“In Vitro” Ovule Culture to Improve Genetic Variability in Hydrangea macrophylla
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Crossing Procedure
2.3. In Vitro Ovule Culture
2.4. Genetic Analysis
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClintock, E. A monograph of the genus Hydrangea. Proc. Calif. Acad. Sci. 1957, 29, 147–256. [Google Scholar]
- Reed, S.M.; Riedel, G.M.; Pooler, M.R. Verification and establishment of H. macrophylla ‘Kardinal’ × H. paniculata ‘Brussels Lace’ interspecific hybrids. J. Environ. Hort. 2001, 19, 85–88. [Google Scholar] [CrossRef]
- Reed, S.M. Floral characteristics of a Hydrangea macrophylla × H. paniculata hybrid. In Proceedings of the Southern Nursery Association Research Conference, Atlanta, GA, USA, 13 December 2004; Volume 49, pp. 580–582. [Google Scholar]
- Rinehart, T.A.; Scheffler, B.E.; Reed, S.M. Genetic diversity estimates for the gens Hydrangea and development of a molecular key based on SSRs. J. Amer. Soc. Hort. Sci. 2006, 131, 787–797. [Google Scholar] [CrossRef]
- Rinehart, T.A.; Wadl, P.A.; Staton, M.E. An update on Hydrangea macrophylla breeding targets and genomics. Acta Hort. 2018, 1191, 217–224. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Van Dién, M.P.; Van Kleinwee, M.G.M.; Franken, J.; Bino, R.J. Application of in vitro pollination, ovary culture and embryo rescue for overcoming incongruity barriers in interspecific Lilium crosses. Plant Sci. 1991, 74, 115–126. [Google Scholar] [CrossRef]
- Jones, K.D.; Reed, S.M.; Rinehart, T.A. Wide crosses in the Hydrangeaceae: Dichroa febrifuga × Hydarngea macrophylla. Proc. South. Nurs. Assn. Res. Conf. 2006, 51, 577–579. [Google Scholar]
- Kudo, N.; Niimi, Y. Production of interspecific hybrids between Hydrangea macrophylla f. hortensia (Lam.) Rehd. and H. arborescens L. J. Jpn. Soc. Hort. Sci. 1999, 68, 428–439. [Google Scholar] [CrossRef]
- Kudo, N.; Niimi, Y. Production of interspecific hybrid plants through cotyledonary segment culture of embryos derived from crosses between Hydrangea macrophylla f. hortensia (Lam.) Rehd. and H. arborescens L. J. Jpn. Soc. Hort. Sci. 1999, 68, 803–809. [Google Scholar] [CrossRef]
- Reed, S.M. Development of an in ovolo embryo culture procedure for Hydrangea. J. Environ. Hort. 2000, 18, 34–39. [Google Scholar] [CrossRef]
- Cai, M.; Wang, K.; Luo, L.; Pan, H.T.; Zhang, Q.X.; Yang, Y.Y. Production of interspecific hybrids between Hydrangea macrophylla and Hydrangea arborescens via ovary culture. HortScience 2015, 50, 1765–1769. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Van Laere, K.; De Riek, J.; Van Huylenbroeck, J. Overcoming interspecific barriers in ornamental plant breeding. In Floriculture, Ornamental and Plant Biotechnology; Da Silva, J.A.T., Ed.; Global Science Books: London, UK, 2006; Volume I, pp. 540–551. [Google Scholar]
- Eeckhaut, T.; Van Huylenbroeck, J.; Van Laere, K.; Van Bockstaele, E. Interspecific hybridization in woody ornamentals: How to deal with barriers? Acta Hortic. 2006, 725, 117–126. [Google Scholar] [CrossRef]
- Lazzereschi, S.; Nesi, B.; Pecchioli, S.; Grassotti, A. Costituzione, selezione e propagazione di ibridi interspecifici di Lilium. Acta Italus Hortus 2012, 6, 188. [Google Scholar]
- Nesi, B.; Lazzereschi, S.; Pecchioli, S.; Pacifici, S.; Venturieri, A.G.; Burchi, G. Development of complementary techniques applied to Hydrangea hybridizations. Acta Hortic. 2015, 1104, 341–348. [Google Scholar] [CrossRef]
- Venturieri, G.A.; Nesi, B.; Lazzereschi, S.; Pecchioli, S.; Burchi, G. Development of pollination and in vitro germination techniques to improve the hybridization in Hydrangea spp. Adv. Hort. Sci. 2017, 31, 45–51. [Google Scholar]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Pan, M.; Van Staden, J. The effect of activated charcoal and auxins on root formation by hypocotyl segments of Daucus carota. S. Afr. J. Bot. 2002, 68, 349–356. [Google Scholar] [CrossRef]
- Greer, S.P.; Rinehart, T.A. Dormancy and germination in vitro response of Hydrangea macrophylla and Hydrangea paniculata seed to light, cold-treatment and gibberellic acid. J. Environ. Hortic. 2010, 28, 41–47. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Nicoloso, F.T.; Erig, A.C.; Martins, C.F.; Russowski, D. Micro propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Rev. Bras. Pl. Med. 2001, 3, 11–18. [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, L. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hempel, P.; Hohe, A.; Tränkner, C. Molecular Reconstruction of an old pedigree of diploid and triploid Hydrangea macrophylla genotypes. Front. Plant Sci. 2018, 18, 429. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Wadl, P.A.; Hadziabdic, D.; Wang, X.; Trigiano, R.N. Analyzing microsatellites using the QIAxcel system. Methods Mol. Biol. 2013, 1006, 223–243. [Google Scholar] [CrossRef]
- Tallòn, C.I.; Porras, I.; Peréz-Tornero, O. High efficiency in vitro organogenesis from mature tissue explants of Citrus macrophylla and C. aurantium. Vitr. Cell. Dev. Biol. Plant 2013, 49, 145–155. [Google Scholar] [CrossRef]
- Takamura, Y.; Asano, C.; Hikage, T.; Hatakeyama, K.; Takahata, Y. Production of interspecific hybrids between Japanese gentians and wild species of Gentiana. Breed. Sci. 2019, 69, 680–687. [Google Scholar] [CrossRef]
- Johansson, L. Effects of activated charcoal in anther cultures. Physiol. Plant. 1983, 59, 397–403. [Google Scholar] [CrossRef]
- Weatherhead, M.A.; Bourdon, J.; Henshan, G.G. Effect of activated charcoal as an additive plant tissue culture media. Zeitschrift Pflanzenphysiologie 1979, 94, 399–406. [Google Scholar] [CrossRef]
- Paek, K.Y.; Yeung, E.C. The effects of 1-naphthalene acetic acid and N6-benzyladenine on the growth of Cymbidium forrestii rhizomes in vitro. Plant Cell Tissue Org. Cult. 1991, 24, 65–71. [Google Scholar] [CrossRef]
- Kim, C.; Oh, J.; Chung, J.; Burrell, A.; Byrne, D. Somatic embryogenesis and plant regeneration from in-vitro-grown leaf explants of rose. HortScience 2004, 39, 1378–1380. [Google Scholar] [CrossRef]
- Reed, S.M.; Rinehart, T.A. Simple sequence repeat marker analysis of genetic relationships within Hydrangea macrophylla. J. Am. Soc. Hort. Sci. 2007, 132, 341–351. [Google Scholar] [CrossRef]
- Sukhikh, N.; Malyarovskaya, V.; Lidia Samarina, L.; Vinigradova, S. Genetic variation in Hydrangea macrophylla (Thunb.) ser. in Russia based on simple sequence repeat markers. Bangladesh J. Bot. 2018, 47, 937–943. [Google Scholar] [CrossRef]




| Flower Image | Name | Description |
|---|---|---|
 | Amor blue® Breeding goal: pot plant | It is a deciduous shrub cultivated for its showy inflorescences, with ovate glossy toothed dark leaves. The large rounded clusters of mophed flowers are intense blue during the summer time. |
 | Parental CBreeding goal: Europe red | Hydrangea macrophylla with a large-leaved, deciduous shrub species that produces huge, showy pink, tipped with green gold mop-head shaped flowers during the summer. It is common for garden use. |
 | Endless Summer Bloom Star Breeding goal: tropical red | It is an upright, bushy deciduous shrub with broadly ovate, toothed, pointed dark green leaves and, from early summer to fall. It has an endless procession of stunning vivid purple or rose pink colored blooms that sit above strong sturdy red stems. |
 | Koria Breeding goal: Pot plant | It is a lovely lace-cap variety, conspicuous for its serrated bracts that surround a center of blue or pink flowers, set against apple-green foliage. |
 | Forever&Ever® “Light Purple” Breeding goal: Tropical red | It belongs to a group called Forever&Ever® and to Hydrangeas that have proven extended blooming time and excellent hardiness. It produces large, globular, mophead flowers on both old and new wood so do not worry about pruning or unexpected frost. It starts blooming on the first hot days of spring and will continue until the first frost. Deadheading will enhance flowering and speed up the forming of new flower buds. |
 | Rodeo Breeding goal: Europe red | Compact spherical shrub up to about 1–1.5 m tall. Deciduous. Large green leaves. Large spherical inflorescence. Deep pink flower color. Flowering period: July—September. Sunshade. Nourishing soil. |
 | Parental B Breeding goal: Tropical pink | Native to Colombia is a popular variety grown for cut flowers, which can also be used for drying. |
 | Verena® Breeding goal: Europe pink | Popular cut flower which can also be used to dry. Plant height: 50–70 cm. Flower color: light pink. Easy pruning; easily by coloring in autumn tones; blooming richly also at low temperatures; good variety to bluish. |
 | Wedding Ring Breeding goal: Europe pink/blue | Is a trade name for “Fanfare”, a new variety originating from the United States where Hydrangea is the “trend” shrub of the moment. It is a hybrid of the species Hydrangea macrophylla with ball-shaped flowers. This variety is notable for its cold hardiness in winter (it adapts well to USDA Zone 5 winters) compared to many of the commercially available cultivars of H. macrophylla today. |
 | Parental D Breeding goal: Tropical white | Colombian Hydrangea, old varieties imported many years ago in South America, probably from France or the USA, and never correctly identified. Recurrent blooming. |
 | Hybrid 1 Breeding goal: Europe pink/white | New selection tropical white/pink (patent pending material). |
 | Parental A Breeding goal: Europe red | These plants are very beautiful and can be expected to throw out up to 30 strong flower heads during their first flowering season. A wonderful Hydrangea variety with huge flowers often found in high-class florist bouquets, it has massive blooms. |
| Experiment A | |||
|---|---|---|---|
| Female Parentals ♀ (Code) | Male Parentals ♂ (Code) | D.A.P. | |
| Amor blue® (Amor) | x | Endless Summer Bloom Star (ESBlo) | 124 |
| Verena (Vere) | x | Koria (Kori) | 123 |
| Rodeo (Rod) | x | Wedding Ring (Wedd) | 121 |
| Light Purple (Ligh) | x | Koria (Kori) | 138 |
| Parental A (ParA) | x | Parental B (ParB) | 121 |
| Experiment B | |||
| Parental C (ParC) | x | Parental A (ParA) | 134 |
| Parental D (ParD) | x | Hybrid 1 (Hyb1) | 151 |
| Source of Variation | Sprouted Ovules | Live Plants | Total Live Plants | Leaves | Plant Height | Root Length | Height 1st Internode | Plant Weight | Branches |
|---|---|---|---|---|---|---|---|---|---|
| % | n. | mm | g | n. | |||||
| Genotype (G) | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
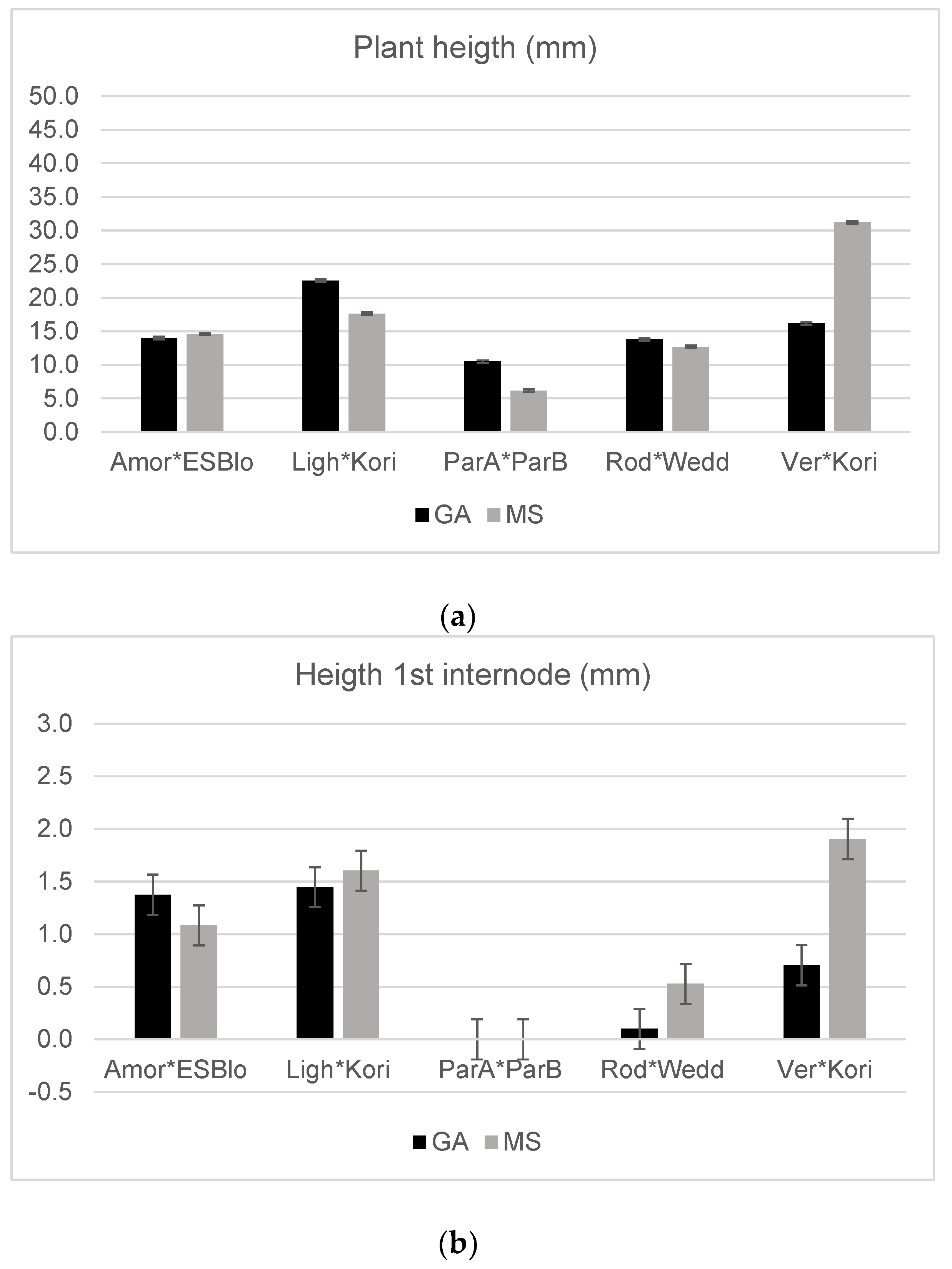
| Amor × ESBlo | 51.32 | 97.50 | 5.63 b | 13.66 | 14.43 | 21.89 | 1.16 | 0.14 | 0.45 |
| Ligh × Kori | 65.42 | 78.71 | 23.75 a | 13.14 | 20.09 | 19.37 | 1.53 | 0.23 | 0.46 |
| ParA × ParB | 78.33 | 93.75 | 2.25 b | 7.58 | 7.25 | 9.83 | 0.00 | 0.12 | 0.01 |
| Rod × Wedd | 77.89 | 83.10 | 6.67 b | 13.75 | 13.26 | 23.31 | 0.31 | 0.16 | 0.57 |
| Vere × Kori | 57.41 | 88.54 | 11.18 b | 13.85 | 23.00 | 16.20 | 1.25 | 0.14 | 0.33 |
| Medium (M) | n.s. | n.s. | n.s. | * | n.s. | ** | n.s. | ** | * |
| GA | 61.50 | 87.08 | 13.94 | 13.89 | 17.34 | 21.62 | 0.89 | 0.20 | 0.53 |
| MS | 65.25 | 87.15 | 10.91 | 12.26 | 17.65 | 16.34 | 1.18 | 0.14 | 0.29 |
| G × M | n.s. | n.s. | n.s. | n.s. | ** | n.s. | ** | n.s. | n.s. |
| Component | Total | % of Variance | % Cumulative |
|---|---|---|---|
| 1 | 3.408 | 37.864 | 37.864 |
| 2 | 1.402 | 15.578 | 53.442 |
| 3 | 1.249 | 13.873 | 67.314 |
| 4 | 0.943 | 10.481 | 77.796 |
| 5 | 0.844 | 9.375 | 87.171 |
| 6 | 0.586 | 6.506 | 93.676 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Plant weight | 0.817 | 0.015 | 0.070 | 0.368 | −0.280 | −0.081 |
| Plant height | 0.775 | −0.454 | −0.161 | −0.135 | 0.190 | 0.069 |
| Total leaves | 0.744 | 0.407 | 0.037 | −0.354 | 0.228 | −0.018 |
| Height 1st internode | 0.726 | −0.476 | −0.083 | −0.318 | 0.172 | 0.180 |
| Root length | 0.681 | 0.246 | −0.242 | 0.287 | 0.197 | −0.506 |
| Total live plants | 0.676 | −0.139 | 0.335 | 0.284 | −0.458 | 0.219 |
| Branches | 0.356 | 0.748 | 0.222 | −0.383 | −0.212 | 0.119 |
| % Live plants | 0.096 | 0.387 | −0.708 | 0.355 | 0.141 | 0.440 |
| % Sprouted ovules | 0.040 | 0.123 | 0.698 | 0.357 | 0.578 | 0.174 |
| Sources of Variation | Sprouted Ovules | Live Plants | Total Live Plants | Leaves | Plant Height | Root Length | Height 1st Internode | Plant Weight | Branches |
|---|---|---|---|---|---|---|---|---|---|
| % | n. | mm | g | n. | |||||
| Parental genotype (G) | n.s. | n.s. | ** | n.s. | ** | n.s. | ** | * | n.s. |
| ParC × ParA | 84.38 | 88.42 | 6.15 | 13.13 | 14.52 | 28.18 | 1.12 | 0.20 | 0.23 |
| ParD × Hyb1 | 87.68 | 83.82 | 2.29 | 15.17 | 27.00 | 34.62 | 1.92 | 0.37 | 0.32 |
| Medium (M) | n.s. | * | * | n.s. | n.s. | * | n.s. | n.s. | n.s. |
| GA | 85.99 | 90.45 | 5.17 | 13.51 | 21.74 | 44.31 | 1.84 | 0.28 | 0.26 |
| MS | 85.81 | 82.39 | 3.63 | 14.58 | 18.84 | 18.66 | 1.16 | 0.27 | 0.29 |
| Activated charcoal (AC) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ** | n.s. | n.s. |
| C0 | 86.74 | 84.01 | 3.89 | 13.62 | 17.88 | 29.77 | 1.00 | 0.26 | 0.18 |
| C1 | 85.10 | 88.49 | 4.84 | 14.49 | 22.49 | 32.44 | 1.95 | 0.29 | 0.36 |
| G × M | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| G × AC | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| M × AC | * | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| G × M × AC | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Sample | Parental | SSR Markers | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| STAB 305_306 | STAB 317_318 | STAB 321_322 | ||||||||
| Amor | ♀ | 130 | 154 | 148 | 151 | |||||
| ESBlo | ♂ | 133 | 136 | 148 | 155 | 137 | 151 | |||
| Amor × ESBlo | Hybrid | 130 | 136 | 148 | 154 | 151 | ||||
| Vere | ♀ | 130 | 136 | 154 | 151 | |||||
| Kori | ♂ | 130 | 136 | 148 | 154 | 151 | ||||
| Vere × Kori | Hybrid | 130 | 136 | 154 | 151 | |||||
| Rod | ♀ | 133 | 136 | 148 | 154 | 148 | 151 | |||
| Wedd | ♂ | 133 | 136 | 154 | 151 | |||||
| Rod × Wedd | Hybrid | 130 | 136 | 154 | 151 | |||||
| Ligh | ♀ | 130 | 133 | 154 | 136 | 151 | ||||
| Kori | ♂ | 130 | 136 | 148 | 154 | 151 | ||||
| Ligh × Kori | Hybrid | 133 | 136 | 154 | 136 | 151 | ||||
| Parental C | ♀ | 130 | 136 | 154 | 151 | |||||
| Parental A | ♂ | 136 | 148 | 154 | 148 | 151 | ||||
| ParC × ParA | Hybrid | 130 | 136 | 148 | 154 | 151 | ||||
| ParD | ♀ | 130 | 136 | 145 | 154 | 151 | ||||
| Hybr1 | ♂ | 133 | 136 | 145 | 154 | 151 | ||||
| ParD × Hyb1 | Hybrid | 133 | 136 | 145 | 148 | 154 | 136 | 151 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesi, B.; Ghiselli, L.; Gori, M.; Natale, R.; Tomiozzo, R.; Mansuino, A.; Biricolti, S. “In Vitro” Ovule Culture to Improve Genetic Variability in Hydrangea macrophylla. Horticulturae 2023, 9, 1028. https://doi.org/10.3390/horticulturae9091028
Nesi B, Ghiselli L, Gori M, Natale R, Tomiozzo R, Mansuino A, Biricolti S. “In Vitro” Ovule Culture to Improve Genetic Variability in Hydrangea macrophylla. Horticulturae. 2023; 9(9):1028. https://doi.org/10.3390/horticulturae9091028
Chicago/Turabian StyleNesi, Beatrice, Lisetta Ghiselli, Massimo Gori, Roberto Natale, Regina Tomiozzo, Andrea Mansuino, and Stefano Biricolti. 2023. "“In Vitro” Ovule Culture to Improve Genetic Variability in Hydrangea macrophylla" Horticulturae 9, no. 9: 1028. https://doi.org/10.3390/horticulturae9091028
APA StyleNesi, B., Ghiselli, L., Gori, M., Natale, R., Tomiozzo, R., Mansuino, A., & Biricolti, S. (2023). “In Vitro” Ovule Culture to Improve Genetic Variability in Hydrangea macrophylla. Horticulturae, 9(9), 1028. https://doi.org/10.3390/horticulturae9091028






