Effect of Aqueous n-Butanol Treatments on Shelf-Life Extension of Longkong Fruit during Ambient Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Treatments
2.2. Quality Assessment
2.2.1. Determination of Pericarp Color Characteristics
2.2.2. Determination of Pericarp Browning Index
2.2.3. Determination of Membrane Permeability
2.2.4. Determination of Malondialdehyde (MDA) Content
2.2.5. Determination of Pericarp Total Phenolic Contents (TPC)
2.2.6. Determination of Reactive Oxygen Species (ROS)
2.2.7. Enzymatic Determinations
Determination of PAL Activity
Determination of PPO Activity
Determination of PLD Activity
Determination of LOX Activity
Determination of SOD Activity
Determination of CAT Activity
Determination of GPX Activity
2.3. Statistical Analysis
3. Results and Discussion
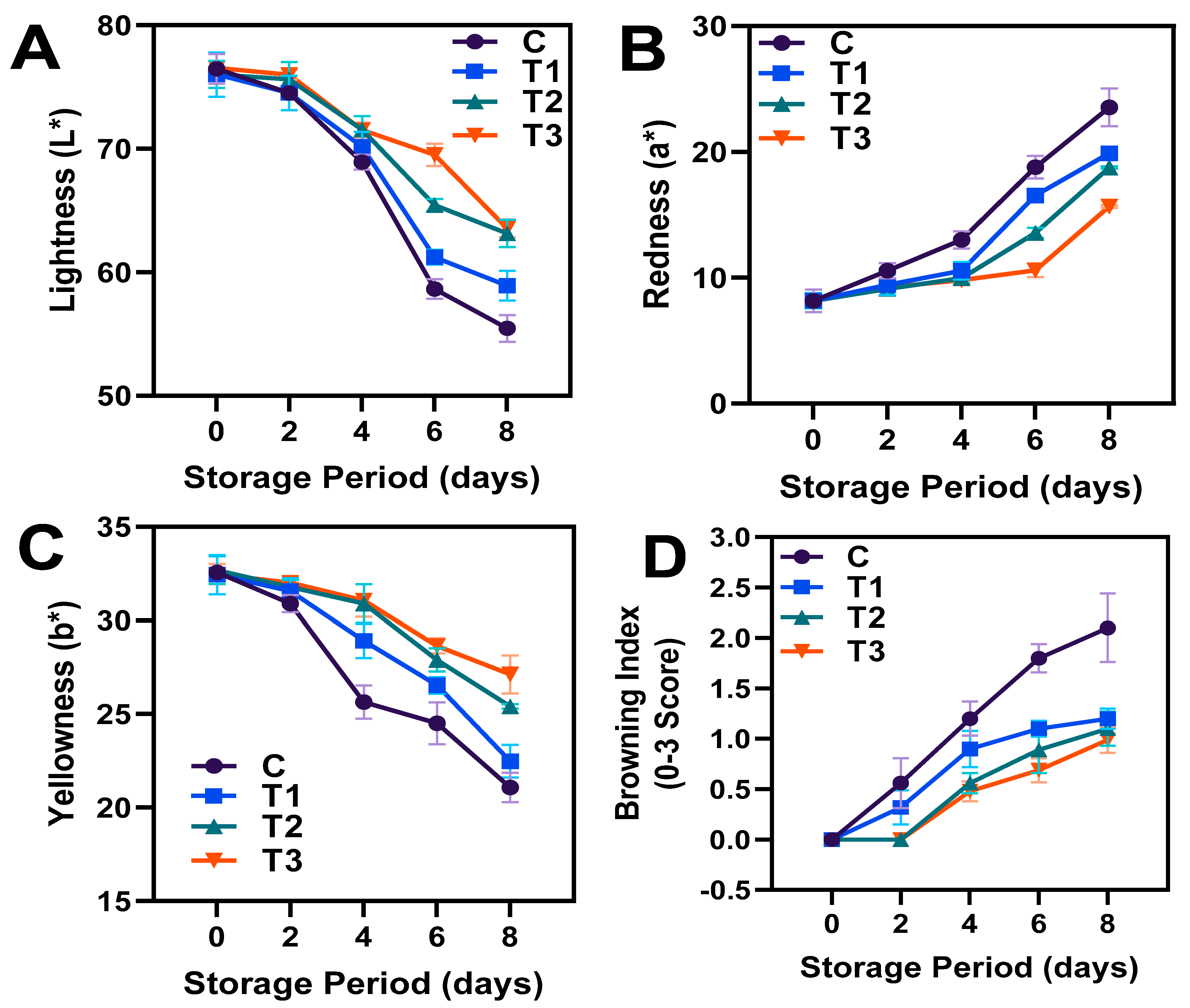
3.1. Pericarp Color Characteristics and Browning Index
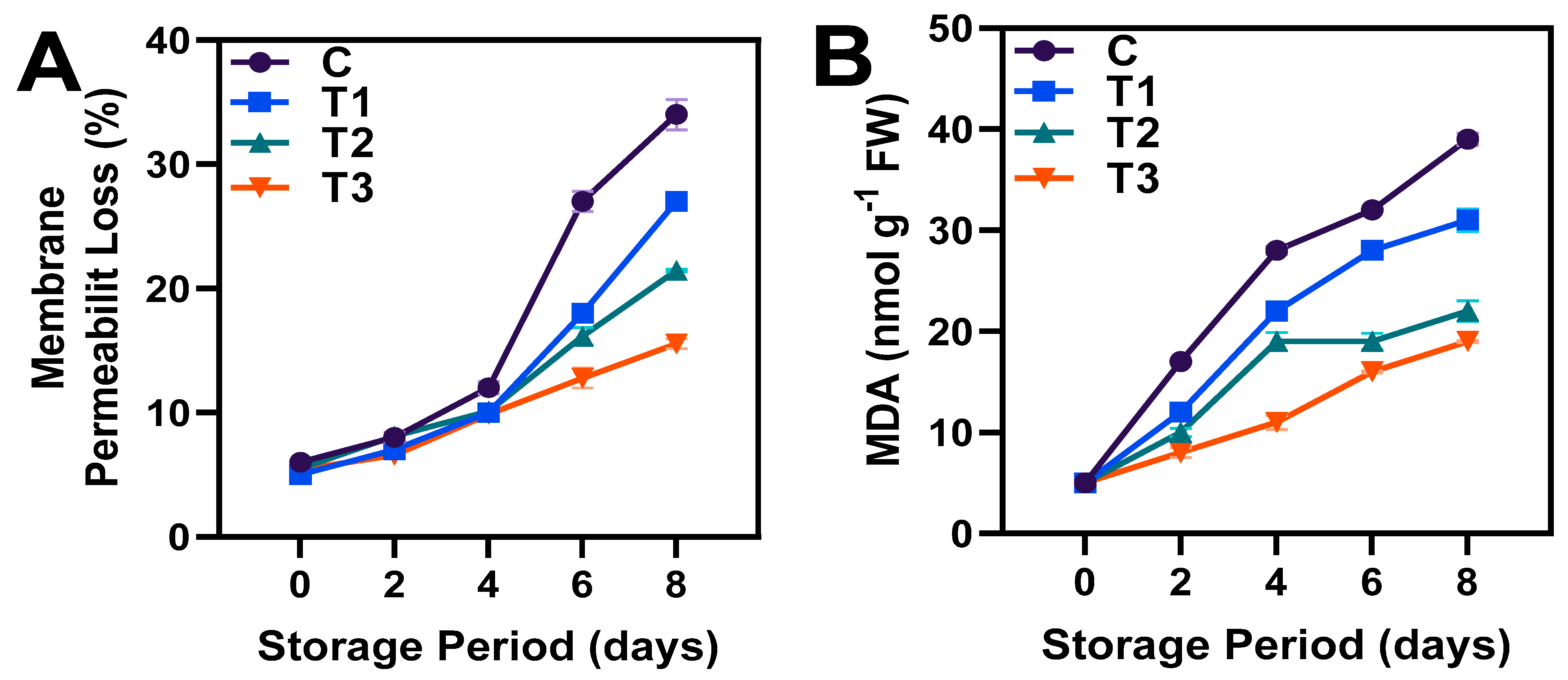
3.2. Membrane Permeability and MDA Content
3.3. Total Phenolic Content and PAL and PPO Activity
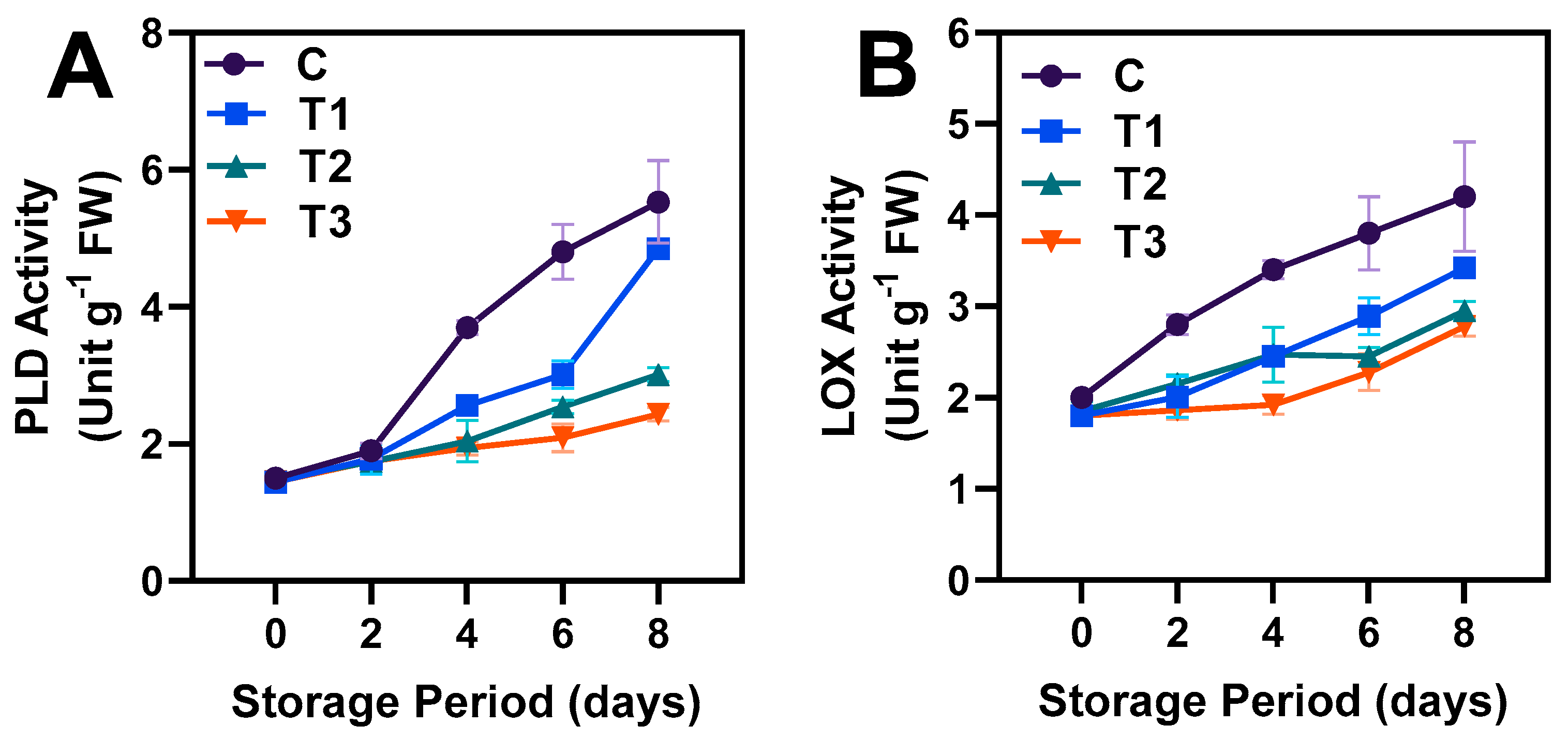
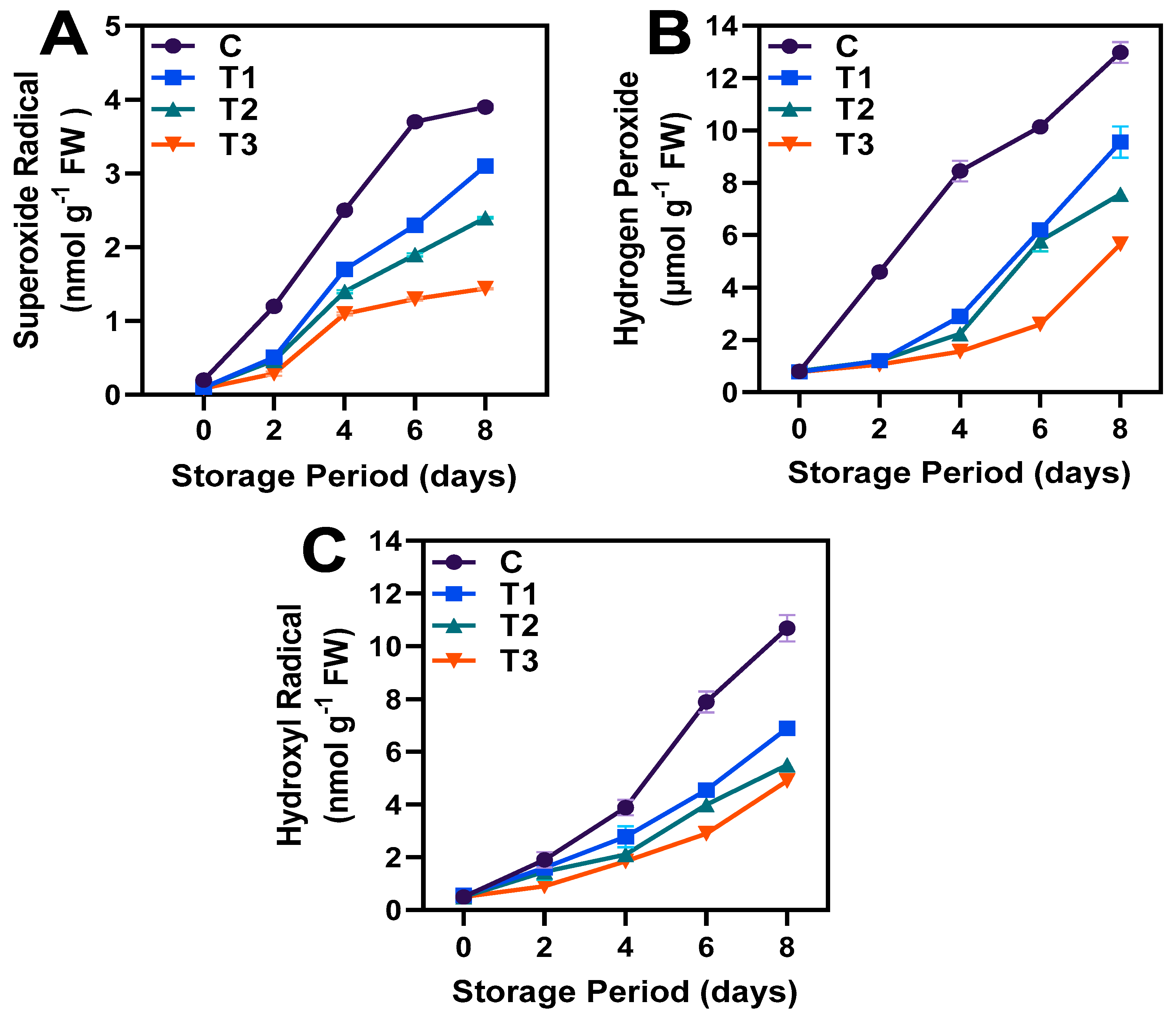
3.4. PLD and LOX Activities and ROS Production
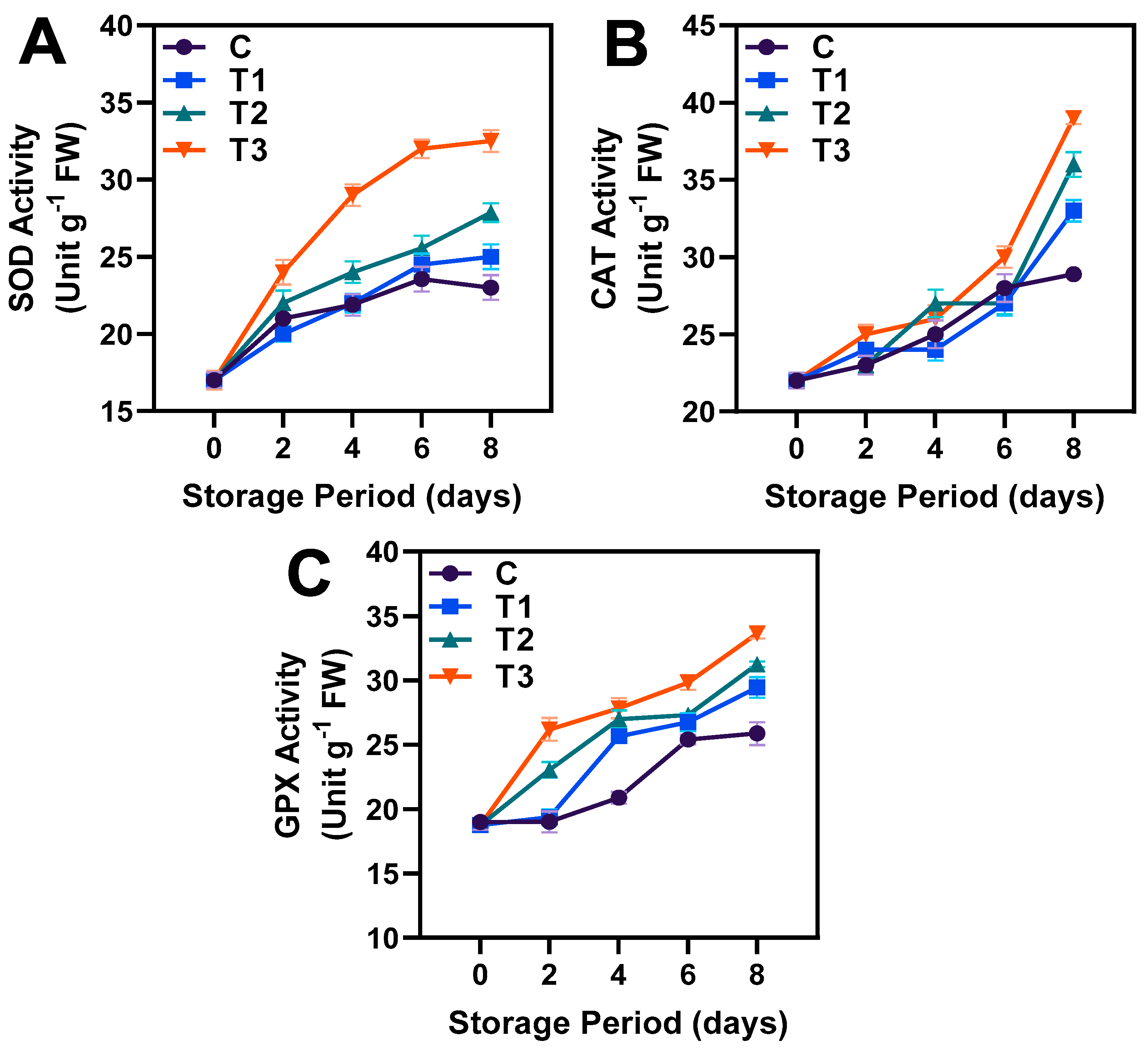
3.5. Antioxidant Enzyme Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangkasanya, S.; Meenune, M. Physical, chemical and sensory qualities of longkong (Aglaia dookkoo Griff.) as affected by storage at different atmospheres. Asian J. Food Sci. Agro-Ind. 2009, 3, 64–74. [Google Scholar]
- Abdallah, M.H.; Mohamed, A.G.; Ibrahim, M.R.S. Lansium domesticum—A fruit with multi-benefits: Traditional uses, phytochemicals, nutritional value, and bioactivities. Nutrients 2022, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Techavuthiporn, C. Langsat—Lansium domesticum. In Exotic Fruits; Rodrigues, S., Silva, E., Brito, E., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 279–283. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Meenune, M. Physiological changes of longkong fruit during different storage conditions. Adv. Environ. Biol. 2014, 8, 362–368. [Google Scholar]
- Noonim, P.; Venkatachalam, K. Combination of salicylic acid and ultrasonication for alleviating chilling injury symptoms of longkong. J. Qual. Saf. 2022, 6, fyab032. [Google Scholar] [CrossRef]
- Lichanporn, I.; Srilaong, V.; Wongs-Aree, C.; Kanlayanarat, S. Postharvest physiology and browning of longkong (Aglaia dookkoo Griff.) fruit under ambient conditions. Postharvest Biol. Technol. 2009, 52, 294–299. [Google Scholar] [CrossRef]
- Sun, J.; Changbao, L.; Prasad, N.K.; You, X. Membrane deterioration, enzymatic browning and oxidative stress in fresh fruits of three litchi cultivars during six-day storage. Sci. Hortic. 2012, 148, 97–103. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, C.S.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef]
- Sun, J.; You, X.; Li, L.; Peng, H.; Su, W.; Li, C.; He, Q.; Liao, F. Effects of a phospholipase D inhibitor on postharvest enzymatic browning and oxidative stress of litchi fruit. Postharvest Biol. Technol. 2011, 62, 288–294. [Google Scholar] [CrossRef]
- Jia, X.; Li, W. Phospholipase D antagonist 1-butanol inhibited the mobilization of triacylglycerol during seed germination in Arabidopsis. Plant Divers. 2018, 40, 292–298. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Sun, J.; Li, C.; Sheng, J.; Zheng, F.; Liao, F.; He, X.; Liu, G.; Ling, D.; et al. Effects of 2-butanol on quality and physiological characteristics of longan fruit stored at ambient temperature. Postharvest Biol. Technol. 2015, 101, 96–102. [Google Scholar] [CrossRef]
- Luo, T.; Yin, F.; Liao, L.; Liu, Y.; Guan, B.; Wang, M.; Lai, T.; Wu, Z.; Shuai, L. Postharvest melatonin treatment inhibited longan (Dimocarpus longan Lour.) pericarp browning by increasing ROS scavenging ability and protecting cytomembrane integrity. Food Sci. Nutr. 2021, 9, 4963–4973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qi, Y.; Li, R.; Yang, Y.; Yan, D.; Liu, X.; Ren, X. Postharvest applications of n-butanol increase greasiness in apple skins by altering wax composition via effects on gene expression. Postharvest Biol. Technol. 2019, 155, 111–119. [Google Scholar] [CrossRef]
- Caihong, L.; Qi, Z.; Qian, L.; Xue, W.; Taledawu, G.; Zuoshan, F.; Jing, W. Effect of N-butanol treatment on chilling injury and quality of postharvest Hami melon fruits. Sci. Technol. Food Ind. 2021, 42, 325–330. [Google Scholar]
- Huang, S.; Bi, Y.; Li, H.; Liu, C.; Wang, X.; Wang, X.; Lei, Y.; Zhang, Q.; Wang, J. Reduction of membrane lipid metabolism in postharvest Hami melon fruits by n-Butanol to mitigate chilling injury and the cloning of phospholipase D-β Gene. Foods 2023, 12, 1904. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Du, R.; Liu, Y.; Ying, T.; Mao, L. Effect of nitric oxide on antioxidative response and proline metabolism in banana during cold storage. J. Agric. Food Chem. 2013, 61, 8880–8887. [Google Scholar] [CrossRef]
- Barman, K.; Siddiqui, W.M.; Patel, V.B.; Prasad, M. Nitric oxide reduces pericarp browning and preserves bioactive antioxidants in litchi. Sci. Hortic. 2014, 171, 71–77. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Shi, J. Inhibitory effects of propyl gallate on browning and its relationship to active oxygen metabolism in pericarp of harvested longan fruit. LWT-Food Sci. Technol. 2015, 60, 1122–1128. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidation stress and some antioxidant systems in acid rain-treated bean plant: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Chumyam, A.; Rachtanapun, P.; Uthaibutra, J.; Saengnil, K. Reduction of reactive oxygen species production and membrane damage during storage of ‘Daw’ longan fruit by chlorine dioxide. Sci. Hortic. 2014, 170, 143–149. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C. ABA effects on ethylene production PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174. [Google Scholar] [CrossRef]
- Jiang, Y. Role of anthocyanins, polyphenol oxidase and phenols in lychee pericarp browning. J. Sci. Food Agric. 2000, 80, 305–310. [Google Scholar] [CrossRef]
- Yi, C.; Qu, H.X.; Jiang, Y.M.; Shi, J.; Duan, X.W.; Joyce, D.C.; Li, Y.B. ATP-induced changes in energy status and membrane integrity of harvested litchi fruit and its relation to pathogen resistance. J. Phytopathol. 2008, 156, 365–371. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liquori, L.; Pizzolongo, F.; Romano, R.; Matteo, M. Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1432. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with lncreased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Modesti, M.; Petriccione, M.; Forniti, R.; Zampella, L.; Scortichini, M.; Mencarelli, F. Methyl jasmonate and ozone affect the antioxidant system and the quality of wine grape during postharvest partial dehydration. Food Res. Int. 2018, 112, 369–377. [Google Scholar] [CrossRef]
- Lichanporn, I.; Techavuthiporn, C.; Wongs-Aree, C. Effect of silver particle-longkong peel extract coating on postharvest decay and browning in longkong fruit. Hortic. J. 2020, 89, 328–336. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, A.M.; Shi, J.; Zhang, S.; Chen, Y.; Wang, H. Hydrogen peroxide-induced pericarp browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.M.; Barrett, M.D. Thermal, high pressure, and electric filed processing effects on plant cell membrane integrity and relevance to fruit and vegetable quality. J. Food Sci. 2010, 75, R121–R130. [Google Scholar] [CrossRef]
- Qu, G.; Ba, L.; Wang, R.; Li, J.; Ma, C.; Ji, N.; Cao, S. Effects of melatonin on blueberry fruit quality and cell wall metabolism during low temperature storage. Food Sci. Technol. 2022, 42, e40822. [Google Scholar] [CrossRef]
- Mohideen, K.; Sudhakar, U.; Balakrishnan, T.; Almasri, A.M.; Al-Ahmari, M.M.; Al Dira, S.H.; Suhluli, M.; Dubey, A.; Mujoo, S.; Khurshid, Z.; et al. Malondialdehyde, an oxidative stress marker in oral squamous cell carcinoma—A systematic review and meta-analysis. Curr. Issues Mol. Biol. 2021, 43, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Motes, M.C.; Pechter, P.; Yoo, M.C.; Wang, S.Y.; Chapman, D.K.; Blancaflor, B.E. Differential effects of two phospholipase D inhibitors, 1-butanol and N-acylethanolamine on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma 2005, 226, 109–123. [Google Scholar] [CrossRef]
- Venkatachalam, K. Bioactive compounds of Longkong fruit (Lansium domesticum Corr.). In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H., Bapat, V., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Aloo, O.S.; Ofosu, K.F.; Oh, H.D. Elicitation: A new prespective into plant chemo-diversity and functional property. Crit. Rev. Food Sci. Nutr. 2021, 22, 4522–4540. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Bukso, M.; Ostrowska-Kolodziejczak, A.; Zaluski, D.; Perkowski, J. trans-Cinnamic and cholorogenic acids affect the secondary metabolic profiles and ergosterol biosynthesis by Fusarium culmorum and F. graminearum Sensu Stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidase in crops: Biochemical physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K. Postharvest physiology and handling of longkong fruit: A review. Fruits 2016, 71, 289–298. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Wei, B.; Cheng, S.; Ji, S. Membrane lipid metabolism in relation to core browning during ambient storage of “Nanguo” pears. Postharvest Biol. Technol. 2020, 169, 111288. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Dai, H.; Wang, Y.; Ji, S.; Kong, X.; Zhang, F.; Zhou, X.; Zhou, Q. Effect of intermittent warming on the quality and lipid metabolism of blueberry (Vaccinium corymbosum L., cv. Duke) fruit. Front. Plant Sci. 2020, 11, 590928. [Google Scholar] [CrossRef]
- Wang, X.; Devaiah, P.S.; Zhang, W.; Welti, R. Signaling functions of phophatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhou, X.; Liu, X.; Wang, J.; Zhou, Q.; Wang, L.; Zhang, Q.; Ji, S. Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’ pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 2016, 117, 1–8. [Google Scholar] [CrossRef]
- Li, L.; Ishdorji, G.; Gibson, B.S. Reactive oxygen species regulation of autophagy in cancer: Implications for cancer treatment. Free Radic. Biol. Med. 2012, 53, 1399–1410. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. The ambiguous aspects of oxygen. Oxygen 2022, 2, 382–409. [Google Scholar] [CrossRef]
- Cho, J.; Seo, M.; Kim, H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol. Cells 2011, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Rossatto, T.; Amaral, N.M.; Benitez, C.L.; Vighi, L.I.; Braga, B.J.E.; Junior, M.M.A.; Maia, C.A.M.; Pinto, S.L. Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol. Mol. Biol. Plants 2017, 23, 865–875. [Google Scholar] [CrossRef]
- Juan, A.C.; Lastra, P.M.J.; Plou, J.F.; Perez-Lebena, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, B.M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, M.S.; Mahmud, A.J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antoxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Tanko, Y.; Abdelaziz, M.M.; Adelaiya, B.A.; Fatitu, M.Y. Effects of n-butanol portion of indigofera pulchra leaves extract on blood glucose levels of Alloxan-induced diabetic and normoglycemic wistar rats. Int. J. Appl. Res. Nat. Prod. 2008, 1, 13–18. [Google Scholar]
- Abu, S.M.; Yakuba, E.O.; Tatah, V.S.; Onuche, J. Antioxidant potency of n-butanol fraction of Ficus glumosa leaves against oxidative stress induced by carbon tetrachloride in the kidney of rats. Nusant. Biosci. 2022, 14, 40–46. [Google Scholar] [CrossRef]
- Chen, J.; He, F.; Liu, S.; Zhou, T.; Baloch, S.; Jiang, C.; Pei, X. Cytoprotective effect of Ligustrum robustum polyphenol extract against hydrogen peroxide-induced oxidative stress via Nrf2 signaling pathway in Caco-2 cells. Evid. Based Complement. Altern. Med. 2019, 2019, 5026458. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenphun, N.; Ali, A.M.M.; Paulraj, B.; Venkatachalam, K. Effect of Aqueous n-Butanol Treatments on Shelf-Life Extension of Longkong Fruit during Ambient Storage. Horticulturae 2023, 9, 938. https://doi.org/10.3390/horticulturae9080938
Charoenphun N, Ali AMM, Paulraj B, Venkatachalam K. Effect of Aqueous n-Butanol Treatments on Shelf-Life Extension of Longkong Fruit during Ambient Storage. Horticulturae. 2023; 9(8):938. https://doi.org/10.3390/horticulturae9080938
Chicago/Turabian StyleCharoenphun, Narin, Ali Muhammed Moula Ali, Balaji Paulraj, and Karthikeyan Venkatachalam. 2023. "Effect of Aqueous n-Butanol Treatments on Shelf-Life Extension of Longkong Fruit during Ambient Storage" Horticulturae 9, no. 8: 938. https://doi.org/10.3390/horticulturae9080938
APA StyleCharoenphun, N., Ali, A. M. M., Paulraj, B., & Venkatachalam, K. (2023). Effect of Aqueous n-Butanol Treatments on Shelf-Life Extension of Longkong Fruit during Ambient Storage. Horticulturae, 9(8), 938. https://doi.org/10.3390/horticulturae9080938








