Key Role of Size and Electronic Configuration on the Sign and Strength of the Magnetic Coupling in a Series of Cu2Ln Trimers (Ln = Ce, Gd, Tb, Dy and Er)
Abstract
:1. Introduction
2. Results and Discussion
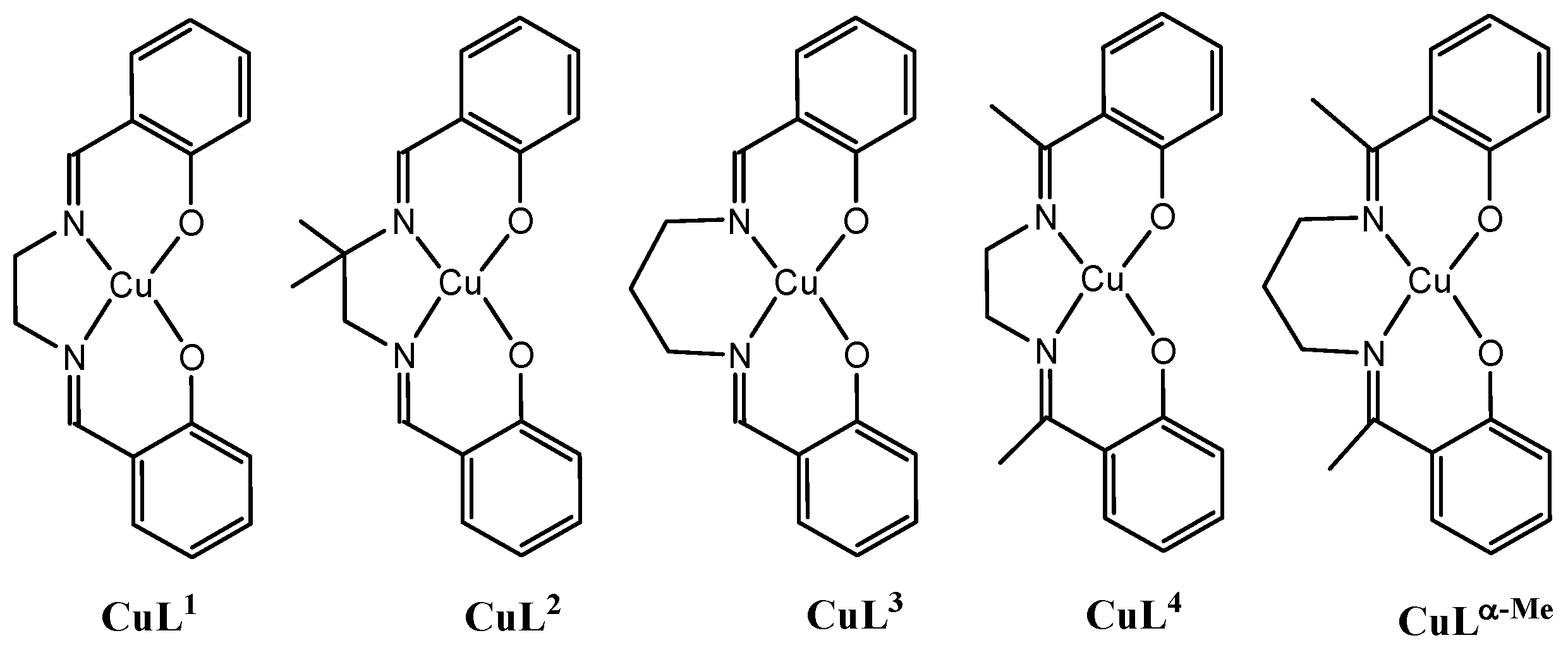
2.1. Syntheses and Spectroscopic Characterizations of the Complexes
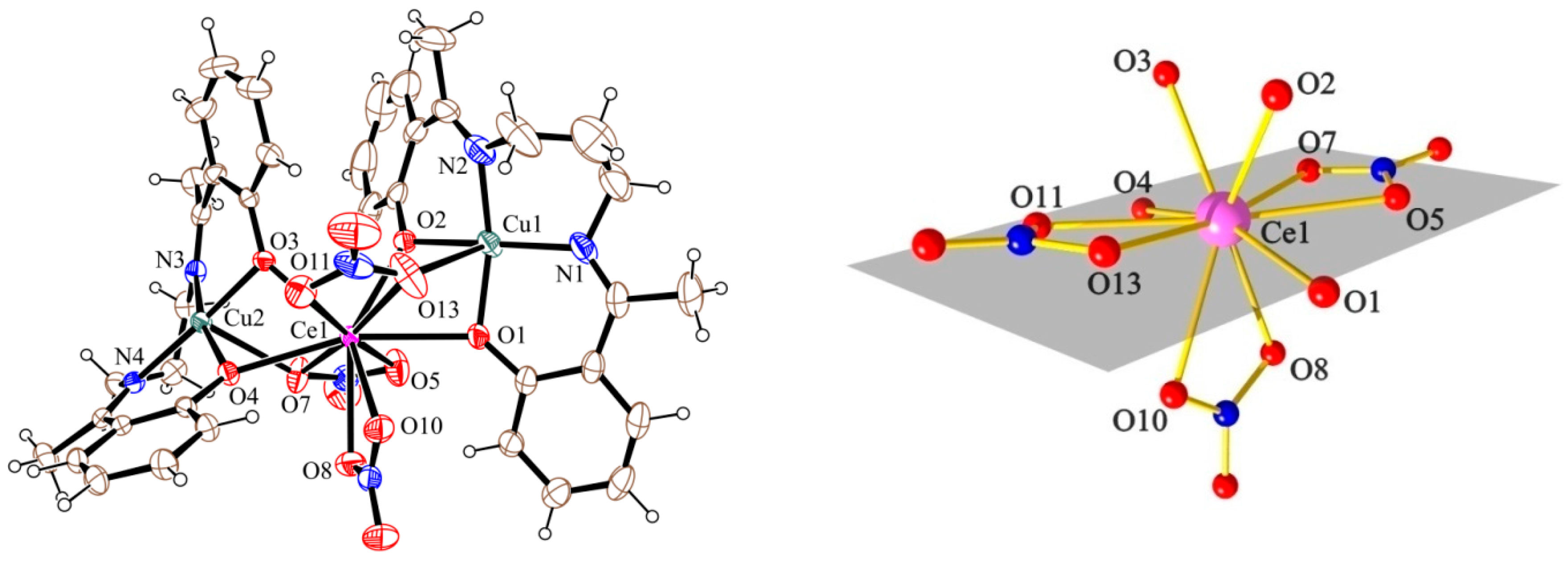
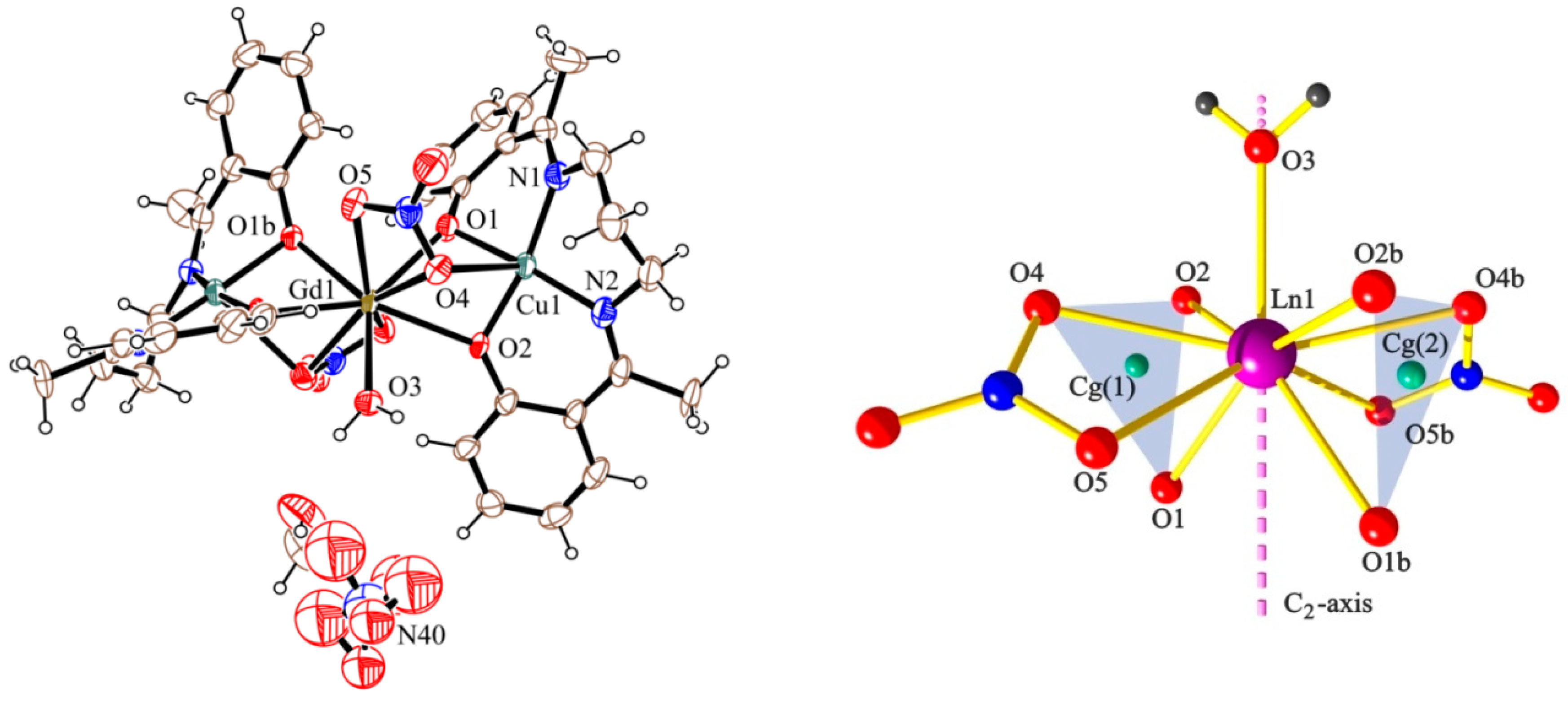
2.2. Description of the Structures
| Compound | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Formula | C38H40N7O13Cu2Ce | C40H50N7O16Cu2Gd | C40H50N7O16Cu2Tb | C40H50N7O16Cu2Dy | C40H50N7O16Cu2Er |
| Formula Weight | 1069.99 | 1169.22 | 1170.90 | 1174.47 | 1179.23 |
| Space group | Pbca | C2/c | C2/c | C2/c | C2/c |
| Crystal system | Orthorhombic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| a/Å | 18.380(4) | 16.112(5) | 16.259(5) | 16.192(5) | 16.257(5) |
| b/Å | 21.084(5) | 19.288(5) | 19.449(5) | 19.380(5) | 19.458(5) |
| c/Å | 21.284(5) | 14.740(5) | 14.826(5) | 14.884(5) | 14.815(5) |
| β/° | 90 | 97.875(5) | 97.882(5) | 97.948(5) | 97.996(5) |
| V/Å3 | 8248(3) | 4538(2) | 4644(2) | 4626(2) | 4641(2) |
| Z | 8 | 4 | 4 | 4 | 4 |
| Dcalc/g·cm−3 | 1.723 | 1.711 | 1.675 | 1.686 | 1.688 |
| μ/mm−1 | 2.181 | 2.451 | 2.490 | 2.586 | 2.776 |
| F(000) | 4296 | 2356 | 2360 | 2364 | 2372 |
| R(int) | 0.0538 | 0.0723 | 0.0701 | 0.0490 | 0.0370 |
| θ range (deg) | 1.75–25.14 | 1.66–25.01 | 1.64–25.14 | 1.65–25.02 | 1.64–25.21 |
| Total reflections | 55203 | 9971 | 25546 | 24215 | 9997 |
| Unique reflections | 7349 | 3829 | 4123 | 4058 | 3967 |
| Data with I > 2σ(I) | 5396 | 2689 | 3370 | 3381 | 3271 |
| R1 a on I > 2σ(I) | 0.0313 | 0.0669 | 0.0464 | 0.0475 | 0.0361 |
| wR2 b (all) | 0.0751 | 0.1819 | 0.1312 | 0.1302 | 0.1079 |
| GOF c on F2 | 1.010 | 0.996 | 1.082 | 1.079 | 1.055 |
| Temp (K) | 293 | 293 | 293 | 293 | 293 |
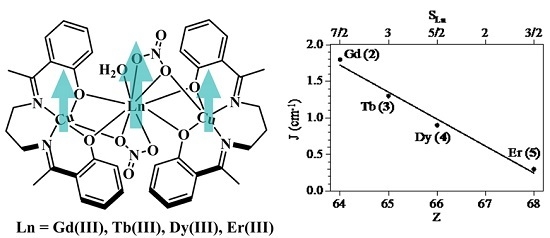
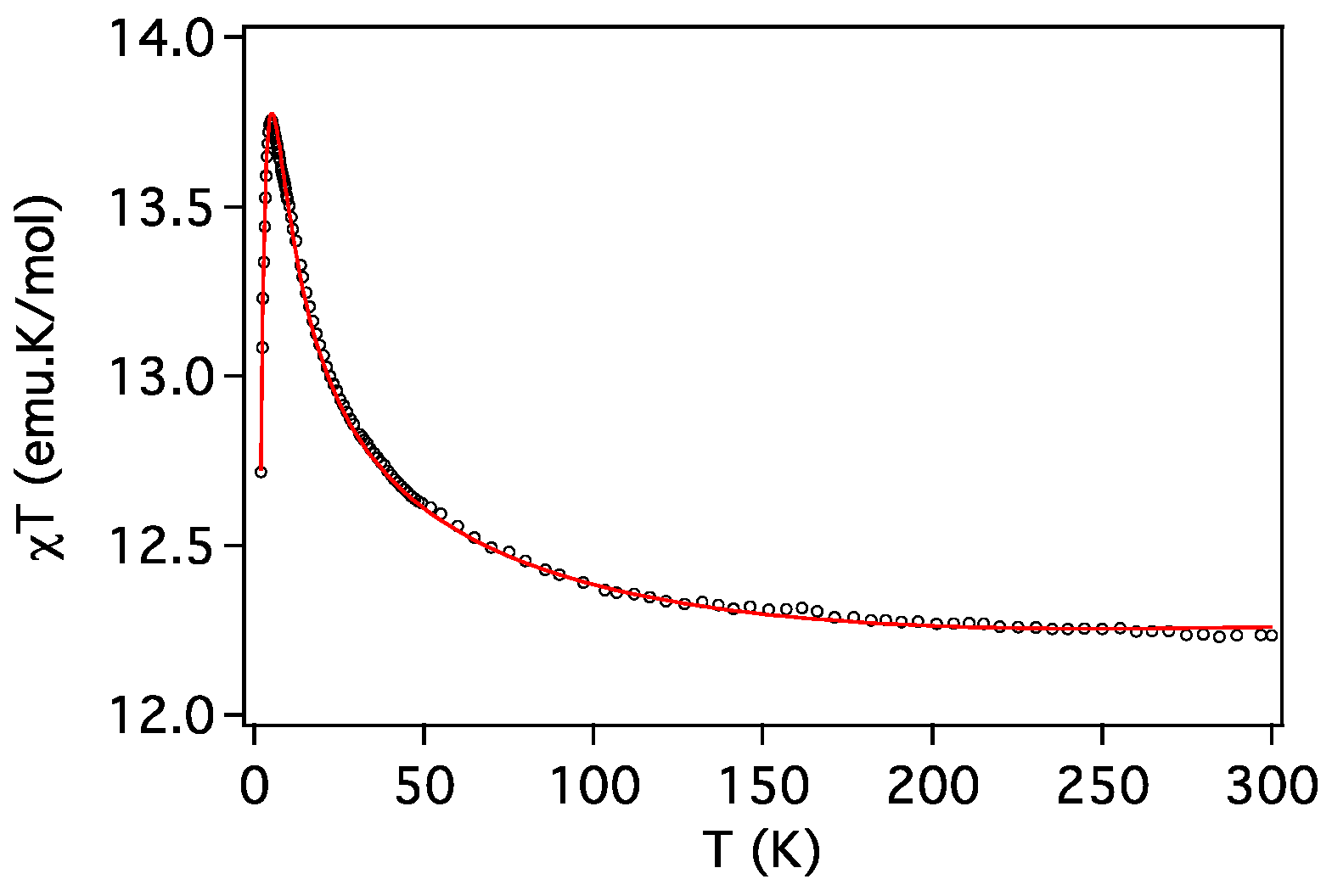
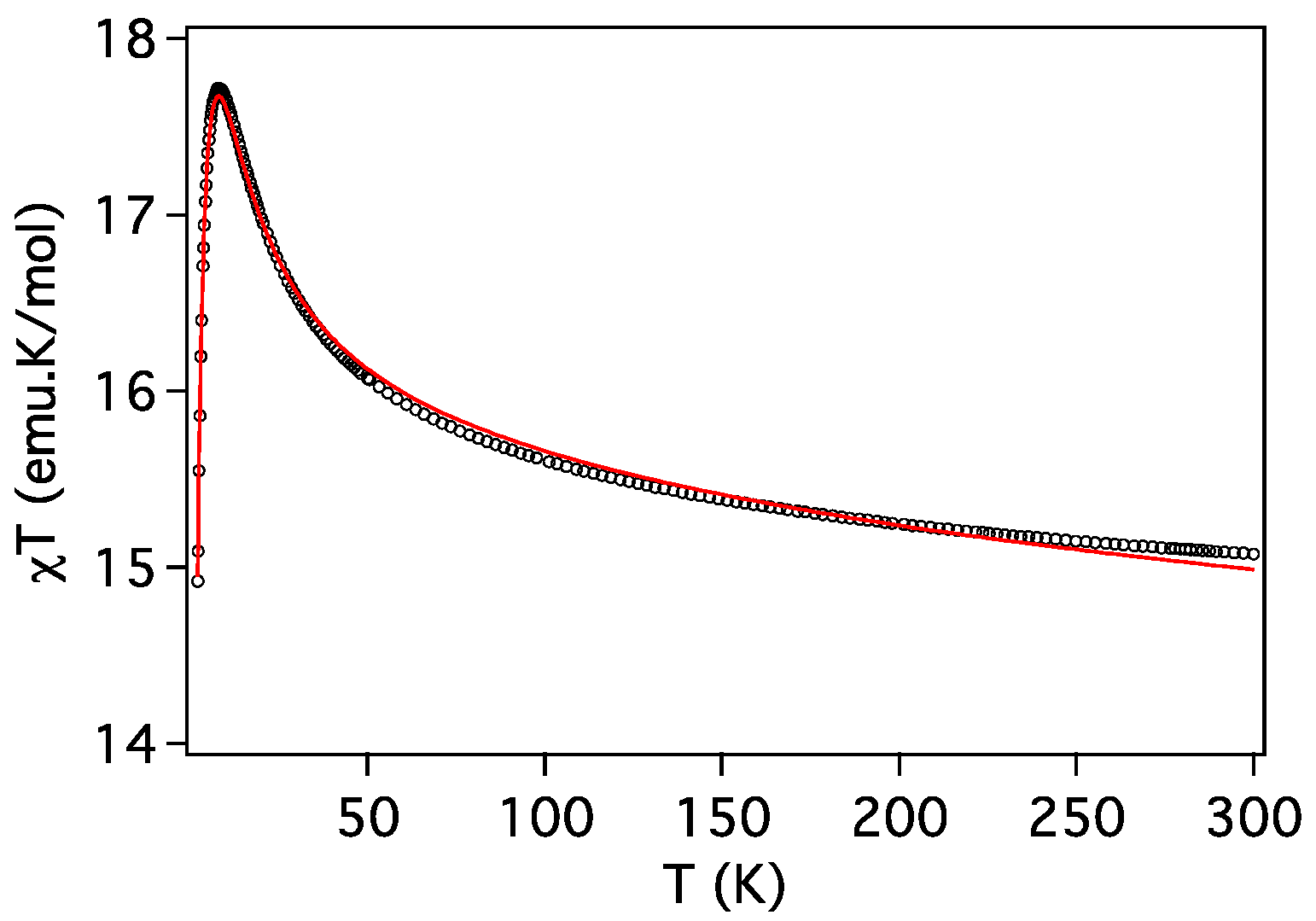
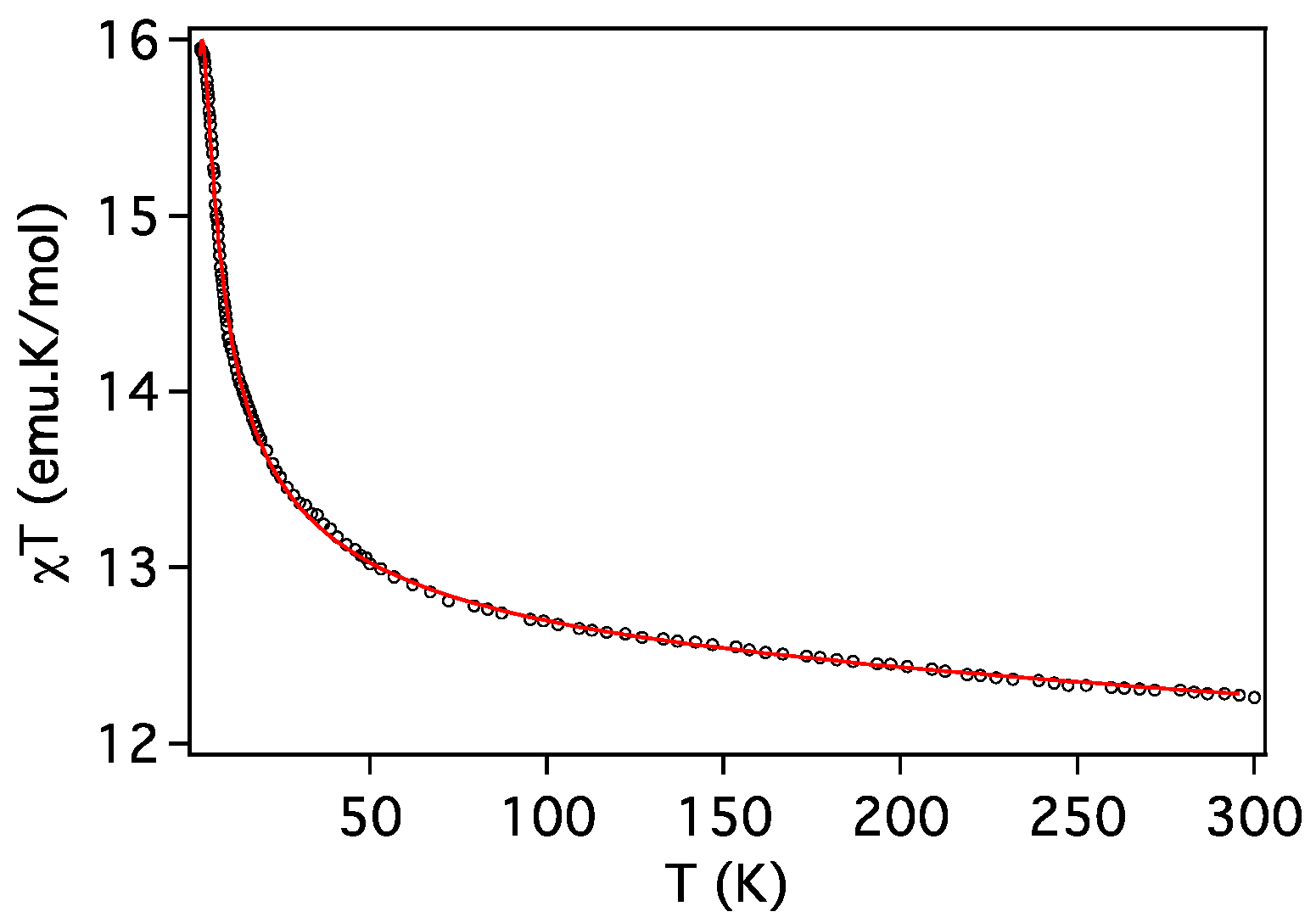
2.3. Magnetic Properties
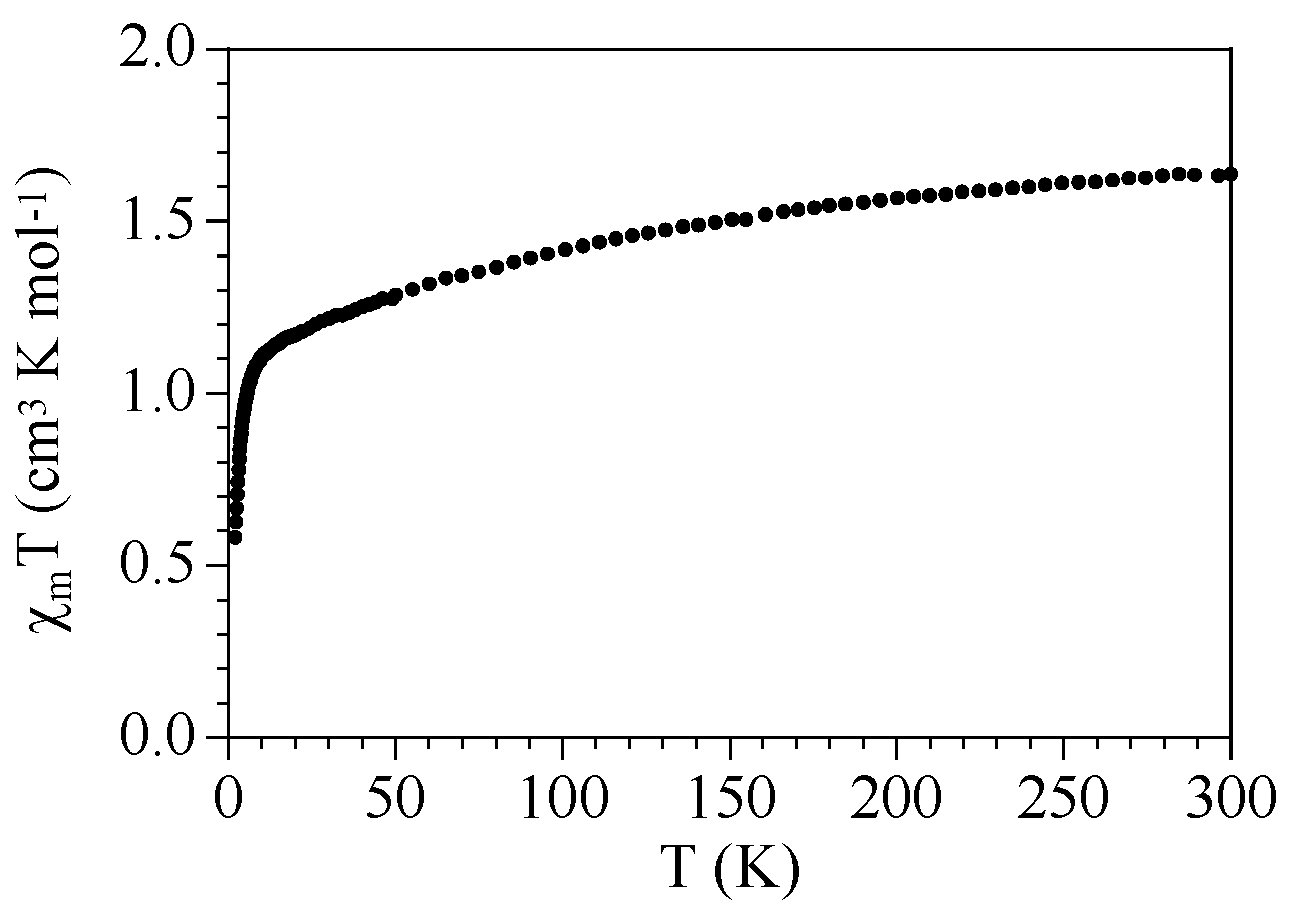
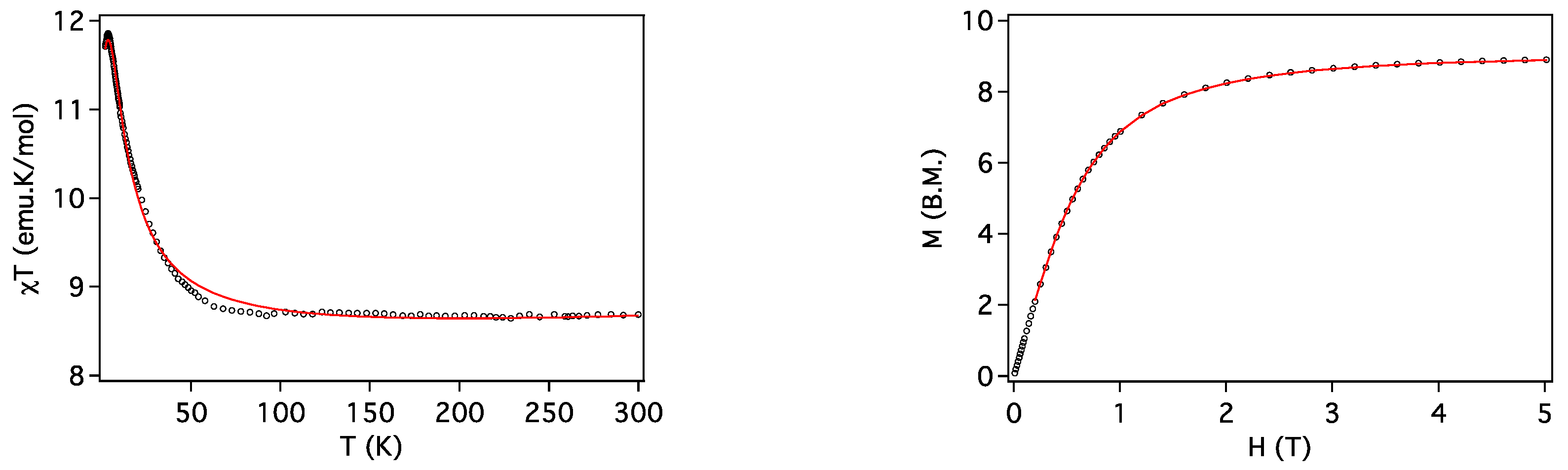
| Compound | ST | gCu | gLn | JCu-Ln (cm−1) | D (cm−1) | Tip (cm3 mol−1) | First Excited Level Energy (cm−1) |
|---|---|---|---|---|---|---|---|
| 2 (Cu2Gd) | 9/2 | 2.036 | 2.0 | 1.81 | 0 | 1 × 10−3 | 12.7 |
| 3 (Cu2Tb) | 7 | 1.990 | 3/2 | 1.27 | 2.0 | 0.7 × 10−3 | 11.8 |
| 4 (Cu2Dy) | 17/2 | 1.991 | 4/3 | 0.88 | 5.1 | 0 | 4.53 |
| 5 (Cu2Er) | 17/2 | 2.131 | 6/5 | 0.31 | 1.4 | 0 | 1.33 |
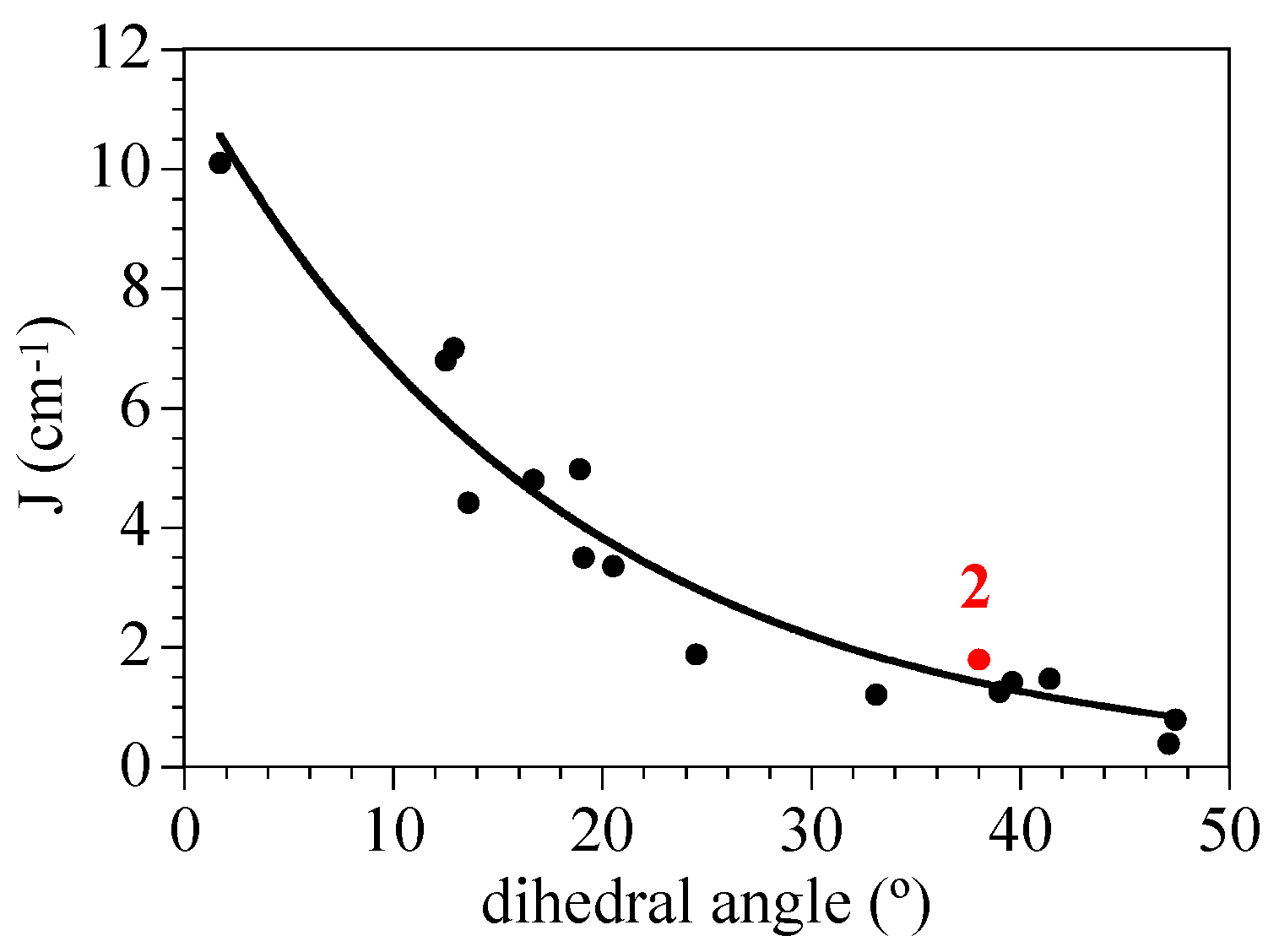
2.4. Magneto-Structural Correlations
| Compound | Cu–O (Å) | Ln–O (Å) | Cu–O–Ln (°) | Cu–O–O–Ln (°) |
|---|---|---|---|---|
| 1 (Cu2Ce) a | 1.901 | 2.612 | 97.2 | |
| 1.932 | 2.431 | 102.5 | 34.5 | |
| 1.940 | 2.464 | 99.6 | 42.7 | |
| 1.912 | 2.581 | 96.4 | ||
| 2 (Cu2Gd) | 1.971 | 2.368 | 97.1 | 38.0 |
| 1.889 | 2.414 | 97.9 | ||
| 3 (Cu2Tb) | 1.973 | 2.346 | 97.9 | 37.4 |
| 1.898 | 2.418 | 97.7 | ||
| 4 (Cu2Dy) | 1.975 | 2.336 | 97.9 | 37.9 |
| 1.900 | 2.410 | 97.5 | ||
| 5 (Cu2Er) | 1.979 | 2.309 | 98.0 | 37.8 |
| 1.900 | 2.401 | 97.2 |
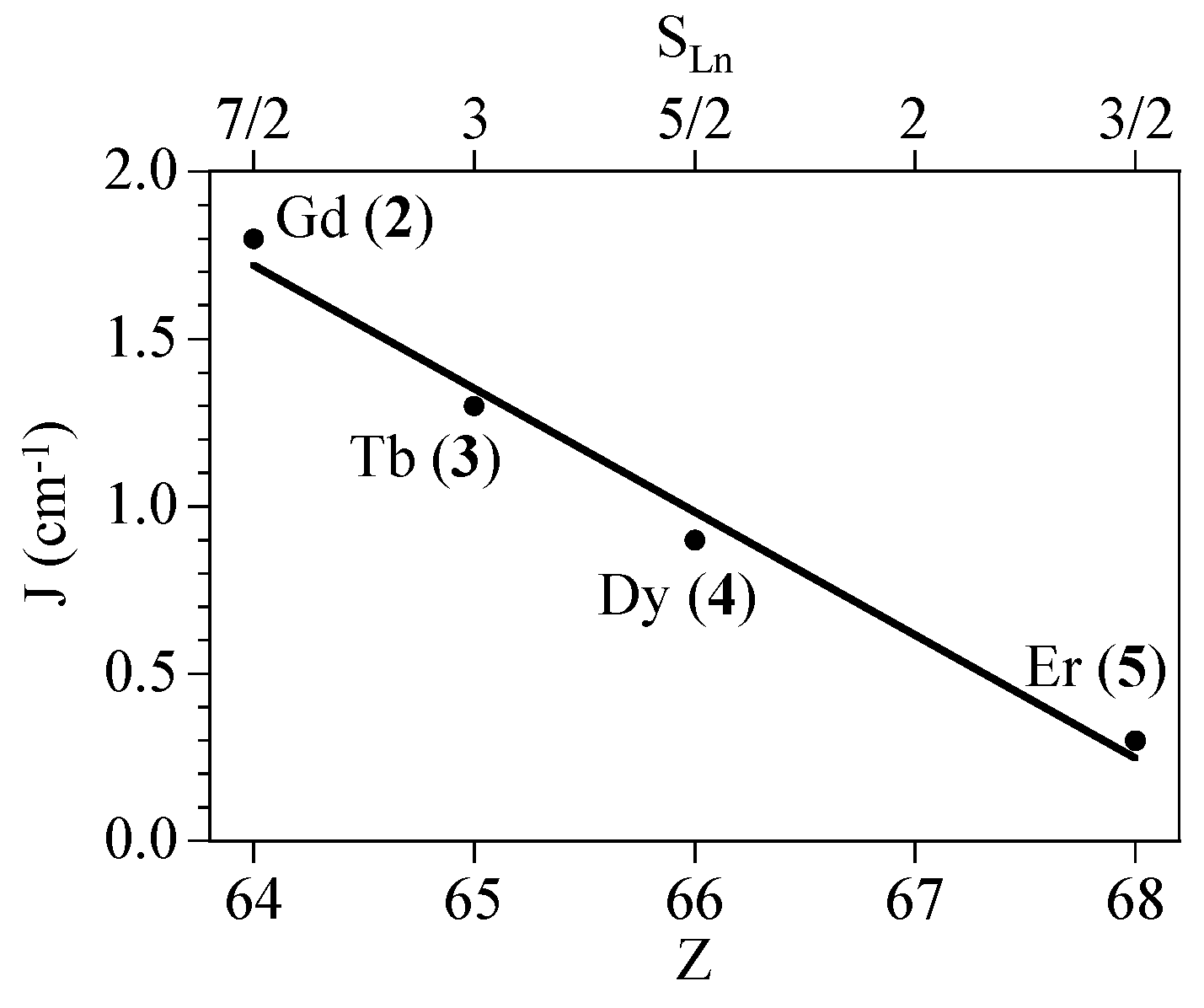
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of the Schiff Base Ligand H2Lα−Me and the Metalloligand [CuLα−Me]
3.3. Synthesis of Complex [(CuLα−Me)2Ce(NO3)3] (1)
3.4. Synthesis of Complexes [(CuLα−Me)2Ln(H2O)(NO3)2]·(NO3)·2(CH3OH) (Ln = Gd for 2, Tb for 3, Dy for 4 and Er for 5)
3.5. Physical Measurements
3.6. Magnetic Model
3.7. Crystallographic Data Collection and Refinement
4. Conclusions
Supplementary Files
Supplementary File 1Supplementary File 2Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A Tetranuclear 3d-4f single molecule magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Paulovič, J.; Cimpoesu, F.; Ferbinteanu, M.; Hirao, K. Mechanism of ferromagnetic coupling in Copper(II)-Gadolinium(III) complexes. J. Am. Chem. Soc. 2004, 126, 3321–3331. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.E.; Hughbanks, T. Magnetic coupling in dinuclear Gd complexes. J. Am. Chem. Soc. 2006, 128, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Benelli, C.; Gatteschi, D. Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Nyui, T.; Ishida, T.; Nogami, T.; Choi, K.-Y.; Nojiri, H. Oximate-bridged trinuclear Dy-Cu-Dy complex behaving as a single-molecule magnet and its mechanistic investigation. J. Am. Chem. Soc. 2006, 128, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.P.; Dahan, F.; Wernsdorfer, W. Heterodinuclear Cu-Tb single-molecule magnet. Inorg. Chem. 2006, 45, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Gatteschi, D.; Sessoli, R. Alternating current susceptibility, high field magnetization, and millimeter band EPR evidence for a ground S = 10 state in [Mn12O12(CH3COO)16(H2O)4]·2CH3COOH·4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5814. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Neese, F.; Pantazis, D.A. What is not required to make a single molecule magnet. Faraday Discuss. 2011, 148, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-R.; Clérac, R. Controlled association of single-molecule magnets (SMMs) into coordination networks: Towards a new generation of magnetic materials. Dalton Trans. 2012, 41, 9569–9586. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.-D.; Long, J.-R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Vostrikova, K.E. High-spin molecules based on metal complexes of organic free radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [Google Scholar] [CrossRef]
- Murakami, R.; Ishida, T.; Yoshii, S.; Nojiri, H. Single-molecule magnet [Tb(hfac)3(2pyNO)] (2pyNO = t-butyl 2-pyridyl nitroxide) with a relatively high barrier of magnetization reversal. Dalton Trans. 2013, 42, 13968–13973. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.K.; Wielechowski, D.P.; Vieru, V.; Chilton, N.F.; Moubaraki, B.; Abrahams, B.F.; Chibotaru, L.F.; Murray, K.S. A CrIII2DyIII2 single-molecule magnet: Enhancing the blocking temperature through 3d magnetic exchange. Angew. Chem. Int. Ed. 2013, 52, 12014–11201. [Google Scholar] [CrossRef] [PubMed]
- Kettles, F.J.; Milway, V.A.; Tuna, F.; Valiente, R.; Thomas, L.H.; Wernsdorfer, W.; Ochsenbein, S.T.; Murrie, M. Exchange interactions at the origin of slow relaxation of the magnetization in TbCu3 and DyCu3 single-molecule magnets. Inorg. Chem. 2014, 53, 8970–8978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-L.; Wu, J.-Y.; Chen, Y.-C.; Mereacre, V.; Powell, A.K.; Ungur, L.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. A heterometallic FeII–DyIII single-molecule magnet with a record anisotropy barrier. Angew. Chem. Int. Ed. 2014, 53, 12966–12970. [Google Scholar] [CrossRef] [PubMed]
- Bencini, A.; Benelli, C.; Caneschi, A.; Carlin, R.L.; Dei, A.; Gatteschi, D. Crystal and molecular structure of and magnetic coupling in two complexes containing gadolinium(III) and copper(II) ions. J. Am. Chem. Soc. 1985, 107, 8128–8136. [Google Scholar] [CrossRef]
- Guillou, O.; Bergerat, P.; Kahn, O.; Bakalbassis, E.; Boubekeur, K.; Batail, P.; Guillot, M. Ferromagnetically coupled GdIIICuII molecular material. Inorg. Chem. 1992, 31, 110–114. [Google Scholar] [CrossRef]
- Andruh, M.; Ramade, I.; Codjovi, E.; Guillou, O.; Kahn, O.; Trombe, J.C. Crystal structure and magnetic properties of [Ln2Cu4] hexanuclear clusters (where Ln = trivalent lanthanide). Mechanism of the Gd(III)-Cu(II) magnetic interaction. J. Am. Chem. Soc. 1993, 115, 1822–1829. [Google Scholar] [CrossRef]
- Sanz, J.L.; Ruiz, R.; Gleizes, A.; Lloret, F.; Faus, J.; Julve, M.; Borrás-Almenar, J.J.; Journaux, Y. Crystal structures and magnetic properties of novel [LnIIICuII4] (Ln = Gd, Dy, Ho) pentanuclear complexes. Topology and ferromagnetic interaction in the LnIII-CuII pair. Inorg. Chem. 1996, 35, 7384–7393. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Nagasato, S.; Sunatsuki, Y.; Matsumoto, N. A cyclic tetranuclear Cu2Gd2 complex with an S = 8 ground state derived from ferromagnetic spin-coupling between copper(II) and gadolinium(III) ions arrayed alternately. Chem. Commun. 2000, 2113–2114. [Google Scholar] [CrossRef]
- Sorace, L.; Sangregorio, C.; Figuerola, A.; Benelli, C.; Gatteschi, D. Magnetic interactions and magnetic anisotropy in exchange coupled 4f-3d systems: A case study of a heterodinuclear Ce3+-Fe3+ cyanide-bridged complex. Chem. Eur. J. 2009, 15, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.M. Rare-earth-iron exchange interaction in the garnets. I. Hamiltonian for anisotropic exchange interaction. Phys. Rev. 1964, 135, A155–A165. [Google Scholar] [CrossRef]
- Levy, P.M. Rare-earth-iron exchange interaction in the garnets. II. Exchange potential for ytterbium. Phys. Rev. 1966, 147, 311–319. [Google Scholar] [CrossRef]
- Kahn, M.L.; Sutter, J.P.; Golhen, S.; Guionneau, P.; Ouahab, L.; Kahn, O.; Chasseau, D. Systematic investigation of the nature of the coupling between a Ln(III) ion (Ln = Ce(III) to Dy(III)) and its aminoxyl radical ligands. Structural and magnetic characteristics of a series of Ln(organic radical)2 compounds and the related Ln(Nitrone)2 derivatives. J. Am. Chem. Soc. 2000, 122, 3413–3421. [Google Scholar]
- Okazawa, A.; Nogami, T.; Nojiri, H.; Ishida, T. Exchange coupling and energy-level crossing in a magnetic chain [Dy2Cu2]n evaluated by high-frequency electron paramagnetic resonance. Chem. Mater. 2008, 20, 3110–3119. [Google Scholar] [CrossRef]
- Dreiser, J.; Piamonteze, C.; Nolting, F.; Pedersen, K.S.; Bendix, J.; Rusponi, S.; Brune, H. XMCD study of the magnetic exchange coupling in a fluoride-bridged Dy-Cr molecular cluster. J. Korean Phys. Soc. 2013, 62, 1368–1371. [Google Scholar] [CrossRef]
- Huang, X.-C.; Vieru, V.; Chibotaru, L.F.; Wernsdorfer, W.; Jiang, S.-D.; Wang, X.-Y. Determination of magnetic anisotropy in a multinuclear TbIII-based single-molecule magnet. Chem. Commun. 2015, 51, 10373–10376. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.W.; Bünzli, J.-C.G. Polynuclear complexes of 3d and 4f transition metals. Synthesis and structure of lanthanide(III) adducts with copper and nickel Schiff's base complexes as ligands. Inorg. Chim. Acta 1985, 109, 185–192. [Google Scholar] [CrossRef]
- Bencini, A.; Benelli, C.; Caneschi, A.; Dei, A.; Gatteschi, D. Crystal and molecular structure and magnetic properties of a trinuclear complex containing exchange-coupled GdCu2 species. Inorg. Chem. 1986, 25, 572–575. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. Structural studies of a dinuclear (Cu, Gd) and two trinuclear (Cu2, Ln) complexes (Ln = Ce, Er). Magnetic properties of two original (Cu, Gd) complexes. New J. Chem. 1998, 22, 1525–1529. [Google Scholar] [CrossRef]
- Kahn, M.L.; Rajendiran, T.M.; Jeannin, Y.; Mathonière, C.; Kahn, O. LnIIICuII Schiff base compounds (Ln = Ce, Gd, Tb, Dy, Ho, Er): Structural and magnetic properties. C. R. Acad. Sci. IIC Chem. 2000, 3, 131–137. [Google Scholar] [CrossRef]
- Novitchi, G.; Shova, S.; Caneschi, A.; Costes, J.-P.; Gdaniec, M.; Stanica, N. Hetero di- and trinuclear Cu-Gd complexes with trifluoroacetate bridges: Synthesis, structural and magnetic studies. Dalton Trans. 2004, 8, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d-4f discrete complexes. Coord. Chem. Rev. 2015, 289–290, 74–122. [Google Scholar] [CrossRef]
- Rosado-Piquer, L.; Sañudo, E.C. Heterometallic 3d-4f single-molecule magnets. Dalton Trans. 2015, 44, 8771–8780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andruh, M. Compartmental Schiff-base ligands-a rich library of tectons in designing magnetic and luminescent materials. Chem. Commun. 2011, 47, 3025–3042. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ida, Y.; Ishida, T.; Ghosh, A. Linker stoichiometry-controlled stepwise supramolecular growth of a flexible Cu2Tb single molecule magnet from monomer to dimer to one-dimensional chain. Cryst. Growth Des. 2014, 14, 2588–2598. [Google Scholar] [CrossRef]
- Ida, Y.; Ghosh, S.; Ghosh, A.; Nojiri, H.; Ishida, T. strong ferromagnetic exchange interactions in hinge-like Dy(O2Cu)2 complexes involving double oxygen bridges. Inorg. Chem. 2015, 54, 9543–9555. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Saha, R.; Ghosh, A. Copper(II)−Mercury(II) heterometallic complexes derived from a salen-type ligand: A new coordination mode of the old Schiff base ligand. Organometallics 2012, 31, 3844–3850. [Google Scholar] [CrossRef]
- Biswas, S.; Naiya, S.; Gómez-García, C.J.; Ghosh, A. Synthesis of the first heterometalic star-shaped oxido-bridged MnCu3 complex and its conversion into trinuclear species modulated by pseudohalides (N3−, NCS− and NCO−): Structural analyses and magnetic properties. Dalton Trans. 2012, 41, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Das, L.K.; Kadam, R.M.; Bauzá, A.; Frontera, A.; Ghosh, A. differences in nuclearity, molecular shapes, and coordination modes of azide in the complexes of Cd(II) and Hg(II) with a “metalloligand” [CuL] (H2L = N,N′-Bis(salicylidene)-1,3-propanediamine): Characterization in solid and in solutions, and theoretical calculations. Inorg. Chem. 2012, 51, 12407–12418. [Google Scholar] [PubMed]
- Das, L.K.; Park, S.-W.; Cho, S.J.; Ghosh, A. An unprecedented “linear-bent” isomerism in tri-nuclear Cu2IIZnII complexes with a salen type di-Schiff base ligand. Dalton Trans. 2012, 41, 11009–11017. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Gómez-García, C.J.; Clemente-Juan, J.M.; Benmansour, S.; Ghosh, A. Supramolecular 2D/3D isomerism in a compound containing heterometallic CuII2CoII nodes and dicyanamide bridges. Inorg. Chem. 2014, 53, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Hazari, A.; Das, L.K.; Bauzá, A.; Frontera, A.; Ghosh, A. The influence of H-bonding on the ‘ambidentate’ coordination behaviour of the thiocyanate ion to Cd(II): A combined experimental and theoretical study. Dalton Trans. 2014, 43, 8007–8015. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Aromí, G.; Gamez, P.; Ghosh, A. The impact of anion-modulated structural variations on the magnetic coupling in trinuclear heterometallic CuII–CoII complexes derived from a salen-type Schiff base ligand. Eur. J. Inorg. Chem. 2014, 2014, 3341–3349. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A, 6th ed.; Wiley: New York, NY, USA, 2008; pp. 1–432. [Google Scholar]
- Bullock, J.I. Infrared spectra of some uranyl nitrate complexes. J. Inorg. Nucl. Chem. 1967, 29, 2257–2264. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Alvarez, S. Stereochemistry of compounds with coordination number ten. Chem. Eur. J. 2009, 15, 7470–7480. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Akine, S.; Matsumoto, T.; Taniguchi, T.; Nabeshima, T. Synthesis, structures, and magnetic properties of tri- and dinuclear copper(II)-Gadolinium(III) complexes of linear oligooxime ligands. Inorg. Chem. 2005, 44, 3270–3274. [Google Scholar] [CrossRef] [PubMed]
- Madalan, A.M.; Avarvari, N.; Fourmigué, M.; Clérac, R.; Chibotaru, L.F.; Clima, S.; Andruh, M. Heterospin systems constructed from [Cu2Ln]3+ and [Ni(mnt)2]1−,2− tectons: First 3p-3d-4f complexes (mnt = maleonitriledithiolato). Inorg. Chem. 2008, 47, 940–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiga, T.; Ohba, M.; Ōkawa, H. A series of trinuclear CuIILnIIICuII complexes derived from 2,6-di(acetoacetyl)pyridine: Synthesis, structure, and magnetism. Inorg. Chem. 2004, 43, 4435–4446. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tong, M.-L.; Chen, X.-M. Synthesis, structures, and magnetic properties of heteronuclear Cu(II)-Ln(III) (Ln = La, Gd, or Tb) complexes. Inorg. Chem. 2005, 44, 8285–8292. [Google Scholar] [CrossRef] [PubMed]
- Fellah, F.Z.C.; Costes, J.-P.; Dahan, F.; Duhayon, C.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- and triheteronuclear Cu-Gd and Cu-Gd-Cu complexes with dissymmetric double bridge. Inorg. Chem. 2008, 47, 6444–6451. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, B.; Miroslaw, B.; Kłak, J. N,N′-bis(5-bromo-2-hydroxy-3-methoxybenzylidene)-1,3-diaminopropane Cu-4f-Cu and Cu-4f complexes: Synthesis, crystal structures and magnetic properties. Polyhedron 2012, 34, 121–128. [Google Scholar] [CrossRef]
- Hu, K.; Wu, S.; Cui, A.; Kou, H. Synthesis, structure, and magnetic properties of heterotrimetallic tetranuclear complexes. Trans. Metal Chem. 2014, 39, 713–718. [Google Scholar]
- Benelli, C.; Borgogelli, E.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Paoli, P.; Rossi, P. Di-maltol-polyamine ligands to form heterotrinuclear metal complexes: Solid state, aqueous solution and magnetic characterization. Dalton Trans. 2013, 42, 5848–5859. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Okazawa, A.; Kojima, N.; Yoshii, S.; Nojiri, H.; Ishida, T. Ferromagnetic exchange couplings showing a chemical trend in Cu-Ln-Cu complexes (Ln = Gd, Tb, Dy, Ho, Er). Inorg. Chem. 2011, 50, 10555–10557. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Biswas, S.; Bauzá, A.; Barceló-Oliver, M.; Frontera, A.; Ghosh, A. Use of metalloligands [CuL] (H2L = salen type di-Schiff bases) in the formation of heterobimetallic copper(II)-uranyl complexes: Photophysical investigations, structural variations, and theoretical calculations. Inorg. Chem. 2013, 52, 7508–7523. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. High-nuclearity magnetic clusters: Generalized spin hamiltonian and its use for the calculation of the energy levels, bulk magnetic properties, and in elastic neutrón scattering spectra. Inorg. Chem. 1999, 38, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. MAGPACK1 A package to calcúlate the energy levels, bulk magnetic properties, and inelastic neutrón scattering spectra of high nuclearity spin clusters. J. Comput. Chem. 2001, 22, 985–991. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SAINT, Version 6.02, SADABS; Version 2.03; BrukerAXS, Inc.: Madison, WI, USA, 2002. [Google Scholar]
- Sheldrick, G.M. SHELXS 97, Program for Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL 97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Gómez García, C.J.; Clemente-Juan, J.M.; Ghosh, A. Key Role of Size and Electronic Configuration on the Sign and Strength of the Magnetic Coupling in a Series of Cu2Ln Trimers (Ln = Ce, Gd, Tb, Dy and Er). Magnetochemistry 2016, 2, 2. https://doi.org/10.3390/magnetochemistry2010002
Ghosh S, Gómez García CJ, Clemente-Juan JM, Ghosh A. Key Role of Size and Electronic Configuration on the Sign and Strength of the Magnetic Coupling in a Series of Cu2Ln Trimers (Ln = Ce, Gd, Tb, Dy and Er). Magnetochemistry. 2016; 2(1):2. https://doi.org/10.3390/magnetochemistry2010002
Chicago/Turabian StyleGhosh, Soumavo, Carlos J. Gómez García, Juan M. Clemente-Juan, and Ashutosh Ghosh. 2016. "Key Role of Size and Electronic Configuration on the Sign and Strength of the Magnetic Coupling in a Series of Cu2Ln Trimers (Ln = Ce, Gd, Tb, Dy and Er)" Magnetochemistry 2, no. 1: 2. https://doi.org/10.3390/magnetochemistry2010002
APA StyleGhosh, S., Gómez García, C. J., Clemente-Juan, J. M., & Ghosh, A. (2016). Key Role of Size and Electronic Configuration on the Sign and Strength of the Magnetic Coupling in a Series of Cu2Ln Trimers (Ln = Ce, Gd, Tb, Dy and Er). Magnetochemistry, 2(1), 2. https://doi.org/10.3390/magnetochemistry2010002