Fruit Phenology of Two Hazelnut Cultivars and Incidence of Damage by Halyomorpha halys in Treated and Untreated Hazel Groves
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Location, and Plant Materials
2.2. Brown Marmorated Stink Bugs Occurrence: Monitoring Activities
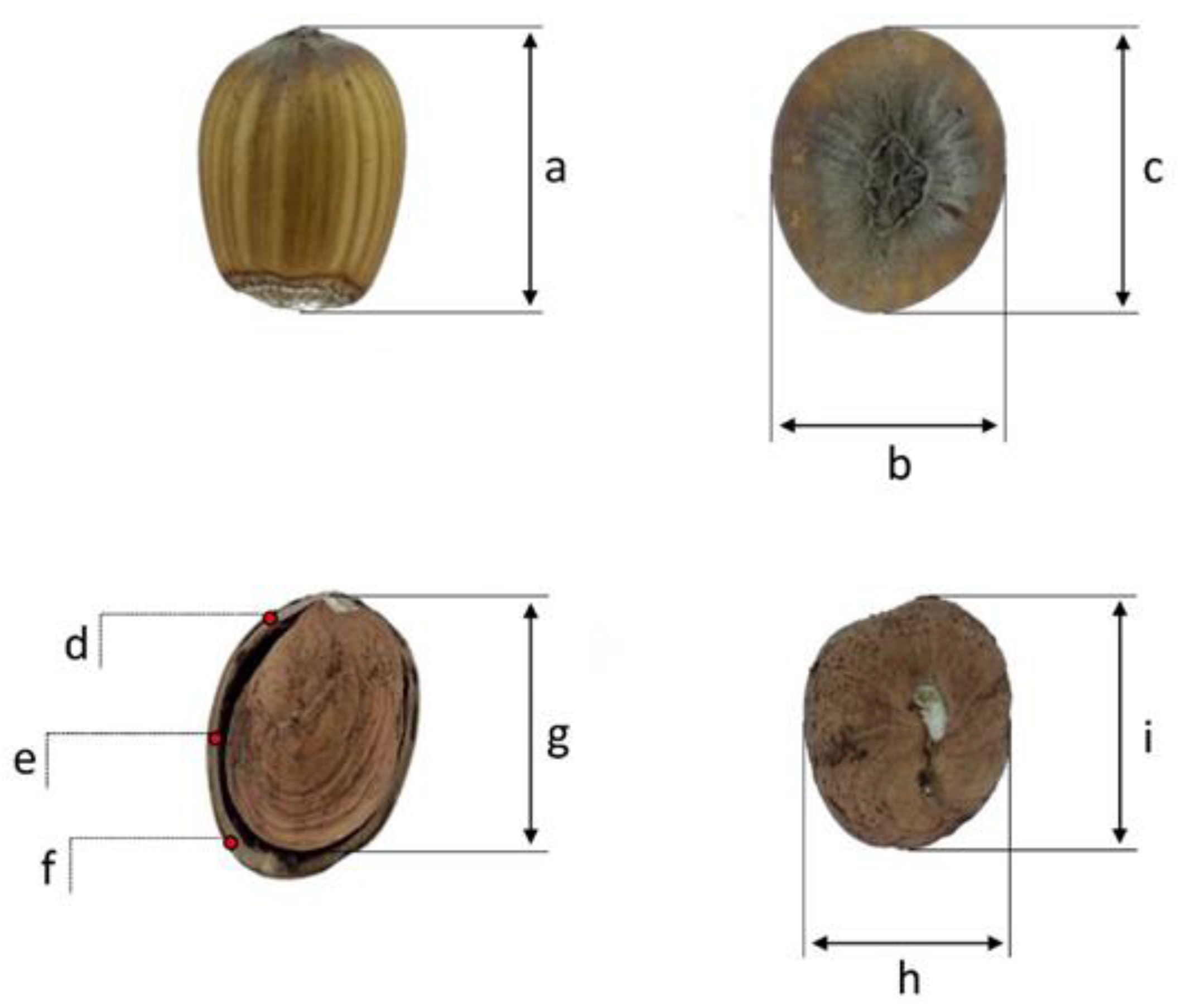
2.3. Fruit Growth and Phenological Classification
2.4. Harvest and Hazelnut Damage Assessment
2.5. Statistical Analysis
3. Results
3.1. Stink Bug Occurrence
3.2. Fruit Growth
3.3. Harvest and Hazelnut Damage Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Climate Change 2007: The Physical Science Basis; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2007; Volume 4, p. 333. [Google Scholar]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A.; Grinnell, J.; Elton, C. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. S2), 19729–19736. [Google Scholar] [CrossRef]
- De Benedetta, F.; Gargiulo, S.; Miele, F.; Figlioli, L.; Innangi, M.; Audisio, P.; Nugnes, F.; Bernardo, U. The spread of Carpophilus truncatus is on the razor’s edge between an outbreak and a pest invasion. Sci. Rep. 2022, 12, 18841. [Google Scholar] [CrossRef]
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef]
- Sweeney, J.; Rassati, D.; Meurisse, N.; Hurley, B.; Duan, J.; Stauffer, C.; Battisti, A. Special issue on invasive pests of forests and urban trees: Pathways, early detection, and management. J. Pest. Sci. 2019, 92, 1–2. [Google Scholar] [CrossRef]
- Gargiulo, S.; Nugnes, F.; de Benedetta, F.; Bernardo, U. Bactrocera latifrons in Europe: The importance of the right attractant for detection. Bull. Insectology 2021, 74, 311–320. [Google Scholar]
- Capinha, C.; Essl, F.; Porto, M.; Seebens, H. The worldwide networks of spread of recorded alien species. Proc. Natl. Acad. Sci. USA 2023, 120, e2201911120. [Google Scholar] [CrossRef]
- Pace, R.; Ascolese, R.; Miele, F.; Russo, E.; Griffo, R.V.; Bernardo, U.; Nugnes, F. The bugs in the bags: The risk associated with the introduction of small quantities of fruit and plants by airline passengers. Insects 2022, 13, 617. [Google Scholar] [CrossRef]
- Encyclopedia Britannica. Available online: https://www.britannica.com/science/Mediterranean-climate (accessed on 23 February 2023).
- Stoeckli, S.; Felber, R.; Haye, T. Current distribution and voltinism of the brown marmorated stink bug, Halyomorpha halys, in Switzerland and its response to climate change using a high-resolution CLIMEX model. Int. J. Biometeorol. 2020, 64, 2019–2032. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Streito, J.C.; Chartois, M.; Pierre, É.; Dusoulier, F.; Armand, J.M.; Gaudin, J.; Rossi, J.P. Citizen science and niche modeling to track and forecast the expansion of the brown marmorated stinkbug Halyomorpha halys (Stål, 1855). Sci. Rep. 2021, 11, 11421. [Google Scholar] [CrossRef]
- Giovannini, L.; Mazza, G.; Chitarra, W.; Sabbatini-Peverieri, G.; Sonnati, C.; Roversi, P.F.; Nerva, L. New insights from the virome of Halyomorpha halys (Stål, 1855). Virus Res. 2022, 316, 198802. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Avila, G.A.; Zhang, F.; Guo, L.F.; Sandanayaka, M.; Mi, Q.Q.; Shi, S.S.; Zhang, J.P. Field cage assessment of feeding damage by Halyomorpha halys on kiwifruit orchards in China. J. Pest. Sci. 2020, 93, 953–963. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 23 February 2023).
- Singldinger, B.; Dunkel, A.; Hofmann, T. The cyclic diarylheptanoid asadanin as the main contributor to the bitter off-taste in hazelnuts (Corylus avellana L.). J. Agric. Food Chem. 2017, 65, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Istat. Available online: https://www.istat.it/ (accessed on 22 February 2023).
- Cianferoni, F.; Graziani, F.; Dioli, P.; Ceccolini, F. Review of the occurrence of Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) in Italy, with an update of its European and world distribution. Biologia 2018, 73, 599–607. [Google Scholar] [CrossRef]
- Regione Campania, Decreto Dirigenziale Numero 28 del 20 March 2019; BURC Regione Campania: Napoli, Italy, 2019.
- Cesari, M.; Maistrello, L.; Ganzerli, F.; Dioli, P.; Rebecchi, L.; Guidetti, R. A pest alien invasion in progress: Potential pathways of origin of the brown marmorated stink bug Halyomorpha halys populations in Italy. J. Pest. Sci. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Maistrello, L.; Vaccari, G.; Caruso, S.; Costi, E.; Bortolini, S.; Macavei, L.; Foca, G.; Ulrici, A.; Bortolotti, P.P.; Nannini, R.; et al. Monitoring of the invasive Halyomorpha halys, a new key pest of fruit orchards in northern Italy. J. Pest. Sci. 2017, 90, 1231–1244. [Google Scholar] [CrossRef]
- Costi, E.; Haye, T.; Maistrello, L. Surveying native egg parasitoids and predators of the invasive Halyomorpha halys in northern Italy. J. Appl. Entomol. 2019, 143, 299–307. [Google Scholar] [CrossRef]
- Hedstrom, C.S.; Shearer, P.W.; Miller, J.C.; Walton, V.M. The effects of kernel feeding by Halyomorpha halys (Hemiptera: Pentatomidae) on commercial hazelnuts. J. Econ. Entomol. 2014, 107, 1858–1865. [Google Scholar] [CrossRef]
- Hamidi, R.; Calvy, M.; Valentie, E.; Driss, L.; Guignet, J.; Thomas, M.; Tavella, L. Symptoms resulting from the feeding of true bugs on growing hazelnuts. Entomol. Exp. Appl. 2022, 170, 477–487. [Google Scholar] [CrossRef]
- Tavella, L.; Arzone, A.; Miaja, M.L.; Sonnati, C. Influence of bug (Heteroptera, Coreidae and Pentatomidae) feeding activity on hazelnut in northwestern Italy. Acta Hortic. 2001, 556, 461–468. [Google Scholar] [CrossRef]
- Singldinger, B.; Dunkel, A.; Bahmann, D.; Bahmann, C.; Kadow, D.; Bisping, B.; Hofmann, T. New taste-active 3-(O-β-d-Glucosyl)-2-oxoindole-3-acetic acids and diarylheptanoids in cimiciato-infected hazelnuts. J. Agric. Food Chem. 2018, 66, 4660–4673. [Google Scholar] [CrossRef] [PubMed]
- Squara, S.; Stilo, F.; Cialiè Rosso, M.; Liberto, E.; Spigolon, N.; Genova, G.; Castello, G.; Bicchi, C.; Cordero, C. Corylus avellana L. Aroma blueprint: Potent odorants signatures in the volatilome of high quality hazelnuts. Front. Plant Sci. 2022, 13, 840028. [Google Scholar] [CrossRef]
- Memoli, A.; Albanese, D.; Esti, M.; Lombardelli, C.; Crescitelli, A.; di Matteo, M.; Benucci, I. Effect of bug damage and mold contamination on fatty acids and sterols of hazelnut Oil. Eur. Food Res. Technol. 2017, 243, 651–658. [Google Scholar] [CrossRef]
- Turan, A. Effect of the damages caused by the green shield bug (Palomena prasina L.) on the qualitative traits of hazelnuts. Grasas Aceites 2021, 72, 1135192. [Google Scholar]
- Viggiani, G. Nematodi, Acari e Insetti Dannosi al Nocciuolo; Annali della Facoltà di Scienze Agrarie dell’Università di Napoli: Napoli, Italy, 1972; p. 6. [Google Scholar]
- Bosco, L.; Moraglio, S.T.; Tavella, L. Halyomorpha halys, a serious threat for hazelnut in newly invaded areas. J. Pest. Sci. 2018, 91, 661–670. [Google Scholar] [CrossRef]
- Pollini, A. Manuale di Entomologia Applicata; Edagricole-Edizioni Agricole: Bologna, Italy, 2013. [Google Scholar]
- Ak, K.; Uluca, M.; Tunçer, C. Distribution and population density of Halyomorpha halys (Stål, 1855) (Hemiptera: Pentatomidae) in Black Sea Region of Türkiye. Turk. J. Zool. 2023, 47, 120–129. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J. Econ. Entomol. 2009, 102, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Hamilton, G.C.; Shearer, P.W. Seasonal phenology and monitoring of the non-native Halyomorpha halys (Hemiptera: Pentatomidae) in soybean. Environ. Entomol. 2011, 40, 231–238. [Google Scholar] [CrossRef]
- Leskey, T.C.; Lee, D.; Short, B.D.; Wright, S.E. Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. J. Econ. Entomol. 2012, 105, 1726–1735. [Google Scholar] [CrossRef]
- Leskey, T.C.; Short, B.D.; Lee, D. Efficacy of insecticide residues on adult Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) mortality and injury in apple and peach orchards. Pest. Manag. Sci. 2014, 70, 1097–1104. [Google Scholar] [CrossRef]
- Leskey, T.C.; Short, B.D.; Butler, B.R.; Wright, S.E. Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål), in mid-Atlantic tree fruit orchards in the United States: Case studies of commercial management. Psyche 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Suckling, D.M.; Levy, M.C.; Roselli, G.; Mazzoni, V.; Ioriatti, C.; Deromedi, M.; Cristofaro, M.; Anfora, G. Live traps for adult brown marmorated stink bugs. Insects 2019, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.; Roselli, G.; Sasso, R.; Cristofaro, M.; Chen, S.; Jermy, M.; Anfora, G. The tunnel trap: Aerodynamic Design Principles for Improved Brown Marmorated Stink Bug Trapping. In Proceedings of the International Congress on Biological Invasions, Christchurch, New Zealand, 1–4 May 2023. [Google Scholar]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.Y.; Aldrich, J.R.; Vermillion, K.E.; Siegler, M.A.; Shirali, S.; Guzman, F.; Leskey, T.C. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Vandervoet, T.F.; Bellamy, D.E.; Anderson, D.; MacLellan, R. Trapping for early detection of the brown marmorated stink bug, Halyomorpha halys, in New Zealand. N. Z. Plant Protect. Soc. 2019, 72, 36–43. [Google Scholar] [CrossRef]
- Wyniger, D.; Kment, P. Key for the separation of Halyomorpha halys (Stål) from similar-appearing pentatomids (Insecta: Heteroptera: Pentatomidae) occurring in Central Europe, with new Swiss records. J. Swiss Entomol. Soc. 2010, 83, 261–270. [Google Scholar]
- UNECE, U.S. DDP-04 Concerning the Marketing and Commercial Quality Control of Hazelnut Kernels; United Nations: New York, NY, USA, 2010. [Google Scholar]
- Silva, A.P.; Ribeiro, R.M.; Santos, A.; Rosa, E. Blank fruits in hazelnut (Corylus avellana L.) Cv. “Butler”: Characterization and influence of climate. J. Hortic. Sci. 1996, 71, 709–720. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- De Benedetta, F.; Pica, F.; Improta, A.; Turrà, D.; Vinale, F.; Giaccone, M.; Lisanti, M.T.; Bernardo, U. Relationship between Halyomorpha halys (Stål) Population Density, Its Damage, and Phenology of Three Hazelnut Cultivars (Corylus avellana L). In Proceedings of the XXVII Congresso Nazionale Italiano di Entomologia (CNIE), Palermo, Italy, 12–16 June 2023. [Google Scholar]
- Ak, K.; Tuncer, C.; Baltaci, A.; Eser, U.; Saruhan, I. Incidence and severity of stink bugs damage on kernels in turkish hazelnut orchards. Acta Hortic. 2018, 1226, 407–411. [Google Scholar] [CrossRef]
- Bergh, J.C.; Morrison III, W.R.; Joseph, S.V.; Leskey, T.C. Characterizing spring emergence of adult Halyomorpha halys using experimental overwintering shelters and commercial pheromone traps. Entomol. Exp. Appl. 2017, 162, 336–345. [Google Scholar] [CrossRef]
- Leskey, T.C.; Agnello, A.; Bergh, J.C.; Dively, G.P.; Hamilton, G.C.; Jentsch, P.; Khrimian, A.; Krawczyk, G.; Kuhar, T.P.; Lee, D.H.; et al. Attraction of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to traps baited with semiochemical stimuli across the United States. J. Econ. Entomol. 2015, 44, 746–756. [Google Scholar] [CrossRef]
- Weber, D.C.; Morrison, W.R., III; Khrimian, A.; Rice, K.B.; Leskey, T.C.; Rodriguez-Saona, C.; Nielsen, A.L.; Blaauw, B.R. Chemical ecology of Halyomorpha halys: Discoveries and applications. J. Pest Sci. 2017, 90, 989–1008. [Google Scholar] [CrossRef]
- Smith, J.R.; Hesler, S.P.; Loeb, G.M. Potential impact of Halyomorpha halys (Hemiptera: Pentatomidae) on grape production in the finger lakes region of New York. J. Entomol. Sci. 2014, 49, 290–303. [Google Scholar] [CrossRef]
- Preti, M.; Montanari, M.; Landi, M.; Cavazza, F.; Franceschelli, F.; Mirossevich, L.; Nannini, R.; Bortolotti, P.P. Screening in open field and laboratory condition of pyrethroids insecticide activity against Halyomorpha halys. In Proceedings of the Emilia-Romagna, Atti, Giornate Fitopatologiche, Chianciano Terme, Italy, 6–9 March 2018; Volume 1, pp. 403–414. [Google Scholar]
- Yao, X.; Min, H.; Lü, Z.; Yuan, H. Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2006, 42, 120–126. [Google Scholar] [CrossRef]
- Al-Rajab, A.J.; Alhababy, A.M.; Alfaifi, T. Persistence of imidacloprid, acetamiprid and methomyl in qat leaves. Hell. Plant Prot. J. 2016, 9, 51–59. [Google Scholar] [CrossRef]


| Date | Commercial Name and Manufacturer | Active Substance | Concentration of a.i. in the Formulate | Quantity of the Formulate/Hectare |
|---|---|---|---|---|
| 2 May 2022 | Kestrel® (Nufarm, Melbourne, Australia) | Acetamiprid | 200 g/L | 0.4 (L/hm2) |
| 6 June 2022 | Sparviero® (Sipcam, Pero, Italy) | Lambda-cyhalothrin | 100 g/L | 0.125 (mL/hm2) |
| 21 July 2022 | Kaimo® Sorbie (Nufarm, Melbourne, Australia) | Lambda-cyhalothrin | 50 g/kg | 0.3 (kg/hm2) |
| Cultivar | Cimiciato Hazelnuts | |
|---|---|---|
| NI | IPM | |
| San Giovanni (SG) | 4 ± 0.45 | 1.9 ± 0.35 |
| Tonda Romana (TR) | 2.3 ± 0.30 | 1.1 ± 0.28 |
| Factors | Cimiciato Incidence | Shriveled Incidence | ||||
|---|---|---|---|---|---|---|
| p | df | F | p | df | F | |
| Cultivar | 0.001 | 1 | 12.813 | ns | 1 | 0.671 |
| Pesticide | <0.001 | 1 | 22.326 | ns | 1 | 0.953 |
| Cultivar vs. Pesticide | ns | 1 | 1.661 | ns | 1 | 0.106 |
| SG–IPM vs. SG–NI | 0.002 | 1 | 13.734 | ns | 1 | 0.600 |
| TR–IPM vs. TR–NI | 0.009 | 1 | 8.640 | ns | 1 | 0.360 |
| SG–IPM vs. TR–IPM | ns | 1 | 3.236 | ns | 1 | 0.600 |
| SG–NI vs. TR–NI | 0.005 | 1 | 9.966 | ns | 1 | 0.360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Benedetta, F.; Giaccone, M.; Pica, F.; Lisanti, M.T.; Vinale, F.; Turrà, D.; Giacca, G.M.; Bernardo, U. Fruit Phenology of Two Hazelnut Cultivars and Incidence of Damage by Halyomorpha halys in Treated and Untreated Hazel Groves. Horticulturae 2023, 9, 727. https://doi.org/10.3390/horticulturae9060727
de Benedetta F, Giaccone M, Pica F, Lisanti MT, Vinale F, Turrà D, Giacca GM, Bernardo U. Fruit Phenology of Two Hazelnut Cultivars and Incidence of Damage by Halyomorpha halys in Treated and Untreated Hazel Groves. Horticulturae. 2023; 9(6):727. https://doi.org/10.3390/horticulturae9060727
Chicago/Turabian Stylede Benedetta, Flavia, Matteo Giaccone, Feliciana Pica, Maria Tiziana Lisanti, Francesco Vinale, David Turrà, Gianpaolo Maria Giacca, and Umberto Bernardo. 2023. "Fruit Phenology of Two Hazelnut Cultivars and Incidence of Damage by Halyomorpha halys in Treated and Untreated Hazel Groves" Horticulturae 9, no. 6: 727. https://doi.org/10.3390/horticulturae9060727
APA Stylede Benedetta, F., Giaccone, M., Pica, F., Lisanti, M. T., Vinale, F., Turrà, D., Giacca, G. M., & Bernardo, U. (2023). Fruit Phenology of Two Hazelnut Cultivars and Incidence of Damage by Halyomorpha halys in Treated and Untreated Hazel Groves. Horticulturae, 9(6), 727. https://doi.org/10.3390/horticulturae9060727








