Abstract
The coordination reaction of the [Dy(hfac)3(H2O)2] units (hfac− = 1,1,1,5,5,5-hexafluoroacetylacetonate) with the [8′-(Diphenoxylphosphinyl)[1,1′-binaphthalen]-8-yl]diphenoxylphosphine oxide ligand (L) followed by a crystallisation in a 1:3 CH2Cl2:n-hexane solvent mixture led to the isolation of a new polymorph of formula [(Dy(hfac)3((S)-L))3]n (1). The X-ray structure on single crystal of 1 revealed the formation of a mono-dimensional coordination polymer with three crystallographically independent DyIII centres, which crystallised in the polar chiral P21 space group. Ac magnetic measurements highlighted single-molecule magnet behaviour under both zero and 1000 Oe applied magnetic field with magnetic relaxation through quantum tunneling of the magnetisation (QTM, zero field only) and Raman processes. Despite the three crystallographically independent DyIII centres adopting a distorted D4d coordination environment, a single slow magnetic relaxation contribution was observed at a slower rate than its previously studied [(Dy(hfac)3((S)-L))]n (2) polymorph.
1. Introduction
Single-molecule magnets (SMMs) have fascinated communities of chemists and physicists for the last 30 years because they display slow magnetic relaxation with magnetic bistability and quantum behaviours at low temperatures [1,2]. The latter opens the door to potential applications in molecular spintronics [3,4,5,6,7], quantum computing [8,9,10,11,12] and magneto-optics [13]. The discovery of lanthanide SMMs [14] with blocking temperatures for the reversal magnetisation up to 80 K [15], push back SMMs into the race of applications in high-density data storage [16,17].
To magnetism, another physical property can be added to achieve multi-property materials [18,19,20,21,22,23,24,25]. In this context, the addition of the chirality is probably the most popular choice because it leads to the observation of multiferroic materials [26,27,28,29], for instance when ferromagnetism cohabits with ferroelectricity and magneto-chiral anisotropy [30,31,32,33]. In the particular case of SMM behaviour, the addition of chirality could lead to the observation of circularly polarised luminescent SMMs [34,35,36,37], ferroelectric SMMs [38,39,40,41,42], magnetoelectric coupling in SMMs [43] and magneto-chiral SMMs [44,45]. A few years ago, some of us demonstrated that chirality can indirectly modulate the SMM behaviour through the change of dipolar interaction due to different crystal packings in the racemic SMM mixture and enantiopure SMM [46].
Polymorphism could also play a crucial role in the modulation of the SMM behaviour because it could induce changes in the intermolecular interaction (dipolar magnetic interaction), and local environment of the metal (crystal field) [47,48].
Recently, the (chir)optical and magnetic properties of [Dy(hfac)3((S)-L)]n (2) (L = Diphenoxylphosphinyl)[1,1′-binaphthalen]-8-yl]diphenoxylphosphine oxide) (Scheme 1) were studied [49,50]. A new polymorph of formula [(Dy(hfac)3((S)-L))3]n (1) was then isolated, and its magnetic properties are presented here and compared to those of 2.
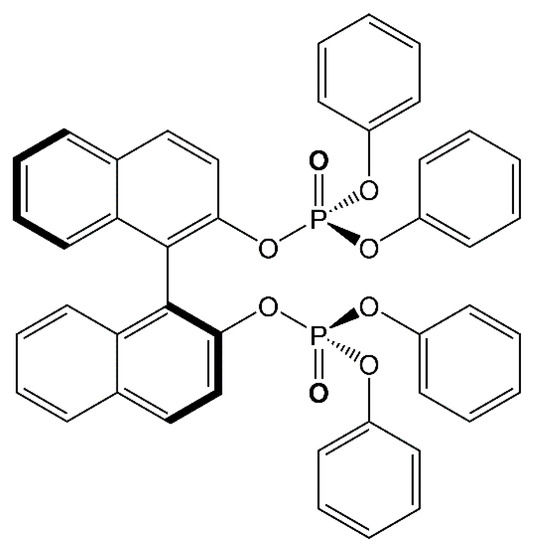
Scheme 1.
Molecular structure of (S)-L.
2. Results and Discussion
2.1. Synthesis
The coordination reaction between the ligand (S)-L and the precursor Dy(hfac)3(H2O)2 led to the formation of a mono-dimensional polymer of formula [Dy(hfac)3((S)-L)]n (2) [49]. The polycrystalline powder of the latter polymer was obtained by slow diffusion of a large excess of n-hexane into a CH2Cl2 solution of 2 (CH2Cl2/n-hexane 1:40), while a slow evaporation from a solution of the reagents in CH2Cl2 quantitatively yielded the new polymorph of formula [(Dy(hfac)3((S)-L))3]n (1) as single crystal suitable for X-ray diffraction study and confirmed by PXRD studies.
2.2. X-ray Structures
XRD studies on single crystal revealed that the form of this further product crystallises in the space group P21 (No. 4) with formula [(Dy(hfac)3((S)-L))3]n (1) (Table S1), while our previous work concluded that the mono-dimensional polymorph 2 crystallised in the chiral orthorhombic space group C2221 (N°20)49. It is worthwhile to note that the P21 space group is polar and chiral, which is a prerequisite for magnetoelectric coupling due to the combination of ferroelectric behaviour with magnetostrictive effects [43]. Three (S)-L ligands and three Dy(hfac)3 fragments compose the asymmetric unit (Figure 1).
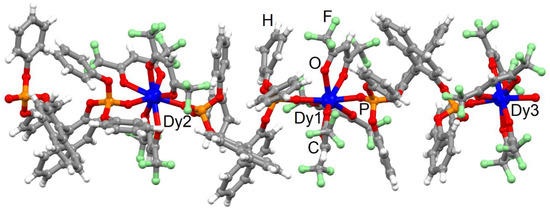
Figure 1.
Asymmetric unit for the complex [(Dy(hfac)3((S)-L))3]n (1). Dy = blue, O = red, C = grey, P = orange, F = green and H = white.
Each DyIII centre is octa-coordinated by six oxygen atoms from three hfac− ligands and two oxygen atoms from two phosphate groups of two different (S)-L ligands. The repetition of the asymmetric unit gives rise to a mono-dimensional coordination polymer. SHAPE [51] analysis suggests that all the first metal coordination spheres significantly deviate from the closest D4d coordination polyhedra, although with different degrees of distortion (Table S2). Indeed, the Dy1 and Dy3 centres, respectively, show distortion degree values of 0.469 and 0.657, while Dy2 adopts a D4d surrounding with a distortion degree of 0.814 close to the deviation from the C2v symmetry (0.997) (Table S2). The average bond and angle values are similar for each Dy(hfac)3((S)-L) fragment. Around each DyIII centre, the Dy-OP=O lengths (2.31(2) Å) are found to be slightly shorter than the Dy-Ohfac distances (2.34(7) Å). The average Dy-OP=O length (range from 2.34(9) to 2.38(7) Å) and P=OO-Dy-OP=O angle values (range from 141.9(8) to 146.3(7)°) are similar between the [Dy(hfac)3((S)-L)] fragments. The dihedral angle between the naphthyl groups have an average value of 85.6(4)°. The shortest intra- and inter-chain Dy-Dy distances have values of 12.144(2) Å and 11.296(2) Å, respectively. The crystal packing is driven by the formation of π-CH, F⋯F (2.718 Å) and F⋯H (2.493 Å) interactions between the polymeric chains (Figure S1). Since it was not possible to obtain 2 crystals of satisfactory quality for XRD structural determination [49], the straight relationship between structure and magnetic properties drives us to deeply compare two polymorphic structures with DyIII and EuIII centres. Therefore, the data at 150 K for the isostructural [Eu(hfac)3((S)-L)]n were used. In the latter, the coordination environment is similar, with an identical bridging mode of the (S)-ligand. SHAPE analysis supported a more regular D4d symmetry of the coordination sphere (SAPR-8 = 0.300) than what has been observed in 1. The P=OO-Dy-OP=O angle values (145.3(3)°) and dihedral angle between the naphthyl groups (77.3°) are similar in both polymorphs. The crystal packing of the 2 polymorph is also driven by very similar π-CH, F⋯F (2.718 Å) and F⋯H (2.545 Å) interactions, leading to the shortest intra- and inter-molecular Dy⋯Dy distances, respectively, equal to 12.647(2) and 11.466(2) Å, which are values close to those observed in the 1 polymorph. In summary, on one side, the SHAPE analysis demonstrated a distortion degree of the dysprosium coordination spheres stronger in 1 than for 2, while on the other side, both crystal packings are comparable. Therefore, neither the symmetry of the coordination spheres nor the crystal packing can reasonably explain the difference of magnetic behaviour. A deeper analysis of the coordination sphere of the DyIII ion in both polymorphs shows (i) close values for the angles between the plans formed by the hfac− ligand (blue one depicted in Figure S2) and the P=OO-Dy-OP=O fragment (drawn in orange in the Figure S2) for both polymorphs 2 (20.6(3)°) and 1 (18.6(7)°) and (ii) significant difference of angle values calculated between the two plans formed by the two “face-to-face” hfac− ligands (drawn in green on Figure S2) for both polymorphs 2 (12.3(5)°) and 1 (26.2(9)°). In other words, the closest ideal symmetry for coordination sphere in both polymorphs is the same regardless of whether the arrangements of the ligands around the DyIII centre are different, leading to different electronic distribution, and then modification in the magnetic properties could be expected.
Finally, the phase purity for the 1 polymorph was checked by powder X-ray diffraction (PXRD) at room temperature. This study confirmed that the single crystal structure of 1 corresponds to the bulk material and its phase purity. Both PXRD patterns of 2 and 1 were compared together with their calculated ones (from single crystal data), showing that no trace of 2 polymorph was detected in the sample of 1 (Figure S3).
2.3. Magnetic Properties
2.3.1. Static Magnetic Measurements
In Figure S4, the experimental temperature dependence of χMT is reported for both 2 and 1 polymorphs for comparison. It should be noted that the magnetic data are reported for 3 DyIII centres for 1 to be in agreement with the proposed formula [(Dy(hfac)3((S)-L))3]n. For 1, the room temperature value of 43.22 cm3 K mol−1 is close to the expected one of 42.51 cm3 K mol−1 for three isolated DyIII ions in 6H15/2 ground multiplets [52]. On cooling down the system, the χMT curve decreases monotonically down to 36.93 cm3 K mol−1 at 2 K. No increase in the χMT product is observed at low temperatures, conversely to the 2 case. This highlights the presence of different dipolar interactions in the two compounds even if the shortest intra- and inter-molecular Dy⋯Dy distances were found to be similar in both polymorphs. Indeed, relative orientation of the main component of the magnetic anisotropy has a crucial role in the intensity and nature of the dipolar magnetic interaction. The field-dependence of magnetisation were measured at 2 K between 0 and 5 T for both polymorphs (Figure S5). At high fields, the magnetisation value of 1 is 15.93 Nβ, about three times the value for 2, and far from the expected saturated value for three DyIII ions of 30 Nβ, a sign of significant magnetic anisotropy.
2.3.2. Dynamic Magnetic Measurements
The 1 polymorph exhibits slow relaxation of the magnetisation in zero applied field (Figure 2a and Figure S6). The complex 1 relaxes about 100 times slower than 2, which was previously published at 2 K. The normalised Argand plot for 1 indicates that the observed slow magnetisation relaxation represents approximately all the signal detected in the static measurements (Figure S7). The dynamic data can be fitted with an extended Debye model (Figure S8 and Table S3). The observed thermal dependence of the extracted relaxation times (τ) could be fitted (Equation (1)) with a combination of QTM and Orbach processes using τTI = 1.57(4) × 10−3 s, τ0 = 1.7(2) × 10−5 s and Δ = 23.6(6) K (Figure S9).
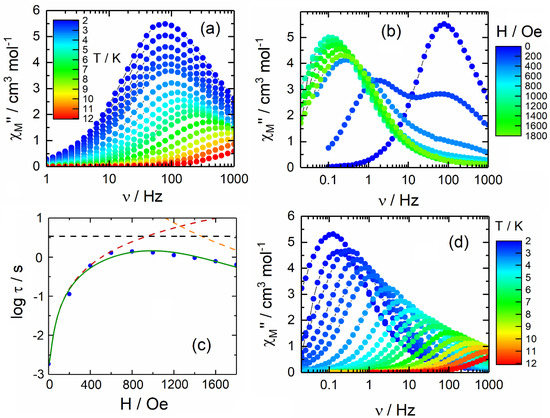
Figure 2.
(a) Frequency dependence of χM″ at zero applied magnetic field for 1 in the temperature range of 2–12 K. (b) Frequency dependence of χM″ for 1 at 2 K in the 0–1800 Oe field range. (c) Field dependence of the relaxation time at 2 K for 1 with representation of the best fit curve (full green line) and the QTM (red dashed line), direct (orange dashed line) and thermally activated (black dashed line) contributions. (d) Frequency dependence of χM″ at 1000 Oe applied magnetic field for 1 in the temperature range 2–12 K.
However, the thermal energy of the system for temperatures ≤12 K does not appear sufficient for activating this Orbach relaxation pathway. A more reasonable fitting procedure could consider a combination of QTM and Raman processes (Figure 3 and Figure S10). The best fit parameters are τTI = 1.57(4) × 10−3 s, C = 4.0(3) s−1 K−n and n = 3.16(3). In the case of a pure acoustic phonons (lattice vibrations) Raman process, the expected n value is 9 for Kramers ions [53], but the involvement of optical phonons (molecular vibrations) [8,15] can induce the diminution of n between 2 and 7 [54,55,56].
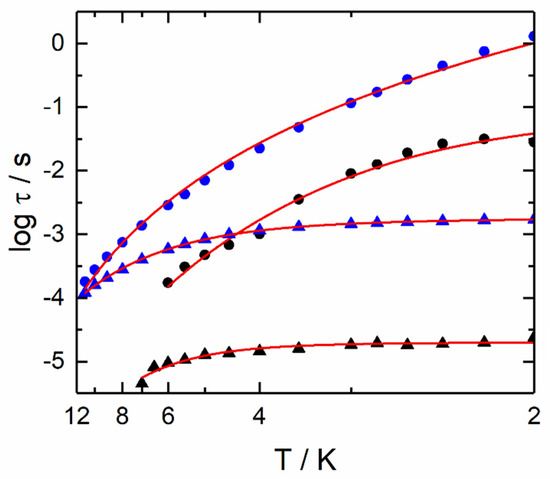
Figure 3.
Temperature variation of the relaxation time for 1 (blue spots, temperature range 2–12 K) and 2 (black spots, temperature range 2–7 K) in zero (triangles) and 1000 Oe (full circles) applied magnetic field.
Under a static magnetic field (Figure 2b and Figure S11), the out-of-phase maxima start to move to lower frequencies. While 2 displays a complicated relaxation dynamic, for the polymorph 1, the field dependence of the magnetic susceptibility displays a classic behaviour. Two contributions can be distinguished for the low magnetic field values usually attributed to the presence of dipolar magnetic interactions between the DyIII magnetic centres [57]. By applying field values higher than 500 Oe, only one contribution is significant. Thus, despite the presence of three independent DyIII centres, only one contribution to the magnetisation relaxation is detected once the dipolar magnetic interactions are destroyed. The relaxation times are extracted from the χM curves using two contributions for low magnetic field values (0–400 Oe) and a single contribution for the highest magnetic field values (600–1800 Oe) (Figure S12 and Table S4). The fit output is superimposed on the argand plot in Figure S13. The field dependence of the extracted can be fit by Equation (2) (Figure 2c) [58,59].
The fit parameters are B1 = 5(1) × 102 s−1, B2 = 2.1(6) × 10−3 Oe−2, A = 3.2(7) × 10−14 s−1 K−1 Oe−4 and k(T) = 3(1) × 10−1 s−1. The value of 1000 Oe was selected to study the in-field temperature-dependent behaviour of 1 (Figure 2d and Figure S14) because it is close to the optimal field and analogous to the field used in the studies for polymorph 2 [49]. Under 1000 Oe at 2 K, the complex 1 relaxes about times slower than the slowest contribution of the polymorph 2. Such difference has already been observed under zero applied field. Thus, the two polymorphs appear to respond in a similar way to the applied field (Figure 3). The normalised Argand plot for 1 indicates that the observed slow magnetization relaxation under an applied magnetic field represents approximately all the signal detected in the static measurements (Figure S15). The data for 1 can be interpreted with an extended Debye model (Figure S16, Table S5), accounting for only one contribution to the magnetisation relaxation in the temperature range of 2–11 K (Figure 3).
The fit output is superimposed on the normalised argand plot (Figure S15). Based on the fit of the log(τ) vs. H curve (Figure 2c), no remaining QTM nor Direct processes are expected to play a major role in the magnetic relaxation. Thus, only k(T) (thermally activated Raman and Orbach processes) (Equation (2)) should be involved in the magnetic relaxation mechanism. Moreover, as previously mentioned for ac study under zero applied magnetic field, the thermal energy of the system cannot activate a similar Orbach mechanism in the studied temperatures range. Conversely, a Raman process can be satisfactorily employed to interpret the experimental log(τ) vs. T plot (Figure 3). The best fit parameters are C = 2.6(3) s−1 K−n and n = 5.22(8). Therefore, the tunnelling phenomena observed at zero field are efficiently suppressed by applying a static dc magnetic field of 1000 Oe. The values for the Raman process is similar in both zero and applied field for temperature ranging from 612 K. Conversely to 2, no direct pathways seem to significantly participate in the magnetisation relaxation dynamic.
3. Conclusions
In this article, a new polymorph was isolated from the reaction between Dy(hfac)3(H2O)2 and [8′-(Diphenoxylphosphinyl)[1,1′-binaphthalen]-8-yl]diphenoxylphosphine oxide ligand (L). The single crystal X-ray structure of [(Dy(hfac)3(L))3]n (1) revealed that three crystallographically independent DyIII centres composed the asymmetric unit. 1 displayed slow magnetic relaxation in both zero and applied dc magnetic field. Despite the high level of distortion for the D4d coordination sphere around the DyIII ions, the polymorph 1 displayed a slower relaxation of its magnetisation compared to the polymorph 2 which involves a more regular D4d surrounding. This observation was attributed to different arrangement of the coordinated ligands and a more favorable electronic distribution for 1 than 2, demonstrating once more that the electronic distribution is more decisive than the coordination symmetry. Surprisingly, the polymorph 1 displayed one contribution for the slow magnetisation relaxation, whereas 2 displayed multi-contributions under the same applied magnetic field and this despite the presence of three different crystallographic DyIII centres for 1. The observed magnetic behaviour appears counterintuitive, and currently it cannot be fully justified. However, different magnetic dynamic properties as a consequence of differences in the dipolar interactions for similar systems were already documented [46]. This work underlines the effect of subtle structural variations on SMM behaviour.
Interestingly, the polymorph 1 crystallised in the polar chiral space group P21 and involves the hfac− ligands, which could display keto-enol transformation, allowing the switching of molecular polarity. In other words, all the needed prerequisites to observe ferroelectricity are reached [60,61,62]. Thus, 1 appears to be a promising candidate to the exhibition of magnetoelectric coupling [43].
4. Materials and Methods
4.1. Synthesis, General Procedures and Materials
The precursor [Dy(hfac)3(H2O)2] (hfac− = 1,1,1,5,5,5-hexafluoroacetylacetonate anion) [63] and [8′-(Diphenoxylphosphinyl)[1,1′-binaphthalen]-8-yl]diphenoxylphosphine oxide (L) [64] were synthesised following previously reported methods. All other reagents were commercially available and used without further purification.
4.2. Synthesis of the Ligand [8′-(Diphenoxylphosphinyl)[1,1′-binaphthalen]-8-yl]diphenoxylphosphine Oxide (L)
Phenol in a quantity of 1.12 g (11.9 mmol) and 4.7 mL (33.7 mmol) of Et3N were added to a solution containing 0.52 mL (5.96 mmol) of PCl3 in 100 mL of anhydrous toluene at 0 °C under argon atmosphere. After stirring the mixture for 30 min, 0.85 g (2.97 mmol) of (S)-BINOL were added at −10 °C. The mixture was stirred for 1 h at room temperature, then filtered over Celite. The solvent was removed at reduced pressure. The residue was dissolved in EtOAc, washed with H2O, an aqueous 5% NaOCl solution and brine. The organic phase was dried with MgSO4, then the solvent was removed at reduced pressure. The residue was purified by column chromatography on silica gel using petroleum ether/EtOAc (7:3) as the eluent. 0.84 g, 38% yield for (S)-L. 1H-NMR (300 MHz, CDCl3): δ = 7.93 (d, J = 9.0 Hz, 2 H), 7.87 (d, J = 8.1 Hz, 2 H), 7.78 (d, J = 9.0 Hz, 2 H), 7.43–7.37 (m, 2 H), 7.25–7.06 (m, 10 H), 7.00–6.98 (m, 6 H), 6.88–6.85 (m, 4 H), 6.57–6.54 (m, 4 H). 13C-NMR (75 MHz, CDCl3): δ = 150.3 (d, JCP = 8.2 Hz), 150.1 (d, JCP = 7.5 Hz), 146.7 (d, JCP = 6.7 Hz), 133.6, 131.2, 130.5, 129.7, 129.5, 128.1, 127.3, 126.2, 125.8, 125.4 (d, JCP = 1.5 Hz), 125.2 (d, JCP = 1.5 Hz), 121.7 (d, JCP = 9.0 Hz), 120.0 (d, JCP = 5.2 Hz), 119.6 (d, JCP = 5.2 Hz), 119.2 (d, JCP = 1.5 Hz). for (S)-L (c = 1.0, CHCl3).
4.3. Synthesis of Complex [(Dy(hfac)3(L))3]n (1)
[Dy(hfac)3(H2O)216.4 mg (0.02 mmol) was added to a solution containing 15 mg (0.02 mmol) of (S)-L in 2 mL of CH2Cl2. After min of stirring, the solution was slowly evaporated, leading to a colourless and microcrystalline residue. 28.8 mg, 94% yield for 1. I.R. (KBr, range 1800–400 cm−1): 1654 (s), 1591 (m), 1556 (m), 1528 (m), 1490 (s), 1476 (m), 1459 (m), 1254 (s), 1222 (s), 1208 (s), 1194 (s), 1160 (s), 1145 (s), 1102 (m), 1078 (m), 1029 (m), 1015 (m), 1002 (m), 994 (m), 968 (m), 952 (w), 903 (w), 871 (w), 849 (w), 815 (w), 809 (w), 797 (m), 777 (m), 762 (w), 752 (m), 742 (w), 704 (w), 687 (m), 661 (m), 616 (w), 586 (m), 560 (w), 549 (w), 526 (w), 526(w), 513 (w), 500(w), 495 (w), 482 (w), 469 (w) cm−1. Slow evaporation from a solution in CH2Cl2/n-hexane (1:3) led to single crystals suitable for structural X-ray studies. Anal. Calcd (%) for C177H104Dy3F54O42P6: C 46.15, H 2.26; found: C 46.32, H 2.35.
4.4. Crystallography
A single crystal of 1 was mounted on a D8 VENTURE Bruker-AXS diffractometer for data collection (MoKα radiation source, λ = 0.71073 Å) at 150 K, from the Diffractometric Centre X (CDIFX), University of Rennes 1, France (Table S1). The SHELXT program [65] was used to solve the structures with a direct method and refinements were done with the SHELXL-14/7 program [66] using a full matrix least-squares method on F2. The CCDC number is 2112160 for compound 1. X-ray diffraction (XRD) patterns were recorded at room temperature in the 2θ range of 5–30° with a step size of 0.026° and a scan time per step of 600 s using a PANalytical X’Pert Pro diffractometer (Cu-L2,L3 radiation, λ = 1.5418 Å, 40 kV, 40 mA, PIXcel 1D detector). Data collector and HighScore Plus softwares were used, respectively, for recording and analysis of the patterns.
4.5. Physical Measurements
The elemental analyses of the compounds were performed at the Centre Régional de Mesures Physiques de l’Ouest, Rennes. The static susceptibility measurements were performed on solid polycrystalline samples with a Quantum Design MPMS-XL SQUID magnetometer. The following values of magnetic field were used: 0.2 kOe, 2 kOe and 10 kOe, respectively, for the temperature range of 2–20 K, 20–80 K and 80–300 K in order to prevent any saturation effect. The ac magnetic susceptibility measurements were both performed on a Quantum Design MPMS-XL SQUID magnetometer (1–1000 Hz frequency range). Immobilised selected and crunched single crystals were employed to realize the magnetic measurements, and the latter were all corrected for the diamagnetic contribution as calculated with Pascal’s constants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/magnetochemistry7110150/s1, Figure S1: Fragments of two chains for the complex (S)-1 (view along the axis a). (Dy = blue, O = red, C = dark grey, H = light grey, P = orange, F = green). Figure S2: Coordination sphere of a DyIII ion of 1. P=O, the two face-to-face hfac− and the third hfac− ligands are drawn in orange, green and blue, respectively. Figure S3: Superposition of experimental powder X-ray diffraction patterns from (S)-2 and (S)-1 measured at 300 K and simulated from (S)-2 [1] and (S)-1 single-crystal data obtained at 150 K. Figure S4: Temperature dependence of the χMT products for (S)-2 [1] and (S)-1 (given for the three crystallographically independent DyIII centres) in the temperature range of 2–300 K. Figure S5: Field variation of the magnetisation for (S)-2 [1] and (S)-1 (given for the three crystallographically independent DyIII centres) in the field range of 0–5 T. Figure S6: Frequency dependence of χ′M zero field for (S)-1 in the temperature range of 1–12 K. Figure S7: Normalised argand plot of experimental (dots) and fit Debye (black lines) data for (S)-1 at zero applied field in the temperature range 2–11 K. Figure S8: Frequency dependence of the in-phase (χM′) and out-of-phase (χM″) components of the ac susceptibility measured on powder at 2.8 K and 0 Oe with the best fitted curves (red lines) for (S)-1. Figure S9: Temperature dependence of τ (blue spots) for (S)-1 at zero applied magnetic field in the temperature range 2–11 K. The best fit curve is depicted as a full green line, and the Orbach and QTM contributions are represented, respectively, in a black dashed line and a red dashed line. Figure S10: Temperature dependence of τ (blue spots) for (S)-1 at zero applied magnetic field in the temperature range 2–11 K. The best fit curve is depicted as a full green line and the Raman and QTM contributions are represented, respectively, in a black dashed line and a red dashed line. Figure S11: Frequency dependence of χM′ for 1 at 2 K in the field range of 0–1800 Oe. Figure S12: Frequency dependence of the in-phase (χM′) and out-of-phase (χM″) components of the ac susceptibility measured on powder at 2 K and 1000 Oe with the best fitted curves (red lines) for (S)-1. Figure S13: Argand plot of experimental (colored plots) and fit data (black lines) for 1 at 2 K in the field range 0–3000 Oe. Figure S14: Frequency dependence of χM′ under an applied magnetic field of 1000 Oe for (S)-1 in the temperature range of 1–12 K. Figure S15: Normalised argand plot of experimental (coloured plots) and fit data (black lines) for Dy3 under an applied magnetic field of 1000 Oe in the temperature range 2–11 K. Figure S16. Frequency dependence of the in-phase (χM′) and out-of-phase (χM″) components of the ac susceptibility measured on powder at 2.8 K and 1000 Oe with the best fitted curves (red lines) for (S)-1. Table S1: X-ray crystallographic data for 1. Table S2: SHAPE analysis of the coordination polyhedra around the lanthanideIII centres in 1. Table S3: Best fitted parameters (χT, χS, α and τ) with the extended Debye model for compound 1 at 0 Oe in the temperature range 2–11 K. Table S4: Best fitted parameters (χT,1, χS, τ1, α1 χT,2, τ2, χT,2 and α2) with the extended Debye model for compound 1 at 2 K in the field range 0–1800 Oe. Table S5. Best fitted parameters (χT, χS, α and τ) with the extended Debye model for compound 1 at 1 kOe in the temperature range 2–11 K.
Author Contributions
C.A.M., B.L. and G.A. performed the organic syntheses and performed the coordination chemistry and crystallisations; V.D. realised the single crystal X-ray diffraction experiments and refined the X-ray structures; C.A.M. and O.C. performed and analyzed the magnetic measurements. C.L. discussed the idea and the results and commented on the manuscript. F.P. conceived and designed the experiments and contributed to the writing of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by CNRS, Université de Rennes 1, and the European Commission through the ERC-CoG 725184 MULTIPROSMM (project No. 725184).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data are available for this article.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study, the collection, analyses or interpretation of data, the writing of the manuscript or the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PXRD | Powder X-Ray Diffraction |
| SQUID | Superconducting Quantum Interference Device |
| SMM | Single Molecule Magnet |
| QTM | Quantum Tunneling of the Magnetisation |
| CH2Cl2 | Dichloromethane |
| hfac− | 1,1,1,5,5,5-hexafluoroacetylacetonate |
References
- Sessoli, R.; Tsai, H.L.; Schake, A.R.; Wang, S.Y.; Vincent, J.B.; Folting, K.; Gatteschi, D.; Christou, G.; Hendrickson, D.N. High-spin molecules: [Mn12O12(O2CR)16(H2O)4]. J. Am. Chem. Soc. 1993, 115, 1804–1816. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Ariciu, A.-M.; McAdams, S.; Weihe, H.; Bendix, J.; Tuna, F.; Piligkos, S. Toward Molecular 4f Single-Ion Magnet Qubits. J. Am. Chem. Soc. 2016, 138, 5801–5804. [Google Scholar] [CrossRef] [PubMed]
- Bogani, F.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Vincent, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Balestro, F. Electronic read-out of a single nuclear spin using a molecular spin transistor. Nature 2012, 488, 357–360. [Google Scholar] [CrossRef]
- Ganzhorn, M.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Strong spin–phonon coupling between a single-molecule magnet and a carbon nanotube nanoelectromechanical system. Nat. Nanotechnol. 2013, 8, 165–169. [Google Scholar] [CrossRef]
- Cornia, A.; Seneor, P. The molecular way. Nat. Mater. 2017, 16, 505–506. [Google Scholar] [CrossRef]
- Thiele, S.; Balestro, F.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Electrically driven nuclear spin resonance in single-molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Ardavan, A.; Rival, O.; Morton, J.J.L.; Blundell, S.J.; Tyryshkin, A.M.; Timco, G.A.; Winpenny, R.E.P. Will Spin-Relaxation Times in Molecular Magnets Permit Quantum Information Processing? Phys. Rev. Lett. 2007, 98, 057201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamp, P.C.E.; Gaita-Ariño, A. Spin-based quantum computers made by chemistry: Hows and whys. J. Mater. Chem. 2009, 19, 1718–1730. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Pérez, M.J.; Cardona-Serra, S.; Schlegel, C.; Moro, F.; Alonso, P.J.; Prima-García, H.; Clemente-Juan, J.M.; Evangelisti, M.; Gaita-Arino, A.; Sesé, J.; et al. Gd-Based Single-Ion Magnets with Tunable Magnetic Anisotropy: Molecular Design of Spin Qubits. Phys. Rev. Lett. 2012, 108, 247213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sessoli, R.; Boulon, M.-E.; Caneschi, A.; Mannini, M.; Poggini, L.; Wilhelm, F.; Rogalev, A. Strong magnetochiral dichroism in a paramagnetic molecular helix observed by hard X-rays. Nat. Phys. 2015, 11, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.-M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Affronte, M. Molecular nanomagnets for information technologies. J. Mater. Chem. 2009, 19, 1731–1737. [Google Scholar] [CrossRef]
- Coronado, E.; Forment-Aliaga, A.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-Garcia, C.J.; Martinéz-Ferrero, E.; Nuez, A.; Romero, F.M. Multifunctional molecular materials. Solid State Sci. 2003, 5, 917–924. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-Saiz, C.; Martí-Gastaldo, C. Crystal engineering of multifunctional molecular materials. In Engineering of Crystalline Materials Properties; Novoa, J.J., Braga, D., Addadi, L., Eds.; NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Dordrecht, The Netherlands, 2008; pp. 173–191. [Google Scholar]
- Coronado, E.; Palacio, F.; Veciana, J. Molecule–Based Magnetic Materials. Angew. Chem. Int. Ed. 2003, 42, 2570–2572. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Sánchez, C. Functional Hybrids Materials; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Fahmi, A.; Pietsch, T.; Mendoza, C.; Cheval, N. Functional hybrid materials. Mater. Today 2009, 12, 44–50. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent progress in hybrid materials science. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
- Sanchez, C.; Shea, K.J.; Kitagawa, S. Recent progress in hybrid materials science. Chem. Soc. Rev. 2011, 40, 471–472. [Google Scholar] [CrossRef]
- Ouahab, L. Multifunctional Molecular Materials; Taylor and Francis Group CRC Press: New York, NY, USA, 2012. [Google Scholar]
- Scott, J.F. Applications of Modern Ferroelectrics. Science 2007, 315, 954–959. [Google Scholar] [CrossRef]
- Tsymbal, E.Y.; Kohlstedt, H. Tunneling Across a Ferroelectric. Science 2006, 313, 181–183. [Google Scholar] [CrossRef]
- Cheong, S.-W.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, R.; Spaldin, N.A. Multiferroics: Progress and prospects in thin films. Nat. Mater. 2007, 6, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.L.J.A.; Raupach, E. Observation of magneto-chiral dichroism. Nature 1997, 390, 493–494. [Google Scholar] [CrossRef]
- Barron, L.D. Chirality, magnetism and light. Nature 2000, 405, 895–896. [Google Scholar] [CrossRef] [Green Version]
- Train, C.; Gheorghe, R.; Krstic, V.; Chamoreau, L.; Ovnesyan, N.S.; Rikken, G.L.J.A.; Gruselle, M.; Verdaguer, M. Strong magneto-chiral dichroism in enantiopure chiral ferromagnets. Nat. Mater. 2008, 7, 729–734. [Google Scholar] [CrossRef]
- Bordács, S.; Kézsmárki, I.; Szaller, D.; Demkó, L.; Kida, N.; Murakawa, H.; Onose, Y. Chirality of matter shows up via spin excitations. Nat. Phys. 2012, 8, 734–738. [Google Scholar] [CrossRef] [Green Version]
- El Rez, B.; Liu, J.; Béreau, V.; Duhayon, C.; Horino, Y.; Suzuki, T.; Coolen, L.; Sutter, J.-P. Concomitant emergence of circularly polarized luminescence and single-molecule magnet behavior in chiral-at-metal Dy complex. Inorg. Chem. Front. 2020, 7, 4527–4534. [Google Scholar] [CrossRef]
- Huizi-Rayo, U.; Zabala-Lekuona, A.; Terenzi, A.; Cruz, C.M.; Cuerva, J.M.; Rodriguez-Diéguez, A.; Garcia, J.A.; Seco, J.M.; San Sebastian, E.; Cepeda, J. Influence of thermally induced structural transformations on the magnetic and luminescence properties of tartrate-based chiral lanthanide organic-frameworks. J. Mater. Chem. C. 2020, 8, 8243–8256. [Google Scholar] [CrossRef]
- Gendron, F.; Di Pietro, S.; Abad Galan, L.; Riobé, F.; Placide, V.; Guy, L.; Zinna, F.; Di Bari, L.; Bensalah-Ledoux, A.; Guyot, Y.; et al. Luminescence, chiroptical, magnetic and ab initio crystal-field characterizations of an enantiopure helicoidal Yb(III) complex. Inorg. Chem. Front. 2021, 8, 914–926. [Google Scholar] [CrossRef]
- Lefeuvre, B.; Mattei, C.A.; Flores Gonzalez, J.; Gendron, F.; Dorcet, V.; Riobé, F.; Lalli, C.; Le Guennic, B.; Cador, O.; Maury, O.; et al. Solid-State near-Infrared Circularly Polarized Luminescence from Chiral YbIII-Single-Molecule Magnet. Chem. Eur. J. 2021, 27, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-P.; Wang, T.-W.; Li, C.-H.; Liu, D.-S.; Li, Y.-Z.; You, X.-Z. Single-ion magnets based on mononuclear lanthanide complexes with chiral Schiff base ligands [Ln(FTA)3L] (Ln = Sm, Eu, Gd, Tb and Dy). Chem. Commun. 2010, 46, 2929–2931. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Chen, C.-L.; Gao, Y.-L.; Liu, C.-M.; Feng, X.-L.; Gui, Y.-H.; Fang, S.-M. Modulation of Homochiral DyIII Complexes: Single–Molecule Magnets with Ferroelectric Properties. Chem.–A Eur. J. 2012, 18, 14632–14637. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Chen, C.-L.; Xiao, H.-P.; Wang, A.-L.; Liu, C.-M.; Zheng, X.; Gao, L.-J.; Yang, X.-G.; Fang, S.-M. Luminescent, magnetic and ferroelectric properties of noncentrosymmetric chain-like complexes composed of nine-coordinate lanthanide ions. Dalton Trans. 2013, 42, 15325–15347. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Rouquette, J.; Thibaud, J.-M.; Ferreira, R.A.S.; Carlos, L.D.; Donnadieu, B.; Vieru, V.; Chibotaru, L.F.; Konczewicz, L.; Haines, J.; et al. A High–Temperature Molecular Ferroelectric Zn/Dy Complex Exhibiting Single–Ion–Magnet Behavior and Lanthanide Luminescence. Angew. Chem. Int. Ed. 2015, 54, 2236–2240. [Google Scholar] [CrossRef]
- Li, X.-L.; Hu, M.; Yin, Z.; Zhu, C.; Liu, C.-M.; Xiao, H.-P.; Fang, S. Enhanced single-ion magnetic and ferroelectric properties of mononuclear Dy(III) enantiomeric pairs through the coordination role of chiral ligands. Chem. Commun. 2017, 53, 3998–4001. [Google Scholar] [CrossRef]
- Long, J.; Ivanov, M.S.; Khomchenko, V.A.; Mamontova, E.; Thibaud, J.-M.; Rouquette, J.; Beaudhuin, M.; Granier, D.; Ferreira, R.A.S.; Carlos, L.D.; et al. Room temperature magnetoelectric coupling in a molecular ferroelectric ytterbium(III) complex. Science 2020, 367, 671–676. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, S.; Wang, H.; Dou, J.; Jiang, J. Magnetochiral dichroism in chiral mixed (phthalocyaninato)(porphyrinato) rare earth triple-decker SMMs. Inorg. Chem. Front. 2014, 1, 167–171. [Google Scholar] [CrossRef]
- Atzori, M.; Dhbaibi, K.; Douib, H.; Grasser, M.; Dorcet, V.; Breslavetz, I.; Paillot, K.; Cador, O.; Rikken, G.L.J.A.; Le Guennic, B.; et al. Helicene-Based Ligands Enable Strong Magneto-Chiral Dichroism in a Chiral Ytterbium Complex. J. Am. Chem. Soc. 2021, 143, 2671–2675. [Google Scholar] [CrossRef]
- Ou-Yang, J.-K.; Saleh, N.; Fernandez Garcia, G.; Norel, L.; Pointillart, F.; Guizouarn, T.; Cador, O.; Totti, F.; Ouahab, L.; Crassous, J.; et al. Improved slow magnetic relaxation in optically pure helicene-based DyIII single molecule magnets. Chem. Commun. 2016, 52, 14474–14477. [Google Scholar] [CrossRef] [PubMed]
- Pointillart, F.; Bernot, K.; Poneti, G.; Sessoli, R. Crystal Packing Effects on the Magnetic Slow Relaxation of Tb(III)-Nitronyl Nitroxide Radical Cyclic Dinuclear Clusters. Inorg. Chem. 2012, 51, 12218–12229. [Google Scholar] [CrossRef]
- Kishi, Y.; Pointillart, F.; Lefeuvre, B.; Riobé, F.; Le Guennic, B.; Golhen, S.; Cador, O.; Maury, O.; Fujiwara, H.; Ouahab, L. Isotopically enriched polymorphs of dysprosium single molecule magnets. Chem. Commun. 2017, 53, 3575–3578. [Google Scholar] [CrossRef]
- Mattei, C.A.; Montigaud, V.; Gendron, F.; Denis-Quanquin, S.; Dorcet, V.; Giraud, N.; Riobé, F.; Argouarch, G.; Maury, O.; Le Guennic, B.; et al. Solid-state versus solution investigation of a luminescent chiral BINOL-derived bisphosphate single-molecule magnet. Inorg. Chem. Front. 2021, 8, 947–962. [Google Scholar] [CrossRef]
- Mattei, C.A.; Montigaud, V.; Dorcet, V.; Riobé, F.; Argouarch, G.; Maury, O.; Le Guennic, B.; Cador, O.; Lalli, C.; Pointillart, F. Luminescent dysprosium single-molecule magnets made from designed chiral BINOL-derived bisphosphate ligands. Inorg. Chem. Front. 2021, 8, 963–976. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Departament de Quimica Fisica, Departament de Quimica Inorganica, and Institut de Quimica Teorica i Computacional–Universitat dè Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH: Weinhem, Germany, 1993. [Google Scholar]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Clarendon Press: Oxford, UK, 1970. [Google Scholar]
- Singh, A.; Shrivastava, K.N. Optical-acoustic two-phonon relaxation in spin systems. Phys. Status Solidi B 1979, 95, 273–277. [Google Scholar] [CrossRef]
- Shirivastava, K.N. Theory of Spin-Lattice Relaxation. Phys. Status Solidi B 1983, 177, 437. [Google Scholar] [CrossRef]
- Evans, P.; Reta, D.; Whitehead, G.F.S.; Chilton, N.F.; Mills, D.P. Bis-Monophospholyl Dysprosium Cation Showing Magnetic Hysteresis at 48 K. J. Am. Chem. Soc. 2019, 141, 19935–19940. [Google Scholar] [CrossRef] [Green Version]
- Pointillart, F.; Bernot, K.; Golhen, S.; Le Guennic, B.; Guizouarn, T.; Ouahab, L.; Cador, O. Magnetic memory in an Isotopically Enriched and Magnetically Isolated Mononuclear Dysprosium Complex. Angew. Chem. Int. Ed. 2015, 54, 1504–1507. [Google Scholar] [CrossRef]
- Pointillart, F.; Flores Gonzalez, J.; Montigaud, V.; Tesi, L.; Cherkasov, V.; Le Guennic, B.; Cador, O.; Ouahab, L.; Sessoli, R.; Kuropatov, V. Redox- and solvato-magnetic switching in a tetrathiafulvalene-based triad single-molecule magnet. Inorg. Chem. Front. 2020, 7, 2322–2334. [Google Scholar] [CrossRef]
- Car, P.-E.; Perfetti, M.; Mannini, M.; Favre, A.; Caneschi, A.; Sessoli, R. Giant field dependence of the low temperature relaxation of the magnetization in a dysprosium(iii)–DOTA complex. Chem. Commun. 2011, 47, 3751–3753. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-W.; Song, Y.-M.; Wang, G.-X.; Ye, Q.; Xiong, R.-G.; Akutagawa, T.; Nakamura, T.; Chan, P.W.H.; Huang, S.D. Dielectric Anisotropy of a Homochiral Trinuclear Nickel(II) Complex. J. Am. Chem. Soc. 2007, 129, 5346–5347. [Google Scholar] [CrossRef]
- Li, X.-L.; Chen, K.; Liu, Y.; Wang, Z.-X.; Wang, T.-W.; Zuo, J.-L.; Li, -Z.; Wang, Y.; Zhu, J.-S.; Liu, J.-M.; et al. Molecule-Based Ferroelectric Thin Films: Mononuclear Lanthanide Enantiomers Displaying Room-Temperature Ferroelectric and Dielectric Properties. Angew. Chem. Int. Ed. 2007, 46, 6820–6823. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tokunaga, Y.; Giovannetti, G.; Piccozzi, S.; Itoh, H.; Shimano, R.; Kumai, R.; Tokura, Y. Above-room-temperature ferroelectricity in a single-component molecular crystal. Nature 2010, 463, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Ngo Ndimba, A.; Roisnel, T.; Argouarch, G.; Lalli, C. Harvesting New Chiral Phosphotriesters by Phosphorylation of BINOL and Parent Bis-phenols. Synthesis 2019, 51, 865–873. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).