Metabolomics Reveals Specific Metabolic Changes in Sweet Cherries (Prunus avium L.) Subjected to Postharvest Treatment with Melatonin after Mechanical Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Treatment with Melatonin
2.3. Induction of Pitting Damage
2.4. Targeted and Untargeted Metabolomic Analyses
2.5. Statistical Analysis
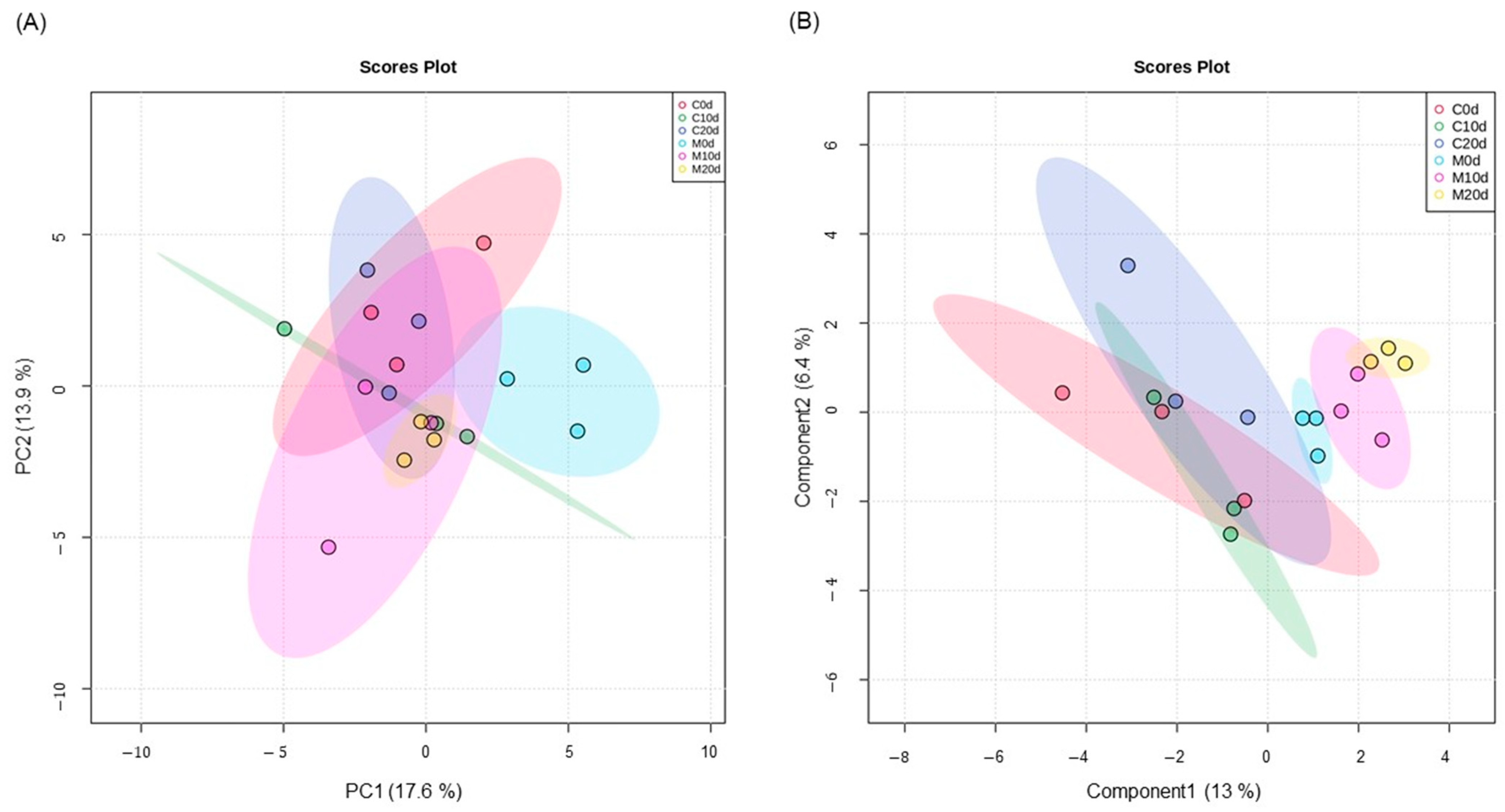
3. Results
3.1. Severity of Pitting Damage
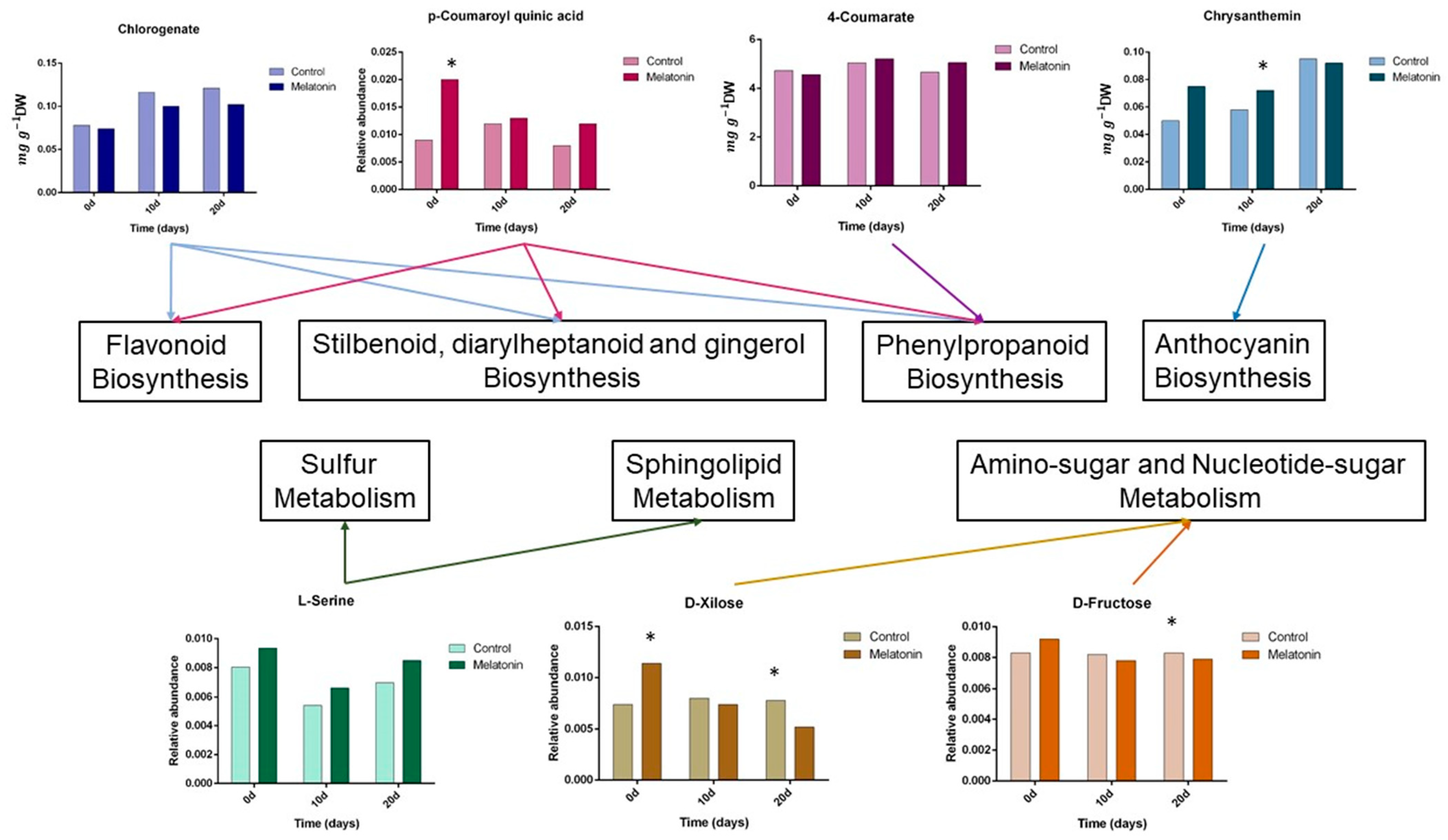
3.2. Metabolic Changes after Melatonin Treatment and Pitting Damage
4. Discussion
4.1. Severity of Pitting Damage
4.2. Metabolic Changes after Melatonin Treatment and Pitting Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Zhi, H.; Wang, Y. Cooperative effects of pre-harvest calcium and gibberellic acid on tissue calcium content, quality attributes, and in relation to postharvest disorders of late-maturing sweet cherry. Sci. Hortic. 2019, 246, 123–128. [Google Scholar] [CrossRef]
- Valenzuela, L. Copefrut.com. Available online: https://www.copefrut.com/wp-content/themes/copefrut/img/revistas/2020_N1.pdf (accessed on 18 July 2022).
- Ropelewska, E.; Popińska, W.; Sabanci, K.; Aslan, M.F. Cultivar identification of sweet cherries based on texture parameters determined using image analysis. J. Food Process Eng. 2021, 44, 13724. [Google Scholar] [CrossRef]
- Ropelewska, E.; Sabanci, K.; Aslan, M.F. Discriminative Power of Geometric Parameters of Different Cultivars of Sour Cherry Pits Determined Using Machine Learning. Agriculture 2021, 11, 1212. [Google Scholar] [CrossRef]
- Sabanci, K.; Aslan, M.F.; Ropelewska, E. Benchmarking analysis of CNN models for pits of sour cherry cultivars. Eur. Food Res. Technol. 2022, 248, 2441–2449. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Kappel, F.; Toivonen, P.; Stan, S.; Mckenzie, D. Resistance of sweet cherry cultivars to fruit surface pitting. Can. J. Plant Sci. 2006, 86, 1197–1202. [Google Scholar] [CrossRef]
- Fuentealba, C.; Ejsmentewicz, T.; Campos-Vargas, R.; Saa, S.; Aliaga, O.; Chirinos, R.; Campos, D.; Pedreschi, R. Cell wall and metabolite composition of sweet cherry fruits from two cultivars with contrasting susceptibility to surface pitting during storage. Food Chem. 2021, 342, 128307. [Google Scholar] [CrossRef]
- Ponce, E.; Alzola, B.; Cáceres, N.; Gas, M.; Ferreira, C.; Vidal, J.; Chirinos, R.; Campos, D.; Rubilar, M.; Campos-Vargas, R.; et al. Biochemical and phenotypic characterization of sweet cherry (Prunus avium L.) cultivars with induced surface pitting. Postharvest Biol. Technol. 2021, 175, 111494. [Google Scholar] [CrossRef]
- Zhi, H.; Dong, Y. Effect of hydrogen sulfide on surface pitting and related cell wall metabolism in sweet cherry during cold storage. J. Appl. Bot. Food Qual. 2018, 91, 109–113. [Google Scholar]
- Michailidis, M.; Karagiannis, E.; Polychroniadou, C.; Tanou, G.; Karamanoli, K.; Molassiotis, A. Metabolic features underlying the response of sweet cherry fruit to postharvest UV-C irradiation. Plant Physiol. Biochem. 2019, 144, 49–57. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Karamanoli, K.; Lazaridou, A.; Minas, I.S.; Molassiotis, A. Postharvest responses of sweet cherry fruit and stem tissues revealed by metabolomic profiling. Plant Physiol. Biochem. 2018, 127, 478–484. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in energy metabolism accompanying pitting in blueberries stored at low temperature. Food Chem. 2014, 164, 493–501. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; García, K.; Méndez, M.A.; González, M.; Meisel, L.A.; Defilippi, B.G.; Del Pozo, T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin accumulation in sweet cherry and its influence on fruit quality and antioxidant properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Torçuk, A.Z.; Özer, C. Influence of melatonin treatments on fruit quality and storage life of sweet cherry cv. ‘Sweetheart’. Erwerbs-Obstbau 2022, 64, 127–133. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of melatonin treatment on sweet cherry tree yield and fruit quality. Agronomy 2021, 12, 3. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.; Casa, R.; Reiter, R.J.; Xu, Y.; Lin, X.; Luo, Z.; Li, L. Melatonin confers enhanced polyamine metabolism and cell tolerance in Vitis vinifera against oxidative damage: Quantitative proteomic evidence. Postharvest Biol. Technol. 2022, 184, 111756. [Google Scholar] [CrossRef]
- Fuentealba, C.; Hernández, I.; Saa, S.; Toledo, L.; Burdiles, P.; Chirinos, R.; Campos, D.; Brown, P.; Pedreschi, R. Colour and in vitro quality attributes of walnuts from different growing conditions correlate with key precursors of primary and secondary metabolism. Food Chem. 2017, 232, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Investigations on sweet cherry phenolic degradation during thermal treatment based on fluorescence spectroscopy and inactivation kinetics. Food Bioproc. Technol. 2016, 9, 1706–1715. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef]
- Cao, L.; Jin, X.; Zhang, Y.; Zhang, M.; Wang, Y. Transcriptomic and metabolomic profiling of melatonin treated soybean (Glycine max L.) under drought stress during grain filling period through regulation of secondary metabolite biosynthesis pathways. PLoS ONE 2020, 15, e0239701. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Yu, Y.; Shi, T.C.; Fu, Y.S.; Zhao, T.; Zhang, Z.W. Melatonin treatment of pre-veraison grape berries modifies phenolic components and antioxidant activity of grapes and wine. Food Sci. Technol. 2019, 39, 35–42. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin may increase disease resistance and flavonoid biosynthesis through effects on DNA methylation and gene expression in grape berries. BMC Plant Biol. 2020, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, J.; Wang, S.; Song, T.; Zhang, J.; Yao, Y. Application of melatonin promotes anthocyanin accumulation in crabapple leaves. Plant Physiol. Biochem. 2019, 142, 332–341. [Google Scholar] [CrossRef]
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009, 14, 237–241. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Xie, X.B.; LI, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Env. 2012, 35, 1884–1897. [Google Scholar] [CrossRef]
- Bandoly, M.; Grichnik, R.; Hilker, M.; Steppuhn, A. Priming of anti-herbivore defence in Nicotiana attenuata by insect oviposition: Herbivore-specific effects. Plant Cell Environ. 2016, 39, 848–859. [Google Scholar] [CrossRef]
- Huby, E.; Napier, J.A.; Baillieul, F.; Michaelson, L.V.; Dhondt-Cordelier, S. Sphingolipids: Towards an integrated view of metabolism during the plant stress response. New Phytol. 2019, 225, 659–670. [Google Scholar] [CrossRef]
- Venable, M.E. Elevation of Ceramide in Senescence: Role of Sphingolipid Metabolism. In Tumor Dormancy, Quiescence, and Senescence; Hayat, M.A., Ed.; Aging, Cancer, and Noncancer Pathologies; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 81–88. [Google Scholar]
- Huang, D.; Tian, W.; Feng, J.; Zhu, S. Interaction between nitric oxide and storage temperature on sphingolipid metabolism of postharvest peach fruit. Plant Physiol. Biochem. 2020, 151, 60–68. [Google Scholar] [CrossRef]
- Cantrel, C.; Vazquez, T.; Puyaubert, J.; Reze, N.; Lesch, M.; Kaiser, W.M.; Dutilleul, C.; Guillas, I.; Zachowski, A.; Baudouin, E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011, 189, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Guillas, I.; Puyaubert, J.; Baudouin, E. Nitric oxide-sphingolipid interplays in plant signalling: A new enigma from the Sphinx? Front. Plant Sci. 2013, 4, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.M.; Snider, A.J.; Obeid, L.M.; Luberto, C.; Hannun, Y.A. Probing de novo sphingolipid metabolism in mammalian cells utilizing mass spectrometry. J. Lipid Res. 2018, 59, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Demir, N. Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus Avium L.) cultivars grown in Turkey. J. Food Sci. 2015, 80, C564–C570. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; López-Corrales, M.; González-Gómez, D. Physicochemical and bioactive properties evolution during ripening of ‘Ambrunés’ sweet cherry cultivar. LWT 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Guo, X.; Luo, T.; Han, D.; Zhu, D.; Li, Z.; Wu, Z.; Wu, Z. Multi-omics analysis revealed room temperature storage affected the quality of litchi by altering carbohydrate metabolism. Sci. Hortic. 2022, 293, 110663. [Google Scholar] [CrossRef]
- Param, N.; Zoffoli, J.P. Genotypic differences in sweet cherries are associated with the susceptibility to mechanical damage. Sci. Hortic. 2016, 211, 410–419. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, I.; Ponce, E.; Vidal, J.; Chirinos, R.; Campos, D.; Pedreschi, R.; Fuentealba, C. Metabolomics Reveals Specific Metabolic Changes in Sweet Cherries (Prunus avium L.) Subjected to Postharvest Treatment with Melatonin after Mechanical Stress. Horticulturae 2023, 9, 940. https://doi.org/10.3390/horticulturae9080940
Hernández I, Ponce E, Vidal J, Chirinos R, Campos D, Pedreschi R, Fuentealba C. Metabolomics Reveals Specific Metabolic Changes in Sweet Cherries (Prunus avium L.) Subjected to Postharvest Treatment with Melatonin after Mechanical Stress. Horticulturae. 2023; 9(8):940. https://doi.org/10.3390/horticulturae9080940
Chicago/Turabian StyleHernández, Ignacia, Excequel Ponce, Juan Vidal, Rosana Chirinos, David Campos, Romina Pedreschi, and Claudia Fuentealba. 2023. "Metabolomics Reveals Specific Metabolic Changes in Sweet Cherries (Prunus avium L.) Subjected to Postharvest Treatment with Melatonin after Mechanical Stress" Horticulturae 9, no. 8: 940. https://doi.org/10.3390/horticulturae9080940
APA StyleHernández, I., Ponce, E., Vidal, J., Chirinos, R., Campos, D., Pedreschi, R., & Fuentealba, C. (2023). Metabolomics Reveals Specific Metabolic Changes in Sweet Cherries (Prunus avium L.) Subjected to Postharvest Treatment with Melatonin after Mechanical Stress. Horticulturae, 9(8), 940. https://doi.org/10.3390/horticulturae9080940






