Current Application of Magnetic Materials in the Dental Field
Abstract
1. Introduction:
2. Forms of Magnetic Particles in Dental Research
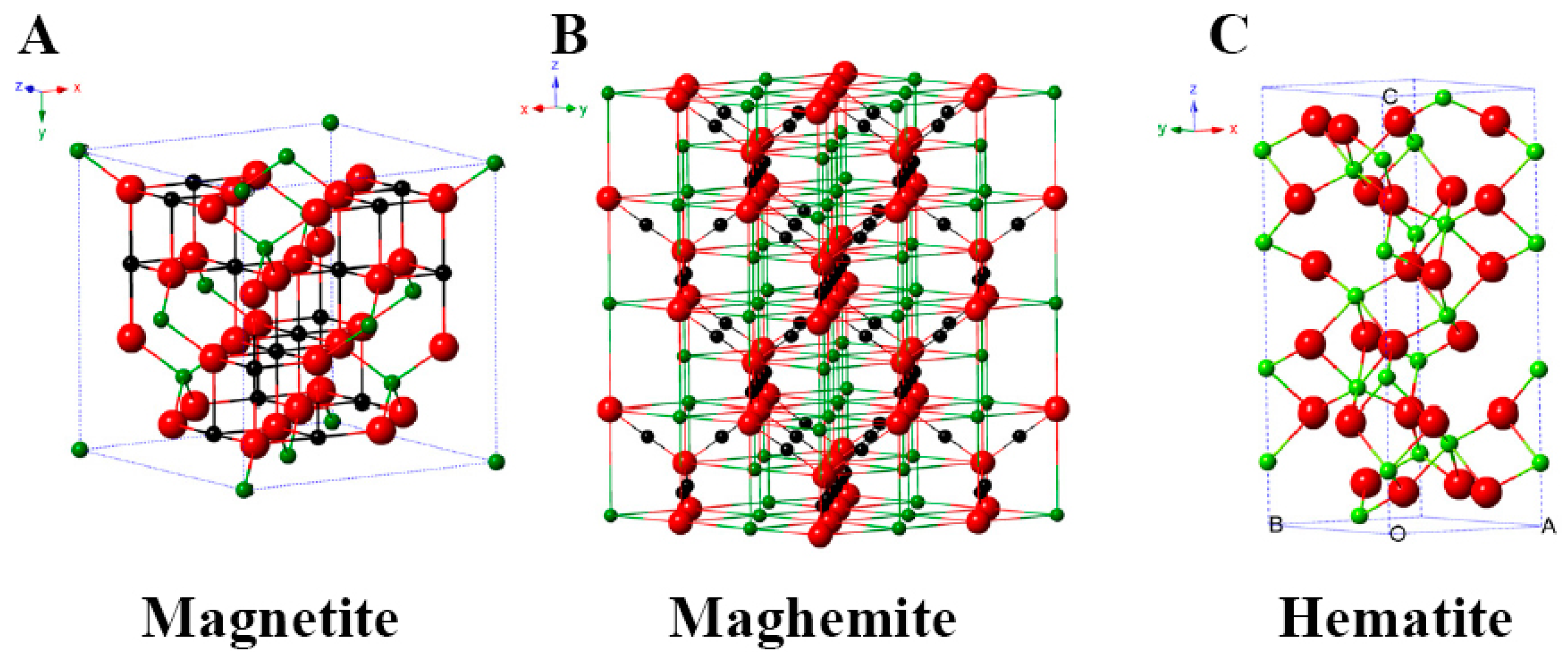
2.1. Magnetite Iron Oxide
2.2. Maghemite Iron Oxide
2.3. Hematite Iron Oxide
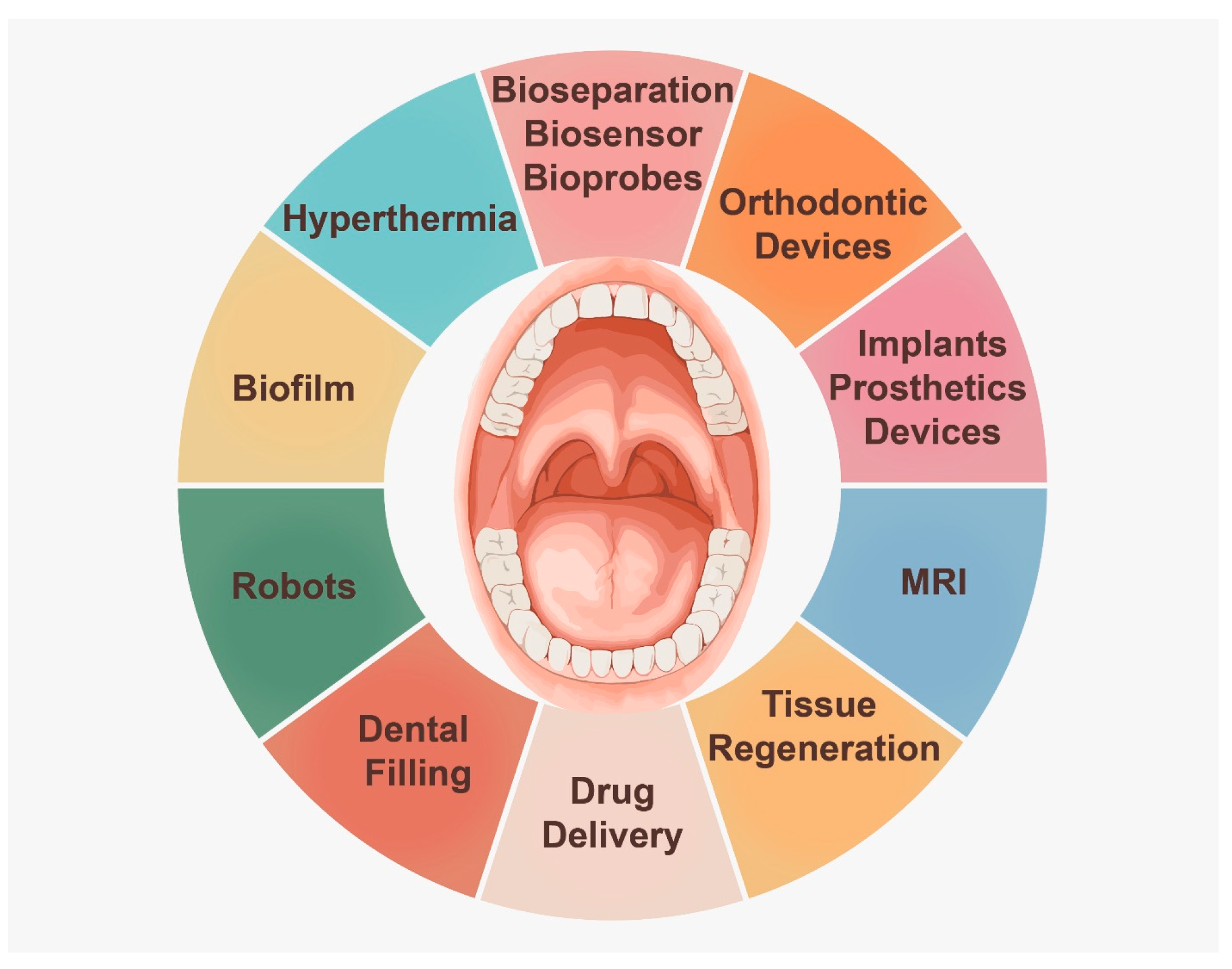
3. Applications of Magnetic Particles
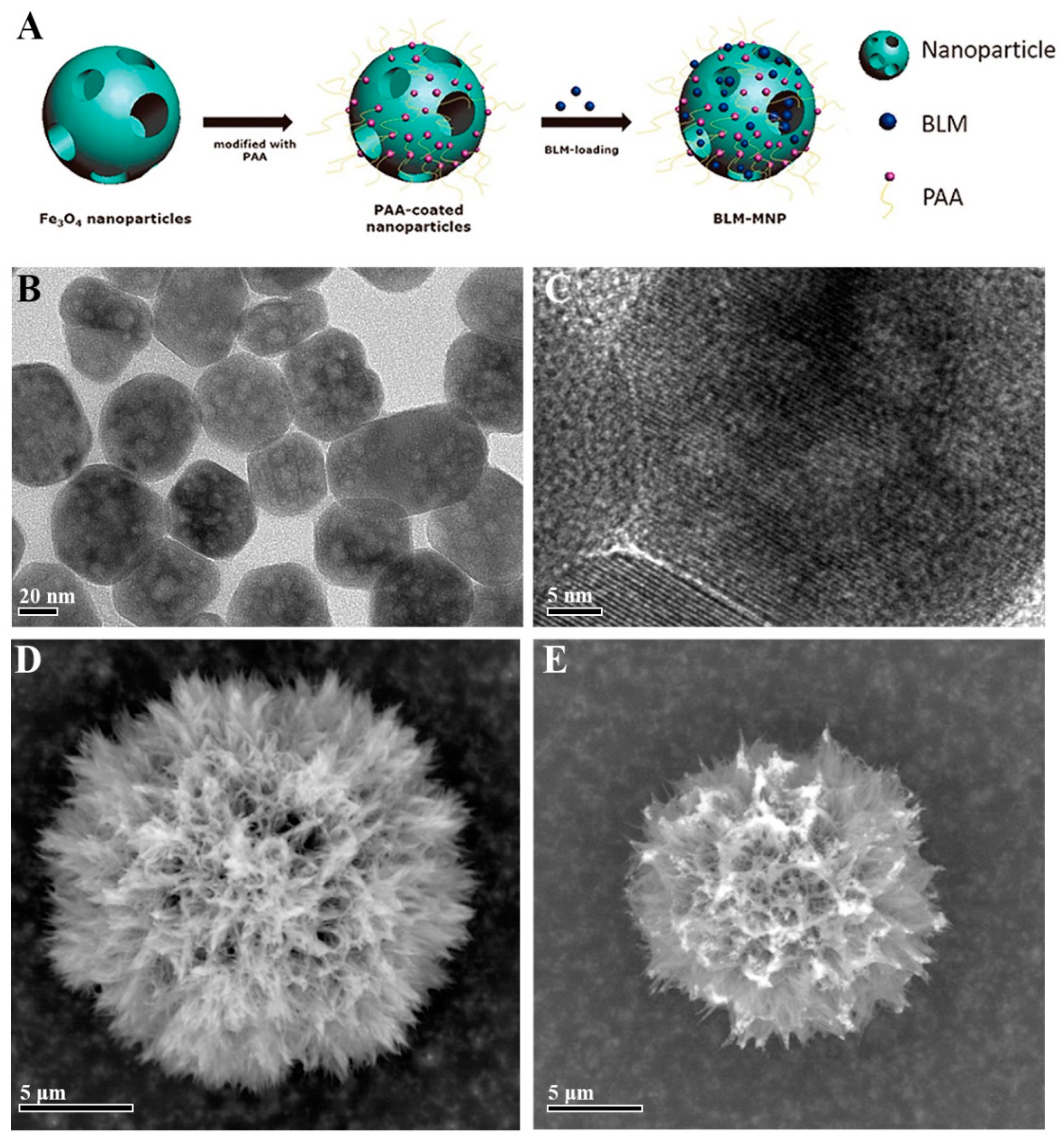
3.1. Drug Delivery
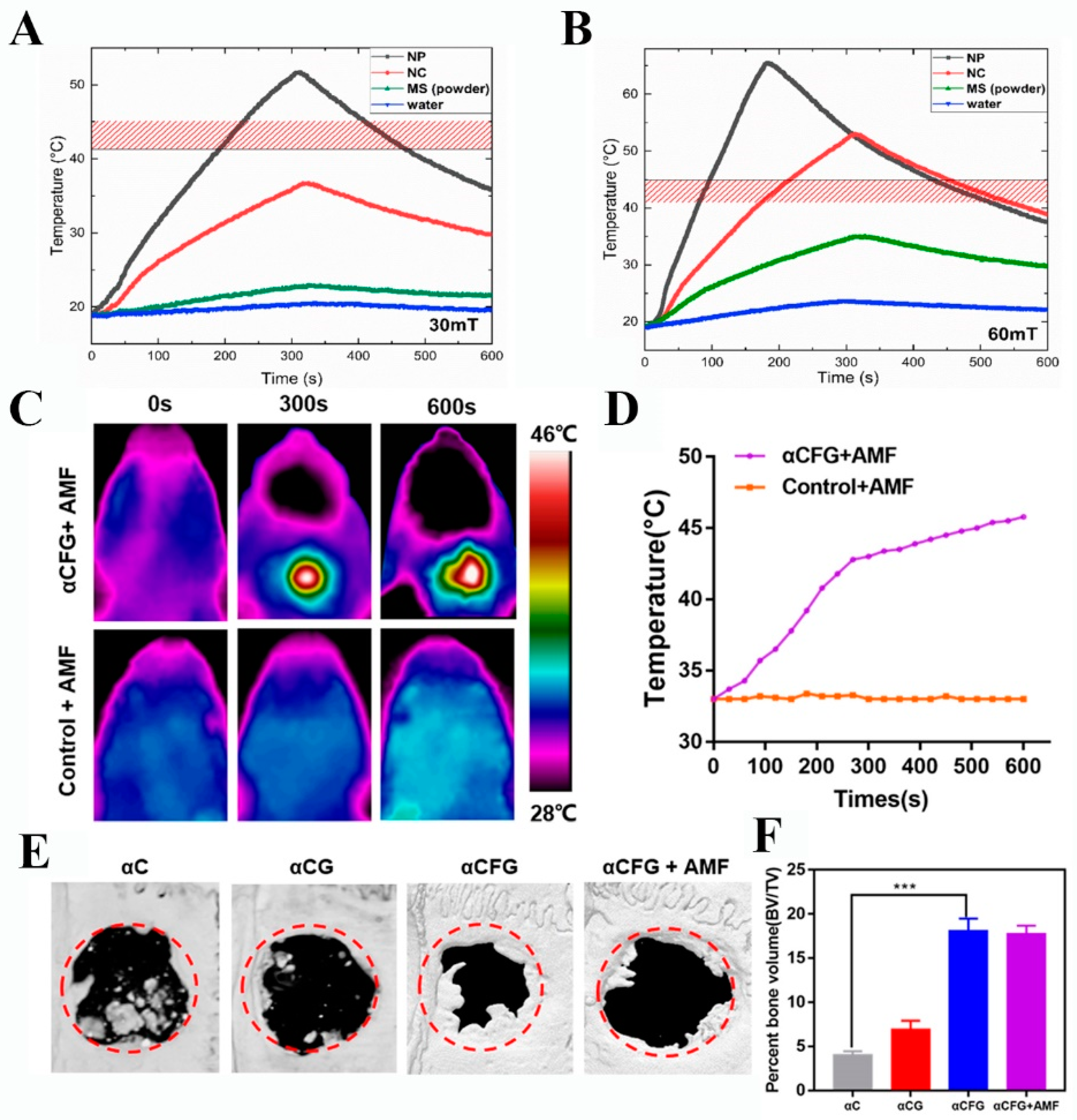
3.2. Hyperthermia
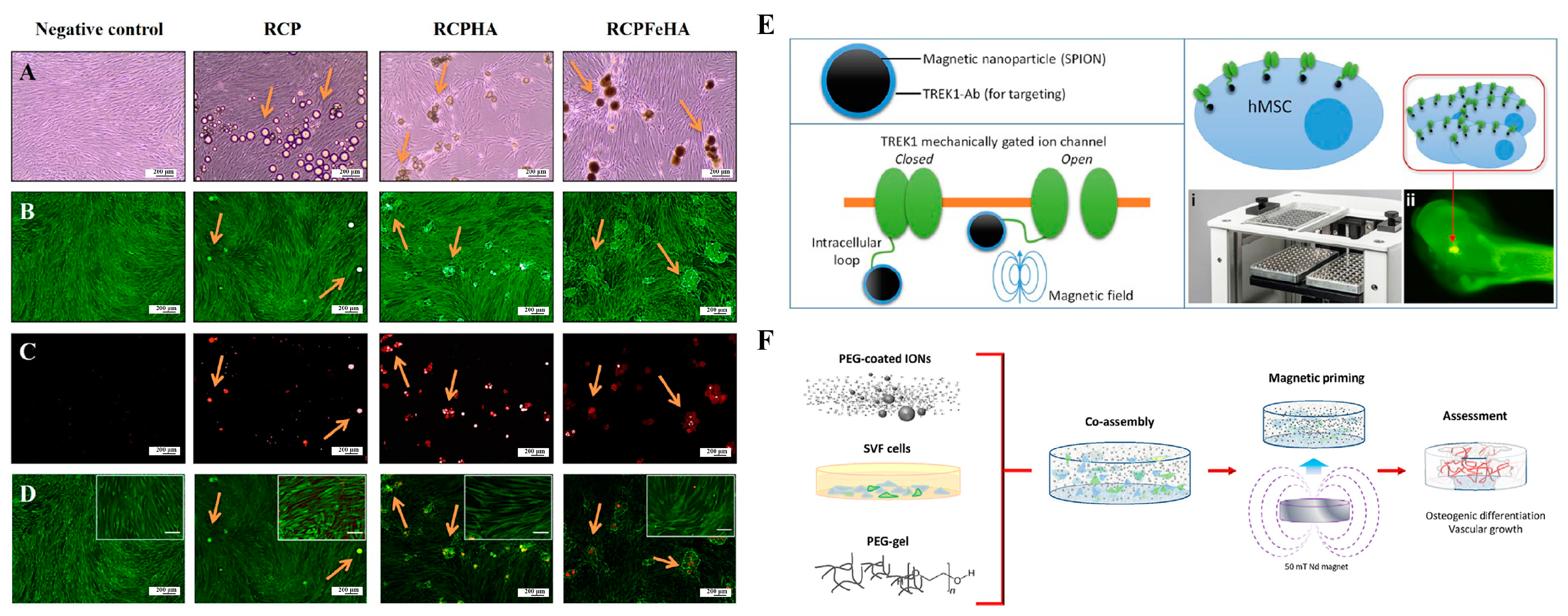
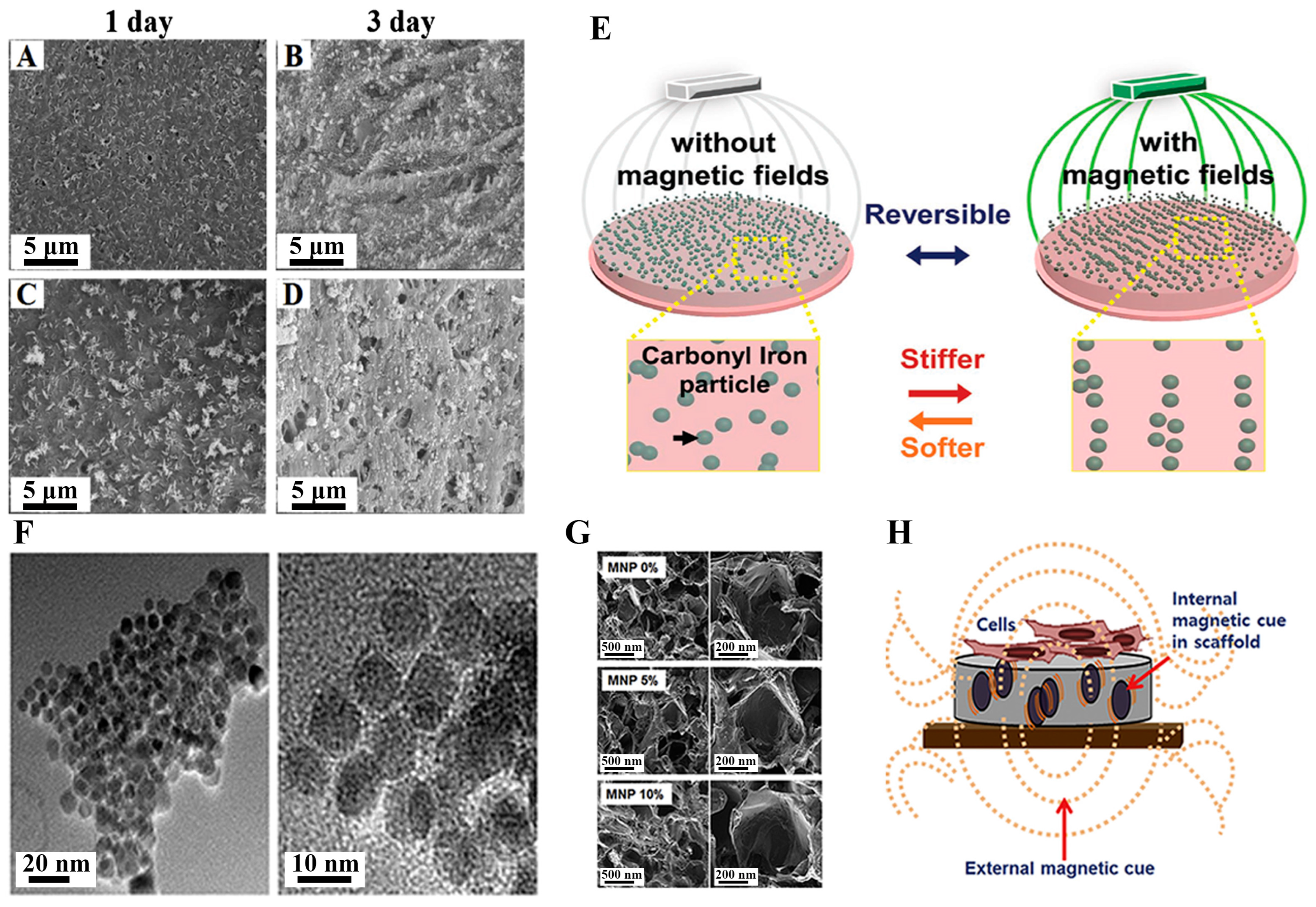
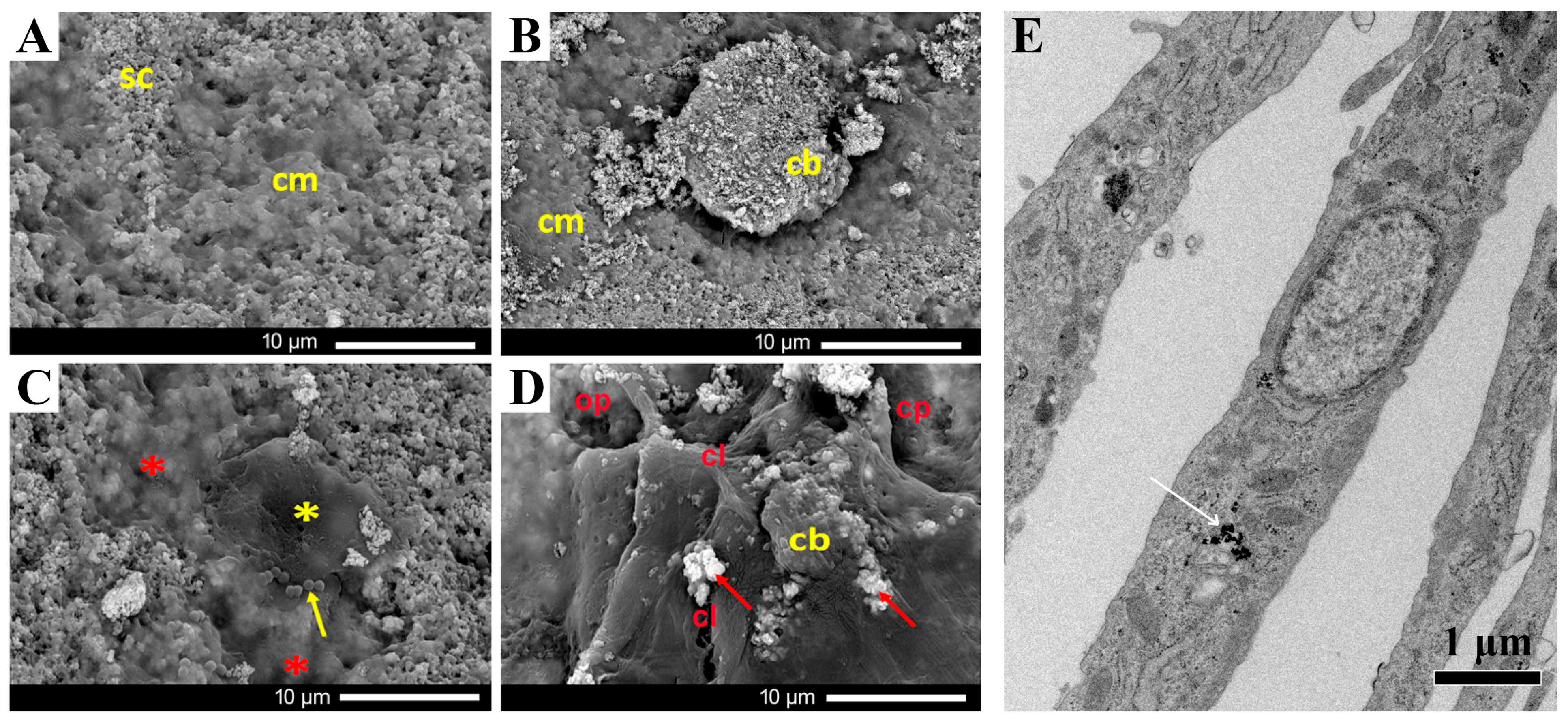
3.3. Bone Regeneration
3.4. Skin Regeneration
3.5. Tooth Structure Regeneration
3.6. Magnetic Resonance Imaging (MRI)
3.7. Bioseparation
3.8. Biosensors and Bioprobes
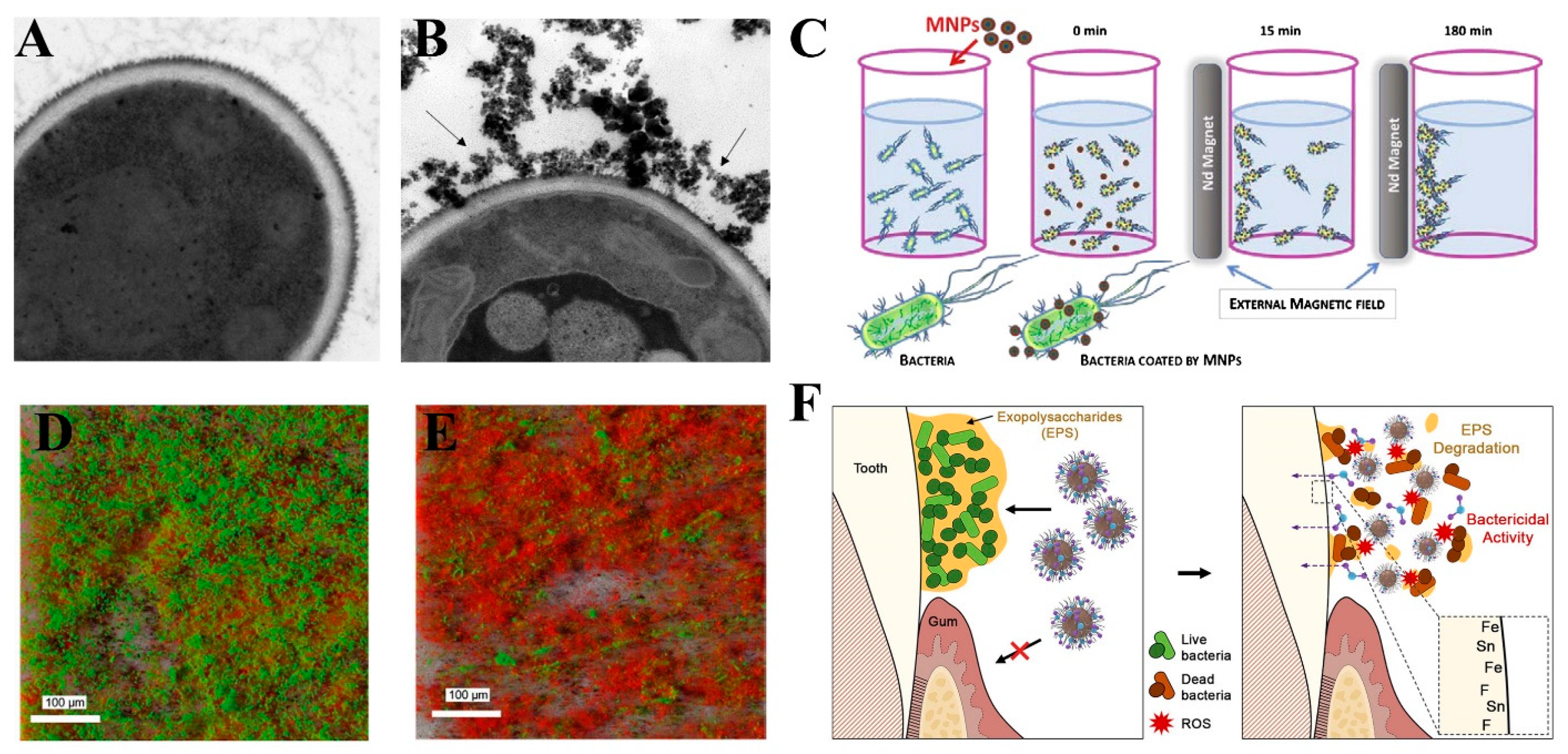
3.9. Biofilm
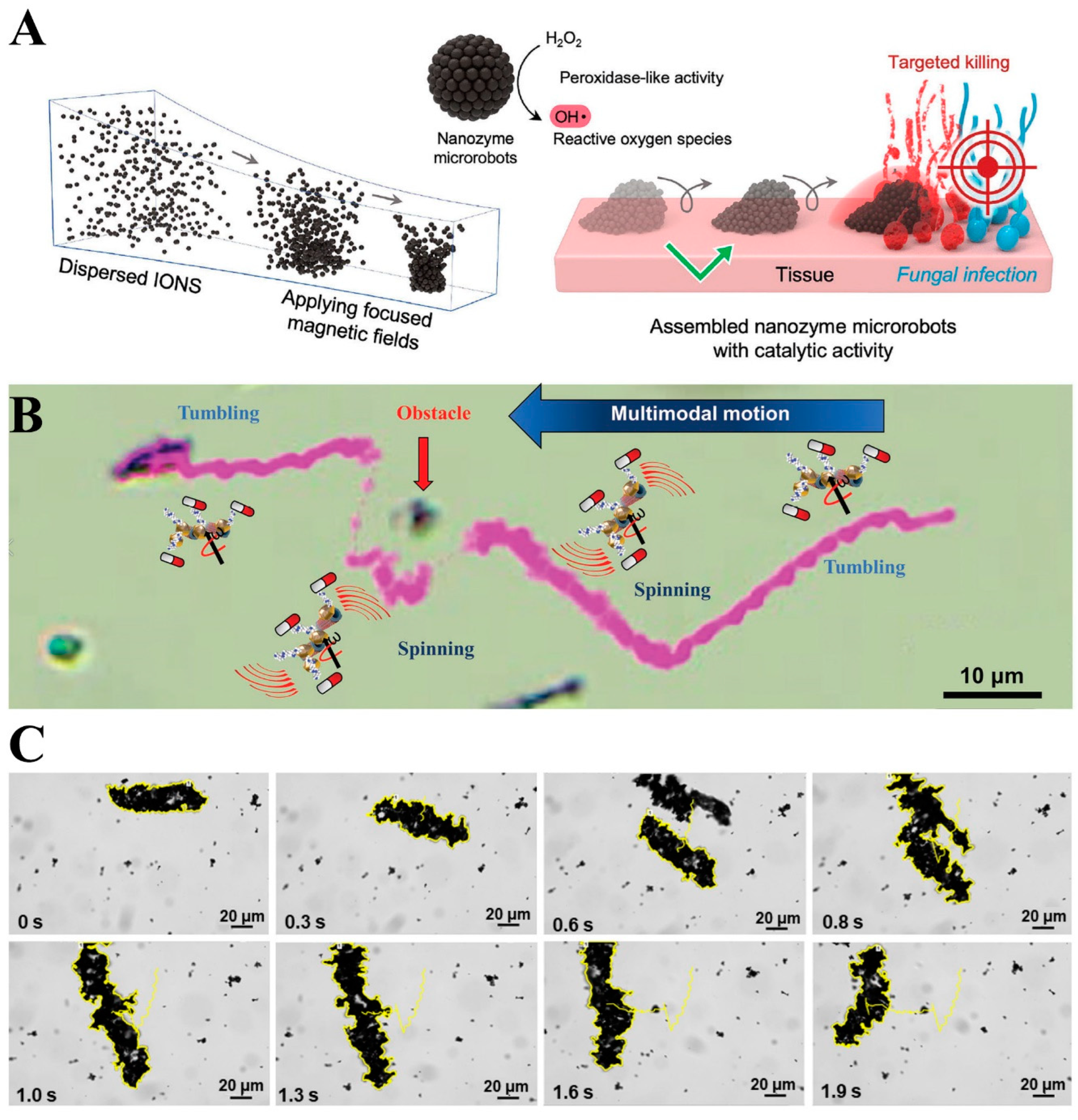
3.10. Robotic Research
3.11. Dental Filling
4. Potential Toxicity of Magnetic Particles
5. Magnets in Dental Clinical Practice
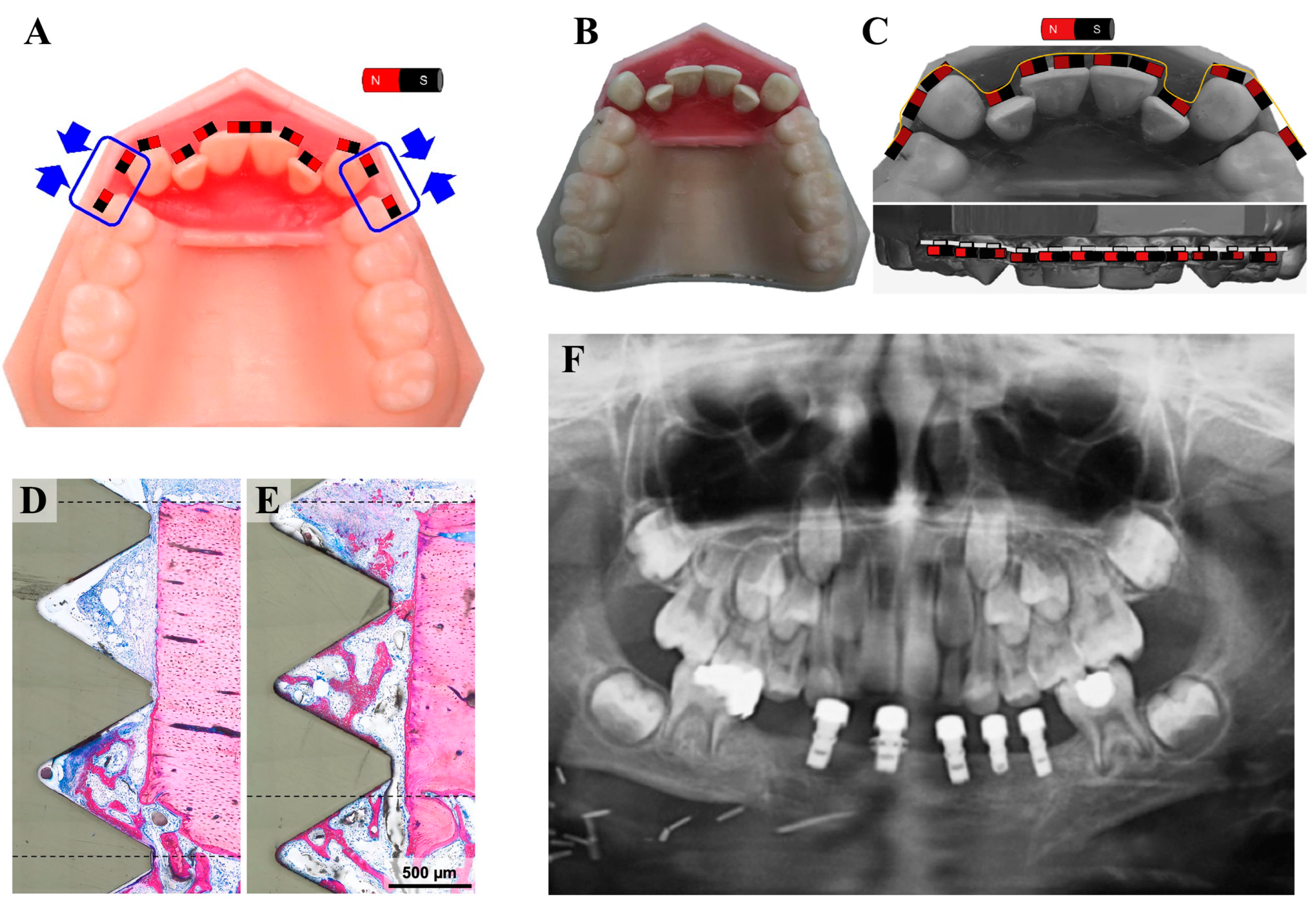
5.1. Magnetic Orthodontic Devices
5.2. Magnetic Implants and Prosthetics
6. Future Prospects
6.1. Dental Research Aspects
6.2. Dental Clinical Aspects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montoya, C.; Roldan, L.; Yu, M.C.L.; Valliani, S.; Ta, C.; Yang, M.B.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Shearer, A.; Montazerian, M.; Mauro, J.C. Modern definition of bioactive glasses and glass-ceramics. J. Non-Cryst. Solids 2023, 608, 8. [Google Scholar] [CrossRef]
- Li, X.M.; Wei, J.R.; Aifantis, K.E.; Fan, Y.B.; Feng, Q.L.; Cui, F.Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Rinne, C.A.; Schmidt, J.C.; Dagassan-Berndt, D.; Walter, C. Evaluation of magnetic resonance imaging for diagnostic purposes in operative dentistry-A systematic review. Clin. Oral Investig. 2020, 24, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Ajkidkarn, P.; Ritprajak, P.; Injumpa, W.; Porntaveetus, T.; Insin, N. Synthesis, characterization, drug release and transdentinal delivery studies of magnetic nanocubes coated with biodegradable poly(2-(dimethyl amino) ethyl methacrylate). J. Magn. Magn. Mater. 2017, 427, 235–240. [Google Scholar] [CrossRef]
- Farzin, A.; Fathi, M.; Emadi, R. Multifunctional magnetic nanostructured hardystonite scaffold for hyperthermia, drug delivery and tissue engineering applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Kida, I.; Esaki, M.; Akasaka, T.; Uo, M.; Hosono, T.; Sato, Y.; Jeyadevan, B.; Kuboki, Y.; Morita, M.; et al. Biodistribution imaging of magnetic particles in mice: X-ray scanning analytical microscopy and magnetic resonance imaging. Bio-Med. Mater. Eng. 2009, 19, 213–220. [Google Scholar] [CrossRef]
- Dasari, A.; Xue, J.Y.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef]
- Rivas, J.; Bañobre-López, M.; Piñeiro-Redondo, Y.; Rivas, B.; López-Quintela, M.A. Magnetic nanoparticles for application in cancer therapy. J. Magn. Magn. Mater. 2012, 324, 3499–3502. [Google Scholar] [CrossRef]
- Fluksman, A.; Lafuente, A.; Braunstein, R.; Steinberg, E.; Friedman, N.; Yekhin, Z.; Roca, A.G.; Nogues, J.; Hazan, R.; Sepulveda, B.; et al. Modular Drug-Loaded Nanocapsules with Metal Dome Layers as a Platform for Obtaining Synergistic Therapeutic Biological Activities. ACS Appl. Mater. Interfaces 2023, 15, 50330–50343. [Google Scholar] [CrossRef] [PubMed]
- Injumpa, W.; Ritprajak, P.; Insin, N. Size-dependent cytotoxicity and inflammatory responses of PEGylated silica-iron oxide nanocomposite size series. J. Magn. Magn. Mater. 2017, 427, 60–66. [Google Scholar] [CrossRef]
- Man, H.B.; Ho, D. Diamond as a nanomedical agent for versatile applications in drug delivery, imaging, and sensing. Phys. Status Solidi A-Appl. Mater. Sci. 2012, 209, 1609–1618. [Google Scholar] [CrossRef]
- Beeran, A.E.; Fernandez, F.B.; Nazeer, S.S.; Jayasree, R.S.; John, A.; Anil, S.; Vellappally, S.; Al Kheraif, A.A.A.; Varma, P.R.H. Multifunctional nano manganese ferrite ferrofluid for efficient theranostic application. Colloid Surf. B-Biointerfaces 2015, 136, 1089–1097. [Google Scholar] [CrossRef]
- Uskokovic, V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. Part B 2011, 96B, 152–191. [Google Scholar] [CrossRef]
- Choukrani, G.; Maharjan, B.; Park, C.H.; Kim, C.S.; Sasikala, A.R.K. Biocompatible superparamagnetic sub-micron vaterite particles for thermo-chemotherapy: From controlled design to in vitro anticancer synergism. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 106, 110226. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.J.; Sun, I.C.; Lee, S.C.; Kim, K.; Kwon, I.C.; Ahn, C.H. Development of a pH sensitive nanocarrier using calcium phosphate coated gold nanoparticles as a platform for a potential theranostic material. Macromol. Res. 2012, 20, 319–326. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zhou, R.H.; Yang, Y.T.; Peng, S.L.; Xiao, D.X.; Kong, T.T.; Cai, X.X.; Zhu, B.F. Aptamer-mediated synthesis of multifunctional nano-hydroxyapatite for active tumour bioimaging and treatment. Cell Prolif. 2021, 54, e13105. [Google Scholar] [CrossRef]
- Park, J.H.; Im, K.H.; Lee, S.H.; Kim, D.H.; Lee, D.Y.; Lee, Y.K.; Kim, K.M.; Kim, K.N. Preparation and characterization of magnetic chitosan particles for hyperthermia application. J. Magn. Magn. Mater. 2005, 293, 328–333. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moaca, E.A.; Watz, C.G.; Pînzaru, S.C.; Tudoran, L.B.; Nekvapil, F.; Avram, S.; Dehelean, C.A.; Cretu, M.O.; et al. Development and Characterization of Fe3O4@Carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef]
- Sulaiman, N.H.; Ghazali, M.J.; Majlis, B.Y.; Yunas, J.; Razali, M. Superparamagnetic calcium ferrite nanoparticles synthesized using a simple sol-gel method for targeted drug delivery. Bio-Med. Mater. Eng. 2015, 26, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, F.; Calabrese, L.; Caprì, A.; Currò, M.; Borsellino, C.; Bonaccorsi, L.; Fabiano, V.; Ientile, R.; Proverbio, E. Development and Characterization of Silane Coated Miniaturize NdFeB Magnets in Dentistry. Sci. Adv. Mater. 2017, 9, 1141–1145. [Google Scholar] [CrossRef]
- Kim, E.C.; Leesungbok, R.; Lee, S.W.; Hong, J.Y.; Ko, E.J.; Ahn, S.J. Effects of static magnetic fields on bone regeneration of implants in the rabbit: Micro-CT, histologic, microarray, and real-time PCR analyses. Clin. Oral Implant. Res. 2017, 28, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Michels, R.; Kampleitner, C.; Dobsak, T.; Doppelmayer, K.; Heimel, P.; Lettner, S.; Tangl, S.; Gruber, R.; Benfatti, C.A.M. Impact of a Static Magnetic Field on Early Osseointegration: A Pilot Study in Canines. Materials 2023, 16, 1846. [Google Scholar] [CrossRef] [PubMed]
- Siadat, H.; Bassir, S.H.; Alikhasi, M.; Shayesteh, Y.S.; Khojasteh, A.; Monzavi, A. Effect of Static Magnetic Fields on the Osseointegration of Immediately Placed Implants: A Randomized Controlled Clinical Trial. Implant. Dent. 2012, 21, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kuwajima, Y.; Ishida, Y.; Lee, C.; Mayama, H.; Satoh, K.; Ishikawa-Nagai, S. 3D digital analysis of magnetic force-driven orthodontic tooth movement. Heliyon 2019, 5, e02861. [Google Scholar] [CrossRef] [PubMed]
- Wongsarat, W.; Sarapirom, S.; Aukkaravittayapun, S.; Jotikasthira, D.; Boonyawan, D.; Yu, L.D. Plasma immersion ion implantation and deposition of DLC coating for modification of orthodontic magnets. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2012, 272, 346–350. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proano, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Niu, L.J.; Zhang, G.M.; Xian, G.; Ren, Z.J.; Wei, T.; Li, Q.G.; Zhang, Y.; Zou, Z.G. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Kong, J.; Lim, A.; Yoon, C.; Jang, J.H.; Ham, H.C.; Han, J.; Nam, S.; Kim, D.; Sung, Y.E.; Choi, J.; et al. Electrochemical Synthesis of NH3 at Low Temperature and Atmospheric Pressure Using a γ-Fe2O3 Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 10986–10995. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, G.L.; Wang, L.D. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015, 269, 443–451. [Google Scholar] [CrossRef]
- Yu, Y.X.; Tan, W.; An, D.Q.; Wang, X.W.; Liu, A.; Zou, W.X.; Tang, C.J.; Ge, C.Y.; Tong, Q.; Sun, J.F.; et al. Insight into the SO2 resistance mechanism on γ-Fe2O3 catalyst in NH3-SCR reaction: A collaborated experimental and DFT study. Appl. Catal. B-Environ. 2021, 281, 119544. [Google Scholar] [CrossRef]
- Quan, H.Y.; Cheng, B.C.; Xiao, Y.H.; Lei, S.J. One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem. Eng. J. 2016, 286, 165–173. [Google Scholar] [CrossRef]
- Lu, X.F.; Chen, X.Y.; Zhou, W.; Tong, Y.X.; Li, G.R. α-Fe2O3@PANI-Core Shell Nanowire Arrays as Negative Electrodes for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 14843–14850. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.K.; Si, G.K.; Li, Y.; Zhang, S.W.; Deng, X.L.; Xu, X.J. Oxygen vacancy defects engineering on Ce-doped α-Fe2O3 gas sensor for reducing gases. Sens. Actuators B Chem. 2020, 302, 127165. [Google Scholar] [CrossRef]
- Bhatti, M.M.; Sait, S.M.; Ellahi, R. Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid. Pharmaceuticals 2022, 15, 1352. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.Z.; Lee, Y.W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic Nanoparticles: Synthesis, Stabilization, Functionalization, Characterization, and Applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Saleh, A.; Rezaee, M.; Ghambarian, M.; Hassani, R. A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal. Chim. Acta 2010, 659, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Zhuang, L.; Lin, Y.; Yan, M.D.; Lv, J.H.; Li, X.L.; Lin, H.; Zhu, P.; Lin, Q.P.; Xu, Y. Novel drug delivery system based on hollow mesoporous magnetic nanoparticles for head and neck cancers-targeted therapy in vitro and in vivo. Am. J. Cancer Res. 2020, 10, 350–364. [Google Scholar] [PubMed]
- Jin, L.; Wang, Q.; Chen, J.; Wang, Z.; Xin, H.; Zhang, D. Efficient Delivery of Therapeutic siRNA by Fe3O4 Magnetic Nanoparticles into Oral Cancer Cells. Pharmaceutics 2019, 11, 615. [Google Scholar] [CrossRef]
- Luo, D.; Shahid, S.; Hasan, S.M.; Whiley, R.; Sulzhorulzov, G.B.; Cattell, M.J. Controlled release of chlorhexidine from a HEMA-UDMA resin using a magnetic field. Dent. Mater. 2018, 34, 764–775. [Google Scholar] [CrossRef]
- Tokajuk, G.; Niemirowicz, K.; Deptuła, P.; Piktel, E.; Cieśluk, M.; Wilczewska, A.Z.; Dąbrowski, J.R.; Bucki, R. Use of magnetic nanoparticles as a drug delivery system to improve chlorhexidine antimicrobial activity. Int. J. Nanomed. 2017, 12, 7833–7846. [Google Scholar] [CrossRef]
- Patricio, T.M.F.; Mumcuoglu, D.; Montesi, M.; Panseri, S.; Witte-Bouma, J.; Garcia, S.F.; Sandri, M.; Tampieri, A.; Farrell, E.; Sprio, S. Bio-inspired polymeric iron-doped hydroxyapatite microspheres as a tunable carrier of rhBMP-2. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 119, 14. [Google Scholar]
- Xue, J.; Li, X.; Li, Q.; Lyu, J.; Wang, W.; Zhuang, L.; Xu, Y. Magnetic drug-loaded osteoinductive Fe3O4/CaCO3 hybrid microspheres system: Efficient for sustained release of antibiotics. J. Phys. D Appl. Phys. 2020, 53, 245401. [Google Scholar] [CrossRef]
- Henstock, J.R.; Rotherham, M.; El Haj, A.J. Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells. J. Tissue Eng. 2018, 9, 2041731418808695. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef] [PubMed]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia-a promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Somvanshi, S.B.; Jadhav, S.A.; Gawali, S.S.; Zakde, K.; Jadhav, K.M. Core-shell structured superparamagnetic Zn-Mg ferrite nanoparticles for magnetic hyperthermia applications. J. Alloys Compd. 2023, 947, 169574. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D-Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Kazeli, K.; Athanasiadou, A.; Makridis, A.; Malletzidou, L.; Vourlias, G.; Kontonasaki, E.; Lymperaki, E.; Angelakeris, M. Synthesis and characterization of a novel multifunctional magnetic bioceramic nanocomposite. Ceram. Int. 2023, 49, 24650–24659. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Z.; Zhang, T.; Zhang, Q.; Chen, Y.; Xie, Y.; Lei, J.; Cai, L. Biphasic Injectable Bone Cement with Fe3O4/GO Nanocomposites for the Minimally Invasive Treatment of Tumor-Induced Bone Destruction. ACS Biomater. Sci. Eng. 2019, 5, 5833–5843. [Google Scholar] [CrossRef]
- Kawashita, M. Development and evaluation of the properties of functional ceramic microspheres for biomedical applications. J. Ceram. Soc. Jpn. 2018, 126, 1–7. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, D.; Li, Y.; Xie, W.; Wang, X.; Tao, L.; Wei, Y.; Wang, X.; Zhao, L. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci. China Life Sci. 2018, 61, 448–456. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Zhu, Y.; Gelinsky, M.; Chang, J.; Cuniberti, G.; Albrecht, V.; Friis, T.; Xiao, Y. Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater. 2011, 7, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, Y.; Yu, L.; Lin, K.; Wang, X. Magnetic Hyperthermia–Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Regeneration Bioactivity by 3D-Printing Composite Scaffolds. Adv. Funct. Mater. 2020, 30, 1907071. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Liu, W.W.; Li, P.; Zhao, H.; Deng, X.L.; Wang, H.L. Electric/Magnetic Intervention for Bone Regeneration: A Systematic Review and Network Meta-Analysis. Tissue Eng. Part B Rev. 2023, 29, 217–231. [Google Scholar] [CrossRef]
- Okada, R.; Yamato, K.; Kawakami, M.; Kodama, J.; Kushioka, J.; Tateiwa, D.; Ukon, Y.; Zeynep, B.; Ishimoto, T.; Nakano, T.; et al. Low magnetic field promotes recombinant human BMP-2-induced bone formation and influences orientation of trabeculae and bone marrow-derived stromal cells. Bone Rep. 2021, 14, 100757. [Google Scholar] [CrossRef]
- Xu, H.; Hao, S.; Zhou, J. Magnetically Actuated Scaffolds to Enhance Tissue Regeneration. In Nanotechnology in Regenerative Medicine and Drug Delivery Therapy; Xu, H., Gu, N., Eds.; Springer: Singapore, 2020; pp. 1–38. [Google Scholar]
- Aydin, N.; Bezer, M. The effect of an intramedullary implant with a static magnetic field on the healing of the osteotomised rabbit femur. Int. Orthop. 2011, 35, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Kawaguchi, H.; Shimoaka, T.; Iwasaka, M.; Ueno, S.; Ozawa, H.; Nakamura, K.; Hoshi, K. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 2002, 17, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, M.C.; Ponzoni, D.; Langie, R.; Artuzi, F.E.; Puricelli, E. Effects of a buried magnetic field on cranial bone reconstruction in rats. J. Appl. Oral Sci. 2016, 24, 162–170. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.T.; Chen, J.D.; Su, J.C.; Zhi, X.; Pan, P.P.; Zou, L.; Zhang, Q.Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloid Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Abdeen, A.A.; Lee, J.; Bharadwaj, N.A.; Ewoldt, R.H.; Kilian, K.A. Temporal Modulation of Stem Cell Activity Using Magnetoactive Hydrogels. Adv. Healthc. Mater. 2016, 5, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.M.; Zhang, F.M.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.Q.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, S423–S433. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Yu, Y.; Lu, Y.; He, B.; Liu, M.; Zhuang, L.; Xu, Y.; Li, W. In Situ Rapid-Formation Sprayable Hydrogels for Challenging Tissue Injury Management. Adv. Mater. 2024, 36, 2400310. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.H.; Su, J.C. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 20. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, G.; Du, G.; Zhan, D.; Cong, Y.; Cheng, Y.; Fu, J. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B Biointerfaces 2013, 103, 318–325. [Google Scholar] [CrossRef]
- Fan, M.; Yan, J.X.; Tan, H.P.; Miao, Y.T.; Hu, X.H. Magnetic biopolymer nanogels via biological assembly for vectoring delivery of biopharmaceuticals. J. Mater. Chem. B 2014, 2, 8399–8405. [Google Scholar] [CrossRef]
- Drobota, M.; Vlad, S.; Gradinaru, L.M.; Bargan, A.; Radu, I.; Butnaru, M.; Rimbu, C.M.; Ciobanu, R.C.; Aflori, M. Composite Materials Based on Gelatin and Iron Oxide Nanoparticles for MRI Accuracy. Materials 2022, 15, 3479. [Google Scholar] [CrossRef] [PubMed]
- Fallahiarezoudar, E.; Ahmadipourroudposht, M.; Idris, A.; Yusof, N.M.; Marvibaigi, M.; Irfan, M. Characterization of maghemite (gamma-Fe2O3)-loaded poly-L-lactic acid/thermoplastic polyurethane electrospun mats for soft tissue engineering. J. Mater. Sci. 2016, 51, 8361–8381. [Google Scholar] [CrossRef]
- Farag, M.M.; Beherei, H.; Al-Rashidy, Z.M.; Farag, D.B.E.; Salem, Z.A. Dental pulp stem cell viability and osteogenic potential assessment of new Mg-phosphate magnetic bioceramic nanoparticles. J. Mater. Res. 2022, 37, 595–607. [Google Scholar] [CrossRef]
- Koto, W.; Shinohara, Y.; Kitamura, K.; Wachi, T.; Makihira, S.; Koyano, K. Porcine Dental Epithelial Cells Differentiated in a Cell Sheet Constructed by Magnetic Nanotechnology. Nanomaterials 2017, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M.Q. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Wu, A.G.; Chen, X.Y. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Yang, L.J.; Gao, J.H.; Chen, X.Y. Structure-Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, e1804567. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Güvener, N.; Dou, W.; Alghamdi, H.S.; Camargo, W.A.; Cremers, J.G.O.; Borm, P.J.A.; Heerschap, A.; Oosterwijk, E.; Jansen, J.A.; et al. A theranostic dental pulp capping agent with improved MRI and CT contrast and biological properties. Acta Biomater. 2017, 62, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L.; Wei, L. Application of dental nanomaterials: Potential toxicity to the central nervous system. Int. J. Nanomed. 2015, 10, 3547–3565. [Google Scholar]
- Melancon, M.P.; Lu, W.; Zhong, M.; Zhou, M.; Liang, G.; Elliott, A.M.; Hazle, J.D.; Myers, J.N.; Li, C.; Jason Stafford, R. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials 2011, 32, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.K.; Tai, M.F.; Chu, H.H.; Chen, S.T.; Li, H.; Lai, D.M.; Hsieh, S.T.; Wang, J.L.; Liu, H.M. Magnetic nanoparticle labeling of mesenchymal stem cells without transfection agent: Cellular behavior and capability of detection with clinical 1.5 T magnetic resonance at the single cell level. Magn. Reson. Med. 2007, 58, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Kim, K.S. Magnetic nanoparticles for bioseparation. Korean J. Chem. Eng. 2017, 34, 589–599. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Fraga-García, P.; Berensmeier, S. Bio-nano interactions: Binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater. Res. 2021, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Wang, P.P.; Li, L.L. Applications of Bacterial Magnetic Nanoparticles in Nanobiotechnology. J. Nanosci. Nanotechnol. 2016, 16, 2164–2171. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S.H. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Song, Y.S.; Ku, J.H. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol. Urodyn. 2007, 26, 584–593. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Li, M.; Xia, N.; Huang, Q.; Do, H.; Liu, Y.N.; Zhou, F. Carboxymethylated dextran-coated magnetic iron oxide nanoparticles for regenerable bioseparation. J. Nanosci. Nanotechnol. 2011, 11, 10187–10192. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.A.; Hauser, J.L.; Allen, A.C.; Lindquist, K.P.; Ramirez, A.P.; Oliver, S.; Zhang, J.Z. Fe3O4@SiO2 Nanoparticles Functionalized with Gold and Poly(vinylpyrrolidone) for Bio-Separation and Sensing Applications. ACS Appl. Nano Mater. 2018, 1, 1406–1412. [Google Scholar] [CrossRef]
- Arora, S.; Cooper, P.R.; Ratnayake, J.T.; Friedlander, L.T.; Rizwan, S.B.; Seo, B.; Hussaini, H.M. A critical review of in vitro research methodologies used to study mineralization in human dental pulp cell cultures. Int. Endod. J. 2022, 55, 3–13. [Google Scholar] [CrossRef]
- Calenic, B.; Ishkitiev, N.; Yaegaki, K.; Imai, T.; Costache, M.; Tovaru, M.; Tovaru, S.; Parlatescu, I. Characterization of oral keratinocyte stem cells and prospects of its differentiation to oral epithelial equivalents. Rom. J. Morphol. Embryol. 2010, 51, 641–645. [Google Scholar] [PubMed]
- Calenic, B.; Ishkitiev, N.; Yaegaki, K.; Imai, T.; Kumazawa, Y.; Nasu, M.; Hirata, T. Magnetic separation and characterization of keratinocyte stem cells from human gingiva. J. Periodontal Res. 2010, 45, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, D.; Zhan, Q.; Li, X.; Shan, P.; Hu, Y.; Ding, H.; Wang, Y.; Zhang, L.; Zhang, Y.; et al. Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics 2019, 9, 7680–7696. [Google Scholar] [CrossRef]
- Hu, J.; Xie, M.; Wen, C.Y.; Zhang, Z.L.; Xie, H.Y.; Liu, A.A.; Chen, Y.Y.; Zhou, S.M.; Pang, D.W. A multicomponent recognition and separation system established via fluorescent, magnetic, dualencoded multifunctional bioprobes. Biomaterials 2011, 32, 1177–1184. [Google Scholar] [CrossRef]
- Rudolf, B.; Salmain, M.; Wilczewska, A.Z.; Kubicka, A.; Misztalewska, I.; Fischer-Durand, N. Fabrication of multifunctional magnetic nanoparticles bearing metallocarbonyl probes and antibodies. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 142–151. [Google Scholar] [CrossRef]
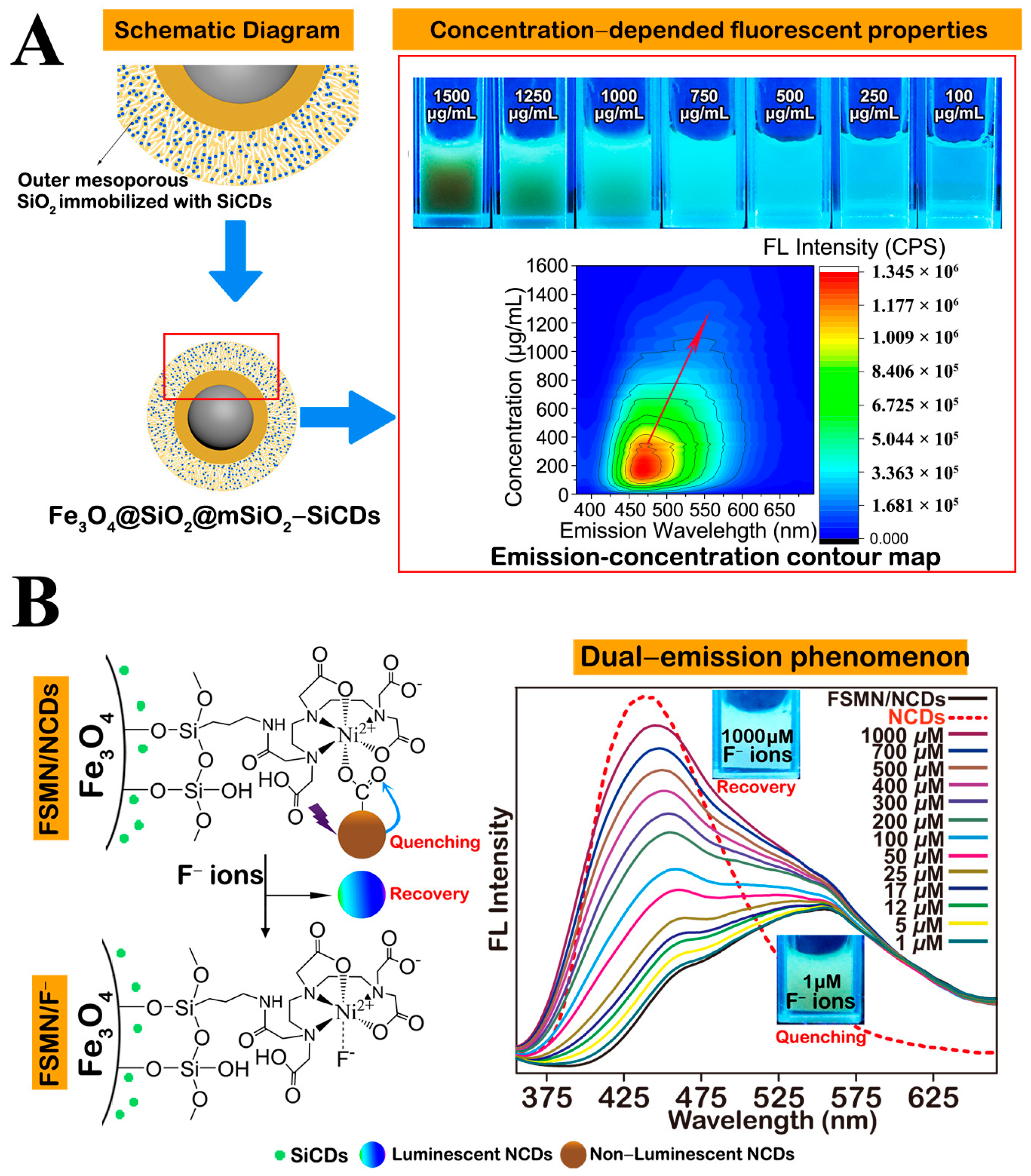
- Li, X.; Wang, W.; Li, Q.; Lin, H.; Xu, Y.; Zhuang, L. Design of Fe3O4@SiO2@mSiO2-organosilane carbon dots nanoparticles: Synthesis and fluorescence red-shift properties with concentration dependence. Mater. Des. 2018, 151, 89–101. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Li, Q.; Xue, J.; Xu, Y.; Zhuang, L. Recyclable Magnetic Fluorescent Fe3O4@SiO2 Core–Shell Nanoparticles Decorated with Carbon Dots for Fluoride Ion Removal. ACS Appl. Nano Mater. 2021, 4, 3062–3074. [Google Scholar] [CrossRef]
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Zavabet, A.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; McConville, C.F.; et al. Antibacterial Liquid Metals: Biofilm Treatment via Magnetic Activation. ACS Nano 2020, 14, 802–817. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Swiecicka, I.; Wilczewska, A.Z.; Markiewicz, K.H.; Surel, U.; Kulakowska, A.; Namiot, Z.; Szynaka, B.; Bucki, R.; Car, H. Growth arrest and rapid capture of select pathogens following magnetic nanoparticle treatment. Colloid Surf. B-Biointerfaces 2015, 131, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, H.J.; Kim, J.A.; Lee, S.H.; Kim, J.H.; Yoon, J.; Park, T.H. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 2011, 84, 41–45. [Google Scholar] [CrossRef]
- Taylor, E.; Webster, T.J. Reducing infections through nanotechnology and nanoparticles. Int. J. Nanomed. 2011, 6, 1463–1473. [Google Scholar]
- Huang, Y.; Liu, Y.; Pandey, N.K.; Shah, S.; Simon-Soro, A.; Hsu, J.C.; Ren, Z.; Xiang, Z.; Kim, D.; Ito, T.; et al. Iron oxide nanozymes stabilize stannous fluoride for targeted biofilm killing and synergistic oral disease prevention. Nat. Commun. 2023, 14, 6087. [Google Scholar] [CrossRef]
- Baskaran, P.; Udduttula, A.; Uthirapathy, V. Development and characterisation of novel Ce-doped hydroxyapatite-Fe3O4 nanocomposites and their in vitro biological evaluations for biomedical applications. Iet Nanobiotechno. 2018, 12, 138–146. [Google Scholar] [CrossRef]
- Wang, B.; Handschuh-Wang, S.; Shen, J.; Zhou, X.C.; Guo, Z.G.; Liu, W.M.; Pumera, M.; Zhang, L. Small-Scale Robotics with Tailored Wettability. Adv. Mater. 2023, 35, e2205732. [Google Scholar] [CrossRef]
- Yu, J.F.; Wang, B.; Du, X.Z.; Wang, Q.Q.; Zhang, L. Ultra-extensible ribbon-like magnetic microswarm. Nat. Commun. 2018, 9, 3260. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.J.; Babeer, A.; Liu, Y.; Ren, Z.; Wu, J.; Issadore, D.A.; Stebe, K.J.; Lee, D.; Steager, E.; Koo, H. Surface Topography-Adaptive Robotic Superstructures for Biofilm Removal and Pathogen Detection on Human Teeth. ACS Nano 2022, 16, 11998–12012. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.J.; Yoon, S.; Babeer, A.; Liu, Y.; Ren, Z.; Xiang, Z.; Miao, Y.; Cormode, D.P.; Chen, C.; Steager, E.; et al. Nanozyme-Based Robotics Approach for Targeting Fungal Infection. Adv. Mater. 2024, 36, 2300320. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martinez, C.C.; Zelenka, J.; Klima, K.; Kubanova, M.; Ruml, T.; Pumera, M. Multimodal-Driven Magnetic Microrobots with Enhanced Bactericidal Activity for Biofilm Eradication and Removal from Titanium Mesh. Adv. Mater. 2023, 35, 2300191. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Zelenka, J.; Klima, K.; Mayorga-Burrezo, P.; Hoang, L.; Ruml, T.; Pumera, M. Swarming Magnetic Photoactive Microrobots for Dental Implant Biofilm Eradication. ACS Nano 2022, 16, 8694–8703. [Google Scholar] [CrossRef]
- Craciunescu, I.; Ispas, G.M.; Ciorita, A.; Leostean, C.; Illes, E.; Turcu, R.P. Novel Magnetic Composite Materials for Dental Structure Restoration Application. Nanomaterials 2023, 13, 1215. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.H.; Ai, H.; Chen, X.Y. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Fischer, H.C.; Chan, W.C.W. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef]
- Saravanan, J.; Nair, A.; Krishna, S.S.; Viswanad, V. Nanomaterials in biology and medicine: A new perspective on its toxicity and applications. Drug Chem. Toxicol. 2024, 18. [Google Scholar] [CrossRef]
- Somasundaran, P.; Fang, X.; Ponnurangam, S.; Li, B. Nanoparticles: Characteristics, Mechanisms and Modulation of Biotoxicity. Kona Powder Part. J. 2010, 28, 38–49. [Google Scholar] [CrossRef]
- Wang, W.P.; He, S.L.; Hong, T.T.; Zhang, Y.M.; Sui, H.; Zhang, X.; Ma, Y.N. Synthesis, self-assembly, and in vitro toxicity of fatty acids-modified Bletilla striata polysaccharide. Artif. Cells Nanomed. Biotechnol. 2017, 45, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Yang, V.C. Magnetic brain tumor targeting and biodistribution of long-circulating PEG-modified, cross-linked starch-coated iron oxide nanoparticles. Biomaterials 2011, 32, 6291–6301. [Google Scholar] [CrossRef]
- Liu, Y.P.; Xia, Q.Y.; Liu, Y.; Zhang, S.Y.; Cheng, F.; Zhong, Z.H.; Wang, L.; Li, H.X.; Xiao, K. Genotoxicity assessment of magnetic iron oxide nanoparticles with different particle sizes and surface coatings. Nanotechnology 2014, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Connell, J.J.; Payne, C.; Patrick, P.S.; Baker, R.; Yu, Y.C.; Siow, B.; Zaw-Thin, M.; Kalber, T.L.; Pankhurst, Q.A.; et al. Scalable magnet geometries enhance tumour targeting of magnetic nano-carriers. Mater. Des. 2020, 191, 108610. [Google Scholar] [CrossRef]
- Shetake, N.G.; Ali, M.; Kumar, A.; Bellare, J.; Pandey, B.N. Theranostic magnetic nanoparticles enhance DNA damage and mitigate doxorubicin-induced cardio-toxicity for effective multi-modal tumor therapy. Biomater. Adv. 2022, 142, 213147. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2021, 15, 167–204. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kuwajima, Y.; Lee, C.; Ogawa, K.; Da Silva, J.D.; Ishikawa-Nagai, S. Effect of Magnet Position on Tipping and Bodily Tooth Movement in Magnetic Force-Driven Orthodontics. Materials 2020, 13, 3588. [Google Scholar] [CrossRef]
- Aksu, A.E.; Dursun, E.; Calis, M.; Ersu, B.; Safak, T.; Tozum, T.F. Intraoral Use of Extraoral Implants for Oral Rehabilitation of a Pediatric Patient After Resection of Ewing Sarcoma of the Mandible and Reconstruction with Iliac Osteocutaneous Free Flap. J. Craniofacial Surg. 2014, 25, 930–933. [Google Scholar] [CrossRef]
- Florian, G.; Gabor, A.R.; Nicolae, C.A.; Iacobescu, G.; Stanica, N.; Marasescu, P.; Petrisor, I.; Leulescu, M.; Degeratu, S.; Gîngu, O.; et al. Physical properties (thermal, thermomechanical, magnetic, and adhesive) of some smart orthodontic wires. J. Therm. Anal. Calorim. 2018, 134, 189–208. [Google Scholar] [CrossRef]
- Meral, O.; Yüksel, S. Skeletal and dental effects during observation and treatment with a magnetic device. Angle Orthod. 2003, 73, 716–722. [Google Scholar] [PubMed]
- Han, X.; Lu, H.; Li, S.; Xu, Y.; Zhao, N.; Xu, Y.; Zhao, W. Cell morphologic changes and PCNA expression within craniofacial sutures during monkey Class III treatment. Orthod. Craniofacial Res. 2016, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Feng, J.; Hu, Z.; Chen, R.J.; Shen, G. Effects of a novel magnetic orthopedic appliance (MOA-III) on the dentofacial complex in mild to moderate skeletal class III children. Head Face Med. 2015, 11, 10. [Google Scholar] [CrossRef]
- Frederick, D. Magnetically Implantable Prosthetic Device and Method to Shorten Healing Time, Enhance Bone Fusion, and Retard Bacterial Growth. U.S. Patent No. 8,475,167, 2 July 2009. [Google Scholar]
- Nalabothu, P.; Verna, C.; Benitez, B.K.; Dalstra, M.; Mueller, A.A. Load Transfer during Magnetic Mucoperiosteal Distraction in Newborns with Complete Unilateral and Bilateral Orofacial Clefts: A Three-Dimensional Finite Element Analysis. Appl. Sci. 2020, 10, 7728. [Google Scholar] [CrossRef]
- Akin, H.; Coskun, M.E.; Akin, E.G.; Ozdemir, A.K. Evaluation of the attractive force of different types of new-generation magnetic attachment systems. J. Prosthet. Dent. 2011, 105, 203–207. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, X. Current Application of Magnetic Materials in the Dental Field. Magnetochemistry 2024, 10, 46. https://doi.org/10.3390/magnetochemistry10070046
Yu Y, Li X. Current Application of Magnetic Materials in the Dental Field. Magnetochemistry. 2024; 10(7):46. https://doi.org/10.3390/magnetochemistry10070046
Chicago/Turabian StyleYu, Yilin, and Xiaolei Li. 2024. "Current Application of Magnetic Materials in the Dental Field" Magnetochemistry 10, no. 7: 46. https://doi.org/10.3390/magnetochemistry10070046
APA StyleYu, Y., & Li, X. (2024). Current Application of Magnetic Materials in the Dental Field. Magnetochemistry, 10(7), 46. https://doi.org/10.3390/magnetochemistry10070046







