Effect of Bat Guano and Biochar on Okra Yield and Some Soil Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Trial Layout and Treatments
2.3. Field Plot Management
2.4. Sampling Soils and Plant Tissues and Field Measurements
2.5. Pot Experiment
2.6. Laboratory Analyses
2.7. Data Analysis
3. Results
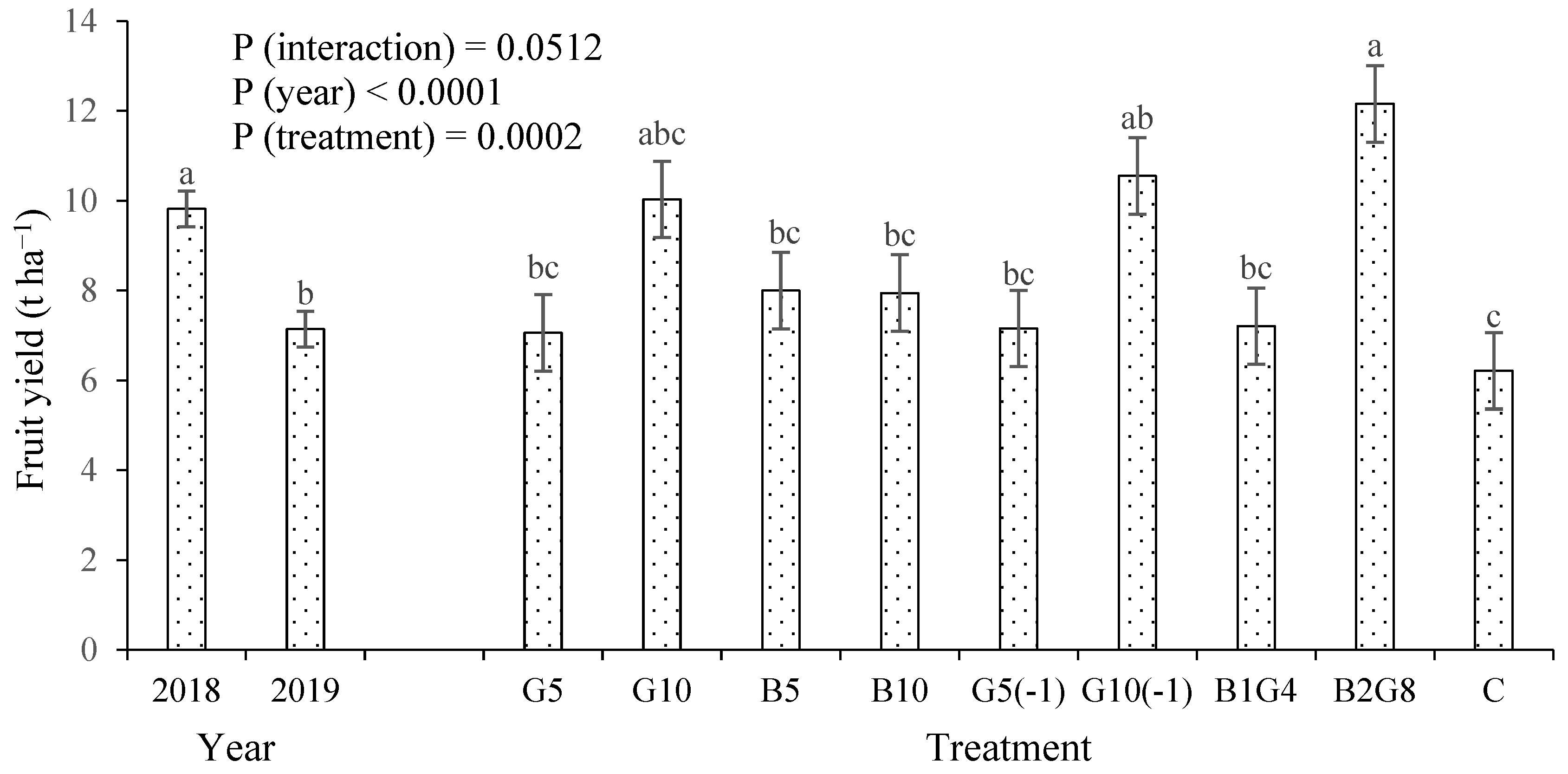
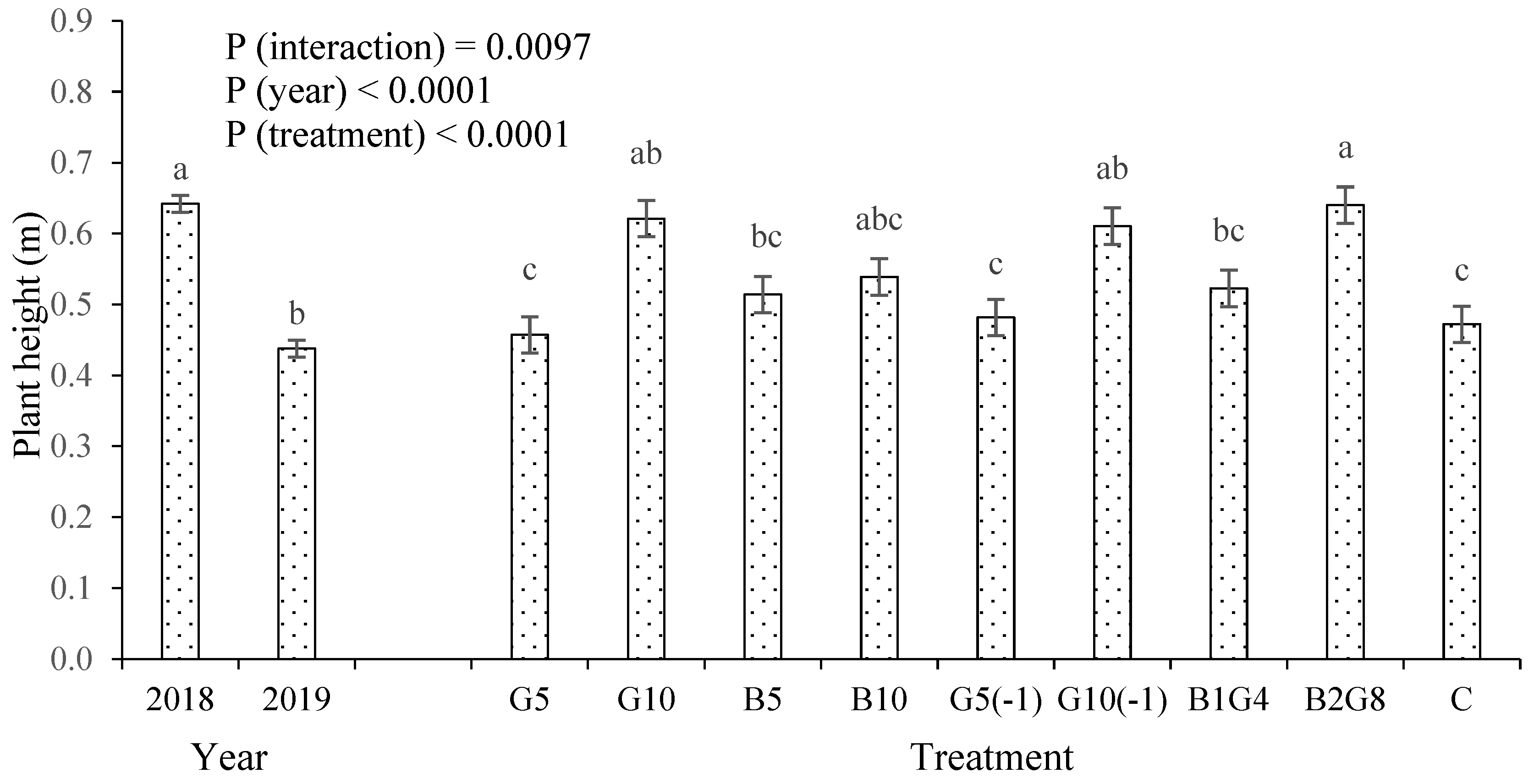
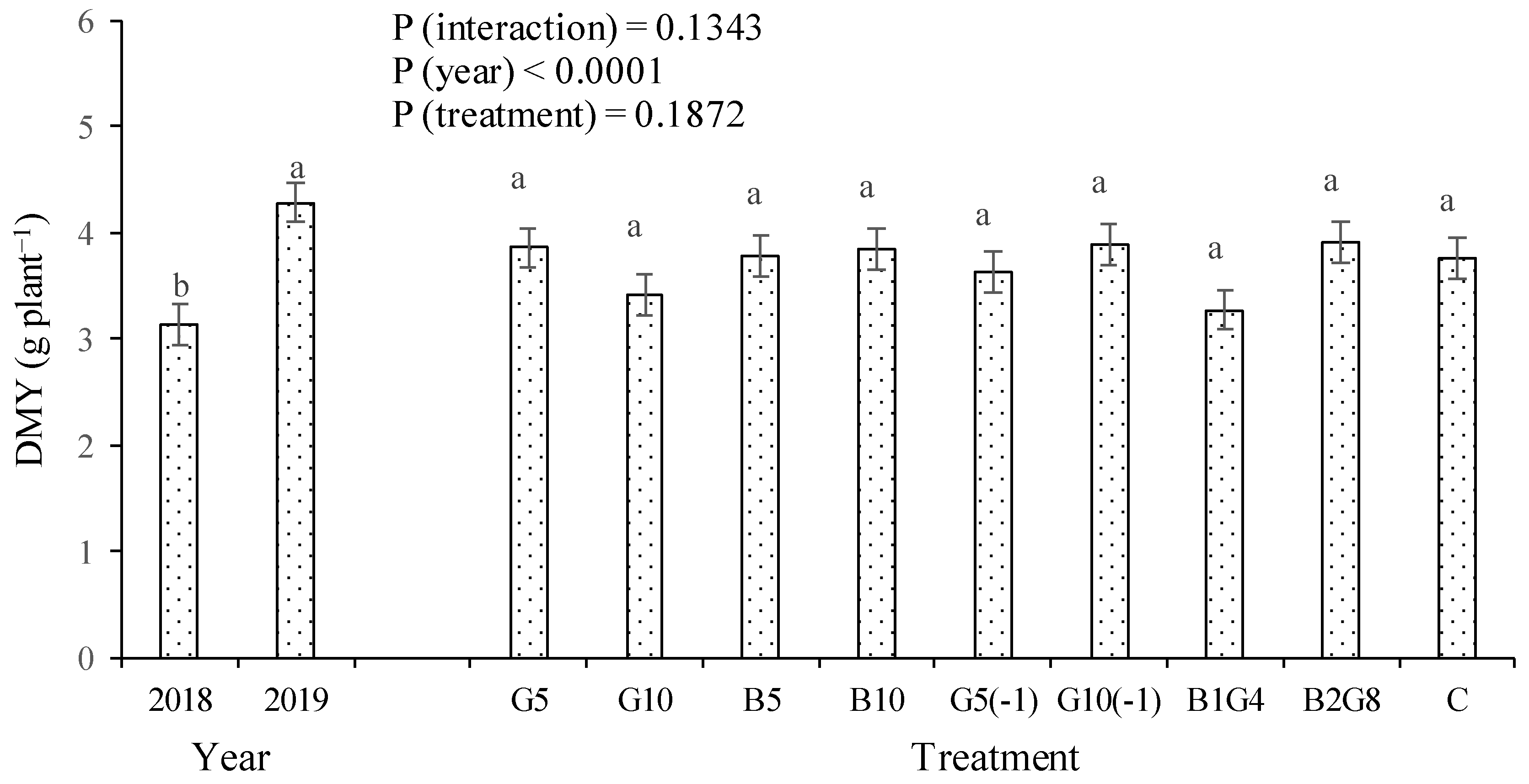
3.1. Okra Fruit Yield and Plant Height
3.2. Plant Nutritional Status and Nutrient Recovery
3.3. Soil Properties
3.4. Growth and Nutrient Uptake by Cabbage Grown in Pots as a Biological Index of Soil Nutrient Availability
4. Discussion
4.1. Guano Increased Crop Growth and Yield, but Biochar Did Not
4.2. Okra Plant Took Up Higher Amounts of Nutrients than Those Released by Guano
4.3. Biochar Increased Total Soil Organic Carbon and Guano Increased the Easily Oxidizable Carbon
4.4. Potted Cabbage Showed a Low Residual Effect for Guano and Nil for Biochar
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT: Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 May 2023).
- Kshash, B.H. Okra grower’s knowledge of cultivation practices: A case study. Int. J. Veg. Sci. 2017, 23, 80–86. [Google Scholar] [CrossRef]
- Al-Shawi, A.A.A.; Hameed, M.F.; Hussein, K.A.; Thawini, H.K. Review on the “biological applications of okra polysaccharides and prospective research”. Future J. Pharm. Sci. 2021, 7, 102. [Google Scholar] [CrossRef]
- Chaemsawang, W.; Prasongchean, W.; Papadopoulos, K.I.; Ritthidej, G.; Sukrong, S.; Wattanaarsakit, P. The effect of okra [Abelmoschus esculentus (L.) Moench] seed extract on human cancer cell lines delivered in its native form and loaded in polymeric micelles. Int. J. Biomater. 2019, 2019, 9404383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, R.; Wang, H.; Chen, J.; Tu, Z. Structural properties, bioactivities, and applications of polysaccharides from okra [Abelmoschus esculentus (L.) Moench]: A review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef] [PubMed]
- Sing, R.; Saurav, S.; Kumar, G.; Kumar, A.; Kumar, D.; Diwakar, D.K.; Kumar, S.; Raj, R.; Kumar, K.; Kumar, A.; et al. A review on anti-diabetic properties of lady’s finger (Abelmoschus esculentus L.) plant. Int. J. Pharm. Life Sci. 2021, 12, 13–16. [Google Scholar]
- Moosavi, S.A.; Aghaalikhani, M.; Ghobadian, B.; Fayyazi, E. Okra: A potential future bioenergy crop in Iran. Renew. Sustain. Energy Rev. 2018, 93, 517–524. [Google Scholar] [CrossRef]
- Aboyeji, C.M.; Dahunsi, S.O.; Olaniyan, D.O.; Dunsin, O.; Adekiya, A.O.; Olayanju, A. Performance and quality attributes of okra [Abelmoschus esculentus (L.) Moench] fruits grown under soil applied Zn-fertilizer, green biomass and poultry manure. Sci. Rep. 2021, 11, 8291. [Google Scholar] [CrossRef]
- Hoffmann, M.P.; Swanepoel, C.M.; Nelson, W.C.D.; Beukes, D.J.; van der Laan, M.; Hargreaves, J.N.G.; Rötter, R.P. Simulating medium-term effects of cropping system diversification on soil fertility and crop productivity in southern Africa. Eur. J. Agron. 2020, 119, 126089. [Google Scholar] [CrossRef]
- Bese, D.; Zwane, E.; Cheteni, P. The use of sustainable agricultural methods amongst smallholder farmers in the Eastern Cape province, South Africa. Afr. J. Sci. Technol. Innov. Dev. 2021, 13, 261–271. [Google Scholar] [CrossRef]
- Krause, A.; Rotter, V.S. Recycling improves soil fertility management in smallholdings in Tanzania. Agriculture 2018, 8, 31. [Google Scholar] [CrossRef]
- Gowing, J.W.; Golicha, D.D.; Sanderson, R.A. Integrated crop-livestock farming offers a solution to soil fertility mining in semi-arid Kenya: Evidence from Marsabit County. Int. J. Agric. Sustain. 2020, 18, 492–504. [Google Scholar] [CrossRef]
- Karagöz, K.; Hanay, A. Effects of bat guano on some yield parameters of wheat. Acad. J. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef]
- Ünal, M.; Can, O.; Can, B.A.; Poyraz, K. The effect of bat guano applied to the soil in different forms and doses on some plant nutrient contents. Commun. Soil Sci. Plant Anal. 2018, 49, 708–716. [Google Scholar] [CrossRef]
- Ojobor, S.A.; Omovie-Stephen, O.F. Influence of formulated palm mill effluent and bat guano mixture on maize performance and soil chemical properties in Delta State, Nigeria. Indian J. Agric. Res. 2022, 56, 28–32. [Google Scholar] [CrossRef]
- Oladele, S.; Adeyemo, A.; Awodun, M.; Ajayi, A.; Fasina, A. Effects of biochar and nitrogen fertilizer on soil physicochemical properties, nitrogen use efficiency and upland rice (oryza sativa) yield grown on an Alfisol in Southwestern Nigeria. Int. J. Recycl. Org. 2019, 8, 295–308. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.M.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and Arbuscular mycorrhizal fungi mediated enhanced drought tolerance in Okra (Abelmoschus esculentus) plant growth, root morphological traits and physiological properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Saliha, B.B.; Kannan, P.; Vellaikumar, S. Effect of biochar composite and organic sources on soil properties and yield of bhendi (Abelmoschus esculentus L.). J. Appl. Nat. Sci. 2021, 13, 1198–1205. [Google Scholar] [CrossRef]
- Ibrahim, I.I. Efficacy of biochar and npk fertilizer on soil properties and yield of okra (Abelmeschus esculentus L.) in Guinea Savanna region of Nigeria. J. Environ. Bioremed. Toxicol. 2022, 5, 6–10. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- MAE. Perfil do Distrito de Vilankulo Província de Inhambane; Ministério da Administração Estatal: Vilankulo, Mozanbique, 2014. [Google Scholar]
- CLIMATE DATA. Dados Climáticos para Vilanculos (1991–2021). Available online: https://pt.climate-data.org/africa/mocambique/inhambane/vilanculos-52395/ (accessed on 15 May 2023).
- WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Wijnhoud, G.D. Os Solos dos Locais dos Ensaios “on Farm” (Mapira, Mexoeira e Feijão Jugo) nos Distritos de Mabote e Vilanculos; INIA: Maputo, Mozambique, 1998. [Google Scholar]
- Chun, Y.; Lee, S.K.; Yoo, H.Y.; Kim, S.W. Recent advancements in biochar production according to feedstock classification, pyrolysis conditions, and applications: A review. BioResources 2021, 16, 6512–6547. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants; Julius Kühn-Institut: Quedlinburg, Germany, 2018. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Technical Paper 9; ISRIC; FAO: Rome, Italy, 2002. [Google Scholar]
- Jones, J.J. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Available Micronutrients (Cu, Fe, Mn, Zn) and Heavy Metals (Ni, Pb, Cd), DTPA Extraction Method; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/3/cc0048en/cc0048en.pdf (accessed on 9 May 2023).
- Temminghoff, E.E.; Houba, V.J. Plant Analysis Procedures, 2nd ed.; Temminghoff, E.E., Houba, V.J., Eds.; Kluwer Academic Publishers: London, UK, 2004. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: In an Introduction to Nutrient Management, 8th ed.; Pearson, Inc.: Chennai, India, 2017. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Global Edition: London, UK, 2017. [Google Scholar]
- Dimande, P.; Arrobas, M.; Rodrigues, M.Â. Under a tropical climate and in sandy soils, bat guano mineralises very quickly, behaving more like a mineral fertiliser than a conventional farmyard manure. Agronomy 2023, 13, 1367. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Zwieten, L.V.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in agriculture—A systematic review of 26 global meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Sheoran, S.; Jhariya, M.K.; Bhatt, R.; Yadav, G.S.; Rao, C.S. Soil organic carbon restoration in India programs, policies and thrust Areas. In Soil Organic Matter and Feeding the Future; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Rodrigues, M.A.; Garmus, T.; Arrobas, M.; Gonçalves, A.; Silva, E.; Rocha, L.; Pinto, L.; Brito, C.; Martins, S.; Vargas, T.; et al. Combined biochar and organic waste have little effect on chemical soil properties and plant growth. Span. J. Soil Sci. 2019, 9, 199–211. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Torres, L.N.D.; Damo, L.; Raimundo, S.; Sartor, L.; Cassol, L.C.; Arrobas, M. Nitrogen use efficiency and crop yield in four successive crops following application of biochar and zeolites. J. Soil Sci. Plant Nutr. 2021, 21, 1053–1065. [Google Scholar] [CrossRef]
- Arrobas, M.; Decker, J.V.; Feix, B.L.; Godoy, W.I.; Casali, C.A.; Correia, C.M.; Rodrigues, M.A. Biochar and zeolites did not improve phosphorus uptake or crop productivity in a field trial performed in an irrigated intensive farming system. Soil Use Manag. 2021, 38, 564–575. [Google Scholar] [CrossRef]
- MacKinnon, D.; Pavlovič, V.; Čeh, B.; Naglič, B.; Pavlovič, M. The impact of weather conditions on alpha-acid content in hop (Humulus lupulus L.) cv. Aurora. Plant Soil Environ. 2020, 66, 519–525. [Google Scholar] [CrossRef]
- Afonso, S.; Dias, M.I.; Ferreira, I.C.F.R.; Arrobas, M.; Cunha, M.; Barros, L.; Rodrigues, M.Â. The phenolic composition of hops (Humulus lupulus L.) was highly influenced by cultivar and year and little by soil liming or foliar spray rich in nutrients or algae. Horticulturae 2022, 8, 385. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar]
- Bryson, G.; Mills, H.A.; Sasseville, D.N.; Jones, J.B., Jr.; Barker, A.V. Plant Analysis Handbook III. A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing Inc.: Athens, GA, USA, 2014. [Google Scholar]
- Bouchet, A.-S.; Laperch, A.; Bissuel-Belaygue, C.; Snowdon, R.; Nesi, N.; Stahl, A. Nitrogen use efficiency in rapeseed. A Review. Agron. Sustain. Dev. 2016, 36, 38. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.A. The effect of nitrogen applications on the growth of young olive trees and nitrogen use efficiency. Turk. J. Agric. For. 2020, 44, 278–289. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Fox, R.H.; Rayner, J.H. Interactions between fertilizer nitrogen and soil nitrogen—The so-called “priming effect”. J. Soil Sci. 1985, 36, 425–444. [Google Scholar] [CrossRef]
- Schnier, H.F. Nitrogen-15 recovery fraction in flooded tropical rice as affected by added nitrogen interaction. Eur. J. Agron. 1994, 3, 161–167. [Google Scholar] [CrossRef]
- Jalpa, L.; Mylavarapu, R.S.; Hochmuth, G.J.; Wright, A.L.; van Santen, E. Apparent recovery and efficiency of nitrogen fertilization in tomato grown on sandy soils. Horttechnology 2020, 30, 204–211. [Google Scholar] [CrossRef]
- Singh, B.P.; Noack, S.R.; Fang, Y.; Mehra, P.; Page, K.; Dang, Y.P. Crop residue management for improving soil carbon storage, nutrient availability and use efficiency. In Soil and Fertilizers: Managing the Environmental Footprint; Lal, R., Ed.; CRC Press: Denver, CO, USA, 2020; pp. 29–66. [Google Scholar]
- Lopes, J.I.; Gonçalves, A.; Brito, C.; Martins, S.; Pinto, L.; Moutinho-Pereira, J.; Raimundo, S.; Arrobas, M.; Rodrigues, M.A.; Correia, C.M. inorganic fertilization at high n rate increased olive yield of a rainfed orchard but reduced soil organic matter in comparison to three organic amendments. Agronomy 2021, 11, 2172. [Google Scholar] [CrossRef]
- Misra, P.; Gautam, N.; Elangovan, V. Bat guano: A rich source of macro and microelements essential for plant growth. Ann. Plant Soil Res. 2019, 21, 82–86. [Google Scholar]
- Audra, P.; Heresanu, V.; Barriquand, L.; Boutchich, E.K.M.; Jaillet, S.; Pons-Branchu, E.; Bosák, P.; Cheng, H.; Edwards, R.L.; Renda, M. Bat guano minerals and mineralization processes in Chameau Cave, Eastern Morocco. Int. J. Speleol. 2021, 50, 91–109. [Google Scholar] [CrossRef]
- Chaney, K. Phosphate fertilizers (Cap 6). In Ullmann’s Agrochemicals 1; Wiley-VCH, Velag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Majumdar, K.; Sanyal, S.K.; Dutta, S.K.; Satyanarayana, T.; Singh, V.K. Nutrient mining: Addressing the challenges to soil resources and food security. In Biofortification of Food Crops; Singh, U., Praharaj, C., Singh, S., Singh, N., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Henao, J.; Baanante, C. Agricultural Production and Soil Nutrient Mining in Africa: Implications for Resource Conservation and Policy Development; IFDC—An International Center for Soil Fertility and Agricultural Development: Muscle Shoals, AL, USA, 2006. [Google Scholar]
- Zavale, H.; Matchaya, G.; Vilissa, D.; Nhemachena, C.; Nhlengethwa, S.; Wilson, D. Dynamics of the fertilizer value chain in Mozambique. Sustainability 2020, 12, 4691. [Google Scholar] [CrossRef]
- Nalivata, P.; Kibunja, C.; Mutegi, J.; Tetteh, F.; Tarfa, B.; Dicko, M.K.; Ouattara, K.; Cyamweshi, R.A.; Nouri, M.K.; Bayu, W.; et al. Integrated soil fertility management in sub-Saharan Africa. In Fertilizer Use Optimization in Sub-Saharan Africa; Wortmann, C.S., Sones, K., Eds.; CABI: Wallingford, UK, 2017; pp. 25–39. [Google Scholar] [CrossRef]
- Gupta, U.C. Boron. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC: Boca Raton, FL, USA, 2007; pp. 241–277. [Google Scholar]
- Lopes, J.I.; Arrobas, M.; Raimundo, S.; Gonçalves, A.; Brito, C.; Martins, S.; Pinto, L.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.A. Photosynthesis, yield, nutrient availability and soil properties after biochar, zeolites or mycorrhizal inoculum application to a mature rainfed olive orchard. Agriculture 2022, 12, 171. [Google Scholar] [CrossRef]
- Godbold, D.L.; Hoosbeek, M.R.; Lukac, M.; Cotrufo, M.F.; Janssens, I.A.; Ceulemans, R.; Polle, A.; Velthorst, E.J.; Scarascia-Mugnozza, G.; De Angelis, P.; et al. Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil 2006, 281, 15–24. [Google Scholar] [CrossRef]
- Silva, E.; Arrobas, M.; Gonçalves, A.; Martins, S.; Raimundo, S.; Pinto, L.; Brito, C.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.A. A controlled-release fertilizer improved soil fertility but not olive tree performance. Nutr. Cycl. Agroecosyst. 2021, 120, 1–15. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Cui, H.J.; Wang, M.K.; Fu, M.L.; Ci, E. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed to ferrihydrite using rice straw-derived biochar. J. Soils Sediments 2011, 11, 1135–1141. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Chen, C.R.; Gray, E.M.; Boyd, S.E.; Hong, Y.; Zhang, D.K. Roles of biochar in improving phosphorus availability in soils; a phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Eichler-Löbermann, B.; Gaj, R.; Schnug, E. Improvement of soil phosphorus availability by green fertilization with catch crops. Commun. Soil Sci. Plant Anal. 2009, 40, 70–81. [Google Scholar] [CrossRef]
- Arrobas, M.; Rodrigues, M.A. Agronomic evaluation of a fertiliser with D-CODER technology, a new mechanism for the slow release of nutrients. J. Agric. Sci. Technol. 2013, 15, 409–419. [Google Scholar]
- Rodrigues, M.Â.; Dimande, P.; Pereira, E.L.; Ferreira, I.Q.; Freitas, S.; Correia, C.M.; Moutinho-Pereira, J.; Arrobas, M. Early-maturing annual legumes: An option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosyst. 2015, 103, 153–166. [Google Scholar] [CrossRef]
| Temperature (°C) | ||||
|---|---|---|---|---|
| Month | Minimum | Average | Maximum | Precipitation (mm) |
| January | 24.3 | 26.9 | 30.0 | 142 |
| February | 24.3 | 26.9 | 30.0 | 151 |
| March | 23.7 | 26.3 | 29.5 | 85 |
| April | 21.6 | 24.6 | 28.1 | 40 |
| May | 19.3 | 22.7 | 26.6 | 20 |
| June | 17.7 | 21.3 | 25.3 | 14 |
| July | 17.1 | 20.6 | 24.6 | 13 |
| August | 18.0 | 21.6 | 25.7 | 8 |
| September | 19.7 | 23.1 | 27.2 | 11 |
| October | 21.0 | 24.3 | 28.3 | 23 |
| November | 22.6 | 25.6 | 29.2 | 67 |
| December | 23.9 | 26.6 | 29.8 | 102 |
| Soil Properties | 2018 | 2019 |
|---|---|---|
| Organic carbon (g kg−1) | 4.2 ± 0.24 | 11.1 ± 1.64 |
| pH (H2O) | 6.6 ± 0.15 | 6.8 ± 0.18 |
| Extract. P (mg P2O5 kg−1) | 41.8 ± 8.56 | 75.1 ± 17.94 |
| Extract. K (mg K2O kg−1) | 87.2 ± 10.07 | 90.4 ± 17.57 |
| Exchang. Ca (cmolc kg−1) | 3.1 ± 0.19 | 5.4 ± 0.56 |
| Exchang. Mg (cmolc kg−1) | 1.0 ± 0.15 | 1.4 ± 0.13 |
| Exchang. K (cmolc kg−1) | 0.3 ± 0.03 | 0.2 ± 0.04 |
| Exchang. Na (cmolc kg−1) | 0.6 ± 0.13 | 0.7 ± 0.11 |
| Exchang. acidity (cmolc kg−1) | 0.1 ± 0.06 | 0.2 ± 0.06 |
| CEC (cmolc kg−1) | 5.1 ± 0.33 | 7.9 ± 0.53 |
| Sand | 89.5 ± 0.87 | 84.6 ± 1.03 |
| Silt | 2.2 ± 0.51 | 6.3 ± 0.91 |
| Clay | 8.2 ± 0.76 | 9.2 ± 0.86 |
| Texture | Loamy-sand | Loamy-sand |
| Properties | Guano | Biochar | ||
|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | |
| Moisture (%) | 9.1 ± 1.50 | 8.0 ± 1.73 | 35.5 ± 3.70 | 33.9 ± 2.71 |
| Organic carbon (g kg−1) | 59.8 ± 2.47 | 57.5 ± 2.87 | 534.5 ± 14.12 | 538.2 ± 16.53 |
| pH (H2O) | 7.5 ± 0.17 | 7.3 ± 0.20 | 9.2 ± 0.24 | 9.3 ± 0.20 |
| Nitrogen (g kg−1) | 3.3 ± 0.40 | 4.2 ± 0.47 | 3.3 ± 0.28 | 5.0 ± 0.35 |
| Phosphorus (g kg−1) | 10.1 ± 1.65 | 8.4 ± 1.01 | 0.8 ± 0.10 | 0.9 ± 0.09 |
| Boron (mg kg−1) | 13.7 ± 2.55 | 15.5 ± 3.59 | 28.5 ± 2.70 | 34.6 ± 3.92 |
| Potassium (g kg−1) | 2.9 ± 0.20 | 3.9 ± 0.67 | 3.6 ± 0.52 | 4.0 ± 0.59 |
| Calcium (g kg−1) | 0.7 ± 0.08 | 0.5 ± 0.06 | 4.3 ± 0.68 | 4.8 ± 0.34 |
| Magnesium (g kg−1) | 0.9 ± 0.06 | 1.1 ± 0.15 | 1.6 ± 0.17 | 1.9 ± 0.24 |
| Iron (mg kg−1) | 28,188.0 ± 2720.97 | 45,606.2 ± 4732.90 | 3637.3 ± 539.37 | 5679.6 ± 316.57 |
| Manganese (mg kg−1) | 168.2 ± 17.59 | 286.3 ± 71.07 | 364.1 ± 34.16 | 388.5 ± 43.65 |
| Zinc (mg kg−1) | 109.7 ± 33.04 | 112.6 ± 19.19 | 27.2 ± 5.06 | 42.1 ± 8.39 |
| Copper (mg kg−1) | 72.8 ± 14.29 | 113.3 ± 13.07 | 72.2 ± 27.81 | 23.6 ± 4.60 |
| Leaf N | Leaf P | Leaf K | Leaf B | |
|---|---|---|---|---|
| g kg−1 | g kg−1 | g kg−1 | mg kg−1 | |
| Year | ||||
| 2018 | 20.8 b | 4.1 a | 15.9 b | 56.9 b |
| 2019 | 30.3 a | 3.3 b | 20.0 a | 65.2 a |
| Treatment | ||||
| G5 | 26.1 a | 3.5 bcd | 16.9 a | 62.5 abc |
| G10 | 26.4 a | 4.2 ab | 19.2 a | 67.5 ab |
| B5 | 24.1 a | 3.4 bcd | 16.8 a | 55.0 bc |
| B10 | 25.4 a | 3.0 d | 16.1 a | 54.1 bc |
| G5(-1) | 25.6 a | 4.1 abc | 18.0 a | 63.7 abc |
| G10(-1) | 26.4 a | 4.6 a | 17.6 a | 70.7 a |
| B1G4 | 25.3 a | 3.4 cd | 20.5 a | 59.2 abc |
| B2G8 | 26.0 a | 3.8 abcd | 17.8 a | 65.0 abc |
| C | 24.6 a | 3.1 d | 18.4 a | 52.2 c |
| 1 LLSR | 25 | 3 | 17 | 20 |
| 2 HLSR | 45 | 6 | 30 | 50 |
| p (interaction) | 0.9998 | 0.0054 | 0.9319 | 0.0742 |
| p (year) | <0.0001 | <0.0001 | 0.0006 | 0.0001 |
| p (treatment) | 0.4831 | <0.0001 | 0.7295 | 0.0005 |
| SE (year) | 0.382 | 0.087 | 0.775 | 1.375 |
| SE (treatment) | 0.810 | 0.184 | 1.645 | 2.917 |
| N Recovery | P Recovery | K Recovery | B Recovery | |
|---|---|---|---|---|
| kg ha−1 | kg ha−1 | kg ha−1 | g ha−1 | |
| Year | ||||
| 2018 | 138.0 b | 34.1 a | 164.5 b | 304.8 a |
| 2019 | 144.1 a | 20.7 b | 184.2 a | 216.9 b |
| Treatment | ||||
| G5 | 139.3 b | 23.8 b | 164.1 c | 242.5 bcd |
| G10 | 160.3 a | 35.1 a | 208.6 a | 320.7 ab |
| B5 | 135.0 b | 24.4 b | 171.4 bc | 237.6 cd |
| B10 | 129.3 b | 22.9 b | 146.9 c | 229.3 d |
| G5(-1) | 130.2 b | 26.0 b | 153.1 c | 258.2 abcd |
| G10(-1) | 158.8 a | 37.6 a | 216.2 a | 329.3 a |
| B1G4 | 133.7 b | 23.0 b | 165.4 c | 231.6 d |
| B2G8 | 158.9 a | 36.5 a | 206.6 ab | 316.7 abc |
| C | 124.0 b | 17.3 b | 137.0 c | 181.6 d |
| p (interaction) | 0.2840 | 0.0105 | 0.1342 | 0.2171 |
| p (year) | 0.0228 | <0.0001 | 0.0007 | <0.0001 |
| p (treatment) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| SE (year) | 1.832 | 0.903 | 3.739 | 8.064 |
| SE (treatment) | 3.886 | 1.916 | 7.932 | 17.107 |
| ANR (%) | APR (%) | AKR (%) | ABR (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| G5 | 146.8 | 45.2 | 19.9 | 10.2 | 298.4 | 83.1 | 136.2 | 51.7 |
| G10 | 112.2 | 101.0 | 18.6 | 23.9 | 294.4 | 182.7 | 127.1 | 84.0 |
| B5 | --- | --- | --- | --- | --- | --- | --- | --- |
| B10 | --- | --- | --- | --- | --- | --- | --- | --- |
| G5(-1) | 42.0 | 32.1 | 28.7 | 11.1 | 216.1 | 21.2 | 195.0 | 44.5 |
| G10(-1) | 88.4 | 111.9 | 24.8 | 23.1 | 347.5 | 185.9 | 159.2 | 68.1 |
| B1G4 | 107.0 | 42.8 | 20.0 | 13.6 | 445.1 | 69.1 | 153.9 | 40.9 |
| B2G8 | 127.3 | 127.0 | 40.2 | 14.3 | 459.3 | 147.9 | 190.9 | 70.2 |
| C | --- | --- | --- | --- | --- | --- | --- | --- |
| TOC | EOC | P (P2O5) | ||
|---|---|---|---|---|
| g kg−1 | g kg−1 | pH(H2O) | mg kg−1 | |
| Year | ||||
| 2018 | 9.2 b | 3.9 b | 6.8 a | 34.7 b |
| 2019 | 13.2 a | 10.5 a | 6.9 a | 64.5 a |
| Treatment | ||||
| G5 | 10.5 bc | 6.5 bc | 6.9 a | 46.0 bcd |
| G10 | 11.6 ab | 8.2 a | 6.9 a | 63.4 ab |
| B5 | 12.1 ab | 6.3 c | 6.9 a | 44.3 bcd |
| B10 | 13.6 a | 7.7 a | 6.9 a | 46.6 bcd |
| G5(-1) | 10.2 bc | 7.5 ab | 6.8 a | 55.9 abc |
| G10(-1) | 10.7 bc | 8.0 a | 6.8 a | 66.7 a |
| B1G4 | 10.4 bc | 6.4 bc | 6.9 a | 38.3 cd |
| B2G8 | 13.0 a | 8.0 a | 6.9 a | 53.3 abc |
| C | 8.7 c | 6.4 c | 6.7 a | 31.9 d |
| p (interaction) | 0.0005 | <0.0001 | 0.8636 | 0.0036 |
| p (year) | <0.0001 | <0.0001 | 0.0893 | <0.0001 |
| p (treatment) | <0.0001 | <0.0001 | 0.8875 | <0.0001 |
| SE (year) | 0.210 | 0.112 | 0.041 | 1.999 |
| SE (treatment) | 0.446 | 0.237 | 0.088 | 4.241 |
| Ca2+ | Mg2+ | K+ | CEC | |
|---|---|---|---|---|
| cmolc kg−1 | cmolc kg−1 | cmolc kg−1 | cmolc kg−1 | |
| Year | ||||
| 2018 | 3.27 b | 0.85 b | 0.31 a | 5.01 b |
| 2019 | 5.07 a | 1.04 a | 0.29 a | 6.94 a |
| Treatment | ||||
| G5 | 3.52 d | 0.91 a | 0.30 a | 5.25 cd |
| G10 | 4.15 bcd | 0.99 a | 0.32 a | 6.12 bc |
| B5 | 4.53 b | 0.87 a | 0.28 a | 6.19 bc |
| B10 | 5.36 a | 0.99 a | 0.32 a | 7.27 a |
| G5(-1) | 3.65 cd | 0.91 a | 0.29 a | 5.36 cd |
| G10(-1) | 4.03 bcd | 1.04 a | 0.27 a | 5.92 bcd |
| B1G4 | 4.34 bc | 0.94 a | 0.29 a | 6.13 bc |
| B2G8 | 4.53 b | 1.00 a | 0.29 a | 6.40 ab |
| C | 3.43 d | 0.86 a | 0.32 a | 5.12 d |
| p (interaction) | <0.0001 | 0.3630 | 0.4843 | 0.0013 |
| p (year) | <0.0001 | <0.0001 | 0.3764 | <0.0001 |
| p (treatment) | <0.0001 | 0.1609 | 0.9579 | <0.0001 |
| SE (year) | 0.079 | 0.023 | 0.015 | 0.097 |
| SE (treatment) | 0.168 | 0.050 | 0.032 | 0.206 |
| Tissue N | Tissue P | Tissue K | Tissue B | |
|---|---|---|---|---|
| g kg−1 | g kg−1 | g kg−1 | mg kg−1 | |
| Year | ||||
| 2018 | 15.6 a | 3.3 a | 28.1 a | 32.4 b |
| 2019 | 11.0 b | 2.2 b | 18.4 b | 35.0 a |
| Treatment | ||||
| G5 | 14.7 a | 3.2 ab | 25.6 a | 34.6 ab |
| G10 | 15.8 a | 3.5 a | 26.0 a | 37.7 a |
| B5 | 13.6 a | 2.8 ab | 24.8 a | 28.7 b |
| B10 | 14.9 a | 2.9 ab | 27.7 a | 31.0 ab |
| G5(-1) | 15.0 a | 3.2 ab | 24.8 a | 35.0 ab |
| G10(-1) | 13.8 a | 3.2 ab | 24.2 a | 35.2 ab |
| B1G4 | 14.9 a | 2.9 ab | 28.7 a | 30.9 ab |
| B2G8 | 14.6 a | 3.3 a | 26.5 a | 33.7 ab |
| C | 14.8 a | 2.7 b | 27.2 a | 28.5 b |
| p (interaction) | 0.8894 | 0.2959 | 0.7914 | 0.5688 |
| p (year) | <0.0001 | <0.0001 | <0.0001 | 0.0302 |
| p (treatment) | 0.8841 | 0.0017 | 0.8276 | 0.0038 |
| SE (year) | 0.366 | 0.058 | 0.994 | 1.184 |
| SE (treatment) | 1.201 | 0.194 | 1.667 | 2.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimande, P.; Arrobas, M.; Rodrigues, M.Â. Effect of Bat Guano and Biochar on Okra Yield and Some Soil Properties. Horticulturae 2023, 9, 728. https://doi.org/10.3390/horticulturae9070728
Dimande P, Arrobas M, Rodrigues MÂ. Effect of Bat Guano and Biochar on Okra Yield and Some Soil Properties. Horticulturae. 2023; 9(7):728. https://doi.org/10.3390/horticulturae9070728
Chicago/Turabian StyleDimande, Paulo, Margarida Arrobas, and Manuel Ângelo Rodrigues. 2023. "Effect of Bat Guano and Biochar on Okra Yield and Some Soil Properties" Horticulturae 9, no. 7: 728. https://doi.org/10.3390/horticulturae9070728
APA StyleDimande, P., Arrobas, M., & Rodrigues, M. Â. (2023). Effect of Bat Guano and Biochar on Okra Yield and Some Soil Properties. Horticulturae, 9(7), 728. https://doi.org/10.3390/horticulturae9070728






