Abstract
In organic phytosanitary management of vineyards, it is crucial to understand the available pathogen control alternatives in order to progress towards a more sustainable form of viticulture. The objective of this study was to evaluate the efficacy of seven biofungicides registered in Chile for the prevention and treatment of Botrytis Bunch Rot (BBR), and to test a novel fungicide composed of chitosan and riboflavin (CH-RF). Biofungicides including Trichoderma spp., Aureobasidium pullulans, and Melaleuca alternifolia were evaluated using biotests with grapevine berries. Registered products (specifically biological control agents) significantly reduced the in vitro growth of B. cinerea. However, the degree of pathogen control varied significantly among products in grapevine berries of the Chardonnay cultivar, and disease incidence and severity changed depending on the inoculation time. High control effectiveness was achieved with two biofungicides, A. pullulans (average efficacy 34%) and M. alternifolia oil (average efficacy 29%). In vitro tests showed CH-RF significantly reduced mycelial growth of B. cinerea. Noticeable differences between the new CH-RF fungicide (incidence under 50% and infection score under 1) and A. pullulans- and M. alternifolia-based products were detected in grapevine berries. Therefore, the new experimental formulation CH-RF constitutes a promising alternative for the control of B. cinerea and provides a basis for further research.
1. Introduction
Botrytis cinerea Pers. is a polyphagous fungus that causes Botrytis Bunch Rot (BBR) in grapes (Vitis vinifera L.). The pathogen can survive saprophytically on dead plants and/or plant debris and produce dormant overwintering structures, notably sclerotia [1]. BBR is responsible for considerable economic losses in many crops, including grapes, in both field and storage conditions. Specifically, in wine grapes, the disease can result in a decrease in yield and a reduction in wine quality [2,3]. The epidemiology of the disease is mostly determined by factors such as seasonal weather patterns [4,5], cultivar susceptibility [6], genetic structure of pathogen populations [7], host plant ontogenic resistance and vigor [8,9,10], and bunch structure and micro-climatic conditions [11,12].
The control of BBR is mainly based on the occasional and/or more regular use of synthetic fungicides according to the vineyard management and production system (organic, biodynamic, conventional, wine, and table grapes) [13,14]; nevertheless, the development of resistant populations has been observed in vineyards worldwide [15]. Moreover, the high frequency of synthetic fungicides applications could cause severe problems to human health and/or to the environment [16]. In grapevine cultivation, various practices can directly or indirectly influence disease development, by modifying both berry defense mechanisms and/or the microclimate of the vine [10,17]. Some agronomic practices significantly reduce the disease, such as avoiding excessive nitrogen and water application, leaf removal around bunches area, and thinning of the berries [8,18,19]. In addition, biological control by using products based on microorganisms or natural compounds is considered an attractive and sustainable option [20,21].
Furthermore, among the active ingredients or products of high interest, chitosan (CH), and chitosan-based products, have been highlighted for a high potential to control several plant diseases [22]. The naturally occurring polymer CH is a linear polysaccharide of glucosamine and N-acetylglucosamine units, obtained by deacetylation of chitin following exposure to NaOH solutions or chitinase [23]. Because of its antimicrobial activity, CH can interfere with spore germination and mycelial growth of phytopathogenic fungi, including B. cinerea [24,25]. It may also activate plant defenses [26,27]. The antimicrobial activity of CH varies according to its molecular weight, degree of deacetylation, pH of the chitosan solution, and the susceptibility of the target organism [28]. Due to its multifunctional activity, CH has been proposed as a pre- and post-harvest fungicide [29,30], and such CH applications have been reported to effectively control the BBR in table grapes [31].
To date, original studies have reported an increasing trend in the use of Photodynamic Inactivation (PDI) [32,33]. PDI is a technique that relies on the occurrence of non-thermal photophysical and photochemical reactions, requiring light and photosensitizers (PSs) in the presence of oxygen [34]. The mechanism of action of PDI in microorganisms is based on the application and subsequent accumulation of a PS on the pathogenic cell followed by light irradiation to generate reactive oxygen species (ROS) [35,36,37]. Specifically, PS is a molecule that interacts with molecular oxygen to create ROS after being excited by light at a specific wavelength. Following light irradiation, ROS generated oxidized biological targets resulting in microbial cell death [38]. Singlet oxygen (1O2), a strong oxidant and relatively long-lived compared to free radicals, is extremely reactive and can interact with many biological substrates, inducing oxidative damage and ultimately cell death [39]. Cell membrane sterol oxidation by singlet oxygen is described as one of the possible mechanisms of PDI antifungal action [40]. However, only a few studies have been published with CH in combination with a PS, including the following: (i) the use of CH conjugates in PDI against bacteria [41]; (ii) the antimicrobial effect of physical disinfection with a mixture of chlorophyllin and CH, on Listeria monocytogenes and B. cinerea, in wheat seeds and strawberries, respectively [42]. In the present study, the photosensitizer associated with CH is vitamin B2, also known as riboflavin (RF) [35]. Several vitamins, such as riboflavin and thiamine, have also been reported to induce systemic acquired resistance in some crop plants [43]. In this study, we present a new molecule based on a CH conjugate with RF as photosensitizer being tested as a fungicidal formulation to control B. cinerea.
In recent years, the use of biofungicides that rely on microbial biocontrol agents (BCAs) and natural compounds (NCs) products has been on the rise due to growing public concerns about the potential risk of pesticide residues in food and their negative impacts on the environment and/or human health [44,45,46]. Regarding registered BCAs and NCs, in augmentative biological control of diseases, recent research has focused on different aspects, including different modes of action, legal considerations (such as the registration process), and the potential risks associated with pathogen resistance development against biocontrol agents [47,48,49,50]. The possible mechanisms behind the antagonism of BCAs against pathogen fungi may include antimicrobial secondary metabolite production, secretion of lytic and fungal cell wall degrading enzymes, and competition for nutrients and space [51]. In addition, depending on the conditions and type of BCAs, one or more of these interconnected mechanisms may be observed, with variable contributions to decrease pathogen populations [52].
Research has also focused on assessing the efficacy of various experimental and registered BCAs and NCs products to control BBR in both table and wine grapes [17,47,53,54,55]. Interestingly, all these studies showed high variability in efficacy for controlling the disease. Therefore, a major key objective in the development and implementation of biological control products is to improve the ability of the control agent to survive and successfully control the disease under a wider array of conditions and with minimal variability [56]. Another main point of interest is exploring the curative potential of biocontrol products, as there have been only a limited number of studies that have examined the curative effects of biocontrol products against fungal pathogens [57].
Thus, our main objectives were as follows. The first objective was to test and compare both preventive and curative effects of various biofungicides registered in Chile to control B. cinerea. For this, it was necessary to address the critical issue of the variability in efficacy of these biofungicides using standard biotests with Chardonnay grapevine berries. Second, under laboratory-controlled conditions, the objective was to compare the efficacy of two biofungicides commonly used in phytosanitary programs for grapes in Chile, using both as control, with the new candidate biocontrol fungicide molecule CH-RF.
2. Materials and Methods
2.1. B. cinerea Strain Used in Laboratory Biotests
The B. cinerea pathogenic strain B05.10 that was used was provided by the Center for Plant Biotechnology of the Andrés Bello University. This strain has been characterized as virulent on grapevine berries [58]. The pathogen was grown on potato dextrose agar (PDA; Difco Inc., New York, NY, USA) plates for 10 days at 22 °C for routine cultivation. First, the conidial suspensions were prepared by flooding the plates with sterile water containing 0.05% (v/v) Tween-80 and gently scraping the agar surface with a sterile scalpel. Next, the suspension was passed through two layers of sterilized gauze (autoclaved at 120 °C for 20 min), and the conidia were enumerated with a hemocytometer (BOECO, Hamburg, Germany) under a light microscope (Olympus CX31, Tokio, Japan). Finally, the conidial suspension was diluted with sterile water to a final concentration of 1 × 106 conidia mL−1 for the biotests.
2.2. CH-RF Molecule Description
The preparation of CH-RF molecule was described in detail in the literature [35]. Commercially available CH (Sigma-Aldrich, St. Louis, MO, USA; ThermoFisher, Waltham, MA, USA) was subjected to salt-assisted microwave hydrolysis to reduce its molecular weight and increase water solubility. The deacetylation degree of hydrolyzed CH was 86%. The conjugate was synthetized in three steps: CH thiolation, synthesis of the RF-PMPI ad-duct, and the conjugation of these two products to produce the CH-RF chemical conjugate.
2.3. In Vitro and In Vivo Efficacy Level of Registered BCAs and NCs Biofungicides
Seven products were used in all the biotests including five BCAs and two NCs (Table 1).

Table 1.
Biological control agents (BCAs) and natural compounds (NCs) applied in the biotests.
Most commercial formulations of BCAs and NCs tested are registered in Chile for application on table and wine grapes, except for PRO, which has not yet been registered. Therefore, the viability of the BCAs was assessed and confirmed by plating the product suspensions on PDA (Difco Inc., New York, NY, USA). For testing in vitro BCAs and NCs activity against B. cinerea, 0.5 L of a solution of each product was prepared according to the doses recommended by manufacturers. The area of a Petri plate (90 mm × 16.2 mm) was calculated to add an equivalent quantity of product to the dose recommended by spraying 1 ha. The solutions were placed in Petri plates containing 12 mL of PDA, gently spread on the plate using a sterile Drigalski spatula, and dried in air for 30 min. In succession, 10 µL of a conidial suspension (1 × 106 conidia mL−1) of B. cinerea was applied in the center of each plate. The fungal activity was assessed for 6 days. As a control, Petri plates inoculated only with the pathogen were used. The mean radial growth of the fungus was determined by measuring the fungal colony diameters on days 2, 4, and 6. The treatments were replicated five times. Plates were incubated at 21 ± 1 °C. For the data analysis, only the growth of B. cinerea was considered. The biotest was repeated twice.
For assessing in vivo BCAs and NCs activity, berries of wine grape cv. Chardonnay were used. Bunches were collected in 2021 and 2022 from an organic vineyard located at Viña Emiliana (33°21′52″ S 71°18′31″ W, 312 m.a.s.l.) in the Valparaiso region, Chile. Bunches were transported to the laboratory in a cooler, rinsed under tap water for 4 min, disinfected with 2/3 of distilled water and 1/3 of 5% sodium hypochlorite to remove epiphytic microflora, and finally rinsed again with sterile water. Healthy berries, with the pedicel intact, were cut from the rachis and pooled together. Three biotests were performed to assess the effect of organic products according to the time of application. In the first one, organic products were sprayed in boxes containing 10 berries according to each product’s label 24 h and 48 h before the inoculation with the pathogen (to assess preventive effect). Grapevine berries were inoculated by placing 10 µL of B. cinerea conidial suspension (1 × 106 conidia mL−1) on each berry and drying in the air for 1 h. In the second one, boxes were sprayed and immediately after, the pathogen was inoculated into each berry. In the third biotest, the inoculation of the berries with the pathogen was carried out 24 h and 48 h prior to spraying the organic products (to assess the curative effect). Boxes (20 × 10 × 5 cm) were stored at 21 ± 1 °C and 95 to 100% relative humidity (RH) for 5 days. Berries sprayed with sterile water and then inoculated by conidial suspension were used as a control. Four replicates were used for the application of each treatment. Berries were assessed at 5 days dpi. The experiment was performed twice. For all assays, berries were assessed, individually, as healthy or rotten, and disease incidence was calculated as the percentage of rotten berries. The severity of symptoms was ranked according to a scale as follows: 0 = no infection, 1 = tiny spot, 2 = one infected spot, 3 = two to four infected spots, 4 = <50% of the berry surface infected with typical sporulation, and 5 = >50% of the berry surface infected with typical sporulation [59].
2.4. Effect of CH-RF on Wine Grape Berries
The effect of the new molecule on the mycelial growth of B. cinerea was assessed in vitro using PDA amended with different concentrations of the molecule 0.5% (w/v) and 0.7% (w/v). Solutions were prepared as follows, 0.55 g and 0.77 g of powder CH-RF were weighed, and then, added to 100 mL of sterile PDA. The mixtures were homogenized by stirring for 3 h at 60 °C, at 240 rpm using a magnetic stirrer (Thermo Scientific Cimarec™, Waltham, MA, USA). pH was adjusted to 5.5 using 1 M sodium hydroxide. The CH-RF solution was added to 90 mm Petri plates to yield a total volume of 12 mL plate− 1. Ten µL of B. cinerea conidial suspension (1 × 106 conidia mL−1) was placed in the center of each Petri plate. PDA without CH-RF served as the control. Two replicates of three plates were used for each chitosan concentration. The inoculated plates were incubated at 21 ± 1 °C in a light:dark cycle 12:12 h. After incubation for 5 days, the growth of the fungus was determined by measuring mycelial colony diameters.
The effect of CH-RF was assessed on wine grape berries, using the Chardonnay cultivar. Bunches were treated as described above before use. The biotests were carried out by selecting healthy berries with the pedicel intact attached. Treatments were performed as follows: (i) CH-RF 0.7% (w/v) and 2% (w/v), (ii) TIM as NC, and (iii) BOT as BCA. For testing the light effect, berries sprayed with CH-RF were placed under white and blue light for 30 min. Biofungicides were applied according to the dose indicated by manufacturers. Berries were inoculated by adding a 10 µL droplet of B. cinerea conidial suspension (1 × 106 conidia mL−1) and drying in the air for 1 h. Incubation took place in the boxes (20 × 10 × 5 cm) stored at 21 ± 1 °C and 95 to 100% relative humidity (RH) for 7 days. Berries sprayed with sterile water and then inoculated by conidial suspension were used as a control. Four replicates were used for the application of each treatment. Berries were assessed at 5 days dpi as described above. The experiment was performed twice.
2.5. Statistical Analysis
Analysis of variance was performed using JMP Pro 14 (SAS Institute Inc., Cary, NC, USA) for all datasets. Tukey’s test (p = 0.05) was used to analyze further significant differences between treatments. For severity (infection score) analysis, a Kruskal–Wallis Test (p < 0.01) was performed, and significant differences were evaluated using a Dunn’s test.
3. Results
3.1. Anti-Botrytis Effect of Registered BCAs and NCs Products
When evaluating the in vitro growth of B. cinerea on PDA plates, the biofungicides showed inhibitory effect on the mycelial growth in all treatments. Significant differences (p < 0.05) between different products were observed when compared to the control. The treatments with BOT, MAM, and PUV reduced the B. cinerea mycelial growth to 49.2, 50.2, and 51.9 mm, respectively. PRO, TIM, TRI, and TIF also showed mycelial growth values ranging between 77.1 and 66.9 mm compared to 85 mm of the control (Figure 1).
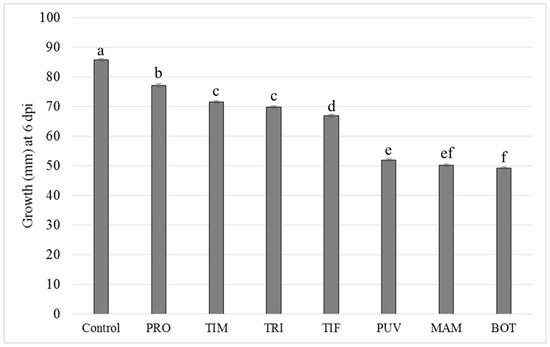
Figure 1.
Mycelial growth (mm) of Botrytis cinerea on PDA media containing biofungicides (Problad PRO, Timorex TIM, Trichonativa TRI, Tifi TIF, Puelche-VTO PUV, Mamull MAM, and Botector BOT). The results were subjected to ANOVA at p < 0.05. Different letters indicate significant differences according to Tukey’s HSD test (p ≤ 0.05). Error bars show the standard error (SE).
Furthermore, different biotests were performed by inoculating B. cinerea on grapevine berries. Biofungicides were applied onto the berries as follows: (i) 48 and 24 h before the inoculum, (ii) inoculum and products application on the same day, and (iii) 24 and 48 h after the inoculum. In all biotests, disease incidence was the lowest when TIM and BOT were sprayed onto the berries except for the 48 h before the inoculum. BOT showed significant differences (p < 0.05) compared to the control and the other biofungicides. For each of the three-treatment strategies, i.e., at 48 and 24 h before inoculation, and inoculation with simultaneous product application, BOT biofungicide resulted in the lowest disease severity: 48.7, 51.2, and 50.0% respectively. On the other hand, in each curative treatment strategy, 24 and 48 h after inoculation, TIM caused the lowest disease incidence with 47.5 and 61.3%, respectively. Overall, PRO, TRI, PUV, MAM, and TIF showed no significant differences compared to the control (Figure 2).
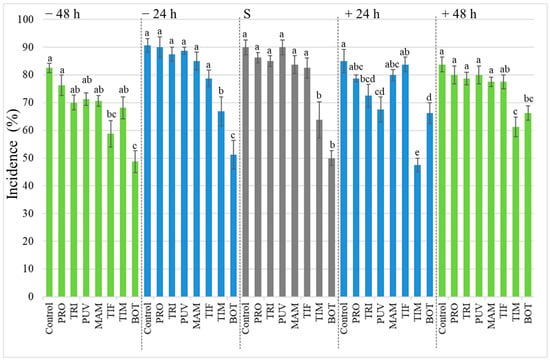
Figure 2.
Botrytis cinerea incidence (%) in wine grapes (cv. Chardonnay) following preventive (−48 h, −24 h, green and blue bars at left), simultaneous application (S, grey bars), and curative (+24 h, +48 h, green and blue bars at right) treatments. The biofungicides (Problad PRO, Trichonativa TRI, Puelche-VTO PUV, Mamull MAM, Tifi TIF, Timorex TIM, and Botector BOT) application was carried out 24 h and 48 h prior to pathogen inoculation and 24 h and 48 h after inoculation. The results were subjected to ANOVA at p = 0.05. Different letters indicate significant differences according to Tukey’s HSD test (p ≤ 0.05). Error bars show the SE.
Disease severity was measured as infection score and analyzed by the Kruskal–Wallis and Dunn’s tests. The two treatments sprayed with BOT and TIM always significantly reduced the disease severity, except for the TIM treatment 48 h before inoculation. Overall, PRO, TRI, PUV, MAM, and TIF were not significantly different from the control; however, TIF was significantly different from the control when sprayed 48 h before the inoculation (Figure 3).
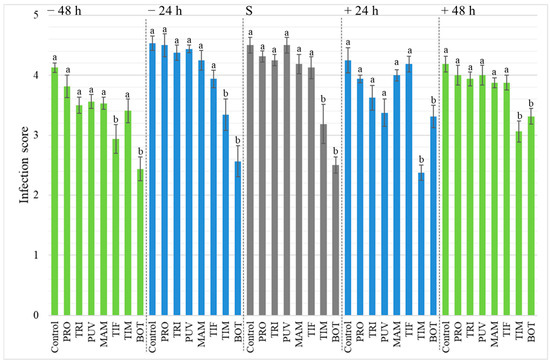
Figure 3.
Botrytis cinerea severity in diseased berries (infection score) in wine grapes cv. Chardonnay following to preventive (−48 h, −24 h, green and blue bars at left), simultaneous application (S, grey bars), and curative (+24 h, +48 h, green and blue bars at right) treatments. The biofungicides (Problad PRO, Trichonativa TRI, Puelche-VTO PUV, Mamull MAM, Tifi TIF, Timorex TIM, and Botector BOT) application was carried out 24 and 48 h prior to pathogen inoculation and 24 and 48 h after inoculation. Infection was scored at 5 dpi from 0 to 5 (see Section 2.5 in Materials and Methods). The results were subjected to a Kruskal–Wallis Test (p < 0.01). Different letters indicate significant differences according to Dunn’s test. Error bars show the SE.
The efficacy of biofungicides, based on the disease incidence, is summarized in Table 2.

Table 2.
Efficacy a of the different biofungicides to control Botrytis cinerea incidence (Problad PRO, Trichonativa TRI, Puelche-VTO PUV, Mamull MAM, Tifi TIF, Timorex TIM, and Botector BOT).
TIM and BOT were found as the most effective biofungicides compared to the other products except for the TIF treatment applied 48 h before the pathogen artificial inoculation.
3.2. Anti-Botrytis Effect of CH-RF
The in vitro effects of two concentrations of CH-RF molecule on the radial growth of B. cinerea are shown in Figure 4.
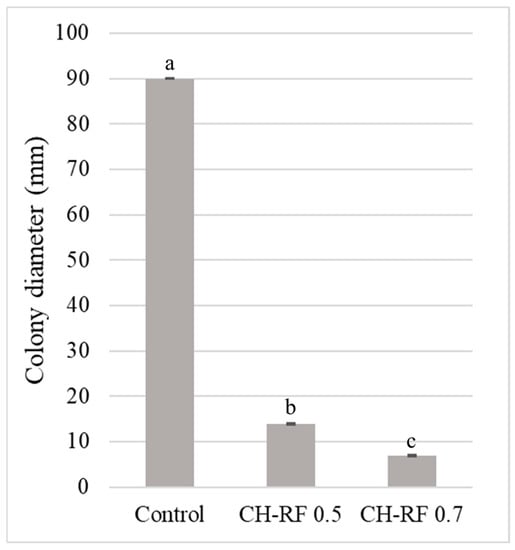
Figure 4.
Mycelial growth (mm) of B. cinerea on PDA media supplemented with 0.5% w/v and 0.7% w/v of Chitosan-Riboflavin molecule (CH-RF) after 5 days post inoculation (dpi). The results were subjected to an ANOVA at p = 0.05. Different letters indicate significant differences according to Tukey’s HSD test (p < 0.05). Error bars show the SE.
Both concentrations tested significantly inhibited B. cinerea growth. The highest colony diameter was observed in the control, while the lowest was obtained with the 0.7% w/v concentration of CH-RF. A total of 0.5% w/v and 0.7% w/v concentrations reduced the pathogen growth 84.5 and 91.4%, respectively.
Results from the in vivo preliminary biotests (in the early stages of biofungicide development) with table grapes (cv. Flame Seedless) are shown in Supplementary Materials (Figure S1). The effect of the new CH-RF molecule on both B. cinerea growth and colonization in the grape berries was compared with the two biofungicides with the highest efficacy, i.e., TIM and BOT, in previous biotests. The disease incidence was significantly reduced by CH-RF, whatever its form at the two concentrations tested, compared with TIM and BOT. Similarly, the application of the CH-RF molecule, whatever the form tested, resulted in a significantly lower infection score, i.e., under 1, compared with the two reference biofungicides, TIM and BOT (Figure 5).
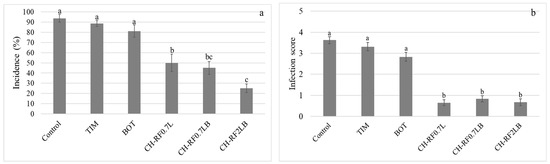
Figure 5.
(a) Incidence (%) and (b) severity (infection score) of Botrytis cinerea in wine grapes cv. Chardonnay following treatment with two organic products (Timorex TIM and Botector BOT), CH-RF at 0.7% w/v concentration (CH-RF0.7L), CH-RF at 0.7% w/v concentration with an adjuvant (CH-RF0.7LB), and CH-RF at 2% w/v concentration with an adjuvant (CH-RF2LB). Incidence was measured at 5 dpi. The results were subjected to ANOVA at p = 0.05. Different letters indicate significant differences according to Tukey’s HSD test (p ≤ 0.05). Error bars show the SE. Infection was scored at 5 dpi from 0 to 5 (see Section 2.5 in Materials and Methods). The results were subjected to a Kruskal–Wallis Test (p < 0.01). Different letters indicate significant differences according to Dunn’s test. Error bars show the SE.
The efficacy of biofungicides tested is compared in Table 3.

Table 3.
Efficacy a of two organic products (Timorex TIM and Botector BOT), and CH-RF molecule at different concentrations b in the control of Botrytis cinerea.
TIM and BOT treatments resulted with the lowest efficacies, 5 and 13%, respectively. In contrast, CH-RF molecule at 2% w/v of concentration showed the highest efficacy, reaching 73%.
4. Discussion
Our first in vitro trials with the Chitosan-Riboflavin (CH-RF) molecule showed a significant reduction in the mycelial growth of the pathogen compared to the control without any fungicide. This was in keeping with similar other positive results reported concerning this new conjugate [35]. By using grapevine berries cv. Chardonnay, obtained directly from the field, and cold stored at 5 °C for just a few weeks, the results were much better with an increased efficiency compared with the biofungicides used as standard controls (BOT and TIM). In addition, we found that higher concentrations of the molecule (CH-RF 2% w/v) effectively reduced disease severity, possibly due to the enhanced effect of CH when it is combined with other organic products, i.e., riboflavin [60]. TIM and BOT low efficacies in these biotests (Table 3) could be due to some factors that can affect the effectiveness of BCAs and NCs, notably nutrient availability, which is increased favoring the pathogen at the surface of overripe grape berries [20].
Chitosan conjugates have been broadly assessed and have showed a good effect controlling fungal diseases including BBR. The protective effect of chitosan against B. cinerea has been reported by many authors on different plant species including grapevines [61]. For example, CH at coating concentrations of 0.5% w/v and 1.0% w/v are sufficient to reduce incidence and severity disease in table grapes (cv. Italia) [31]. In addition, Photodynamic inactivation (PDI) using photosensitizers (PSs) appears to be an innovative strategy to enhance the antifungal effect of chitosan. In this study, we use riboflavin (RF) as PS since this molecule exhibits fluorescence and photosensitivity and can absorb UV light and visible light ranging from 200 to 500 nm. Furthermore, RF is generally considered safe, and poses minimal safety risk when it is used as conjugate with other molecules [62]. In a previous work, Dibona and Fuentealba [35] demonstrated CH-RF conjugate exhibit a high efficacy against Penicillium digitatum, due mainly to the increased and localized ROS generation that inactivates fungal cells, being promissory also for application in citrus. The results obtained herein should be considered preliminary, given that they correspond to biotests carried out in a controlled-conditions environment. The efficiency of PDI can be affected by several variables, including the nature of the microorganisms, characteristics of the photosensitizer (PS), duration of light exposure, and those related to the environment [63]. Therefore, further studies under vineyard conditions should be carried out in the near future.
Some microbial and botanicals’ active ingredients have been registered as biofungicides in Europe, the USA, and worldwide. Nevertheless, biofungicides inconsistency in terms of their efficacy is often claimed to be a strong limiting factor for their implementation in practice [17,47]. For example, field tests using different registered biofungicides with the same anti-Botrytis management strategy have shown significant or not significant results to control BBR according to year and application site [54]. For in vitro and in vivo biotests, we studied six registered products and one in process of registration as a product used for controlling the pathogen. In vitro assays showed a notable variability between biofungicides in the efficacy to control B. cinerea growth. When comparing BCAs- and NCs-based products, the former seems to be more effective. BCAs-based products showed a better performance, possibly due to the nutrition content of the medium. Composition of agar medium may enhance and trigger the modes of action of the BCAs products against the pathogen. BCAs act also by competing with the pathogen for nutrients, in addition to producing antifungal compounds and/or inducing plant resistance [46,64]. On the contrary, NCs products only involve active ingredient(s) from the plant that control the pathogen through antibiosis, i.e., mainly by releasing secondary metabolites with antimicrobial effect and/or inducing or eliciting plant defense mechanisms [65]. For in vivo biotests, a high variability in the efficacy of both BCAs and NCs products to control BBR was noticeable. A low efficacy to control the disease with four BCAs products and one NCs-based product was observed. These BCAs biofungicides are based mainly on Trichoderma spp., a proved BBR control microbial agent [46]. Artificial inoculation allowed us to remove the effects associated with varying inoculum dose and/or timing of the fungal infection, which may influence BBR development and therefore BCAs-based biofungicides efficacy [66,67]. Our findings suggest that cases of successful control of BCA in field conditions reported in literature could be explained by induced systemic resistance, potentially stimulated by the biological control agent (BCA), although we did not investigate this aspect in our study. Interestingly, under our controlled conditions, two biofungicides appeared to be most effective against the disease. BOT, based on BCAs (Aureobasidium pullulans strains), and the TIM product, based on the NC Melaleuca alternifolia oil, showed noticeable stability according to the time of application. BOT showed the highest efficacy when it was applied preventively, i.e., before the pathogen inoculation, but also simultaneously, that is, application at the same time with the pathogen. This may be explained since multiple modes of action of A. pullulans may be active against the pathogen, including competition for nutrients and space, production of cell wall-degrading enzymes, synthesis of antifungal compounds, and mycoparasitism [68]. Our results are consistent with some other published studies demonstrating different efficacies of the BOT biofungicide under different conditions. For example, Fedele et al. [67] demonstrated a significant control of BOT against B. cinerea under controlled conditions, and Galli et al. [69] highlighted a reduction in the incidence of B. cinerea under field conditions. Notably, variations in temperature and relative humidity, which markedly affect the growth of microorganisms such as A. pullulans, may interfere with the control efficacy according to the different experimental environments tested [21]. For example, Rotolo et al. [47] found an efficacy of 11% based on incidence for A. pullulans in field trial using table grapes (cv. Red Globe and Italia), and a study conducted by Calvo-Garrido et al. [54] showed an efficacy based on incidence of 18 and 17% for A. pullulans in a multiple field test using four wine grape varieties.
The fungicidal activity of the essential Tea Tree Oil (TTO) against fungal pathogens is derived from its ability to disrupt the permeability barrier presented by the cellular membrane structures of living organisms [70]. TIM biofungicide has shown inhibitory effects on fungal pathogens including B. cinerea. Li et al. [71] showed that TTO alters mycelial morphology and cellular ultrastructure of B. cinerea causing an inhibitory effect on the pathogen growth. In addition, our findings suggest also, very interestingly, a possible curative effect of TIM when applied 24 h after the pathogen inoculation. Similarly, such a curative effect, rarely shown with biocontrol product and not so often tested, was also observed for controlling another major fungal plant pathogen, i.e., Mycosphaerella fijiensis [72]. Moreover, NC product based on Band of Sweet Lupinus albus has showed a high efficacy to control B. cinerea in strawberries [73], but there is little information about the product’s behavior on grapevines. Our finding could be explained by the dose of the product since Monteiro et al. [73] suggested that the doses of BLAD-oligomer required for fungal inhibition in vitro are higher than those usually required for other products.
Overall, the use of biofungicides (BOTs and NCs) shows promise for the control of B. cinerea in grapes, offering a more sustainable and environmentally friendly alternative to conventional fungicides. The use of these products could be summarized as follows: (i) the product is applied to the grapes either as a preventive measure or after the onset of disease symptoms; (ii) the product interacts with the plant and/or the fungal pathogen, leading to the inhibition of fungal growth or the stimulation of plant defense mechanisms; (iii) the efficacy of the biofungicide is evaluated through various methods [74]; and (iv) the results are analyzed, and the biofungicide’s potential for use is evaluated based on its efficacy, cost-effectiveness, and environmental impact.
5. Conclusions
These evaluations have provided important information about the properties, safety, and potential applications of the chitosan-riboflavin (CH-RF) molecule, helpful for determining its suitability for further development and use. Further investigations of this new molecule, under different conditions of application and under different agronomical and environmental vineyard conditions, are needed to constitute a promising source of knowledge and to set up strategies in the near future to propose this molecule as a new fungicide against BBR. Biofungicides application to control BBR in grapevines depends on several factors: the stage of the grapevine growth, e.g., berries maturity stage, the weather conditions, and the disease pressure. The results show that a high variability exists to control BBR between products commercially available in Chile. Our findings suggest that some microbial biofungicides (BCAs) and natural compounds biofungicides (NCs) represent an alternative in organic viticulture programs against B. cinerea but is very important to know the appropriate moments for its application (phenological states, preventive or curative, etc.).
6. Patents
Patent application PCT/CL2020/050154 “photoactive biofungicide” USA: No. 17/784,797 (13 June 2022). European Patent Office 20899708.0 (11 July 2022). Brazil: No. BR 11 2022 011326 (9 June 2022).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9070746/s1, Figure S1 shows (a) Disease Incidence (%) and (b) Disease Severity (Infection score) caused by B. cinerea in table grapes (cv. Flame Seedless) according to Chitosan-Riboflavin molecule (CH-RF) 0.7% w/v exposed to white led light (CH-RF0.7L), CH-RF 0.7% w/v exposed to natural light (CH-RF 0.7N), CH-RF 0.9% expose to white led light (CH-RF0.9L), and CH-RF 0.9% w/v exposed to natural light (BS0.9N). Infection was scored at 5 dpi from 0 to 5 (see Section 2.5 in Materials and Methods). The results were subjected to a Kruskal-Wallis Test (p < 0.01). Different letters indicate significant differences according to Dunn’s test. Error bars show the SE.
Author Contributions
Conceptualization, H.V.-G., M.H.-D. and M.F.; methodology, H.V.-G., M.F. and M.H.-D.; software, M.H.-D.; validation, H.V.-G., M.H.-D., M.F., D.F., D.S., L.D.-V. and B.L.; formal analysis, H.V.-G., M.H.-D. and M.F.; investigation, M.H.-D., B.I., L.D.-V. and B.J.; resources, H.V.-G. and D.F.; data curation, H.V.-G. and M.H.-D.; writing—original draft preparation, M.H.-D.; writing—review and editing, H.V.-G., M.F. and B.L.; visualization, H.V.-G., M.H.-D. and M.F.; supervision, H.V.-G. and M.F.; project administration, H.V.-G.; funding acquisition, H.V.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Nacional de Investigación y Desarrollo, Project grant number 1191244.
Data Availability Statement
Data will be made available on genuine request.
Conflicts of Interest
Authors declare no conflicts of interest.
References
- Elmer, P.A.G.; Michailides, T.J. Epidemiology of Botrytis cinerea in Orchard and Vine Crops. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Jourdes, M.; Pasquier, G.; Fermaud, M.M.; Gény, L.; Rey, P.P.; Donoche, B.; Teissedre, P.L. Assessment of grey mould (Botrytis cinerea) impact on phenolic and sensory quality of Bordeaux grapes, musts and wines for two consecutive vintages. Aust. J. Grape Wine Res. 2012, 18, 215–226. [Google Scholar] [CrossRef]
- Mundy, D.; Agnew, R.; Wood, P. Grape tendrils as an inoculum source of Botrytis cinerea in vineyards—A review. N. Z. Plant Prot. 2012, 65, 218–227. [Google Scholar] [CrossRef]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Rossi, V. Environmental conditions affect Botrytis cinerea infection of mature grape berries more than the strain or transposon genotype. Phytopathology 2015, 105, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, E.; Caffi, T.; Ciliberti, N.; Rossi, V. A Mechanistic Model of Botrytis cinerea on Grapevines That Includes Weather, Vine Growth Stage, and the Main Infection Pathways. PLoS ONE 2015, 10, e0140444. [Google Scholar] [CrossRef]
- Pañitrur-De La Fuente, C.; Valdés-Gómez, H.; Roudet, J.; Acevedo-Opazo, C.; Verdugo-Vasquez, N.; Araya-Alman, M.; Lolas, M.; Moreno, Y.; Fermaud, M. Classification of winegrape cultivars in Chile and France according to their susceptibility to Botrytis cinerea related to fruit maturity. Aust. J. Grape Wine Res. 2018, 24, 145–157. [Google Scholar] [CrossRef]
- Martínez, F.; Dubos, B.; Fermaud, M. The role of saprotrophy and virulence in the population dynamics of Botrytis cinerea in vineyards. Phytopathology 2005, 95, 692–700. [Google Scholar] [CrossRef]
- Valdés-Gómez, H.; Fermaud, M.; Roudet, J.; Calonnec, A.; Gary, C. Grey mould incidence is reduced on grapevines with lower vegetative and reproductive growth. Crop Prot. 2008, 27, 1174–1186. [Google Scholar] [CrossRef]
- Deytieux-Belleau, C.; Geny, L.; Roudet, J.; Mayet, V.; Donèche, B.; Fermaud, M. Grape berry skin features related to ontogenic resistance to Botrytis cinerea. Eur. J. Plant Pathol. 2009, 125, 551–563. [Google Scholar] [CrossRef]
- Pañitrur-De la Fuente, C.; Valdés-Gómez, H.; Roudet, J.; Verdugo-Vásquez, N.; Mirabal, Y.; Laurie, V.F.; Goutouly, J.-P.; Acevedo-Opazo, C.; Fermaud, M. Vigor thresholded NDVI is a key early risk indicator of Botrytis bunch rot in vineyards. OENO One 2020, 52, 279–297. [Google Scholar] [CrossRef]
- Latorre, B.; Elfar Aedo, K.D.; Ferrada, E.E. Gray mold caused by Botrytis cinerea limits grape production in Chile. Cienc. Investig. Agrar. 2015, 42, 305–330. [Google Scholar] [CrossRef]
- Hill, G.N.; Beresford, R.M.; Evans, K.J. Automated analysis of aggregated datasets to identify climatic predictors of botrytis bunch rot in wine grapes. Phytopathology 2019, 109, 84–95. [Google Scholar] [CrossRef]
- Fermaud, M.; Smits, N.; Merot, A.; Roudet, J.; Thiéry, D.; Wery, J.; Delbac, L. New multipest damage indicator to assess protection strategies in grapevine cropping systems. Aust. J. Grape Wine Res. 2016, 22, 450–461. [Google Scholar] [CrossRef]
- Merot, A.; Fermaud, M.; Gosme, M.; Smits, N. Effect of conversion to organic farming on pest and disease control in French vineyards. Agronomy 2020, 10, 1047. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanisms of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Mundy, D.C. A review of the direct and indirect effects of nitrogen on botrytis bunch rot in wine grapes. N. Z. Plant Prot. 2008, 61, 306–310. [Google Scholar] [CrossRef]
- Würz, D.A.; Rufato, L.; Bogo, A.; Allebrandt, R.; de Bem, B.P.; Marcon Filho, J.L.; Fontanella Brighenti, A.; Bonin, B.F. Effects of leaf removal on grape cluster architecture and control of Botrytis bunch rot in Sauvignon Blanc grapevines in Southern Brazil. Crop Prot. 2020, 131, 105079. [Google Scholar] [CrossRef]
- Haidar, R.; Calvo-Garrido, C.; Roudet, J.; Gautier, T.; Deschamps, A.; Fermaud, M. In vitro and in vivo screening of antagonistic bacterial strains from vineyards to control Botrytis cinerea in grapevine tissues. In Proceedings of the III International Symposium on Postharvest Pathology: Using Science to Increase Food Availability, Bari, Italy, 7–11 June 2015; Volume 1144, pp. 85–92. [Google Scholar] [CrossRef]
- Nicot, P.C.; Stewart, A.; Bardin, M.; Elad, Y. Biological control and biopesticide suppression of Botrytis-incited diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: Cham, Switzerland, 2016; pp. 165–187. [Google Scholar] [CrossRef]
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Wood, P.N.; Hoyte, S.M. Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol. 2010, 59, 882–890. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Sivakumar, D. Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: Eliciting, antimicrobial and film-forming properties. Front. Microbiol. 2018, 9, 2745. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, Z.; Moret, A.; Garcés, S. Assessment of chitosan for inhibition of Colletotrichum sp. on tomatoes and grapes. Crop Prot. 2009, 28, 36–40. [Google Scholar] [CrossRef]
- El-Ghaouth, J.A.; Grenier, J.; Benhamou, N. Suppression of Pythium aphanidermatum and induction of defense reactions. Phytopathology 1994, 84, 313–320. [Google Scholar] [CrossRef]
- Amborabé, B.E.; Bonmort, J.; Fleurat-Lessard, P.; Roblin, G. Early events induced by chitosan on plant cells. J. Exp. Bot. 2008, 59, 2317–2324. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Han, X.; Du, Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2007, 87, 220–228. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Liu, W. Effects of chitin and its derivative chitosan on postharvest decay of fruits: A review. Int. J. Mol. Sci. 2011, 12, 917–934. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Divenere, D.; Salerno, M. Effects of pre-and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Pei, J.; Xue, F.; Cui, X.; Xiong, X.; Li, C. The application of photodynamic inactivation to microorganisms in food. Food Chem. X 2021, 12, 100150. [Google Scholar] [CrossRef]
- Braga, G.Ú.; Silva-Junior, G.J.; Brancini, G.T.; Hallsworth, J.E.; Wainwright, M. Photoantimicrobials in agriculture. J. Photochem. Photobiol. B Biol. 2022, 235, 112548. [Google Scholar] [CrossRef]
- Penha, C.B.; Bonin, E.; da Silva, A.F.; Hioka, N.; Zanqueta, É.B.; Nakamura, T.U.; Mikcha, J.M.G. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT-Food Sci. Technol. 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Dibona-Villanueva, L.; Fuentealba, D. Novel chitosan–riboflavin conjugate with visible light-enhanced antifungal properties against Penicillium digitatum. J. Agric. Food Chem. 2021, 69, 945–954. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural photosensitizers in antimicrobial photodynamic therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Do Prado-Silva, L.; Brancini, G.T.; Braga, G.Ú.; Liao, X.; Ding, T.; Sant’Ana, A.S. Antimicrobial photodynamic treatment (aPDT) as an innovative technology to control spoilage and pathogenic microorganisms in agri-food products: An updated review. Food Control 2022, 132, 108527. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Fitzgerald, F. (Ed.) Photodynamic Therapy (PDT); Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2017. [Google Scholar]
- Böcking, T.; Barrow, K.D.; Netting, A.G.; Chilcott, T.C.; Coster, H.G.; Höfer, M. Effects of singlet oxygen on membrane sterols in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000, 267, 1607–1618. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef]
- Luksiene, Z.; Buchovec, I. Impact of chlorophyllin-chitosan coating and visible light on the microbial contamination, shelf life, nutritional and visual quality of strawberries. Innov. Food Sci. Emerg. Technol. 2019, 52, 463–472. [Google Scholar] [CrossRef]
- Sathiyabama, M. Biopolymeric nanoparticles as a nanocide for crop protection. In Nanoscience for Sustainable Agriculture; Springer: Cham, Switzerland, 2019; pp. 139–152. [Google Scholar] [CrossRef]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Alternatives to synthetic fungicides for Botrytis cinerea management in vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Filinger, S.; Walker, A.S. Chemical Control and Resistance Management of Botrytis Diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 189–216. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Rotolo, C.; De Miccolis Angelini, R.M.; Dongiovanni, C.; Pollastro, S.; Fumarola, G.; Di Carolo, M.; Perrelli, D.; Natale, P.; Faretra, F. Use of biocontrol agents and botanicals in integrated management of Botrytis cinerea in table grape vineyards. Pest Manag. Sci. 2018, 74, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.F.; Lopes, E.A.; Moraes, A.R.F.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Valente, V.M.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, B.N.; Verma, S.; Choudhary, P.; Das, S.; Chakdar, H.; Murugan, K.; Goswami, S.K.; Saxena, A.K. Bioactive antifungal metabolites produced by Streptomyces amritsarensis V31 help to control diverse phytopathogenic fungi. Braz. J. Microbiol. 2021, 52, 1687–1699. [Google Scholar] [CrossRef]
- Pellan, L.; Dieye, C.A.T.; Durand, N.; Fontana, A.; Strub, C.; Schorr-Galindo, S. Biocontrol agents: Toolbox for the screening of weapons against Mycotoxigenic Fusarium. J. Fungi 2021, 7, 446. [Google Scholar] [CrossRef]
- Elad, Y. TRICHODEX: Commercialization of Trichoderma harzianum T39—A case study. In Agro Report, Biopesticides: Trends and Opportunities; PJB Publications Ltd.: Richmond, UK, 2001; pp. 45–50. [Google Scholar]
- Calvo-Garrido, C.; Roudet, J.; Aveline, N.; Davidou, L.; Dupin, S.; Fermaud, M. Microbial antagonism toward Botrytis bunch rot of grapes in multiple field tests using one Bacillus ginsengihumi strain and formulated biological control products. Front. Plant Sci. 2019, 10, 105. [Google Scholar] [CrossRef]
- Calvo, H.; Roudet, J.; Gracia, A.; Venturini, M.; Novello, V.; Fermaud, M. Comparison of efficacy and modes of action of two high-potential biocontrol Bacillus strains and commercial biocontrol products against Botrytis cinerea in table grapes. OENO One 2021, 55, 228–243. [Google Scholar] [CrossRef]
- Droby, S.; Lichter, A. Post-Harvest Botrytis Infection: Etiology, Development and Management. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 349–367. [Google Scholar] [CrossRef]
- Köhl, J.; Scheer, C.; Holb, I.J.; Masny, S.; Molhoek, W. Toward an integrated use of biological control by Cladosporium cladosporioides H39 in apple scab (Venturia inaequalis) management. Plant Dis. 2015, 99, 535–543. [Google Scholar] [CrossRef]
- Silva-Moreno, E.; Brito-Echeverría, J.; López, M.; Ríos, J.; Balic, I.; Campos-Vargas, R.; Polanco, R. Effect of cuticular waxes compounds from table grapes on growth, germination and gene expression in Botrytis cinerea. World J. Microbiol. Biotechnol. 2016, 32, 74. [Google Scholar] [CrossRef]
- Ozkan, R.; Smilanick, J.L.; Karabulut, O.A. Toxicity of ozone gas to conidia of Penicillium digitatum, Penicillium italicum, and Botrytis cinerea and control of gray mold on table grapes. Postharvest Biol. Technol. 2011, 60, 47–51. [Google Scholar] [CrossRef]
- Kanetis, L.; Exarchou, V.; Charalambous, Z.; Goulas, V. Edible coating composed of chitosan and Salvia fruticosa Mill. extract for the control of grey mould of table grapes. J. Sci. Food Agric. 2017, 97, 452–460. [Google Scholar] [CrossRef]
- De Bona, G.S.; Vincenzi, S.; De Marchi, F.; Angelini, E.; Bertazzon, N. Chitosan induces delayed grapevine defense mechanisms and protects grapevine against Botrytis cinerea. J. Plant Dis. Prot. 2021, 128, 715–724. [Google Scholar] [CrossRef]
- Su, L.; Huang, J.; Li, H.; Pan, Y.; Zhu, B.; Zhao, Y.; Liu, H. Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int. J. Biol. Macromol. 2021, 172, 231–240. [Google Scholar] [CrossRef]
- Wang, D.; Kyere, E.; Ahmed Sadiq, F. New Trends in Photodynamic Inactivation (PDI) Combating Biofilms in the Food Industry—A Review. Foods 2021, 10, 2587. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Haidar, R.; Roudet, J.; Gautier, T.; Fermaud, M. Pre-selection in laboratory tests of survival and competition before field screening of antagonistic bacterial strains against Botrytis bunch rot of grapes. Biol. Control 2018, 124, 100–111. [Google Scholar] [CrossRef]
- Das, K.; Tiwari, R.K.S.; Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J. Med. Plant Res. 2010, 4, 104–111. [Google Scholar] [CrossRef]
- Coertze, S.; Holz, G. Surface colonization, penetration, and lesion formation on grapes inoculated fresh or after cold storage with single airborne conidia of Botrytis cinerea. Plant Dis. 1999, 83, 917–924. [Google Scholar] [CrossRef]
- Fedele, G.; Brischetto, C.; Rossi, V. Biocontrol of Botrytis cinerea on grape berries as influenced by temperature and humidity. Front. Plant Sci. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Bozoudi, D.; Tsaltas, D. The Multiple and Versatile Roles of Aureobasidium pullulans in the Vitivinicultural Sector. Fermentation 2018, 4, 85. [Google Scholar] [CrossRef]
- Galli, V.; Romboli, Y.; Barbato, D.; Mari, E.; Venturi, M.; Guerrini, S.; Granchi, L. Indigenous Aureobasidium pullulans strains as biocontrol agents of Botrytis cinerea on grape berries. Sustainability 2021, 13, 9389. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Effects and possible mechanism of tea tree oil against Botrytis cinerea and Penicillium expansum in vitro and in vivo test. Can. J. Microbiol. 2017, 63, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, M.; Sanches, E.; Barbier, M. Curative and suppressive activities of essential tea tree oil against fungal plant pathogens. Agronomy 2020, 10, 609. [Google Scholar] [CrossRef]
- Monteiro, S.; Carreira, A.; Freitas, R.; Pinheiro, A.M.; Ferreira, R.B. A nontoxic polypeptide oligomer with a fungicide potency under agricultural conditions which is equal or greater than that of their chemical counterparts. PLoS ONE 2015, 10, e0122095. [Google Scholar] [CrossRef]
- Altieri, V.; Rossi, V.; Fedele, G. Efficacy of preharvest application of biocontrol agents against gray mold in grapevine. Front. Plant Sci. 2023, 14, 865. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).