Use of Light Spectra for Efficient Production of PLBs in Temperate Terrestrial Orchids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Explant Preparation
2.2. Media and Culture Conditions
2.3. Germination and Acclimatization
2.4. Statistical Analysis
3. Results
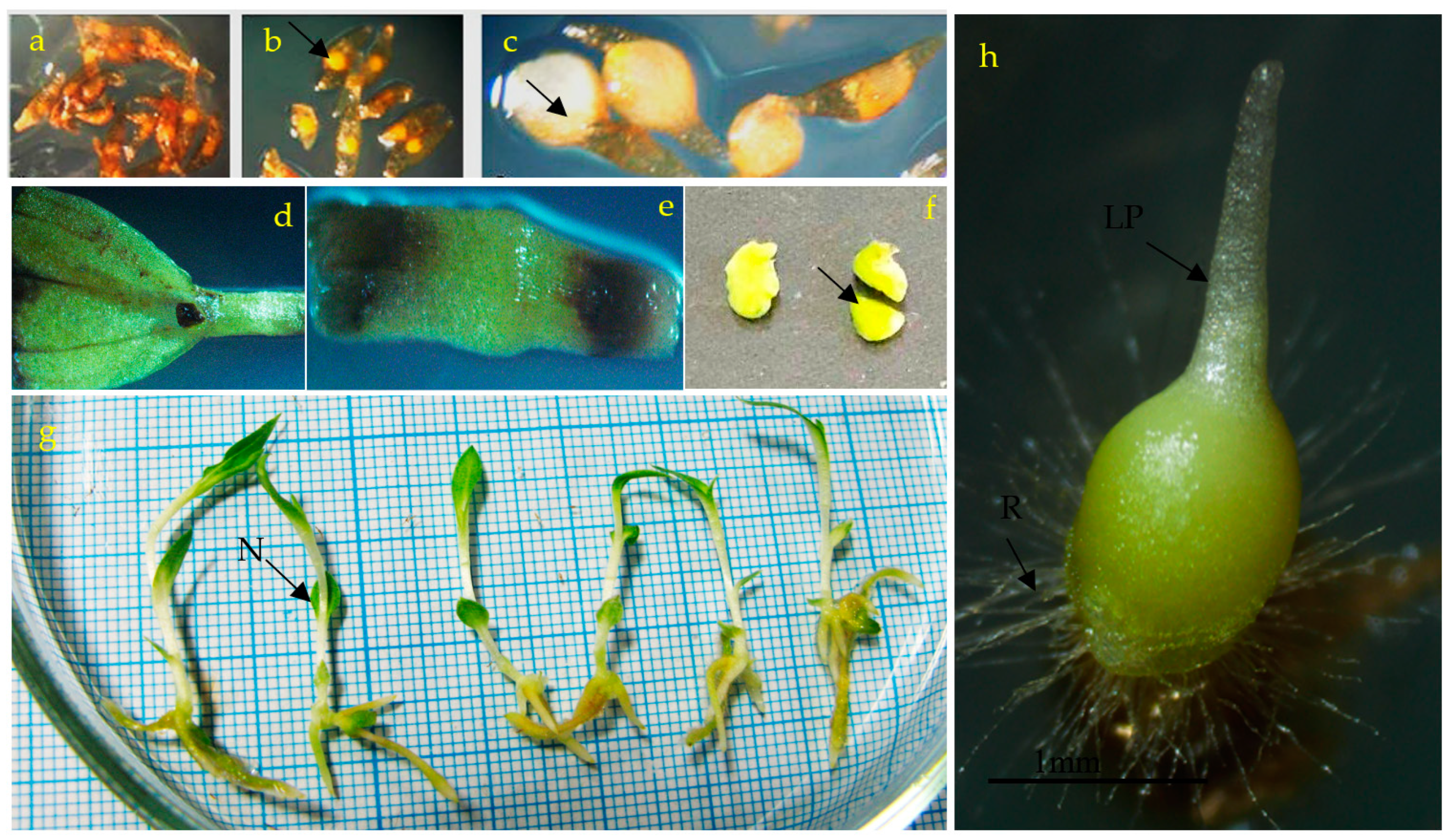
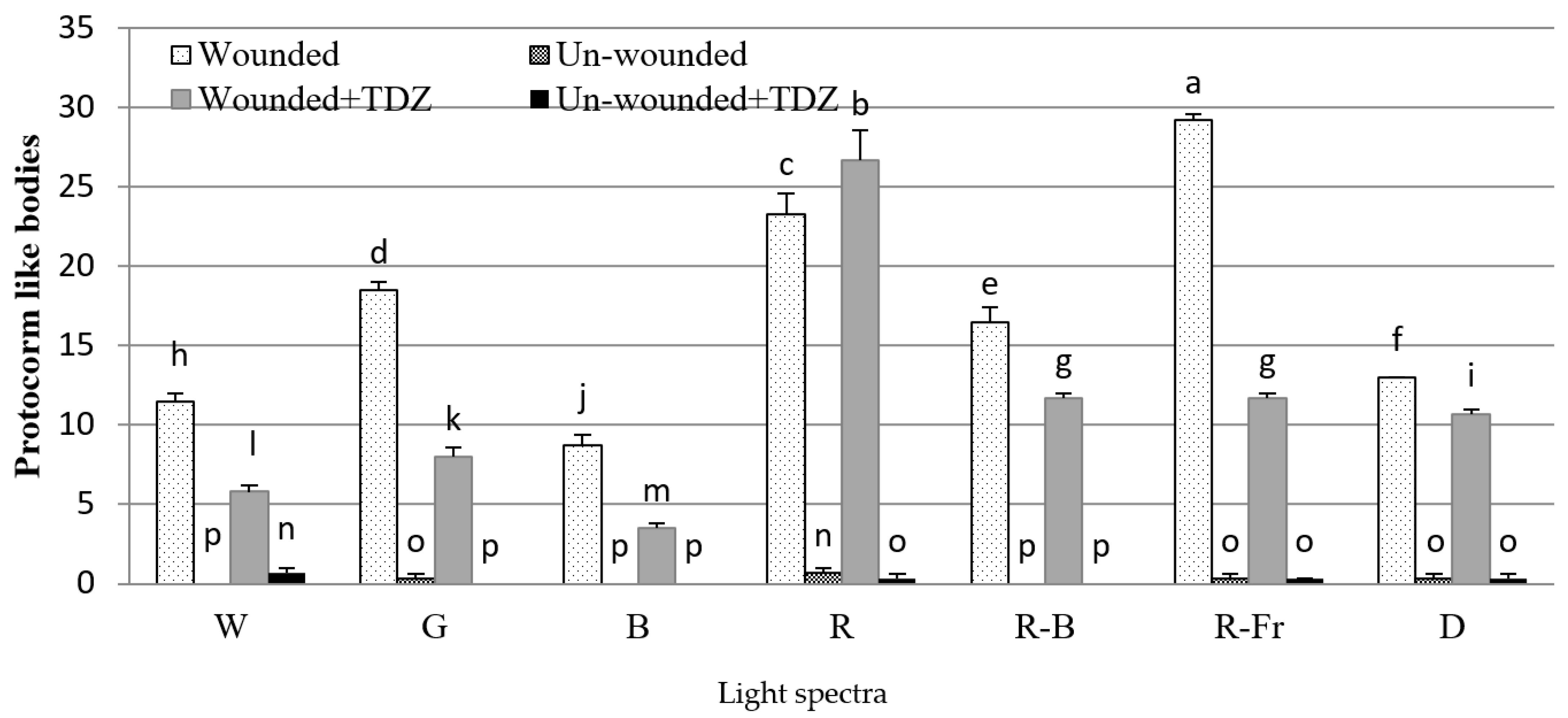
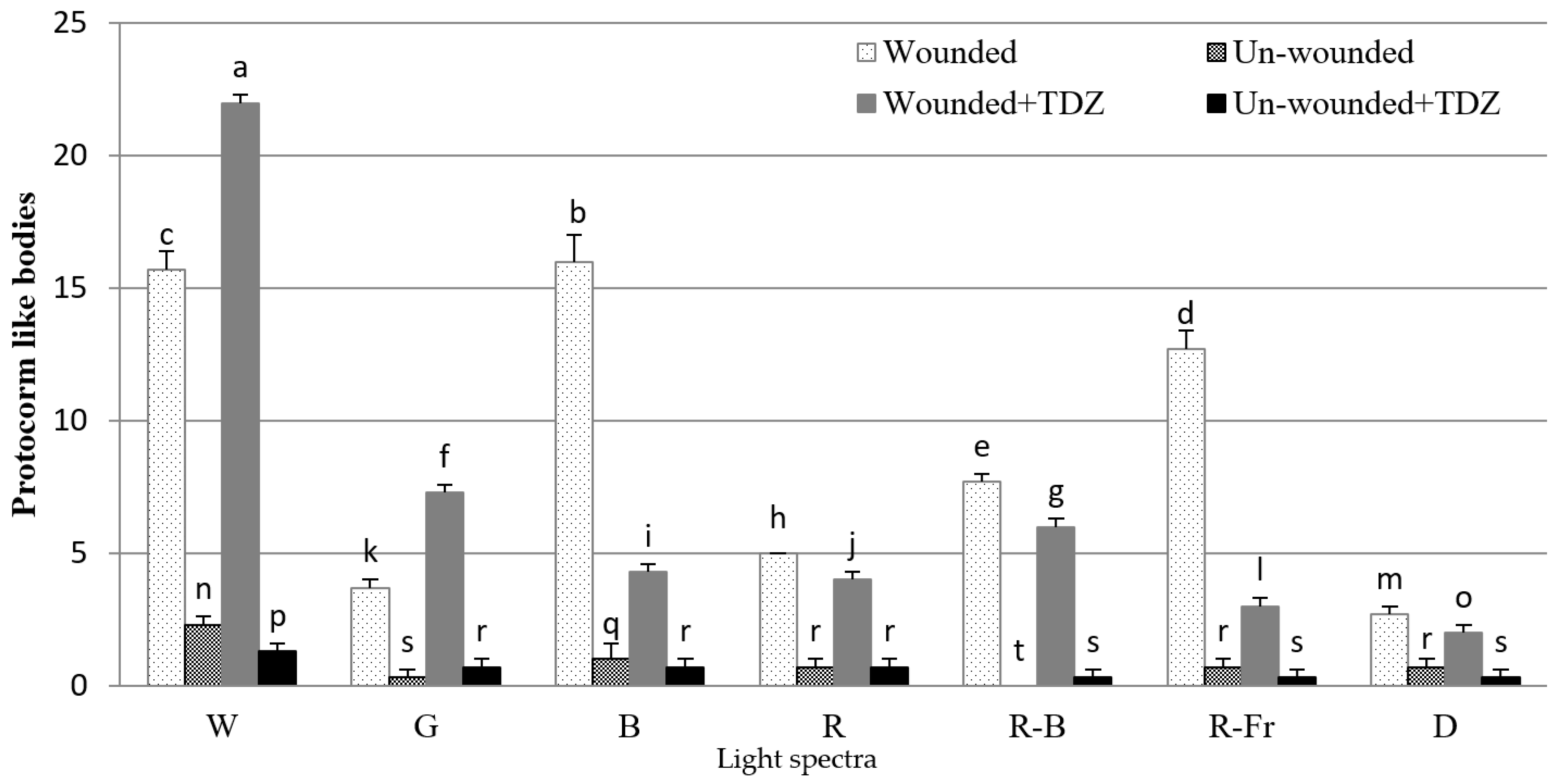
3.1. Effects of PGRs, Explant Type, and Wounding on DSE
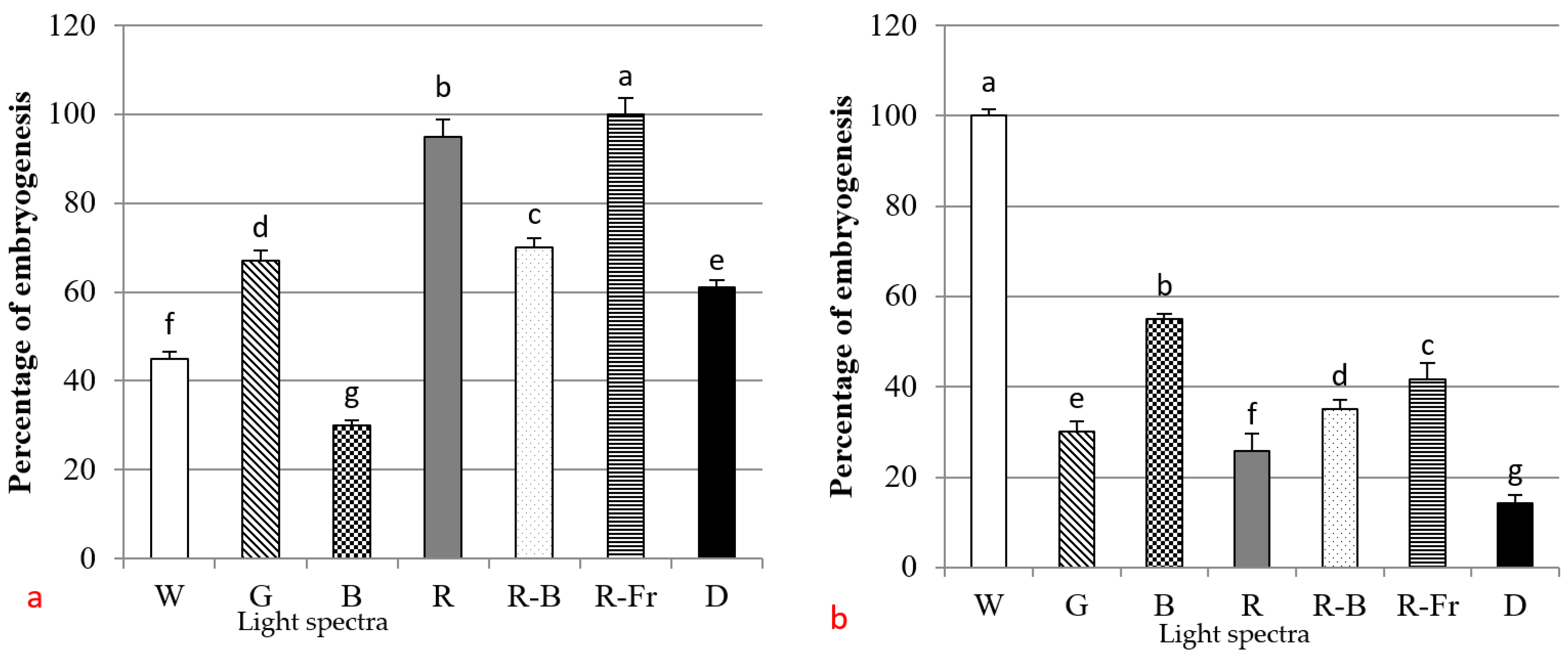
3.2. Induction of SE under Different Light Spectra
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gow, W.P.; Chen, J.T.; Chang, W.C. Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis orchids. Acta Physiol. Plant. 2008, 30, 500–507. [Google Scholar] [CrossRef]
- Ghorbani, A.; Barbara, G.; Farzaneh, N.; Hugo, B. Wild orchid tuber collection in Iran: A wake-up call for conservation. Biodivers. Conserv. 2014, 23, 2749–2760. [Google Scholar] [CrossRef]
- Starin, D. Salepi extinction, salepi survival: How a change in ingredients could help safeguard orchids. Am. Orch. Soc. Bull. 2012, 81, 490–494. [Google Scholar]
- Sezik, E. Destroying of Ophrys species to obtain salep in Turkey. J. Eur. Orch. 2006, 38, 290–295. [Google Scholar]
- Moradi, S.; Dianati Daylami, S.; Arab, M.; Vahdati, K. Direct somatic embryogenesis in Epipactis veratrifolia, a temperate terrestrial orchid. J. Hortic. Sci. Biotechnol. 2017, 92, 88–97. [Google Scholar] [CrossRef]
- Jalal, J.S. Endemic orchids of peninsular India: A review. J. Threat. Taxa 2012, 15, 3415–3425. [Google Scholar] [CrossRef]
- Sherif, N.; Ahamed, J.H.; Franklin, B.; Senthil, K.; Rao, M.V. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult. 2018, 132, 303–316. [Google Scholar] [CrossRef]
- Kumar, S.; Narender, S.; Manisha, M. Micropropagation of Simmondsia chinensis (Link) Schneider through enhanced axillary branching from nodal segments. J. Plant Biol. 2009, 36, 75–81. [Google Scholar]
- Dogan, Y.; Suleyman, B.; Gungor, A.; Hasan, H.M. the use of wild edible plants in western and central Anatolia (Turkey). Econ. Bot. 2004, 58, 684–690. [Google Scholar] [CrossRef]
- Stewart, S.L.; Kane, M.E. Asymbiotic seed germination and in vitro seedling development of Habenaria macroceratitis (Orchidaceae) a rare Florida terrestrial orchid. Plant Cell Tissue Organ Cult. 2006, 86, 147–158. [Google Scholar] [CrossRef]
- Pant, B.; Thapa, D. In vitro mass propagation of an epiphytic orchid, Dendrobium primulinum lindl through shoot tip culture. Afr. J. Biotechnol. 2012, 11, 9970–9974. [Google Scholar] [CrossRef]
- Tanaka, M.; Hasegawa, A.; Goi, M. Studies on the clonal propagation of monopodial orchids by tissue culture I. Formation of protocorm-like bodies from leaf tissues in Phalaenopsis and Vanda. J. Jap. Soc. Hort. Sci. 1975, 44, 47–58. [Google Scholar]
- Kosir, P.; Skof, S.; Luthar, Z. Direct shoot regeneration from nodes of Phalaenopsis orchids. Acta Agric. Slov. 2004, 83, 233–242. [Google Scholar]
- Mahendran, G.; Bai, V.N. Direct somatic embryogenesis and plant regeneration from seed derived protocorms of Cymbidium bicolor Lindl. Sci. Hortic. 2012, 135, 40–44. [Google Scholar] [CrossRef]
- Chen, J.T.; Chang, W.C. Effects of auxins and cytokinins on direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramsey’. J. Plant Growth Regul. 2001, 34, 229–232. [Google Scholar] [CrossRef]
- Mulgund, G.S.; Nataraja, K.; Malabadi, R.B.; Kumar, S.V. TDZ induced in vitro propagation of an epiphytic orchid Xenikophyton smeeanum. Plant Biol. 2011, 1, 235–241. [Google Scholar]
- Arditti, J. Micropropagation of Orchids; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Mengxi, L.; Zhigang, X.; Yang, Y.; Yijie, F. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Kozai, T.; Niu, G.; Van Nguyen, U. Photosynthetic characteristics of coffee (Coffea arabusta) plantlets in vitro in response to different CO2 concentrations and light intensities. Plant Cell Tissue Organ Cult. 1998, 55, 133–139. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H. Responses of strawberry plantlets cultured in vitro under superbright red and blue lightemitting diodes (LEDs). Plant Cell Tissue Organ Cult. 2003, 73, 43–52. [Google Scholar] [CrossRef]
- Cybularz-Urban, T.; Hanus-Fajerska, E.; Swiderski, A. Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Crac. Ser. Bot. 2007, 49, 113–118. [Google Scholar]
- Cavallaro, V.; Pellegrino, A.; Muleo, R.; Forgione, I. Light and plant growth regulators on in vitro proliferation. Plants 2022, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.L.; Murthy, H.N.; Paek, K.Y. Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’. Sci. Hortic. 2002, 94, 365–370. [Google Scholar] [CrossRef]
- Aalifar, M.; Arab, M.; Aliniaeifard, S.; Dianati, S.; Mehrjerdi, M.Z.; Limpens, E.; Serek, M. Embryogenesis efficiency and genetic stability of Dianthus caryophyllus embryos in response to different light spectra and plant growth regulators. Plant Cell Tissue Organ Cult. 2019, 139, 479–492. [Google Scholar] [CrossRef]
- Jao, R.C.; Chien-Chou, L.; Wei, F.; Sen-Fuh, C. Effects of red light on the growth of Zantedeschia plantlets in vitro and tuber formation using light-emitting diodes. HortScience 2005, 40, 436–438. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Biswal, A.K.; Pattanayak, G.K.; Pandey, S.S.; Leelavathi, S.; Reddy, V.S.; Govindjee; Tripathy, B.C. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in Tobacco. Plant Physiol. 2012, 159, 433–449. [Google Scholar] [CrossRef]
- Zhou, M.; Guan, Q.; Wei, Y.; Zhang, Z. Effects of Sucrose Concentration and light intensity on growth and photosynthesis of ginger plantlets in vitro. Chin. J. Appl. Environ. Biol. 2008, 14, 356–361. [Google Scholar]
- Torne, J.M.; Moysset, L.; Santos, M.; Simón, E. Effects of light quality on somatic embryogenesis in Araujia sericifera. Physiol. Plant. 2001, 111, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yeung, E.C.; Paek, K.Y. Endoreduplication in Phalaenopsis is affected by light quality from light-emitting diodes during somatic embryogenesis. Plant Biotechnol. Rep. 2010, 4, 303–309. [Google Scholar] [CrossRef]
- Le, V.T.; Tanaka, M. Effects of red and blue light-emitting diodes on callus induction, callus proliferation, and protocorm-like body formation from callus in Cymbidium orchid. Environ. Control. Biol. 2004, 42, 57–64. [Google Scholar]
- Chen, J.R.; Wu, L.; Hu, B.W.; Yi, X.; Liu, R.; Deng, Z.N.; Xiong, X.Y. The influence of plant growth regulators and light quality on somatic embryogenesis in China rose. J. Plant Growth Regul. 2014, 33, 295–304. [Google Scholar] [CrossRef]
- Rodríguez-Sahagún, A.; Acevedo-Hernández, G.; Rodríguez-Domínguez, J.M.; Rodríguez-Garay, B.; Cervantes-Martínez, J.; Castellanos-Hernández, O.A. Effect of light quality and culture medium on somatic embryogenesis of Agave tequilana Weber, Azul. Plant Cell Tissue Organ Cult. 2011, 104, 271–275. [Google Scholar] [CrossRef]
- Mose, W.; Indrianto, A.; Purwantoro, A.; Semiarti, E. The influence of thidiazuron on direct somatic embryo formation from various types of explant in Phalaenopsis amabilis (L.) blume orchid. Hayati J. Biosci. 2017, 24, 201–205. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Dam, A.; Karmakar, J.; Bandyopadhyay, T.K. Direct somatic embryogenesis and genetic homogeneity assessment of regenerated plants of Anthurium andraeanum Linden cv. Fantasia. Vitr. Cell. Dev. Biol. Plant 2016, 52, 512–519. [Google Scholar] [CrossRef]
- Bakhshaie, M.; Babalar, M.; Mirmasoumi, M.; Khalighi, A. Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss an endangered species. Plant Cell Tissue Organ Cult. 2010, 102, 229–235. [Google Scholar] [CrossRef]
- Prange, A.N.S.; Bartsch, M.; Serek, M.; Winkelmann, T. Regeneration of different Cyclamen species via somatic embryogenesis from callus, suspension cultures and protoplasts. Sci. Hortic. 2010, 125, 442–450. [Google Scholar] [CrossRef]
- Santarem, E.R.; Pelissier, B.; Finer, J.J. Effect of explant orientation, pH, solidifying agent and wounding on initiation of soybean somatic embryos. Vitr. Cell. Dev. Biol. Plant 1997, 33, 13–19. [Google Scholar] [CrossRef]
- Rojas-Herrera, R.; Loyola-Vargas, V. Induction of a class acidic chitinase in foliar explants of (Coffea arabica L.) during somatic embryogenesis and wounding. Plant Sci. 2002, 163, 705–711. [Google Scholar] [CrossRef]
- Dianati, S.; Kafi, M.; Mirmaoumi, M.; Mozaffarian, V.; Salami, A.R. Introduce the suitable medium for asymbiotic germination of Epipactis veratrifolia. J. Crops Improv. 2014, 15, 161–177. [Google Scholar] [CrossRef]
- Bustam, B.M.; Dixon, K.; Bunn, E. Proliferation and harvesting of secondary protocorms as a novel means for improving propagation of terrestrial orchids. Aust. J. Bot. 2015, 62, 614–621. [Google Scholar] [CrossRef]
- Gangaprasad, A.N.; Decruse, W.S.; Seeni, S.; Menon, S. Micropropagation and restoration of the endangered Malabar daffodil orchid Ipsea malabarica. Lindleyana 1999, 14, 38–46. [Google Scholar]
- Romero, C.; Cuba-Díaz, M.; Silva, R. In vitro culture of Chloraea gavilu Lindl., an endemic terrestrial orchid from Chile. Plant Biosyst. 2018, 152, 612–620. [Google Scholar] [CrossRef]
- Showalter, A.M.; Bell, J.N.; Cramer, C.L.; Bailey, J.A.; Varner, J.E.; Lamb, C.J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc. Natl. Acad. Sci. USA 1985, 82, 6551–6555. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Ashwath, N.; Midmore, D.J. Effects of genotype, explant orientation, and wounding on shoot regeneration in tomato. Vitr. Cell. Dev. Biol. Plant. 2005, 41, 457–464. [Google Scholar] [CrossRef]
- Chamandoosti, F. The relationship between plant growth regualtors for organogenesis and phenolic compound in cotton. Asian J. Dev. Biol. 2010, 2, 16–22. [Google Scholar]
- Vahdati, K.; Jariteh, M.; Niknam, V.; Mirmasoumi, M.; Ebrahimzadeh, H. Somatic embryogenesis and embryo maturation in Persian walnut. Acta Hortic. 2006, 705, 199–205. [Google Scholar] [CrossRef]
- Thomas, C.; Jiménez, V.M. Mode of action of plant hormones and plant growth regulators during induction of somatic embryogenesis: Molecular aspects. Somat. Embryog. 2005, 23, 157–175. [Google Scholar] [CrossRef]
- Singh, C.; Raj, S.R.; Jaiswal, P.; Patil, V.; Punwar, B.; Chavda, J.; Subhash, N. Effect of plant growth regulators on in vitro plant regeneration of sandalwood Santalum album L. via organogenesis. Agrofor. Syst. 2016, 90, 281–288. [Google Scholar] [CrossRef]
- Gow, W.P.; Jen-Tsung, C.; Wei-Chin, C. Enhancement of direct somatic embryogenesis and plantlet growth from leaf explants of Phalaenopsis by adjusting culture period and explant length. Acta Physiol. Plant. 2010, 32, 621–627. [Google Scholar] [CrossRef]
- Ponert, J.; Vosolsobě, S.; Kmecová, K.; Lipavská, H. European orchid cultivation–from seed to mature plant. Eur. J. Environ. Sci. 2011, 26, 5–15. [Google Scholar] [CrossRef]
- Chen, L.R.; Chen, J.T.; Chang, W.C. Efficient production of protocorm-like bodies and plant regeneration from flower stalk explants of the sympodial orchid Epidendrum radicans. Vitr. Cell. Dev. Biol. Plant. 2002, 38, 441–445. [Google Scholar] [CrossRef]
- Shibli, R.A.; Ajlouni, M. Somatic embryogenesis in the endemic black iris. Plant Cell Tissue Organ Cult. 2000, 61, 15–21. [Google Scholar] [CrossRef]
- Kuo, H.L.; Chen, J.T.; Chang, W.C. Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. Vitr. Cell. Dev. Biol. Plant. 2005, 41, 453–461. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Shahinul Islam, S.M. Assessment of antibacterial and antifungal activities of the extracts of Rhynchostylis retusa Blume-A Medicinal Orchid. WJPPS 2015, 2, 74–87. [Google Scholar]
- Wenck, A.R.; Conger, B.V.; Trigiano, R.N.; SAMS, C.E. Inhibition of somatic-embryogenesis in orchardgrass by endogenous cytokinins. Plant Physiol. 1988, 88, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Chen, J.T.; Chang, W.C. Plant regeneration through direct somatic embryogenesis from leaf explants of Dendrobium. Biol. Plant. 2007, 2, 346–350. [Google Scholar] [CrossRef]
- Kamal, M.M.; Shimasaki, K.; Akter, N. Effect of light emutting diode (LED) lamps and N-Acetylglucosamine (NAG) on organogenesis in protocorm-like bodies (PLBs) of a Cymbidium hybrid cultured in vitro. Plant Tissue Cult. Biotechnol. 2014, 24, 273–297. [Google Scholar] [CrossRef]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1921. [Google Scholar] [CrossRef]
- Lotfi, M.; Mars, M.; Werbrouck, S. Optimizing pear micropropagation and rooting with light emitting diodes and trans-cinnamic acid. Plant Growth Regul. 2019, 88, 173–180. [Google Scholar] [CrossRef]
- Michler, C.H.; Lineberger, R.D. Effects of light on somatic embryo development and abscisic levels in carrot suspension cultures. Plant Cell Tissue Organ Cult. 1987, 11, 189–207. [Google Scholar] [CrossRef]
- Golovatskaya, I.F.; Karnachuk, R.A. Role of green light in physiological activity of plants. Russ. J. Plant Physiol. 2015, 62, 727–740. [Google Scholar] [CrossRef]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action spectra for phytochrome A-and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.J.; Haque, S.M.; Kazuhiko, S. Application of chondroitin sulfate on organogenesis of two Cymbidium spp. under different sources of lights. Not. Sci. Biol. 2016, 8, 156–160. [Google Scholar] [CrossRef]
- Lavee, S.; Glenda, M. The effect of growth-regulating substances and light on olive callus growth in vitro. J. Exp. Bot. 1969, 20, 604–614. [Google Scholar] [CrossRef]
- Lazzeri, P.; David, F.H.; Glenn, B.C. Soybean somatic embryogenesis: Effects of hormones and culture manipulations. Plant Cell Tissue Organ Cult. 1987, 10, 197–208. [Google Scholar] [CrossRef]
- Gatica, A.; Andrés, M.; Griselda, A.; Ana, M.E. direct somatic embryogenesis in Coffea arabica L. Caturra and Catuaí: Effect of tricontanol, light condition, and medium consistency. Agron. Costarric. 2008, 5, 203–212. [Google Scholar]
- Schuerger, C.; Christopher, S.; Brown, C. Anatomical features of pepper plants Capsicum annuum grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Morini, S.; Bellocchi, G. Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tissue Organ Cult. 1998, 53, 91–98. [Google Scholar] [CrossRef]





| Plant | Concentration of PGRs (mg/L) | Explant Type | PLBs Formation (%) | PLBs Growth (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDZ | NAA | BA | Kin | Protocorm | Leaf | Node | Crown | |||
| tiEpipactis verafolia | 0 | 0 | 0 | 0 | × | - | - | - | 0 | 0 |
| 3 | 0 | 0 | 0 | × | - | - | - | 100 a | 100 a | |
| 0 | 0 | 1 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0 | 1 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 0 | 2 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0 | 2 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 0 | 3 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0 | 3 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 0.5 | 0 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0.5 | 0 | 0 | - | - | × | - | 25 b | 25 d | |
| 0 | 0.5 | 1 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0.5 | 1 | 0 | - | - | × | - | 25 b | 75 b | |
| 0 | 0.5 | 2 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0.5 | 2 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 0.5 | 3 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0.5 | 3 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 1 | 0 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 1 | 0 | 0 | - | - | × | - | 38 a | 20 d | |
| 0 | 1 | 1 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 1 | 1 | 0 | - | - | × | - | 25 b | 50 c | |
| 0 | 1 | 2 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 1 | 2 | 0 | - | - | × | - | 18 c | 75 b | |
| 0 | 1 | 3 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 1 | 3 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 0 | 1 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0 | 1 | 0 | - | - | × | - | 0 | 0 | |
| 0 | 0 | 2 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0 | 2 | 0 | - | - | × | - | 25 b | 50 c | |
| 0 | 0 | 3 | 0 | - | × | - | - | 0 | 0 | |
| 0 | 0 | 3 | 0 | - | - | × | - | 13 c | 100 a | |
| Dactylorhiza umberosa | 0 | 0 | 0 | 0 | × | - | - | - | 0 | 0 |
| 3 | 0 | 0 | 0 | × | - | - | - | 100 a | 90 b | |
| 0 | 0 | 0 | 1 | × | - | - | - | 0 | 0 | |
| 0 | 0 | 0 | 1.5 | × | - | - | - | 25 c | 75 b | |
| 0 | 0 | 0 | 2 | × | - | - | - | 50 b | 100 a | |
| 2 | 0 | 2 | 0 | - | - | - | × | 0 | 0 | |
| 1 | 0.5 | 0 | 0 | - | - | - | × | 0 | 0 | |
| 0 | 0.5 | 2 | 0 | - | - | - | × | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naderi Boldaji, H.; Dianati Daylami, S.; Vahdati, K. Use of Light Spectra for Efficient Production of PLBs in Temperate Terrestrial Orchids. Horticulturae 2023, 9, 1007. https://doi.org/10.3390/horticulturae9091007
Naderi Boldaji H, Dianati Daylami S, Vahdati K. Use of Light Spectra for Efficient Production of PLBs in Temperate Terrestrial Orchids. Horticulturae. 2023; 9(9):1007. https://doi.org/10.3390/horticulturae9091007
Chicago/Turabian StyleNaderi Boldaji, Hossein, Shirin Dianati Daylami, and Kourosh Vahdati. 2023. "Use of Light Spectra for Efficient Production of PLBs in Temperate Terrestrial Orchids" Horticulturae 9, no. 9: 1007. https://doi.org/10.3390/horticulturae9091007
APA StyleNaderi Boldaji, H., Dianati Daylami, S., & Vahdati, K. (2023). Use of Light Spectra for Efficient Production of PLBs in Temperate Terrestrial Orchids. Horticulturae, 9(9), 1007. https://doi.org/10.3390/horticulturae9091007








