Abstract
We assessed the emergence, growth, phenotypic plasticity and quality of both landrace and commercial tomato fruits under conditions of elevated temperature and increased CO2 concentrations [CO2]. Four growth chambers were used in which temperature and [CO2] differed: LTLC (30 °C, 400 µmol CO2 mol−1), LTHC (30 °C, 1200 µmol CO2 mol−1), HTLC (40 °C, 400 µmol CO2 mol−1) and HTHC (40 °C, 1200 µmol CO2 mol−1). The acronyms indicate the following: LT, low temperature; HT, high temperature; LC, low [CO2]; and HC, high [CO2]. Elevated temperatures significantly affected emergence in both genotypes, with the rate decreasing below 35% at HT compared to over 95% at LT. At HT, seedlings died before producing true leaves. This increase in temperature negatively affected plant growth, though HC produced some compensatory growth promotion. Regarding HT and CO2 interactions, HC failed to counteract the negative impacts of HT. The commercial variety showed a higher relative distance plasticity index (RDPI) under HT, whereas the landrace showed greater plasticity in plant height under HC. The largest fruit sizes were observed at LT, whereas no fruits were found at HTLC. Elevated temperature at HC resulted in enhanced total phenol content and increased antioxidant activity in the fruits.
1. Introduction
Climate change poses a significant challenge to agricultural production, largely due to the global increase in average temperatures, which negatively affects the growth and development of crops [1]. Predictions indicate that over the next century, atmospheric CO2 concentrations [CO2] could surpass 1000 µmol CO2 mol−1, resulting in an average temperature rise of approximately 5 °C [2]. While numerous studies have investigated the separate effects of temperature and [CO2] on plants [1,3], research examining their combined effect remains limited [4].
While higher [CO2] can boost plant growth, the potential temperature rises linked to climate change pose a threat to global food security, which could lead to the extinction of certain plant species, subsequently disrupting food production chains [5]. Therefore, addressing the challenges of high-temperature stress is crucial. Plant response to temperature varies across genotypes and developmental stages [6]. In that respect, Jarvis et al. [5] suggest that species that fail to adapt to changing conditions risk extinction, resulting in genetic erosion. Given this context, research on the effects of high temperatures and increased [CO2] should prioritize the study of landrace genotypes.
Vegetables rank among the categories most susceptible to climate change impacts [7]. Tomatoes (Solanum lycopersicum), known as one of the world’s key horticultural crops [8], have experienced production disruptions due to changing climate patterns [9]. A critical phase in their cultivation is the nursery stage: superior seedling quality can enhance root growth, nutrient absorption, photosynthetic activity and overall crop yield [10]. Yet, studies indicate that temperatures exceeding 34 °C adversely impact germination, whereas those over 32 °C inhibit tomato seedling growth [11]. Conversely, certain studies indicate that elevated [CO2] may enhance seedling resilience to higher temperatures [12]. In this regard, plant growth, phenotypic plasticity and fruit quality parameters are an alternative to evaluate the plant responses to environmental factors. Thus, phenotypic plasticity based on phenotypic distances among individuals of a given species exposed to different environments (relative distance plasticity index, RDPI) allows statistical comparisons between species or populations within species [13].
Therefore, rising temperatures are anticipated to affect the initial growth phases of horticultural crops and alter their phenotypic adaptability, potentially leading to reduced fruit quality [4]. This raises an important question: Could high atmospheric [CO2] maintain the positive effects on growth, phenotypic plasticity and fruit quality in tomato plants grown in high temperatures? Thus, the aim of this study was to determine the emergence, growth, phenotypic plasticity and fruit quality of tomatoes under the combined effect of high temperature and elevated [CO2].
2. Materials and Methods
2.1. Location, Plant Material and Crop Management
This research was conducted in the experimental area of the National Technological of Mexico/Technological Institute of Conkal, Yucatán, Mexico. Fruit analyses were performed at the Center for Research and Assistance in Technology and Design of Jalisco’s Yucatán campus. We used two tomato genotypes: a landrace genotype named ‘C40’ from Campeche, Mexico, which is characterized by an indeterminate growth and the production of kidney-type fruits in a warm–subhumid climate; and a commercially temperate-climate variety, with an indeterminate growth, called ‘Moneymaker’ (MM), which produces ball-type fruits. The nutritional needs of the plants were catered to using the Steiner nutrient solution. Insecticides, acaricides and fungicides were preventively applied.
2.2. Growth Chambers and Treatments
We used four growth chambers for this research, each measuring 3 m in length, 2.5 m in width and 2.2 m in height. These chambers, enclosed and constructed with transparent glass, were placed beneath a greenhouse white plastic roof. Beginning at 06:30 h, small values of photosynthetically photon flux density (PPFD) were detected, which reached 1200 µmol m−2 s−1 by 12:00 h. This value persisted until 15:00 h, after which PPFD decreased until darkness at 18:30 h. Each chamber’s temperature was managed using a 12,000 BTU air conditioner (Split Mirage, model X2, Merida, Mexico), which was modified with an external thermostat situated above the plant canopy. The atmospheric [CO2] was governed by a Telair sensor (T6713) integrated with an Arduino microprocessor (Telaire 7001, St. Marys, PA, USA). This setup activated a solenoid valve attached to a CO2 cylinder hose. A dehumidifier (Hisense, DH50K1W, Mexico City, Mexico) regulated the relative humidity (RH). To ensure uniform air distribution inside the chambers, a 12-inch rotating fan was used. We monitored the chambers’ climatic conditions using a datalogger (HOBO H08-004-02, Onset Computer Corp., Bourne, MA, USA). The chambers were set up with the following conditions: LTLC (control: 30 °C and 400 µmol CO2 mol−1), LTHC (30 °C and 1200 µmol CO2 mol−1), HTLC (40 °C and 400 µmol CO2 mol−1) and HTHC (40 °C and 1200 µmol CO2 mol−1). The acronyms indicate the following: LT, low temperature; HT, high temperature; LC, low [CO2]; and HC, high [CO2]. During nighttime, a temperature of 26 °C and RH of 70% were maintained across all chambers. The control chamber’s temperature and [CO2] were determined by Yucatan’s average diurnal temperature and atmospheric [CO2]. Across all treatments, both temperature and atmospheric [CO2] exhibited a variation of ±3 °C and ±55 µmol CO2 mol−1, respectively.
2.3. Seedling Emergence and Growth Parameters
Seedling emergence was calculated by counting for 14 days from sowing, where the emergence percentage was the total number of germinated seeds at the end of the experiment, divided by the total number of sown seeds, multiplied by 100 [14]. All growth parameters in seedlings were carried out at 35 (seedling stage) days after sowing (DAS) in the same seedlings used in the emergence parameters. To evaluate growth parameters in plants at 120 DAS (fruiting stage), a different batch of seedlings was used, i.e., a batch of seedlings grown in the same conditions (from sowing to 40 DAS) allocated to each treatment. The height, leaf area and total biomass were calculated according to [3].
2.4. Phenotypic Plasticity
Phenotypic plasticity was evaluated in seedlings and plants. The relative distance plasticity index (RDPI) was used to calculate the phenotypic plasticity of tomato plants facing an increase in temperature and [CO2] [13]. The mean of RDPI was calculated with the average of all the variables in the same environmental factor, and the difference in the plasticity index (Δ(30 °C—400 µmol CO2 mol−1)−other treatments) was calculated by increasing LTLC (control) to other treatments (LTHC, HTLC and HTHC) [15]. Values of RDPI ranged from 0 to 1, with a value of 1 indicating the highest phenotypic plasticity. The growth parameters were used in all RDPI calculations.
2.5. Fruit Traits
The diameter and length of the fruits were measured using a caliper, and the weight was measured using a digital balance. No fruits were produced in the treatment with HTLC.
2.6. Extraction and Determination of Phenolic Compounds
Phenolic compounds were extracted from tomato fruits using an ultrasonic device (Model GEX130PB, Sonics & Materials, Newtown, CT, USA) operated at 20 kHz and 130 W. Freeze-dried samples (0.5 g) were combined with 25 mL of 50% ethanol and these mixtures were sonicated for 12.5 min at 80% amplitude using a 13 mm diameter probe. A water bath ensured the temperature remained below 50 °C. Following sonication, the mixtures were centrifuged at 4500 rpm for 20 min at a temperature of 4 °C. The resulting supernatants were filtered and stored under freezing conditions for further analysis [16]. To quantify the total polyphenol content, the Folin and Ciocalteu [17] method was employed. First, 250 µL of Folin–Ciocalteau reagent (1 N) was added to 20 µL of the tomato extract. This mixture was allowed to stand for 8 min, after which 1250 µL of 7.5% Na2CO3 and 480 µL of distilled water was added. These solutions were left to react for 30 min, and their absorbance was measured at 760 nm using a spectrophotometer (Thermo Fisher Scientific, Biomate 3S, Madison, WI, USA). Calibration was carried out with a gallic acid curve ranging from 50 to 600 ppm. The results were expressed in terms of mg of gallic acid equivalents (GAE) per g of the dried sample.
2.7. Determination of Antioxidant Activity by DPPH
The uptake of 2,2-diphenyl-1-picrylhydrazil (DPPH) radicals was determined by analyzing the reduction in DPPH according to [18]; 100 µL of the tomato extract was added to 2900 µL of the 0.1 mM solution of DPPH in methanol. The solution was left to react for 30 min and the measurement was carried out at a wavelength of 517 nm. The antioxidant activity (AA) was expressed as mg Trolox equivalents g−1 db based on a calibration curve of 0.1 to 0.5 mM Trolox, and the percentage inhibition was determined according to the following equation:
where Aa = absorbance of the blank of the DPPH solution, Ab = absorbance of the mixture containing DPPH and the sample, and Ac = absorbance of the blank solution.
2.8. Experimental Design and Statistical Analyses
The experimental design was completely random with a bi-factorial arrangement, air temperature (30 and 40 °C) and [CO2] (400 and 1200 µmol CO2 mol−1). In the evaluation of seed emergence and seedling growth, 100 seeds per species were used as an experimental unit, with four repetitions per treatment. To evaluate growth and phenotypic plasticity, 20 seedlings and 10 plants were used as experimental units. Percentage data were transformed with the arcsine of the square root. A two-way analysis of variance (two-way ANOVA, p ≤ 0.05) was performed for all data. Where significant differences were found, a means comparison test (Tukey, p ≤ 0.05) was conducted. Statistical analyses were conducted with Infostat and plotted with SigmaPlot 11.0.
3. Results
3.1. Seedling Emergence
In both the landrace (C40) and commercial (MM) genotypes, elevated temperature affected the seedling emergence, whereas [CO2] showed no significant effect. At 30 °C, genotype C40 showed seedling emergence three days after sowing (Figure 1A). The MM genotype began its emergence a day later (Figure 1C). Emergence percentages were significantly high: 95% for C40 and 97.5% for MM at 400 µmol CO2 mol−1. Under higher [CO2], these percentages were slightly lower: 87.5% for C40 and 90% for MM (Figure 1B,D). On the other hand, at 40 °C, the C40 genotype emergence started at 4 DAS (Figure 1A). The MM genotype had a delayed emergence, starting at 9 DAS (Figure 1C). However, the emergence percentages were significantly low for all treatments, with all falling below 35% of the total emergence (Figure 1B,D).

Figure 1.
Daily and total emergence of seedlings of landrace (C40) tomato (A,B) and commercial (MM) tomato (C,D), cultivated under different temperatures and [CO2]. LTLC = 30 °C and 400 µmol CO2 mol−1; LTHC = 30 °C and 1200 µmol CO2 mol−1; HTLC = 40 °C and 400 µmol CO2 mol−1; HTHC = 40 °C and 1200 µmol CO2 mol−1. Data are means ± SE; * = statistically significant differences (2-way ANOVA, p ≤ 0.05, n = 200). Different letters indicate statistically significant differences among treatments (Tukey, p ≤ 0.05, n = 100).
3.2. Growth Parameters in Seedling and Plant
Growth parameters in seedlings could only be assessed for the 30 °C treatments due to the low emergence (Figure 1A–D) and high mortality of seedlings in the 40 °C treatments. Analyzing seedling height in both genotypes showed similar trends, but LTHC seedlings showed greater height (5.4 and 5.29 cm at C40 and MM, respectively). Under LTLC, the height was 3.45 cm for C40 and 3.82 cm for MM. Therefore, the elevated [CO2] led to an increase in seedling height by 36.2% for C40 and 27.8% for MM (Figure 2A,B). Both leaf area and seedling biomass showed similar trends to seedling height as [CO2] increased. Under higher [CO2], the leaf area for C40 expanded by 62.5% and by 48% for MM (Figure 2C,D). In parallel, the biomass increased by 80% for C40 and 75% for MM (Figure 2E,F).
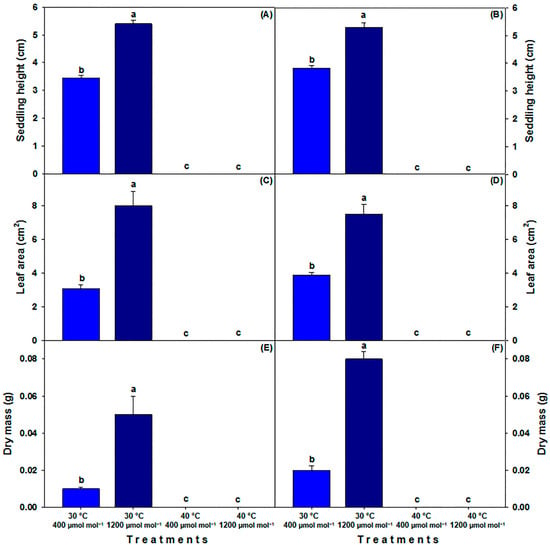
Figure 2.
Height, leaf area and dry mass of seedlings (35 DAS) of landrace (C40) tomato (A,C,E) and commercial (MM) tomato (B,D,F) cultivated under different temperatures and [CO2]. LTLC = 30 °C and 400 µmol CO2 mol−1; LTHC = 30 °C and 1200 µmol CO2 mol−1; HTLC = 40 °C and 400 µmol CO2 mol−1; HTHC = 40 °C and 1200 µmol CO2 mol−1. Data are means ± SE; different letters indicate statistically significant differences among treatments (Tukey, p ≤ 0.05, n = 20).
In adult plants, the height of the C40 genotype significantly increased to 141 cm at 30 °C—1200 µmol CO2 mol−1 treatment, whereas other treatments showed no significant differences (Figure 3A). For MM, distinct height differences were observed between plants at 30 °C—1200 µmol CO2 mol−1 (139 cm) and those at 40 °C—400 µmol CO2 mol−1 (120 cm) (Figure 3B). Regarding leaf area in C40, all treatments showed a significantly larger area ranging from 905 to 1027 cm2 compared to the HTHC treatment, where seedlings had an area of only 495 cm2 (Figure 3C). In the MM genotype, leaf areas at 30 °C with values of 1246 cm2 at LC and 1443 cm2 at HC were significantly greater than those at HT, where the area was 485 cm2 and 748 cm2 for LC and HC, respectively (Figure 3D). A consistent observation across both genotypes was the increased carbon gain in dry biomass under HC. Under LTHC, both C40 (36.5 g) and MM (51.6 g) showed higher values compared to their respective controls (29.5 g for C40 and 33.8 g for MM; Figure 3E,F). For C40, HT did not hinder biomass growth compared to the control. In contrast, MM showed a decline in biomass at HTLC (24.8 g) compared to its control (Figure 3E). Nevertheless, for MM, even under HT, HC helped biomass (36.8 g) remain comparable to its control value (33.8 g) (Figure 3F).
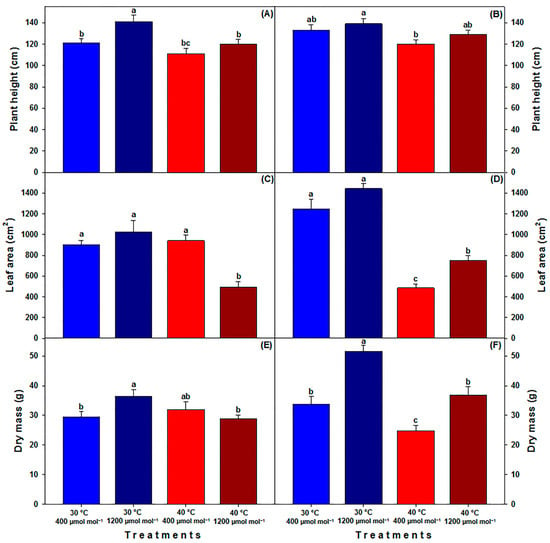
Figure 3.
Height, leaf area and dry mass of adult plants (120 DAS) of landrace (C40) tomato (A,C,E) and commercial (MM) tomato (B,D,F) cultivated under different temperatures and [CO2]. LTLC = 30 °C and 400 µmol CO2 mol−1; LTHC = 30 °C and 1200 µmol CO2 mol−1; HTLC = 40 °C and 400 µmol CO2 mol−1; HTHC = 40 °C and 1200 µmol CO2 mol−1. Data are means ± SE; different letters indicate statistically significant differences among treatments (Tukey, p ≤ 0.05, n = 10).
3.3. Relative Distance Plasticity Index (RDPI)
Values of RDPI were low for plant height (0.04; Figure 4A). No significant differences were found between the genotypes when considering the temperature effect (p ≤ 0.05). However, for both leaf area and biomass, RDPI values in MM (0.43 and 0.15, respectively) were statistically greater than those in C40 (0.04 and 0.03) (Figure 4C,E). Assessing the influence of [CO2] revealed that height RDPI in C40 (0.08) was significantly higher than that in MM (0.03) (Figure 4B). As for the leaf area, HC did not result in any significant differences between the genotypes (Figure 4D). Conversely, for biomass, MM showed a higher RDPI (0.2) than C40 (0.1) (Figure 4F).
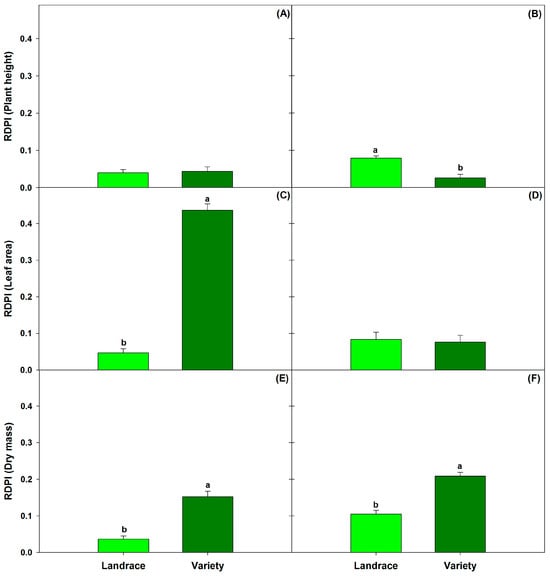
Figure 4.
Relative distance plasticity index (RDPI) of height, leaf area and dry mass per effect of temperature (A,C,E) and [CO2] (B,D,F). Data are means ± SE; different letters indicate statistically significant differences among genotypes (Landrace: C40 and Variety: MM) (Tukey, p ≤ 0.05, n = 10).
Both temperature and [CO2] with the RDPI values in the growth variables were low (<0.14), with no significant statistical differences among treatments (Figure 5A); however, when control values were used to calculate the difference in the plasticity index of growth parameters (the differences between control and the rest of treatments), positive changes were observed in leaf area and height with HC. However, these values became negative with HT. Under HC, certain growth variables showed plasticity, but this adaptability was absent under HT (Figure 5B).
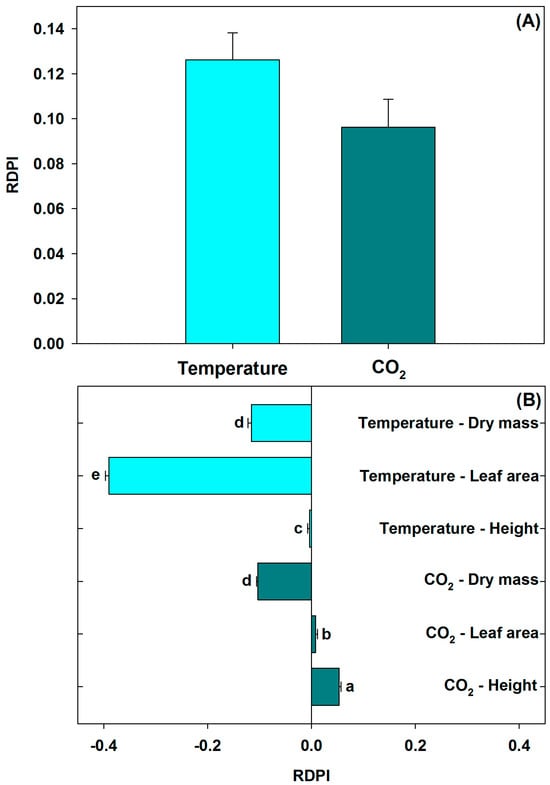
Figure 5.
(A) Relative distance plasticity index (RDPI) of tomato plants in response to temperature and [CO2], and (B) difference in the plasticity index of growth parameters (Δ(30 °C—400 µmol CO2 mol−1) − other treatments). Data are means ± SE. Different letters indicate statistically significant differences (Tukey, p ≤ 0.05, n = 20) among growth parameters by environmental treatment.
3.4. Fruit Quality
Overall, genotype MM produced fruits that were morphologically larger compared to the C40 genotype. Nonetheless, a consistent trend was observed across both genotypes, with fruits from the LT treatments showing statistically greater length, width and weight (p ≤ 0.05) compared to those under the HTHC treatment. It is worth noting that no fruit developed under HTLC, highlighting the adverse impact of elevated temperatures on fruit formation. When [CO2] was increased to 1200 µmol CO2 mol−1 at 30 °C (LTHC), there was no observable change compared to the control (LTLC). Furthermore, fruits from the HTHC treatment did not match the control in terms of quality and size (Figure 6A–F).
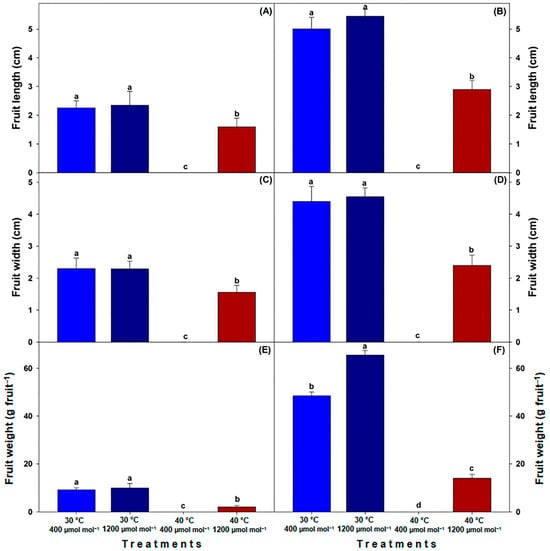
Figure 6.
Length, width and weight of fruits of landrace (C40) tomato (A,C,E) and commercial (MM) tomato (B,D,F) cultivated under different temperatures and [CO2]. LTLC = 30 °C and 400 µmol CO2 mol−1; LTHC = 30 °C and 1200 µmol CO2 mol−1; HTLC = 40 °C and 400 µmol CO2 mol−1; HTHC = 40 °C and 1200 µmol CO2 mol−1. Data are means ± SE; different letters indicate statistically significant differences among treatments (Tukey, p ≤ 0.05, n = 20).
For the total phenol content (TPC) in C40 fruits, no significant differences were found between the two 30 °C treatments (3.8 and 5.1 mg GAE g−1 dw in LC and HC, respectively), but HTHC treatment (6.1 mg GAE/g dw) statistically surpassed both LTLC and LTHC treatments (Figure 7A). For MM fruits, treatment with HTHC resulted in the highest TPC (8.5 mg GAE g−1 dw), which significantly exceeded the other treatments (4.6 and 3.5 mg GAE g−1 dw in LTLC and LTHC, respectively) (Figure 7B). Regarding antioxidant activity (AA), the highest levels were observed in the fruits under the HTHC treatment, yielding 5.8% DPPH inhibition for C40 and 5.5% for MM. The two LT treatments followed closely, ranging from 4% to 4.4% DPPH inhibition (Figure 7A,B).
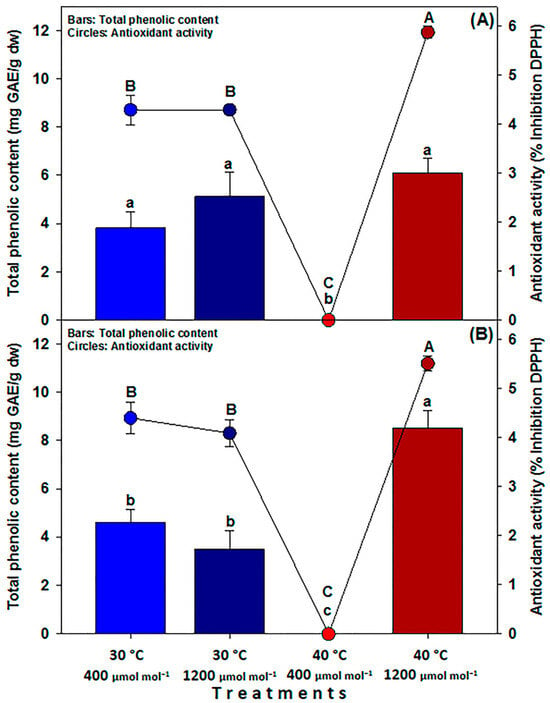
Figure 7.
Total phenolic content (TPC) and antioxidant activity (AA) of fruits of landrace (C40) tomato (A) and commercial (MM) tomato (B) cultivated under different temperatures and [CO2]. LTLC = 30 °C and 400 µmol CO2 mol−1; LTHC = 30 °C and 1200 µmol CO2 mol−1; HTLC = 40 °C and 400 µmol CO2 mol−1; HTHC = 40 °C and 1200 µmol CO2 mol−1. Data are means ± SE; different capital (TPC) and lowercase (AA) letters indicate statistically significant differences among treatments (Tukey, p ≤ 0.05, n = 20).
4. Discussion
In this study, for both tomato seedlings and plants, the increase in growth variables was caused by elevated [CO2]. Rising atmospheric CO2 is known to amplify photosynthetic activity, curb photorespiration and reduce stomatal conductance [19]. For instance, tomato plants grown at 550 µmol CO2 mol−1 were found to be 24% taller than their counterparts at 380 µmol CO2 mol−1 [20]. In seedlings, regardless of CO2 concentration, high temperatures negatively affected biomass across genotypes. In contrast, in the present research, mature plants showed higher resilience to elevated air temperatures, with the sole exception of MM plants under HTLC, which lagged behind the control in terms of biomass. The highest biomass accrual was recorded at LTHC (30 °C—1200 µmol CO2 mol−1).
Elevated temperatures detrimentally impact seed emergence, with temperatures over 35 °C being known to inhibit tomato seed germination [6,11]. In fact, germination can be entirely halted when temperatures climb over 40 °C [21]. This heat stress curtails cell wall elongation and embryonic cell differentiation, potentially due to protein degradation [22], which in turn suppresses protein synthesis pivotal for germination [23]. While elevated CO2 levels were not found to significantly influence germination, which is consistent with prior research [24,25], responses to CO2 can differ across species [26] and even genotypes [27]. The benefits of higher CO2 levels become evident when the first true leaves become photosynthetically functional, but not during germination [4].
In a high-temperature environment (HT), seedlings died out a few days after emergence. While the seedlings continued their growth for some days relying on cotyledons, they perished before sprouting their first true leaves. However, seedlings transferred to 40 °C chambers after reaching 35 DAS (displaying at least five pairs of true leaves) persisted in their growth. The transition from cotyledons to true leaves emerged as a vulnerable juncture for tomato seedlings. Exposing plants to temperatures exceeding their optimal levels can result in halted growth, damage or even death [28]. Increased CO2 typically results in larger leaf areas in both seedlings and mature plants [29]. Plant height is a growth parameter not affected by elevated temperature [4]; however, plant height and other growth parameters (leaf area and biomass) were affected under high [CO2]. Notably, the C40 genotype, native to a warm region and presumably adapted to such climates, produced smaller leaves than MM, a strategy used to curtail water loss [30]. In a study with tomato plants, the effect of high temperature was different among varieties, but treatments with elevated CO2 increased the biomass in all genotypes [31]. In this regard, high-temperature stress decreased nutrient absorption, as well as absorption levels and assimilation of proteins in tomatoes [32,33]. Past research indicated that while separate temperature and CO2 effects on nutrient assimilation can vary, their combined effects are likely to show the positives of increased CO2 overshadowed by the negatives of elevated temperatures [34].
Phenotypic plasticity refers to a genotype’s ability to display varied phenotypes across different environments. One way to assess this is via RDPI. In this study, RDPI values diverged based on genotype; specifically, the commercial variety MM showed high RDPI as temperature increased, whereas the landrace genotype C40 showed notable plasticity only in plant height with increased [CO2]. When these values were compared to a control treatment, growth parameters displayed less plasticity under elevated temperatures, but showed slight plasticity increases in leaf area and height under elevated CO2 conditions; genotype MM showed more pronounced phenotypic plasticity than C40. However, only in certain characteristics did high CO2 treatments surpass the control. Studies, such as one on a multiparent tomato population, underscore the utility of phenotypic plasticity investigations in discerning plant adaptability mechanisms to diverse stress conditions, thereby aiding in the selection of top-performing varieties [35]. Our findings suggest that the greater plasticity in MM may result from prolonged selective breeding, which has anchored genes essential for diverse environments. Some theories propose that plasticity stems from allele expression variations contingent on the environment, inferring separate genetic controls for a trait’s average phenotype and its plasticity [36,37]. To delve deeper into the genetic underpinnings of phenotypic plasticity, a study encompassing two Solanum species in varied environments was undertaken [38]. It was found that over 7000 genes altered their expression patterns in response to environmental changes. This means growth conditions can influence a genotype’s transcriptome, adjusting splice variant numbers based on the environment [38]. Using phenotypic plasticity as a benchmark can effectively pinpoint genotypes with robust growth potential within the genotype-environment interplay [35].
Regarding fruit production, MM generally bore larger fruits than C40. Nevertheless, both genotypes showed similar patterns. Optimal fruit values were recorded at 30 °C treatments, whereas the HTLC treatment bore no fruits. Moreover, fruits from the HC treatment did not outperform control conditions. A parallel study on two Capsicum species subjected to analogous temperature and CO2 conditions echoed these findings, noting the absence of fruit production under high temperatures and enriched CO2. It was inferred that temperatures of 40 °C escalated flower abortion, negatively impacting fruit yield [4]. Another tomato study highlighted that elevated CO2 levels augmented fruit quantity and weight per plant [39]. Typically, high CO2 bolsters plant biomass growth due to increased carbon availability for photosynthesis. However, when temperatures soar, the positive impacts of CO2 might be overshadowed by the detrimental effects of elevated temperatures on the plant [3].
Both total phenolic content (TPC) and antioxidant activity (AA) showed similar patterns. While CO2 levels did not significantly alter these values, high temperatures (HTHC) did elevate both TPC and AA. One study examining the combination of shade netting and low-cost greenhouse technology found that high temperatures raised TPC values. This increase was attributed to elevated PPFD [40]. However, in our study, PPFD remained consistent across all experimental conditions, suggesting that changes in TPC and AA primarily result from temperature variations. Notably, under high temperatures (40 °C), we only assessed fruits exposed to increased CO2 due to the absence of fruits in control seedlings. Another tomato study found that fruit quality was more influenced by temperature than PPFD [41]. Similarly, a research project evaluating a variety of tomato genotypes concluded that areas with warmer temperatures yielded better-quality fruits with higher TPC values. They proposed that this might be a plant’s adaptive response to thermal stress [42]. For other vegetables cultivated in tropical climates, even if photosynthetic enzymes do not always contribute to biomass, they might still support alternative metabolic pathways crucial for species survival [3]. This could be the scenario with TPC and AA. Under thermal stress or high radiation, plants may produce and allocate phenols—ranging from simple to complex compounds—to safeguard their tissues. Many of these phenols can also show antioxidant properties [18].
5. Conclusions
An increase in air temperature to 40 °C adversely affected emergence, seedling growth, adult plant development, phenotypic plasticity and fruit size. However, it did enhance total phenols and antioxidant activity. Notably, when the temperature was raised to 40 °C while keeping atmospheric [CO2] at 400 µmol CO2 mol−1, fruits were absent. Conversely, an elevated [CO2] of 1200 µmol CO2 mol−1 at 30 °C facilitated growth. Yet, the advantages of this elevated CO2 level could not offset the detrimental impacts of the 40 °C temperature. These negative outcomes are particularly evident in seedling metrics and fruit quality, suggesting that these stages might be especially vulnerable in anticipated climate change scenarios. Our findings offer valuable insights for predicting tomato plant responses in comparable environmental contexts. However, to obtain a comprehensive understanding of the plant, it is essential to consider other factors such as gas exchange, leaf photochemistry and proteomics. This would provide a more complete picture in anticipation of imminent climate change.
Author Contributions
Conceptualization, R.G., W.T., R.H.A.-N. and C.D.-l.-P.; methodology, M.O.-R., N.P., J.C.C.-B. and R.G.; software, M.O.-R. and J.F.P.; validation, R.G., W.T., R.H.A.-N. and C.D.-l.-P.; formal analysis, N.P., J.C.C.-B. and R.G.; investigation, M.O.-R.; resources, M.O.-R., N.P. and R.G.; data curation, M.O.-R. and J.F.P.; writing—original draft preparation, M.O.-R.; writing—review and editing, M.O.-R., J.F.P. and R.G.; visualization, W.T., R.H.A.-N., C.D.-l.-P., N.P. and J.C.C.-B.; supervision, R.G.; project administration, R.G.; funding acquisition, R.G. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Humanities, Science and Technology (CONAHCyT, Mexico), project 286756.
Data Availability Statement
The data presented in this study are available upon request from the correspondence author.
Acknowledgments
We thank the National Council of Humanities, Science and Technology (CONAHCyT, Mexico) for the financial support granted to Project 286756 “Elucidation of the role of protein D1 in the photosynthesis of tropical vegetables in climate change scenarios” and for the postgraduate scholarship to the first author. Likewise, thanks to Geovanny Ayora, Oscar Palma and Roberth Us Santamaria for programming the sensors and for the technical assistance provided in the growth chambers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Z.; Shimizu, H.; Yagasaki, Y.; Ito, S.; Zheng, Y.; Zhou, G. Interactive effects of elevated CO2, drought, and warming on plants. J. Plant Growth Regul. 2013, 32, 692–707. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/ (accessed on 12 May 2023).
- Garruña-Hernández, R.; Orellana, R.; Larque-Saavedra, A.; Canto, A. Understanding the physiological responses of a tropical crop (Capsicum Chinense Jacq.) at high temperature. PLoS ONE 2014, 9, e111402. [Google Scholar] [CrossRef]
- Pereyda-González, J.M.; De-la-Peña, C.; Tezara, W.; Zamora-Bustillos, R.; Andueza-Noh, R.H.; Noh-Kú, J.G.; Carrera-Marín, M.; Garruña, R. High temperature and elevated CO2 modify phenology and growth in pepper plants. Agronomy 2022, 12, 1836. [Google Scholar] [CrossRef]
- Jarvis, A.; Lane, A.; Hijmans, R.J. The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ. 2008, 126, 13–23. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Garruña-Hernández, R.; Pereyda-González, J.; Oliva-Ruíz, M.; Castillo-Collí, M.; Ríos-Bolívar, F.; Cetina-Escalante, R. Hortalizas tropicales: Súper plantas ante el cambio climático. Bioagrociencias 2021, 14, 46–55. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.Q.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z.H. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest. Sci. (2004) 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Atif, M.J.; Jellani, G.; Malik, M.H.A.; Saleem, N.; Ullah, H.; Khan, M.Z.; Ikram, S. Different growth media effect the germination and growth of tomato seedlings. Sci. Technol. Dev. 2016, 35, 123–127. [Google Scholar] [CrossRef]
- van Dam, B.; Goffau, M.; van Lidt de Jeude, J.; Naika, S. Cultivation of Tomato: Production, Processing and Marketing, 4th ed.; Agromisa/CTA: Wageningen, The Netherlands, 2005; pp. 6–84. [Google Scholar]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Hernández-Pinto, C.; Garruña, R.; Andueza-Noh, R.; Hernández-Núñez, E.; Zavala-León, M.J.; Pérez-Gutiérrez, A. Almacenamiento postcosecha de frutos: Alternativa para mejorar la calidad fisiológica de semillas de chile habanero. Biociencias 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Valladares, F.; Chico, J.; Aranda, I.; Balaguer, L.; Dizengremel, P.; Manrique, E.; Dreyer, E. The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 2002, 16, 395–403. [Google Scholar] [CrossRef]
- Covarrubias-Cárdenas, A.G.; Martínez-Castillo, J.I.; Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; García-Cruz, N.U.; Pacheco, N. Antioxidant capacity and Uplc-Pda Esi-Ms phenolic profile of Stevia rebaudiana dry powder extracts obtained by ultrasound assisted extraction. Agronomy 2018, 8, 170. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Vázquez-Flota, F.; Miranda-Ham, M.d.L.; Monforte-González, M.; Gutiérrez-Carbajal, G.; Velázquez-García, C.; Nieto-Pelayo, Y. La biosíntesis de capsaicinoides, el principio picante del chile. Rev. Fitotec. Mex. 2007, 30, 353–360. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 Effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. Proc. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Mamatha, H.; Srinivasa Rao, N.K.; Laxman, R.H.; Shivashankara, K.S.; Bhatt, R.M.; Pavithra, K.C. Impact of elevated CO2 on growth, physiology, yield, and quality of tomato (Lycopersicon Esculentum Mill) cv. Arka Ashish. Photosynthetica 2014, 52, 519–528. [Google Scholar] [CrossRef]
- Vallejo-Cabrera, F.A.; Estrada-Salazar, E.I. Producción de Hortalizas de Clima Cálido, 1st ed.; Universidad Nacional de Colombia: Cali, Colombia, 2004; pp. 51–63. [Google Scholar]
- Vierling, E. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Blum, A.; Klueva, N.; Nguyen, H.T. Wheat cellular thermotolerance is related to yield under heat stress. Euphytica 2001, 117, 117–123. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Seed composition, seedling emergence and earl seedling vigour of red kidne bean seed produced at elevated temperature and carbon dioxide. J. Agron. Crop. Sci. 2009, 195, 148–156. [Google Scholar] [CrossRef]
- Way, D.A.; Ladeau, S.L.; McCarthy, H.R.; Clark, J.S.; Oren, R.; Finzi, A.C.; Jackson, R.B. Greater seed production in elevated CO2 is not accompanied by reduced seed quality in Pinus taeda L. Glob. Chang. Biol. 2010, 16, 1046–1056. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A. The Influence of elevated CO2 and temperature on seed germination and emergence from soil. Field Crop. Res. 1993, 34, 147–157. [Google Scholar] [CrossRef]
- Andalo, C.; Godelle, B.; Lefranc, M.; Mousseau, M.; Till-Bottraud, I. Elevated CO2 decreases seed germination in Arabidopsis thaliana. Glob. Chang. Biol. 1996, 2, 129–135. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating breeding for heat tolerance in tomato (Solanum lycopersicum L.): An integrated approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Delgado, V.; Magdaleno, J.; Ayala, O.; Garfias, D. Calidad de semillas de tres variedades nativas y una comercial de tomate producidas bajo temperaturas altas. Rev. Chapingo Ser. Hortic. 2018, 24, 215–227. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Wen, J.; Jensen, N.B.; dos Santos, T.M.; Wu, Z.; Rosenqvist, E.; Ottosen, C.-O. Interactive effects of elevated CO2 concentration and combined heat and drought stress on tomato photosynthesis. BMC Plant Biol. 2020, 20, 260. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient-uptake and-assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Heckathorn, S.A.; Giri, A.; Mishra, S.; Bista, D. Heat stress and roots. Clim. Chang. Plant Abiotic Stress Toler. 2013, 6, 109–136. [Google Scholar] [CrossRef]
- Jayawardena, D.M.; Heckathorn, S.A.; Bista, D.R.; Mishra, S.; Boldt, J.K.; Krause, C.R. Elevated CO2 plus chronic warming reduce nitrogen uptake and levels or activities of nitrogen-uptake and -assimilatory proteins in tomato roots. Physiol. Plant 2017, 159, 354–365. [Google Scholar] [CrossRef]
- Diouf, I.; Derivot, L.; Koussevitzky, S.; Carretero, Y.; Bitton, F.; Moreau, L.; Causse, M. Genetic basis of phenotypic plasticity and genotype x environment interaction in a multi-parental tomato population. J. Exp. Bot. 2020, 71, 5365–5376. [Google Scholar] [CrossRef]
- Scheiner, S.M. Genetics and Evolution of Phenotypic Plasticity. Annu. Rev. Ecol. Syst. 1993, 24, 35–68. [Google Scholar] [CrossRef]
- Via, S.; Gomulkiewicz, R.; De Jong, G.; Scheiner, S.M.; Schlichting, C.D.; Van Tienderen, P.H. Adaptive phenotypic plasticity: Consensus and controversy. Trends Ecol. Evol. 1995, 5, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Weng, L.; Li, M.; Xiao, H. Response of gene expression and alternative splicing to distinct growth environments in tomato. Int. J. Mol. Sci. 2017, 18, 475. [Google Scholar] [CrossRef]
- Pan, T.; Ding, J.; Qin, G.; Wang, Y.; Xi, L.; Yang, J.; Li, J.; Zhang, J.; Zou, Z. Interaction of Supplementary Light and CO2 Enrichment Improves Growth, Photosynthesis, Yield, and Quality of Tomato in Autumn through Spring Greenhouse Production. HortScience 2019, 54, 246–252. [Google Scholar] [CrossRef]
- Angmo, P.; Phuntsog, N.; Namgail, D.; Chaurasia, O.P.; Stobdan, T. Effect of shading and high temperature amplitude in greenhouse on growth, photosynthesis, yield and phenolic contents of tomato (Lycopersicum Esculentum Mill.). Physiol. Mol. Biol. Plants 2021, 27, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Riga, P.; Anza, M.; Garbisu, C. Tomato quality is more dependent on temperature than on photosynthetically active radiation. J. Sci. Food Agric. 2008, 88, 158–166. [Google Scholar] [CrossRef]
- Maršić-Kacjan, N.; Gašperlin, L.; Abram, V.; Budič, M.; Vidrih, R. Quality parameters and total phenolic content in tomato fruits regarding cultivar and microclimatic conditions. Turk. J. Agric. For. 2011, 35, 185–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).