Methyl Jasmonate Elicitation for In Vitro Lycorine Accumulation in Three Zephyranthes Species and Comparative Analysis of Tissue-Cultured and Field Grown Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sterilization Method
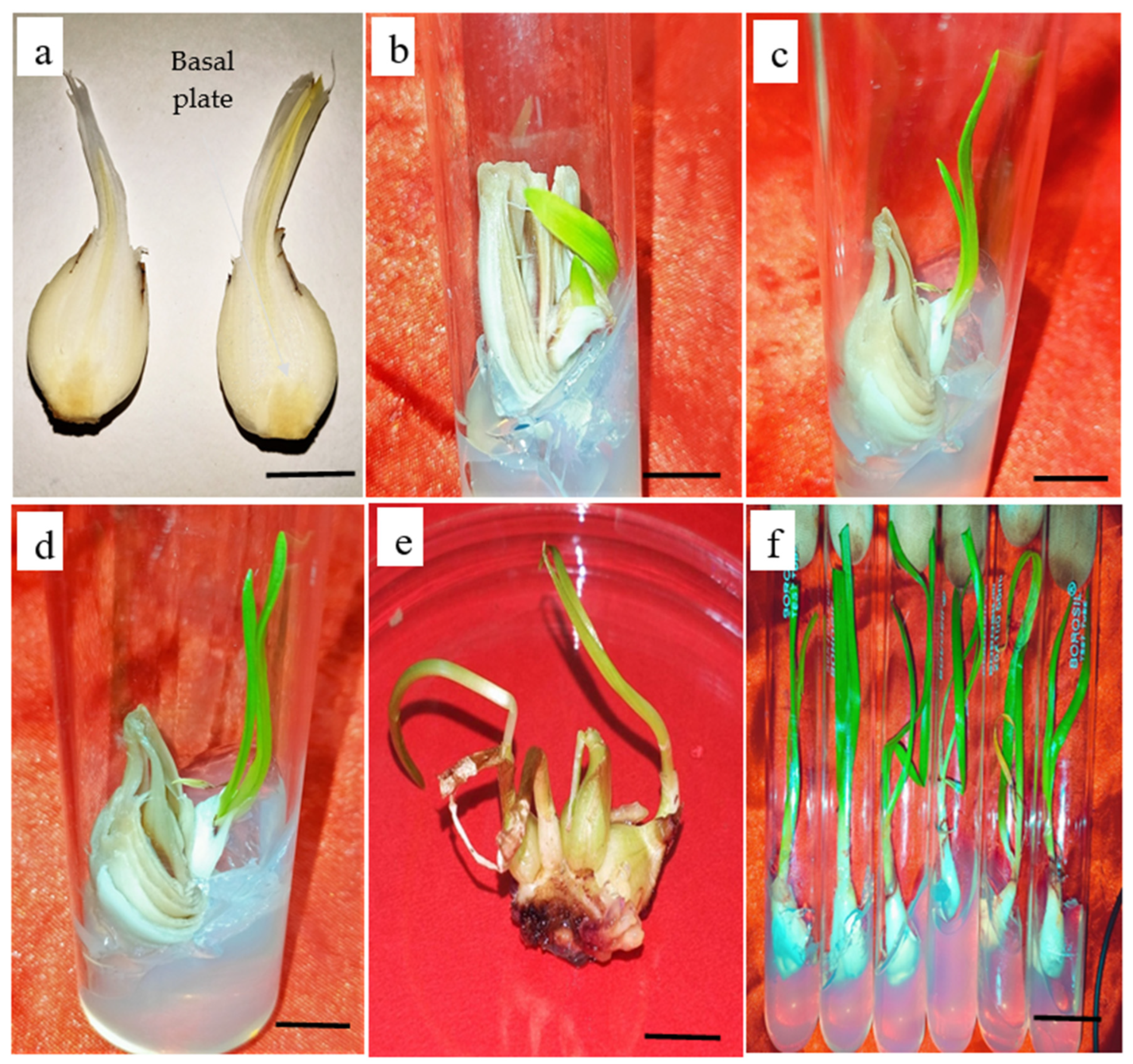
2.2. In Vitro Shoot Induction
2.3. Rooting
2.4. Elicitor Preparation and Dosage
2.5. Extraction Procedure for High Performance Thin Layer Chromatography (HPTLC)
2.5.1. Stock Solution and Sample Extraction Procedure
2.5.2. HPTLC Instrumentation and Chromatographic Conditions
2.6. Biochemical Analysis
2.6.1. Estimation of Sugar
2.6.2. Estimation of Proline
2.6.3. Estimation of Protein
2.7. Antioxidant Enzyme Assay
2.8. Statistical Analysis
3. Results
3.1. Disinfectants and Surface Sterilization of Explants
3.2. Influence of Plant Growth Regulators (PGRs) on Direct Shoot Regeneration and Bulblet Formation
3.3. Auxins and Root Induction
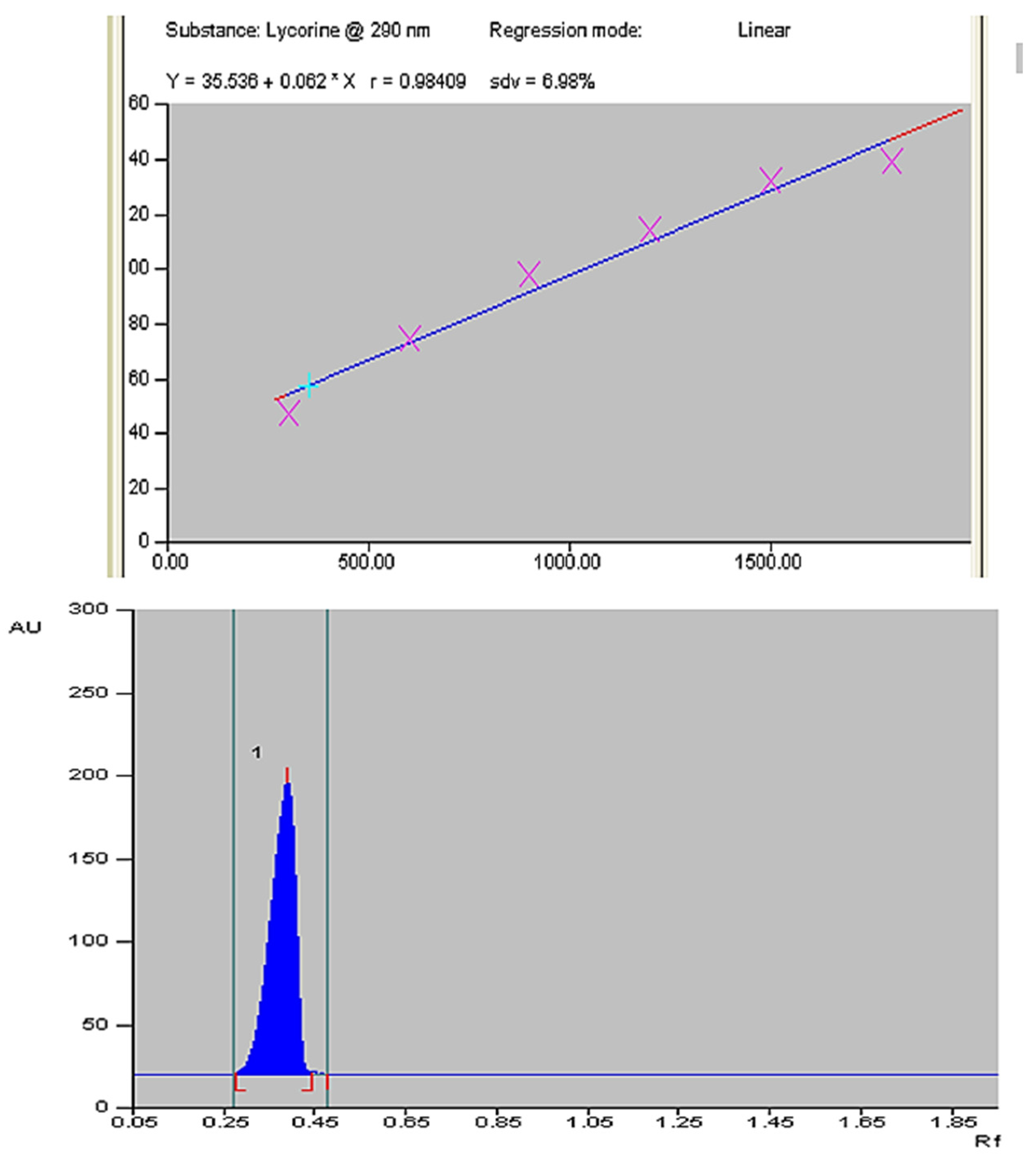
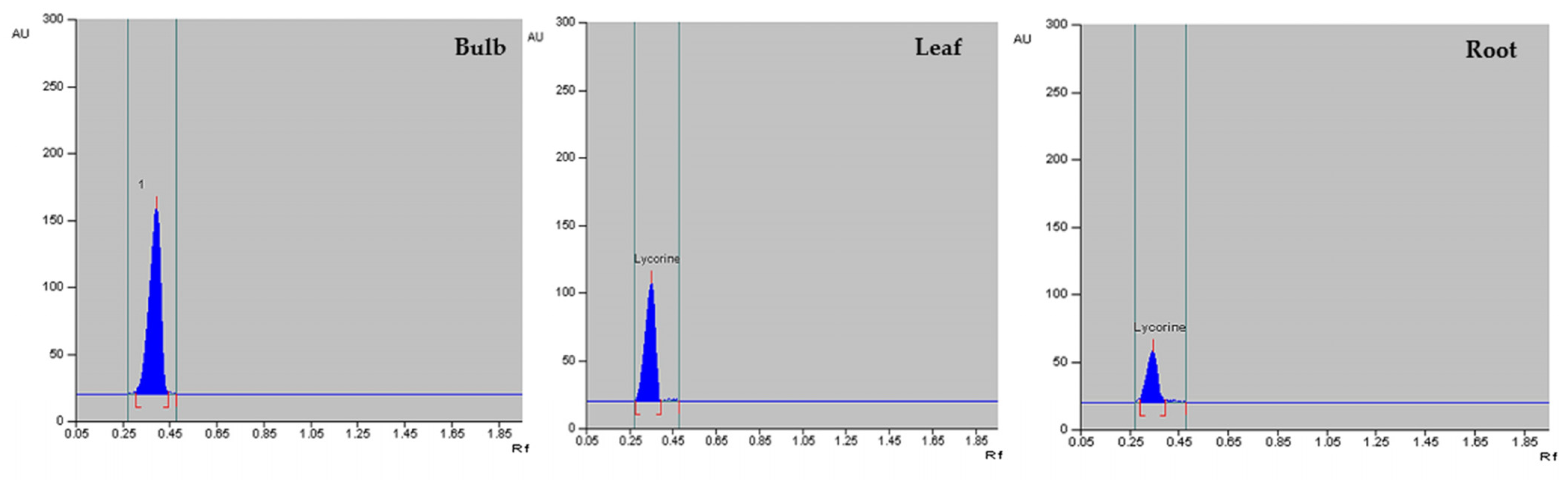
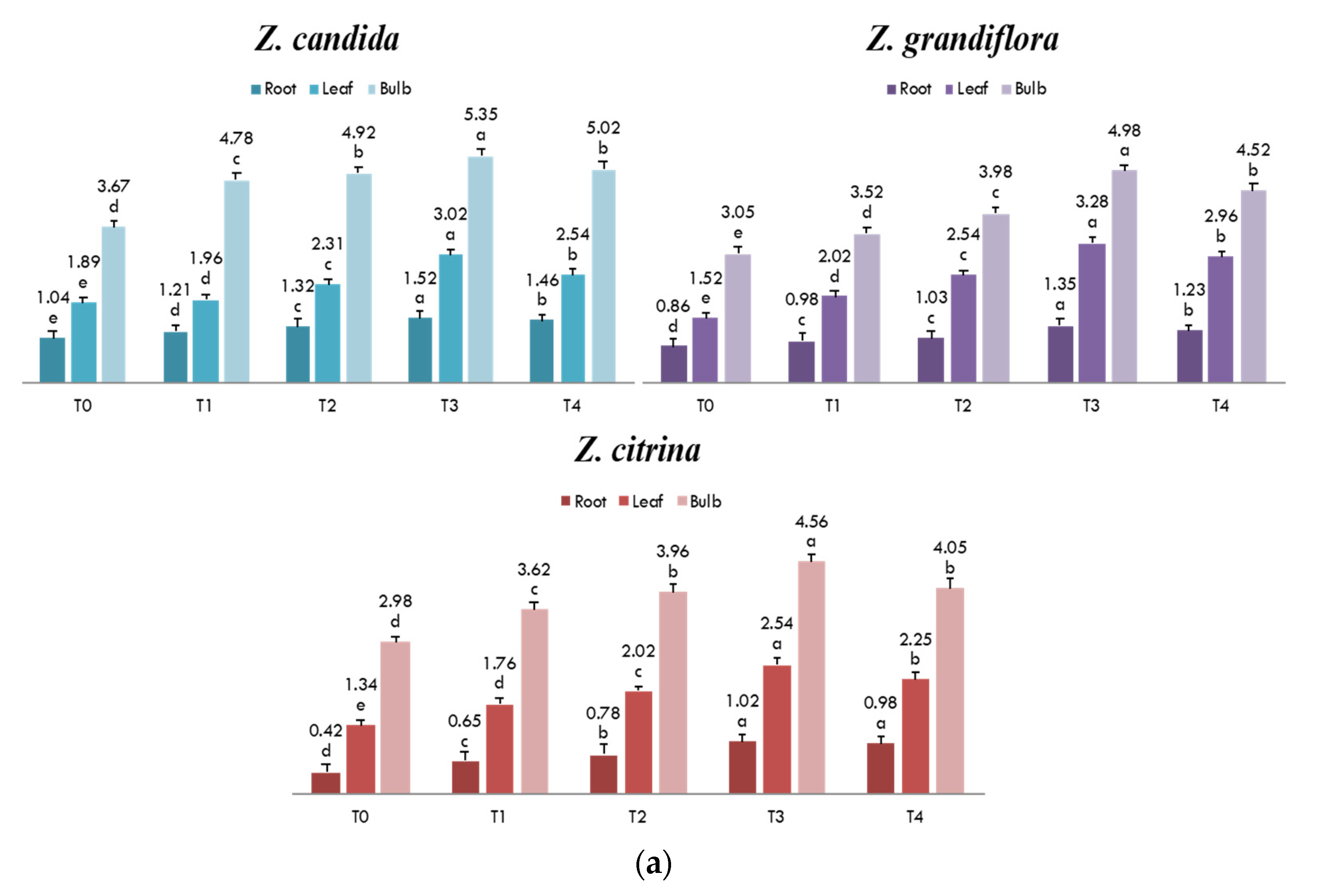
3.4. Quantification of Lycorine in Field Grown Z. grandiflora, Z. candida and Z. citrina Plants
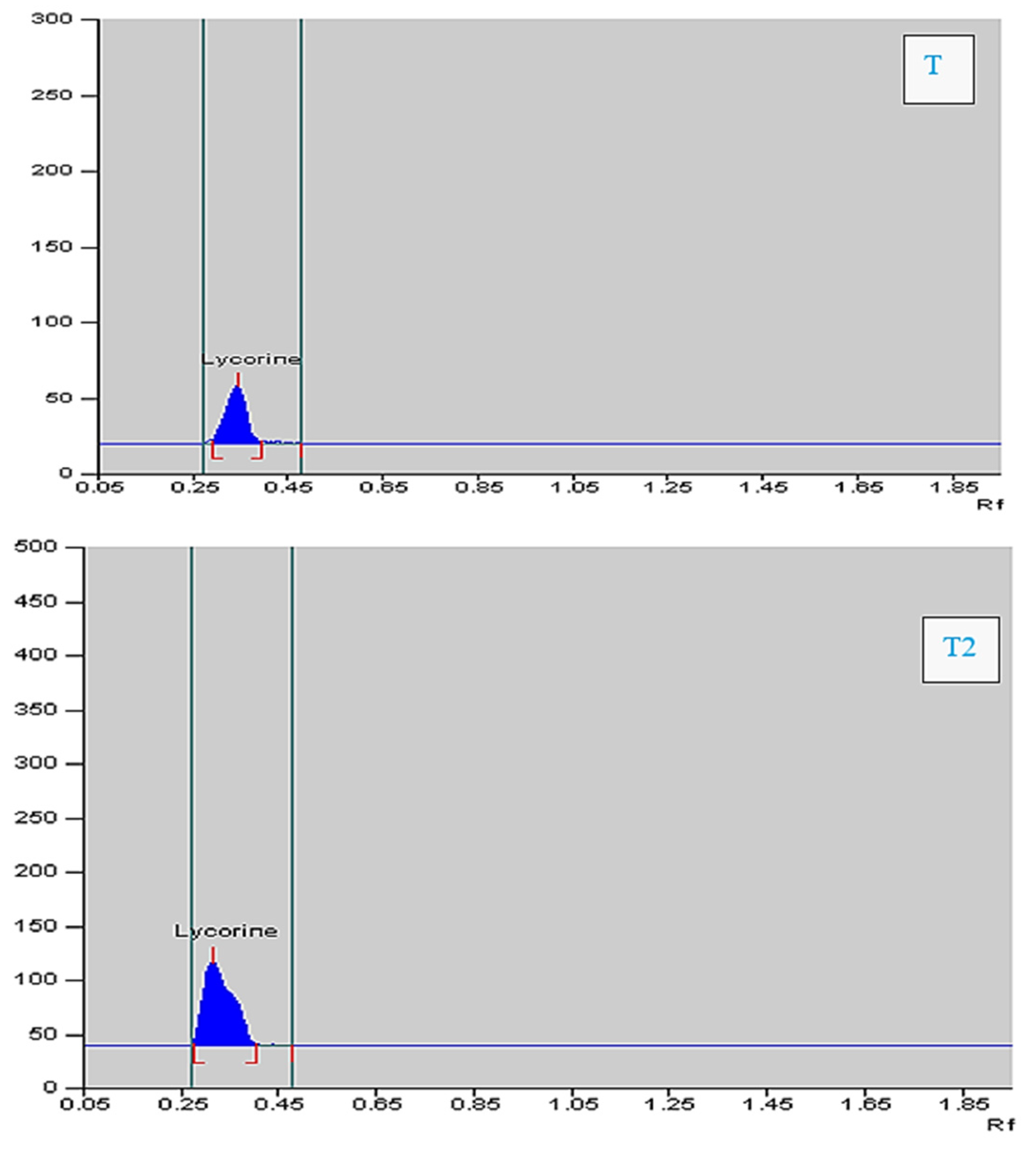
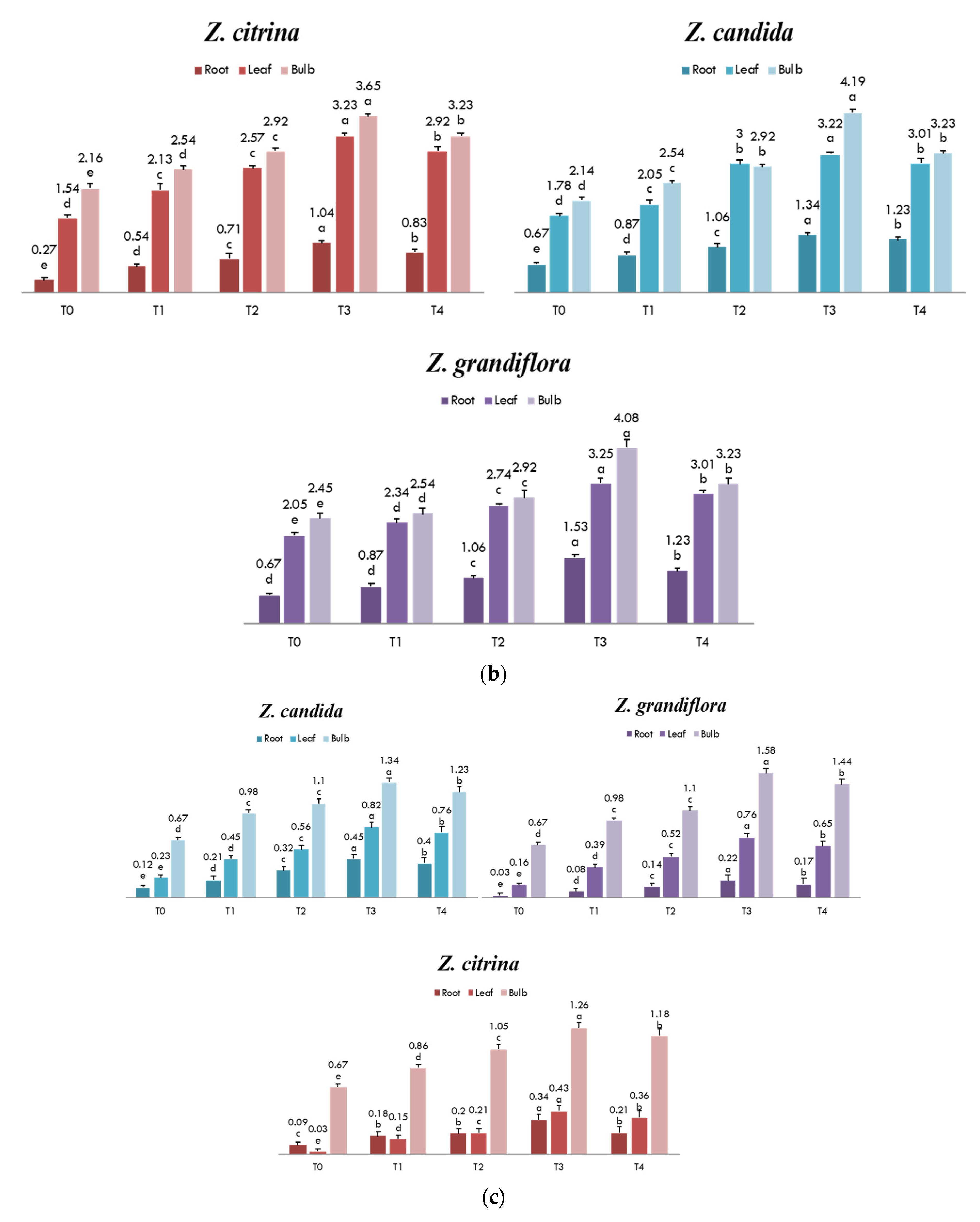
3.5. Different Doses of MJ on Lycorine Yield
3.6. Biochemical Attributes in Response to MJ Treatment
3.7. MJ Treatment and Antioxidant Enzymes Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Rungsung, W.; Ratha, K.; Dutta, S.; Dixit, A.K.; Hazra, J. Secondary Metabolites of Plants in Drugs Discovery. World J. Pharm. Res. 2015, 4, 604–613. [Google Scholar]
- Katoch, D.; Singh, B. Phytochemistry and Pharmacology of Genus Zephyranthes. Med. Aromat Plants 2015, 4, 212. [Google Scholar]
- Jin, Z. ChemInform Abstract: Amaryllidaceae and Sceletium Alkaloids. ChemInform 2013, 44. [Google Scholar] [CrossRef]
- Syeed, R.; Mujib, A.; Malik, M.Q.; Mamgain, J.; Ejaz, B.; Gulzar, B.; Zafar, N. Mass Propagation through Direct and Indirect Organogenesis in Three Species of Genus Zephyranthes and Ploidy Assessment of Regenerants through Flow Cytometry. Mol. Biol. Rep. 2021, 48, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yao, G. Amaryllidaceae and Sceletium Alkaloids. Nat. Prod. Rep. 2019, 36, 1462–1488. [Google Scholar] [CrossRef]
- Desgagné-Penix, I. Biosynthesis of Alkaloids in Amaryllidaceae Plants: A Review. Phytochem. Rev. 2021, 20, 409–431. [Google Scholar] [CrossRef]
- Khalifa, M.F.; Attia, E.Z.; Fahim, J.R.; Kamel, M.S. An Overview on the Chemical and Biological Aspects of Lycorine Alkaloid. J. Adv. Biomed. Pharm. Sci. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Jin, Y.H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a Non-Nucleoside RNA Dependent RNA Polymerase Inhibitor, as Potential Treatment for Emerging SARSvirus Infections. Phytomedicine 2021, 86, 153–440. [Google Scholar] [CrossRef]
- Wang, G.-P.; Yu, X.-D.; Sun, Y.-W.; Jones, H.D.; Xia, L.-Q. Generation of Marker- and/or Backbone-Free Transgenic Wheat Plants via Agrobacterium-Mediated Transformation. Front. Plant Sci. 2016, 7, 1324. [Google Scholar] [CrossRef]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, Chemotaxonomy and Chemoecology of Amaryllidaceae Alkaloids. Alkaloids Chem. Biol. 2020, 83, 113–185. [Google Scholar] [CrossRef]
- Zafar, N.; Mujib, A.; Ali, M.; Tonk, D.; Gulzar, B.; Malik, M.Q.; Mamgain, J.; Sayeed, R. Cadmium Chloride (CdCl2) Elicitation Improves Reserpine and Ajmalicine Yield in Rauvolfia Serpentina as Revealed by High-Performance Thin-Layer Chromatography (HPTLC). 3 Biotech 2020, 10, 344. [Google Scholar] [CrossRef]
- Saiman, M.Z.; Miettinen, K.; Mustafa, N.R.; Choi, Y.H.; Verpoorte, R.; Schulte, A.E. Metabolic Alteration of Catharanthus Roseus Cell Suspension Cultures Overexpressing Geraniol Synthase in the Plastids or Cytosol. Plant Cell Tissue Organ Cult. 2018, 134, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced Flavonoid Production in Hairy Root Cultures of Scutellaria Bornmuelleri by Elicitor Induced Over-Expression of MYB7 and FNSΠ2 Genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef]
- Naik, P.M.; Manohar, S.H.; Praveen, N.; Upadhya, V.; Murthy, H.N. Evaluation of Bacoside A Content in Different Accessions and Various Organs of Bacopa monnieri (L.) Wettst. J. Herbs Spices Med. Plants 2012, 18, 387–395. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Khonakdari, R.; Rezadoost, M.; Heydari, H.; Mirjalili, R. Effect of Photoperiod and Plant Growth Regulators on in Vitro Mass Bulblet Proliferation of Narcissus tazzeta L. (Amaryllidaceae), a Potential Source of Galantamine. Plant Cell Tissue Organ Cult 2020, 142, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; Simlat, M.; Morańska, E.; Skrzypek, E.; Warchoł, M.; Tarakemeh, A.; Laurain-Mattar, D. Exogenous Melatonin Stimulated Amaryllidaceae Alkaloid Biosynthesis in in Vitro Cultures of Leucojum aestivum L. Ind. Crop. Prod. 2019, 138, 111458. [Google Scholar] [CrossRef]
- Malik, M.Q.; Mujib, A.; Gulzar, B.; Zafar, N.; Syeed, R.; Mamgain, J.; Ejaz, B. Kanchan Enrichment of Alliin in Different in Vitro Grown Tissues of Allium Sativum through CdCl2 Elicitation as Revealed by High Performance Thin Layer Chromatography (HPTLC). Ind. Crop. Prod. 2020, 158, 113007. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Ali, M.; Mujib, A.; Gulzar, B.; Zafar, N. Essential Oil Yield Estimation by Gas Chromatography-Mass Spectrometry (GC-MS) after Methyl Jasmonate (MeJA) Elicitation in In Vitro Cultivated Tissues of Coriandrum sativum L. 3 Biotech 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nabi, N.; Singh, S.; Saffeullah, P. Responses of in Vitro Cell Cultures to Elicitation: Regulatory Role of Jasmonic Acid and Methyl Jasmonate: A Review. Vitr. Cell. Dev. Biol. Plant 2021, 57, 341–355. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-Jasmonate and Salicylic Acid as Potent Elicitors for Secondary Metabolite Production in Medicinal Plants: A Review. J. Pharmacogn. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Wang, R.; Xu, S.; Wang, N.; Xia, B.; Jiang, Y.; Wang, R. Transcriptome Analysis of Secondary Metabolism Pathway, Transcription Factors, and Transporters in Response to Methyl Jasmonate in Lycoris Aurea. Front. Plant Sci. 2016, 7, 1971. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Song, L.; Huang, X. Effects of β-Cyclodextrin and Methyl Jasmonate on the Production of Vindoline, Catharanthine, and Ajmalicine in Catharanthus Roseus Cambial Meristematic Cell Cultures. Appl. Microbiol. Biotechnol. 2015, 99, 7035–7045. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Pongkitwitoon, B.; Pathomwichaiwat, T.; Viboonjun, U.; Prathanturarug, S. Effects of Methyl Jasmonate on the Growth and Triterpenoid Production of Diploid and Tetraploid Centella asiatica (L.) Urb. Hairy Root Cultures. Sci. Rep. 2019, 9, 18665. [Google Scholar] [CrossRef]
- Wu, X.-H.; Fan, M.-Z.; Li, X.-F.; Piao, X.-C.; Gao, R.; Lian, M.-L. Involvement of Putrescine, Nitric Oxide, and Hydrogen Peroxide in Methyl Jasmonate-Induced Ginsenoside Synthesis in Adventitious Root Cultures of Panax Ginseng C.A. Meyer. J. Plant Growth Regul. 2021, 40, 1440–1449. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dey, P.M. Oligosaccharides. In Methods in Plant Biochemistry; Elsevier: Amsterdam, The Netherlands, 1990; pp. 189–218. [Google Scholar]
- Bates, L.; Waldren, P.P.; Teare, J.D. Rapid Determination of Free Proline of Water Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for Quantification of Microgram Quantities of Protein, Utilizing the Principle of Protein Dye Binding. Anal. Biochem. 1976, 72, 248–541. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate Specific Peroxidase in Spinach Chloro-Plasts. Plant Cell Physiol. 1981, 22, 867–888. [Google Scholar]
- García-Fortea, E.; García-Pérez, A.; Gimeno-Páez, E.; Martínez-López, M.; Vilanova, S.; Gramazio, P.; Prohens, J.; Plazas, M. Ploidy Modification for Plant Breeding Using in Vitro Organogenesis: A Case in Eggplant. Methods Mol. Biol. 2021, 2264, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, B.; Mujib, A.; Rajam, M.V.; Frukh, A.; Zafar, N. Identification of Somatic Embryogenesis (SE) Related Proteins through Label-Free Shotgun Proteomic Method and Cellular Role in Catharanthus roseus (L.) G. Don. Plant Cell Tissue Organ Cult. 2019, 137, 225–237. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Chakraborty, D.; Dewanjee, S.; Bhattacharya, S. Clonal Propagation of Zephyranthes Grandi-Flora Using Bulbs as Explants. Biol. Plant 2010, 54, 793–797. [Google Scholar] [CrossRef]
- Saeed, W.; Naseem, S.; Gohar, D.; Ali, Z. Efficient and Reproducible Somatic Embryogenesis and Micropropa-Gation in Tomato via Novel Structures-Rhizoid Tubers. PLoS ONE 2019, 14, e0215929. [Google Scholar] [CrossRef]
- Taha, L.S.; Sayed, S.S.; Farahat, M.M. El-Sayed IM. In Vitro Culture and Bulblets Induction of Asiatic Hybrid Lily’red Alert’. J. Biol. Sci. 2018, 18, 84–91. [Google Scholar] [CrossRef]
- Dragassaki, M.; Economou, A.S.; Vlahos, J.C. Bulblet Formation In Vitro and Plantlet Survival Extra Vitrum in Pancratium maritimum L. Acta Hortic. 2003, 616, 347–352. [Google Scholar] [CrossRef]
- Rice, L.J.; Finnie, J.F.; Van Staden, J. In Vitro Bulblet Production of Brunsvigia Undulate from Twin-Scales. S. Afr. J. Bot. 2011, 77, 305–312. [Google Scholar] [CrossRef]
- Youssef, N.M.; Shaaban, S.A.; Ghareeb, Z.F.; Taha, L.S. In Vitro Bulb Formation of Direct and Indirect Regeneration of Lilium Orientalis Cv. “Starfighter” Plants. Bull. Natl. Res. Cent. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Mujib, A.; Banerjee, S.; Maqsood, M.; Ghosh, P. Organogenesis and Plant Regeneration in Zephyranthes Rosea Lindl.: Histological and Chromosomal Study. Plant Biosyst. 2014, 148, 492–498. [Google Scholar] [CrossRef]
- Cai, J.; Ma, Y.; Hu, P.; Zhang, Y.; Chen, J.; Li, X. Elicitation of Furanocoumarins in Changium Smyrnioides Suspension Cells. Plant Cell Tissue Organ Cult. 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The Effects of Jasmonic Acid and Methyl Jasmonate on Rosmarinic Acid Production in Mentha x Piperita Cell Suspension Cultures. Plant Cell Tissue Organ Cult. 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of Ginsenoside and Gene Expres-Sion Related to Triterpene Biosynthesis in Ginseng Hairy Root Cultures Elicited by Methyl Jasmonate. Plant Cell Tissue Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screen-Ing of Amaryllidaceae Alkaloids in Bulbs and Tissue Cultures of Narcissus Papyraceus and Four Varieties of N. Tazetta. J. Pharm. Biomed. Anal. 2019, 172, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Pesaraklu, A.; Radjabian, T.; Salami, S.A. Methyl Jasmonate and Ag+ as Effective Elicitors for Enhancement of Phenolic Acids Contents in Salvia Officinalis and Salvia Verticillata, as Two Traditional Medicinal Plants. South Afr. J. Bot. 2021, 141, 105–115. [Google Scholar] [CrossRef]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.-H.; Verslues, P.E. Dynamic Proline Metabolism: Importance and Regulation in Water Limited Environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef]
- Andi, S.A.; Gholami, M.; Ford, C.M. The Effect of Methyl Jasmonate and Light Irradiation Treatments on the Stilbenoid Biosynthetic Pathway in Vitis Vinifera Cell Suspension Cultures. Nat. Prod. Res. 2018, 32, 909–917. [Google Scholar] [CrossRef]
- Fugate, K.K.; Lafta, A.M.; Eide, J.D.; Li, G.; Lulai, E.C.; Olson, L.L.; Deckard, E.L.; Khan, M.F.R.; Finger, F.L. Methyl Jasmonate Alleviates Drought Stress in Young Sugar Beet (Beta vulgaris L.) Plants. J. Agron. Crop. Sci. 2018, 204, 566–576. [Google Scholar] [CrossRef]
- Shilpha, J.; Satish, L.; Kavikkuil, M.; Largia, M.; Ramesh, M. Methyl Jasmonate Elicits the Solasodine Pro-Duction and Antioxidant Activity in Hairy Root Cultures of Solanum trilobatum L. Ind. Crop. Prod. 2015, 71, 54–64. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Kapil Dev, G.; Jeyaraj, M.; Rajesh, M.; Arjunan, A.; Muthuselvam, M.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Increased Production of Withanolide A, Withanone, and Withaferin A in Hairy Root Cultures of Withania somnifera (L.) Dunal Elicited with Methyl Jasmonate and Salicylic Acid. Plant Cell Tissue Organ Cult. 2013, 114, 121–129. [Google Scholar] [CrossRef]
- Abou-Donia, A.H.; Toaima, S.M.; Hammoda, H.M.; Shawky, E. New Rapid Validated HPTLC Method for the Determina-Tion of Lycorine in Amaryllidaceae Plants Extracts. Chromatographia 2007, 65, 497–500. [Google Scholar] [CrossRef] [PubMed]


| PGRs and Concentrations | fDSR | Bmean | |||
|---|---|---|---|---|---|
| BAP | NAA | Z. grandiflora | Z. candida | Z. citrina | |
| - | - | 40.2 ± 1.24 f | 47.7 ± 1.03 e | 43.2 ± 1.32 d | 0 |
| 0.5 | - | 44.3 ± 1.36 e | 50.3 ± 1.06 d | 46.5 ± 1.67 d | 1.01 ± 0.01 e |
| 0.5 | 0.5 | 50.8 ± 2.21 d | 55.2 ± 1.22 c | 49.5 ± 1.90 d | 1.06 ± 0.01 e |
| 1.0 | - | 58.6 ± 2.38 d | 60.2 ± 1.38 b | 58.0 ± 2.08 c | 2.08 ± 0.01 d |
| 1.0 | 0.5 | 67.5 ± 1.42 c | 66.3 ± 2.01 b | 60.8 ± 2.78 c | 3.03 ± 0.02 c |
| 2.0 | - | 75.6 ± 3.44 b | 67.5 ± 2.78 b | 68.6 ± 2.80 b | 5.32 ± 0.04 b |
| 2.0 | 0.5 | 85.5 ± 3.48 a | 73.9 ± 3.08 a | 76.5 ± 3.09 a | 7.46 ± 0.06 a |
| 2.5 | - | 73.6 ± 3.45 b | 64.0 ± 2.07 b | 65.6 ± 2.55 b | 4.87 ± 0.05 c |
| 2.5 | 0.5 | 47.5 ± 2.54 e | 58.3 ± 2.54 c | 43.7 ± 2.21 d | 2.05 ± 0.04 d |
| 3.0 | - | 40.6 ± 1.33 f | 36.3 ± 1.26 f | 35.7 ± 1.02 e | 1.07 ± 0.02 e |
| 3.0 | 0.5 | 37.5 ± 1.36 g | 32.5 ± 1.32 f | 30.8 ± 1.01 f | 1.66 ± 0.01 e |
| Auxins Concentrations (mg·L−1) | Root Induction Frequency (FRI) | Mean Number of Roots (MR) | |
|---|---|---|---|
| IBA | NAA | ||
| 1 | - | 60.67 ± 1.24 d | 5.22 ± 0.02 c |
| 1 | 0.25 | 68.8 ± 1.08 c | 5.05 ± 0.04 c |
| 2 | - | 80.66 ± 3.43 a | 8.32 ± 1.28 a |
| 2 | 0.25 | 73.55 ± 2.56 b | 6.03 ± 1.09 b |
| 3 | - | 62.05 ± 1.67 d | 5.43 ± 0.03 c |
| 3 | 0.25 | 52.01 ± 1.10 e | 2.76 ± 0.01 d |
| Parts Used | Z. candida | Z. grandiflora | Z. citrina |
|---|---|---|---|
| Bulb | 1.93 ± 0.04 a | 1.87 ± 0.04 a | 1.62 ± 0.03 a |
| Leaf | 0.98 ± 0.02 b | 0.83 ± 0.03 b | 0.65 ± 0.01 b |
| Root | 0.43 ± 0.01 c | 0.50 ± 0.01 c | 0.38 ± 0.01 c |
| Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Bulb | 1.97 ± 0.04 d | 2.33 ± 0.03 c | 2.74 ± 0.05 a | 1.53 ± 0.04 b | 1.45 ± 0.02 b |
| Leaf | 1.08 ± 0.02 c | 1.26 ± 0.01 b | 1.53 ± 0.05 a | 1.14 ± 0.02 b | 1.03 ± 0.02 c |
| Root | 0.53 ± 0.01 a | 0.62 ± 0.01 b | 0.77 ± 0.01 a | 0.33 ± 0.01 c | 0.45 ± 0.01 d |
| Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Bulb | 1.87 ± 0.04 c | 1.94 ± 0.03 b | 2.15 ± 0.04 a | 1.64 ± 0.02 c | 1.62 ± 0.01 d |
| Leaf | 0.83 ± 0.03 c | 0.96 ± 0.02 b | 1.03 ± 0.04 a | 0.76 ± 0.01 b | 0.73 ± 0.01 d |
| Root | 0.50 ± 0.01 c | 0.65 ± 0.02 b | 0.74 ± 0.01 a | 0.46 ± 0.01 b | 0.45 ± 0.01 c |
| Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Bulb | 1.62 ± 0.03 c | 1.96 ± 0.04 b | 2.08 ± 0.05 a | 1.73 ± 0.04 d | 1.65 ± 0.04 e |
| Leaf | 0.65 ± 0.01 c | 0.78 ± 0.01 b | 0.86 ± 0.02 a | 0.56 ± 0.03 d | 0.42 ± 0.02 d |
| Root | 0.38 ± 0.01 d | 0.45 ± 0.01 c | 0.66 ± 0.01 a | 0.35 ± 0.02 b | 0.33 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syeed, R.; Mujib, A.; Dewir, Y.H.; Malik, M.Q.; Bansal, Y.; Ejaz, B.; Mamgain, J.; Hakiman, M.; Alsughayyir, A. Methyl Jasmonate Elicitation for In Vitro Lycorine Accumulation in Three Zephyranthes Species and Comparative Analysis of Tissue-Cultured and Field Grown Plants. Horticulturae 2023, 9, 832. https://doi.org/10.3390/horticulturae9070832
Syeed R, Mujib A, Dewir YH, Malik MQ, Bansal Y, Ejaz B, Mamgain J, Hakiman M, Alsughayyir A. Methyl Jasmonate Elicitation for In Vitro Lycorine Accumulation in Three Zephyranthes Species and Comparative Analysis of Tissue-Cultured and Field Grown Plants. Horticulturae. 2023; 9(7):832. https://doi.org/10.3390/horticulturae9070832
Chicago/Turabian StyleSyeed, Rukaya, Abdul Mujib, Yaser Hassan Dewir, Moien Qadir Malik, Yashika Bansal, Bushra Ejaz, Jyoti Mamgain, Mansor Hakiman, and Ali Alsughayyir. 2023. "Methyl Jasmonate Elicitation for In Vitro Lycorine Accumulation in Three Zephyranthes Species and Comparative Analysis of Tissue-Cultured and Field Grown Plants" Horticulturae 9, no. 7: 832. https://doi.org/10.3390/horticulturae9070832
APA StyleSyeed, R., Mujib, A., Dewir, Y. H., Malik, M. Q., Bansal, Y., Ejaz, B., Mamgain, J., Hakiman, M., & Alsughayyir, A. (2023). Methyl Jasmonate Elicitation for In Vitro Lycorine Accumulation in Three Zephyranthes Species and Comparative Analysis of Tissue-Cultured and Field Grown Plants. Horticulturae, 9(7), 832. https://doi.org/10.3390/horticulturae9070832









