Synthesis, X-Ray Crystal Structures, and Magnetic Properties of a Series of Trinuclear Rare-Earth Hepta-Chloride Clusters †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Syntheses of Complex RE3
2.3. Crystal Structure Determination
2.4. Magnetic Measurements
2.5. Ab Initio Calculations
3. Results and Discussion
3.1. Syntheses and Structural Characterization
3.2. Static-Field Magnetic Susceptibilities for RE3
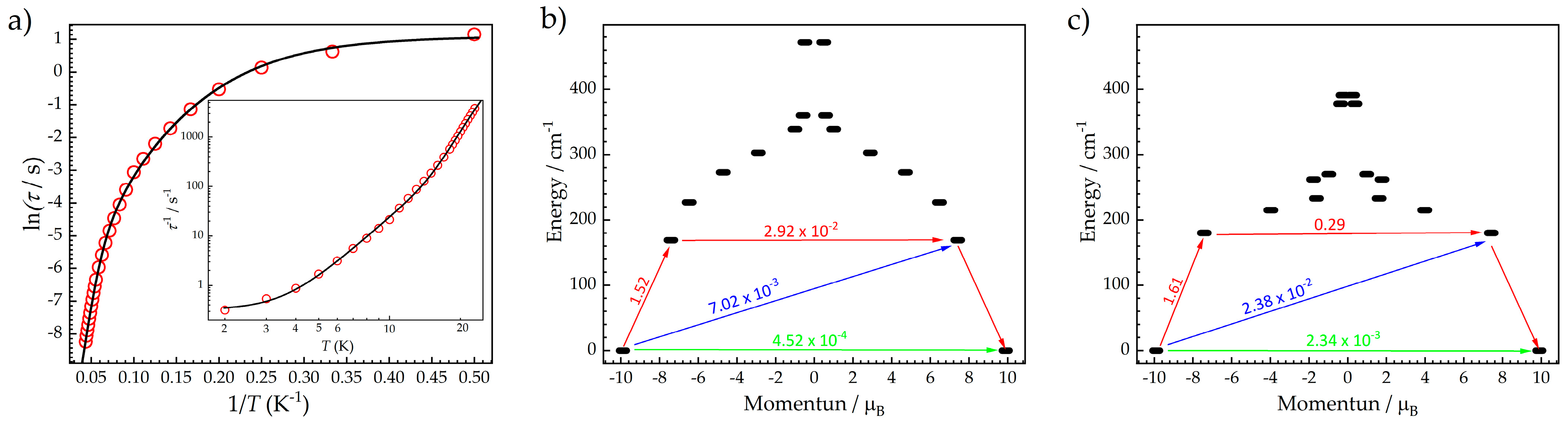
3.3. Dynamic Magnetic Susceptibilities for Dy3
3.4. Ab Initio Calculations for Dy3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, G.; Birmingham, J.M. Cyclopentadienyl compounds of Sc, Y, La, Ce and some lanthanide elements. J. Am. Chem. Soc. 1954, 76, 6210. [Google Scholar] [CrossRef]
- Zimmermann, M.; Anwander, R. Homoleptic rare-earth metal complexes containing Ln–C σ-Bonds. Chem. Rev. 2010, 110, 6194–6259. [Google Scholar] [CrossRef]
- Harriman, K.L.M.; Murugesu, M. An organolanthanide building block approach to single-molecule magnets. Acc. Chem. Res. 2016, 49, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, M.; Guo, F.; Hou, Z. Half-sandwich rare-earth-catalyzed olefin polymerization, carbometalation, and hydroarylation. Acc. Chem. Res. 2015, 48, 2209–2220. [Google Scholar] [CrossRef]
- Day, B.M.; Guo, F.-S.; Layfield, R.A. Cyclopentadienyl ligands in lanthanide single-molecule magnets: One ring to rule them all? Acc. Chem. Res. 2018, 51, 1880–1889. [Google Scholar] [CrossRef]
- Ortu, F. Rare earth starting materials and methodologies for synthetic chemistry. Chem. Rev. 2022, 122, 6040–6116. [Google Scholar] [CrossRef]
- Münzfeld, L.; Gillhuber, S.; Hauser, A.; Lebedkin, S.; Hädinger, P.; Knöfel, N.D.; Zovko, C.; Gamer, M.T.; Weigend, F.; Kappes, M.M.; et al. Synthesis and properties of cyclic sandwich compounds. Nature 2023, 620, 92–96. [Google Scholar] [CrossRef]
- Gould, C.A.; McClain, K.R.; Reta, D.; Kragskow, J.G.C.; Marchiori, D.A.; Lachman, E.; Choi, E.-S.; Analytis, J.G.; Britt, R.D.; Chilton, N.F.; et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal-metal bonding. Science 2022, 375, 198–202. [Google Scholar] [CrossRef] [PubMed]
- McClain, K.R.; Kwon, H.; Chakarawet, K.; Nabi, R.; Kragskow, J.G.C.; Chilton, N.F.; Britt, R.D.; Long, J.R.; Harvey, B.G. A trinuclear gadolinium cluster with a three-center one-electron bond and an S = 11 ground state. J. Am. Chem. Soc. 2023, 145, 8996–9002. [Google Scholar] [CrossRef]
- Wedal, J.C.; Anderson-Sanchez, L.M.; Dumas, M.T.; Gould, C.A.; Beltrán-Leiva, M.J.; Celis-Barros, C.; Páez-Hernández, D.; Ziller, J.W.; Long, J.R.; Evans, W.J. Synthesis and crystallographic characterization of a reduced bimetallic yttrium ansa-metallocene hydride complex, [K(crypt)][(μ-CpAn)Y(μ-H)]2 (CpAn = Me2Si[C5H3(SiMe3)-3]2), with a 3.4 Å yttrium-yttrium distance. J. Am. Chem. Soc. 2023, 145, 10730–10742. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Deng, C.; Sun, R.; Fu, P.-X.; Wang, B.-W.; Gao, S.; Huang, W. Two-electron oxidations at a single cerium center. J. Am. Chem. Soc. 2023, 145, 22466–22474. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; McClain, K.R.; Kragskow, J.G.C.; Staab, J.K.; Ozerov, M.; Meihaus, K.R.; Harvey, B.G.; Choi, E.S.; Chilton, N.F.; Long, J.R. Coercive fields exceeding 30 T in the mixed-valence single-molecule magnet (CpiPr5)2Ho2I3. J. Am. Chem. Soc. 2024, 146, 18714–18721. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, X.-L.; Wu, Q.-Y.; Liu, K.; Wang, W.; Hu, K.-q.; Mei, L.; Chai, Z.-f.; Gibson, J.K.; Yu, J.-p.; et al. 4f/5d hybridization induced single-electron delocalization in an azide-bridged dicerium complex. J. Am. Chem. Soc. 2024, 146, 7088–7096. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.T.; Zivkovic, I.; Scopelliti, R.; Mazzanti, M. Molecular Complex of Tb in the +4 Oxidation State. J. Am. Chem. Soc. 2019, 141, 9827–9831. [Google Scholar] [CrossRef]
- Willauer, A.R.; Palumbo, C.T.; Scopelliti, R.; Zivkovic, I.; Douair, I.; Maron, L.; Mazzanti, M. Stabilization of the Oxidation State +IV in Siloxide-Supported Terbium Compounds. Angew. Chem. Int. Ed. 2020, 59, 3549–3553. [Google Scholar] [CrossRef]
- Willauer, A.R.; Palumbo, C.T.; Fadaei-Tirani, F.; Zivkovic, I.; Douair, I.; Maron, L.; Mazzanti, M. Accessing the +IV Oxidation State in Molecular Complexes of Praseodymium. J. Am. Chem. Soc. 2020, 142, 5538–5542. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.T.; Popov, I.A.; Russo, D.R.; Bacsa, J.; Batista, E.R.; Yang, P.; Telser, J.; La Pierre, H.S. Design, Isolation, and Spectroscopic Analysis of a Tetravalent Terbium Complex. J. Am. Chem. Soc. 2019, 141, 13222–13233. [Google Scholar] [CrossRef]
- Xue, T.; Yang, Q.-S.; Li, L.; Chang, X.-Y.; Ding, Y.-S.; Zheng, Z. Supramolecular assemblies of tetravalent terbium complex units: Syntheses, structure, and materials properties. Chem. Sci. 2025, 16, 6805–6811. [Google Scholar] [CrossRef] [PubMed]
- Boggiano, A.C.; Studvick, C.M.; Roy Chowdhury, S.; Niklas, J.E.; Tateyama, H.; Wu, H.; Leisen, J.E.; Kleemiss, F.; Vlaisavljevich, B.; Popov, I.A.; et al. Praseodymium in the formal +5 oxidation state. Nat. Chem. 2025. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Jiang, X.-L.; Li, L.; Xu, C.-Q.; Li, J.; Zheng, Z. Atomically precise semiconductor clusters of rare-earth tellurides. Nat. Synth. 2024, 3, 655–661. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y.-S.; Zheng, Z. Lanthanide-based molecular magnetic semiconductors. Angew. Chem. Int. Ed. 2024, 63, e202410019. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Jiao, T.; Sun, Z.; Mishra, A.; Zhuo, Q.; Nishiura, M.; Hou, Z.; Cong, X. Regio- and stereoselective hydroalkynylation of internal alkynes with terminal alkynes by half-sandwich rare-earth catalysts. J. Am. Chem. Soc. 2025, 147, 6149–6161. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mishra, A.; Jiao, T.; Chang, W.; Lou, S.-J.; Nishiura, M.; Cong, X.; Hou, Z. Heteroatom-assisted regio- and stereoselective hydrosilylation of unsymmetric internal alkynes by scandium catalyst. Angew. Chem. Int. Ed. 2025, 64, e202502665. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, R.M.; Gálico, D.A.; Chartrand, D.; Suturina, E.A.; Murugesu, M. Toward opto-structural correlation to investigate luminescence thermometry in an organometallic Eu(II) complex. J. Am. Chem. Soc. 2022, 144, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, R.M.; Gálico, D.A.; Chartrand, D.; Murugesu, M. Ligand effects on the emission characteristics of molecular Eu(II) luminescence thermometers. J. Am. Chem. Soc. 2024, 146, 34118–34129. [Google Scholar] [CrossRef]
- Kundu, K.; White, J.R.K.; Moehring, S.A.; Yu, J.M.; Ziller, J.W.; Furche, F.; Evans, W.J.; Hill, S. A 9.2-GHz clock transition in a Lu(II) molecular spin qubit arising from a 3,467-MHz hyperfine interaction. Nat. Chem. 2022, 14, 392–397. [Google Scholar] [CrossRef]
- Smith, P.W.; Hruby, J.; Evans, W.J.; Hill, S.; Minasian, S.G. Identification of an X-Band clock transition in Cp’3Pr− enabled by a 4f25d1 configuration. J. Am. Chem. Soc. 2024, 146, 5781–5785. [Google Scholar] [CrossRef]
- Nodaraki, L.E.; Ariciu, A.-M.; Huh, D.N.; Liu, J.; Martins, D.O.T.A.; Ortu, F.; Winpenny, R.E.P.; Chilton, N.F.; McInnes, E.J.L.; Mills, D.P.; et al. Ligand effects on the spin relaxation dynamics and coherent manipulation of organometallic La(II) potential qudits. J. Am. Chem. Soc. 2024, 146, 15000–15009. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A dysprosium metallocene single-molecule magnet functioning at the axial limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; He, M.; Huang, G.-Z.; Giblin, S.R.; Billington, D.; Heinemann, F.W.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Discovery of a dysprosium metallocene single-molecule magnet with two high-temperature orbach processes. Inorg. Chem. 2022, 61, 6017–6025. [Google Scholar] [CrossRef]
- Jin, P.-B.; Luo, Q.-C.; Gransbury, G.K.; Vitorica-Yrezabal, I.J.; Hajdu, T.; Strashnov, I.; McInnes, E.J.L.; Winpenny, R.E.P.; Chilton, N.F.; Mills, D.P.; et al. Thermally stable terbium(II) and dysprosium(II) bis-amidinate complexes. J. Am. Chem. Soc. 2023, 145, 27993–28009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Q.-C.; Zheng, Y.-Z. Organolanthanide single-molecule magnets with heterocyclic ligands. Angew. Chem. Int. Ed. 2024, 63, e202407016. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.-C.; Zheng, J.; Peng, X.-H.; Tong, M.-L.; Mansikkamäki, A.; Guo, F.-S. Isolation and single-molecule magnetism of neutral rare-earth(III) sandwich complexes supported by compact aromatic rings. Sci. China Chem. 2025. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tong, M.-L. Single-molecule magnets beyond a single lanthanide ion: The art of coupling. Chem. Sci. 2022, 13, 8716–8726. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-S.; Xu, L.; Xiong, J.; Yuan, Q.; Liu, T.; Wang, B.-W.; Gao, S. Low-coordinate single-ion magnets by intercalation of lanthanides into a phenol matrix. Angew. Chem. Int. Ed. 2018, 57, 4673–4676. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Chen, Y.; Wang, B.-W.; Wang, Z.-M.; Gao, S. Assembling high-temperature single-molecule magnets with low-coordinate bis(amido) dysprosium unit [DyN2]+ via Cl–K–Cl linkage. CCS Chem. 2020, 2, 362–368. [Google Scholar] [CrossRef]
- Sun, R.; Wang, C.; Wang, B.-W.; Wang, Z.-M.; Chen, Y.-F.; Tamm, M.; Gao, S. Low-coordinate bis(imidazolin-2-iminato) dysprosium(III) single-molecule magnets. Inorg. Chem. Front. 2023, 10, 485–492. [Google Scholar] [CrossRef]
- Sun, R.; Li, C.-Y.; Huang, Z.-N.; Wang, C.; Chen, Y.; Sun, H.-L.; Wang, Z.-M.; Wang, B.-W.; Gao, S. One is better than two: Improved performance in mono(imidazolin-2-iminato) dysprosium(III) single-molecule magnets featuring short Dy–N bond. CCS Chem. 2024. [CrossRef]
- Emerson-King, J.; Gransbury, G.K.; Whitehead, G.F.S.; Vitorica-Yrezabal, I.J.; Rouzières, M.; Clérac, R.; Chilton, N.F.; Mills, D.P. Isolation of a bent dysprosium bis(amide) single-molecule magnet. J. Am. Chem. Soc. 2024, 146, 3331–3342. [Google Scholar] [CrossRef]
- Benner, F.; Jena, R.; Odom, A.L.; Demir, S. Magnetic hysteresis in a dysprosium bis(amide) complex. J. Am. Chem. Soc. 2025, 147, 8156–8167. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Wang, Y.-F.; Wang, Y.-X.; Tang, M.-J.; Yin, B. Ab initio prediction of key parameters and magneto-structural correlation of tetracoordinated lanthanide single-ion magnets. Phys. Chem. Chem. Phys. 2023, 25, 18387–18399. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wang, Y.-F.; Yin, B. Theoretical study of pentacoordinated lanthanide single-ion magnets via ab initio electronic structure calculation. Magnetochemistry 2025, 11, 3. [Google Scholar] [CrossRef]
- Zhang, P.; Luo, Q.-C.; Zhu, Z.; He, W.; Song, N.; Lv, J.; Wang, X.; Zhai, Q.-G.; Zheng, Y.-Z.; Tang, J. Radical-bridged heterometallic single-molecule magnets incorporating four lanthanoceniums. Angew. Chem. Int. Ed. 2023, 62, e202218540. [Google Scholar] [CrossRef]
- Bajaj, N.; Mavragani, N.; Kitos, A.A.; Chartrand, D.; Maris, T.; Mansikkamäki, A.; Murugesu, M. Hard single-molecule magnet behavior and strong magnetic coupling in pyrazinyl radical-bridged lanthanide metallocenes. Chem 2024, 10, 2484–2499. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Chen, Q.-W.; Ding, Y.-S.; Zhu, X.-F.; Wang, B.-W.; Pan, F.; Zheng, Z. Enhancement of single-molecule magnet properties by manipulating intramolecular dipolar interactions. Adv. Sci. 2025, 12, 2409730. [Google Scholar] [CrossRef]
- David, G.; Le Guennic, B.; Reta, D. Promoting exchange coupling in (CpiPr5)2Gd2X3 complexes. Chem. Commun. 2024, 60, 11988–11991. [Google Scholar] [CrossRef]
- Li, L.; Xue, T.-J.; Ding, Y.-S.; Zheng, Z. Rare-earth chalcogenidotetrachloride clusters (RE3ECl4, RE = Dy, Gd, Y.; E = S, Se, Te): Syntheses and materials properties. J. Mater. Chem. C 2024, 12, 16506–16514. [Google Scholar]
- Zhang, P.; Benner, F.; Chilton, N.F.; Demir, S. Organometallic lanthanide bismuth cluster single-molecule magnets. Chem. 2022, 8, 717–730. [Google Scholar] [CrossRef]
- Shima, T.; Nishiura, M.; Hou, Z. Tetra-, penta-, and hexanuclear yttrium hydride clusters from half-sandwich bis(aminobenzyl) complexes containing various cyclopentadienyl ligands. Organometallics. 2011, 30, 2513–2524. [Google Scholar] [CrossRef]
- Wu, J.; Demeshko, S.; Dechert, S.; Meyer, F. Hexanuclear [Cp*Dy]6 single-molecule magnet. Chem. Commun. 2020, 56, 3887–3890. [Google Scholar] [CrossRef]
- Shen, Q.; Qi, M.; Lin, Y. Synthesis and X-ray crystal structure of a monopentamethylcyclopentadienyl derivative of gadolinium {Na(μ-THF)[Cp*Gd(THF)]2(μ-Cl)2}2·6THF. J. Organomet. Chem. 1990, 399, 247–254. [Google Scholar] [CrossRef]
- Huang, W.; Upton, B.M.; Khan, S.I.; Diaconescu, P.L. Synthesis and characterization of paramagnetic lanthanide benzyl complexes. Organometallics. 2013, 32, 1379–1386. [Google Scholar] [CrossRef]
- Watson, P.L.; Whitney, J.F.; Harlow, R.L. (Pentamethylcyclopentadienyl)ytterbium and -lutetium complexes by metal oxidation and metathesis. Inorg. Chem. 1981, 20, 3271–3278. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Li Manni, G.; Fdez. Galván, I.; Alavi, A.; Aleotti, F.; Aquilante, F.; Autschbach, J.; Avagliano, D.; Baiardi, A.; Bao, J.J.; Battaglia, S.; et al. The OpenMolcas web: A community-driven approach to advancing computational chemistry. J. Chem. Theory. Comput. 2023, 19, 6933–6991. [Google Scholar] [CrossRef]
- Roos, B.O.; Lindh, R.; Malmqvist, P.-Å.; Veryazov, V.; Widmark, P.-O. Main group atoms and dimers studied with a new relativistic ANO basis set. J. Phys. Chem. A 2004, 108, 2851–2858. [Google Scholar] [CrossRef]
- Roos, B.O.; Lindh, R.; Malmqvist, P.-Å.; Veryazov, V.; Widmark, P.-O. New relativistic ANO basis sets for transition metal atoms. J. Phys. Chem. A 2005, 109, 6575–6579. [Google Scholar] [CrossRef] [PubMed]
- Ungur, L.; Chibotaru, L.F. Ab Initio Crystal Field for Lanthanides. Chem. Eur. J. 2017, 23, 3708–3718. [Google Scholar] [CrossRef]
- Chibotaru, L.F.; Ungur, L.; Soncini, A. The origin of nonmagnetic kramers doublets in the ground state of dysprosium triangles: Evidence for a toroidal magnetic moment. Angew. Chem. Int. Ed. 2008, 47, 4126–4129. [Google Scholar] [CrossRef] [PubMed]
- Ungur, L.; Van den Heuvel, W.; Chibotaru, L.F. Ab initio investigation of the non-collinear magnetic structure and the lowest magnetic excitations in dysprosium triangles. New J. Chem. 2009, 33, 1224–1230. [Google Scholar] [CrossRef]
- Du, S.; Yin, J.; Chi, Y.; Xu, L.; Zhang, W.-X. Dual functionalization of white phosphorus: Formation, characterization, and reactivity of rare-earth-metal cyclo-P3 complexes. Angew. Chem. Int. Ed. 2017, 56, 15886–15890. [Google Scholar] [CrossRef]
- Dubé, T.; Gambarotta, S.; Yap, G.P.A.; Conoci, S. Preparation and characterization of two mixed-valence samarium octameric clusters. Organometallics 2000, 19, 115–117. [Google Scholar] [CrossRef]
- Gromada, J.; Mortreux, A.; Chenal, T.; Ziller, J.W.; Leising, F.; Carpentier, J.-F. Neodymium alkoxides: Synthesis, characterization and their combinations with dialkylmagnesiums as unique systems for polymerization and block copolymerization of ethylene and methyl methacrylate. Chem. Eur. J. 2002, 8, 3773–3788. [Google Scholar] [CrossRef]
- Dietrich, H.M.; Schuster, O.; Törnroos, K.W.; Anwander, R. Heterobimetallic half-lanthanidocene clusters: Novel mixed tetramethylaluminato/chloro coordination. Angew. Chem. Int. Ed. 2006, 45, 4858–4863. [Google Scholar] [CrossRef]
- Evans, W.J.; Champagne, T.M.; Davis, B.L.; Allen, N.T.; Nyce, G.W.; Johnston, M.A.; Lin, Y.-C.; Khvostov, A.; Ziller, J.W. Structural studies of mono(pentamethylcyclopentadienyl)lanthanide complexes. J. Coord. Chem. 2006, 59, 1069–1087. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, P. A basis for lanthanide single-molecule magnets. In Lanthanide Single Molecule Magnets; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–39. [Google Scholar]
- Jiang, S.-D.; Wang, B.-W.; Gao, S. Advances in lanthanide single-ion magnets. In Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 111–141. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Han, T.; Ding, Y.-S.; Li, Z.-H.; Yu, K.-X.; Zhai, Y.-Q.; Chilton, N.F.; Zheng, Y.-Z. A dichlorido-bridged dinuclear Dy(III) single-molecule magnet with an effective energy barrier larger than 600 K. Chem. Commun. 2019, 55, 7930–7933. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium(III) single-molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef] [PubMed]
- Reta, D.; Chilton, N.F. Uncertainty estimates for magnetic relaxation times and magnetic relaxation parameters. Phys. Chem. Chem. Phys. 2019, 21, 23567–23575. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Du, S.-N.; Ruan, Z.-Y.; Zhao, X.-J.; Chen, Y.-C.; Liu, J.-L.; Tong, M.-L. Aggregation-induced suppression of quantum tunneling by manipulating intermolecular arrangements of magnetic dipoles. Aggregate 2024, 5, e441. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Chen, Q.-W.; Huang, J.-Q.; Zhu, X.-F.; Zheng, Z. Sandwich-type single-molecule magnets complexes of Er(III) with ansa-cyclooctatetraenyl ligands. Chin. J. Chem. 2024, 42, 3099–3106. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, Y.; Zhu, X.; Wang, B.; Zheng, Z. Tuning intramolecular dipole interactions in dinuclear single-molecule magnetic complexes of Er(III) with monosubstituted cyclooctatetraenide ligands. J. Rare. Earth. 2024. [CrossRef]






| RE(III) | S | L | J | g | χTValue (cm3 mol−1 K) | Magnetic Moments (μB) | |||
| Expected | Experimental | Saturated | Experimental | ||||||
| 300 K | 300 K | 2 K | |||||||
| Gd | 7/2 | 0 | 7/2 | 2 | 7.87 | 8.04 | 7.03 | 7 | 6.92 |
| Tb | 3 | 3 | 6 | 3/2 | 11.82 | 11.86 | 7.86 | 9 | 4.74 |
| Dy | 5/2 | 5 | 15/2 | 4/3 | 14.17 | 14.03 | 10.59 | 10 | 4.69 |
| Ho | 2 | 6 | 8 | 5/4 | 14.07 | 14.75 | 9.17 | 10 | 4.86 |
| Er | 3/2 | 6 | 15/2 | 6/5 | 11.48 | 11.48 | 4.49 | 9 | 5.09 |
| Tm | 1 | 5 | 6 | 7/6 | 7.15 | 7.11 | 1.83 | 7 | 3.14 |
| Yb | 1/2 | 3 | 7/2 | 8/7 | 2.57 | 2.52 | 1.20 | 4 | 1.80 |
| Energy (cm−1) | g1 | g2 | g3 | Wavefunction |
|---|---|---|---|---|
| 0.00 | 1.04 × 10−3 | 1.67 × 10−3 | 19.81 | 98.9%|± 15/2⟩… |
| 168.911 | 6.26 × 10−2 | 9.52 × 10−2 | 17.64 | 58.2%| ± 13/2⟩ + 14.5%| ± 11/2⟩ + 14.5%| ± 9/2⟩ + 7.9%| ± 7/2⟩… |
| 227.038 | 1.60 × 10−2 | 2.86 × 10−2 | 14.49 | 37.3%| ± 13/2⟩ + 17.1%| ± 9/2⟩ + 16%| ± 11/2⟩ + 16%| ± 7/2⟩… |
| 273.431 | 3.53 | 4.72 | 10.07 | 48.1%| ± 11/2⟩ + 16.8%| ± 5/2⟩ + 13.3%| ± 7/2⟩ + 10.8%| ± 3/2⟩… |
| 302.717 | 2.56 | 5.62 | 10.56 | 42.8%| ± 9/2⟩ + 18.8%| ± 7/2⟩ + 11.9%| ± 11/2⟩ + 10.8%| ± 3/2⟩… |
| 338.500 | 0.14 | 2.65 | 13.20 | 30.6%| ± 5/2⟩ + 25.8%| ± 1/2⟩ + 25.6%| ± 7/2⟩+8.1%| ± 9/2⟩… |
| 360.075 | 0.56 | 1.97 | 14.98 | 39.1%| ± 3/2⟩ + 20.2%| ± 5/2⟩ + 16.1%| ± 1/2⟩ + 10.9%| ± 7/2⟩… |
| 472.122 | 2.36 × 10−2 | 3.85 × 10−2 | 19.77 | 42.9%| ± 1/2⟩ + 28.9%| ± 3/2⟩ + 15.6%| ± 5/2⟩ + 7.5%| ± 7/2⟩… |
| Energy (cm−1) | g1 | g2 | g3 | Wavefunction |
|---|---|---|---|---|
| 0.00 | 6.24 × 10−3 | 9.61 × 10−3 | 19.84 | 99.5%| ± 15/2⟩… |
| 180.303 | 0.80 | 0.85 | 16.23 | 73.3%| ± 13/2⟩ + 8.8%| ± 5/2⟩ + 5.8%| ± 11/2⟩ + 5.6%| ± 7/2⟩… |
| 214.563 | 10.20 | 7.12 | 2.75 | 31.6%|± 9/2⟩ + 23.4%| ± 11/2⟩ + 11.2%| ± 3/2⟩ + 10.7%| ± 13/2⟩… |
| 233.352 | 0.68 | 4.13 | 8.94 | 31.4%| ± 11/2⟩ + 21.6%| ± 7/2⟩ + 11.9%| ± 9/2⟩+10.6%| ± 13/2⟩… |
| 261.853 | 10.85 | 7.67 | 1.04 | 46.8%| ± 7/2⟩+42.4%| ± 9/2⟩ + 4.3%| ± 5/2⟩ +3.3%| ± 11/2⟩… |
| 270.398 | 8.77 | 5.42 | 0.48 | 44.4%| ± 5/2⟩ + 30.6%| ± 11/2⟩ + 16.9%| ± 3/2⟩ + 3.6%| ± 13/2⟩… |
| 378.250 | 0.58 | 2.73 | 14.23 | 53%| ± 3/2⟩ + 32.6%| ± 5/2⟩ + 5%| ± 1/2⟩ + 4.5%| ± 7/2⟩… |
| 390.606 | 0.39 | 3.26 | 15.02 | 68.9%| ± 1/2⟩ + 11.5%| ± 7/2⟩ + 8.8%| ± 9/2⟩ + 8.2%| ± 3/2⟩… |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Ding, Y.-S.; Li, L.; Zheng, Z. Synthesis, X-Ray Crystal Structures, and Magnetic Properties of a Series of Trinuclear Rare-Earth Hepta-Chloride Clusters. Magnetochemistry 2025, 11, 38. https://doi.org/10.3390/magnetochemistry11050038
Pan Y, Ding Y-S, Li L, Zheng Z. Synthesis, X-Ray Crystal Structures, and Magnetic Properties of a Series of Trinuclear Rare-Earth Hepta-Chloride Clusters. Magnetochemistry. 2025; 11(5):38. https://doi.org/10.3390/magnetochemistry11050038
Chicago/Turabian StylePan, Yingying, You-Song Ding, Lei Li, and Zhiping Zheng. 2025. "Synthesis, X-Ray Crystal Structures, and Magnetic Properties of a Series of Trinuclear Rare-Earth Hepta-Chloride Clusters" Magnetochemistry 11, no. 5: 38. https://doi.org/10.3390/magnetochemistry11050038
APA StylePan, Y., Ding, Y.-S., Li, L., & Zheng, Z. (2025). Synthesis, X-Ray Crystal Structures, and Magnetic Properties of a Series of Trinuclear Rare-Earth Hepta-Chloride Clusters. Magnetochemistry, 11(5), 38. https://doi.org/10.3390/magnetochemistry11050038







