Abstract
Saline water is used in floriculture as an alternative to freshwater in arid regions such as Saudi Arabia (SA). However, salt stress considerably accelerates serious physio-biochemical changes associated with a decline in plant establishment. Recently, humic acid (HA) foliar spraying has induced plant stress tolerance in the era of climate change; however, its precise roles in the floriculture industry within saline conditions are not yet well documented. A factorial pot experiment throughout the 2022/2023 season was conducted in the Nursery of Sustainability and Environmental Developmental Department, King Saud University, Riyadh, SA, to evaluate the potential effects of HA (0, 500, 1000 and 2000 mg/L) on growth, flowering and some physiological characteristics of Ivy geranium (Pelargoniumpeltatum) plants irrigated with saline water (230 “control”, 2000 and 4000 mg/L NaCl). Irrigation with saline water markedly inhibited plant growth, flowering attributes, the chlorophyll index, as well as macro and micro-nutrient levels, but increased the content of iron, sodium and proline in plant shoots relative to plants irrigated with non-salinized water. However, HA mainly at 1000 mg/L significantly improved plant growth, flowering capacity, nutrient status, proline accumulation and chlorophyll index under salinized or non-salinized irrigation water. Additionally, spraying of HA concentrations (500, 1000 and 2000 mg/L) under normal or salinity conditions significantly increased shoot sodium content relative to non-treated plants under such salinity levels. Our findings highlight the significance of HA concentrations (500, 1000 and 2000 mg/L) in improving the salt tolerance of Ivy geranium. Within the scarcity of irrigation water, it is recommended to irrigate Ivy geranium with saline water up to 4000 mg/L NaCl associated with spraying HA concentrations in special 1000 mg/L.
1. Introduction
Ornamental and flowering plants (OFP) take an imperative place in the horticultural business as they are utilized in gardening, roads, landscaping and as cut flowers [1,2]. Growing OFP is a dynamically developing and profitable sector of plant production. The global market of OFP moves 250 to 400 billion dollars annually in the European Union, USA and Japan [3,4]. Due to the variety of temperatures, soil and flora in Saudi Arabia (SA), the OFP agribusiness has the potential to expand, resulting in a rise in the production of native and foreign species. Pelargonium is a genus of 400 species widely distributed worldwide. It comes in the third rank within potted OFP with USD 2.5 billion yearly production [5,6]. In addition to the bioremediation capacity, they may also be grown in harsh environments, such as saline and calcareous soil [7]. Recently, the study of exploiting saline water on Ivy geranium (P. peltatum (L.) L’Hér. ex Aiton) plant development and flowering has been very scarce and needs more investigation.
Water consumptionis the main constraint to OFP production (each one kg plant dry mass needs 100–350 kg of water) [8]. So, the utilization of marginal water resources in irrigation ranging between 1000–6000 mg/L NaCl [9], such as recycled or salinized water, has been encouraged by the growth in population and agricultural production along with the decline of high-quality water sources, particularly in arid areas like SA [10,11]. Irrigation with saline water has a drastic impact on soil–water–plant relations, i.e., intermittently deteriorating soil resources, strictly hampering the regular physio-biochemical pathways, alongside plant retardation, and initiating salty soil [12,13,14]. Crop development, flowering, productivity and primary carbon metabolism are all adversely impacted by high salt levels due to osmotic effects, nutritional imbalances and oxidative stress [14,15,16]. Additionally, salinity induces ion toxicity and nutritional imbalance due to the excessive uptake of sodium [11,12]. This makes it urgent to improve salt tolerance through spraying with attenuating substances like ions, plant growth substances and biostimulants [16,17,18,19,20,21].
Humic acid (HA) represents the prime component of organic humus [22,23], and its structure is still a matter of discussion [24,25]. HA is assumed to be complex aromatic macromolecules with amino acids, amino sugars, peptides and aliphatic complexes elaborate in connections among the aromatic groups. The hypothetical structure for HA contains free and bound phenolic OH groups, quinone structures, nitrogen and oxygen as bridge units, and COOH groups variously located on aromatic rings [26]. HA application directly or indirectly improved OFP establishment due to its positive role in accelerating several plant metabolic pathways and mitigating the drastic impact of environmental stresses; meanwhile, its precise mechanisms in lessening stress injury have not been well documented [27]. Amir and Hani [28] indicate that foliar spraying with HA increased biological yield, chlorophyll and carotenoid content, as well as essential oil yield of Dracocephalum moldavica L. plants, and the highest positive effect was observed under 400 mg/L HA. Nofal et al. [29] found that the spraying of HA significantly increased Erantheumum pilchellum plant height, leaf number per plant, fresh and dry weight, flower diameter and chlorophyll content. Hammam et al. [30] revealed that spraying geranium plants with HA significantly increased plant height, shoot fresh and dry weight, proline concentration and water use efficiency. Previous research has suggested that the beneficial effects of HA can be attributed to the activation of several metabolic enzymes, improving plant water status, maintaining ion and redox homeostasis and promoting secondary metabolite assimilation [31]. HA also helps plants absorb nutrients, and it is particularly crucial for the movement and availability of micronutrients [32]. Recently, Ennab et al. [33] recorded that the use of HA as soil addition and/or foliar spraying increased significantly macro and micro-nutrient contents as well as chlorophyll and proline concentration associated with improving plant growth trials.
Little is understood about the potential role of HA foliar spraying in alleviating salt injury in Ivy geranium plants. Subsequently, the existing study’s goal was to assess the influence of HA concentration on growth, flowering and some other physiological attributes of salt-affected Ivy geranium plants. In addition, the opportunity to investigate the possible application of saline water to Ivy geranium plants is expected to open new avenues for the development of the ornamental plant industry in SA.
2. Materials and Methods
2.1. Experimental Layout
The two factorial pot trials were carried out in an automated greenhouse of the Nursery of Sustainability and Environmental Developmental Department, King Saud University, Riyadh, SA from 15 December 2022 to 25 April 2023 for assessing the effect of irrigation with saline water (230 ‘tap water control’, 2000 and 4000 mg/L NaCl), HA foliar application (0, 500, 1000 and 2000 mg/L) and their interactions (3 saline water levels x 4 HA concentration) on Ivy geranium plant growth, flowering and some other physiological trials. The experiment had 12 treatments with 6 replicates (pots, one plant per pot). The garden soil was sandy in texture (88.22% silt, 8.78% clay and 3% sand), with pH 7.64, EC 1.47 dSm−1, bulk density 1.40 g/cm3, cation exchange capacity 35.94 meq/100 g soil, organic matter 1.82%, available N 57.20 mg/kg soil, available P 7.93 mg/kg soil and available potassium 120.16 mg/kg soil.
Terminal cuttings of Ivy geranium were taken from the F1 seed mother plants (Kim variety, Blocompic, Holland, MI, USA) on 15 December 2022 and then their bases were dipped in rooting hormone (Rhizopona, 0.8%, Schutz Company, Briogeton, NJ, USA) and consequently planted in 10 cm plastic pots containing peat moss and perlite for rooting. The 35 days of homogenous rooted cuttings were planted in 25 cm plastic pots with 5 kg garden soil. The seedlings were endorsed to establish for 14 days under irrigation with a nutrient solution (Sangral NPK 20:20:20, SQM Europe, Antwerp, NV, Belgium) before the initiation of saline water irrigation treatments. Three levels of saline water (230 ‘tap water control’, 2000 and 4000 mg/L NaCl) were used for irrigation every 3 days throughout the experimental time at 80% of soil field capacity. HA levels (0, 500, 1000 and 2000 mg/L) were sprayed 5 times at intervals of 15 days starting on 6 February 2023.
Six plants (90 days from planting) from each treatment (every 2 plants represent one replicate) were used for recorded morphological and flowering attributes as well as some physiological characteristics.
2.2. Vegetative Growth
Vegetative growth attributes were determined, including stem length (cm), stem diameter (cm), number of leaves/plant, as well as shoot fresh and dry weights/plant (g). Additionally, leaf area per plant (cm2) was estimated using leaf area meter LI-3000 COR (Walz Co., Forest Grove, OR, USA).
2.3. Flowering Attributes
The numbers of inflorescences plant−1, inflorescence diameter (cm), inflorescence stock length (cm), as well as inflorescences fresh and dry weights (g), were also recorded at 90 days from planting.
2.4. Physiological Growth Characteristics
Leaf greenness (SPAD) or chlorophyll content index was estimated with a SPAD-502 Plus chlorophyll meter (Konica Minolta, Tokyo, Japan). Nine measurements were occupied per leaf and averaged to provide a single record per leaf.
Proline concentration in the plant shoot was estimated following the protocol of Bates et al. [34] using a ninhydrin reagent. An aliquot of fresh leaf tissues was extracted by aqueous sulfosalicylic acid. The extract was combined with acid ninhydrin reagent for 1 h in a boiling water bath. The chromophore was collected by toluene; the optical density was measured at 520 nm; and proline concentration (μg/g fresh weight ‘FW’) was determined based on the calibration curve by proline.
Nitrogen (N) and phosphorus (P) contents were extracted from shoot dry weight with 5 mL of H2SO4 at 100 °C for 2 h; an aliquot of H2SO4/HClO3 mix was dispensed dropwise; subsequently, the digestible was chilled for 15 min at Laboratory Temperature (22–24 °C) following Association of Official Analytical Chemists (A.O.A.C.) protocol [35]. N content was measured with the micro-Kjeldahl outline. The technique of Cooper [36] was used for the estimation of P with a phosphate standard curve. Meanwhile, potassium (K), calcium (Ca), magnesium (Mg), manganese (Mn), copper (Cu), iron (Fe), zinc (Zn) and sodium (Na) were extracted by acid digestion (70% nitric acid and 30% hydrochloric acid) in a Milestone MLA 1200 Mega microwave digestion device, then assessed using iCAPTM 7000 Plus Series ICP-OES (Thermo ScientificTM, Waltham, MA, USA), following A.O.A.C. scheme [35].
2.5. Statistical Analysis
Data was exposed to the two-way analysis of variance (ANOVA) using the CoHort Software, version number 6.303 statistical package (CoHort software, 2006; Birmingham, UK) to evaluate the role of humic acid in mitigating salt injury. When significant (p ≤ 0.05), a comparison of means (Tukey test) was implemented. The data existing are mean with standard error (SE). The statistical significance was considered as * p ≤ 0.05, ** p ≤ 0.01; and *** p ≤ 0.001.
3. Results
3.1. Growth Attributes
Ivy geranium plant growth significantly (p ≤ 0.05) decreased by increasing salinity levels up to 4000 mg/L in irrigation water relative to irrigation with tape water (230 mg/L NaCl)(Figure 1a, Table 1). Irrigation with 2000 mg/L NaCl strongly reduced stem length, leaf area per plant and shoot dry weight (50–57%) and had less effect on stem diameter, leaf number plant and shoot fresh weight (15–29%). When treated with 4000 mg/L NaCl dramatically decreased stem length, leaf number/plant, leaf area and shoot dry weight (61–71%) and had less effect on stem diameter and shoot fresh weight (35–40%), respectively, over non-salinized control plants (Figure 1a, Table 1).
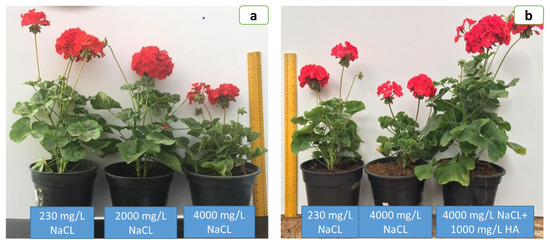
Figure 1.
Effect of saline water (a) and role of 1000 mg/L humic acid (HA) on mitigation of the drastic effect of 4000 mg/L NaCl (b) on Ivy geranium plant growth attributes at full flowering (90 days from planting).

Table 1.
Effect of humic acid (HA), salinity and their interactions on Ivy geranium plant growth attributes at 90 days from planting.
Application of HA concentrations significantly (p ≤ 0.05) increased all growth attributes of Ivy geranium plants over untreated plants. The uppermost values of vegetative trials were obtained and recorded once spraying with 1000 mg/L HA, following 500 mg/L HA, and finally by 2000 mg/L HA. HA at 1000 mg/L significantly boosted (p ≤ 0.05) stem length, stem diameter, leaves number/plant, leaf area, shoot fresh and dry weights by 98.6, 57.2, 97.4, 81.7, 43.2and 81.6%, respectively, over 0 mg/L HA treated plants.
Application of HA concentration gave an encouraging impact and growth improvements at all levels of salt stress and consequently performed as growth stimulants. Accordingly, it is possibly mentioned that HA levels could be lessened by the detrimental impacts of salty water (2000 and 4000 mg/L NaCl), which boosted entire growth trials of Ivy geranium plants under salinity. HA at 1000 mg/L attained the greatest tolerance against severe salinity (4000 mg/L) and enhanced plant growth attributes (Figure 1b, Table 1).
3.2. Flowering Attributes
Salinity levels up to 4000 mg/L significantly (p ≤ 0.05) decreased all flowering characteristics, over control plants. The lowermost values were achieved under 4000 mg/L which decreased inflorescence number/plant, inflorescence stalk length, inflorescence diameter and inflorescence fresh and dry weights by 49.4, 67.0, 62.8, 60.9 and 74.4%, respectively, over the control Ivy geranium plant (Table 2).

Table 2.
Effect of humic acid (HA), salinity and their interactions on Ivy geranium plant flowering attributes at 90 days from planting.
Alternatively, the application of HA boosted flowering attributes. Spraying with 1000 mg/L HA provided the maximum values of flowering characteristics, the increment was 51.7, 90.9, 72.9, 127.2 and 134.9% for inflorescence number/plant, inflorescence stalk length, inflorescence diameter, inflorescence fresh and dry weights, respectively, as compared with untreated plants (Table 2).
The interactive impact of saline water and HA on flowering attributes indicates that the highest inflorescence number plant−1, inflorescence stalk length, inflorescence diameter and inflorescence fresh and dry weights were obtained from the treatment of tap water plus 1000 mg/L HA over all treatments. Within severe salinity (4000 mg/L) utilization of 1000 mg/L HA increased inflorescence number/plant, inflorescence stalk length, inflorescence diameter, inflorescence fresh and dry weights by 7%, respectively, over non-treated plants that irrigated with 4000 mg/L NaCl (Table 2).
3.3. Chlorophyll
The value of chlorophyll index was individually affected (p ≤ 0.05) by saline water and/or HA spray (Table 3). The maximum chlorophyll level (SPAD) was achieved from Ivygeranium irrigated with tape water. Increasing salinity levels induced a significant decline in SPAD value. This reduction reached 37% for geranium irrigated with 4000 mg/L NaCl relative to plants irrigated with tap water.

Table 3.
Effect of humic acid (HA), salinity and their interactions on Ivy geranium plant chlorophyll (mg/g FW) and proline (µg/g FW) concentration at 90 days from planting.
Conversely, HA spraying caused a substantial rise in chlorophyll index in ivy geranium plants over nontreated plants (0 mg/L HA). The greatest values of chlorophyll were recorded once plants were sprayed with 1000 mg/L, followed by 500 mg/L and, finally, 2000 mg/L relative to untreated plants (Table 3).
Likewise, the data in Table 3 reveal that HA as a biostimulant mitigated the harmful impacts of saline water on the chlorophyll index in Ivy geranium plants. HA at 1000 mg/L was utmost effective in this respect and increased chlorophyll by 52.7 and 54.5% as compared with untreated plants (0 mg/L HA) that were irrigated with 2000 and 4000 mg/L NaCl, respectively.
3.4. Proline
Considerable concentrations of free proline were observed under salinity and HA compared with control plants (Table 3). Irrigation with saline water up to 4000 mg/L induced the hyperaccumulation of proline in Ivy geranium plant shoots. Severe salinity produced 67.3% greater proline concentration compared to non-salinized plants (Table 3).
Proline accumulation was raised by the supplementation of HA concentrations as compared to untreated plants. The more effective concentration of HA was 1000 mg/L compared to untreated plants (Table 3).
Regarding interactive effects, spraying HA substantially induced proline accretion in Ivy geranium under saline and non-saline conditions over plants with no HA addition (Table 3). The ultimate concentration of proline was recognized with 1000 mg/L HA spraying together with severe salinity, which boosted proline by 120%.
3.5. Ion Content
Ion contents (macro and micro-nutrient) were significantly (p ≤ 0.05) affected by salinity, HA and their combinations (Table 4 and Table 5). Data recorded that salinity stress progressively decreased all ions except Fe and Na, which is raised with increasing salinity stress. The lowest content of N (54.8%), P (50.7%), K (48.2%), Ca (45.1), Mg (45.9), Mn (36.8%), Cu (33.5%) and Zn (35.6%) was recorded under 4000 mg/L NaCl over nonsalinized plants. Meanwhile, 4000 mg/L NaCl significantly (p ≤ 0.05) increased Fe and Na by 44.3 and 61.3% relative to non-salinized treatment.

Table 4.
Effect of humic acid (HA), salinity, and their interactions on Ivy geranium macro-nutrient concentration (mg/total dry weight) at 90 days from planting.

Table 5.
Effect of humic acid (HA), salinity and their interactions on Ivy geranium micro-nutrient concentration (mg/total dry weight) at 90 days from planting.
On the other hand, HA concentrations foliar spraying increased all ion concentrations except Na which decreased in the shoot of the Ivy geranium plant over non-treated plants. The most effective concentration of HA in increasing N, P, K, Ca, Mg, Mn, Cu, Fe and Zn, as well as decreased Na, was 1000 mg/L over nontreated plants (Table 4 and Table 5).
Foliar spray with HA concentrations under all levels of saline water distinctly invalidates their determinantal impact on ion content. The foremost effective concentration was 1000 mg/L HA, which increased N (66.9%), P (35.8%), K (34.6%), Ca (164.6%), Mg (179.2%), Mn (44.8%), Cu (36.0%), Fe (38.0%) and Zn (113.3%), meanwhile declining Na (27.1%) compared with unsprayed severe salt-affected plants.
4. Discussion
Reduced agricultural water consumption and increased water use efficiency are needed globally to protect water for human use [37]. This disorder encourages the adoption of some alternative water resources for irrigation, such as saline water, which hastens the onset of salinized soil. Accordingly, some researchers have shown that foliar spraying of HA concentrations is able to play a substantial function in boosting plant establishment by increasing a plant’s aptitude to endure stress tolerance [38,39]. Results from the present study show that salinity stress reduced Ivy geranium growth and flowering as well as ion (N, P, K, Ca, Mg, Mn, Cu and Zn) and chlorophyll but raised Fe, Na and proline. Alternatively, HA in special 1000 mg/L considerably boosted all examined characteristics while minimizing Na accumulation.
The injury impact of salinity on vegetative growth trials was established previously [12,14]. These depressive effects might be attributed to the distribution of bio-physiological pathways and molecular modifications, i.e., photosynthesis, nutrient balance, reactive oxygen species (ROS) buildup and alterations in salt injury in different plants. Additionally, salinity stress may be hindering ion absorption resulting from the occurrence of Na and chloride ions in irrigation water or the permeability of these ions to the plant tissues, which consecutively causes ionic toxicity alongside a decline in the vegetative growth characteristics of plants [40]. Additionally, salinity induces hormonal imbalance that participates in cellular division and enlargement that negatively affects plant growth [41,42]. HA supplementation normally increases plant growth and lessens salinity injury [43,44]. The motivating impact of HA under normal or stressful circumstances is devoted to hastening photosynthesis pathways and enhancing photo-assimilation translocation in plants [45]. HA application improved hormonal and ROS balance [46]; as well as activation of antioxidant enzymes and accelerating organic solute accumulation [47], which was ultimately reflected in plant growth [22]. Moreover, the encouraging role of HA can be linked to its impact on boosting interior carbon dioxide concentration and leaf thickness, improving cell water maintenance and boosting water use efficiency [30]. Additionally, HAs’ positive effect could be due to the hormone-like activity or may be connected to encouraging indole acetic acid assimilation, which accelerates cell division and enlargement as well as eradicating ROS [48]. Additionally, gibberellic acid (GA)-like substances and activity in humic substances have been reported since the 1990s [49], accordingly, the accumulation of gibberellin may accelerate the cell elongation-related genes that are induced cell elongation [50], raise cell permeability and recover the absorption of nutrients [51,52].
A current study proved that flowering attributes markedly decreased with saline water, which was confirmed previously in different plants [13,53]. Therefore, salt-affected plants may lessen flowering intensity, delay flowering and shorten the flowering period [8]. Flower stalk length is an energetic quality feature of OFP as it impacts the commercial importance of cut flower crops. Flower length and diameter, stem thickness and length were considerably decreased by raising the salinity level over control, which was approved by Kucukahmetler [54]. According to Ahmad et al. [55], the application of saline water blocks the vascular system and eventually restricts water uptake. The reduction in flowering due to salinity may be attributable to the decline of plant photosynthesis through the variations in chlorophyll levels and components and the destruction of chloroplasts [56]. Moreover, it hinders photochemical activities and reduces the Calvin cycle enzyme activities [57], modifying the concentration of hormones straight intricate in flowering, such as abscisic and jasmonic acids [58]. HA not only encouraged vegetative growth but also floral attributes as a greater number of florets per spike were formed by plants treated with HA. Current findings are harmonized with the results of Nofal et al. [29] and Baldotto and Baldotto [44] who stated that HA improved the flowering of several ornamental and flowering plants when used at higher concentrations. These findings confirmed that HA spraying improved spike length, which established the function of HA in enhancing ion uptake and sequentially improved spike length and whole flower quality. Parallel outcomes of enhancement in ion uptake, particularly of N, P and S by the activity of HA, have also been stated by Atiyeh et al. [59] and Arancon et al. [60]. Additionally, given the presence of GA-like compounds and activity in HS that have been reported since the 1990s, the stimulating effect of HA on blooming may be the result of the buildup of GA that accelerates flowering development [49].
It could be concluded that, from the current outcomes, chlorophyll content considerably declined under saline water up to 4000 mg/L. The degeneration in chlorophyll, once salinity occurs, could result from the drop in chlorophyll biosynthetic or boosted enzymatic chlorophyll deprivation [61], in addition, the degeneration of the thylakoid membranes and devastation of chlorophyll by diverse ROS, and alterations in chlorophyll protein complexes [62]. Moreover, salinity may induce a deterioration in chlorophyll biosynthesis intermediation and decrease the expression of ChlD, Chl Hand Chl I-1 gene encoding subunits of Mg-chelatase [63,64]. As indicated in the current findings and earlier research, utilization of HA has been recorded to improve chlorophyll accumulation in plants within stress or non-stress circumstances [65,66]. This increase may be attributed to the rise in cytokinin assimilation, which accelerates chloroplast differentiation and chlorophyll biosynthesis and declines its degradation [67]. Additionally, HA probably keeps chlorophyll biosynthesis via the protection of the sulphydryl group and boosts Mg absorption and accumulation. The rise in chlorophyll levels by HA spraying might be caused by the hastening of N and NO3uptake, improving N metabolism and assembly of protein [68]. Humic acid additionally increased N and K uptakes, which are elaborated in chloroplast differentiation and chlorophyll assimilation [69].
Within stress conditions, plants possess several strategies including a hyperaccumulation of organic osmolytes like proline without interfering with metabolic pathways to withstand stress conditions [70,71]. Current findings proved that irrigation with saline water with or without HA spraying significantly increased proline accumulation in plant tissues. Numerous occupations are anticipated for proline buildup within stress factor and/or HA spraying including osmotic adjustment, protein and enzyme stabilization, and ROS scavenging, besides acting as a reservoir of energy and N for exploitation [72]. Moreover, Bellinger et al. [73] suggested that the rise of proline in salt-affected plants might be deduced as a tolerance strategy of osmotic regulation and/or buildup of the extra ammonium created by salinity. Proline buildup can be explicated by the greater inhibitory rate of proline dehydrogenase and proline oxidase [71]. Yet, it is also possible to find a lessening in the proline production in plants caused by its fast breakdown upon stress reprieve. The breakdown products deliver reducing agents that support mitochondrial oxidative phosphorylation and generation of adenosine triphosphate (ATP) for rescue from stress and repairing stress-induced injury [74]. Moreover, HA appears to have an encouraging effect on enzyme activity and secondary plant metabolism [22], as well as plant respiration and photosynthesis, which in turn affect carbohydrate levels [75] and amino acid metabolism [76].
Salinity normally induces ion imbalance by declines in N, P, K, Mg, Ca, Mn, Cu and Zn associated with excess accretion of Fe and Na, which was confirmed previously [11,12,20]. The drastic impacts of salinity on plant nutritional status may be attributed to a decline in nutrient uptake and/or transport as well as ion toxicity [77]. The deterioration of root development within salinity could be one of the reasons behind the decline in plants’ ion content [78]. Under salinity, the decrease in either N or P may result from the antagonism between both chloride and nitrate [79] or phosphate [80] molecules, respectively. The prevention of plant K uptake is chiefly caused by the physical and chemical similarities between K and Na that induced the competition on major binding sites [81]. Additionally, there is an antagonism between Ca and Mg with Na which affects membrane properties and causes a degeneration of membrane integrity and selectivity [82]. The current findings displayed that ions’ content was progressively increased by HA concentrations, nevertheless, Na was reduced. These findings were consistent with those achieved by Ennab et al. [33] and Sahar et al. [83]. Additionally, HA maintained an extraordinary level of acid phosphate activity that increased phosphate activity holds for improved plant P uptake [84]. HA has been described to improve plant nutrient uptake due to the improving permeability of root membranes [85]. The findings additionally revealed that HA spraying possibly will lessen the destructive impact of salinity by maintaining leaf water status, dropping the uptake of Na and Cl [86], increasing Ca and K, motivating chloroplast development and improving phloem loading [87]. Also, HA has been displayed to improve plant membrane permeability, stimulating the uptake and translocation of nutrients and increasing root development [88]. Additionally, HA usually retained ATPase and Na/H antiport, which facilitate Na compartmentation under salinity [23]. Fernandez et al. [89] revealed that foliar spraying of leonardite extracts (as a natural source of HA) motivated shoot growth and promoted the buildup of several ions. It was stated that HA encourages H+-ATPase activity in the plasma membrane and stimulates plant growth via the rise in lateral root emergence and whole root absorbance [90]. The rise in nitrogen by HA application may be attributed to the enhancement of nitrogen assimilation enzymes like nitrate reductase and nitrite reductase [91].
5. Conclusions
The current findings indicated that irrigating Ivy geranium plants with saline water up to 4000 mg/L NaCl adversely affected vegetative and flowering growth attributes, alongside decreasing nutrient contents (except Fe and Na). However, HA application special at 1000 mg/L helped in recovering plant growth and flowering to levels comparable to those of control plants.
Author Contributions
Conceptualization, K.M.E., F.A.A. and M.A.A.-Y.; methodology, K.M.E. and M.A.A.-Y.; software, K.M.E.; validation, K.M.E., F.A.A. and M.A.A.-Y.; formal analysis, K.M.E., F.A.A. and M.A.A.-Y.; investigation, K.M.E. and M.A.A.-Y.; resources, K.M.E., F.A.A. and M.A.A.-Y.; data curation, K.M.E.; writing—original draft preparation, K.M.E., F.A.A. and M.A.A.-Y.; writing—review and editing, K.M.E. and F.A.A.; visualization, K.M.E.; supervision, F.A.A.; project administration, F.A.A.; funding acquisition, K.M.E., F.A.A. and M.A.A.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-576-1).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keles, B.; Ertürk, Y. Advantages of microorganism containing biological fertilizers and evaluation of their use in ornamental plants. Int. J. Agric. For. Life Sci. 2021, 5, 189–197. Available online: http://dergipark.gov.tr/ijafls (accessed on 23 July 2021).
- Rocha, C.S.; Rocha, D.C.; Kochi, L.Y.; Carneiro, D.N.M.; Dos Reis, M.V.; Gomes, M.P. Phytoremediation by ornamental plants: A beautiful and ecological alternative. Environ. Sci. Pollut. Res. 2022, 29, 3336–3354. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.F.; Sanchez, C. Genetic modification; the development of transgenic ornamental plant varieties. Plant Biotech. J. 2012, 10, 891–903. [Google Scholar] [CrossRef]
- Azadi, P.; Bagheri, H.; Nalousi, A.M.; Nazari, F.; Chandler, S.F. Current status and biotechnological advances in genetic engineering of ornamental plants. Biotechnol. Adv. 2016, 34, 1073–1090. [Google Scholar] [CrossRef]
- Mamba, B.; Wahome, P.K. Propagation of geranium (Pelargonium hortorum) using different rooting medium components. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 497–500. [Google Scholar] [CrossRef]
- Taylor, M.D.; Nelson, P.V.; Frantz, J.M.; Rufty, T.W. Phosphorus deficiency in Pelargonium: Effects on nitrate and ammonium uptake and acidity generation. J. Plant Nut. 2010, 33, 701–712. [Google Scholar] [CrossRef]
- Krishnaraj, S.; Dan, T.; Saxena, P. A fragrant solution to soil remediation. Inter. J. Phytoremed. 2000, 2, 117–132. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Carrion, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by means of saline water irrigation as related to salinity tolerance. Sci. Hort. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Al-Omran, A.M.; Aly, A.A.; Halim, M.K. Status and New Development on the Use of Brackish Water for Agricultural Production in the Near East Saudi Arabia Country Report; United Nations, Food and Agriculture (FAO), Regional Office for the Near East (RNF): Rome, Italy, 2012; Available online: https://www.fao.org/neareast/events/view/ru/c/249312/2012 (accessed on 23 July 2021).
- Niu, G.; Rodriguez, D. Response of bedding plants to saline water irrigation. HortSci 2010, 45, 628–636. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Al-Mana, F.A.; Algahtani, A.M.; Alotaibi, M.A. Effect of irrigation with saline magnetized water and different soil amendments on growth and flower production of Calendula officinalis L. plants. Saudi J. Bio. Sci. 2020, 27, 3072–3078. [Google Scholar] [CrossRef]
- Amarin, R.; Kafawin, O.; Ayad, J.; Al-Zyoud, F.; Ghidan, A. Effect of saline water irrigation and growing media on growth, physiological and mineral parameters of clove pink Dianthus caryophyllus. Jor. J. Agric. Sci. 2020, 16, 55–62. [Google Scholar] [CrossRef]
- Anny Mrudhula, K.; Venkata Subbaiah, G.; Sambaiah, A.; Sunil Kumar, M. Performance of flower and medicinal plants with saline irrigation water through drip system. Pharma Innov. J. 2021, 10, 1514–1519. Available online: https://www.thepharmajournal.com/archives/2021/vol10issue8/PartU/10-7-439-247.pdf (accessed on 23 July 2021).
- Banon, D.; Lorente, B.; Ortuño, M.F.; Bañón, S.; Sanchez-Blanco, M.J.; Alarcon, J. Effects of saline irrigation on the physiology and ornamental quality of Euphorbia Ascot Rainbow and its relationship with salinity indexes based on the bulk electrical conductivity. Sci. Hort. 2022, 305, 111406. [Google Scholar] [CrossRef]
- Kim, H.; Fonseca, J.M.; Choi, J.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Farouk, S.; Arafa, S.A. Mitigation of salinity stress in canola plants by sodium nitroprusside application. Span. J. Agric. Res. 2018, 16, e0802. [Google Scholar] [CrossRef]
- Helaly, M.N.; Farouk, S.; Arafa, S.A.; Amhimmid, N.B.I.A. Inducing salinity tolerance of rosemary (Rosmarinus officinalis L.) plants by chitosan or zeolite application. Asian J. Adv. Agric. Res. 2018, 5, 1–20. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J. Soil Sci. Plant Nutr. 2019, 19, 887–899. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- El-Banna, M.F.; AL-Huqail, A.A.; Farouk, S.; Belal, B.E.A.; El-kenawy, M.A.; Abd El-Khalek, A. Morpho-physiological and anatomical alterations of salt-affected thompson seedless grapevine (Vitis vinifera L.) to brassinolide spraying. Horticulturae 2022, 8, 568. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; García, A.C.; Fuentes, M.; Baigorri, R.; Mora, V.; García Mina, J.M. Hypothetical framework integrating the main mechanism involved in the promoting action of rhizospherichumic substances on plant root- and shoot-growth. App. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Baigorri, R.; Fuentes, M.; Gonzalez-Gaitano, G.; Garcia-Mina, J.M. Simultaneous presence of diverse molecular patterns in humic substances in solution. J. Phys. Chem. B 2007, 111, 10577–10582. [Google Scholar] [CrossRef] [PubMed]
- Baigorri, R.; Fuentes, M.; Gonzalez-Gaitano, G.; García-Mina, J.M. Analysis of molecular aggregation in humic substances in solution. Colloids Surf. A 2007, 302, 301–306. [Google Scholar] [CrossRef]
- Nyoman Rupiasih, N.; Vidyasagar, P.B. A review: Compositions, structures, properties and applications of humic substances. J. Adv. Inter. Sci. Technol. 2005, 8, 16–25. Available online: https://www.researchgate.net/publication/236347209_A_Review_Compositions_Structures_Properties_and_Applications_of_Humic_Substances (accessed on 23 July 2021).
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzedbased products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18. [Google Scholar] [CrossRef]
- Amir, H.S.; Hani, A. Effect of ethanol and humic acid foliar spraying on morphological traits, photosynthetic pigments and quality and quantity of essential oil content of Dracocephalummoldavica L. Iran. J. Plant Physiol. 2017, 8, 2299–2306. [Google Scholar]
- Nofal, E.M.S.; Menesi, F.A.; EL-Bably, S.Z.; Abd EL Rahman, M. Effect of NPK and humic acid on growth, flowering and chemical composition of (blue sake) Erantheumumpulchellum Andrews plant. App. Ecol. Environ. Res. 2020, 18, 2555–2567. [Google Scholar] [CrossRef]
- Hammam, K.A.; AwadAlla, S.S.S.; Noreldin, T. Response of growth, yield and essential oil of geranium plants to surface irrigation and humic acid treatments. Asian Plant Res. J. 2021, 7, 39–56. [Google Scholar] [CrossRef]
- Hagagg, L.F.; Shahin, M.F.M.; Mustafa, N.S.; Merwad, M.A.; Khalil, F.H. Influence of using humic acid during full bloom and fruit set stages on productivity and fruit quality of ‘Kalamata’ olive trees. J. App. Sci. Res. 2013, 9, 2287–2292. Available online: https://www.aensiweb.com/old/jasr/jasr/2013/2287-2292.pdf (accessed on 1 March 2013).
- Bohme, M.; Thilua, H. Influence of mineral and organic treatments in the rhizosphere on the growth of tomato plants. Acta Hortic. 1997, 450, 161–168. [Google Scholar] [CrossRef]
- Ennab, H.A.; Mohamed, A.H.; El-Hoseiny, H.M.; Omar, A.A.; Hassan, I.F.; Gaballah, M.S.; Khalil, S.E.; Mira, A.M.; Abd El-Khalek, A.F.; Alam-Eldein, S.M. Humic acid improves the resilience to salinity stress of drip-irrigated Mexican lime trees in saline clay soils. Agronomy 2023, 13, 1680. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Cooper, T.G. The Tools of Biochemistry; A Wiley-Interscience Publication John Wiley and Sons: New York, NY, USA, 1977. [Google Scholar]
- Costa, J.M.; Ortuno, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; Pascale, S.D.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ennab, H.; Alam-Eldein, S.M. Biostimulants foliar application to improve growth, yield, and fruit quality of ‘Valencia’ orange trees under deficit irrigation conditions. J. Am. Pom. Soc. 2020, 74, 118–134. Available online: https://www.pubhort.org/aps/74/v74_n3_a1.htm (accessed on 1 July 2020).
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Hashem, A.; Abd Allah, E.F.; Ahmad, P. Arbuscular mycorrhiza in crop improvement under environmental stress. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: Cambridge, MA, USA; London, UK, 2014; pp. 69–95. [Google Scholar] [CrossRef]
- Kozminska, A.; Al Hassan, M.; Kumar, D.; Oprica, L.; Martinelli, F.; Grigore, M.N.; Vicente, O.; Boscaiu, M. Characterizing the effects of salt stress in Calendula officinalis L. J. Appl. Bot. Food Qual. 2017, 90, 323–329. [Google Scholar] [CrossRef]
- Li, G.; Evens, M.R. Humic acid substrate treatments and foliar spray application effects on root growth and development of seedlings. HortSci 2000, 35, 434. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Baldotto, L.E.B. Gladiolus development in response to bulb treatment with different concentrations of humic acids. Rev. Ceres Viçosa 2013, 60, 138–142. [Google Scholar] [CrossRef]
- Aydine, A.; Kant, C.; Turan, M. Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agric. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Can, W.; Kafi, M.; Babalar, M.; Xia, Y. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Li, H.; Kong, F.; Tang, T.; Luo, Y.; Gao, H.; Xu, J.; Xing, G.; Li, L. Physiological and transcriptomic analyses revealed that humic acids improve low-temperature stress tolerance in zucchini (Cucurbita pepo L.) seedlings. Plants 2023, 12, 548. [Google Scholar] [CrossRef]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaro, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology altering the expression of genes involved in primary metabolism, growth and development. Envir. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
- Savy, D.; Canellas, L.; Vinci, G.; Cozzolino, V.; Piccolo, A. Humic-like water-soluble lignins from giant reed (Arundo donax L.) display hormone-like activity on plant growth. J. Plant Growth Regul. 2017, 36, 995–1001. [Google Scholar] [CrossRef]
- Xiong, H.; Lu, D.; Li, Z.; Wu, J.; Ning, X.; Lin, W.; Bai, Z.; Zheng, C.; Sun, Y.; Chi, W.; et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Comm. 2023, 4, 100597. [Google Scholar] [CrossRef]
- Zandonadi, D.; Santos, M.; Dobbss, L.; Fb, O.; Canellas, L.; Binzel, M.; Façanha, A. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 2010, 231, 1025–1036. [Google Scholar] [CrossRef]
- Jindo, K.; Canellas, L.P.; Albacete, A.; Figueiredo dos Santos, L.; Frinhani Rocha, R.L.; Carvalho Baia, D.; Oliveira Aguiar Canellas, N.; Goron, T.L.; Olivares, F.L. Interaction between humic substances and plant hormones for phosphorous acquisition. Agronomy 2020, 10, 640. [Google Scholar] [CrossRef]
- Ashour, H.A.; El-Attar, A.B. Morphological and physiological responses of silvery (Leucophyllum frutescens) to water deficient and irrigation water salinity stresses. J. Hort. Sci. Ornamen. Plants 2017, 9, 1–16. [Google Scholar]
- Kucukahmetler, O. The effects of salinity on yield and quality of ornamental plants and cut flowers. Acta Hortic. 2002, 573, 407–414. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.A.; Qasim, M.; Ahmad, R. Growth, yield and quality of Rosa hybrida L. as influenced by NaCl salinity. J. Ornam. Hortic. Plants 2013, 3, 143–153. Available online: https://jornamental.rasht.iau.ir/article_513390_8543f77e6319e13c7dee80836060c0e7.pdf (accessed on 23 July 2021).
- Lyengar, E.R.; Reddy, M.P. Photosynthesis in highly salt tolerant plants. In Handbook of Photosynthesis; Pesserkali, M., Ed.; Marshal Dekar: Baten Rose, LA, USA, 1996; pp. 897–909. [Google Scholar]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Current Sci. 2004, 86, 408–421. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Edwards, C.A.; Metzger, J.D.; Lee, S.; Arancon, N.Q. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Biores. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Lee, S.; Edwards, C.A.; Atiyeh, R. Effect of humic acids derived from cattle, food and paper-waste vermicompost on growth of green house plants. Pedobiologia 2003, 47, 741–744. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef]
- Siddiqui, M.; Alamri, S.; Al-Khaishany, M.; Khan, M.; Al-Amri, A.; Ali, H.; Alaraidh, I.; Alsahli, A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Ghamdi, A.A.M. Sodium nitroprusside application enhances drought tolerance in marjoram herb by promoting chlorophyll biosynthesis, sustaining ion homeostasis, and enhancing osmotic adjustment capacity. Arab. J. Geosci. 2021, 14, 430. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A.; El-Gamal, S.M.A. Potential role of biochar and silicon in improving physio-biochemical and yield characteristics of borage plants under different irrigation regimes. Plants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Hassanein, R.A.; El Khawas, S.A.; Khafaga, H.S.; Abd El-Nabe, A.S.; Abd Elrady, A.S. Amelioration of drought stress on physiological performance of pearl millet (Pennisetum americanum) plant grown under saline condition using potassium humate and silicon source. Egypt J. Exp. Biol. 2017, 13, 57–68. [Google Scholar] [CrossRef]
- El-Hoseiny, H.M.; Helaly, M.N.; Elsheery, N.I.; Alam-Eldein, S.M. Humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. HortSci 2020, 55, 1026–1037. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Zamarreño, A.M.; Spíchal, L.; García-Mina, J.M. Root ABA and H+ -ATPase are key players in the root and shoot growth-promoting action of humic acids. Plant Direct. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Kafi, M.; Fang, P. Photosynthetic activity and N metabolism of lettuce as affected by humic acid. Inter. J. Veg. Sci. 2012, 18, 182–189. [Google Scholar] [CrossRef]
- Berbara, R.L.; García, A.C. Humic substances and plant defense metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Parvaiz, A., Mohd, R.W., Eds.; Springer: New York, NY, USA, 2014; pp. 297–319. [Google Scholar]
- Hammam, K.A.; AwadAlla, S.S.S. Mitigation of saline water stress on french lavender (Lavandula dentata L.) plants. J. Hort. Sci. Ornamen. Plants 2020, 12, 8–16. [Google Scholar]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Amini, S.; Ghobadi, C.; Yamchi, A. Proline accumulation and osmotic stress: An overview of P5CS gene in plants. J. Plant Mol. Biol. Breed. 2015, 3, 44–55. [Google Scholar] [CrossRef]
- Bellinger, Y.; Bensaoud, A.; Larher, F. Physiological significance of proline accumulation, a trait of use to breeding for stress tolerance. In Physiology–Breeding of Winter Cereals for Stressed Mediterranean Environment; Acevedo, E., Conesa, A.P., Monneveux, P., Srivastava, J.P., Eds.; INRA: Paris, France, 1991; pp. 449–458. [Google Scholar]
- Fichman, Y.; Gerdes, S.Y.; Kovács, H.; Szabados, L.; Zilberstein, A.; Csonka, L.N. Evolution of proline biosynthesis: Enzymology, bioinformatics, genetics, and transcriptional regulation. Biol. Rev. 2015, 90, 1065–1099. [Google Scholar] [CrossRef]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]
- Boehme, M.; Schevtschenko, J.; Pinker, I. Iron supply of cucumbers in substrate culture with humate. Acta Hort. 2005, 41, 329–335. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2012, 352, 353–362. [Google Scholar] [CrossRef]
- Sahar, M.Z.; El-Quesni, F.E.M.; Mazhar, A.A.M. Influence of potassium humate on growth and chemical constituents of Thuja orientalis L. seedlings. Ozean J. App. Sci. 2009, 2, 73–78, CorpusID: 99039569. Available online: https://api.semanticscholar.org/ (accessed on 1 November 2015).
- Malcum, R.L.; Vaughum, D. Humic substances and phosphatase activities in plant tissues. Soil Biochem. 1999, 11, 253–259. [Google Scholar] [CrossRef]
- Mesut, C.K.; Onder, T.; Metin, T.; Burcu, T. Phosphorus, and humic acid application alleviate salinity stress of pepper seedling. Afr. J. Biotech. 2010, 9, 5845–5851. Available online: http://www.academicjournals.org/AJB (accessed on 21 August 2013).
- Nabati, D.A. Responses of two grass species to plant regulators, fertilizer N, chelated Fe, salinity and water stress. Ph.D. Thesis, Crop and Soil Environmental Science Deptrtment, Virginia Tech, Blacksburg, VA, USA, 1994. [Google Scholar]
- Demir, D.; Günes, A.; Inal, A.; Alpaslan, M. Effects of humic acids on the yield and mineral nutrition of cucumber (Cucumis sativus L.) grown with different salinity levels. Acta Hort. 2014, 492, 95–104. [Google Scholar] [CrossRef]
- Vaughan, D.; Macdonald, I.R. Effects of humic acid on protein synthesis and ion uptake in beet. J. Exp. Bot. 2005, 22, 400–410. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Benlock, M.; Barranco, D.; Duenas, A.; Ganan, J.A.G. Response of olive trees to foliar application of humic substances extracted from leonardite. Sci. Hortic. 1996, 66, 191–200. [Google Scholar] [CrossRef]
- Schmidt, W.; Santi, S.; Pinton, R.; Varanini, Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil 2007, 300, 259–267. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).