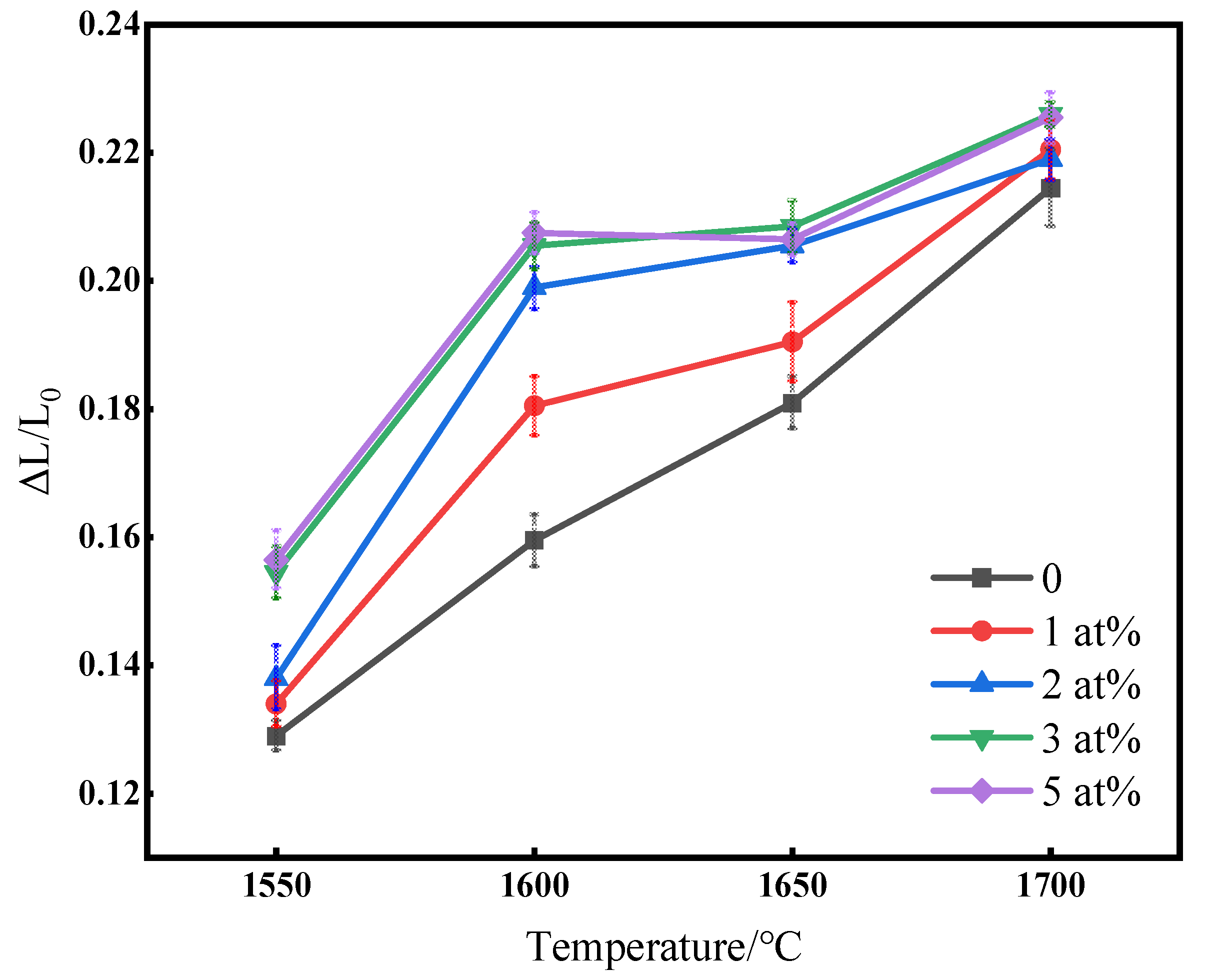
Figure 3 shows the change in linear shrinkage of (Tb0.7Lu0.3)2O3 ceramics with varying ZrO2 contents after hydrogen sintering from 1550 °C to 1700 °C. As shown in the figure, linear shrinkage increases with increased temperature. The shrinkage of the samples increases with increased zirconia content at the same sintering temperature. The linear shrinkage rate of the sample with no additional sintering additives increased linearly in the range of 1550 °C to 1700 °C. However, when ZrO2 was added, a plateau gradually appeared around the temperature of 1600 °C~1650 °C, and the curve of those samples with zirconia as a sintering aid could be divided into two sections. Before 1600 °C, the sample shrank rapidly, and the curve slope was relatively high. When the temperature exceeded 1600 °C, the slope was reduced with increased Zr4+ doping, proving that ZrO2 can improve the densification rate and that ceramics can be rapidly densified below 1600 °C with sufficient zirconia content. The shrinkage curves were nearly identical when the doping content of Zr4+ exceeded 3 at%.
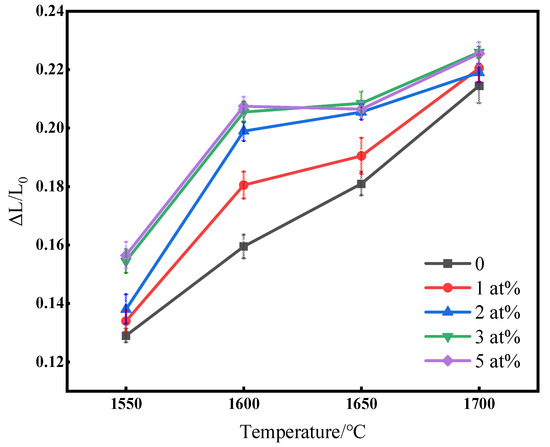
Figure 3.
Linear shrinkage rate with varying ZrO2 contents after hydrogen sintering from 1550 °C to 1700 °C.
According to the results presented above, we presintered the ceramics in a hydrogen atmosphere at 1550 °C, combined with hot isostatic pressing sintering at 1575 °C to make the samples fully dense. Tb2O3 undergoes severe phase transformation at 1600 °C [12,13], so a relatively low sintering temperature was chosen to avoid this transformation.
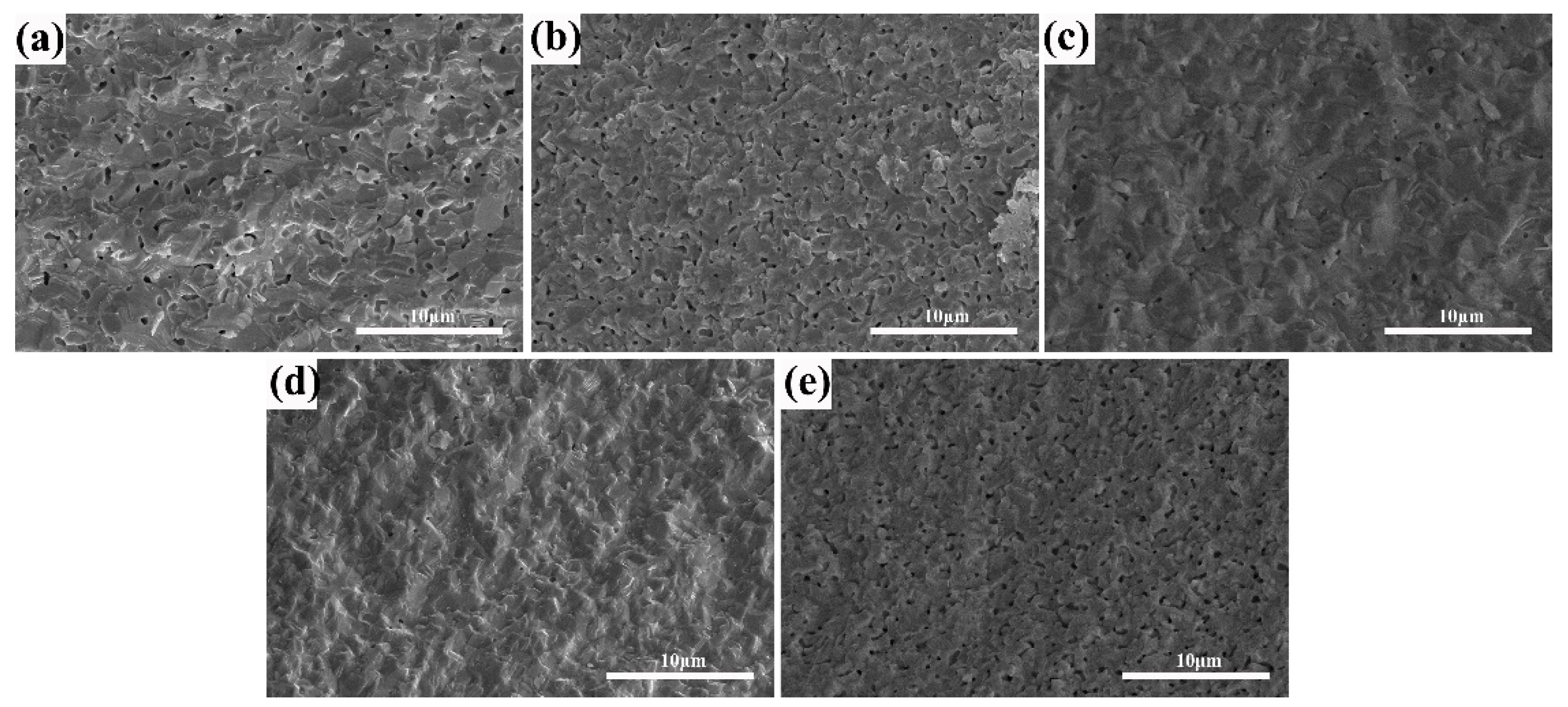
The microstructure of (Tb0.7Lu0.3)2O3 ceramics after hydrogen sintering at 1550 °C with varying ZrO2 contents is shown in Figure 4. With an increase in Zr4+ content from 0 to 3 at%, the pores become smaller, suggesting that the addition of ZrO2 can effectively reduce the sintering barrier of (Tb0.7Lu0.3)2O3 ceramics and promote pore elimination during presintering. Subsequently, the phenomenon of pore shrinkage became indistinct when the addition amount increased to 4 at%. As shown in Figure 4e, the porosity and pore size increased considerably, suggesting that excessive zirconia is unfavorable for pore elimination during the presintering process.
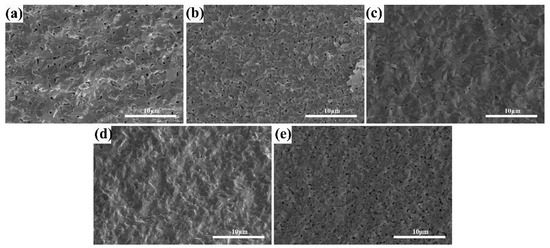
Figure 4.
SEM photomicrographs of the fractured surfaces of the (Tb0.7Lu0.3)2O3 ceramics after 1500 °C hydrogen sintering with varying ZrO2 contents: (a) 0 at%, (b) 2 at%, (c) 3 at%, (d) 4 at%, (e) 5 at%.
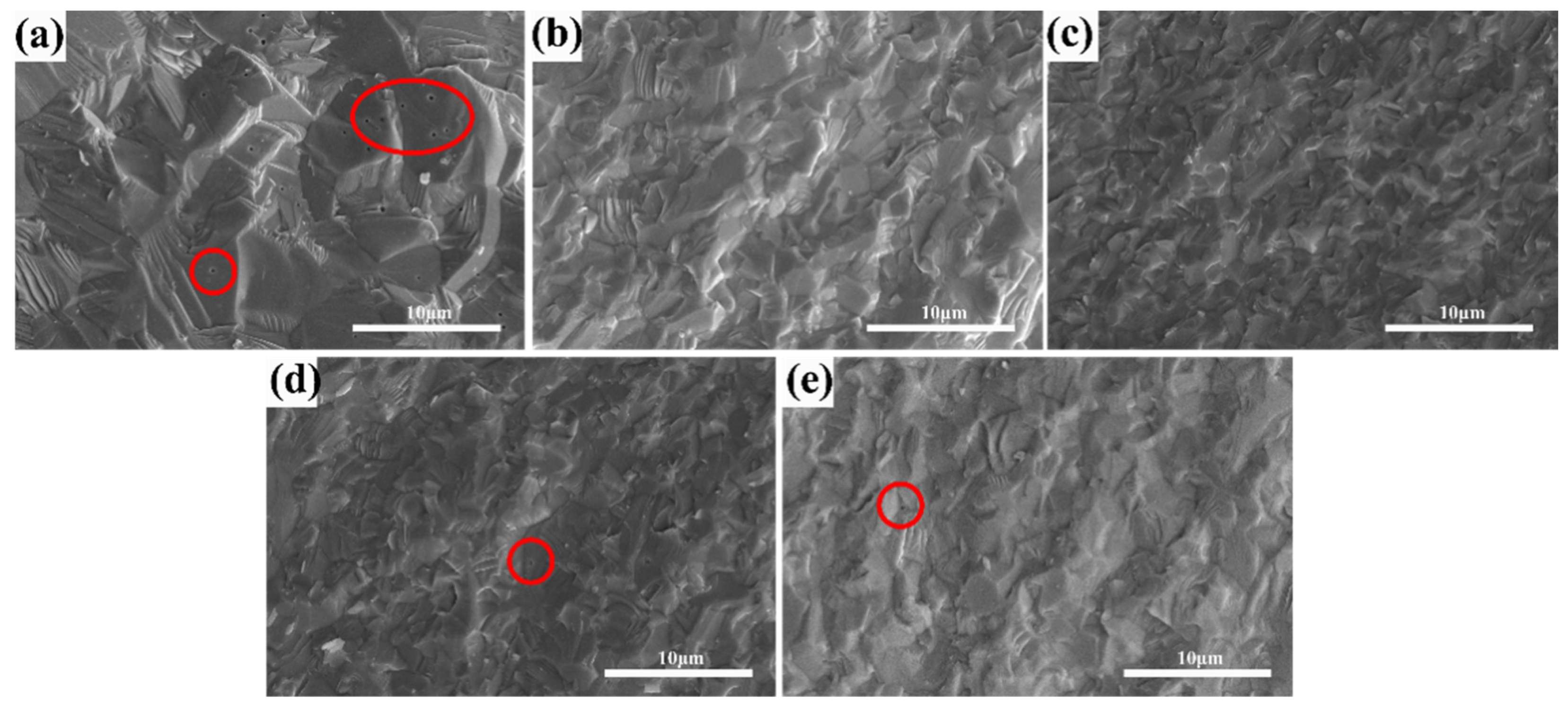
Figure 5 shows SEM image of the ceramics with varying ZrO2 contents after hot isostatic pressing sintering. The samples showed mainly transcrystalline fractures, and all samples presented with high density. As shown in Figure 5a, a large number of intragranular pores were observed, with a relatively large grain size.
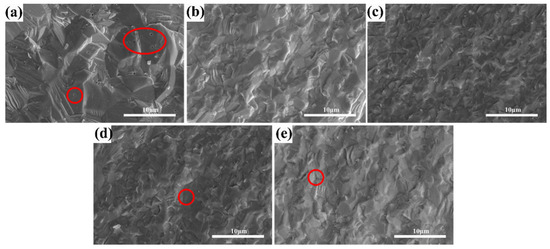
Figure 5.
SEM micrographs of the fractured surfaces of the (Tb0.7Lu0.3)2O3 ceramics after 1575 °C hot isostatic pressing sintering with varying ZrO2 contents (The pores were marked in red circles): (a) 0 at%, (b) 2 at%, (c) 3 at%, (d) 4 at%, (e) 5 at%.
On the contrary, no obvious pores can be observed in Figure 5b,c. When Zr content exceeds 3 at%, some intragranular pores can be observed in Figure 5d,e. The grain size decreases from 2.23 μm to 1.92 μm with increased ZrO2 content, which could be attributed to the doping effect of ZrO2, which has been reported in many other sesquioxide ceramic sintering processes [26,27,28,29]. During the high-temperature sintering process of sesquioxide ceramics, the sintering aid, ZrO2, can enter the lattice of sesquioxide as Zr4+, as shown in Formulae (2) and (3). It can inhibit the rapid growth of ceramic grains, avoid the formation of intracrystalline pores and contribute to the full densification of ceramics. As shown in the figure, this effect is closely related to the concentration of zirconia. When the concentration is 3 at% or less, the densification of zirconia is considerable, but a further increase in the content can result in densifying hazards.
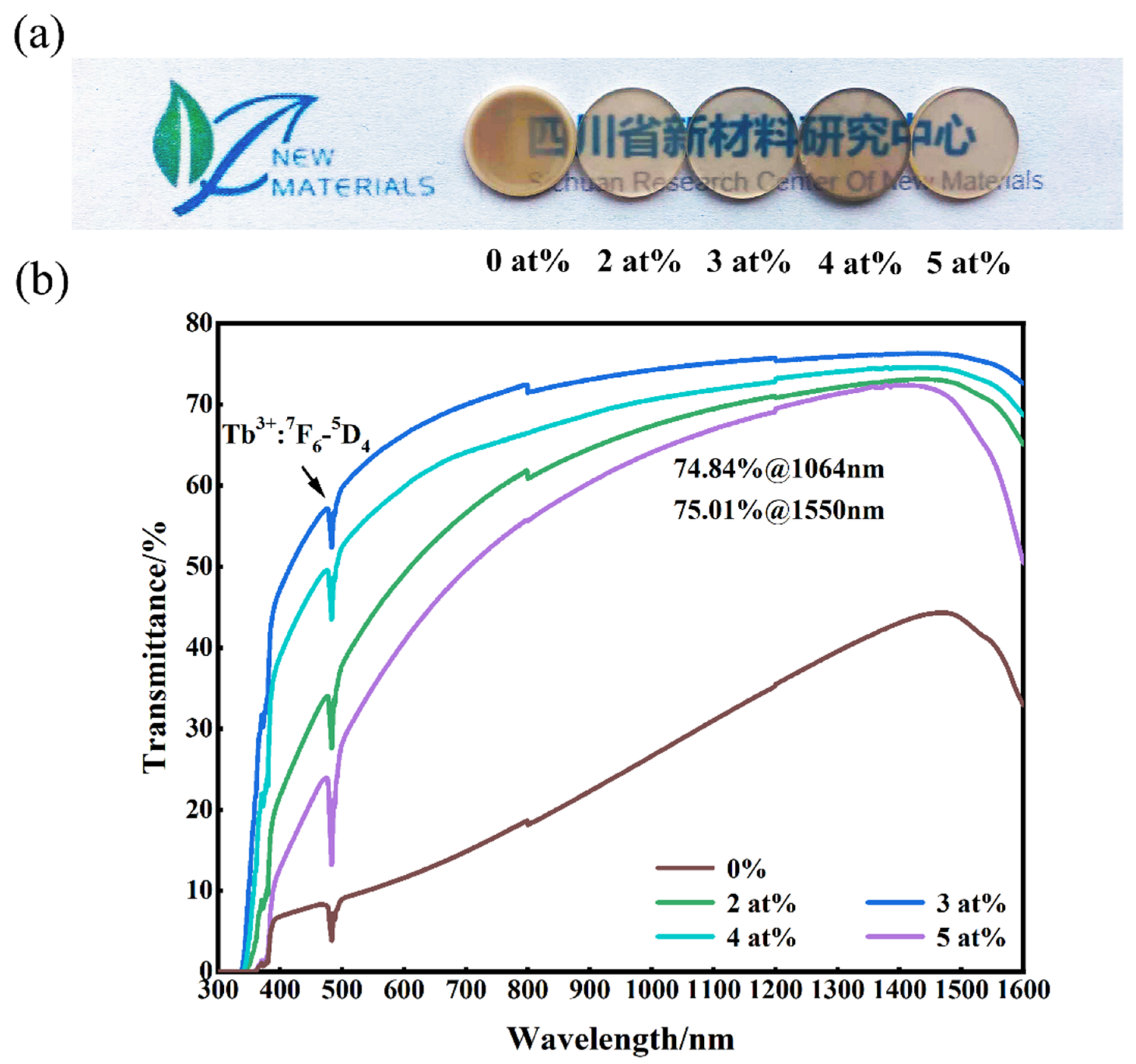
Figure 6 shows the in-line transmittance and appearance of (Tb0.7Lu0.3)2O3 transparent ceramics with varying contents of ZrO2 additive. Figure 6a shows that the samples sintered with ZrO2 exhibited sufficient optical quality, so the words below the ceramics could be clearly seen, and the optical quality was better than that of the ceramic sample without additive. This result is also confirmed by the transmittance curve of the sample shown in Figure 6b. The sample without sintering additives has the lowest transmittance, which could be related to the intracrystalline pores, as shown in Figure 5. With increased Zr4+ content, the transparency first increased and then decreased, which is also related to the effect of zirconia. When zirconia content is excessive, it changes the sintering properties of the material, simultaneously affecting the internal microstructure (as shown in Figure 4 and Figure 5), ultimately reducing the transparency. The ceramic sample with 3 at% ZrO2 addition has the best optical quality, with a transparency of 74.96% at 1064 nm and 75.01% at 1550 nm. However, the transparency decreases with a further increase in Zr4+ content to 4 at%, which could be related to the change in the internal microstructure of the ceramics. All the transmission curves show obvious emission peaks at 483 nm, in association with the Tb3+ energy transition from 7F6 to 5D4 [11].
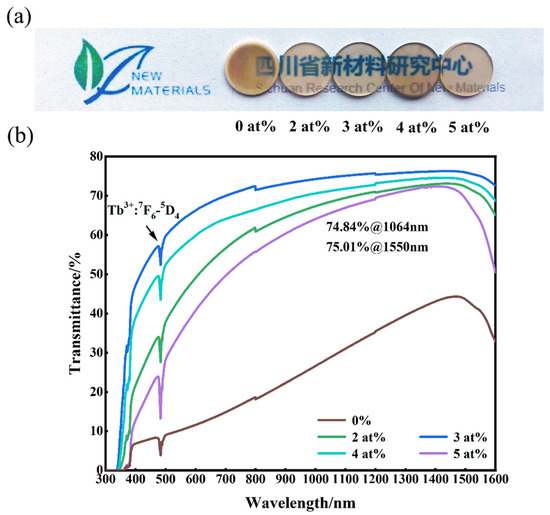
Figure 6.
Photograph (a), and in-line transmittances curves (b) of the mirror–polished (Tb0.7Lu0.3)2O3 ceramics sintered at 1550 °C followed by HIP at 1575 °C. (Zirconia content from left to right is 0 at%, 2 at%, 3 at%, 4 at% and 5 at%).
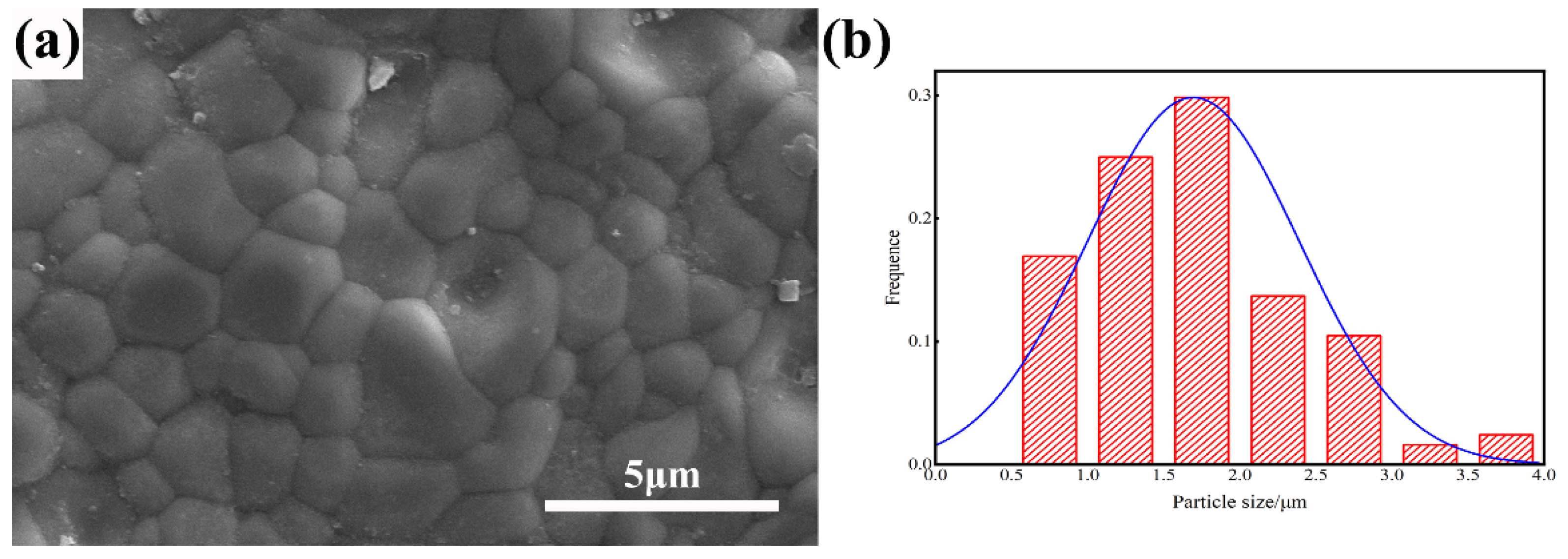
Figure 7a shows the microstructure of 3 at% ZrO2 doped (Tb0.7Lu0.3)2O3 ceramics. The fracture surface exhibited a pore-free structure, and the grains were tightly bound to each other. In addition, no secondary phase grain was observed on the grain boundary. As shown in Figure 5b, the particle size distribution of 3 at% Zr: (Tb0.7Lu0.3)2O3 is mainly in the range of 1.0–2 μm, and the average grain size is approximately 1.74 μm.
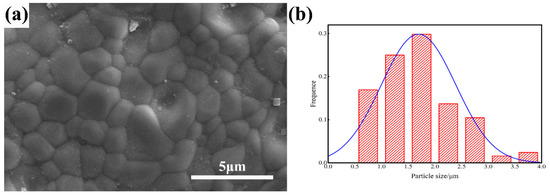
Figure 7.
SEM micrographs of the surface of 3 at% Zr: (Tb0.7Lu0.3)2O3 ceramic after hot isostatic pressing sintering (a) and the particle size distribution (b).
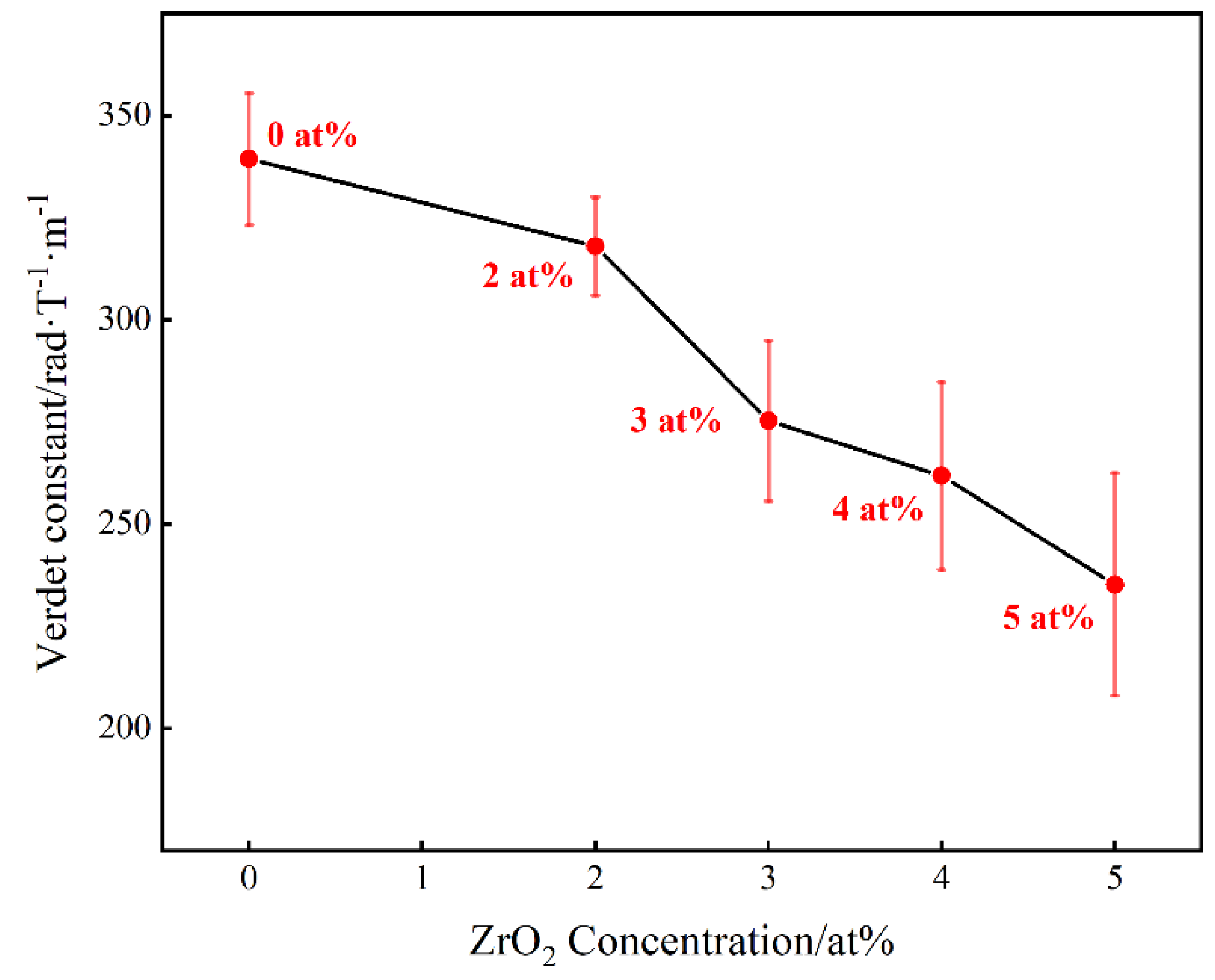
Figure 8 shows the Verdet constant of (Tb0.7Lu0.3)2O3 ceramics with varying Zr4+ contents at the 650 nm wavelength at room temperature. The Verdet constants for the ceramic samples were calculated using the following formula:
where V is the Verdet constant, θ is the rotation angle of the light vector and L is the length of the magneto-optical material. All samples were polished to 2 mm. As shown in the figure, the Vedet constant decreases from 339.36 rad·T−1·m−1 to 235.22 rad·T−1·m−1 with an increase in Zr4+ content from 0 at% to 5 at%. The Verdet constants of (Tb0.7Lu0.3)2O3 ceramics changed depending on the Zr4+ concentration. According to the literature [30,31], for Tb3+ ions, there are unpaired free electrons on the 4f electron layer, which produce an uncompensated magnetic moment in the magnetic field, which is the source of the magnetism. In the external magnetic field, electrons are prone to 4f8−4f75d transitions, which correspond to 7F6–7D5 level transition, demonstrating strong magnetism.
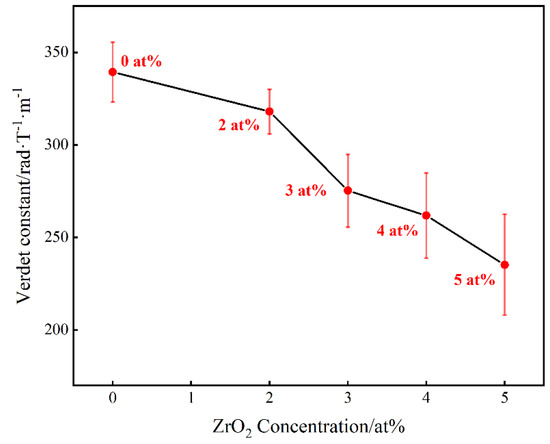
Figure 8.
Verdet constant of Zr: (Tb0.7Lu0.3)2O3 ceramics versus Zr4+ concentration at the 650 nm wavelength at room temperature.
This phenomenon can be explained as follows. A large number of lattice vacancies and defects are produced with the addition of ZrO2, which may affect the electron transition of Tb3+, with an eventual decrease in the Verdet constant. Although the Verdet constant decreases with increased Zr4+ content, the Verdet constant of (Tb0.7Lu0.3)2O3 ceramics with 3 at% ZrO2 is 275.28 rad·T−1·m−1, which is still about 2.05 times that of a commercial TGG single crystal.
4. Conclusions
Highly transparent (Tb0.7Lu0.3)2O3 ceramics were prepared using ZrO2 as a sintering additive a via hot isostatic pressing sintering process. With increased Zr4+ content, the grain size decreases; the transparency of the sample increases first and then decreases. The inline transmittance of 3 at% ZrO2 doped (Tb0.7Lu0.3)2O3 ceramics reached 74.84% at 1064 nm. The addition of ZrO2 can effectively reduce the sintering barrier of (Tb0.7Lu0.3)2O3 ceramics, inhibit the abnormal growth of grains and promote the discharge of pores. The average grain size of the ceramics with 3 at% ZrO2 added is approximately 1.74 μm. The Verdet constant of 3 at% ZrO2-doped ceramics is 275.28 rad·T−1·m−1, which is still approximately 2.05 times that of a commercial TGG single crystal.
Author Contributions
Conceptualization, W.J.; Methodology, Y.X., T.X. and W.J.; Data analysis, Y.X., Y.W. and P.L.; Resources, B.K., W.J. and B.M.; Supervision, B.K. and W.J.; Writing—original draft, Y.X.; Writing—review and editing, W.J. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Development Foundation of China Academy of Engineering Physics and the National Natural Science Foundation of China (Grant No. 51902234).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayramian, A.; Armstrong, J.; Beer, G.; Campbell, R.; Chai, B.; Cross, R.; Erlandson, A.; Fei, Y.; Freitas, B.; Kent, R.; et al. High-average-power femto-petawatt laser pumped by the Mercury laser facility. J. Opt. Soc. Am. B 2008, 25, B57–B61. [Google Scholar] [CrossRef]
- Mueller, G.; Amin, R.S.; Guagliardo, D.; McFeron, D.; Lundock, R.; Reitze, D.H.; Tanner, D. Method for compensation of thermally induced modal distortions in the input optical components of gravitational wave interferometers. Class. Quantum Gravity 2002, 19, 1793–1801. [Google Scholar] [CrossRef]
- Khazanov, E.; Andreev, N.; Mal’Shakov, A.; Palashov, O.; Poteomkin, A.; Sergeev, A.; Shaykin, A.; Zelenogorsky, V.; Ivanov, I.; Amin, R.; et al. Compensation of thermally induced modal distortions in Faraday isolators. IEEE J. Quantum Electron. 2004, 40, 1500–1510. [Google Scholar] [CrossRef]
- Dai, J.; Li, J. Promising magneto-optical ceramics for high power Faraday isolators. Scr. Mater. 2018, 155, 78–84. [Google Scholar] [CrossRef]
- Yasuhara, R.; Nozawa, H.; Yanagitani, T.; Motokoshi, S.; Kawanaka, J. Temperature dependence of thermo-optic effects of single-crystal and ceramic TGG. Opt. Express 2013, 21, 31443–31452. [Google Scholar] [CrossRef]
- Chen, Z.; Hang, Y.; Wang, X.; Hong, J. Fabrication and characterization of TGG crystals containing paramagnetic rare-earth ions. Solid State Commun. 2016, 241, 38–42. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Z.; Feng, G.; Wang, S.; Xu, L.; Wu, S. Preparation and properties of novel Tb3Sc2Al3O12 (TSAG) magneto-optical transparent ceramic. J. Eur. Ceram. Soc. 2021, 41, 195–201. [Google Scholar] [CrossRef]
- Furuse, H.; Yasuhara, R. Magneto-optical characteristics of holmium oxide (Ho2O3) ceramics. Opt. Mater. Express 2017, 7, 827–833. [Google Scholar] [CrossRef]
- Yakovlev, A.; Snetkov, I.; Permin, D.; Balabanov, S.; Palashov, O. Faraday rotation in cryogenically cooled dysprosium based (Dy2O3) ceramics. Scr. Mater. 2018, 161, 32–35. [Google Scholar] [CrossRef]
- Veber, P.; Velázquez, M.; Gadret, G.; Rytz, D.; Peltz, M.; Decourt, R. Flux growth at 1230 °C of cubic Tb2O3 single crystals and characterization of their optical and magnetic properties. CrystEngComm 2014, 17, 492–497. [Google Scholar] [CrossRef]
- Ibáñez, J.; Sans, J.Á.; Cuenca-Gotor, V.; Oliva, R.; Gomis, Ó.; Rodríguez-Hernández, P.; Muñoz, A.; Rodríguez-Mendoza, U.; Velázquez, M.; Veber, P.; et al. Structural and Lattice-Dynamical Properties of Tb2O3 under Compression: A Comparative Study with Rare Earth and Related Sesquioxides. Inorg. Chem. 2020, 59, 9648–9666. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, I.; Yakovlev, A.; Starobor, A.; Balabanov, S.; Permin, D.; Rostokina, E.; Palashov, O. Thermo-optical properties of terbium sesquioxide (Tb2O3) ceramics at room temperature. Opt. Lett. 2021, 46, 3592–3595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jing, W.; Li, W.; Xu, T.; Kang, B.; Liu, X.; Xin, Y.; Luo, P.; Yang, N.; Mei, B. Synthesis of highly sinterable Tb2O3 powders by spray coprecipitation for transparent ceramics: The influence of ammonium hydrogen carbonate to metal ions molar ratio. Opt. Mater. 2022, 132, 112795. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Wang, J.; Wang, D.; Han, D.; Zhang, J.; Wang, S. Phase transformation process of Tb2O3 at elevated temperature. Scr. Mater. 2019, 171, 108–111. [Google Scholar] [CrossRef]
- Lee, C.; Vashishtha, S.; Sayal, A.; Weaver, J.F. Oxidation of a c-Tb2O3 (111) thin film by the sequential formation of stoichiometric phases. Surf. Sci. 2019, 694, 121555. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Wang, J.; Wang, D.; Han, D.; Zhang, J.; Wang, S. Preparation of (Tb1-xLux)2O3 transparent ceramics by solid solution for magneto-optical application. J. Eur. Ceram. Soc. 2021, 41, 2818–2825. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Xu, J.; Tian, T.; Jia, R.; Wang, Z. Fabrication and magneto-optical property of yttria stabilized Tb2O3 transparent ceramics. J. Eur. Ceram. Soc. 2019, 39, 5005–5009. [Google Scholar] [CrossRef]
- Zhu, L.; Park, Y.; Gan, L.; Go, S.; Kim, H.; Kim, J.; Ko, J. Fabrication and characterization of highly transparent Er: Y2O3 ceramics with ZrO2 and La2O3 additives. Ceram. Int. 2017, 43, 13127–13132. [Google Scholar] [CrossRef]
- Yi, Q.; Zhou, S.; Teng, H.; Lin, H.; Hou, X.; Jia, T. Structural and optical properties of Tm: Y2O3 transparent ceramic with La2O3, ZrO2 as composite sintering aid. J. Eur. Ceram. Soc. 2012, 32, 381–388. [Google Scholar] [CrossRef]
- Xie, J.; Mao, X.; Zhu, Q.; Jiang, B.; Zhang, L. Influence of synthesis conditions on the properties of Y2O3-MgO nanopowders and sintered nanocomposites. J. Eur. Ceram. Soc. 2017, 37, 4095–4101. [Google Scholar] [CrossRef]
- Snetkov, I.L.; Permin, D.A.; Balabanov, S.S.; Palashov, O.V. Wavelength dependence of Verdet constant of Tb3+: Y2O3 ceramics. Appl. Phys. Lett. 2016, 108, 161905. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L.; Makikawa, S.; Yahagi, A. Polycrystalline (TbxY1-x)2O3 Faraday rotator. Opt. Lett. 2017, 42, 4399–4401. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Wang, J.; Luo, D.; Dong, Z.L.; Kong, L.B.; Tang, D.Y. Low-level sintering aids for highly transparent Yb: Y2O3 ceramics. J. Alloy. Compd. 2017, 695, 1414–1419. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Liu, Z.; Li, X.; Tian, F.; Zhu, D.; Yang, Z.; Wu, L.; Li, J. Fabrication of Dy2O3 Transparent Ceramics by Vacuum Sintering Using Precipitated Powders. Magnetochemistry 2021, 7, 6. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the concentrations of imperfections in solids. J. Phys. Chem. Solids 1958, 5, 208–223. [Google Scholar] [CrossRef]
- Bokuniaeva, A.O.; Vorokh, A.S. Estimation of particle size using the Debye equation and the Scherrer formula for polyphasic TiO2 powder. J. Phys. Conf. Ser. 2019, 1410, 012057. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, S.; Li, Y.; Li, W. Effect of ZrO2 on the sinterability and spectral properties of (Yb0.05Y0.95)2O3 transparent ceramic. Opt. Mater. 2010, 32, 920–923. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, G.; Shimai, S.; Zhang, J.; Wang, S. ZrO2-doped Y2O3 transparent ceramics via slip casting and vacuum sintering. J. Eur. Ceram. Soc. 2010, 30, 2139–2143. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Mao, X.; Zhu, Q.; Xie, J.; Feng, M.; Jiang, B.; Zhang, L. Investigation of optical, mechanical, and thermal properties of ZrO2-doped Y2O3 transparent ceramics fabricated by HIP. Ceram. Int. 2018, 44, 1362–1369. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, Z. Magneto-optical glass mixed with Tb3+ ions: High Verdet constant and luminescence properties. J. Lumin. 2021, 231, 117804. [Google Scholar] [CrossRef]
- Yin, H.; Gao, Y.; Gong, Y.; Buchanan, R.; Song, J.; Li, M. Wavelength dependence of Tb3+ doped magneto-optical glass Verdet constant. Ceram. Int. 2018, 44, 10929–10933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).