Revisiting the Potential Functionality of the MagR Protein
Abstract
:1. Introduction
2. Results
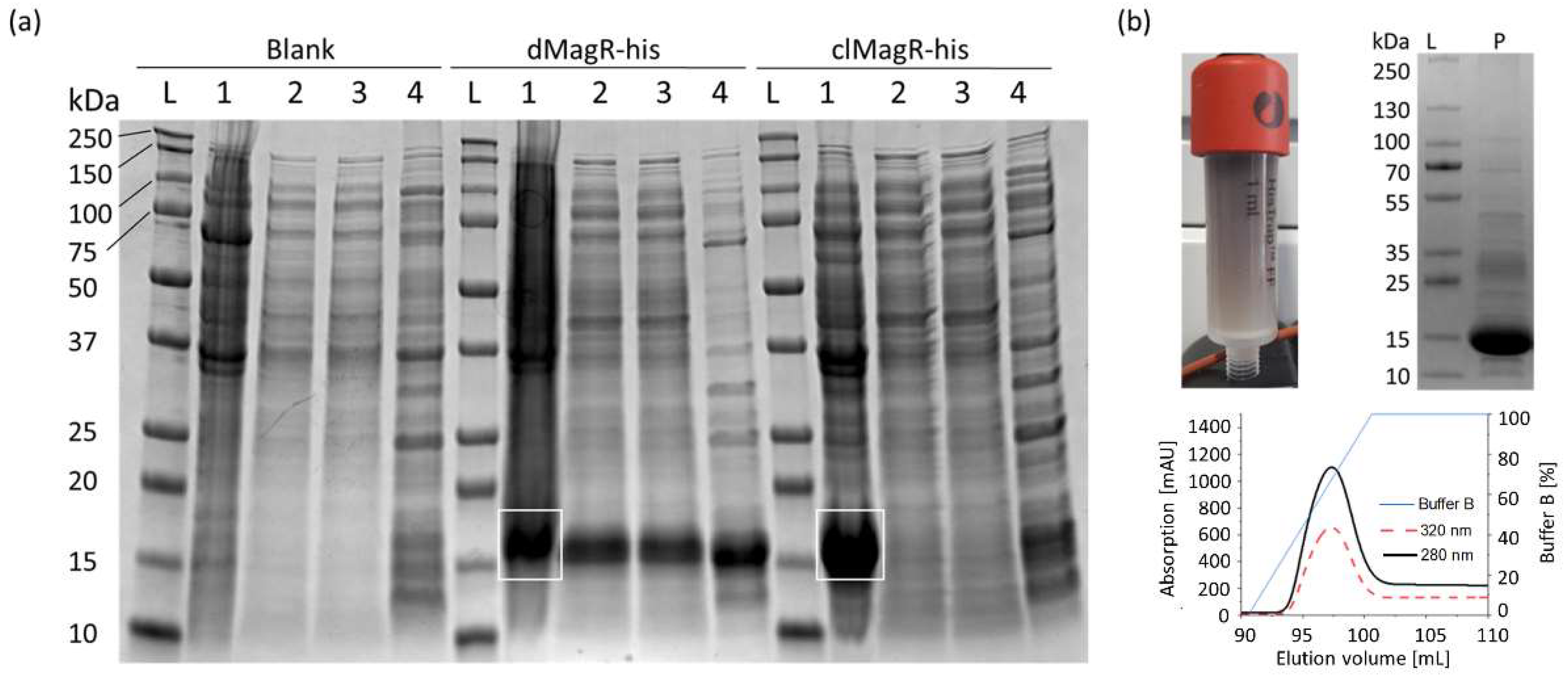
2.1. Evaluation of MagR Capture from a Complex Matrix
2.2. Potential of MagR to Magnetize Bacterial Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Putative Magnetic Proteins
4.3. Bacterial Strains, Plasmids and Cloning
4.4. Expression of Target Proteins
4.5. Capture of Target Proteins by Magnetite Beads
4.6. Purification of Target Proteins by Immobilized Metal Affinity Chromatography
4.7. Expression of dMagR and Preparation for Cell Magnetization Measurement
4.8. Magnetization Measurements of Whole Cells
4.9. Gel Electrophoresis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konhauser, K.O.; Kappler, A.; Roden, E.E. IRON IN MICROBIAL METABOLISMS. Elements 2011, 7, 89–93. [Google Scholar] [CrossRef]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rietzschel, N.; Stehling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Mühlenhoff, U. The role of mitochondria in cellular iron–sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1823, 1491–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, V.D.; Lill, R. Biogenesis of cytosolic and nuclear iron–sulfur proteins and their role in genome stability. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 1528–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandelia, M.-E.; Lanz, N.; Booker, S.J.; Krebs, C. Mössbauer spectroscopy of Fe/S proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1853, 1395–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, L.; Wang, F.; Zhang, J.; Liu, G.; Gao, B.; Wei, D. Novel Application of Magnetic Protein: Convenient One-Step Purification and Immobilization of Proteins. Sci. Rep. 2017, 7, 13329. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xu, H.; Liu, Z.; Sun, T.; Yuan, C.; Yang, Y.; Guo, J.; Xie, H. Magnetic immobilization of a quorum sensing signal hydrolase, AiiA. Microbiologyopen 2019, 8, e00797. [Google Scholar] [CrossRef] [Green Version]
- Pang, K.; You, H.; Chen, Y.; Chu, P.; Hu, M.; Shen, J.; Guo, W.; Xie, C.; Lu, B. MagR Alone Is Insufficient to Confer Cellular Calcium Responses to Magnetic Stimulation. Front. Neural Circuits 2017, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, X.; Ye, J.; Zhao, D.; Zhang, S.-J. Magnetogenetics: Remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor. Sci. Bull. 2015, 60, 2107–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, M. Physical limits to magnetogenetics. eLife 2016, 5, e17210. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer, M.; Mouritsen, H. A room-temperature ferrimagnet made of metallo-proteins? bioRxiv 2016, 8, e094607. [Google Scholar]
- Xiao, D.-W.; Hu, W.-H.; Cai, Y.; Zhao, N. Magnetic Noise Enabled Biocompass. Phys. Rev. Lett. 2020, 124, 128101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekarsky, A.; Spadiut, O. Intrinsically Magnetic Cells: A Review on Their Natural Occurrence and Synthetic Generation. Front. Bioeng. Biotechnol. 2020, 8, 573183. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Jorgensen, P.; Moran, U.; Weber, G.; Springer, M. BioNumbers—The database of key numbers in molecular and cell biology. Nucleic Acids Res. 2010, 38, D750–D753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilks, J.C.; Slonczewski, J.L. pH of the Cytoplasm and Periplasm of Escherichia coli: Rapid Measurement by Green Fluorescent Protein Fluorimetry. J. Bacteriol. 2007, 189, 5601–5607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moerz, S.T.; Huber, P. pH-Dependent Selective Protein Adsorption into Mesoporous Silica. J. Phys. Chem. C 2015, 119, 27072–27079. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Beilschmidt, L.K.; De Choudens, S.O.; Fournier, M.; Sanakis, I.; Hograindleur, M.-A.; Clémancey, M.; Blondin, G.; Schmucker, S.; Eisenmann, A.; Weiss, A.; et al. ISCA1 is essential for mitochondrial Fe4S4 biogenesis In Vivo. Nat. Commun. 2017, 8, 15124. [Google Scholar] [CrossRef] [PubMed]
- Ollagnier-de-Choudens, S.; Mattioli, T.; Takahashi, Y.; Fontecave, M. Iron-sulfur cluster assembly: Characterization of IscA and evidence for a specific and functional complex with ferredoxin. J. Biol. Chem. 2001, 276, 22604–22607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadiut, O.; Leitner, C.; Salaheddin, C.; Varga, B.; Vertessy, B.G.; Tan, T.-C.; Divne, C.; Haltrich, D. Improving thermostability and catalytic activity of pyranose 2-oxidase from Trametes multicolor by rational and semi-rational design. FEBS J. 2009, 276, 776–792. [Google Scholar] [CrossRef] [PubMed]

| dMagR (+ his6) | clMagR (+ his6) | |
|---|---|---|
| NCBI Reference Sequence | NP_573062.1 | XP_005508102.2 |
| Number of amino acids in native protein | 129 (137) | 130 (138) |
| Molecular weight [Da] * | 14,009.29 (15,074.41) | 14,081.14 (15,146.26) |
| Theoretical isoelectric point * | 9.25 (9.06) | 7.18 (6.82) |
| Grand average of hydrophobicity (GRAVY) * | −0.078 (−0.211) | −0.268 (−0.390) |
| Bacterial Strain | Plasmid | Protein | Strain Nomenclature |
|---|---|---|---|
| BL21(DE3) | pET-21a(+) | dMagR | BL21-dMagR |
| dMagR-his | BL21-dMagR-his | ||
| clMagR | BL21-clMagR | ||
| clMagR-his | BL21-clMagR-his | ||
| Pyranose-2-oxidase | BL21-Blank |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekarsky, A.; Michor, H.; Spadiut, O. Revisiting the Potential Functionality of the MagR Protein. Magnetochemistry 2021, 7, 147. https://doi.org/10.3390/magnetochemistry7110147
Pekarsky A, Michor H, Spadiut O. Revisiting the Potential Functionality of the MagR Protein. Magnetochemistry. 2021; 7(11):147. https://doi.org/10.3390/magnetochemistry7110147
Chicago/Turabian StylePekarsky, Alexander, Herwig Michor, and Oliver Spadiut. 2021. "Revisiting the Potential Functionality of the MagR Protein" Magnetochemistry 7, no. 11: 147. https://doi.org/10.3390/magnetochemistry7110147
APA StylePekarsky, A., Michor, H., & Spadiut, O. (2021). Revisiting the Potential Functionality of the MagR Protein. Magnetochemistry, 7(11), 147. https://doi.org/10.3390/magnetochemistry7110147






