A Spin Crossover Transition in a Mn(II) Chain Compound
Abstract
:1. Introduction
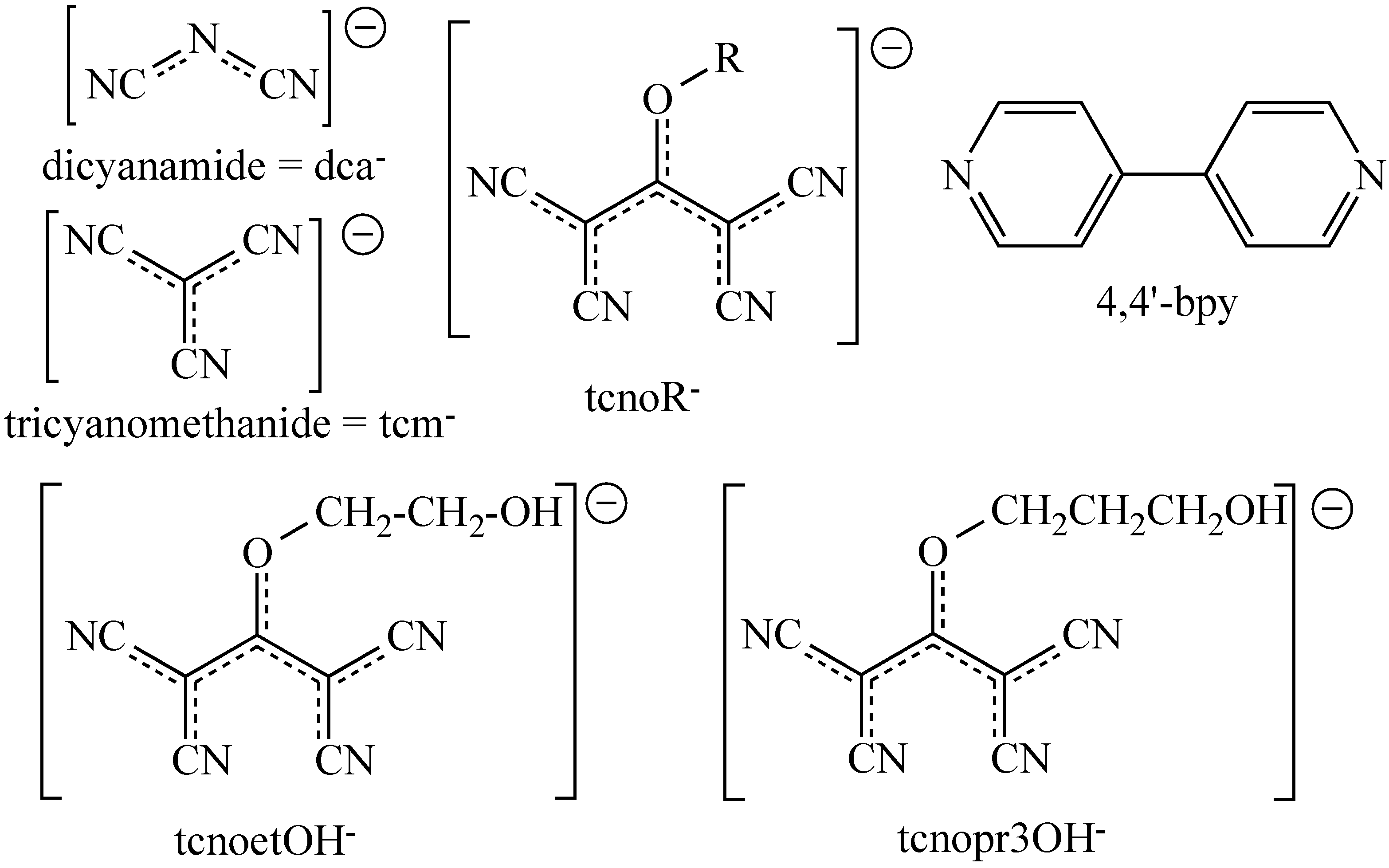
2. Results and Discussion
2.1. Syntheses and Spectroscopic Characterizations of the Complexes
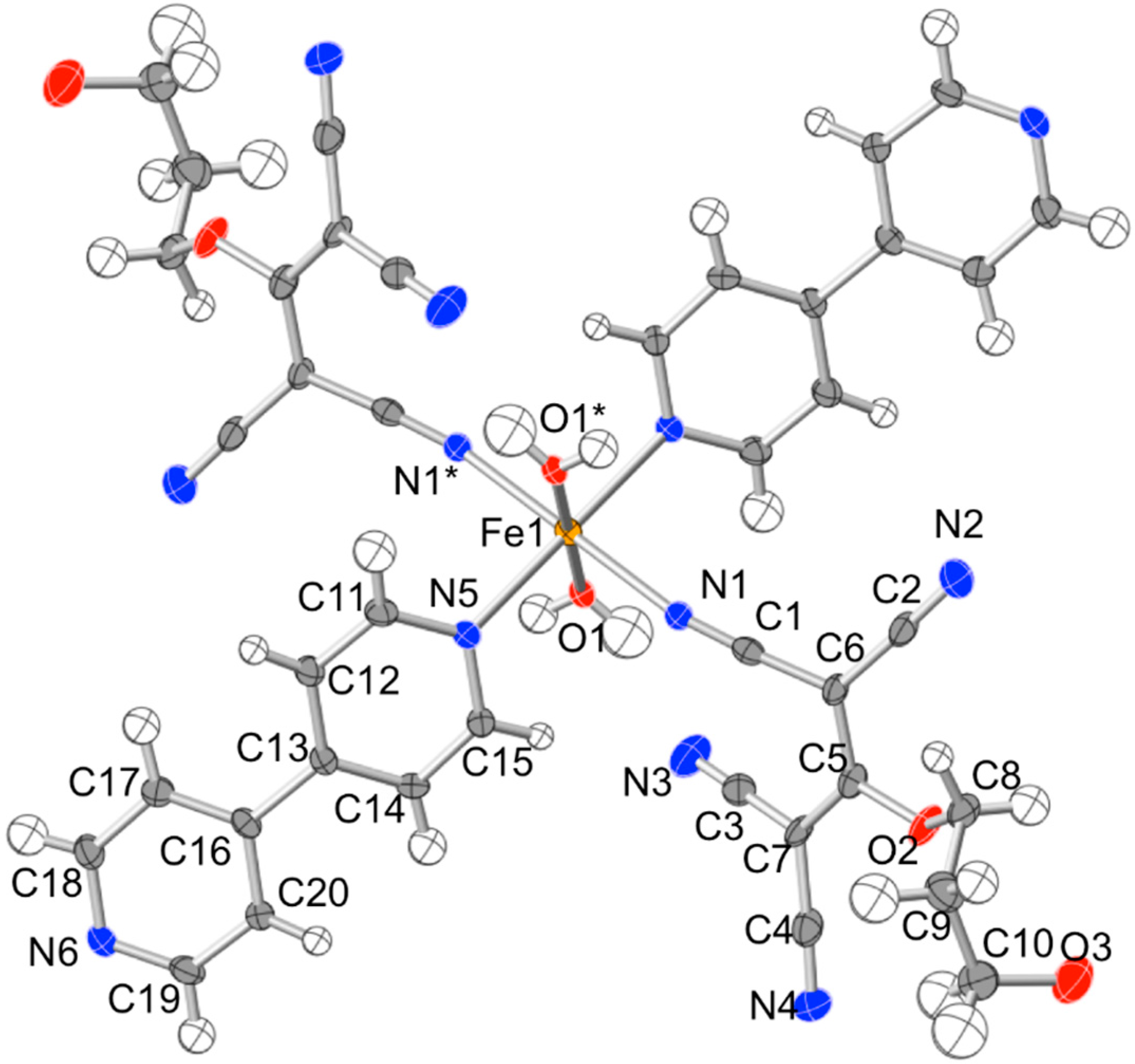
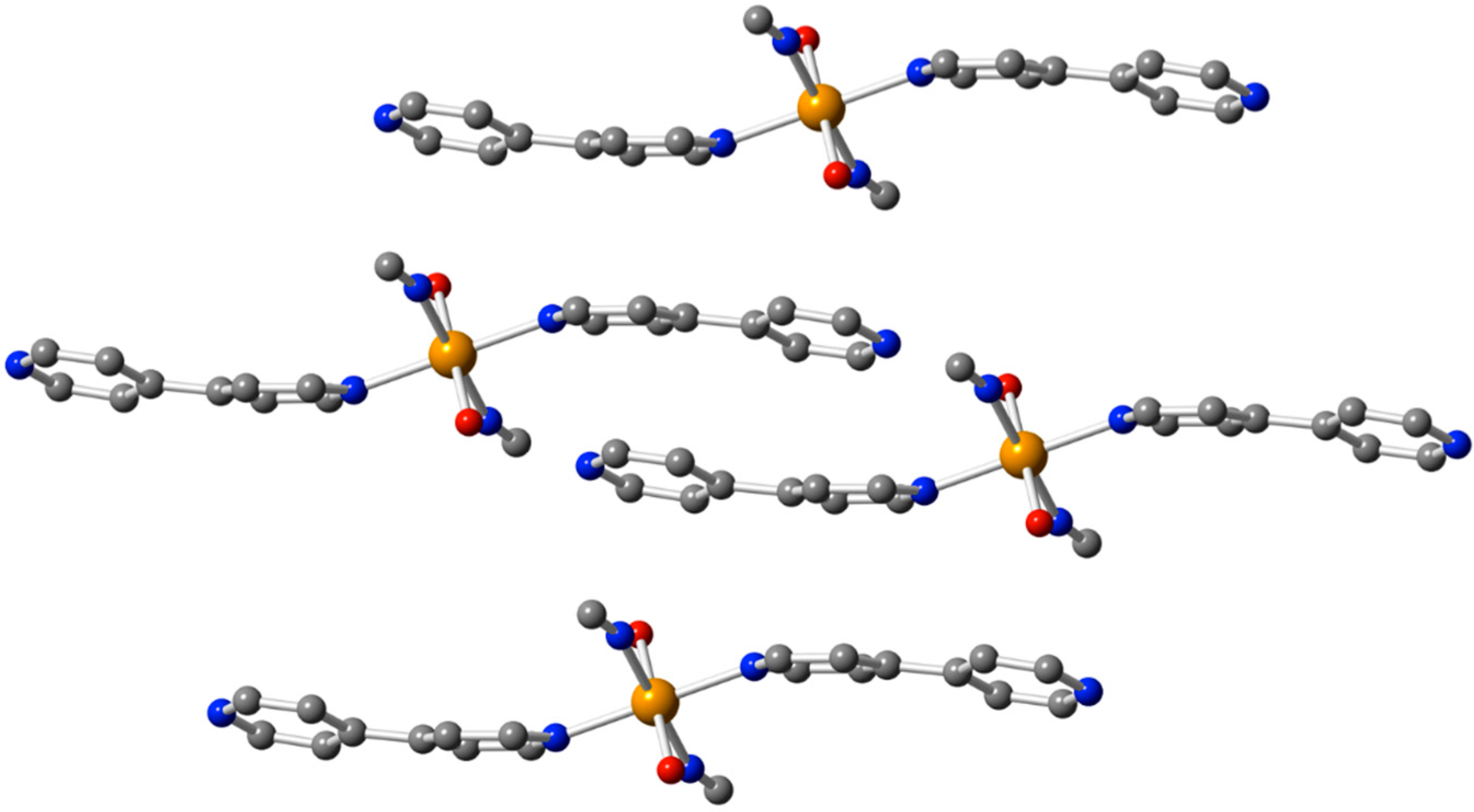
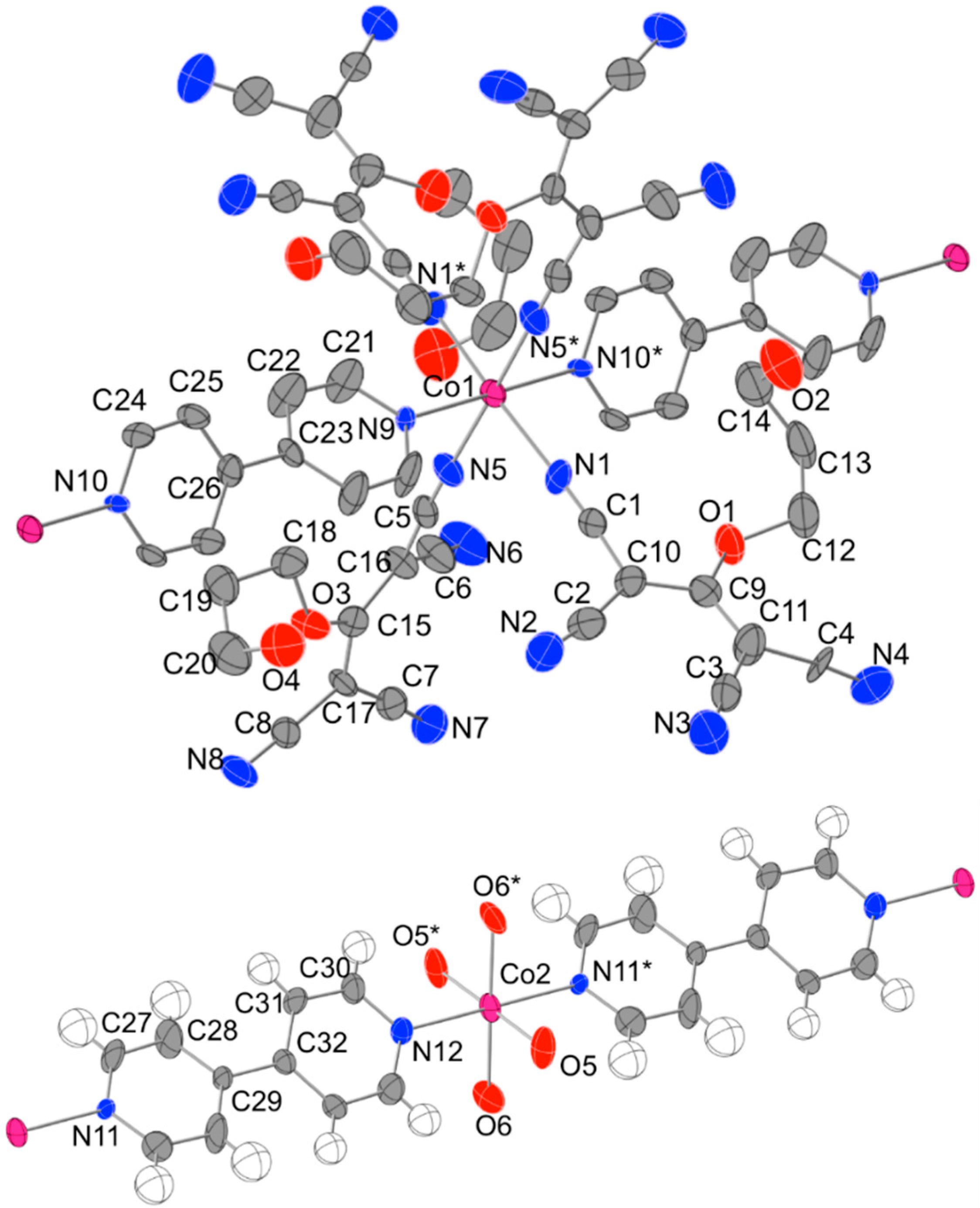
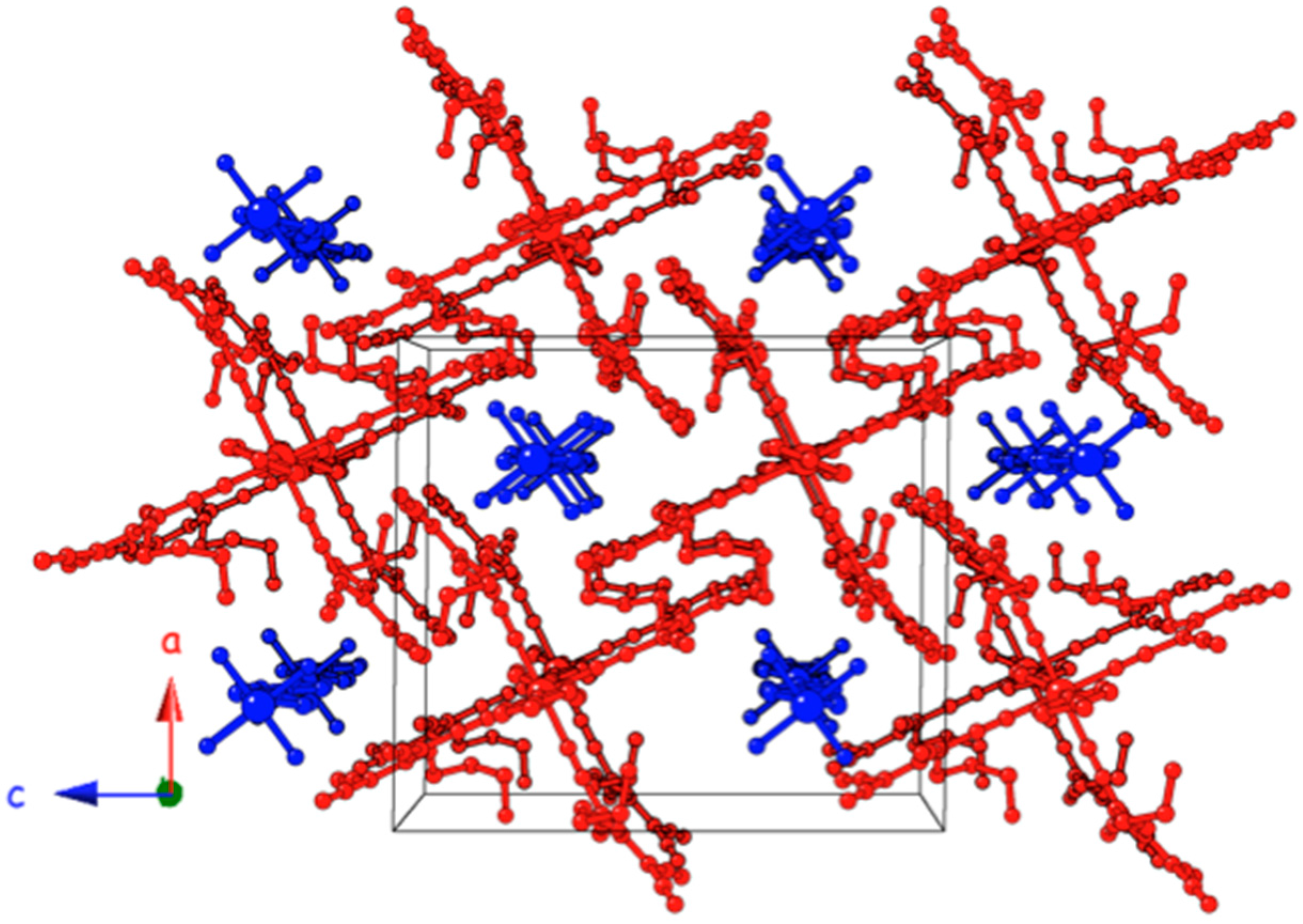
2.2. Description of the Structures
| Compound | Atoms | Distance (Å) | Atoms | Angle (°) |
|---|---|---|---|---|
| 1 | Fe–N1 | 2.182(2) | O1–Fe–N1 | 90.77(7) |
| Fe–N5 | 2.213(2) | O1–Fe–N5 | 90.15(6) | |
| Fe–O1 | 2.089(2) | N1–Fe–N5 | 91.46(7) | |
| 2 | Mn1–N1 | 2.083(2) | N10–Mn1–N9 | 180 |
| Mn1–N5 | 2.109(2) | N5–Mn1–N9 | 89.10(5) | |
| Mn1–N9 | 2.180(2) | N1–Mn1–N10 | 88.36(5) | |
| Mn1–N10 | 2.140(2) | N1–Mn1–N5 | 91.14(8) | |
| Mn2–N11 | 2.150(2) | N12–Mn2–N11 | 180 | |
| Mn2–N12 | 2.132(2) | O5–Mn2–N11 | 90.07(5) | |
| Mn2–O5 | 2.108(2) | O6–Mn2–N11 | 91.65(7) | |
| Mn2–O6 | 2.075(2) | O6–Mn2–O5 | 91.11(14) | |
| 3 | Co1–N1 | 2.082(4) | N10–Co1–N9 | 180 |
| Co1–N5 | 2.106(4) | N5–Co1–N9 | 88.90(11) | |
| Co1–N9 | 2.153(5) | N1–Co1–N10 | 88.32(10) | |
| Co1–N10 | 2.144(5) | N1–Co1–N5 | 91.32(14) | |
| Co2–N11 | 2.146(5) | N12–Co2–N11 | 180 | |
| Co2–N12 | 2.129(5) | O5–Co2–N11 | 90.30(11) | |
| Co2–O5 | 2.107(3) | O6–Co2–N11 | 89.52(11) | |
| Co2–O6 | 2.072(3) | O6–Co2–O5 | 91.11(14) |
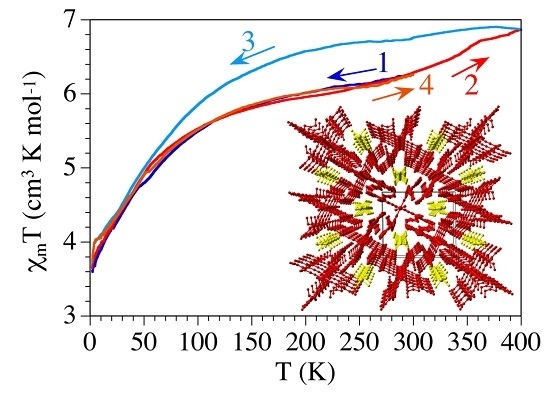
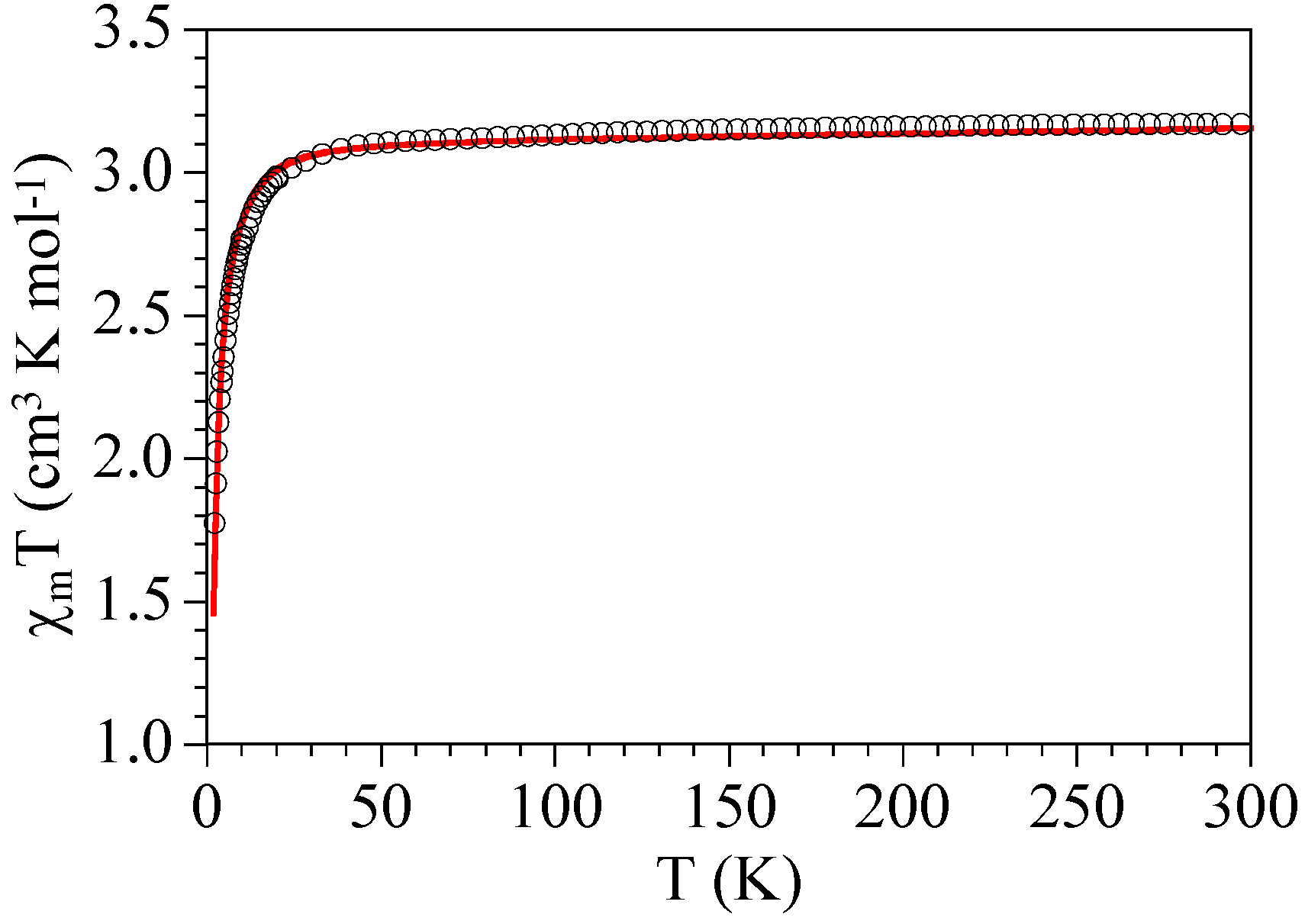
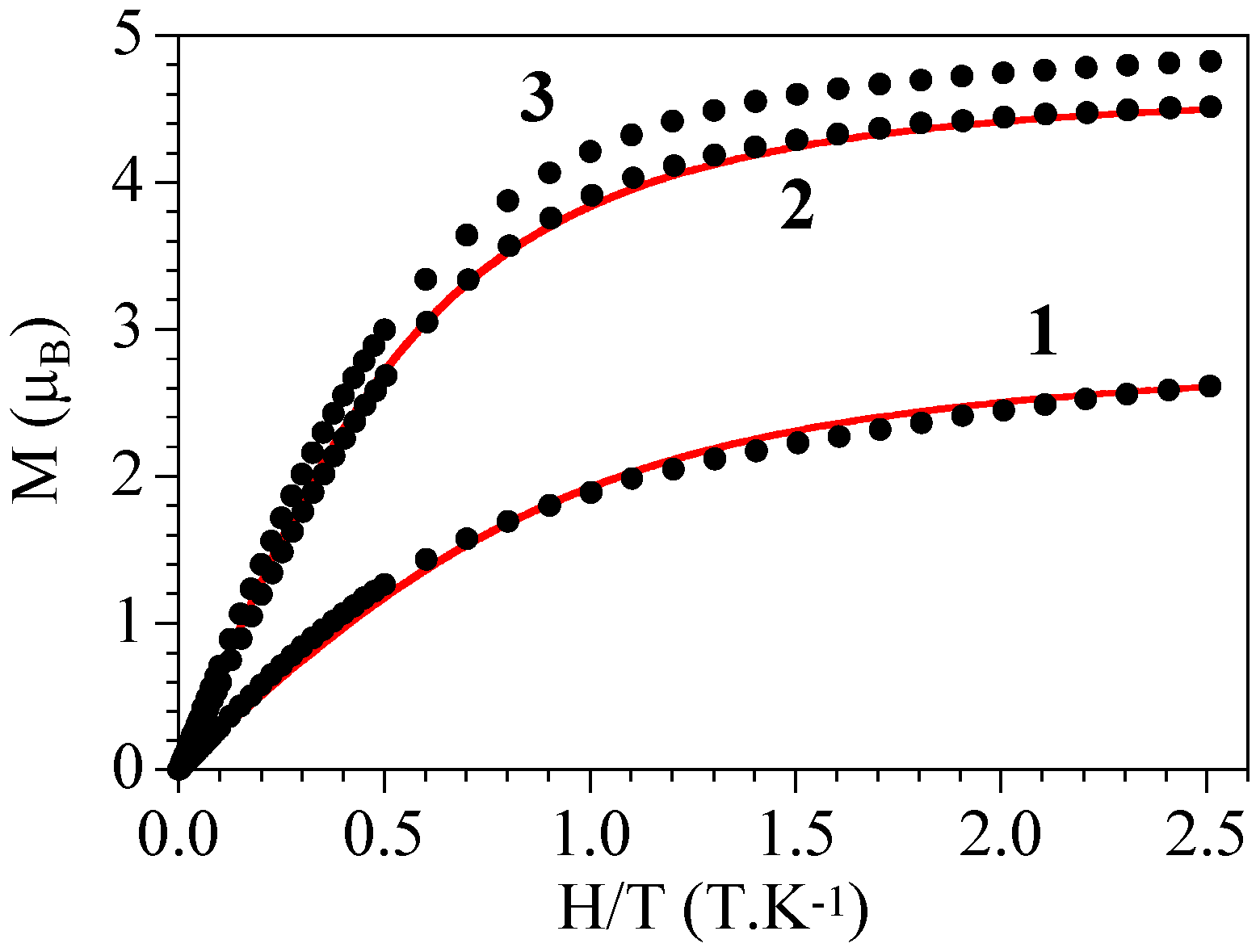
2.3. Magnetic Properties
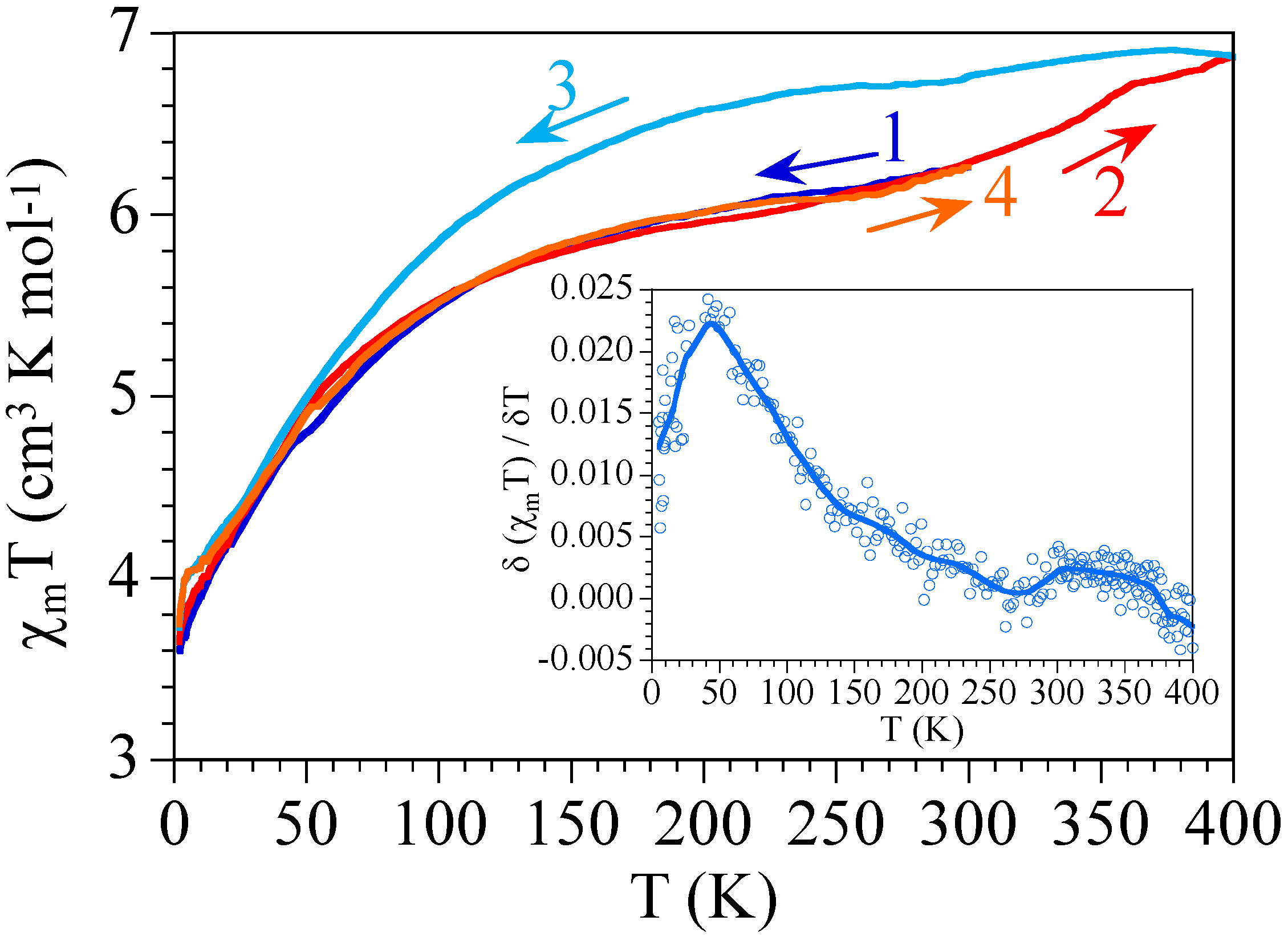
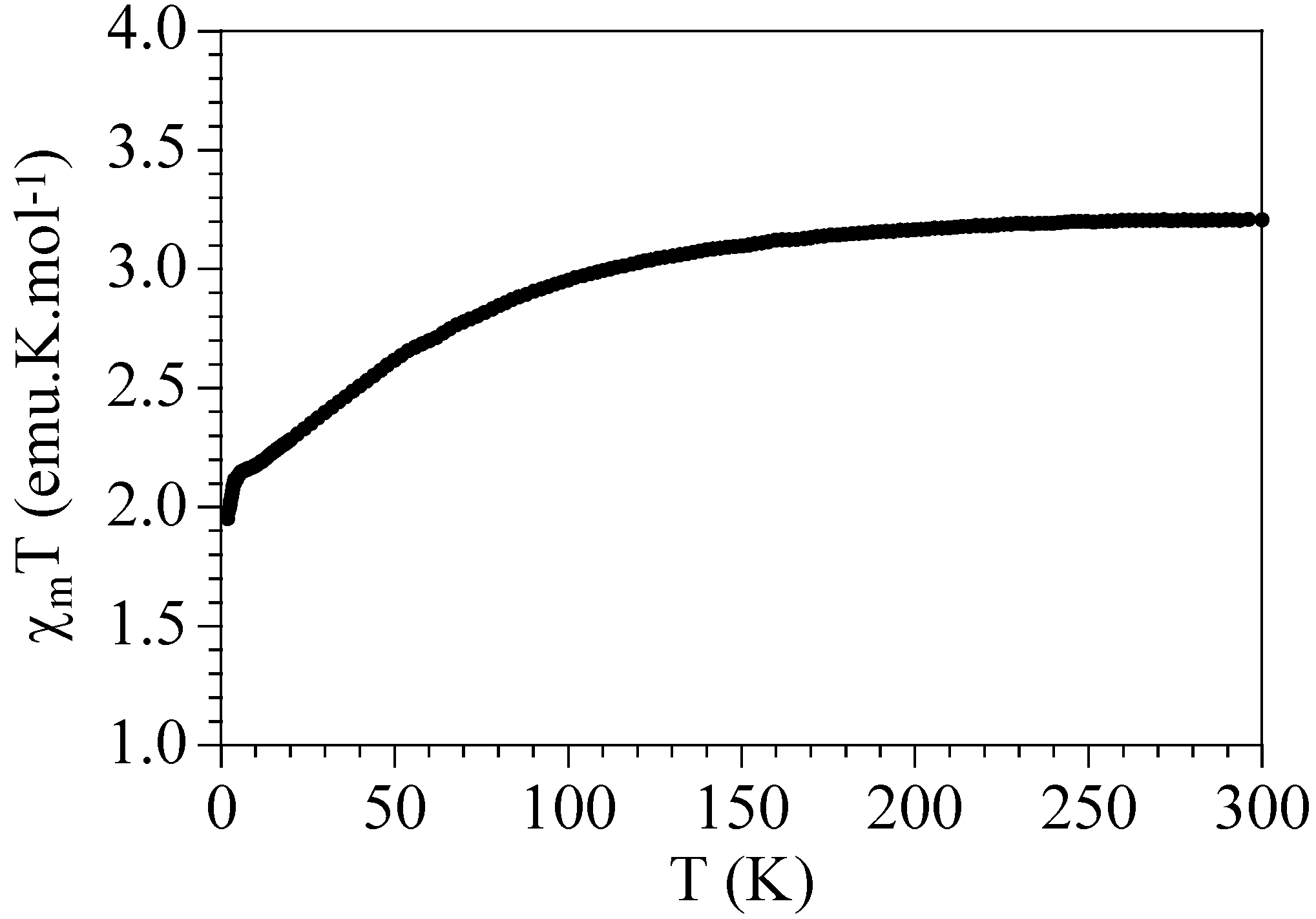
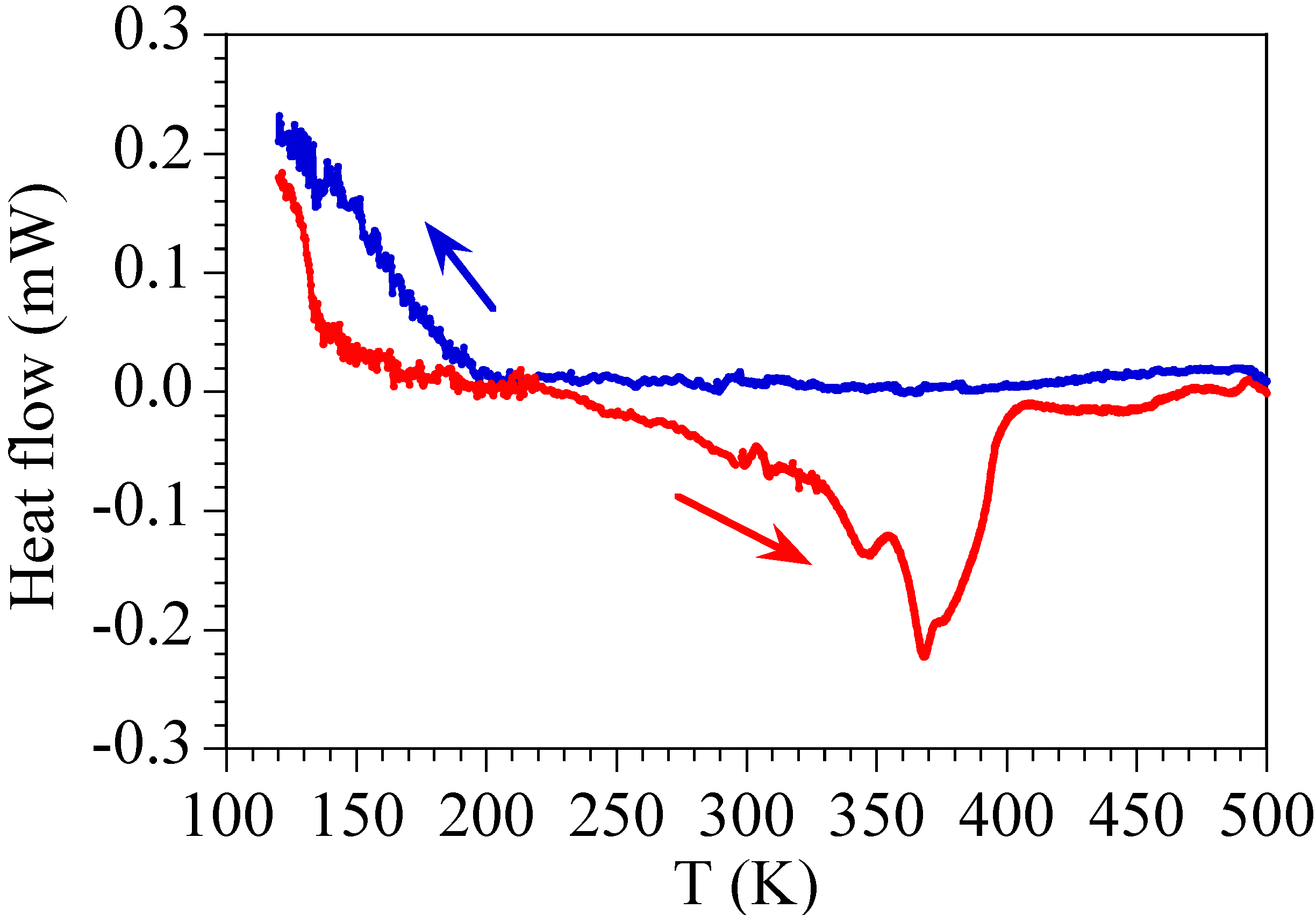
2.4. Differential Scanning Calorimetry
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of the 1,1,3,3-Tetracyano-2-(3-hydroxypropoxy)-propenide (tcnopr3OH−) Ligand
3.3. Synthesis of Compound [FeII(tcnopr3OH)2(H2O)2(4,4′-bpy)2] (1)
3.4. Synthesis of [M(H2O)4(µ-4,4′-bpy)][M(tcnoprOH)4(µ-4,4′-bpy)].3H2O (MII = Mn (2) and Co (3))
3.5. Physical Measurements
3.6. Crystallographic Data Collection and Refinement
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Formula | C40H34FeN8O6 | C60H58Mn2N20O15 | C60H58Co2N20O15 |
| F. Wt. | 778.58 | 1409.14 | 1417.12 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P-1 | P2/n | P2/n |
| a (Å) | 8.4903(2) | 16.1494(4) | 16.2245(8) |
| b (Å) | 9.4790(4) | 11.3855(2) | 11.3941(4) |
| c (Å) | 12.3652(5) | 18.0358(4) | 18.0389(8) |
| α (°) | 93.553(1) | 90.00 | 90.00 |
| β (°) | 105.405(3) | 90.594(2) | 90.693(5) |
| γ (°) | 91.720(3) | 90.00 | 90.00 |
| V (Å3) | 956.43(6) | 3316.05(12) | 3334.5(3) |
| Z | 2 | 2 | 2 |
| T (K) | 170 | 120 | 170 |
| ρcalc (g.cm−3) | 1.449 | 1.411 | 1.411 |
| μ (cm−1) | 0.460 | 0.461 | 0.577 |
| F(000) | 432 | 1456 | 1464 |
| q range (°) | 2.85–25.40 | 2.26–32.68 | 2.88–26.94 |
| Total reflections | 5153 | 126001 | 21361 |
| Unique reflections | 2339 | 11694 | 5381 |
| Rint | 0.0205 | 0.0899 | 0.0808 |
| Data with I > 2σ(I) | 1878 | 5479 | 2116 |
| Nv | 336 | 469 | 469 |
| a R1 | 0.029 | 0.0473 | 0.0441 |
| b wR2 | 0.0688 | 0.1341 | 0.1142 |
| c GooF | 1.000 | 0.867 | 0.875 |
| Δρmax,min (eÅ−3) | +0.263 | +0.887 | +0.360 |
| Δρmax,min (eÅ−3) | −0.197 | −0.577 | −0.213 |
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, Y.; Jiang, F.; Hong, M. Magnetic lanthanide–transition-Metal organic–inorganic Hybrid Materials: From Discrete Clusters to Extended Frameworks. Coord. Chem. Rev. 2009, 253, 2814–2834. [Google Scholar] [CrossRef]
- Weng, D.; Wang, Z.; Gao, S. Framework-Structured Weak Ferromagnets. Chem. Soc. Rev. 2011, 40, 3157–3181. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benmansour, S.; Espallargas, G.M.; Clemente-León, M.; Abhervé, A.; Gómez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A Family of Layered Chiral Porous Magnets Exhibiting Tunable Ordering Temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Givaja, G.; Amo-Ochoa, P.; Gómez-García, C.J.; Zamora, F. Electrical Conductive Coordination Polymers. Chem. Soc. Rev. 2012, 41, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Carlos, L.D.; Paz, F.A.A.; Ananias, D. Luminescent Multifunctional Lanthanides-Based Metal-Organic Frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, P.; Biswas, S.; Chattopadhyay, S.; Diaz, C.; Gómez-García, C.J.; Ghosh, A. Synthesis, Crystal Structure and Magnetic Properties of Two Alternating Double µ1,1 and µ1,3 Azido Bridged Cu(II) and Ni(II) Chains. Dalton Trans. 2014, 43, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, C.; Koner, S. Structural and Magnetic Studies on Copper(II) Azido Complexes. Coord. Chem. Rev. 2010, 254, 2933–2958. [Google Scholar] [CrossRef]
- Escuer, A.; Aromí, G. Azide as a Bridging Ligand and Magnetic Coupler in Transition Metal Clusters. Eur. J. Inorg. Chem. 2006, 2006, 4721–4736. [Google Scholar] [CrossRef]
- Escuer, A.; Esteban, J.; Perlepes, S.P.; Stamatatos, T.C. The Bridging Azido Ligand as a Central Player in High-Nuclearity 3d-Metal Cluster Chemistry. Coord. Chem. Rev. 2014, 275, 87–129. [Google Scholar] [CrossRef]
- Gu, Z.; Zuo, J.; You, X. A Three-Dimensional Ferromagnet Based on Linked Copper-Azido Clusters. Dalton Trans. 2007, 4067–4072. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.N.; Nihei, M.; Oshio, H. Cyanide-Bridged Molecular Squares? the Building Units of Prussian Blue. Eur. J. Inorg. Chem. 2011, 2011, 3031–3042. [Google Scholar] [CrossRef]
- Biswas, S.; Gómez-García, C.J.; Clemente-Juan, J.M.; Benmansour, S.; Ghosh, A. Supramolecular 2D/3D Isomerism in a Compound Containing Heterometallic CuII2CoII Nodes and Dicyanamide Bridges. Inorg. Chem. 2014, 53, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.R.; Chesman, A.S.R.; Murray, K.S.; Deacon, G.B.; Batten, S.R. The Chemistry and Complexes of Small Cyano Anions. Chem. Commun. 2011, 47, 10189–10210. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Murray, K.S. Structure and Magnetism of Coordination Polymers Containing Dicyanamide and Tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Okawa, H. Design of Metal-Complex Magnets. Syntheses and Magnetic Properties of Mixed-Metal Assemblies {NBu4[MCr(ox)3]}x (NBu4+ = Tetra(n-Butyl)Ammonium Ion; ox2− = Oxalate Ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Kumar, G.; Gupta, R. Molecularly Designed Architectures—The Metalloligand Way. Chem. Soc. Rev. 2013, 42, 9403–9453. [Google Scholar] [CrossRef] [PubMed]
- Das, L.K.; Gómez-García, C.J.; Drew, M.G.B.; Ghosh, A. Playing with Different Metalloligands [NiL] and Hg to [NiL] Ratios to Tune the Nuclearity of Ni(II)-Hg(II) Complexes: Formation of di-, tri-, hexa- and nona-Nuclear Ni-Hg Clusters. Polyhedron 2015, 87, 311–320. [Google Scholar] [CrossRef]
- Das, L.K.; Gómez-García, C.; Ghosh, A. Influence of the Central Metal Ion in Controlling the Self-Assembly and Magnetic Properties of 2D Coordination Polymers Derived from (NiL)2M]2+ Nodes (M = Ni, Zn and Cd) (H2L = Salen-Type Di-Schiff Base) and Dicyanamide Spacers. Dalton Trans. 2015, 44, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Thetiot, F.; Triki, S.; Pala, J.S.; Gómez-García, C.J. New Coordination Polymers with a 2,2-Dicyano-1-Ethoxyethenolate (Dcne−) Bridging Ligand: Syntheses, Structural Characterisation and Magnetic Properties of [M(Dcne)2(H2O)2] (M = MnII, FeII, CoII, NiII and ZnII) and [Cu(Dcne)2(H2O)]. J. Chem. Soc. Dalton Trans. 2002, 1687–1693. [Google Scholar] [CrossRef]
- Benmansour, S.; Setifi, F.; Triki, S.; Salaun, J.Y.; Vandevelde, F.; Sala-Pala, J.; Gómez-García, C.J.; Roisnel, T. New Multidimensional Coordination Polymers with µ2- and µ3-Dcno Cyano Carbanion Ligand {dcno− = [(NC)2CC(O)O(CH2)2OH]−}. Eur. J. Inorg. Chem. 2007, 186–194. [Google Scholar] [CrossRef]
- Benmansour, S.; Setifi, F.; Gómez-García, C.J.; Triki, S.; Coronado, E.; Salaun, J.Y. A Novel Polynitrile Ligand with Different Coordination Modes: Synthesis, Structure and Magnetic Properties of the Series [M(tcnoprOH)2(H2O)2] (M = Mn, Co and Cu) (tcnoprOH− = [(NC)2CC(OCH2CH2CH2OH)C(CN)2]−). J. Mol. Struct. 2008, 890, 255–262. [Google Scholar] [CrossRef]
- Atmani, C.; Setifi, F.; Benmansour, S.; Triki, S.; Marchivie, M.; Salaun, J.Y.; Gómez-García, C.J. New Planar Polynitrile Dianion and its First Coordination Polymer with Unexpected Short M···M Contacts (Tcno2− = [(NC)2CC(O)C(CN)2]2−). Inorg. Chem. Commun. 2008, 11, 921–924. [Google Scholar] [CrossRef]
- Kremer, C.; Melián, C.; Torres, J.; Juanicó, M.P.; Lamas, C.; Pezaroglo, H.; Manta, E.; Schumann, H.; Pickardt, J.; Girgsdies, F.; et al. Synthesis, Structure and Magnetic Properties of Mn(II) and Cu(II) Complexes with the Dicyano-Acetic Acid Methyl Ester Anion. Inorg. Chim. Acta 2001, 314, 83–90. [Google Scholar] [CrossRef]
- Thétiot, F.; Triki, S.; Pala, J.S. Polynitriles as Ligands: New Coordination Polymers with the 1,1,3,3-Tetracyano-2-Ethoxypropenide (Tcnp−) Bridging Ligand. Polyhedron 2003, 22, 1837–1843. [Google Scholar] [CrossRef]
- Triki, S.; Pala, J.S.; Decoster, M.; Molinié, P.; Toupet, L. Novel Infinite Three-Dimensional Networks with Highly Conjugated Polynitrile Ligands: Syntheses, Crystal Structures, and Magnetic Properties of [Cu{C[C(CN)2]3}(H2O)2]n and [Cu{C[C(CN)2]3}(en)]n (en = NH2CH2CH2NH2). Angew. Chem. Int. Ed. 1999, 38, 113–115. [Google Scholar] [CrossRef]
- Benmansour, S.; Atmani, C.; Setifi, F.; Triki, S.; Marchivie, M.; Gómez-García, C.J. Polynitrile Anions as Ligands: From Magnetic Polymeric Architectures to Spin Crossover Materials. Coord. Chem. Rev. 2010, 254, 1468–1478. [Google Scholar] [CrossRef]
- Benmansour, S.; Setifi, F.; Triki, S.; Gómez-García, C.J. Linkage Isomerism in Coordination Polymers. Inorg. Chem. 2012, 51, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Setifi, F.; Gómez-García, C.J.; Triki, S.; Coronado, E. New Coordination Polymers Based on a Novel Polynitrile Ligand: Synthesis, Structure and Magnetic Properties of the Series [M(tcnoetOH)2(4,4′-Bpy)(H2O)2] (tcnoetOH− =[(NC)2CC(OCH2CH2OH)C(CN)2]−; M = Fe, Co and Ni). Inorg. Chim. Acta 2008, 361, 3856–3862. [Google Scholar] [CrossRef]
- Shannon, R. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- O’Connor, C.J. Magnetochemistry-Advances in Theory and Experimentation. Prog. Inorg. Chem. 1982, 29, 203–283. [Google Scholar]
- Boca, R. Zero-Field Splitting in Metal Complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Basu, P.; Chakravorty, A. Low-Spin Tris(Quinone Oximates) of Manganese(II,III). Synthesis, Isomerism, and Equilibria. Inorg. Chem. 1992, 31, 4980–4986. [Google Scholar] [CrossRef]
- Karmakar, S.; Choudhury, S.; Chakravorty, A. Thioether Binding of Low-Spin Bivalent Manganese. A MS2N4 Family Furnished by New Hexadentate Thioether-Oxime-Azo Ligands (M = MnII, FeII, FeIII). Inorg. Chem. 1994, 33, 6148–6153. [Google Scholar] [CrossRef]
- Ganguly, S.; Karmakar, S.; Pal, C.; Chakravorty, A. Regiospecific Oximato Coordination at the Oxygen Site: Ligand Design and Low-Spin Mn and Fe Species. Inorg. Chem. 1999, 38, 5984–5987. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Majumdar, P.; Goswami, S. Low-Spin Manganese(II) and Cobalt(III) Complexes of N-Aryl-2-Pyridylazophenylamines: New Tridentate N,N,N-Donors Derived from Cobalt Mediated Aromatic Ring Amination of 2-(Phenylazo)Pyridine. Crystal Structure of a Manganese(II) Complex. Dalton Trans. 2000, 1703–1708. [Google Scholar] [CrossRef]
- Knof, U.; Weyhermuller, T.; Wolter, T.; Wieghardt, K. Synthesis of Low Spin [Mn(L2)2]I2·2MeOH and [Cu(L1)] Via Condensation of S-Methylisothiosemicarbazide and Pentane-2,4-Dione in the Presence of Air. J. Chem. Soc. Chem. Commun. 1993, 726–728. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Basu, P.; Pal, S.; Chakravorty, A. Synthesis and Structure of a Trinuclear Manganese(II) Complex Containing Low-Spin Metal. J. Chem. Soc. Dalton Trans. 1990, 3829–3833. [Google Scholar] [CrossRef]
- Cotton, F.A.; Monchamp, R.; Henry, R.; Young, R. The Preparation and Properties of a New Pentacyanomanganesenitric Oxide Anion, [Mn(CN)5NO]2−, and some Observations on Other Pentacyanonitrosyl Complexes. J. Inorg. Nucl. Chem. 1959, 10, 28–38. [Google Scholar] [CrossRef]
- Entley, W.R.; Girolami, G. New Three-Dimensional Ferrimagnetic Materials: K2Mn[Mn(CN)6], Mn3[Mn(CN)6]2·12H2O, and CsMn[Mn(CN)6]·1/2H2O. Inorg. Chem. 1994, 33, 5165–5166. [Google Scholar] [CrossRef]
- Ohba, M.; Ōkawa, H. Synthesis and Magnetism of Multi-Dimensional Cyanide-Bridged Bimetallic Assemblies. Coord. Chem. Rev. 2000, 198, 313–328. [Google Scholar] [CrossRef]
- Goodwin, H.A.; Gutlich, P. Spin Crossover in Transition Metal Compounds II Thermal Spin Crossover in Mn(II), Mn(III), Cr(II) and Co(III) Coordination Compounds. Top. Curr. Chem. 2004, 234, 49–62. [Google Scholar]
- Scheuermayer, S.; Tuna, F.; Bodensteiner, M.; Scheer, M.; Layfield, R.A. Spin Crossover in Phosphorus- and Arsenic-Bridged Cyclopentadienyl-Manganese(II) Dimers. Chem. Commun. 2012, 48, 8087–8089. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Tokoro, H.; Utsunomiya, M.; Mizuno, M.; Abe, M. Observation of Spin Transition in an Octahedrally Coordinated Manganese(II) Compound. J. Phys. Chem. B 2002, 106, 2423–2425. [Google Scholar] [CrossRef]
- Dupouy, G.; Marchivie, M.; Triki, S.; Sala-Pala, J.; Gómez-García, C.J.; Pillet, S.; Lecomte, C.; Letard, J.F. Photoinduced HS State in the First Spin-Crossover Chain Containing a Cyanocarbanion as Bridging Ligand. Chem. Commun. 2009, 3404–3406. [Google Scholar] [CrossRef] [PubMed]
- Atmani, C.; El Hajj, F.; Benmansour, S.; Marchivie, M.; Triki, S.; Conan, F.; Patinec, V.; Handel, H.; Dupouy, G.; Gómez-García, C.J. Guidelines to Design New Spin Crossover Materials. Coord. Chem. Rev. 2010, 254, 1559–1569. [Google Scholar] [CrossRef]
- Dupouy, G.; Triki, S.; Marchivie, M.; Cosquer, N.; Gómez-García, C.J.; Pillet, S.; Bendeif, E.; Lecomte, C.; Asthana, S.; Létard, J.F. Cyanocarbanion-Based Spin-Crossover Materials: Photocrystallographic and Photomagnetic Studies of a New Iron(II) Neutral Chain. Inorg. Chem. 2010, 49, 9358–9368. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Peng, J.; Gómez-García, C.J.; Benmansour, S.; Gu, X.J. Influence of Metal Ions on the Structures of Keggin Polyoxometalate-Based Solids: Hydrothermal Syntheses, Crystal Structures and Magnetic Properties. J. Solid State Chem. 2006, 179, 253–265. [Google Scholar] [CrossRef]
- Humphrey, S.M.; Alberola, A.; Gómez-García, C.J.; Wood, P.T. A New Co(II) Coordination Solid with Mixed Oxygen, Carboxylate, Pyridine and Thiolate Donors Exhibiting Canted Antiferromagnetism with TC ≈ 68 K. Chem. Commun. 2006, 1607–1609. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, C.J.; Coronado, E.; Borrás-Almenar, J.J. Magnetic Characterization of Tetranuclear Copper(II) and Cobalt(II) Exchange-Coupled Clusters Encapsulated in Heteropolyoxotungstate Complexes. Study of the Nature of the Ground States. Inorg. Chem. 1992, 31, 1667–1673. [Google Scholar] [CrossRef]
- Liu, H.S.; Gómez-García, C.J.; Peng, J.; Sha, J.Q.; Wang, L.X.; Yan, Y.C. A Co-Monosubstituted Keggin Polyoxometalate with an Antenna Ligand and Three Cobalt(II) Chains as Counterion. Inorg. Chim. Acta 2009, 362, 1957–1962. [Google Scholar] [CrossRef]
- Lloret, F.; Julve, M.; Cano, J.; Ruiz-García, R.; Pardo, E. Magnetic Properties of Six-Coordinated High-Spin Cobalt(II) Complexes: Theoretical Background and its Application. Inorg. Chim. Acta 2008, 361, 3432–3445. [Google Scholar] [CrossRef]
- Mosconi, E.; Yum, J.; Kessler, F.; Gómez García, C.J.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Grätzel, M.; de Angelis, F. Cobalt electrolyte/dye Interactions in Dye-Sensitized Solar Cells: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 171.33.55; Oxford Diffraction Ltd.: Oxford, UK, 2004.
- Sheldrick, M. SHELX97: Programs for Crystal Structure Analysis; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- International Tables for X-ray Crystallography; Kyn och Press: Birmingham, UK, 1975.
- Crysalis-Ccd, Version 170; Oxford Diffraction Ltd.: Oxford, UK, 2002.
- Crysalis-Red, Version 170; Oxford Diffraction Ltd.: Oxford, UK, 2002.
- Farrugia, L.J. WinGX Suite for Small-Molecule Single-Crystal Crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmansour, S.; Triki, S.; Gómez-García, C.J. A Spin Crossover Transition in a Mn(II) Chain Compound. Magnetochemistry 2016, 2, 1. https://doi.org/10.3390/magnetochemistry2010001
Benmansour S, Triki S, Gómez-García CJ. A Spin Crossover Transition in a Mn(II) Chain Compound. Magnetochemistry. 2016; 2(1):1. https://doi.org/10.3390/magnetochemistry2010001
Chicago/Turabian StyleBenmansour, Samia, Smail Triki, and Carlos J. Gómez-García. 2016. "A Spin Crossover Transition in a Mn(II) Chain Compound" Magnetochemistry 2, no. 1: 1. https://doi.org/10.3390/magnetochemistry2010001
APA StyleBenmansour, S., Triki, S., & Gómez-García, C. J. (2016). A Spin Crossover Transition in a Mn(II) Chain Compound. Magnetochemistry, 2(1), 1. https://doi.org/10.3390/magnetochemistry2010001