Green Synthesis of Unsaturated Fatty Acid Mediated Magnetite Nanoparticles and Their Structural and Magnetic Studies
Abstract
1. Introduction
2. Materials and Methods
Synthesis of MNPs
3. Characterization of MNPs
3.1. FTIR Analysis
3.2. TGA Analysis
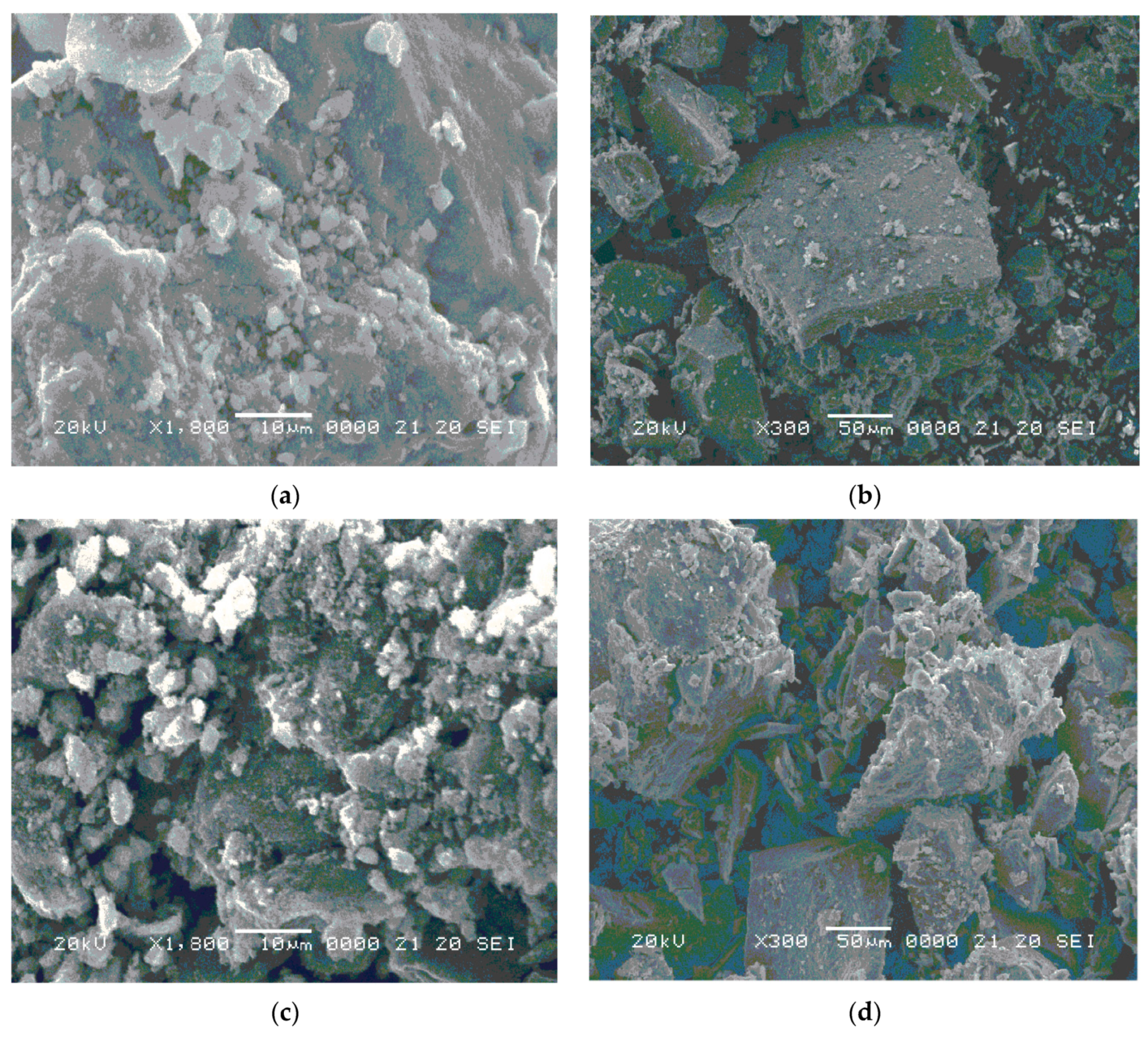
3.3. SEM Analysis
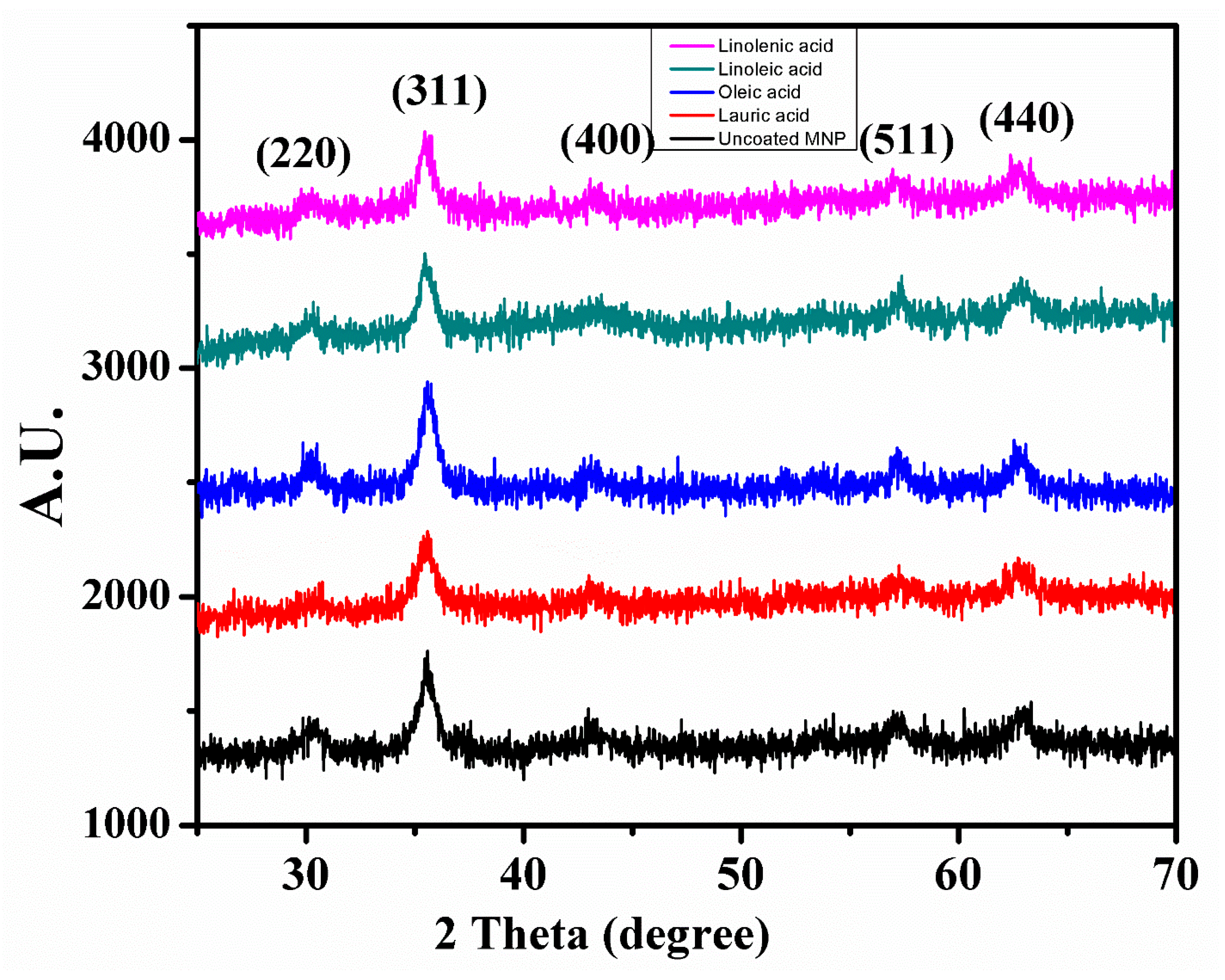
3.4. XRD Analysis
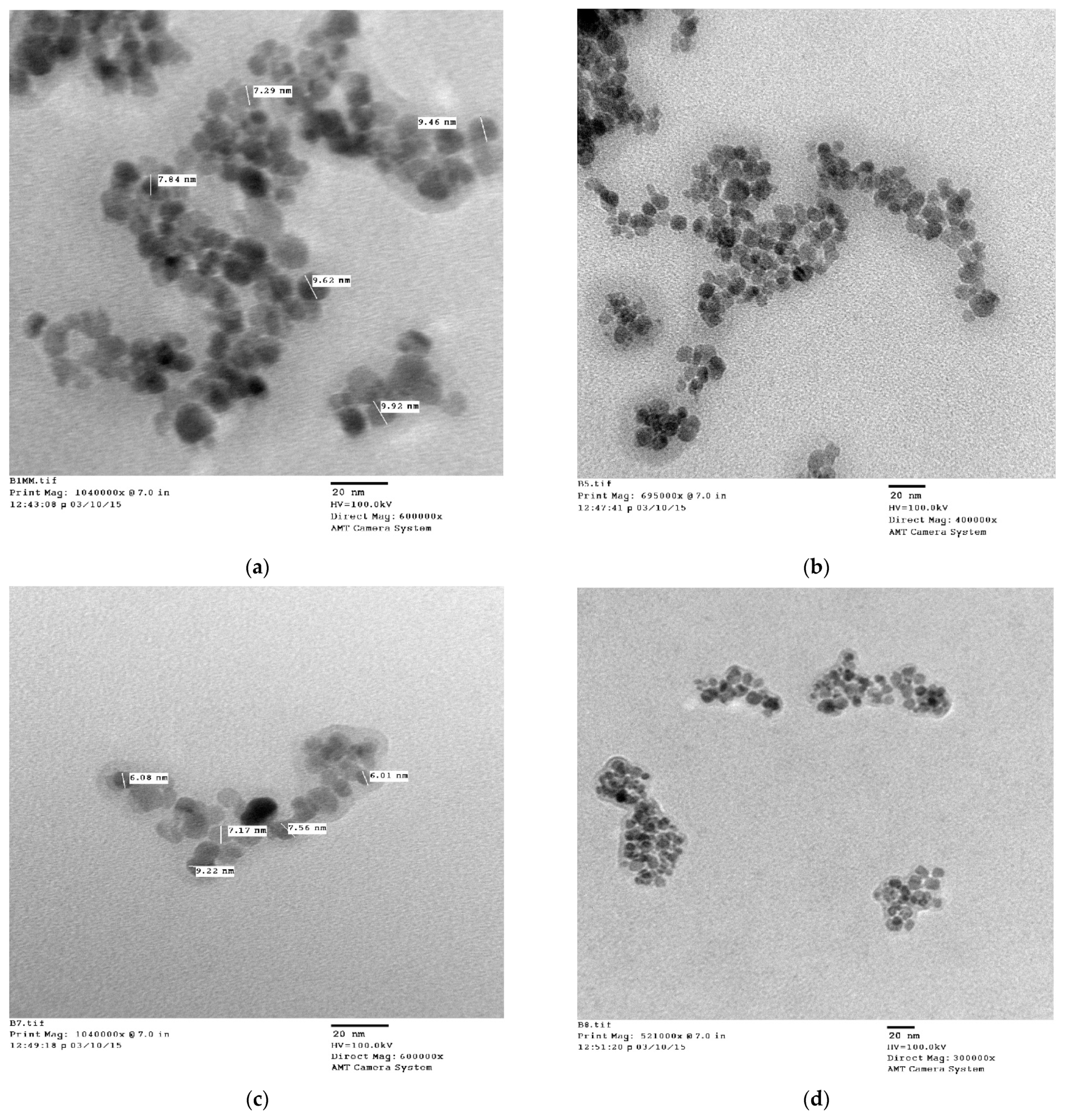
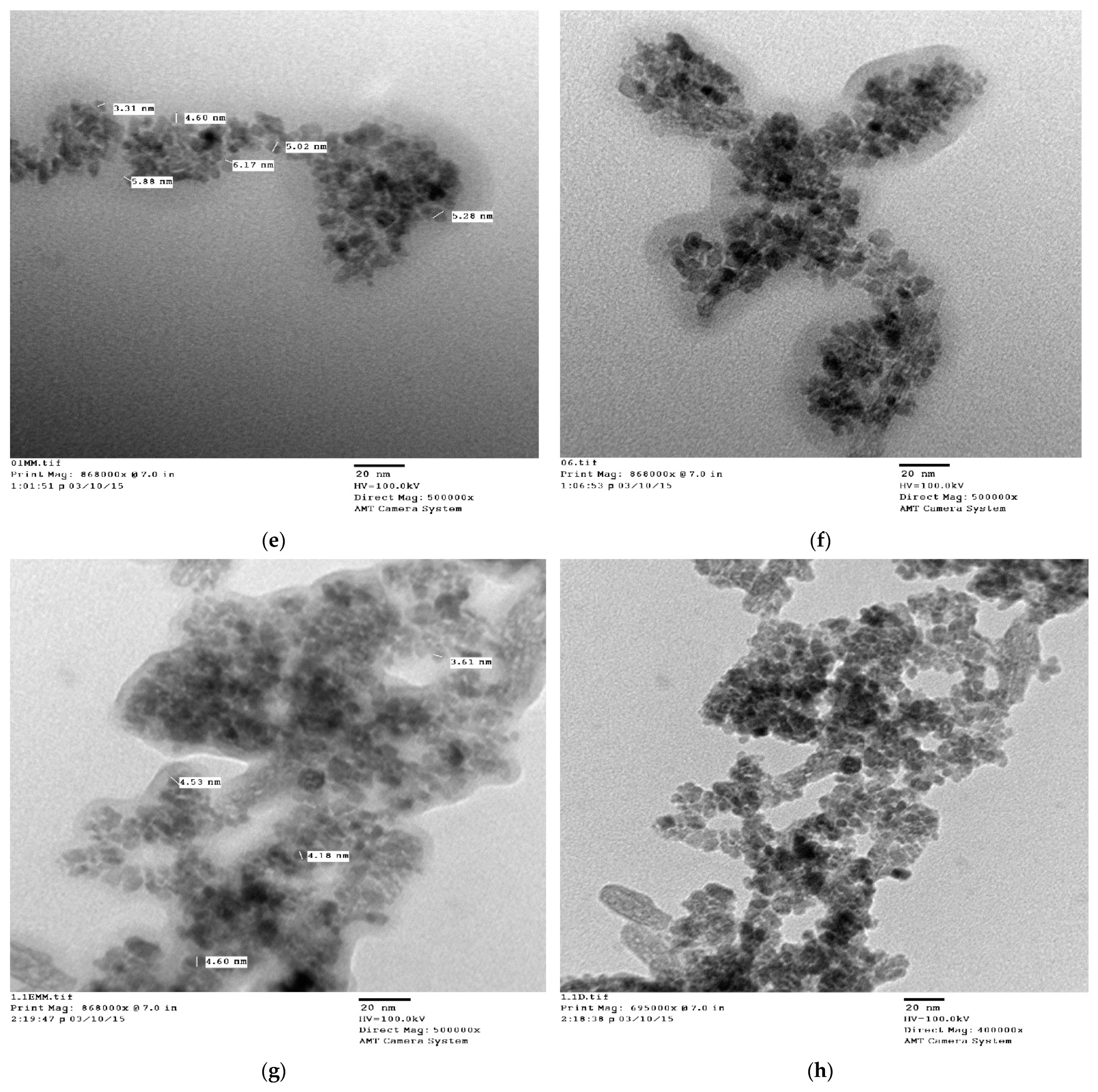
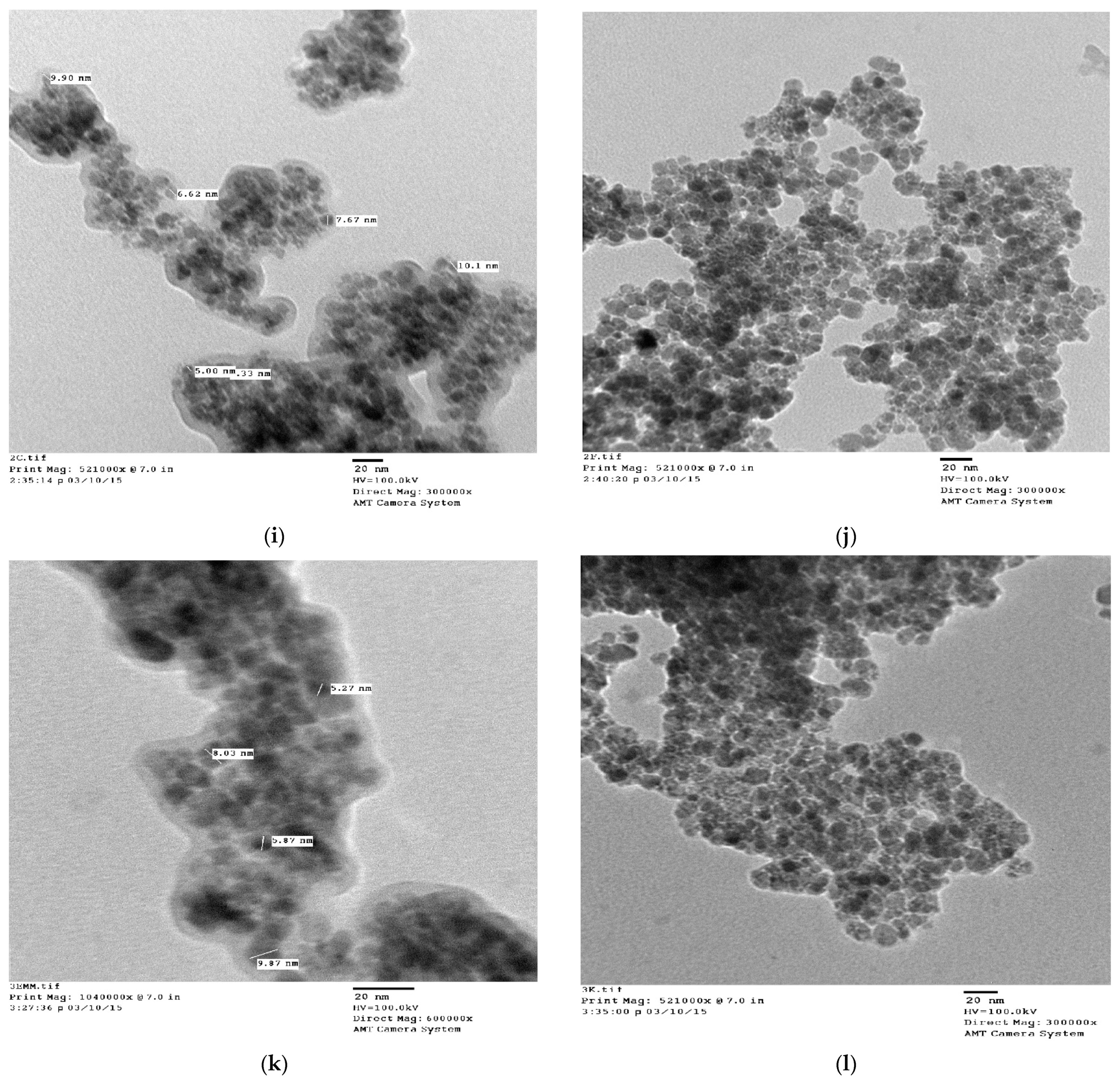
3.5. TEM Analysis
4. Result and Discussion
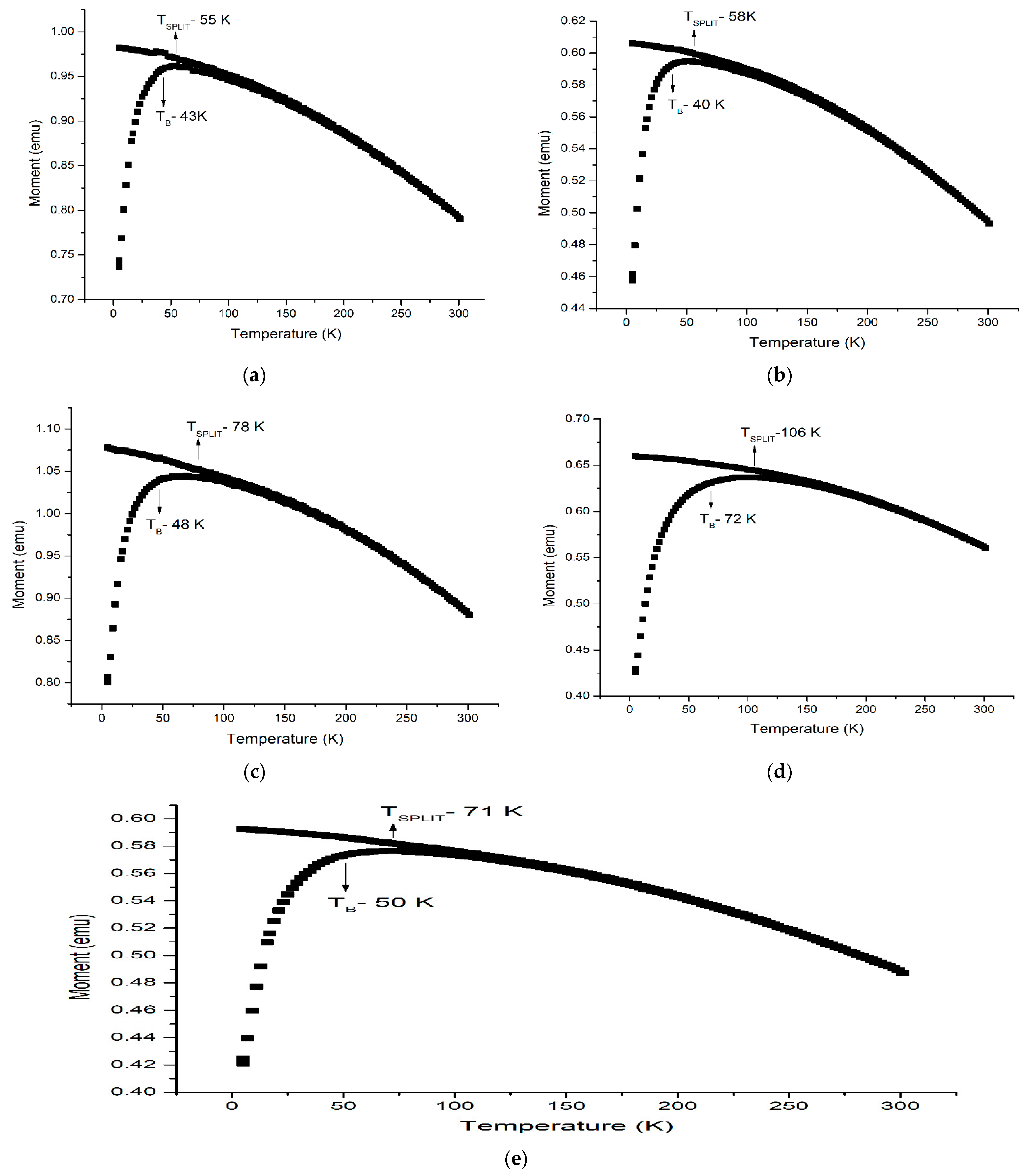
Magnetic Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Justin, C.; Philip, S.A.; Samrot, A.V. Synthesis and characterization of superparamagnetic iron-oxide nanoparticles (SPIONs) and utilization of SPIONs in X-ray imaging. Appl. Nanosci. 2017, 7, 463–475. [Google Scholar] [CrossRef]
- Kamali, M.S.; Ericsson, T.; Wäppling, R. Characterization of iron oxide nanoparticles by Mössbauer spectroscopy. Thin Solid Films 2006, 515, 721–723. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green Synthesis of Magnetic Nanoparticles Using Satureja hortensis Essential Oil toward Superior Antibacterial/Fungal and Anticancer Performance. Biomed. Res. Int. 2021, 2021, 8822645. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Sbargoud, K.; Allard, E.; Larpent, C. Magnetic separation of fatty acids with iron oxide nanoparticles and application to extractive deacidification of vegetable oils. Green Chem. 2012, 14, 1786–1795. [Google Scholar] [CrossRef]
- Ahghari, M.R.; Soltaninejad, V.; Maleki, A. Synthesis of nickel nanoparticles by a green and convenient method as a magnetic mirror with antibacterial activities. Sci. Rep. 2020, 10, 12627. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Mahmad Rozi, S.K.; Bakhshaei, S.; Abdul Manan, N.S.; Mohamad, S. Superhydrophobic magnetic nanoparticle-free fatty acid regenerated from waste cooking oil for the enrichment of carcinogenic polycyclic aromatic hydrocarbons in sewage sludges and landfill leachates. RSC Adv. 2016, 6, 87719–87729. [Google Scholar] [CrossRef]
- Lenin, R.; Joy, P.A. Studies on the role of unsaturation in the fatty acid surfactant molecule on the thermal conductivity of magnetite nanofluids. J. Colloid Interface Sci. 2017, 506, 162–168. [Google Scholar] [CrossRef]
- Valenzuela, R.; Fuentes, M.C.; Parra, C.; Baeza, J.; Durán, N.; Sharma, S.K.; Knobel, M.; Freer, J. Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method. J. Alloy. Compd. 2009, 488, 227–231. [Google Scholar] [CrossRef]
- Vereda, F.; de Vicente, J.; Hidalgo-Álvarez, R. Influence of a Magnetic Field on the Formation of Magnetite Particles via Two Precipitation Methods. Langmuir 2007, 23, 3581–3589. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Q.; Qu, S. Synthesis and Characterization of Uniform and Crystalline Magnetite Nanoparticles via Oxidation-precipitation and Modified co-precipitation Methods. Int. J. Electrochem. Sci. 2013, 8, 3786–3793. [Google Scholar]
- Etemadifar, R.; Kianvash, A.; Arsalani, N.; Abouzari-Lotf, E.; Hajalilou, A. Green synthesis of superparamagnetic magnetite nanoparticles: Effect of natural surfactant and heat treatment on the magnetic properties. J. Mater. Sci. Mater. Electron. 2018, 29, 17144–17153. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Gnanamoorthy, G.; Choudhary, N.; Khan, S.H.; Gupta, N.; Kamyab, H.; Bach, Q.-V. A novel synthesis and characterization of polyhedral shaped amorphous iron oxide nanoparticles from incense sticks ash waste. Environ. Technol. Innov. 2020, 20, 101089. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gnanamoorthy, G.; Ali, D.; Bera, S.P.; Roy, A.; Kumar, G.; Choudhary, N.; Kalasariya, H.; Basnet, A. Cytotoxicity, Removal of Congo Red Dye in Aqueous Solution Using Synthesized Amorphous Iron Oxide Nanoparticles from Incense Sticks Ash Waste. J. Nanomater. 2022, 2022, 5949595. [Google Scholar] [CrossRef]
- Yadav, V.; Ali, D.; Khan, S.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.; Thai, V.; Hussain, S.; Manhrdas, S. Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe 3 O 4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Meng, X.; Li, L.; Ye, Q.; van de Voort, F. Fourier Transform Infrared (FTIR) Spectroscopy as a Utilitarian Tool for the Routine Determination of Acidity in Ester-Based Oils. J. Agric. Food Chem. 2015, 63, 8333–8338. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, S.; Satish, M.; Yadav, A.; Anish Madhavan, A. Thermal analysis of Fe2O3- Myristic acid nanocomposite for latent heat storage. Mater. Today Proc. 2020, 43, 3795–3798. [Google Scholar] [CrossRef]
- Gholoobi, A.; Abnous, K.; Ramezani, M.; Shandiz, F.H.; Darroudi, M.; Ghayour-Mobarhan, M.; Meshkat, Z. Synthesis of γ-Fe2O3 Nanoparticles Capped with Oleic Acid and their Magnetic Characterization. Iran J. Sci. Technol. Trans. A Sci. 2018, 42, 1889–1893. [Google Scholar] [CrossRef]
- Rudolph, M.; Erler, J.; Peuker, U.A. A TGA-FTIR perspective of fatty acid adsorbed on magnetite nanoparticles-Decomposition steps and magnetite reduction. Colloids Surf. A Physicochem. Eng. Asp. 2012, 397, 16–23. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. Biogenic synthesis of maghemite nanoparticles (γ-Fe2O3) using Tridax leaf extract and its application for removal of fly ash heavy metals (Pb, Cd). Mater. Today Proc. 2018, 5, 20704–20710. [Google Scholar] [CrossRef]
- Arévalo, P.; Isasi, J.; Caballero, A.C.; Marco, J.F.; Martín-Hernández, F. Magnetic and structural studies of Fe3O4 nanoparticles synthesized via coprecipitation and dispersed in different surfactants. Ceram. Int. 2017, 43, 10333–10340. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein MZ bin Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Wegmann, M.; Scharr, M. Synthesis of Magnetic Iron Oxide Nanoparticles. In Precision Medicine; Deigner, H.-P., Kohl, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 8; pp. 145–181. [Google Scholar] [CrossRef]
- Joos, A.; Rümenapp, C.; Wagner, F.E.; Gleich, B. Characterisation of iron oxide nanoparticles by Mössbauer spectroscopy at ambient temperature. J. Magn. Magn. Mater. 2016, 399, 123–129. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gnanamoorthy, G.; Yadav, K.K.; Ali, I.H.; Bagabas, A.A.; Choudhary, N.; Yadav, S.; Suriyaprabha, R.; Islam, S.; Modi, S.; et al. Utilization of Incense Stick Ash in Hydrometallurgy Methods for Extracting Oxides of Fe, Al, Si, and Ca. Materials 2022, 15, 1879. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Yetim, N.K.; Kurşun, F. Magnetic and Structural Characterization of Inorganic/Organic coated Fe3O4 Nanoparticles. J. Mater. Electron. Devices 2021, 2, 12–18. [Google Scholar]
- Corrêa, B.S.; Costa, M.S.; Cabrera-Pasca, G.A.; Sena, C.; Pinto, R.H.H.; Silva, A.P.S.; Junior, R.N.C.; Ishida, L.; Ramon, J.G.A.; Freitas, R.S.; et al. High-saturation magnetization in small nanoparticles of Fe3O4 coated with natural oils. J. Nanopart. Res. 2020, 22, 68. [Google Scholar] [CrossRef]
- Barbeta, V.B.; Jardim, R.F.; Kiyohara, P.K.; Effenberger, F.B.; Rossi, L.M. Magnetic properties of Fe3O4 nanoparticles coated with oleic and dodecanoic acids. J. Appl. Phys. 2010, 107, 073913. [Google Scholar] [CrossRef]
- Salvador, M.; Marqués-Fernández, J.L.; Martínez-García, J.C.; Fiorani, D.; Arosio, P.; Avolio, M.; Brero, F.; Balanean, F.; Guerrini, A.; Sangregorio, C.; et al. Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy. Nanomaterials 2022, 12, 205. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, H.; Huo, D.; Tan, W. Room-temperature magnetoresistive and magnetocaloric effect in La1-xBa xMnO3compounds: Role of Griffiths phase with ferromagnetic metal cluster above Curie temperature. J. Appl. Phys. 2022, 131, 043901. [Google Scholar] [CrossRef]
- Yadav, V.K.; Singh, B.; Gacem, A.; Yadav, K.K.; Gnanamoorthy, G.; Alsufyani, T.; Hussein, H.S.; Awwad, N.S.; Verma, R.; Inwati, G.K.; et al. Development of Novel Microcomposite Materials from Coal Fly Ash and Incense Sticks Ash Waste and Their Application for Remediation of Malachite Green Dye from Aqueous Solutions. Water 2022, 14, 3871. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gacem, A.; Choudhary, N.; Rai, A.; Kumar, P.; Yadav, K.K.; Abbas, M.; Khedher, N.B.; Awwad, N.S.; Barik, D.; et al. Status of Coal-Based Thermal Power Plants, Coal Fly Ash Production, Utilization in India and Their Emerging Applications. Minerals 2022, 12, 1503. [Google Scholar] [CrossRef]
- Pare, B.; Barde, V.S.; Solanki, V.S.; Agarwal, N.; Yadav, V.K.; Alam, M.M.; Gacem, A.; Alsufyani, T.; Khedher, N.B.; Park, J.-W.; et al. Green Synthesis and Characterization of LED-Irradiation-Responsive Nano ZnO Catalyst and Photocatalytic Mineralization of Malachite Green Dye. Water 2022, 14, 3221. [Google Scholar] [CrossRef]





| Sr. No. | MNPs Coated by | Shape | Area | Lattice Parameter | Particle Size (nm) | |
|---|---|---|---|---|---|---|
| By XRD | By TEM | |||||
| 1. | Uncoated | Cubic | 263.72 | 8.36577 × 10−10 | 15 | ~16 |
| 2. | Lauric acid | Cubic | 193.32 | 8.35144 × 10−10 | 12 | ~14 |
| 3. | Oleic acid | Cubic | 307.80 | 8.37072 × 10−10 | 10 | ~12 |
| 4. | Linoleic acid | Cubic | 221.86 | 8.3763 × 10−10 | 8 | ~10 |
| 5. | Linolenic acid | Cubic | 207.76 | 8.3763 × 10−10 | 6 | ~8 |
| MNPs Coated by | MS (emu/g) | HC (Oe) | MR (emu/g) | TB (K) | TP (K) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 K | 100 K | 300 K | 10 K | 100 K | 300 K | 10 K | 100 K | 300 K | |||
| Uncoated | 66 | 65 | 56 | 145 | 36 | 0 | 15 | 7 | 0 | 43 | 55 |
| Lauric acid | 57 | 55 | 47 | 152 | 100 | 0 | 16 | 10 | 0 | 40 | 58 |
| Oleic acid | 51 | 49 | 37 | 152 | 73 | 0 | 14 | 3 | 0 | 48 | 78 |
| Linoleic acid | 52 | 50 | 44 | 214 | 39 | 0 | 16 | 4 | 0 | 72 | 106 |
| Linolenic acid | 51 | 49 | 41 | 161 | 0 | 0 | 13 | 0 | 0 | 50 | 71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.K.; Fanan, A.; Ali, D.; Solanki, V.S.; Pare, B.; Almutairi, B.O.; Agrawal, N.; Yadav, N.; Pareek, V.; Yadav, V.K. Green Synthesis of Unsaturated Fatty Acid Mediated Magnetite Nanoparticles and Their Structural and Magnetic Studies. Magnetochemistry 2022, 8, 174. https://doi.org/10.3390/magnetochemistry8120174
Das AK, Fanan A, Ali D, Solanki VS, Pare B, Almutairi BO, Agrawal N, Yadav N, Pareek V, Yadav VK. Green Synthesis of Unsaturated Fatty Acid Mediated Magnetite Nanoparticles and Their Structural and Magnetic Studies. Magnetochemistry. 2022; 8(12):174. https://doi.org/10.3390/magnetochemistry8120174
Chicago/Turabian StyleDas, Amlan Kumar, Apoorva Fanan, Daoud Ali, Vijendra Singh Solanki, Brijesh Pare, Bader O. Almutairi, Neha Agrawal, Neera Yadav, Vikram Pareek, and Virendra Kumar Yadav. 2022. "Green Synthesis of Unsaturated Fatty Acid Mediated Magnetite Nanoparticles and Their Structural and Magnetic Studies" Magnetochemistry 8, no. 12: 174. https://doi.org/10.3390/magnetochemistry8120174
APA StyleDas, A. K., Fanan, A., Ali, D., Solanki, V. S., Pare, B., Almutairi, B. O., Agrawal, N., Yadav, N., Pareek, V., & Yadav, V. K. (2022). Green Synthesis of Unsaturated Fatty Acid Mediated Magnetite Nanoparticles and Their Structural and Magnetic Studies. Magnetochemistry, 8(12), 174. https://doi.org/10.3390/magnetochemistry8120174








