Abstract
The reaction of nitronyl nitroxide biradical NITPhMeImbis(5-(2-methylimidazole)-1,3-bis(1′-oxyl-3′-oxido-4′,4′,5′,5′-tetramethyl-4,5-hydro-1H-imidazol-2-yl)-benzene) with Dy(hfac)3 and La(hfac)3 (hfac = hexafluoroacetylacetonate) afforded a heterolanthanide complex [Dy0.56La1.44(hfac)7(NITPhMeImbisH)] (1). In this complex, the biradical NITPhMeImbis ligand chelates one Ln(III) ion via its two neighboring NO units and simultaneously binds the La(III) ion through another NO group to form a dinuclear structure. Direct current (dc) magnetic measurement shows the dominant ferromagnetic couplings in Complex 1. Spin dynamics studies exhibit visible frequency-dependent peaks of χ″ signals under a dc field evidenced by field-induced magnetic relaxation behavior, which is a combination of Orbach and QTM processes, giving the Ueff, τ0 and τQTM values of 15.14 K, 3.04 × 10−7 s and 3.61 × 10−4 s, respectively.
1. Introduction
Due to the high magnetic moments and strong magnetic anisotropies, lanthanides are of great interest for designing single molecule magnets (SMMs) [1,2,3,4], which have potential applications in high-density memory storage [5], the quantum sciences [6,7,8] and molecular spintronics [9,10]. High performance SMMs have been achieved by the use of lanthanides, which show blocking temperatures of the magnetization (TB) near liquid nitrogen temperatures [11,12]. Despite these great advances, it is still a challenge to enhance magnet behaviors of SMMs toward practical application. To develop performant SMMs, one of the promising strategies is to design Ln-radical architectures in which strong magnetic exchanges between the Ln centers and the radicals could be achieved, leading to effectively suppress the quantum tunneling of magnetization (QTM) of Ln ions and thus toward improving the performance of SMMs [13]. For instance, a series of Ln-radical species exhibiting remarkably high TB and large coercive fields has been obtained using N-based radicals [14,15,16,17].
On the other hand, recently, another approach, with the aim of enhancing lanthanide SMM properties, is to design Ln-Ln′ heterolanthanide complexes that combine two kinds of magnetic anisotropy in one molecule, offering a promising pathway to SMMs with improved performances [18,19,20,21,22]. However, the synthesis of heterolanthanide molecular complexes is difficult due to the similar chemistries of lanthanides and still remains challenging. So far, different synthetic approaches have been developed to obtain Ln-Ln′ heterolanthanide complexes in which the distinct lanthanide ions stay in selected sites, e.g., by step-by-step approaches [23,24,25,26] or connecting preformed lanthanide containing species [27,28,29]. Using these synthetic procedures, a few heterolanthanide SMMs have been reported [20,30]. However, the heterolanthanide SMMs involving nitronyl nitroxide radical remain unexplored. The nitronyl nitroxide–heterolanthanide complexes will provide a new way for improving the magnetic performances of the magnetic system in which three different spins behave in a synergistic way. Therefore, in this study, we use nitronyl nitroxide biradical NITPhMeImbis (5-(2-methylimidazole)-1,3-bis(1′-oxyl-3′-oxido-4′,4′,5′,5′-tetramethyl-4,5-hydro-1H-imidazol-2-yl)-benzene, Scheme 1) to construct a heterolanthanide system. The NITPhMeImbis nitronyl nitroxide biradical could construct Ln-Ln′ complexes as a step-by-step approach since it could firstly chelate a Ln ion by two neighboring NIT moieties and then the remaining NO units could be ligated to another different Ln′ ion. In this study, Dy(III) and La(III) ions were chosen to construct heterolanthanide-radical complex because the Dy(III) ion is a Kramer ion with strong magnetic anisotropy and the diamagnetic La(III) ion could decrease undesirable intermolecular dipole interactions. Accordingly, one DyLa heterolanthanide binuclear complex [Dy0.56La1.44(hfac)7(NITPhMeImbisH)] (NITPhMeImbisH = 5-(2-methyl-3-hydroimidazolium)-1,3-bis(1′-oxyl-3′-oxido-4′,4′,5′,5′-tetramethyl-4,5-hydro-1H-imidazol-2-yl)-benzene) was achieved, which exhibits magnetic relaxation behavior.
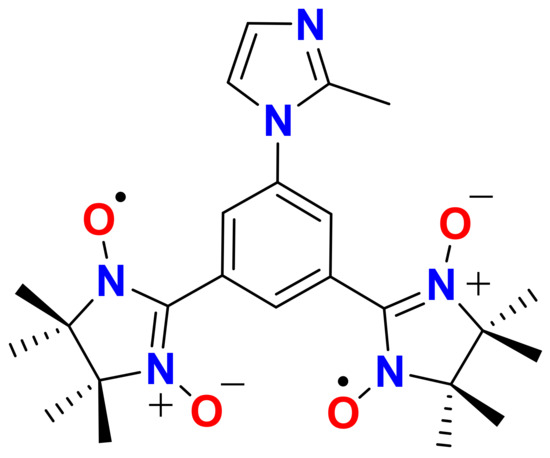
Scheme 1.
NITPhMeImbis ligand.
2. Results and Discussion
2.1. Synthesis and Crystal Structure
The nitronyl nitroxide biradical ligand NITPhMeImbis was used to prepare a DyLa heterolanthanide complex by step-by-step strategy. The NITPhMeImbis biradical was firstly coordinated with Dy(hfac)3 via two adjacent NO units of the biradical to a form mononuclear Dy complex and then the resulting mononuclear precursor was ligated to La(hfac)3 using uncoordinated NO groups. As a result, a heterolanthanide binuclear DyLa complex [Dy0.56La1.44(hfac)7(NITPhMeImbisH)] with a statistical distribution was obtained.
Complex 1 crystallizes in the monoclinic P21/c space group. As shown in Figure 1, the NITPhMeIm ligand chelates one Ln(hfac)3 unit through its two adjacent NO units and meanwhile coordinates with one La(hfac)4 unit via another NO group to lead to a dinuclear structure. It should be noted that one Ln atom is octa-coordinated with three hfac coligands while the other is in nona-coordinated with four hfac coligands. Thus, to charge balance, the N atom of imidazole ring in the biradical ligand was protonated.
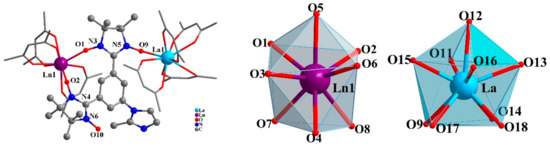
Figure 1.
The binuclear structure of Complex 1 (left). Coordination polyhedra of Ln1 and La in Complex 1 (right).
Continuous shape measure analysis (CShM) [31] was performed to elucidate the geometry of the Ln1(III) and La(III) ion. The octa-coordinated Ln1(III) ion exhibits a capped trigonal prism(C2v) geometry while the nona-coordinated La(III) ion exhibits a spherical capped square antiprism(C4v) geometry (Table S3). The octa-coordinated Ln1 is bonded by two O atoms from two NO groups of the biradical and six O atoms from three hfac coligands. Two Ln1-Orad bond lengths are 2.443(5) and 2.471(7) Å, respectively, and the Ln–Ohfac bond distances vary from 2.378(8) to 2.462(6) Å. These bond lengths are obviously longer than those which exist in similar nitronyl nitroxide biradical-DyIII complexes [32,33,34]. As for this, it thus seems reasonable to consider this Ln coordination site to be occupied by either the DyIII and LaIII ions. The La atom is nine-coordinated by one O atom of the NO group (La–Orad: 2.512(5) Å) and eight O atoms of the four hfac ligands (La–Ohfac: 2.415(6)–2.598(6)Å). The average La–Ohfac bond distance is 2.498(6) Å, which is close to those in reported LaIII complexes with hfac coligands [35,36,37]. Inductively coupled plasma (ICP) measurements were therefore conducted to investigate the composition of Complex 1. The result reveals a ratio of 2.59:1 for LaIII: DyIII and the Ln1 site shows a statistical distribution of the Dy and La ions.
The bond angles of Ln1–O1–N3 and Ln1–O2–N4 are 145.0(5)° and 148.6(6)°, respectively, and the torsion angles of Ln1–O1–N3–C1 and Ln1–O2–N4–C8 are |−72.06(1)°| and 62.45(2)°, respectively. The N5–O9–La bond angle is 142.0(5)° and the La–O9–N5–C1 torsion angle is |−85.65°|. For Complex 1, the dihedral angles between the benzene ring and the two NIT planes are 26.68(1)° and 29.48(4)°, respectively, while the dihedral angle formed by the imidazole and phenyl rings is 60.44(3)°. In the dinuclear molecule, the distance between two LnIII ions is 8.736(7) Å while the nearest intermolecular Ln---Ln(Ln1⋯La) distance is 12.653(7) Å. The stacking diagram is depicted in Figure S3, and the closest distance for non-coordinating NO groups is 9.450(2) Å.
2.2. Magnetic Properties
Direct current (dc) magnetic data of Complex 1 were measured under 1000 Oe between 2 K and 300 K. As seen in Figure 2a, at 300 K, the found χMT value was 8.59 cm3 Kmol−1 at room temperature, which is lower than that expected for one Dy(III) in the eight-coordinated Ln site and one La(III) in the nine-coordinated site (Dy(III): 6H15/2, S = 5/2, L = 5, g = 4/3, C = 14.17 cm3 K mol−1; La(III): diamagnetic) plus two radicals (S = 1/2); however, this value is close to the theoretical values (8.68 cm3 K mol−1) for mixed La(III) and Dy(III) ions with a ratio of 2.59:1 plus two radicals. This suggests that the La(III) ion is also found in the eight-coordinated Ln site. As the temperature decreases, the χMT value gradually increases and reaches the maximum of 11.80 cm3 K mol−1 at 3 K and then slightly decreases to 11.80 cm3 K mol−1 at 2 K, indicating the presence of ferromagnetic coupling in the system. In Complex 1, the magnetic exchange between the Dy(III) ion and the coordinated NO unit is usually ferromagnetic [38,39]. Magnetic communication between two NIT units via the Ln(III) ion is expected to be antiferromagnetic while exchange coupling between two NIT moieties through m-phenyl ring is expected to be ferromagnetic based on spin polarization [40]. The isothermal M vs. H plot for Complex 1 has been determined to be in the range of 0−7 T at 2 K (Figure 2b). The value of M increases with the magnetic field and reaches a value of 5.16 Nβ at 70 kOe, which is lower than the anticipated saturation value, implying the existence of magnetic anisotropy of Complex 1.
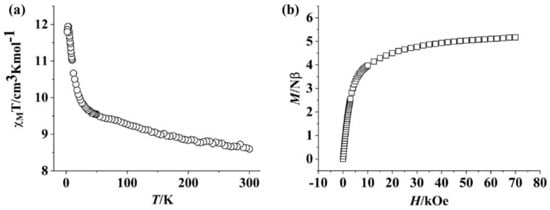
Figure 2.
(a) χMT vs. T curve for Complex 1. (b) M vs. H plot for Complex 1 at 2 K.
To explore the potential magnetic relaxation behavior of Complex 1, ac susceptibility measurements without an applied dc field were performed on Complex 1. Complex 1 displays pronounced frequency-dependent χ″ signals between 2 K and 10 K in the absence of a dc magnetic field, demonstrating the existence of magnetic relaxation behavior (Figure S4). However, there were no peaks to be found, suggesting significant quantum tunneling (QTM) of the magnetization in Complex 1 [13]. Consequently, the frequency-dependent ac magnetic susceptibilities of Complex 1 were measured at 2 K within a dc field range of 400–2400 Oe for suppressing QTM (Figure S5). The τ values obtained by fitting the ac susceptibility data through the Debye mode [41] were plotted vs. H (Figure S6) and then the optimized field was determined to be 800 Oe. Under the optimum dc field of 800 Oe, the χ″ signals exhibit distinct peaks in the range of 2.0 to 3.6 K (Figure 3). The relaxation time (τ) and the corresponding distribution parameter (α) were extracted by fitting the frequency-dependent ac data through the generalized Debye model [41]. The resulting lnτ versus T plot exhibits a linear temperature dependence at high temperature, but the derivation is observed at low temperature (Figure 4a). Consequently, the best fitting was achieved by considering the Orbach and QTM processes, i.e., τ−1 = τQTM−1 + τ0−1exp(−Ueff/kBT), leading to the anisotropic energy barrier Ueff/kB = 15.14 K with τ0 = 3.04 × 10−7 s and τQTM = 3.61 × 10−4 s. The resulting τ0 value of 3.04 × 10−7 falls in the characteristic range for SMMs (10−5–10−12 s) [2,42,43,44]. The Cole–Cole plot displays an almost semicircular shape (Figure 4a) and the obtained α is within the limit of 0.32–0.52 indicating the middle distribution of relaxation time. It is appealing to compare Complex 1 with the related Dy-nitronyl nitroxide biradical complexes in the literature. The related magnetic and structural parameters are summarized in Table 1. As seen, the magnetic relaxation behaviors of Dy-biradical complexes are very sensitive to the ligand field around the Dy atom as well as the ligand substituents. The energy barrier of Complex 1 remains superior, which could be ascribed to weak intermolecular dipole interactions arising from diamagnetic La(III) ions.
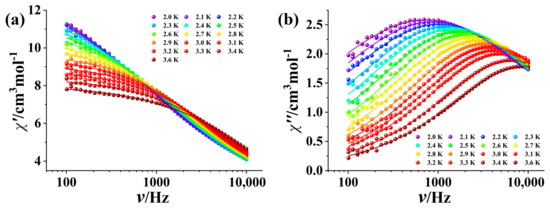
Figure 3.
Frequency-dependent χ′ (a) and χ″ (b) curves for Complex 1 under the 800 Oe dc field. (The solid lines are the fitting results of the Debye model.)
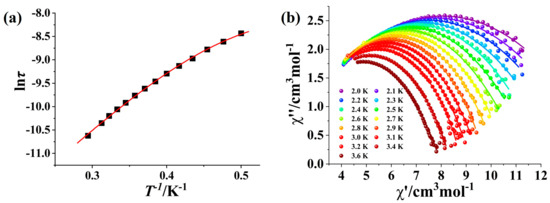
Figure 4.
(a) The ln τ versus 1/T plot for Complex 1 (the red line is the fitting result of the combined Orbach and QTM processes). (b) Cole–Cole curves for Complex 1 under the 800 Oe dc field (the solid lines are the fitting results of the Debye model).

Table 1.
Structural and magnetic parameters for related nitronyl nitroxide biradical-Dy complexes.
3. Experimental Section
3.1. Materials and Characterizations
All reactions were carried out using commercial grade solvents without further purification. The specific synthesis process of nitronyl nitroxide biradical NITPhMeImbis [32] and Ln(hfac)3·2H2O [50] were prepared following previously reported methods. Elemental analyses were carried out by Perkin-Elmer 240 elemental analyzer. IR spectra were recorded on a Bruker Tensor 27 spectrometer with the samples prepared as KBr discs in the 4000–400 cm−1 range. Powder X-ray diffraction (PXRD) patterns were recorded with a Rigaku Ultima IV diffractometer. Inductively coupled plasma–optical emission spectrometry (ICP-OES) was performed on SPECTRO-BLUE to verify the composition of the complex. Magnetization measurements were conducted using a Quantum Design model MPMS3 SQUID and PPMS-9 magnetometers. DC magnetic susceptibility data were corrected using Pascal’s constants [51].
3.2. Synthesis of [Dy0.56La1.44(hfac)7(NITPhMeImbisH)]
A total of 0.01 mmol of Dy(hfac)3·2H2O (8.2 mg) in dry n-heptane (20 mL) was refluxed for 3 h. Then 8 mL of CH2Cl2 containing 4.7 mg (0.01 mmol) of NITPhMeImbis was added and reacted for 15 min. Subsequently, 0.02 mmol of La(hfac)3·2H2O(16.0 mg) in 8.0 mL of n-heptane, in which the hydration water molecules had been azeotropically removed, was dropped in the above solution. The resulting solution was then allowed to cool to room temperature and filtered, and the dark purple crystals suitable for crystallographic characterization were obtained after five days. Yield 56%; C59H40F42Dy0.56La1.44N6O18 (2210.00 g mol−1); Elem. Anal. (%) found (calcd.): C, 32.24 (32.06); N, 3.70 (3.80); H, 1.69 (1.81).
[Dy0.56La1.44(hfac)7(NITPhMeImbisH)] (1): Yield 56%; C59H39F42DyLaN6O18 (2219.37 g mol−1); Elem. Anal. (%) found (calcd.): C, 32.24 (31.93); N, 3.70 (3.79); H, 1.69 (1.77). FT-IR (cm−1): 1648 (s), 1605 (w), 1554 (w), 1527 (w), 1492 (m), 1369 (m), 1249 (s), 1195 (s), 1129 (s), 796 (s), 740 (m), 659 (s), 580 (s) and 547 (m).
For FT-IR spectra (Figure S1), the peaks at 1648 cm−1 and 796 cm−1 could be assigned to the stretching and bending vibrations of C=O from hfac- ligands, respectively. The C-F stretching vibrations of the CF3 group appear at 1249, 1195 and 1129 cm−1 while the peak at 659 cm−1 is associated with the bending vibration of C–F from the hfac- ligand. The peaks at 1605, 1527 and 1492 cm−1 result from the stretching vibration of C=C (benzene ring) while those at 1554 and 1369 cm−1 could be assigned to the stretching vibration of the C=N and N–O groups, respectively [50]. The recorded experimental PXRD pattern of Complex 1 is in good agreement with the simulated pattern from single-crystal X-ray diffraction (Figure S2).
3.3. X-Ray Crystallography
Diffraction intensity data collection of Complex 1 was made on a Rigaku HyPix diffractometer with graphite monochromated Cu-Kα radiation (λ = 1.54184 Å) at a temperature of 113 K. Direct methods were utilized to solve crystal structures of Complex 1 using SHELXS-2018 and SHELXL-2018 [52,53]. All of the non-H atoms were refined anisotropically, and the H atom positions were given geometrically. To rationalize some disordered F and C atoms, commands of ISOR, SIMU and DELU were used. Crystallographic data and selected bond distances and angles are given in Table S1 and Table S2, respectively.
CCDC-2427073 contains the supplementary crystallographic data for Complex 1. The data can be obtained free of charge from the Cambridge Structural Database through www.ccdc.cam.ac.uk/structures (accessed on 28 March 2025)
4. Conclusions
We have successfully synthesized one DyLa heterolanthanide binuclear complex [Dy0.56La1.44(hfac)7(NITPhMeImbisH) using a nitronyl nitroxide biradical ligand. In this heterolanthanide complex, one Ln(III) ion is chelated by two neighboring NO units of nitronyl nitroxide biradical while the La(III) ion is coordinated by another NO group. Interestingly, DyLa heterolanthanide binuclear complex exhibits field-induced magnetic behavior. To the best of our knowledge, this DyLa radical-heterolanthanide represents the first heterolanthanide complex involving nitronyl nitroxide radical. This work demonstrates that the radical-heterolanthanide approach is a promising way for designing new radical-Ln magnetic relaxation systems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/magnetochemistry11040026/s1. Table S1: The crystallographic data and refinement parameters for 1; Table S2: Selected bond lengths [Å] and bond angles [°] for Complex 1; Table S3: SHAPE analysis for the Ln coordination spheres for 1; Figure S1: The IR spectra of Complex 1; Figure S2: Powder X-ray diffraction (PXRD) patterns for Complex 1 at room temperature; Figure S3: Crystal packing diagram of Complex 1; Figure S4: Frequency dependences of the χ′ (left) and χ″ (right) components of the ac susceptibility for 1 at 2 K in the absence of an applied dc field; Figure S5: Frequency dependences of the χ′ (left) and χ″ (right) components of the ac susceptibility for Complex 1 at 2 K under different dc fields (The solid lines are the fitting results of the Debye model); Figure S6: The τ vs. H plot for Complex 1 at 2.0 K under the applied dc field.
Author Contributions
Synthesis and characterization of the materials: Y.Z., J.X. and C.J.; measurement and analysis of dc and ac magnetic data: J.X.; writing: Y.Z. and J.X.; supervision and writing–review: Y.M. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 22371138 and 21773122).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [PubMed]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Affronte, M. Molecular nanomagnets for information technologies. J. Mater. Chem. 2009, 19, 1731–1737. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef]
- Troiani, F.; Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev. 2011, 40, 3119–3129. [Google Scholar] [CrossRef]
- Aromí, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [PubMed]
- Randall McClain, K.; Gould, C.A.; Chakarawet, K.; Teat, S.J.; Groshens, T.J.; Long, J.R.; Harvey, B.G. High-temperature magnetic blocking and magneto-structural correlations in a series of dysprosium(III) metallocenium single-molecule magnets. Chem. Sci. 2018, 9, 8492–8503. [Google Scholar] [PubMed]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar]
- Demir, S.; Gonzalez, M.I.; Darago, L.E.; Evans, W.J.; Long, J.R. Giant coercivity and high magnetic blocking temperatures for N23− radical-bridged dilanthanide complexes upon ligand dissociation. Nat. Commun. 2017, 8, 2144. [Google Scholar]
- Mavragani, N.; Errulat, D.; Gálico, D.A.; Kitos, A.A.; Mansikkamäki, A.; Murugesu, M. Radical-Bridged Ln4 Metallocene Complexes with Strong Magnetic Coupling and a Large Coercive Field. Angew. Chem. Int. Ed. 2021, 60, 24206–24213. [Google Scholar]
- Zhang, P.; Luo, Q.-C.; Zhu, Z.; He, W.; Song, N.; Lv, J.; Wang, X.; Zhai, Q.-G.; Zheng, Y.-Z.; Tang, J. Radical-Bridged Heterometallic Single-Molecule Magnets Incorporating Four Lanthanoceniums. Angew. Chem. Int. Ed. 2023, 62, e202218540. [Google Scholar]
- Bajaj, N.; Mavragani, N.; Kitos, A.A.; Chartrand, D.; Maris, T.; Mansikkamäki, A.; Murugesu, M. Hard single-molecule magnet behavior and strong magnetic coupling in pyrazinyl radical-bridged lanthanide metallocenes. Chem 2024, 10, 2484–2499. [Google Scholar]
- Sato, R.; Suzuki, K.; Sugawa, M.; Mizuno, N. Heterodinuclear Lanthanoid-Containing Polyoxometalates: Stepwise Synthesis and Single-Molecule Magnet Behavior. Chem. Eur. J 2013, 19, 12982–12990. [Google Scholar]
- Leng, J.-D.; Liu, J.-L.; Zheng, Y.-Z.; Ungur, L.; Chibotaru, L.F.; Guo, F.-S.; Tong, M.-L. Relaxations in heterolanthanide dinuclear single-molecule magnets. Chem. Commun. 2013, 49, 158–160. [Google Scholar]
- Aguilà, D.; Velasco, V.; Barrios, L.A.; González-Fabra, J.; Bo, C.; Teat, S.J.; Roubeau, O.; Aromí, G. Selective Lanthanide Distribution within a Comprehensive Series of Heterometallic [LnPr] Complexes. Inorg. Chem. 2018, 57, 8429–8439. [Google Scholar] [CrossRef]
- Li, Q.-W.; Liu, J.-L.; Jia, J.-H.; Leng, J.-D.; Lin, W.-Q.; Chen, Y.-C.; Tong, M.-L. Fluorescent single-ion magnets: Molecular hybrid (HNEt3)[DyxYb1−x(bpyda)2] (x = 0.135−1). Dalton Trans. 2013, 42, 11262–11270. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, E.; Arauzo, A.; Fuertes, S.; Navarro-Spreafica, L.; Sevilla, P.; Fernández Cortés, H.; Settineri, N.; Teat, S.J.; Sañudo, E.C. Luminescent and magnetic [TbEu] 2D metal–organic frameworks. Dalton Trans. 2023, 52, 7258–7270. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Nicodème, F. Structure-Based Description of a Step-by-Step Synthesis of Homo- and Heterodinuclear (4f, 4f ′) Lanthanide Complexes. Inorg. Chem. 2003, 42, 6556–6563. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Glover, P.B.; Solomons, M.C.; Pikramenou, Z. Purely Heterometallic Lanthanide(III) Macrocycles through Controlled Assembly of Disulfide Bonds for Dual Color Emission. J. Am. Chem. Soc. 2011, 133, 1033–1043. [Google Scholar]
- Zhu, P.; Pan, N.; Li, R.; Dou, J.; Zhang, Y.; Cheng, D.Y.Y.; Wang, D.; Ng, D.K.P.; Jiang, J. Electron-Donating Alkoxy-Group-Driven Synthesis of Heteroleptic Tris(phthalocyaninato) Lanthanide(III) Triple-Deckers with Symmetrical Molecular Structure. Chem. Eur. J 2005, 11, 1425–1432. [Google Scholar] [CrossRef]
- Artizzu, F.; Quochi, F.; Serpe, A.; Sessini, E.; Deplano, P. Tailoring functionality through synthetic strategy in heterolanthanide assemblies. Inorg. Chem. Front 2015, 2, 213–222. [Google Scholar]
- Le Roy, J.J.; Cremers, J.; Thomlinson, I.A.; Slota, M.; Myers, W.K.; Horton, P.H.; Coles, S.J.; Anderson, H.L.; Bogani, L. Tailored homo- and hetero- lanthanide porphyrin dimers: A synthetic strategy for integrating multiple spintronic functionalities into a single molecule. Chem. Sci. 2018, 9, 8474–8481. [Google Scholar] [CrossRef]
- Placidi, M.P.; Villaraza, A.J.L.; Natrajan, L.S.; Sykes, D.; Kenwright, A.M.; Faulkner, S. Synthesis and Spectroscopic Studies on Azo-Dye Derivatives of Polymetallic Lanthanide Complexes: Using Diazotization to Link Metal Complexes Together. J. Am. Chem. Soc. 2009, 131, 9916–9917. [Google Scholar] [CrossRef]
- Natrajan, L.S.; Villaraza, A.J.L.; Kenwright, A.M.; Faulkner, S. Controlled preparation of a heterometallic lanthanide complex containing different lanthanides in symmetrical binding pockets. Chem. Commun. 2009, 6020–6022. [Google Scholar] [CrossRef]
- Douib, H.; Flores Gonzalez, J.; Speed, S.; Montigaud, V.; Lefeuvre, B.; Dorcet, V.; Riobé, F.; Maury, O.; Gouasmia, A.; Le Guennic, B.; et al. Modulation of the magnetic and photophysical properties in 3d–4f and 4f–4f′ heterobimetallic complexes involving a tetrathiafulvalene-based ligand. Dalton Trans. 2022, 51, 16486–16496. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Xie, J.; Han, J.; Huang, X.; Jin, C.; Li, L.; Sutter, J.-P. Enhancing the Magnetization Blocking Energy of Biradical-Metal System by Merging Discrete Complexes into One-Dimensional Chains. Chem. Eur. J 2023, 29, e202203852. [Google Scholar] [PubMed]
- Bernot, K.; Pointillart, F.; Rosa, P.; Etienne, M.; Sessoli, R.; Gatteschi, D. Single molecule magnet behaviour in robust dysprosium–biradical complexes. Chem. Commun. 2010, 46, 6458–6460. [Google Scholar]
- Xi, L.; Han, J.; Huang, X.; Li, L. Nitronyl Nitroxide Biradical-Based Binuclear Lanthanide Complexes: Structure and Magnetic Properties. Magnetochemistry 2020, 6, 48. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Guo, J.; Sun, G.; Li, L. Cu–Ln compounds based on nitronyl nitroxide radicals: Synthesis, structure, and magnetic and fluorescence properties. CrystEngComm 2016, 18, 9345–9356. [Google Scholar]
- Wang, X.-L.; Xu, P.-P. Synthesis and Crystal Structure of a Lanthanum-Nitronyl Nitroxide Complex. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 918–921. [Google Scholar]
- Ramade, I.; Kahn, O.; Jeannin, Y.; Robert, F. Design and Magnetic Properties of a Magnetically Isolated GdIIICuII Pair. Crystal Structures of [Gd(hfa)3Cu(salen)], [Y(hfa)3Cu(salen)], [Gd(hfa)3Cu(salen)(Meim)], and [La(hfa)3(H2O)Cu(salen)] [hfa = Hexafluoroacetylacetonato, salen = N,N‘-Ethylenebis(salicylideneaminato), Meim = 1-Methylimidazole]. Inorg. Chem. 1997, 36, 930–936. [Google Scholar]
- Xi, L.; Li, H.; Sun, J.; Ma, Y.; Tang, J.; Li, L. Designing Multicoordinating Nitronyl Nitroxide Radical Toward Multinuclear Lanthanide Aggregates. Inorg. Chem. 2020, 59, 443–451. [Google Scholar]
- Han, J.; Jin, C.; Wang, X.; Huang, X.; Song, H.; Xie, J.; Li, L. Magnetic Relaxation in Unique Nitronyl Nitroxide Biradical-Ln–Cu Chains with Ln-bis(NIT)–Cu-bis(NIT)–Ln Units. Dalton Trans. 2023, 52, 6853–6859. [Google Scholar]
- Catala, L.; Le Moigne, J.; Kyritsakas, N.; Rey, P.; Novoa, J.J.; Turek, P. Towards a Better Understanding of the Magnetic Interactions within m-Phenylene α-Nitronyl Imino Nitroxide Based Biradicals. Chem. Eur. J 2001, 7, 2466–2480. [Google Scholar] [CrossRef]
- Aubin, S.M.J.; Sun, Z.; Pardi, L.; Krzystek, J.; Folting, K.; Brunel, L.-C.; Rheingold, A.L.; Christou, G.; Hendrickson, D.N. Reduced Anionic Mn12 Molecules with Half-Integer Ground States as Single-Molecule Magnets. Inorg. Chem. 1999, 38, 5329–5340. [Google Scholar]
- Zheng, Y.Z.; Lan, Y.; Anson, C.E.; Powell, A.K. Anion-perturbed magnetic slow relaxation in planar {Dy4} clusters. Inorg. Chem. 2008, 47, 10813–10815. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-M.; Li, H.-Y.; Zhang, Y.-Q.; Yang, E.-C.; Zhao, X.-J. Magnetic Relaxation Dynamics of a Centrosymmetric Dy2 Single-Molecule Magnet Triggered by Magnetic-Site Dilution and External Magnetic Field. Inorg. Chem. 2017, 56, 5611–5622. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Jing, P.; Xi, L.; Lu, J.; Han, J.; Huang, X.; Jin, C.; Xie, J.; Li, L. Regulating Spin Dynamics of Nitronyl Nitroxide Biradical Lanthanide Complexes through Introducing Different Transition Metals. Chem.—Asian J. 2021, 16, 793–800. [Google Scholar] [CrossRef]
- Xi, L.; Sun, J.; Wang, K.; Lu, J.; Jing, P.; Li, L. Slow magnetic relaxation in CoII–LnIII heterodinuclear complexes achieved through a functionalized nitronyl nitroxide biradical. Dalton Trans. 2020, 49, 1089–1096. [Google Scholar] [CrossRef]
- Wang, K.; Sun, J.; Xi, L.; Lu, J.; Jing, P.; Li, L. Heterometallic Ln–Cu complexes derived from a phenyl pyrimidyl substituted nitronyl nitroxide biradical. Dalton Trans. 2019, 48, 14383–14389. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Li, X.; Li, T.; Tian, L.; Liu, Z.Y.; Wang, X.G. A series of heterospin complexes based on lanthanides and pyridine biradicals: Synthesis, structure and magnetic properties. RSC Adv. 2015, 5, 17131–17139. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Tian, L.; Liu, Z.Y.; Wang, X.G. A family of rare earth complexes with chelating furan biradicals: Syntheses, structures and magnetic properties. RSC Adv. 2015, 5, 74864–74873. [Google Scholar] [CrossRef]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A Family of Rare-Earth-Based Single Chain Magnets: Playing with Anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).