Before or after Planting? Mycorrhizal and Bacterial Biostimulants and Extracts in Intense Strawberry (Fragaria × ananassa Duch.) Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Extraction and Determination of Sugars and Organic Acids
2.3. Ascorbic Acid Extraction and Determination
2.4. Extraction of Phenolic Compounds
2.5. Determination of Individual Phenolic Compounds
2.6. Chemicals and Products
2.7. Statistical Analyses
3. Results and Discussion
3.1. Strawberry Vegetative Performance
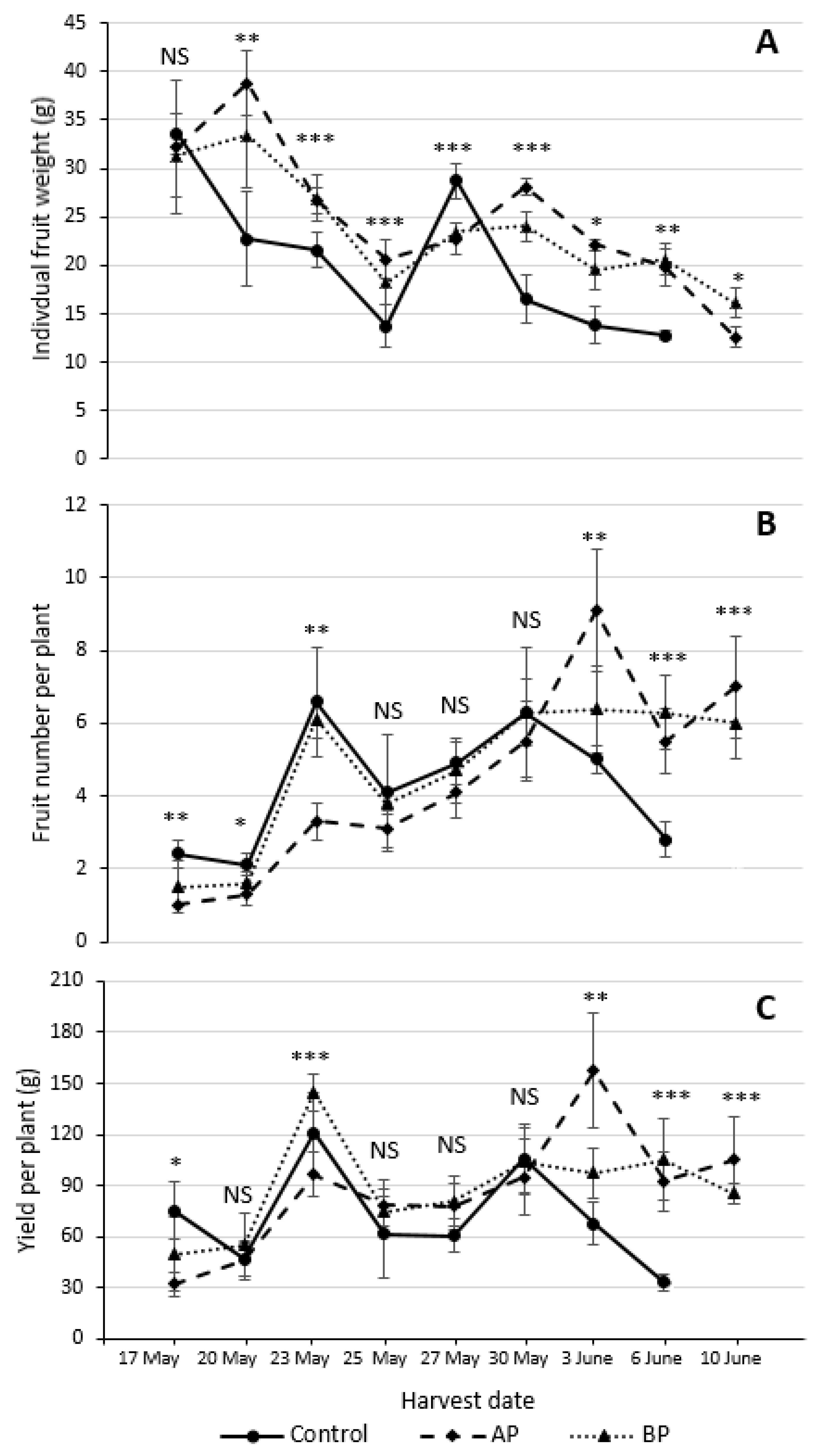
3.2. Strawberry Reproductive Performance and Fruit Measurements
3.3. The Content of Sugars, Organic Acids, and Sugar/Acid Ratio of Strawberry Fruits
3.4. Phenolic Profile of Strawberry Fruits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paparozzi, E.T.; Meyer, G.E.; Schlegel, V.; Blankenship, E.E.; Adams, S.A.; Conley, M.E.; Loseke, B.; Read, P.E. Strawberry Cultivars Vary in Productivity, Sugars and Phytonutrient Content When Grown in a Greenhouse during the Winter. Sci. Hortic. 2018, 227, 1–9. [Google Scholar] [CrossRef]
- Al-Helal, I.M.; Abdel-Ghany, A.M. Responses of Plastic Shading Nets to Global and Diffuse PAR Transfer: Optical Properties and Evaluation. NJAS-Wagening. J. Life Sci. 2010, 57, 125–132. [Google Scholar] [CrossRef]
- de los Santos, B.; Medina, J.J.; Miranda, L.; Gómez, J.A.; Talavera, M. Soil Disinfestation Efficacy against Soil Fungal Pathogens in Strawberry Crops in Spain: An Overview. Agronomy 2021, 11, 526. [Google Scholar] [CrossRef]
- Samtani, J.B.; Ajwa, H.A.; Weber, J.B.; Browne, G.T.; Klose, S.; Hunzie, J.; Fennimore, S.A. Evaluation of Non-Fumigant Alternatives to Methyl Bromide for Weed Control and Crop Yield in California Strawberries (Fragaria ananassa L.). Crop Prot. 2011, 30, 45–51. [Google Scholar] [CrossRef]
- Storer, K.; Kendall, S.; White, C.; Roques, S.; Berry, P.A. Review of the Function, Efficacy and Value of Biostimulant Products Available for UK Cereals and Oilseeds; Agricultural and Horticulture Development Board (AHDB): Coventry, UK, 2016. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum Extract Application on Plant Growth, Fruit Yield and Soil Microbial Communities of Strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- de Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and Their Role in Improving Plant Growth under Abiotic Stresses. In Biostimulants in Plant Science; Mirmajlessi, S.M., Radhakrishnan, R., Eds.; IntechOpen: London, UK, 2019; pp. 3–16. [Google Scholar]
- Porras, M.; Barrau, C.; Arroyo, F.T.; Santos, B.; Blanco, C.; Romero, F. Reduction of Phytophthora cactorum in Strawberry Fields by Trichoderma Spp. and Soil Solarization. Plant Dis. 2007, 91, 142–146. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Tomić, J.; Pešaković, M.; Milivojević, J.; Karaklajić-Stajić, Ž. How to Improve Strawberry Productivity, Nutrients Composition, and Beneficial Rhizosphere Microflora by Biofertilization and Mineral Fertilization? J. Plant Nutr. 2018, 41, 2009–2021. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Donati, I. Promoting strawberry (Fragaria × ananassa) stress resistance, growth, and yield using native bacterial biostimulants. Agronomy 2023, 13, 529. [Google Scholar] [CrossRef]
- Farahi, M.H.; Aboutalebi, A.; Eshghi, S.; Dastyaran, M.; Yosefi, F. Foliar Application of Humic Acid on Quantitative and Qualitative Characteristics of ’ Aromas ’ Strawberry in Soilless Culture. Agric. Commun. 2013, 1, 13–16. [Google Scholar]
- Saidimoradi, D.; Ghaderi, N.; Javadi, T. Salinity Stress Mitigation by Humic Acid Application in Strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2019, 256, 108594. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First Fruit in Season: Seaweed extract and silicon advance organic strawberry (Fragaria × ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Sekmen Cetinel, A.H.; Gokce, A.; Erdik, E.; Cetinel, B.; Cetinkaya, N. The effect of Trichoderma citrinoviride treatment under salinity combined to Rhizoctonia solani infection in strawberry (Fragaria × ananassa Duch.). Agronomy 2021, 11, 1589. [Google Scholar] [CrossRef]
- Trzciński, P.; Frąc, M.; Lisek, A.; Przybył, M.; Frąc, M.; Sas-Paszt, L. Growth promotion of raspberry and strawberry plants by bacterial inoculants. Acta Sci. Pol. Hortorum Cultus 2021, 20, 71–82. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Nam, M.H.; Park, M.S.; Kim, H.G.; Yoo, S.J. Biological control of strawberry Fusarium wilt caused by Fusarium oxysporum f. sp. fragariae using Bacillus velezensis BS87 and RK1 formulation. J. Microbiol. Biotechnol. 2009, 19, 520–524. [Google Scholar] [CrossRef]
- Simkova, K.; Veberic, R.; Hudina, M.; Grohar, M.C.; Ivancic, T.; Smrke, T.; Pelacci, M.; Jakopic, J. Variability in ‘Capri’ Everbearing Strawberry Quality during a Harvest Season. Foods 2023, 12, 1349. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The Strawberry: Composition, Nutritional Quality, and Impact on Human Health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Hancock, J.F. Strawberries, 2nd ed.; Springer: Dordrecht, The Netherlands, 2020; pp. 445–455. [Google Scholar]
- Cervantes, L.; Ariza, M.T.; Miranda, L.; Lozano, D.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Stability of fruit quality traits of different strawberry varieties under variable environmental conditions. Agronomy 2020, 10, 1242. [Google Scholar] [CrossRef]
- Palmieri, L.; Masuero, D.; Martinatti, P.; Baratto, G.; Martens, S.; Vrhovsek, U. Genotype-by-environment effect on bioactive compounds in strawberry (Fragaria × ananassa Duch.). J. Sci. Food Agric. 2017, 97, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.A.; Denaxa, N.-K.; Damvakaris, T. Strawberry fruit quality attributes after application of plant growth stimulating compounds. Sci. Hortic. 2009, 119, 138–146. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, B.G.; Khan, F.; Bhujel, A.; Jaihuni, M.; Kim, N.E.; Moon, B.E.; Kim, H.T. Influence of different growing media on the growth and development of strawberry plants. Heliyon 2021, 7, e07170. [Google Scholar] [CrossRef] [PubMed]
- Cocco, C.; Andriolo, J.L.; Cardoso, F.L.; Erpen, L.; Schmitt, O.J. Crown size and transplant type on the strawberry Yield. Sci. Agric. 2011, 68, 489–493. [Google Scholar] [CrossRef]
- Fagherazzi, A.F.; Suek Zanin, D.; Soares Dos Santos, M.F.; Martins de Lima, J.; Welter, P.D.; Francis Richter, A.; Regianini Nerbass, F.; Anneliese Kretzschmar, A.; Rufato, L.; Baruzzi, G. Initial crown diameter influences on the fruit yield and quality of strawberry Pircinque. Agronomy 2021, 11, 184. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Aguiar, N.O.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2012, 353, 209–220. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma applications on strawberry plants modulate the physiological processes positively affecting fruit production and quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; D’Errico, G.; et al. Effect of Trichoderma bioactive metabolite treatments on the production, quality, and protein profile of strawberry fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef] [PubMed]
- Vestberg, M.; Kukkonen, S.; Saari, K.; Parikka, P.; Huttunen, J.; Tainio, L.; Devos, N.; Weekers, F.; Kevers, C.; Thonart, P.; et al. Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl. Soil Ecol. 2004, 27, 243–258. [Google Scholar] [CrossRef]
- Khan, F.; Kim, N.E.; Bhujel, A.; Jaihuni, M.; Lee, D.H.; Basak, J.K.; Kim, H.T. Assessment of combined Trichoderma-enriched biofertilizer and nutrients solutions on the growth and yield of strawberry plants. J. Biosyst. Eng. 2021, 46, 225–235. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Sas-Paszt, L.; Mikiciuk, M.; Derkowska, E.; Trzciński, P.; Głuszek, S.; Lisek, A.; Wera-Bryl, S.; Rudnicka, J. Mycorrhizal frequency, physiological parameters, and yield of strawberry plants inoculated with endomycorrhizal fungi and rhizosphere bacteria. Mycorrhiza 2019, 29, 489–501. [Google Scholar] [CrossRef]
- Shafir, S.; Dag, A.; Bilu, A.; Abu-Toamy, M.; Elad, Y. Honey bee dispersal of the biocontrol agent Trichoderma harzianum T39: Effectiveness in suppressing Botrytis cinerea on strawberry under field conditions. Eur. J. Plant Pathol. 2006, 116, 119–128. [Google Scholar] [CrossRef]
- Khalid, S.; Qureshi, K.M.; Hafiz, I.A.; Khan, K.S.; Qureshi, U.S. Effect of organic amendments on vegetative growth, fruit and yield quality of strawberry. Pakistan. J. Agric. Resour. 2013, 26, 104–112. [Google Scholar]
- Palencia, P.; Martínez, F.; Pestana, M.; Oliveira, J.A.; Correia, P.J. Effect of Bacillus velezensis and Glomus intraradices on fruit quality and growth parameters in strawberry soilless growing system. Hortic. J. 2015, 84, 122–130. [Google Scholar] [CrossRef]
- Garzoli, S.; Cairone, F.; Carradori, S.; Mocan, A.; Menghini, L.; Paolicelli, P.; Ak, G.; Zengin, G.; Cesa, S. Effects of processing on polyphenolic and volatile composition and fruit quality of Clery strawberries. Antioxidants 2020, 9, 632. [Google Scholar] [CrossRef]
- Weber, N.; Koron, D.; Jakopic, J.; Veberic, R.; Hudina, M.; Basa Cesnik, H. Influence of nitrogen, calcium and nano-fertilizer on strawberry (Fragaria × ananassa Duch.) fruit inner and outer quality. Agronomy 2021, 11, 997. [Google Scholar] [CrossRef]
- Błaszczyk, J.; Nawrocki, J.; Łysiak, G.P. The effect of the method of plant protection on the quality of remontant strawberry cultivars grown in a gutter system under covers. Agriculture 2022, 12, 2041. [Google Scholar] [CrossRef]
- Krüger, E.; Josuttis, M.; Nestby, R.; Toldam-Andersen, T.B.; Carlen, C.; Mezzetti, B. Influence of growing conditions at different latitudes of Europe on strawberry growth performance, yield and quality. J. Berry Res. 2012, 2, 143–157. [Google Scholar] [CrossRef]
- Milivojević, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and antioxidant properties of cultivated and wild Fragaria and Rubus berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A Novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Schmitzer, V.; Sircelj, H.; Stampar, F.; Slatnar, A. Physico-chemical characterization of Cornus kousa Burg. fruit: Determining optimal maturity for fresh consumption. J. Sci. Food Agric. 2021, 101, 778–785. [Google Scholar] [CrossRef]
- Yoshida, Y.; Koyama, H.; Tamura, H. Color and Anthocyanin Composition of Strawberry Fruit. Changes during Fruit Development and Differences among Cultivars, with Special Reference to the Occurrence of Pelargonidin 3-malonylglucoside. J. Jpn. Soc. Hort. Sci. 2002, 71, 355–361. [Google Scholar] [CrossRef]
- Pastrana, A.M.; Borrero, C.; Pérez, A.G.; Avilés, M. Soilborne pathogens affect strawberry fruit flavor and quality. Plant Sci. 2023, 326, 111533. [Google Scholar] [CrossRef]
- Morais, M.C.; Mucha, Â.; Ferreira, H.; Gonçalves, B.; Bacelar, E.; Marques, G. Comparative study of plant growth-promoting bacteria on the physiology, growth and fruit quality of strawberry. J. Sci. Food Agric. 2019, 99, 5341–5349. [Google Scholar] [CrossRef]
| Treatment | Root Length (cm) | Root Weight (g) | Canopy Height (cm) | Canopy Weight (g) | Crown Number |
|---|---|---|---|---|---|
| Control | 21.35 ± 4.54 | 42.93 ± 14.82 b | 46.99 ± 2.98 b | 286.7 ± 93.8 b | 3.2 ± 0.8 b |
| AP | 20.11 ± 6.63 | 59.67 ± 11.29 a | 50.28 ± 2.22 a | 347.2 ± 47.0 a | 4.3 ± 1.3 a |
| BP | 17.98 ± 6.76 | 52.13 ± 13.00 ab | 49.83 ± 3.35 a | 301.9 ± 73.8 ab | 4.3 ± 1.2 a |
| Significance | NS | ** | ** | * | * |
| Treatment | Harvest Date | Fruit Height (mm) | Fruit Diameter 1 (mm) | Fruit Diameter 2 (mm) |
|---|---|---|---|---|
| Control | 17 May | 51.6 ± 3.9 | 43.1 ± 4.0 | 36.6 ± 2.5 |
| AP | 47.8 ± 10.9 | 42.6 ± 1.47 | 38.5 ± 1.5 | |
| BP | 49.2 ± 5.6 | 42.8 ± 5.2 | 36.4 ± 2.5 | |
| Significance | NS | NS | NS | |
| Control | 20 May | 46.5 ± 4.0 b | 36.4 ± 4.5 b | 32.9 ± 2.1 b |
| AP | 51.9 ± 4.8 a | 46.1 ± 5.0 a | 35.6 ± 2.2 a | |
| BP | 51.4 ± 3.8 a | 42.9 ± 4.4 a | 37.3 ± 2.2 a | |
| Significance | ** | *** | *** | |
| Control | 23 May | 46.5 ± 4.1 b | 35.3 ± 2.6 b | 33.6 ± 2.3 b |
| AP | 51.8 ± 4.3 a | 37.8 ± 3.3 a | 35.5 ± 3.3 a | |
| BP | 50.2 ± 3.4 a | 37.9 ± 3.7 a | 35.6 ± 1.4 a | |
| Significance | ** | * | * | |
| Control | 25 May | 37.2 ± 2.7 b | 27.9 ± 2.1 b | 26.8 ± 2.1 c |
| AP | 43.6 ± 3.1 a | 33.2 ± 2.1 a | 32.6 ± 2.0 a | |
| BP | 41.5 ± 3.0 a | 32.3 ± 4.6 a | 30.2 ± 1.8 b | |
| Significance | *** | *** | *** | |
| Control | 27 May | 47.5 ± 2.9 | 41.4 ± 4.3 a | 36.6 ± 2.5 a |
| AP | 46.3 ± 2.7 | 35.6 ± 2.7 b | 32.9 ± 1.8 b | |
| BP | 46.5 ± 3.4 | 36.4 ± 2.4 b | 34.1 ± 2.1 b | |
| Significance | NS | *** | *** | |
| Control | 30 May | 38.2 ± 2.9 c | 31.5 ± 2.2 b | 33.0 ± 2.3 b |
| AP | 46.6 ± 2.3 a | 39.0 ± 2.9 a | 35.3 ± 2.5 a | |
| BP | 43.9 ± 4.6 b | 37.3 ± 3.7 a | 34.0 ± 2.8 ab | |
| Significance | *** | *** | * | |
| Control | 3 June | 34.9 ± 2.6 b | 30.7 ± 3.1 b | 29.0 ± 2.9 c |
| AP | 42.6 ± 4.0 a | 35.6 ± 2.4 a | 34.2 ± 2.3 a | |
| BP | 42.5 ± 2.8 a | 34.7 ± 2.3 a | 32.2 ± 2.7 b | |
| Significance | *** | *** | *** | |
| Control | 6 June | 32.0 ± 3.2 b | 30.4 ± 2.5 b | 27.9 ± 2.4 b |
| AP | 37.4 ± 2.7 a | 35.5 ± 1.9 a | 33.5 ± 1.6 a | |
| BP | 38.2 ± 2.5 a | 36.1 ± 2.2 a | 34.0 ± 2.0 a | |
| Significance | *** | *** | *** | |
| Control | 10 June | - | - | - |
| AP | 31.5 ± 4.6 b | 30.3 ± 2.5 b | 28.0 ± 2.4 b | |
| BP | 34.8 ± 3.3 a | 33.1 ± 4.3 a | 30.3 ± 3.7 a | |
| Significance | * | * | * |
| Treatment | Harvest Date | L * | C | h° | a * | b * |
|---|---|---|---|---|---|---|
| Control | 17 May | 36.0 ± 2.8 | 47.8 ± 4.1 | 33.4 ± 2.8 | 39.8 ± 2.5 | 26.4 ± 4.0 |
| AP | 36.5 ± 1.3 | 50.3 ± 3.5 | 34.7 ± 1.3 | 41.4 ± 2.9 | 28.7 ± 2.3 | |
| BP | 36.6 ± 1.8 | 49.4 ± 2.7 | 33.7 ± 2.3 | 41.0 ± 1.9 | 27.4 ± 2.7 | |
| Significance | NS | NS | NS | NS | NS | |
| Control | 20 May | 35.7 ± 2.0 | 48.3 ± 3.5 | 34.0 ± 1.7 | 40.0 ± 2.2 | 27.0 ± 3.0 |
| AP | 35.9 ± 2.3 | 48.6 ± 3.5 | 34.7 ± 1.5 | 40.0 ± 2.5 | 27.7 ± 2.8 | |
| BP | 37.3 ± 1.9 | 50.2 ± 3.1 | 34.9 ± 2.2 | 41.1 ± 2.0 | 28.7 ± 3.0 | |
| Significance | NS | NS | NS | NS | NS | |
| Control | 23 May | 35.0 ± 1.6 | 46.3 ± 3.7 | 33.2 ± 2.1 ab | 38.7 ± 2.8 | 25.3 ± 2.6 |
| AP | 36.0 ± 1.5 | 47.9 ± 2.7 | 33.5 ± 1.4 a | 39.9 ± 1.9 | 26.5 ± 2.3 | |
| BP | 34.6 ± 1.9 | 46.7 ± 3.5 | 32.2 ± 2.1 b | 39.5 ± 2.3 | 25.0 ± 3.2 | |
| Significance | NS | NS | * | NS | NS | |
| Control | 25 May | 34.8 ± 4.1 | 47.3 ± 4.8 | 35.6 ± 1.5 a | 38.4 ± 3.7 | 27.5 ± 3.3 |
| AP | 35.6 ± 2.3 | 45.6 ± 4.5 | 33.6 ± 1.7 b | 38.5 ± 3.0 | 25.9 ± 3.3 | |
| BP | 36.2 ± 2.1 | 47.3 ± 3.3 | 34.1 ± 2.2 b | 39.1 ± 2.0 | 26.6 ± 3.0 | |
| Significance | NS | NS | * | NS | NS | |
| Control | 27 May | 34.0 ± 2.1 | 44.6 ± 3.3 | 32.1 ± 2.6 | 37.7 ± 2.2 | 23.7 ± 3.2 |
| AP | 35.0 ± 1.3 | 46.4 ± 2.8 | 33.5 ± 1.9 | 38.6 ± 1.9 | 25.7 ± 2.7 | |
| BP | 34.8 ± 2.0 | 45.6 ± 3.3 | 32.6 ± 2.1 | 38.4 ± 2.1 | 24.7 ± 3.1 | |
| Significance | NS | NS | NS | NS | NS | |
| Control | 30 May | 33.0 ± 2.7 | 41.7 ± 5.2 b | 32.1 ± 2.7 b | 35.9 ± 3.9 ab | 22.3 ± 4.3 b |
| AP | 34.5 ± 2.0 | 45.0 ± 3.8 a | 33.2 ± 1.8 ab | 37.6 ± 2.6 a | 24.7 ± 3.1 ab | |
| BP | 34.4 ± 1.2 | 44.4 ± 3.1 ab | 34.2 ± 1.2 a | 34.6 ± 4.2 b | 25.3 ± 2.2 a | |
| Significance | NS | * | * | * | * | |
| Control | 3 June | 32.7 ± 1.2 | 43.2 ± 2.3 a | 32.0 ± 1.9 | 36.6 ± 2.2 a | 22.9 ± 1.5 ab |
| AP | 32.1 ± 2.3 | 42.1 ± 3.9 a | 33.0 ± 1.1 | 35.3 ± 3.1 a | 23.4 ± 2.9 a | |
| BP | 31.7 ± 2.3 | 39.0 ± 5.3 b | 32.7 ± 1.9 | 32.7 ± 4.3 b | 21.1 ± 3.3 b | |
| Significance | NS | * | NS | ** | * | |
| Control | 6 June | 34.7 ± 1.8 | 45.2 ± 3.5 | 33.7 ± 1.3 | 37.6 ± 2.6 | 25.2 ± 2.6 ab |
| AP | 33.5 ± 2.2 | 43.2 ± 4.1 | 32.6 ± 1.9 | 36.3 ± 3.0 | 23.3 ± 3.0 b | |
| BP | 34.5 ± 2.0 | 46.0 ± 4.1 | 33.7 ± 1.6 | 38.2 ± 2.8 | 25.7 ± 3.4 a | |
| Significance | NS | NS | NS | NS | * | |
| Control | 10 June | - | - | - | - | - |
| AP | 33.5 ± 1.6 b | 43.1 ± 2.9 b | 32.0 ± 1.2 b | 36.5 ± 2.2 b | 22.2 ± 4.1 b | |
| BP | 35.9 ± 2.2 a | 46.2 ± 3.6 a | 33.7 ± 1.8 a | 38.4 ± 2.5 a | 25.7 ± 3.0 a | |
| Significance | ** | * | ** | * | * |
| Treatment | Harvest Date | Fruit Firmness (kg cm−1) | Total Soluble Solids (°Brix) | Sugar/Organic Acid |
|---|---|---|---|---|
| Control | 17 May | 0.87 ± 0.31 | 8.28 ± 0.94 | 4.11 ± 0.41 a |
| AP | 1.06 ± 0.24 | 7.68 ± 0.34 | 3.43 ± 0.33 b | |
| BP | 0.89 ± 0.34 | 7.85 ± 1.27 | 4.08 ± 0.25 a | |
| Significance | NS | NS | * | |
| Control | 20 May | 0.84 ± 0.49 | 7.80 ± 0.47 a | 3.74 ± 0.55 |
| AP | 0.68 ± 0.26 | 7.25 ± 0.39 b | 3.47 ± 0.31 | |
| BP | 0.79 ± 0.33 | 7.80 ± 0.43 a | 3.74 ± 0.24 | |
| Significance | NS | ** | NS | |
| Control | 23 May | 0.91 ± 0.41 | 8.22 ± 0.84 a | 4.29 ± 0.46 |
| AP | 0.73 ± 0.51 | 7.53 ± 0.54 b | 4.21 ± 0.86 | |
| BP | 0.70 ± 0.33 | 7.67 ± 0.41 b | 4.16 ± 0.53 | |
| Significance | NS | * | NS | |
| Control | 25 May | 1.12 ± 0.26 | 7.96 ± 0.78 a | 3.06 ± 0.41 b |
| AP | 1.19 ± 0.34 | 6.72 ± 1.26 b | 4.08 ± 0.79 a | |
| BP | 1.07 ± 0.18 | 7.48 ± 0.70 a | 4.05 ± 0.52 a | |
| Significance | NS | ** | * | |
| Control | 27 May | 0.76 ± 0.41 | 8.89 ± 0.68 a | 4.26 ± 0.83 a |
| AP | 0.83 ± 0.37 | 7.09 ± 0.63 b | 3.30 ± 0.24 b | |
| BP | 0.73 ± 0.32 | 7.44 ± 0.64 b | 3.63 ± 0.80 ab | |
| Significance | NS | *** | * | |
| Control | 30 May | 1.25 ± 0.32 a | 8.65 ± 0.97 a | 3.44 ± 0.29 |
| AP | 1.26 ± 0.21 b | 7.58 ± 0.88 b | 3.79 ± 0.56 | |
| BP | 1.28 ± 0.33 a | 8.23 ± 1.01 ab | 3.41 ± 0.32 | |
| Significance | NS | * | NS | |
| Control | 3 June | 0.48 ± 0.31 | 9.33 ± 1.27 a | 3.69 ± 0.63 ab |
| AP | 0.54 ± 0.23 | 7.31 ± 1.41 b | 3.34 ± 0.41 b | |
| BP | 0.51 ± 0.27 | 7.59 ± 0.81 b | 4.19 ± 0.37 a | |
| Significance | NS | *** | * | |
| Control | 6 June | 1.00 ± 0.30 | 8.26 ± 1.28 | 3.29 ± 0.60 |
| AP | 1.07 ± 0.27 | 7.58 ± 1.07 | 3.36 ± 0.54 | |
| BP | 1.05 ± 0.24 | 7.77 ± 0.86 | 3.12 ± 0.21 | |
| Significance | NS | NS | NS | |
| Control | 10 June | - | - | - |
| AP | 0.76 ± 0.33 | 7.97 ± 1.19 | 3.62 ± 0.70 | |
| BP | 0.74 ± 0.31 | 7.68 ± 0.62 | 3.19 ± 0.29 | |
| Significance | NS | NS | NS |
| Treatment | Harvest Date | Total Sugar Content (g kg−1) | Sucrose (g kg−1) | Glucose (g kg−1) | Fructose (g kg−1) |
|---|---|---|---|---|---|
| Control | 17 May | 52.92 ± 3.11 | 11.06 ± 1.80 | 19.47 ± 1.39 a | 22.39 ± 1.04 a |
| AP | 49.22 ± 3.47 | 13.47 ± 1.66 | 16.58 ± 1.12 b | 19.17 ± 1.29 b | |
| BP | 53.04 ± 5.06 | 12.14 ± 2.73 | 18.99 ± 1.72 a | 21.92 ± 1.49 a | |
| Significance | NS | NS | * | ** | |
| Control | 20 May | 50.09 ± 3.81 a | 11.56 ± 1.17 | 17.90 ± 1.49 a | 20.63 ± 1.50 a |
| AP | 43.04 ± 2.42 b | 9.76 ± 0.82 | 15.17 ± 0.89 b | 18.11 ± 1.02 b | |
| BP | 45.57 ± 3.98 ab | 10.82 ± 1.84 | 15.89 ± 1.01 b | 18.86 ± 1.14 b | |
| Significance | * | NS | ** | * | |
| Control | 23 May | 50.91 ± 3.93 a | 11.37 ± 1.87 | 18.14 ± 1.12 a | 21.40 ± 1.19 a |
| AP | 44.39 ± 5.43 b | 10.77 ± 2.13 | 15.35 ± 1.73 b | 18.27 ± 1.96 b | |
| BP | 46.02 ± 1.87 ab | 11.52 ± 1.57 | 15.76 ± 0.32 b | 18.74 ± 0.36 b | |
| Significance | * | NS | ** | ** | |
| Control | 25 May | 45.34 ± 3.05 | 10.19 ± 1.45 | 15.97 ± 0.84 | 19.17 ± 0.88 |
| AP | 45.54 ± 5.51 | 11.96 ± 1.85 | 15.26 ± 1.77 | 18.33 ± 1.99 | |
| BP | 47.95 ± 6.53 | 12.79 ± 2.49 | 15.97 ± 2.07 | 19.19 ± 2.33 | |
| Significance | NS | NS | NS | NS | |
| Control | 27 May | 61.34 ± 6.73 a | 17.61 ± 2.51 a | 20.13 ± 2.22 a | 23.61 ± 2.37 a |
| AP | 44.55 ± 3.89 b | 9.90 ± 1.77 c | 15.85 ± 1.46 b | 18.80 ± 1.68 b | |
| BP | 48.17 ± 7.64 b | 13.50 ± 3.26 b | 15.85 ± 2.15 b | 18.82 ± 2.44 b | |
| Significance | ** | ** | ** | ** | |
| Control | 30 May | 50.43 ± 4.25 | 12.26 ± 2.76 | 17.33 ± 0.95 | 20.83 ± 1.08 |
| AP | 51.84 ± 11.89 | 14.21 ± 6.69 | 17.27 ± 2.72 | 20.36 ± 2.94 | |
| BP | 44.92 ± 3.59 | 11.11 ± 1.80 | 15.50 ± 1.22 | 18.31 ± 1.36 | |
| Significance | NS | NS | NS | NS | |
| Control | 3 June | 49.52 ± 4.78 a | 12.03 ± 3.12 | 17.14 ± 1.01 a | 20.35 ± 0.95 a |
| AP | 41.22 ± 3.38 b | 9.83 ± 2.05 | 14.22 ± 1.11 b | 17.16 ± 1.19 b | |
| BP | 48.69 ± 3.16 a | 12.78 ± 3.37 | 16.50 ± 0.62 a | 19.41 ± 0.73 a | |
| Significance | *** | NS | *** | *** | |
| Control | 6 June | 45.52 ± 6.28 | 11.46 ± 2.65 a | 15.41 ± 1.88 | 18.65 ± 1.90 |
| AP | 40.01 ± 3.58 | 8.73 ± 1.11 b | 14.11 ± 1.43 | 17.17 ± 1.52 | |
| BP | 41.86 ± 1.87 | 11.29 ± 1.51 ab | 13.77 ± 1.10 | 16.80 ± 1.32 | |
| Significance | NS | * | NS | NS | |
| Control | 10 June | - | - | - | - |
| AP | 43.58 ± 2.37 a | 12.84 ± 1.62 | 14.01 ± 0.42 a | 16.72 ± 0.46 | |
| BP | 38.01 ± 2.76 b | 10.21 ± 3.42 | 12.28 ± 0.52 b | 15.52 ± 1.58 | |
| Significance | ** | NS | *** | NS |
| Treatment | Harvest Date | Total Content of Detected Organic Acids (g kg−1) | Citric Acid (g kg−1) | Malic Acid (g kg−1) | Fumaric Acid (g kg−1) | Shikimic Acid (g kg−1) | Ascorbic Acid (g kg−1) |
|---|---|---|---|---|---|---|---|
| Control | 17 May | 13.41 ± 1.61 ab | 8.86 ± 1.08 ab | 4.09 ± 0.50 | 0.009 ± 0.001 | 0.035 ± 0.004 | 0.42 ± 0.02 a |
| AP | 14.70 ± 0.40 a | 9.77 ± 0.17 a | 4.52 ± 0.26 | 0.01 ± 0.001 | 0.037 ± 0.002 | 0.36 ± 0.01 b | |
| BP | 13.37 ± 0.57 b | 8.74 ± 0.48 b | 4.20 ± 0.22 | 0.009 ± 0.002 | 0.035 ± 0.002 | 0.38 ± 0.04 ab | |
| Significance | * | * | NS | NS | NS | * | |
| Control | 20 May | 13.91 ± 1.47 a | 9.23 ± 0.81 | 4.25 ± 0.65 a | 0.008 ± 0.000 a | 0.036 ± 0.003 a | 0.38 ± 0.03 |
| AP | 12.81 ± 0.65 ab | 8.83 ± 0.59 | 3.55 ± 0.25 b | 0.009 ± 0.001 ab | 0.030 ± 0.003 b | 0.39 ± 0.03 | |
| BP | 12.53 ± 0.58 b | 8.55 ± 0.37 | 3.58 ± 0.19 b | 0.008 ± 0.000 b | 0.030 ± 0.003 b | 0.36 ± 0.04 | |
| Significance | * | NS | * | * | ** | NS | |
| Control | 23 May | 12.31 ± 0.90 | 8.08 ± 0.67 | 3.80 ± 0.20 a | 0.009 ± 0.001 a | 0.034 ± 0.003 a | 0.38 ± 0.03 |
| AP | 11.06 ± 1.35 | 8.07 ± 0.54 | 2.60 ± 1.31 b | 0.008 ± 0.001 b | 0.029 ± 0.003 b | 0.35 ± 0.03 | |
| BP | 11.54 ± 1.25 | 7.76 ± 1.00 | 3.37 ± 0.25 ab | 0.008 ± 0.000 ab | 0.030 ± 0.002 b | 0.37 ± 0.03 | |
| Significance | NS | NS | * | * | * | NS | |
| Control | 25 May | 15.33 ± 1.25 a | 9.84 ± 0.75 a | 5.06 ± 0.51 a | 0.010 ± 0.001 a | 0.040 ± 0.004 a | 0.38 ± 0.02 |
| AP | 11.80 ± 2.18 b | 8.23 ± 1.26 b | 3.15 ± 1.19 b | 0.008 ± 0.001 b | 0.027 ± 0.003 b | 0.38 ± 0.07 | |
| BP | 12.26 ± 0.87 b | 8.55 ± 1.10 ab | 3.28 ± 0.93 b | 0.008 ± 0.001 b | 0.029 ± 0.003 b | 0.40 ± 0.04 | |
| Significance | ** | * | * | ** | *** | NS | |
| Control | 27 May | 15.06 ± 1.49 | 10.10 ± 1.01 | 4.48 ± 0.56 | 0.009 ± 0.001 | 0.037 ± 0.004 | 0.43 ± 0.04 a |
| AP | 13.88 ± 0.41 | 8.80 ± 0.31 | 4.65 ± 0.36 | 0.009 ± 0.002 | 0.032 ± 0.003 | 0.39 ± 0.02 ab | |
| BP | 13.97 ± 2.31 | 9.25 ± 1.72 | 4.29 ± 0.60 | 0.009 ± 0.001 | 0.037 ± 0.005 | 0.38 ± 0.03 b | |
| Significance | NS | NS | NS | NS | NS | * | |
| Control | 30 May | 15.12 ± 1.08 | 10.10 ± 1.13 | 4.56 ± 0.27 | 0.009 ± 0.001 | 0.032 ± 0.003 | 0.42 ± 0.04 |
| AP | 14.00 ± 1.24 | 9.14 ± 0.56 | 4.41 ± 0.77 | 0.009 ± 0.002 | 0.034 ± 0.003 | 0.41 ± 0.04 | |
| BP | 13.62 ± 1.50 | 9.13 ± 1.23 | 4.06 ± 0.35 | 0.009 ± 0.001 | 0.031 ± 0.003 | 0.39 ± 0.05 | |
| Significance | NS | NS | NS | NS | NS | NS | |
| Control | 3 June | 14.08 ± 1.94 | 9.02 ± 1.33 | 4.55 ± 0.80 | 0.009 ± 0.001 | 0.034 ± 0.004 a | 0.46 ± 0.04 a |
| AP | 12.84 ± 1.48 | 8.35 ± 0.91 | 4.05 ± 0.56 | 0.009 ± 0.001 | 0.030 ± 0.004 ab | 0.41 ± 0.04 b | |
| BP | 12.08 ± 0.89 | 7.63 ± 0.47 | 4.00 ± 0.43 | 0.006 ± 0.003 | 0.029 ± 0.003 b | 0.41 ± 0.03 b | |
| Significance | NS | NS | NS | NS | * | * | |
| Control | 6 June | 14.44 ± 1.49 a | 9.91 ± 1.19 a | 4.03 ± 0.25 | 0.008 ± 0.001 | 0.029 ± 0.004 a | 0.47 ± 0.06 |
| AP | 12.52 ± 1.30 b | 8.35 ± 1.01 b | 3.67 ± 0.26 | 0.008 ± 0.001 | 0.025 ± 0.003 b | 0.47 ± 0.04 | |
| BP | 13.87 ± 0.95 ab | 9.54 ± 0.79 ab | 3.88 ± 0.23 | 0.007 ± 0.001 | 0.026 ± 0.002 ab | 0.42 ± 0.04 | |
| Significance | * | * | NS | NS | * | NS | |
| Control | 10 June | - | - | - | - | - | - |
| AP | 12.73 ± 2.01 | 7.99 ± 1.50 | 4.32 ± 0.50 | 0.007 ± 0.001 | 0.027 ± 0.004 | 0.39 ± 0.08 | |
| BP | 12.42 ± 1.86 | 8.07 ± 1.35 | 3.93 ± 0.57 | 0.006 ± 0.001 | 0.025 ± 0.004 | 0.38 ± 0.03 | |
| Significance | NS | NS | NS | NS | NS | NS |
| Treatment | Harvest Date | Total Content of Detected Phenolic Compounds (mg kg−1) | Hydroxycinnamic Acids (mg kg−1) | Hydroxybenzoic Acids (mg kg−1) | Flavan-3-ols (mg kg−1) | Flavonols (mg kg−1) | Anthocyanins (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Control | 17 May | 952.9 ± 42.5 a | 8.60 ± 0.53 a | 12.87 ± 1.20 | 467.0 ± 21.1 a | 59.34 ± 4.90 a | 405.1 ± 31.5 a |
| AP | 769.9 ± 45.8 b | 7.30 ± 0.85 b | 13.32 ± 1.47 | 387.8 ± 24.8 b | 52.94 ± 4.11 b | 308.3 ± 42.0 b | |
| BP | 824.0 ± 36.2 b | 7.38 ± 0.57 b | 12.88 ± 0.57 | 414.2 ± 19.7 b | 47.81 ± 2.19 b | 341.5 ± 29.7 b | |
| Significance | *** | * | NS | *** | ** | ** | |
| Control | 20 May | 837.3 ± 19.7 a | 7.64 ± 0.61 a | 14.35 ± 1.52 | 408.1 ± 22.4 a | 52.93 ± 2.75 a | 354.3 ± 26.3 b |
| AP | 782.4 ± 37.8 b | 6.27 ± 0.65 b | 12.80 ± 1.11 | 335.7 ± 16.5 c | 20.96 ± 2.24 c | 406.7 ± 28.3 a | |
| BP | 769.7 ± 41.8 b | 6.95 ± 0.88 ab | 13.59 ± 1.17 | 368.8 ± 26.1 b | 43.60 ± 3.58 b | 336.8 ± 30.2 b | |
| Significance | * | * | NS | *** | *** | ** | |
| Control | 23 May | 989.5 ± 62.0 a | 8.63 ± 0.40 a | 14.86 ± 2.06 | 491.8 ± 29.8 a | 68.19 ± 7.07 a | 406.0 ± 35.3 |
| AP | 783.1 ± 34.0 c | 6.55 ± 0.76 b | 14.00 ± 1.68 | 348.2 ± 28.4 c | 36.29 ± 4.23 c | 378.1 ± 28.5 | |
| BP | 865.5 ± 37.3 b | 7.36 ± 0.79 b | 13.66 ± 1.34 | 410.3 ± 27.1 b | 45.54 ± 4.48 b | 388.7 ± 27.0 | |
| Significance | *** | ** | NS | *** | *** | NS | |
| Control | 25 May | 1091 ± 53.4 a | 9.60 ± 1.28 a | 20.32 ± 1.31 a | 501.1 ± 19.4 a | 70.11 ± 7.39 a | 489.4 ± 33.9 a |
| AP | 831.6 ± 46.1 b | 7.66 ± 0.78 b | 15.73 ± 1.51 b | 349.5 ± 29.3 c | 49.18 ± 4.87 b | 409.5 ± 25.1 b | |
| BP | 887.9 ± 35.1 b | 8.57 ± 0.45 ab | 16.77 ± 1.68 b | 393.6 ± 21.8 b | 53.36 ± 2.90 b | 415.7 ± 26.7 b | |
| Significance | *** | * | ** | *** | *** | ** | |
| Control | 27 May | 896.7 ± 49.8 | 7.28 ± 0.72 b | 15.38 ± 1.36 b | 454.7 ± 27.0 a | 67.53 ± 7.67 a | 351.8 ± 32.3 b |
| AP | 926.5 ± 33.1 | 8.40 ± 0.67 a | 20.77 ± 2.36 a | 359.9 ± 33.8 b | 46.69 ± 2.91 b | 490.7 ± 18.1 a | |
| BP | 914.3 ± 55.1 | 8.18 ± 0.63 ab | 19.23 ± 1.63 a | 380.6 ± 30.2 b | 47.94 ± 4.29 b | 458.4 ± 35.1 a | |
| Significance | NS | * | ** | *** | *** | *** | |
| Control | 30 May | 1235 ± 49.0 a | 9.93 ± 0.61 a | 22.32 ± 2.12 a | 580.9 ± 30.9 a | 52.23 ± 3.46 a | 570.0 ± 32.0 a |
| AP | 1074 ± 42.2 b | 9.18 ± 0.65 ab | 19.93 ± 1.16 b | 494.9 ± 36.0 b | 50.14 ± 5.71 ab | 500.0 ± 34.7 b | |
| BP | 985.5 ± 40.2 c | 8.86 ± 0.72 b | 21.30 ± 1.78 ab | 430.3 ± 24.8 c | 44.30 ± 4.05 b | 480.8 ± 30.0 b | |
| Significance | *** | * | * | *** | * | ** | |
| Control | 3 June | 1008 ± 55.9 b | 8.09 ± 0.64 b | 18.25 ± 0.88 a | 467.8 ± 36.2 ab | 42.01 ± 3.01 b | 472.1 ± 33.3 b |
| AP | 1171 ± 82.1 a | 10.00 ± 0.62 a | 20.12 ± 1.71 a | 514.1 ± 36.0 a | 44.57 ± 5.76 b | 582.6 ± 41.7 a | |
| BP | 947.3 ± 82.4 b | 6.96 ± 0.57 c | 15.19 ± 1.98 b | 431.4 ± 44.5 b | 52.91 ± 5.29 a | 440.9 ± 37.1 b | |
| Significance | ** | *** | ** | * | * | *** | |
| Control | 6 June | 1057 ± 59.8 b | 8.30 ± 1.13 | 17.44 ± 1.31 b | 515.8 ± 33.3 | 46.51 ± 6.40 b | 469.7 ± 29.0 c |
| AP | 1143 ± 69.0 ab | 9.13 ± 1.29 | 20.78 ± 1.74 a | 508.4 ± 32.2 | 44.95 ± 4.23 b | 559.3 ± 37.9 a | |
| BP | 1151 ± 61.9 a | 9.47 ± 1.02 | 18.75 ± 1.06 b | 551.7 ± 39.0 | 59.16 ± 3.21 a | 512.4 ± 20.8 b | |
| Significance | * | NS | ** | NS | *** | ** | |
| Control | 10 June | - | - | - | - | - | - |
| AP | 1089 ± 60.6 a | 9.53 ± 0.85 a | 19.47 ± 1.30 a | 460.3 ± 41.4 a | 45.52 ± 2.41 a | 554.1 ± 32.3 a | |
| BP | 874.4 ± 65.1 b | 7.13 ± 0.55 b | 16.64 ± 1.32 b | 381.8 ± 22.7 b | 29.96 ± 1.73 b | 438.9 ± 33.5 b | |
| Significance | *** | *** | ** | ** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitzer, V.; Stampar, F.; Turk, A.; Jakopic, J.; Hudina, M.; Veberic, R.; Smrke, T. Before or after Planting? Mycorrhizal and Bacterial Biostimulants and Extracts in Intense Strawberry (Fragaria × ananassa Duch.) Production. Horticulturae 2023, 9, 769. https://doi.org/10.3390/horticulturae9070769
Schmitzer V, Stampar F, Turk A, Jakopic J, Hudina M, Veberic R, Smrke T. Before or after Planting? Mycorrhizal and Bacterial Biostimulants and Extracts in Intense Strawberry (Fragaria × ananassa Duch.) Production. Horticulturae. 2023; 9(7):769. https://doi.org/10.3390/horticulturae9070769
Chicago/Turabian StyleSchmitzer, Valentina, Franci Stampar, Anze Turk, Jerneja Jakopic, Metka Hudina, Robert Veberic, and Tina Smrke. 2023. "Before or after Planting? Mycorrhizal and Bacterial Biostimulants and Extracts in Intense Strawberry (Fragaria × ananassa Duch.) Production" Horticulturae 9, no. 7: 769. https://doi.org/10.3390/horticulturae9070769
APA StyleSchmitzer, V., Stampar, F., Turk, A., Jakopic, J., Hudina, M., Veberic, R., & Smrke, T. (2023). Before or after Planting? Mycorrhizal and Bacterial Biostimulants and Extracts in Intense Strawberry (Fragaria × ananassa Duch.) Production. Horticulturae, 9(7), 769. https://doi.org/10.3390/horticulturae9070769









