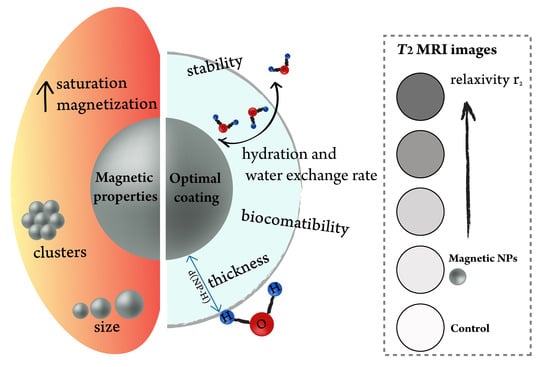
A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents
Abstract
1. Introduction
2. Optimization of the NPs’ Magnetic Properties
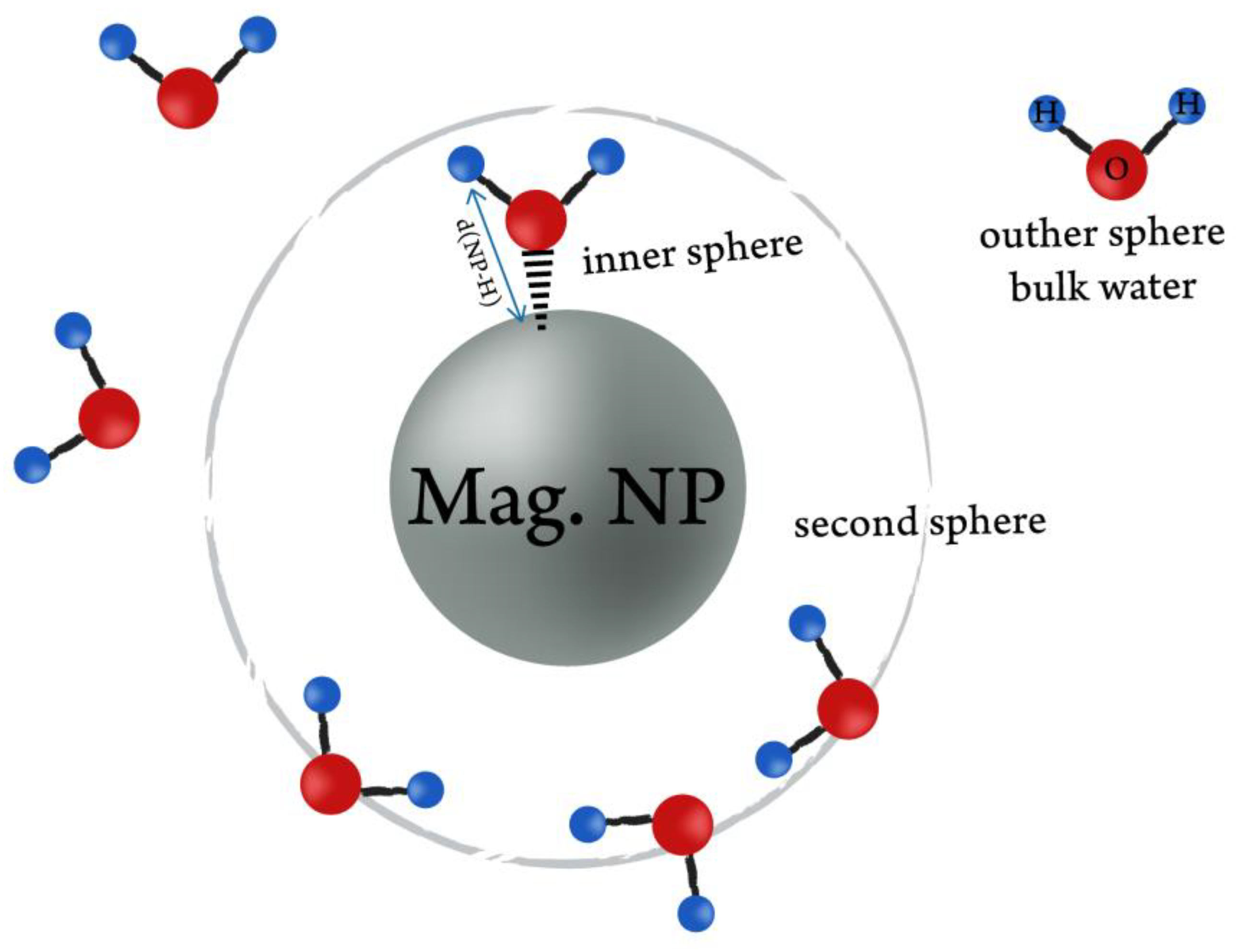
3. Influence of a Coating on Relaxivity
- Thin coating to minimize the distance between a magnetic core and water molecules;
- Highly hydrated surface;
- Fast water exchange rate;
- Ligands containing π-electrons, which increase magnetic field inhomogeneity;
- Biocompatibility of the coating and stability in biologically relevant media. Clinically approved moieties are prioritized due to an easier translation from a lab scale to a market scale.
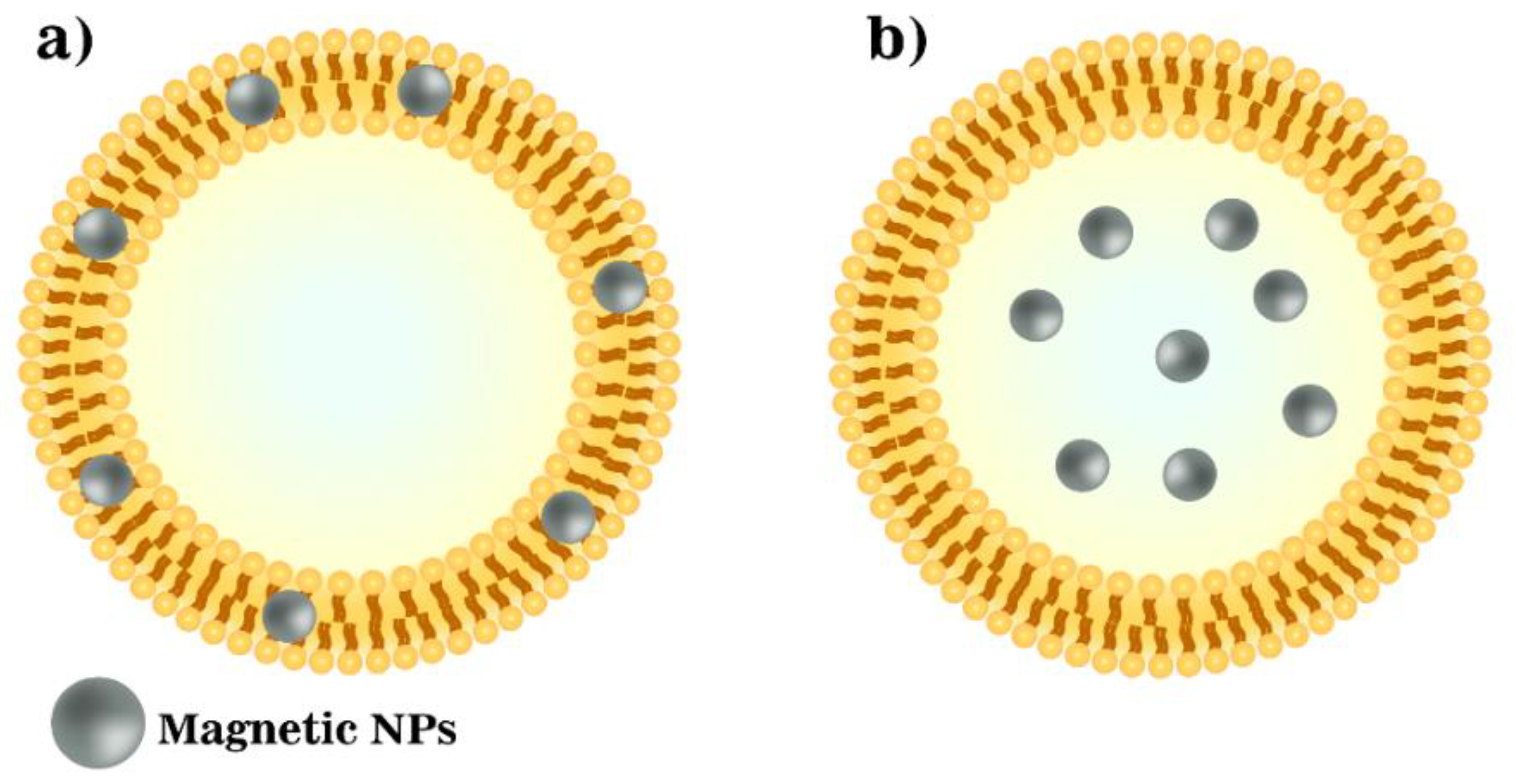
4. Magneto-Liposomes as T2 Contrast Agents
- Transverse relaxivity, which is a function of the water exchange rate and is proportional to the cholesterol content;
- Even though cholesterol reduces the fluidity of a membrane, relaxivity r2 values are important for the stability in vivo;
- Polyethene glycol (PEG) molecules are responsible for a fixed aqueous layer thickness near liposomes, thus assisting water diffusion through the bilayer and maintaining a high hydration number. Therefore, besides the stealth effect, the inclusion of PEGylated phospholipids, such as DSPE-PEG2000, plays an important role in enhancing liposomal hydration states and consequently r2 values [41,42].
5. Biomimetic Nanostructures
6. Conclusions
Funding
Conflicts of Interest
References
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Boros, E.; Gale, E.M.; Caravan, P. MR imaging probes: Design and applications. Dalt. Trans. 2015, 44, 4804. [Google Scholar] [CrossRef]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Daldrup-Link, H.E. Ten things you might not know about iron oxide nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E.; Rydland, J.; Helbich, T.H.; Bjørnerud, A.; Turetschek, K.; Kvistad, K.A.; Kaindl, E.; Link, T.M.; Staudacher, K.; Shames, D.; et al. Quantification of Breast Tumor Microvascular Permeability with Feruglose-enhanced MR Imaging: Initial Phase II Multicenter Trial. Radiology 2003, 229, 885–892. [Google Scholar] [CrossRef]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, S.; Zhang, Y.; Gao, J.; Hong, L.; Wang, X.; Wu, W.; Jiang, X. Ultra-high relaxivity iron oxide nanoparticles confined in polymer nanospheres for tumor MR imaging. J. Mater. Chem. B 2015, 3, 5702–5710. [Google Scholar] [CrossRef]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef]
- Frey, N.A.; Sun, S. Magnetic Nanoparticle for Information Storage Applications. In Inorganic Nanoparticles: Synthesis, Applications, and Perspectives; CRC Press: Boca Raton, FL, USA, 2011; pp. 33–68. [Google Scholar]
- Thanh, N.T.K. Magnetic Nanoparticles: From Fabrication to Clinical Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Şimşek, T.; Özcan, Ş. Effective magnetic anisotropy enhancement of FePt nanocrystals through shape control. J. Magn. Magn. Mater. 2014, 351, 47–51. [Google Scholar] [CrossRef]
- Koksharov, Y.A. Magnetism of Nanoparticles: Effects of Size, Shape, and Interactions. In Magnetic Nanoparticles; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp. 197–254. ISBN 9783527627561. [Google Scholar]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Phys. Rev. Lett. 1994, 72, 282–285. [Google Scholar] [CrossRef]
- Wu, X.W.; Liu, C.; Li, L.; Jones, P.; Chantrell, R.W.; Weller, D. Nonmagnetic shell in surfactant-coated FePt nanoparticles. J. Apply. Phys. 2004, 95, 6810–6812. [Google Scholar] [CrossRef]
- Tanaka, Y.; Saita, S.; Maenosono, S. Influence of surface ligands on saturation magnetization of FePt nanoparticles. Appl. Phys. Lett. 2008, 92, 47–50. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Shin, T.; Choi, Y.; Kim, S.; Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Kayandan, S.; Lin, Y.-N.; Kelly, D.F.; House, M.J.; Woodward, R.C.; St Pierre, T.G.; Riffle, J.S.; Davis, R.M. Toward design of magnetic nanoparticle clusters stabilized by biocompatible diblock copolymers for T₂-weighted MRI contrast. Langmuir 2014, 30, 1580–1587. [Google Scholar] [CrossRef]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Lévy, M.; Bacri, J.C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and MRI contrast agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef]
- Kostevšek, N.; Šturm, S.; Serša, I.; Sepe, A.; Bloemen, M.; Verbiest, T.; Kobe, S.; Žužek Rožman, K. “Single-” and “multi-core” FePt nanoparticles: From controlled synthesis via zwitterionic and silica bio-functionalization to MRI applications. J. Nanoparticle Res. 2015, 17, 464. [Google Scholar] [CrossRef]
- Caravan, P.; Farrar, C.T.; Frullano, L.; Uppal, R. Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T1 contrast agents. Contrast Media Mol. Imaging 2009, 4, 89–100. [Google Scholar] [CrossRef]
- Kostevšek, N.; Abramovič, I.; Hudoklin, S.; Kreft, M.E.; Serša, I.; Sepe, A.; Vidmar, J.; Šturm, S.; Spreitzer, M.; Ščančar, J.; et al. Hybrid FePt/SiO2/Au nanoparticles as a theranostic tool: In vitro photo-thermal treatment and MRI imaging. Nanoscale 2018, 10, 1308–1321. [Google Scholar] [CrossRef]
- Zeng, J.; Jing, L.; Hou, Y.; Jiao, M.; Qiao, R.; Jia, Q.; Liu, C.; Fang, F.; Lei, H.; Gao, M. Anchoring group effects of surface ligands on magnetic properties of Fe3O4 nanoparticles: Towards high performance MRI contrast agents. Adv. Mater. 2014, 26, 2694–2698. [Google Scholar] [CrossRef]
- Damodaran, K.V.; Merz, K.M.; Gaber, B.P. Structure and Dynamics of the Dilauroylphosphatidylethanolamine Lipid Bilayer. Biochemistry 1992, 31, 7656–7664. [Google Scholar] [CrossRef]
- Dumas, S.; Jacques, V.; Sun, W.-C.; Troughton, J.S.; Welch, J.T.; Chasse, J.M.; Schmitt-Willich, H.; Caravan, P. High relaxivity magnetic resonance imaging contrast agents. Part 1. Impact of single donor atom substitution on relaxivity of serum albumin-bound gadolinium complexes. Invest. Radiol. 2010, 45, 600–612. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar]
- Shen, S.; Huang, D.; Cao, J.; Chen, Y.; Zhang, X.; Guo, S.; Ma, W.; Qi, X.; Ge, Y.; Wu, L. Magnetic liposomes for light-sensitive drug delivery and combined photothermal–chemotherapy of tumors. J. Mater. Chem. B 2019, 7, 1096–1106. [Google Scholar] [CrossRef]
- Carvalho, A.; Goncalves, M.C.; Martins, M.B.F.; Meixedo, D.; Feio, G. Relaxivities of magnetoliposomes: The effect of cholesterol. Magn. Reson. Imaging 2013, 31, 610–612. [Google Scholar] [CrossRef]
- Skouras, A.; Mourtas, S.; Markoutsa, E.; De Goltstein, M.-C.; Wallon, C.; Catoen, S.; Antimisiaris, S.G. Magnetoliposomes with high USPIO entrapping efficiency, stability and magnetic properties. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 572–579. [Google Scholar] [CrossRef]
- Garnier, B.; Tan, S.; Miraux, S.; Bled, E.; Brisson, A.R. Optimized synthesis of 100 nm diameter magnetoliposomes with high content of maghemite particles and high MRI effect. Contrast Media Mol. Imaging 2012, 7, 231–239. [Google Scholar] [CrossRef]
- Marie, H.; Lemaire, L.; Franconi, F.; Lajnef, S.; Frapart, Y.-M.; Nicolas, V.; Frebourg, G.; Trichet, M.; Menager, C.; Lesieur, S. Superparamagnetic Liposomes for MRI Monitoring and External Magnetic Field-Induced Selective Targeting of Malignant Brain Tumors. Adv. Funct. Mater. 2015, 25, 1258–1269. [Google Scholar] [CrossRef]
- Faria, M.R.; Cruz, M.M.; Gonçalves, M.C.; Carvalho, A.; Feio, G.; Martins, M.B.F. Synthesis and characterization of magnetoliposomes for MRI contrast enhancement. Int. J. Pharm. 2013, 446, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.S.; Fortin, J.P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef]
- Béalle, G.; Di Corato, R.; Kolosnjaj-Tabi, J.; Dupuis, V.; Clément, O.; Gazeau, F.; Wilhelm, C.; Ménager, C. Ultra magnetic liposomes for MR imaging, targeting, and hyperthermia. Langmuir 2012, 28, 11834–11842. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Ma, J.; Li, Q.; Li, Y.; Zhou, X.; Zhao, D.; Song, H.; Chen, Q.; Zhu, X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J. Control. Release 2018, 272, 145–158. [Google Scholar] [CrossRef]
- Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T₂ Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, 1209. [Google Scholar] [CrossRef]
- Ferrauto, G.; Delli Castelli, D.; Di Gregorio, E.; Terreno, E.; Aime, S. LipoCEST and cellCEST imaging agents: Opportunities and challenges. WIREs Nanomed. Nanobiotechnol. 2016, 8, 602–618. [Google Scholar] [CrossRef]
- Carvalho, A.; Gonçalves, M.C.; Corvo, M.L.; Martins, M.B.F. Development of New Contrast Agents for Imaging Function and Metabolism by Magnetic Resonance Imaging. Magn. Reson. Insights 2017, 10. [Google Scholar] [CrossRef]
- Carvalho, A.; Martins, M.B.F.; Corvo, M.L.; Feio, G. Enhanced contrast efficiency in MRI by PEGylated magnetoliposomes loaded with PEGylated SPION: Effect of SPION coating and micro-environment. Mater. Sci. Eng. C 2014, 43, 521–526. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Shen, M. Hydrothermal Synthesis and Functionalization of Iron Oxide Nanoparticles for MR Imaging Applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Li Zhao, H.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall water-soluble and biocompatible magnetic iron oxide nanoparticles as positive and negative dual contrast agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Scheu, R.; Rankin, B.M.; Chen, Y.; Jena, K.C.; Ben-Amotz, D.; Roke, S. Charge asymmetry at aqueous hydrophobic interfaces and hydration shells. Angew. Chem. Int. Ed. 2014, 126, 9714–9717. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef]
- Phua, K.K.L.; Boczkowski, D.; Dannull, J.; Pruitt, S.; Leong, K.W.; Nair, S.K. Whole Blood Cells Loaded with Messenger RNA as an Anti-Tumor Vaccine. Adv. Healthc. Mater. 2014, 3, 837–842. [Google Scholar] [CrossRef]
- Antonelli, A.; Sfara, C.; Manuali, E.; Bruce, I.J.; Magnani, M. Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine 2011, 6, 211–223. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Xu, J.H.; Cai, B.; Yu, G.T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.S.; et al. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef]
- Antonelli, A.; Pacifico, S.; Sfara, C.; Tamma, M.; Magnani, M. Ferucarbotran-loaded red blood cells as long circulating MRI contrast agents: First in vivo results in mice. Nanomedicine 2018, 13, 675–687. [Google Scholar] [CrossRef]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef]

| Reference (NPs location) | Ex Vitro Suspension Only (Formulation, Fe Concentration Range and Maximum r2 Value) | In vivo (Concentration Used and Administration Route) |
|---|---|---|
| [30] Shen et al., 14 nm IO NPs in the core | DPPC/Chol (80/20 mol%) 0–0.8 mM r2 = 20.49 | Intravenous injection of 200 µL of MLs with a Fe3O4 concentration of 2 mg/mL + magnet next to the tumor. Tumor appeared 59% darker. |
| [31] Carvalho et al., 6 nm IO NPs core | Soybean PC + Chol 0–2.5 mM Max r2 value = 143.69 without Chol | / |
| [32] Skouras et al., size not specified, in the core | Concentration range not specified, only relaxivities shown | / |
| [33] Garnier et al., 7 nm iron oxide (IO) NPs in the core | DOPC/Chol (75/25 mol%) 0–0.8 mM Maximum r2 value = 323 | / |
| [34] Marie et al., 13 nm IO NPs in the core | EPC/DSPE-polyethene glycol (PEG)2000/Rho-PE = 94/5/1 0–0.2 mM Maximum r2 value= 259 | Intravenous injection of 200 µL MLs (122.5 µmoles lipids and 533 µmoles IO per kg) + magnet next to the tumor. Tumor appeared darker |
| [35] Faria et al., 11 nm IO NPs in the core | SPC/Chol = 1/0.5 no conc. ranges Agar phantoms–T2 images slightly darker | / |
| [36] Martina et al., 17 nm IO NPs in the core | Egg-PC/DSPE-PEG2000 (95/5) 0.02–10 mM Maximum r2 value = 130 | Intravenous of 200 μL of MLs (20 mM total lipid and 25 mM Fe). Tumor was 22% brighter on T1 image. |
| [37] Béalle et al., 7 nm IO NPs in the core | DPPC/DSPC (90/10) 0–1 mM max r2 value = 267.9 | DPPC/DSPC/Rhod-PE/DSPE-PEG (94/10/1/5) 100 µL of MLs with 0.1 mM Fe retro-orbital venous sinus injection + magnet next to the tumor. Darker contrast observed in the tumor |
| [38] Guo et al., 4 nm IO NPs in the bilayer | DPPC/Chol/SA/DSPE-MPEG2000-MTX 0–1 mM T2-weighted images Maximum r2 value = 60.06 | Intravenous injection of 0.2 mL of 2 mg/kg (DOX equivalent dose) + tumor next to the tumor. Colored T2 images |
| [39] Martínez-González et al., 5 nm hydrophobic IO NPs in the bilayer Hydrophilic NPs not in the liposomes, but forming branched–linear clusters | DMPC, DMPC/Chol, DMPC-PS, DOPC, DOPC/Chol, DOPC-PS 0–0.12 mM Maximum r2 value = 995 for DOPC-PS | / |
| Abbreviation | Chemical Name |
|---|---|
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine |
| DSPC | 1,2-distearoyl-sn-glycero-3-phophatidylcholine |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DSPE-PEG2000 | N-[carbonyl-methoxy(polyethylene glycol)-2000]-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt |
| Egg-PC | L-α-phosphatidylcholine |
| SPC | soybean phosphatidylcholine |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostevšek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry 2020, 6, 11. https://doi.org/10.3390/magnetochemistry6010011
Kostevšek N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry. 2020; 6(1):11. https://doi.org/10.3390/magnetochemistry6010011
Chicago/Turabian StyleKostevšek, Nina. 2020. "A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents" Magnetochemistry 6, no. 1: 11. https://doi.org/10.3390/magnetochemistry6010011
APA StyleKostevšek, N. (2020). A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry, 6(1), 11. https://doi.org/10.3390/magnetochemistry6010011