Assessment of Fatty Acid Composition, Bioactive Compounds, and Mineral Composition in Hazelnut Genetic Resources: Implications for Nutritional Value and Breeding Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preparation
2.3. Fatty Acids Composition
2.4. Bioactive Compounds
2.4.1. Total Phenolics
2.4.2. Total Flavonoids
2.4.3. Antioxidant Activity
2.5. Mineral Composition
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
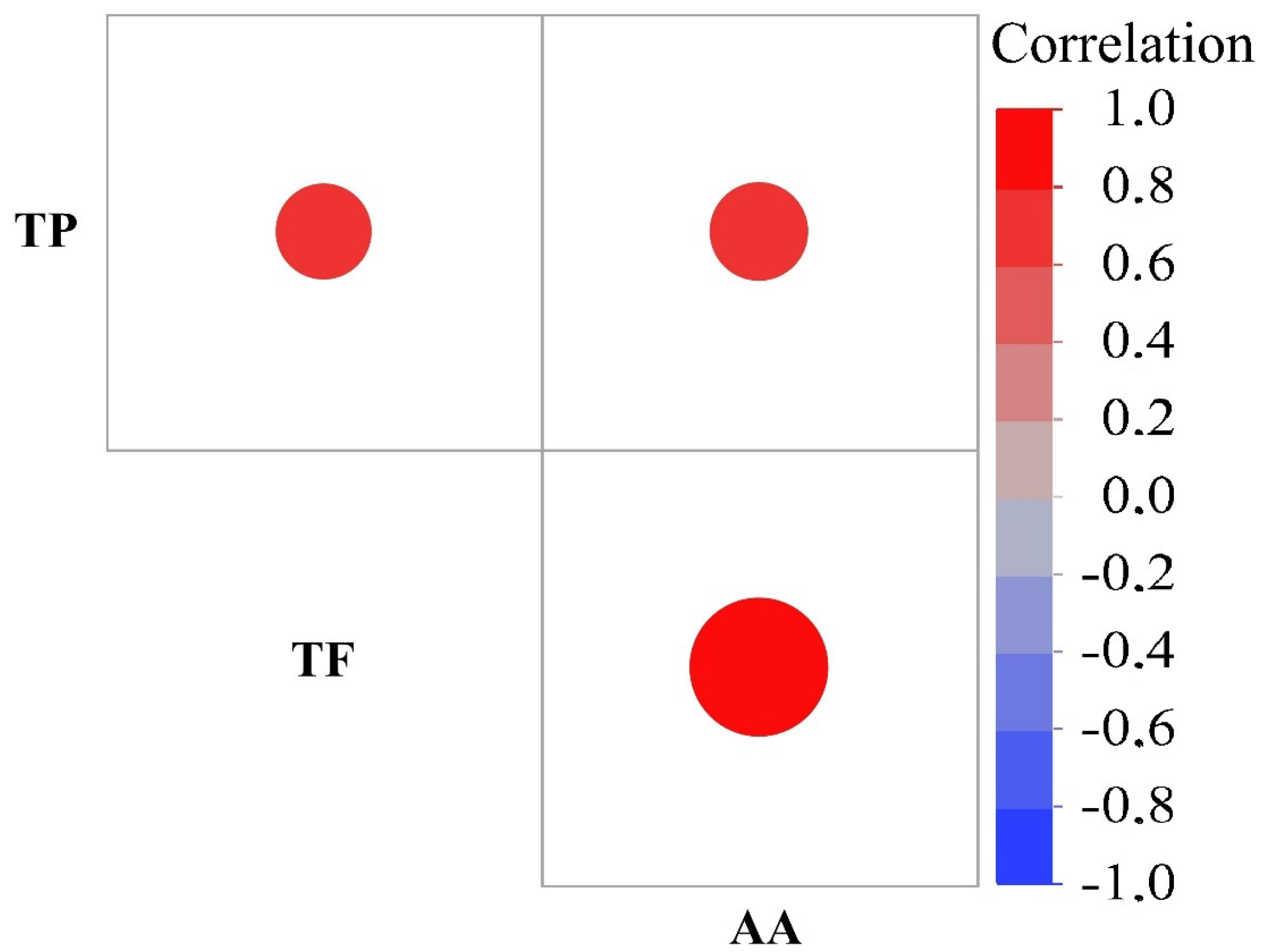
3.2. Bioactive Compounds
3.3. Mineral Composition
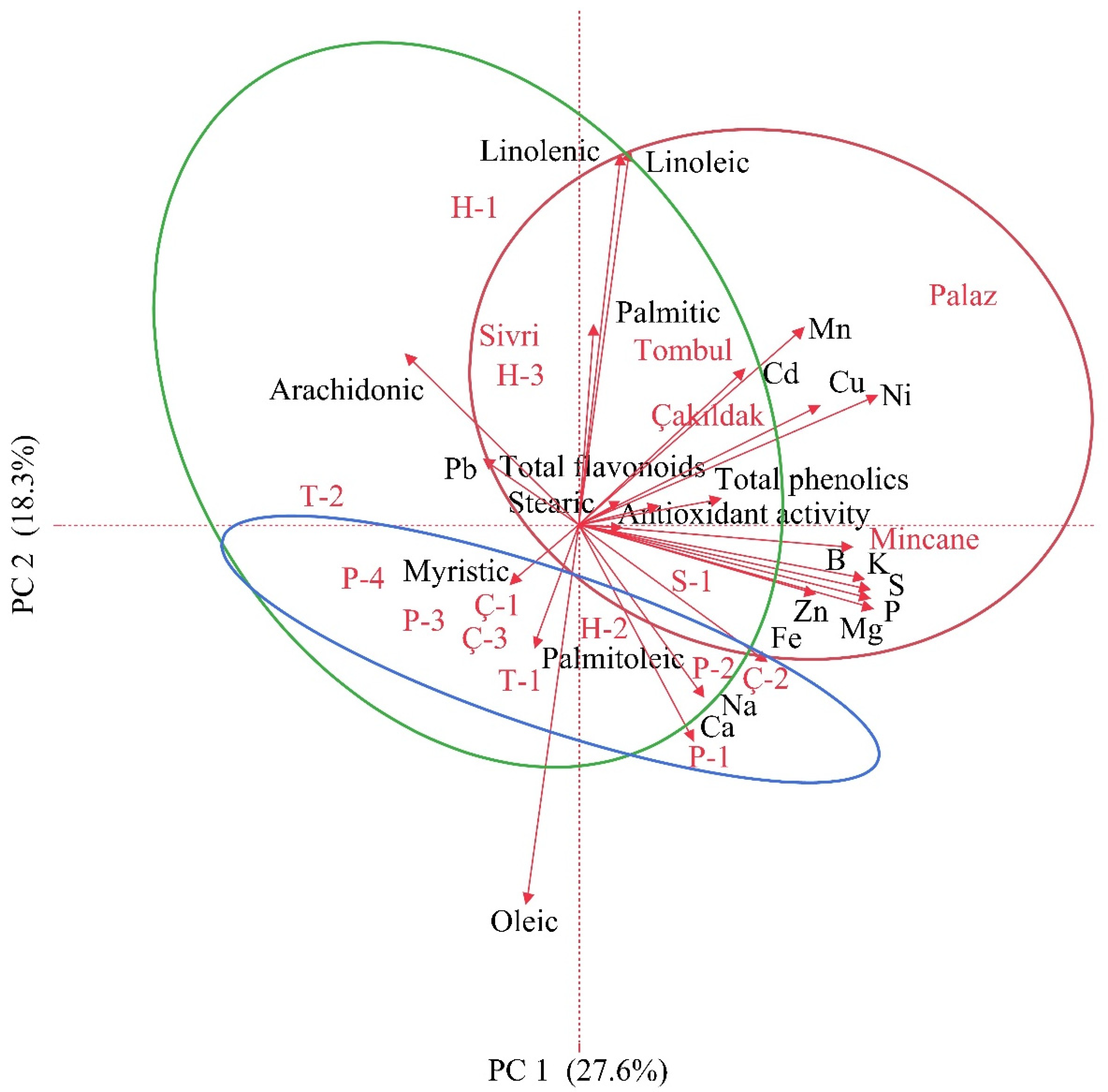
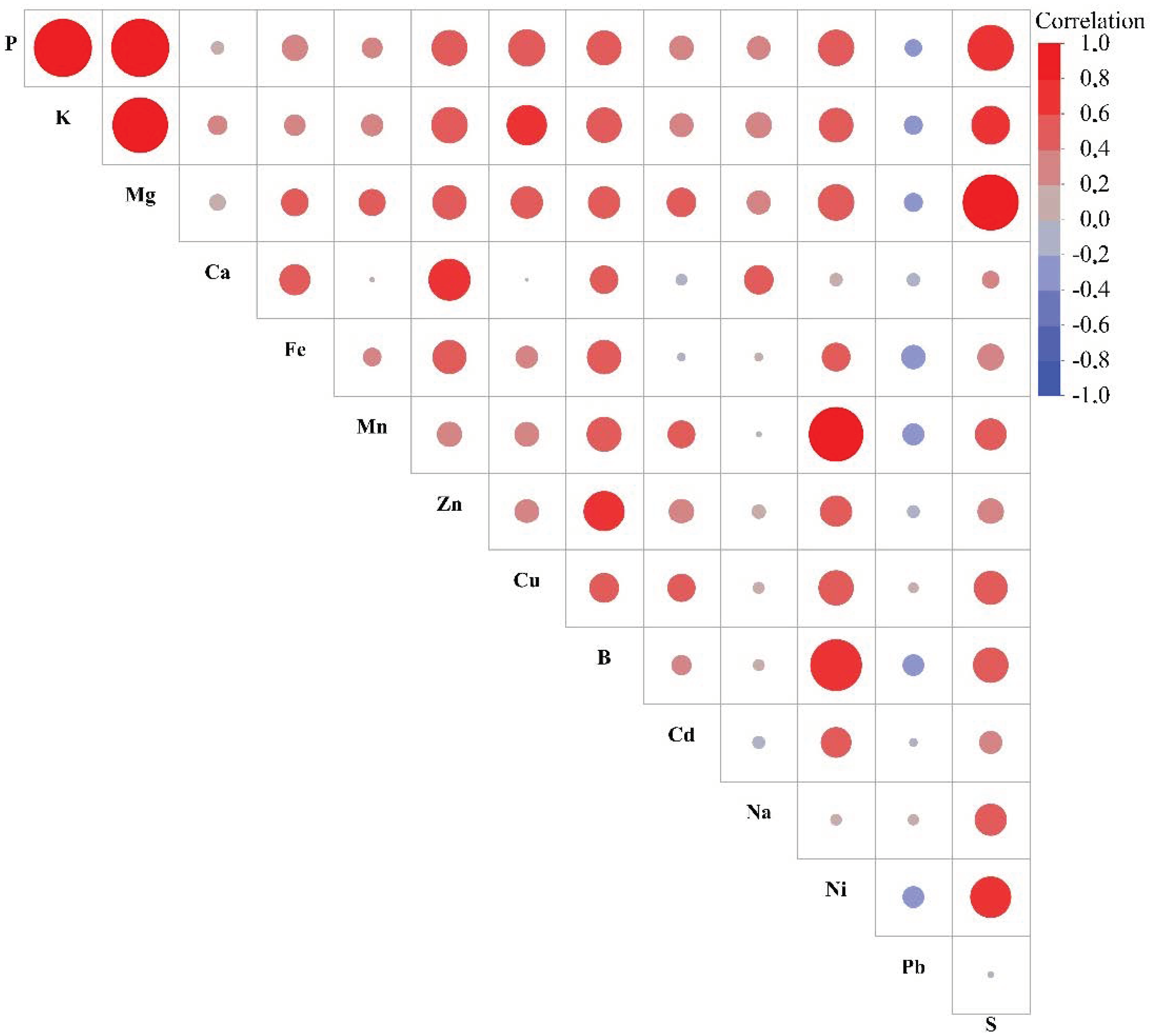
3.4. Principal Component and Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Özbek, S. Fındık Yetiştiriciliği. Özel Meyvecilik. Çukurova Üniversitesi Ziraat Fakültesi Yayınları 1978, 128, 286–321. [Google Scholar]
- Whitcher, I.N.; Wen, J. Phylogeny and biogeography of Corylus (Betulaceae): Inferences from ITS sequences. Syst. Bot. 2001, 26, 283–298. [Google Scholar]
- İslam, A. Fındık ıslahında gelişmeler. Akad. Ziraat Derg. 2019, 8, 167–174. [Google Scholar] [CrossRef]
- Balta, F.; Balta, M.F.; Karadeniz, T. The evaluations on preselection of the hazelnut ‘Tombul’and ‘Palaz’cultivars grown in Carsamba and Terme (Samsun) districts. Acta Hortic. 1997, 445, 109–118. [Google Scholar] [CrossRef]
- Karadeniz, T.; Bostan, S.Z.; Tuncer, C.; Tarakçıoğlu, C. Fındık Yetiştiriciliği. In Ziraat Odası Başkanlığı Bilimsel Yayınlar Serisi; Ofset Matbaa: Ordu, Türkiye, 2009; p. 126. [Google Scholar]
- Gökirmak, T.; Mehlenbacher, S.A.; Bassil, N.V. Characterization of European hazelnut (Corylus avellana) cultivars using SSR markers. Genet. Resour. Crop. Evol. 2009, 56, 147–172. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A.; Molnar, T.J. Hazelnut breeding. Plant Breed. Rev. 2021, 45, 9–141. [Google Scholar]
- Karakaya, O.; Yaman, İ.; Kırkaya, H.; Uzun, S.; Kaya, T.; Balta, M.F. Effect of cluster drop intensity on nut traits, biochemical properties, and fatty acids composition in the ‘Çakıldak’ hazelnut cultivar. Erwerbs Obstbau 2023, 65, 785–793. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agricultural Organization of the United Nations. Hazelnuts—Production Statistics in 2021. Available online: https://fao.org (accessed on 11 August 2023).
- Bacchetta, L.; Rovira, M.; Tronci, C.; Aramini, M.; Drogoudi, P.; Silva, A.P.; Solar, A.; Avanza, D.; Botta, R.; Valentini, N.; et al. A multidisciplinary approach to enhance the conservation and use of hazelnut Corylus avellana L. genetic resources. Genet. Resour. Crop Evol. 2015, 62, 649–663. [Google Scholar] [CrossRef]
- Demir, T.; Beyhan, N. Samsun ilinde yetiştirilen fındıkların seleksiyonu üzerine bir araştırma. Turk. J. Agric. For. 2000, 24, 173–183. [Google Scholar]
- Turan, A.; İslam, A. Fındığın ekolojik istekleri. In Fındık Yetiştiriciliği, 1st ed.; İslam, A., Ed.; Yeşiller grafik tasarım reklam ve matbaacılık, Fatsa: Ordu, Türkiye, 2020; pp. 27–29. [Google Scholar]
- Alasalvar, C.; Pelvan, E.; Topal, B. Effects of roasting on oil and fatty acid composition of Turkish hazelnut varieties (Corylus avellana L.). Int. J. Food Sci. Nutr. 2010, 61, 630–642. [Google Scholar] [CrossRef]
- Platteau, C.; De Loose, M.; De Meulenaer, B.; Taverniers, I. Quantitative detection of HN (Corylus avellana) in cookies: ELISA versus real-time PCR. J. Agric. Food Chem. 2011, 59, 11395–11402. [Google Scholar] [CrossRef] [PubMed]
- Moscetti, R.; Frangipane, M.T.; Monarca, D.; Cecchini, M.; Massantini, R. Maintaining the quality of unripe, fresh hazelnuts through storage under modified atmospheres. Postharvest Biol. Technol. 2012, 65, 33–38. [Google Scholar] [CrossRef]
- Yılmaz, M.; Karakaya, O.; Balta, M.F.; Balta, F.; Yaman, İ. Çakıldak fındık çeşidinde iç meyve iriliğine bağlı olarak biyokimyasal özelliklerin değişimi. Akad. Zir. Derg. 2019, 8, 61–70. [Google Scholar] [CrossRef]
- Di Nunzio, M. Hazelnuts as source of bioactive compounds and health value underestimated food. Curr. Res. Nutr. Food Sci. 2019, 7, 17–28. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Ye, Z.Q.; Zhang, F.; Han, J.J.; Yang, T.; Wang, Z.Z.; Zhang, Y. Nutshell Extracts of Xanthoceras sorbifolia: A New Potential Source of Bioactive Phenolic Compounds as a Natural Antioxidant and Immunomodulator. J. Agric. Food Chem. 2018, 66, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.H.; Adzib, M.S.M.; Ilham, Z. Potential antioxidants from crude extracts of roselle seeds and cashew nut shells for biodiesel storage stability improvement. In Proceedings of the AIP Conference, Jawa Barat, Indonesia, 29–30 April 2019; American Institute of Physics Inc.: Bandung, Indonesia, 2019; Volume 2129, p. 020087. [Google Scholar]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as natural antioxidants: Sources, extraction and applications in food, cosmetics and drugs. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Springer Nature: Singapore, 2019; pp. 197–235. [Google Scholar]
- Kamala Kumari, P.V.; Akhila, S.; Srinivasa Rao, Y.; Rama Devi, B. Alternative to artificial preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic compounds: Functional properties, impact of processing and bioavailability. Phenolic Compd. Biol. Act 2017, 8, 1–24. [Google Scholar]
- Luo, S.; Lenon, G.B.; Gill, H.; Yuen, H.; Yang, A.W.; Hung, A.; Nguyen, L.T. Do the natural chemical compounds interact with the same targets of current pharmacotherapy for weight management? A review. Curr. Drug Targets 2019, 20, 399–411. [Google Scholar] [CrossRef]
- Duman, E.; Özcan, M.M. The influence of industrial refining stages on the physico-chemical properties, fatty acid composition and sterol contents in hazelnut oil. J. Food Sci. Technol. 2020, 57, 2501–2506. [Google Scholar] [CrossRef]
- Balık, H.İ. Bioactive Compounds and Fatty Acid Composition of New Turkish Hazelnut Cultivars. Int. J. Fruit Sci. 2021, 21, 106–114. [Google Scholar] [CrossRef]
- Gülsoy, E.; Kaya, E.D.; Türkhan, A.; Bulut, M.; Koyuncu, M.; Güler, E.; Sayın, F.; Muradoglu, F. The Effect of Altitude on Phenolic, Antioxidant and Fatty Acid Compositions of Some Turkish Hazelnut (Coryllus avellana L.) Cultivars. Molecules 2023, 28, 5067. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of bioactive compounds from hazelnuts and walnuts shells: Quantitative–qualitative analysis and chromatographic purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Lelli, V.; Molinari, R.; Merendino, N.; Timperio, A.M. Detection and Comparison of Bioactive Compounds in Different Extracts of Two Hazelnut Skin Varieties, Tonda Gentile Romana and Tonda Di Giffoni, Using a Metabolomics Approach. Metabolites 2021, 11, 296. [Google Scholar] [CrossRef]
- Müller, A.K.; Helms, U.; Rohrer, C.; Möhler, M.; Hellwig, F.; Glei, M.; Schwerdtle, T.; Lorkowski, S.; Dawczynski, C. Nutrient Composition of Different Hazelnut Cultivars Grown in Germany. Foods 2020, 9, 1596. [Google Scholar] [CrossRef]
- Azizov, F.; Atayeva, V.; Khalilov, Z.; Mustafayev, N.; Imanlı, H. Chemical Composition and Biological Active Substances from Hazelnut Green Leafy Covers. JOTCSA 2022, 9, 999–1006. [Google Scholar] [CrossRef]
- Karaosmanoğlu, H. Lipid characteristics, bioactive properties, and mineral content in hazelnut grown under different cultivation systems. J. Food Process. Preserv. 2022, 46, 16717. [Google Scholar] [CrossRef]
- Sengul, S. The Effect of Different Harvest Date and Altitude on Chemical Composition, Antioxidant Capacity and Quality Parameters of Hazelnut Oil. Master’s Thesis, Ordu University, Institute of Science, Ordu, Türkiye, 2019. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ustaoğlu, F.; Tepe, Y. Water quality and sediment contamination assessment of Pazarsuyu stream, Turkey using multivariate statistical methods and pollution indicators. ISWCR 2019, 7, 47–56. [Google Scholar] [CrossRef]
- Balta, M.F.; Yarılgaç, T.; Aşkın, M.A.; Kuçuk, M.; Balta, F.; Özrenk, K. Determination of fatty acid compositions, oil contents and some quality traits of hazelnut genetic resources grown in eastern Anatolia of Turkey. J. Food Compos. Anal. 2006, 19, 681–686. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Piotrowska, A. Morphological traits, kernel composition and sensory evaluation of hazelnut (Corylus avellana L.) cultivars grown in Poland. Agronomy 2019, 9, 703. [Google Scholar] [CrossRef]
- Özdemir, M.; Açkurt, F.; Yildiz, M.; Biringen, G.; Gürcan, T.; Löker, M. Effect of roasting on some nutrients of hazelnuts (Corylus avellena L.). Food Chem. 2001, 73, 185–190. [Google Scholar] [CrossRef]
- Granata, M.U.; Bracco, F.; Gratani, L.; Catoni, R.; Corana, F.; Mannucci, B.; Sartori, F.; Martino, E. Fatty acid content profile and main constituents of Corylus avellana kernel in wild type and cultivars growing in Italy. Nat. Pro. Res. 2017, 31, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Bakhshi, D.; Ghazvini, R.F.; Majd, D.J.; Pourghayoumi, M. Evaluation of fatty acid content and nutritional properties of selected native and imported hazelnut (Corylus avellana L.) varieties grown in Iran. J. App. Bot. Food Qual. 2014, 87, 104–107. [Google Scholar]
- Çetin, N.; Yaman, M.; Karaman, K.; Demir, B. Determination of some physicomechanical and biochemical parameters of hazelnut (Corylus avellana L.) cultivars. Turk. J. Agric. For. 2020, 44, 439–450. [Google Scholar] [CrossRef]
- Hicks, K.B.; Moreau, R.A. Phytosterols and phytostanols: Functional food cholesterol busters. Food Technol. 2001, 55, 63–67. [Google Scholar]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Nut and kernel traits and chemical composition of hazelnut (Corylus avellana L.) cultivars. J. Sci. Food Agric. 2008, 88, 1091–1098. [Google Scholar] [CrossRef]
- Arcoleo, G. Caratteristiche e composizione di nocciole di alcune varietà coltivate in Sicilia. (Characteristics and composition of some varieties of hazelnuts cultivate in Sicily, The Italian magazine of fatty substances). Riv. Ital. Sostanze Grasse 1991, 68, 257–260. [Google Scholar]
- Serra Bonvehí, J.; Ventura Coll, F. A chemical study of the mineral fraction of Tarragona hazelnuts (Corylus avellana L.). Acta Aliment. 1997, 26, 243–253. [Google Scholar]
- Cristofori, V.; Bertazza, G.; Bignami, C. Changes in kernel chemical composition during nut development of three Italian hazelnut cultivars. Fruits 2015, 70, 311–322. [Google Scholar] [CrossRef]
- Salas-Salvadò, J.; Megias, I. Health and tree nuts: Scientific evidence of disease prevention. Acta Hortic. 2005, 686, 507–514. [Google Scholar] [CrossRef]
- Karaosmanoğlu, H.; Ustun, N.S. Fatty acids, tocopherol and phenolic contents of organic and conventional grown hazelnuts. J. Agric. Sci. Technol. 2021, 23, 167–177. [Google Scholar]
- Król, K.; Gantner, M.; Piotrowska, A.; Hallmann, E. Effect of climate and roasting on polyphenols and tocopherols in the kernels and skin of six hazelnut cultivars (Corylus avellana L.). Agriculture 2020, 10, 36. [Google Scholar] [CrossRef]
- Sali, K.; Öztürk, B.; Akgün, M.; Ateş, U. Effect of drying methods on the sensory attributes of hazelnut cultivars in different sizes throughout the storage. Turk. J. Food Agric. Sci. 2022, 4, 69–75. [Google Scholar] [CrossRef]
- Schmitzer, V.; Slatnar, A.; Veberic, R.; Stampar, F.; Solar, A. Roasting affects phenolic composition and antioxidative activity of hazelnuts (Corylus avellana L.). J. Food Sci. 2011, 76, S14–S19. [Google Scholar] [CrossRef]
- Özdemir, I.O.; Tuncer, C.; Solmaz, F.G.; Ozturk, B. The impact of green shield bug (Palomena prasina [Hemiptera: Pentatomidae]) infestation on antioxidant enzyme activities in hazelnut (Corylus avellana L. cvs. ‘Tombul, ‘Palaz ’and ‘Çakıldak’). Erwerbs Obstbau 2023, 65, 767–773. [Google Scholar] [CrossRef]
- Jaćimović, S.; Kiprovski, B.; Ristivojević, P.; Dimić, D.; Nakarada, Đ.; Dojčinović, B.; Sikora, V.; Teslić, N.; Pantelić, N.Đ. Chemical composition, antioxidant potential, and nutritional evaluation of cultivated Sorghum Grains: A combined experimental, theoretical, and multivariate analysis. Antioxidants 2023, 12, 1485. [Google Scholar] [CrossRef]
- Ozdemir, F.; Akinci, I. Physical and nutritional properties of four major commercial Turkish hazelnut varieties. J. Food Eng. 2004, 63, 341–347. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Botu, M.; Trandafir, I. The mineral source for human nutrition of nuts in different hazelnut (Corylus avellana L.) cultivars. Not. Bot. Hortic. Agrobot. 2013, 41, 250–254. [Google Scholar] [CrossRef]
- Fennema, O.R. Food Chemistry, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1985; p. 991. [Google Scholar]
- Wei, L.; Zhai, Q. The dynamics and correlation between nitrogen, phosphorus, potassium and calcium in a hazelnut fruit during its development. Front Agric. China 2010, 4, 352–357. [Google Scholar] [CrossRef]
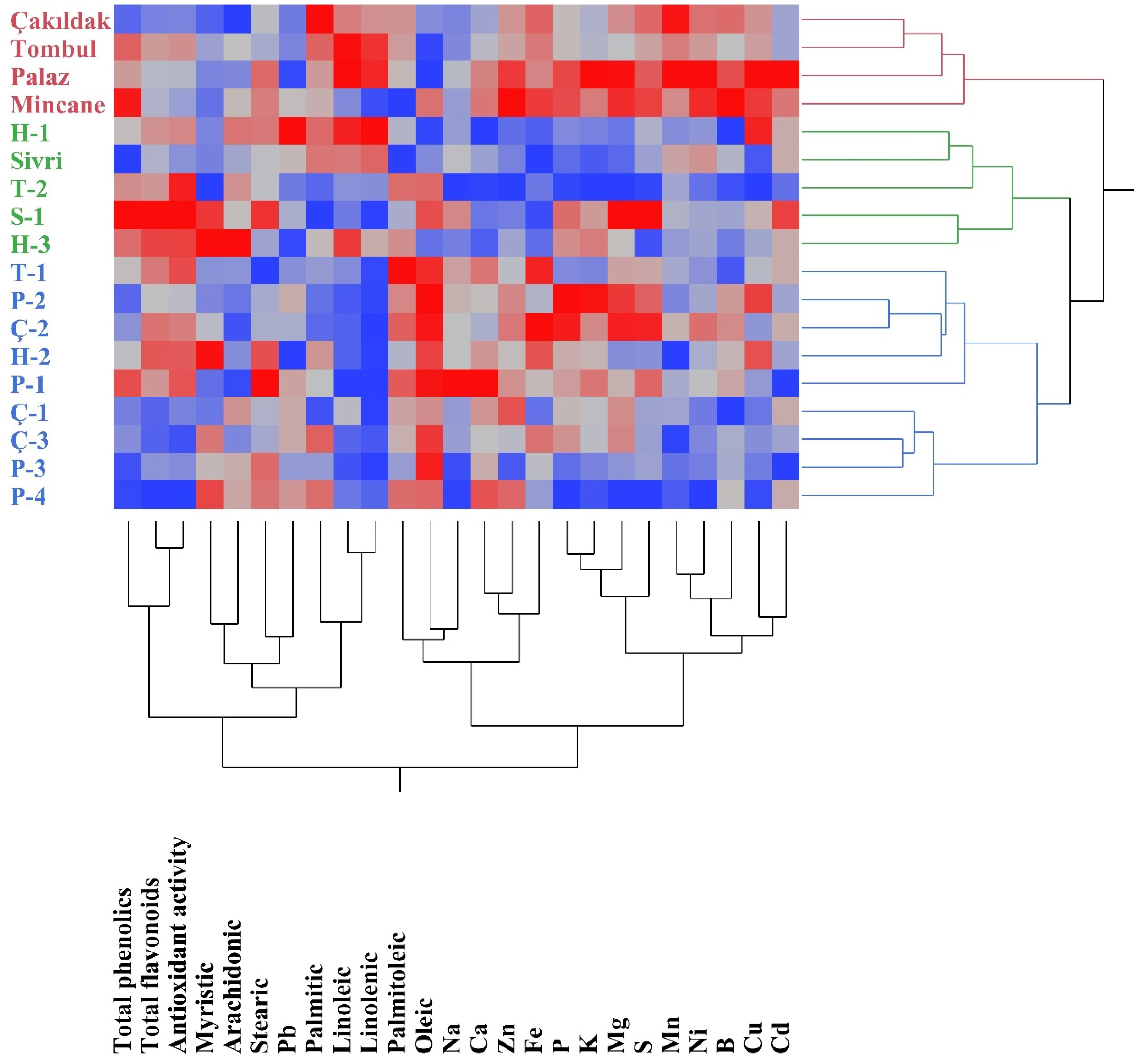
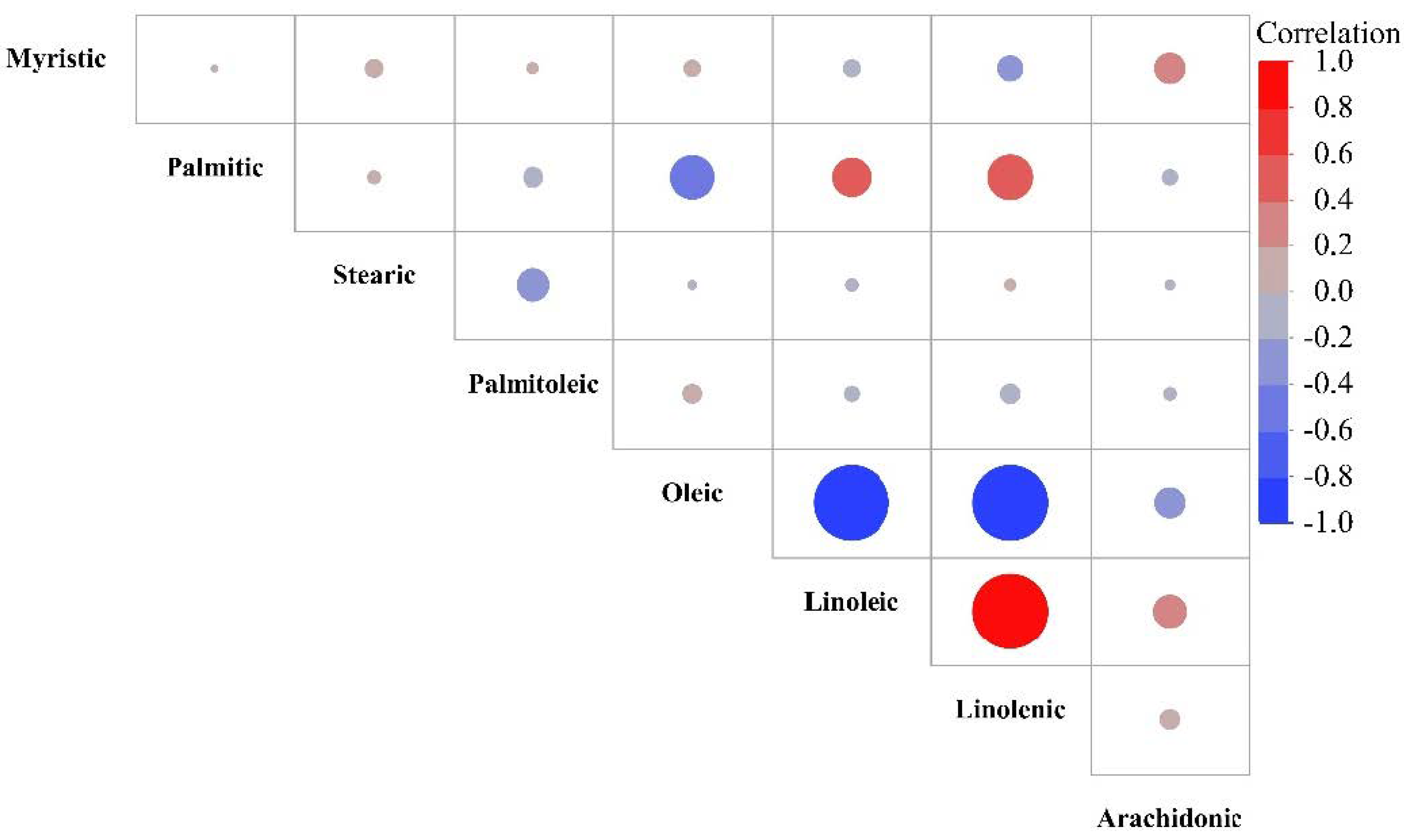
| Cultivars/ Accessions | Oleic | Linoleic | Palmitic | Stearic | Linolenic | Palmitoleic | Arachidonic | Myristic | SFA | PUFA | MUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Çakıldak | 77.10 h z | 13.33 d | 6.09 a | 2.41 e | 0.96 d | 0.05 b | 0.03 b | 0.04 f | 8.54 ab | 14.32 e | 77.14 h |
| Mincane | 83.97 fg | 7.64 f | 5.42 cde | 2.76 d | 0.11 gh | 0.02 d | 0.04 ab | 0.04 f | 8.22 a–d | 7.79 h | 83.99 fg |
| Palaz | 70.47 j | 19.19 a | 5.51 bcd | 2.85 cd | 1.86 b | 0.05 b | 0.03 ab | 0.04 f | 8.40 abc | 21.08 a | 70.52 j |
| Sivri | 76.92 h | 13.56 d | 5.66 bc | 2.44 e | 1.33 c | 0.02 d | 0.04 ab | 0.04 f | 8.14 bcd | 14.93 d | 76.94 h |
| Tombul | 71.18 j | 19.03 a | 5.73 b | 2.17 fg | 1.75 b | 0.05 b | 0.04 ab | 0.05 e | 7.95 de | 20.82 a | 71.23 j |
| Ç-1 | 83.10 g | 9.67 e | 4.78 g | 2.27 ef | 0.05 h | 0.05 b | 0.04 ab | 0.04 f | 7.09 g | 9.76 f | 83.14 g |
| Ç-2 | 86.85 ab | 5.85 hi | 4.90 fg | 2.22 fg | 0.05 h | 0.05 b | 0.03 ab | 0.05 e | 7.17 fg | 5.93 jk | 86.90 ab |
| Ç-3 | 85.79 bcd | 6.02 h | 5.75 b | 2.16 fg | 0.14 gh | 0.05 b | 0.03 ab | 0.07 c | 7.97 cde | 6.19 j | 85.83 bcd |
| H-1 | 71.24 j | 18.06 b | 5.71 b | 2.78 d | 2.08 a | 0.04 c | 0.05 ab | 0.04 f | 8.53 ab | 20.19 b | 71.28 j |
| H-2 | 85.46 cde | 5.83 hi | 5.53 bcd | 2.96 bc | 0.06 h | 0.04 c | 0.03 ab | 0.09 a | 8.58 a | 5.92 jk | 85.50 cde |
| H-3 | 74.83 i | 16.80 c | 5.37 de | 2.08 g | 0.72 e | 0.05 b | 0.06 a | 0.09 a | 7.55 ef | 17.57 c | 74.88 i |
| P-1 | 86.88 ab | 4.35 k | 5.34 de | 3.26 a | 0.05 h | 0.05 b | 0.03 ab | 0.04 f | 8.64 a | 4.43 m | 86.93 ab |
| P-2 | 87.16 a | 5.52 ij | 4.94 fg | 2.17 fg | 0.08 gh | 0.05 b | 0.03 ab | 0.04 f | 7.15 fg | 5.64 kl | 87.21 a |
| P-3 | 86.59 abc | 5.21 j | 5.16 ef | 2.85 cd | 0.06 h | 0.04 c | 0.04 ab | 0.06 d | 8.06 cd | 5.31 l | 86.63 abc |
| P-4 | 84.37 ef | 6.77 g | 5.69 b | 2.80 cd | 0.20 g | 0.05 b | 0.04 ab | 0.08 b | 8.57 ab | 7.00 i | 84.42 ef |
| S-1 | 85.09 def | 6.92 g | 4.68 g | 3.09 ab | 0.06 h | 0.04 c | 0.04 ab | 0.08 b | 7.85 de | 7.02 i | 85.13 def |
| T-1 | 86.19 a–d | 7.66 f | 5.16 ef | 0.76 h | 0.09 gh | 0.06 a | 0.03 ab | 0.05 e | 5.96 h | 7.79 h | 86.25 a–d |
| T-2 | 84.27 efg | 7.93 f | 4.88 g | 2.42 e | 0.36 f | 0.05 b | 0.04 ab | 0.03 g | 7.34 fg | 8.33 g | 84.33 efg |
| Significance | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** |
| LSD (0.05) | 1.22 | 0.37 | 0.27 | 0.17 | 0.13 | 0.009 | 0.029 | 0.009 | 0.44 | 0.50 | 1.23 |
| Cultivars/ Accessions | Total Phenolics (mg/100 g) | Total Flavonoids (mg/100 g) | Antioxidant Activity (mmol/100 g) |
|---|---|---|---|
| Çakıldak | 333.3 h z | 10.5 h | 0.29 j |
| Mincane | 546.2 a | 12.3 g | 0.33 h |
| Palaz | 440.8 d | 12.5 g | 0.37 g |
| Sivri | 299.1 j | 12.3 g | 0.31 i |
| Tombul | 486.7 c | 13.7 f | 0.42 f |
| Ç-1 | 354.3 g | 9.5 i | 0.29 j |
| Ç-2 | 370.1 f | 14.6 cd | 0.44 e |
| Ç-3 | 365.9 fg | 9.4 i | 0.22 k |
| H-1 | 413.8 e | 13.9 ef | 0.43 ef |
| H-2 | 407.3 e | 15.2 bc | 0.47 d |
| H-3 | 477.1 c | 15.7 b | 0.49 c |
| P-1 | 503.1 b | 13.8 ef | 0.47 d |
| P-2 | 333.2 h | 12.8 g | 0.38 g |
| P-3 | 314.2 i | 11.2 h | 0.31 i |
| P-4 | 307.3 ij | 8.1 j | 0.19 l |
| S-1 | 557.3 a | 17.0 a | 0.53 a |
| T-2 | 451.6 d | 13.8 f | 0.51 b |
| Significance | *** | *** | *** |
| LSD (0.05) | 13.82 | 0.67 | 0.01 |
| Cultivars/ Accessions | K | P | Ca | Mg | Na | S | Mn | Zn | Fe | Cu | B | Ni | Pb | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Çakıldak | 2226 e z | 1922 g | 1707 j | 1567 bc | 242 fg | 211 c | 154 b | 20.0 ef | 23.2 cde | 10.4 d | 4.90 c | 1.83 c | 0.10 fg | 0.02 de |
| Mincane | 2730 bc | 2268 b | 2207 c | 1732 a | 250 efg | 220 bc | 89 d | 25.5 a | 24.5 bc | 12.1 bc | 7.06 a | 2.45 b | 0.18 cde | 0.04 bc |
| Palaz | 3383 a | 2281 b | 2152 d | 1778 a | 259 def | 214 c | 158 a | 23.7 b | 22.1 e | 13.0 a | 5.60 b | 2.72 a | 0.05 gh | 0.06 a |
| Sivri | 1637 g | 1479 i | 1689 j | 1188 fg | 261 de | 178 ef | 75 e | 14.3 j | 14.6 j | 6.8 j | 2.81 gh | 1.53 d | 0.19 cd | 0.03 cd |
| Tombul | 2256 e | 1929 g | 1820 h | 1476 cd | 243 fg | 197 d | 95 c | 19.5 fg | 22.6 de | 10.0 de | 3.03 fg | 1.48 d | 0.11 f | 0.02 de |
| Ç-1 | 2321 e | 1934 fg | 2071 e | 1567 bc | 249 efg | 178 ef | 48 h | 22.6 bc | 16.8 h | 7.5 i | 0.68 l | 0.52 ij | 0.22 bc | 0.03 cd |
| Ç-2 | 2654 bc | 2412 a | 1766 i | 1752 a | 262 de | 227 ab | 68 f | 21.2 de | 25.9 a | 8.3 h | 4.24 d | 1.85 c | 0.15 def | 0.03 cd |
| Ç-3 | 2302 e | 2048 de | 1865 g | 1579 bc | 250 efg | 177 ef | 9 m | 17.8 h | 22.8 de | 8.1 hi | 2.69 hi | 0.63 hi | 0.22 bc | 0.02 de |
| H-1 | 1906 f | 1624 h | 1142 m | 1236 ef | 250 efg | 184 e | 38 j | 12.9 k | 16.0 hi | 12.6 ab | 0.66 l | 0.76 gh | 0.54 a | 0.03 cd |
| H-2 | 2417 de | 1967 efg | 2066 e | 1311 ef | 263 de | 171 f | 7 m | 18.4 gh | 23.7 cd | 11.7 c | 3.27 f | 0.95 f | 0.04 h | 0.02 de |
| H-3 | 2732 bc | 2062 d | 1317 l | 1483 c | 240 gh | 147 g | 43 i | 16.5 i | 16.7 h | 8.7 gh | 1.78 j | 0.88 fg | 0.05 gh | 0.03 cd |
| P-1 | 2773 b | 2016 def | 2701 a | 1507 c | 412 a | 211 c | 51 gh | 20.2 ef | 20.6 f | 8.4 h | 3.66 e | 1.10 e | 0.23 bc | 0.00 f |
| P-2 | 3349 a | 2464 a | 1884 g | 1710 ab | 275 cd | 212 c | 35 j | 20.6 ef | 19.6 fg | 12.0 bc | 3.92 e | 0.90 f | 0.21 bc | 0.02 de |
| P-3 | 1919 f | 1488 i | 1966 f | 1334 de | 223 hi | 178 ef | 27 k | 11.7 l | 20.0 fg | 7.7 i | 2.63 hi | 0.55 ij | 0.13 ef | 0.00 f |
| P-4 | 1583 g | 1252 j | 2375 b | 1053 g | 219 i | 140 g | 18 l | 21.9 cd | 18.7 g | 6.5 j | 3.11 f | 0.01 k | 0.26 b | 0.03 cd |
| S-1 | 2561 cd | 2166 c | 1452 k | 1787 a | 308 b | 232 a | 53 g | 14.0 jk | 15.2 ij | 9.7 ef | 2.52 i | 0.90 f | 0.15 def | 0.05 ab |
| T-1 | 1923 f | 1626 h | 2220 c | 1531 c | 289 c | 196 d | 50 gh | 17.9 h | 25.2 ab | 9.2 fg | 1.11 k | 0.72 h | 0.12 f | 0.03 cd |
| T-2 | 1444 g | 1292 j | 1172 m | 1050 g | 218 i | 143 g | 48 h | 9.9 m | 17.1 h | 6.2 j | 0.98 k | 0.44 j | 0.10 fg | 0.01 ef |
| Significance | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| LSD (0.05) | 198.8 | 86.50 | 34.8 | 143.9 | 18.0 | 9.53 | 4.08 | 1.24 | 1.37 | 0.64 | 0.29 | 0.13 | 0.05 | 0.015 |
| Variables | Components | ||||||
|---|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 | |
| Total phenolics | 0.3989 | 0.0627 | 0.6466 * | 0.1560 | −0.0471 | −0.1907 | 0.1765 |
| Total flavonoids | 0.2186 | 0.0455 | 0.8946 * | −0.0713 | −0.1080 | −0.2197 | 0.0992 |
| Antioxidant activity | 0.1129 | −0.0064 | 0.9018 * | −0.1907 | −0.2370 | −0.1872 | 0.1122 |
| Myristic | −0.1899 | −0.1416 | 0.2719 | 0.2352 | 0.7003 * | −0.1356 | 0.3938 |
| Palmitic | 0.0431 | 0.4993 | −0.5786 * | 0.0158 | −0.0496 | −0.1643 | 0.5016 * |
| Stearic | 0.1067 | 0.0511 | −0.0095 | 0.8215 * | −0.1413 | −0.2323 | 0.1558 |
| Palmitoleic | −0.1269 | −0.3002 | 0.1675 | −0.6328 * | −0.0215 | 0.3210 | 0.3923 |
| Oleic | −0.1503 | −0.9552 * | 0.0018 | 0.0802 | 0.0074 | −0.0579 | −0.1713 |
| Linoleic | 0.1456 | 0.9425 * | 0.0489 | −0.1740 | 0.0365 | 0.0923 | 0.1220 |
| Linolenic | 0.1203 | 0.9302 * | −0.0554 | −0.0589 | −0.2125 | 0.0883 | 0.1480 |
| Arachidonic | −0.4951 * | 0.4264 | 0.3967 | 0.1194 | 0.4381 | 0.0934 | 0.0052 |
| P | 0.8373 * | −0.1849 | 0.2247 | 0.0474 | 0.2197 | 0.2135 | −0.0333 |
| K | 0.8198 * | −0.1343 | 0.2012 | 0.0930 | 0.2158 | 0.2217 | 0.1044 |
| Mg | 0.8466 * | −0.2094 | 0.1832 | −0.0693 | 0.1662 | 0.2417 | −0.1929 |
| Ca | 0.3278 | −0.5393 * | −0.4862 | 0.0640 | −0.0503 | −0.0064 | 0.3375 |
| Fe | 0.5374 * | −0.3425 | −0.3119 | −0.4513 | 0.0535 | −0.1988 | 0.2173 |
| Mn | 0.6469 * | 0.4952 | −0.2054 | −0.2467 | −0.1910 | −0.1817 | −0.1928 |
| Zn | 0.6779 * | −0.1726 | −0.4328 | −0.0186 | 0.2801 | 0.2177 | 0.1531 |
| Cu | 0.6903 * | 0.2985 | 0.1316 | 0.1162 | 0.0275 | 0.1901 | 0.2204 |
| B | 0.7844 * | −0.0568 | −0.3826 | 0.2193 | 0.0787 | −0.3276 | −0.0208 |
| Cd | 0.4756 * | 0.3885 | 0.1391 | 0.1027 | 0.4180 | 0.2002 | −0.2262 |
| Na | 0.3574 | −0.4318 | 0.3045 | 0.1856 | −0.5128 * | 0.1190 | 0.3318 |
| Ni | 0.8597 * | 0.3253 | −0.1276 | −0.0121 | −0.0766 | −0.2582 | −0.1580 |
| Pb | −0.2684 | 0.1606 | −0.1054 | 0.4139 | −0.3685 | 0.6635 * | 0.0576 |
| S | 0.8372 * | −0.1631 | 0.1203 | 0.0537 | −0.3040 | 0.1283 | −0.2074 |
| Eigen value | 6.9 | 4.6 | 3.6 | 1.8 | 1.7 | 1.3 | 1.3 |
| Variance (%) | 27.6 | 18.3 | 14.5 | 7.3 | 7.0 | 5.4 | 5.0 |
| Cumulative (%) | 27.6 | 46.0 | 60.5 | 67.8 | 74.8 | 80.1 | 85.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaman, M.; Balta, M.F.; Karakaya, O.; Kaya, T.; Necas, T.; Yildiz, E.; Dirim, E. Assessment of Fatty Acid Composition, Bioactive Compounds, and Mineral Composition in Hazelnut Genetic Resources: Implications for Nutritional Value and Breeding Programs. Horticulturae 2023, 9, 1008. https://doi.org/10.3390/horticulturae9091008
Yaman M, Balta MF, Karakaya O, Kaya T, Necas T, Yildiz E, Dirim E. Assessment of Fatty Acid Composition, Bioactive Compounds, and Mineral Composition in Hazelnut Genetic Resources: Implications for Nutritional Value and Breeding Programs. Horticulturae. 2023; 9(9):1008. https://doi.org/10.3390/horticulturae9091008
Chicago/Turabian StyleYaman, Mehmet, Mehmet Fikret Balta, Orhan Karakaya, Tuncay Kaya, Tomas Necas, Ercan Yildiz, and Emine Dirim. 2023. "Assessment of Fatty Acid Composition, Bioactive Compounds, and Mineral Composition in Hazelnut Genetic Resources: Implications for Nutritional Value and Breeding Programs" Horticulturae 9, no. 9: 1008. https://doi.org/10.3390/horticulturae9091008
APA StyleYaman, M., Balta, M. F., Karakaya, O., Kaya, T., Necas, T., Yildiz, E., & Dirim, E. (2023). Assessment of Fatty Acid Composition, Bioactive Compounds, and Mineral Composition in Hazelnut Genetic Resources: Implications for Nutritional Value and Breeding Programs. Horticulturae, 9(9), 1008. https://doi.org/10.3390/horticulturae9091008






