Dormancy Characteristics of Euphorbia maculata L. Seeds and Strategies for Their Effective Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Properties and Morphological Characteristics
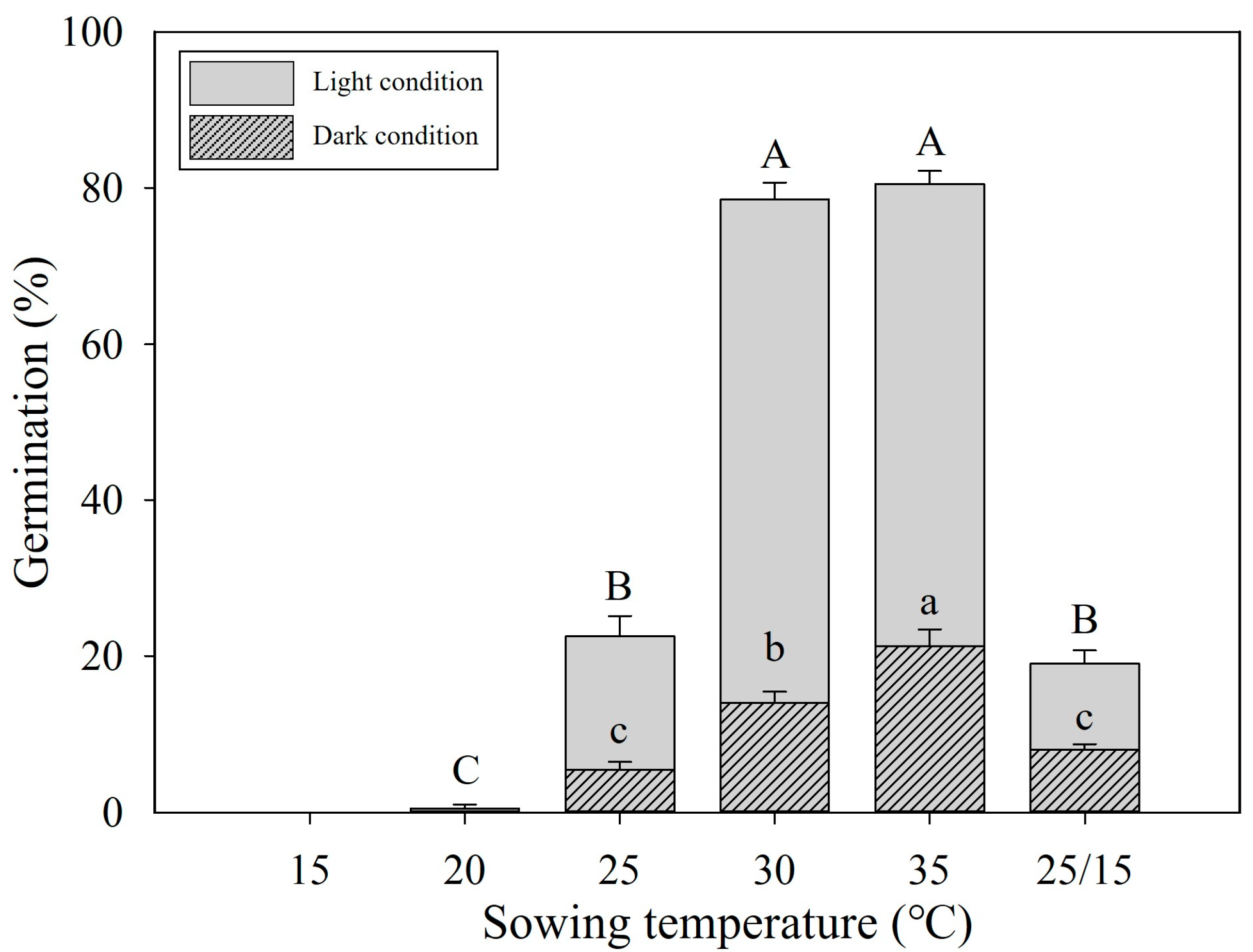
2.3. Seed Germination under Various Temperatures
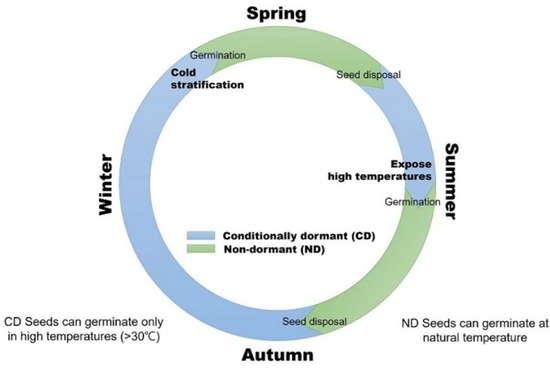
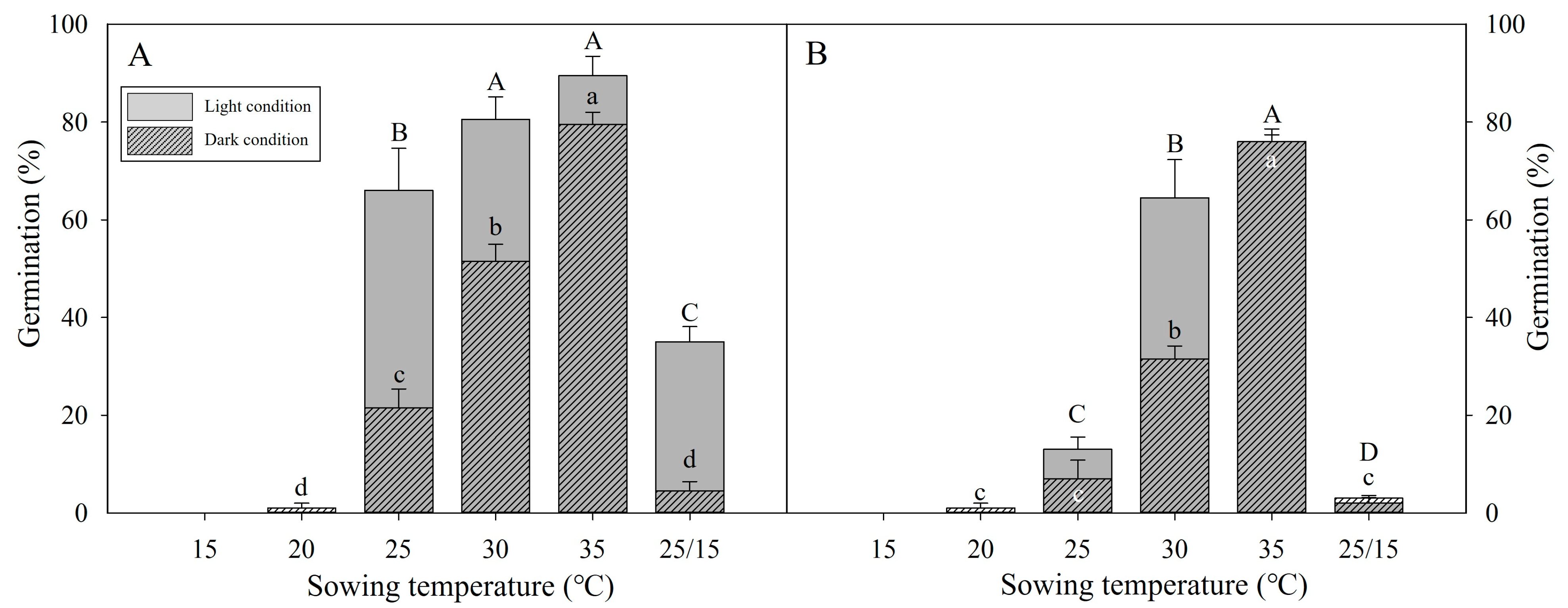
2.4. Stratification (Temperature Treatment) for Dormancy Release
2.5. High-Temperature Exposure for Dormancy Release
2.6. Maintaining Dormancy Release Status during Storage
2.7. Statistical Analyses
3. Results
3.1. Seed Properties
3.2. Optimal Germination Temperature
3.3. Stratification Can Expand Germination Temperatures
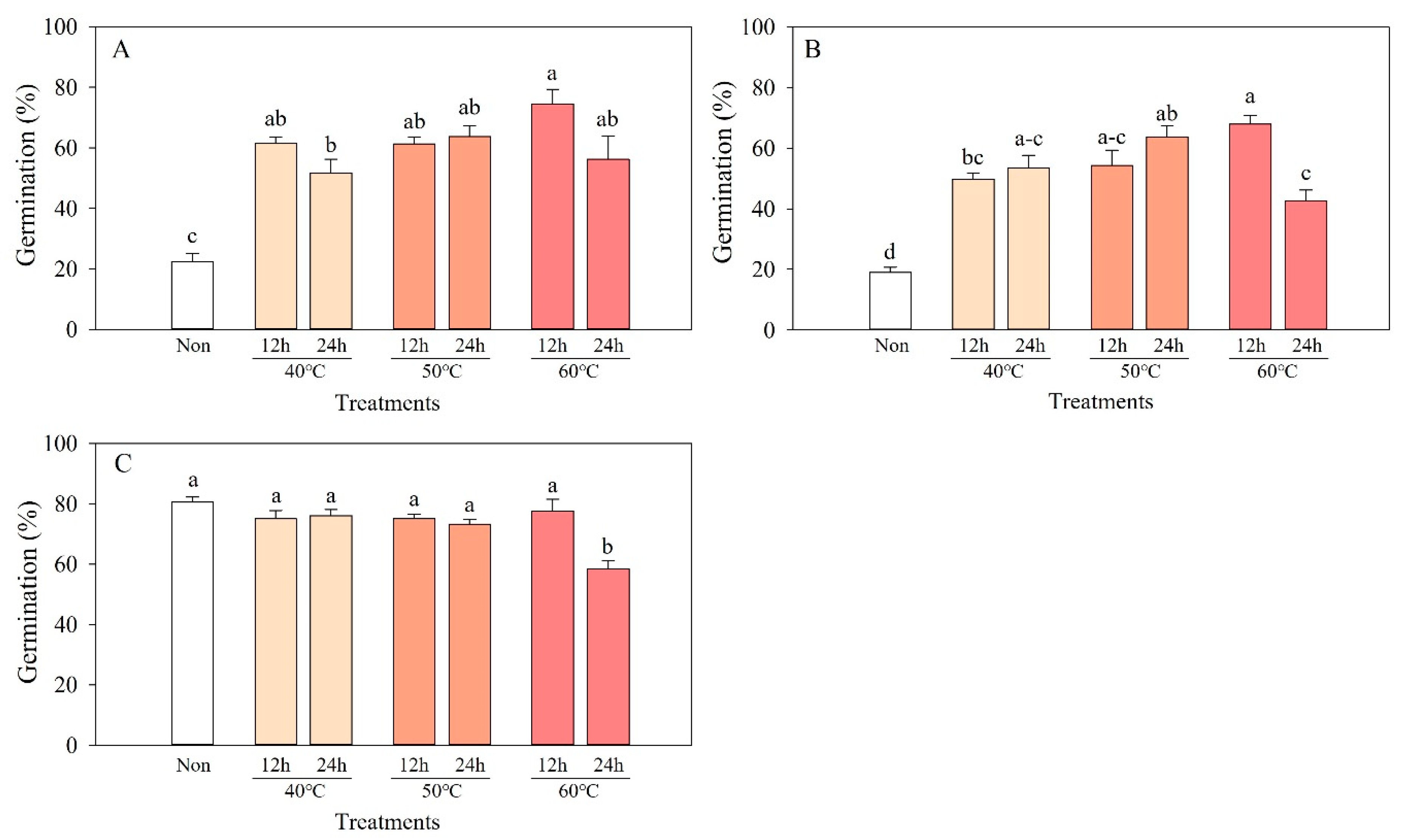
3.4. Effect of High Temperatures on Dormancy and Germination
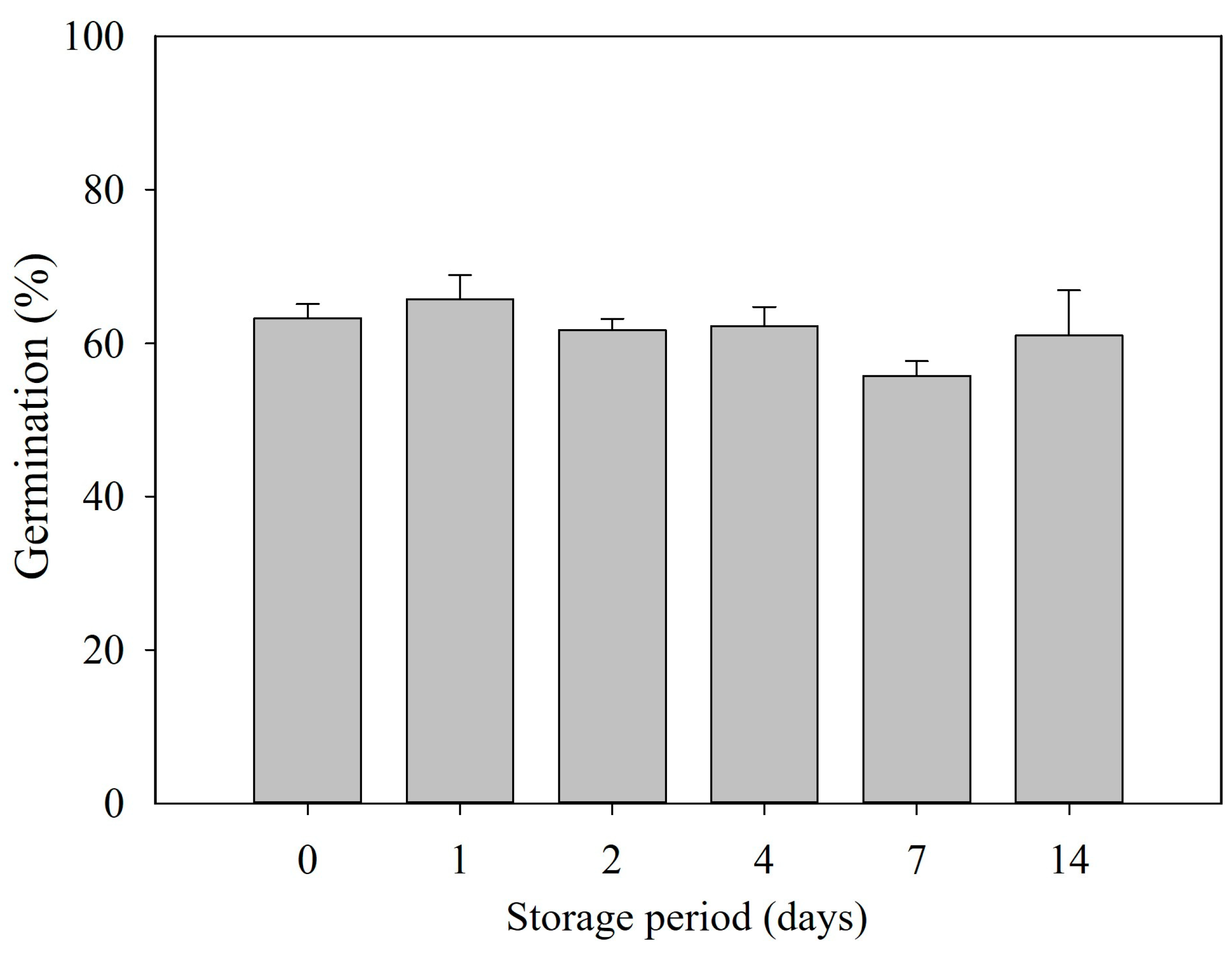
3.5. Maintain Dormancy Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805. [Google Scholar] [CrossRef] [PubMed]
- Masondo, N.A.; Makunga, N.P. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. S. Afr. J. Bot. 2019, 126, 40–57. [Google Scholar] [CrossRef]
- Patil, P.S.; Shettigar, R. An advancement of analytical techniques in herbal research. J. Adv. Sci. Res. 2010, 1, 8–14. [Google Scholar]
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, N.; Saggoo, M.I.S. Conservation strategies for medicinal plants in the face of environmental challenges. In Environmental Challenges and Medicinal Plants: Sustainable Production Solutions under Adverse Conditions; Springer International Publishing: Cham, Switzerland, 2022; pp. 461–485. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Frodin, D.G. History and concepts of big plant genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A.; Carter, S. World Checklist and Bibliography of Euphorbiaceae (with Pandaceae); Royal Botanic Gardens, Kew: Richmond, UK, 2000. [Google Scholar]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef]
- Pascal, O.A.; Bertrand, A.E.V.; Esaïe, T.; Sylvie, H.A.M.; Eloi, A.Y. A review of the ethnomedical uses, phytochemistry and pharmacology of the Euphorbia genus. J. Pharm. Innov. 2017, 6, 34. [Google Scholar] [CrossRef]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Galvez, J.; Zarzuelo, A.; Crespo, M.E.; Lorente, M.D.; Ocete, M.A.; Jiménez, J. Antidiarrhoeic activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta Med. 1993, 59, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Jain, V.; Wani, A.R. Euphorbia hirta as a gold mine of high-value phytochemicals: A comprehensive review of its pharmacological activities and possible role against SARS-CoV-2. Biomed. Res. Ther. 2022, 9, 4930–4949. [Google Scholar] [CrossRef]
- Kumar, S.; Malhotra, R.; Kumar, D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn. Rev. 2010, 4, 58–61. [Google Scholar] [CrossRef]
- Lanhers, M.C.; Fleurentin, J.; Dorfman, P.; Mortier, F.; Pelt, J.M. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 1991, 57, 225–231. [Google Scholar] [CrossRef]
- Ogbulie, J.N.; Ogueke, C.C.; Okoli, I.C.; Anyanwu, B.N. Antibacterial activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta. Afr. J. Biotechnol. 2007, 6, 1544–1548. [Google Scholar]
- Kwon, S.U.; Cha, J.Y.; Lee, H.Y.; Xin, M.; Ji, S.J.; Kim, D.K.; Park, D.S.; Pyo, M.K.; Lee, Y.M. Chloroform fraction of Euphorbia maculata has antiplatelet activity via suppressing thromboxane B2 formation. Mol. Med. Rep. 2015, 11, 4255–4261. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Lee, S.H.; Jang, H.D.; Lee, Y.M.; Kim, Y.H. Evaluation of the anti-osteoporosis and antioxidant activities of phenolic compounds from Euphorbia maculata. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 573–579. [Google Scholar] [CrossRef]
- Rakotondrabe, T.F.; Fan, M.X.; Zhang, Y.L.; Guo, M.Q. Simultaneous screening and analysis of anti-inflammatory and antiproliferative compounds from Euphorbia maculata combining bio-affinity ultrafiltration with multiple drug targets. J. Anal. Test. 2022, 6, 98–110. [Google Scholar] [CrossRef]
- Matsunaga, S.; Tanaka, R.; Akagi, M. Triterpenoids from Euphorbia maculata. Phytochemistry 1988, 27, 535–537. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.L.; Tang, M.Y.; Feng, B.M.; Pei, Y.H.; Yasukawa, K. Triterpenoids from Euphorbia maculata and their anti-inflammatory effects. Molecules 2018, 23, 2112. [Google Scholar] [CrossRef]
- Park, S.C.; Son, D.Y. Inhibitory effects of Euphorbia supina Rafin on the production of pro-inflammatory mediator by LPS-stimulated RAW 264.7 macrophages. J. Korean Soc. Food Sci. Nutr. 2011, 40, 486–492. [Google Scholar] [CrossRef]
- Rhim, T. Comparison of radical scavenging, anticytotoxic, and anti-inflammatory effects of Euphorbia maculata and E. supina. J. Environ. Sci. Int. 2016, 25, 1131–1142. [Google Scholar] [CrossRef]
- Hong, H.K.; Kwak, J.H.; Kang, S.C.; Lee, J.W.; Park, J.H.; Ahn, J.W.; Kang, H.S.; Choung, E.S.; Zee, O.P. Antioxidative constituents from the whole plants of Euphorbia supina. Korean J. Pharmacogn. 2008, 39, 260–264. [Google Scholar]
- Alinezhad, H.; Pakzad, K. Green synthesis of copper oxide nanoparticles with an extract of Euphorbia maculata and their use in the Biginelli reaction. Org. Prep. Proc. Int. 2020, 52, 319–327. [Google Scholar] [CrossRef]
- Pakzad, K.; Alinezhad, H.; Nasrollahzadeh, M. Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram. Int. 2019, 45, 17173–17182. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Jang, B.K.; Park, K.; Lee, S.Y.; Lee, H.; Song, S.K.; Kim, J.; Lee, C.H.; Cho, J.S. Comparison of the seed dormancy and germination characteristics of six Clematis species from South Korea. Sci. Hortic. 2023, 307, 111488. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva–Baskin classification system for primary seed dormancy. Seed Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Allen, P.S.; Meyer, S.E. Ecological aspects of seed dormancy loss. Seed Sci. Res. 1998, 8, 183–192. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; Rubio de Casas, R.; NESCent Germination Working Group. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef] [PubMed]
- van Klinken, R.D.; Goulier, J.B. Habitat-specific seed dormancy-release mechanisms in four legume species. Seed Sci. Res. 2013, 23, 181–188. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Leubner-Metzger, G. Seed dormancy and weed emergence: From simulating environmental change to understanding trait plasticity, adaptive evolution, and population fitness. J. Exp. Bot. 2021, 72, 4181–4185. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ohnishi, Y. Significance of the simultaneous growth of vegetative and reproductive organs in the prostrate annual Chamaesyce maculata (L.) Small (Euphorbiaceae). Ecol. Res. 2006, 21, 91–99. [Google Scholar] [CrossRef]
- Cho, H. Effects of road components and roadside vegetation on temperature reduction in Seoul considering air, wet-bulb globe, and surface temperatures. Sustainability 2022, 14, 16663. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Timing of seed germination in the weedy summer annual Euphorbia supina. Bartonia 1979, 46, 63–68. [Google Scholar] [CrossRef]
- Asgarpour, R.; Ghorbani, R.; Khajeh-Hosseini, M.; Mohammadvand, E.; Chauhan, B.S. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015, 63, 502–510. [Google Scholar] [CrossRef]
- Milberg, P.; Andersson, L. Does cold stratification level out differences in seed germinability between populations? Plant Ecol. 1998, 134, 225–234. [Google Scholar] [CrossRef]
- Aiello, N.; Lombardo, G.; Giannì, S.; Scartezzini, F.; Fusani, P. The effect of cold stratification and of gibberellic acid on the seed germination of wild musk yarrow [Achillea erba-rotta subsp. moschata (Wulfen) I. Richardson] populations. J. Appl. Res. Med. Aromat. Plants 2017, 7, 108–112. [Google Scholar] [CrossRef]
- Hu, X.W.; Ding, X.Y.; Baskin, C.C.; Wang, Y.R. Effect of soil moisture during stratification on dormancy release in seeds of five common weed species. Weed Res. 2018, 58, 210–220. [Google Scholar] [CrossRef]
- Farooq, S.; Onen, H.; Ozaslan, C.; El-Shehawi, A.M.; Elseehy, M.M. Characteristics and methods to release seed dormancy of two ground cherry (Physalis) species. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100337. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Mimicking the natural thermal environments experienced by seeds to break physiological dormancy to enhance seed testing and seedling production. Seed Sci. Technol. 2022, 50, 21–29. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Ecophysiology of secondary dormancy in seeds of Ambrosia artemisiifolia. Ecology 1980, 61, 475–480. [Google Scholar] [CrossRef]
- Visser, M.; Beaugendre, A. Conditional dormancy of Stipa lagascae (Poaceae) bulk-harvested on seed increase plots in South Tunisia: A reassessment and a surprise. Plant Ecol. Evol. 2019, 152, 450–459. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Does seed dormancy play a role in the germination ecology of Rumex crispus? Weed Sci. 1985, 33, 340–343. [Google Scholar] [CrossRef]
- Engelbrecht, M.; Bochet, E.; García-Fayos, P. Mucilage secretion: An adaptive mechanism to reduce seed removal by soil erosion? Biol. J. Linn. Soc. Lond. 2014, 111, 241–251. [Google Scholar] [CrossRef]
- Kreitschitz, A. Biological properties of fruit and seed slime envelope: How to live, fly, and not die. In Functional Surfaces in Biology; Gorb, S.N., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 11–30. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, C.C.; Baskin, J.M.; Liu, G.; Huang, Z. Seed mucilage improves seedling emergence of a sand desert shrub. PLoS ONE 2012, 7, e34597. [Google Scholar] [CrossRef]
- Phan, J.L.; Burton, R.A. New insights into the composition and structure of seed mucilage. Annu. J. Plant Rev. Online 2018, 1, 63–104. [Google Scholar] [CrossRef]
- Baker, K.S.; Steadman, K.J.; Plummer, J.A.; Merritt, D.J.; Dixon, K.W. Dormancy release in Australian fire ephemeral seeds during burial increases germination response to smoke water or heat. Seed Sci. Res. 2005, 15, 339–348. [Google Scholar] [CrossRef]
- Zirondi, H.L.; Silveira, F.A.O.; Fidelis, A. Fire effects on seed germination: Heat shock and smoke on permeable vs. impermeable seed coats. Flora 2019, 253, 98–106. [Google Scholar] [CrossRef]
- Moon, K.H.; Song, E.Y.; Wi, S.H.; Seo, H.H.; Hyun, H.N. Generation of daily temperature data using monthly mean temperature and precipitation data. Korean J. Agric. For. Meteorol. 2018, 20, 252–261. [Google Scholar]
- Suzuki, N.; Teranishi, S. Phenology and life cycle of the annual, Chamaesyce maculata (L.) Small (Euphorbiaceae), with multiple overlapping generations in Japan. Ecol. Res. 2005, 20, 425–432. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Temperature requirements for after-ripening in buried seeds of four summer annual weeds. Weed Res. 1987, 27, 385–389. [Google Scholar] [CrossRef]
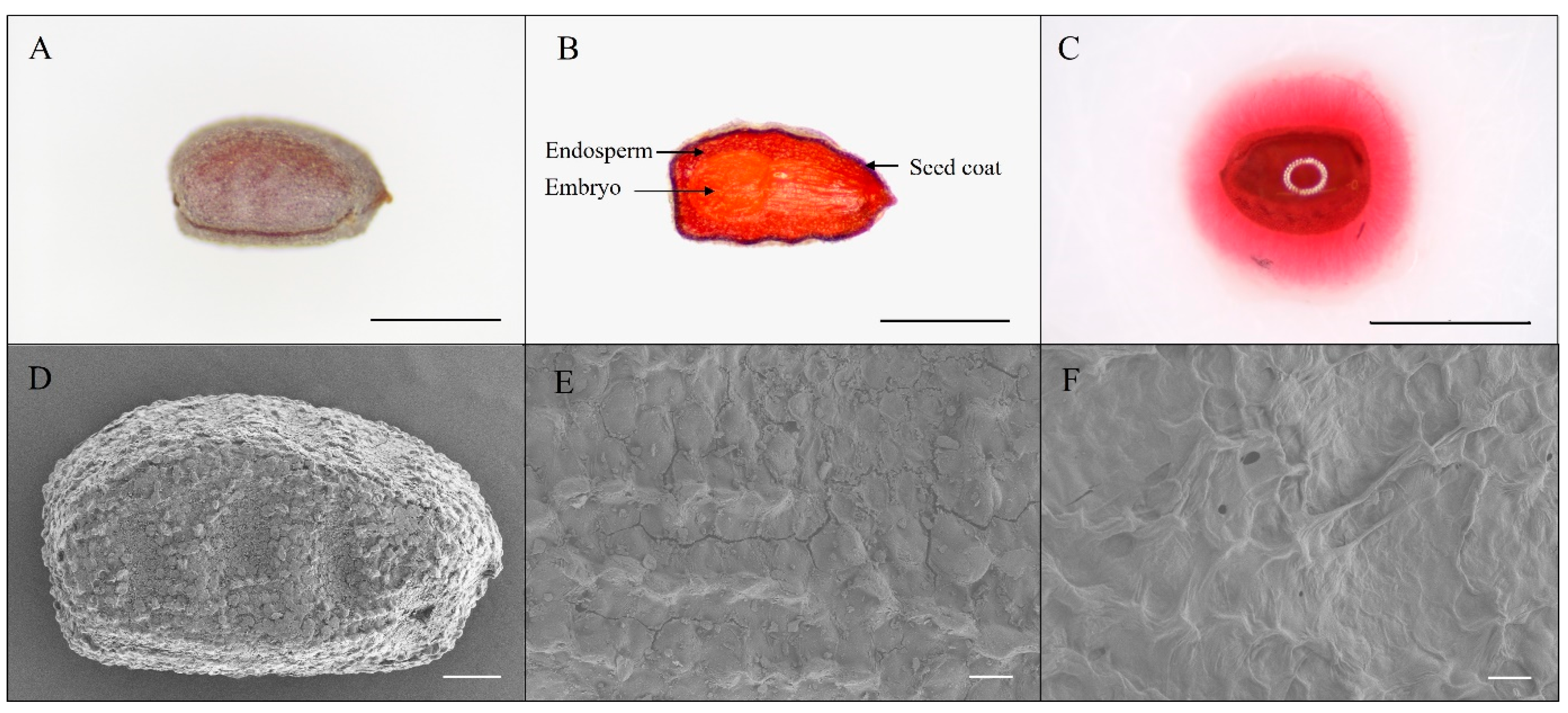
| Length (mm) | Width (mm) | Weight 1 (g) | E:S 2 Ratio | Viability (%) |
|---|---|---|---|---|
| 0.855 ± 0.012 | 0.505 ± 0.005 | 0.112 ± 0.003 | 0.84 ± 0.01 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Lee, H.; Jang, B.-K.; Cho, J.-S. Dormancy Characteristics of Euphorbia maculata L. Seeds and Strategies for Their Effective Germination. Horticulturae 2023, 9, 990. https://doi.org/10.3390/horticulturae9090990
Park K, Lee H, Jang B-K, Cho J-S. Dormancy Characteristics of Euphorbia maculata L. Seeds and Strategies for Their Effective Germination. Horticulturae. 2023; 9(9):990. https://doi.org/10.3390/horticulturae9090990
Chicago/Turabian StylePark, Kyungtae, Hamin Lee, Bo-Kook Jang, and Ju-Sung Cho. 2023. "Dormancy Characteristics of Euphorbia maculata L. Seeds and Strategies for Their Effective Germination" Horticulturae 9, no. 9: 990. https://doi.org/10.3390/horticulturae9090990
APA StylePark, K., Lee, H., Jang, B.-K., & Cho, J.-S. (2023). Dormancy Characteristics of Euphorbia maculata L. Seeds and Strategies for Their Effective Germination. Horticulturae, 9(9), 990. https://doi.org/10.3390/horticulturae9090990