Abstract
Psidium friedrichsthalianum (Myrtaceae) is a small tree with antioxidant activity in its fruits and antimicrobial activity in its leaves and thin branches. The present study analyzed the seasonal variability in the yield and essential oil composition of a P. friedrichsthalianum population in Belém, Brazil. Essential oils were obtained by hydrodistillation and analyzed by gas chromatography (GC) coupled to mass spectrometer (MS) and flame ionization detector (FID). Chemometric analyses were carried out to verify the climatic influence on the production and composition of the essential oil. The average oil yield in the dry season (August–February) was 0.5 ± 0.0%, and in the rainy season (March–May), it was 0.8 ± 0.0%, with statistical differentiation. There was a moderate correlation between oil yield and the collection area’s relative humidity (r = 0.63). The PCA and HCA analyses did not show differentiation between the P. friedrichsthalianum oil samples during the dry and rainy seasons. However, the class of monoterpene hydrocarbons presented a negative correlation with temperature (r = −0.81) and humidity (−0.80) of the sampled area. In the PCA and HCA studies, the samples were classified into three groups: Group I (leaf oils) was characterized by a higher content of α-pinene (6.3–18.0%), β-elemene (9.9–14.8%), caryophyllene oxide (4.3–16.3%), and β-pinene (4.8–13.4%). Group II (leaf oils) was defined by a higher content of selin-11-en-4-α-ol (4.6–15.6%), β-elemene (9.9–14.8%), α-pinene (6.3–18.0%), and E-caryophyllene (3.1–8.7%). Group III (fruits volatile concentrate) was characterized by a higher content of α-pinene (17.6%), α-terpineol (13.7%), and selin-11-en-4-α-ol (10.0%). There was significant seasonal variability in P. friedrichsthalianum, whose responses are directly linked to abiotic factors such as precipitation, insolation, humidity, and temperature.
1. Introduction
Myrtaceae has around 1200 species, comprising 29 genera of trees, shrubs, and sub-shrubs, emphasizing Eugenia, Myrciaria, and Psidium found in Brazilian territory. Many scientific reports about its pharmacological and cosmetic applications are present in the literature [1,2]. The Psidium genus comprises 266 species, widely distributed worldwide in tropical and subtropical regions. In Brazil, Psidium has 60 species of trees, from large to small sizes. Among them, P. guineense Sw., known as “araçá-mirim”, P. acutangulum Mart. ex DC., popularly called “araçá-pera”, and P. guajava L., the traditional “guava”, all used in the treatment of coughs, diarrhea, stomach pain, vomiting, fever, and flu [3,4].
Myrtaceae essential oils have high variability in volatile compounds and have outstanding biological activities [1]. Essential oils from Psidium species have antiproliferative, antioxidant, fungicidal, antibacterial, phytotoxic, larvicidal, anti-inflammatory, and cytotoxic properties [1,5]. In addition, Psidium oils are abundant in terpene compounds, with great emphasis on monoterpene hydrocarbons limonene and α-pinene [6,7].
Psidium friedrichsthalianum (O. Berg) Nied. (syn. Calyptropsidium friedrichsthalianum O. Berg), known as “Costa Rican guava” or “sour guava”, is a small tree with fruits with antioxidant activity and widely used to prepare juices, jellies, and sweets [8,9]. This species originates from Central America, but its cultivation is currently carried out in several tropical countries, including Colombia, Brazil, and Ecuador [10]. Phytochemical and pharmacological studies with leaf and bark extracts of P. friedrichsthalianum reported significant antimicrobial potential [11].
The present work aimed to analyze the seasonal variability in a Psidium friedrichsthalianum population sampled in Belém, Pará state, Brazil, based on the analysis of yield and composition of its essential oils from August 2021 to May 2022 (10 months), using chemometric tools.
2. Materials and Methods
2.1. Plant Material and Climatic Data
The leaves (250 g) and fruits (100 g) of a cultivated population of Psidium friedrichsthalianum were randomly collected in Belém city, Pará state, Brazil (coordinates: 1°26′14.2″ S/48°26′30.2″ W). The mature leaves for the seasonal study were sampled on day 10 of each month, at 8 am, from August 2021 to May 2022. For its volatile concentrate analysis, the fruits were collected in November 2021, the month of fruiting of the species. Plant identification was performed by comparison with an authentic specimen of Psidium friedrichsthalianum. A specimen sample (MSF001848) was incorporated into the Herbarium Marlene Freitas da Silva at Universidade do Estado do Pará, Belém, State of Pará, Brazil. The specimen was collected in agreement with Brazilian laws concerning the protection of biodiversity (Sisgen A47AD8F).
The climatic parameters (insolation, relative air humidity, and rainfall precipitation) of the mentioned area were obtained for each month from the website of the Instituto Nacional de Meteorologia (INMET, http://www.inmet.gov.br/portal/, accessed on 5 December 2022), of Brazilian Government (INMET, 2022). Meteorological data were recorded through the automatic station A-201, located in Belém, Pará state, Brazil, equipped with a Vaisala system, model MAWS 301 (Vaisala Corporation, Helsinki, Finland).
2.2. Essential Oil and Volatile Concentrate Extraction
The leaves were dried for seven days at room temperature, then pulverized. The dried leaves (100 g) were subjected to hydrodistillation (in duplicate) using a Clevenger-type apparatus (3 h). The dry plant weights were used to calculate the oil yields (in duplicate). The moisture content of the leaves samples was calculated in an infrared moisture balance for water loss measurement. The fresh fruits were cut, homogenized (20 g), and subjected to a distillation-simultaneous extraction (DES) system using a Nickerson and Likens type extractor, in addition to water (150 mL) and n-pentane (2 mL) as solvents, for 2 h (in duplicate), to obtain its volatile concentrate (Vc). The oils (leaves) and the volatile concentrate (fruits) were stored in dark bottles for later chromatographic analysis.
2.3. Oils and Volatile Concentrate Composition Analysis
The analyses of the oils and volatile concentrate were performed by GC-MS. A Shimadzu instrument Model QP-2010 ultra (Shimadzu, Tokyo, Japan) was used. An Rtx-5MS (30 m × 0.25 mm; 0.25 μm film thickness) fused silica capillary column (Restek, Bellafonte, PA, USA) was used as the stationary phase. The carrier gas was helium adjusted to 1.0 mL/min at 57.5 Kpa. One μL of n-hexane solution (oil and volatile concentrate, 5 μL: n-hexane, 500 μL) was injected in split mode (split ratio 1:20). The injector and interface temperature was 250 °C, oven programmed temperature was 60 to 240 °C (3 °C/min), followed by an isotherm of 10 min. EIMS (electron ionization mass spectrometry) at 70 eV. The ion source temperature was 200 °C. The mass spectra were obtained by scanning every 0.3 s. The mass fragments were from 35 to 400 m/z. The retention index was calculated for all components using C8-C40 n-alkanes series (Sigma-Aldrich, Milwaukee, WI, USA) according to the van den Dool and Kratz linear equation [12]. Individual components were identified by comparing their retention indices and mass spectra (molecular mass and fragmentation pattern) with those in the GCMS-Solution system libraries [13,14]. The quantitative data regarding the volatile constituents were obtained using a Shimadzu GC 2010 Series, operated under similar conditions to the Shimadzu GC-MS system. The relative amounts of individual components were calculated by peak-area normalization using the flame ionization detector (GC-FID). GC-FID and GC-MS analyses were performed in duplicate.
2.4. Statistical Analysis
The statistical analysis was performed according to Santos et al. [6]. The significance was assessed by a Tukey test (p < 0.05). The GraphPad Prism software, version 5.0 was used to calculate the Pearson correlation coefficients (r). The principal component analysis (PCA) was applied to the oil components (>3.0%). The hierarchical cluster analysis (HCA) was carried out considering the Euclidean distance and the Ward linkage [15].
3. Results and Discussion
3.1. Seasonal Effect on Oil Yields
Climatic factors, such as insolation, precipitation, temperature, and relative humidity, were monitored from August 2021 to May 2022 to evaluate their influence on the production and composition of the essential oil of P. friedrichsthalianum. The insolation values varied between 105.4 (March) and 256.1 h (August), the monthly precipitation from 163.4 (October) to 527.4 mm (March), the temperature from 25.9 °C (January) to 27.6 °C (October), and the relative air humidity from 82.1% (October) to 93.0% (April). The dry period in the region where the plant occurs comprised the months from August to February, with an average precipitation of 253.7 ± 58.4 mm, and the rainy period from March to May, with an average precipitation of 472.5 ± 60.2 mm (Figure 1). In previous work, in the seasonal study of the essential oil composition of Lippia alba, the dry period occurred from August to February, and the rainy period from March to May [16].
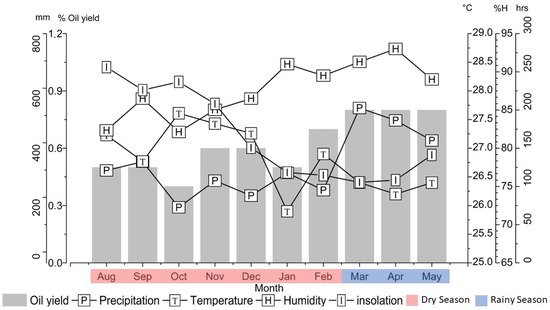
Figure 1.
Relationship between climatic factors and Psidium friedrichsthalianum leaves essential oil yield during the seasonal study.
The climate in the Brazilian Amazon is represented only by the dry and rainy seasons. With a hot and humid climate, the Amazon region has the highest rainfall from December to April, characterized by the rainy season, and the lowest rainfall from June to November, represented by the dry season. The year’s remaining months are considered transition periods between these two seasons [17,18]. However, from one year to another, these two seasons may change depending on the atmospheric phenomena that affect tropical regions [19].
In the present seasonal study, the leaves essential oil yields of P. friedrichsthalianum ranged from 0.4% (October) to 0.8% (March to May), averaging 0.6 ± 0.1% for the annual period (Figure 1). The essential oil yield showed a significant difference (Tukey, p < 0.05) during the dry (0.5 ± 0.0%) and rainy (0.8 ± 0.0%) periods. Concerning the climatic factors vs. the essential oil yield, no significant correlation was observed (Tukey, p > 0.05) with the temperature (r = −0.32), while with the relative humidity (r = 0.63), the oil yield showed a moderate correlation. There was also a strong and negative correlation between the oil yield and the insolation (r = −0.70), as seen in Table 1.

Table 1.
Correlation between the yield, principal components, and classes of compounds of the Psidium friedrichsthalianum oil and the climatic factors.
A previous study evaluating the effect of seasonality on the leaves essential oil of a Psidium acutangulum DC. population, collected in Belém, Pará, Brazil, did not show a significant difference in the oils yield between the dry period (0.7 ± 0.3%) and the rainy period (0.9 ± 0.2%) [6]. On the other hand, during the seasonal study of Psidium salutare (Kunth). O. Berg leaves in Northeast Brazil, its leaf oil yield showed different percentages during the dry (0.15%) and rainy (0.73%) seasons, with no significant correlation with the precipitation [20].
3.2. Seasonal Effect on P. friedrichsthalianum Oil Composition
Table 2 lists eighty-nine (89) chemical constituents identified by GC and GC-MS in the EOs from the leaves and volatile concentrate of P. friedrichsthalianum, in ascending order of their respective retention indices. These constituents comprise about 89.5% of the oils analyzed in the seasonal study and 85.0% of the components from the fruits’ volatile concentrate. The predominant classes of compounds in the leaf oil samples were sesquiterpene hydrocarbons (19.1% to 45.7%), followed by oxygenated sesquiterpenes (18.8 to 39.4%), monoterpene hydrocarbons (13.2 to 34.6%), and oxygenated monoterpenes (1.3 to 9.6%). As for the volatile concentrate of the fruits, there was a predominance of monoterpene hydrocarbons (31.2%), followed by oxygenated sesquiterpenes (20.9%), sesquiterpene hydrocarbons (17.5%), and oxygenated monoterpenes (15.4%). The main constituents identified in the leaf oils of the seasonal study were α-pinene (6.3 to 18.0%), caryophyllene oxide (4.3 to 16.3%), selin-11-en-4-α-ol (4.6 to 15.6%), β-elemene (9.9 to 14.9%), β-pinene (4.8 to 13.4%), bicyclogermacrene (6.1 to 7.3%), linalool (0.1 to 7.1%), and spathulenol (1.9 to 5.9%). In the volatile concentrate of the fruits, there was a predominance of α-pinene (17.6%), α-terpineol (13.7%), selin-11-en-4-α-ol (10.0%), β-pinene (7.1%), β-selinene (6.0%), and E-caryophyllene (5.0%). The chemical structures of these compounds are shown in Figure 2.

Table 2.
Seasonal study of leaves essential oils (19 August–20 May) and fruits volatile concentrate (19 November) composition of P. friedrichsthalianum.
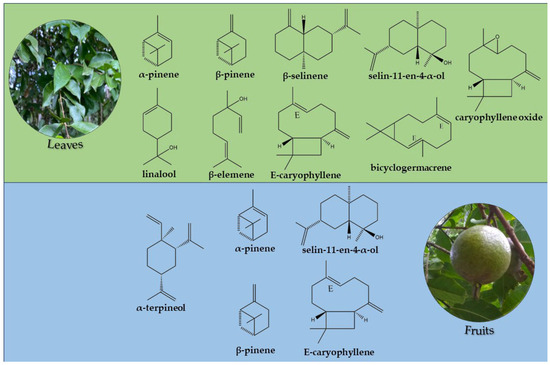
Figure 2.
Chemical structures of main constituents identified in the oils and fruits of P. friedrichsthalianum.
The chemical constituents that significantly correlated with climatic factors were α-pinene with insolation (r = −0.70), the caryophyllene oxide with precipitation (r = 0.63), the β-elemene with mean temperature (r = 0.86), relative humidity (r = 0.88), and precipitation (r = 0.60), the α-terpineol with temperature (−0.99), relative humidity (−0.99), insolation (r = −0.66), and precipitation (r = −0.65), the β-selinene with temperature (−0.75), relative humidity (−0.72), and insolation (r = −0.59). The constituents that did not significantly correlate with climatic factors were selin-11-en-4-α-ol and β-pinene. On the other hand, the class of oxygenated monoterpenes showed a strong negative and significant correlation with temperature (r = −0.79) and relative humidity (−0.78) (see Table 1).
3.3. Multivariate Analysis of P. friedrichsthalianum
Hierarchical cluster analysis (HCA) and principal component analysis (PCA) were plotted with volatile constituents above 3%. Applying hierarchical cluster analysis (HCA) provided the dendrogram shown in Figure 3, which presents the P. friedrichsthalianum oil volatiles in three groups and zero similarity. Group I comprised oils from August, November, January, February, March, and April. Group II included September, October, December, and May oils. Group III concerned only the volatile concentrate of the fruits.
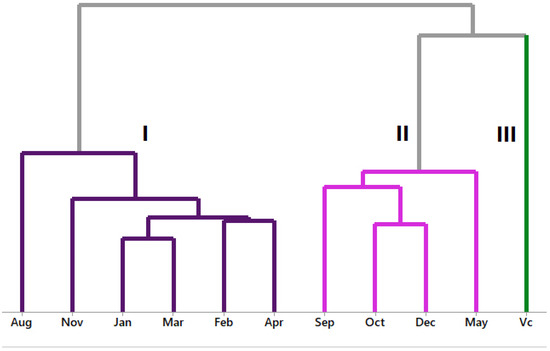
Figure 3.
HCA analysis of main volatiles from P. friedrichsthalianum.
Principal Component Analysis (PCA) (Figure 4) clarified 79.81% of the data variability. The PC1 component explained 36.33% and was positively correlated with α-pinene (r = −0.11), α-terpineol (r = −0.50), and β-selinene (r = −0.39). The PC2 component explained 24.54% and showed a negative correlation with α-pinene (r = −0.13), β-pinene (r = −0.09), β-elemene (r = −0.07), α-copaene (r = −0.10), and spathulenol (r = −0.45). The PC3 component explained 18.94% of the data and showed a positive correlation with α-pinene (r = 0.68), β-pinene (r = 0.60), α-terpineol (r = 0.14), β-elemene (r = 0.03), and E-caryophyllene (r = 0.19). As with the HCA, the analysis of the PCA confirmed the formation of three distinct groups. Group I was characterized by the highest content of α-pinene (6.3–18.0%), β-elemene (9.9–14.9%), caryophyllene oxide (4.3–16.3%), and β-pinene (4.8–13.4%). Group II was characterized by the highest content of selin-11-en-4-α-ol (4.6–15.6%), β-elemene (9.9–14.9%), α-pinene (6.3–18.0%), and E-caryophyllene (3.1–8.7%). Group III was characterized by the highest content of α-pinene (17.6%), α-terpineol (13.7%), and Selin-11-en-4-α-ol (10.0%).
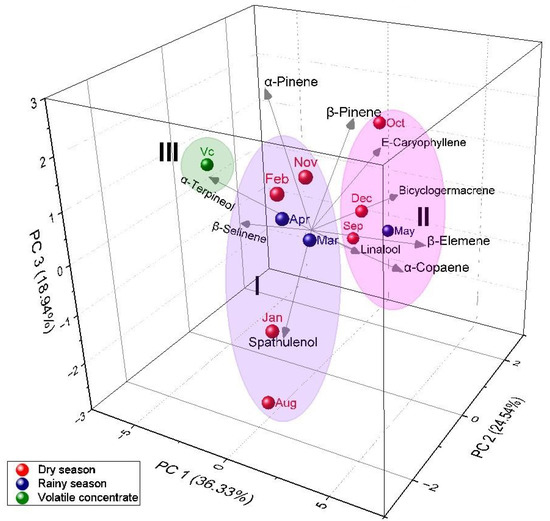
Figure 4.
PCA analysis of main volatiles from P. friedrichsthalianum.
PCA and HCA analyses did not differentiate between P. friedrichsthalianum oil samples during dry and rainy seasons. A previous study about the seasonality of Psidium acutangulum leaves essential oils from Brazil showed no sample separation from dry and rainy seasons [6]. Indeed, some species show variation in the constituents contents but cannot be separated in chemometric analyses due to their metabolism not correlating with climatic parameters or other factors, biotic or abiotic, which may interfere with metabolic pathways [6,15]. However, correlations were observed between climatic parameters and constituents of oils and their classes of compounds, as mentioned before (see Table 1).
About eighteen Psidium species are grown worldwide, and the chemical compositions of more than one hundred of their essential oils have been reported in the literature, with significant variability of volatile constituents and according to seasonality and collection sites [1]. Previously, the essential oils composition of the leaves and the volatile concentrate of the fruits of P. friedrichsthalianum were reported: The leaves of a specimen collected in San Jose, Costa Rica, having E-caryophyllene, α- and β-pinene, and β-elemene as main constituents [21], the fruits of a specimen sampled in Havana, Cuba, with the predominance of E-caryophyllene, α-terpineol, α-pinene, α- and β-selinene, δ-cadinene, and α-copaene [22], and the leaves of a specimen collected in Alegre, Espírito Santo, Brazil, having E-caryophyllene, caryophyllene oxide, α-humulene, and α-copaene as significant components [23].
The extracts of leaves and fruits of P. friedrichsthalianum, the Costa Rican guava, proved to be a rich source of phenolic compounds, mainly quercetin derivatives, and proanthocyanidins derived from epicatechin units, besides other compounds such as ellagitannins, and benzophenones [24,25].
4. Conclusions
The main constituents identified in the leaf oils of the seasonal study of Psidium friedrichsthalianum were α-pinene, caryophyllene oxide, selin-11-en-4-α-ol, β-elemene, β-pinene, bicyclogermacrene, linalool, and spathulenol. In the volatile concentrate of the fruits, there was a predominance of α-pinene, α-terpineol, selin-11-en-4-α-ol, β-pinene, β-selinene, and E-caryophyllene. The essential oil exhibited a significantly strong correlation with humidity and insolation, and the constituents of the oil were correlated with climatic parameters. Furthermore, the class of monoterpene hydrocarbons showed a moderate negative correlation with temperature and humidity. Thus, the present study contributes to the knowledge on the chemical variability of P. friedrichsthalianum essential oils.
Author Contributions
Conceptualization, P.L.B.F. and J.G.S.M.; formal analysis, P.V.L.S., E.d.N.S.d.C., W.M.O.d.N. and J.d.A.N.; writing—original draft preparation, P.V.L.S., E.d.N.S.d.C., J.d.A.N. and R.H.V.M.; writing—review and editing, P.L.B.F. and J.G.S.M. Project administration, P.L.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PAPQ (Programa de Apoio à Publicação Qualificada), Propesp, UFPA.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), to Universidade Federal do Pará (PIBIC-UFPA) for providing scholarships to E.d.N.S.d.C., and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing scholarships to J.d.A.N.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, R.C.E.; Costa, J.S.D.; Figueiredo, R.O.D.; Setzer, W.N.; Silva, J.K.R.D.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. [Google Scholar] [CrossRef]
- Psidium, L. GBIF Backbone Taxonomia. In Conjunto de Dados Da Lista de Verificação; NoSecretariado Do GBIF: Palikir, Micronesia, 2022. [Google Scholar]
- Da Costa, J.S.; Andrade, W.M.S.; de Figueiredo, R.O.; Santos, P.V.L.; Freitas, J.J.d.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Chemical Composition and Variability of the Volatile Components of Myrciaria Species Growing in the Amazon Region. Molecules 2022, 27, 2234. [Google Scholar] [CrossRef]
- Juárez-Vázquez, M.D.C.; Carranza-Álvarez, C.; Alonso-Castro, A.J.; González-Alcaraz, V.F.; Bravo-Acevedo, E.; Chamarro-Tinajero, F.J.; Solano, E. Ethnobotany of Medicinal Plants Used in Xalpatlahuac, Guerrero, México. J. Ethnopharmacol. 2013, 148, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, Anti-Inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Santos, P.V.L.; Ellen de Nazaré, S.; de Sousa Barroso, A.; Mourão, R.H.V.; Setzer, W.N.; da Silva, J.K.; do Nascimento, W.M.O.; da Costa, J.S.; Figueiredo, P.L.B. Chemometric Analysis of the Seasonal Variation in the Essential Oil Composition of Psidium acutangulum Growing in the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104528. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids from Brazil. Nat. Prod. Commun. 2021, 16, 1934578X2199615. [Google Scholar] [CrossRef]
- Gentil, D.F.d.O.; Ferreira, S.A.d.N.; Rebouças, E.R. Germination of Psidium friedrichsthalianum (O. Berg) Nied. Seeds under Different Temperature and Storage Conditions. J. Seed Sci. 2018, 40, 246–252. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Díaz, C.; Vaillant, F.; Tamayo-Castillo, G. Oral Administration of Costa Rican Guava (Psidium friedrichsthalianum) Juice Induces Changes in Urinary Excretion of Energy-Related Compounds in Wistar Rats Determined by 1H NMR. NFS J. 2020, 20, 48–57. [Google Scholar] [CrossRef]
- Cuadrado-Silva, C.T.; Pozo-Bayón, M.Á.; Osorio, C. Targeted Metabolomic Analysis of Polyphenols with Antioxidant Activity in Sour Guava (Psidium friedrichsthalianum Nied.) Fruit. Molecules 2017, 22, 11. [Google Scholar] [CrossRef]
- Miranda-Cruz, E.; Espinosa-Moreno, J.; Centurión-Hidalgo, D.; Velázquez-Martínez, J.R.; Alor-Chávez, M.d.J. Antimicrobial Activity of Psidium friedrichsthalianum L., Pterocarpus hayesii L., Tynanthus guatemalensis L. and Spondias purpurea L. Extracts. Bol. Latinoam. Caribe Plantas Med. Aromat. 2012, 11, 354–361. [Google Scholar]
- Van Den Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Da Cruz, E.d.N.S.; Peixoto, L.d.S.; da Costa, J.S.; Mourão, R.H.V.; do Nascimento, W.M.O.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonal Variability of a Caryophyllane Chemotype Essential Oil of Eugenia patrisii Vahl Occurring in the Brazilian Amazon. Molecules 2022, 27, 2417. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.d.S.P.; Santos da Cruz, E.d.N.; de Araújo Guimarães, B.; Setzer, W.N.; Veras Mourão, R.H.; do Rosário da Silva, J.K.; Silva da Costa, J.; Baia Figueiredo, P.L. Chemometric Analysis of the Seasonal Variation in the Essential Oil Composition and Antioxidant Activity of a New Geraniol Chemotype of Lippia alba (Mill.) N.E.Br. Ex Britton & P. Wilson from the Brazilian Amazon. Biochem. Syst. Ecol. 2022, 105, 104503. [Google Scholar] [CrossRef]
- De Loureiro, R.S.; Saraiva, J.M.; Saraiva, I.; Senna, R.C.; Fredó, A.S. Estudo Dos Eventos Extremos de Precipitação Ocorridos Em 2009 No Estado Do Pará. Rev. Bras. Meteorol. 2014, 29, 83–94. [Google Scholar] [CrossRef]
- Da Costa, J.S.; da Cruz, E.d.N.; Setzer, W.N.; da Silva, J.K.d.R.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules 2020, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced In Situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef]
- De Macêdo, D.G.; Souza, M.M.A.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; dos Santos, A.T.L.; da Cruz, R.P.; da Costa, J.G.M.; Rodrigues, F.F.G.; Quintans-junior, L.J.; da Silva Almeida, J.R.G.; et al. Effect of Seasonality on Chemical Profile and Antifungal Activity of Essential Oil Isolated from Leaves Psidium salutare (Kunth) O. Berg. PeerJ 2018, 6, e5476. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Landrum, L.R. Volatile Leaf Oils of American Myrtaceae. III. Psidium cattleianum Sabine, P. friedrichsthalianum (Berg) Niedenzu, P. guajava L., P. guineense Sw., and P. sartorianum (Berg) Niedenzu. J. Essent. Oil Res. 1995, 7, 187–190. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Vázquez, C. Characterization of Volatiles in Costa Rican Guava [Psidium friedrichsthalianum (Berg) Niedenzu] Fruit. J. Agric. Food Chem. 2002, 50, 6023–6026. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; de Souza Santos, E.; de Oliveira Bernardes, C.; da Silva Ferreira, M.F.; Ferreira, A.; Tuler, A.C.; Carvalho, J.A.M.; Pinheiro, P.F.; Praça-Fontes, M.M. Phytochemical Analysis and Effect of the Essential Oil of Psidium L. Species on the Initial Development and Mitotic Activity of Plants. Environ. Sci. Pollut. Res. 2019, 26, 26216–26228. [Google Scholar] [CrossRef]
- Flores, G.; Dastmalchi, K.; Wu, S.B.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D’Armiento, J.M.; Kennelly, E.J. Phenolic-Rich Extract from the Costa Rican Guava (Psidium friedrichsthalianum) Pulp with Antioxidant and Anti-Inflammatory Activity. Potential for COPD Therapy. Food Chem. 2013, 141, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garbanzo, C.; Rodríguez, L.; Pérez, A.M.; Mayorga-Gross, A.L.; Vásquez-Chaves, V.; Fuentes, E.; Palomo, I. Anti-Platelet Activity and Chemical Characterization by UPLC-DAD-ESI-QTOF-MS of the Main Polyphenols in Extracts from Psidium Leaves and Fruits. Food Res. Int. 2021, 141, 110070. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).