Morphology, Anatomy, Micromorphology, and Palynology of the Squirrel’s Foot Fern, Davallia mariesii (Davalliaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Materials
2.2. Morphological Analyses
2.3. Anatomical Analyses
2.4. Micromorphological and Palynological Analyses
3. Results
3.1. Morphological Characteristics
3.2. Anatomical Characteristics
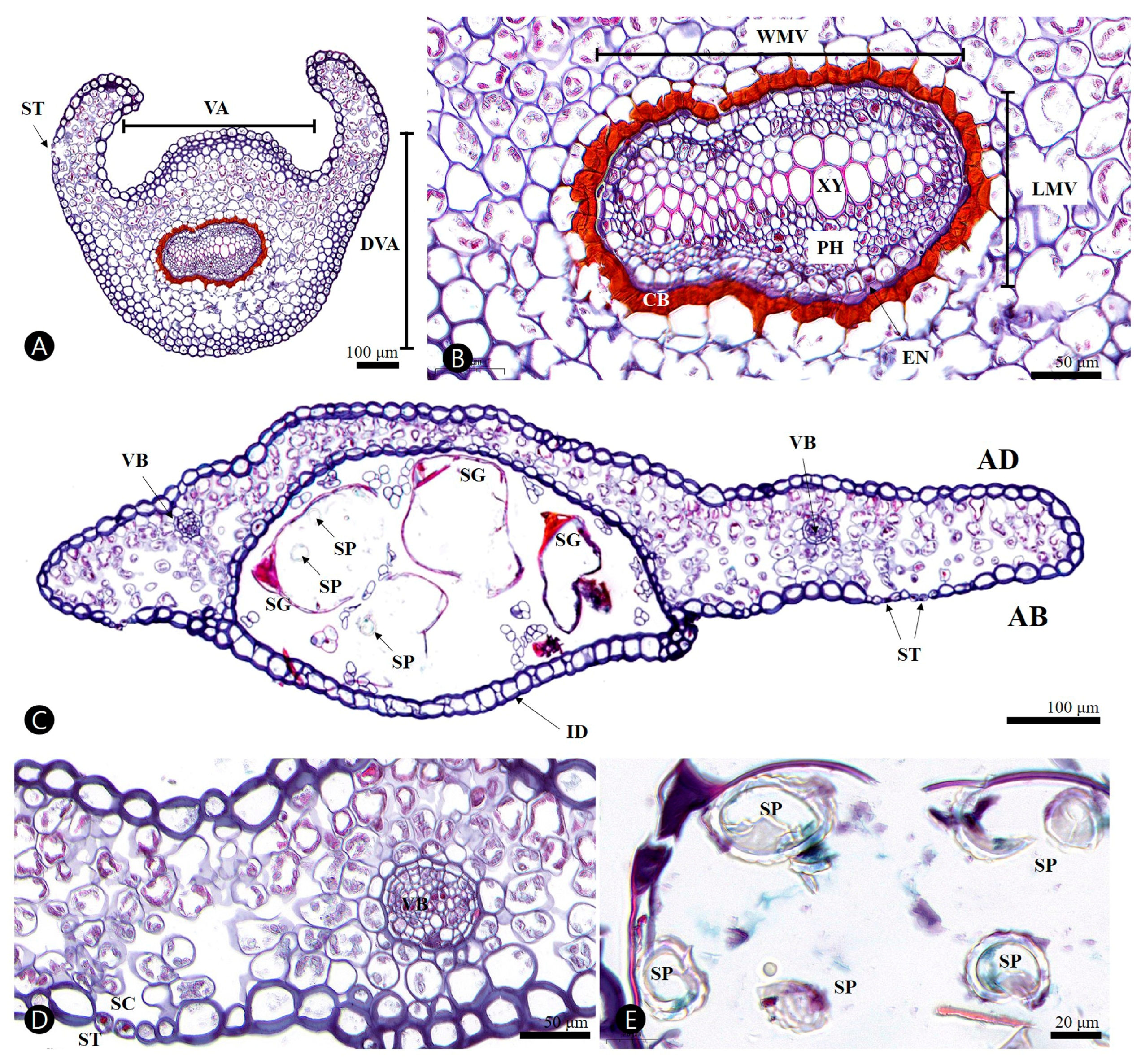
3.2.1. Stipe
3.2.2. Ultimate Segment (of Pinnule)
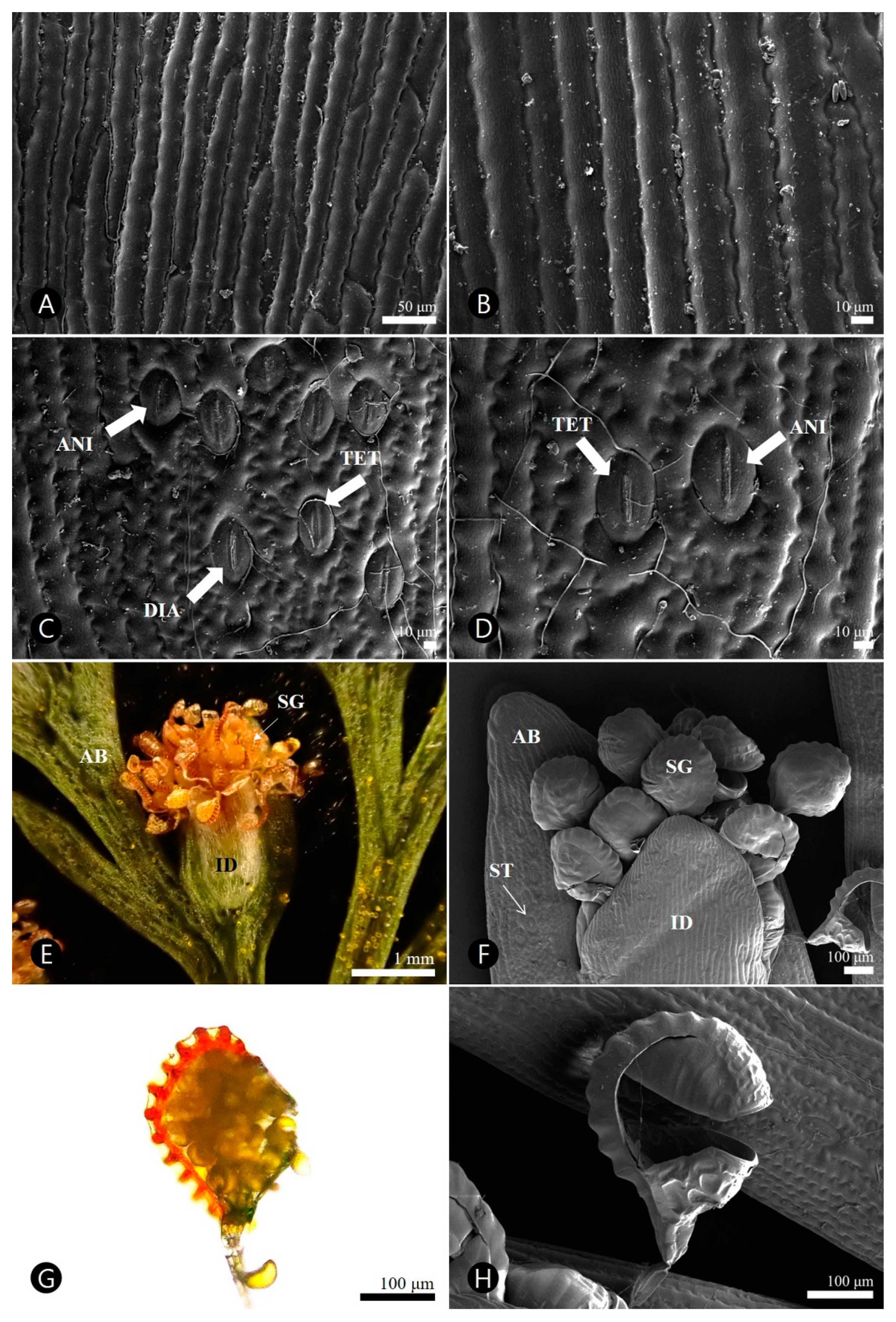
3.3. Micromorphological Characteristics
3.3.1. Pinnule
3.3.2. Sorus
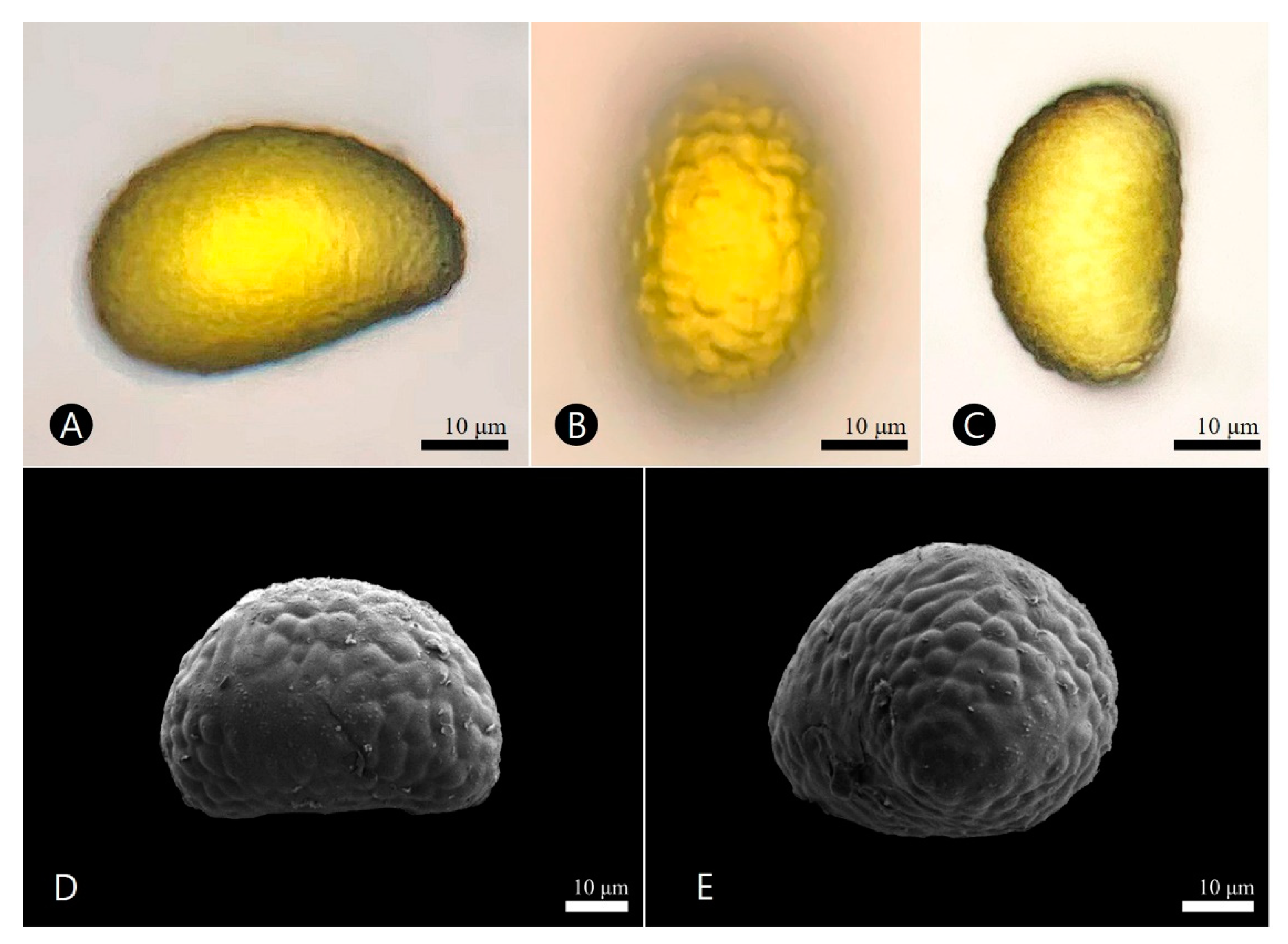
3.4. Palynological Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kramer, K.U. Davalliaceae. In The Families and Genera of Vascular Plants: Pteridophytes and Gymnosperms; Kubitzki, K., Ed.; Springer: Heidelberg/Berlin, Germany, 1990; Volume 1, pp. 74–80. [Google Scholar]
- Nooteboom, H.P. Davalliaceae of China. Acta Phytotax. Sin. 1996, 34, 162–179. [Google Scholar]
- Wu, S.H. Davalliaceae. In Flora Reipublicae Popularis Sinicae; Wu, S.H., Wang, C.H., Eds.; Science Press: Beijing, China, 1999; Volume 6, pp. 161–199. [Google Scholar]
- Von Konrat, M.J.; Braggins, J.E.; de Lange, P.J. Davallia (Pteridophyta) in New Zealand, including description of a new subspecies of D. tasmanii. N. Z. J. Bot. 1999, 37, 579–593. [Google Scholar] [CrossRef]
- Xing, F.W.; Wang, F.G.; Nooteboom, H.P. Davalliaceae. In Flora of China (Pteridophytes); Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, UK, 2013; pp. 749–757. [Google Scholar]
- Tsutsumi, C.; Chen, C.W.; Larsson, A.; Hirayama, Y.; Kato, M. Phylogeny and classification of Davalliaceae on the basis of chloroplast and nuclear markers. Taxon 2016, 65, 1236–1248. [Google Scholar] [CrossRef]
- Sun, B.Y.; Davalliaceae, M.R. Schomb. In Flora of Korea: Pteridophytes and Gymnosperms; Flora of Korea Editorial Committee, Ed.; The National Institute of Biological Resources: Incheon, Republic of Korea, 2015; Volume I, p. 148. [Google Scholar]
- Ma, X.D.; Wang, A.H.; Wang, F.G.; He, C.M.; Liu, D.M.; Gerstberger, P.; Xing, F.W. A revised classification of Chinese Davalliaceae based on new evidence from molecular phylogenetics and morphological characteristics. PLoS ONE 2018, 13, e0206345. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.J.; Wang, N.N.; Zhang, L.G.; Guo, Y.Z.; Shi, W.Z. Evaluation of the effects of active fractions of Chinese medicine formulas on IL-1beta, IL-6, and TNF-alpha release from ANA-1 murine macrophages. J. Ethnopharmacol. 2016, 179, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Peng, S.W.; Imtiyaz, Z.; Ho, C.W.; Chiou, W.F.; Lee, M.H. In vivo and in vitro evaluation of the osteogenic potential of Davallia mariesii T. Moore ex Baker. J. Ethnopharmacol. 2021, 264, 113126. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii Moore. I. Isolation and structures of davallialactone and a new flavanone glucuronide. Chem. Pharm. Bull. 1990, 38, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Tezuka, Y.; Yamashita, H.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii Moore. V. Isolation and structures of davallin, a new tetrameric proanthocyanidin, and two new phenolic glycosides. Chem. Pharm. Bull. 1993, 41, 1491–1497. [Google Scholar] [CrossRef][Green Version]
- Gunasinghe, Y.H.K.I.S.; Rathnayake, I.V.N.; Deeyamulla, M.P. Plant and plant associated microflora: Potential bioremediation option of indoor air pollutants. Nepal J. Biotechnol. 2021, 9, 63–74. [Google Scholar] [CrossRef]
- White, R.A. Comparative anatomical studies of the ferns. Ann. Mo. Bot. Gard. 1974, 61, 379–387. [Google Scholar] [CrossRef]
- Lin, B.L.; DeVol, C.D. The use of stipe characters in fern taxonomy I. Taiwania 1977, 22, 91–99. [Google Scholar]
- Lin, B.L.; DeVol, C.D. The use of stipe characters in fern taxonomy II. Taiwania 1978, 23, 77–95. [Google Scholar]
- Lee, C.-S. A taxonomical study of Korean Pteridaceae on the morphology of leaf epidermis. Korean J. Plant Taxon. 1988, 18, 275–290. [Google Scholar] [CrossRef]
- Sen, U.; De, B. Structure and ontogeny of stomata in ferns. Blumea 1992, 37, 239–261. [Google Scholar]
- Bondada, B.; Tu, C.; Ma, L. Surface structure and anatomical aspects of Chinese brake fern (Pteris vittata; Pteridaceae). Brittonia 2006, 58, 217–228. [Google Scholar] [CrossRef]
- Chuang, Y.-Y.; Liu, H.-Y. Leaf epidermal morphology and its systematic implications in Taiwan Pteridaceae. Taiwania 2003, 48, 60–71. [Google Scholar]
- Shah, S.N.; Ahmad, M.; Zafar, M.; Ullah, F.; Zaman, W.; Mazumdar, J.; Khuram, I.; Khan, S.M. Leaf micromorphological adaptations of resurrection ferns in northern Pakistan. Flora 2019, 255, 1–10. [Google Scholar] [CrossRef]
- Giacosa, J.R.; Morbelli, M.; Giudice, G. Spore morphology and wall ultrastructure of Anemia Swartz species (Anemiaceae) from Argentina. Rev. Palaeobot. Palynol. 2012, 174, 27–38. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, C.W. Spore morphology of the genus Dryopteris Adans. (Dryopteridaceae) in Korea. J. Plant Biol. 2014, 57, 302–311. [Google Scholar] [CrossRef]
- Giacosa, J.R. Spore morphology and wall ultrastructure of Lomariocycas (Blechnaceae) species from America. Rev. Palaeobot. Palynol. 2019, 269, 55–63. [Google Scholar] [CrossRef]
- Yañez, A.; Marquez, G.J.; Morbelli, M.A. Spore morphology and ultrastructure of Dennstaedtiaceae from Paranaense Phytogeographic Province I.: Genus Dennstaedtia. Rev. Palaeobot. Palynol. 2016, 224, 181–194. [Google Scholar] [CrossRef]
- Shah, S.N.; Ahmad, M.; Zafar, M.; Hadi, F.; Khan, M.N.; Noor, A.; Malik, K.; Rashid, N.; Iqbal, M. Spore morphology and leaf epidermal anatomy as a taxonomic source in the identification of Asplenium species from Malakand division Pakistan. Microsc. Res. Tech. 2020, 83, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.A.; White, R.A. Frond articulation in species of Polypodiaceae and Davalliaceae. Am. Fern J. 1967, 57, 78–88. [Google Scholar] [CrossRef]
- Ma, X.; He, C.; Wang, F.; Wang, A.; Xing, F. Structural characteristics of leaf epidermis and their systematic significance in Davalliaceae. Plant Sci. J. 2015, 33, 438–447. [Google Scholar]
- Tsutsumi, C.; Kato, M. Morphology and evolution of epiphytic Davalliaceae scales. Botany 2008, 86, 1393–1403. [Google Scholar] [CrossRef]
- Wang, F.G.; Liu, H.M.; He, C.M.; Yang, D.M.; Xing, F.W. Taxonomic and evolutionary implications of spore ornamentation in Davalliaceae. J. Syst. Evol. 2015, 53, 72–81. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company, Inc.: London, UK, 1940; 530p. [Google Scholar]
- Wilkinson, H.P. The plant surface (mainly leaf). In Anatomy of the Dicotyledons, 2nd ed.; Metcalfe, C.R., Chalk, L., Eds.; Clarendon Press: Oxford, UK, 1979; Volume 1, pp. 97–165. [Google Scholar]
- Sen, T.; Sen, U.; Holttum, R.E. Morphology and anatomy of the genera Davallia, Araiostegia and Davallodes, with a discussion on their affinities. Kew Bull. 1972, 27, 217–243. [Google Scholar] [CrossRef]
- Hernández-Hernández, V.; Terrazas, T.; Mehltreter, K.; Angeles, G. Studies of petiolar anatomy in ferns: Structural diversity and systematic significance of the circumendodermal band. Bot. J. Linn. Soc. 2012, 169, 596–610. [Google Scholar] [CrossRef]
- Nooteboom, H.P. Notes on Davalliaceae II. A revision of the genus Davallia. Blumea 1994, 39, 151–214. [Google Scholar]
- Liu, H.; Schneider, H. Evidence supporting Davallia canariensis as a Late Miocene relict endemic to Macaronesia and Atlantic Europe. Aust. Syst. Bot. 2013, 26, 378–385. [Google Scholar] [CrossRef]
- Noraini, T.; Ruzi, A.R.; Nadiah, N.; Nisa, R.N.; Maideen, H.; Solihani, S.N. Stipe anatomical characteristics in some Davallia (Davalliaceae) species in Malaysia. Sains Malays. 2012, 41, 53–62. [Google Scholar]
- Tan, J.M.P.; Banaticla-Hilario, M.C.; Malabrigo, P.; Angeles, M.D.; Buot, I.E. Anatomical examination of the petiole of eupolypods I (Polypodiales). Biodiversitas J. Biol. Divers. 2020, 21, 1767–1777. [Google Scholar]
- Russow, E. Vergleichende Untersuchungen betreffend der Histologie (Histographie und Histogenie) der Vegetativen und Sporenbildenden Organe und die Entwicklung der Sporen der Leitbündel-Kryptogamen, mit Berücksichtigung der Histologie der Phanerogamen Ausgehend von der Betrachtung der Marsiliaceae; Mémoires de l’Académie Impériale des Sciences de St. Petersbourg, sér. 7; Académie Impériale des Sciences: Saint Petersburg, Russia, 1872; Volume 19, 207p. [Google Scholar]
- Ogura, Y. Comparative Anatomy of the Vegetative organs of the Pteridophytes; Borntraeger: Berlin, Germany, 1972. [Google Scholar]
- Moran, R.C. Monograph of the neotropical fern genus Polybotrya (Dryopteridaceae). Ill. Nat. Hist. Surv. Bull. 1987, 34, 1–138. [Google Scholar] [CrossRef]
- Hernández-Hernández, V.; Terrazas, T.; Stevenson, D.W.M. Ontogeny of Ctenitis melanosticta and Diplazium expansum fronds with emphasis on the circumendodermal band. Feddes Repert. 2009, 120, 426–442. [Google Scholar] [CrossRef]
- Van Fleet, D.S. Histochemistry and function of the endodermis. Bot. Rev. 1961, 27, 165–220. [Google Scholar] [CrossRef]
- Niklas, K.J. Research review: A mechanical perspectiveon foliage leaf form and function. New Phytol. 1999, 143, 19–31. [Google Scholar] [CrossRef]
- Shah, S.N.; Celik, A.; Ahmad, M.; Ullah, F.; Zaman, W.; Zafar, M.; Malik, K.; Rashid, N.; Iqbal, M.; Sohail, A.; et al. Leaf epidermal micromorphology and its implications in systematics of certain taxa of the fern family Pteridaceae from northern Pakistan. Microsc. Res. Tech. 2019, 82, 317–332. [Google Scholar] [CrossRef]
- Sen, T. The evidence of stomatal development on the relationships between Davallia and genera associated with it by recent authors. Ann. Bot. 1986, 58, 663–677. [Google Scholar] [CrossRef]
- Huang, T.-C. Spore Flora of Taiwan; Meitai Color Print Co., Ltd.: Taipei, Taiwan, 1981. [Google Scholar]
- Moy, C.J. Variations of fern spore ultrastructure as reflections of their evolution. Grana 1988, 27, 39–51. [Google Scholar] [CrossRef]
- Tryon, A.F.; Lugardon, B. Spores of the Pteridophyta; Springer: New York, NY, USA, 1991. [Google Scholar]
- Sofiyanti, N.; Isda, M.N.; Juliantari, E.; Suriatno, R.; Pranata, S. The inventory and spore morphology of ferns from Bengkalis Island, Riau Province, Indonesia. Biodivers. J. Biol. Divers. 2019, 20, 3223–3236. [Google Scholar] [CrossRef]
- Conran, J.G.; Kaulfuss, U.; Bannister, J.M.; Mildenhall, D.C.; Lee, D.E. Davallia (Polypodiales: Davalliaceae) macrofossils from early Miocene Otago (New Zealand) with in situ spores. Rev. Palaeobot. Palynol. 2010, 162, 84–94. [Google Scholar] [CrossRef]

| Our Study | Flora of Korea | |
|---|---|---|
| Plants | 9.4–34.0 cm tall | - |
| Rhizome | 6.1–58 cm × 3.2–9.8 mm | 3–5 mm in diam. |
| Scale | 1.6–9.7 × 0.1–0.8 mm | 5–8 mm long |
| Frond | 8.1–38.5 cm long | 15–35 cm long |
| Stipe | 3.6–15.7 cm × 0.3–2.1 mm | 5–15 cm long |
| Blade | 4.5–22.8 × 5.2–26.3 cm | 10–20 × 8–15 cm |
| Pinnae | 5–17 pairs | 6–12 pairs |
| Primary upper and middle pinna | 1.0–5.2 × 0.5–2.4 cm | - |
| Primary basal pinna | 2.5–13.0 × 1.5–7.5 cm | - |
| Ultimate segments | 1.5–6.0 × 0.5–3.0 mm | 1–2 mm wide |
| Indusium | 0.9–2.0 × 0.4–1.0 mm | - |
| Davallia mariesii | |
|---|---|
| Stipe | |
| Outline of stipe | circular with wing |
| Length of ventral axis (VA) (μm) | 468.5–(559.2)–718.0 |
| Length of dorsiventral axis (DVA) (μm) | 501.2–(585.6)–627.1 |
| Epidermal thickness-abaxial surface (μm) | 7.5–(10.5)–18.0 |
| Epidermal thickness-adaxial surface (μm) | 11.6–(15.2)–21.3 |
| Arrangement of cortex | isometric |
| Outline of stipe vascular bundle | open arc |
| Width of main vascular bundle (WMV) (μm) | 171.5–(213.5)–274.6 |
| Length of main vascular bundle (LMV) (μm) | 114.1–(133.5)–156.3 |
| Circumendodermal band type | continuous ring |
| Circumendodermal band proportion | 1/4 to 1/2 |
| Circumendodermal band thickness (μm) | 6.9–(9.8)–11.7 |
| Endodermis thickness (μm) | 2.0–(3.7)–5.4 |
| Blade (of ultimate segment) | |
| Epidermal thickness-abaxial surface (μm) | 7.1–(13.1)–19.7 |
| Epidermal thickness-adaxial surface (μm) | 11.8–(15.1)–23.0 |
| Indusium epidermal thickness (μm) | 15.0–(21.3)–25.1 |
| Mesophyll thickness (non-indusium area) (μm) | 61.2–(99.4)–149.6 |
| Diameter of vascular bundle (μm) | 25.2–(44.3)–70.6 |
| Adaxial Side | Abaxial Side | |
|---|---|---|
| Epidermal cell arrangement | Elongated | elongated |
| Anticlinal cell wall | straight, slightly undulate | undulate to sinuate |
| Periclinal cell wall | striate, convex | striate, convex |
| Stomata type | Absent | diacytic, anisocytic, tetracytic |
| Stomata length (μm) | Absent | 35.6–(46.3)–51.4 |
| Stomata width (μm) | Absent | 25.9–(31.4)–36.6 |
| Stomata area (μm2) | Absent | 912.5–(1178.5)–1410.9 |
| Stomatal ledge | Absent | lip-shaped |
| Davallia mariesii | |
|---|---|
| Polar axes (μm) | 34.3–(38.8)–42.2 |
| Equatorial axes (μm) | 39.3–(50.8)–55.0 |
| Shape | ellipsoidal |
| Exine thickness (μm) | 2.3–(3.4)–5.1 |
| Aperture | monolete |
| Exine ornamentation | verrucate colliculate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Choi, G.; Song, J.-H. Morphology, Anatomy, Micromorphology, and Palynology of the Squirrel’s Foot Fern, Davallia mariesii (Davalliaceae). Horticulturae 2023, 9, 939. https://doi.org/10.3390/horticulturae9080939
Yang S, Choi G, Song J-H. Morphology, Anatomy, Micromorphology, and Palynology of the Squirrel’s Foot Fern, Davallia mariesii (Davalliaceae). Horticulturae. 2023; 9(8):939. https://doi.org/10.3390/horticulturae9080939
Chicago/Turabian StyleYang, Sungyu, Goya Choi, and Jun-Ho Song. 2023. "Morphology, Anatomy, Micromorphology, and Palynology of the Squirrel’s Foot Fern, Davallia mariesii (Davalliaceae)" Horticulturae 9, no. 8: 939. https://doi.org/10.3390/horticulturae9080939
APA StyleYang, S., Choi, G., & Song, J.-H. (2023). Morphology, Anatomy, Micromorphology, and Palynology of the Squirrel’s Foot Fern, Davallia mariesii (Davalliaceae). Horticulturae, 9(8), 939. https://doi.org/10.3390/horticulturae9080939







