Abstract
Trewia nudiflora Linn. is a valuable forest resource due to its economic, ethnomedicinal, and ecological properties; however, its allelopathic potential is undocumented. Therefore, this research was designed to investigate the allelopathic impacts of T. nudiflora leaf extracts on alfalfa (Medicago sativa L.) and barnyard grass (Echinochloa crus-galli (L.) P. Beauv.) growth, as well as to isolate and to identify the active allelopathic substances responsible for these effects. A bioassay experiment with eight different treatments (0.1, 0.3, 1, 3, 10, 30, 100, and 300 mg dry weight (DW) equivalents of T. nudiflora extracts in mL−1) was carried out. The results showed that the growth of both plants decreased with increasing contents of T. nudiflora extracts, with the effect depending on the plant species and reaching its peak at a concentration of a 300 mg DW equivalent of T. nudiflora extract in mL−1. Active substances were isolated and identified using an HPLC system, which revealed the presences of methyl gallate and pinoresinol in aqueous methanol extracts of T. nudiflora. The shoot and root lengths of the alfalfa and the barnyard grass decreased significantly when they were treated with the methyl gallate and the pinoresinol. The allelopathic inhibition increased with increasing compound concentrations, with the root growth being more sensitive to the pinoresinol than to the methyl gallate, and it was significantly higher at the concentration of 10 mM. These results indicated that the T. nudiflora leaf extracts limited the growth of the treated plants, and the methyl gallate and pinoresinol in the extracts may have caused the inhibition of the T. nudiflora extracts. Thus, the leaf extracts of T. nudiflora and the substances methyl gallate and pinoresinol could be incorporated into sustainable agricultural practices or used to develop bioherbicides that would promote sustainable weed management practices.
1. Introduction
Trewia nudiflora Linn., a member of the Euphorbiaceae family, is a fast-growing tree species widely used in traditional medicine. The tree is medium- to large-sized, growing up to 25 to 35 m in height with a straight trunk. The leaves are ovate and glossy green and can be up to 15 cm in length (Figure 1A,B). The tree produces clusters of small, white, fragrant flowers in the early spring months. The fruit is a small, yellowish-brown drupe that contains several seeds. The bark, leaves, and roots of this plant are utilized for treating flatulence; promoting wound healing; managing sputum; and relieving stomach-related issues, rheumatism, excessive bile, gout, and edema [1,2,3,4]. Various members of the Euphorbiaceae family are known to possess different bioactive compounds, some of which could be used for controlling weed growth. Previous studies have shown that certain species within this family produce allelopathic substances that can impede the growths of different plant species [5,6]. Moreover, chemical analysis of T. nudiflora has revealed that this plant contains various categories of active substances, including flavonoids, phenols, tannins, saponins, and other phytochemicals with potential biological effects [7]. Different phytochemicals of T. nudiflora have been documented for their antioxidant, anticancer, insecticidal, cerebroprotective, and antimicrobial properties [8,9,10,11]. Furthermore, studies have indicated that extracts from T. nudiflora leaves have shown cytotoxic properties [12]. While extensive research has been conducted on the bioactivity of T. nudiflora, its allelopathic effects have yet to be established.

Figure 1.
T. nudiflora leaf (A) and tree (B).
Allelopathy is a natural phenomenon that involves the interaction of plants and other organisms through the synthesis and release of bioactive compounds known as allelochemicals [13]. Allelochemicals are secondary metabolites traced in different plant parts such as leaves, roots, flowers, fruits, bark, and seeds and released into the surrounding soil, water (via leaching, root secretions, and litter decomposition), or air (via volatilization) [14,15]. When released, allelochemicals significantly affect the growth and development of nearby plants, reducing competition for resources and growing space [13,16,17,18]. Many studies have explored the inhibitory properties of allelochemicals from diverse plants against weed growth, suggesting their potential use for weed biomanagement [19,20,21]. Several medicinal plant species, including the Eucalyptus sp., Chrysanthemoides sp., and Euphorbia sp., have been investigated for their allelopathic substances, such as 1,8-cineole, isomenthol, and α-terpineol, which have been reported to suppress the growth of Solanum elaeagnifolium weed species [22]; p-coumaric acid, phloridzin, catechin, and ferulic acid, which have been reported to suppress the growth of Isotoma axillaris [23]; and β-sitosterol, taraxasterol, germanicol, α-amyrin, stigmasterol, and β-amyrin, which have been reported to suppress the growths of Lactuca sativa and Sorghum bicolor [6]. Over the past few years, researchers have examined the allelopathic properties of various medicinal plant species and the bioactive compounds they contain in order to identify natural substances with allelopathic effects that can inhibit the growth of weeds, potentially serving as alternatives to synthetic herbicides. The utilization of allelopathic plants, along with their extracts and compounds, holds great promise for improving agricultural yields and controlling weed growth. Previous researchers have demonstrated the effectiveness of incorporating this in different forms, including mulch, intercropping, cover crops, and soil drenching [21,24]. Hence, it is necessary to identify and assess candidate plant species with allelopathic potential, along with the specific compounds involved. T. nudiflora leaves have displayed a wide range of biological activities, but their allelopathic properties remain unverified. Therefore, the aim of this study was to determine the allelopathic potential of T. nudiflora leaf extracts and isolate and identify the active substances responsible for the allelopathic effect.
2. Materials and Methods
2.1. Trewia nudiflora Samples and Test Plant Species
Fresh T. nudiflora leaves were harvested from Sirajganj, Bangladesh, during May–June 2020 (latitude: 24°38′30.12″ N and longitude: 89°39′0.00″ E). After washing, the leaves were subsequently dried in shade before being ground into a fine powder (GM 200 Laboratory Grinder; Retsch, D-42781 Haan, Germany) and stored at 2 °C for further use. The allelopathic effects of T. nudiflora were investigated in a growth experiment using a dicot, alfalfa (Medicago sativa L.), and a monocot, barnyard grass (Echinochloa crus-galli (L.) P. Beauv.). Alfalfa was selected based on its established seedling growth, while barnyard grass was selected due to its widespread presence as a prevalent weed in crop fields worldwide [25].
2.2. Extraction of Trewia nudiflora Leaves for the Bioassay Experiment
The extraction of T. nudiflora started with soaking 100 g of its leaf powder in 1 L of 70% aqueous methanol for 48 h. The resulting mixture was filtered through a No. 2 filter paper (Toyo Roshi Kaisha Ltd., Tokyo, Japan), and the remaining residue was subjected to another 24 h of extraction using 1 L of methanol and filtered again. The two filtered solutions were combined and subjected to evaporation using a rotary evaporator at 40 °C (Model RE 200; Yamato Scientific Co., Ltd., Tokyo, Japan) to obtain the crude extract. The crude extracts were diluted with methanol to prepare eight different treatment concentrations (0.1, 0.3, 1, 3, 10, 30, 100, and 300 mg DW equivalents of T. nudiflora extracts in mL−1). The treatment concentrations were added to the papers (No. 2) in Petri plates (28 mm) and allowed to dry. Each Petri dish was then prepared by placing ten alfalfa seeds and ten barnyard grass seedlings (sprouted at 25 °C for 48 h) inside and moistening them with 0.6 mL of Tween 20, a polyoxyethylene sorbitan monolaurate solution (0.05% (v/v)). The control group was moistened with the Tween 20 solution instead of the T. nudiflora extract solution. The Petri plates were then moved to a growth chamber and kept in the dark at 25 °C for 48 h. The growth inhibition percentages were calculated by analyzing the differences in the plant lengths between the treated and the control group.
2.3. Method for Isolating and Purifying Active Allelopathic Substances
T. nudiflora leaf powder (2.9 kg) was used to prepare an aqueous residue through the extraction process, as has been described in the preceding Section 2.2. To isolate active substances, we used a cress bioassay that would separate the active fraction at each step. The resulting residue was then subjected to partitioning with ethyl acetate (EtOAc) after its pH was balanced to 7.0. The partitioning involved six repetitions using equal amounts of EtOAc each time. The residue of the EtOAc fraction was divided by a column containing 60 g of silica gel, with a mesh size of 70–230 (Nacalai Tesque, Kyoto, Japan). The column was eluted gradually with elevating concentrations of EtOAc in n-hexane, with each step consisting of a 10% increase in EtOAc volume and a total volume of 150 mL per step and methanol (300 mL). Fraction F6 (eluted with 70% EtOAc) showed a greater inhibitory effect and was separated with a Sephadex LH-20 column (100 g; GE Healthcare, Uppsala, Sweden). Purification involved elution with 20 (F1), 40 (F2), 60 (F3), and 80% (F4) aqueous methanol (v/v; 150 mL for each step) and methanol (F5) (300 mL). Fraction F3 (eluted with 60% aqueous methanol) showed inhibitory activity and was fractionated using a reverse-phase C18 cartridge (1.2 × 6.5 cm; YMC Co., Ltd., Kyoto, Japan) eluted with 15 mL of 20–90% (v/v) aqueous methanol in each step and finally with 30 mL of methanol. Fractions F1 and F3, which were obtained from elution with 20 and 40% aqueous methanol, respectively, showed inhibitory activity and were selected for purification using reverse-phase HPLC (500 × 10 mm I.D., S-5µm, 12 nm; YMC Co., Ltd.). The eluents consisted of 15 and 45% (v/v) of aqueous methanol, respectively, and the flow rate was 1.5 mL min−1. Inhibitory activity was observed at retention times of 61–72 and 68–92 min. The peak was further subjected to purification with a 3 µm column (4.6 I.D. × 250 mm; Inertsil ODS-3, HP 3 µm; GL Sciences Inc., Tokyo, Japan). The eluents were 10 and 40% (v/v), respectively, aqueous methanol, with a 0.5 mL min−1 flow rate, and eluted at retention times of 64–90 and 50–55 min, respectively, at a 220 nm wavelength.
2.4. Bioactivity of the Identified Substances
To conduct a bioassay, five concentrations, 0.1, 0.3, 1, 3, and 10 mM, of two substances were formulated by diluting them with methanol. The solutions were added to No. 2 filter paper and allowed to dry in a draft chamber. Petri plates were prepared with ten alfalfa seeds, barnyard grass seedlings, and a control. The seeds were hydrated with 0.6 mL Tween 20 (0.05% v/v) and transferred to the growth chamber. After 48 h of treatment, the alfalfa and barnyard grass growths were compared with that of the control to compute the growth inhibition percentage for each concentration of the two substances.
2.5. Analysis
The data gathered from the CRD study were analyzed using a one-way ANOVA and Tukey’s test to identify any statistically significant distinctions between treatments at p = 0.05. The statistical software IBM SPSS 16.0 [26] was used to conduct data analysis, while GraphPad Prism 6.0 was used to determine the I50 values (required concentrations to achieve 50% growth reduction). The T. nudiflora extract bioassay was performed twice, with three replications (n = 60) for each treatment, while the bioassays carried out with the allelopathic substances had three replications (n = 30) for each treatment.
3. Results
3.1. Effects of Extracts from Trewia nudiflora Leaves on Test Plants
The extracts of T. nudiflora strongly suppressed the growths of the alfalfa and the barnyard grass, with corresponding increases in inhibition as the doses of the extract increased. (Figure 2, Figure 3 and Figure 4). For alfalfa, the inhibition was observed at a concentration of 0.3 mg DW, with more than 65% growth reduction at a concentration of a 1 mg DW equivalent of T. nudiflora extracts in mL–1. Shoot growth reductions of 66.85, 79.44, and 89.07% and root growth reductions of 67.03, 81.11, and 87.3% were observed at concentrations of 3, 10, and 30 mg DW equivalents of T. nudiflora extracts in mL−1, respectively. At extract concentrations of 100 and 300 mg DW, the alfalfa growth was completely inhibited. For barnyard grass, growth inhibition was observed at a concentration of 0.1 mg DW, with an 18% inhibition of shoot growth at a 0.3 mg DW equivalent of T. nudiflora extracts in mL−1. However, shoot growth reduction was 4.95% with the extract concentration of 1 mg DW, and the reduction gradually increased with increasing extract concentration. Shoot growth reductions of 19.42, 38.44, and 84.97% and root growth reductions of 77.80, 89.05, and 99.87% were observed at concentrations of 10, 30, and 100 mg DW equivalents of T. nudiflora extracts in mL−1, respectively. At an extract concentration of 300 mg DW, the barnyard grass growth was completely inhibited by the T. nudiflora extracts. Table 1 shows that for a 50% decrease in the alfalfa and barnyard grass growth, concentrations of 0.53–40.48 mg DW of T. nudiflora extracts were required. The I50 values indicated that the alfalfa was more susceptible to the T. nudiflora extracts than the barnyard grass, with concentrations of 0.53 and 0.60 mg DW causing a 50% decrease in alfalfa development while concentrations of 1.24 and 40.48 mg DW were required for the same effect on the barnyard grass.

Figure 2.
Effects of eight concentrations of T. nudiflora extracts on the growths of alfalfa and barnyard grass (control, 0.1, 0.3, 1, 3, 10, 30, 100, and 300 mg DW equivalents of T. nudiflora extracts in mL−1).
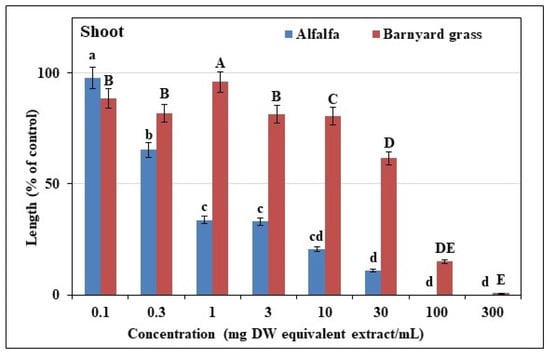
Figure 3.
Different doses of T. nudiflora leaf extracts inhibited the treated plants’ shoot growth. Means ± SEs from three replicates of two separate tests are shown, and the vertical bars depict the mean standard errors. Different letters represent the differences between the T. nudiflora-treated and control groups (Tukey’s HSD at p ≤ 0.05).
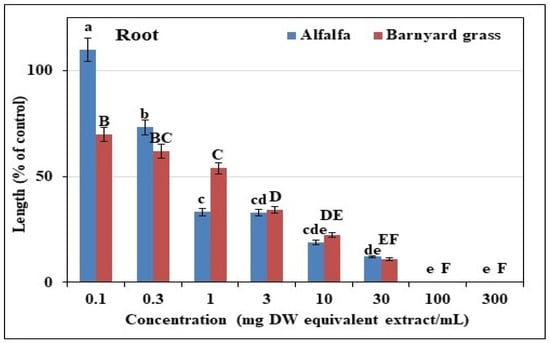
Figure 4.
Different doses of T. nudiflora leaf extract inhibited the treated plants’ root growth. Means ± SEs from three replicates of two separate tests; vertical bars depict the mean standard errors. Different letters represent the differences between the T. nudiflora-treated and control groups (Tukey’s HSD at p ≤ 0.05).

Table 1.
The concentrations of T. nudiflora extracts needed to suppress the growth of alfalfa and barnyard grass by 50% (I50 values).
3.2. Characterization of the Isolated Compounds
To characterize the isolated substances, HRESIMS analysis was conducted using a Thermo Scientific Orbitrap Exploris 240 mass spectrometer. NMR spectroscopic data were obtained at a 500 MHz optical rotation, and the substances were colorless powders. Substance 1 was analyzed, and its molecular formula was determined to be C8H8O5 with HRESIMS m/z 183.0298 [M−H]− (calculated for C8H7O5 183.0299); (500 MHz, CD3OD). The 1H NMR spectrum of this substance as measured in CD3OD showed two aromatic proton signals at δH 7.04 (2H, s) and one methyl proton signal at δH 3.81 (3H, s). Substance 1 was identified as methyl gallate, which was in agreement with published data [27] (Figure 5a).
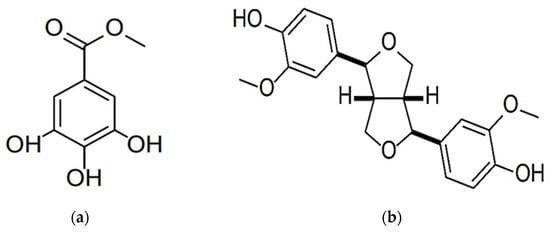
Figure 5.
The molecular structures of methyl gallate (a) and pinoresinol (b) identified and characterized from T. nudiflora leaf extracts.
Substance 2 was analyzed, and its molecular formula was determined to be C20H22O6 with HRESIMS m/z 357.1336 [M−H]− (calculated for C20H21O6 357.1344); [α]D23 = +18.2 (c 0.13, CHCl3). The 1H NMR spectrum of this substance as measured in CDCl3 showed six aromatic proton signals at δH 6.90 (2H, d, J = 1.7), 6.89 (2H, d, J = 8.1), and 6.82 (2H, dd, J = 8.1, 1.7); two methyl proton signals at δH 3.91 (6H, s); four methine proton signals at δH 4.74 (2H, d, J = 4.3) and 4.24 (2H, m); four methylene proton signals at δH 3.88 (2H, dd, J = 9.3, 3.6) and 3.10 (2H, dd, J = 6.8, 3.6); and two hydroxy proton signals at δH 5.60 (2H, s). The 1H NMR spectrum of substance 2 was identified as pinoresinol, which was in agreement with published data [28] (Figure 5b).
3.3. Bioactivities of the Two Substances (Methyl Gallate and Pinoresinol)
The bioactivities of the methyl gallate and the pinoresinol were studied against alfalfa and barnyard grass seedling growth. The results, depicted in Figure 6 and Figure 7, revealed significant growth inhibition in the two test plants treated with both substances, and this effect increased with increasing concentrations. Statistical analysis indicated that the observed effects were significant at doses of 0.1 mM for each compound (p ≤ 0.05). The application of 0.3 mM of methyl gallate resulted in significant growth inhibition of alfalfa and barnyard grass, with shoot and root growth inhibitions of 65 and 50.8%, respectively, for alfalfa and 37.9 and 46.3%, respectively, for barnyard grass. Pinoresinol, at the same concentration, resulted in greater growth inhibition, with shoot and root growth inhibitions of 68.9 and 80.9%, respectively, for alfalfa and 58.9 and 86.1%, respectively, for barnyard grass. At 1.0 mM, the methyl gallate inhibited the alfalfa shoot and root growth by more than 85% and the barnyard grass shoot and root growth by 44.1 and 54.2%, respectively, while the pinoresinol reduced the shoot and root growth for alfalfa by 83.8 and 93.6%, respectively, and for barnyard grass by 72 and 97.7%, respectively. The concentration of 3.0 mM of both substances resulted in further growth inhibition, with 93 and 94.2% inhibition, respectively, of the alfalfa shoot and root growth by the methyl gallate and 89.1 and 94.1% inhibition, respectively, by the pinoresinol as well as 56.3 and 79.8% inhibition, respectively, of the barnyard grass shoot and root growth by the methyl gallate and 89.6 and 98.7% inhibition, respectively, by the pinoresinol. At concentrations of 10 mM, the methyl gallate and pinoresinol completely inhibited the alfalfa shoot and root growth and the barnyard grass root growth. With the same doses, the barnyard grass shoot growth was inhibited by 78.9 and 99.4% of the control by the methyl gallate and pinoresinol, respectively. The I50 values were 0.065–0.293 mM for the alfalfa and 0.080–2.051 mM for the barnyard grass (Table 2). The I50 values of the alfalfa and barnyard grass roots were 2.2, 3.6, and 2.7 times lower than those of the shoots, respectively, indicating that the roots were more sensitive to methyl gallate and pinoresinol, except for the alfalfa roots to methyl gallate. Based on the I50 values, the pinoresinol exhibited stronger inhibition than the methyl gallate for both plant species.
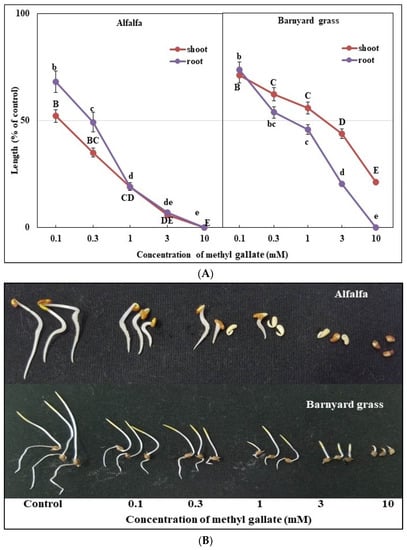
Figure 6.
Different doses (0.1, 0.3, 1, 3, and 10 mM) of methyl gallate inhibited treated plants’ growth (A). Alfalfa and barnyard grass seedlings treated with five concentrations of methyl gallate (B). Means ± SEs of three replications. Vertical bars depict the mean standard errors. Different letters represent the differences between the control and the methyl gallate treatments (Tukey’s HSD at p ≤ 0.05).
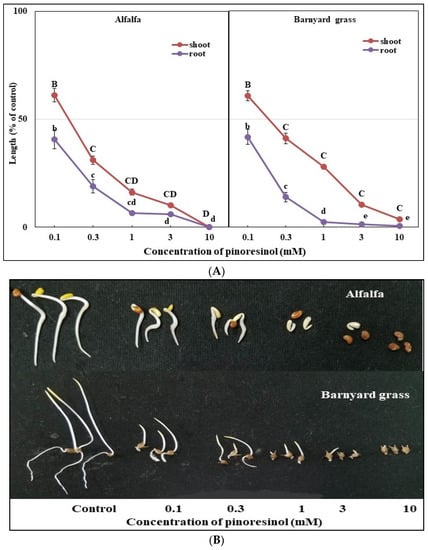
Figure 7.
Different doses (0.1, 0.3, 1, 3, and 10 mM) of pinoresinol inhibited the treated plants’ growth (A). Alfalfa and barnyard grass seedlings treated with five concentrations of pinoresinol (B). Means ± SEs of three replications. Vertical bars depict the mean standard errors. Different letters represent the differences between the control and the pinoresinol treatments (Tukey’s HSD at p ≤ 0.05).

Table 2.
I50 values of methyl gallate and pinoresinol, identified from aqueous methanol extracts of T. nudiflora for alfalfa and barnyard grass growth inhibition.
4. Discussion
The development of both the alfalfa and barnyard grass seedlings was limited by the T. nudiflora extracts, and this inhibitory effect became more evident with increasing contents of the extracts (Figure 2 and Figure 3). The concentration required for a 50% reduction in shoot growth for alfalfa was lower than that for root growth, while for barnyard grass, the concentration required for root growth was lower than that for shoot growth (Table 1). Similar inhibitory effects were recorded in the growths of alfalfa and barnyard grass when they were exposed to the leaf extracts of Jatropha curcas, a plant species of the family Euphorbiaceae, in a concentration-dependent manner [29]. Various plant species have been reported to have concentration-dependent inhibitory effects on weed growth, caused by allelopathic substances in their extracts [30,31,32]. In our prior investigation, two phytotoxic substances were identified from the leaf extracts of T. nudiflora [33]. Thus, allelochemicals may be the cause of the growth-limiting actions of T. nudiflora extracts. We fractionated the extracts using chromatography and identified the two phenolic substances, methyl gallate and pinoresinol, through spectral analysis. Phenolics are a diverse group of plant secondary metabolites that have a variety of biological functions and have been shown to be toxic to plant growth [34,35,36]. They are ubiquitous in plants and are synthesized through the shikimate pathway [34,37]. Different plant phenolics, such as ferulic acid, syringic acid, coumaric acid, gallic acid, and vanillic acid, have been reported to inhibit the growths of weed species [38,39].
Methyl gallate, also known as methyl 3,4,5 trihydroxybenzoate, is a type of gallo-tannin that belongs to the phenolic group and has three hydroxyl groups on an aromatic hydrocarbon. It is commonly found in various plant species, including Geranium niveum [40], Paeonia suffruticosa [41], Acer saccharlnum [42], and Acacia farnesiana [43]. Methyl gallate has exhibited antioxidant, anti-inflammatory, and anticancer properties and is used as a preservative in the food industry due to its antimicrobial activity, as reported in the literature [44]. Studies have shown that methyl gallate from the species Mangifera indica and Caesalpinia mimosoides has inhibited a range of plant growth. Its bioactivity is linked to three hydroxyl groups on its phenolic ring and its short alkyl chain, which may contribute to its allelopathic properties [45,46].
On the other hand, pinoresinol is a type of lignan that belongs to the group of polyphenolic compounds. It is made up of two phenylpropanoid units that are linked by a β-β’ bond, and each unit consists of two aromatic rings with a hydroxyl group at the para position and a methoxy group at the meta position [47]. This compound is naturally found in various plants, such as Forsythia koreana [48], the Brassica sp., Sesamum indicum [49], and the Olea sp. [50], as well as in animals such as Pieris rapae larvae [51]. Pinoresinol has been reported to have anti-inflammatory [52], anticancer [53], antioxidant [54], and antifungal [55] activities. Scavo et al. [56] and Kato-Noguchi et al. [57] have demonstrated that pinoresinol extracted from Cynara spp. and Osmanthus spp. has inhibited the growth of cress, wheat, and Italian ryegrass. 3,4-Dihydroxyphenylethanol, a polyphenol identified from the leaf extracts of A. reticulata, was reported to impair the growths of two weed species [58]. However, the inhibitory effect of pinoresinol is not well-understood. Oliva et al. [59] reported that plant lignans suppress cell division and cause abnormal chromosome configurations in the meristematic cells of onion root tips. Moreover, pinoresinol exhibits antibacterial and antifungal activity by disrupting bacterial cell membranes and interacting with ergosterol in fungal cell membranes, leading to destabilization and cell lysis [55,60].
Table 2 shows that the efficacies of methyl gallate and pinoresinol vary among plant species and compounds, with root growth being more susceptible to the compounds than shoot growth. The degree of sensitivity depends on the concentration of each compound. Phenolic acids from Buchloe dactyloides showed similar dose-dependent inhibition of root growth to that of the treated plants [61]. The root growth exhibited greater sensitivity to the substances compared to the shoot growth, which is potentially attributable to the enhanced permeability of radicles, which results in elevated oxidative stress and hindered plant growth [62,63]. Benzoic acid has been reported to alter the morphology and internal arrangement of cells in mustard roots, while ferulic acid interferes with enzymatic activity and promotes cell wall stiffening and lignin production, limiting cell expansion and elongation [64,65]. Phenolic acid has exhibited varying growth-inhibiting effects among different plant species [66,67], possibly due to variations in the inherent physiologies and metabolisms of the target plants, which responded differently to distinct structural groups of the compounds [68,69,70]. The I50 values indicated greater growth inhibition by pinoresinol than methyl gallate. This greater growth inhibition can be attributed to the methoxy group on the aromatic ring of pinoresinol, which, in addition to the hydroxyl group, likely contributes to more potent growth inhibition of alfalfa and barnyard grass compared to methyl gallate [71,72]. However, this study is the first to isolate methyl gallate and pinoresinol from T. nudiflora leaf extracts and demonstrate their allelopathic properties. It has been demonstrated that using Secale cereale, Oryza sativa, and Carum carvi as mulch restricts the spread of diverse invasive weeds by releasing phytotoxic compounds. Furthermore, employing these plants as mulch has been found to enhance yields [24,73,74]. Weed Zap and Weed Warriors are some commercially available herbicides derived from diverse botanical origins [75]. Accordingly, T. nudiflora and its individual substances, methyl gallate and pinoresinol, could be used to develop eco-friendly herbicides that promote sustainable agricultural practices.
5. Conclusions
The results of this experiment demonstrated that the extracts from T. nudiflora leaves significantly inhibited the growths of alfalfa and barnyard grass, indicating their strong allelopathic effects. With the increase in the T. nudiflora extracts, the growth of the seedlings decreased. Active allelopathic substances, namely methyl gallate and pinoresinol, were detected in the extracts, and these substances may be potential allelochemicals responsible for inhibiting the growth of both treated plants. Notably, this experiment represents the initial assessment of the allelopathic impacts of T. nudiflora as well as the identification of its active compounds, namely methyl gallate and pinoresinol, from its leaf. However, the allelopathic effects were solely assessed in controlled lab settings. Therefore, additional research is necessary to delve into the mechanisms and actions of the T. nudiflora extracts and the compounds involved. The results of such research could provide a basis for developing potential bioherbicides.
Author Contributions
Conceptualization, M.R.K. and H.K.-N.; methodology, M.R.K., S.T., T.T. and H.K.-N.; software, M.R.K.; validation, S.T., T.T. and H.K.-N.; formal analysis, M.R.K.; investigation, M.R.K.; data curation, H.K.-N.; writing (original draft preparation), M.R.K.; writing (review and editing), H.K.-N.; visualization, M.R.K.; supervision, H.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
The Japanese government provided funding for this research through a Ministry of Education, Culture, Sports, Science and Technology (MEXT) scholarship (Grant Number MEXT-203626).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Dennis Murphy from The United Graduate School of Agricultural Sciences (UGAS), Ehime University, Japan, for correcting the English of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ram, R.; Mehrotra, B.N.; Sinha, S.; Pant, P.; Sheth, R. Compendium of Indian Medicinal Plants; CDRI Lucknow and National Institute of Science Communication: New Delhi, India, 2004. [Google Scholar]
- Kulju, K.K.M.; Sierra, S.E.C.; Van Welzen, P.C. Re-shaping Mallotus [part 2]: Inclusion of Neotrewia, Octospermum and Trewia in Mallotus ss (Euphorbiaceae ss). Blumea-Biodiversity. Evol. Biogeogr. 2007, 52, 115–136. [Google Scholar] [CrossRef][Green Version]
- Trewia nudiflora. Flora of China. Available online: https://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200013883 (accessed on 2 June 2021).
- Sultana, R.; Milon, M.M.M.; Kader, M.A.; Parvin, S.; Masud, G.M. Trewia nudiflora: A potential source of new drugs. J. Phytopharm. 2022, 11, 421–424. [Google Scholar] [CrossRef]
- Nasrine, S.; El-taher, S.E.D.H. Allelopathic effect of Euphorbia guyoniana aqueous extract and their potential uses as natural herbicides. Sains Malays. 2013, 42, 1501–1504. [Google Scholar]
- Da Silva, U.P.; Furlani, G.M.; Demuner, A.J.; da Silva, O.L.M.; Varejão, E.V.V. Allelopathic activity and chemical constituents of extracts from roots of Euphorbia heterophylla L. Nat. Prod. Res. 2018, 33, 2681–26844. [Google Scholar] [CrossRef]
- Ripa, F.A.; Hossain, M.J.; Munira, M.S.; Roy, A.; Riya, F.H.; Alam, F.; Khidir, E.B. Phytochemical and pharmacological profiling of Trewia nudiflora Linn. leaf extract deciphers therapeutic potentials against thrombosis, arthritis, helminths, and insects. Open Chem. 2022, 20, 1304–1312. [Google Scholar] [CrossRef]
- Powell, R.G.; Weisleder, D.; Smith, C.R., Jr. Novel maytansinoid tumor inhibitors from Trewia nudiflora: Trewiasine, de-hydrotrewiasine, and demethyltrewiasine. J. Org. Chem. 1981, 46, 4398–4403. [Google Scholar] [CrossRef]
- Kumar, K.P.; Sastry, V.G. Protective of Trewia nudiflora against ischemic stroke in experimental rats. Inter. J. Pharmacother. 2012, 2, 7–12. [Google Scholar]
- Balakrishnan, N.; Srivastava, M.; Tiwari, P. Preliminary phytochemical analysis and DPPH free radical scavenging activity of Trewia nudiflora Linn. roots and leaves. Pak. J. Biol. Sci. 2013, 16, 1403–1406. [Google Scholar] [CrossRef][Green Version]
- Esan, V.; Elanchezhiyan, C.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Marimuthu, G. Toxicity of Trewia nudiflora-mediated silver nanoparticles on mosquito larvae and non-target aquatic fauna. Toxin Rev. 2022, 41, 229–236. [Google Scholar] [CrossRef]
- Begum, Y. Antibacterial, antioxidant and cytotoxic activities of Trewia nudiflora. Pharma Tutor. 2016, 4, 37–41. Available online: http://www.pharmatutorjournal.com/index.php/pt/article/view/332 (accessed on 1 January 2016).
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Kruse, M.; Strandberg, M.; Strandberg, B. Ecological Effects of Allelopathic Plants—A Review; NERI Technical Report; National Environmental Research Institute: Roskilde, Denmark, 2000; p. 315. [Google Scholar]
- Bertin, C.; Yang, X.H.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; Van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Yuan, L.; Li, J.; Van Kleunen, M. Competition induces negative conspecific allelopathic effects on seedling recruitment. Ann. Bot. 2022, 130, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, P.B.S. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides—Current Research and Case Studies in Use; IntechOpen: London, UK, 2013; Volume Chapter 20, pp. 518–542. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Liu, D.L.; Stanton, R. Phytotoxic activity and chemical composition of aqueous volatile fractions from Eucalyptus species. PLoS ONE 2014, 9, e93189. [Google Scholar] [CrossRef]
- Al Harun, M.A.Y.; Johnson, J.; Uddin, M.N.; Robinson, R.W. Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed). PLoS ONE 2015, 10, e0139992. [Google Scholar] [CrossRef]
- Devasinghe, D.A.U.D.; Premarathne, K.P.; Sangakkara, U.R. Weed management by rice straw mulching in direct seeded lowland rice (Oryza sativa L.). Trop. Agric. Res. 2011, 22, 263–272. [Google Scholar] [CrossRef]
- Khatun, R.; Kato-Noguchi, H. Piper longum L. leaf extracts, a candidate allelopathic plant that suppressed the growth of six test plants, could be a source of potent phytotoxic compounds. Res. Crop. 2022, 23, 874–880. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 16.0; IBM Corp: Armonk, NY, USA, 2007. [Google Scholar]
- Kiss, A.K.; Derwińska, M.; Dawidowska, A.; Naruszewicz, M. Novel biological properties of Oenothera paradoxa defatted seed extracts: Effects on metallopeptidase activity. J. Agric. Food Chem. 2008, 56, 7845–7852. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, S.; Kishimoto, T.; Uraki, Y.; Okamoto, T.; Ubukata, M. Low molecular weight lignin suppresses activation of NF-κB and HIV-1 promoter. Bioorg. Med. Chem. 2008, 16, 2645–2650. [Google Scholar] [CrossRef]
- Islam, A.M.; Kato-Noguchi, H. Allelopathic prospective of Ricinus communis and Jatropha curcas for bio-control of weeds. Acta Agric. Scand. B Soil Plant Sci. 2013, 63, 731–739. [Google Scholar] [CrossRef]
- Das, K.R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic potential and identification of phytotoxic substances in Cassia alata Linn. leaves. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 479–488. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Karami, A.; Haghighi, T.M.; Alizadeh, S.; Maggi, F. Phytotoxic potential and phenolic profile of extracts from Scrophularia striata. Plants 2021, 10, 135. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Senna garrettiana leaves and identification of potent phytotoxic substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Khatun, M.R.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. The allelopathic effects of Trewia nudiflora leaf extracts and its identified substances. Plants 2023, 12, 1375. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Jacob, J.; Sarada, S. Role of phenolics in allelopathic interactions. Allelopath. J. 2012, 29, 215–230. [Google Scholar]
- Fatholahi, S.; Karimmojeni, H.; Ehsanzadeh, P. Phenolic compounds and allelopathic activities of ancient emmer wheats: Perspective for non-chemical weed control scenarios. Acta Physiol. Plant. 2020, 42, 135. [Google Scholar] [CrossRef]
- Favaretto, A.; Schefer-Basso, S.M.; Perez, N.B. Allelopathy in Poaceae species present in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 22. [Google Scholar] [CrossRef]
- Rob, M.M.; Hossen, K.; Khatun, M.R.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and application of bioactive compounds from Garcinia xanthochymus Hook. for weed management. Appl. Sci. 2021, 11, 2264. [Google Scholar] [CrossRef]
- Šćepanović, M.; Košćak, L.; Šoštarčić, V.; Pismarović, L.; Milanović-Litre, A.; Kljak, K. Selected phenolic acids inhibit the initial growth of Ambrosia artemisiifolia L. Biology 2022, 11, 482. [Google Scholar] [CrossRef]
- Calzada, F.; Cerda-García-Rojas, C.M.; Meckes, M.; Cedillo-Rivera, R.; Bye, R.; Mata, R. Geranins A and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum. J. Nat. Prod. 1999, 62, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Jung, H.J.; Park, C.H.; Yokozawa, T.; Jeong, J.C. Root bark of Paeonia suffruticosa extract and its component methyl gallate possess peroxynitrite scavenging activity and anti-inflammatory properties through NF-κB inhibition in LPS-treated mice. Molecules 2019, 24, 3483. [Google Scholar] [CrossRef]
- Abou-Zaid, M.M.; Lombardo, D.A.; Nozzolillo, C. Methyl gallate is a natural constituent of maple (genus Acer) leaves. Nat. Prod. Res. 2009, 23, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Heredia, N.; del Camacho-Corona, M.R.; García, S. Isolation, characterization and mode of antimicrobial action against Vibrio cholera of methyl gallate isolated from Acacia farnesiana. J. Appl. Microbiol. 2013, 115, 1307–1316. [Google Scholar] [CrossRef]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef]
- Suzuki, M.; Khan, M.S.I.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and an allelopathic substance in mango leaves. Acta. Agric. Scand. B Soil Plant Sci. 2017, 67, 37–42. [Google Scholar] [CrossRef]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic activity of leaf and stem extracts and identification of a phytotoxic substance from Caesalpinia mimosoides Lamk. Theor. Exp. Plant Physiol. 2018, 30, 129–139. [Google Scholar] [CrossRef]
- Yue, F.; Lan, W.; Zhang, L.; Lu, F.; Sun, R.; Ralph, J. Efficient synthesis of pinoresinol, an important lignin dimeric model compound. Front. Energy Res. 2021, 9, 640337. [Google Scholar] [CrossRef]
- Jung, H.W.; Mahesh, R.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Park, Y.K. Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci. Lett. 2010, 480, 215–220. [Google Scholar] [CrossRef]
- Milder, I.E.; Arts, I.C.; Van de Putte, B.; Venema, D.P.; Hollman, P.C. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.C.; Del Campo, M.L.; Grant, J.B.; Weibel, D.B.; Smedley, S.R.; Bolton, K.L.; Meinwald, J.; Eisner, T. Pinoresinol: A lignol of plant origin serving for defense in a caterpillar. Proc. Natl. Acad. Sci. USA 2006, 103, 15497–15501. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Debouche, C.; Raas, T.; Larondelle, Y. Among plant lignans, pinoresinol has the strongest anti-inflammatory properties in human intestinal Caco-2 cells. J. Nutr. 2012, 142, 1798–1805. [Google Scholar] [CrossRef]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involves a selective activation of the ATM–p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef]
- Tebboub, O.; Cotugno, R.; Oke-Altuntas, F.; Bouheroum, M.; Demirtas, Í.; D’Ambola, M.; Vassallo, A. Antioxidant potential of herbal preparations and components from Galactites elegans (All.) Nyman ex Soldano. Evid. Based Complement. Altern. Med. 2018, 2018, 9294358. [Google Scholar] [CrossRef]
- Hwang, B.; Lee, J.; Liu, Q.H.; Woo, E.R.; Lee, D.G. Antifungal effect of (+)-pinoresinol isolated from Sambucus williamsii. Molecules 2010, 15, 3507–3516. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Molinillo, J.M.; Varela, R.M.; Mauromicale, G.; Macias, F.A. The extraction procedure improves the allelopathic activity of cardoon (Cynara cardunculus var. altilis) leaf allelochemicals. Ind. Crops Prod. 2019, 128, 479–487. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hamada, Y.; Kojima, M.; Kumagai, S.; Iwasaki, A.; Suenaga, K. Allelopathic substances of Osmanthus spp. for developing sustainable agriculture. Plants 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.R.; Tojo, S.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity of Annona reticulata L. leaf extracts and identification of three allelopathic compounds for the development of natural herbicides. Agronomy 2022, 12, 2883. [Google Scholar] [CrossRef]
- Oliva, A.; Moraes, R.M.; Watson, S.B.; Duke, S.O.; Dayan, F.E. Aryltetralin lignans inhibit plant growth by affecting the formation of mitotic microtubular organizing centers. Pestic. Biochem. Physiol. 2002, 72, 45–54. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, J.; Li, Z. Antibacterial activity and mechanism of pinoresinol from Cinnamomum camphora leaves against food-related bacteria. Food Control 2017, 79, 192–199. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Harivandi, M.A. Allelopathic effects of phenolic acids detected in buffalograss (Buchloe dactyloides) clippings on growth of annual bluegrass (Poa annua) and buffalograss seedlings. Environ. Exp. Bot. 1998, 39, 159–167. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Z.; Lei, X.U.; Jin-jing, S.; Zhang, Z.H.; Fu, M.L.; Teng, H.Y.; Yi, K.K. Allelochemical p-hydroxybenzoic acid inhibits root growth via regulating ROS accumulation in cucumber (Cucumis sativus L.). J. Integr. Agric. 2020, 19, 518–527. [Google Scholar] [CrossRef]
- Kaur, H.; Kaushik, S. Cellular evidence of allelopathic interference of benzoic acid to mustard (Brassica juncea L.) seedling growth. Plant Physiol. Biochem. 2005, 43, 77–81. [Google Scholar] [CrossRef]
- Dos Santos, W.D.; Ferrarese, M.D.L.L.; Finger, A.; Teixeira, A.C.; Ferrarese-Filho, O. Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J. Chem. Ecol. 2004, 30, 1203–1212. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; González, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Piyatida, P.; Suenaga, K.; Kato-Noguchi, H. Allelopathic potential and chemical composition of Rhinacanthus nasutus extracts. Allelopath. J. 2010, 26, 207–216. [Google Scholar]
- Suzuki, T.; Usui, I.; Tomita-Yokotani, K.; Kono, S.; Tsubura, H.; Miki, Y.; Hasegawa, K. Effects of acid extracts of tomato (Lycopersicon esculentum Mill.) and carrot (Daucus carota L.) wastes from the food industry on the growth of some crops and weeds. Weed Biol. Manag. 2001, 1, 226–230. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Maffei, M.; Bertea, C.M.; Garneri, F.; Scannerini, S. Effect of benzoic acid hydroxy-and methoxy-ring substituents during cucumber (Cucumis sativus L.) germination. I. Isocitrate lyase and catalase activity. Plant Sci. 1999, 141, 139–147. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Synowiec, A.; Mozdzen, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. essential oil: A promising candidate for botanical herbicide against Echinochloa crus-galli (L.) P. Beauv. in maize cultivation. Ind. Crops Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Shiffler, A. Natural Weed Killers: A Guide to Organic Herbicides. Available online: https://www.lawnstarter.com/blog/lawn-care-2/natural-weed-killers-a-guide-to-organic-herbicides/ (accessed on 30 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).