Effects of Light Intensity and Water Stress on Growth, Photosynthetic Characteristics and Plant Survival of Cistus heterophyllus Desf. Subsp. carthaginensis (Pau) M. B. Crespo & Mateo
Abstract
1. Introduction
2. Materials and Methods
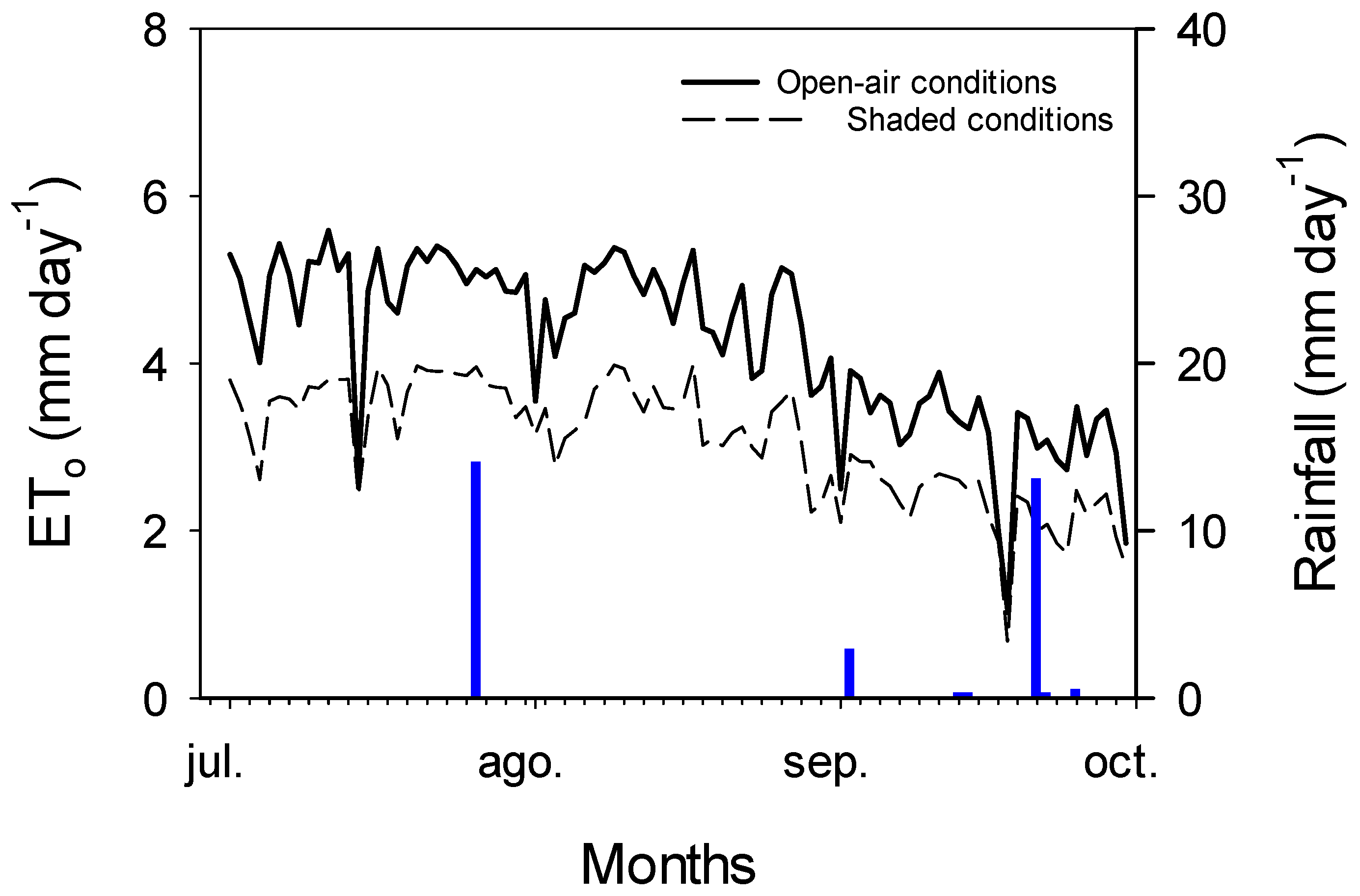
2.1. Plant Material and Experiment Conditions
2.2. Treatments and Experimental Design
2.3. Water Relations Measurements
2.4. Gas Exchange Measurements
2.5. Leaf Temperature Determinations
2.6. Leaf Chlorophyll Content and Colour Parameters
2.7. Leaf Mineral Content and Biomass Parameters and Survival Rate
2.8. Survival Rate and Damaged Plants
2.9. Statistics
3. Results
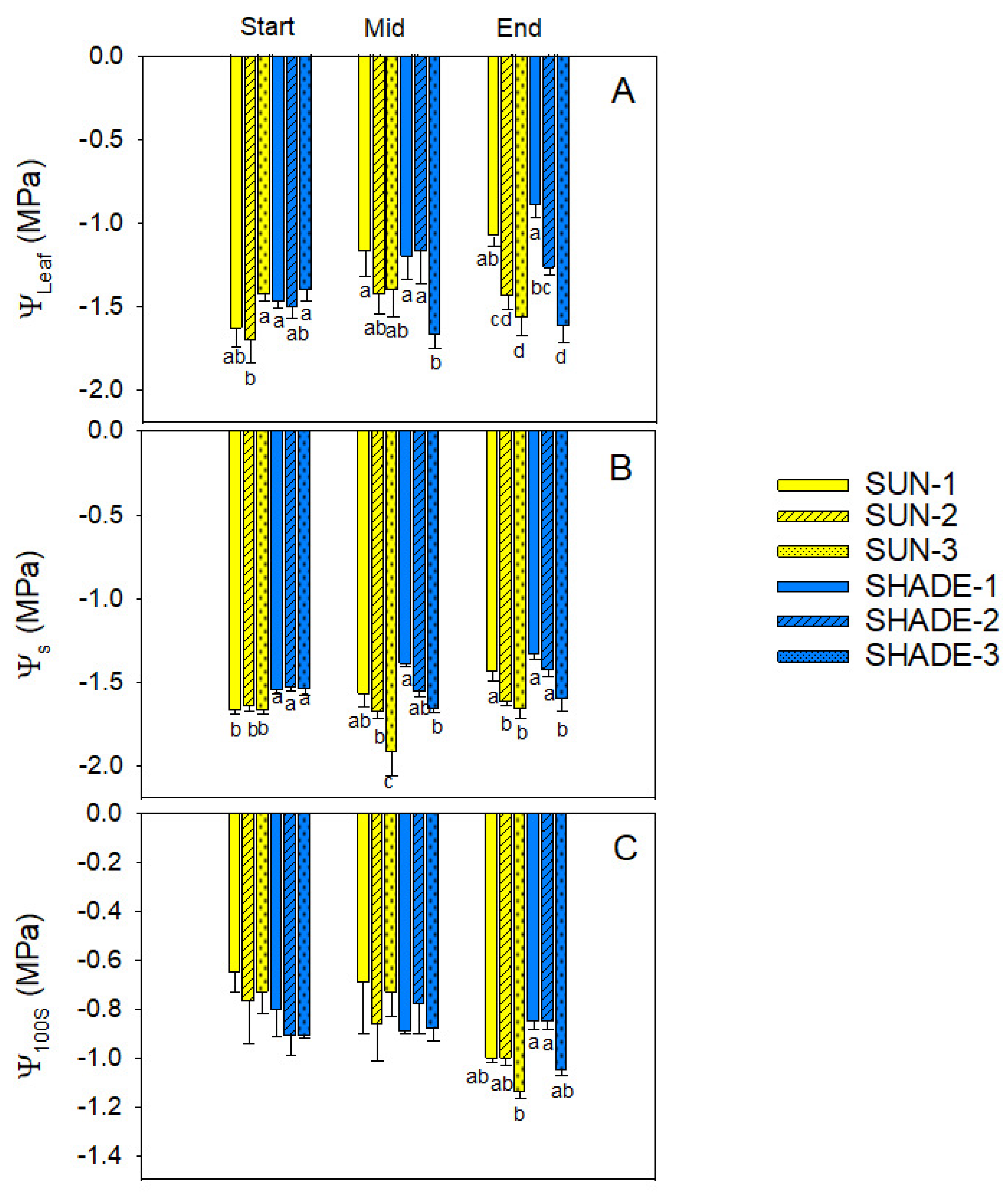
3.1. Water Relations
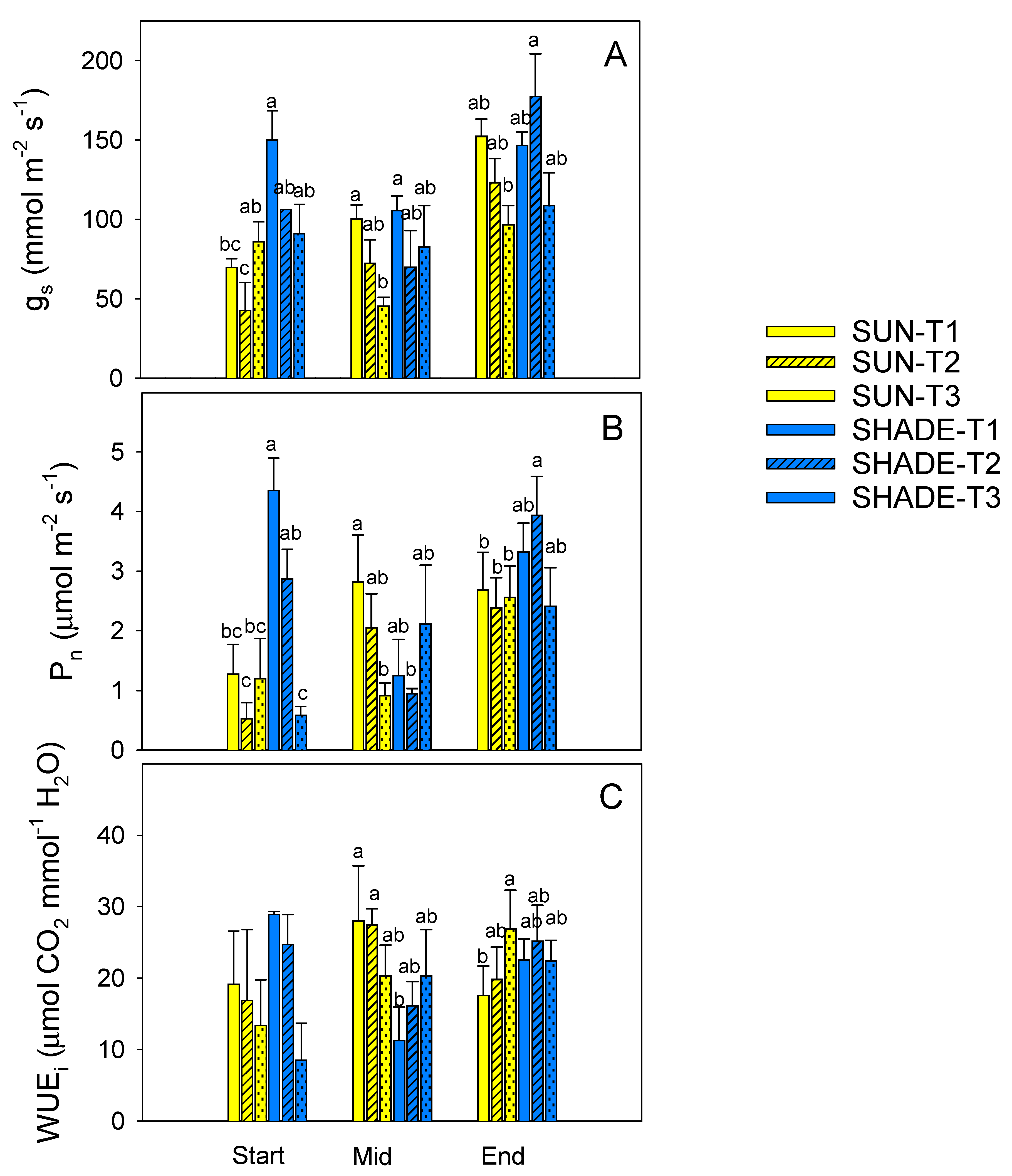
3.2. Gas Exchange
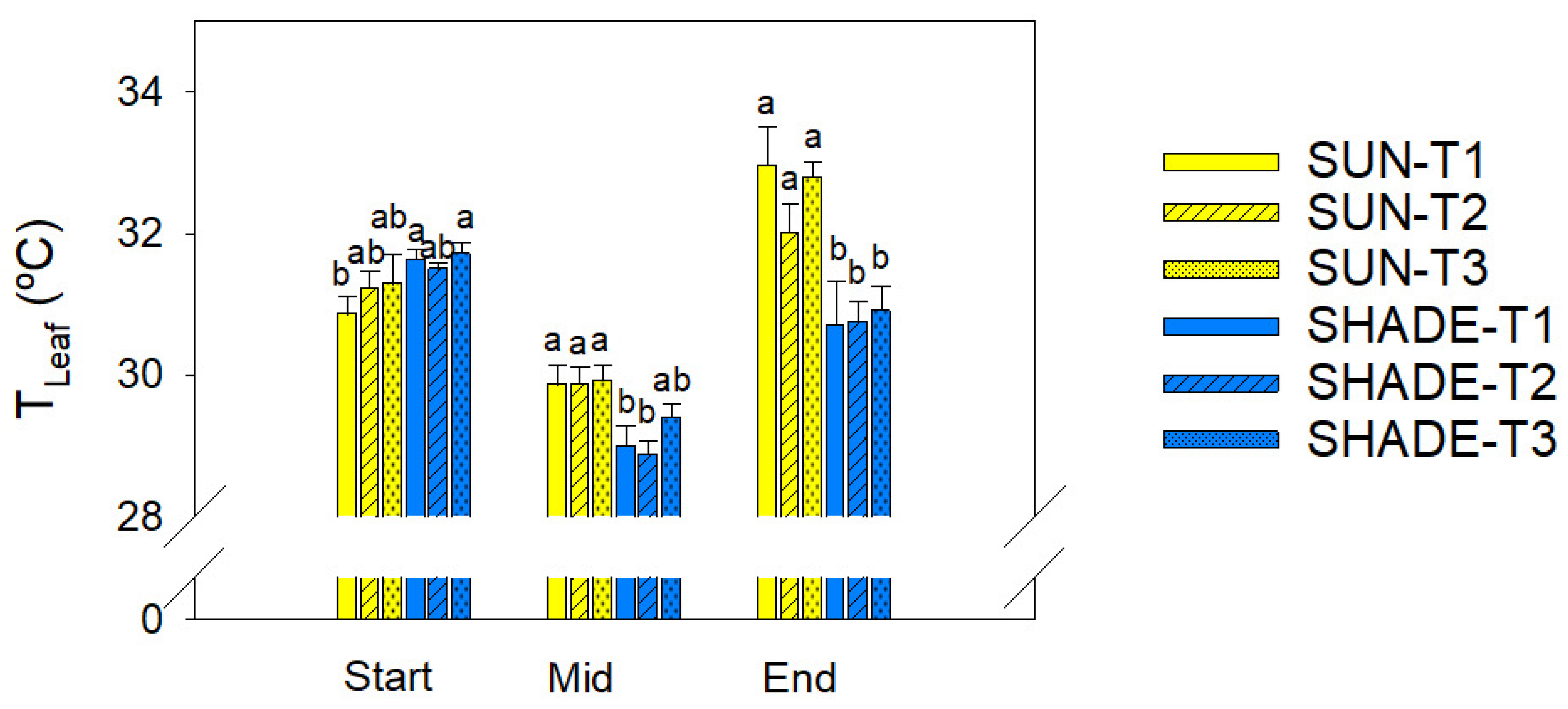
3.3. Leaf Temperature
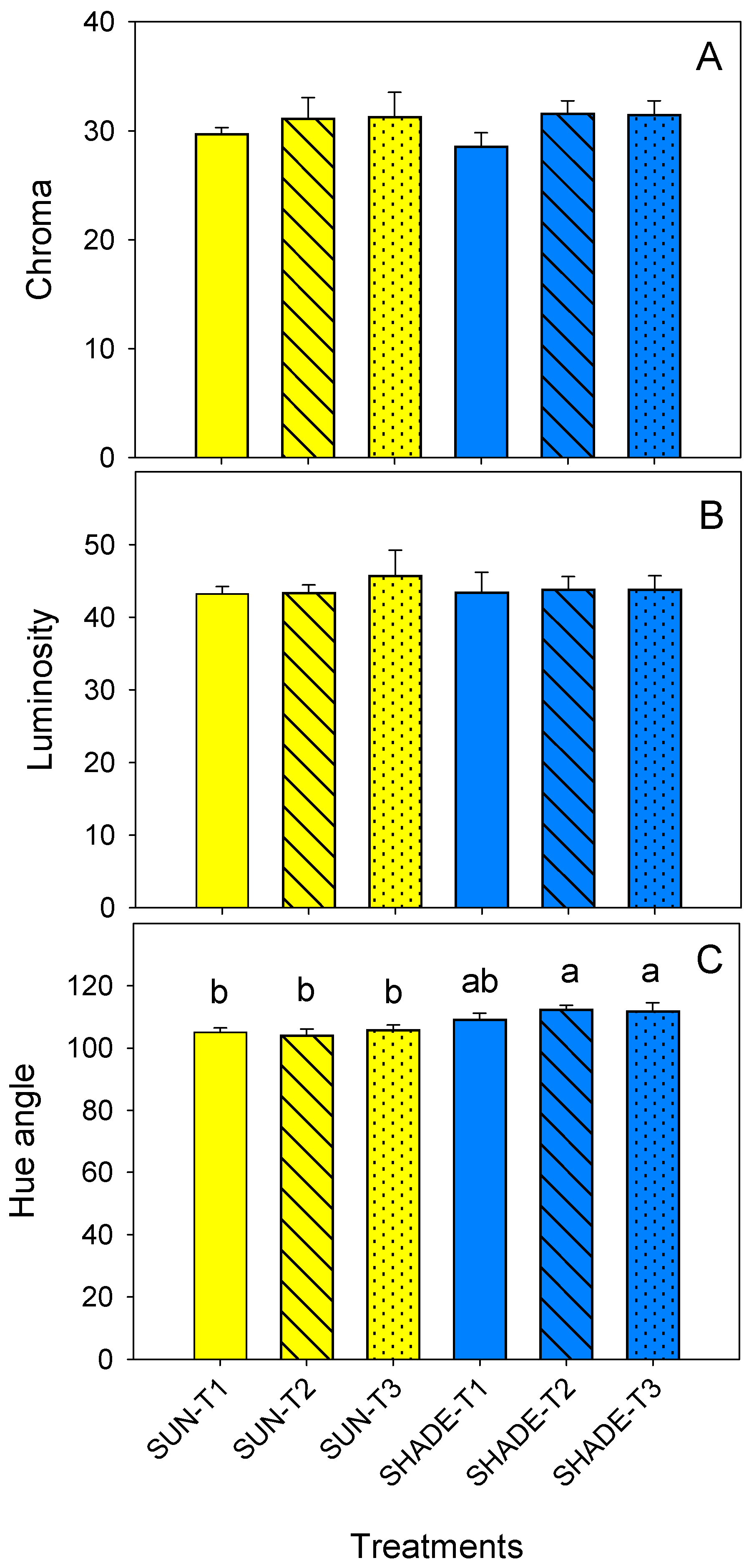
3.4. Leaf Chlorophyll Content and Colour Parameters
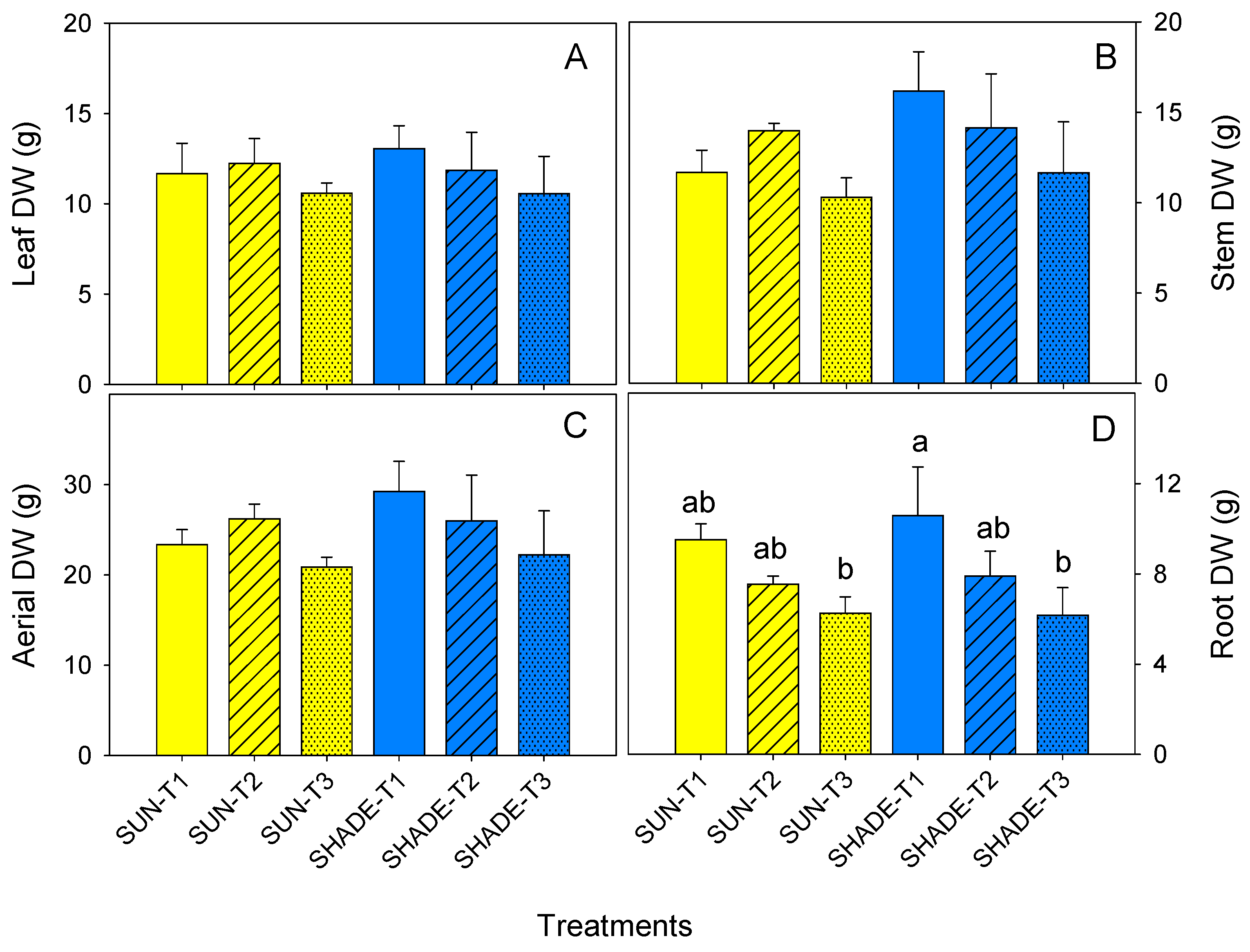
3.5. Biomass Parameters and Growth
3.6. Leaf Mineral Content
3.7. Survival Rate and Damaged Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bachman, S.P.; Nic Lughadha, E.M.; Rivers, M.C. Quantifying progress toward a conservation assessment for all plants. Conserv. Biol. 2018, 32, 516–524. [Google Scholar] [CrossRef]
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green plants in the red: A baseline global assessment for the IUCN sampled Red List Index for plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.; Forest, F.; Halley, J.M.; Moat, J.; Walker, B.E. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Visconti, P.; Di Marco, M.; Martin, T.G.; Rondinini, C.; Rhodes, J.R. Climate change modifies risk of global biodiversity loss due to land-cover change. Biol. Conserv. 2015, 187, 103–111. [Google Scholar] [CrossRef]
- Cardona, C.; Capó, M. First insular population of the critically endangered Cistus heterophyllus subsp. carthaginensis on Cabrera Archipelago National Park (Balearic Islands, Spain). Biodivers. Conserv. 2023, 32, 811–820. [Google Scholar] [CrossRef]
- Werner, O.; Vicente, M.J.; Aguado, M.; Robles, J.; Ros, R.M. Ex situ conservation of Cistus heterophyllus subsp. carthaginensis (Cistaceae) saves the taxon from introgression by Cistus albidus. Genetics 2021, 155, 945–959. [Google Scholar]
- Ferrer, P.P.; Ferrando Pardo, I.; Albert Llana, F.J.; Martínez Granell, V.; Laguna, E. Obtención de Material Vegetal de Reproducción de Cistus Heterophyllus Carthaginensis (Cistaceae), Especie Catalogada en Peligro de Extinción en la Comunidad Valenciana (España); Universidad de Alicante: San Vicente del Raspeig, Spain, 2017. [Google Scholar]
- Jiménez, J.F.; Sánchez Gómez, P.; Rosselló, J.A. Evidencia de introgresión en Cistus heterophyllus subsp. carthaginensis (Cistaceae) a partir de marcadores (Cistaceae) moleculares RAPD. An. Biol. 2007, 29, 2007. [Google Scholar]
- Ferrer, P.P.; Ferrando Pardo, I.; Laguna, E.; Viu, M.; Vallejo, N.; Robles, J.; Díaz, R. Segunda reunión del Grupo de Trabajo “Situación Crítica de Cistus heterophyllus subsp. Carthaginensis”; Consevación Vegetal: Valencia, Spain, 27–28 May 2019. [Google Scholar]
- Vicente Colomer, M.J.; Martínez Sánchez, J.J. La Jara de Cartagena (Cistus heterophyllus). Una Especie en Peligro. Estado Actual de Conocimientos; Universidad Politécnica, CRAI Biblioteca: Cartagena, Spain, 2018. [Google Scholar]
- Navarro-Cano, J.A. Taxonomía, Propagación y Conservación de Cistus heterophyllus Desf.(Cistaceae): Una Planta en Peligro de Extinción en España. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2002. [Google Scholar]
- Navarro-Cano, J.A. Effect of grass litter on seedling recruitment of the critically endangered Cistus heterophyllus in Spain. Flora Morphol. Distrib. Funct. Ecol. 2008, 203, 663–668. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Rivera, D.; González Barberá, G. Induction of seed germination in Cistus heterophyllus (Cistaceae): A rock rose critically endangered in Spain. Res. J. Bot. 2009, 4, 10–16. [Google Scholar] [CrossRef][Green Version]
- Navarro-Cano, J.A.; Robles, J. Reintroducciones y Conservación Ex Situ en la Región de Murcia. In La Jara de Cartagena (Citrus heterophyllus), una Especie en Peligro: Estado Actual de Conocimiento; Universidad Politécnica de Cartagena: Cartagena, Spain, 2018; pp. 144–160. [Google Scholar]
- Tardieu, F. Drought perception by plants do cells of droughted plants experience water stress? Plant Growth Regul. 1996, 20, 93–104. [Google Scholar] [CrossRef]
- Arve, L.E.; Torre, S.; Olsen, J.E.; Tanino, K.K. Stomatal responses to drought stress and air humidity. In Abiotic Stress in Plants-Mechanisms and Adaptations; IntechOpen: London, UK, 2011. [Google Scholar]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.S.M.A.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar]
- Givnish, T.J. Adaptation to sun and shade: A whole-plant perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Gucci, R.; Xiloyannis, C.; Flore, J.A. Gas exchange parameters, water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol. Plant. 1991, 83, 497–505. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; Sousa, C.D.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.M.; Chaves, M.M.; Jones, H.G. Optimizing thermal imaging as a technique for detecting stomatal closure induced by drought stress under greenhouse conditions. Physiol. Plant. 2006, 127, 507–518. [Google Scholar] [CrossRef]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2004; Volume 41, pp. 107–163. [Google Scholar]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G. Reporting of objective colour measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Rodrıguez, P.; Morales, M.A.; Ortuño, M.F.; Torrecillas, A. Comparative growth and water relations of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Sci. 2002, 162, 107–113. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Falara, V.; Pateraki, I.; López-Carbonell, M.; Cela, J.; Kanellis, A.K. Physiological and molecular responses of the isoprenoid biosynthetic pathway in a drought-resistant Mediterranean shrub, Cistus creticus exposed to water deficit. J. Plant Physiol. 2009, 166, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Redondo-Gómez, S.; Gratani, L.; Mateos-Naranjo, E. Highlighting the differential role of leaf paraheliotropism in two Mediterranean Cistus species under drought stress and well-watered conditions. J. Plant Physiol. 2017, 213, 199–208. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Shahid, M.A.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Grant, O.M.; Tronina, Ł.; García-Plazaola, J.I.; Esteban, R.; Pereira, J.S.; Chaves, M.M. Resilience of a semi-deciduous shrub, Cistus salvifolius, to severe summer drought and heat stress. Funct. Plant Biol. 2014, 42, 219–228. [Google Scholar] [CrossRef]
- Puglielli, G.; Gratani, L.; Varone, L. Leaf rolling as indicator of water stress in Cistus incanus from different provenances. BioRxiv 2017. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Chaves-Barrantes, N.F.; Gutiérrez-Soto, M.V. Respuestas al estrés por calor en los cultivos. I. Aspectos moleculares, bioquímicos y fisiológicos. Agron. Mesoam. 2017, 28, 237–253. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, M.F.; Lorente, B.; Hernández, J.A.; Sánchez-Blanco, M.J. Mycorrhizal inoculation on compost substrate affects nutritional balance, water uptake and photosynthetic efficiency in Cistus albidus plants submitted to water stress. Rev. Bras. Bot. 2018, 41, 299–310. [Google Scholar] [CrossRef]
- González, L.M.; Estrada, A.; Zaldivar, N.; Argentel, L. Tolerancia a la sequía en diferentes variedades de trigo sobre la base de algunas variables del régimen hídrico y la concentración de pigmentos en estadía de plántula. Rev. Cienc. Téc. Agropecu. 2007, 16, 45–49. [Google Scholar]
- Lorente, B.; Zugasti, I.; Ortuño, M.F.; Nortes, P.; Bañón, S.; Hernández, J.A.; Sánchez-Blanco, M.J. Substrate composition affects the development of water stress and subsequent recovery by inducing physiological changes in Cistus albidus plants. Plant Physiol. Biochem. 2021, 158, 125–135. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Mira-García, A.B.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Leaf water relations in lime trees grown under shade netting and open-air. Plants 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Torre, S.; Gori, A.; Brunetti, C.; Centritto, M.; Ferrini, F.; Tattini, M. Dissecting adaptation mechanisms to contrasting solar irradiance in the mediterranean shrub Cistus incanus. Int. Mol. Sci. 2019, 20, 3599. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C.; Döll, M.; Fietz, H.J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U.; Döll, M. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosyn. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef]
- Rezai, S.; Etemadi, N.; Nikbakht, A.; Yousefi, M.; Majidi, M.M. Effect of Light Intensity on Leaf Morphology, Photosynthetic Capacity, and Chlorophyll Content inSage (Salvia officinalis L.). Hortic. Sci. Technol. 2018, 36, 46–57. [Google Scholar]
- Chang, P.T.; Hsieh, C.C.; Jiang, Y.L. Responses of ‘Shih Huo Chuan’ pitaya (Hylocereus polyrhizus (Weber) Britt. & Rose) to different degrees of shading nets. Sci. Hortic. 2016, 198, 154–162. [Google Scholar]
- Puglielli, G.; Varone, L.; Gratani, L.; Catoni, R. Specific leaf area variations drive acclimation of Cistus salvifolius in different light environments. Photosynthetica 2017, 55, 31–40. [Google Scholar] [CrossRef]
- Fini, A.; Ferrini, F.; Frangi, P.; Amoroso, G.; Giordano, C.; Bonzi, L. Growth, leaf gas exchange and leaf anatomy of three ornamental shrubs grown under different light intensities. Eur. J. Hortic. Sci. 2010, 75, 113–117. [Google Scholar]
- Chérel, I.; Gaillard, I. The complex fine-tuning of K+ fluxes in plants in relation to osmotic and ionic abiotic stresses. Int. J. Mol. Sci. 2019, 20, 715. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Ammar, M.S.; Nevein, A. Effects of shade level and microenvironment on vegetative growth, physiological and biochemical characteristics of transplanted cucumber (Cucumis sativus). Arch. Agric. Environ. Sci. 2017, 2, 361–368. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]

| L Week−1 Plant−1 | ||||
|---|---|---|---|---|
| Irrigation Treatments | 24 March | 1 July | 20 Sept | 29 Sept |
| T1 | 1.02 | 2.05 | 1.54 | 1.02 |
| T2 | 1.02 | 1.17 | 0.88 | 0.59 |
| T3 | 1.02 | 0.59 | 0.44 | 0.29 |
| Macronutrients (g 100 g−1) | |||||||
|---|---|---|---|---|---|---|---|
| Ca | K | Mg | Na | P | S | ||
| SUN-T1 | 1.40 | 0.66 bc | 0.45 abc | 0.20 ab | 0.20 ab | 0.28 b | |
| SUN-T2 | 1.52 | 0.72 bc | 0.52 a | 0.29 a | 0.16 b | 0.49 a | |
| SUN-T3 | 1.44 | 0.55 c | 0.50 ab | 0.26 ab | 0.21 ab | 0.45 a | |
| SHADE-T1 | 1.17 | 0.89 ab | 0.40 bc | 0.16 ab | 0.26 a | 0.18 b | |
| SHADE-T2 | 1.01 | 1.07 a | 0.35 c | 0.04 b | 0.22 ab | 0.25 b | |
| SHADE-T3 | 1.41 | 0.75 bc | 0.51 ab | 0.23 ab | 0.22 ab | 0.48 a | |
| Significance | P | ns | ** | * | * | * | *** |
| Radiation | SUN | 1.45 a | 0.65 b | 0.49 a | 0.25 | 0.19 b | 0.40 a |
| SHADE | 1.20 b | 0.90 a | 0.42 b | 0.14 | 0.24 a | 0.30 b | |
| Irrigation | T1 | 1.29 | 0.70 ab | 0.42 | 0.18 | 0.23 | 0.23 b |
| T2 | 1.27 | 0.90 a | 0.44 | 0.17 | 0.19 | 0.37 a | |
| T3 | 1.42 | 0.65 b | 0.45 | 0.20 | 0.22 | 0.47 a | |
| Radiation | * | *** | * | ns | * | * | |
| Significance | Irrigation | ns | ** | ns | ns | ns | *** |
| Interaction | ns | ns | ns | ns | ns | * | |
| Micronutrients (mg kg−1) | |||||||
| B | Cu | Fe | Mn | Ni | Zn | ||
| SUN-T1 | 22.24 b | 1.61 | 316.5 ab | 52.84 c | 0.94 ab | 36.25 b | |
| SUN-T2 | 21.14 b | 1.68 | 288.9 b | 69.98 abc | 0.93 ab | 35.43 b | |
| SUN-T3 | 20.67 b | 1.67 | 354.4 a | 74.39 ab | 1.14 a | 33.63 b | |
| SHADE-T1 | 28.21 a | 2.12 | 224.1 c | 64.54 bc | 0.75 b | 51.45 a | |
| SHADE-T2 | 24.84 a | 1.87 | 151.4 d | 58.97 bc | 0.67 b | 55.02 a | |
| SHADE-T3 | 25.83 a | 1.61 | 224.7 c | 87.79 a | 0.87 ab | 43.50 ab | |
| Significance | P | * | ns | *** | * | * | * |
| Radiation | SUN | 21.35 b | 1.65 | 319.9 a | 65.7 | 1.01 a | 35.10 b |
| SHADE | 26.29 a | 1.90 | 200.1 b | 70.4 | 0.76 b | 49.99 a | |
| Irrigation | T1 | 25.22 | 1.87 | 270.3 a | 58.7 b | 0.85 | 43.85 |
| T2 | 23.00 | 1.78 | 220.2 b | 64.5 b | 0.80 | 45.23 | |
| T3 | 23.25 | 1.64 | 289.5 a | 81.1 a | 1.01 | 38.57 | |
| Radiation | *** | ns | *** | ns | * | *** | |
| Significance | Irrigation | ns | ns | * | ** | ns | ns |
| Interaction | ns | ns | ns | ns | ns | ns | |
| Survival Rate (%) | Damaged Plants (%) | |
|---|---|---|
| SUN-T1 | 96.55 | 18.97 |
| SUN-T2 | 96.49 | 17.54 |
| SUN-T3 | 94.74 | 19.30 |
| SHADE-T1 | 88.33 | 25.00 |
| SHADE-T2 | 96.15 | 13.46 |
| SHADE-T3 | 92.31 | 21.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Bellot, M.J.; Sánchez-Blanco, M.J.; Lorente, B.; Vicente-Colomer, M.J.; Ortuño, M.F. Effects of Light Intensity and Water Stress on Growth, Photosynthetic Characteristics and Plant Survival of Cistus heterophyllus Desf. Subsp. carthaginensis (Pau) M. B. Crespo & Mateo. Horticulturae 2023, 9, 878. https://doi.org/10.3390/horticulturae9080878
Gómez-Bellot MJ, Sánchez-Blanco MJ, Lorente B, Vicente-Colomer MJ, Ortuño MF. Effects of Light Intensity and Water Stress on Growth, Photosynthetic Characteristics and Plant Survival of Cistus heterophyllus Desf. Subsp. carthaginensis (Pau) M. B. Crespo & Mateo. Horticulturae. 2023; 9(8):878. https://doi.org/10.3390/horticulturae9080878
Chicago/Turabian StyleGómez-Bellot, María José, María Jesús Sánchez-Blanco, Beatriz Lorente, María José Vicente-Colomer, and María Fernanda Ortuño. 2023. "Effects of Light Intensity and Water Stress on Growth, Photosynthetic Characteristics and Plant Survival of Cistus heterophyllus Desf. Subsp. carthaginensis (Pau) M. B. Crespo & Mateo" Horticulturae 9, no. 8: 878. https://doi.org/10.3390/horticulturae9080878
APA StyleGómez-Bellot, M. J., Sánchez-Blanco, M. J., Lorente, B., Vicente-Colomer, M. J., & Ortuño, M. F. (2023). Effects of Light Intensity and Water Stress on Growth, Photosynthetic Characteristics and Plant Survival of Cistus heterophyllus Desf. Subsp. carthaginensis (Pau) M. B. Crespo & Mateo. Horticulturae, 9(8), 878. https://doi.org/10.3390/horticulturae9080878








