Abstract
This study was designed to bridge extant research gaps regarding the vulnerable and protected local endemic Campanula pelviformis, a wild edible green traditionally consumed in Crete (Greece) with agro-alimentary and medicinal interest as well as ornamental value. The C. pelviformis ecological profile was generated using the climate and temperature conditions prevailing in its wild habitats through mapping of natural distribution linked with online bioclimatic databases in geographical information systems. We tested the germination of seeds from five wild-growing populations at four different temperatures (10, 15, 20 and 25 °C) and under different light conditions (light/dark and darkness), and we performed fertilization trails [integrated nutrient management (INF), chemical fertilization (ChFe), control] examining morphological and physiological characteristics, above- and below-ground macro- and micronutrients and phenol contents, as well as their antioxidant capacity. We found population and temperature effects on seed germination with their interaction being statistically significant. Campanula pelviformis germinated better at 10 and 15 °C (>85% for all populations) with no preference on light conditions (98.75% and 95% in light and dark conditions). The INF application increased root dry mass, chlorophyll content index and chlorophyll fluorescence compared to other treatments and was beneficial for macro- and micronutrient concentrations in above-ground parts compared to previously studied wild-growing material, while below-ground parts were positively impacted by both fertilization types. Total phenols and antioxidant capacity were both increased by ChFe fertilization. The data furnished herein permitted the re-evaluation and upgrade of its sustainable exploitation potential in different economic sectors.
1. Introduction
Campanula spp. (Campanulaceae) are widely recognized plant species for their ornamental value, and many of them are frequently used as ornamental plants around the world [1]. In fact, Campanula glomerata L., Campanula medium L., Campanula cochleariifolia Lam. are used as garden plants; Campanula isophylla Moretti, Campanula carpatica Jacq., Campanula rotundifolia L., Campanula pyramidalis L. are used as potted plants, while C. medium, Campanula persicifolia L., C. pyramidalis, and C. glomerata are used for cut flowers. To date, there is an ongoing quest for new Campanula species with interesting or promising ornamental potential in this sector [1,2].
Apart from the horticultural value, species within the genus Campanula are also being exploited for agro-alimentary purposes in different regions [3]. For example, local Spanish residents use raw roots of C. rapunculus L. in salads for their nutritional benefits, and Italians collect the same plant using its raw roots or aerial parts in salads [3,4]. Furthermore, Campanula pelviformis Lam. has been reported as a wild green traditionally consumed as food in the eastern part of Crete [5,6]. In fact, the phytochemical profile of C. pelviformis has been recently analyzed, and several isolated compounds have been found to possess pharmaceutical properties, thus rendering it as an emerging medicinal plant [6]. These findings render C. pelviformis as a trustworthy source of diverse phytonutrients and bioactive compounds [6].
The genus Campanula comprises 448 taxa (species and subspecies) worldwide (https://powo.science.kew.org/, accessed on 19 April 2023), most of which are perennials (occasionally annuals or biennials), often thriving in rocky areas, slopes, or grounds; many Campanula members are often range-restricted or endemic plants in different regions, thus associated with high biological value [7]. In Greece, there are 95 wild-growing Campanula taxa, and most of them are exclusively found in crevices of limestone cliffs growing as chasmophytes [8]. Of these, 11 taxa are to be found in Crete, with 8 being local single-island endemics [8,9]. C. pelviformis is included as a local endemic plant in the extraordinary plant diversity of Crete [9], which involves numerous single-island endemic taxa that are still neglected and underutilized plants (NUPs) [2,10,11,12]. Previous studies have shown that the exploitation of such unique phytogenetic resources holds great potential for different economic sectors, such as in agriculture, medicinal-cosmetics, and ornamental horticulture [1,2,11,12,13,14]. Furthermore, conservation efforts have been increased to date to achieve effective protection for local endemic NUPs, especially for those threatened with extinction [2]. Significant information on both the economic and biological value of threatened local endemic NUPs has been gathered through species-specific studies [2,11,12,13], and basic research gaps related to their propagation and ex-situ cultivation protocols have been addressed during the last decade [2,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].
Applied research to develop appropriate propagation methods for local endemic NUPs aiming to re-enforce threatened populations in the wild has primarily been driven by their appreciated biological rarity and priceless ecological value [2,10]. However, this has also been often coupled by potential economic benefits associated with their sustainable utilization and concomitant exploitation [2,11,12]. The effectiveness of these two research directions can be facilitated by information on the environmental conditions associated with targeted NUPs reflecting their adaptation in natural habitats and their ecological preferences [2,15,16,17,18,19,20,22,23,24,25,26,28]. Such data can be utilized to adjust or improve their propagation conditions, for example, through seed propagation [30], which is the most economical and efficient propagation method for many plant species [31]. Furthermore, for the ex situ conservation of targeted NUPs, and particularly for threatened species originating from the challenging terrain of the Mediterranean basin, it is crucial to achieve successful propagation and cultivation allowing for the production of plant stocks [2,32]. Various cultural practices have been employed in the past with reasonable ex situ cultivation and acclimatization results in terms of morphological and physiological traits, thus leading to the conclusion that ex situ cultivation can contribute significantly to the production of high-quality plants [33]. The application of fertilizers during the propagation stage in the nursery has been shown to increase the nutrient reserves in propagated plants for population restoration efforts, thus rendering fertilization as an efficient method leading to enhanced establishment success in their natural habitat [34].
Species-wise, this study is focused on Campanula pelviformis, a vulnerable [35] and range-restricted local endemic plant of eastern Crete, which is protected by the Greek Presidential Decree 67/1981, although it is traditionally consumed as a wild edible green [6], thus meriting conservation concern. Given that many species from genus Campanula are already used for ornamental and ethnobotanical purposes [1,3,4,6], C. pelviformis also takes advantage of its sustainable exploitation, since high demand of such unique resources may lead to excessive collection in the wild, risking the degradation of genetic pools and local biodiversity. Context-wise, this study was designed to bridge extant research gaps regarding the studied focal species. The aims of this research were to: (i) define the optimal temperature and light conditions required for the successful seed germination of C. pelviformis, examining seed responses of different wild-growing populations from low and semi-mountainous areas of Crete; (ii) identify the preferred ecological conditions of C. pelviformis based on its natural distribution; (iii) compare the impact chemical fertilizers and integrated nutrient management on the growth of seedlings and estimate their nutrient content; (iv) assess the overall phenolic content and antioxidant capacity of its aerial parts; and in the light of all the aforementioned, (v) reassess the sustainable utilization potential of C. pelviformis in the horticultural-ornamental, agro-alimentary and medicinal sectors.
2. Materials and Methods
2.1. Plant Characteristics
Campanula pelviformis (Figure 1) is a hemicryptophyte with a restricted range occurring only in the eastern part of the island of Crete, Greece [8]. This local endemic species thrives in rocky slopes and ledges, stony dry places or walls in villages and rocky road embankments, spanning an elevation range from sea level to 850 m above sea level [8]. Currently, it is considered as Vulnerable taxon [35] and a protected plant species due to its inclusion in the Greek Presidential Decree 67/1981 (https://portal.cybertaxonomy.org/flora-greece/cdm_dataportal/taxon/332301ee-177e-4f9f-95b9-62fcea5e9714, accessed 29 June 2023).
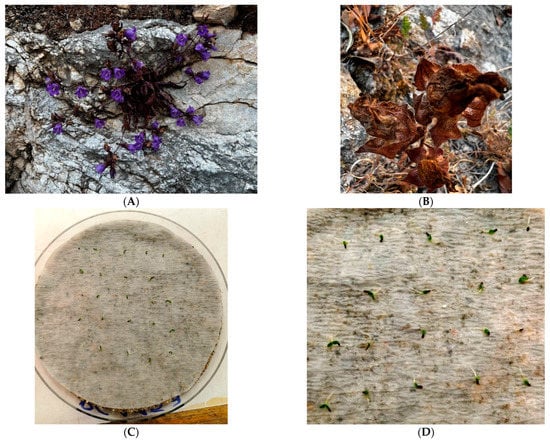
Figure 1.
Plant individual of Campanula pelviformis as chasmophyte on vertical rocky crevices of Kalo Chorio (Lasithi, Greece) in full flowering (A), ripe fruits collected in Mt Thrypti (B), seed germination in Petri dishes (C) and individual germinated seeds (D).
2.2. GIS Ecological Profiling
The natural occurrence points of C. pelviformis derived from a previously published study [36]. To create the ecological profile in geographical information systems (GIS) for this species, historical (1970–2000) climate and precipitation data as well as similar values regarding 19 bioclimatic variables (minimum, maximum, and average values for every month) with a pixel size 30 sec and spatial resolution of 1 km2, were obtained from the WorldClim database (https://www.worldclim.org/data/worldclim21.html, accessed on 15 June 2023) and were linked with the raster file containing its natural distribution points in Crete (n = 16).
2.3. Seed Collection and Storage
Mature seeds from approximately five to ten wild-growing individuals of five wild-growing populations thriving in different altitudes were collected by hand during the summer of 2019 and the summer of 2020, prior dispersal (Table 1). As C. pelviformis is both a protected and threatened species, the seeds were collected after applying for a special permission, and the collection permits (182336/879 of 16 May 2019 and 64886/2959 of 6 July 2020) were issued by the Greek Ministry of Environment and Energy. Upon collection, the collected capsules of each population were spread out on filter papers in laboratory conditions and left for a week to dry out. Then, the seeds were cleaned manually from capsule and/or stem remnants, and they were dry-stored in glass containers at 3–5 °C before experimentation.

Table 1.
Geographical details about the seed collections performed and IPEN (International Plant Exchange Network) accession numbers of Campanula pelviformis seed lots collected from lowland and semi-mountainous areas of Eastern Crete, Greece.
2.4. Germination Tests
The germination experiments were conducted in April 2022 at the Laboratory of Floriculture, School of Agriculture, Aristotle University of Thessaloniki (Thermi, Greece). Prior to experimentation, measurements of seeds’ weight, length and width were conducted for each population. The weights of 1000 seeds for each population were measured using a laboratory scale with four decimals, and photos from 30 seeds per population were taken for calculating their length and width using the ImageJ software (ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA). Based on the above measurements, the seeds from lowland populations used in the experiments had a 1000-seed weight of 0.099 g for GR-BBGK-1-20,365, 0.07 g for GR-BBGK-1-19,1056 and 0.042 g for GR-BBGK-1-19,1194, whereas both semi-mountainous populations had a lower 1000-seed weight of 0.02 g in both cases. The average length/width of the seeds from lowland populations ranged from 0.615/0.283 mm (GR-BBGK-1-19,1194) to 0.691/0.358 mm (GR-BBGK-1-20,365). In comparison, the semi-mountainous populations showed an average length/width of 0.592/0.284 mm (GR-BBGK-1-19,1187A) and 0.583/0.280 mm (GR-BBGK-1-19,1187B).
The temperature experiments employed seeds from five distinct populations originating from various altitudes, as described above, and evaluated their seed responses to four constant temperatures of 10, 15, 20 and 25 °C within four different temperature-controlled growth chambers. For each temperature treatment, we tested four replications of 20 seeds (n = 4) placed in 9 cm sterile plastic Petri dishes with two filter papers moistened with distilled water. All Petri dishes were randomly placed on the shelves of the growth chambers with the photoperiod adjusted at 12 h light/12 h dark. During the experimental period, all filter papers were kept regularly moist. Every five days for a period of 45 days, all germinated seeds were totaled and removed. The criterion used for determining seed germination of an individual seed was the evident emergence of radicle protrusion (at least 2 mm) from the seed coat.
After completion of the temperature-related germination experiments, new experiments were carried out to investigate the light requirements for C. pelviformis seed germination. The population GR-BBGK-1-19,1194 with the highest gemination percentages and the temperature of 15 °C for seed germination were selected for these experiments. The same experimental design/protocol was followed, and Petri dishes were chambered under alternating light/dark (12 h light/ 12 h dark photoperiod) and continuous dark conditions. For the treatment in the dark, each Petri dish was covered with aluminum foil, which was opened only for less than a few minutes to allow scoring for seed germination and was immediately reclosed. For each treatment (light/dark, dark), the mean germination time (MGT) was calculated as the average of four replications consisted of 20 seeds each. The MGT was calculated for each replication per treatment according to the following equation:
where n is the number of seeds which germinated on day D, and D is the number of days counted from the beginning of the test [37].
2.5. Plant Production
The seeds of C. pelviformis GR-BBGK-1-19,1056 (Table 1) were used for a pilot germination experiment performed in December 2020 aiming at plant production. Specifically, three germinated seeds were placed in each plastic pot (6 × 6 × 6.5 cm) filled with a 3:1 ratio (v/v) substrate of sphagnum peat moss (TS2, Klasmann-Deilmann GmbH, Geeste, Germany) and perlite (Perloflor Hellas, Volos, Greece); the latter substrate was carefully topped with river sand, and all the containers were placed randomly on a greenhouse bench. After seedling emergence and the fully developed cotyledons, the seedlings were thinned so that one seedling remained per pot. In total, there were 36 pots with one seedling each. The potted seedlings were periodically irrigated to maintain the proper moisture conditions for further growth until developing a potent root system. Three months after sowing (in the middle of March 2021), all seedlings were carefully transplanted into larger pots (8.5 × 8.5 × 9.5 cm). The substrate of the larger pots was soil, sphagnum peat moss (TS2, Klasmann-Deilmann GmbH, Geeste, Germany) and perlite (Perloflor Hellas, Volos, Greece) in a ratio of 4:5:1 (v/v). To assess the soil fertility before the substrate preparation, a soil sample of approximately 1.5 kg was chemically and mechanically analyzed (Table 2).

Table 2.
Chemical and physical properties of the soil used for Campanula pelviformis seedling development.
After transplantation, a total of 36 seedlings were randomly divided into 3 groups of 12 plants each (n = 12) to employ 3 different fertilization treatments: seedling group with integrated nutrient management (INM) applied; a second group with chemical fertilization (ChFe) applied; and a third group with no applied fertilization (control). Both types of fertilizers were foliar spray applications using a low-pressure electric sprayer system; the plants were sprayed with the INM and ChFe treatment solutions until saturation, and the control group was sprayed only with tap-water. The INM fertilization consisted of a nutrient solution of THEOCOPPER at 7 mL/L, THEOCAL at 1.5 g/L, THEOFAST at 5 mL/L (THEOFRASTOS fertilizers, Korinthos, Greece), 10-47-10 (AGRI.FE.M. LTD Fertilizers, Athens, Attica, Greece) at 3.2 g/L, K2SO4 (0-0-52, AGRI.FE.M. LTD Fertilizers, Greece) at 2.07 g/L, micronutrients (Plex Mix, AGRI.FE.M. LTD Fertilizers, Athens, Attica, Greece) at 1.5 mL/L and MgSO4 (Mg 25.6%, AGRI.FE.M. LTD Fertilizers, Greece) at 0.6 g/L [21,27]. For the conventional fertilization (ChFe), the nutrient solution consisted of NH4NO3 (34,4-0-0, Neofert®, Neochim PLC, Dimitrovgrad, Bulgaria) at 2.7 g/L, Ca(NO3)2 (NITROCAL, Agrohimiki, Patras, Greece) at 1.7 g/L, 10-47-10 at 3.2 g/L, K2SO4 (0-0-52) at 2.27 g/L, micronutrients Plex Mix at 1.5 mL/L and MgSO4 (Mg 25.6%) at 0.6 g/L [21,27]. Both fertilization treatments obtained the same units of macronutrients at ratio of 15-15-15, Mg at 1.5%, Ca at 4.6% and the same amounts of micronutrients. The INM treatment consisted of an additional amount of copper at 1% w/v, polysaccharides, organic nitrogen (at urea and amino acid form) and a foliar biostimulator (THEOFAST) compared to the ChFe treatment. The application of fertilization treatments was performed every week from late March 2021 until the middle of June 2021, and the plants were irrigated every 2–3 days depending on ambient temperature conditions. The plants were grown inside an unheated greenhouse of the Laboratory of Floriculture, School of Agriculture, located in the farm of Aristotle University of Thessaloniki (Thermi, Greece).
2.6. Morphological and Physiological Measurements of Seedlings
At the end of June, we evaluated the effect of fertilization treatment on morphological and physiological variables of C. pelviformis. In each plant individual per treatment, we measured the main shoot height (SH) using a metal ruler, root collar diameter (RCD) using a digital calliper and the shoot number. In addition, we measured the root dry biomass (RDB) and the above-ground dry biomass (AGDB) in four randomly sampled plants per treatment. All dry weights were defined after drying of the materials in the oven at 74 °C for 48 h.
Along with the above-mentioned, gas exchange parameters, chlorophyll content and fluorescence were determined in the fertilized and control seedlings of C. pelviformis. A chlorophyll meter CCM 200 (Opti-sciences, Tyngsboro, MA, USA) was used to calculate the chlorophyll content index (CCI) based on the ratio of transmittance measurement at 660 and 940 nm [38]. The chlorophyll fluorescence parameter Fv/Fm was measured by OS30p+ Rapid Plant Stress Screening Device (Opti-sciences, Tyngsboro, MA, USA). Generally, Fv/Fm is the ratio of variable (v) to maximum (m) fluorescence after dark-adaptation, thus representing the maximum photochemical efficiency of photosystem II, which is commonly used for detection of various stress conditions [39]. For each fertilization treatment, 13 measurements with CCM200 and 10 measurements with OS30p+ were performed on fully expanded young leaves of the C. pelviformis plant individuals.
2.7. Plant Tissue Analyses of Seedlings
After drying in the oven at 74 °C for 48 h, the tissue samples of the C. pelviformis plant individuals were finely ground to pass a 40-mesh sieve. More precisely, for each treatment the above-ground part (leaves and shoots) and the below-ground part (roots) of the dried plants were separately grounded to record the respective tissue nutrient concentration. Three samples of fine powder were formed for each plant part (either above-ground or below-ground), which corresponded to the three fertilization treatments (ChFe, INM, control) examined. Subsequently, from each fine powdered and homogenized sample, three subsamples of ca. 0.25 g each were randomly taken (n = 3), and each subsample was disorganized by the method of wet oxidation until a transparent solution was obtained using a triple acid mixture of H2SO4, HNO3 and HClO4 in a ratio of 5:1:1 at 80 °C [40]. The solutions resulting from the digested samples were analyzed colorimetrically for the total P (phosphorus) determination according to the molybdenum blue method by using a Shimadzu spectrophotometer model UV-1201V [41]. The potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), copper (Cu), iron (Fe), zinc (Zn) and manganese (Mn) concentrations were assessed by atomic absorption spectroscopy (Perkin-Elmer Analyst 300), while total nitrogen (N) was determined by the Kjeldahl method [42].
2.8. Total Phenol Content and Antioxidant Capacity
Fresh leaves from C. pelviformis plant individuals were selected per fertilization treatment at the end of the experiment and were grounded to powder by liquid nitrogen. The amount of 0.1 g of lyophilized leaves was extracted by adding 1 mL methanol, was vortexed for 20 min and was centrifuged at 10,000× g at room temperature for 5 min. The upper liquid portion was separated from the solid residue, was transferred to a new vial, and was stored under −20 °C until analyzed.
We employed the Folin–Ciocalteu method [43] for the determination of total phenol content (TPC) in samples. Each sample (0.5 mL) was diluted in MeOH (1:50 v/v) with the addition of 2.5 mL of the Folin–Ciocalteu solution (1:10 v/v). Two (2) mL of Na2CO3 (7.5% w/v) solution were also added after 6 min, and the whole mixture was vortexed. Finally, the prepared sample mixtures were placed in a water bath for 5 min at 50 °C (LabTech Digital Water Bath, Gurgaon, India). Analyses of all samples were conducted by recording their absorbance at 760 nm by a Helios Alpha spectrophotometer (Thermo Spectronic, Cambridge, UK). We used a gallic acid calibration curve (100, 50, 25, 12.5 and 0 g/mL) to calculate the TPC as mg of gallic acid equivalents per g of fresh weight (mg GAE/g FW).
We employed the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method [43] for the determination of antioxidant capacity (AC). In 0.2 mL of samples dissolved in 80% v/v MeOH (1:20 v/v), the amount of 2.8 mL 4% w/v DPPH was added, vortexed and incubated in darkness for 30 min at room temperature. The AC of the samples was recorded at 517 nm in a spectrophotometer, was compared to an ascorbic acid calibration curve (0.1, 0.05, 0.025, 0.01 and 0 g/mL), thus was expressed in mg of ascorbic acid equivalents per g of fresh weight (mg AAE/g FW).
The final readings of TPC and AC determination of each C. pelviformis sample represented the mean of three biological replications, and they were shown as mean ± standard error. Analysis of variation (ANOVA) was used for comparisons of means using SPSS 27 (IBM Corp., Armonk, NY, USA) and Duncan’s multiple range test at p < 0.05.
2.9. Statistical Analysis
In the germination experiment, the experimentation employed a completely randomised design with two factors (different populations, and incubation temperature). The germination data at 25 °C was not further analysed since none of the seeds germinated for populations GR-BBGK-1-19,1194; GR-BBGK-1-19,1187A; GR-BBGK-1-19,1187B, or the percentages of the germinated seeds were very low, i.e., <6% in populations GR-BBGK-1-20,365 and GR-BBGK-1-19,1056. Therefore, in the statistical analysis, the levels of the factors were: five for the populations and three for the incubation temperature (5 × 3 factorial design). The data were analysed using ANOVA in the frame of the procedure general linear model (GLM) [44], and the means were compared using the LSD test at a significance level p ≤ 0.05. In the remaining statistical analyses, the comparisons of the means were made using the Duncan’s test at a significance level p ≤ 0.05 [45]. All statistical analyses were performed with SPSS 21.0 (SPSS, Inc., IBM Corp., Armonk, NY, USA).
3. Results
3.1. Seed Germination Success
3.1.1. Effects of Temperature Treatments
The population (p = 0.002) and temperature (p = 0.000) factors as well as their interaction (p = 0.000) had a significant effect on seed germination of C. pelviformis.
The most successful germination was achieved at 10 and 15 °C for populations GR-BBGK-1-19,1194 and GR-BBGK-1-19,1187A (97.5% and 92.5%, respectively), while populations GR-BBGK-1-20,365 (96.25%) and GR-BBGK-1-19,1187B (95%) exhibited higher germination percentage at 15 and 10 °C, respectively, than at 20 °C (Figure 2). For population GR-BBGK-1-19,1056, temperature had no statistically significant impact on seed germination (Figure 2).
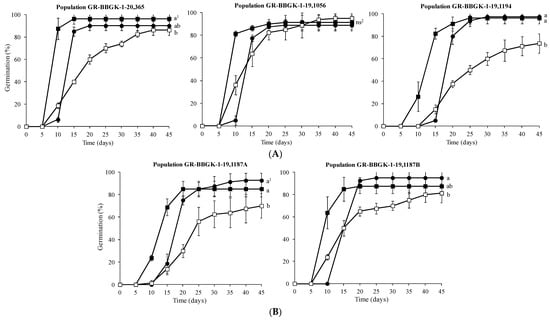
Figure 2.
Cumulative germination percentage diagrams of Campanula pelviformis seeds of the five populations ((A): lowland populations; (B): semi-mountainous populations, see Table 1) incubated at 10 (●), 15 (■) and 20 (□). The comparisons were made using the LSD test. 1—In each population, means were statistically different at p < 0.05 when not sharing a common letter (n = 4); ns2—non-significant differences (n = 4).
Regarding the germination speed of C. pelviformis seeds, it was observed that in all populations the seeds germinated faster at 15 °C in comparison with the rest of the temperature treatments (Figure 2). In fact, population GR-BBGK-1-20,365, GR-BBGK-1-19,1056 and GR-BBGK-1-19,1187B recorded higher rates of germination (>60%) on the 10th day (Figure 2). Furthermore, the germination of seeds at 20 °C was quite gradual compared to the rest of the examined temperatures for all populations (Figure 2).
The temperature level of 10 °C had a significant impact on seed germination of C. pelviformis, with seeds of GR-BBGK-1-19,1194 population exhibiting higher germination percentage compared to those of population GR-BBGK-1-19,1056 (Table 3). At 15 °C, the seeds of the lowland populations GR-BBGK-1-20,365 and GR-BBGK-1-19,1194 achieved higher germination percentages than those of the semi-mountainous populations GR-BBGK-1-19,1187A and GR-BBGK-1-19,1187B (Table 3). The temperature level of 20 °C notably reduced all seed germination percentages of the examined populations, particularly in GR-BBGK-1-19,1187A and GR-BBGK-1-19,1194. However, in the same temperature the seeds of the lowland population GR-BBGK-1-19,1056 germinated at their highest percentage and those of population GR-BBGK-1-19,1187B still showed 81.25% germination (see Table 3).

Table 3.
Effect of Campanula pelviformis populations from lowland and semi-mountainous areas on the seed germination percentage for each incubation temperature with high seed germination. Means ± standard deviation values are given.
3.1.2. Effects of Light Treatments
No statistically significant results were observed in terms of mean germination percentage and mean germination time (MGT) between C. pelviformis seeds germinated under alternating light/dark and continuous dark conditions. Germination percentages were high for both treatments (98.75% in light/dark and 95% in dark) (Table 4), and the examined seeds showed similar behaviour regarding MGT under different light conditions (10.95 days in light/dark and 11.32 days in dark conditions).

Table 4.
Effect of light conditions on germination of Campanula pelviformis population GR-BBGK-1-19,1194 seeds. Means and standard deviation values are given.
3.1.3. Ecological Profiling
To better understand the biological circle of C. pelviformis, an ecological profile was created in GIS depicting the temperature and precipitation needs of the wild-growing populations (Supplementary Material Table S1).
The results of the temperature-related attributes showed that C. pelviformis populations are naturally adapted to a relatively warm winter season (mean temperature of coldest quarter = 11.12 ± 0.87 °C; minimum temperature of coldest month = 7.63 ± 0.86 °C), with the lowest average temperatures recorded in January (10.77 ± 0.89 °C) and February (10.69 ± 0.86 °C). The average temperature is shown to rise gradually from March (11.96 ± 0.84 °C) until June (22.71 ± 0.70 °C). Onwards in mid-summer (July–August), the average temperature steadily rises to 26 °C. After the summer season, the average temperature decreases from 22.72 ± 0.79 °C in September to 12.25 ± 0.88 °C in December (winter season). The aforementioned factors, combined with the absence of extreme temperatures in its natural distribution sites (Tmin of Tmin = 5.71 °C in February, Tmax of Tmax = 30.29 °C in July) and the low mean diurnal range (6.86 ± 0.13 °C), may indicate that C. pelviformis is naturally adapted in relatively warm environments with regards to temperature.
Historical precipitation data depicted a seasonality of rainfall in the natural range of C. pelviformis. Specifically, high precipitation was recorded from December (120.09 ± 11.06 mm) until February (98.31 ± 8.99 mm), with the highest precipitation values recorded in January (134.03 ± 12.20 mm). From March (75.16 ± 7.74 mm) onwards, the mean of precipitation was shown to decrease until May (16.90 ± 2.75 mm), leading to summer season, which is the driest period with scarce precipitation (4.54 ± 1.22 mm in June, 1.68 ± 0.60 mm in July and 2.00 ± 0.52 mm in August). After summer, the mean of precipitation was shown to rise again from 17.42 ± 1.20 mm in September to 72.80 ± 4.80 mm in November, initiating the wet season.
3.2. Seedlings’ Growth
The parameters main shoot height (p = 0.085), root collar diameter (p = 0.827), number of shoots (p = 0.146), and above-ground dry biomass (p = 0.086) were not affected by the applications of fertilizers (Table 5), whereas fertilizing had impact on root dry biomass (p = 0.005), chlorophyll content index (p = 0.000), and chlorophyll fluorescence (p = 0.017) of C. pelviformis plant individuals (Table 5). As far as the root dry biomass is concerned, control plants showed significantly lower values compared to INM fertilized plants, whereas no significant difference was observed between plants fertilized with INM and ChFe (Table 5). The non-fertilized plants recorded the lowest values of chlorophyll content, and the plants fertilized with INM had the highest values (Table 5). The chlorophyll fluorescence of fertilized plants regardless of the type of fertilizer was significantly higher compared to that of the non-fertilized plants of C. pelviformis (Table 5).

Table 5.
Effect of fertilization (INM: integrated nutrient management; ChFe: chemical fertilization) on morphological and physiological characteristics of Campanula pelviformis plant individuals. Means ± standard deviation values are provided.
3.3. Leaf Nutrient Concentration
Macronutrient concentrations in leaves and shoots of C. pelviformis (Table 6) were generally affected by fertilization (p = 0.000 for N; p = 0.002 for P; p = 0.000 for K; p = 0.000 for Ca; p = 0.000 for magnesium; p = 0.000 for Na). The macronutrient content of C. pelviformis leaves and shoots showed a trend for increased concentration of N, K, Ca, Mg and Na in plants treated with INM compared to unfertilized plants (control) or ChFe, while P was detected in the highest concentration in the leaves and shoots of untreated plants (Table 6).

Table 6.
Macronutrient concentrations in above-ground (leaves and shoots) and below-ground (roots) parts of cultivated Campanula pelviformis plant individuals in greenhouse with regards to fertilization (INM: integrated nutrient management; ChFe: chemical fertilization; Control: absence of INM or CHFe, only water).
In the roots of the plant, fertilization affected all macronutrient concentrations (p = 0.000 for N; p = 0.004 for Pl; p = 0.000 for K; p = 0.000 for Ca; p = 0.005 for Mg) except Na (p = 0.198). INM fertilization showed increased contents of N, Ca and Mg compared to ChFe and control plants, while the application of INF decreased P and K compared to the other two treatments.
The fertilization affected the concentration of micronutrients (Table 7) in the above-ground parts (p = 0.000 for Fe; p = 0.008 for Mn; p = 0.000 for Zn; p = 0.000 for Cu) of cultivated C. pelviformis. A remarkable 10-fold increase was detected in the concentration of Cu in plants treated with INM compared to control plants (Table 7). A high increase was also noticed in the concentration of Zn, but no statistical differences were found regarding Mn between the plants treated with INM and ChFe. The concentration of Fe was increased in ChFe plants compared to the other two treatments.

Table 7.
Micronutrient concentration in above-ground (leaves and shoots) and below-ground (roots) parts of Campanula pelviformis plant individuals with regards to fertilization (INM: integrated nutrient management; ChFe: chemical fertilization; Control: only water).
Similarly, root micronutrients were also affected by INF fertilization (p = 0.052 for Fe; p = 0.025 for Mn; p = 0.001 for Cu; p = 0.525 for Zn). INF fertilization increased the concentration of Cu, while ChFe and control plants had higher concentration for Mn (Table 7).
3.4. Total Phenol Content and Antioxidant Capacity
The presence of total phenols was detected in all samples of C. pelviformis, but it was more prominent and significantly higher in the fertilized samples, especially those treated with ChFe (Table 8). The same trend was observed for the AC of the samples, where the one with the most potent antioxidant capacity was again the samples from fertilized plants with ChFe.

Table 8.
Effect of fertilization (INM: integrated nutrient management; ChFe: chemical fertilization; Control: only water) on the concentration of total phenols and antioxidant capacity of Campanula pelviformis young leaves, expressed in mg gallic acid equivalents (GAE) and ascorbic acid equivalents (AAE) per g of fresh (FW), respectively.
4. Discussion
4.1. Seed Germination Protocols
The results of this study suggested a strong temperature effect (p = 0.0001) in the germination of C. pelviformis seeds in line with previous studies examining other members of the genus Campanula [32]. The highest percentages (>85%) of seed germination were observed at 10 and 15 °C, while high rates were even recorded at 20 °C (>70%). Similar results have been recently reported for another chasmophytic local endemic plants of Crete, namely Campanula saxatilis L. subsp. saxatilis [43]. Previous studies have indicated that various taxa of the genus Campanula can germinate even at 25 °C under specific pretreatment methods [32], i.e., 99% for Campanula merxmuelleri Phitos, 91% for Campanula pangea Hatvig, and 80% for C. glomerata. However, no pretreatments were tested herein for C. pelviformis, and at this temperature, C. pelviformis seed germination was quite low and was not included in the analysis.
The investigation herein attempted, for the first time, to examine the germination of seeds collected from different populations of C. pelviformis from lowlands (three populations) and semi-mountainous areas (two populations) of Eastern Crete, Greece. This study reports, for the first time, on the combined influence of altitude and temperature in seed germination behavior of C. pelviformis as recorded for the different populations examined. Although individual effects were not considered herein, this study provides evidence on variation in seed germination of lowland C. pelviformis populations; for example, the seeds of population GR-BBGK-1-19,1194 achieved higher germination percentage compared to population GR-BBGK-1-19,1056 at 10 °C, while the latter showed its highest seed germination at 20 °C. Some variation was also detected between the two semi-mountainous populations of C. pelviformis examined herein from Mt. Thrypti (the second highest mountain in the Lasithi region of Crete), resulting in different germination percentages but similar decrease rates. In general, it is well established that differences may exist in the germination characteristics (rate and percentage of germination) of a taxon’s populations [30]. Previous studies have indicated that other Balkan endemic Campanula species may have quite different germination responses related to temperatures depending on the altitude of original collections; for example: (i) 10%, 11.1%, and 61.1% at 5, 15 and 25 °C, respectively, in C. spatulata Sm. seeds from intermediate (900 m) altitudes compared to 0%, 21.1% and 2.2% at 5, 15 and 25 °C, respectively, in seeds collected from high (1850 m) altitudes [46]: (ii) 0%, 64.4% and 65.6% at 5, 15 and 25 °C, respectively, in C. lingulata Waldst. & Kit. seeds collected from lowlands (450 m) compared to 0%, 54.4% and 30% at 5, 15 and 25 °C, respectively, for seeds originating from plants of intermediate (850 m) altitudes; and (iii) 0%, 56.7% and 16.7% at 5, 15 and 25 °C, respectively, for seeds of plants from higher (1300 m) altitudes as well as 0%, 36.7% and 4.4% at 5, 15 and 25 °C, respectively, for seeds of subalpine altitudes (1700 m) close to timberline [46]. It should be noted, however, that the above-mentioned examples refer to rather common and widespread in Greece Campanula members which are Balkan endemics, with broad ecological adaptations and different habitat preferences [46]. As such, these findings should not be directly compared with other range-restricted chasmophytic Campanula members with narrow niche confined in small geographical territories such as C. pelviformis studied herein (single-island endemic of Crete). Undoubtedly, it would be useful to conduct further experimentation in freshly collected seeds to draw safer conclusions in relation to the germination requirements of the species in concern and in fact this presents another research line that we will soon follow.
Regarding the light requirements for C. pelviformis seed germination, the findings herein suggest that this taxon has no specific preference, as high percentages of germination were achieved in both light treatments (98.75% in alternating light/dark conditions and 95% in darkness), thus aligning with previous studies found in grey literature (Antonidaki-Giatromanolaki as cited by Koutsouvoulou et al. [32]). On the other hand, the seeds of several Campanula taxa clearly exhibit a preference for light for their germination [32], i.e., C. andrewsii A. DC. subsp. andrewsii (93% in light and 26% in darkenss), C. cretica (A. DC.) A. Dietr. (99% in light and 24% in darkness), C. spatulata subsp. spruneriana (Hampe) Hayek (90% in light 60% in darkness), C. spatulata subsp. spatulata (92% in light and 63% in darkness), and C. versicolor Andrews (85% in light and 38% in darkness).
Based on the available data, it appears that the seeds of C. pelviformis germinate best at temperatures of 10 and 15 °C. Consequently, its natural germination in the wild habitats is expected to occur between late autumn and early winter, where the mean monthly air temperature may range between 10 and 15 °C as indicated by the GIS-derived ecological profile. During this period, the increased soil moisture due to high amount of precipitation is probably expected to create the ideal conditions for the germination of seeds (Supplementary Material Table S1). Furthermore, this timing of seed germination probably allows the growth of seedlings through winter to develop into fresh rosettes of C. pelviformis by February when local inhabitants of Crete traditionally collect this wild edible green from the wild [6].
C. pelviformis is widely used in the eastern part of Crete (Lasithi), and young rosettes of leaves are sourced directly from the wild habitats at the end of winter or early in spring together with other wild edible plants. This represents a very localized old tradition in the Lasithi area; for example, C. pelviformis is not mentioned as an edible wild green by Pieroni et al. [47] in their study in central Crete, although it occurs in Heraklion prefecture [8]. This wild gathering of C. pelviformis is mainly being performed to prepare the typical local culinary preparation called “tsigarolachana” (meaning to stir in olive oil). The “tsigarolachana” can be served in everyday life with other basic food ingredients (fish, other seafood, or meat), can be cooked in casserole with all the latter and/or with eggs and other common vegetables. Ultimately, “tsigarolachana” is also the basic filling (together with other wild greens) for pot pies with handmade dough [6]. The daily use and consumption of wild ‘chórta’ (or hórta) including C. pelviformis is linked with the traditional Mediterranean (Cretan) diet, which is associated with high adult life expectancy (longest in the world) and decreased rates of coronary heart disease, certain types of cancer, and other chronic diseases [47]. Among other wild greens, C. pelviformis is considered as a medicinal plant of high nutritional value [6]. In the Lasithi area, there are many regular open markets selling wild herbs and chórta in bunches, especially at the end of winter and beginning of spring, at very high prices equivalent to those of high-quality meat. It should be noted, however, that the high demand of such localized products even at local scales can trigger over-collection from range-restricted wild populations, thus jeopardizing local genetic pools of the local Cretan endemic C. pelviformis. Therefore, the ex situ propagation and cultivation with fertilization regimes as presented herein, for the first time, may offer a sustainable exploitation strategy able to satisfy any future demand regarding the traditionally consumed nutritious rosettes of C. pelviformis.
4.2. Fertilization and Morphological-Physiological Traits
Previous studies have indicated that fertilization applications can enhance the plant photosynthetic rate [48]. The findings herein suggested that the photosynthetic rate such as chlorophyll content index and chlorophyll fluorescence had positive impact from the INF treatment. Similar outcomes had been observed in other rare local endemic species such as the Tunisian endemics Teucrium luteum (Mill.) Degen subsp. gabesianum (S. Puech) Greuter or Marrubium aschersonii Magnus, where fertilization treatments led to an increased photosynthetic rate [22,28]. Additionally, another three Cretan endemic species namely Lomelosia minoana (P. H. Davis) Greuter & Burdet subsp. minoana and subsp. asterusica (Greuter) Greuter & Burdet, and Eryngium ternatum Poir. were affected in the same direction regarding these physiological characteristics from the application of fertilization regimes [43]. More specifically, the INF treatment that resulted in this study in increased root dry biomass in C. pelviformis in a similar pattern to the observations related with M. aschersonii, while L. minoana subsp. asterusica and subsp. minoana showed a negative impact following the INF treatment [28].
4.3. Nutrient Content and Absorption
The effect of fertilization on ex situ cultivated C. pelviformis plants is the first study to date, describing the content and the absorption of macro- and micronutrients. Both INM and ChFe fertilization schemes resulted positively regarding most of the measured parameters compared to the control. The INM fertilization showed statistically significant higher values in chlorophyll content index (CCI) and root dry biomass (RDB) compared to the ChFe and control; however, ChFe resulted in higher values for the rest of the parameters measured, but these were not statistically significant (Table 5).
Tissue analysis of the above-ground parts (leaves, stems) indicated a trend of the INM fertilization to lead to a higher nutrient content and absorption for N, K, Ca, Mg, Na, Zn and Cu compared to the ChFe and control; however, ChFe and control plants resulted in higher values only for Fe and P, respectively (Table 6 and Table 7).
Tissue analysis for the below-ground part (roots) indicated that INM fertilization showed higher nutrient content and absorption for N, Ca, Mg, and Cu compared to the ChFe and control; however, both ChFe and control resulted in higher values for P, K, and Mn compared to INM (Table 6 and Table 7).
Measurements conducted on the leaves of other local endemic plants of Crete revealed that L. minoana subsp. minoana benefited from the INF application in terms of all macronutrients examined except for Na [43]. E. ternatum showed benefits in terms of N and K, while L. minoana subsp. asterusica exhibited high N and P levels. Additionally, in terms of micronutrient measurements in plant leaves of these taxa, the INF fertilization resulted in increased Cu levels for L. minoana subsp. minoana, Mn and Cu levels for E. ternatum, as well as in all micronutrients except Mn for L. minoana subsp. asterusica [43]. It is well known that Cu is an important micronutrient in photosynthesis and in carbon metabolism [49,50], but it can also be toxic to plants in excess quantities [51]. In the current study, it is worth noting a 10-fold increase and 58.6–64.7% higher amounts of Cu through INM foliar application for the tissue analysis of above-ground parts and below-ground parts of C. pelviformis, respectively, as compared to the ChFe and control plants. The latter means that Cu moves systemically in plant tissues and, moreover, is translocated downstream to the root system of C. pelviformis plants, without showing any toxicity effects. Since Cu plays an important role in controlling fungal diseases in plants [52], the high amounts of Cu in C. pelviformis leaves, stems and roots probably act as protective agent against fungal pathogens. Undoubtedly, more experiments are needed to investigate the beneficial properties of Cu absorption in different developmental stages of C. pelviformis prior to further conclusions.
By analyzing the concentrations of nutrients in different plant organs, valuable insights are obtained that enhance the understanding of plant health and ecological adaptation, as well as specific needs in terms of fertilization [53]. At the same time, identifying nutrient deficiencies in plants can contribute significantly to the optimization of precision agricultural technology, forming appropriate strategies for applying nutrients to plants through fertilization [54,55]. This approach ensures both economic and environmental benefits which are very important aspects of modern agriculture [56].
This study also showcased the beneficial effect of fertilization and the ease of responsiveness of the cultivated germplasm to fertilization, and it can provide evidence on the nutritional superiority of the cultivated germplasm. The latter, if sustainably exploited and managed, reveals a noteworthy economic potential for new product development with high added value which can be marketed in an exclusive way to address the commercial demand for C. pelviformis rosettes at least at local (Crete) or national (Greece) scales [2]. In the light of the above-mentioned, INF fertilization appears to offer promising prospects for enhancing cultivation practices for C. pelviformis when preparing nursery stocks, either for population re-introduction purposes or sustainable exploitation strategies.
4.4. Total Phenol Content, Antioxidant Capacity and Fertilization Protocols
The study herein provides, for the first time, an insight on the macro- and micronutrient profiles of the ex situ cultivated C. pelviformis and on the effect of fertilization regimes in TPC and AC of C. pelviformis. The presence of total phenols was evidenced in all samples of ex situ cultivated C. pelviformis regardless of fertilization, but they were prominent and significantly higher in fertilized samples, especially those treated with ChFe. The same trend was observed for the AC of the cultivated samples of C. pelviformis.
Considering other Cretan endemic plant species examined to date, the ChFe application has been shown to have a positive impact on both total phenols and antioxidant capacity for L. minoana subsp. minoana and E. ternatum, similar to the patterns observed in C. pelviformis, while total phenol content is reported to increase in plants without any fertilization application; antioxidant capacity is reported as negatively affected by the INF application for L. minoana subsp. asterusica [43]. Previous research on other Campanula species has shown that the presence of total phenolics and significant antioxidant activity is a fact. For instance, previous studies [57] demonstrated strong antioxidant activity in methanol extracts of whole plants of C. alliariifolia Willd. Similarly, other studies [58] investigated, in a comparative way, the antioxidant activity of 15 Campanula species from Palestine, concluding that C. sulphurea Boiss. and C. sidoniensis Boiss. & C.I.Blanche show comparatively the best DPPH radical scavenging activity. In a separate study, the phytochemical analysis of C. latifolia L. has reported very high levels of DPPH radical scavenging activity in both the aerial parts and roots of the species [59]; however, comparatively lower concentrations of total phenols have been detected in the aerial parts (0.053 mg Gallic acid/g extract) and root extracts (0.037 mg Gallic acid/g extract) of C. latifolia compared to the concentrations found in fresh leaves of C. pelviformis in the present study.
4.5. Re-Evaluation of Feasibility and Readiness Timescale for Sustainable Exploitation
The data furnished in this study may facilitate future conservation efforts and population re-introduction actions in the wild habitats and also pave the way for the sustainable utilization of C. pelviformis. In this sense, the amount of data furnished in this study shed light on the urgent need to re-evaluate the feasibility and readiness timescale of value chain establishment for the species of concern. The medicinal potential (Level I evaluation) of C. pelviformis has increased considerably to above average to high potential (68.2%) due to newly furnished data on isolated phytochemical compounds with medicinal properties [6], coupled with evidence on its nutritive value in terms of macro- and micronutrients (both Tsiftsoglou et al. [6] and this study), thus rendering C. pelviformis as a noteworthy medicinal plant of agro-alimentary interest.
Furthermore, the feasibility of value chain establishment for C. pelviformis (Level II evaluation in Krigas et al. [2]) was improved from 43.06% [2] to 66.67% after re-evaluation of related attributes in light of newly furnished data. This upgrade affected the readiness timescale for its sustainable exploitation (Level III evaluation) from achievable in long-term to achievable in medium-term (see Table 9). This re-evaluation was based on the change of threat status for C. pelvifromis (Vulnerable according to Kougioumtzis et al. [35]), which resulted in a score of four compared to zero; the successful seed germination, resulting in a score of six compared to zero; the definition of its cultivation needs in terms of watering and fertilization (score six from four), resulting in full cultivation protocols (score of six instead of two); and the documentation of its extant commercial prices as wild edible greens gathered from the wild [6].

Table 9.
Overview of the multifaceted evaluation regarding Campanula pelviformis (see Krigas et al. [2] which is revisited herein in light of the data furnished in this study. Previous assessments and upgrades of individual scores after the data presented herein are discerned with right arrow (→).
5. Conclusions
This study provided for the first time comprehensive information on the abiotic environmental conditions of the C. pelviformis based on its distribution in natural habitats of Crete, Greece. The GIS ecological profile developed offers valuable insights into the successful ex situ conservation and cultivation of this vulnerable local endemic species, as well as guidance for its adaptation in anthropogenic environments for conservation purposes. The results of the seed trials clearly demonstrated the effective seed germination of C. pelviformis achieved herein for the first time, thus marking a significant step towards its in situ conservation and its potential sustainable utilization for noteworthy ornamental, agro-alimentary and medicinal properties. The experimental cultivation achieved in this study for the first time provided valuable insights into the beneficial effects of different types of fertilization in morphological-physiological characteristics, in phytochemicals such as total phenols and antioxidant capacity as well as in terms of increased contents of macro- and micro-nutrients contents as documented in the above-ground compared to wild-growing plants as well as in the below-ground part of C. pelviniformis which was studied herein for the first time. All the data furnished in this multidisciplinary investigation built to bridge extant applied research gaps was finally exploited to provide an update on the feasibility and readiness timescale for the sustainable exploitation of C. pelviformis, thus further facilitating the creation of a new value chain for the ex situ cultivated C. pelviformis. The latter is expected to alleviate the collection pressure exerted to the traditionally harvested wild-growing populations of this remarkable yet vulnerable local endemic plant species of Crete.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9080877/s1, Table S1: Ecological profile across the natural distribution range of Campanula pelviformis wild-growing populations in Crete, Greece.
Author Contributions
Conceptualization, S.H., N.K., I.A. and G.T.; data curation, N.K., I.A., S.K., E.P. (Elias Pipinis), E.P. (Evgenia Papaioannou), E.D. and G.T.; formal analysis, I.A., E.P. (Elias Pipinis), E.P. (Evgenia Papaioannou), N.K., S.K. and S.H.; investigation, I.A., N.K., S.H., E.D., E.P. (Elias Pipinis), E.P. (Evgenia Papaioannou), E.K. (Eleftherios Karapatzak), S.K., P.T., E.K. (Emmanouil Koundourakis), E.C. and G.T.; methodology, I.A., N.K., S.H., E.P. (Elias Pipinis), E.P. (Evgenia Papaioannou), S.K., E.D., E.K. (Eleftherios Karapatzak), P.T., E.K. (Emmanouil Koundourakis), E.C. and G.T.; project administration, S.H., N.K. and G.T.; resources, N.K., S.H. and S.K.; software, I.A.; supervision, N.K., S.H. and G.T.; validation, N.K., I.A., E.K. (Eleftherios Karapatzak), E.D. and G.T.; visualization, I.A., E.P. (Elias Pipinis) and N.K.; writing—original draft, I.A., E.P. (Elias Pipinis), S.H., N.K., E.K. (Eleftherios Karapatzak) and G.T.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data supporting the results of this study are included in the manuscript, and the datasets are available upon request.
Acknowledgments
The fieldwork of N.K., G.T., E.D. and I.A. has been partially supported by the MULTI-VAL-END project (2018–2022) entitled “Multifaceted Valorisation of single-country Endemic plants of Crete, Greece, Tunisia and Rif, Morocco for sustainable exploitation in the agro-alimentary, horticultural-ornamental and medicinal-cosmetic sectors” (ARIMNet2 2017 Transnational Joint Call).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scariot, V.; Seglie, L.; Caser, M.; Devecchi, M. Evaluation of Ethylene Sensitivity and Postharvest Treatments to Improve the Vase Life of Four Campanula Species. Eur. J. Hortic. Sci. 2008, 73, 166–170. Available online: https://www.researchgate.net/publication/230691067_Evaluation_of_ethylene_sensitivity_and_postharvest_treatments_to_improve_the_vase_life_of_four_Campanula_species (accessed on 3 July 2023).
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Müller, W.E.; Galli, C. Local Mediterranean Food Plants and Nutraceuticals; Karger Medical and Scientific Publishers: Basel, Switzerland, 2006; Volume 59, ISSN 1660-0347. [Google Scholar]
- Tsiftsoglou, O.S.; Lagogiannis, G.; Psaroudaki, A.; Vantsioti, A.; Mitić, M.N.; Mrmošanin, J.M.; Lazari, D. Phytochemical analysis of the aerial parts of Campanula pelviformis Lam. (Campanulaceae): Documenting the dietary value of a local endemic plant of Crete (Greece) traditionally used as wild edible green. Sustainability 2023, 15, 7404. [Google Scholar] [CrossRef]
- Cellinese, N.; Smith, S.A.; Edwards, E.J.; Kim, S.T.; Haberle, R.C.; Avramakis, M.; Donoghue, M.J. Historical biogeography of the endemic Campanulaceae of Crete. J. Biogeogr. 2009, 36, 1253–1269. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora Part 1 (Text & Plates) & Part 2 (Maps), 1st ed.; Englera 33 (1 & 2); Botanic Garden and Botanical Museum: Berlin, Germany; Freie Universität: Berlin, Germany, 2016; ISSN 0170-4818. [Google Scholar]
- Menteli, V.; Krigas, N.; Avramakis, M.; Turland, N.; Vokou, D. Endemic plants of Crete in electronic trade and wildlife tourism: Current patterns and implications for conservation. J. Biol. Res. Thessalon. 2019, 26, 10. [Google Scholar] [CrossRef]
- Padulosi, S.; Thompson, J.; Rudebjer, P. Fighting Poverty, Hunger and Malnutrition with Neglected and Underutilized Species (NUS): Needs, Challenges and the Way Forward; Biodiversity International: Rome, Italy, 2013; Available online: https://hdl.handle.net/10568/68927 (accessed on 3 July 2023).
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- Rivera, D.; Obón, C.; Heinrich, M.; Inocencio, C.; Verde, A.; Fajardo, J. Gathered Mediterranean food plants—Ethnobotanical investigations and historical development. In Local Mediterranean Food Plants and Nutraceuticals; Heinrich, M., Müller, W.E., Galli, C., Eds.; Karger Publishing: Basel, Switzerland, 2006; Volume 59, pp. 18–74. [Google Scholar] [CrossRef]
- Scariot, V.; Seglie, L.; Gaino, W.; Devecchi, M. Evaluation of European native bluebells for sustainable floriculture. Acta Hortic. 2012, 937, 273–280. [Google Scholar] [CrossRef]
- Gkika, P.I.; Krigas, N.; Menexes, G.; Eleftherohorinos, I.G.; Maloupa, E. Conservation of the rare Erysimum naxense Snogerup and the threatened Erysimum krendlii Polatschek: Effect of temperature and light on seed germination. Open Life Sci. 2013, 8, 1194–1203. [Google Scholar] [CrossRef][Green Version]
- Grigoriadou, K.; Krigas, N.; Maloupa, E. GIS-facilitated in vitro propagation and ex situ conservation of Achillea occulta. Plant Cell Tissue Organ Cult. 2011, 107, 531–540. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol. Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex-situ conservation needs and its future sustainable exploitation as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Maloupa, E.; Tsoktouridis, G. Propagation and ex-situ conservation of Lomelosia minoana subsp. minoana and Scutellaria hirta-two ornamental and medicinal Cretan endemics (Greece). Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12168. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; El Haissoufi, M.; Pipinis, E.; Kostas, S.; Libiad, M.; Khabbach, A.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; et al. GIS-Facilitated seed germination and multifaceted evaluation of the Endangered Abies marocana Trab. (Pinaceae) enabling conservation and sustainable exploitation. Plants 2021, 10, 2606. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2021, 12, 94. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Bourgou, S.; Ben Haj Jilani, I.; Ben Othman, W.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; et al. DNA Barcoding, GIS-Facilitated seed germination and pilot cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian local endemic with potential medicinal and ornamental value. Biology 2022, 11, 462. [Google Scholar] [CrossRef]
- Khabbach, A.; Libiad, M.; El Haissoufi, M.; Bourgou, S.; Megdiche-Ksouri, W.; Lamchouri, F.; Ghrabi-Gammar, Z.; Menteli, V.; Vokou, D.; Tsoktouridis, G.; et al. Electronic commerce of the endemic plants of Northern Morocco (Mediterranean Coast-Rif) and Tunisia over the Internet. Bot. Sci. 2021, 100, 139–152. [Google Scholar] [CrossRef]
- Krigas, N.; Mouflis, G.; Grigoriadou, K. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants 2021, 10, 580. [Google Scholar] [CrossRef]
- Krigas, N.; Karapatzak, E.; Panagiotidou, M.; Sarropoulou, V.; Samartza, I.; Karydas, A.; Damianidis, C.K.; Najdovski, B.; Teofilovski, A.; Mandzukovski, D.; et al. Prioritizing plants around the cross-border area of Greece and the Republic of North Macedonia: Integrated conservation actions and sustainable exploitation potential. Diversity 2022, 14, 570. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the Vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; et al. Facilitating conservation and bridging gaps for the sustainable exploitation of the Tunisian local endemic plant Marrubium aschersonii (Lamiaceae). Sustainability 2022, 14, 1637. [Google Scholar] [CrossRef]
- Sefi, O.; Bourgou, S.; Megdiche-Ksouri, W.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Krigas, N.; Ghrabi-Gammar, Z. Bioactivities and phenolic composition of Limonium boitardii Maire and L. cercinense Brullo & Erben (Plumbaginaceae): Two Tunisian strict endemic plants. Inter. J. Environ. Health Res. 2022, 32, 2496–2511. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; ISBN 978-0-12-416677-6. [Google Scholar]
- Macdonald, B. Practical Woody Plant Propagation for Nursery Growers; Timber Press Inc.: Portland, OR, USA, 2006; p. 669. ISBN 9780881920628. [Google Scholar]
- Koutsovoulou, K.; Daws, M.I.; Thanos, C.A. Campanulaceae: A family with small seeds that require light for germination. Ann. Bot. 2014, 113, 135–143. [Google Scholar] [CrossRef] [PubMed]
- O’Reill, C.; Arnott, J.T.; Owens, J.N. Effects of photoperiod and moisture availability on shoot growth, seedling morphology, and cuticle and epicuticular wax features of container-grown western hemlock seedlings. Can. J. Forest Res. 1989, 19, 122–131. [Google Scholar] [CrossRef]
- Buendia-Velazquez, M.V.; Lopez-Lopez, M.A.; Cetina-Alcala, V.M.; Diakite, L. Substrates and nutrient addition rates affect morphology and physiology of Pinus leiophylla seedlings in the nursery stage. iForest 2016, 10, 115–120. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Lazarina, M.; Kallimanis, A.S.; Dimopoulos, P.; Psaralexi, M.; Michailidou, D.E.; Sgardelis, S.P. Patterns and drivers of species richness and turnover of neo-endemic and palaeo-endemic vascular plants in a Mediterranean hotspot: The case of Crete, Greece. J. Biol. Res.-Thessaloniki 2019, 26, 12. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Cessna, S.; Demmig-Adams, B.; Adams, W.W., III. Exploring photosynthesis and plant stress using inexpensive chlorophyll fluorometers. J. Nat. Resourc. Life Sci. Educ. 2010, 39, 22–30. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical analysis. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publication: Oxford, UK, 1986; pp. 285–344. ISBN 9780632003211. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Total phosphorus. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. Available online: https://acsess.onlinelibrary.wiley.com/doi/pdf/10.2134/agronmonogr9.2.2ed.frontmatter (accessed on 3 July 2023).
- Bremmer, J.M.; Mulvaney, C.S. Nitrogen–Total. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. Available online: https://acsess.onlinelibrary.wiley.com/doi/pdf/10.2134/agronmonogr9.2.2ed.frontmatter (accessed on 3 July 2023).
- Hatzilazarou, S.; Kostas, S.; Pipinis, E.; Anestis, I.; Papaioannou, E.; Aslanidou, V.; Tsoulpha, P.; Avramakis, M.; Krigas, N.; Tsoktouridis, G. GIS-Facilitated Seed Germination, Fertilization Effects on Growth, Nutrient and Phenol Contents and Antioxidant Potential in Three Local Endemic Plants of Crete (Greece) with Economic Interest: Implications for Conservation and Sustainable Exploitation. Horticulturae 2023, 9, 335. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures in Agricultural Research, 2nd ed.; Wiley: New York, NY, USA, 1984; ISBN 0-471-87092-7. [Google Scholar]
- Klockars, A.; Sax, G. Multiple Comparisons; Sage Publications: Newbury Park, CA, USA, 1986; p. 87. ISBN 0-8039-2051-2. [Google Scholar]
- Blionis, G.; Vokou, D. Reproductive attributes of Campanula populations from Mt Olympos, Greece. Plant Ecol. 2005, 178, 77–88. [Google Scholar] [CrossRef]
- Pieroni, A.; Sulaiman, N.; Sõukand, R. Chorta (wild greens) in Central Crete: The bio-cultural heritage of a hidden and resilient ingredient of the Mediterranean Diet. Biology 2022, 11, 673. [Google Scholar] [CrossRef]
- Ziegler, C.; Dusenge, M.E.; Nyirambangutse, B.; Zibera, E.; Wallin, G.; Uddling, J. Contrasting dependencies of photosynthetic capacity on leaf nitrogen in early-and late-successional tropical montane tree species. Front. Plant Sci. 2020, 11, 500479. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Dalcorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 10, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. 8-Functions of Mineral Nutrients: Macronutrients. In Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; pp. 229–312. [Google Scholar]
- Zhu, Q.; Zhang, M.; Ma, Q. Copper-based foliar fertilizer and controlled release urea improved soil chemical properties, plant growth and yield of tomato. Sci. Hortic. 2012, 143, 109–114. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Luo, F.; Wu, L.; Zhang, Y.; Lan, T. Determination and analysis of leaf P and K concentrations of several plant species in Jinan city. E3S Web Conf. 2018, 53, 03055. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. Detection of nutrition deficiencies in plants using proximal images and machine learning: A review. Comput. Electron. Agric. 2019, 162, 482–492. [Google Scholar] [CrossRef]
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Fahey, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant-soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Pastuszka-Wozniak, J.; Zubik, M.; Krzyszczak, J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, M.U.; Gurkan, E.; Tuzlaci, E. Chemical composition and antioxidant activity of Campanula alliariifolia. Nat. Prod. Res. 2008, 22, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Abualhasan, M. Comparison in vitro of Antioxidant Activity between Fifteen Campanula Species (Bellflower) from Palestinian Flora. Pharmacogn. J. 2015, 7, 276–279. [Google Scholar] [CrossRef]
- Moosavi, S.R.; Ardekani, M.R.S.; Vazirian, M.; Lamardi, S.N.S. Campanula latifola, Giant Bellflower: Ethno-Botany, Phytochemical and Antioxidant Evaluation. Tradit. Integr. Med. 2018, 3, 113–119. Available online: https://jtim.tums.ac.ir/index.php/jtim/article/view/168 (accessed on 3 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).