A B-Box Transcription Factor CoBBX24 from Camellia oleifera Delays Leaf Senescence and Enhances Drought Tolerance in Arabidopsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.3. Isolation and Sequence Analysis of CoBBX24
2.4. Subcellular Localization Analysis of CoBBX24
2.5. Transactivation Activity Analysis of CoBBX24
2.6. Transformation of Arabidopsis
2.7. ABA Treatment of Transgenic Arabidopsis
2.8. Determination of Chlorophyll Content
2.9. Statistical Analysis
3. Results
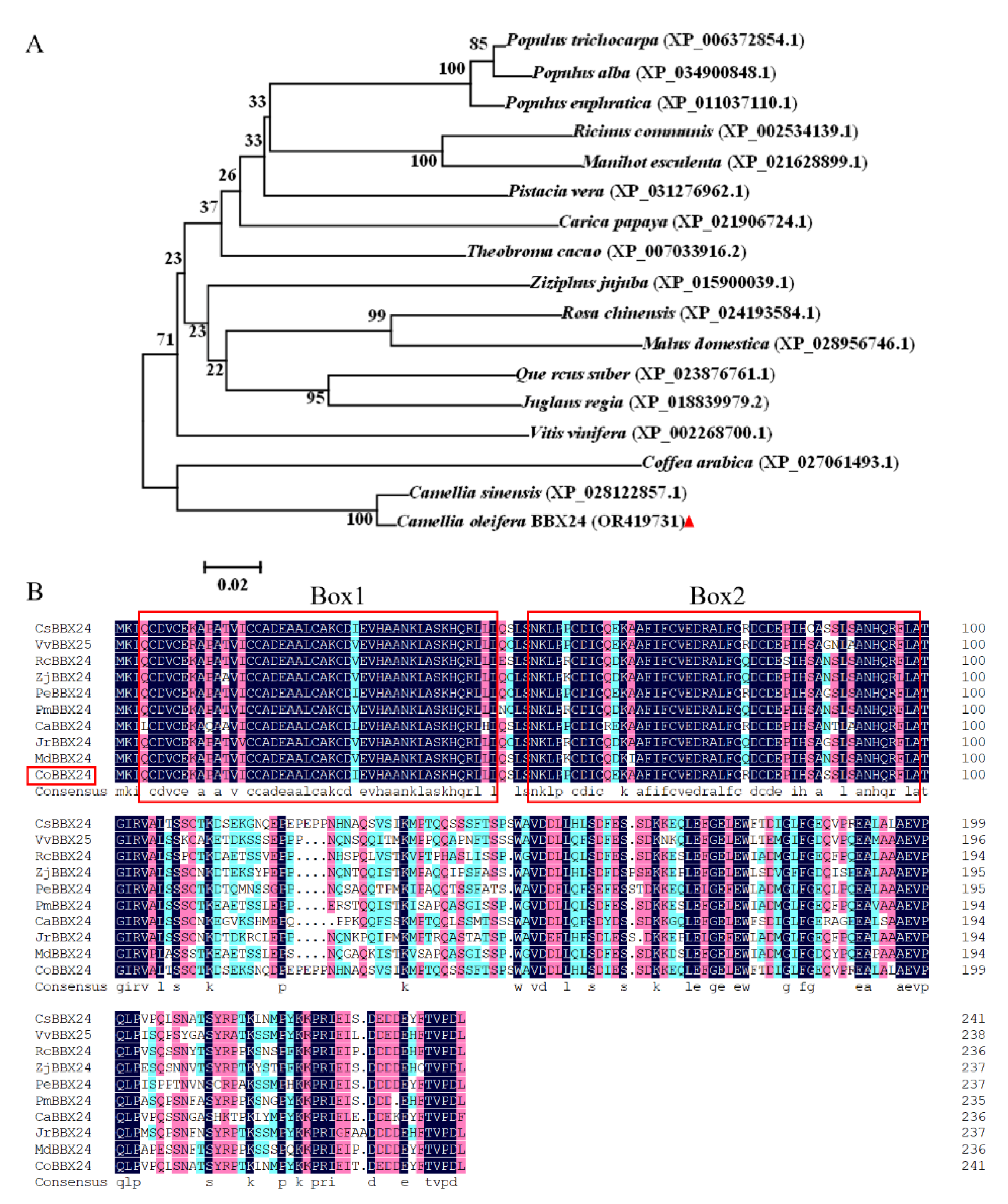
3.1. Cloning and Sequence Analysis of CoBBX24
3.2. Subcellular Localization, Transcriptional Activation, and Transcriptional Profiling of CoBBX24
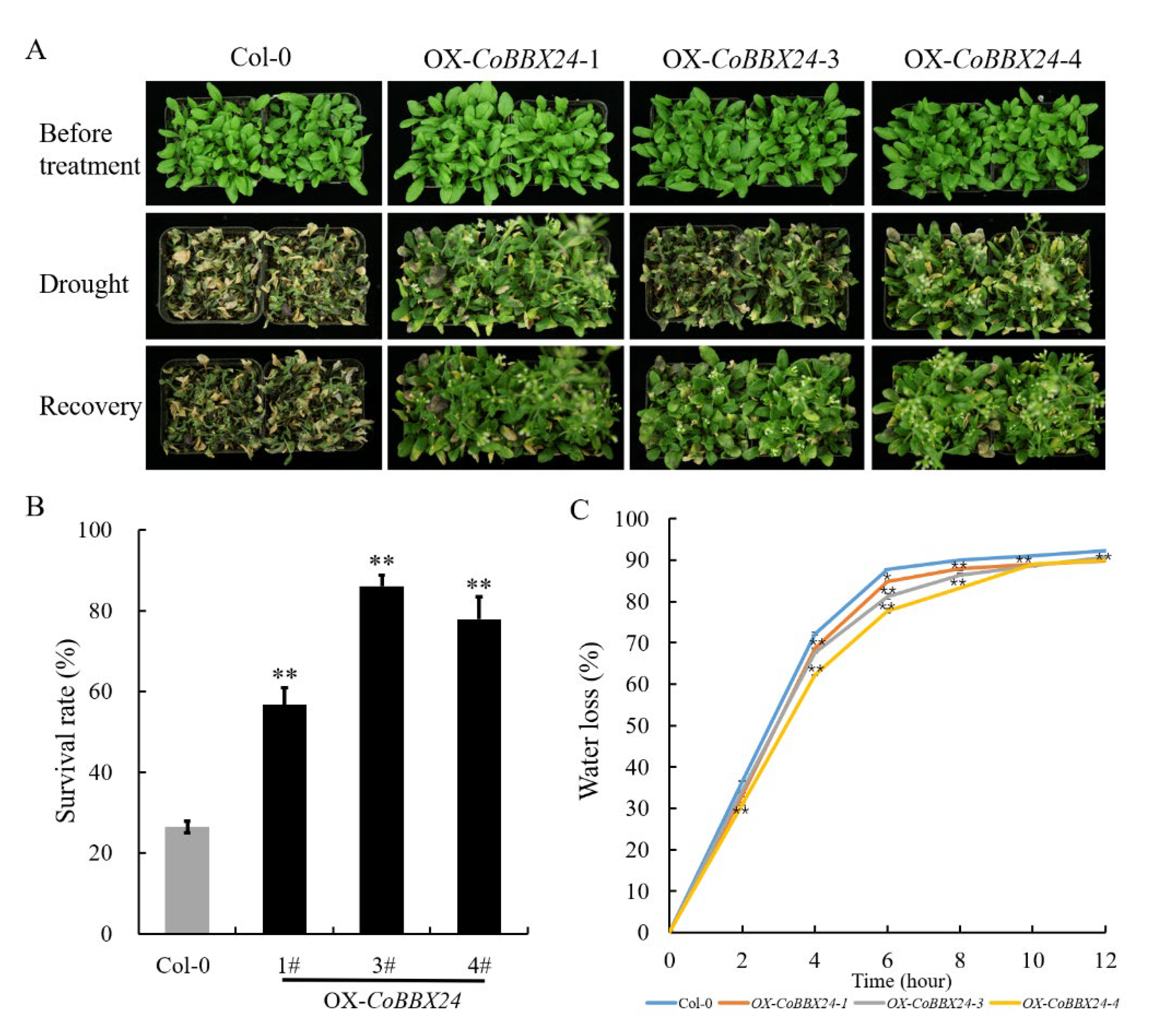
3.3. Overexpression of CoBBX24 Confers Drought Tolerance in Arabidopsis
3.4. Overexpression of CoBBX24 in Arabidopsis Improves ABA Sensitivity
3.5. Overexpression of CoBBX24 Reduces ABA-Induced Leaf Senescence in Arabidopsis
3.6. CoBBX24 Regulates the Transcription of SAGs and CCGs in Arabidopsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf senescence: Systems and dynamics aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Hong, S.W.; Whang, S.S.; Lim, P.O.; Nam, H.G.; Koo, J.C. Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol. 2011, 52, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.R.; Gao, M.; Li, Y.F.; Zhang, J.L.; Su, H.; Cao, M.; Liu, Z.J.; Zhang, X.C.; Zhao, B.; Guo, Y.D.; et al. The transcription factor SlWRKY37 positively regulates jasmonic acid- and dark-induced leaf senescence in tomato. J. Exp. Bot. 2022, 73, 6207–6225. [Google Scholar] [CrossRef]
- Janack, B.; Sosoi, P.; Krupinska, K.; Humbeck, K. Knockdown of WHIRLY1 affects drought stress-induced leaf senescence and histone modifications of the senescence-associated gene HvS40. Plants 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Guo, Y.S.; Joan, H.I.; Tu, Y.G.; Adil, M.F.; Sehar, S.; Zhao, D.G.; Shamsi, I.H. iTRAQ-based comparative proteomic analysis reveals high temperature accelerated leaf senescence of tobacco (Nicotiana tabacum L.) during flue-curing. Genomics 2020, 112, 3075–3088. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.W.; Sang, L.J.; Xie, H.L.; Chai, M.F.; Wang, Z.Y. Comparative transcriptome analysis of salt stress-induced leaf senescence in Medicago truncatula. Front. Plant Sci. 2021, 12, 666660. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Hao, Y.J. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation. Plant Biotechnol. J. 2019, 17, 2231–2233. [Google Scholar] [CrossRef] [PubMed]
- Quirino, B.F.; Noh, Y.S.; Himelblau, E.; Amasino, R.M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- Smith, L.M. Salicylic acid, senescence, and heterosis. Plant Physiol. 2019, 180, 3–4. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhao, Y.; Liu, X.C.; Peng, J.Y.; Guo, H.W.; Luo, J.C. LSD 2.0: An update of the leaf senescence database. Nucleic Acids Res. 2014, 42, 1200–1205. [Google Scholar] [CrossRef]
- Gao, S.; Gao, J.; Zhu, X.Y.; Song, Y.; Li, Z.P.; Ren, G.D.; Zhou, X.; Kuai, B.K. ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, Z.L.; Gao, J.H.; Xing, L.; Cao, M.J.; Yu, C.M.; Hu, Y.L.; You, J.; Shi, H.T.; Zhu, Y.F.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Cheng, Q.; Zhao, Y.T.; Wu, F.F.; Mu, B.B.; Gao, J.P.; Yang, L.; Yan, J.L.; Zhang, H.F.; Cui, X.; et al. The abscisic acid-responsive element binding factors MAPKKK18 module regulates abscisic acid-induced leaf senescence in Arabidopsis. J. Biol. Chem. 2023, 299, 103060. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, J.M.; Kang, S.K.; Kim, S.G.; Park, C.M. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 2011, 233, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Wu, S.X.; Chen, J.Y.; Wang, X.Y.; Gao, J.; Ren, G.D.; Kuai, B.K. NYEs/SGRs-mediated chlorophyll degradation is critical for detoxification during seed maturation in Arabidopsis. Plant J. 2017, 92, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.X.; Li, Z.P.; Yang, L.F.; Xie, Z.K.; Chen, J.Y.; Zhang, W.; Liu, T.Q.; Gao, S.; Gao, J.; Zhu, Y.H.; et al. NON-YELLOWING2 (NYE2), a close paralog of NYE1, plays a positive role in chlorophyll degradation in Arabidopsis. Mol. Plant 2016, 9, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morita, R.; Katsuma, S.; Nishimura, M.; Tanaka, A.; Kusaba, M. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J. 2009, 57, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Ito, H.; Hu, X.Y.; Tanaka, A. Accumulation of the NON-YELLOW COLORING 1 protein of the chlorophyll cycle requires chlorophyll b in Arabidopsis thaliana. Plant J. 2015, 81, 586–596. [Google Scholar] [CrossRef]
- Kang, J.; Peng, Y.F.; Xu, W.F. Crop root responses to drought stress: Molecular mechanisms, nutrient regulations, and interactions with microorganisms in the rhizosphere. Int. J. Mol. Sci. 2022, 23, 9310. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Marias, D.E.; Meinzer, F.C.; Still, C. Impacts of leaf age and heat stress duration on photosynthetic gas exchange and foliar nonstructural carbohydrates in Coffea arabica. Ecol. Evol. 2016, 7, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Xiong, L.Z. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life. Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Crocco, C.D.; Botto, J.F. BBX proteins in green plants: Insights into their evolution, structure, feature and functional diversification. Gene 2013, 531, 44–52. [Google Scholar] [CrossRef]
- Yan, H.L.; Marquardt, K.; Indorf, M.; Jutt, D.; Kircher, S.; Neuhaus, G. Nuclear localization and interaction with COP1 are required for STO/BBX24 function during photomorphogenesis. Plant Physiol. 2011, 156, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, S.; Song, Z.T.; Jiang, Y.; Han, J.J.; Lu, S.J.; Li, L.; Liu, J.X. Two B-Box domain proteins, BBX18 and BBX23, interact with ELF3 and regulate thermomorphogenesis in Arabidopsis. Cell Rep. 2018, 25, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Crocco, C.D.; Holm, M.; Yanovsky, M.J.; Botto, J.F. AtBBX21 and COP1 genetically interact in the regulation of shade avoidance. Plant J. 2010, 64, 551–562. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Zhai, H.; Gao, S.P.; Yang, L.; Wang, Z.; Xu, Y.T.; Huo, J.X.; Ren, Z.T.; Zhao, N.; et al. IbBBX24 promotes the jasmonic acid pathway and enhances fusarium wilt resistance in sweet potato. Plant Cell. 2020, 32, 1102–1123. [Google Scholar] [CrossRef]
- Dong, J.J.; Zhao, C.L.; Zhang, J.; Ren, Y.C.; He, L.H.; Tang, R.M.; Wang, W.B.; Jia, X.Y. The sweet potato B-box transcription factor gene IbBBX28 negatively regulates drought tolerance in transgenic Arabidopsis. Front. Genet. 2022, 13, 1077958. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, X.; Gao, X.R.; Dai, Z.R.; Cui, Y.F.; Zhi, Y.H.; Liu, Q.C.; Zhai, H.; Gao, S.P.; et al. The IbBBX24-IbTOE3-IbPRX17 module enhances abiotic stress tolerance by scavenging reactive oxygen species in sweet potato. New Phytol. 2002, 233, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Shen, J.Q.; Xu, Y.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice. J. Exp. Bot. 2016, 67, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Cheng, H.; Cheng, P.L.; Wang, C.M.; Li, J.Y.; Liu, Y.; Song, A.P.; Chen, S.M.; Chen, F.D.; Wang, L.K.; et al. The BBX gene CmBBX22 negatively regulates drought stress tolerance in chrysanthemum. Hortic. Res. 2022, 9, uhac181. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Zhang, X.W.; Liu, Y.J.; Zhang, J.C.; Wang, X.F.; You, C.X.; Hao, Y.J. MdABI5 works with its interaction partners to regulate abscisic acid-mediated leaf senescence in apple. Plant J. 2021, 105, 1566–1581. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, C.L.; Li, H.L.; Wang, G.L.; You, C.X. Apple SINA E3 ligase MdSINA3 negatively mediates JA-triggered leaf senescence by ubiquitinating and degrading the MdBBX37 protein. Plant J. 2022, 111, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Li, Q.F.; Björn, L.O.; He, J.X.; Li, S.S. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 2012, 22, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Chiriotto, T.S.; Saura-Sánchez, M.; Barraza, C.; Botto, J.F. BBX24 increases saline and osmotic tolerance through ABA signaling in Arabidopsis seeds. Plants 2023, 12, 2392. [Google Scholar] [CrossRef]
- Yang, Y.J.; Ma, C.; Xu, Y.J.; Wei, Q.; Imtiaz, M.; Lan, H.B.; Gao, S.; Cheng, L.N.; Wang, M.Y.; Fei, Z.J.; et al. A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell 2014, 26, 2038–2054. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Coutu, C.; Brandle, J.; Brown, D.; Brown, K.; Miki, B.; Simmonds, J.; Hegedus, D.D. pORE: A modular binary vector series suited for both monocot and dicot plant transformation. Transgenic Res. 2007, 16, 771–781. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, Y.X.; Wang, H.B.; Wang, J.J.; Song, A.P.; Hu, Y.H.; Chen, S.M.; Jiang, J.F.; Chen, F.D. The heterologous expression of a chrysanthemum TCP-P transcription factor CmTCP14 suppresses organ size and delays senescence in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 115, 239–248. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Osaki, M.; Takebe, M.; Shinano, T.; Wasaki, J. Endogenous hormones and expression of senescence-related genes in different senescent types of maize. J. Exp. Bot. 2005, 56, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.Z.; Niu, Y.H.; Li, W.J.; Zhang, D.M. Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence. J. Exp. Bot. 2008, 59, 1295–1304. [Google Scholar] [CrossRef]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.H. The Arabidopsis B-box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef]
- Lippuner, V.; Cyert, M.S.; Gasser, C.S. Two classes of plant cDNA clones differentially complement yeast calcineurin mutants and increase salt tolerance of wild-type yeast. J. Biol. Chem. 1996, 271, 12859–12866. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Takano, T. Salt tolerance-related protein STO binds to a Myb transcription factor homologue and confers salt tolerance in Arabidopsis. J. Exp. Bot. 2003, 54, 2231–2237. [Google Scholar] [CrossRef]
- Kiełbowicz-Matuk, A.; Rey, P.; Rorat, T. Interplay between circadian rhythm, time of the day and osmotic stress constraints in the regulation of the expression of a Solanum Double B-box gene. Ann. Bot. 2014, 113, 831–842. [Google Scholar] [CrossRef]
- Woo, H.R.; Masclaux-Daubresse, C.; Lim, P.O. Plant senescence: How plants know when and how to die. J. Exp. Bot. 2018, 69, 715–718. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Gan, S.S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2012, 35, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, X.Y.; Wang, L.C.; Xu, J.; Zhang, X.L. GhTZF1 regulates drought stress responses and delays leaf senescence by inhibiting reactive oxygen species accumulation in transgenic Arabidopsis. Plant Mol. Biol. 2014, 85, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.J.; Ma, Q.; Zhang, C.; Wang, C.C.; Wei, H.L.; Wang, H.T.; Yu, S.X. The cotton GhWRKY91 transcription factor mediates leaf senescence and responses to drought stress in transgenic Arabidopsis thaliana. Front. Plant Sci. 2019, 29, 1352. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.L.; Liu, T.; Deng, Z.C.; Zhang, Z.L.; Wang, Q.; Wang, W.F.; Li, W.; Guo, Y.F. Characterization of NAC transcription factor NtNAC028 as a regulator of leaf senescence and stress responses. Front. Plant Sci. 2022, 13, 941026. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, J.; Yao, L.Y.; Ren, G.D.; Zhu, X.Y.; Gao, S.; Qiu, K.; Zhou, X.; Kuai, B.K. The role of ANAC072 in the regulation of chlorophyll degradation during age- and dark-induced leaf senescence. Plant Cell Rep. 2016, 35, 1729–1741. [Google Scholar] [CrossRef]
- Yang, J.D.; Worley, E.; Udvardi, M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 2014, 26, 4862–4874. [Google Scholar] [CrossRef]
- Qi, T.C.; Wang, J.J.; Huang, H.; Liu, B.; Gao, H.; Liu, Y.L.; Song, S.S.; Xie, D.X. Regulation of jasmonate-induced leaf senescence by antagonism between bHLH subgroup IIIe and IIId factors in Arabidopsis. Plant Cell 2015, 27, 1634–1649. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.R.; Tan, S.Y.; Li, Z.H. Transcription factors-regulated leaf senescence: Current knowledge, challenges and approaches. Int. J. Mol. Sci. 2023, 24, 9245. [Google Scholar] [CrossRef]
- Soon, F.F.; Ng, L.M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 2012, 335, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Kang, K.; Shim, Y.; Yoo, S.C.; Paek, N.C. Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol. 2022, 188, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Min, Z.; Wang, X.H.; Fang, Y.L.; Zhang, J.X. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.M.; Xie, Y.Z.; Yang, W.B.; Lv, Q.; Chen, L.P.; Li, J.T.; Meng, Y.; Li, L.Q.; Li, X.J. Membrane-bound transcription factor TaNTL1 positively regulates drought stress tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2022, 182, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Z.; Chen, Z.Z.; Liu, Y.; Zhang, H.R.; Zhang, M.; Liu, Q.; Hong, X.H.; Zhu, J.K.; Gong, Z.Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010, 63, 417–429. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, Z.; Wu, Y.; Gao, Y.; Zhang, L.; Yu, C.; Ye, S.; Liu, W. A B-Box Transcription Factor CoBBX24 from Camellia oleifera Delays Leaf Senescence and Enhances Drought Tolerance in Arabidopsis. Horticulturae 2023, 9, 991. https://doi.org/10.3390/horticulturae9090991
Liu Y, Zhu Z, Wu Y, Gao Y, Zhang L, Yu C, Ye S, Liu W. A B-Box Transcription Factor CoBBX24 from Camellia oleifera Delays Leaf Senescence and Enhances Drought Tolerance in Arabidopsis. Horticulturae. 2023; 9(9):991. https://doi.org/10.3390/horticulturae9090991
Chicago/Turabian StyleLiu, Yanan, Zhiguo Zhu, Yang Wu, Yinxiang Gao, Lisha Zhang, Changshuai Yu, Sicheng Ye, and Wenxin Liu. 2023. "A B-Box Transcription Factor CoBBX24 from Camellia oleifera Delays Leaf Senescence and Enhances Drought Tolerance in Arabidopsis" Horticulturae 9, no. 9: 991. https://doi.org/10.3390/horticulturae9090991
APA StyleLiu, Y., Zhu, Z., Wu, Y., Gao, Y., Zhang, L., Yu, C., Ye, S., & Liu, W. (2023). A B-Box Transcription Factor CoBBX24 from Camellia oleifera Delays Leaf Senescence and Enhances Drought Tolerance in Arabidopsis. Horticulturae, 9(9), 991. https://doi.org/10.3390/horticulturae9090991




