Taxonomic Comparison, Antioxidant and Antibacterial Activities of Three Ebenus pinnata Ait. ecotypes (Fabaceae) from Algeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Whole Plant Morphology Analysis
2.3. Reagents Required for Pollen and Meiosis Study
2.3.1. Fixative Solution
2.3.2. Lactopropionic Orcein Preparation
2.3.3. Cotton Blue Preparation
2.4. Pollen Grains Size and Shape
2.5. Meiosis Analysis
2.6. Pollen Fertility Assessment
2.7. Determination of Antioxidant Activity
2.7.1. Chemical Reagents
2.7.2. Plant Extract Preparation
2.7.3. Total Phenolic Content
2.7.4. DPPH Free Radical Scavenging Assay
2.7.5. Reducing Power Assay
2.8. Screening of the Antibacterial Activity
2.8.1. Bacterial Strains
2.8.2. Antibacterial Test
2.9. Statistical Evaluation
3. Results
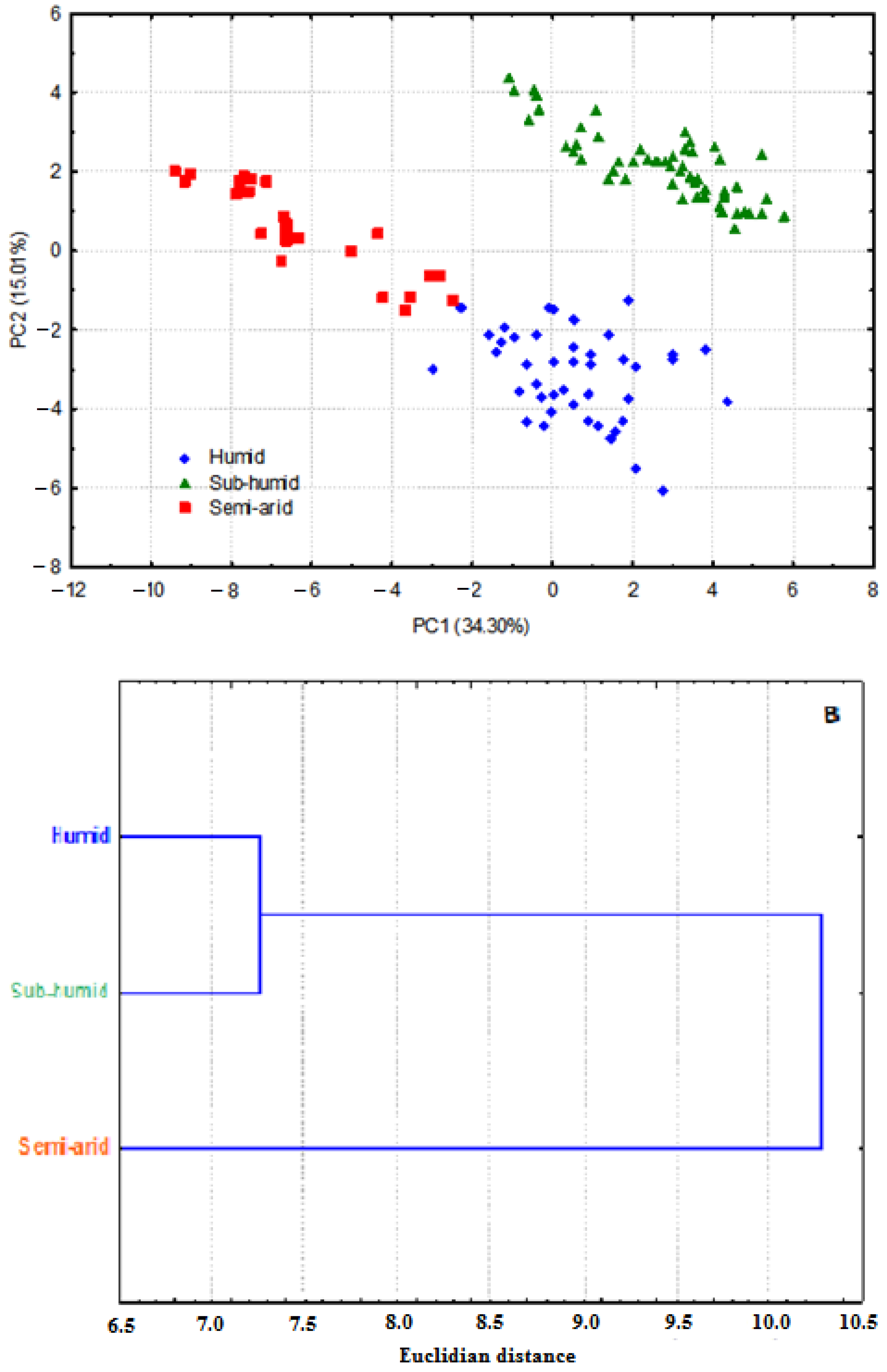
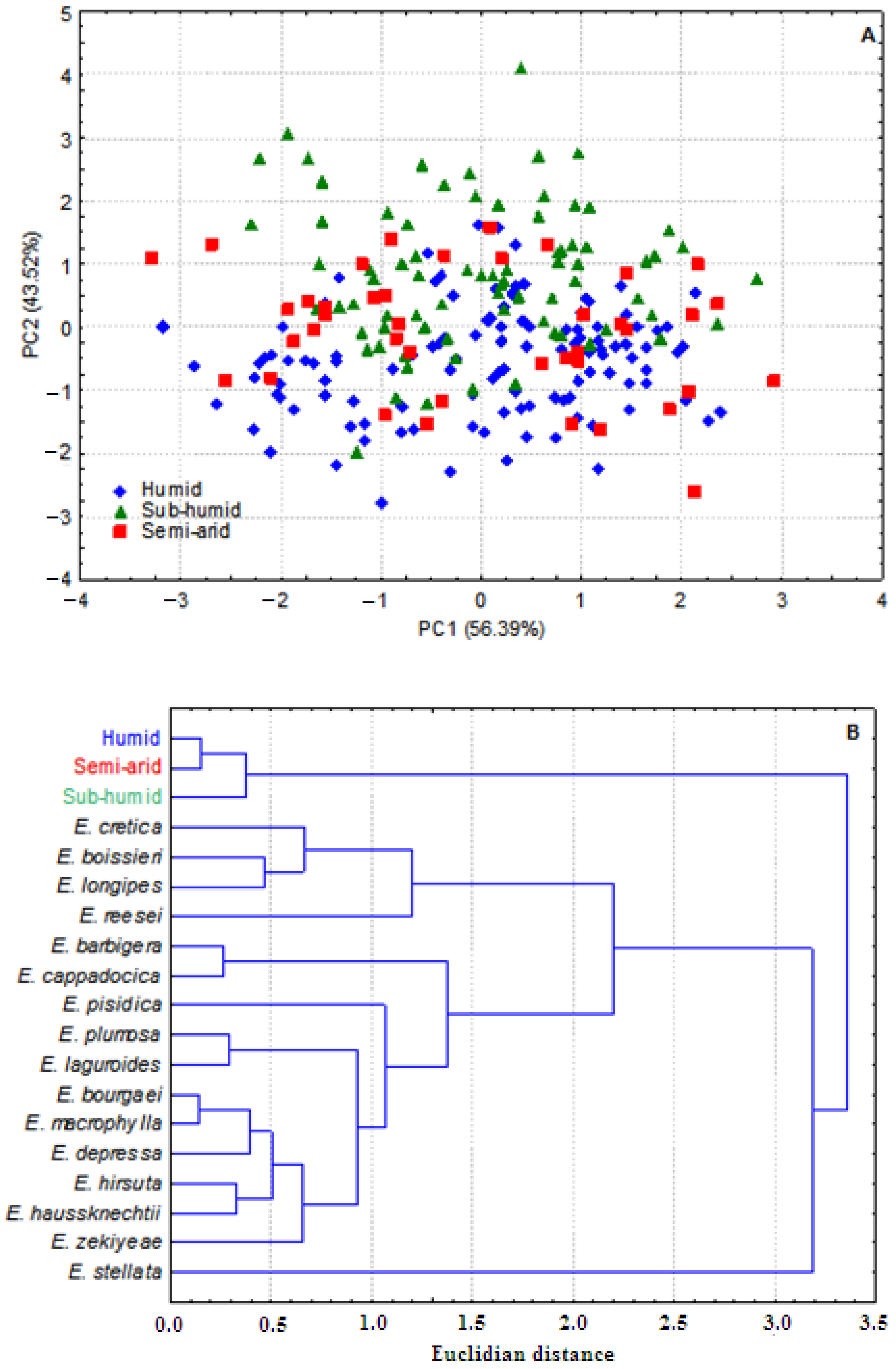
3.1. Whole Plant Morphology
3.2. Pollen Grains Size
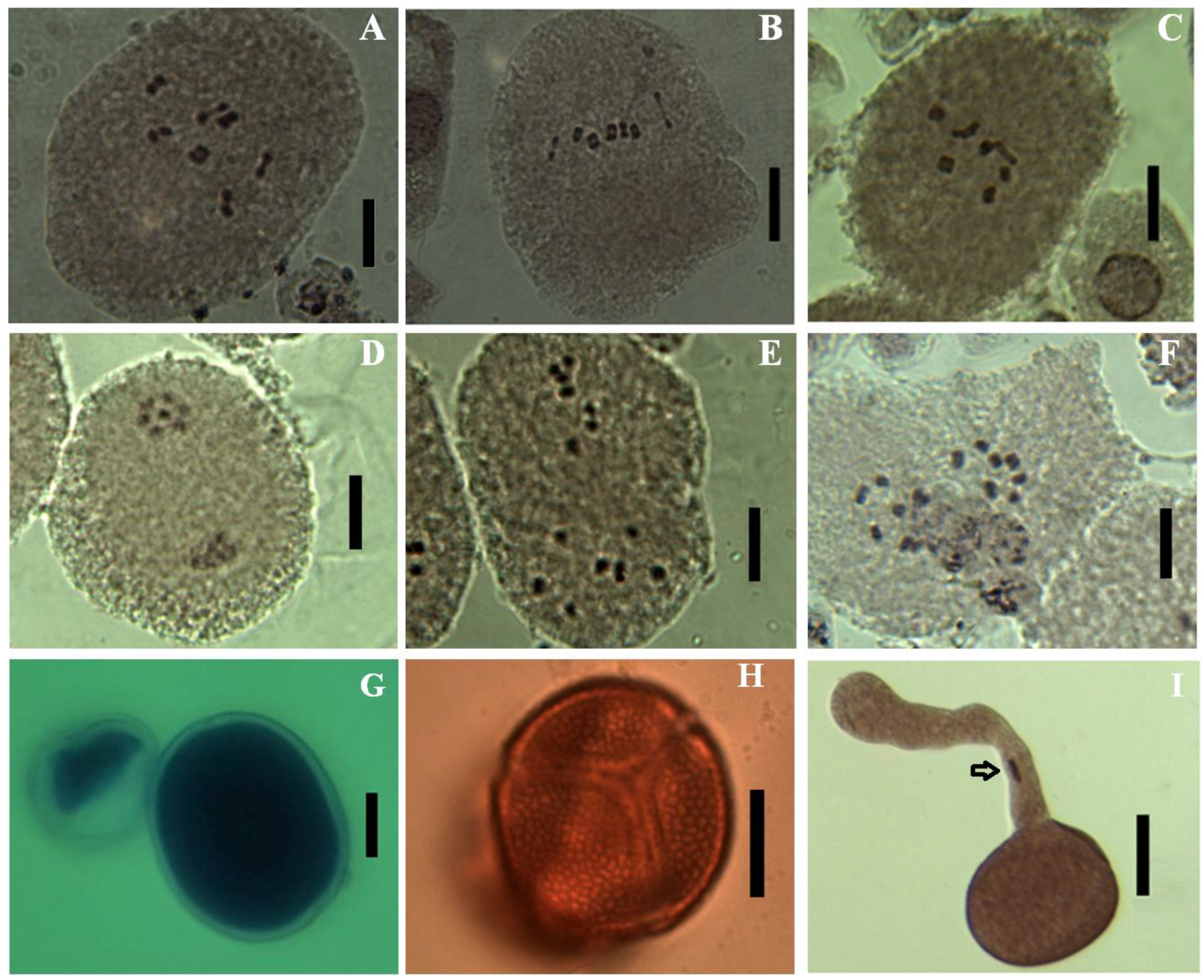
3.3. Chromosome Numbers, Meiotic Abnormalities and Pollen Fertility
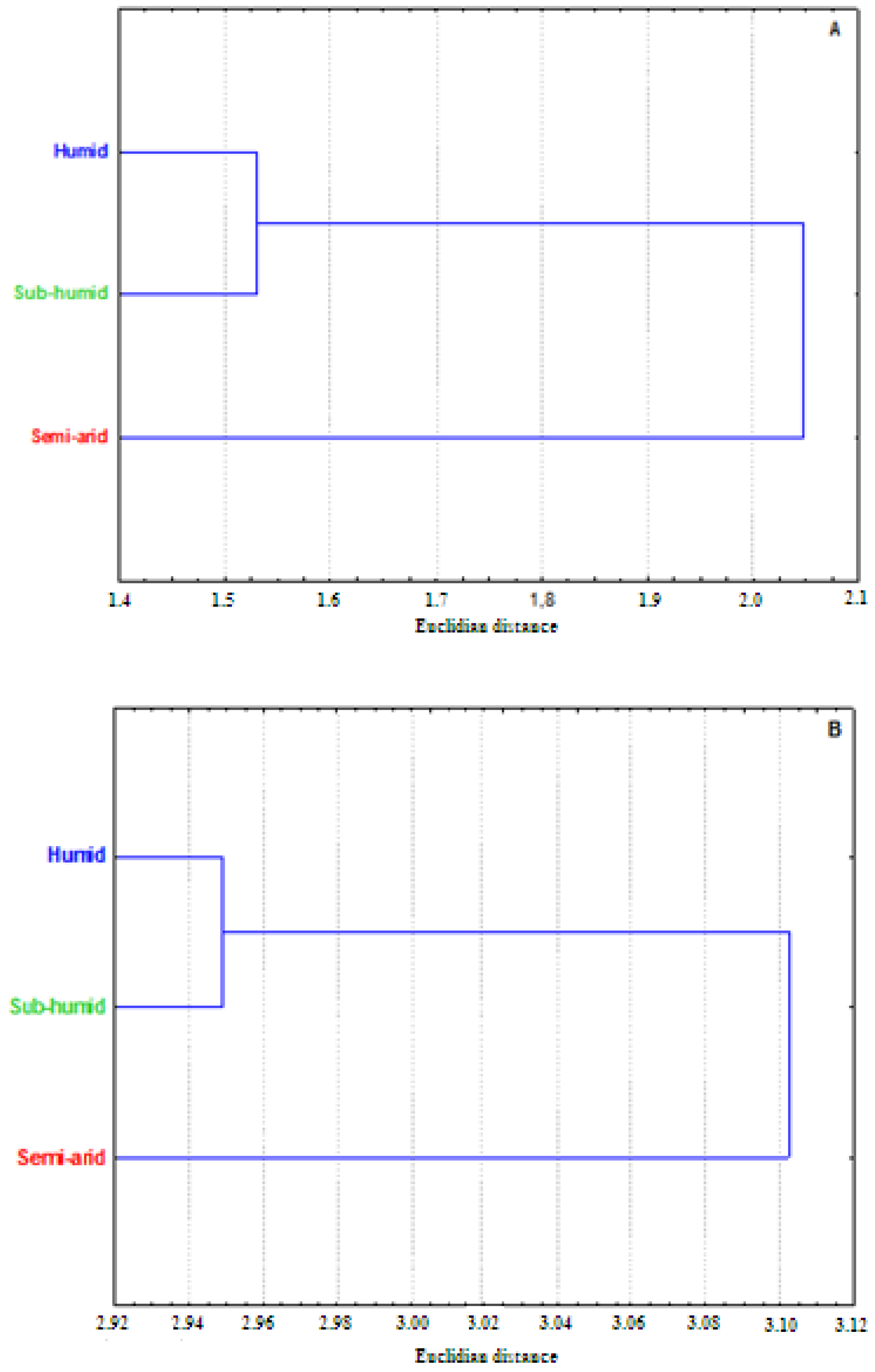
3.4. Antioxidant Activity
3.5. Antibacterial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Aytaç, Z. The genus Ebenus L. (Leguminosae/Fabaceae) in Turkey. Karaca Arbor. Mag. 2000, 5, 145–171. [Google Scholar]
- Aytaç, Z.; Yildirim, H. Ebenus zekiyeae (Fabaceae), a new species from Turkey. Ann. Bot. Fenn. 2018, 55, 25–29. [Google Scholar] [CrossRef]
- Aytaç, Z.; Suludere, Z.; Pınar, M. Examination of the leaflets hairs and stoma structures with the electron microscope of the genus Ebenus L. (Leguminosae) in Turkey. Biodicon 2015, 8, 32–50. [Google Scholar]
- Boissier, E. Flora Orientalis: Save Enumeratio Plantarum in Oriente a Græcia et Ægypto ad Indfiæ Fines Hucusque Observatarum; NCSU Libraries: Genevae, Switzerland, 1872; Volume 2. [Google Scholar]
- Dobignard, A.; Chatelain, C. Index Synonymique de la Flore D’afrique du Nord. Dicotyledoneae: Fabaceae-Nymphaeaceae; Conservatoire et Jardin botaniques de la ville de Genève et ECWP: Genève, Switzerland, 2012; Volume 4, 431p. [Google Scholar]
- Huber-Morath, A. 1970: Ebenus L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK; Volume 3, pp. 590–596.
- Amirahmadi, A.; Kazampour-Osaloo, S.; Moein, F.; Kaveh, A.; Maassoumi, A.A. Molecular systematics of the tribe Hedysareae (Fabaceae) based on nrDNA ITS and plastid trnL-F and matK sequences. Plant Syst. Evol. 2014, 300, 729–747. [Google Scholar] [CrossRef]
- Kaveh, A.; Kazampour-Osaloo, S. Estimation of Ebenus species divergence time based on nrDNA ITS and matK cpDNA sequences. In Proceedings of the 4th National Congress of Plants, Tehran, Iran, 12–13 May 2015. [Google Scholar]
- Ghanavati, F.; Amirabadizadeh, H. Pollen grain morphology in Iranian Hedysareae (Fabaceae). Crop Breed. J. 2012, 2, 25–33. [Google Scholar] [CrossRef]
- Aytaç, Z.; Ünal, F.; Pinar, M.N. Morphological, palynological, and cytotaxonomical study of Ebenus longipes Boiss. & Bal. and E. argentea Siehe ex Bornm. (Leguminosae) from Turkey. Isr. J. Plant Sci. 2000, 48, 321–326. [Google Scholar] [CrossRef]
- Pinar, N.M.; Vural, C.; Zytac, Z. Pollen morphology of Ebenus L. (Leguminosae: Subfamily Papilionoideae) in Turkey. Pak. J. Bot. 2000, 32, 303–310. [Google Scholar]
- Halbritter, H.; Auer, W.; Igersheim, A.; Ebenus cretica. In PalDat-A Palynological Database. 2020. Available online: https://www.paldat.org/pub/Ebenus_cretica/305777;jsessionid=B9B8A714C47DE9C0CB28F0595C4D0398 (accessed on 25 January 2021).
- Molero, J.; Montserrat-Marti, J.M. Números cromosomáticos de plantas marroquíes. Collect. Bot. 1986, 16, 351–354. [Google Scholar]
- Aksoy, H.; Ünal, F.; Aytaç, Z. Karyological study on four endemic Ebenus L. taxa (Leguminosae) in Turkey. Caryologia 2001, 54, 307–311. [Google Scholar] [CrossRef]
- Gadoum, N.; Hamma, A. Etude Cytogénétique de L’espèce Ebenus pinnata Aiton (Fabaceae) du Golfe de Béjaïa et de la Vallée de la Soummam. Master’s Thesis, Université Abderrahmane Mira de Bejaia, Algérie, North Africa, 2016; 58p. Available online: http://univ-bejaia.dz/dspace/123456789/10254 (accessed on 8 July 2023).
- Hadawat, A.K.; Madani, S. Etude Cytogénétique de Quelques Populations d’Ebenus pinnata Ait. (Fabacées) de la Région de Béjaïa. Master’s Thesis, Université Abderrahmane Mira de Bejaia, Algérie, North Africa, 2022; 49p. Available online: http://univ-bejaia.dz/dspace/123456789/21618 (accessed on 8 July 2023).
- Uyar, Z.; Böke, N.; Türkay, E.; Koz, Ö.; Yas, A.I.; Kırmızıgül, S. Flavonoid glycosides and methylinositol from Ebenus haussknechtii. Nat. Prod. Res. 2006, 20, 999–1007. [Google Scholar] [CrossRef]
- Özdemir, E.; Alpınar, K. An ethnobotanical survey of medicinal plants in western part of central Taurus Mountains: Aladaglar (Nigde–Turkey). J. Ethnopharmacol. 2015, 166, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kültür, S.; Gürdal, B.; Sari, A.; Melikoğlu, G. Traditional herbal remedies used in kidney diseases in Turkey: An overview. Turk. J. Bot. 2021, 45, 269–287. [Google Scholar] [CrossRef]
- Ceylan, R.; Katani, J.; Zengina, G.; Mati, S.; Aktumsek, A.; Boroja, T.; Stanic, S.; Vladimir Mihailovic, V.; Guler, G.O.; Boga, M.; et al. Chemical and biological fingerprints of two Fabaceae species (Cytisopsis dorycniifolia and Ebenus hirsuta): Are they novel sources of natural agents for pharmaceutical and food formulations? Ind. Crops Prod. 2016, 84, 254–262. [Google Scholar] [CrossRef]
- Bektas, E.; Kaltalioglu, K.; Sahin, H.; Turkmen, Z.; Kandemir, A. Analysis of phenolic compounds, antioxidant and antimicrobial properties of some endemic medicinal plants. Int. J. Second. Metab. 2018, 5, 75–86. [Google Scholar] [CrossRef]
- İmir, N.; Aydemir, E.; Şimşek, E.; Göktürk, R.; Yesilada, E.; Fişkin, K. Cytotoxic and immunomodulatory effects of Ebenus boissieri Barbey on breast cancer cells. Genet. Mol. Res. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simsek, E.; Imir, N.; Aydemir, E.R.; Gokturk, R.S.; Yesilada, E.; Fiskin, K. Caspase-mediated apoptotic effects of Ebenus boissieri Barbey extracts on human cervical cancer Cell Line HeLa. Pharmacogn. Mag. 2017, 13, 254–259. [Google Scholar] [CrossRef]
- Aydemir, E.A.; Simsek, E.; Imir, N.; Göktürk, R.S.; Yesilada, E.; Fiskin, K. Cytotoxic and apoptotic effects of Ebenus boissieri Barbey on human lung cancer Cell Line A549. Pharmacogn. Mag. 2015, 11 (Suppl. 1), S37. [Google Scholar] [CrossRef][Green Version]
- Kounadi, S.; Aligiannis, N.; Pongratz, I.; Lelovas, P.; Ismini, D.; Skaltsounis, A. Estrogenic activity of the methanolic extract of Ebenus cretica L. Planta Medica 2011, 77, PM168. [Google Scholar] [CrossRef]
- Dontas, I.; Kounadi, S.; Aligiannis, N.; Galanos, A.; Skaltsounis, A.; Lelovas, P. Plant extract administration and mild daily exercise increase bone density of ovariectomized rats. In Abstracts of 14th FELASA congress 2019, PC41, p. 157. Lab. Anim. 2019, 53, 28–203. [Google Scholar]
- Mitrocotsa, D.; Skaltsounis, A.-L.S.; Harvala, C.; Tillequin, F. Flavonoid and terpene glycosides from European Ebenus species. Biochem. Syst. Ecol. 1999, 27, 305–307. [Google Scholar] [CrossRef]
- Khodaparast, A.; Sayyah, M.; Sardari, S. Anticonvulsant activity of hydroalcoholic extract and aqueous fraction of Ebenus stellata in mice. Iran. J. Basic Med. Sci. 2012, 15, 811–819. [Google Scholar]
- Zameer, S.; Ali, S.; Gulmeena Tareen, R.B. Identification of volatile constituents and antimicrobial activity of Ebenus stellata. Gu J. Phytosciences 2022, 2, 214–222. [Google Scholar]
- Zemouri, T.; Chikhoune, A.; Benmouhoub, H.; Sahnoune, M. Taxonomic Comparison, Antioxidant and Antibacterial Activities of Three Ecotypes of Ebenus pinnata Ait. (Fabaceae) from Algeria. Preprints 2023, 2023071339. [Google Scholar] [CrossRef]
- Parra, R.; Valdés, B.; Gordillo, I.; Venanzi, R. Meditenanean chromosome number reports. Flora Mediterr. 1999, 9, 323–387. [Google Scholar]
- Braham, H.; Ben Jannet, H.; Castedo, L.; Mighri, Z. Isolation, for the first time, of a flavonoid glycoside and the (±)-catechin from the aerial parts of Ebenus pinnata. J. De La Société Chim. De Tunis. 2004, 6, 153–160. [Google Scholar]
- Abreu, P.M.; Braham, H.; Ben Jannet, H.; Mighri, Z.; Matthew, S. Antioxidant compounds from Ebenus pinnata. Fitoterapia 2007, 78, 32–34. [Google Scholar] [CrossRef]
- Mebarki, A. Hydrologie des Bassins de l’Est Algérien: Ressources en Eau, Aménagement et Environnement. Ph.D. Thesis, Université Mentouri de Constantine, Algérie, North Africa, 2005; 360p. [Google Scholar]
- Mertens, T.R.; Hamnersmith, R.L. Genetic Laboratory Investigations; Eleventh, Ed.; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Herzi, N.; Bouajila, J.; Camy, S.; Romdhane, M.; Condoret, J.S. Comparison of different methods for extraction from Tetraclinis articulata: Yield, chemical composition and antioxidant activity. Food Chem. 2013, 141, 3537–3545. [Google Scholar] [CrossRef]
- Dyer, A.F. The use of lactopropionic orcein in rapid squash. Methods for chromosome preparations. Stain. Technol. 1963, 38, 85–90. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu RGAEent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- NCCLS (National Committee for Clinical Laboratory Standards). Performance Standard for Antimicrobial Disc Susceptibility Tests; Approved Standard; Villanova, P.A., Ed.; Publication M2-A5; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1993. [Google Scholar]
- Statistica, version 8.0. Data analysis software system. Statsof, Inc.: Tulsa, OK, USA, 2007.
- Knight, C.A.; Clancy, R.B.; Götzenberger, L.; Dann, L.; Beaulieu, J.M. On the relationship between pollen size and genome size. J. Bot. 2010, 2010, 612017. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alrumman, S.A. Methomyl, imbraclaobrid and clethodim induced cytomixis and syncytes behaviors in PMCs of Pisum sativum L: Causes and outcomes. Saudi J. Biol. Sci. 2022, 29, 103390. [Google Scholar] [CrossRef] [PubMed]
- Nouioua, W.; Gaamoune, S. Antioxidant, antimicrobial and anti-inflammatory activities development of methanol extracts of some species growing in the massif of Boutaleb, Setif, Algeria. Int. J. Pharm. Nat. Med. 2018, 6, 15–20. [Google Scholar]
- Zhang, Y.; Wang, Z. Phenolic composition and antioxidant activities of two Phlomis species: A correlation study. Comptes Rendus Biol. 2009, 332, 816–826. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Rabeta, M.S.; Nur Faraniza, R. Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of Garcinia atrovirdis and Cynometra cauliflora. Int. Food Res. J. 2013, 20, 1691–1696. [Google Scholar]
- Kabtni, S.; Sdouga, D.; Bettaib Rebey, I.; Save, M.; Trifi-Farah, N.; Fauconnier, M.L.; Marghali, S. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 2020, 10, 8293. [Google Scholar] [CrossRef]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef] [PubMed]
| Climate stage | Humid | Sub-humid | Semi-arid |
| Locality name | Kherrata | Semaoun | Boudjelil |
| Population Code | H | SH | SA |
| GPS Localization | 36° 31′33.3″ N 5° 16′49.73″ | 36° 37′27.16″ N 4° 49′7.20″ E | 36° 22′11″ N 4° 26′48.30″ E |
| Altitude/ Exposure | 612 m/East | 195 m/West | 270 m/East |
| Soil | Red clay | Brown clay | White ground |
| Plant formation | Road bank | Garrigue, Grassland | Grassland, Sparse garrigue |
| Rainfall 1 (mm) | 800–1000 | 600–800 | 600–800 |
| Number of plants | 44 | 55 | 27 |
| Sampling date/ Plant codes | 08/06/2015/H01-H20 03/06/2015/H21-H35 15/07/2015/H236-H44 | 20/05/2015/SH01-SH15 09/06/2015/SH16-SH25 09/06/2015/SH26- SH34 20/05/2015/SH35-SH47 28/06/2016 SH48-SH55 | 13/05/2015/SA01-SA13 07/06/2015/SA14-SA27 |
| No | Coding | Character Name | Type 1 | Unit |
|---|---|---|---|---|
| 1 | DBP | Diameter at the base of the plant | C | cm |
| 2 | LLS | Length of the longest stem | C | cm |
| 3 | NS | Number of stems | D | Stems |
| 4 | DLS | Diameter of the longest stem | C | mm |
| 5 | LIN | Length of the third internode | C | cm |
| 6 | NI | Number of inflorescences (racemes) | D | Raceme |
| 7 | LS1 | Length of stipules | C | mm |
| 8 | WS | Width of stipules | C | mm |
| 9 | LL | Length of the leaf (3rd node) | C | cm |
| 10 | WL | Width of the leaf (3rd node) | C | cm |
| 11 | LP | Length of the petiole | C | cm |
| 12 | NPL | Number of pairs of leaflets (3rd node) | D | Pairs |
| 13 | LNLP | Lowest number of leaflets pairs | D | Pairs |
| 14 | HNLP | Highest number of leaflets pairs | D | Pairs |
| 15 | LLB | Length of the leaflet blade | C | cm |
| 16 | WLB | Width of the leaflet blade | C | mm |
| 17 | LIP | Length of the inflorescence peduncle (3rd node) | C | cm |
| 18 | HI | Height of the inflorescence | C | cm |
| 19 | DI | Diameter of the inflorescence | C | cm |
| 20 | LNF | Lowest number of flowers | D | Flower |
| 21 | HNF | Highest number of flowers | D | Flower |
| 22 | LFB | Length of the flower bract | C | mm |
| 23 | WFB | Width of the flower bract | C | mm |
| 24 | LC1 | Length of the flower calyx | C | mm |
| 25 | LC2 | Length of the corolla | C | mm |
| 26 | TLW | Total length of the wing | C | mm |
| 27 | WWB | Width of the wing blade | C | mm |
| 28 | WBW | Width at the base of the wing | C | mm |
| 29 | WMW | Width at the middle of the wing | C | mm |
| 30 | TWK | Total width of the keel | C | mm |
| 31 | LWPK | Length of the widest part of the keel | C | mm |
| 32 | TLK | Total length of the keel | C | mm |
| 33 | TLS | Total length of the standard | C | mm |
| 34 | LSB | Length of the standard blade | C | mm |
| 35 | WSB | Width of the standard blade | C | mm |
| 36 | LSP | Length of the standard ‘petiolule’ | C | mm |
| 37 | LCM | Length of the calyx at maturity | C | mm |
| 38 | LCT | Length of the calyx tube at maturity | C | mm |
| 39 | LHCT | Length of hairs at the base of the calyx teeth | C | mm |
| 40 | LPM | Length of the pod at maturity | C | mm |
| 41 | WPM | Width of the pod at maturity | C | mm |
| 42 | LS2 | Length of the seed | C | mm |
| 43 | WS | Width of the seed | C | mm |
| 44 | LR | Length of the radicle | C | mm |
| Extra variables (Not included in multivariate analyses) | ||||
| 45 | P | Polar diameter of pollen grains | C | µm |
| 46 | E | Equatorial diameter of pollen grains | C | µm |
| 47 | P/E | Rate of P and E diameters of pollen grains | C | |
| 48 | PxE | Product of P * E. | C | µm2 |
| 49 | PF | Pollen fertility rate | C | % |
| No | Trait Code | R1 | R2 | Humid | Sub-Humid | Semi-Arid |
|---|---|---|---|---|---|---|
| 1 | DBP | 0.49 | −0.24 | 0.73 ± 0.19 B | 0.73 ± 0.33 B | 0.55 ± 0.25 A |
| 2 | LLS | 0.80 | 0.10 | 68.22 ± 18.38 B | 89.28 ± 28.93 C | 44.41 ± 19.79 A |
| 3 | NS | 0.23 | −0.60 | 9.55 ± 6.52 B | 4.65 ± 2.47 A | 3.11 ± 2.41 A |
| 4 | DLS | 0.47 | −0.41 | 4.19 ± 1.12 B | 3.75 ± 1.01 AB | 3.16 ± 1.11 A |
| 5 | LIN | 0.67 | 0.16 | 5.27 ± 1.04 B | 6.53 ± 1.57 C | 4.36 ± 1.58 A |
| 6 | NI | 0.52 | −0.39 | 57.57 ± 28.25 B | 47.89 ± 32.23 B | 17.89 ± 23.80 A |
| 7 | LS1 | 0.66 | 0.17 | 10.98 ± 1.67 A | 13.60 ± 2.74 B | 10.10 ± 2.71 A |
| 8 | WS | 0.56 | −0.13 | 4.22 ± 0.75 B | 4.41 ± 0.74 B | 3.64 ± 1.00 A |
| 9 | LL | 0.77 | −0.05 | 10.01 ± 2.06 B | 10.85 ± 2.60 B | 6.91 ± 1.93 A |
| 10 | WL | 0.67 | −0.06 | 5.19 ± 0.80 B | 5.55 ± 0.88 B | 4.64 ± 0.66 A |
| 11 | LP | 0.73 | 0.03 | 4.56 ± 1.21 B | 5.23 ± 1.61 B | 2.96 ± 0.98 A |
| 12 | NPL | 0.43 | −0.07 | 4.73 ± 0.50 B | 4.78 ± 0.50 B | 4.11 ± 0.97 A |
| 13 | LNLP | −0.18 | −0.29 | 2.80 ± 0.55 B | 2.45 ± 0.50 A | 2.81 ± 0.68 B |
| 14 | HNLP | 0.43 | −0.23 | 4.98 ± 0.15 B | 4.91 ± 0.35 B | 4.52 ± 0.89 A |
| 15 | LLB | 0.70 | 0.03 | 2.45 ± 0.33 B | 2.66 ± 0.35 C | 2.15 ± 0.36 A |
| 16 | WLB | 0.22 | −0.63 | 7.77 ± 1.51 B | 6.14 ± 1.21 A | 6.18 ± 1.46 A |
| 17 | LIP | 0.56 | −0.31 | 22.00 ± 3.17 B | 21.29 ± 3.56 B | 18.20 ± 3.94 A |
| 18 | HI | 0.43 | −0.49 | 5.21 ± 1.41 B | 4.40 ± 0.75 A | 3.88 ± 1.61 A |
| 19 | DI | 0.33 | −0.16 | 2.44 ± 0.31 A | 2.40 ± 0.25 A | 2.30 ± 0.31 A |
| 20 | LNF | 0.24 | 0.24 | 11.84 ± 8.05 A | 16.64 ± 7.17 B | 13.56 ± 9.89 AB |
| 21 | HNF | 0.66 | −0.41 | 56.43 ± 11.83 C | 49.44 ± 9.47 B | 33.93 ± 17.42 A |
| 22 | LFB | 0.08 | 0.29 | 8.15 ± 0.62 A | 8.60 ± 0.89 B | 8.46 ± 1.04 AB |
| 23 | WFB | 0.06 | −0.12 | 3.29 ± 0.32 A | 3.25 ± 0.18 A | 3.19 ± 0.43 A |
| 24 | LC1 | −0.05 | 0.25 | 12.72 ± 0.94 A | 13.21 ± 0.90 B | 13.38 ± 1.06 B |
| 25 | LC2 | 0.13 | −0.25 | 8.21 ± 0.44 A | 8.05 ± 0.24 A | 7.98 ± 0.70 A |
| 26 | TLW | 0.40 | 0.87 | 1.79 ± 0.08 A | 3.54 ± 0.13 C | 2.40 ± 0.07 B |
| 27 | WWB | 0.45 | 0.85 | 1.37 ± 0.09 A | 2.45 ± 0.07 C | 1.67 ± 0.05 B |
| 28 | WBW | −0.67 | 0.23 | 1.10 ± 0.10 A | 1.14 ± 0.06 A | 1.38 ± 0.04 B |
| 29 | WMW | −0.02 | 0.89 | 0.43 ± 0.05 A | 0.89 ± 0.07 C | 0.83 ± 0.07 B |
| 30 | TWK | −0.86 | −0.34 | 4.21 ± 0.23 B | 3.62 ± 0.10 A | 4.99 ± 0.09 C |
| 31 | LWPK | −0.86 | −0.25 | 3.47 ± 0.26 B | 3.12 ± 0.08 A | 4.14 ± 0.08 C |
| 32 | TLK | −0.81 | −0.29 | 6.36 ± 0.36 B | 5.94 ± 0.11 A | 6.99 ± 0.08 C |
| 33 | TLS | −0.89 | −0.02 | 6.65 ± 0.45 B | 6.30 ± 0.23 A | 8.04 ± 0.08 C |
| 34 | LSB | −0.86 | −0.29 | 5.13 ± 0.47 B | 4.49 ± 0.12 A | 6.06 ± 0.05 C |
| 35 | WSB | −0.87 | −0.39 | 4.67 ± 0.36 B | 3.36 ± 0.16 A | 6.04 ± 0.10 C |
| 36 | LSP | −0.31 | 0.38 | 1.72 ± 0.47 A | 1.81 ± 0.16 A | 2.02 ± 0.10 B |
| 37 | LCM | −0.42 | 0.67 | 13.19 ± 0.48 A | 14.50 ± 0.89 B | 15.26 ± 0.18 C |
| 38 | LCT | 0.04 | 0.81 | 2.80 ± 0.14 A | 3.29 ± 0.12 C | 3.19 ± 0.12 B |
| 39 | LHCT | −0.15 | 0.22 | 3.27 ± 0.05 A | 3.28 ± 0.07 A | 3.29 ± 0.03 A |
| 40 | LPM | −0.85 | 0.14 | 5.09 ± 0.09 A | 5.08 ± 0.11 A | 5.99 ± 0.10 B |
| 41 | WPM | −0.71 | 0.40 | 3.04 ± 0.10 A | 3.15 ± 0.13 B | 3.46 ± 0.07 C |
| 42 | LS2 | −0.87 | −0.06 | 2.35 ± 0.07 B | 2.19 ± 0.10 A | 3.00 ± 0.08 C |
| 43 | WS | −0.85 | 0.06 | 2.05 ± 0.05 A | 2.02 ± 0.05 A | 2.34 ± 0.05 B |
| 44 | LR | −0.77 | −0.34 | 2.14 ± 0.05 B | 2.02 ± 0.07 A | 2.25 ± 0.05 C |
| 45 | P | NA | NA | 17.95 ± 1.10 Ab | 19.47 ± 1.27 Bb | 18.43 ± 1.08 Ab |
| 46 | E | NA | NA | 12.80 ± 1.18 Aa | 13.03 ± 0.99 Aa | 12.84 ± 1.37 Aa |
| 47 | P/E | NA | NA | 1.41 ± 0.10 A | 1.50 ± 0.11 B | 1.45 ± 0.15 AB |
| 48 | PxE | NA | NA | 230.56 ± 31.60 A | 254.31 ± 30.81 B | 237.16 ± 32.74 A |
| 49 | PF | NA | NA | 94.04 ± 2.64 A | 95.01 ± 2.02 A | 94.97 ± 2.11 A |
| Parameters/Strains | Sample 1 (Humid) | Sample 2 (Sub–Humid) | Sample 3 (Semi–Arid) | |
|---|---|---|---|---|
| Antioxident activity 1 | TPC (mg GAE/g EXT) | 50.32–51.33 50.79 ± 0.51 A | 50.93–53.02 52.04 ± 1.05 A | 56.49–57.40 56.89 ± 0.46 B |
| DPPH (mg GAE/g EXT) | 68.62–72.74 71.18 ± 2.24 A | 82.93–88.56 86.39 ± 3.02 B | 93.53–97.54 95.67 ± 2.02 C | |
| RP (mg GAE/g EXT) | 10.27–12.52 11.09 ± 1.24 A | 16.45–17.94 17.21 ± 0.75 B | 25.64–26.16 25.88 ± 0.26 C | |
| Antioxidant activity 2 | Escherichia coli | 10–13 11.50 ± 2.12 A | 11–13 12.00 ± 1.41 A | 10–13 11.50 ± 2.12 A |
| Staphylococcus aureus | 10–10.4 10.20 ± 0.28 A | 8.5–10 9.50 ± 0.87 A | 9–12 10.50 ± 1.50 A | |
| Pseudomonas aeruginosa | 8.8–11 9.90 ± 1.56 A | 11.5–12 11.75 ± 0.35 A | 8.5–10 9.25 ± 1.06 A | |
| Methicillin-resistant S.aureus (MRSA) | 9–10.4 9.80 ± 0.72 A | 9–10 9.60 ± 0.53 A | 10–11 10.33 ± 0.58 A | |
| Vibrio cholerae | 9–10 9.50 ± 0.71 A | 8–11.2 9.60 ± 2.26 A | 9–14.5 11.75 ± 3.89 A | |
| Salmonella typhi | 9.6–11 10.53 ± 0.81 A | 8.9–13 11.30 ± 2.14 A | 10.3–13 11.77 ± 1.36 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemouri, T.; Chikhoune, A.; Benmouhoub, H.; Sahnoune, M. Taxonomic Comparison, Antioxidant and Antibacterial Activities of Three Ebenus pinnata Ait. ecotypes (Fabaceae) from Algeria. Horticulturae 2023, 9, 879. https://doi.org/10.3390/horticulturae9080879
Zemouri T, Chikhoune A, Benmouhoub H, Sahnoune M. Taxonomic Comparison, Antioxidant and Antibacterial Activities of Three Ebenus pinnata Ait. ecotypes (Fabaceae) from Algeria. Horticulturae. 2023; 9(8):879. https://doi.org/10.3390/horticulturae9080879
Chicago/Turabian StyleZemouri, Tassadit, Amirouche Chikhoune, Hassina Benmouhoub, and Mohamed Sahnoune. 2023. "Taxonomic Comparison, Antioxidant and Antibacterial Activities of Three Ebenus pinnata Ait. ecotypes (Fabaceae) from Algeria" Horticulturae 9, no. 8: 879. https://doi.org/10.3390/horticulturae9080879
APA StyleZemouri, T., Chikhoune, A., Benmouhoub, H., & Sahnoune, M. (2023). Taxonomic Comparison, Antioxidant and Antibacterial Activities of Three Ebenus pinnata Ait. ecotypes (Fabaceae) from Algeria. Horticulturae, 9(8), 879. https://doi.org/10.3390/horticulturae9080879






