Enriched Synthesis of Magnetosomes by Expanding the Magnetospirillum magneticum AMB-1 Culture at Optimal Iron Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
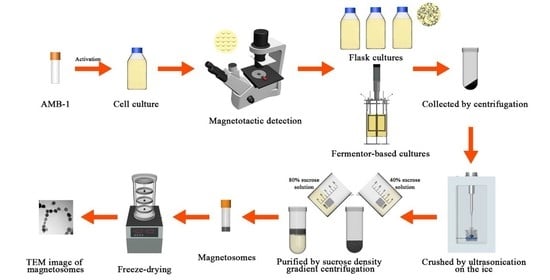
2.2. Culture of the AMB-1 Magnetotactic Bacteria
2.3. Comparison of AMB-1 in the Flask and Fermenter-Based Cultures
2.4. Optimization of Iron Content in the Fermenter Culture
2.5. Characterization of Magnetosomes
3. Results and Discussion
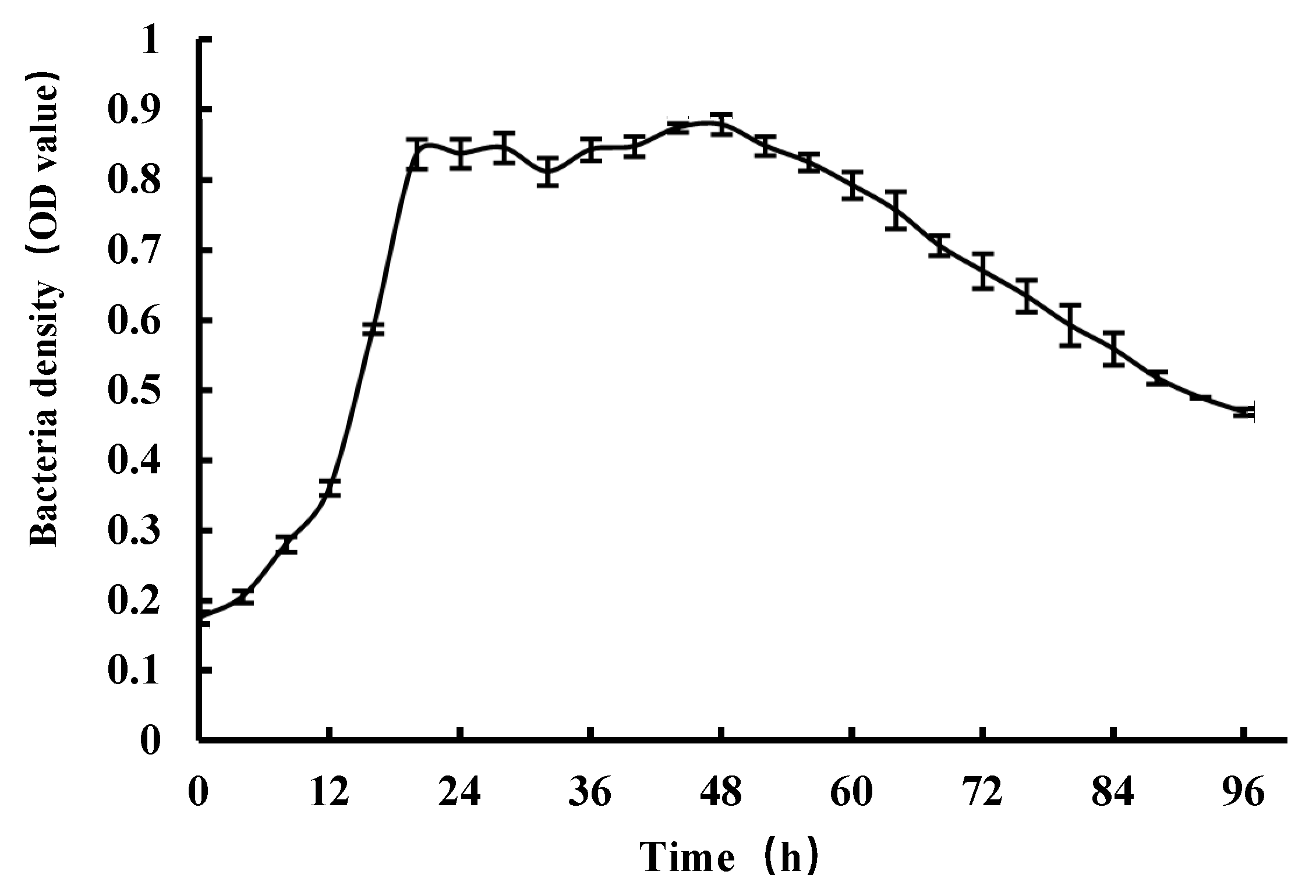
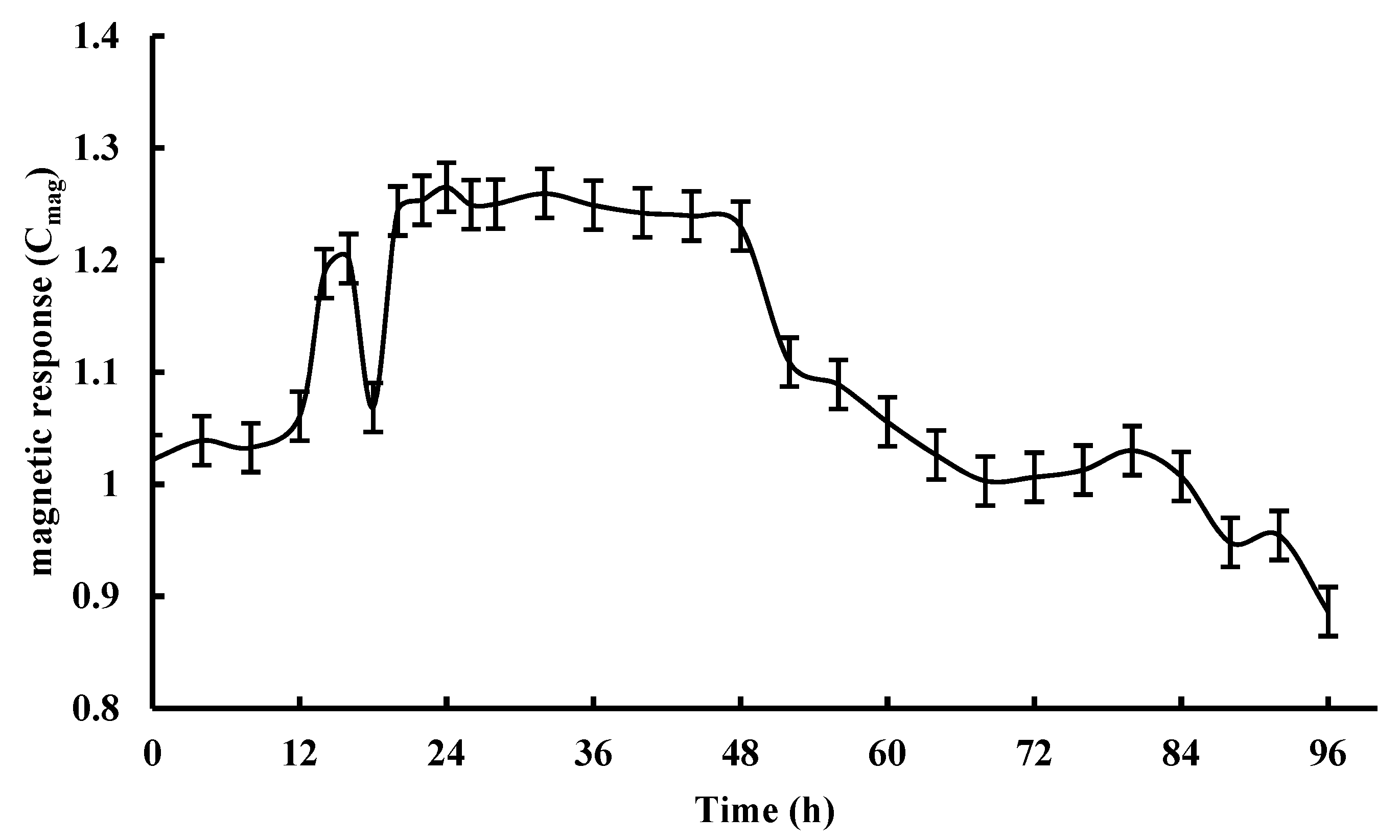
3.1. The Culture Conditions of the AMB-1 Magnetotactic Bacteria
3.2. Yields of Magnetosomes by Flask and Fermenter Culture Approaches
3.3. Optimization of Iron Source Content
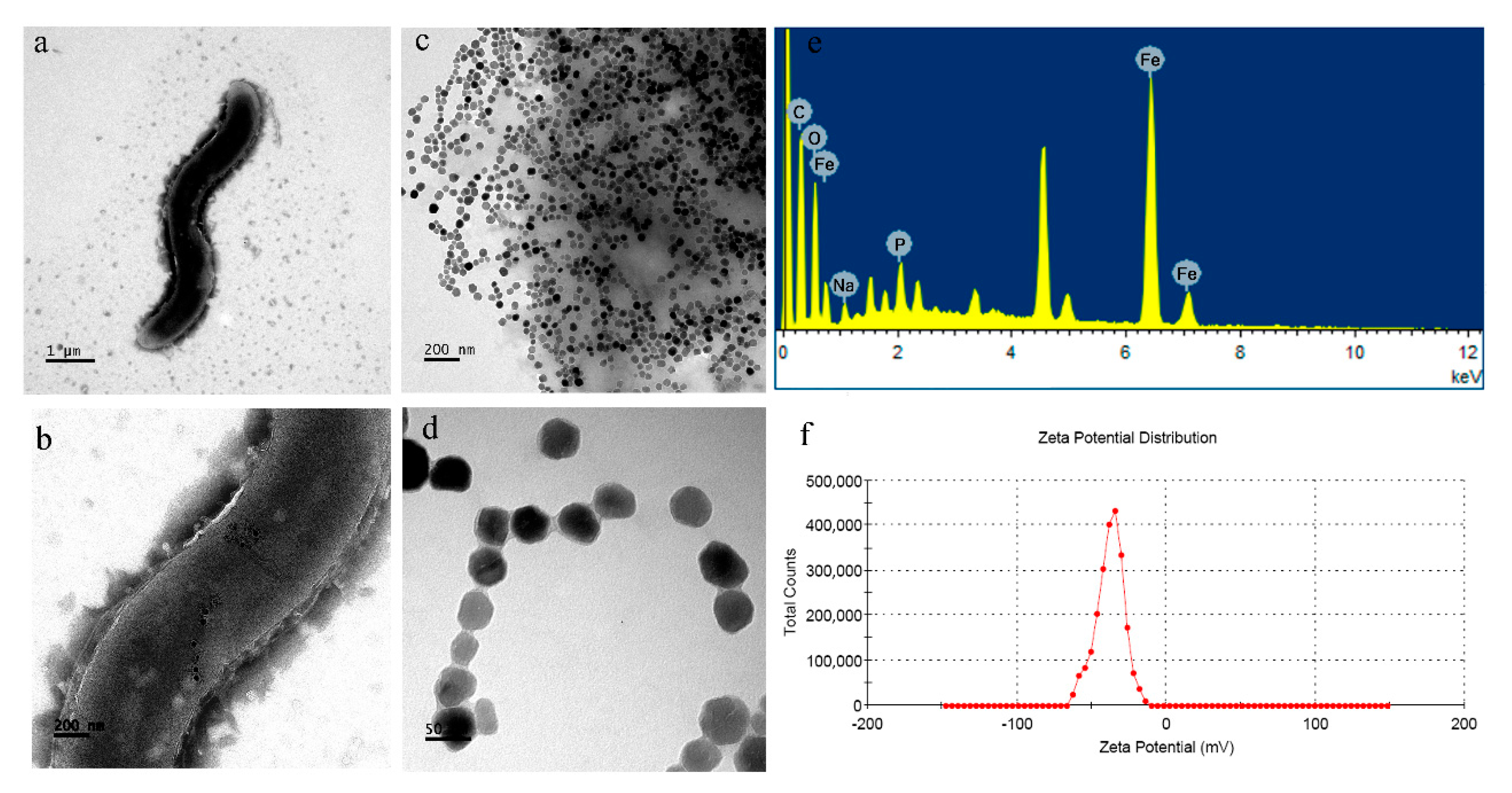
3.4. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blakemore, R.P. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230. [Google Scholar] [CrossRef]
- Jacob, J.J.; Suthindhiran, K. Magnetotactic bacteria and magnetosomes-scope and challenges. Mater. Sci. Eng. C 2016, 68, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Bazylinski, D.A.; Frankel, R.B.; Heywood, B.R.; Mann, S.; King, J.W.; Donaghay, P.L.; Hanson, A.K. Controlled biomineralization of magnetite (Fe3O4) and greigite (Fe3S4) in a magnetotactic bacterium. Appl. Environ. Microbiol. 1995, 61, 3232–3239. [Google Scholar] [CrossRef]
- Farina, M.; Kachar, B.; Lins, U.; Broderick, R.; de Barros, H.L. The observation of large magnetite (Fe3O4) crystals from magnetotactic bacteria by electron and atomic force microscopy. J. Microsc. 1994, 173, 1–8. [Google Scholar] [CrossRef]
- Le Nagard, L.; Zhu, X.; Yuan, H.; Benzerara, K.; Bazylinski, D.A.; Fradin, C.; Besson, A.; Swaraj, S.; Stanescu, S.; Belkhou, R.; et al. Magnetite Magnetosome Biomineralization in Magnetospirillum magneticum strain AMB-1: A Time Course Study. Chem. Geol. 2019, 530, 119348. [Google Scholar] [CrossRef]
- Flies, C.B.; Jonkers, H.M.; de Beer, D.; Bosselmann, K.; Böttcher, M.E.; Schüler, D. Diversity and vertical distribution of magnetotactic bacteria along chemical gradients in freshwater microcosms. FEMS Microbiol. Ecol. 2005, 52, 185–195. [Google Scholar] [CrossRef]
- Tan, S.M.; Ismail, M.H.; Cao, B. Biodiversity of magnetotactic bacteria in the tropical marine environment of Singapore revealed by metagenomic analysis. Environ. Res. 2021, 194, 110714. [Google Scholar] [CrossRef]
- Xing, W.; Hu, H.; Zhang, Y.; Zhao, D.; Wang, W.; Pan, H.; Zhang, S.; Yan, L. Magnetotactic bacteria diversity of and magnetism contribution to sediment in Wudalianchi volcanic barrier lakes, NE China. Sci. Total Environ. 2020, 718, 137348. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, X.; Xu, J.; Zhang, W.; Guo, Y. Removal of hexavalent chromium from wastewater using magnetotactic bacteria. Sep. Purif. Technol. 2014, 136, 10–17. [Google Scholar] [CrossRef]
- Yan, L.; Da, H.; Zhang, S.; López, V.M.; Wang, W. Bacterial magnetosome and its potential application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef]
- Hafsi, M.; Preveral, S.; Hoog, C.; Herault, J.; Perrier, G.; Lefèvre, C.; Michel, H.; Pignol, D.; Doyen, J.; Pourcher, T.; et al. RGD-functionalized magnetosomes are efficient tumor radioenhancers for X-rays and protons. Nanomedicine 2019, 23, 102084. [Google Scholar] [CrossRef]
- Yadav, A.; Gerislioglu, B.; Ahmadivand, A.; Kaushik, A.; Cheng, G.J.; Ouyang, Z.; Wang, Q.; Yadav, V.S.; Mishra, Y.K.; Wu, Y.; et al. Controlled self-assembly of plasmon-based photonic nanocrystals for high performance photonic technologies. Nano Today 2021, 37, 101072. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I. Yield cultivation of magnetotactic bacteria and magnetosomes: A review. J. Basic Microbiol. 2017, 57, 643–652. [Google Scholar] [CrossRef]
- Claus, L.; Schüler, D. Biogenic nanoparticles: Production, characterization, and application of bacterial magnetosomes. J. Phys. Condens. Matter. 2006, 18, S2815. [Google Scholar]
- Heyen, U.; Schüler, D. Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl. Microbiol. Biotechnol. 2003, 61, 536–544. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tsujimura, N.; Kamiya, S. Enhancement of magnetic particle production by nitrate and succinate fed-batch culture of Magnetospirillum sp. AMB-1. Biotechnol. Tech. 1996, 10, 495–500. [Google Scholar] [CrossRef]
- Matsunaga, T.; Togo, H.; Kikuchi, T.; Tanaka, T. Production of luciferase magnetic particle complex by recombinant Magnetospirillum sp. AMB-1. Biotechnol. Bioeng. 2000, 70, 704–709. [Google Scholar] [CrossRef]
- Yang, C.; Takeyama, H.; Matsunaga, T. Iron feeding optimization and plasmid stability in production of recombinant bacterial magnetic particles by Magnetospirillum magneticum AMB-1 in fed-batch culture. J. Biosci. Bioeng. 2001, 91, 213–216. [Google Scholar] [CrossRef]
- Marcano, L.; García-Prieto, A.; Muñoz, D.; Barquin, L.F.; Orue, I.; Alonso, J.; Muela, A.; Fdez-Gubieda, M.L. Influence of the bacterial growth phase on the magnetic properties of magnetosomes synthesized by Magnetospirillum gryphiswaldense. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, S.; Liu, H.; Wang, W.; Chen, P.; Li, H. Optimization of magnetosome production by Acidithiobacillus ferrooxidans using desirability function approach. Mat. Sci. Eng. C 2016, 59, 731–739. [Google Scholar] [CrossRef]
- Alphandéry, E.; Guyot, F.; Chebbi, I. Preparation of chains of magnetosomes, isolated from Magnetospirillum magneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int. J. Pharm. 2012, 434, 444–452. [Google Scholar] [CrossRef]
- Schüler, D.; Uhl, R.; Bäuerlein, E. A simple light scattering method to assay magnetism in Magnetospirillum gryphiswaldense. FEMS Microbiol. Lett. 1995, 132, 139–145. [Google Scholar] [CrossRef]
- Lefevre, C.T.; Song, T.; Yonnet, J.P.; Wu, L.F. Characterization of Bacterial Magnetotactic Behaviors by Using a Magnetospectrophotometry Assay. Appl. Environ. Microbiol. 2009, 75, 3835–3841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, J.; Liu, X.; Liu, W.; Qiu, G. Extraction of Magnetosome from Acidithiobacillus ferrooxidans. Biomagnetism 2005, 5, 7–10. [Google Scholar]
- Fu, G.; Jiang, W.; Li, Y.; Sun, J.; Wang, Z.; Zhang, Y.; Pan, F.; Liu, W.; Luo, Y. Electron microscopic observation of magnetosome formation in magnetospirillum gryphiswaldense and its purification. China J. Mod. Med. 2004, 14, 45–49. [Google Scholar]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J. Control. Release 2017, 262, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, M.; Dai, S.; Peng, K.; Jia, R. Characterization of magnetotactic bacteria and their magnetosomes isolated from Tieshan iron ore in Hubei Province of China. Mater. Sci. Eng. C 2006, 26, 597–601. [Google Scholar] [CrossRef]
| Flask-Type | Fermenter-Type | |
|---|---|---|
| Wet weight/mg | 37.1 | 78.8 |
| Dry weight/mg | 0.3 | 2.2 |
| Yield/mg L−1 | 0.3 | 0.7 |
| 0.02 μmol L−1 | 0.2 μmol L−1 | 2 μmol L−1 | |
|---|---|---|---|
| Wet weight/mg | 190.1 | 235.0 | low |
| Dry weight/mg | 22.7 | 63.3 | low |
| Yield/mg L−1 | 7.6 | 21.1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Kankala, R.K.; Yu, R.; Liu, H.; Liu, Y. Enriched Synthesis of Magnetosomes by Expanding the Magnetospirillum magneticum AMB-1 Culture at Optimal Iron Concentration. Magnetochemistry 2021, 7, 115. https://doi.org/10.3390/magnetochemistry7080115
Hong Y, Kankala RK, Yu R, Liu H, Liu Y. Enriched Synthesis of Magnetosomes by Expanding the Magnetospirillum magneticum AMB-1 Culture at Optimal Iron Concentration. Magnetochemistry. 2021; 7(8):115. https://doi.org/10.3390/magnetochemistry7080115
Chicago/Turabian StyleHong, Yazhen, Ranjith Kumar Kankala, Ruqi Yu, Huaqing Liu, and Yuangang Liu. 2021. "Enriched Synthesis of Magnetosomes by Expanding the Magnetospirillum magneticum AMB-1 Culture at Optimal Iron Concentration" Magnetochemistry 7, no. 8: 115. https://doi.org/10.3390/magnetochemistry7080115
APA StyleHong, Y., Kankala, R. K., Yu, R., Liu, H., & Liu, Y. (2021). Enriched Synthesis of Magnetosomes by Expanding the Magnetospirillum magneticum AMB-1 Culture at Optimal Iron Concentration. Magnetochemistry, 7(8), 115. https://doi.org/10.3390/magnetochemistry7080115