Response of Dahlia Photosynthesis and Transpiration to High-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. High-Temperature Stress Treatment
2.3. Index Determination and Method
2.3.1. Heat Injury Index
2.3.2. Recovery Index
2.3.3. Determination of Stomatal Characteristics
2.3.4. Determination of Water Potential
2.3.5. Determination of Photosynthetic Pigments
2.3.6. Determination of Chl Fluorescence Parameters
2.3.7. Determination of Photosynthetic Parameters
2.4. Data Analysis
3. Results
3.1. External Morphology
3.2. Stomatal Characteristics
3.3. Leaf Water Potential
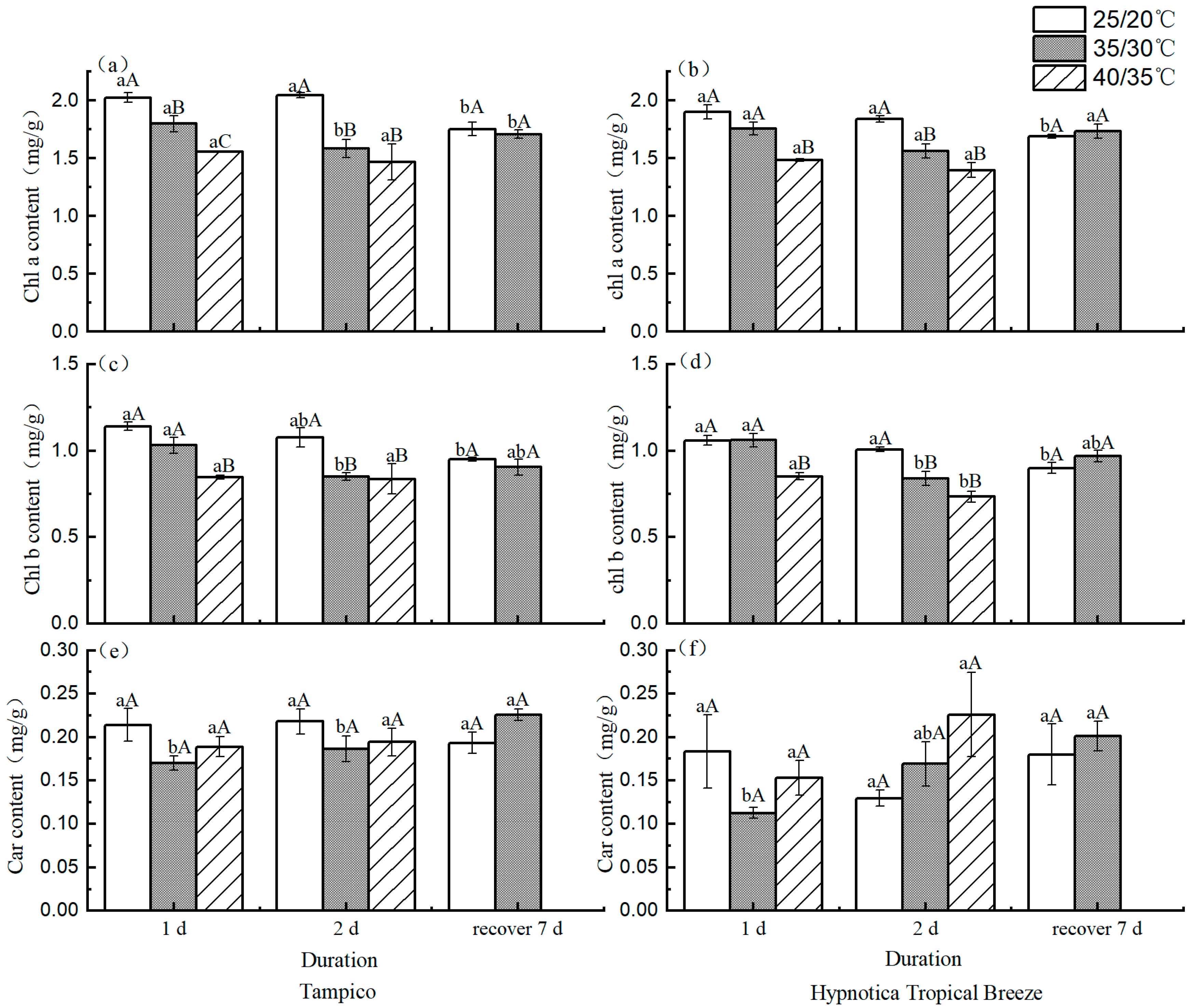
3.4. Photosynthetic Pigment Content
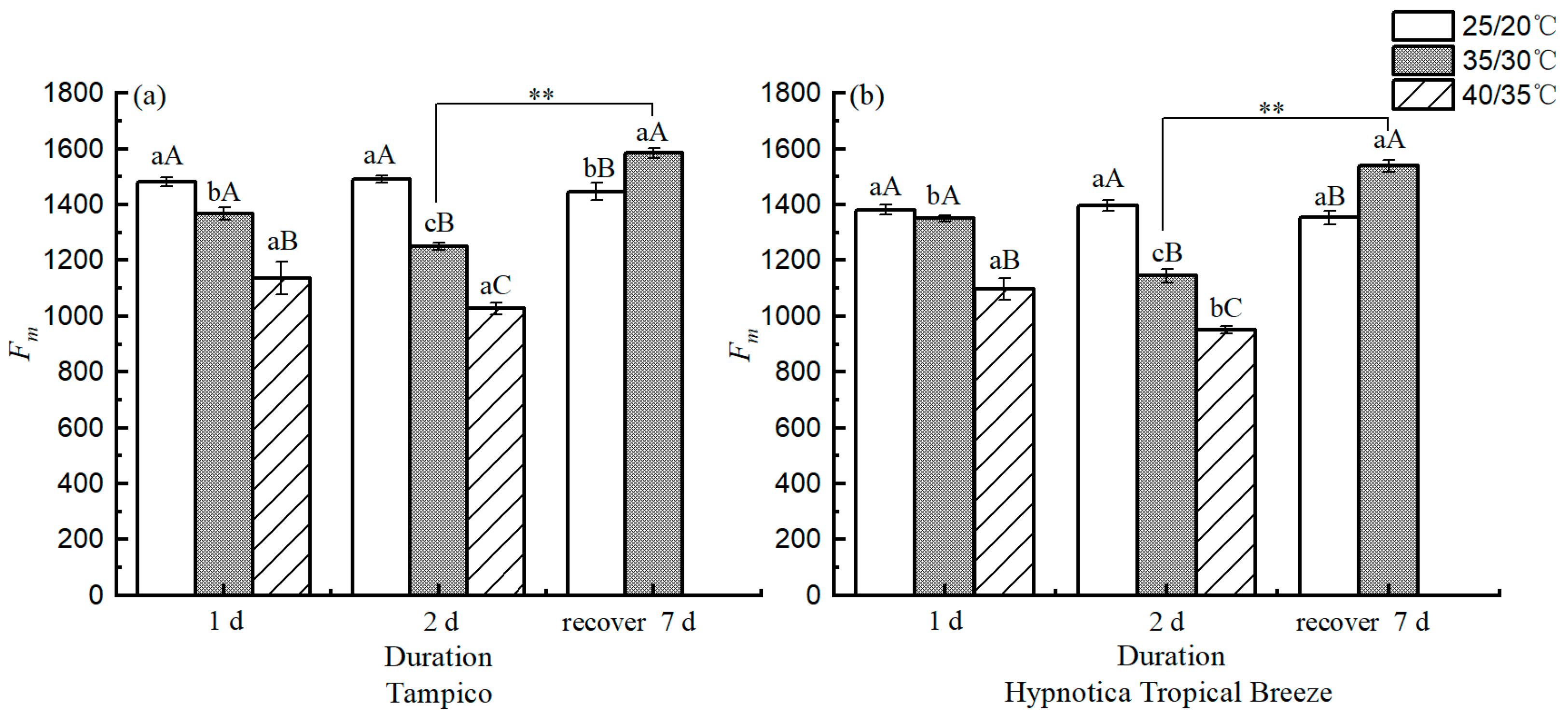
3.5. Chl fluorescence Parameters
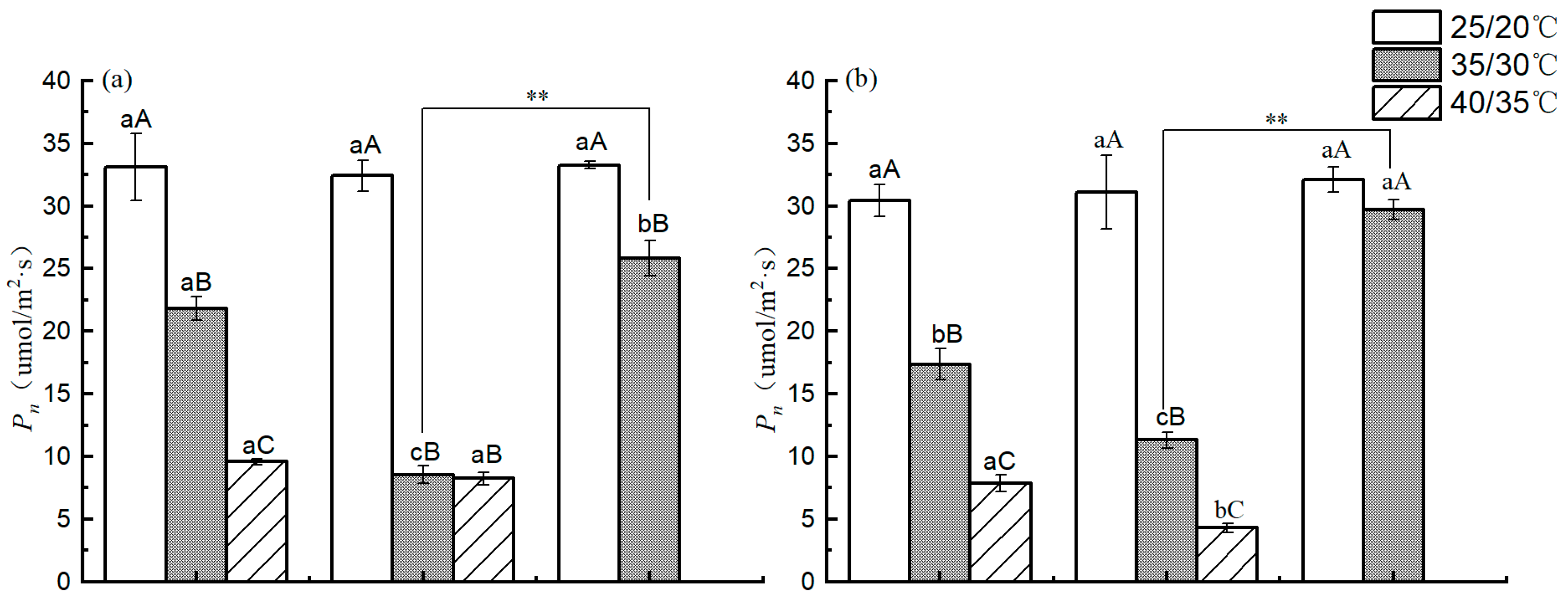
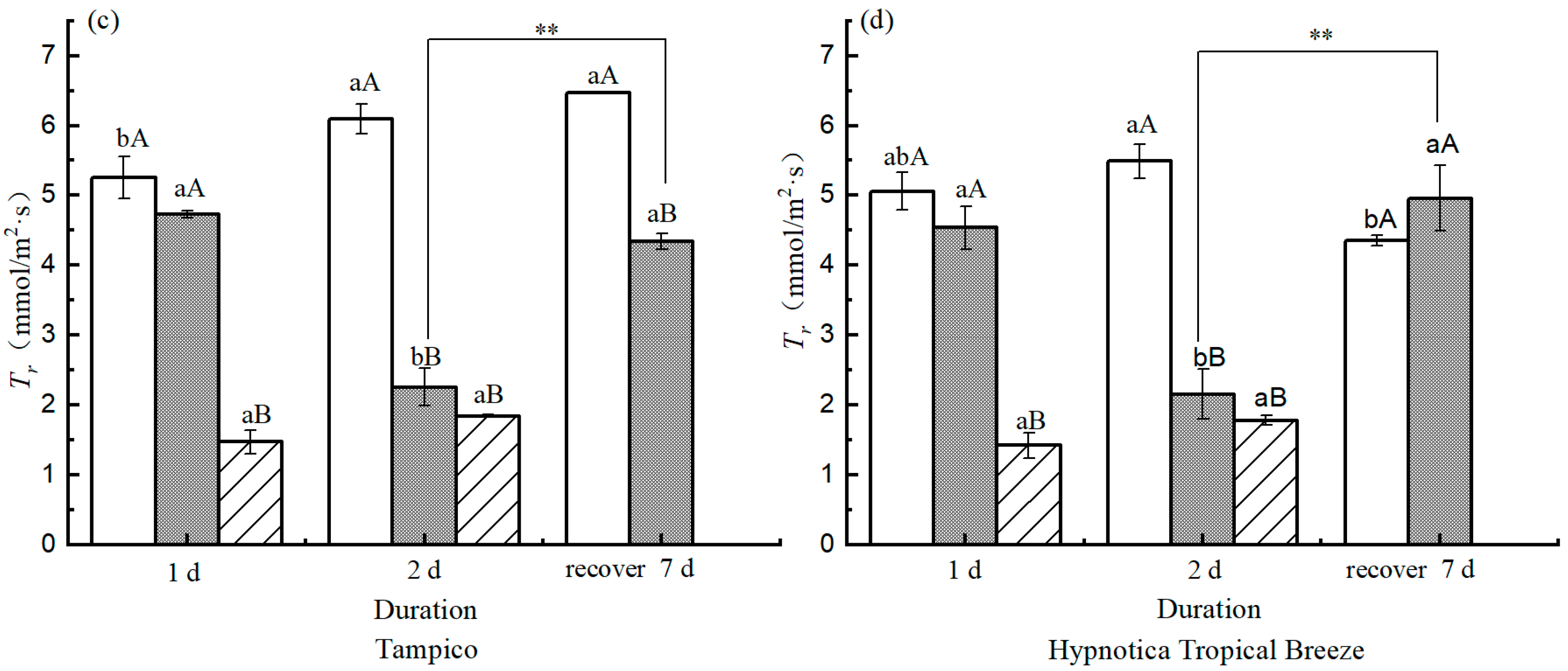
3.6. Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grünig, M.; Mazzi, D.; Calanca, P.; Karger, D.N.; Pellissier, L. Crop and Forest Pest Metawebs Shift towards Increased Linkage and Suitability Overlap under Climate Change. Commun. Biol. 2020, 3, 233. [Google Scholar] [CrossRef] [PubMed]
- Walliser, B.; Lucaciu, C.R.; Molitor, C.; Marinovic, S.; Nitarska, D.A.; Aktaş, D.; Rattei, T.; Kampatsikas, I.; Stich, K.; Haselmair-Gosch, C. Dahlia Variabilis Cultivar ‘Seattle’ as a Model Plant for Anthochlor Biosynthesis. Plant Physiol. Biochem. 2021, 159, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Baburao, D.S.; Kullur, L.R.; Manavi, G.H.; Prasad, V.M. Evaluation of Different Hybrids for Growth and Tuberous Root Parameters of Flowers of Dahlia (Dahlia Variabilis L.) Grown Under Allahabad Agroclimatic Condition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2013–2016. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Kohila, S.; Gomathi, R. Adaptive Physiological and Biochemical Response of Sugarcane Genotypes to High-Temperature Stress. Indian J. Plant Phys. 2018, 23, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.T.; Li, F.P.; Gu, H.H. Effects of High Temperature on Photosynthetic Capacity in the Leaves of Creepers. Scienceasia 2020, 46, 436. [Google Scholar] [CrossRef]
- Lundmark, T.; Bergh, J.; Strand, M.; Koppel, A. Seasonal Variation of Maximum Photochemical Efficiency in Boreal Norway Spruce Stands. Trees 1998, 13, 63–67. [Google Scholar] [CrossRef]
- Wang, X.; Shi, X.; Zhang, R.; Zhang, K.; Shao, L.; Xu, T.; Li, D.; Zhang, D.; Zhang, J.; Xia, Y. Impact of Summer Heat Stress Inducing Physiological and Biochemical Responses in Herbaceous Peony Cultivars (Paeonia Lactiflora Pall.) from Different Latitudes. Ind. Crop. Prod. 2022, 184, 115000. [Google Scholar] [CrossRef]
- Pallas, J.E., Jr.; Michel, B.E.; Harris, D.G. Photosynthesis, Transpiration, Leaf Temperature, and Stomatal Activity of Cotton Plants under Varying Water Potentials. Plant Physiol. 1967, 42, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Zandalinas, S.I.; Fichman, Y.; Sen, S.; Zeng, S.; Gómez-Cadenas, A.; Joshi, T.; Fritschi, F.B.; Mittler, R. Differential Regulation of Flower Transpiration during Abiotic Stress in Annual Plants. New Phytol. 2022, 235, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, C.; Gu, S.; Ma, Q.; Zhang, Y.; Li, X.; Shi, K. Stomatal Movements are Involved in Elevated CO2—Mitigated High Temperature Stress in Tomato. Physiol. Plant 2019, 165, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, X.; Liu, X.; Cheng, C.; Guo, W.; Zhong, C.; Atak, A. Effects of Short—Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.). Biology 2022, 11, 1686. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration Increases under High-temperature Stress: Potential Mechanisms, Trade-offs and Prospects for Crop Resilience in a Warming World. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Huang, Y.C.; Liu, C.H.; Jinn, T.L. Using Silicon Polymer Impression Technique and Scanning Electron Microscopy to Measure Stomatal Aperture, Morphology, and Density. Bio-protocol 2017, 7, e2449. [Google Scholar] [PubMed]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme Heat Increases Stomatal Conductance and Drought-induced Mortality Risk in Vulnerable Plant Species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Krich, C.; Mahecha, M.D.; Migliavacca, M.; De Kauwe, M.G.; Griebel, A.; Runge, J.; Miralles, D.G. Decoupling between Ecosystem Photosynthesis and Transpiration: A Last Resort against Overheating. Environ. Res. Lett. 2022, 17, 044013. [Google Scholar] [CrossRef]
- Gu, K.; Geng, X.; Yue, Y.; Ozaki, Y. Contribution of Keeping More Stable Anatomical Structure under High Temperature to Heat Resistance of Rhododendron Seedlings. J. Fac. Agr. Kyushu Univ. 2016, 61, 273–279. [Google Scholar] [CrossRef]
- Nazdar, T.; Tehranifar, A.; Nezami, A.; Nemati, H.; Samiei, L. Physiological and Anatomical Responses of Calendula (Calendula Officinalis L.) Cultivars to Heat-Stress Duration. J. Hortic. Sci. Biotechnol. 2019, 94, 400–411. [Google Scholar] [CrossRef]
- Saleem, M.A.; Malik, W.; Qayyum, A.; Ul-Allah, S.; Ahmad, M.Q.; Afzal, H.; Amjid, M.W.; Ateeq, M.F.; Zia, Z.U. Impact of Heat Stress Responsive Factors on Growth and Physiology of Cotton (Gossypium Hirsutum L.). Mol. Biol. Rep. 2021, 48, 1069–1079. [Google Scholar] [CrossRef]
- Leuzinger, S.; Körner, C. Tree Species Diversity Affects Canopy Leaf Temperatures in a Mature Temperate Forest. Agric. For. Meteorol. 2007, 146, 29–37. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Siddique, K.H.M.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and Characterization of Contrasting Genotypes/Cultivars for Developing Heat Tolerance in Agricultural Crops: Current Status and Prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef]
- Sunarti, S.; Ginting, C.N.; Ginting, S.F. Processing of Red Dahlia Tubers in Produce Inulin Extract and Material Proximate Testing. Nusant. Sci. Technol. Proc. 2022, 34–38. [Google Scholar] [CrossRef]
- Saravanan, Y.; Devaraj, B.S.; Velusamy, N.K.; Soundirarajan, P.S.; Kandaswamy, K. Phytochemical Extracts of Leucas Aspera and Dahlia Pinnata Exhibit Antimicrobial Properties in Escherichia Coli and Enterococcus Faecalis. Curr. Biotechnol. 2020, 9, 297–303. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhattacharya, S.; Biswas, M. Evaluation of Analgesic Activity of Dahlia Pinnata Leaf Extracts in Swiss Albino Mice. J. Adv. Pharm. Educ. Res. 2013, 3, 556–558. [Google Scholar]
- Lehnert, E.M.; Walbot, V. Sequencing and de Novo Assembly of a Dahlia Hybrid Cultivar Transcriptome. Front. Plant Sci. 2014, 5, 340. [Google Scholar] [CrossRef][Green Version]
- Azuma, M.; Onozaki, T.; Ichimura, K. Effects of Bacterial Proliferation and Soluble Carbohydrate Levels on the Vase Life of Cut Dahlia (Dahlia Variabilis) Flowers. Hortic. J. 2019, 88, 106–115. [Google Scholar] [CrossRef]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The Physiological Functions and Pharmaceutical Applications of Inulin: A Review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef]
- Zhao, C.L. Study on the Technique and Mechanisms of Chemical Regulation of Heat Resistance in Dahlia. Master’s Thesis, Soochow University, Suzhou, China, 2018. [Google Scholar]
- Jia, K.Z.; Chen, G.L. Tolerance of Different Eggplant Varieties at Seedling Stage to High Temperature Stress. Chin. J. Ecol. 2005, 4, 398–401. [Google Scholar]
- Ohsumi, A.; Kanemura, T.; Homma, K.; Horie, T.; Shiraiwa, T. Genotypic Variation of Stomatal Conductance in Relation to Stomatal Density and Length in Rice (Oryza Sativa L.). Plant Prod. Sci. 2007, 10, 322–328. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.Y.; Zhang, Y.C.; Yin, L.Q.; Li, Q.Z.; Zhao, H.H. Heat Tolerance Evaluation of Different Varieties of Heuchera spp. Based on Chlorophyll Fluorescence Parameters. J. Cent. South Univ. 2021, 41, 1–10 + 71. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Yu, X.; Du, W.; Li, H.; Sun, Y.; Sun, H.; Ruan, C. Role of Xanthoceras Sorbifolium MYB44 in Tolerance to Combined Drought and Heat Stress via Modulation of Stomatal Closure and ROS Homeostasis. Plant Physiol. Biochem. 2021, 162, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C. Relationship Between Rice Water Status and Heat Resistance and Its Mechanisms. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Yang, Z.; Lee, K. Photosynthetic and Physiological Responses to High Temperature in Grapevine (Vitis Vinifera L.) Leaves during the Seedling Stage. J. Hortic. Sci. Biotechnol. 2017, 92, 2–10. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive Oxygen Species, Oxidative Signaling and the Regulation of Photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Yang, W.; Sun, Y.; Chen, S.; Jiang, J.; Chen, F.; Fang, W.; Liu, Z. The Effect of Exogenously Applied Nitric Oxide on Photosynthesis and Antioxidant Activity in Heat Stressed Chrysanthemum. Biol. Plant. 2011, 55, 737–740. [Google Scholar] [CrossRef]
- Hasanuzzaman, M. (Ed.) Approaches to the Remediation of Inorganic Pollutants; Springer Singapore: Singapore, 2021; ISBN 9789811562204. [Google Scholar]
- Shao, Y.; Li, S.; Gao, L.; Sun, C.; Hu, J.; Ullah, A.; Gao, J.; Li, X.; Liu, S.; Jiang, D. Magnesium Application Promotes Rubisco Activation and Contributes to High-Temperature Stress Alleviation in Wheat during the Grain Filling. Front. Plant Sci. 2021, 12, 675582. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M. Melatonin-Mediated Photosynthetic Performance of Tomato Seedlings under High-Temperature Stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.H.; Wang, H.R.; Zhang, B.N.; Gao, S.X.; Wang, Z.H.; Wang, Y.; Zhang, H.H.; Sun, G.Y. Elevated Air Temperature Damage to Photosynthetic Apparatus Alleviated by Enhanced Cyclic Electron Flow around Photosystem I in Tobacco Leaves. Ecotoxicol. Environ. Saf. 2020, 204, 111136. [Google Scholar] [CrossRef]
- Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S. Changes in Photosynthetic Characteristics of Paeonia Suffruticosa under High Temperature Stress. Agronomy 2022, 12, 1203. [Google Scholar] [CrossRef]
- Li, G.; Chen, T.; Feng, B.; Peng, S.; Tao, L.; Fu, G. Respiration, Rather than Photosynthesis, Determines Rice Yield Loss under Moderate High-Temperature Conditions. Front. Plant Sci. 2021, 12, 678653. [Google Scholar] [CrossRef] [PubMed]
- Viljevac Vuletić, M.; Mihaljević, I.; Tomaš, V.; Horvat, D.; Zdunić, Z.; Vuković, D. Physiological Response to Short-Term Heat Stress in the Leaves of Traditional and Modern Plum (Prunus Domestica L.) Cultivars. Horticulturae 2022, 8, 72. [Google Scholar] [CrossRef]







| Variety Name | Basic Information |
|---|---|
| Tampico | Plant height: 29.99 ± 0.75 cm; number of branches per plant: 8–10; stem thickness: 8.67 ± 0.21 mm; large leaves and small number of leaves per plant; large flower diameter; number of flowers open at one time 4–5. |
| Hypnotica Tropical Breeze | Plant height: 27.35 ± 0.45 cm: number of branches per plant: 10–14; stem thickness: 7.82 ± 0.25 mm; small leaves and large number of leaves per plant; small flower diameter; number of flowers open at one time: 6–8. |
| Treatment | Heat Injury Index (%) | Recovery Index (%) | ||
|---|---|---|---|---|
| Tampico | Hypnotica Tropical Breeze | Tampico | Hypnotica Tropical Breeze | |
| 25/20 °C 1 d | 0.00 | 0.00 | — | — |
| 25/20 °C 2 d | 0.00 | 0.00 | 100.00 | 100.00 |
| 35/30 °C 1 d | 5.00 | 5.00 | — | — |
| 35/30 °C 2 d | 12.50 | 15.00 | 100.00 | 100.00 |
| 40/35 °C 1 d | 17.50 | 25.00 | — | — |
| 40/35 °C 2 d | 35.00 | 60.00 | 15.00 | 0.00 |
| Variety Name | Tampico | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | 25/20 °C | 35/30 °C | 40/35 °C | ||||||
| Duration (d) | 1 | 2 | Recover 7 | 1 | 2 | Recover 7 | 1 | 2 | Recover 7 |
| Leaf heat damage symptom level | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 3 |
| Variety Name | Hypnotica Tropical Breeze | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | 25/20 °C | 35/30 °C | 40/35 °C | ||||||
| Duration (d) | 1 | 2 | Recover 7 | 1 | 2 | Recover 7 | 1 | 2 | Recover 7 |
| Leaf heat damage symptom level | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-J.; Zhang, Y.-C.; Niu, S.-C.; Hao, L.-H.; Yu, W.-B.; Chen, D.-F.; Xiang, D.-Y. Response of Dahlia Photosynthesis and Transpiration to High-Temperature Stress. Horticulturae 2023, 9, 1047. https://doi.org/10.3390/horticulturae9091047
Liu J-J, Zhang Y-C, Niu S-C, Hao L-H, Yu W-B, Chen D-F, Xiang D-Y. Response of Dahlia Photosynthesis and Transpiration to High-Temperature Stress. Horticulturae. 2023; 9(9):1047. https://doi.org/10.3390/horticulturae9091047
Chicago/Turabian StyleLiu, Jing-Jing, Ying-Chan Zhang, Shan-Ce Niu, Li-Hong Hao, Wen-Bin Yu, Duan-Fen Chen, and Di-Ying Xiang. 2023. "Response of Dahlia Photosynthesis and Transpiration to High-Temperature Stress" Horticulturae 9, no. 9: 1047. https://doi.org/10.3390/horticulturae9091047
APA StyleLiu, J.-J., Zhang, Y.-C., Niu, S.-C., Hao, L.-H., Yu, W.-B., Chen, D.-F., & Xiang, D.-Y. (2023). Response of Dahlia Photosynthesis and Transpiration to High-Temperature Stress. Horticulturae, 9(9), 1047. https://doi.org/10.3390/horticulturae9091047






