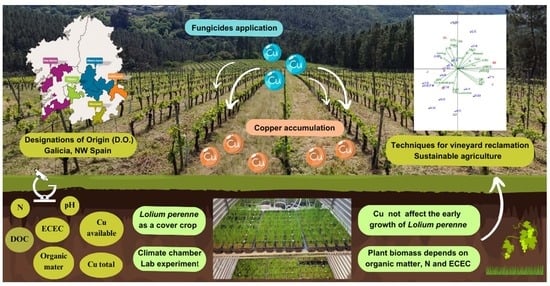
Early Growth Assessment of Lolium perenne L. as a Cover Crop for Management of Copper Accumulation in Galician Vineyard Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Selection and Sampling of Vineyard Soils
2.2. Soil Characterization
2.3. Total and Available Cu Content in Vineyard Soils
2.4. Plant Material and Pot Experiments
2.5. Determination of Cu in L. perenne Shoots
2.6. Statistical Analysis
3. Results and Discussion
3.1. General Characterization of the Vineyard Soils
3.2. Total and Available Cu Content and Extraction Efficiency (ExEf) in Vineyard Soils
3.3. Biomass of L. perenne, Copper Content, and the Bioaccumulation Factor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xunta de Galicia Autorizaciones Para Plantaciones de Viñedo (2016–2045). (In Spanish). Available online: https://mediorural.xunta.gal/es/temas/explotaciones/vinedo/autorizaciones-para-plantaciones-de-vina (accessed on 1 July 2023).
- Denomination of Origen Ribeiro. Available online: https://www.ribeiro.wine (accessed on 14 August 2023).
- Denomination of Origen Valdeorras. Available online: https://dovaldeorras.tv (accessed on 14 August 2023).
- Denomination of Origen Rías Baixas. Available online: https://doriasbaixas.com (accessed on 14 August 2023).
- Denomination of Origen Ribeira Sacra. Available online: https://ribeirasacra.org (accessed on 14 August 2023).
- Denomination of Origen Monterrei. Available online: https://domonterrei.wine (accessed on 14 August 2023).
- Rodríguez Guitián, M.A.; Ramil Rego, P. Clasificaciones Climáticas Aplicadas a Galicia: Revisión Desde Una Perspectiva Biogeográfica. Recursos Rurais 2007, 3, 31–53. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Cardoso, R.M.; Soares, P.M.M.; Cancela, J.J.; Pinto, J.G.; Santos, J.A. Integrated Analysis of Climate, Soil, Topography and Vegetative Growth in Iberian Viticultural Regions. PLoS ONE 2014, 9, e108078. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.V.; Bulle, C.; Levasseur, A.; Deschênes, L. Regionalized Terrestrial Ecotoxicity Assessment of Copper-Based Fungicides Applied in Viticulture. Sustainability 2018, 10, 2522. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; González-Feijoo, R.; Campillo-Cora, C.; Fernández-Calviño, D.; Arenas-Lago, D. Risk Assessment and Limiting Soil Factors for Vine Production—Cu and Zn Contents in Vineyard Soils in Galicia (Rías Baixas D.O.). Agronomy 2023, 13, 309. [Google Scholar] [CrossRef]
- Macías, F. Proceso de Contaminación y Recuperación de Suelos. Manten. Ing. Ind. Y De Edif. 2000, 135, 15–25. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420093704. [Google Scholar]
- Macías, F.; Calvo de Anta, R. Niveles Genéricos de Referencia de Metales Pesados y Otros Elementos Traza en los Suelos de Galicia; Xunta de Galicia: Santiago de Compostela, Spain, 2009; ISBN 978-84-453-4664-8.
- Arenas-Lago, D.; Vega, F.A.; Silva, L.F.O.; Andrade, M.L. Copper Distribution in Surface and Subsurface Soil Horizons. Environ. Sci. Pollut. Res. 2014, 21, 10997–11008. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper Bioavailability, Uptake, Toxicity and Tolerance in Plants: A Comprehensive Review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The Effect of Excess Copper on Growth and Physiology of Important Food Crops: A Review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Karimi, B.; Masson, V.; Guilland, C.; Leroy, E.; Pellegrinelli, S.; Giboulot, E.; Maron, P.A.; Ranjard, L. Ecotoxicity of Copper Input and Accumulation for Soil Biodiversity in Vineyards. Environ. Chem. Lett. 2021, 19, 2013–2030. [Google Scholar] [CrossRef]
- Boso, S.; Alonso-Villaverde, V.; Gago, P.; Santiago, J.L.; Martínez, M.C. Susceptibility of 44 Grapevine (Vitis vinifera L.) Varieties to Downy Mildew in the Field. Aust. J. Grape Wine Res. 2011, 17, 394–400. [Google Scholar] [CrossRef]
- Pham, N.T.H.; Babcsányi, I.; Balling, P.; Farsang, A. Accumulation Patterns and Health Risk Assessment of Potentially Toxic Elements in the Topsoil of Two Sloping Vineyards (Tokaj-Hegyalja, Hungary). J. Soils Sediments 2022, 22, 2671–2689. [Google Scholar] [CrossRef]
- Pietrzak, U.; McPhail, D.C. Copper Accumulation, Distribution and Fractionation in Vineyard Soils of Victoria, Australia. Geoderma 2004, 122, 151–166. [Google Scholar] [CrossRef]
- Eon, P.; Robert, T.; Goutouly, J.P.; Aurelle, V.; Cornu, J.Y. Cover Crop Response to Increased Concentrations of Copper in Vineyard Soils: Implications for Copper Phytoextraction. Chemosphere 2023, 329, 138604. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Fernández-Calviño, D.; Pérez-Rodríguez, P.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Copper and Zinc in Rhizospheric Soil of Wild Plants Growing in Long-Term Acid Vineyard Soils. Insights on Availability and Metal Remediation. Sci. Total Environ. 2019, 672, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Lopes, C.M. Influence of Cover Crop on Water Use and Performance of Vineyard in Mediterranean Portugal. Agric. Ecosyst. Environ. 2007, 121, 336–342. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper Accumulation in Agricultural Soils: Risks for the Food Chain and Soil Microbial Populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef]
- Komárek, M.; Száková, J.; Rohošková, M.; Javorská, H.; Chrastný, V.; Balík, J. Copper Contamination of Vineyard Soils from Small Wine Producers: A Case Study from the Czech Republic. Geoderma 2008, 147, 16–22. [Google Scholar] [CrossRef]
- Madejón, P.; Ramírez-Benítez, J.E.; Corrales, I.; Barceló, J.; Poschenrieder, C. Copper-Induced Oxidative Damage and Enhanced Antioxidant Defenses in the Root Apex of Maize Cultivars Differing in Cu Tolerance. Environ. Exp. Bot. 2009, 67, 415–420. [Google Scholar] [CrossRef]
- Aarya, K.R.; Mathew, A.M. Effect of Copper Based Fungicides on the Properties of Red Soils. Int. J. Sci. Res. Eng. Dev. 2020, 3, 856–860. [Google Scholar]
- Ortega, P.; Sánchez, E.; Gil, E.; Matamoros, V. Use of Cover Crops in Vineyards to Prevent Groundwater Pollution by Copper and Organic Fungicides. Soil Column Studies. Chemosphere 2022, 303, 134975. [Google Scholar] [CrossRef]
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Coll, P.; Cortet, J.; Le Cadre, E.; d’Errico, G.; et al. Vineyard Management and Its Impacts on Soil Biodiversity, Functions, and Ecosystem Services. Front. Ecol. Evol. 2022, 10, 850272. [Google Scholar] [CrossRef]
- Dumitriu Gabur, G.D.; Gabur, I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Cotea, V.V.; Teodosiu, C. Investigating Six Common Pesticides Residues and Dietary Risk Assessment of Romanian Wine Varieties. Foods 2022, 11, 2225. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Fouillet, E.; Delière, L.; Flori, A.; Rapidel, B.; Merot, A. Diversity of Pesticide Use Trajectories during Agroecological Transitions in Vineyards: The Case of the French DEPHY Network. Agric. Syst. 2023, 210, 103725. [Google Scholar] [CrossRef]
- Ruggieri, F.; Coulon-Leroy, C.; Mazé, A. How Can Collective Action Support the Agroecological Transition in Geographical Indication Vineyards? Insights from the Loire Valley Wine Area. Sustainability 2023, 15, 9371. [Google Scholar] [CrossRef]
- Coll, P.; Le Cadre, E.; Blanchart, E.; Hinsinger, P.; Villenave, C. Organic Viticulture and Soil Quality: A Long-Term Study in Southern France. Appl. Soil Ecol. 2011, 50, 37–44. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. A Review: Soil Management, Sustainable Strategies and Approaches to Improve the Quality of Modern Viticulture. Agronomy 2021, 11, 2359. [Google Scholar] [CrossRef]
- Abad, J.; de Mendoza, I.H.; Marín, D.; Orcaray, L.; Santesteban, L.G. Cover Crops in Viticulture. A Systematic Review (2): Implications on Vineyard Agronomic Performance. OENO One 2021, 55, 1–27. [Google Scholar] [CrossRef]
- Abad, J.; Hermoso De Mendoza, I.; Marín, D.; Orcaray, L.; Santesteban, L.G. Cover Crops in Viticulture. A Systematic Review (1): Implications on Soil Characteristics and Biodiversity in Vineyard. OENO One 2021, 55, 295–312. [Google Scholar] [CrossRef]
- Pardini, A.; Faiello, C.; Longhi, F.; Mancuso, S.; Snowball, R. Cover Crop Species and Their Management in Vineyards and Olive Groves. Adv. Hortic. Sci. 2002, 16, 225–234. [Google Scholar]
- Scavo, A.; Fontanazza, S.; Restuccia, A.; Pesce, G.R.; Abbate, C.; Mauromicale, G. The Role of Cover Crops in Improving Soil Fertility and Plant Nutritional Status in Temperate Climates. A Review. Agron. Sustain. Dev. 2022, 42, 93. [Google Scholar] [CrossRef]
- Abad, F.J.; Marín, D.; Imbert, B.; Virto, I.; Garbisu, C.; Santesteban, L.G. Under-Vine Cover Crops: Impact on Physical and Biological Soil Proprieties in an Irrigated Mediterranean Vineyard. Sci. Hortic. 2023, 311, 111797. [Google Scholar] [CrossRef]
- Santibáñez, C.; Verdugo, C.; Ginocchio, R. Phytostabilization of Copper Mine Tailings with Biosolids: Implications for Metal Uptake and Productivity of Lolium Perenne. Sci. Total Environ. 2008, 395, 1–10. [Google Scholar] [CrossRef]
- Dineen, M.; McCarthy, B.; Ross, D.; Ortega, A.; Dillon, P.; Van Amburgh, M.E. Characterization of the Nutritive Value of Perennial Ryegrass (Lolium perenne L.) Dominated Pastures Using Updated Chemical Methods with Application for the Cornell Net Carbohydrate and Protein System. Anim. Feed Sci. Technol. 2021, 272, 114752. [Google Scholar] [CrossRef]
- Healy, M.G.; Ryan, P.C.; Fenton, O.; Peyton, D.P.; Wall, D.P.; Morrison, L. Bioaccumulation of Metals in Ryegrass (Lolium perenne L.) Following the Application of Lime Stabilised, Thermally Dried and Anaerobically Digested Sewage Sludge. Ecotoxicol. Environ. Saf. 2016, 130, 303–309. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Serran, S.; Lauzon, J.; Janovicek, K.; Deen, B.; Th Ilakarathna, M.S.; Serran, S.; Janovicek, K.; Deen, B.; Lauzon, J. Management of Manure Nitrogen Using Cover Crops. Agron. J. 2015, 107, 1595–1607. [Google Scholar] [CrossRef]
- Giese, G.; Velasco-Cruz, C.; Roberts, L.; Heitman, J.; Wolf, T.K. Complete Vineyard Floor Cover Crops Favorably Limit Grapevine Vegetative Growth. Sci. Hortic. 2014, 170, 256–266. [Google Scholar] [CrossRef]
- Smit, H.J.; Tas, B.M.; Taweel, H.Z.; Tamminga, S.; Elgersma, A. Effects of Perennial Ryegrass (Lolium perenne L.) Cultivars on Herbage Production, Nutritional Quality and Herbage Intake of Grazing Dairy Cows. Grass Forage Sci. 2005, 60, 297–309. [Google Scholar] [CrossRef]
- Guitián, F.; Carballas, T. Técnicas de Análisis de Suelos; Pico Sacro: Santiago de Compostela, Spain, 1976. [Google Scholar]
- Day, R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis, Part 1; Black, C., Evans, D.D., White, J.L., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Sánchez-Monedero, M.A.; Roig, A.; Martínez-Pardo, C.; Cegarra, J.; Paredes, C. A Microanalysis Method for Determining Total Organic Carbon in Extracts of Humic Substances. Relationships between Total Organic Carbon and Oxidable Carbon. Bioresour. Technol. 1996, 57, 291–295. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Duquette, M. A Simple Barium Chloride Method for Determining Cation Exchange Capacity and Exchangeable Cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a Rhizosphere-Based Method with Other One-Step Extraction Methods for Assessing the Bioavailability of Soil Metals to Wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Trought, M.C.T.; Dixon, R.; Mills, T.; Greven, M.; Agnew, R.; Mauk, J.L.; Praat, J.P. The Impact of Differences in Soil Texture within a Vineyard on Vine Vigour, Vine Earliness and Juice Composition. J. Int. Sci. Vigne Vin 2008, 42, 62–72. [Google Scholar] [CrossRef]
- Trentin, E.; Cesco, S.; Pii, Y.; Valentinuzzi, F.; Celletti, S.; Feil, S.B.; Zuluaga, M.Y.A.; Ferreira, P.A.A.; Ricachenevsky, F.K.; Stefanello, L.O.; et al. Plant Species and PH Dependent Responses to Copper Toxicity. Environ. Exp. Bot. 2022, 196, 104791. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Andrade, M.L. A Versatile Parameter for Comparing the Capacities of Soils for Sorption and Retention of Heavy Metals Dumped Individually or Together: Results for Cadmium, Copper and Lead in Twenty Soil Horizons. J. Colloid Interface Sci. 2008, 327, 275–286. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; López-Periago, E.; Novoa-Muñoz, J.C.; Arias-Estévez, M. Short-Scale Distribution of Copper Fractions in a Vineyard Acid Soil. Land Degrad. Dev. 2008, 19, 190–197. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; López-Periago, E.; Arias-Estéves, M. Changes in Copper Content and Distribution in Young, Old and Abandoned Vineyard Acid Soils Due to Land Use Changes. Land Degrad. Dev. 2008, 19, 165–177. [Google Scholar] [CrossRef]
- Parat, C.; Chaussod, R.; Lévéque, J.; Dousset, S.; Andreux, F. The Relationship between Copper Accumulated in Vineyard Calcareous Soils and Soil Organic Matter and Iron. Eur. J. Soil Sci. 2002, 53, 663–670. [Google Scholar] [CrossRef]
- Duplay, J.; Semhi, K.; Errais, E.; Imfeld, G.; Babcsanyi, I.; Perrone, T. Copper, Zinc, Lead and Cadmium Bioavailability and Retention in Vineyard Soils (Rouffach, France): The Impact of Cultural Practices. Geoderma 2014, 230–231, 318–328. [Google Scholar] [CrossRef]
- Marín-Martínez, A.; Sanz-Cobeña, A.; Bustamante, M.A.; Agulló, E.; Paredes, C. Effect of Organic Amendment Addition on Soil Properties, Greenhouse Gas Emissions and Grape Yield in Semi-Arid Vineyard Agroecosystems. Agronomy 2021, 11, 1477. [Google Scholar] [CrossRef]
- Jez, E.; Pellegrini, E.; Contin, M. Copper Bioavailability and Leaching in Conventional and Organic Viticulture under Environmental Stress. Appl. Sci. 2023, 13, 2595. [Google Scholar] [CrossRef]
- Filipović, L.; Defterdarović, J.; Chen, R.; Krevh, V.; Gerke, H.H.; Baumgartl, T.; Kovač, Z.; Ondrašek, G.; Ružičić, S.; He, H.; et al. Leached Copper Correlation with Dissolved Organic Carbon in Sloped Vineyard Soil. Water 2023, 15, 800. [Google Scholar] [CrossRef]
- Ouédraogo, F.; Cornu, J.Y.; Janot, N.; Nguyen, C.; Sourzac, M.; Parlanti, E.; Denaix, L. Do DOM Optical Parameters Improve the Prediction of Copper Availability in Vineyard Soils? Environ. Sci. Pollut. Res. 2022, 29, 29268–29284. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Rey, P.; Vázquez-Rowe, I.; Quinteiro, P.; Rafael, S.; Gonçalves, C.; Moreira, M.T.; Feijoo, G.; Arroja, L.; Dias, A.C. Regionalizing Eco-Toxicity Characterization Factors for Copper Soil Emissions Considering Edaphic Information for Northern Spain and Portuguese Vineyards. Sci. Total Environ. 2019, 686, 986–994. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Pateiro-Moure, M.; López-Periago, E.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Copper Distribution and Acid-Base Mobilization in Vineyard Soils and Sediments from Galicia (NW Spain). Eur. J. Soil Sci. 2008, 59, 315–326. [Google Scholar] [CrossRef]
- Baragaño, D.; Gallego, J.L.R.; Baleriola, G.; Forján, R. Effects of Different In Situ Remediation Strategies for an As-Polluted Soil on Human Health Risk, Soil Properties, and Vegetation. Agronomy 2020, 10, 759. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Cutillas-Barreiro, L.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cu Immobilization and Lolium perenne Development in an Acid Vineyard Soil Amended with Crushed Mussel Shell. Land Degrad. Dev. 2017, 28, 762–772. [Google Scholar] [CrossRef]
- Kgaladi, M.; Masotla, L.; Melato, F.A.; Mokgalaka-Fleischmann, S. Extraction Potential of Lolium perenne L. (Perennial Rye Grass) for Metals in Landfill Soil: Its Tolerance and Defense Strategies. Minerals 2023, 13, 873. [Google Scholar] [CrossRef]
- De Conti, L.; Cesco, S.; Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Melo, G.W.B.; Ceretta, C.A.; Trentin, E.; Marques, A.C.R.; Brunetto, G. Iron Fertilization to Enhance Tolerance Mechanisms to Copper Toxicity of Ryegrass Plants Used as Cover Crop in Vineyards. Chemosphere 2020, 243, 125298. [Google Scholar] [CrossRef]
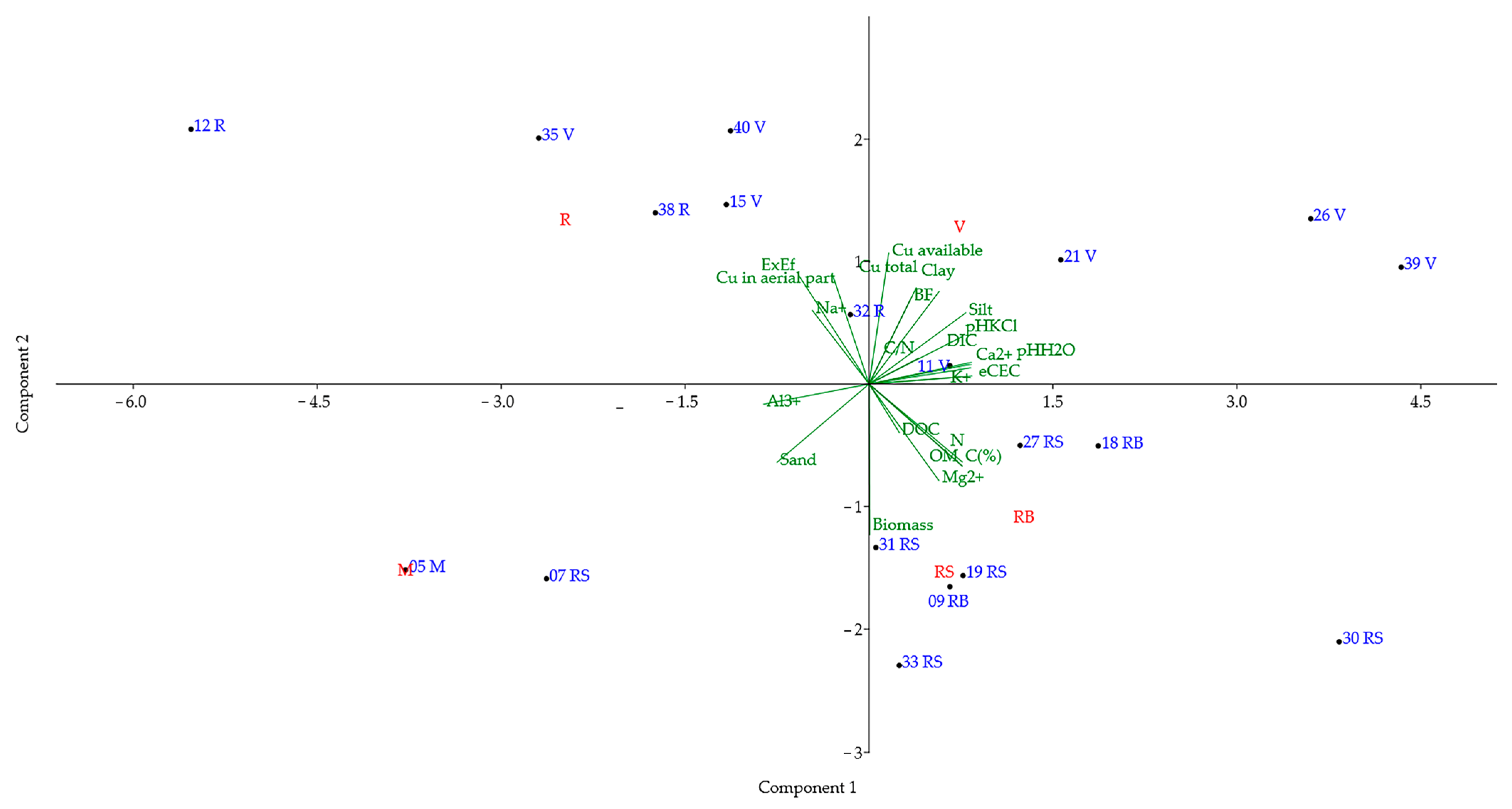
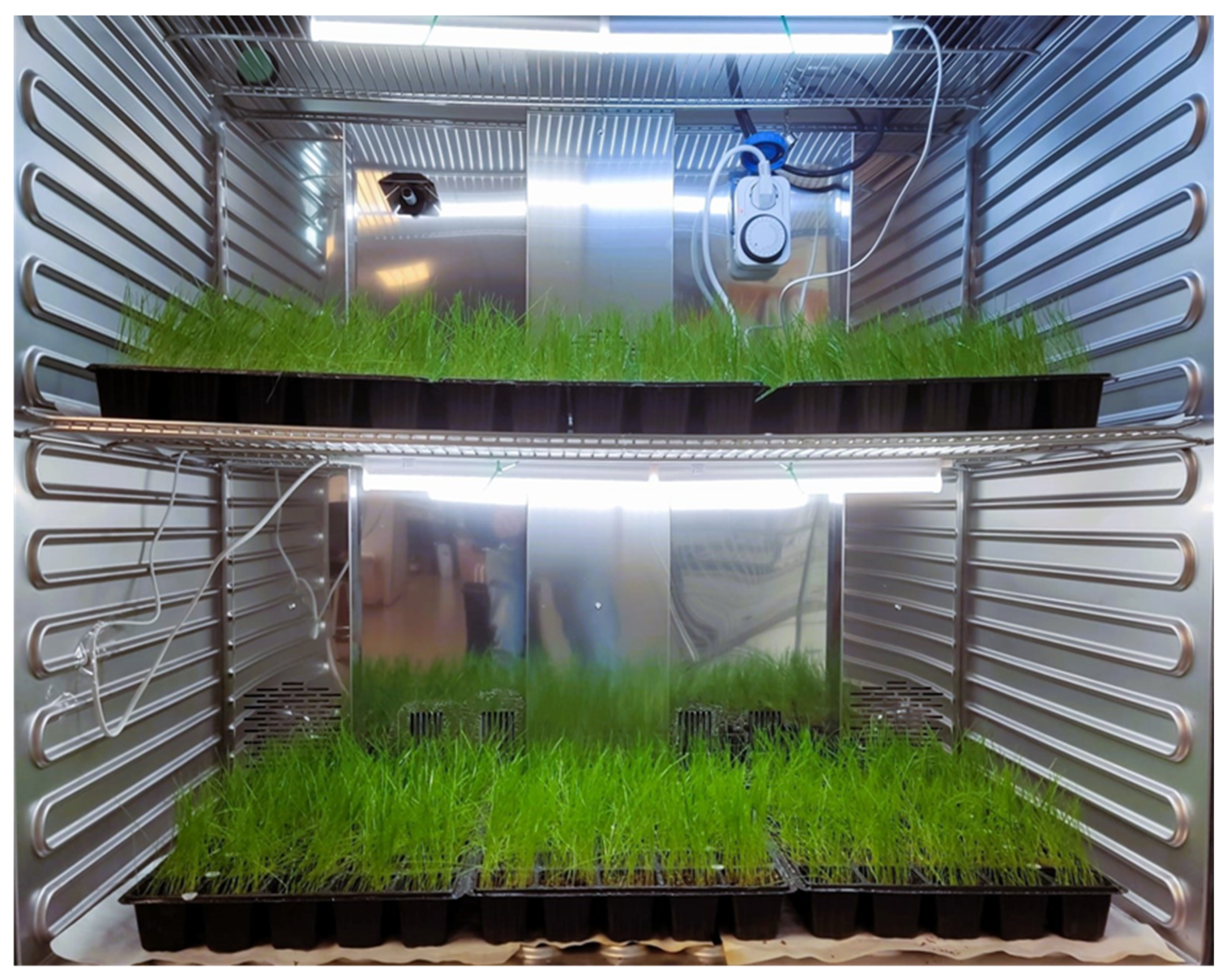
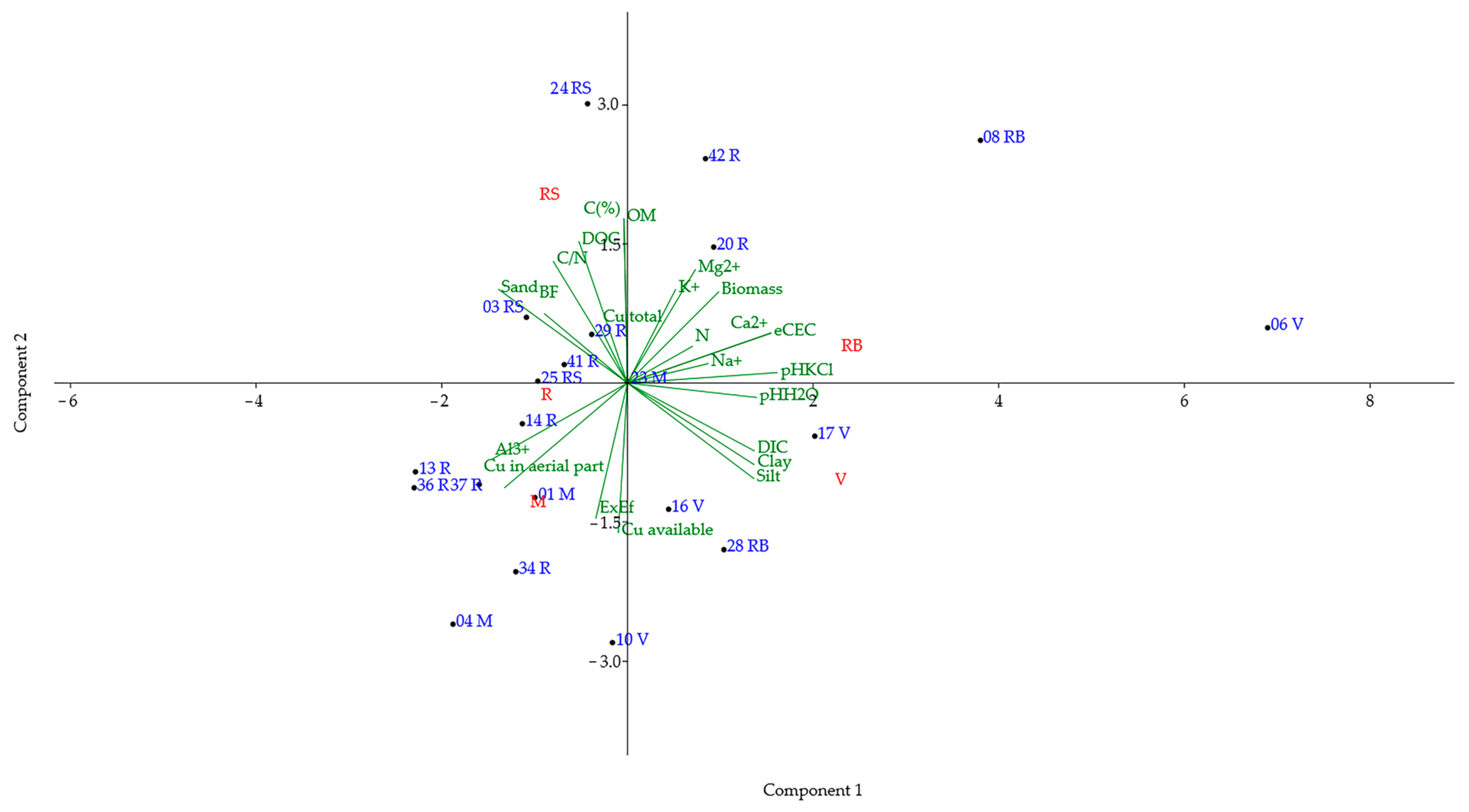
| Soil | Sampling Area | D.O. | Municipality | Soil | Sampling Area | D.O. | Municipality |
|---|---|---|---|---|---|---|---|
| 1 M | VR | Monterrei | Vilardevós | 22 RS | VR | Ribeira Sacra | Sober |
| 2 RB | VS | Rías Baixas | Salvaterra de Miño | 23 M | VR | Monterrei | Verín |
| 3 RS | VR | Ribeira Sacra | A Pobra de Trives | 24 RS | VR | Ribeira Sacra | Parada de Sil |
| 4 M | VR | Monterrei | Monterrei | 25 RS | VR | Ribeira Sacra | A Teixeira |
| 5 M | VS | Monterrei | Oímbra | 26 V | VS | Valdeorras | Vilamartín de Valdeorras |
| 6 V | VR | Valdeorras | O Barco de Valdeorras | 27 RS | VS | Ribeira Sacra | Parada de Sil |
| 7 RS | VS | Ribeira Sacra | A Pobra de Trives | 28 RB | VR | Rías Baixas | Arbo |
| 8 RB | VR | Rías Baixas | Tomiño | 29 R | VR | Ribeiro | Sober |
| 9 RB | VS | Rías Baixas | Meis | 30 RS | VS | Ribeira Sacra | Sober |
| 10 V | VR | Valdeorras | Vilamartín de Valdeorras | 31 RS | VS | Ribeira Sacra | Sober |
| 11 V | VS | Valdeorras | Petín | 32 R | VS | Ribeiro | Beade |
| 12 R | VS | Ribeiro | Ribadavia | 33 RS | VS | Ribeira Sacra | Parada de Sil |
| 13 R | VR | Ribeiro | Ribadavia | 34 R | VR | Ribeiro | Arnoia |
| 14 R | VR | Ribeiro | Cenlle | 35 V | VS | Valdeorras | A Rúa |
| 15 V | VS | Valdeorras | Rubiá | 36 R | VR | Ribeiro | Toén |
| 16 V | VR | Valdeorras | Rubiá | 37 R | VR | Ribeiro | Toén |
| 17 V | VR | Valdeorras | Rubiá | 38 R | VS | Ribeiro | Sober |
| 18 RB | VS | Rías Baixas | Arbo | 39 V | VS | Valdeorras | Rubiá |
| 19 RS | VS | Ribeira Sacra | Sober | 40 V | VS | Valdeorras | Vilamartín de Valdeorras |
| 20 R | VR | Ribeiro | Sober | 41 R | VR | Ribeiro | Toén |
| 21 V | VS | Valdeorras | O Barco de Valdeorras | 42 R | VR | Ribeiro | Toén |
| Soil | S.a. | pHH2O | pHKCl | C(%) | OM | N | C/N | DOC | DIC | Ca2+ | Mg2+ | K+ | Na+ | Al3+ | eCEC | Sand | Silt | Clay | Texture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | - | mg kg−1 | cmol(+) kg−1 | (%) | |||||||||||||||
| 1 M | VR | 5.35 | 3.31 | 2.62 | 4.52 | 0.21 | 12.61 | 20.36 | 0.09 | 0.06 | 0.44 | 0.57 | 0.05 | 2.27 | 3.38 | 33.06 | 49.94 | 17.01 | silt loam |
| 2 RB | VS | 7.37 | 5.42 | 4.48 | 7.72 | 0.27 | 16.77 | 26.20 | 0.79 | 13.48 | 1.40 | 0.79 | 0.09 | 0.00 | 15.75 | 46.54 | 35.87 | 17.59 | loam |
| 3 RS | VR | 6.37 | 4.03 | 1.62 | 2.79 | 0.12 | 13.07 | 25.16 | 0.40 | 0.26 | 0.56 | 0.62 | 0.03 | 0.37 | 1.83 | 69.68 | 16.57 | 13.74 | sandy loam |
| 4 M | VR | 6.46 | 4.18 | 1.07 | 1.84 | 0.08 | 12.63 | 16.12 | 0.50 | 0.18 | 0.70 | 0.44 | 0.05 | 0.00 | 1.38 | 72.99 | 14.27 | 12.75 | sandy loam |
| 5 M | VS | 4.86 | 2.94 | 1.73 | 2.98 | 0.13 | 13.31 | 27.85 | 0.19 | 0.14 | 0.73 | 0.75 | 0.06 | 1.69 | 3.37 | 72.09 | 15.06 | 12.85 | sandy loam |
| 6 V | VR | 8.02 | 7.32 | 2.84 | 4.90 | 0.37 | 7.70 | 28.58 | 11.47 | 18.00 | 0.76 | 0.38 | 0.04 | 0.00 | 19.18 | 44.82 | 36.10 | 19.08 | loam |
| 7 RS | VS | 5.76 | 3.75 | 2.54 | 4.38 | 0.20 | 12.68 | 35.29 | 0.22 | 0.13 | 0.44 | 0.59 | 0.04 | 1.34 | 2.54 | 69.68 | 16.57 | 13.74 | sandy loam |
| 8 RB | VR | 7.71 | 5.88 | 3.04 | 5.24 | 0.18 | 17.04 | 18.74 | 3.63 | 11.56 | 1.07 | 0.72 | 0.31 | 0.00 | 13.65 | 55.42 | 28.63 | 15.95 | sandy loam |
| 9 RB | VS | 6.87 | 4.69 | 3.78 | 6.52 | 0.29 | 13.27 | 14.92 | 0.51 | 7.64 | 1.15 | 0.96 | 0.10 | 0.02 | 9.87 | 64.65 | 19.03 | 16.32 | sandy loam |
| 10 V | VR | 5.45 | 2.96 | 1.30 | 2.24 | 0.11 | 11.91 | 17.70 | 0.01 | 0.10 | 0.40 | 1.05 | 0.03 | 0.89 | 2.48 | 32.52 | 52.00 | 15.49 | silt loam |
| 11 V | VS | 7.73 | 6.09 | 3.94 | 6.79 | 0.14 | 28.23 | 23.70 | 2.06 | 6.16 | 1.25 | 0.57 | 0.03 | 0.00 | 8.01 | 65.45 | 21.25 | 13.30 | sandy loam |
| 12 R | VS | 4.87 | 3.75 | 0.67 | 1.16 | 0.05 | 13.07 | 19.34 | 0.02 | 0.32 | 0.84 | 0.53 | 0.42 | 2.23 | 4.34 | 70.15 | 16.20 | 13.65 | sandy loam |
| 13 R | VR | 4.83 | 3.81 | 0.62 | 1.07 | 0.05 | 12.53 | 18.14 | 0.00 | 0.29 | 0.66 | 0.52 | 0.44 | 1.93 | 3.84 | 70.15 | 16.20 | 13.65 | sandy loam |
| 14 R | VR | 5.68 | 4.27 | 1.32 | 2.28 | 0.10 | 13.78 | 18.87 | 0.07 | 0.10 | 0.33 | 0.66 | 0.03 | 0.70 | 1.81 | 61.47 | 24.14 | 14.40 | sandy loam |
| 15 V | VS | 5.77 | 3.99 | 1.39 | 2.40 | 0.15 | 9.50 | 25.07 | 0.05 | 0.42 | 0.42 | 0.61 | 0.03 | 1.12 | 2.60 | 49.56 | 24.31 | 26.13 | sandy clay loam |
| 16 V | VR | 6.48 | 4.67 | 1.45 | 2.50 | 0.16 | 9.06 | 34.04 | 0.52 | 0.50 | 0.44 | 0.47 | 0.03 | 0.00 | 1.44 | 49.56 | 24.31 | 26.13 | sandy clay loam |
| 17 V | VR | 6.48 | 4.26 | 1.63 | 2.81 | 0.16 | 10.19 | 30.21 | 0.24 | 4.68 | 1.42 | 0.45 | 0.06 | 0.00 | 6.61 | 39.11 | 43.88 | 17.01 | loam |
| 18 RB | VS | 7.75 | 5.90 | 2.62 | 4.52 | 0.22 | 11.93 | 35.11 | 2.70 | 11.76 | 1.17 | 0.71 | 0.04 | 0.21 | 13.89 | 52.80 | 31.60 | 15.59 | sandy loam |
| 19 RS | VS | 5.70 | 4.93 | 3.82 | 6.59 | 0.24 | 15.62 | 115.21 | 0.47 | 4.95 | 2.58 | 0.84 | 0.01 | 0.33 | 8.38 | 61.49 | 26.47 | 12.04 | sandy loam |
| 20 R | VR | 5.55 | 5.33 | 1.96 | 3.38 | 0.17 | 13.10 | 54.36 | 1.93 | 4.77 | 1.66 | 0.94 | 0.01 | 0.34 | 7.38 | 62.16 | 23.10 | 14.74 | sandy loam |
| 21 V | VS | 7.78 | 6.47 | 1.74 | 3.00 | 0.14 | 12.57 | 34.53 | 6.91 | 12.64 | 0.76 | 0.55 | 0.04 | 0.64 | 14.62 | 53.30 | 32.06 | 14.64 | sandy loam |
| 22 RS | VR | 4.61 | 4.49 | 3.75 | 6.47 | 0.23 | 15.99 | 101.82 | 0.34 | 4.45 | 1.45 | 0.68 | 0.01 | 0.97 | 7.56 | 56.97 | 31.71 | 11.32 | sandy loam |
| 23 M | VR | 6.77 | 4.81 | 1.50 | 2.59 | 0.12 | 12.84 | 20.46 | 2.81 | 0.47 | 0.52 | 0.67 | 0.04 | 0.63 | 2.32 | 54.81 | 30.78 | 14.40 | sandy loam |
| 24 RS | VR | 6.19 | 4.63 | 2.97 | 5.12 | 0.21 | 14.15 | 35.18 | 1.33 | 7.20 | 0.55 | 0.54 | 0.07 | 0.00 | 8.35 | 68.25 | 17.84 | 13.91 | sandy loam |
| 25 RS | VR | 6.19 | 3.81 | 1.95 | 3.36 | 0.14 | 14.19 | 28.93 | 0.15 | 0.28 | 0.51 | 0.67 | 0.03 | 0.47 | 1.95 | 61.92 | 24.12 | 13.96 | sandy loam |
| 26 V | VS | 7.29 | 5.49 | 2.76 | 4.76 | 0.22 | 12.56 | 40.78 | 4.34 | 11.80 | 1.25 | 1.25 | 0.04 | 0.00 | 14.35 | 30.96 | 50.04 | 19.00 | silt loam |
| 27 RS | VS | 7.60 | 5.98 | 1.87 | 3.22 | 0.14 | 13.54 | 31.28 | 4.08 | 6.80 | 1.87 | 1.80 | 0.13 | 0.05 | 10.65 | 65.83 | 19.52 | 14.66 | sandy loam |
| 28 RB | VR | 6.47 | 4.08 | 0.90 | 1.55 | 0.10 | 9.50 | 20.18 | 0.03 | 0.12 | 0.25 | 0.66 | 0.02 | 0.15 | 1.20 | 35.17 | 47.55 | 17.28 | loam |
| 29 R | VR | 5.29 | 4.70 | 1.35 | 2.33 | 0.11 | 12.74 | 43.68 | 0.32 | 3.12 | 0.54 | 0.79 | 0.01 | 0.33 | 4.79 | 61.09 | 24.18 | 14.73 | sandy loam |
| 30 RS | VS | 5.94 | 5.59 | 5.22 | 9.00 | 0.33 | 15.61 | 127.07 | 6.58 | 13.96 | 2.53 | 0.96 | 0.01 | 0.33 | 17.79 | 56.97 | 31.71 | 11.32 | sandy loam |
| 31 RS | VS | 5.64 | 4.88 | 3.06 | 5.28 | 0.22 | 14.01 | 75.12 | 0.53 | 4.29 | 1.12 | 0.79 | 0.01 | 0.33 | 6.54 | 59.69 | 27.55 | 12.76 | sandy loam |
| 32 R | VS | 7.03 | 5.97 | 1.27 | 2.19 | 0.10 | 12.37 | 26.63 | 2.46 | 9.68 | 0.77 | 0.77 | 0.04 | 0.00 | 11.26 | 65.68 | 20.50 | 13.82 | sandy loam |
| 33 RS | VS | 6.26 | 4.10 | 3.59 | 6.19 | 0.25 | 14.44 | 37.39 | 0.61 | 6.40 | 1.21 | 0.78 | 0.04 | 0.00 | 8.43 | 68.25 | 17.84 | 13.91 | sandy loam |
| 34 R | VR | 6.29 | 4.28 | 1.05 | 1.81 | 0.08 | 12.65 | 6.72 | 0.29 | 0.20 | 0.32 | 0.43 | 0.05 | 0.32 | 1.31 | 64.53 | 20.51 | 14.96 | sandy loam |
| 35 V | VS | 5.96 | 3.39 | 1.09 | 1.88 | 0.11 | 9.59 | 22.85 | 0.02 | 0.21 | 0.45 | 0.80 | 0.04 | 0.48 | 1.98 | 61.45 | 24.60 | 13.95 | sandy loam |
| 36 R | VR | 6.10 | 3.57 | 1.10 | 1.90 | 0.09 | 12.51 | 20.50 | 0.92 | 0.22 | 0.69 | 0.71 | 0.05 | 0.92 | 2.59 | 70.15 | 16.20 | 13.65 | sandy loam |
| 37 R | VR | 4.85 | 3.79 | 1.45 | 2.50 | 0.13 | 11.23 | 18.69 | 0.04 | 0.08 | 0.22 | 0.54 | 0.03 | 1.25 | 2.12 | 47.95 | 37.67 | 14.37 | loam |
| 38 R | VS | 4.91 | 4.31 | 1.85 | 3.19 | 0.15 | 12.37 | 63.78 | 0.22 | 2.52 | 0.42 | 0.86 | 0.01 | 0.52 | 4.33 | 61.09 | 24.18 | 14.73 | sandy loam |
| 39 V | VS | 6.11 | 5.85 | 3.61 | 6.22 | 0.37 | 9.80 | 62.94 | 1.34 | 18.88 | 0.65 | 2.58 | 0.12 | 0.00 | 22.23 | 42.22 | 35.88 | 21.89 | clay loam |
| 40 V | VS | 6.01 | 3.89 | 2.06 | 3.55 | 0.09 | 22.81 | 18.72 | 0.05 | 0.19 | 0.88 | 0.62 | 0.02 | 0.20 | 1.92 | 57.14 | 29.42 | 13.44 | sandy clay loam |
| 41 R | VR | 5.00 | 4.00 | 2.20 | 3.79 | 0.16 | 13.63 | 30.21 | 0.42 | 0.22 | 0.33 | 0.56 | 0.03 | 0.61 | 1.75 | 42.78 | 43.17 | 14.05 | loam |
| 42 R | VR | 5.03 | 4.31 | 3.12 | 5.38 | 0.25 | 12.26 | 49.26 | 0.24 | 0.56 | 0.63 | 0.78 | 0.04 | 0.01 | 2.03 | 43.70 | 41.21 | 15.09 | loam |
| Soil | Sampling Area | Total Cu | Available Cu | ExEf | Soil | Sampling Area | Total Cu | Available Cu | ExEf |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | (%) | mg kg−1 | mg kg−1 | (%) | ||||
| 1 M | VR | 16.4 | 4.2 | 25.8 | 22 RS | VR | 129.4 | 4.3 | 3.3 |
| 2 RB | VS | 33.1 | 3.7 | 11.0 | 23 M | VR | 135.6 | 2.2 | 1.6 |
| 3 RS | VR | 34.2 | 2.0 | 5.7 | 24 RS | VR | 153.2 | 1.2 | 0.8 |
| 4 M | VR | 42.3 | 21.3 | 50.4 | 25 RS | VR | 164.3 | 19.4 | 11.8 |
| 5 M | VS | 44.5 | 2.4 | 5.3 | 26 V | VS | 166.9 | 1.4 | 0.8 |
| 6 V | VR | 49.3 | 3.2 | 6.5 | 27 RS | VS | 170.4 | 1.4 | 0.8 |
| 7 RS | VS | 60.7 | 2.8 | 4.6 | 28 RB | VR | 182.5 | 7.5 | 4.1 |
| 8 RB | VR | 64.3 | 7.5 | 11.6 | 29 R | VR | 187.6 | 8.3 | 4.4 |
| 9 RB | VS | 65.7 | 2.4 | 3.6 | 30 RS | VS | 192.3 | 8.1 | 4.2 |
| 10 V | VR | 66.8 | 23.7 | 35.5 | 31 RS | VS | 200.8 | 7.6 | 3.8 |
| 11 V | VS | 69.5 | 11.1 | 15.9 | 32 R | VS | 201.8 | 1.5 | 0.8 |
| 12 R | VS | 73.6 | 10.0 | 13.6 | 33 RS | VS | 204.0 | 3.4 | 1.6 |
| 13 R | VR | 74.6 | 3.2 | 4.3 | 34 R | VR | 204.0 | 24.0 | 11.8 |
| 14 R | VR | 82.4 | 6.7 | 8.1 | 35 V | VS | 216.0 | 13.7 | 6.4 |
| 15 V | VS | 104.0 | 0.4 | 0.4 | 36 R | VR | 219.4 | 15.2 | 7.0 |
| 16 V | VR | 109.2 | 2.3 | 2.1 | 37 R | VR | 229.8 | 4.2 | 1.8 |
| 17 V | VR | 114.0 | 12.0 | 10.5 | 38 R | VS | 230.8 | 11.5 | 5.0 |
| 18 RB | VS | 117.5 | 2.3 | 1.9 | 39 V | VS | 248.8 | 15.1 | 6.1 |
| 19 RS | VS | 117.7 | 7.8 | 6.7 | 40 V | VS | 268.4 | 18.7 | 7.0 |
| 20 R | VR | 122.2 | 18.2 | 14.9 | 41 R | VR | 285.5 | 7.6 | 2.7 |
| 21 V | VS | 126.7 | 3.1 | 2.5 | 42 R | VR | 292.9 | 8.0 | 2.7 |
| SAMPLING AREA: VS | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pHH2O | pHKCl | C(%) | OM | N | C/N | DOC | DIC | Ca2+ | Mg2+ | K+ | Na+ | Al3+ | eCEC | Sand | Silt | Clay | Total Cu | Avail. Cu | ExEf | Bm | Cu a.p. | BF | |
| Total Cu | −0.13 | 0.02 | −0.06 | −0.06 | −0.03 | −0.03 | 0.24 | −0.06 | 0.04 | −0.01 | 0.34 | −0.27 | −0.29 | 0.03 | 0.00 | 0.03 | −0.08 | - | - | - | - | - | - |
| Avail. Cu | −0.19 | −0.27 | −0.22 | −0.22 | −0.28 | 0.14 | −0.08 | −0.26 | −0.25 | −0.09 | 0.06 | −0.09 | −0.10 | −0.26 | 0.02 | 0.04 | −0.19 | 0.25 | - | - | - | - | - |
| ExEf | −0.08 | −0.26 | −0.15 | −0.15 | −0.22 | 0.11 | −0.23 | −0.18 | −0.23 | −0.10 | −0.13 | 0.04 | 0.10 | −0.23 | −0.06 | 0.12 | −0.14 | −0.41 | 0.63 | - | - | - | - |
| Bm | 0.26 | 0.35 | 0.50 | 0.50 | 0.56 | −0.01 | 0.20 | 0.30 | 0.53 | 0.27 | 0.36 | 0.03 | −0.25 | 0.54 | 0.03 | −0.02 | −0.02 | 0.13 | −0.31 | −0.30 | - | - | - |
| Cu a.p. | −0.17 | −0.30 | −0.25 | −0.25 | −0.28 | −0.11 | −0.20 | −0.23 | −0.24 | −0.19 | −0.09 | 0.13 | 0.26 | −0.23 | −0.05 | 0.06 | 0.00 | 0.18 | 0.31 | 0.29 | −0.17 | - | - |
| BF | 0.10 | 0.04 | −0.07 | −0.07 | 0.02 | −0.23 | −0.13 | 0.03 | 0.02 | −0.11 | −0.02 | −0.02 | 0.08 | 0.01 | −0.09 | −0.06 | 0.53 | −0.10 | −0.55 | −0.34 | 0.05 | −0.08 | - |
| SAMPLING AREA: VR | |||||||||||||||||||||||
| pHH2O | pHKCl | C(%) | OM | N | C/N | DOC | DIC | Ca2+ | Mg2+ | K+ | Na+ | Al3+ | eCEC | Sand | Silt | Clay | Total Cu | Avail. Cu | ExEf | Bm | Cu a.p. | BF | |
| Total Cu | −0.23 | −0.03 | −0.01 | −0.01 | 0.02 | −0.04 | 0.23 | −0.10 | −0.04 | −0.08 | 0.28 | −0.27 | −0.29 | −0.06 | −0.11 | 0.16 | −0.10 | - | - | - | - | - | - |
| Avail. Cu | −0.18 | −0.27 | −0.22 | −0.22 | −0.27 | 0.14 | −0.07 | −0.25 | −0.24 | −0.09 | 0.06 | −0.09 | −0.10 | −0.26 | 0.02 | 0.04 | −0.19 | 0.22 | - | - | - | - | - |
| ExEf | −0.04 | −0.24 | −0.16 | −0.16 | −0.22 | 0.11 | −0.23 | −0.16 | −0.21 | −0.08 | −0.12 | 0.04 | 0.11 | −0.20 | −0.03 | 0.07 | −0.13 | −0.42 | 0.62 | - | - | - | - |
| Bm | 0.21 | 0.33 | 0.51 | 0.51 | 0.57 | −0.02 | 0.21 | 0.27 | 0.48 | 0.25 | 0.36 | 0.02 | −0.28 | 0.49 | 0.00 | 0.01 | −0.02 | 0.16 | −0.30 | −0.30 | - | - | - |
| Cu a.p. | −0.13 | −0.28 | −0.26 | −0.26 | −0.29 | −0.11 | −0.20 | −0.22 | −0.22 | −0.17 | −0.09 | 0.14 | 0.26 | −0.20 | −0.02 | 0.02 | 0.00 | 0.11 | 0.31 | 0.30 | −0.18 | - | - |
| BF | 0.13 | 0.05 | −0.08 | −0.08 | 0.01 | −0.22 | −0.13 | 0.04 | 0.04 | −0.09 | −0.01 | −0.01 | 0.08 | 0.03 | −0.06 | −0.09 | 0.53 | −0.13 | −0.55 | −0.32 | 0.03 | −0.07 | - |
| Soils with total Cu contents below phytotoxic level (<100 mg kg−1) | |||||||||||||||||||||||
| pHH2O | pHKCl | C(%) | OM | N | C/N | DOC | DIC | Ca2+ | Mg2+ | K+ | Na+ | Al3+ | eCEC | Sand | Silt | Clay | Total Cu | Avail. Cu | ExEf | Bm | Cu a.p. | BF | |
| Total Cu | −0.11 | 0.07 | −0.26 | −0.26 | −0.35 | 0.19 | −0.27 | −0.05 | −0.09 | −0.01 | 0.14 | 0.41 | −0.01 | −0.09 | 0.33 | −0.32 | −0.31 | 0.19 | −0.24 | −0.09 | −0.30 | −0.44 | |
| Avail. Cu | −0.05 | −0.18 | −0.32 | −0.33 | −0.43 | 0.10 | −0.47 | −0.16 | −0.25 | −0.15 | 0.16 | −0.10 | −0.16 | −0.29 | −0.19 | 0.25 | −0.24 | 0.19 | 0.87 | −0.65 | 0.11 | −0.78 | |
| ExEf | −0.05 | −0.23 | −0.25 | −0.25 | −0.30 | 0.00 | −0.44 | −0.17 | −0.27 | −0.18 | −0.02 | −0.21 | −0.08 | −0.31 | −0.27 | 0.32 | −0.13 | −0.24 | 0.87 | −0.62 | 0.37 | −0.53 | |
| Bm | 0.40 | 0.41 | 0.58 | 0.58 | 0.62 | 0.11 | 0.45 | 0.44 | 0.50 | 0.34 | 0.04 | −0.07 | −0.14 | 0.52 | 0.03 | −0.09 | 0.31 | −0.09 | −0.65 | −0.62 | −0.52 | 0.36 | |
| Cu a.p. | −0.31 | −0.22 | −0.22 | −0.22 | −0.26 | −0.11 | −0.29 | −0.29 | −0.16 | 0.10 | −0.35 | 0.45 | 0.40 | −0.10 | −0.03 | 0.04 | −0.01 | −0.30 | 0.11 | 0.37 | −0.52 | 0.16 | |
| BF | −0.21 | −0.20 | 0.19 | 0.19 | 0.30 | −0.25 | 0.14 | −0.16 | −0.01 | 0.04 | 0.08 | 0.05 | 0.30 | 0.04 | 0.03 | −0.07 | 0.21 | −0.44 | −0.78 | −0.53 | 0.36 | 0.16 | |
| Soils with total Cu contents above phytotoxic level (>100 mg kg−1) | |||||||||||||||||||||||
| pHH2O | pHKCl | C(%) | OM | N | C/N | DOC | DIC | Ca2+ | Mg2+ | K+ | Na+ | Al3+ | eCEC | Sand | Silt | Clay | Total Cu | Avail. Cu | ExEf | Bm | Cu a.p. | BF | |
| Total Cu | −0.40 | −0.31 | 0.05 | 0.05 | 0.02 | 0.22 | −0.13 | −0.25 | −0.14 | −0.33 | 0.20 | 0.09 | −0.06 | −0.15 | −0.04 | 0.15 | −0.26 | 0.34 | −0.02 | 0.24 | 0.17 | −0.40 | |
| Avail. Cu | −0.27 | −0.36 | −0.15 | −0.15 | −0.18 | 0.20 | −0.08 | −0.34 | −0.25 | −0.09 | 0.03 | −0.07 | −0.01 | −0.24 | 0.18 | −0.12 | −0.20 | 0.34 | 0.90 | −0.19 | 0.08 | −0.59 | |
| ExEf | −0.19 | −0.24 | −0.14 | −0.14 | −0.15 | 0.11 | 0.01 | −0.27 | −0.20 | 0.12 | −0.05 | −0.14 | −0.03 | −0.18 | 0.17 | −0.12 | −0.18 | −0.02 | 0.90 | −0.24 | −0.04 | −0.54 | |
| Bm | 0.15 | 0.33 | 0.51 | 0.51 | 0.59 | −0.08 | 0.21 | 0.22 | 0.50 | 0.23 | 0.41 | 0.31 | −0.48 | 0.50 | 0.00 | 0.04 | −0.08 | 0.24 | −0.19 | −0.24 | −0.02 | −0.01 | |
| Cu a.p. | 0.00 | −0.19 | −0.19 | −0.19 | −0.21 | −0.17 | −0.13 | 0.07 | −0.06 | −0.18 | −0.14 | 0.01 | 0.13 | −0.08 | 0.25 | −0.27 | −0.01 | 0.17 | 0.08 | −0.04 | −0.02 | 0.04 | |
| BF | 0.23 | 0.10 | −0.14 | −0.14 | −0.05 | −0.26 | −0.21 | 0.10 | 0.04 | −0.13 | −0.05 | 0.15 | 0.17 | 0.03 | −0.06 | −0.15 | 0.56 | −0.40 | −0.59 | −0.54 | −0.01 | 0.04 | |
| Soils Sampled in Vineyard Rows (VR) | ||||
|---|---|---|---|---|
| PC | Eigenvalues | % Variance | ||
| 1 | 9.694 | 42.148 | ||
| 2 | 6.602 | 28.704 | ||
| 3 | 4.591 | 19.961 | ||
| 4 | 2.113 | 9.187 | ||
| PC 1 | PC 2 | PC 3 | PC 4 | |
| 01 M | −0.996 | −1.236 | 1.575 | −2.04 |
| 03 RS | −1.088 | 0.709 | 0.088 | −1.138 |
| 04 M | −1.88 | −2.599 | 0.503 | −1.008 |
| 06 V | 6.897 | 0.597 | 3.817 | −0.17 |
| 08 RB | 3.803 | 2.62 | −0.558 | −2.253 |
| 10 V | −0.162 | −2.797 | −0.864 | −0.111 |
| 13 R | −2.284 | −0.959 | −1.835 | −0.988 |
| 14 R | −1.133 | −0.439 | −0.843 | −0.569 |
| 16 V | 0.444 | −1.362 | 1.669 | 0.488 |
| 17 V | 2.018 | −0.574 | 0.966 | 0.639 |
| 20 R | 0.929 | 1.467 | 0.339 | 1.837 |
| 22 RS | −0.869 | 4.436 | 2.025 | 0.986 |
| 23 M | −0.002 | 0.016 | −0.086 | −0.797 |
| 24 RS | −0.429 | 3.015 | 1.459 | −1.004 |
| 25 RS | −0.965 | 0.02 | −0.749 | 0.356 |
| 28 RB | 1.04 | −1.798 | −1.276 | 0.29 |
| 29 R | −0.385 | 0.525 | −1.048 | 1.197 |
| 34 R | −1.203 | −2.035 | −1.472 | 0.632 |
| 36 R | −2.298 | −1.131 | −1.351 | 1.14 |
| 37 R | −1.597 | −1.095 | −0.764 | 0.521 |
| 41 R | −0.681 | 0.199 | −0.723 | 0.715 |
| 42 R | 0.841 | 2.421 | −0.872 | 1.276 |
| Soils sampled in vineyard strains (VS) | ||||
| PC | Eigenvalues | % variance | ||
| 1 | 11.487 | 49.945 | ||
| 2 | 6.112 | 26.572 | ||
| 3 | 2.988 | 12.992 | ||
| 4 | 2.413 | 10.491 | ||
| PC 1 | PC 2 | PC 3 | PC 4 | |
| 05 M | −3.78 | −1.518 | −0.729 | −1.402 |
| 07 RS | −2.631 | −1.589 | −0.893 | −0.893 |
| 09 RB | 0.659 | −1.654 | −1.645 | 0.38 |
| 11 V | 0.658 | 0.151 | 0.728 | −1.347 |
| 12 R | −5.53 | 2.082 | 0.442 | 0.96 |
| 15 V | −1.164 | 1.466 | −3.879 | −1.874 |
| 18 RB | 1.869 | −0.505 | −1.437 | 0.937 |
| 19 RS | 0.767 | −1.564 | 2.058 | −0.358 |
| 21 V | 1.563 | 1.015 | −1.037 | 0.877 |
| 26 V | 3.602 | 1.35 | −0.988 | 0.577 |
| 27 RS | 1.232 | −0.501 | −0.16 | 0.633 |
| 30 RS | 3.834 | −2.102 | 3.193 | 1.38 |
| 31 RS | 0.055 | −1.336 | 1.092 | −0.187 |
| 32 R | −0.153 | 0.567 | −0.544 | 1.561 |
| 33 RS | 0.246 | −2.295 | −0.033 | 0.28 |
| 35 V | −2.694 | 2.01 | 0.576 | −0.04 |
| 38 R | −1.743 | 1.399 | 1.421 | −0.157 |
| 39 V | 4.34 | 0.954 | 0.34 | 0.652 |
| 40 V | −1.13 | 2.069 | 1.494 | −1.979 |
| Soil | Sampling Area | Biomass | Cu in Aerial Part | BF | Soil | Sampling Area | Biomass | Cu in Aerial Part | BF |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | (%) | mg kg−1 | mg kg−1 | (%) | ||||
| 1 M | VR | 0.769 ± 0.048 | 0.023 ± 0.002 | 0.55 | 22 RS | VR | 0.690 ± 0.004 | 0.011 ± 0.001 | 0.26 |
| 2 RB | VS | 0.731 ± 0.030 | 0.015 ± 0.001 | 0.41 | 23 M | VR | 0.679 ± 0.036 | 0.013 ± 0.001 | 0.59 |
| 3 RS | VR | 0.744 ± 0.010 | 0.009 ± 0.001 | 0.47 | 24 RS | VR | 0.831 ± 0.127 | 0.015 ± 0.001 | 1.27 |
| 4 M | VR | 0.621 ± 0.021 | 0.017 ± 0.004 | 0.08 | 25 RS | VR | 0.724 ± 0.099 | 0.013 ± 0.001 | 0.07 |
| 5 M | VS | 0.858 ± 0.078 | 0.011 ± 0.008 | 0.46 | 26 V | VS | 0.837 ± 0.017 | 0.015 ± 0.001 | 1.05 |
| 6 V | VR | 0.847 ± 0.048 | 0.009 ± 0.003 | 0.27 | 27 RS | VS | 0.859 ± 0.103 | 0.012 ± 0.001 | 0.85 |
| 7 RS | VS | 0.83 ± 0.103 | 0.008 ± 0.002 | 0.30 | 28 RB | VR | 0.778 ± 0.099 | 0.014 ± 0.001 | 0.18 |
| 8 RB | VR | 0.883 ± 0.077 | 0.013 ± 0.005 | 0.17 | 29 R | VR | 0.699 ± 0.103 | 0.012 ± 0.001 | 0.15 |
| 9 RB | VS | 0.847 ± 0.016 | 0.013 ± 0.003 | 0.55 | 30 RS | VS | 0.99 ± 0.166 | 0.015 ± 0.004 | 0.18 |
| 10 V | VR | 0.643 ± 0.042 | 0.014 ± 0.002 | 0.06 | 31 RS | VS | 0.979 ± 0.042 | 0.013 ± 0.001 | 0.17 |
| 11 V | VS | 0.788 ± 0.006 | 0.014 ± 0.001 | 0.12 | 32 R | VS | 0.922 ± 0.011 | 0.015 ± 0.001 | 1.00 |
| 12 R | VS | 0.695 ± 0.031 | 0.017 ± 0.001 | 0.17 | 33 RS | VS | 1.061 ± 0.053 | 0.012 ± 0.001 | 0.37 |
| 13 R | VR | 0.685 ± 0.053 | 0.016 ± 0.001 | 0.51 | 34 R | VR | 0.765 ± 0.019 | 0.019 ± 0.001 | 0.08 |
| 14 R | VR | 0.691 ± 0.012 | 0.013 ± 0.001 | 0.19 | 35 V | VS | 0.643 ± 0.046 | 0.019 ± 0.001 | 0.14 |
| 15 V | VS | 0.672 ± 0.052 | 0.012 ± 0.001 | 2.98 | 36 R | VR | 0.708 ± 0.037 | 0.025 ± 0.008 | 0.16 |
| 16 V | VR | 0.654 ± 0.019 | 0.016 ± 0.002 | 0.70 | 37 R | VR | 0.727 ± 0.073 | 0.016 ± 0.002 | 0.37 |
| 17 V | VR | 0.746 ± 0.027 | 0.013 ± 0.002 | 0.10 | 38 R | VS | 0.645 ± 0.057 | 0.017 ± 0.001 | 0.15 |
| 18 RB | VS | 0.855 ± 0.006 | 0.014 ± 0.001 | 0.60 | 39 V | VS | 0.957 ± 0.047 | 0.013 ± 0.003 | 0.09 |
| 19 RS | VS | 0.605 ± 0.227 | 0.012 ± 0.001 | 0.15 | 40 V | VS | 0.569 ± 0.038 | 0.011 ± 0.001 | 0.06 |
| 20 R | VR | 0.732 ± 0.009 | 0.014 ± 0.001 | 0.07 | 41 R | VR | 0.689 ± 0.087 | 0.013 ± 0.001 | 0.17 |
| 21 V | VS | 0.613 ± 0.049 | 0.012 ± 0.001 | 0.40 | 42 R | VR | 0.961 ± 0.002 | 0.012 ± 0.001 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Blanco, R.; Arias-Estévez, M.; Fernández-Calviño, D.; Arenas-Lago, D. Early Growth Assessment of Lolium perenne L. as a Cover Crop for Management of Copper Accumulation in Galician Vineyard Soils. Horticulturae 2023, 9, 1029. https://doi.org/10.3390/horticulturae9091029
Vázquez-Blanco R, Arias-Estévez M, Fernández-Calviño D, Arenas-Lago D. Early Growth Assessment of Lolium perenne L. as a Cover Crop for Management of Copper Accumulation in Galician Vineyard Soils. Horticulturae. 2023; 9(9):1029. https://doi.org/10.3390/horticulturae9091029
Chicago/Turabian StyleVázquez-Blanco, Raquel, Manuel Arias-Estévez, David Fernández-Calviño, and Daniel Arenas-Lago. 2023. "Early Growth Assessment of Lolium perenne L. as a Cover Crop for Management of Copper Accumulation in Galician Vineyard Soils" Horticulturae 9, no. 9: 1029. https://doi.org/10.3390/horticulturae9091029
APA StyleVázquez-Blanco, R., Arias-Estévez, M., Fernández-Calviño, D., & Arenas-Lago, D. (2023). Early Growth Assessment of Lolium perenne L. as a Cover Crop for Management of Copper Accumulation in Galician Vineyard Soils. Horticulturae, 9(9), 1029. https://doi.org/10.3390/horticulturae9091029